- 1Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy
- 2Unit of Pediatric Genetics and Immunology, Department of Pediatrics, University of Messina, Messina, Italy
- 3Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Childhood asthma is actually defined as a heterogeneous disease, including different clinical variants and partially sharing similar immune mechanisms. Asthma management is mainly focused on maintaining the control of the disease and reducing the risk of adverse outcomes. Most children achieve good control with standard therapies, such as low doses of inhaled corticosteroids (ICS) and/or one or more controller. These medications are targeted to suppress bronchial inflammation and to restore airway responsiveness. However, they are not disease-modifying and do not specifically target inflammatory pathways of asthma; in addition, they are not significantly effective in patients with severe uncontrolled asthma. The aim of this review is to update knowledge on current and novel therapeutic options targeted to immunomodulate inflammatory pathways underlying pediatric asthma, with particular reference on biologic therapies.
Introduction
Asthma represents a major health problem in the pediatric population worldwide. Childhood asthma is actually defined as a heterogeneous disease, including different clinical variants (phenotypes) and partially sharing similar immune mechanisms (1). Asthma management is mainly focused on maintaining the control of the disease and reducing the risk of asthma-related exacerbations and deaths (2). Most children achieve good control with standard therapies, such as low doses of inhaled corticosteroids (ICS) and/or one or more controller (2). These medications are targeted to suppress bronchial inflammation and to restore airway responsiveness. However, they are not disease-modifying and the inflammation return on their discontinuation; in addition, they are not significantly effective in patients with severe uncontrolled asthma (3, 4).
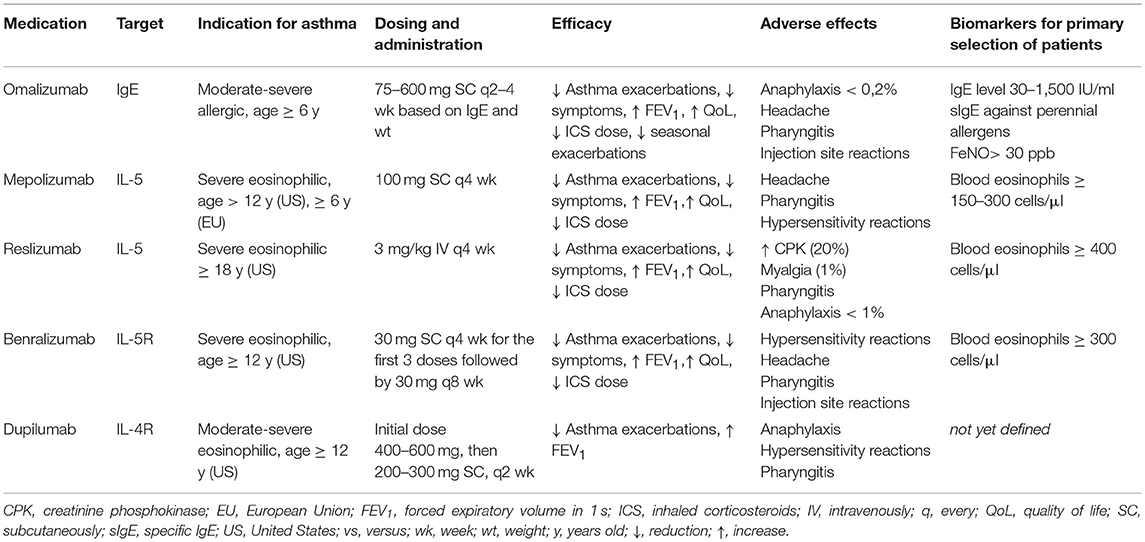
A number of individualized therapies, such as biologics, are available as add-on treatment in adult asthmatic patients with severe uncontrolled symptoms (5). When considering children, only few biologics have been approved and there is limited experience in this population (6) (Table 1).
Cellular and Molecular Mechanisms in Asthma
Asthma phenotypes are closely related to airway inflammatory pathways (endotypes), which are determined by numerous cell types, mediators, and immune pathways (7, 8). Two major distinct inflammatory endotypes have been recognized so far: T (Type) 2 and non-T2 endotype.
Eosinophilic inflammation is predominant in T2 endotype and is driven by allergy in more than a half of patients (9). When exposed to allergens and/or to microbes and pollutants, airway epithelial cells release cytokine mediators such as interleukin (IL)-33, IL-25, and thymic stromal lymphopoietin (TSLP)—the so called “alarmins”—that initiate multiple signaling pathways (10). While interleukin (IL)-33 and IL-25 mainly activate type 2 innate lymphoid cells (ILC2s), TSLP also primes dendritic cells (DCs) to promote T2 immunity by activating CD4+ Th2 cells and B cells. CD4+ Th2 cells are the principal driver of eosinophilic airway inflammation by generating abundant quantities of IL-4, IL-5, and IL-13 (Th2 cytokines) (7). IL-4 activates B cells to differentiate into plasma cells that generate immunoglobulin E (IgE) required for mast cell responses to allergens; IL-5 promotes eosinophil differentiation and survival; IL-13, IL-4, and other inflammatory mediators promote goblet cell overexpression, increased mucus secretion, as well as airway hyperresponsiveness, then contributing to the hallmarks of asthma pathophysiology (7, 11–13). In allergic asthma, allergen-specific IgE contributes to the amplification of this inflammatory pathway by inducing a delayed phase reaction characterized by the massive influx of eosinophils and other inflammatory cells (7). Moreover, IgE seems to be directly involved in the pathogenesis of airway remodeling, since the expression of its receptors has been recently demonstrated on airway smooth muscle cells (14, 15).
