- 1Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
- 2Ludeman Family Center for Women’s Health Research, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
- 3Division of Endocrinology, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
- 4Rocky Mountain Regional Department of Veterans Affairs Medical Center (VAMC), Aurora, CO, United States
- 5Division of General Internal Medicine, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
- 6Division of Cardiology, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
Type 2 diabetes (T2D) has been rising in prevalence over the past few decades in the US and worldwide. T2D contributes to significant morbidity and premature mortality, primarily due to cardiovascular disease (CVD). Exercise is a major cornerstone of therapy for T2D as a result of its positive effects on glycemic control, blood pressure, weight loss and cardiovascular risk as well as other measures of health. However, studies show that a majority of people with T2D do not exercise regularly. The reasons given as to why exercise goals are not met are varied and include physiological, psychological, social, cultural and environmental barriers to exercise. One potential cause of inactivity in people with T2D is impaired cardiorespiratory fitness, even in the absence of clinically evident complications. The exercise impairment, although present in both sexes, is greater in women than men with T2D. Women with T2D also experience greater perceived exertion with exercise than their counterparts without diabetes. These physiological barriers are in addition to constructed societal barriers including cultural expectations of bearing the burden of childrearing for women and in some cultures, having limited access to exercise because of additional cultural expectations. People at risk for and with diabetes more commonly experience unfavorable social determinants of health (SDOH) than people without diabetes, represented by neighborhood deprivation. Neighborhood deprivation measures lack of resources in an area influencing socioeconomic status including many SDOH such as income, housing conditions, living environment, education and employment. Higher indices of neighborhood deprivation have been associated with increased risk of all-cause, cardiovascular and cancer related mortality. Unfavorable SDOH is also associated with obesity and lower levels of physical activity. Ideally regular physical activity should be incorporated into all communities as part of a productive and healthy lifestyle. One potential solution to improve access to physical activity is designing and building environments with increased walkability, greenspace and safe recreational areas. Other potential solutions include the use of continuous glucose monitors as real-time feedback tools aimed to increase motivation for physical activity, counseling aimed at improving self-efficacy towards exercise and even acquiring a dog to increase walking time. In this narrative review, we aim to examine some traditional and novel barriers to exercise, as well as present evidence on novel interventions or solutions to overcome barriers to increase exercise and physical activity in all people with prediabetes and T2D.
1 Introduction
