- Institute of Preventive Pediatrics, TUM Department of Sport and Health Sciences, Technical University of Munich, Munich, Germany
A physically active lifestyle can prevent cardiovascular disease. Exercise intervention studies in children and adolescents that aim to increase physical activity have resulted in reduced vascular wall thickening and improve cardiovascular function. Here we review the literature that explores the correlations between physical activity, health-related physical fitness, and exercise interventions with various measures of vascular structure and function in children and adolescents. While several of these studies identified improvements in vascular structure in response to physical activity, these associations were limited to studies that relied on questionnaires. Of concern, these findings were not replicated in studies featuring quantitative assessment of physical activity with accelerometers. Half of the studies reviewed reported improved vascular function with increased physical activity, with the type of vascular measurement and the way physical activity was assessed having an influence on the reported relationships. Similary, most of the studies identified in the literature report a beneficial association of health-related physical fitness with vascular structure and function. Overall, it was difficult to compare the results of these studies to one another as different methodologies were used to measure both, health-related physical fitness and vascular function. Likewise, exercise interventions may reduce both arterial wall thickness and increased vascular stiffness in pediatric populations at risk, but the impact clearly depends on the duration of the intervention and varies depending on the target groups. We identified only one study that examined vascular structure and function in young athletes, a group of particular interest with respect to understanding of cardiovascular adaptation to exercise. In conclusion, future studies will be needed that address the use of wall:diameter or wall:lumen-ratio as part of the evaluation of arterial wall thickness. Furthermore, it will be critical to introduce specific and quantitative measurements of physical activity, as intensity and duration of participation likely influence the effectiveness of exercise interventions.
Introduction
Cardiovascular disease (CVD) is currently the leading cause of death worldwide; in 2030, CVD may be the major underlying factor in 22.2 million deaths per year (1). To prevent CVD, the American Heart Association recommends adopting a physically active lifestyle, healthy diet and the avoidance of tobacco use (2). Cardiovascular (CV) parameters, including intima-media thickness (IMT) and pulse-wave-velocity (PWV) as subclinical risk maker for CVD, should be monitored at an early age to identify and follow children and adolecents who are at higher risk for CV events (3).
Carotid IMT (cIMT) is an important surrogate marker of subclinical atherosclerosis. It can be assessed using ultrasound at a young age and long before atherosclerotic symptoms occur. Although the incidence of carotid plaques is low in this age group, cIMT may be elevated in children and adolescents with obesity (4, 5), hypertension (6), diabetes (7), cancer (8), or some types of congenital heart disease (9).
The Association for European Paediatric Cardiology Working Group on Cardiovascular Prevention recommends measuring cIMT in pediatric populations using high-resolution ultrasound in two different angels after the carotid bulb of the left and right common carotid artery with a slightly stretched neck and head turned 45° to the opposite side (10). IMT can also be measured at the abdominal aorta as well as at the femoral artery. Of note, arterial diameters can also be measured; these values will permit one to calculate IMT:diameter-ratio (alternatively wall:lumen-ratio) which expresses the relation of wall thickness (IMT) to vascular diameter or lumen, respectively. These ratios are helpful for determining the full impact of physical activity (PA) and other interventions on vascular structure and function.
Vascular compliance is a measure of arterial function that describes the physical adaptation to the blood volume ejected by the left ventricle (LV) and reflects the ability to maintain constant blood flow (11). Vascular stiffness is the reciprocal of vascular compliance and is defined by different parameters such as elastic modulus or pulse wave velocity (PWV). Vascular stiffness can be measured with ultrasound, oscillometric devices, photoplethysmography, and/or applanation tonometry applied to the aorta, femoral, brachial or carotid arteries. Parameters measured with this devices include compliance, distensibility, stiffness index, reflection index, elastic modulus, augmentation index (AI), and PWV. Pediatric populations with elevated cardiovascular risk can present reduced compliance or increased stiffness, for example children with hypertension (12), obesity (12, 13), hypercholesterolemia (14), metabolic syndrome (15), diabetes (16), and congenital heart disease (17).
PA and health-related physical fitness (HRPF) are both associated with a lower risk of CVD in adulthood (18, 19). The results of several recent studies suggest a critical role for PA and HRPF in promoting healthy vascular structure and function in children and adolescents (20, 21). Interventions that include endurance or resistance exercises have also been introduced in an attempt to improve impaired vascular structure and function in pediatric populations at risk (22, 23).
This review provides an overview of the relationship between PA and HRPF with vascular structure and function in children and adolescents. Exercise is a planned and structured PA to improve activity level and HRPF. Therefore, this review further discusses the effect of exercise on vascular structure and function in intervention studies and young athletes. We searched in several databases for articles published between 2005 and June 2019 using the keywords “intima-media thickness,” “arterial structure,” “arterial diameter,” “vascular function,” “arterial stiffness,” “exercise,” “physical activity,” “physical fitness,” “cardiorespiratory fitness,” “athlete,” “child*,” or “adolesc*.” We did not include a discussion of biochemical parameters of endothelial function in the review.
Physical Activity
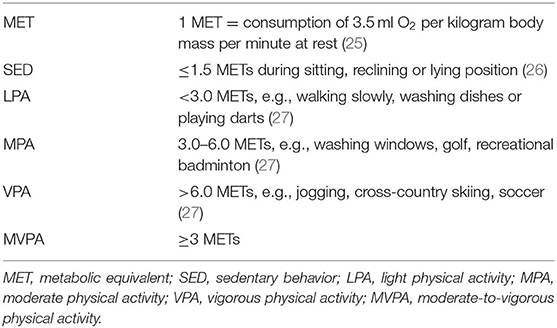
PA is any movement of the body that is produced by skeletal muscles and that requires energy expenditure (24). Regular PA has a cardioprotective effect on health not only in youth but also later in life. Therefore, children should adopt a physically active lifestyle at an early age and maintain it throughout life. The World Health Organization recommends that children and adolescents undertake moderate-to-vigorous physical activity (MVPA) for at least 60 min per day (Table 1) (24). MVPA is commonly assessed as self-reporting using specific questionnaires. While this method is simple, the data that result may be inaccurate due to recall bias (28). Accelerometer and related wearable instruments permit collection of more objective data, as these devices do not depent on self-assessment. PA measurements vary by design and as per the individual goal for the research program (29).
Vascular Structure
We identified six published studies that explored correlations between PA and vascular structure (Table 2); four of these reports are included in the literature review published in 2016 by Cayres et al. (41). Three of the six studies used accelerometers to measure PA (20, 33, 37) and three relied on questionnaires (31, 35, 36).