Non-T2 asthma is marked by a neutrophilic cellular infiltrate or few cells (pauci-granulocytic). The neutrophilic inflammation is mainly the results of a mixed Th1 and Th17 cytokine milieu (IL-8, IL-17A, IL-22) triggered by infections and/or inhaled pollutants, while the pauci-granulocytic inflammatory profile is still largely unknown (13). A role of systemic and metabolic inflammation has also been supposed to contribute to non-T2 endotype, since it is prevalent in obese and older patients (16).
This dual endotype categorization has proven to be clinically relevant and, in particular, eosinophilia directly correlates with corticosteroid response, onset of disease and symptoms (17). The identification of the inflammatory endotype and the prediction of a specific treatment response rely on validated non-invasive biomarkers, such as eosinophils (in blood and sputum), IgE, fractional exhaled nitric oxide (FeNO), and serum periostin, which are available in clinical practice and are related to T2 asthma endotype (7, 17–19). Even though both pathways may coexist in some few patients, the T2 endotype is found in the majority of asthma patients, in particular in children (7). Thus, the development of novel biologic treatments has been focused on this component of the inflammatory pathways.
Immunomodulating Approaches in Pediatric Asthma
One of the primary aims of asthma treatment is the reversal of existing airway inflammation, hence, the therapeutic strategies have focused on either reducing inflammatory cells and mediators or blocking their effects.
The local immune effects of ICS in asthmatic airways include anti-inflammatory gene activation and switching off inflammatory gene expression which affect the synthesis of inflammatory and anti-inflammatory cytokines/chemokines, receptors, enzymes, and adhesion molecules, and result in decreased inflammatory cell survival and recruitment (20). Moreover, ICS increase the β2-receptor expression, function, and signaling, thus, prevent the development of tolerance to β2-agonists in asthmatic patients treated with β-agonist bronchodilators (21). ICS also act by decreasing vascular permeability and the release of secretagogue from macrophages, reducing local edema and mucus secretion. Finally, the prevention of the cytotoxic effect of the major basic protein (MBP) released from eosinophils is the most common ICS-mediated eosinopenic effects (20).
However, the ICS fail to inhibit leukotriene-induced airway inflammation, thus, the use of leukotrienes (LT) modifiers can be crucial in asthma management, offering additional clinical benefit. Two different types of LT modifiers have been identified: LT synthesis inhibitors and cysteinyl leukotriene receptor (CysLT) antagonists. By interrupting the 5-lipoxygenase pathway, the LT synthesis inhibitors hinder the synthesis of all leukotrienes. The CysLT antagonists influence the bronchoconstrictor and pro-inflammatory activity of cysteinyl leukotrienes (LTC4, LTD4 LTE4) within the asthmatic airway (22).
Novel interventional approaches to modulate the pathogenic immune response have demonstrated significant benefits in preventing the development of asthma and in treating established asthmatic disease (23).
Allergen Immunotherapy
Currently, allergen immunotherapy (AIT) is the only disease-modifying treatment strategy for allergic patients (24). It is proven to be the only therapy that alters the natural history of allergic disease, prevents its progression and the development of new sensitizations and may even delay the development of asthma in patients with allergic rhinitis (25). AIT demonstrated to induce a persistent immunological and clinical tolerance toward the causal allergen, through molecular mechanisms involving both innate and adaptive immunity (26–28). In particular, AIT upregulates allergen-specific T-regulatory (Treg) cells and B-regulatory (Breg) cells, inhibiting the activation of CD4+ Th2 lymphocytes, suppressing allergic inflammation and shifting toward a Type 1-mediated immune response, releasing cytokines, interleukin (IL)-10 and transforming growth factor-β (TGF-β) (27, 29–31). Several studies showed a significant reduction in asthma symptoms, reduction in use of medications and improvement in bronchial hyperreactivity following AIT (32). In children, randomized clinical trials (RCTs) and meta-analyses confirmed the clinical effectiveness of AIT in asthma, possibly even in long-term (33, 34); AIT may also contribute to delay or prevent the onset of asthma in children (35–37). More recently, after the positive clinical results of a Phase III clinical trial evaluating the treatment of asthma with standardized quality (SQ) house dust mite (HDM) sublingual (SLIT)-tablet, GINA (Global Initiative for Asthma) endorsed this specific SLIT product in adolescents and adults with mild-to-moderate and controlled HDM-asthma (2). According to this recommendation, severe asthma represents a clinical contraindication for AIT (2, 38). Although biologics in severe asthma and AIT in allergic diseases target two different populations, biologic therapies have been coupled to AIT to treat asthmatic patients at high risk of adverse reactions in a novel experimental therapeutic approach (39).
Biologic Therapies
The development of the biological drugs has revolutionized the therapeutic approach to asthma, particularly in patients with severe disease and resistant to standard treatment. These drugs are characterized by an innovative and highly selective mechanism of action, based on the targeted inhibition of specific molecular or cellular targets directly involved in the pathogenesis of airway inflammation (5).
