- 1Department of Human Anatomy and Psychobiology, Faculty of Medicine, University of Murcia, Murcia, Spain
- 2Institute of Biomedical Research of Murcia (IMIB), Virgen de la Arrixaca University Hospital, University of Murcia, Murcia, Spain
- 3Department of Histology and Anatomy, Faculty of Medicine, University of Miguel Hernández, Sant Joan Alacant, Spain
- 4Neurological Unit, University Hospital “Santa Lucia”, Cartagena, Spain
- 5Pharmacy Unit, Nursing Home Residence “La Florida”, Alicante, Spain
Verapamil is a phenylalkylamine class calcium channel blocker that for half a century has been used for the treatment of cardiovascular diseases. Nowadays, verapamil is also considered as a drug option for the treatment of several neurological and psychiatric disorders, such as cluster headache, bipolar disorders, epilepsy, and neurodegenerative diseases. Here, we review insights into the potential preventive and therapeutic role of verapamil on Alzheimer’s disease (AD) based on limited experimental and clinical data. Pharmacological studies have shown that verapamil has a wide therapeutic spectrum, including antihypertensive, anti-inflammatory, and antioxidative effects, regulation of the blood-brain barrier function, due to its effect on P-glycoprotein, as well as adjustment of cellular calcium homeostasis, which may result in the delay of AD onset or ameliorate the symptoms of patients. However, the majority of the AD individuals are on polypharmacotherapy, and the interactions between verapamil and other drugs need to be considered. Therefore, for an appropriate and successful AD treatment, a personalized approach is more than necessary. A well-known narrow pharmacological window of verapamil efficacy may hinder this approach. It is therefore important to note that the verapamil efficacy may be conditioned by different factors. The onset, grade, and brain distribution of AD pathological hallmarks, the time-sequential appearances of AD-related cognitive and behavioral dysfunction, the chronobiologic and gender impact on calcium homeostasis and AD pathogenesis may somehow be influencing that success. In the future, such insights will be crucial for testing the validity of verapamil treatment on animal models of AD and clinical approaches.
Introduction
Alzheimer’s disease (AD) is one of the most widespread types of irreversible dementia among the older population. It is clinically characterized by a progressive cognitive decline (e.g. memory loss, difficulties in learning new tasks, problems with attention and orientation), behavioral changes (e.g. anxiety, depression, and sleep–wake cycle alterations), and inability to maintain activities of daily living (Lyketsos et al., 2011; Atri, 2019).
The early-onset form of AD (age < 65 years) is distinguished by a rapid progression and is linked to the dominant mutations in amyloid precursor protein (APP) and presenilin proteins 1 and 2 (PS 1 and PS2). Mutation in the apolipoprotein E (ApoE) gen, is related to the sporadic late-onset variation of AD (age > 65 years). This form, present in the majority of AD population, is considered multifactorial, since both genetic predisposition and several other factors contribute to the progression of the disease. Excepting aging as the major determinant, other risk factors are more modifiable including lifestyle habits (mental, physical, and social activities, smoking, nutrition), cardiovascular risk factors (obesity, diabetes mellitus, hypertension, etc.), and head injury (Hersi et al., 2017).
The main AD neuropathological features are the presence of extracellular amyloid deposits (senile plaques) in the brain parenchyma and cerebral blood vessels as well as intracellular neurofibrillary tangles accumulation (Love and Miners, 2016; Cline et al., 2018). Amyloid deposits are composed of abnormally folded amyloid β (Aβ) peptides with 40 or 42 amino acids, produced by sequential cleavage of the transmembrane APP, via β and ƴ-secretase enzymes. Intracellular neurofibrillary tangles are formed by an accumulation of hyperphosphorylated and misfolded tau protein. The neuropathological hallmarks are accompanied by extensive microglia cells and astrocyte activation, the key event in neuroinflammation (McGeer et al., 1989; Eikelenboom et al., 1994; Popović et al., 1998a; Henaka et al., 2015; Kim et al., 2019). These cells produce cytokines (interleukins (ILs), tumor necrosis factors (TNFs), transforming growth factors (TGFs), and interferons (IFN). In AD, some of them have pro-inflammatory (IL-1β, TNFα, and IFN-γ), anti-inflammatory (IL-4, IL-10, and TGF-β) or even both properties (IL-6) (McGeer and McGeer, 2010; Frost et al., 2019).
Both Aβ peptides and PS are implicated in the intracellular Ca2+ dyshomeostasis (Khachaturian, 1989; Bezprozvanny and Mattson, 2008; Demuro et al., 2010; Alzheimer’s Association Calcium Hypothesis, 2017; Pchitskaya et al., 2018). The Aβ peptides produce an excessive elevation in intracellular Ca2+ through the formation of Ca2+-permeable pores in the plasma membrane or increasing Ca2+ influx via activation of L-type Ca2+ channels and N-methyl-D-aspartate (NMDA) receptors. Mutated PSs may form passive Ca2+-leak channels on the endoplasmic reticulum, and together with enhanced ryanodine and inositol 1,4,5-trisphosphate receptors function, augment Ca2+ level in the cytoplasm and mitochondria (Wang and Zheng, 2019). The dysregulation of cellular Ca2+ homeostasis in aging and AD leads to mitochondrial dysfunction, increased production of reactive oxygen species, autophagy, impaired synaptic plasticity, reduced long-term potentiation (LTP), enhanced long-term depression, synaptic loss, cell death, and eventually cognitive decline (Disterhoft et al., 1994; LaFerla, 2002; Thibault et al., 2007; Murchison et al., 2009; Tönnies and Trushina, 2017; Peineau et al., 2018; Liu and Li, 2019). Therefore, targeting the disturbed calcium homeostasis is one plausible option for the prevention and therapy of AD.
Verapamil (C27H38N2O4), a generic name for iproveratril, which was discovered in the mid-1960s, belongs to the first generation of the phenylalkylamine class of calcium channel antagonists (Fleckenstein, 1977; Nayler and Dillon, 1986), and it is commercialized as a racemic mixture of levo (S) and dextro (R) enantiomers. Verapamil causes dilatation of the main coronary and systemic arteries and decreases myocardial contractility (Fleckenstein, 1977; Nayler and Dillon, 1986). Therefore, for many years it has been deployed as a treatment option for cardiovascular diseases, such as hypertension (Lewis et al., 1978; Midtbø et al., 1986), supraventricular tachyarrhythmias (Schamroth et al., 1972; Krikler & Spurrell, 1974), and angina pectoris (Livesley et al., 1973; Parodi et al., 1979). Remarkably, verapamil has been also used as a drug option for the treatment of hypertrophic and keloid scars (Ahuja and Chatterjee, 2014; Verhiel et al., 2015), obesity-associated autophagy defects and fatty liver pathologies (Park and Lee, 2014), osteoarthritis (Matta et al., 2015), cluster headache (Meyer and Hardenberg, 1983; Tfelt-Hansen and Tfelt-Hansen, 2009; Leone et al., 2017; Petersen et al., 2019), bipolar disorders (Wisner et al., 2002; Cipriani et al., 2016; Dubovsky, 2018), type 2 diabetes (Yin et al., 2017; Carvalho et al., 2018; Carnovale et al., 2019), chronic rhinosinusitis (Miyake et al., 2018), Peyronie’s disease (Russo et al., 2018), tuberculosis (Rayasam and Balganesh, 2015; Song and Wu, 2016), epilepsy (Nicita et al., 2016; Turner and Perry, 2017), and reversible cerebral vasoconstriction syndrome (Cappelen-Smith et al., 2017). However, verapamil has expressed ambiguous effects in Parkinson’s disease (García-Albea et al., 1993; Pasternak et al., 2012) and dementia (Maxwell et al., 1999; Yaser et al., 2005; Nimmrich and Eckert, 2013).
The aim of this mini review is to bring together data about the potential effects of verapamil on the prevention and therapy of AD, and highlights some concerns for future research.
Alzheimer’s Disease And Verapamil
Experimental Findings
Ex Vivo Studies
It has been demonstrated that verapamil ameliorated the neurotoxicity caused by Aβ and reduces the Aβ1–40 oligomer levels by improving their efflux from the LS-180 cells, via P-glycoprotein up-regulation (Abuznait et al., 2011). Verapamil also restores the microtubule-binding activity of tau and ameliorates the level of oxidative stress in the SH-SY5Y neuroblastoma cell line exposed to Aβ1–42 (Melone et al., 2018). Physiological studies indicated that the Aβ25–35-induced depression of LTP in a hippocampal slice preparation may be attenuated by verapamil (Freir et al, 2003). Furthermore, this drug protected MC65 neuroblastoma cells from the APP C-terminal fragment-induced neurotoxicity (Anekonda et al., 2011). However, Whitson and Appel (1995) demonstrated the inefficiency of verapamil in the prevention of Aβ1–40 neurotoxicity in a pure hippocampal neuronal culture, suggesting that glial-neuronal interaction is important for the preventive effect of verapamil. The rise of Ca2+ concentration was inhibited by verapamil in a human microglia cells culture treated with Aβ25–35 (Silei et al., 1999).