The global prevalence of type 2 diabetes (T2D) is rising, increasing from 6.2% in 2007 to 10.5% in 2021. Prevalence is predicted to continue to rise to an estimated 12.2% by the year 2045 (1, 2). This increasing prevalence is problematic since T2D confers a 2-4 times increased risk of all-cause mortality compared to the general population (3). The majority of premature mortality in T2D is due to cardiovascular disease (CVD), the leading cause of death in T2D (3, 4). CVD includes disease related to pathology within the heart and vasculature including but not limited to coronary artery disease, heart failure, hypertension, cerebrovascular accidents and peripheral artery disease (5). One major modifiable risk factor related to cardiovascular mortality is cardiorespiratory fitness (CRF). CRF is a measure of the circulatory and respiratory systems’ ability to supply oxygen to skeletal muscle during sustained exercise (6). Maximum oxygen uptake during peak exercise (VO2peak) is the gold standard measure of CRF and is a strong predictor of all-cause mortality in T2D as well as many other disease states (7–9). Lower CRF is also predictive of short-term CVD events and increased risk of sudden cardiac death (10, 11). Physical inactivity, which results in decreased CRF, is an additional independent risk factor for all-cause mortality (12, 13). Fortunately, CRF can be improved through exercise (14, 15). In addition to the influence of exercise on CRF, and therefore mortality, exercise has also been shown to have beneficial effects on HbA1c, hypertension and body mass index (BMI) in type 2 diabetes (T2D) making it a cornerstone of treatment (16, 17). Similarly, exercise is beneficial to the general population and in people with prediabetes as it lowers the risk of developing T2D (18, 19). Major medical societies including the American Diabetes Association (ADA), American College of Cardiology (ACC), American Heart Association (AHA) and American College of Sports Medicine (ACSM) include exercise recommendations in their guidelines as a risk modifier to reduce CVD risk. It is recommended that all adults participate in 150 minutes or more per week of moderate intensity aerobic exercise, such as biking, dancing or brisk walking or 75 minutes or more per week of vigorous intensity exercise, such as running, singles tennis or swimming laps in addition to 2-3 sessions per week of resistance training exercise (20–22). Unfortunately, as noted, people with T2D exercise less than the general population and the majority do not meet this recommendation (23–27). Even when people with T2D participate in exercise programs, few maintain these changes long term (28, 29). The contributors to decreased exercise participation include the universal societal barriers to exercise, such as busy schedules and competing interests influencing motivation, in addition to those specific to people with T2D. Several qualitative studies have also explored reasons why people with T2D exercise less (30, 31). Answers reflect a wide variety of obstacles including physiological barriers such as “physical discomfort,” psychological barriers such as “lack of motivation,” social barriers like “lack of childcare” and environmental barriers including “weather” (30–32). In this narrative review, we will explore the physiological and societal frameworks behind these hurdles to better understand exercise patterns in those with T2D. We will also explore novel, evidenced-based solutions required to overcome each of these barriers to increase exercise for people with prediabetes and T2D (Figure 1).
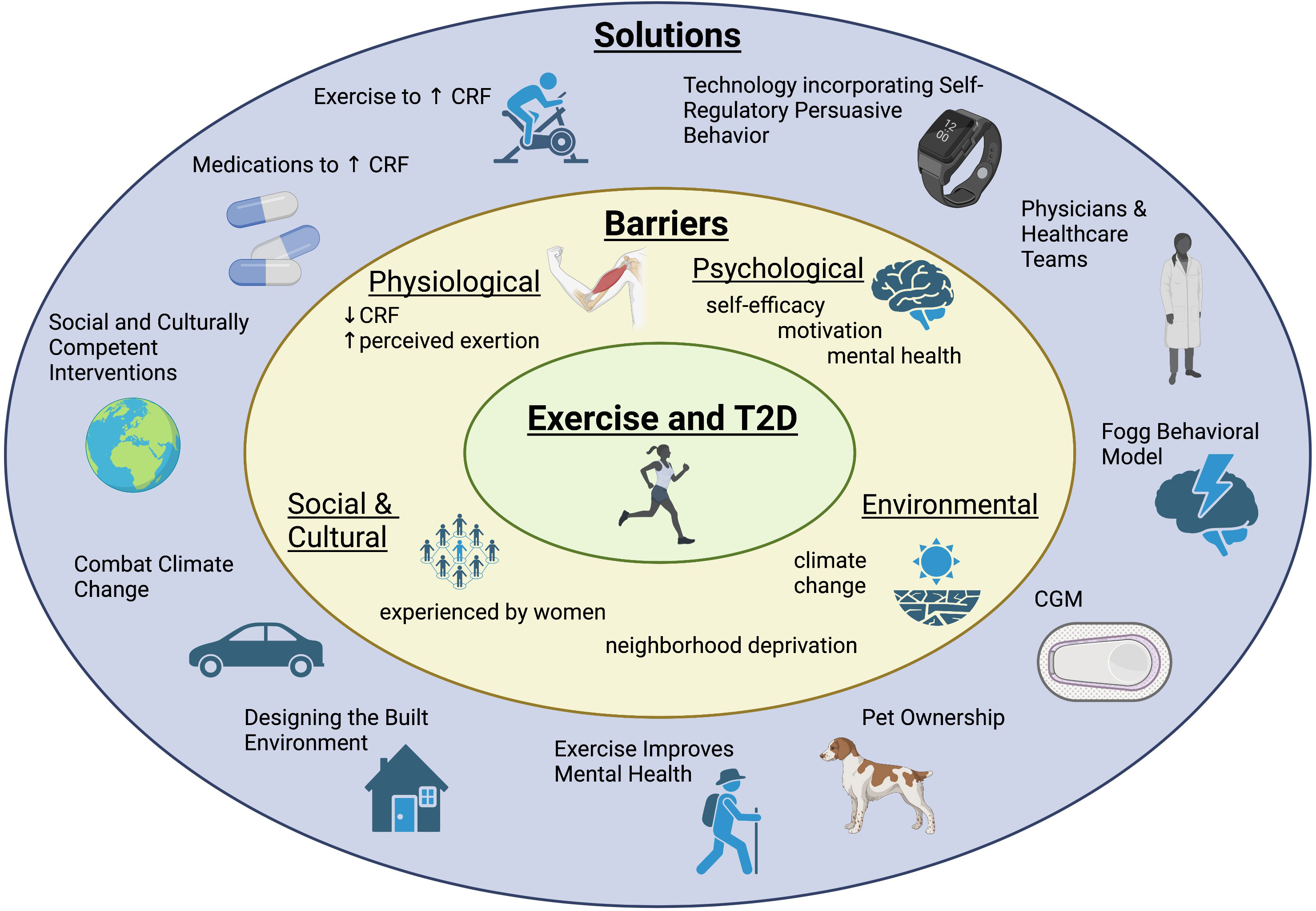
Figure 1 Adults with prediabetes and type 2 diabetes face a variety of barriers to exercise. This diagram highlights a summary of those barriers as well as novel interventions and solutions to overcome obstacles and increase exercise for adults with prediabetes and type 2 diabetes.
2 Physiological barriers to exercise in T2D and potential solutions
