- 1Department of Integrative Biology, University of Wisconsin-Madison, Madison, WI, United States
- 2Department of Entomology, University of Wisconsin-Madison, Madison, WI, United States
Conservation biological control (CBC) seeks to promote the occurrence of natural enemies of agricultural pests by managing habitat to provide key resources in and around farm fields. In particular, vegetation diversity may help ensure temporal resource continuity such that natural enemies are less likely to experience detrimental gaps or bottlenecks as they move through and use different habitats. While the conceptual value of resource continuity has long been recognized by CBC researchers and practitioners, empirical studies have tended to focus on snapshots in space and time. Here we review how continuity of trophic (food) and structural (shelter) resources affect natural enemy conservation and pest control outcomes within farm fields and across agricultural landscapes. Key trophic resources include alternative prey and non-prey food (such as floral nectar and pollen), which can bolster natural enemy nutrition when pests are scarce. Vegetative and non-vegetative structural resources can protect enemies when crop fields are disturbed and provide important overwintering habitat in temperate regions. Within fields, non-crop plantings such as wildflower strips or beetle banks are the most popular habitat management strategies, but temporal intercropping, asynchronous planting/harvesting, and the construction of artificial shelters have high potential to contribute to resource continuity. Analogously, semi-natural habitat at the landscape scale may contribute to resource continuity in some cases, but crop diversity, asynchrony, and urban habitat can also be important. Simultaneous consideration of resource diversity and continuity could generate better predictions and more targeted management interventions for particular pest and enemy assemblages. Future research should strive to expand our understanding of natural enemy resource requirements in space and time.
Introduction
Farmers, scientists, and policymakers are increasingly looking for ways to “ecologically intensify” agricultural production to meet the needs of human populations while minimizing negative effects on the environment and protecting biodiversity (Bommarco et al., 2013; Tittonell, 2014; Kleijn et al., 2019). Habitat management is often promoted as a promising strategy for managing insect pests while avoiding the downsides of indiscriminate insecticide use (Landis et al., 2000; Gurr et al., 2017). This typically entails diversifying fields and landscapes to minimize the occurrence of herbivores and promote their natural enemies, an approach known as conservation biological control (CBC; Begg et al., 2017). CBC constitutes a shift from the presently dominant “curative” approach to pest control, focused on the use of pesticides once pest problems arise, to a preventative paradigm that relies on biodiversity conservation to support agricultural production. Yet such a shift requires agroecological approaches supported by theoretical underpinnings and a technical infrastructure that enable ecological intensification in ways that are good for farming and the broader environment.
Many principles and practices associated with CBC are thousands of years old (Shields et al., 2019). For example, there are records of farmers in fourth century China manipulating weaver ant nests in citrus orchards to protect the fruit from pests (Huang and Yang, 1987), and indigenous farmers across the tropics engage in various cultural practices to avoid pest outbreaks (Morales, 2002). However, formal scientific investigations by ecologists and entomologists are relatively young (Shields et al., 2019). A classic paper by Root (1973) sparked significant interest in the “enemies hypothesis,” which posits that predators and parasitoids should benefit more from plant diversity than their herbivore prey, increasing the ratio of natural enemies to pests and providing top-down control. This early formulation explicitly recognized the potential importance of resource continuity in time for natural enemies. Root (1973) writes:
“A greater diversity of prey/host species and microhabitats is available within complex environments, such as most natural, compound communities. As a result, relatively stable populations of generalized predators and parasites can persist in these habitats because they can exploit the wide variety of herbivores which become available at different times.”
However, most studies and syntheses of CBC research have ignored temporal dynamics and focused on snapshots of insect populations in space (i.e., in focal crop fields) and at particular times (i.e., during the growing season of the focal crop). Furthermore, researchers frequently assume but rarely measure or directly account for the resource complementation in time that Root (1973) described.
While some studies have demonstrated that diverse fields (Letourneau et al., 2011; Dassou and Tixier, 2016) and landscapes (Chaplin-Kramer et al., 2011; Dainese et al., 2019) can promote more natural enemies and fewer pests than monocropped systems, it is by no means a guarantee (Tscharntke et al., 2016; Karp et al., 2018). Uncertainty about the effectiveness of habitat management, high risk aversion, and perception of non-crop habitat as a likely source of pests make farmers wary of adopting preventative pest management approaches (Salliou and Barnaud, 2017; Chaplin-Kramer et al., 2019; Shields et al., 2019). The challenge for agroecologists is to improve the scientific basis for habitat management while accounting for the context-dependency of pest and natural enemy dynamics (Settele and Settle, 2018). Temporal resource patterns are increasingly recognized as a crucial aspect of agroecosystem context, with many calling for more rigorous consideration in CBC research (Welch and Harwood, 2014; Schellhorn et al., 2015; Haan et al., 2020; Spiesman et al., 2020).
Here, we review the role of temporal resource continuity—and its opposite, discontinuity—for CBC in agricultural systems. While temporal resource patterns are likely to be important across agroecosystems globally, a persistent bias in CBC research toward the developed world (Wyckhuys et al., 2013; Peñalver-Cruz et al., 2019) makes examples from tropical regions scarce; thus, the empirical cases we draw upon come primarily from temperate regions, with a few key exceptions. We begin by outlining a conceptual framework for understanding how and in what instances temporal continuity may be important for facilitating desirable pest and enemy population dynamics. We then use this framework to summarize published studies that explicitly consider the temporal dimensions of different types of resources and habitat management strategies. Our systematic review of the literature focuses on top-down control by natural enemies, but we acknowledge that temporal resource patterns are also highly relevant to bottom-up processes (i.e., Root's “resource concentration hypothesis”; Root, 1973); accordingly, we include a brief discussion of these considerations. We conclude by proposing a new framework for predicting and evaluating the effects of heterogeneous resources on arthropods in agroecosystems that distinguishes temporal considerations from diversity per se, and offering recommendations for future research.
Conceptual Framework: Defining Resources, Continuity, and Scale
The collection of organisms that function as “natural enemies” of crop pests is incredibly broad and diverse, ranging from vertebrates to viruses. Even within the narrower grouping of arthropod natural enemies on which we focus here, species have considerable variation in their life history traits including diet breadth, mobility, voltinism, longevity, and habitat requirements. Accordingly, the particular resources in question, as well as the spatial and temporal scales relevant to patterns of resource continuity are highly context dependent. Nevertheless, the ecological processes underlying resource use and population dynamics are largely generalizable.
In simplified agroecosystems with just one or few annual crop types, resource scarcity is likely for significant portions of the year or growing season (e.g., before planting or after harvest), even if these resources may be occasionally abundant (i.e., a resource pulse; Ostfeld and Keesing, 2000; Yang et al., 2008). This situation creates discontinuity for organisms in need of resources over extended periods of time (Figure 1A). In contrast, complex, diversified, and/or perennial systems may include multiple types of crop or non-crop vegetation with different phenologies, providing more continuous resources over time (Figure 1B). Promoting the successful development and persistence of abundant natural enemy populations in agroecosystems thus requires “linking the resource chain” (Schellhorn et al., 2015) through time by ensuring that the appropriate trophic and structural resources are locally or regionally accessible—that is, within farm fields or in the surrounding landscape. This principle has been recognized for a different group of beneficial arthropods, wild and managed bees, and such “feast-famine” conditions have been shown to be important for pollinators and pollination services (Mallinger et al., 2016; Dolezal et al., 2019; Hemberger et al., 2020). Similar temporal dynamics are likely to be consequential for natural enemies and pest control (Schellhorn et al., 2015).
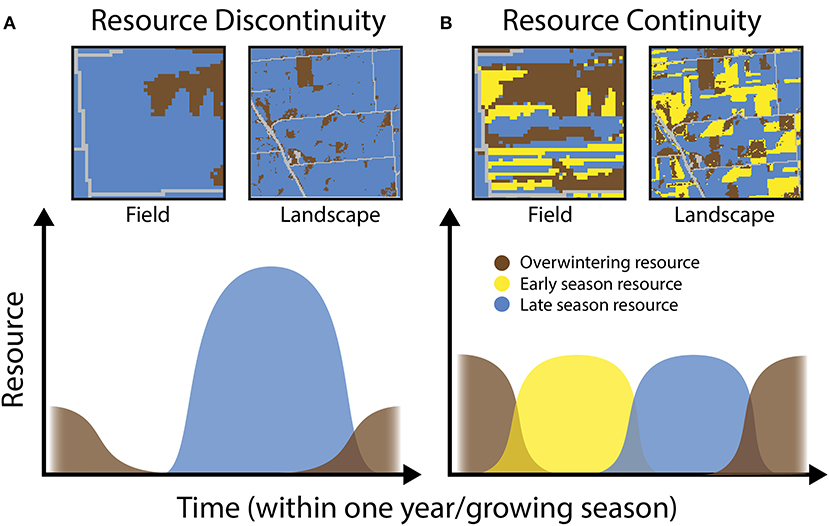
Figure 1. Conceptual representation of how resources may vary through time in heterogeneous agroecosystems. (A) Depicts hypothetical low-continuity scenarios in a single farm field or an entire agricultural landscape. In these scenarios, natural enemy populations are limited by overwintering habitat and experience a resource gap in the early season. (B) Depicts high-continuity scenarios where enemies have sufficient resources available at all times.
In the short term (i.e., the span of a single growing season), ensuring temporal resource continuity could be beneficial for promoting early recruitment of natural enemies to subsequent crops. The importance of early predation or parasitism for effective pest suppression is well-established in theoretical predator-prey population models (Ekbom et al., 1992; van der Werf, 1995). Thus, manipulating resources to attract and maintain natural enemies within crop fields could provide farmers with immediate pest control benefits. In the longer term, resource continuity is important for shoring up the stability of natural enemies by reducing gaps and bottlenecks that may result in the failed development of entire generations and ultimately reduced population sizes (Schellhorn et al., 2015).
Like all organisms, arthropod natural enemies rely upon two broad categories of resources in order to carry out their life cycles: food, or trophic resources, and shelter, or structural resources. In addition to crop pests, trophic resources include alternative prey as well as non-prey food such a floral nectar and pollen (Figure 2; section Trophic Resources). Structural resources include both short term refugia from disturbance as well as longer-term shelter such as overwintering sites (Figure 2; section Structural Resources). Because natural enemies are mobile, traversing multiple resource patches within and/or across generations, individuals, or populations may benefit from the ability to acquire resources from multiple patches of the same habitat type (landscape supplementation), while some may necessitate distinct resources from spatially segregated habitats (landscape complementation; Dunning et al., 1992). For many arthropods, particular trophic or structural resources requirements may vary across life stages or seasons. For example, a parasitoid wasp may feed and develop inside a caterpillar during its larval stage but benefit from nectar as an adult (varied trophic resources requirements). Alternatively, predatory beetles may forage in herbaceous vegetation as both larvae and adults during warm months but aggregate in wooded areas to overwinter (varied structural resource requirements). Proponents of CBC frequently recognize the relevance of organism movement from natural vegetation to crops, but spillover in the opposite direction is equally important from the perspective of continuous resource access and population persistence (Rand et al., 2006; Blitzer et al., 2012).