Verapamil pre-treatment protects IMR-32 cells against the scopolamine-induced cytotoxicity, attenuates oxidative stress, and prevents mitochondrial damage (Ponne et al., 2019). It also augments the expression of genes involved in the cholinergic function (mACR1), Ca2+-dependent memory-related genes (CREB1, CREBBP, BDNF), and synaptic plasticity (GAP43, SYP) that were downregulated by scopolamine (Ponne et al., 2019). Nevertheless, the lipopolysaccharide neurotoxicity in mesencephalic dopaminergic neurons that are dependent on microglia activation and augmented production of pro-inflammatory factors (superoxide, NO, and TNF-α) may be abolished by verapamil treatment (Liu et al., 2011). Similarly, verapamil significantly reduces IFN-γ;-induced neurotoxicity of human astrocytes to SH-SY5Y neuroblastoma cells (Hashioka et al., 2012). Moreover, both R and S-isomers of verapamil show equivalent potency to reduce the microglia-mediated neurotoxicity, even though the R-isomer has much less activity to block L-type calcium channels than the S counterpart (Liu et al., 2011).
In Vivo Studies
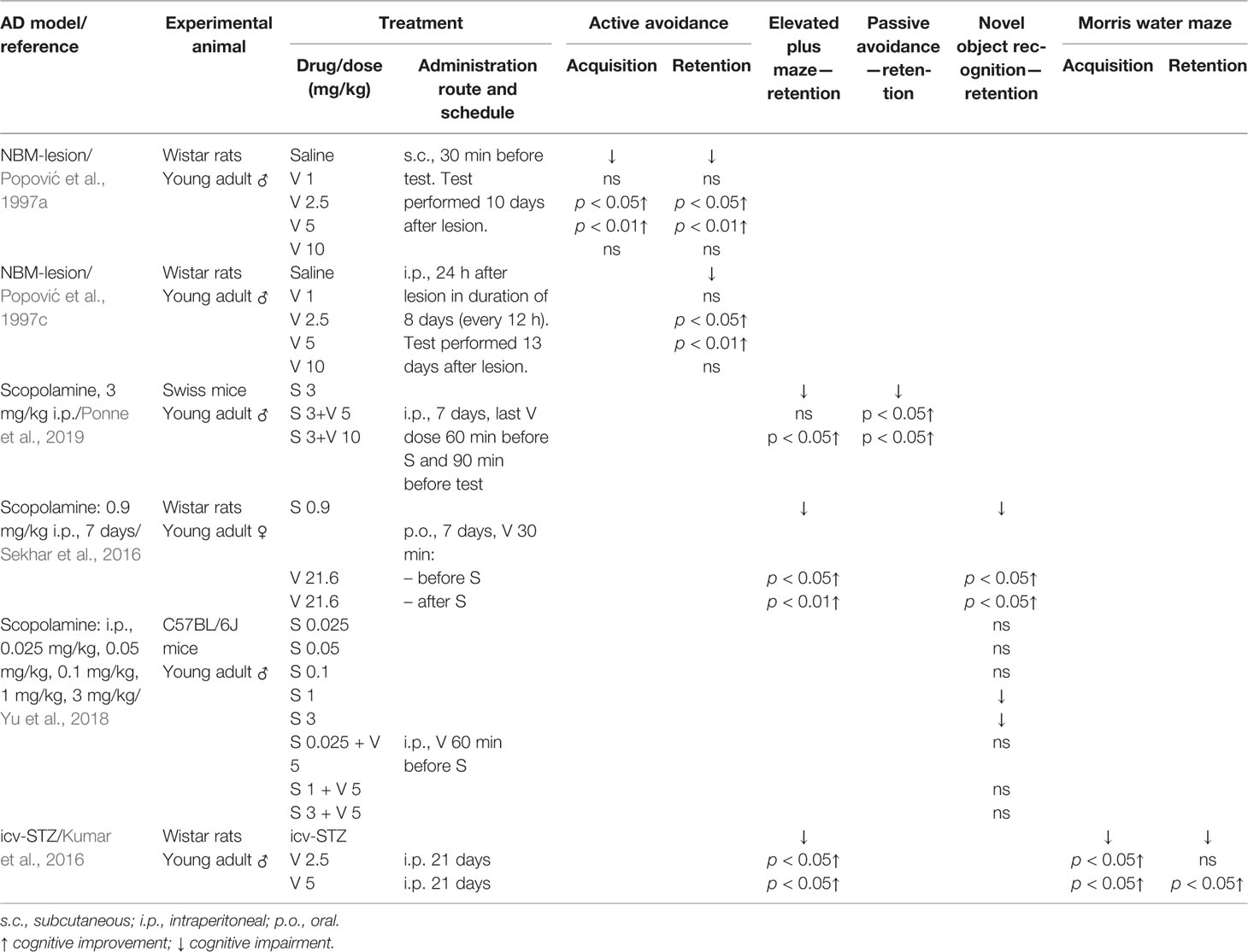
Table 1 Effect of verapamil (V) on learning and memory in three animal models of Alzheimer’s disease (AD): nucleus basalis magnocellularis (NBM) lesion, scopolamine (S) treatment, and intracerebroventricular injection (icv) of streptozotocin (STZ).
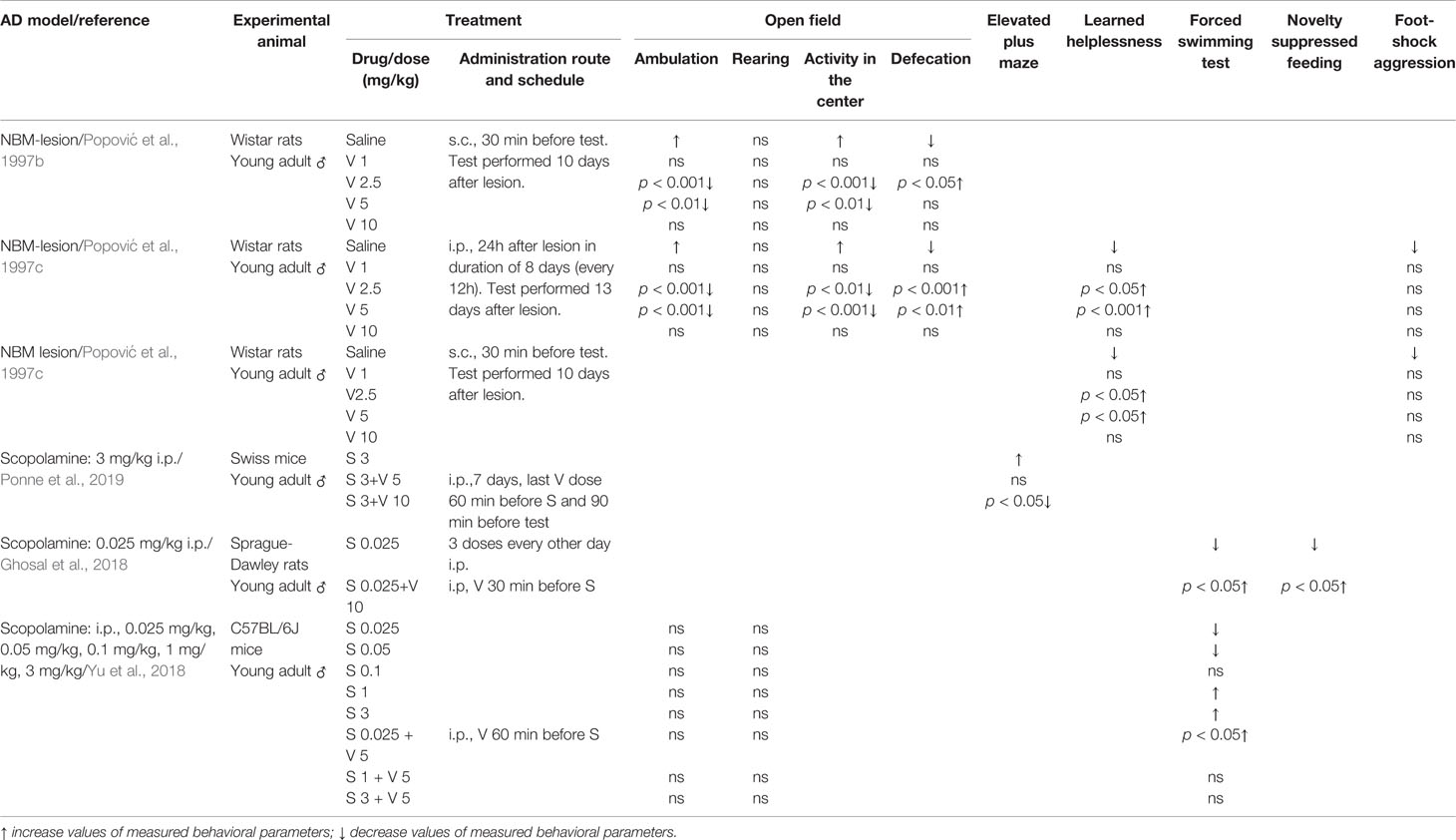
Table 2 Effect of verapamil (V) on anxiety-like behavior (open field and elevated plus maze tests), depressive-like behavior (learned helplessness, forced swimming and novelty suppressed tests) and aggression (foot-shock aggression) in two animal models of Alzheimer’s disease (AD): nucleus basalis magnocellularis (NBM) lesion and scopolamine (S).