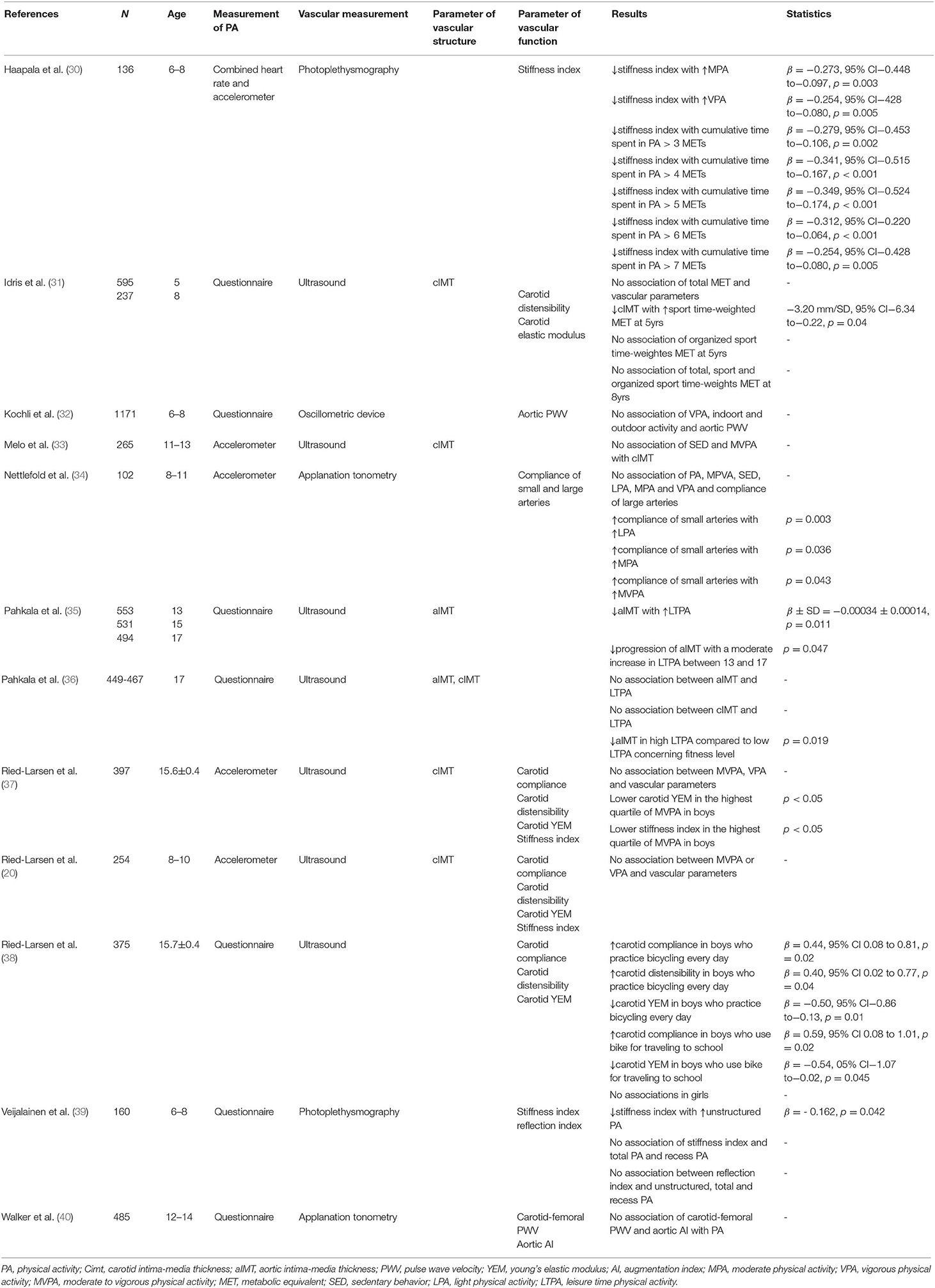
Table 2. Overview of studies that evaluated associations of physical activity and vascular structure and function.
Among the results of the questionnaire-based studies, Idris et al. (31) found no relationship between time-weighted metabolic equivalents (MET, Table 1) and cIMT but identified lower cIMT in association with higher values of time-weighted sports-related MET among the 5-year old participants. Likewise, Pahkala et al. (35) found that leisure-time physical activity (LTPA) had a beneficial impact on aortic IMT (aIMT); specifically, a moderate increase in LTPA among sedentary 13 and 15 year olds was associated with a significant decreased progression of aIMT. In a similar study, Pahkala et al. (36) reported no direct association between LTPA and cIMT or aIMT, although 17-year olds with high levels of LTPA overall experienced lower levels of aIMT compared to those with low LPTA in relation to their fitness level.
By contrast, among the studies that made use of accelerometers, Ried-Larsen et al. (20, 37) reported no correlation between MVPA or vigorous PA (VPA) and cIMT in a study of 8- to-10-year old Danish children; analogous results were obtained from adolescents with a mean age of 15.6 ± 0.4 years. Similary, Melo et al. (33) found that sedentary behavior (SED) and MVPA were not definitively related to cIMT in 11- to-13-year-old Portuguese children and adolescents.
In summary, we conclude that studies that used questionnaires identified correlations between PA and healthy vascular structure, but no such relationship emerged from studies that employed accelerometers. As such, it is clear that associations between PA and vascular structure may depend all or in part on the method used to assess activity. Nonetheless, three out of six studies reported beneficial associations between PA and cIMT or aIMT.
Vascular Function
We identified eight studies that examined the relationships between PA and vascular function; five of these studies used questionnaires (31, 32, 38–40) and three (20, 30, 34) used accelerometers to assess PA in children and adolescents.
Moderate PA (MPA), VPA and cumulative time spent in PA, specifically data documenting individuals achieving above 3–7 METs, were inversely associated with stiffness index, measured at the finger tip by pulse contour analysis. Of note, children participating in PA who achieved 3 METs/day presented with lower stiffness index values (30). Nettlefold et al. (34) found no association between PA and compliance of the large arteries, but did report higher compliance of small arteries with increased time spent in light PA (LPA), MPA, or MVPA per day. Likewise, Ried-Larsen et al. (38) found that boys who ride bicycles every day have lower young's elastic modulus, higher distensibility, and higher arterial compliance compared to boys who ride fewer than three times per week. Likewise, boys who traveled to school by bicycle had higher compliance and lower young's elastic modulus compared to those who use passive transportation (38).
In other studies, more time spent in unstructured PA was related to lower stiffness index in children between 6 and 8 years of age (39). However, Ried-Larsen et al. (37) did not identify a direct association between time spent in MVPA and carotid compliance, distensibility, young's elastic modulus, and stiffness index; there was also no statistical relationship between VPA and compliance, distensibility, young's elastic modulus, and stiffness index. However, boys classified in the highest quartile of MVPA had significantly lower young's elastic modulus and stiffness index compared to boys in the lowest quartile.