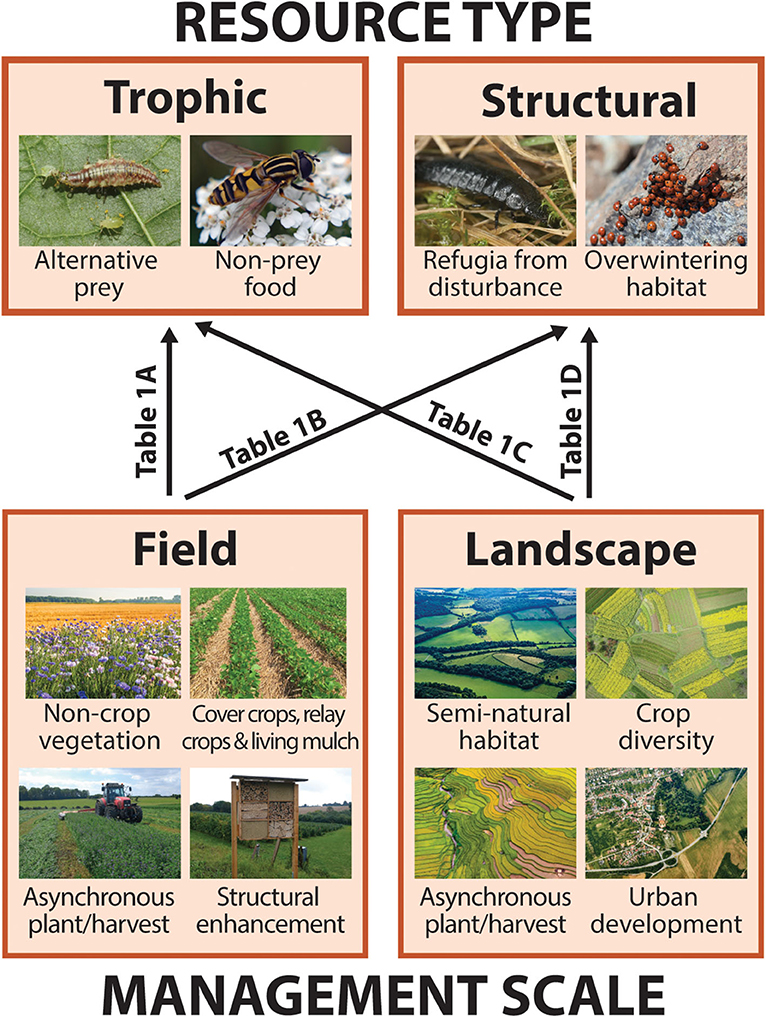
Figure 2. Graphical schematic of the relationships between key natural enemy resources and habitat features that may contribute to their continuity or discontinuity at two management scales (described in section Conceptual Framework: Defining Resources, Continuity, and Scale). Boxes correspond to subsequent sections detailing how and in what instances different resource types, trophic (i.e., food) or structural (i.e., shelter), may be important for natural enemies (section Resources for Natural Enemies: Trophic and Structural), and management features that contribute to resource continuity both within-fields and across agricultural landscapes (section Creating Continuity: Habitat Features in Fields and Landscapes). Arrow labels refer to rows in Table 1 listing examples of studies that describe each of four scale-by-resource interactions. Photos via Shutterstock, standard license.
As mentioned above, the life history traits of the arthropod enemies in a particular agroecosystem will dictate the relevant spatial and temporal extents of resource access and use. For example, the distance over which a species is able to disperse or forage has substantial bearing on the scale at which habitat patches could feasibly contribute to temporal resource continuity; large-bodied species with strong flight ability would be influenced by conditions at greater spatial extents than small, ground-dwelling species. Similarly, a long-lived species with a single generation per year would require a different duration of resource access in order for conditions of “temporal continuity” to be met than a short-lived species with many generations per year. In other words, it remains crucial to take an “organism's eye view” of the world when determining ecologically relevant scales of investigation and manipulation (Wiens, 1989).
At the same time, we identify two scales relevant to farmers and other land stewards for temporal resource management in agroecosystems: within a single crop field (field level) and across multiple fields and adjacent non-crop areas (landscape level). Field scale management features that have the potential to generate or increase temporal resource continuity for natural enemies include cover crops, relay crops, and living mulches, non-crop plantings, and structural enhancements such as overwintering shelters (Figure 2; section In-Field Features). In studies of the landscape ecology of predator-prey interactions, so-called “semi-natural habitat,” or non-crop vegetation around farm fields, is the landscape-level feature most often considered to enhance natural enemy populations and pest control outcomes (Chaplin-Kramer et al., 2011; Karp et al., 2018; Dainese et al., 2019), but others may include landscape-scale crop diversity, asynchronous planting/harvesting, and urban development (Figure 2; section Landscape Features).
Importantly, resource type and management scale interact to affect natural enemy populations. That is, continuities or discontinuities can arise in both trophic and structural resources at field or landscape scales (Figure 2 and Table 1). In the following sections, we explore each of these interactions by highlighting examples from a systematic review of the peer-reviewed literature that relate temporal resource continuity and natural enemies in agroecosystems. For simplicity, we describe resource type and management scale separately, with examples of particular scale-by-resource combinations throughout.
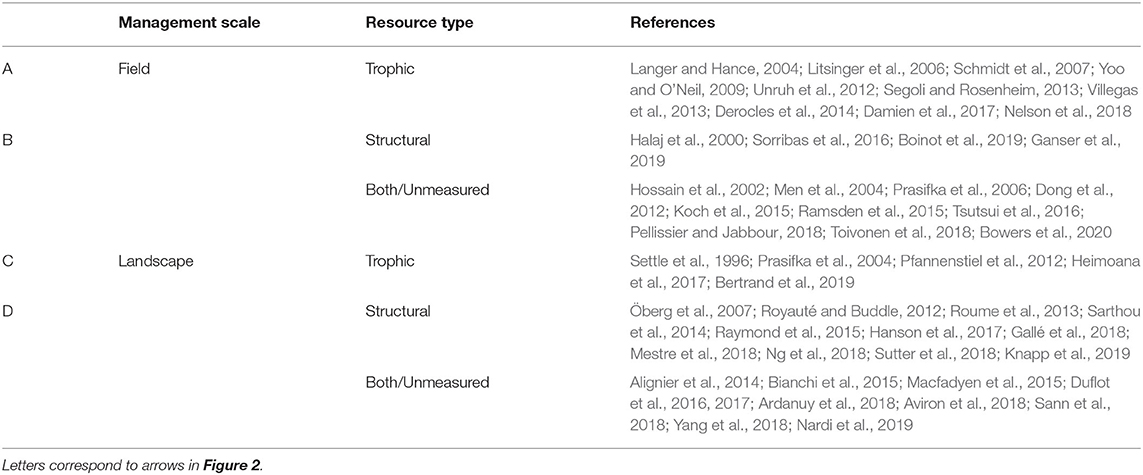
Table 1. Representative studies of temporal resource continuity for natural enemies in agroecosystems across trophic and structural resource types (sections Trophic Resources and Structural Resources) and landscape and field scales (sections In-Field Features and Landscape Features).
To conduct our review, we used ISI Web of Science to search peer-reviewed English-language literature through February 2020. To capture research on pest control, we used the topic terms “pest suppression” OR “pest control” OR “pest regulation” OR “biological control” OR “biocontrol” OR “natural enem*”; to capture temporal dimensions, we used “continu*” OR “complement*” OR “perennial” OR “tempor*” OR “asynch*” OR “early season” OR “late season” OR “overwinter*”; to capture habitat management features at multiple scales we used “habitat” OR “cover crop*” OR “relay crop*” OR “living mulch” OR “fallow” OR “landscape diversi*” OR “landscape complexity.” This search returned 752 results. We then reviewed titles and abstracts for relevance, resulting in a final set of 55 papers. From these we extracted the geographic location (country or U.S. state) in which field work was conducted, the cropping system, the scale(s) (field or landscape) of manipulation or observation, the habitat feature(s) observed or manipulated, the resource type(s) (trophic or structural) considered, the pest and natural enemy group(s) studied, and a brief summary of the main findings (Supplementary Table 1).
Resources For Natural Enemies: Trophic and Structural
Trophic Resources
For the purpose of pest control, the most relevant trophic resources that natural enemies consume are the pests themselves. While facilitating larger or more continuous pest populations could provide an ample food supply for enemies, this is obviously an undesirable situation for crop production. On the other hand, secondary pests or non-pest prey may contribute to the stability of biocontrol without increasing crop damage. The presence of alternative prey is sometimes shown to disrupt effective biocontrol if generalist predators prefer to consume alternative prey compared to pests (Koss and Snyder, 2005). Yet, it has also been hypothesized that the early presence of alternative prey (i.e., a temporally complementary resource for enemies) could build up predator populations to such an extent that large population size compensates for reduced individual predation (Harwood and Obrycki, 2005). In other words, temporal separation in the presence of alternative prey and primary pests may contribute to “apparent competition” between prey species (Langer and Hance, 2004; Blitzer and Welter, 2011), mitigating the negative effect of preferential feeding on non-pests. Similarly, a modeling study by Spiesman et al. (2020) showed that fields or landscapes that contain resource patches with non-overlapping phenologies and distinct specialist prey communities could avoid the build-up of large pest populations by providing continuous food for mobile generalist predators at the landscape scale.
Temporally complementary alternative prey can occur in the focal crop itself or in adjacent vegetation. A study in Indiana (USA) soybean fields found that minute pirate bugs benefited from the presence of thrips early in the growing season and prevented soybean aphid outbreaks later (Yoo and O'Neil, 2009). Prasifka et al. (2004) used stable isotope analysis to show that lady beetles feeding on aphids in grain sorghum emigrated to nearby cotton fields when the sorghum senesced, and remained in cotton even in the absence of aphids; when aphids were present in cotton they switched their diets, enhancing biocontrol. Similarly, leafroller parasitoids in Washington (USA) fruit orchards benefited from rose and strawberry plantings that provided a key overwinter host (Pfannenstiel et al., 2012; Unruh et al., 2012). Adjacent vegetation may also provide alternative prey that keep natural enemies near crop fields during periods of disturbance such as harvest (Villegas et al., 2013) or pesticide application (Heimoana et al., 2017). Nevertheless, in some cases the prey species found in adjacent vegetation may be inadequate alternative prey for agriculturally-relevant natural enemies, and therefore fail to contribute to temporal resource continuity and improved pest control outcomes (Derocles et al., 2014).