Biologics for T2 Asthma in Children
Anti-IgE
The pharmacological blockade of IgE represents a milestone in the field of biologic treatments for severe asthma (40). Omalizumab is the first available humanized monoclonal anti-IgE with the pediatric indication (age ≥ 6 years) for severe asthma (41). It is indicated as add-on treatment for children with severe allergic asthma with elevated serum IgE (>30 and <1,500 IU/ml) and serum IgE positivity for at least one aeroallergen (42). Omalizumab is recommended to be administered as a subcutaneous (SC) injection every 2–4 weeks based on body weight and serum IgE level (43). After binding circulating IgE, omalizumab decreases IgE levels, inhibits IgE binding with its receptors, and downregulates the expression of IgE receptors on mast cells, basophils and dendritic cells (41). Overall, this results in decreased release of inflammatory mediators related to the allergic response. Several RCTs consolidated the efficacy and safety of omalizumab in the pediatric population (44–47), leading to its final registration more than 10 years ago. Omalizumab demonstrated to be effective in reducing the number of asthma exacerbations requiring oral corticosteroids (OCS), and the need of hospitalizations in severe asthmatic children; these effects resulted in improvement of asthma control and quality of life of these children and their families (48). A significant decrease in the number of seasonal exacerbations triggered by respiratory viruses has been also recently reported in treated subjects, probably due to the restoration of antiviral defenses (in particular type I interferon production) (47, 49, 50). Observational studies conducted in children with poorly controlled asthma demonstrated a significant improvement in asthma control as well as a huge decrease in exacerbation and hospitalization rates over 2 years of therapy (51–54); the impact of this biologic was also observed on the discontinuation of daily OCS, the decrease of ICS dose and a slight improvement of lung function (51–54). Safety data derived from clinical trials, observational studies and post-marketing analyses showed that omalizumab is characterized by a very good profile of safety and tolerability in children and adolescents (55–59). In particular, injection site reactions, usually of mild-to-moderate severity and short in duration, were the most reported side effects (55, 56); anaphylactic events have not been observed in pediatric studies, unlike those in adults and adolescents (51–53, 59). Finally, there is no evidence to support an increased risk of malignancy in patients treated with omalizumab (57, 58); however, a long-term monitoring of treated patients is still required to confirm the good safety profile.
Despite the widespread clinical use of omalizumab in the pediatric population, a number of questions remain unanswered based on available scientific data. The profile of the best responding patient phenotype has not been identified yet: having severe asthma with multiple allergic comorbidities associated with raised blood eosinophil count, high levels of total IgE and fractional exhaled nitric oxide (FeNO) seem to be predictive of a positive clinical response in the pediatric population (60, 61). Age < 6 years, IgE > 1,500 IU/ml, and non-allergic severe asthma together represent the current limit for omalizumab use in children, as well as in adolescents and adults. Preliminary studies have been conducted in non-allergic children (62) and children with excessively high IgE levels (63) with positive encouraging results; a single study on uncontrolled asthmatic children <6 years is actually ongoing (Preventing Asthma in High Risk Kids study, NCT02570984) with the aim to evaluate the disease-modifying effect of anti-IgE therapy. The optimal duration of omalizumab therapy has not been determined, but it is considered an effective treatment approach to continue treatment in responders for at least 2 years, based on observational data in children (64, 65). Finally, its long-lasting effect after suspension has been not yet clearly defined. The definition of targeted courses of therapy may represent the starting point for optimizing the cost-effectiveness of this biologic treatment in the pediatric population.
IL-5
Mepolizumab, a murine humanized IgG1 monoclonal antibody, was the first anti eosinophil-targeted molecular therapy to be validated in patients with severe asthma. Mepolizumab acts against circulating IL-5, preventing the IL-5/IL-5Rα interaction on the surface of eosinophils, and, thereby, affecting the release and growth of eosinophils (66).
In 2015, mepolizumab has been approved as add-on maintenance therapeutic option for the treatment of severe eosinophilic asthma in patients who are 12 years and older, and then in pediatric population 6 years old and above (67). The recommended dose of mepolizumab is 100 mg for adults and children older than 12 years of age, and 40 mg for children aged 6 to 11 years old, both administered subcutaneously (66, 67).
Mepolizumab has demonstrated favorable efficacy profile in decreasing the number of asthma exacerbations, improving lung function, asthma control and quality-of-life (QoL) scores, as well as significantly reducing OCS use (68–70). Interestingly, all these outcomes were maintained over time, although, lung function, expressed in terms of forced expiratory volume in 1 s (FEV1), was gradually decreasing to approximately baseline, reflecting a stabilization of lung function over the course of the treatment period (71–73).
Treatment response criteria and duration of therapy have been the subject of considerable debate. Exacerbation rate, OCS treatment, blood eosinophil count, and lung function have been proposed as treatment response criteria (74). Besides, the decision to continue mepolizumab treatment should be annually evaluated and based on assessment of at least 50% reduction in exacerbation frequency (75).
Regarding safety, mepolizumab appeared well-tolerated, with the most commonly described adverse events being injection-site reactions, airway infections, exacerbations of asthma, headaches, and fatigue (69, 70, 72, 76).
There is limited data on the safety of mepolizumab in children. One case of histiocytic necrotizing lymphadenitis and varicella have been reported by Food and Drug Administration (FDA) in postmarket surveillance of adverse events. However, the association between mepolizumab and these two events still remains uncertain (77).
IL-4/13
IL-4 and IL-13 are crucial Th2 cytokines directly involved in the inflammatory remodeling occurring in the airways of asthmatic patients (78). Ig switching from class M to E antibodies, airway recruitment of eosinophils, basophils, lymphocytes, and monocytes are the principle effects mediated by IL-4 and IL-13. Also, while IL-4 mediates polarization and maintenance of T2-type immune response, IL-13 induces airway goblet cell hyperplasia.