By contrast, carotid distensibility and carotid elastic modulus were associated with neither total time-weighted MET, sports time-weighted MET nor organized sport time-weighted MET in children (31). Furthermore, parameters relating to vascular stiffness were not associated with time spent in MVPA in a daily basis and VPA in children and adolescents (20). VPA (categorized in tertiles) had no impact on carotid-femoral PWV nor on aortic AI (40). Kochli et al. (32) also found no association between aortic PWV and VPA, indoor or outdoor activity.
In conclusion, of the eight studies that explored the association between PA and vascular stiffness, four reported improved vascular function with increased PA; two of these studies reported accelerometer findings and two used questionnaires. Of importance, none of the studies identified any unfavorable associations between PA and vascular stiffness. The two studies which used photoplethysmography reported significant lower stiffness indices in association with higher levels of PA (30, 39). The four studies reported ultrasound measurements of vascular parameters and the study which used PA questionnaires all report an inverse correlation between PA and vascular stiffness (38); this result was not reported in the two studies which used accelerometers for measuring PA (20, 37). Of the studies which used applanation tonometry to measure vascular stiffness, only Nettlefold et al. (34) reported better vascular elasticity with increasing PA; of note, this study featured accelerometer findings to report PA in contrast to the report from Walker et al. (40) which used a questionnaire. Hence, the type of vascular measurement employed and the means by which PA was assessed may have an influence on the relationships reported. Of note, the number of study participants in each study may also have an influence on the results obtained.
Health-Related Physical Fitness
HRPF includes cardiorespiratory fitness (CRF), muscular endurance, muscular strength, flexibility, and body composition and is an important indicator of health and well-being (42). The following studies focus on CRF or strength and include healthy children and adolescents only and exclude those with chronic conditions including overweight, obesity or diabetes.
Vascular Structure
The majority of the studies included in this review used ergometers to measure HRPF (Table 3) and include results from single trails that include a 20 m shuttle run, handgrip strength test, curl-ups, and push-ups.
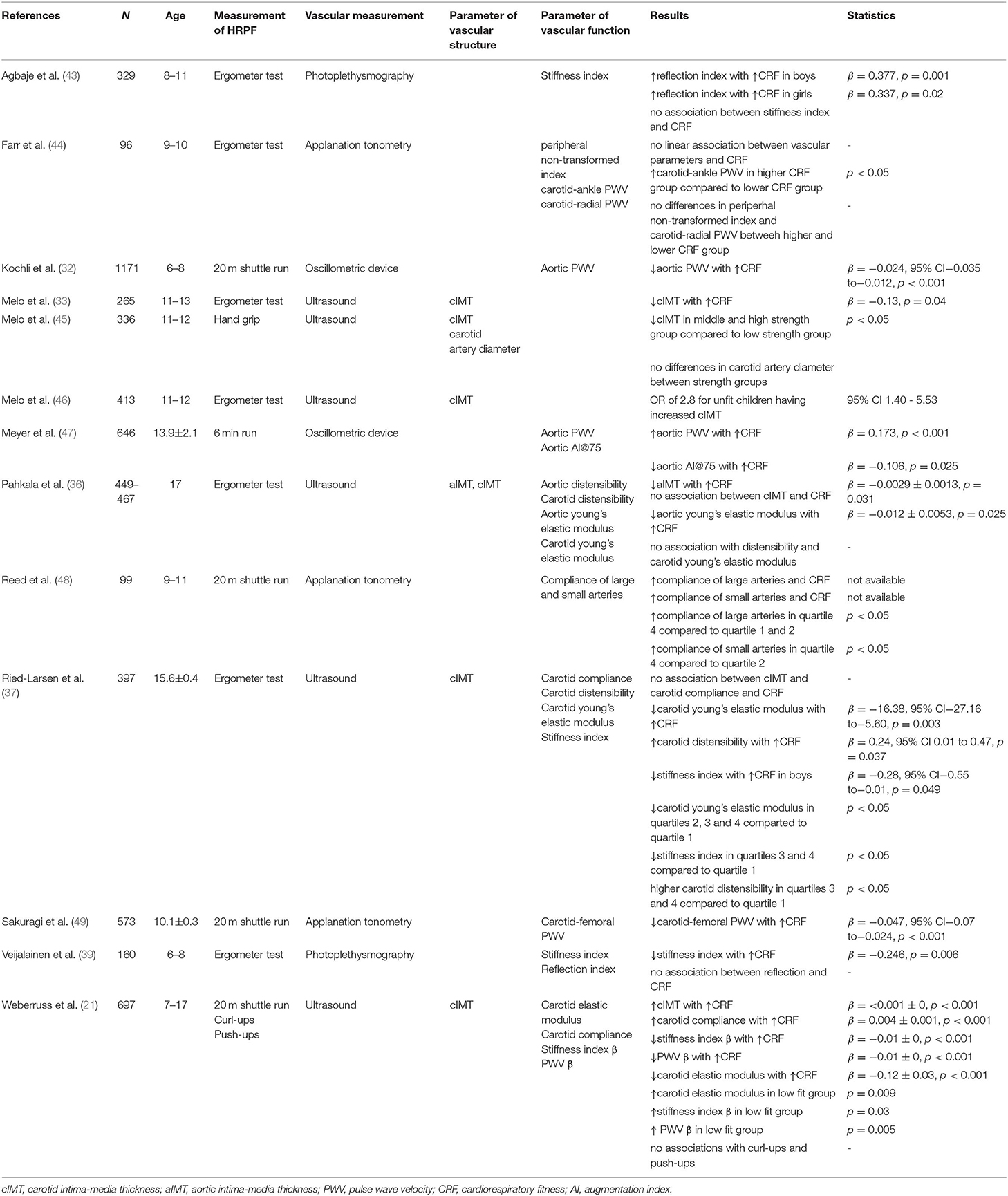
Table 3. Overview of studies that evaluated associations of health-related physical fitness and vascular structure and function.
Among Portuguese children between the ages of 11 and 13 years, cIMT was inversely associated with CRF independent of age, sex, maturity, SED, and MVPA (33) and values of maximum oxygen uptake revealed an inverse correlation with aIMT (36). Furthermore, participants identified as “low-fit” at the age of 11 experienced accelerated progression of aIMT between ages of 11 and 17 years (36).
By contrast, in a study involving 697 children and adolescents between 7 and 17 years, Weberruss et al. (21) found a significant positive correlation between cIMT and CRF. CRF was measured with a 20m shuttle run, which means that the participant runs between two lines (20m distance) with a given speed. The speed is stepwise increased and the time will be stopped if the participant does not touch the line in the given speed. In contrast, Pahkala et al. (36) observed no associations between these parameters; diminished CRF was not associated with increased risk of having cIMT. No association of a maximal load test on a cycle ergometer and cIMT was reported in Danish adolescents (37) but Melo et al. (46) reported an OR of 2.8 in unfit children for having a cIMT ≥ 75th percentile.