Non-prey foods such as nectar, pollen, seeds, and fungi may also be vital to the energetic and nutritional requirements of natural enemies, with some species even requiring non-prey food to complete their life cycles (Wäckers et al., 2005; Lundgren, 2009). Plant- and fungus-derived foods can be especially important for predator and parasitoid subsistence when prey are scarce (e.g., Eubanks and Denno, 1999). Floral resources such as nectar and pollen have been well-studied in the CBC literature, especially for parasitoids (Tylianakis et al., 2004; Lee and Heimpel, 2005, 2008), but temporal dimensions are not often considered explicitly. Continuous access to floral resources has been shown to benefit parasitoids (Segoli and Rosenheim, 2013) and hoverflies (van Rijn et al., 2013) in lab settings, with implications for how flowers are managed in the field. For example, Segoli and Rosenheim (2013) show that leafhopper parasitoids in wine grape vineyards are sugar-limited, especially in autumn, and suggest that planting late-season flowers could enhance their biocontrol potential.
The importance of phenologically complementary floral resources has also been demonstrated recently for natural enemies at the landscape scale. Bertrand et al. (2019) quantified pollen use by a lady beetle and lacewing species throughout the course of a growing season in German and Swiss agricultural landscapes. They observed a clear shift from tree-derived to herbaceous pollen over time, and found that the majority of pollen came from non-crop plants even in areas dominated by farmland. This indicates that diverse, temporally continuous non-prey food is a key resource for natural enemy populations in agricultural landscapes.
Finally, natural enemies themselves may function as “alternative prey” in some cases (i.e., intraguild predation or cannibalism; Rosenheim et al., 1995). Although theory predicts that such antagonistic interactions between enemies should have negative consequences for biocontrol, this prediction is infrequently borne out in practice (Janssen et al., 2006; Rosenheim and Harmon, 2006). From the perspective of temporal resource continuity, intraguild predation could be beneficial if the presence of intraguild prey acts as an additional trophic resource that enables the persistence of the intraguild predator during times of extraguild prey scarcity; however, we did not encounter any examples of this phenomenon in our literature search.
Structural Resources
In addition to food, natural enemies require appropriate habitat structure for growth & development, sheltering from predation and disturbance, reproduction, and in temperate climate zones, overwintering (Landis et al., 2000). Gontijo (2019) recently reviewed the engineering of natural enemy shelters to enhance CBC in crop fields, highlighting the importance that vegetative and artificial structures can have in providing suitable microclimatic conditions and protection from intraguild predation and pesticide exposure, in addition to providing supplemental food resources (discussed in section Trophic Resources above). While sheltering can improve conditions for predators in the middle of the growing season—such as protecting them from desiccation in high sun conditions (Diehl et al., 2012)—it may be especially important during periods when crop fields are bare or sparse. For example, Tsutsui et al. (2016) found that spiders in Japanese rice agroecosystems relied on the complementary use of irrigation and drainage ditches during periods when paddies were dry, suggesting that providing essential microhabitats could be important at particular times of the season.
Because highly intensified crop fields provide little suitable substrate outside of the growing season, overwintering habitat is likely to be a key limiting structural resource for natural enemies in temperate agroecosystems. In studies from European oilseed rape landscapes, overwintering spider density was found to be significantly higher in natural areas than crop fields (Mestre et al., 2018), and ground beetle-to-pollen beetle ratio was greatest in forest edges, especially those with high litter cover and compact soil (Sutter et al., 2018). In the absence of semi-natural landscape features, in-field enhancements (see section In-Field Features) have the potential to provide supplemental overwintering habitat to natural enemies. In one study from Switzerland, perennial wildflower strips were found to host significantly more overwintering spiders, ground beetles, rove beetles, and hoverflies than adjacent wheat fields, but plowing strips during the overwintering period reversed any benefits they provided (Ganser et al., 2019). In an alley cropped agroforestry system, Boinot et al. (2019) found that more predators, and disturbance-sensitive ground beetle species in particular, overwintered in understory vegetation strips than crop alleys, suggesting that the structural complexity created by the trees could enhance biocontrol services during the growing season. Finally, even in perennial systems where cropland itself may be a suitable overwintering habitat for some natural enemy species, supplementary habitat may be valuable for others. For example, several species of lacewings in Spanish fruit orchards tended to overwinter in nearby shelterbelts and disperse to fruit trees the following spring, while others remained on fruit trees year-round (Sorribas et al., 2016).
In summary, both trophic and structural resources within and outside of crop fields that complement the availability of prey are essential for sustaining long-lived, mobile natural enemies in agroecosystems. Agricultural systems that retain such temporally complementary resources are likely to have a greater potential for pest suppression within a crop by supporting robust natural enemy communities through periods of low pest abundance.
Creating Continuity: Habitat Features in Fields and Landscapes
In-Field Features
Within individual farms or crop fields, there are a variety of habitat features and management techniques that could provide natural enemies with temporally continuous trophic and structural resources. Within a farm, local non-crop vegetation has long been studied for its potential value to beneficial insects and may be especially important before crops begin growing and after they are harvested. Grassy field margins, wildflower plantings, and beetle banks, are common examples of such non-crop features. This vegetation can often simultaneously offer both food and shelter for natural enemies. Ramsden et al. (2015) evaluated the relative importance of alternative prey, floral resources, and overwintering habitat provided by managed field margins to flying natural enemies of aphids in winter wheat by manipulating the type of vegetation present. They found that floral resources had the strongest effect, significantly increasing wheat aphid parasitism rates, as well as the abundance of hoverflies, lady beetles, and lacewings, particularly at the beginning of the growing season. Perennial wildflower strips have also been documented to support ground dwelling predators early in the season and facilitate their subsequent movement to adjacent barley better than non-flowering grasses (Toivonen et al., 2018). Yet in some cases, vegetation phenology may be more important than floral resources per se. Comparing the value of riparian buffers planted with cool- vs. warm-season grass mixes for natural enemies in maize and soybean, Nelson et al. (2018) expected the warm season plantings to perform better due to the greater abundance of flowering species included in the seed mix. However, they found that cool season grasses promoted earlier, more abundant ground- and canopy-dwelling enemies in crop fields. They attributed this to phenological differences between plantings, positing that the cool season grasses provide more continuous substrate and beneficial microhabitat for prey and predators early in the season. It is important to note that while managed non-crop vegetation frequently promotes early-season natural enemy abundance in the planting itself, benefits do not always spill over to the adjacent crop (Pellissier and Jabbour, 2018).
Cover crops, relay crops, and living mulches are within-field vegetation management strategies that could also promote temporal resource continuity. Cover crops are regularly promoted for their soil-building (Blanco-Canqui et al., 2015) and weed suppression (Osipitan et al., 2018) properties, but may also provide valuable habitat for beneficial insects outside of the focal crop growing season. Bowers et al. (2020) show that rye and clover cover crops increase early season recruitment of natural enemies to Georgia (USA) cotton fields and decrease thrips abundance, while rye cover crops also decreased boll injury by stink bugs. In France, flowering brassica cover crops increased parasitism rates of aphids in adjacent cereals, likely attributable to the early nectar resources they provide (Damien et al., 2017). Even when cover crops fail to enhance natural enemy recruitment to crop fields (Fox et al., 2016), they have been shown to depress pest populations by other mechanisms in some cases (Hooks et al., 2013; Koch et al., 2015).
In relay cropping systems, crops that have different phenologies are planted in the same field, and the first (early season or fast growing) crop is harvested before the second (late season or slow growing) crop. This strategy has proven especially effective in promoting early recruitment of lady beetles to control aphids in wheat-cotton (Men et al., 2004) and rye-wheat (Dong et al., 2012) relay cropping systems in China, as well as soybean planted into an alfalfa living mulch in Iowa (USA) (Schmidt et al., 2007). Alfalfa-clover living mulch has also been effective in increasing ground beetle abundance and predation of the European corn borer in an Iowa maize-soy-forage rotation (Prasifka et al., 2006).
For crops that can accommodate multiple harvest dates, such as alfalfa and other forages, asynchronous strip harvesting may promote the persistence of natural enemy populations in the field throughout the harvest season. This practice was popularized in California alfalfa fields (Stern et al., 1964; Summers, 1976) and its benefits for enemy conservation and pest suppression have been extensively documented in Australian production systems (Hossain et al., 2000, 2001, 2002). Similar results have been found in other parts of the world (Samu, 2003; Rakhshani et al., 2010).
Finally, non-vegetative within-field enhancements have the potential to provide resources for natural enemies and keep them around in the absence of crops. Halaj et al. (2000) note that the establishment of straw shelters in crop fields is a millennia-old practice used by Chinese farmers to create a refuge for spiders during periods of disturbance. They found dramatic increases in predator abundance and diversity in shelters compared to open fields, as well as one-third less insect damage to soybean seedlings near shelters. The use of artificial shelters for structural resource continuity is generally uncommon but has received particular attention in orchard systems (Horton et al., 2002; Horton, 2004; Kawashima and Jung, 2010; Yanik et al., 2011).
Landscape Features
At the landscape scale, semi-natural habitat (i.e., non-crop vegetation patches) is perhaps the most investigated feature presumed to benefit natural enemy conservation and biocontrol services through its combined effects on both trophic and structural resource continuity for natural enemies. Semi-natural habitats are expected to improve temporal continuity because they are comprised of long-lived, perennial species that undergo minimal disturbance. Studies often measure the proportion of semi-natural landcover in a given area (e.g., 1 km radius) surrounding a crop field and relate this attribute to pest or enemy responses. The amount of surrounding semi-natural habitat sometimes correlates with the early-season abundance of natural enemies in crop fields (Alignier et al., 2014; Bianchi et al., 2015; Raymond et al., 2015; Wilson et al., 2017), suggesting its function as overwintering habitat and potential contribution to temporal resource continuity. Semi-natural habitat here is inferred to be a proxy for some limiting trophic or structural resources at low levels within crop fields themselves. However, these potentially limiting resources are rarely measured directly. When resources are measured, there tends to be substantial local heterogeneity in the quality of semi-natural habitat (Sarthou et al., 2014; Holland et al., 2016; Bartual et al., 2019), and this discrepancy could partially explain why it is an inconsistent predictor of CBC outcomes (Karp et al., 2018).
In addition to habitat amount, landscape configuration strongly affects pests, enemies, and crop yield, though temporal dimensions remain largely under-explored (Haan et al., 2020). One robust finding across temperate agricultural landscapes is that habitat edges tend to support more diverse, abundant ground beetle (Roume et al., 2013; Duflot et al., 2017; Ng et al., 2018; Knapp et al., 2019) and spider communities (Öberg et al., 2007, 2008; Royauté and Buddle, 2012; Mestre et al., 2018) in and around cereal fields, particularly early in the growing season. This observation demonstrates the contribution of semi-natural vegetation in patchy landscapes to overwintering habitat and timely recruitment of predators to crop fields (Bertrand et al., 2016; Gallé et al., 2018).