2.1 Barrier: reduced cardiorespiratory fitness
Physical symptoms are among the most reported barriers to exercise in people with T2D (23–27, 30). The majority of people with T2D are sedentary with as many as 39% of patients with T2D reporting either “physical discomfort” or being “too tired” as their primary reason for not exercising at recommended levels (23–27, 30). However, there are other reasons why people with T2D might not exercise. One potential reason is that people with T2D have impaired peak exercise performance compared to activity and weight similar controls without diabetes, a deficit that appears to be more pronounced in women than men (33, 34). Specifically, on graded exercise testing, sedentary individuals with uncomplicated T2D displayed 24% less maximal walking time and 20% lower VO2peak than age and activity similar controls without diabetes, regardless of glycemic control (35). In terms of sex differences, women with T2D had a greater deficit in VO2peak versus women without diabetes, a 24% reduction, when compared to men with T2D versus men without diabetes, a 16% reduction (33). This limitation is particularly significant since lower CRF, measured by VO2peak, strongly correlates with increased cardiovascular mortality, and may explain the higher mortality rate in women with uncomplicated T2D compared to men with uncomplicated T2D (15). The mechanism by which this impairment occurs is complex and multifactorial. Physiological mechanisms of the impaired oxygen consumption are being explored and include a combination of metabolic, cardiac and peripheral factors including insulin resistance, mitochondrial dysfunction, vascular endothelial dysfunction and cardiac dysfunction (36). No single mechanism fully explains the deficit in CRF, but the sum of cardiac, skeletal muscle, circulatory and metabolic abnormalities of these impairments likely contribute to physical symptoms hindering exercise performance in people with T2D.
2.2 Barrier: greater perceived exertion
Greater perceived exertion is one of the most commonly cited reasons why people with T2D do not exercise (30). Women with T2D were compared to controls without diabetes to determine differences in perceived exertion (37). Rate of perceived exertion (RPE), a measure of exercise exertion, was measured in women with uncomplicated T2D by the self-reported Borg scale during submaximal bicycling exercise and compared to RPE in obese and lean women without diabetes. The group with T2D perceived higher rates of exertion compared to both obese and lean women without diabetes. In another study, RPE was recorded during every minute of maximal stress testing in people with T2D to determine if subjective perception of moderate exercise intensity correlated with target heart rate for moderate exercise intensity (38). Almost half of individuals were unable to accurately perceive moderate intensity exercise (as defined by heart rate) which further suggests limitations in perception of exertion during exercise in T2D. Those who were unable to appropriately identify moderate intensity exercise were more likely to be female, African American or Hispanic as well as have a higher BMI and lower fitness level. Increased perception of exercise exertion compared to people without diabetes remains a physiological barrier to exercise in T2D. This difference in perception is poorly understood and further research is needed to identify mechanisms to explain this phenomenon. It is possible that interventions, either pharmacological or otherwise, that decrease perceived exercise exertion could support greater engagement in exercise behaviors.