Muscular strength has also been evaluated as a parameter contributing to HRPF. Among 11- to 12-year old Portuguese children, carotid artery diameter did not differ between children with low, middle or high muscle strength index; but children with middle to high muscle strength index as measured using a handgrip test showed lower values of cIMT compared to those measured in children with low muscular strength (45). By contrast, muscular strength determined in a series of German children and adolescents (push-ups and curl-ups) was not associated with CRF (21).
Taken together, the literature reveals a significant relationship between HRPF and vascular structure in population-based studies that include children and adolescents; in general, higher performance in HRPF tasks corresponds to lower levels of IMT. One study did reveal an inverse correlation between CRF and aIMT but not with cIMT. The authors hypothesize that structural alterations such as IMT may begin in the aorta and progress in the carotid artery later in life (36, 50).
Interestingly, the association between HRPF and vascular structure has occurred primarily in children and adolescents with increased cardiovascular risk; by contrast, the participants in most published studies are their healthy peers. For example, Ried-Larsen et al. (37) enrolled only healthy participants and excluded those who were chronically ill; this may explain the absence of associations observed between cIMT and CRF in this study. Arteries of fit children and adolescents may undergo adaptation to exercise, a process which may have an impact on smooth muscle cells in the vascular media; this adaptation may explain the positive relationship of cIMT to CRF observed by Weberruss et al. (21). Nevertheless, at this time there is no clear association between HRPF (evaluated as muscular strength) and vascular structure; further investigation is warranted.
Vascular Function
We identified ten studies which explored the association between HRPF and arterial function in children and adolescents; five studies featured a (maximal) ergometer test, one included a 6-min run, three studies included the 20 m shuttle run, and one study explored responses to a combination of the 20 m shuttle run, curl-ups and push-ups. The studies applied numerous methods to measure vascular stiffness (ultrasound, oscillometric device, photoplethysmography, applanation tonometry) and included a wide range of stiffness parameters.
In the first group of studies, CRF was inversely and therefore beneficially associated with aortic PWV but the association became non-significant when the model was further adjusted for blood pressure (32). Carotid PWV (21) and carotid-femoral PWV (49) were both inversely related to CRF adjusted for blood pressure. Farr et al. (44) reported no direct correlation between CRF and carotid-ankle PWV, carotid-radial PWV or peripheral non-transformed index but when devided into a higher and lower group of CRF, subjects in the higher group of CRF had higher carotid-ankle-PWV compared to the lower group. By contrast, another study reported increased aortic PWV in association with higher CRF in children and adolescents between the ages of 11 and 17 years (47). Three of four studies observed a significant favorable association between CRF and stiffness index (21, 37, 39, 43).
Two studies used non-invasive photoplethysmography and assessed reflection index as parameter of vascular function; one identified no correlations between the reflection index and CRF (39), one reported a positive association between reflection index and CRF in boys and girls (43). Pahkala et al. (36) reported an inverse correlation between CRF and young's elastic modulus for the aorta but not for the carotid artery among a group of 17-years olds. Furthermore, Weberruss et al. (21) documented a favorable association between CRF and carotid elastic modulus while Ried-Larsen et al. (37) reported a significant inverse correlation between CRF and carotid elastic modulus.
By contrast, aortic distensibility was not associated with CRF (36). Two studies investigated the associations between carotid distensibility and CRF; only Ried-Larsen et al. (37) reported a positive relationship (36, 37). Two of the three studies documented a positive association between carotid arterial compliance and CRF (21, 37, 48), while aortic AI@75 (the AI at a standardized heart rate of 75 beats per minute) was inversely associated with CRF (47).
Only one study investigated the relationship between muscle strength (push-up and curl-ups) and CRF; no associations with vascular function were identified when evaluating these parameters (21).
Several studies compared levels of CRF to specific stiffness parameters. Reed et al. (48) evaluated this relationship and found significantly lower compliance in large arteries in the first (up to 10 laps) and second quartile (21 to 32 laps) compared to the highest quartile (more than 43 laps) of CRF, recorded with a 20 m shuttle run; compliance of small arteries was lower in the second quartile of CRF compared to the fourth quartile.
Ried-Larsen et al. (37) divided CRF in four quartiles and observed significant lower carotid young's elastic modulus in the second to fourth quartiles compared to the first quartile; these results indicate stiffer arteries at lower fitness levels. Likewise, stiffness index was higher in the first quartile compared to the third and fourth quartile, and adolescents in CRF quartiles three and four showed significantly higher carotid compliance compared to adolescents within the first quartile. In a related study, very fit children and adolescents (>80th percentile) had lower elastic modulus, stiffness index and carotid PWV than low fit (<20th percentile) children and adolescents (21).
To summarize, most of the studies reported a beneficial association between vascular stiffness and CRF among children and adolescents; these findings are consistent with those reported in studies of, young, middle-aged and older adults (51–53). Nevertheless, it was difficult to compare studies to one another because of different methods used to measure both HRPF and vascular stiffness. Of interest, all studies, in which the 20 m shuttle run was used to assess HRPF, revealed a favorable association between stiffness parameters and HRPF.
Exercise
Children and adolescents with obesity or hypertension typically have impaired arterial structure and function. Several groups have introduced intervention methods in an attempt to alter the vascular architecture among subjects in these groups. Besides, exercise also refers to children and adolescents who perform at a sustained level in organized sports club activities.
Vascular Structure
Garcia-Hermoso et al. (54) published a comprehensive meta-analysis that included six studies that focused on the impact of aerobic, resistance or both aerobic and resistance exercises on vascular structure in obese populations aged between 6 and 18 years. Four of these studies (three aerobic, one aerobic, and resistance exercises) resulted in reductions in cIMT. Overall, the results indicate the changes in cIMT that result from exercise interventions were small to moderate (g = −0.306; 95% CI :−0.540 to −0.072, p = 0.011) with higher impact achieved in response to the longer interventions. Cayres et al. (41) also conclude that there are beneficial effects of exercise interventions on cIMT, but note that there are very few studies that examine interventions in healthy, non-obese populations.
In addition to these observations, there are several more recent interventional studies investigated that explore the impact of exercise on vascular structure (Table 4). For example, obese boys were subjected to a 12-week high-intensity intermittent training (HIIT) of 8 × 2 min at 90% peak power output or a supra-HIIT of 2 × 20 s at 170% peak power output; these strategies resulted in significant reductions in cIMT of 0.02 mm in both intervention groups, but had no impact on the diameter of the brachial artery (55).