Non-crop habitat is not the only landscape feature that may promote desirable pest and enemy dynamics in agricultural landscapes. The heterogeneity of farmland itself is increasingly recognized for its relevance to biodiversity conservation (Perfecto et al., 2019; Sirami et al., 2019) and ecosystem service provisioning (Vasseur et al., 2013; Cohen and Crowder, 2017; Redlich et al., 2018). In particular, crop diversity at the landscape scale could offer temporally complementary resource patches to mobile generalists that can make use of different habitats throughout the growing season, as well as “bridge” semi-natural habitat and annual cropland by providing connectivity in time and space. For example, Nardi et al. (2019) used network analysis to show that while forest habitats hosted spider communities distinct from those in annual crop fields, perennial crops and meadows played a key role in facilitating dispersal across agricultural landscapes. Studying ground beetles in maize, Aviron et al. (2018) found that the presence of semi-natural areas did not enhance farmland species, but connectivity to winter cereal crops promoted short-winged species, whereas Duflot et al. (2016) saw no evidence of complementation between cereal and maize fields. In some cases, spatio-temporal resource complementation may benefit generalist pests but not predators (Macfadyen et al., 2015; Ardanuy et al., 2018). Finally, diverse crop types can act as temporally complementary sources of natural enemy population from distinct functional groups throughout the growing season. One study illustrating this point in Swedish agricultural landscapes found that predators emerged and dispersed early in the season from sugar beet fields, while later in the season grasslands were an important spider source and wheat fields were an important rove beetle source (Hanson et al., 2017).
In regions with year-long growing seasons, asynchronous planting of a focal crop can be an effective way to ensure resource continuity for natural enemies across the landscape. There is a longstanding debate in theoretical models and pest management policy about the value of synchronous vs. asynchronous planting for pest control, with the classic example coming from tropical rice systems (Ives and Settle, 1997). Settle et al. (1996) demonstrated that generalist predators were more abundant, and pest suppression was improved, with asynchronous planting and in the presence of alternative, detritivorous prey that provided continuous trophic resources and boosted their early population size. Subsequent work in tropical rice systems has corroborated these results, showing that asynchronous planting is sometimes more beneficial for natural enemies of rice pests than crop diversity or semi-natural habitat (Litsinger et al., 2006; Dominik et al., 2018; Sann et al., 2018).
Recently, an additional landscape element that has received scientific attention and which has the potential to affect resource continuity and pest control is the presence of developed or urban habitat. Long written-off as irrelevant to conservation, cities are increasingly recognized as remarkably complex, heterogenous patchworks that harbor abundant and diverse insect communities (New, 2015) and have the potential to support insect-mediated ecosystem services (Gardiner et al., 2013; Lin et al., 2015). Urban habitat features are important for pest and enemy dynamics within small urban agroecosystems (Gardiner et al., 2014; Egerer et al., 2017; Gardiner and Harwood, 2017; Philpott and Bichier, 2017), but may also influence nearby natural (Spear et al., 2018), and agricultural areas. Yang et al. (2018) found that lady beetle abundance in wheat fields was correlated with the proportion of dwellings in the surrounding area, but only in the early season, likely because human structures provide valuable overwintering habitat to beetles. Urban warming may also contribute to earlier emergence and faster development times in cities, but these effects seem more pronounced for pests than natural enemies (Dale and Frank, 2014; Meineke et al., 2014).
Overall, a variety of habitat features at both the field and landscape scales could promote or interfere with natural enemies' continuous access to key resources. Beyond the non-crop or semi-natural vegetation present in agroecosystems, crop diversity, management schedules, and non-vegetative structures may also be advantageous targets for manipulation to improve conservation and pest control.
Bottom-up Processes and Resource Discontinuity for Pests
The research summarized above primarily focuses on the value of resource continuity for predators and parasitoids, beneficial species whose presence is desirable in agroecosystems. However, it is worth noting that temporal resource patterns can also be manipulated to generate resource discontinuities for undesirable species. One example of this is practiced at the field scale, where the rotation of annual crops has been used for thousands of years to disrupt the inter-annual life cycles of pathogens and insect pests (Bullock, 1992). Longer, more diverse crop rotations have been shown to decrease insect pest pressure in a variety of crops including canola (Harker et al., 2015), maize (Brust and King, 1994), and potato (Hare, 1990; Kabaluk and Vernon, 2000). In some cases, pests may evolve resistance to simple rotation schemes (e.g., corn rootworm in North America; Gray et al., 2009). Although crop rotation could potentially disrupt resource continuity for top-down control within crop fields, some studies have found neutral or positive effects on natural enemies (O'Rourke et al., 2008; Dunbar et al., 2016). The consequences of crop rotation for pests and enemies at the landscape scale are poorly characterized, but nevertheless a potentially important temporal consideration for CBC (Rusch et al., 2013; Bertrand et al., 2016).
Other cultural controls can interfere with the habitat requirements of insect pests. For example, plastic or biodegradable mulches can be used to alter the microclimate within crop canopies and on the soil surface, deterring or killing insect herbivores (Kasirajan and Ngouajio, 2012). Similarly, the isolation or removal of infested fruits can interrupt resource access for pests (Chouinard et al., 2016; Leach et al., 2018). Non-crop vegetation could also be managed to disrupt resource continuity for pests, such as by removing alternative host plants in the landscape. For example, Parry et al. (2019) demonstrate that exotic weeds in found in alfalfa fields and pasture in Australia act as early season hosts for a native hemipteran pest, the Rutherglen bug, and suggest that reducing weeds in these habitats could disrupt temporal resource continuity and facilitate better landscape-scale management. Similarly, soybean aphids and their overwinter host European buckthorn constitute two key pillars of an “invasional meltdown” North America; removing buckthorn in the landscape could promote the suppression of soybean aphid as well as other co-invaders (Heimpel et al., 2010).
Discussion
Diversity and Continuity: The “What” and “When” of Resources in Agroecosystems
In Root's (1973) initial formulation of the enemies hypothesis, improved temporal resource continuity for natural enemies is a corollary to plant diversity. In other words, one reason agroecosystem diversification is presumed to be beneficial for natural enemy conservation and top-down pest suppression is because it decreases the likelihood enemies will encounter a period of resource scarcity, allowing populations to persist and grow. This is one among several potential mechanisms by which diversification may benefit natural enemies (e.g., nutritional enhancement provided by more diverse diets; Root, 1973; Russell, 1989). Disentangling the contributions to natural enemy response of resource diversity per se and resources continuity could be a fruitful direction for CBC research. For example, natural enemy diets could be manipulated in a factorial experiment crossing high and low temporal continuity with high and low nutritional diversity, measuring physiological, developmental, or survivorship outcomes. Clarifying the mechanisms by which diversification is likely to benefit enemies in specific contexts could inform more useful habitat management schemes that address relevant resource deficiencies.
When considering resource continuity and resource diversity separately, four broad types of agroecosystems are apparent (Figure 3A). In low diversity systems such as crop monocultures, resources may be either ephemeral (as is the case for the commercial production of many annual crops around the world) or long-lasting (as in orchards or perennial forage crops, for example). Similarly, high diversity systems can encompass mixtures of plants with similar phenologies (“synchronous polyculture,” such as many classic companion plants) or temporally distinct phenologies (“asynchronous polyculture,” such as relay or cover crops). Given the findings from studies on resource pulse-consumer interactions in natural ecosystems (Ostfeld and Keesing, 2000; Yang et al., 2010), we would predict that the nature of the effects of resource patterns in agroecosystems will depend on the specific life history traits and resource requirements of relevant pest and enemy species. Accordingly, resource continuity and/or diversity could in some cases be manipulated to facilitate optimal pest management outcomes. We describe the application of this framework for three relevant traits: habitat/diet breadth (generalists vs. specialist; Figure 3B), voltinism (univoltine vs. multivoltine; Figure 3C), and mobility (high vs. low mobility; Figure 3D).
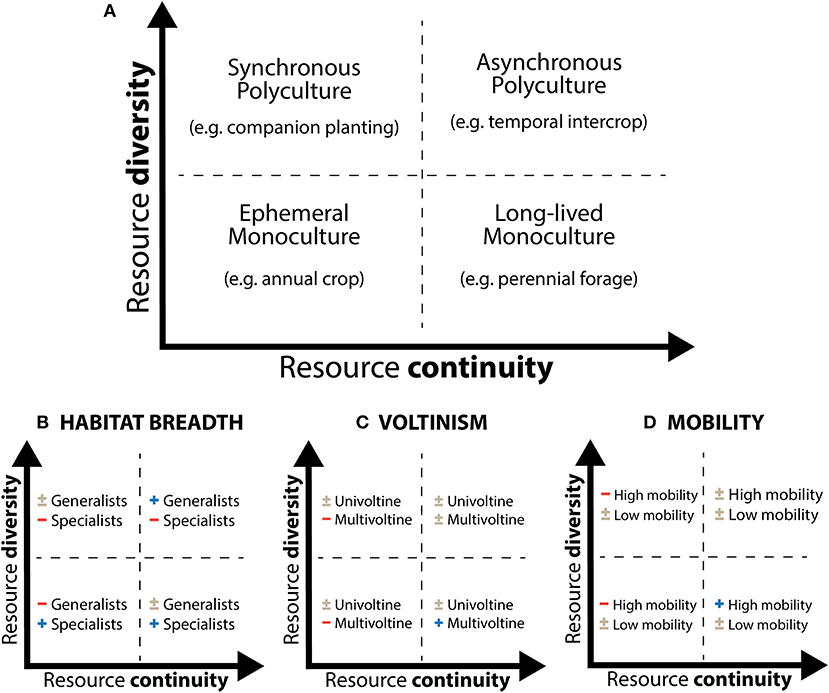
Figure 3. Framework for analyzing resource patterns in agroecosystems incorporating both diversity and continuity dimensions. (A) Depicts four broad agroecosystem types in each quadrant. (B–D) Speculate how different arthropod life history traits (habitat/diet breadth, voltinism, and mobility) may interact with resource conditions. Symbols correspond to expectations of positive (+), negative (–), or mixed effects (±) on species with a given life history trait.
We expect that habitat generalists are likely perform better relative to specialists in cropping systems with heterogeneous, temporally complementary resources (Figure 3B, top right quadrant) because, all else being equal, they have the ability to move and exploit a diversity of resources in habitats that become available at different points in time. In contrast, specialists are more likely to perform better in simplified systems (Figure 3B, bottom left quadrant) because they are well-adapted to such ephemeral environments (Wissinger, 1997). Results in diverse but fleeting (Figure 3B, top left quadrant) or homogenous perennial systems may be more variable (Figure 3B, bottom right quadrant). This suggests that effective management of pests with field or landscape diversification practices would be more likely under scenarios in which the targeted pests are specialists (and only occur in the crop or limited number of alternative habitats) that are attacked by generalist natural enemies which can bolster their populations by accessing resources in a diversity of habitats. In contrast, diversification may be less effective if key pests and enemies have similar habitat or diet breadths.
An organism's voltinism, or the number of generations it completes in a year, is also relevant to how a population may respond to changes in resources over time. For univoltine species, adequate capture of resources may in some cases be achieved even when trophic resources are fleeting—as long as food is available during the organism's phenological growth and development window. Individuals may remain in their dormant life stage for the rest of the year (provided adequate structural resources) when trophic resources are not available. Multivoltine species, on the other hand, require host plants (in the case of pests) or prey (in the case of enemies) at multiple time points (for each generation), and thus stand to suffer more from resource gaps (Figure 3C, left quadrants). Enhancing trophic resource continuity may therefore be more likely to improve pest control outcomes when natural enemies are multivoltine. Univoltine natural enemies, on the other hand, would not be as sensitive to variability in trophic continuity or diversity (since they are presumably adapted to coincide with their prey), but enhancing structural resource continuity may benefit univoltine enemies if they are limited by appropriate substrate for their non-feeding (e.g., overwintering) phase. The consequences of resource diversity per se would be a function of diet breadth (i.e., Figure 3B), rather than voltinism.