Nucleus basalis of Meynert (in non-human animals correspond to the nucleus basalis magnocellularis-NBM) is composed of numerous cholinergic neurons that are affected in AD (Whitehouse et al., 1981; Rinne et al., 1987). Therefore, the lesion of this area was taken as an animal model of AD. Studies from our group with rats supported that verapamil, given in doses of 2.5 and 5 mg/kg, ameliorated the effect of NBM electrolytic lesions on learning and memory in active avoidance test (Popović et al., 1997a), anxiety behavior in open field test (Popović et al., 1997b), learned helplessness-induced depression (Popović et al., 1998b), and body temperature (Popović et al., 1998c). Findings that acute verapamil treatment (5 and 10 mg/kg), in NBM-lesioned rats, abolished cold restraint induced gastric petechiae, while in control rats, it diminished gastric erosion formation, suggest that the verapamil effect may be modified by stress (Popović et al., 1999). The long-term treatment with verapamil in NBM-lesioned rats prevented the impairments in active avoidance memory, open field behavior, and performance in learning helplessness tests (Popović et al., 1997c). Neither acute nor chronic verapamil treatment improved foot-shock induced aggression in NBM-lesioned rats (Popović et al., 1997c; Popović et al., 1998b, respectively). Moreover, morphological studies indicated that long-term verapamil treatment (2.5, 5, and 10 mg/kg/12 h) produced a significant neuroprotection on NADPH-diaphorase and ChAT-immunopositive cortical neurons in NBM lesioned rats (Caballero-Bleda et al., 2001; Popović et al., 2006). The above-mentioned studies indicated that verapamil expresses an inverted U-shape mode of action (Tables 1 and 2).
Systemic administration of scopolamine, a non-selective muscarinic receptor antagonist, has been considered as a pharmacological model of AD (Kwon et al., 2010; Pandareesh and Anand, 2013; Xiao et al., 2014). Long-term verapamil treatment, in a dose of 10 mg/kg (but not in a dose of 5 mg/kg), attenuated the increased mouse locomotion induced by scopolamine (Ponne et al., 2019). However, verapamil in both used doses, improved memory deficit in the elevated plus maze task and passive avoidance test, reversed the increased acetylcholinesterase (AChE) activity, and preserved the mouse brain from the oxidative stress induced by scopolamine (Ponne et al., 2019). Prophylactic and curative treatment by verapamil significantly abolished scopolamine-induced impairment in both the elevated plus maze test and the novel object recognition task in female rats (Sekhar et al., 2016). The above-mentioned treatment significantly enhanced oxidative stress markers that were decreased by scopolamine and reduced the enhanced AChE level. Furthermore, verapamil, given in a dose of 10 mg/kg, prevented the anti-depressive effect of scopolamine in rats tested in forced swim and novelty suppressed feeding tests. In the same animals, verapamil blocked the scopolamine-induced increase of the brain-derived neurotrophic factor (BDNF)/TrkB activation in the prefrontal cortex (Ghosal et al., 2018). Similarly, verapamil (5 mg/kg) significantly blockaded: 1) the scopolamine antidepressant-like effect, in mice tested in a forced swimming test; and 2) scopolamine upregulated the effect on BDNF and neuropeptide VGF (nonacronymic) content, in the hippocampus and prefrontal cortex (Yu et al., 2018). However, verapamil failed to abolish scopolamine-induced amnesia in the novel object recognition test (Yu et al., 2018) (Tables 1 and 2). Our recent study, focused on a passive avoidance task in rats, demonstrated that verapamil, at the dose that per se does not affect the memory consolidation, significantly reversed the memory consolidation improvement induced by scopolamine (Giménez de Bejar et al., 2017).
Intracerebroventricular injection (icv) of streptozotocin (STZ) has been described as an experimental model for sporadic AD (Kumar et al., 2016). In this rat model, chronic verapamil treatment (2.5 and 5 mg/kg) significantly improved memory in both the elevated plus maze test and the Morris water maze task (Kumar et al., 2016). Moreover, at mentioned doses, verapamil attenuated AChE activity in the hippocampus and frontal cortex. However, only the higher verapamil dose attenuated the oxidative stress damage and the level of TNF-α in the hippocampus and frontal cortex of icv-STZ-injected rats (Kumar et al., 2016) (Table 1). At the same dose, verapamil significantly reduced number of apoptotic pyramidal cells at the CA3 hippocampal region.
The icv administration of peptide Aβ25–35 in adult Wistar rats causes depression of LTP in the hippocampal CA1 area. This depression is significantly reversed by intraperitoneal administration of 1 mg/kg of verapamil, and with less efficacy at 10 mg/kg (Freir et al., 2003).
Verapamil (1 mg/kg/day), given orally (drinking water) for 3 months, inhibits Aβ formation and Ser202/Thr205 phosphorylation of tau by blocking the TXNIP/ROS/p38 MAPK pathway in the hippocampus of 5xFAD transgenic mice (Melone et al., 2018).
Clinical Findings
Studies on AD patient brains have shown that phenylalkylamines receptor binding parameters, KD (affinity) and Bmax (density), are not affected in frontal, temporal, parietal, and occipital cortices, hippocampus, striatum, and thalamus (Sen et al., 1993), suggesting, altogether, that clinical trials with verapamil should not be impeded by the functionality of these receptors. One limited prospective study indicated that the use of verapamil neither significantly reduced the risk of developing AD nor had an effect on the risk of all-cause mortality (Yaser et al., 2005).
Particularities of Mechanism of Action of Verapamil: Pros and Cons
Although initially considered as an L-type Ca2+ channel blocker (Cav1.2 and Cav1.3 alpha subunits), verapamil also binds the alpha subunits of P/Q type (Cav2.1), N-type (Cav2.2), R-type (Cav2.3), and T-type (Cav3.1 and Cav3.2) calcium channels (Ishibashi et al., 1995; Cai et al., 1997; Dobrev et al., 1999; Tarabova et al., 2007; Kuryshev et al., 2014). Both Cav1.2 and Cav1.3 alpha subunits are similarly upregulated at the surface level in rat cultured hippocampal neurons exposed to the Aβ25–35 (Kim and Rhim, 2011). On one hand, a study performed in 2, 4- and 11-month transgenic mice, overexpressing hAβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations, indicated that the expression of Cav1.2 α1-subunits in reactive astrocytes, is related to the increased amyloid-β load in the plaques (Daschil et al., 2013). On the other hand, a blockade of the Cav3.1 T-type channel reduces non-amyloidogenic processing and produces higher levels of Aβ peptide in the brain of 3xTg-AD mice aged 14 to 16 months (Rice et al., 2014). The pathological, aggregated form of Aβ1–40, reduced Ca2+ channel current density in cortical neurons, via an action on N-type calcium channels (Ramsden et al., 2002), as well as Aβ1–42 globulomer inhibited presynaptic P/Q calcium currents in hippocampal cells (Nimmrich et al., 2008). These findings pinpointed that verapamil may have a beneficial effect on L-type Ca2+ channels but not on P/Q-type, N-type and T-type Ca2+ channels.
However, verapamil, as a small conductance calcium-activated potassium channels (SK channel) antagonist (Tao et al., 2013), may have a dual effect: improve memory but fail to have a neuroprotective role in AD (Lam et al., 2013).
Besides its general action as inhibitor of the transmembrane influx of extracellular calcium ions, verapamil, in several brain regions (e.g. cerebral cortex, hippocampus, hypothalamus), acts as an antagonist of muscarinic (Baumgold, 1986; Popova et al., 1990), α- and β-adrenergic (Galzin and Langer, 1983; Staneva-Stoytcheva et al., 1990; Staneva-Stoytcheva et al., 1992), dopaminergic (Sitges and Guarneros, 1998), serotoninergic (Taylor and Defeudis, 1984; Adachi and Shoji, 1986; Green et al., 1990; Popova et al., 1991; Shad and Saeed, 2007), and GABAergic receptors (Staneva-Stoytcheva et al., 1991). Thus, it is likely from these findings and the impairments of cholinergic (Hampel et al., 2018), GABAergic (Li et al., 2016), and monoaminergic transmission (Šimić et al., 2017) in AD disease that verapamil could modify the neurotransmission in AD.