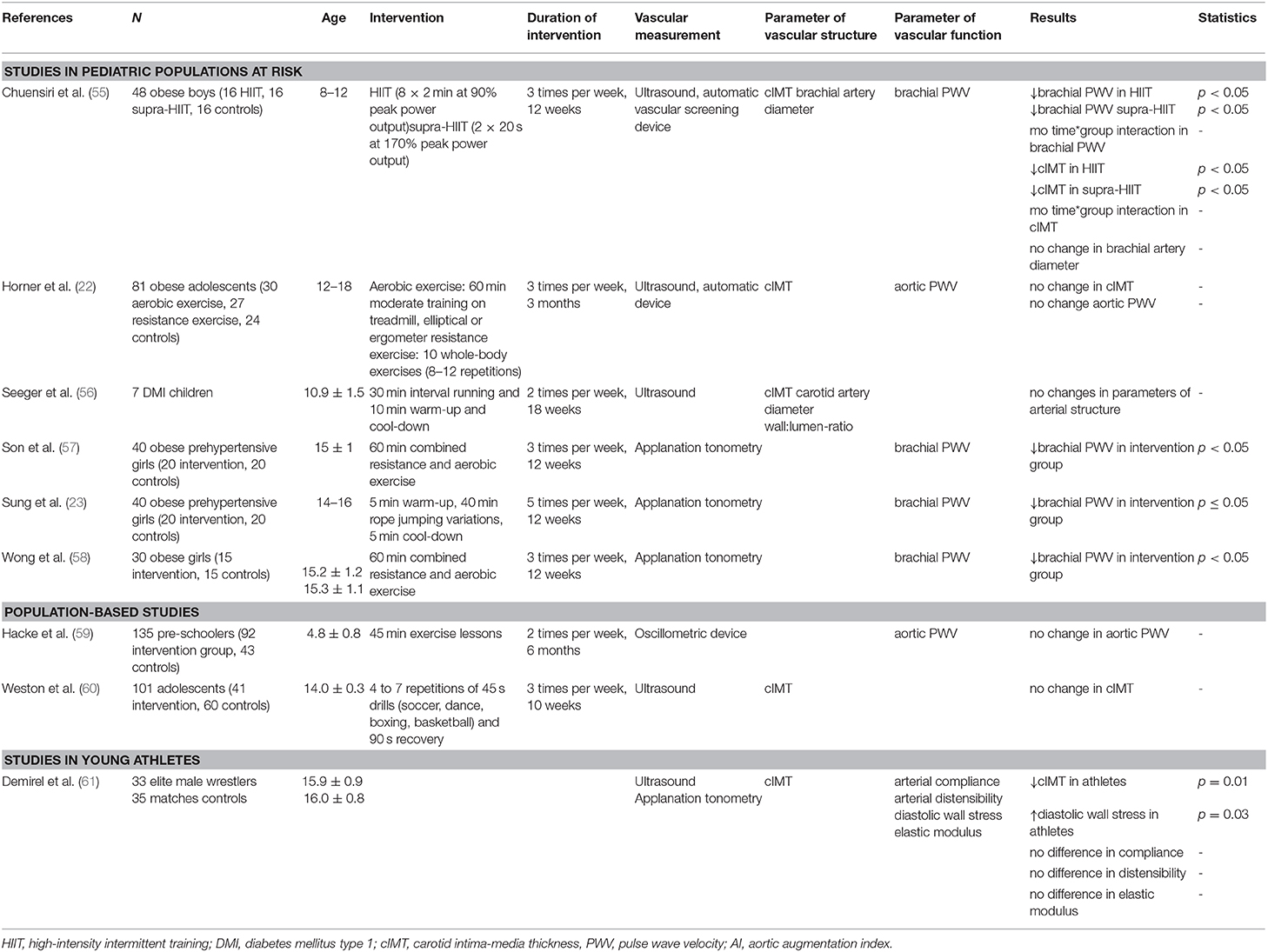
Table 4. Overview of studies investigating the impact of exercise on vascular structure and function.
By contrast, seven children with type 1 diabetes mellitus were enrolled in an 18-week interval running intervention (30 min intervention, 10 min cool-down, two sessions per week); no significant changes in carotid artery diameter, cIMT or wall:lumen-ratio were detected (56).
To the best of our knowledge, there is only one published study that investigated the impact of an exercise intervention (high-intensity interval training) in a school-based population; this study also revealed no significant reduction in cIMT after 10 weeks (60). However, in young adolescent male wrestlers, cIMT measurements were significantly lower when compared to age- and sex-matched controls (61).
Taken together, the published results suggest that exercise interventions in groups of young participants may result in reduced thickening of the arterial walls, but the overall impact depends on duration and intensity of the intervention and the nature of the target groups. Given the current prevalence of overweight and obesity among children and adolescents, future population-based studies are needed to determine definitively whether exercise leads to an improved vascular health in these populations.
Vascular Function
Exercise interventions on obese and/or prehypertensive children and adolescents have shown promising results toward the goal of reducing arterial stiffness. The study noted earlier in which 48 obese boys were challenged with a 12-week HIIT or supra-HIIT resulted in significant reductions in brachial PWV in both intervention groups (55). In another study, a combined resistance and aerobic exercise intervention (50 min, three times per week) in prehypertensive adolescent girls resulted in significant reductions in brachial-ankle PWV after 12 weeks (57). Similarly, a rope-jumping intervention (50 min, five times per week) resulted in reduced brachial-ankle PWVs in a study with obese prehypertensive girls (23). Likewise, Wong et al. (58) found that combined resistance and aerobic exercise (60 min, 3 times per week) in obese girls led to a significant reduction of brachial PWV compared to a control group after three months.
By contrast, Horner et al. (22) found no change in aortic PWV in obese adolescents who participated in aerobic (treadmill training, 60–75% of VO2peak) or resistance (whole-body exercises) interventions for a period of three months. Moreover, Hacke et al. (59) implemented preschool exercise lessons (45 min, two times per week for six months) but was unable to detect any improvement in aortic PWV in the intervention group compared to controls.
We identified only one study that evaluated vascular function in young athletes; this study reported no significant differences in arterial compliance, distensibility and elastic modulus among young adolescent male wrestlers vs. age- and sex-matched controls (61).
In summary, four out of five studies that we evaluated were conducted in children and adolescents with increased cardiovascular risk (obesity, hypertension), reported reductions in vascular stiffness after an exercise intervention. As such, we can conclude that exercise interventions with at least moderate levels of intensity can reduce vascular stiffness among pediatric populations at risk. Horner et al. (22) and Hacke et al. (59) reported no reductions in body mass index in response to these interventions; improvement in vascular function might be linked to a change in anthropometric parameters. It is also possible that the intensity of their intervention was too low to promote measurable changes in vascular function. The need to implement exercise programs in order to improve the health of an increasing number of inactive children and adolescents is supported globally. Further studies should take into account the intensity and duration of interventions to improve the design and therefore effectiveness of these programs.
Conclusion
In conclusion, PA and HRPF are directly associated with improved vascular structure and function in children and adolescents as determined in population-based studies. Furthermore, interventions in pediatric populations at risk reveal promising results that suggest a role for moderate exercise in correcting early increases in vascular wall thickness and vascular stiffness.