Hence, the possibility of blocking or modulating IL-4 and/or IL-13 aroused great interest among researchers aiming to gain therapeutic benefit in asthma.
Currently, dupilumab is the only available biologic drug targeting both IL-4 and IL-13, approved to treat patients with moderate-to-severe asthma and airway or peripheral eosinophilia (78).
Dupilumab appeared to improve both FEV1 and asthma control as well as to decrease T2-inflammation and asthma exacerbation rate (79, 80). In QUEST trial, patients aged over 12 years with uncontrolled, moderate-to-severe asthma were randomized to receive dupilumab or placebo. Over the 52 week treatment period, dupilumab significantly reduced the severe asthma exacerbations rate, especially in patients showing higher baseline eosinophilia (>300 cells/mm3) and FeNO values major than 25 ppb (81). The Liberty Asthma VENTURE (82) demonstrated the effectiveness of dupilumab in reducing OCS use in 210 patients with CS-dependent severe asthma (dupilumab groups:placebo group = −70.1%:−41.9%, respectively). Moreover, 48% of patients in dupilumab group completely discontinuing OCS use.
In April 2017, a randomized, double-blind, placebo-controlled, parallel group study to evaluate the efficacy and safety of dupilumab in children 6 to <12 years of age with uncontrolled persistent asthma has been started and not yet concluded (83).
Recently, a systematic review by Zayed et al. evaluated the results of four placebo controlled RCTs, assessing dupilumab safety (84); injection site reactions were commonly described in experimental group. A transient blood eosinophilia was also recorded but it was not associated to any consequence or adverse effect.
Biologics directed exclusively against IL-4 or IL-13 have been also investigated, however both pitrakinra (anti-IL-4R) (85) and tralokinumab (86) and lebrikizumab (anti-IL13) (87) failed to show consistent benefits for the treatment of severe and uncontrolled asthma.
Biologics for non-T2 Asthma in Children
Anti IL-25
The evidence of IL-25-mediated Th2 cell differentiation, increase in production of IL-4, IL-5, and IL-13, elevated IgE and IgG levels, eosinophil infiltration, goblet cell hyperplasia and mucus hypersecretion, provided the proof for the role of IL-25 into asthma pathogenesis (88–90). IL-25 is a Th2 cell-derived cytokine belonging to IL-17 family. Following its release from mast cells, eosinophils, basophils, and alveolar macrophages, IL-25 binds IL-17 receptor and induces Th2 cell-mediated inflammatory response in the airways. In a vivo model, IL-25 was able to cause airway inflammation and remodeling as well as hypersensitivity (89, 91). By blocking IL-25, a significant prevention of airway hyperresponsiveness and a minor eosinophil infiltration into the lung tissue as well as goblet cell hyperplasia were also noted (89).
However, neutralizing IL-25 activity showed partial efficacy into modulate the airways smooth muscle (91).
To date, no clinical trials are investigating the potential role of anti-IL-25 in asthmatic patients.
Anti IL-33
Bronchial epithelial cells are also considered the primary source of IL-33, an IL-1-like epithelial-derived cytokine. In response to infectious or inflammatory stimulus, IL-33 binds its receptor ST2 on mast cells; and it stimulates both the Th2-associated cytokines release as well as the Th2/IL-31 and Th17 axis (92). Moreover, acting synergistically with other cytokines such as TSLP and IL-17, IL-33 can induce a pulmonary inflammation, which was found to be glucocorticoid-resistant (93). The critical role of IL-33 was confirmed by GWAS studies showing that IL-33 and ST2 genes were significantly associated with asthma (94). Also, sputum IL-33 values reflected disease severity; higher IL-33 levels were detected in patients with more severe disease (95).
Currently, one phase 1 trial [AMG 282 (RG 6149)] and one phase 2 trial (ANB020) are ongoing in patients affected by asthma (96).
Anti Thymic Stromal Lymphopoietin (TSLP)
Following inflammatory or infectious injury, and/or allergen exposure, lung derived epithelial cells, airway smooth muscle cells, mast cells, macrophages, granulocytes, and dendritic cells, release TSLP, a cytokine belonging to IL-2 family. Via interaction with its receptor, TSLP amplifies the Th2 polarization causing airway and blood eosinophilia, cells recruitment (mast cells, basophils, and dendritic cells), differentiation of naive T cells into Th2 cells, and proinflammatory cytokines release (97). Several genetic analyses have linked TSLP to Th2-polarized immunity and asthma (98). Bronchial epithelial cells from asthmatics patients express higher TSLP levels than healthy subjects, and, moreover, TSLP expression in the bronchial epithelium and submucosa was correlating with basal membrane thickness, thus, also with disease severity (99).
In a double-blind, placebo-controlled study, 31 patients (age range, 8 to 60 years) with mild asthma were randomized to undergo to 3 monthly doses of AMG 157, a human anti-TSLP monoclonal IgG2, or placebo treatment for 12 weeks. When compared to placebo group, AMG 157 group reported a significant decrease in allergen-induced bronchoconstriction and in systemic and airway inflammation (100). Successively, a phase 2, randomized, double-blind, placebo-controlled trial, enrolling adult patients affected by mild to moderate uncontrolled asthma assessed the efficacy and safety of tezepelumab (AMG 157/MEDI9929), an human IgG2 monoclonal antibody. Tezepelumab administration was associated with a minor annualized asthma exacerbation rate and g a higher increase in prebronchodilator FEV1 (101). The percentage of mild to serious adverse events was similar among experimental and placebo arms (101).