As previously mentioned, the distance which a species is able to travel to disperse or forage in large part determines the spatial scale at which resource distribution patterns affect population dynamics. Species' mobility may also matter for the ability of resource manipulation to enhance pest control outcomes by influencing their fidelity to a given area. If highly mobile species (e.g., wind-dispersers, strong fliers, or crawlers) experience resource gaps locally they may leave in search of resources elsewhere (e.g., the harlequin lady beetle; Osawa, 2000; Forbes and Gratton, 2011). If the species in question are pests this dynamic would be desirable, but if they are enemies it could result in reduced top-down control of subsequent pests. In contrast, low-mobility species (e.g., small ground dwellers) may be unable to escape local resource scarcity and die from starvation, or persist long enough to respond to a resource influx (i.e., new crop growth or pest outbreak) when it arrives. Accordingly, engineering resource gaps may be desirable when pests are highly mobile but enemies are not (Figure 3D, left quadrants). As with voltinism, the consequences of resource diversity will depend on whether arthropods can take advantage of few or many resource types in the agroecosystem.
By explicitly assessing the temporal resource dimensions for both natural enemies and their pests, in addition to diversity per se, the conceptual framework presented here could serve as a valuable starting point for testing novel agroecosystem designs for pest management within a field, farm, or landscape.
Research Outlook and Conclusion
The temporal dynamics of food and shelter resources for arthropods can have important consequences for natural enemy conservation and pest control services in agroecosystems. By shifting focus away from habitat features themselves and toward the underlying mechanisms that drive insect-mediated processes and functions, the temporal continuity framework described here can generate more accurate predictions and targeted management interventions for CBC. Within fields, habitat management with temporal complementation in mind could maintain the pest control benefits of diversification while minimizing negative effects of direct plant competition that result in yield losses (Letourneau et al., 2011), since the benefits of diversity are spaced over time. At the landscape scale, it could point to natural enemy conservation strategies that do not necessitate taking land out of production—i.e., by growing phenologically complementary crops rather than just restoring long-lived semi-natural habitats (Schellhorn et al., 2015).
It is important that researchers and practitioners maintain a strong systems approach to CBC that accounts for arthropod dynamics at appropriate spatial extents and temporal resolutions. What happens in crop fields at peak growing season is certainly important, but for pest control service providers it is not the only place or time that matters (Vasseur et al., 2013). Landscape-scale studies rightfully acknowledge the effects that landscape context may have, but it is insufficient to assume what resources the habitat patches surrounding a focal crop field actually provide based on coarse land cover classifications alone (Cohen and Crowder, 2017). Directly measuring these resources, their use, and the movement of natural enemies over time typically provides a clearer picture of the roles that spatial and temporal heterogeneity play in conservation and ecosystem service delivery. Furthermore, more studies that measure resource patterns before planting, after harvest, and during overwintering periods could deepen our understanding of resource gaps and be crucial for achieving natural enemy conservation objectives. Studies focusing on the temporal dimensions of pest and enemy resources are particularly lacking in the tropics and sub-tropics (at least in papers published in English). This is unfortunate, as tropical agroecosystems may be especially poised to take advantage of temporal resource manipulation due to the long (and in some cases continuous) growing season in these regions. Expanding the geographic scope of temporally-focused CBC research would be invaluable for clarifying the idiosyncratic mechanisms that drive arthropod community dynamics in specific local contexts as well as patterns that repeat across the globe.
To facilitate the wider adoption of a pest management paradigm that emphasizes preventing outbreaks rather than treating them after they have occurred, farmers need reliable management techniques that in many cases depend on sufficient natural enemy populations to keep herbivores in check. Ensuring the availability of the limiting resources that these enemies need to persist on farms and in agricultural landscapes requires attention to their continuity over time. By studying and manipulating this resource continuity, CBC research may be able to advance agricultural practices that sustain both people and the diverse organisms on which we depend.
Author Contributions
BI and CG conceived of the review topic and contributed significantly to subsequent drafts. BI researched and wrote the first draft. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by AFRI Grant No. 2018-67013-28060 from the USDA National Institute of Food and Agriculture.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AR declared a past co-authorship with one of the authors CG to the handling editor.
Acknowledgments
We thank Randy Jackson, Matthew Turner, members of the Gratton Lab, the associate editor, and two reviewers for helpful comments on prior versions of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2020.00127/full#supplementary-material
References
Alignier, A., Raymond, L., Deconchat, M., Menozzi, P., Monteil, C., Sarthou, J.-P., et al. (2014). The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 77, 76–82. doi: 10.1016/j.biocontrol.2014.06.006
Ardanuy, A., Lee, M. S., and Albajes, R. (2018). Landscape context influences leafhopper and predatory Orius spp. abundances in maize fields. Agric. For. Entomol. 20, 81–92. doi: 10.1111/afe.12231
Aviron, S., Lalechère, E., Duflot, R., Parisey, N., and Poggi, S. (2018). Connectivity of cropped vs. semi-natural habitats mediates biodiversity: a case study of carabid beetles communities. Agric. Ecosyst. Environ. 268, 34–43. doi: 10.1016/j.agee.2018.08.025
Bartual, A. M., Sutter, L., Bocci, G., Moonen, A.-C., Cresswell, J., Entling, M., et al. (2019). The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 279, 43–52. doi: 10.1016/j.agee.2019.04.009
Begg, G. S., Cook, S. M., Dye, R., Ferrante, M., Franck, P., Lavigne, C., et al. (2017). A functional overview of conservation biological control. Crop Protect. 97, 145–158. doi: 10.1016/j.cropro.2016.11.008
Bertrand, C., Burel, F., and Baudry, J. (2016). Spatial and temporal heterogeneity of the crop mosaic influences carabid beetles in agricultural landscapes. Landscape Ecol. 31, 451–466. doi: 10.1007/s10980-015-0259-4
Bertrand, C., Eckerter, P. W., Ammann, L., Entling, M. H., Gobet, E., Herzog, F., et al. (2019). Seasonal shifts and complementary use of pollen sources by two bees, a lacewing and a ladybeetle species in European agricultural landscapes. J. Appl. Ecol. 56, 2431–2442. doi: 10.1111/1365-2664.13483
Bianchi, F. J. J. A., Walters, B. J., ten Hove, A. L. T., Cunningham, S. A., van der Werf, W., Douma, J. C., et al. (2015). Early-season crop colonization by parasitoids is associated with native vegetation, but is spatially and temporally erratic. Agric. Ecosyst. Environ. 207, 10–16. doi: 10.1016/j.agee.2015.03.018
Blanco-Canqui, H., Shaver, T. M., Lindquist, J. L., Shapiro, C. A., Elmore, R. W., Francis, C. A., et al. (2015). Cover crops and ecosystem services: insights from studies in temperate soils. Agron. J. 107, 2449–2474. doi: 10.2134/agronj15.0086
Blitzer, E. J., Dormann, C. F., Holzschuh, A., Klein, A. M., Rand, T. A., and Tscharntke, T. (2012). Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 146, 34–43. doi: 10.1016/j.agee.2011.09.005
Blitzer, E. J., and Welter, S. C. (2011). Emergence asynchrony between herbivores leads to apparent competition in the field. Ecology 92, 2020–2026. doi: 10.1890/11-0117.1
Boinot, S., Poulmarc'h, J., Mézière, D., Lauri, P.-É., and Sarthou, J.-P. (2019). Distribution of overwintering invertebrates in temperate agroforestry systems: implications for biodiversity conservation and biological control of crop pests. Agric. Ecosyst. Environ. 285:106630. doi: 10.1016/j.agee.2019.106630
Bommarco, R., Kleijn, D., and Potts, S. G. (2013). Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. doi: 10.1016/j.tree.2012.10.012
Bowers, C., Toews, M., Liu, Y., and Schmidt, J. M. (2020). Cover crops improve early season natural enemy recruitment and pest management in cotton production. Biol. Control 141:104149. doi: 10.1016/j.biocontrol.2019.104149
Brust, G. E., and King, L. R. (1994). Effects of crop rotation and reduced chemical inputs on pests and predators in maize agroecosystems. Agric. Ecosyst. Environ. 48, 77–89. doi: 10.1016/0167-8809(94)90077-9
Bullock, D. G. (1992). Crop rotation. CRC. Crit. Rev. Plant Sci. 11, 309–326. doi: 10.1080/07352689209382349