The results of our study are comparable with the findings of adult studies. Here Thijssen et al. (62) showed in a comprehensive review that both physical activity and HRPF are inversely related to cIMT and that movement interventions lead to a significant reduction in wall thickness of the carotid, femoral and brachial arteries. The identical direction of the results was also observed in the correlation between activity, fitness and exercise and vascular function in adults (63–65).
It was difficult to perform critical comparisons among these studies given the variety of methods and measurements employed, especially in studies that measured vascular function. Elmenhorst et al. (66) compared carotid PWV measured by ultrasound and aortic PWV measured by an oscillometric device and found significant lower values of carotid PWV (4.01 ± 0.44 m/s) compared aortic PWV (4.67 ± 0.34, p < 0.001). In addition to physiological and hemodynamic differences between the carotid artery and the aorta, HRPF, PA and exercise may have a varied impact on the function along the arterial tree. Regarding IMT, all included studies that measured the vascular structure used an ultrasound device. However, both the type of ultrasound device and the analysis method used, e.g. B-Mode imaging or radiofrequency multiple M-line analysis, influence the magnitude of IMT and, therefore, limit the comparability of the included studies. Thus, only one technique should be performed in prospective studies (67). Following Skilton et al. (68), the choice of location of IMT measurement—at the carotid artery or the aorta—should be decided depending on the target group and the individual goal of each study.
Likewise, we are unable to comment on the role of exercise in young athletes given the limited numer of studies on this subject. As athletes are of special interest with respect to cardiovascular adaptation to exercise, this target population should be the subject of further investigation focused on the duration and intensity of exercise and its impact on vascular structure and function (69).
Future studies are also needed to address the question of wether IMT:diameter-ratio or wall:lumen-ratio and the role of this parameters determining vascular patency. At this time, most studies evaluate only IMT, a parameter that provides a quantitative assessment of wall thickness but does not take into account any changes in the vessel diameter or lumen in response to HRPF, PA and/or specific exercise interventions. Finally, our findings suggest that individual exercise participation should be evaluated quantitatively because intensity and duration likely influence the effectiveness of these interventions.
Author Contributions
LB conceptualized the study, reviewed the literature, and drafted the manuscript. HW, RO-F, and TS conceptualized the study and provided important input for drafting and revising of the manuscript.
Funding
The German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program supported this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AI, augmentation index; AI@75, augmentation index at heart rate 75/min; aIMT, aortic intima-media thickness; cIMT, carotid intima-media thickness; CRF, cardiorespiratory fitness; CVD, cardiovascular disease; HIIT, high-intensity intermittent training; HRPF, health-related physical fitness; IMT, intima-media thickness; LPA, light physical activity; LTPA, leisure-time physical activity; LV, left ventricle; MET, metabolic equivalent; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; PWV, pulse wave velocity; SED, sedentary behavior; VPA, vigorous physical activity.
References
2. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2019). 140:e596–646. doi: 10.1161/CIR.0000000000000678
3. Juonala M, Viikari JSA, Ronnemaa T, Helenius H, Taittonen L, Raitakari OT. Elevated blood pressure in adolescent boys predicts endothelial dysfunction - The cardiovascular risk in Young Finns Study. Hypertension. (2006) 48:424–30. doi: 10.1161/01.HYP.0000237666.78217.47
4. Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. (2006) 55:113–8. doi: 10.1016/j.metabol.2005.07.016
5. Park MH, Skow A, De Matteis S, Kessel AS, Saxena S, Viner RM, et al. Adiposity and carotid-intima media thickness in children and adolescents: a systematic review. BMC Pediatr. (2015) 15:161. doi: 10.1186/s12887-015-0478-5
6. Day TG, Park M, Kinra S. The association between blood pressure and carotid intima-media thickness in children: a systematic review. Cardiol Young. (2017) 27:1295–305. doi: 10.1017/S1047951117000105
7. Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. (2004) 109:1750–5. doi: 10.1161/01.CIR.0000124725.46165.2C
8. Krawczuk-Rybak M, Tomczuk-Ostapczuk M, Panasiuk A, Goscik E. Carotid intima-media thickness in young survivors of childhood cancer. J Med Imag Radiat. (2017) 61:85–92. doi: 10.1111/1754-9485.12510
9. Reiner B, Oberhoffer R, Hacker AL, Ewert P, Muller J. Carotid intima-media thickness in children and adolescents with congenital heart disease. Can J Cardiol. (2018) 34:1618–23. doi: 10.1016/j.cjca.2018.09.012
10. Dalla Pozza R, Ehringer-Schetitska D, Fritsch P, Jokinen E, Petropoulos A, Oberhoffer R, et al. Intima media thickness measurement in children: a statement from the Association for European Pediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. (2015) 238:380–7. doi: 10.1016/j.atherosclerosis.2014.12.029
11. London GM, Pannier B. Arterial functions: how to interpret the complex physiology. Nephrol Dial Transpl. (2010) 25:3815–23. doi: 10.1093/ndt/gfq614
12. Kulsum-Mecci N, Goss C, Kozel BA, Garbutt JM, Schechtman KB, Dharnidharka VR. Effects of obesity and hypertension on pulse wave velocity in children. J Clin Hypertens. (2017) 19:221–6. doi: 10.1111/jch.12892
13. Cote AT, Phillips AA, Harris KC, Sandor GGS, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children systematic review and meta-analysis. Arterioscl Throm Vasc. (2015) 35:1038–336. doi: 10.1161/ATVBAHA.114.305062
14. Riggio S, Mandraffino G, Sardo MA, Iudicello R, Camarda N, Imbalzano E, et al. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur J Clin Invest. (2010) 40:250–7. doi: 10.1111/j.1365-2362.2010.02260.x
15. Zhang L, Mi J, Li M, Jiang B. Association of metabolic syndrome with arterial compliance in children and adolescents. Front Med China. (2007) 1:68. doi: 10.1007/s11684-007-0014-6
16. Bradley TJ, Slorach C, Mahmud FH, Dunger DB, Deanfield J, Deda L, et al. Early changes in cardiovascular structure and function in adolescents with type 1 diabetes. Cardiovasc Diabetol. (2016) 15:31. doi: 10.1186/s12933-016-0351-3
17. Hacker AL, Reiner B, Oberhoffer R, Hager A, Ewert P, Muller J. Functional outcomes in children with anatomically repaired transposition of the great arteries with regard to congenital ventricular septal defect and coronary pattern. Arch Dis Child. (2019) 104:851–6. doi: 10.1136/archdischild-2018-316444
18. Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. (2017) 390:2643–54. doi: 10.1016/S0140-6736(17)31634-3
19. Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. (2015) 57:306–14. doi: 10.1016/j.pcad.2014.09.011
20. Ried-Larsen M, Grontved A, Moller NC, Larsen KT, Froberg K, Andersen LB. Associations between objectively measured physical activity intensity in childhood and measures of subclinical cardiovascular disease in adolescence: prospective observations from the European Youth Heart Study. Br J Sports Med. (2014) 48:1502–7. doi: 10.1136/bjsports-2012-091958
21. Weberruss H, Pirzer R, Schulz T, Bohm B, Dalla Pozza R, Netz H, et al. Reduced arterial stiffness in very fit boys and girls. Cardiol Young. (2017) 27:117–24. doi: 10.1017/S1047951116000226
22. Horner K, Kuk JL, Barinas-Mitchell E, Drant S, DeGroff C, Lee S. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci. (2015) 27:494–502. doi: 10.1123/pes.2015-0067
23. Sung KD, Pekas EJ, Scott SD, Son WM, Park SY. The effects of a 12-week jump rope exercise program on abdominal adiposity, vasoactive substances, inflammation, and vascular function in adolescent girls with prehypertension. Eur J Appl Physiol. (2019) 119:577–85. doi: 10.1007/s00421-018-4051-4