To date, a new clinical trial evaluating the effects of anti-TSLP in adult patients with asthma (UPSTREAM) is ongoing (102).
Anti IL-17 and Anti-tumor Necrosis Factor (TNF)-α
Several studies demonstrated that IL-17 family of cytokines actively contributes to airway inflammation in non T2 asthma (13). In particular, airway concentration of IL-17 and its related cytokines (IL-17A and IL-25) are upregulated in patients with uncontrolled asthma (13); their levels have been positively correlated to neutrophilic inflammation and asthma severity (13, 103, 104). High levels of serum IL-17 have been also detected in children with asthma and, together with IL-17+ T cells, have been associated with asthma severity in children (105, 106). Likewise, levels of Tumor Necrosis Factor (TNF)-α are increased in either the blood or sputum of patients with neutrophilic asthma, exerting major biological effects on airway inflammation, remodeling, and hyper responsiveness (107, 108). These patients experience persistent symptoms and are prone to frequent exacerbations, which better respond to antibiotics (such as macrolides) rather than to corticosteroids (109). Accordingly, therapeutic strategies to modulate neutrophilic function have been proposed to improve clinical outcomes in non T2 asthma. Cytokine-targeted strategies inhibiting IL-17 and TNF-α receptor signaling both failed to be effective in asthma treatment. Brodalumab (AMG 827), a human anti-IL-17 receptor A monoclonal antibody, demonstrated marginal therapeutic benefit in two Phase 2 studies conducted in adult patients with moderate to severe asthma (110, 111). Golimumab (CNTO148), a human monoclonal antibody against TNF-α, failed to achieve significant treatment effect and demonstrated an unfavorable risk-benefit ratio in adult patients with severe asthma (112).
No ongoing trials are available in adolescents and in children with neutrophilic severe asthma.
Conclusion
Novel biologic therapies are available as add-on treatment for severe and uncontrolled asthma in adult population. However, when considering special patient populations, such as children, limited treatment options have been approved, as omalizumab and mepolizumab. Moreover, uncertainties regarding optimal treatment duration, ability to modify the disease course, approach to discontinuation, and long-lasting effects still remain unsolved. These gaps are deeper in non-T2 asthma for which the clinical development of the biologic drugs is still in primeval stage. Finally, the wide interpersonal variability in response to biologic treatment confirms the complex mechanisms underlying asthma and lets hypothesize that probably not a single biologic but a “cocktails” of biologics could be a more appropriate treatment approach, providing the possibility to block or influence two or more key pathways, thus, representing a novel and promising strategy to immunomodulate asthma.
Author Contributions
All authors made substantial contribution to the conception of the work. RC, AM, TF, and IB reviewed the literature on the subject. AL and SM drafted the final version of the manuscript. AL, SM, and GM revised it critically for important intellectual content. All authors finally approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. (2018) 391:350–400. doi: 10.1016/S0140-6736(17)30879-6
2. Pocket Guide for Asthma Management and Prevention. GINA 2019 update. Available online at: https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf (accessed Apr 20, 2019).
3. Bush A, Fleming L, Saglani S. Severe asthma in children. Respirology. (2017) 22:886–97. doi: 10.1111/resp.13085
4. Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: definition and limits of asthma control in the pediatric population. Front Pediatr. (2018) 6:170. doi: 10.3389/fped.2018.00170
5. Pepper AN, Renz H, Casale TB, Garn H. Biologic therapy and novel molecular targets of severe asthma. J Allergy Clin Immunol Pract. (2017) 5:909–16. doi: 10.1016/j.jaip.2017.04.038
6. Selby L, Saglani S. Severe asthma in children: therapeutic considerations. Curr Opin Allergy Clin Immunol. (2019) 19:132–40. doi: 10.1097/ACI.0000000000000521
7. Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL, et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol. (2018) 31:44–55. doi: 10.1089/ped.2018.0886
8. Manti S, Brown P, Perez MK, Piedimonte G. The role of neurotrophins in inflammation and allergy. Vitam Horm. (2017)104:313–41. doi: 10.1016/bs.vh.2016.10.010
9. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. (2009) 180:388–95. doi: 10.1164/rccm.200903-0392OC
10. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. (2015) 16:45–56. doi: 10.1038/ni.3049
11. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. (2015) 15:57–65. doi: 10.1038/nri3786
12. Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. (2017) 17:665–78. doi: 10.1038/nri.2017.86
13. Samitas K, Zervas E, Gaga M. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulm Med. (2017) 23:48–55. doi: 10.1097/MCP.0000000000000342
14. Redhu NS, Gounni AS. The high affinity IgE receptor (FcεRI) expression and function in airway smooth muscle. Pulm Pharmacol Ther. (2013) 26:86–94. doi: 10.1016/j.pupt.2012.04.004
15. Roth M, Zhong J, Zumkeller C, S'ng CT, Goulet S, Tamm M. The role of IgE-receptors in IgE-dependent airway smooth muscle cell remodelling. PLoS ONE. (2013) 8:e56015. doi: 10.1371/journal.pone.0056015
16. Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. (2012) 142:86–93. doi: 10.1378/chest.11-1838
17. Bush A. Pathophysiological mechanisms of asthma. Front Pediatr. (2019) 7:68. doi: 10.3389/fped.2019.00068
18. Pavlidis S, Takahashi K, Ng Kee Kwong F, Xie J, Hoda U, Sun K, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J. (2019) 53:1800938. doi: 10.1183/13993003.00938-2018
19. Leonardi S, Cuppari C, Lanzafame A, Attardo D, Tardino L, Parisi G, et al. Exhaled breath temperature in asthmatic children. J Biol Regul Homeost Agents. (2015) 29(Suppl. 1):47–54.
20. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. (2011) 163:29. doi: 10.1111/j.1476-5381.2010.01199.x
21. Mak JC, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J Clin Invest. (1995) 96:99–106. doi: 10.1172/JCI118084
22. Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. (2001) 119:1533–46. doi: 10.1378/chest.119.5.1533
23. Licari A, Brambilla I, Sacchi L, Marseglia G, Ciprandi G. Periostin, type 2 biomarker, is not associated with asthma control grade in asthmatic allergic children. Respir Med. (2019) 151:118–20. doi: 10.1016/j.rmed.2019.04.010
24. Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL, et al. New approaches for identifying and testing potential new anti-asthma agents. Expert Opin Drug Discov. (2018) 13:51–63. doi: 10.1080/17460441.2018.1396315
25. Muraro A, Roberts G, Halken S, Agache I, Angier E, Fernandez-Rivas M, et al. EAACI guidelines on allergen immunotherapy: executive statement. Allergy. (2018) 73:739–43. doi: 10.1111/all.13420
26. Tosca MA, Licari A, Olcese R, Marseglia G, Sacco O, Ciprandi G. Immunotherapy and asthma in children. Front Pediatr. (2018) 6:231. doi: 10.3389/fped.2018.00231
27. Ciprandi G, Fenoglio D, Cirillo I, Tosca MA, La Rosa M, Licari A, et al. Sublingual immunotherapy: an update on immunologic and functional effects. Allergy Asthma Proc. (2007) 28:40–3. doi: 10.2500/aap.2007.28.2974
28. La Rosa M, Lionetti E, Leonardi S, Salpietro A, Bianchi L, Salpietro C, et al. Specific immunotherapy in children: the evidence. Int J Immunopathol Pharmacol. (2011) 24:69–78. doi: 10.1177/03946320110240S413
29. van de Veen W, Wirz OF, Globinska A, Akdis M. Novel mechanisms in immune tolerance to allergens during natural allergen exposure and allergen-specific immunotherapy. Curr Opin Immunol. (2017) 48:74–81. doi: 10.1016/j.coi.2017.08.012
30. Mahler V, Esch RE, Kleine-Tebbe J, Lavery WJ, Plunkett G, Vieths S, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. (2019) 143:813–28. doi: 10.1016/j.jaci.2019.01.024
31. Alterio T, Manti S, Colavita L, Marseglia L, Sturiale M, Miraglia Del Giudice M, et al. Sublingual immunotherapy in children: state of art. J Biol Regul Homeost Agents. (2015) 29(Suppl. 1):120–4.
32. Cuppari C, Leonardi S, Manti S, Filippelli M, Alterio T, Spicuzza L, et al. Allergen immunotherapy, routes of administration and cytokine networks: an update. Immunotherapy. (2014) 6:775–86 doi: 10.2217/imt.14.47
33. Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. (2017) 72:1825–48. doi: 10.1111/all.13208
34. Masuyama K, Okamoto Y, Okamiya K, Azuma R, Fujinami T, Riis B, et al. Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet in Japanese children. Allergy. (2018) 73:2352–63. doi: 10.1111/all.13544
35. Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. (2018) 5:275–90. doi: 10.1007/s40521-018-0176-2
36. Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sorensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. (2018) 141:529–38 e13. doi: 10.1016/j.jaci.2017.06.014
37. Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol. (2002) 109:251–6. doi: 10.1067/mai.2002.121317
38. Kristiansen M, Dhami S, Netuveli G, Halken S, Muraro A, Roberts G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2017) 28:18–29. doi: 10.1111/pai.12661
39. Pitsios C, Demoly P, Bilò MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. (2015) 70:897–909. doi: 10.1111/all.12638
40. Ciprandi G, Marseglia GL, Castagnoli R, Valsecchi C, Tagliacarne C, Caimmi S, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. (2015) 11:1321–33. doi: 10.1586/1744666X.2015.1086645
41. Licari A, Marseglia G, Castagnoli R, Marseglia A, Ciprandi G. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov. (2015) 10:1033–42. doi: 10.1517/17460441.2015.1048220
42. Licari A, Marseglia A, Caimmi S, Castagnoli R, Foiadelli T, Barberi S, et al. Omalizumab in children. Paediatr Drugs. (2014) 16:491–502. doi: 10.1007/s40272-014-0107-z
43. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. doi: 10.1183/09031936.00202013
44. Chipps BE, Lanier B, Milgrom H, Deschildre A, Hedlin G, Szefler SJ, et al. Omalizumab in children with uncontrolled allergic asthma: review of clinical trial and real-world experience. J Allergy Clin Immunol. (2017) 139:1431–44. doi: 10.1016/j.jaci.2017.03.002
45. Brodlie M, McKean MC, Moss S, Spencer DA. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch Dis Child. (2012) 97:604–9. doi: 10.1136/archdischild-2011-301570
46. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. (2011) 364:1005–15. doi: 10.1056/NEJMoa1009705
47. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. (2009) 124:1210–16. doi: 10.1016/j.jaci.2009.09.021
48. Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. (2015) 136:1476–85. doi: 10.1016/j.jaci.2015.09.008
49. Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. (2015) 26:551–6. doi: 10.1111/pai.12405
50. Cardet JC, Casale TB. New insights into the utility of omalizumab. J Allergy Clin Immunol. (2019) 143:923–26.e1. doi: 10.1016/j.jaci.2019.01.016
51. Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. (2018) 141:1735–43.e9. doi: 10.1016/j.jaci.2017.07.035
52. Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. (2013) 42:1224–33. doi: 10.1183/09031936.00149812
53. Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. (2015) 46:856–9. doi: 10.1183/09031936.00008115
54. Licari A, Castagnoli R, Denicolò C, Rossini L, Seminara M, Sacchi L, et al. Omalizumab in children with severe allergic asthma: the italian real-life experience. Curr Respir Med Rev. (2017) 13:36–42. doi: 10.2174/1573398X13666170426094536
55. Pitrez PM, de Souza RG, Roncada C, Heinzmann-Filho JP, Santos G, Pinto LA, et al. Impact of omalizumab in children from a middle-income country with severe therapy-resistant asthma: a real-life study. Pediatr Pulmonol. (2017) 52:1408–13. doi: 10.1002/ppul.23845
56. Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. (2003) 91:182–8. doi: 10.1016/S1081-1206(10)62175-8
57. Milgrom H, Fowler-Taylor A, Vidaurre CF, Jayawardene S. Safety and tolerability of omalizumab in children with allergic (IgE-mediated) asthma. Curr Med Res Opin. (2011) 27:163–9. doi: 10.1185/03007995.2010.539502
58. Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. (2012) 129:983–9.e6. doi: 10.1016/j.jaci.2012.01.033
59. Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. (2014) 134:560–7.e4. doi: 10.1016/j.jaci.2014.02.007
60. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. (2014) 1:CD003559. doi: 10.1002/14651858.CD003559.pub4
61. Busse W, Haselkorn T, Rosén K, Trzaskoma BL, Ortiz B, Szefler SJ. Greater treatment benefit with omalizumab in children with increased asthma severity: exploratory analyses from the Inner-City Anti-IgE Therapy for Asthma (ICATA) study. J Allergy Clin Immunol. (2018) 141:AB14. doi: 10.1016/j.jaci.2017.12.045
62. Sesé L, Schneider M, Bourgoin M, Saint-Pierre P, Lambert N, Guiddir T, et al. Asthma with multiple allergic comorbidities is associated with complete response to omalizumab. Clin Exp Allergy. (2019) 49:733–5. doi: 10.1111/cea.13373
63. Bourgoin-Heck M, Amat F, Trouvé C, Bernard A, Magny JP, Lambert N, et al. Omalizumab could be effective in children with severe eosinophilic non-allergic asthma. Pediatr Allergy Immunol. (2018) 29:90–3. doi: 10.1111/pai.12813
64. Wang KY, Sindher SB, Stinson R, DaVeiga SP. Efficacy and safety of omalizumab in pediatric patients with high immunoglobulin E levels: a case series. Allergy Asthma Proc. (2018) 39:289–91. doi: 10.2500/aap.2018.39.4146
65. Deschildre A, Roussel J, Drumez E, Abou-Taam R, Rames C, Le Roux P, et al. Omalizumab discontinuation in children with severe allergic asthma: an observational real-life study. Allergy. (2018) 74:999–1003. doi: 10.1111/all.13678
66. Nucala (mepolizumab) for Injection [Prescribing Information]. Research Triangle Park, NC: GlaxoSmithKline (2015).
67. NUCALA® (mepolizumab) EMA Approval. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/nucala (accessed May 4, 2019).
68. Pavord ID, Kom S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. (2012) 380:651–9. doi: 10.1016/S0140-6736(12)60988-X
69. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. (2014) 371:1198–207. doi: 10.1056/NEJMoa1403290
70. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. (2014) 371:1189–97. doi: 10.1056/NEJMoa1403291
71. Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. (2016) 38:2058–70.e1. doi: 10.1016/j.clinthera.2016.07.010
72. Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. (2018) 143:1742–51.e7. doi: 10.1016/j.jaci.2018.09.033
73. Henriksen DP, Bodtger U, Sidenius K, Maltbaek N, Pedersen L, Madsen H, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis. Eur Clin Respir J. (2018) 5:1536097. doi: 10.1080/20018525.2018.1536097
74. Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. (2018) 18:119. doi: 10.1186/s12890-018-0689-2
75. National Institute for Health and Clinical Excellence. Mepolizumab for Treating Severe Refractory Esoinophilic Asthma. Technol Apprais Guide (2016) (accessed May 12, 2017).
76. Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. (2007) 176:1062–71. doi: 10.1164/rccm.200701-085OC
77. Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology. Nucala (mepolizumab) Pediatric Postmarketing Pharmacovigilance Review (2017).
78. Santini G, Mores N, Malerba M, Mondino C, Anzivino R, Macis G, et al. Dupilumab for the treatmentof asthma. Expert Opin Investig Drugs. (2017) 26:357–66. doi: 10.1080/13543784.2017.1282458
79. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. (2013) 368:2455–66. doi: 10.1056/NEJMoa1304048
80. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. (2016) 388:31–44. doi: 10.1016/S0140-6736(16)30307-5
81. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. (2018) 378:2486–96. doi: 10.1056/NEJMoa1804092
82. Sanofi Evaluation of Dupilumab in Patients With Severe Steroid Dependent Asthma (VENTURE). Available online at: https://clinicaltrials.gov/ct2/show/NCT02528214. NLM identifier: NCT02528214 (accessed May 4, 2019).
83. Sanofi Evaluation of Dupilumab in Children With Uncontrolled Asthma (VOYAGE). Available online at: https://clinicaltrials.gov/ct2/show/NCT02948959. NLM identifier: NCT02948959 (accessed May 4, 2019).