P-glycoprotein is a transmembrane glycoprotein localized in several barriers (e.g. blood-brain barrier (BBB), blood-cerebrospinal fluid barrier (BCSFB)). Its presence on the luminal membrane of the endothelial cells at the BBB (Beaulieu et al., 1997), restricts the entry of an exogenous compound (e.g., drugs, xenobiotics, toxins) into the brain and also regulates the cellular efflux from the brain. Most of the studies support that P-glycoprotein is important for Aβ1–40 and Aβ1–42 clearance, and that dysfunction of this process might lead to AD development (Lam et al., 2001; Vogelgesang et al., 2002; Vogelgesang et al., 2004; Cirrito et al., 2005; Kuhnke et al., 2007; Silverberg et al., 2010). Furthermore, microglia activation and, consequently, secretion of pro-inflammatory cytokines can disturb the P-glycoprotein production and function. On one hand, verapamil as P-glycoprotien inhibitor (Bendayan et al., 2002) may diminish the efflux of Aβ1–40 and Aβ1–42, while, on the other hand, it may restrain the pro-inflammatory processes and permit the entrance of some beneficial drugs, too.
During normal aging, there is a progressive decline in the BBB P-glycoprotein activity, particularly in men but not in women, that can lead to the accumulation of harmful compounds in the brain parenchyma (Bartels, 2011; van Assema et al., 2012a). Furthermore, the decreased BBB P-glycoprotein function in young women, compared with young men, may implicate an increased risk of AD in women (van Assema et al., 2012a). Nowadays, R-[11C]verapamil isomer is being used for the evaluation of the blood-brain barrier function in aging and AD, since its high uptake as radiotracer indicates decreased P-glycoprotein functionality in these conditions (Luurtsema et al., 2003; van Assema et al., 2012b; Chai et al., 2019).
Intriguingly, low and high systolic blood pressure trajectories from mid- to late-life have been associated with cognitive deficit and increased risk of AD (Skoog et al., 1996; Kivipelto et al., 2001; Qiu et al., 2004; McGrath et al., 2017). The daily fluctuations of blood pressure highlight the importance of synchronizing antihypertensive therapy with time of day (Hermida et al., 2005; Hermida et al., 2007). Similarly, the half-life of verapamil during the evening dosing regimen is much longer than the morning treatment (Jespersen et al., 1989). Bearing in mind that intracellular Ca2+ oscillates on a circadian and ultradian rhythm base (Noguchi et al., 2017; Wu et al., 2018) and that circadian rhythm is highly disturbed in AD patients (Coogan et al., 2013) a chronotherapy with verapamil in AD patents should be considered.
Finally, since the age and gender may modify the verapamil pharmacokinetic and pharmacodynamic effect on a stereoselective (S- and R-isomers) base (Schwartz, 1990; Sasaki et al., 1993; Schwartz et al., 1993; Schwartz et al., 1994; Gupta et al., 1995; Schwartz, 1996; Krecic-Shepard et al., 2000; Dadashzadeh et al., 2006), it seems reasonable to consider the Franco and Petrovic (2015) proposal, which suggests testing the efficacy of S- and R-verapamil molecules in AD models separately.
Conclusion
Experimental data in animal models of AD indicated a potential positive effect of verapamil in ameliorating AD-like pathology. However, the lack of clinical findings limits its potential use in AD therapy. Probably, instead of a racemic mixture of both isomers, the equilibrate percent of each isomer should be considered. Moreover, the dose used, daytime therapy schedule, and route of verapamil application should be adjusted depending on individual conditions (gender, age, the severity of AD pathology, person’s chronotype, etc). Multimorbidity increases with aging and most of the AD patients are on polypharmacotherapy. Therefore, a possible interaction between verapamil and other drugs needs to be considered. Bearing in mind that presently, more than eight million people, just in the USA, are currently on verapamil treatment, it would seem that a rational approach to personalized AD therapy will not only be beneficial for patients, but it will also decrease the harmful impact of verapamil on the environment (Saari et al., 2017) and health economics.
Author Contributions
All authors managed the literature searches, have read and approved the final manuscript.
Funding
Funding was provided by the Spanish Ministry of Science, Innovation and Universities and European Regional Development Fund (FEDER; PGC2018-098229-B-100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abuznait, A. H., Cain, C., Ingram, D., Burk, D., Kaddoumi, A. (2011). Up-regulation of P-glycoprotein reduces intracellular accumulation of beta amyloid: investigation of P-glycoprotein as a novel therapeutic target for Alzheimer’s disease. J. Pharm. Pharmacol. 63, 1111–1118. doi: 10.1111/j.2042-7158.2011.01309.x
Adachi, H., Shoji, T. (1986). Characteristics of the inhibition of ligand binding to serotonin receptors in rat brain membranes by verapamil. Jpn. J. Pharmacol. 41, 431–435. doi: 10.1254/jjp.41.431
Ahuja, R. B., Chatterjee, P. (2014). Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 40, 583–588. doi: 10.1016/j.burns.2013.09.029
Alzheimer’s Association Calcium Hypothesis Workgroup (2017). Calcium Hypothesis of Alzheimer’s disease and brain aging: a framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer’s Dement. 13, 178–182 e117. doi: 10.1016/j.jalz.2016.12.006
Anekonda, T. S., Quinn, J. F., Harris, C., Frahler, K., Wadsworth, T. L., Woltjer, R. L. (2011). L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 41, 62–70. doi: 10.1016/j.nbd.2010.08.020
Atri, A. (2019). The Alzheimer’s disease clinical spectrum: diagnosis and management. Med. Clin. North. Am. 103, 263–293. doi: 10.1016/j.mcna.2018.10.009
Bartels, A. L. (2011). Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr. Pham. Des. 17, 2771–2777. doi: 10.2174/138161211797440122
Baumgold, J. (1986). Effects of verapamil on the binding characteristics of muscarinic receptor subtypes. Eur. J. Pharmacol. 126, 151–154. doi: 10.1016/0014-2999(86)90752-1
Beaulieu, E., Demeule, M., Ghitescu, L., Beliveau, R. (1997). P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 326, 539–544. doi: 10.1042/bj3260539
Bendayan, R., Lee, G., Bendayan, M. (2002). Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc. Res. Ech. 57, 365–380. doi: 10.1002/jemt.10090
Bezprozvanny, I., Mattson, M. P. (2008). Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 31, 454–463. doi: 10.1016/j.tins.2008.06.005
Caballero-Bleda, M., Redondo-Aniorte, F. J., Aldeguer-Montiel, A., Popović, N., Popović, M., Puelles, L. (2001). NADPH-diaphorase activity in the frontal cortex of NBM-lesioned rats treated with verapamil. Neurosci. Res. Commun. 28, 115–122. doi: 10.1002/nrc.1011
Cai, D., Mulle, J. G., Yue, D. T. (1997). Inhibition of recombinant Ca2+ channels by benzothiazepines and phenylalkylamines: class-specific pharmacology and underlying molecular determinants. Mol. Pharmacol. 51, 872–881. doi: 10.1124/mol.51.5.872
Cappelen-Smith, C., Calic, Z., Cordato, D. (2017). Reversible cerebral vasoconstriction syndrome: recognition and treatment. Curr. Treat. Options Neurol. 19, 21. doi: 10.1007/s11940-017-0460-7
Carnovale, C., Dassano, A., Mosini, G., Mazhar, F., D´Addio, F., Pozzi, M., et al. (2019). The β−cell effect of verapamil−based treatment in patients with type 2 diabetes: a systematic review. Acta Diabetol. 57, 117–131. doi: 10.1007/s00592-019-01370-1
Carvalho, D. S., de Almeida, A. A., Borges, A. F., Vannucci Campos, D. (2018). Treatments for diabetes mellitus type II: new perspectives regarding the possible role of calcium and cAMP interaction. Eur. J. Pharmacol. 830, 9–16. doi: 10.1016/j.ejphar.2018.04.002
Chai, A. B., Leung, G. K. F., Callaghan, R., Gelissen, I. C. (2019). P-glycoprotein: a role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 287, 612–625. doi: 10.1111/febs.15148
Cipriani, A., Saunders, K., Attenburrow, M. J., Stefaniak, J., Panchal, P., Stockton, S., et al. (2016). A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Mol. Psychiatry 21, 1324–1332. doi: 10.1038/mp.2016.86
Cirrito, J. R., Deane, R., Fagan, A. M., Spinner, M. L., Parsadanian, M., Finn, M. B., et al. (2005). P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290. doi: 10.1172/JCI25247
Cline, E. N., Bicca, M. A., Viola, K. L., Klein, W. L. (2018). The amyloid-β oligomer hypothesis: beginning of the third decade. J. Alzheimer’s Dis. 64 (s1), S567–S610. doi: 10.3233/JAD-179941