2.3 Solution: improving cardiorespiratory fitness through exercise training
If inherently reduced CRF is associated, at least in part, with the physical symptoms that are barriers to exercise in people with T2D, then increasing CRF can be a possible solution to increasing exercise participation. Increasing CRF can be challenging since the best way to accomplish this is through exercise training and/or increased physical activity. In a prospective cohort study of 72,624 individuals with a variety of CVD risk factors, including diabetes, high cholesterol, and hypertension among others, there was a strong association with self-reported physical activity and higher CRF (15). Furthermore, in a different study, women with uncomplicated T2D, who have lower CRF, overweight women without diabetes and lean women without diabetes underwent a 3-month exercise training intervention with pre and post exercise testing to see if CRF would improve with exercise training (14). The exercise intervention consisted of 60 minutes of supervised aerobic exercise 3 times per week to achieve 70-85% maximum heart rate for 3 months. After completing the exercise program, women with T2D showed 28% improvement in VO2peak compared to 5% in overweight women without diabetes and 8% in lean women without diabetes (P<0.05 when T2D group compared to both control groups) representing the ability to modify CRF in T2D with exercise training. Women with T2D started with the lowest CRF and had the greatest increase in CRF with exercise, emphasizing the potential benefits of exercise training in T2D. The U-TURN trial provides an additional example of the benefits of exercise in T2D. Adults with T2D participated in a 12 month lifestyle intervention including 5-6 sessions per week of partially supervised aerobic and resistance exercise, which allowed for graduated autonomy over a period of 4 months and an individualized diet plan focused on macronutrient distribution. The participants in the lifestyle intervention had improved glycemic control compared to the group receiving standard of care alone (39). Participants were interviewed at completion of the study and at a 6-month interval. They stated their main motivators to maintain exercise were “improved fitness and physical form” and “improved well-being” (40). The problem remains that initiating exercise training to improve CRF requires the individual to participate in exercise. Overcoming inertia related to baseline low CRF requires creativity on the part of the individual and their coach or provider. Lessons can be taken from cardiac rehabilitation programs where step one is overcoming deconditioning prior to effective engagement in conditioning or exercise training programs (41). One term for initiating an exercise program in an inactive person is “conditioning” with a focus on gradually increasing activity with stretching, warm-up and cool down.
2.4 Solution: improving cardiorespiratory fitness through medications
Great effort and expense have gone into identifying potential pharmacological strategies to improve CRF. The search for exercise mimetics has been largely ineffective to date. However, some small physiological studies have demonstrated improvements in CRF with medications that have effects that might translate to improving exercise capacity (42, 43). Ideally medications or other strategies to decrease impairment in CRF could set the stage for encouraging individuals to incorporate exercise into daily life. For example, we and others have demonstrated that rosiglitazone, a thiazolidinedione, improves CRF in patients with T2D (42, 43). In 2005 participants with uncomplicated T2D were randomized to rosiglitazone 4mg/day or placebo (42). Exercise capacity, endothelial function and insulin sensitivity were measured at baseline and after 4 months of intervention. Compared to placebo rosiglitazone was associated with an improvement in VO2peak, endothelial function and insulin sensitivity (42). This phenomenon was corroborated in 2007 when a similar study also demonstrated an increase in VO2peak and insulin sensitivity with rosiglitazone versus placebo (43). It’s important to note that increases in CRF are even greater with rosiglitazone plus exercise than either rosiglitazone or exercise alone (44). However, rosiglitazone is not in wide use at this time. Notably, the currently available thiazolidinedione, pioglitazone, also improves CRF (45).