24. World Health Organization. Global Recommendations on Physical Activity for Health. Geneva (2010).
25. Jette M, Sidney K, Blumchen G. Metabolic equivalents (Mets) in exercise testing, exercise prescription, and evaluation of functional-capacity. Clin Cardiol. (1990) 13:555–65. doi: 10.1002/clc.4960130809
26. Sedentary Behaviour Research N. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. (2012) 37:540–2. doi: 10.1139/h2012-024
27. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. (2007) 39:1423–34. doi: 10.1249/mss.0b013e3180616b27
28. Corder K, Ekelund U, Steele RM, Wareham NJ, Brage S. Assessment of physical activity in youth. J Appl Physiol. (2008) 105:977–87. doi: 10.1152/japplphysiol.00094.2008
29. Ndahimana D, Kim E-K. Measurement methods for physical activity and energy expenditure: a review. Clin. Nutr Res. (2017) 6:68–80. doi: 10.7762/cnr.2017.6.2.68
30. Haapala EA, Vaisto J, Veijalainen A, Lintu N, Wiklund P, Westgate K, et al. Associations of objectively measured physical activity and sedentary time with arterial stiffness in pre-pubertal children. Pediatr Exerc Sci. (2017) 29:326–35. doi: 10.1123/pes.2016-0168
31. Idris NS, Evelein AM, Geerts CC, Sastroasmoro S, Grobbee DE, Uiterwaal CS. Effect of physical activity on vascular characteristics in young children. Eur J Prevent Cardiol. (2015) 22:656–64. doi: 10.1177/2047487314524869
32. Kochli S, Endes K, Steiner R, Engler L, Infanger D, Schmidt-Trucksass A, et al. Obesity, high blood pressure, and physical activity determine vascular phenotype in young children. Hypertension. (2019) 73:153–61. doi: 10.1161/HYPERTENSIONAHA.118.11872
33. Melo X, Santa-Clara H, Pimenta NM, Martins SS, Minderico CS, Fernhall B, et al. Intima-media thickness in 11- to 13-year-old children: variation attributed to sedentary behavior, physical activity, cardiorespiratory fitness, and waist circumference. J Phys Act Health. (2015) 12:610–7. doi: 10.1123/jpah.2013-0501
34. Nettlefold L, McKay HA, Naylor PJ, Bredin SS, Warburton DE. The relationship between objectively measured physical activity, sedentary time, and vascular health in children. Am J Hypertens. (2012) 25:914–9. doi: 10.1038/ajh.2012.68
35. Pahkala K, Heinonen OJ, Simell O, Viikari JS, Ronnemaa T, Niinikoski H, et al. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. (2011) 124:1956–63. doi: 10.1161/CIRCULATIONAHA.111.043851
36. Pahkala K, Laitinen TT, Heinonen OJ, Viikari JS, Ronnemaa T, Niinikoski H, et al. Association of fitness with vascular intima-media thickness and elasticity in adolescence. Pediatrics. (2013) 132:e77–84. doi: 10.1542/peds.2013-0041
37. Ried-Larsen M, Grontved A, Froberg K, Ekelund U, Andersen LB. Physical activity intensity and subclinical atherosclerosis in Danish adolescents: the European Youth Heart Study. Scand J Med Sci Sports. (2013) 23:e168–77. doi: 10.1111/sms.12046
38. Ried-Larsen M, Grontved A, Ostergaard L, Cooper AR, Froberg K, Andersen LB, et al. Associations between bicycling and carotid arterial stiffness in adolescents: the European Youth Hearts Study. Scand J Med Sci Spor. (2015) 25:661–9. doi: 10.1111/sms.12296
39. Veijalainen A, Tompuri T, Haapala EA, Viitasalo A, Lintu N, Vaisto J, et al. Associations of cardiorespiratory fitness, physical activity, and adiposity with arterial stiffness in children. Scand J Med Sci Sports. (2016) 26:943–50. doi: 10.1111/sms.12523
40. Walker DJ, MacIntosh A, Kozyrskyj A, Becker A, McGavock J. The associations between cardiovascular risk factors, physical activity, and arterial stiffness in youth. J Phys Act Health. (2013) 10:198–204. doi: 10.1123/jpah.10.2.198
41. Cayres SU, Agostinete RR, de Moura Mello Antunes B, Lira FS, Fernandes RA. Impact of physical exercise/activity on vascular structure and inflammation in pediatric populations: a literature review. J Spec Pediatr Nurs. (2016) 21:99–108. doi: 10.1111/jspn.12149
42. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. (1985) 100:126–31.
43. Agbaje AO, Haapala EA, Lintu N, Viitasalo A, Vaisto J, Khan S, et al. Associations of cardiorespiratory fitness and adiposity with arterial stiffness and arterial dilatation capacity in response to a bout of exercise in children. Pediatr Exerc Sci. (2019) 31:238–47. doi: 10.1123/pes.2018-0145
44. Farr C, Middlebrooke AR, Armstrong N, Barker AR, Fulford J, Mawson DM, et al. Objectively measured aerobic fitness is not related to vascular health outcomes and cardiovascular disease risk in 9-10 year old children. J Sports Sci Med. (2019) 18:513–22.