Coogan, A. N., Schutová, S., Husung, S., Furczyk, K., Baune, B. T., Kropp, P., et al. (2013). The circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunities. Biol. Psychiatry 74, 333–339. doi: 10.1016/j.biopsych.2012.11.021
Dadashzadeh, S., Javadian, B., Sadeghian, S. (2006). The effect of gender on the pharmacokinetics of verapamil and norverapamil in human. Biopharm. Drug Dispos. 27, 329–334. doi: 10.1002/bdd.512
Daschil, N., Obermair, G. J., Flucher, B. E., Stefanova, N., Hutter-Paier, B., Windisch, M., et al. (2013). Cav1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-β plaques in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 37, 439–451. doi: 10.3233/JAD-130560
Demuro, A., Parker, I., Stutzmann, G. E. (2010). Calcium signaling and amyloid toxicity in Alzheimer disease. J. Biol. Chem. 285, 12463–12468. doi: 10.1074/jbc.R109.080895
Disterhoft, J. F., Moyer, J. R., Jr., Thompson, L. T. (1994). The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann. N.Y. Acad. Sci. 747, 382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x
Dobrev, D., Milde, A. S., Andreas, K., Ravens, U. (1999). The effects of verapamil and diltiazem on N-, P- and Q-type calcium channels mediating dopamine release in rat striatum. Br. J. Pharmacol. 127, 576–582. doi: 10.1038/sj.bjp.0702574
Dubovsky, S. L. (2018). Applications of calcium channel blockers in psychiatry: pharmacokinetic and pharmacodynamic aspects of treatment of bipolar disorder. Expert Opin. Drug Metab. Toxicol. 15, 35–47. doi: 10.1080/17425255.2019.1558206
Eikelenboom, P., Zhan, S. S., van Gool, W. A., Allsop, D. (1994). Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol. Sci. 15, 447–450. doi: 10.1016/0165-6147(94)90057-4
Fleckenstein, A. (1977). Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Ann. Rev. Pharmacol. Toxicol. 17, 149–166. doi: 10.1146/annurev.pa.17.040177.001053
Franco, R., Petrovic, M. (2015). Suggesting a way to understand the actual potential of anti-Alzheimer’s disease drugs that show promise in transgenic mouse models. Front. Neurol. 6, 206. doi: 10.3389/fneur.2015.00206
Freir, D. B., Costello, D. A., Herron, C. A. (2003). Aβ25-35-induced depression of long-term potentiation in area CA1 in vivo and in vitro is attenuated by verapamil. J. Neurophysiol. 89, 3061–3069. doi: 10.1152/jn.00992.2002
Frost, G. R., Jonas, L. A., Li, Y.-M. (2019). Friend, foe or both? Immune activity in Alzheimer’s disease. Front. Aging Neurosci. 11, 337. doi: 10.3389/fnagi.2019.00337
Galzin, A. M., Langer, S. Z. (1983). Presynaptic alpha 2-adrenoceptor antagonism by verapamil but not by diltiazem in rabbit hypothalamic slices. Br. J. Pharmacol. 78, 571–577. doi: 10.1111/j.1476-5381.1983.tb08817.x
García-Albea, E., Jiménez-Jiménez, F. J., Ayuso-Peralta, L., Cabrera-Valdivia, F., Vaquero, A., Tejeiro, J. (1993). Parkinsonism unmasked by verapamil. Clin. Neuropharmacol. 16, 263–265. doi: 10.1097/00002826-199306000-00011
Ghosal, S., Bang, E., Yue, W., Hare, B. D., Lepack, A. E., Girgenti, M. J., et al. (2018). Activity-dependent BDNF release is required for the rapid antidepressant actions of scopolamine. Biol. Psychiatry 83, 29–37. doi: 10.1016/j.biopsych.2017.06.017
Giménez de Bejar, V., Caballero Bleda, M., Popović, N., Popović, M. (2017). Verapamil blocks scopolamine enhancement effect on memory consolidation in passive avoidance task in rats. Front. Pharmacol. 8, 566. doi: 10.3389/fphar.2017.00566
Green, A. R., DeSouza, R. J., Davies, E. M., Cross, A. J. (1990). The effects of Ca2+ antagonists and hydralazine on central 5-hydroxytryptamine biochemistry and function in rats and mice. Br. J. Pharmacol. 99, 41–46. doi: 10.1111/j.1476-5381.1990.tb14651.x
Gupta, S. K., Atkinson, L., Tu, T., Longstreth, J. A. (1995). Age and gender related changes in stereoselective pharmacokinetics and pharmacodynamics of verapamil and norverapamil. Br. J. Clin. Pharmacol. 40, 325–331. doi: 10.1111/j.1365-2125.1995.tb04554.x
Hampel, H., Mesulam, M. M., Cuello, A. C., Farlow, M. R., Giacobini, E., Grossber, G. T., et al. (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 141, 1917–1933. doi: 10.1093/brain/awy132
Hashioka, S., Klegeris, A., McGeer, P. L. (2012). Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology 63, 685–691. doi: 10.1016/j.neuropharm.2012.05.033
Henaka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405. doi: 10.1016/S1474-4422(15)70016-5
Hermida, R. C., Ayala, D. E., Calvo, C. (2005). Administration-time-dependent effects of antihypertensive treatment on the circadian pattern of blood pressure. Curr. Opin. Nephrol. Hypertens. 14, 453–459. doi: 10.1097/01.mnh.0000174144.07174.74
Hermida, R. C., Ayala, D. E., Calvo, C., Portaluppi, F., Smolensky, M. H. (2007). Chronotherapy of hypertension: administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv. Drug Deliv. Rev. 59, 923–939. doi: 10.1016/j.addr.2006.09.021
Hersi, M., Irvine, B., Gupta, P., Gomes, J., Birkett, N., Krewski, D. (2017). Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology 61, 143–187. doi: 10.1016/j.neuro.2017.03.006
Ishibashi, H., Yatani, A., Akaike, N. (1995). Block of P-type Ca2+ channels in freshly dissociated rat cerebellar Purkinje neurons by diltiazem and verapamil. Brain Res. 695, 88–91. doi: 10.1016/0006-8993(95)00815-8
Jespersen, C. M., Frederiksen, M., Hansen, J. F., Klitgaard, N. A., Sørum, C. (1989). Circadian variation in the pharmacokinetics of verapamil. Eur. J. Clin. Pharmacol. 37, 613–615. doi: 10.1007/bf00562555
Khachaturian, Z. S. (1989). Calcium, membranes, aging, and Alzheimer’s disease. Introduction and overview. Ann. N. Y. Acad. Sci. 568, 1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x
Kim, S., Rhim, H. (2011). Effects of Amyloid-β peptides on voltage-gated L-type Cav1.2 and Cav1.3 Ca2+ channels. Mol. Cells 32, 289–294. doi: 10.1007/s10059-011-0075-x
Kim, S. H., Noh, M. Y., Kim, H. J., Oh, K. W., Park, J., Lee, S., et al. (2019). A therapeutic strategy for Alzheimer’s disease focused on immune-inflammatory modulation. Dement. Neurocogn. Disord. 18, 33–46. doi: 10.12779/dnd.2019.18.2.33
Kivipelto, M., Helkala, E. L., Laakso, M. P., Hänninen, T., Hallikainen, M., Alhainen, K., et al. (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population-based study. BMJ 322, 1447–1451. doi: 10.1136/bmj.322.7300.1447
Krecic-Shepard, M. E., Barnas, C. R., Slimko, J., Jones, M. P., Schwartz, J. B. (2000). Gender-specific effects on verapamil pharmacokinetics an7d pharmacodynamics in humans. J. Clin. Pharmacol. 40, 219–230. doi: 10.1177/00912700022008883
Krikler, D. M., Spurrell, R. A. J. (1974). Verapamil in the treatment of paroxysmal supraventicular tachycardia. Postgrad. Med. J. 50, 447–453. doi: 10.1136/pgmj.50.585.447
Kuhnke, D., Jedlitschky, G., Grube, M., Krohn, M., Jucker, M., Mosyagin, I., et al. (2007). MDR1-P-Glycoprotein (ABCB1) Mediates transport of Alzheimer’s amyloid-β peptides–implications for the mechanisms of Aβ clearance at the blood-brain barrier. Brain Pathol. 17, 347–353. doi: 10.1111/j.1750-3639.2007.00075.x
Kumar, A., Ekavali, Mishra, J., Chopra, K., Dhull, D. K. (2016). Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacol. (Berl). 233, 137–152. doi: 10.1007/s00213-015-4095-7
Kuryshev, Y. A., Brown, A. M., Duzic, E., Kirsch, G. E. (2014). Evaluating state dependence and subtype selectivity of calcium channel modulators in automated electrophysiology assays. Assay Drug Dev. Technol. 12, 110–119. doi: 10.1089/adt.2013.552
Kwon, S. H., Lee, H. K., Kim, J. A., Hong, S. I., Kim, H. C., Jo, T. H., et al. (2010). Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via antiacetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 649, 210–217. doi: 10.1016/j.ejphar.2010.09.001
LaFerla, F. M. (2002). Calcium dyshomeostasis and intracellular signaling in Alzheimer´s disease. Nat. Rev. Neurosci. 3, 862–872. doi: 10.1038/nrn960
Lam, F. C., Liu, R., Lu, P., Shapiro, A. B., Renoir, J., Sharom, F. J., et al. (2001). β-amyloid efflux mediated by p-glycoprotein. J. Neurochem. 76, 1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x
Lam, J., Coleman, N., Garing, A. L. A., Wulff, H. (2013). The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin. Ther. Targets 17, 1203–1220. doi: 10.1517/14728222.2013.823161
Leone, M., Giustiniani, A., Proietti Cecchini, A. (2017). Cluster headache: present and future therapy. Neurol. Sci. 38, S45–S50. doi: 10.1007/s10072-017-2924-7
Lewis, G. R. J., Morley, K. D., Lewis, B. M., Bones, P. J. (1978). The treatment of hypertension with verapamil. N.Z. Med. J. 87, 351–354.