Chaplin-Kramer, R., O'Rourke, M., Schellhorn, N., Zhang, W., Robinson, B. E., Gratton, C., et al. (2019). Measuring what matters: actionable information for conservation biocontrol in multifunctional landscapes. Front. Sustain. Food Syst. 3:60. doi: 10.3389/fsufs.2019.00060
Chaplin-Kramer, R., O'Rourke, M. E., Blitzer, E. J., and Kremen, C. (2011). A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 14, 922–932. doi: 10.1111/j.1461-0248.2011.01642.x
Chouinard, G., Firlej, A., and Cormier, D. (2016). Going beyond sprays and killing agents: exclusion, sterilization and disruption for insect pest control in pome and stone fruit orchards. Sci. Hortic. 208, 13–27. doi: 10.1016/j.scienta.2016.03.014
Cohen, A. L., and Crowder, D. W. (2017). The impacts of spatial and temporal complexity across landscapes on biological control: a review. Curr. Opin. Insect Sci. 20, 13–18. doi: 10.1016/j.cois.2017.02.004
Dainese, M., Martin, E. A., Aizen, M. A., Albrecht, M., Bartomeus, I., Bommarco, R., et al. (2019). A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 5:eaax0121. doi: 10.1126/sciadv.aax0121
Dale, A. G., and Frank, S. D. (2014). Urban warming trumps natural enemy regulation of herbivorous pests. Ecol. Appl. 24, 1596–1607. doi: 10.1890/13-1961.1
Damien, M., Le Lann, C., Desneux, N., Alford, L., Al Hassan, D., Georges, R., et al. (2017). Flowering cover crops in winter increase pest control but not trophic link diversity. Agric. Ecosyst. Environ. 247, 418–425. doi: 10.1016/j.agee.2017.07.015
Dassou, A. G., and Tixier, P. (2016). Response of pest control by generalist predators to local-scale plant diversity: a meta-analysis. Ecol. Evol. 6, 1143–1153. doi: 10.1002/ece3.1917
Derocles, S. A. P., Ralec, A. L., Besson, M. M., Maret, M., Walton, A., Evans, D. M., et al. (2014). Molecular analysis reveals high compartmentalization in aphid–primary parasitoid networks and low parasitoid sharing between crop and noncrop habitats. Mol. Ecol. 23, 3900–3911. doi: 10.1111/mec.12701
Diehl, E., Wolters, V., and Birkhofer, K. (2012). Arable weeds in organically managed wheat fields foster carabid beetles by resource- and structure-mediated effects. Arthropod Plant Interact. 6, 75–82. doi: 10.1007/s11829-011-9153-4
Dolezal, A. G., Clair, A. L. S., Zhang, G., Toth, A. L., and O'Neal, M. E. (2019). Native habitat mitigates feast–famine conditions faced by honey bees in an agricultural landscape. Proc. Natl. Acad. Sci. U.S.A. 116, 25147–25155. doi: 10.1073/pnas.1912801116
Dominik, C., Seppelt, R., Horgan, F. G., Settele, J., and Václavík, T. (2018). Landscape composition, configuration, and trophic interactions shape arthropod communities in rice agroecosystems. J. Appl. Ecol. 55, 2461–2472. doi: 10.1111/1365-2664.13226
Dong, Z.-K., Gao, F.-J., and Zhang, R.-Z. (2012). Use of ryegrass strips to enhance biological control of aphids by ladybirds in wheat fields. Insect Sci 19, 529–534. doi: 10.1111/j.1744-7917.2011.01499.x
Duflot, R., Ernoult, A., Aviron, S., Fahrig, L., and Burel, F. (2017). Relative effects of landscape composition and configuration on multi-habitat gamma diversity in agricultural landscapes. Agric. Ecosyst. Environ. 241, 62–69. doi: 10.1016/j.agee.2017.02.035
Duflot, R., Ernoult, A., Burel, F., and Aviron, S. (2016). Landscape level processes driving carabid crop assemblage in dynamic farmlands. Popul. Ecol. 58, 265–275. doi: 10.1007/s10144-015-0534-x
Dunbar, M. W., Gassmann, A. J., and O'Neal, M. E. (2016). Impacts of rotation schemes on ground-dwelling beneficial arthropods. Environ. Entomol. 45, 1154–1160. doi: 10.1093/ee/nvw104
Dunning, J. B., Danielson, B. J., and Pulliam, H. R. (1992). Ecological processes that affect populations in complex landscapes. Oikos 65, 169–175. doi: 10.2307/3544901
Egerer, M. H., Arel, C., Otoshi, M. D., Quistberg, R. D., Bichier, P., and Philpott, S. M. (2017). Urban arthropods respond variably to changes in landscape context and spatial scale. J. Urban Ecol. 3:jux001. doi: 10.1093/jue/jux001
Ekbom, B. S., Wiktelius, S., and Chiverton, P. A. (1992). Can polyphagous predators control the bird cherry-oat aphid (Rhopalosiphum padi) in spring cereals?: a simulation study. Entomol. Exp. Appl. 65, 215–223. doi: 10.1111/j.1570-7458.1992.tb00674.x
Eubanks, M. D., and Denno, R. F. (1999). The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 80, 1253–1266. doi: 10.1890/0012-9658(1999)0801253:TECOVI2.0.CO
Forbes, K. J., and Gratton, C. (2011). Stable isotopes reveal different patterns of inter-crop dispersal in two ladybeetle species. Ecol. Entomol. 36, 396–400. doi: 10.1111/j.1365-2311.2011.01268.x
Fox, A. F., Kim, T. N., Bahlai, C. A., Woltz, J. M., Gratton, C., and Landis, D. A. (2016). Cover crops have neutral effects on predator communities and biological control services in annual cellulosic bioenergy cropping systems. Agric. Ecosyst. Environ. 232, 101–109. doi: 10.1016/j.agee.2016.07.003
Gallé, R., Császár, P., Makra, T., Gallé-Szpisjak, N., Ladányi, Z., Torma, A., et al. (2018). Small-scale agricultural landscapes promote spider and ground beetle densities by offering suitable overwintering sites. Landscape Ecol. 33, 1435–1446. doi: 10.1007/s10980-018-0677-1
Ganser, D., Knop, E., and Albrecht, M. (2019). Sown wildflower strips as overwintering habitat for arthropods: effective measure or ecological trap? Agric. Ecosyst. Environ. 275, 123–131. doi: 10.1016/j.agee.2019.02.010
Gardiner, M. M., Burkman, C. E., and Prajzner, S. P. (2013). The value of urban vacant land to support arthropod biodiversity and ecosystem services. Environ. Entomol. 42, 1123–1136. doi: 10.1603/EN12275
Gardiner, M. M., and Harwood, J. D. (2017). Influence of heavy metal contamination on urban natural enemies and biological control. Curr. Opin. Insect Sci. 20, 45–53. doi: 10.1016/j.cois.2017.03.007
Gardiner, M. M., Prajzner, S. P., Burkman, C. E., Albro, S., and Grewal, P. S. (2014). Vacant land conversion to community gardens: influences on generalist arthropod predators and biocontrol services in urban greenspaces. Urban Ecosyst. 17, 101–122. doi: 10.1007/s11252-013-0303-6
Gontijo, L. M. (2019). Engineering natural enemy shelters to enhance conservation biological control in field crops. Biol. Control 130, 155–163. doi: 10.1016/j.biocontrol.2018.10.014
Gray, M. E., Sappington, T. W., Miller, N. J., Moeser, J., and Bohn, M. O. (2009). Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu. Rev. Entomol. 54, 303–321. doi: 10.1146/annurev.ento.54.110807.090434
Gurr, G. M., Wratten, S. D., Landis, D. A., and You, M. (2017). Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 62, 91–109. doi: 10.1146/annurev-ento-031616-035050
Haan, N. L., Zhang, Y., and Landis, D. A. (2020). Predicting landscape configuration effects on agricultural pest suppression. Trends Ecol. Evol. 35, 175–186. doi: 10.1016/j.tree.2019.10.003
Halaj, J., Cady, A. B., and Uetz, G. W. (2000). Modular habitat refugia enhance generalist predators and lower plant damage in soybeans. Environ. Entomol. 29, 383–393. doi: 10.1093/ee/29.2.383
Hanson, H. I., Birkhofer, K., Smith, H. G., Palmu, E., and Hedlund, K. (2017). Agricultural land use affects abundance and dispersal tendency of predatory arthropods. Basic Appl. Ecol. 18, 40–49. doi: 10.1016/j.baae.2016.10.004
Hare, J. D. (1990). Ecology and management of the Colorado potato beetle. Annu. Rev. Entomol. 35, 81–100. doi: 10.1146/annurev.en.35.010190.000501
Harker, K. N., O'Donovan, J. T., Turkington, T. K., Blackshaw, R. E., Lupwayi, N. Z., Smith, E. G., et al. (2015). Canola rotation frequency impacts canola yield and associated pest species. Can. J. Plant Sci. 95, 9–20. doi: 10.4141/cjps-2014-289
Harwood, J. D., and Obrycki, J. J. (2005). “The role of alternative prey in sustaining predator populations,” in Proceedings of the Second International Symposium on Biological Control of Arthropods (Davos), 453–462.
Heimoana, V., Pilkington, L. J., Raman, A., Mitchell, A., Nicol, H. I., Johnson, A. C., et al. (2017). Integrating spatially explicit molecular and ecological methods to explore the significance of non-crop vegetation to predators of brassica pests. Agric. Ecosyst. Environ. 239, 12–19. doi: 10.1016/j.agee.2017.01.008
Heimpel, G. E., Frelich, L. E., Landis, D. A., Hopper, K. R., Hoelmer, K. A., Sezen, Z., et al. (2010). European buckthorn and Asian soybean aphid as components of an extensive invasional meltdown in North America. Biol. Invasions 12, 2913–2931. doi: 10.1007/s10530-010-9736-5
Hemberger, J., Frappa, A., Witynski, G., and Gratton, C. (2020). Saved by the pulse? Separating the effects of total and temporal food abundance on the growth and reproduction of bumble bee microcolonies. Basic Appl. Ecol. 45, 1–11. doi: 10.1016/j.baae.2020.04.004
Holland, J. M., Bianchi, F. J., Entling, M. H., Moonen, A.-C., Smith, B. M., and Jeanneret, P. (2016). Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag. Sci. 72, 1638–1651. doi: 10.1002/ps.4318
Hooks, C. R. R., Hinds, J., Zobel, E., and Patton, T. (2013). Impact of crimson clover dying mulch on two eggplant insect herbivores. J. Appl. Entomol. 137, 170–180. doi: 10.1111/j.1439-0418.2012.01729.x
Horton, D. R. (2004). Phenology of emergence from artificial overwintering shelters by some predatory arthropods common in pear orchards of the Pacific Northwest. J. Entomol. Soc. Br. Colum. 101, 101–108.