To our knowledge other potential exercise mimetics have not shown a direct measured benefit in CRF in people, or have not been studied for this outcome, but may still target the mechanisms to ameliorate impaired exercise capacity. The large NIH multi-center study evaluating the molecular transducers of exercise (MoTrPAC) may inform targets for exercise mimetics since this study is examining the molecular basis of exercise (46).
In animals numerous agents have been shown to either increase VO2peak or endurance but to date, compelling studies to support their use in humans are lacking (47, 48). The enzyme 5’ adenosine monophosphate-activated protein kinase (AMPK) is a critical bioenergetic sensor in the skeletal muscle stimulated and necessary for exercise training responses; many molecular in vitro and animal studies have explored AMPK activators as exercise mimetics (49, 50). Another example, glucagon-like peptide 1 (GLP 1) agonists, have known cardiovascular benefits and have been shown to improve skeletal and cardiac muscle insulin resistance through nitric oxide dependent endothelial dilation in animal and human studies (51–55). Dipeptidyl Peptidase-4 inhibitors, that work on the same incretin pathway as GLP 1 agonists, have been shown in rats to improve mitochondrial adaptation to exercise (56). Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors ameliorate production of advanced glycation end products in animal models, which are pathologic in development of microvascular complications of T2D (57–61). L-arginine has also been demonstrated to improve endothelial dilation and therefore insulin sensitivity in human studies (62, 63). Searching for exercise mimetics is an exciting pursuit; although to date agents have not been discovered to fully mimic the systemic impact of exercise. Targeting and reversing various mechanisms of exercise impairment in T2D may lead to improved CRF and therefore fewer physical symptoms of exertion during exercise and improved exercise self-efficacy. Exercise in combination with agents that activate peroxisome proliferator-activated receptor (PPAR) delta have shown promise as combination therapy in animal studies (47). At this moment more investigation is needed in this area and interventions involving medications in combination with behavior change are most likely to enable durable improvements in CRF.
3 Psychological barriers to exercise in T2D and potential solutions
3.1 Barrier: motivation and self-efficacy
Psychological barriers also contribute to low exercise levels in people with T2D, which have been expressed in interviews as “too boring,” “dislike,” “negative past experiences,” “embarrassed” and “I just feel like giving up” (30, 40). These statements reflect feelings of poor motivation and low self-efficacy, or one’s belief in their ability to perform a behavior to achieve a specific goal (64). It is challenging to disentangle motivation per se from symptoms of depression, which are common in people with diabetes or diabetes distress which is emotional pain related to living with diabetes. Diabetes distress, which is specifically related to the demands of daily life and fear about short-and long-term consequences of diabetes, encumbers many people with diabetes and impacts self-efficacy (65). Considering diabetes distress and high risk for depression is essential when trying to positively impact diabetes self-efficacy.
Qualitative studies assessing barriers to exercise in people with T2D negatively describe low perceived motivation as “lack of will” (45.6-59%) and “lack of time” (20.3-31.5%) in addition to low self-efficacy as “lack of skills” (21%) (66, 67). Both depression and diabetes distress can exaggerate these barriers. Motivation and self-efficacy, influenced by diabetes distress, not only affect exercise, but also many self-management aspects of diabetes including diet, glucose monitoring and medication persistence. In one study, diabetes related distress was found to have a negative impact on self-care behaviors, such as exercise (68). In another study employing the Problem Areas in Diabetes Survey (PAID) to measure diabetes distress, there was also a significant decrease in diabetes self-care behaviors including exercise in those with higher diabetes distress scores (65). This is of particular interest since 36% of people with T2D experience diabetes related distress (69). A cross-sectional analysis of adults with T2D who completed a structured interview regarding barriers to exercise self-reported that feeling a lack of self-efficacy is strongly associated with suboptimal diabetes self-management behaviors. Specifically, in a sample of individuals with diabetes not at goal (HbA1c >7), higher self-efficacy scores in diet, performance of exercise, blood sugar testing and medical treatment were associated with higher ability to reach their goals in relation to diet, exercise, blood sugar testing and taking medications (70). One’s perception of their motivation and self-efficacy reflect components of self-regulatory behavior, a target for overcoming psychological barriers to exercise.