Li, Y., Sun, H., Chen, Z., Xu, H., Bu, G., Zheng, H. (2016). Implications of GABAergic neurotransmission in Alzheimer´s disease. Front. Aging Neurosci. 8, 31. doi: 10.3389/fnagi.2016.00031
Liu, J., Li, L. (2019). Targeting autophagy for the treatment of Alzheimer’s disease: challenges and opportunities. Front. Mol. Neurosci. 12, 203. doi: 10.3389/fnmol.2019.00203
Liu, Y., Lo, Y. C., Qian, L., Crews, F. T., Wilson, B., Chen, H. L., et al. (2011). Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology 60, 373–380. doi: 10.1016/j.neuropharm.2010.10.002
Livesley, B., Catley, P. F., Campbell, R. C., Oram, S. (1973). Double-blind evaluation of verapamil, propranolol and isosorbide dinitrate against placebo in the treatment of angina pectoris. Br. Med. J. 1, 375–378. doi: 10.1136/bmj.1.5850.375
Love, S., Miners, J. S. (2016). Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 131, 645–658. doi: 10.1007/s00401-015-1522-0
Luurtsema, G., Molthoff, C. F., Windhorst, A. D., Smit, J. W., Keizer, H., Boellaard, R., et al. (2003). (R)- and (S)-[11C]verapamil as PET-tracers for measuring P-glycoprotein function: in vitro and in vivo evaluation. Nucl. Med. Biol. 30, 747–751. doi: 10.1016/s0969-8051(03)00078-7
Lyketsos, C. G., Carillo, M. C., Ryan, J. M., Khachaturian, A. S., Trzepacz, P., Amatniek, J., et al. (2011). Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s. Dement. 7, 532–539. doi: 10.1016/j.jalz.2011.05.2410
Matta, C., Zákány, R., Mobasheri, A. (2015). Voltage-dependent calcium channels in chondrocytes: Roles in health and disease. Curr. Rheumatol. Rep. 17, 43. doi: 10.1007/s11926-015-0521-4
Maxwell, C. J., Hogan, D. B., Ebly, E. M. (1999). Calcium-channel blockers and cognitive function in elderly people: results from the Canadian study of health and aging. CMAJ 161, 501–506. doi: 10.1136/bmj.319.7213.806b
McGeer, E. G., McGeer, P. L. (2010). Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J. Alzheimers Dis. 19, 355–361. doi: 10.3233/JAD-2010-1219
McGeer, P. L., Akiyama, H., Itagaki, S., McGeer, E. G. (1989). Immune system response in Alzheimer´s disease. Can. J. Neurol. Sci. 16, 516–527. doi: 10.1017/s0317167100029863
McGrath, E. R., Beiser, A. S., DeCarli, C., Plourde, K. L., Vasan, R. S., Greenber, S. M., et al. (2017). Blood pressure from mid- to late life and risk of incident dementia. Neurology 89, 2447–2454. doi: 10.1212/WNL.0000000000004741
Melone, M. A. B., Dato, C., Paladino, S., Coppola, C., Trebini, C., Giordana, M. T., et al. (2018). Verapamil inhibits Ser202/Thr205 phosphorylation of tau by blocking TXNIP/ROS/p38 MAPK pathway. Pharm. Res. 35:44, 1–14. doi: 10.1007/s11095-017-2276-2
Meyer, J. S., Hardenberg, J. (1983). Clinical effectiveness of calcium entry blockers in prophylactic treatment of migraine and cluster headaches. Headache. 23, 266–277. doi: 10.1111/j.1526-4610.1983.hed2306266.x
Midtbø, K., Hals, O., Lauve, O., van der Meer, J., Storstein, L. (1986). Studies on verapamil in the treatment of essential hypertension: a review. Br. J. Clin. Pharamacol. 21 (Suppl. 2), 165S–171S. doi: 10.1111/j.1365-2125.1986.tb02867.x
Miyake, M. M., Nocera, A., Miyake, M. M. (2018). P-glycoprotein and chronic rhinosinusitis. World J. Otorhinolaryngol. Head Neck Surg. 4, 169–174. doi: 10.1016/j.wjorl.2018.07.002
Murchison, D., McDermott, A. N., LaSarge, C. L., Peebles, K. A., Bizon, J. L., Griffith, W. H. (2009). Enhanced calcium buffering in F344 rat cholinergic basal forebrain neurons is associated with age-related cognitive impairment. J. Neurophysiol. 102, 2194–2207. doi: 10.1152/jn.00301.2009
Nayler, W. G., Dillon, J. S. (1986). Calcium antagonists and their mode of action: an historical overview. Br. J. Clin. Pharmac. 21, 97S–107S. doi: 10.1111/j.1365-2125.1986.tb02859.x
Nicita, F., Spalice, A., Raucci, U., Iannetti, P., Parisi, P. (2016). The possible use of the L-type calcium channel antagonist verapamil in drug-resistant epilepsy. Expert Rev. Neurother. 16, 9–15. doi: 10.1586/14737175.2016.1121097
Nimmrich, V., Eckert, A. (2013). Calcium channel blockers and dementia. Br. J. Pharmacol. 169, 1203–1210. doi: 10.1111/bph.12240
Nimmrich, V., Grimm, C., Draguhn, A., Barghorn, S., Lehmann, A., Schoemaker, H., et al. (2008). Amyloid β oligomers (Aβ1-42 globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J. Neurosci. 28, 788–797. doi: 10.1523/JNEUROSCI.4771-07.2008
Noguchi, T., Leise, T. L., Kingsbury, N. J., Diemer, T., Wang, L. L., Henson, M. A., et al. (2017). Calcium circadian rhythmicity in the suprachiasmatic nucleus: cell autonomy and network modulation. eNeuro 4, 1–12. doi: 10.1523/ENEURO.0160-17.2017
Pandareesh, M. D., Anand, T. (2013). Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol. Neurobiol. 33, 875–884. doi: 10.1007/s10571-013-9952-5
Park, H.-W., Lee, J. H. (2014). Calcium channel blockers as potential therapeutics for obesity-associated autophagy defects and fatty liver pathologies. Autophagy 10, 2385–2386. doi: 10.4161/15548627.2014.984268
Parodi, O., Maseri, A., Simonetti, I. (1979). Management of unstable angina at rest by verapamil. A double-blind crossover study in coronary care unit. Br. Heart J. 41, 167–174. doi: 10.1136/hrt.41.2.167
Pasternak, B., Svanström, H., Nielsen, N. M., Fugger, L., Melbye, M., Hviid, A. (2012). Use of calcium channel blockers and Parkinson’s disease. Am. J. Epidemiol. 175, 627–635. doi: 10.1093/aje/kwr362
Pchitskaya, E., Popugaeva, E., Bezprozvanny, I. (2018). Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70, 87–94. doi: 10.1016/j.ceca.2017.06.008
Peineau, S., Rabiant, K., Pierrefiche, O., Potier, B. (2018). Synaptic plasticity modulation by circulating peptides and metaplasticity: involvement in Alzheimer’s disease. Pharmacol. Res. 130, 385–401. doi: 10.1016/j.phrs.2018.01.018