84. Zayed Y, Kheiri B, Banifadel M, Hicks M, Aburahma A, Hamid K, et al. Dupilumab safety and efficacy in uncontrolled asthma: a systematic review and meta-analysis of randomized clinical trials. J Asthma. (2018). doi: 10.1080/02770903.2018.1520865 [Epub ahead of print].
85. Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol. (2016) 117:121–5. doi: 10.1016/j.anai.2016.05.016
86. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. (2015) 3:692–701. doi: 10.1016/S2213-2600(15)00197-6
87. Hanania N, Korenblat P, Chapman K, Bateman E, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. (2016) 4:781–96. doi: 10.1016/S2213-2600(16)30265-X
88. Yao XJ, Huang KW, Li Y, Zhang Q, Wang JJ, Wang W, et al. Direct comparison of the dynamics of IL-25- and ‘allergen’-induced airways inflammation, remodelling and hypersensitivity in a murine asthma model. Clin Exp Allergy. (2014) 44:765–77. doi: 10.1111/cea.12298
89. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. (2007) 120:1324–31. doi: 10.1016/j.jaci.2007.07.051
90. Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. (2006) 118:606–14. doi: 10.1016/j.jaci.2006.04.051
91. Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. (2011) 128:116–24. doi: 10.1016/j.jaci.2011.03.043
92. Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. (2016) 16:676–89. doi: 10.1038/nri.2016.95
93. Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. (2013) 132:676–85.e13. doi: 10.1016/j.jaci.2013.04.012
94. Savenije OE, Mahachie John JM, Granell R, Kerkhof M, Dijk FN, de Jongste JC, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol. (2014) 134:170–7. doi: 10.1016/j.jaci.2013.12.1080
95. Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. (2013) 50:803–9. doi: 10.3109/02770903.2013.816317
96. Lawrence MG, Steinke JW, Borish L. Cytokine-targeting biologics for allergic diseases. Ann Allergy Asthma Immunol. (2018) 120:376–81. doi: 10.1016/j.anai.2018.01.009
97. Mitchell PD, O'Byrne PM. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol Ther. (2017) 169:104–12. doi: 10.1016/j.pharmthera.2016.06.009
98. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genome wide association study of asthma. N Engl J Med. (2010) 363:1211–21. doi: 10.1056/NEJMoa0906312
99. Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lym-phopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. (2005) 174:8183–90. doi: 10.4049/jimmunol.174.12.8183
100. Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. (2014) 370:2102–10. doi: 10.1056/NEJMoa1402895
101. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. (2017) 377:936–46. doi: 10.1056/NEJMoa1704064
102. Effects of Anti-TSLP in Patients With Asthma (UPSTREAM). NCT02698501. Available online at: https://clinicaltrials.gov/ct2/show/NCT02698501 (accessed May 4, 2019).
103. Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol. (2016) 138:413–20.e6. doi: 10.1016/j.jaci.2015.12.1347
104. Ricciardolo FLM, Sorbello V, Folino A, Gallo F, Massaglia GM, Favatà G, et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J Allergy Clin Immunol. (2017) 140:395–406. doi: 10.1016/j.jaci.2016.10.034
105. Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. (2013) 43:1018–26. doi: 10.1111/cea.12119
106. Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol. (2018) 141:2048–60.e13. doi: 10.1016/j.jaci.2017.08.020
107. Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. (2007) 132:1871–5. doi: 10.1378/chest.07-1047
108. Bruijnzeel PL, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease-relevant neutrophil phenotype? J Leukoc Biol. (2015) 98:549–56. doi: 10.1189/jlb.3VMR1214-600RR
109. Panettieri RA Jr. Neutrophilic and Pauci-immune phenotypes in severe asthma. Immunol Allergy Clin North Am. (2016) 36:569–79. doi: 10.1016/j.iac.2016.03.007
110. Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. (2013) 188:1294–302. doi: 10.1164/rccm.201212-2318OC
111. Study, of Efficacy and Safety of Brodalumab Compared With Placebo in Inadequately Controlled Asthma Subjects With High Bronchodilator Reversibility. ClinicalTrials.gov Identifier: NCT01902290. Available online at: https://clinicaltrials.gov/ct2/show/NCT01902290?term=NCT01902290&rank=1 (accessed May 4, 2019).
Keywords: asthma, children, endotypes, biologics, treatment, omalizumab, mepolizumab
Citation: Licari A, Manti S, Castagnoli R, Marseglia A, Foiadelli T, Brambilla I and Marseglia GL (2019) Immunomodulation in Pediatric Asthma. Front. Pediatr. 7:289. doi: 10.3389/fped.2019.00289
Received: 05 May 2019; Accepted: 27 June 2019;
Published: 12 July 2019.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Klaus Tenbrock, RWTH Aachen Universität, GermanyAntonio Condino-Neto, University of São Paulo, Brazil
Copyright © 2019 Licari, Manti, Castagnoli, Marseglia, Foiadelli, Brambilla and Marseglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia Licari, YS5saWNhcmlAc21hdHRlby5wdi5pdA==
†These authors have contributed equally to this work as co-first authors
 Amelia Licari
Amelia Licari Sara Manti
Sara Manti Riccardo Castagnoli
Riccardo Castagnoli Alessia Marseglia1
Alessia Marseglia1 Thomas Foiadelli
Thomas Foiadelli Gian Luigi Marseglia
Gian Luigi Marseglia