3.2 Solution: technology and self-regulatory behavior
Self-regulatory behavior is the ability to regulate behavior through self-monitoring, judgement of the behavior in relation to personal and environmental standards and appropriately adjusting behaviors to meet goals, and it is well studied (71). Self-regulatory behavior can be applied, often through technology, to strategically overcome psychological barriers to exercise in T2D (72). A meta-analysis of 30 reviews evaluating the most effective techniques for increasing physical activity found that interventions incorporating self-regulatory behavior strategies were associated with increased physical activity and weight loss (73). In one study of adults with prediabetes aimed at increasing and maintaining resistance training, additional action theory tests were conducted to determine which intermediary targets of behavior change were most successful at producing sustained increase in exercise. After 18 months maintenance of behavior change was most influenced by self-regulatory behavior. In another study, self-regulatory behaviors included reporting workouts online after completion, scheduling workouts in advance and participating in active problem solving to overcome barriers to attending workouts (74).
Many recent technological interventions, including smart phone applications, online coaching platforms and fitness technology can help increase physical activity, decrease sedentary time and promote weight loss (75, 76). The most impactful health technologies utilize self-regulatory behavior strategies (77, 78). A systematic review of 26 studies examining technology on the influence of objectively measured physical activity found that the most useful strategies to promote physical activity change included goal setting, real-time feedback, physical activity profiles and social support networking, all factors of self-regulatory behavior (77). In a similar review, also noting the impact of self-regulatory behavior, the authors recommend the next step in advancing fitness technologies is to incorporate cognitive behavior therapy focused on developing positive feelings towards exercise (78). Cognitive behavioral therapy has been incorporated into apps focused on beliefs and barriers surrounding various diabetes-related behaviors. In one randomized controlled trial, people with T2D using an app with cognitive behavioral therapy had significant reductions in HbA1c compared to placebo (79). More exercise specific research in people with T2D is needed to assess and augment the efficacy of cognitive behavioral therapy in fitness technology.
One popular method to incorporate self-regulatory behavior into daily life is through wearable activity trackers. Wearable activity trackers, often watches, harness aspects of self-regulatory behavior including goal setting, most often through monitoring steps per day, and feedback through real-time tracking of physical activity. A 2019 meta-analysis of 26 randomized controlled trials evaluated the impact of wearable activity trackers on physical activity in adults. Compared to nonactivity tracker interventions, participants with wearable activity trackers had a significant increase in daily step count, an average of 627 additional steps/day (80). This increase in physical activity with wearable activity trackers is consistent when studied in people with T2D (81, 82). In one study the use of wearable fitness trackers (FitBit©) in people with T2D showed a statistically significant increase in average weekly minutes of walking from 358 to 507 (p=0.04) (82). In a narrative interview examining influences on behavior change after a 12-month lifestyle intervention for people with T2D, including structured exercise with wearable fitness trackers, participants who maintained lifestyle changes stated they were motivated by feedback from their step tracking device. In another example of self-regulatory behavior, participants were also motivated by being able to reduce their diabetes medications, asserting “I see what I’m doing is working.” (40). Others when interviewed highlighted the importance of social accountability from the coaches and participants, leading to increased feelings of commitment and responsibility to the group. Social community and accountability are principal factors of self-regulatory behavior noted in many studies as a key influence on effectiveness (40, 73, 77, 78). In fact, one meta-analysis of interventions including both physical activity and diet in people at risk for diabetes reported that adding social support to interventions accounted for an additional 3.0kg of weight loss at 12 months compared to those without social support (73).