Petersen, A. S., Barloese, M. C. J., Snoer, A., Soerensen, A. M. S., Jensen, R. H. (2019). Verapamil and cluster headache: Still a mystery. A narrative review of efficacy, mechanisms and perspectives. Headache 59, 1198–1211. doi: 10.1111/head.13603
Ponne, S., Kumar, C. R., Boopathy, R. (2019). Verapamil attenuates scopolamine induced cognitive deficits by averting oxidative stress and mitochondrial injury– a potential agent for Alzheimer’s disease. Metab. Brain Dis. 35, 503–515. doi: 10.1007/s11011-019-00498-x
Popova, J., Staneva-Stoytcheva, D., Mutafova, V. (1990). Effects of the Ca2+-antagonists nifedipine, verapamil, flunarizine and of the calmodulin antagonist trifluoperazine on muscarinic cholinergic receptors in rat cerebral cortex. Gen. Pharmacol. 21, 317–319. doi: 10.1016/0306-3623(90)90830-f
Popova, J., Staneva-Stoytcheva, D., Ivanova, E., Tosheva, T. (1991). The long-term treatment with the Ca2+-antagonists nifedipine, verapamil, flunarizine and with the calmodulin antagonist trifluoperazine decreases the activity of 5-HT1 receptors in rat cerebral cortex and hippocampus. Gen. Pharmacol. 22, 1147–1149. doi: 10.1016/0306-3623(91)90593-u
Popović, M., Popović, N., Jovanova-Nešić, K., Bokonjić, D., Dobrić, S., Kostić V.S. and Rosić, N. (1997a). Effect of physostigmine and verapamil on active avoidance in an experimental model of Alzheimer´s disease. Int. J. Neurosci. 90, 87–97. doi: 10.3109/00207459709000628
Popović, M., Popović, N., Jovanova-Nešić, K., Bokonjić, D., Dobrić, S., Rosić, N. (1997b). Open field behavior in nucleus basalis magnocellularis-lesioned rats treated with physostigmine and verapamil. Int. J. Neurosci. 91, 181–188. doi: 10.3109/00207459708986375
Popović, M., Caballero-Bleda, M., Popović, N., Bokonjić, D., Dobrić, S. (1997c). Neuroprotective effect of chronic verapamil treatment on cognitive and noncognitive deficits in an experimental Alzheimer’s disease in rats. Int. J. Neurosci. 92, 87–97. doi: 10.3109/00207459708986392
Popović, M., Caballero-Bleda, M., Puelles, L., Popović, N. (1998a). Importance of immunological and inflammatory processes in the pathogenesis and therapy of Alzheimer´s disease. Int. J. Neurosci. 95, 203–236. doi: 10.3109/00207459809003341
Popović, M., Popović, N., Bokonjić, D., Dobrić, S., Ugrešić, N., Kostić, V. S. (1998b). Effect of acute physostigmine and verapamil treatment on aggressive and depressive behavior in rats with lesioned nucleus basalis magnocellularis. Neurosci. Res. Commun. 23, 13–22. doi: 10.1002/s1520-6769(199807/08)23:1<13::AID-NRC2>3.0.CO;2-O
Popović, M., Popović, N., Caballero-Bleda, M., Puelles, L. (1998c). Effect of acute verapamil treatment on body temperatura in nucleus basalis magnocellularis-lesioned rats. Neurosci. Res. Commun. 23, 181–187. doi: 10.1002/(SICI)1520-6769(199811/12)23:3<181::AID-NRC6>3.0.CO;2-E
Popović, M., Popović, N., Caballero-Bleda, M. (1999). Effect of acute verapamil treatment on cold restraint-induced gastric lesions in rats with lesioned nucleus basalis magnocellularis. Neurosci. Res. Commun. 25, 163–171. doi: 10.1002/(SICI)1520-6769(199911/12)25:3<163::AID-NRC5>3.0.CO;2-4
Popović, M., Caballero-Bleda, M., Popović, N., Puelles, L., van Groen, T., Witter, M. P. (2006). Verapamil prevents, in a dose-dependent way, the loss of ChAT-immunoreactive neurons in the cerebral cortex following lesions of the rat nucleus basalis magnocellularis. Exp. Brain Res. 170, 368–375. doi: 10.1007/s00221-005-0219-3
Qiu, C., von Strauss, E., Winbland, B., Fratiglioni, L. (2004). Decline in blood pressure over time and risk of dementia. A longitudinal study from the Kungsholmen project. Stroke 35, 1810–1815. doi: 10.1161/01.STR.0000133128.42462.ef
Ramsden, M., Henderson, Z., Pearson, H. A. (2002). Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid beta protein (1-40) is dependent on solubility status. Brain Res. 956, 254–261. doi: 10.1016/s0006-8993(02)03547-3
Rayasam, G. V., Balganesh, T. S. (2015). Exploring the potential of adjunct therapy in tuberculosis. Trends Pharmcol. Sci. 36, 506–513. doi: 10.1016/j.tips.2015.05.005
Rice, R. A., Berchtodl, N. C., Cotman, C. W., Green, K. N. (2014). Age-related downregulation of the CaV3.1 T-type calcium channel as a mediator of amyloid beta production. Neurobiol. Aging 35, 1002–1011. doi: 10.1016/j.neurobiolaging.2013.10.090
Rinne, J., Paljärvi, L., Rinne, U. K. (1987). Neuronal size and density in the nucleus basalis of Meynert in Alzheimer`s disesase. J. Neurol. Sci. 79, 67–76. doi: 10.1016/0022-510X(87)90260-7
Russo, G. I., Milenkovic, U., Hellstrom, W., Levine, L. A., Ralph, D., Albersen, M. (2018). Clinical efficacy of injection and mechanical therapy for Peyronie’s disease: a systematic review of the literature. Eur. Urol. 74, 767–781. doi: 10.1016/j.eururo.2018.07.005
Saari, G. N., Scott, W. C., Brooks, B. W. (2017). Global scanning assessment of calcium channel blockers in the environment: Review and analysis of occurrence, ecotoxicology and hazards in aquatic systems. Chemosphere 189, 466–478. doi: 10.1016/j.chemosphere.2017.09.058
Sasaki, M., Tateishi, T., Ebihara, A. (1993). The effects of age and gender on the stereoselective pharmacokinetics of verapamil. Clin. Pharmacol. Ther. 54, 278.285. doi: 10.1038/clpt.1993.148
Schamroth, L., Krikler, D. M., Garrett, C. (1972). Immediate effects of intravenous verapamil in cardiac arrhythmias. Br. Med. J. 1, 660–664. doi: 10.1136/bmj.1.5801.660
Schwartz, J. B., Troconiz, I. F., Verotta, D., Liu, S., Capili, H. (1993). Aging effects on stereoselective pharmacokinetics and pharmacodynamics of verapamil. J. Pharmacol. Exp. Ther. 265, 690–698.
Schwartz, J. B., Capili, H., Daugherty, J. (1994). Aging of women alters S-verapamil pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 55, 509–517. doi: 10.1038/clpt.1994.64
Schwartz, J. B. (1990). Aging alters verapamil elimination and dynamics: single dose and steady-state responses. J. Pharmacol. Exp. Ther. 255, 364–373.