45. Melo X, Santa-Clara H, Santos DA, Pimenta NM, Minderico CS, Fernhall B, et al. Independent association of muscular strength and carotid intima-media thickness in children. Int J Sports Med. (2015) 36:624–30. doi: 10.1055/s-0034-1398678
46. Melo X, Santa-Clara H, Santos DA, Pimenta NM, Minderico CS, Fernhall B, et al. Linking cardiorespiratory fitness classification criteria to early subclinical atherosclerosis in children. Appl Physiol Nutr Metab. (2015) 40:386–92. doi: 10.1139/apnm-2014-0378
47. Meyer J, Elmenhorst J, Giegerich T, Oberhoffer R, Muller J. Controversies in the association of cardiorespiratory fitness and arterial stiffness in children and adolescents. Hypertens Res. (2017) 40:675–8. doi: 10.1038/hr.2017.19
48. Reed KE, Warburton DER, Lewanczuk RZ, Haykowsky MJ, Scott JM, Whitney CL, et al. Arterial compliance in young children: the role of aerobic fitness. Eur J Cardiov Prev R. (2005) 12:492–7. doi: 10.1097/01.hjr.0000176509.84165.3d
49. Sakuragi S, Abhayaratna K, Gravenmaker KJ, O'Reilly C, Srikusalanukul W, Budge MM, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. (2009) 53:611–6. doi: 10.1161/HYPERTENSIONAHA.108.123364
50. McGill HC Jr., McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, et al. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. (2000) 20:836–45. doi: 10.1161/01.ATV.20.3.836
51. Fernberg U, Fernstrom M, Hurtig-Wennlof A. Arterial stiffness is associated to cardiorespiratory fitness and body mass index in young Swedish adults: the Lifestyle, Biomarkers, and Atherosclerosis study. Eur J Prev Cardiol. (2017) 24:1809–18. doi: 10.1177/2047487317720796
52. Fahs CA, Thiebaud RS, Rossow LM, Loenneke JP, Bemben DA, Bemben MG. Relationships between central arterial stiffness, lean body mass, and absolute and relative strength in young and older men and women. Clin Physiol Func Imaging. (2018) 38:676–80. doi: 10.1111/cpf.12467
53. Chung J, Kim M, Jin Y, Kim Y, Hong J. Fitness as a determinant of arterial stiffness in healthy adult men: a cross-sectional study. J Sport Med Phys Fit. (2018) 58:150–6. doi: 10.23736/S0022-4707.17.06767-6
54. Garcia-Hermoso A, Gonzalez-Ruiz K, Triana-Reina HR, Olloquequi J, Ramirez-Velez R. Effects of exercise on carotid arterial wall thickness in obese pediatric populations: a meta-analysis of randomized controlled trials. Child Obes. (2017) 13:138–45. doi: 10.1089/chi.2016.0265
55. Chuensiri N, Suksom D, Tanaka H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child Obes. (2018) 14:41–9. doi: 10.1089/chi.2017.0024
56. Seeger JP, Thijssen DH, Noordam K, Cranen ME, Hopman MT, Nijhuis-van der Sanden MW. Exercise training improves physical fitness and vascular function in children with type 1 diabetes. Diabetes Obes Metab. (2011) 13:382–4. doi: 10.1111/j.1463-1326.2011.01361.x
57. Son WM, Sung KD, Bharath LP, Choi KJ, Park SY. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens. (2017) 39:546–52. doi: 10.1080/10641963.2017.1288742
58. Wong A, Sanchez-Gonzalez MA, Son WM, Kwak YS, Park SY. The effects of a 12-week combined exercise training program on arterial stiffness, vasoactive substances, inflammatory markers, metabolic profile, and body composition in obese adolescent girls. Pediatr Exerc Sci. (2018) 30:480–6. doi: 10.1123/pes.2017-0198
59. Hacke C, Ketelhut S, Wendt U, Muller G, Schlesner C, Ketelhut K. Effectiveness of a physical activity intervention in preschoolers: a cluster-randomized controlled trial. Scand J Med Sci Sports. (2019) 29:742–52. doi: 10.1111/sms.13390
60. Weston KL, Azevedo LB, Bock S, Weston M, George KP, Batterham AM. Effect of novel, school-based high-intensity interval training (HIT) on cardiometabolic health in adolescents: project FFAB (Fun Fast Activity Blasts) - an exploratory controlled before-and-after trial. PLoS ONE. (2016) 11:e0159116. doi: 10.1371/journal.pone.0159116
61. Demirel A, Baykara M, Koca TT, Berk E, Gencay OA. Comparison of vascular arterial stiffness parameters of adolescent wrestlers with healthy subjects: Is heavy training harmful for wrestlers? J Back Musculoskelet Rehabil. (2019) 32:155–60. doi: 10.3233/BMR-171083
62. Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci. (2012) 122:311–22. doi: 10.1042/CS20110469
63. Tomoto T, Maeda S, Sugawara J. Relation between arterial stiffness and aerobic capacity: importance of proximal aortic stiffness. Eur J Sport Sci. (2017) 17:571–5. doi: 10.1080/17461391.2016.1277787
64. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. (2013) 26:1093–102. doi: 10.1093/ajh/hpt080
65. Ahmadi-Abhari S, Sabia S, Shipley MJ, Kivimaki M, Singh-Manoux A, Tabak A, et al. Physical activity, sedentary behavior, and long-term changes in aortic stiffness: the Whitehall II study. J Am Heart Assoc. (2017) 6:e005974. doi: 10.1161/JAHA.117.005974
66. Elmenhorst J, Weberruss H, Mayr M, Pfister K, Oberhoffer R. Comparison of two measurement devices for pulse wave velocity in children: which tool is useful to detect vascular alterations caused by overweight? Front Pediatr. (2019) 7:334. doi: 10.3389/fped.2019.00334
67. El Jalbout R, Cloutier G, Cardinal MR, Henderson M, Lapierre C, Soulez G, et al. Carotid artery intima-media thickness measurement in children with normal and increased body mass index: a comparison of three techniques. Pediatr Radiol. (2018) 48:1073–9. doi: 10.1007/s00247-018-4144-6
68. Skilton MR, Celermajer DS, Cosmi E, Crispi F, Gidding SS, Raitakari OT, et al. Natural history of atherosclerosis and abdominal aortic intima-media thickness: rationale, evidence, and best practice for detection of atherosclerosis in the young. J Clin Med. (2019) 8:E1201. doi: 10.3390/jcm8081201
Keywords: vascular structure, vascular function and stiffness, health-related physical fitness, physical activity, exercise, children, adolescents
Citation: Baumgartner L, Weberruß H, Oberhoffer-Fritz R and Schulz T (2020) Vascular Structure and Function in Children and Adolescents: What Impact Do Physical Activity, Health-Related Physical Fitness, and Exercise Have? Front. Pediatr. 8:103. doi: 10.3389/fped.2020.00103
Received: 30 September 2019; Accepted: 27 February 2020;
Published: 19 March 2020.
Edited by:
Umberto Morbiducci, Politecnico di Torino, ItalyReviewed by:
Guido Pieles, University of Bristol, United KingdomConstance G. Weismann, Lund University, Sweden
Copyright © 2020 Baumgartner, Weberruß, Oberhoffer-Fritz and Schulz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa Baumgartner, bGlzYS5iYXVtZ2FydG5lckB0dW0uZGU=
 Lisa Baumgartner
Lisa Baumgartner Heidi Weberruß
Heidi Weberruß Renate Oberhoffer-Fritz
Renate Oberhoffer-Fritz Thorsten Schulz
Thorsten Schulz