3.3 Solution: the role of physicians and healthcare teams
Physicians and healthcare team members influence exercise beliefs and behaviors of their patients (40) (23). The healthcare provider’s overall approach to diabetes self-management, education, and support greatly contributes to a tone of encouragement and motivation or judgement and blame. The ADA recommends healthcare providers engage in person-centered assessments to identify and address barriers encountered by patients (83). By using shared decision making and person-first, non-judgmental language, the healthcare provider can establish trust which in turn can be motivating for behavior change in people with diabetes (84). A cluster sampling of 48 general practitioners and their 369 patients with T2D demonstrated that the general practitioner’s level of perceived barriers to patient exercise correlated with the patient’s level of perceived barriers to exercise and low physical activity levels (23). Thinking of exercise as an individualized prescription and anticipating barriers in advance may help both physicians and patients achieve physical activity goals. Instead of counseling generally about 150 minutes of moderate-intensity exercise weekly, one method to individualize exercise prescriptions includes specifying frequency, intensity, time and type (FITT) (85). For example, a patient with T2D and knee osteoarthritis who works full time, could be prescribed moderate intensity swimming for 50 minutes, three days per week in anticipation of potential barriers of knee pain and time traveling to the gym daily. In addition to advice from physicians, several studies have shown the benefit of multidisciplinary teams improving physical activity among patients with T2D (86, 87). A randomized controlled trial measuring sustained 3-year change in physical activity in sedentary patients with T2D in Italy showed patients receiving regular counseling sessions by diabetologists and exercise specialists had higher levels of physical activity when compared to standard care general physician recommendations (86). Similarly in the US, a pragmatic pilot trial established feasibility and acceptable cost, reimbursable by the Medicare Chronic Disease Management Program, of a multidisciplinary program termed Be ACTIVE, which included physical activity tracking with FitBit©, six biweekly calls with a physical activity coach, and three primary care visits to ensure safety among other exercise education (87). This multidisciplinary intervention yielded improved Short Physical Performance Battery scores, which predict reduction in clinical falls, and a non-statistically significant increase in physical activity. Importantly patients appreciated support from their coach and accountability provided by the physical activity tracker (87). Frequent contact with healthcare professionals can encourage physical activity, even if only to reinforce previously implemented positive behavior change (40). A more general approach, such as the 150 min per week recommendation also remains of benefit, given that not all people with T2D may have access to more individualized recommendations.
3.4 Solution: Fogg behavior model
The Fogg Behavior Model, which incorporates elements of self-regulatory behavior, suggests behavior is a result of simultaneous motivation, prompting and ability (88). While the Fogg Behavior Model has not been formally studied in T2D, we examine the individual elements of the Fogg Behavior Model as they have been applied to overcome barriers to exercise in people with T2D (89). A study investigating the effect of motivation in patients with T2D on physical activity showed that higher self-motivation, measured qualitatively as less apathy, less dislike, more support and more knowledge, correlated with higher levels of physical activity and exercise (89). Motivation prompting behavior change is also closely tied with feedback and social accountability (40). Motivation combined with real-time prompts can further impact behavior. A feasibility study of a health application used “just in time” prompts to remind users to initiate runs, walks or strength exercises. With use of prompts users reported increased intrinsic motivation for exercise and increased perceived capability or self-efficacy to exercise (90). Self-efficacy is closely tied with ability, the final ingredient to the Fogg Behavior Model. While one single factor has not been identified to increase self-efficacy related to increased physical activity, a systematic review showed that interventions that utilized more behavior change techniques were associated with increased self-efficacy (91). Additional interventions to increase exercise in those with T2D designed to address all three elements of the Fogg Behavior Model including motivation, prompting and ability simultaneously are needed.