Horton, D. R., Broers, D. A., Hinojosa, T., Lewis, T. M., Miliczky, E. R., and Lewis, R. R. (2002). Diversity and phenology of predatory arthropods overwintering in cardboard bands placed in pear and apple orchards of central Washington state. Ann. Entomol. Soc. Am. 95, 469–480. doi: 10.1603/0013-8746(2002)0950469:DAPOPA2.0.CO
Hossain, Z., Gurr, G. M., and Wratten, S. D. (2000). Effects of harvest on survival and dispersal of insect predators in hay lucerne. Biol. Agric. Hortic. 17, 339–348. doi: 10.1080/01448765.2000.9754854
Hossain, Z., Gurr, G. M., and Wratten, S. D. (2001). Habitat manipulation in lucerne (Medicago sativa L.): strip harvesting to enhance biological control of insect pests. Int. J. Pest Manag. 47, 81–88. doi: 10.1080/09670870151130471
Hossain, Z., Gurr, G. M., Wratten, S. D., and Raman, A. (2002). Habitat manipulation in lucerne Medicago sativa: arthropod population dynamics in harvested and ‘refuge' crop strips. J. Appl. Ecol. 39, 445–454. doi: 10.1046/j.1365-2664.2002.00729.x
Huang, H. T., and Yang, P. (1987). The ancient cultured citrus ant. Bioscience 37, 665–671. doi: 10.2307/1310713
Ives, A. R., and Settle, W. H. (1997). Metapopulation dynamics and pest control in agricultural systems. Am. Nat. 149, 220–246. doi: 10.1086/285988
Janssen, A., Montserrat, M., HilleRisLambers, R., de Roos, A. M., Pallini, A., and Sabelis, M. W. (2006). “Intraguild predation usually does not disrupt biological control,” in Trophic and Guild in Biological Interactions Control, eds J. Brodeur and G. Boivin (Dordrecht: Springer), 21–44. doi: 10.1007/1-4020-4767-3_2
Kabaluk, J. T., and Vernon, R. S. (2000). Effect of crop rotation on populations of Epitrix tuberis (Coleoptera: Chrysomelidae) in potato. J. Econ. Entomol. 93, 315–322. doi: 10.1603/0022-0493-93.2.315
Karp, D. S., Chaplin-Kramer, R., Meehan, T. D., Martin, E. A., DeClerck, F., Grab, H., et al. (2018). Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. U.S.A. 115, E7863–E7870. doi: 10.1073/pnas.1800042115
Kasirajan, S., and Ngouajio, M. (2012). Polyethylene and biodegradable mulches for agricultural applications: a review. Agron. Sust. Dev. 32, 501–529. doi: 10.1007/s13593-011-0068-3
Kawashima, M., and Jung, C. (2010). Artificial ground shelters for overwintering phytoseiid mites in orchards. Exp. Appl. Acarol. 52, 35–47. doi: 10.1007/s10493-010-9347-y
Kleijn, D., Bommarco, R., Fijen, T. P. M., Garibaldi, L. A., Potts, S. G., and van der Putten, W. H. (2019). Ecological intensification: bridging the gap between science and practice. Trends Ecol. Evol. 34, 154–166. doi: 10.1016/j.tree.2018.11.002
Knapp, M., Seidl, M., Knappov,á, J., Macek, M., and Saska, P. (2019). Temporal changes in the spatial distribution of carabid beetles around arable field-woodlot boundaries. Sci. Rep. 9:8967. doi: 10.1038/s41598-019-45378-7
Koch, R. L., Sezen, Z., Porter, P. M., Ragsdale, D. W., Wyckhuys, K. A. G., and Heimpel, G. E. (2015). On-farm evaluation of a fall-seeded rye cover crop for suppression of soybean aphid (Hemiptera: Aphididae) on soybean. Agric. For. Entomol. 17, 239–246. doi: 10.1111/afe.12099
Koss, A. M., and Snyder, W. E. (2005). Alternative prey disrupt biocontrol by a guild of generalist predators. Biol. Control 32, 243–251. doi: 10.1016/j.biocontrol.2004.10.002
Landis, D. A., Wratten, S. D., and Gurr, G. M. (2000). Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 45, 175–201. doi: 10.1146/annurev.ento.45.1.175
Langer, A., and Hance, T. (2004). Enhancing parasitism of wheat aphids through apparent competition: a tool for biological control. Agric. Ecosyst. Environ. 102, 205–212. doi: 10.1016/j.agee.2003.07.005
Leach, H., Moses, J., Hanson, E., Fanning, P., and Isaacs, R. (2018). Rapid harvest schedules and fruit removal as non-chemical approaches for managing spotted wing Drosophila. J. Pest. Sci. 91, 219–226. doi: 10.1007/s10340-017-0873-9
Lee, J. C., and Heimpel, G. E. (2005). Impact of flowering buckwheat on Lepidopteran cabbage pests and their parasitoids at two spatial scales. Biol. Control 34, 290–301. doi: 10.1016/j.biocontrol.2005.06.002
Lee, J. C., and Heimpel, G. E. (2008). Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Animal Ecol. 77, 565–572. doi: 10.1111/j.1365-2656.2008.01355.x
Letourneau, D. K., Armbrecht, I., Rivera, B. S., Lerma, J. M., Carmona, E. J., Daza, M. C., et al. (2011). Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 21, 9–21. doi: 10.1890/09-2026.1
Lin, B. B., Philpott, S. M., and Jha, S. (2015). The future of urban agriculture and biodiversity-ecosystem services: challenges and next steps. Basic Appl. Ecol. 16, 189–201. doi: 10.1016/j.baae.2015.01.005
Litsinger, J. A., Alviola, A. L., Cruz, C. G. D., Canapi, B. L. E. H. B.-A III., and Barrion, A. T. (2006). Rice white stemborer Scirpophaga innotata (Walker) in southern Mindanao, Philippines. II. Synchrony of planting and natural enemies. Int. J. Pest Manage. 52, 23–37. doi: 10.1080/09670870600552463
Macfadyen, S., Kramer, E. A., Parry, H. R., and Schellhorn, N. A. (2015). Temporal change in vegetation productivity in grain production landscapes: linking landscape complexity with pest and natural enemy communities. Ecol. Entomol. 40, 56–69. doi: 10.1111/een.12213
Mallinger, R. E., Gibbs, J., and Gratton, C. (2016). Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees' foraging periods. Landsc. Ecol. 31, 1523–1535. doi: 10.1007/s10980-015-0332-z
Meineke, E. K., Dunn, R. R., and Frank, S. D. (2014). Early pest development and loss of biological control are associated with urban warming. Biol. Lett. 10:20140586. doi: 10.1098/rsbl.2014.0586
Men, X., Ge, F., Yardim, E., and Parajulee, M. (2004). Evaluation of winter wheat as a potential relay crop for enhancing biological control of cotton aphids. Bio Control 49, 701–714. doi: 10.1007/s10526-004-5278-z
Mestre, L., Schirmel, J., Hetz, J., Kolb, S., Pfister, S. C., Amato, M., et al. (2018). Both woody and herbaceous semi-natural habitats are essential for spider overwintering in European farmland. Agric. Ecosyst. Environ. 267, 141–146. doi: 10.1016/j.agee.2018.08.018
Morales, H. (2002). Pest management in traditional tropical agroecosystems: lessons for pest prevention research and extension. Integr. Pest Manag. Rev. 7, 145–163. doi: 10.1023/B:IPMR.0000027502.91079.01
Nardi, D., Lami, F., Pantini, P., and Marini, L. (2019). Using species-habitat networks to inform agricultural landscape management for spiders. Biol. Conserv. 239:108275. doi: 10.1016/j.biocon.2019.108275
Nelson, J. L., Hunt, L. G., Lewis, M. T., Hamby, K. A., Hooks, C. R. R., and Dively, G. P. (2018). Arthropod communities in warm and cool grass riparian buffers and their influence on natural enemies in adjacent crops. Agric. Ecosyst. Environ. 257, 81–91. doi: 10.1016/j.agee.2018.01.019
New, T. R. (2015). Insect Conservation and Urban Environments. Springer International Publishing. doi: 10.1007/978-3-319-21224-1
Ng, K., Barton, P. S., Macfadyen, S., Lindenmayer, D. B., and Driscoll, D. A. (2018). Beetle's responses to edges in fragmented landscapes are driven by adjacent farmland use, season and cross-habitat movement. Landsc. Ecol. 33, 109–125. doi: 10.1007/s10980-017-0587-7
Öberg, S., Ekbom, B., and Bommarco, R. (2007). Influence of habitat type and surrounding landscape on spider diversity in Swedish agroecosystems. Agric. Ecosyst. Environ. 122, 211–219. doi: 10.1016/j.agee.2006.12.034
Öberg, S., Mayr, S., and Dauber, J. (2008). Landscape effects on recolonisation patterns of spiders in arable fields. Agric. Ecosyst. Environ. 123, 211–218. doi: 10.1016/j.agee.2007.06.005
O'Rourke, M. E., Liebman, M., and Rice, M. E. (2008). Ground beetle (Coleoptera: Carabidae) assemblages in conventional and diversified crop rotation systems. Environ. Entomol. 37, 121–130. doi: 10.1603/0046-225X(2008)37121:GBCCAI2.0.CO
Osawa, N. (2000). Population field studies on the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Popul. Ecol. 42, 115–127. doi: 10.1007/PL00011990
Osipitan, O. A., Dille, J. A., Assefa, Y., and Knezevic, S. Z. (2018). Cover crop for early season weed suppression in crops: systematic review and meta-analysis. Agron. J. 110, 2211–2221. doi: 10.2134/agronj2017.12.0752
Ostfeld, R. S., and Keesing, F. (2000). Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol. Evol. 15, 232–237. doi: 10.1016/S0169-5347(00)01862-0
Parry, H. R., Marcora, A., Macfadyen, S., Hopkinson, J., Hulthen, A. D., Neave, M., et al. (2019). A native with a taste for the exotic: weeds and pasture provide year-round habitat for Nysius vinitor (Hemiptera: Orsillidae) across Australia, with implications for area-wide management. Austr. Entomol. 58, 237–247. doi: 10.1111/aen.12391
Pellissier, M. E., and Jabbour, R. (2018). Herbivore and parasitoid insects respond differently to annual and perennial floral strips in an alfalfa ecosystem. Biol. Control 123, 28–35. doi: 10.1016/j.biocontrol.2018.04.014
Peñalver-Cruz, A., Alvarez-Baca, J. K., Alfaro-Tapia, A., Gontijo, L., and Lavandero, B. (2019). Manipulation of agricultural habitats to improve conservation biological control in South America. Neotrop. Entomol. 48, 875–898. doi: 10.1007/s13744-019-00725-1
Perfecto, I., Vandermeer, J. H., and Wright, A. L. (2019). Nature's Matrix: Linking Agriculture, Conservation and Food Sovereignty. 2nd Edn. New York, NY: Routledge.