Schwartz, J. B. (1996). Calcium antagonists in the elderly. A risk-benefit analysis. Drugs Aging 9, 24–36. doi: 10.2165/00002512-199609010-00003
Sekhar, D. S., Shwetha, B. K., Haimavathi, B., Vikram, P. (2016). The effect of calcium channel blockers against scopolamine induced cognitive impairment and oxidative stress. Int. J. Basic Clin. Pharmacol. 5, 2199–2211. doi: 10.18203/2319-2003.ijbcp20163262
Sen, A. P., Boksa, P., Quirion, R. (1993). Brain calcium channel related dihydropyridine and phenylalkylamine binding sites in Alzheimer’s, Parkinson’s and Huntington’s diseases. Brain Res. 611, 216–221. doi: 10.1016/0006-8993(93)90505-H
Shad, K. F., Saeed, S. A. (2007). The metabolism of serotonin in neuronal cells in culture and platelets. Exp. Brain Res. 183, 411–416. doi: 10.1007/s00221-007-1133-7
Silei, V., Fabrizi, C., Venturini, G., Salmona, M., Bugiani, O., Tagliavini, F., et al. (1999). Activation of microglial cells by PrP and beta-amyloid fragments raises intracellular calcium through L-type voltage sensitive calcium channels. Brain Res. 818, 168–170. doi: 10.1016/S0006-8993(98)01272-4
Silverberg, G. D., Messier, A. A., Miller, M. C., Machan, J. T., Majmudar, S. S., Stopa, E. G., et al. (2010). Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J. Neuropathol. Exp. Neurol. 69, 1034–1043. doi: 10.1097/NEN.0b013e3181f46e25
Šimić, G., Babić Leko, M., Wray, S., Harrington, C., Delalle, I., Jovanov-Milošević, N., et al. (2017). Monoaminergic neuropathology in Alzheimer´s disease. Prog. Neurobiol. 151, 101–138. doi: 10.1016/j.pneurobio.2016.04.001
Sitges, M., Guarneros, A. (1998). Chronic verapamil modifies striatal and frontal cortex dopamine levels. Eur. Neuropsychopharmacol. 8, 105–111. doi: 10.1016/s0924-977x(97)00053-9
Skoog, I., Lernfelt, B., Landahl, S., Palmertz, B., Andreasson, L. A., Nilsson, L., et al. (1996). 15-year longitudinal study of blood pressure and dementia. Lancet 347, 1141–1145. doi: 10.1016/s0140-6736(96)90608-x
Song, L., Wu, X. (2016). Development of efflux pump inhibitors in antituberculosis therapy. Int. J. Antimicrob. Agents. 47, 421–429. doi: 10.1016/j.ijantimicag.2016.04.007
Staneva-Stoytcheva, D., Popova, J., Mutafova-Yambolieva, V., Alov, P. (1990). Influence of long-term treatment with the Ca2(+)-antagonists nifedipine, verapamil, flunarizine and with the calmodulin antagonist trifluoperazine on beta-adrenoceptors in rat cerebral cortex. Gen. Pharmacol. 21, 149–152. doi: 10.1016/0306-3623(90)90611-o
Staneva-Stoytcheva, D., Danchev, N., Popov, P. (1991). Changes in benzodiazepine receptors of rat brain after long-term treatment with the Ca2+-antagonists nifedipine, verapamil, flunarizine and with the calmodulin antagonist trifluoperazine. Gen. Pharmacol. 22, 1151–1154. doi: 10.1016/0306-3623(91)90594-V
Staneva-Stoytcheva, D., Danchev, N., Popov, P. (1992). Long-term treatment with different calcium- and calmodulin-antagonists induces changes in rat brain alpha-adrenoceptors. Gen. Pharmacol. 23, 61–63. doi: 10.1016/0306-3623(92)90048-o
Tönnies, E., Trushina, E. (2017). Oxidative stress, synaptic dysfunction and Alzheimer´s disease. J. Alzheimers Dis. 57, 1105–1121. doi: 10.3233/JAD-161088
Tao, L., Liang, M., Wenjun, H., Rui, Z., Miaoling, L., Yan, Y., et al. (2013). The effects of verapamil on SK2 channel in myocardium cells from human chronic atrial fibrillation. Heart 99, A28. doi: 10.1136/heartjnl-2013-303992.086
Tarabova, B., Lacinova, L., Engel, J. (2007). Effects of phyenilalkylamines and benzothiazepines on Cav1.3-mediated Ca2+ currents in neonatal mouse inner hair cells. Eur. J. Pharmacol. 573, 39–48. doi: 10.1016/j.ejphar.2007.06.050
Taylor, J. E., Defeudis, F. V. (1984). Inhibition of [3H]spiperone binding to 5-HT2 receptors of rat cerebral cortex by the calcium antagonists verapamil and D600. Eur. J. Pharmacol. 106, 215–216. doi: 10.1016/0014-2999(84)90703-9
Tfelt-Hansen, P., Tfelt-Hansen, J. (2009). Verapamil for cluster headache. Clinical pharmacology and possible mode of action. Headache 49, 117–125. doi: 10.1111/j.1526-4610.2008.01298.x
Thibault, O., Gant, J. C., Landfield, P. W. (2007). Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6, 307–317. doi: 10.1111/j.1474-9726.2007.00295.x
Turner, A. L., Perry, M. S. (2017). Outside the box: Medications worth considering when traditional antiepileptic drugs have failed. Seizure 50, 173–185. doi: 10.1016/j.seizure.2017.06.022
van Assema, D. M. E., Lubberink, M., Boellaard, R., Schuit, R. C., Windhorst, A. D., Scheltens, P., et al. (2012a). P-glycoprotein function at the blood–brain barrier: Effects of age and gender. Mol. Imaging Biol. 14, 771–776. doi: 10.1007/s11307-012-0556-0
van Assema, D. M. E., Lubberink, M., Boellaard, R., Schuit, R. C., Windhorst, A. D., Scheltens, P., et al. (2012b). Reproducibility of quantitative (R)-[11C]verapamil studies. EJNMMI Res. 2, 1. doi: 10.1186/2191-219X-2-1
Verhiel, S., Piatkowski de Grzymala, A., van der Hulst, R. (2015). Mechanism of action, efficacy, and adverse events of calcium antagonists in hypertrophic scars and keloids: a systematic review. Dermatol. Surg 41, 1343–1350. doi: 10.1097/DSS.0000000000000506
Vogelgesang, S., Cascorbi, I., Schroeder, E., Pahnke, J., Kroemer, H. K., Siegmund, W., et al. (2002). Deposition of Alzheimer’s β-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 12, 535–541. doi: 10.1097/00008571-200210000-00005
Vogelgesang, S., Warzok, R. W., Cascorbi, I., Kunert-Keil, C., Schroeder, E., Kroemer, H. K., et al. (2004). The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 1, 121–125. doi: 10.2174/1567205043332225
Wang, X., Zheng, W. (2019). Ca2+ homeostasis dysregulation in Alzheimer’s disease: a focus on plasma membrane and cell organelles. FASEB J. 33, 6697–6712. doi: 10.1096/fj.201801751R
Whitehouse, P. J., Price, A. W., Clark, A. W., Coyle, J. T., Delong, M. R. (1981). Alzheimer’s disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126. doi: 10.1002/ana.410100203
Whitson, J. S., Appel, S. H. (1995). Neurotoxicity of Aβ amyloid protein in vitro is not altered by calcium channel blockade. Neurobiol. Aging 16, 5–10. doi: 10.1016/0197-4580(95)80002-9
Wisner, K. L., Peindl, K. S., Perel, J. M., Hanusa, B. H., Piontek, C. M., Baab, S. (2002). Verapamil treatment for women with bipolar disorder. Biol. Psychiatry 51, 745–752. doi: 10.1016/s0006-3223(01)01338-5
Wu, Y.-E., Enoki, R., Oda, Y., Huang, Z.-L., Honma, K.-I., Honma, S. (2018). Ultradian calcium rhythms in the paraventricular nucleus and subparaventricular zone in the hypothalamus. PNAS 115, E9469–E9478. doi: 10.1073/pnas.1804300115
Xiao, J., Li, S., Sui, Y., Wu, Q., Li, X., Xie, B., et al. (2014). Lactobacillus casei-01 facilitates the ameliorative effects of proanthocyanidins extracted from lotus seedpod on learning and memory impairment in scopolamine-induced amnesia mice. PloS One 9, e112773. doi: 10.1371/journal.pone.0112773
Yaser, S., Corrada, M., Brookmeyer, R., Kawas, C. (2005). Calcium channel blockers and risk of AD: The Baltimore longitudinal study of aging. Neurobiol. Aging 26, 157–163. doi: 10.1016/j.neurobiolaging.2004.03.009
Yin, T., Kuo, S. C., Chang, Y. Y., Chen, Y. T., Wang, K. K. (2017). Verapamil use is associated with reduction of newly diagnosed diabetes mellitus. J. Clin. Endocrinol. Metab. 102, 2604–2610. doi: 10.1210/jc.2016-3778
Keywords: Alzheimer’s disease, emotion, learning, memory, verapamil
Citation: Popović N, Morales-Delgado N, Vidal Mena D, Alonso A, Pascual Martínez M, Caballero Bleda M and Popović M (2020) Verapamil and Alzheimer’s Disease: Past, Present, and Future. Front. Pharmacol. 11:562. doi: 10.3389/fphar.2020.00562
Received: 15 February 2020; Accepted: 14 April 2020;
Published: 05 May 2020.
Edited by:
Joanna Wieronska, Polish Academy of Sciences, PolandReviewed by:
Olivier Thibault, University of Kentucky, United StatesAgnieszka Basta-Kaim, Polish Academy of Sciences, Poland
Copyright © 2020 Popović, Morales-Delgado, Vidal Mena, Alonso, Pascual Martínez, Caballero Bleda and Popović. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miroljub Popović, bWlyb2xqdWJAdW0uZXM=
 Natalija Popović
Natalija Popović Nicanor Morales-Delgado
Nicanor Morales-Delgado David Vidal Mena
David Vidal Mena Antonia Alonso
Antonia Alonso María Pascual Martínez5
María Pascual Martínez5 María Caballero Bleda
María Caballero Bleda Miroljub Popović
Miroljub Popović