Pfannenstiel, R. S., Mackey, B. E., and Unruh, T. R. (2012). Leafroller parasitism across an orchard landscape in central Washington and effect of neighboring rose habitats on parasitism. Biol. Control 62, 152–161. doi: 10.1016/j.biocontrol.2012.04.006
Philpott, S. M., and Bichier, P. (2017). Local and landscape drivers of predation services in urban gardens. Ecol. Appl. 27, 966–976. doi: 10.1002/eap.1500
Prasifka, J. R., Heinz, K. M., and Winemiller, K. O. (2004). Crop colonisation, feeding, and reproduction by the predatory beetle, Hippodamia convergens, as indicated by stable carbon isotope analysis. Ecol. Entomol. 29, 226–233. doi: 10.1111/j.0307-6946.2004.00585.x
Prasifka, J. R., Schmidt, N. P., Kohler, K. A., O'neal, M. E., Hellmich, R. L., and Singer, J. W. (2006). Effects of living mulches on predator abundance and sentinel prey in a corn–soybean–forage rotation. Environ. Entomol. 35, 1423–1431. doi: 10.1093/ee/35.5.1423
Rakhshani, H., Ebadi, R., Hatami, B., Rakhshani, E., and Gharali, B. (2010). A survey of alfalfa aphids and their natural enemies in Isfahan, Iran, and the effect of alfalfa strip-harvesting on their population. J. Entomol. Soc. Iran 30, 13–28. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?id=217184
Ramsden, M. W., Menéndez, R., Leather, S. R., and Wäckers, F. (2015). Optimizing field margins for biocontrol services: the relative role of aphid abundance, annual floral resources, and overwinter habitat in enhancing aphid natural enemies. Agric. Ecosyst. Environ. 199, 94–104. doi: 10.1016/j.agee.2014.08.024
Rand, T. A., Tylianakis, J. M., and Tscharntke, T. (2006). Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 9, 603–614. doi: 10.1111/j.1461-0248.2006.00911.x
Raymond, L., Ortiz-Martínez, S. A., and Lavandero, B. (2015). Temporal variability of aphid biological control in contrasting landscape contexts. Biol. Control 90, 148–156. doi: 10.1016/j.biocontrol.2015.06.011
Redlich, S., Martin, E. A., and Steffan-Dewenter, I. (2018). Landscape-level crop diversity benefits biological pest control. J. Appl. Ecol. 55, 2419–2428. doi: 10.1111/1365-2664.13126
Root, R. B. (1973). Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monogr. 43, 95–124. doi: 10.2307/1942161
Rosenheim, J. A., and Harmon, J. P. (2006). “The influence of intraguild predation on the suppression of a shared prey population: an empirical reassessment,” in Trophic and Guild in Biological Interactions Control, eds J. Brodeur and G. Boivin (Dordrecht: Springer), 21–44. doi: 10.1007/1-4020-4767-3_1
Rosenheim, J. A., Kaya, H. K., Ehler, L. E., Marois, J. J., and Jaffee, B. A. (1995). Intraguild predation among biological-control agents: theory and evidence. Biol. Control 5, 303–335. doi: 10.1006/bcon.1995.1038
Roume, A., Ouin, A., Raison, L., and Deconchat, M. (2013). Abundance and species richness of overwintering ground beetles (Coleoptera: Carabidae) are higher in the edge than in the centre of a woodlot. Eur. J. Entomol. 108, 615–622. doi: 10.14411/eje.2011.080
Royauté, R., and Buddle, C. M. (2012). Colonization dynamics of agroecosystem spider assemblages after snow-melt in Quebec (Canada). J. Arachnol. 40, 48–58. doi: 10.1636/P11-16.1
Rusch, A., Bommarco, R., Jonsson, M., Smith, H. G., and Ekbom, B. (2013). Flow and stability of natural pest control services depend on complexity and crop rotation at the landscape scale. J. Appl. Ecol. 50, 345–354. doi: 10.1111/1365-2664.12055
Russell, E. P. (1989). Enemies hypothesis: a review of the effect of vegetational diversity on predatory insects and parasitoids. Environ. Entomol. 18, 590–599. doi: 10.1093/ee/18.4.590
Salliou, N., and Barnaud, C. (2017). Landscape and biodiversity as new resources for agro-ecology? Insights from farmers' perspectives. Ecol. Soc. 22:16. doi: 10.5751/ES-09249-220216
Samu, F. (2003). Can field-scale habitat diversification enhance the biocontrol potential of spiders? Pest Manag. Sci. 59, 437–442. doi: 10.1002/ps.635
Sann, C., Theodorou, P., Heong, K. L., Villareal, S., Settele, J., Vidal, S., et al. (2018). Hopper parasitoids do not significantly benefit from non-crop habitats in rice production landscapes. Agric. Ecosyst. Environ. 254, 224–232. doi: 10.1016/j.agee.2017.11.035
Sarthou, J.-P., Badoz, A., Vaissière, B., Chevallier, A., and Rusch, A. (2014). Local more than landscape parameters structure natural enemy communities during their overwintering in semi-natural habitats. Agric. Ecosyst. Environ. 194, 17–28. doi: 10.1016/j.agee.2014.04.018
Schellhorn, N. A., Gagic, V., and Bommarco, R. (2015). Time will tell: resource continuity bolsters ecosystem services. Trends Ecol. Evol. 30, 524–530. doi: 10.1016/j.tree.2015.06.007
Schmidt, N. P., O'Neal, M. E., and Singer, J. W. (2007). Alfalfa living mulch advances biological control of soybean aphid. Environ. Entomol. 36, 416–424. doi: 10.1093/ee/36.2.416
Segoli, M., and Rosenheim, J. A. (2013). Spatial and temporal variation in sugar availability for insect parasitoids in agricultural fields and consequences for reproductive success. Biol. Control 67, 163–169. doi: 10.1016/j.biocontrol.2013.07.013
Settele, J., and Settle, W. H. (2018). Conservation biological control: improving the science base. Proc. Natl. Acad. Sci. U.S.A. 115, 8241–8243. doi: 10.1073/pnas.1810334115
Settle, W. H., Ariawan, H., Astuti, E. T., Cahyana, W., Hakim, A. L., Hindayana, D., et al. (1996). Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 77, 1975–1988. doi: 10.2307/2265694
Shields, M. W., Johnson, A. C., Pandey, S., Cullen, R., González- Chang, M., Wratten, S. D., et al. (2019). History, current situation and challenges for conservation biological control. Biol. Control 131, 25–35. doi: 10.1016/j.biocontrol.2018.12.010
Sirami, C., Gross, N., Baillod, A. B., Bertrand, C., Carrié, R., Hass, A., et al. (2019). Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. U.S.A. 116 16442–16447. doi: 10.1073/pnas.1906419116
Sorribas, J., González, S., Domínguez-Gento, A., and Vercher, R. (2016). Abundance, movements and biodiversity of flying predatory insects in crop and non-crop agroecosystems. Agron. Sust. Dev. 36:34. doi: 10.1007/s13593-016-0360-3
Spear, J. E., Grijalva, E. K., Michaels, J. S., and Parker, S. S. (2018). Ecological spillover dynamics of organisms from urban to natural landscapes. J. Urban Ecol. 4:juy008. doi: 10.1093/jue/juy008
Spiesman, B. J., Iuliano, B. G., and Gratton, C. (2020). Temporal resource continuity increases predator abundance in a metapopulation model: insights for conservation and biocontrol. bioRxiv [Preprint]. doi: 10.1101/2020.03.25.006882
Stern, V., Bosch, R., and Leigh, T. (1964). Strip cutting alfalfa for lygus bug control. Calif. Agric. 18, 4–6.
Summers, C. G. (1976). Population fluctuations of selected arthropods in alfalfa: influence of two harvesting practices. Environ. Entomol 5, 103–110. doi: 10.1093/ee/5.1.103
Sutter, L., Amato, M., Jeanneret, P., and Albrecht, M. (2018). Overwintering of pollen beetles and their predators in oilseed rape and semi-natural habitats. Agric. Ecosyst. Environ. 265, 275–281. doi: 10.1016/j.agee.2018.06.030
Tittonell, P. (2014). Ecological intensification of agriculture—sustainable by nature. Curr. Opin. Environ. Sust. 8, 53–61. doi: 10.1016/j.cosust.2014.08.006
Toivonen, M., Huusela-Veistola, E., and Herzon, I. (2018). Perennial fallow strips support biological pest control in spring cereal in Northern Europe. Biol. Control 121, 109–118. doi: 10.1016/j.biocontrol.2018.02.015
Tscharntke, T., Karp, D. S., Chaplin-Kramer, R., Batáry, P., DeClerck, F., Gratton, C., et al. (2016). When natural habitat fails to enhance biological pest control: five hypotheses. Biol. Conserv. 204, 449–458. doi: 10.1016/j.biocon.2016.10.001
Tsutsui, M. H., Tanaka, K., Baba, Y. G., and Miyashita, T. (2016). Spatio-temporal dynamics of generalist predators (Tetragnatha spider) in environmentally friendly paddy fields. Appl. Entomol. Zool. 51, 631–640. doi: 10.1007/s13355-016-0440-5
Tylianakis, J. M., Didham, R. K., and Wratten, S. D. (2004). Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85, 658–666. doi: 10.1890/03-0222
Unruh, T. R., Pfannenstiel, R. S., Peters, C., Brunner, J. F., and Jones, V. P. (2012). Parasitism of leafrollers in Washington fruit orchards is enhanced by perimeter plantings of rose and strawberry. Biol. Control 62, 162–172. doi: 10.1016/j.biocontrol.2012.04.007
van der Werf, W. (1995). “How do immigration rates affect predator/prey interactions in field crops? Predictions from simple models and an example involving the spread of aphid-borne viruses in sugar beet,” in Arthropod Natural Enemies in Arable Land. 1. Density, Spatial Heterogeneity and Dispersal, eds S. Toft and W. Riedel (Aarhus), 295–312.
van Rijn, P. C. J., Kooijman, J., and Wäckers, F. L. (2013). The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 67, 32–38. doi: 10.1016/j.biocontrol.2013.06.014
Vasseur, C., Joannon, A., Aviron, S., Burel, F., Meynard, J.-M., and Baudry, J. (2013). The cropping systems mosaic: how does the hidden heterogeneity of agricultural landscapes drive arthropod populations? Agric. Ecosyst. Environ. 166, 3–14. doi: 10.1016/j.agee.2012.08.013
Villegas, C. M., Verdugo, J. A., Grez, A. A., Tapia, J., and Lavandero, B. (2013). Movement between crops and weeds: temporal refuges for aphidophagous insects in Central Chile. Ciencia e Investigación Agraria 40, 317–326. doi: 10.4067/S0718-16202013000200007
Wäckers, F. L., Rijn, P. C. J., and van Bruin, J. (2005). Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications. Cambridge, UK: Cambridge University Press.
Welch, K. D., and Harwood, J. D. (2014). Temporal dynamics of natural enemy–pest interactions in a changing environment. Biol. Control 75, 18–27. doi: 10.1016/j.biocontrol.2014.01.004
Wilson, H., Miles, A. F., Daane, K. M., and Altieri, M. A. (2017). Landscape diversity and crop vigor outweigh influence of local diversification on biological control of a vineyard pest. Ecosphere 8:e01736. doi: 10.1002/ecs2.1736
Wissinger, S. A. (1997). Cyclic colonization in predictably ephemeral habitats: a template for biological control in annual crop systems. Biol. Control 10, 4–15. doi: 10.1006/bcon.1997.0543
Wyckhuys, K. A., Lu, Y., Morales, H., Vazquez, L. L., Legaspi, J. C., Eliopoulos, P., et al. (2013). Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 65, 152–167. doi: 10.1016/j.biocontrol.2012.11.010
Yang, L., Zeng, Y., Xu, L., Liu, B., Zhang, Q., and Lu, Y. (2018). Change in ladybeetle abundance and biological control of wheat aphids over time in agricultural landscape. Agric. Ecosyst. Environ. 255, 102–110. doi: 10.1016/j.agee.2017.12.013
Yang, L. H., Bastow, J. L., Spence, K. O., and Wright, A. N. (2008). What can we learn from resource pulses? Ecology 89, 621–634. doi: 10.1890/07-0175.1
Yang, L. H., Edwards, K. F., Byrnes, J. E., Bastow, J. L., Wright, A. N., and Spence, K. O. (2010). A meta-analysis of resource pulse-consumer interactions. Ecol. Monogr. 80, 125–151. doi: 10.1890/08-1996.1
Yanik, E., Unlu, L., and Yucel, A. (2011). The phenology of emergence from artificial overwintering sites by predatory arthropods in pistachio orchards and adjacent habitats. Ekoloji 20, 1–6. doi: 10.5053/ekoloji.2011.781
Keywords: habitat management, natural enemies, agroecology, predator-prey interactions, landscape ecology, entomology
Citation: Iuliano B and Gratton C (2020) Temporal Resource (Dis)continuity for Conservation Biological Control: From Field to Landscape Scales. Front. Sustain. Food Syst. 4:127. doi: 10.3389/fsufs.2020.00127
Received: 31 March 2020; Accepted: 22 July 2020;
Published: 11 September 2020.
Edited by:
Inge Armbrecht, University of Valle, ColombiaReviewed by:
Adrien Rusch, Institut National de la Recherche Agronomique (INRA), FranceMichael Garratt, University of Reading, United Kingdom
Copyright © 2020 Iuliano and Gratton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Iuliano, Yml1bGlhbm9Ad2lzYy5lZHU=
 Benjamin Iuliano
Benjamin Iuliano Claudio Gratton
Claudio Gratton