3.5 Solution: continuous glucose monitoring
The ADA Standards of Care 2023 describe the traditional use of continuous glucose monitors (CGMs). “Real-time continuous glucose monitoring should be offered for diabetes management in adults with diabetes on multiple daily injections or continuous subcutaneous insulin infusion who are capable of using the devices safely,” supported by randomized controlled trials with a level of evidence A (21). Additionally, there is expert consensus that use of glucose monitoring, not specifically CGMs, expands beyond just for those on intense insulin regimens. This consensus is reflected in the ADA Standard of Care statement, “Although blood glucose monitoring in individuals on noninsulin therapies has not consistently shown clinically significant reductions in A1c, it may be helpful when altering nutrition plan, physical activity, and/or medications in conjunction with a treatment adjustment program.” (21). Several studies have shown increased self-efficacy and increased exercise with the use of CGMs in patients with T2D, supporting their use as a self-discovery tool to overcome barriers to exercise in T2D (92–96). A review of CGM use and its impacts on lifestyle change in people with prediabetes and T2D describes increased physical activity, decreased calorie consumption and weight loss with CGM use (92). A pilot randomized controlled trial randomized 13 participants with prediabetes or T2D to an 8-week exercise intervention with or without CGMs as a real-time feedback tool (95). After the initial 8-weeks and at 1 month of follow up, the self-monitoring group utilizing CGMs demonstrated higher self-monitoring, higher self-efficacy to self-monitor and goal setting. They also had higher attendance rates of exercise programs and were more likely to register for additional exercise programs. Another randomized controlled trial of 52 adults with T2D evaluated self-efficacy counseling through incorporating feedback on CGM graphs during physical activity compared to traditional diabetes education that did not include CGM use (93). The group receiving self-efficacy counseling on CGM graphs had a significant increase in moderate activity as well as decreases in sedentary time, HbA1c and BMI. Similar results were reported in another randomized controlled trial that evaluated intermittent CGM use, 3 days per month, in people with T2D over a 3-month period (96). Compared to the control group, instructed to self-monitor blood glucose four times per week, the real-time CGM group had a significant increase in total exercise time per week together with reduction in total calorie intake, weight, BMI and HbA1c. While not a formal guideline, expert consensus, supported by several randomized controlled trials, find that CGMs are beneficial for people with T2D that are not using insulin, by providing real-time feedback that can lead to positive behavior change including increased exercise (97). While promising, cost may be a barrier to broader CGM use and medical coverage for CGM with most providers is currently limited to people using insulin therapy. More research on cost-effectiveness of CGM use to facilitate effective and durable behavior change is needed to determine efficacy of CGM use in a broader set of people with prediabetes and T2D.
3.6 Solution: pet ownership
Pet ownership, particularly dog ownership, promotes increased walking which has been associated with reduced risk for CVD regardless of diabetes status (98, 99). One solution for self-described feelings of low motivation for physical activity, often named as a barrier to physical activity by people with T2D, can thus include dog ownership. Health benefits such as increased physical activity associated with pet ownership are well studied. In one study, dog owners were 34% more likely to achieve at least 150 minutes of exercise per week and 69% more likely to do any leisure-time physical activity compared to non-dog owners (100). In 2013 the AHA released a scientific statement suggesting that pet ownership, particularly dog ownership and increased physical activity were associated with a reduction in CVD, although a causal relationship cannot be determined due to lack of randomized controlled trials. Based on their review of a large body of evidence, pet ownership to reduce risk of CVD is now a Class IIb recommendation, with Level of Evidence B (99). Following this recommendation, the National Health and Nutrition Examination Survey found mixed results that pet ownership of a dog or cat was associated with lower prevalence of systemic hypertension, but not other cardiovascular risk factors including diabetes, heart failure, coronary artery disease or stroke after adjustments compared to non-pet owners (98). More research is needed, ideally ethically conducted randomized controlled trials without risk of harm to animals, to further determine the impact of pet ownership on CVD risk in people with T2D. There was a clear increase in physical activity associated with pet ownership, recently exhibited by puppy acquisition during the COVID-19 pandemic in 2020 (101). If feasible, pet ownership can be considered one solution to increasing physical activity in people with T2D.
3.7 Barrier: mental health and T2D
The increased prevalence of mental health disorders such as depression and anxiety in people with T2D compared to the general population has a significant impact on motivation and self-efficacy (102). Women with T2D have a higher overall prevalence of depression than men with T2D (23.8% vs 12.8%) (103). However, the likelihood of depression is more greatly influenced by diabetes in men compared to women with T2D (OR 1.9 vs 1.3). Additionally, the increased risks of mental health disorders and diabetes are bidirectional. The diagnosis of diabetes increases the risk of developing depression and severe symptoms of depression. Likewise, depression increases the risk of the development of diabetes and its complications (104). While not well understood, this bidirectional relationship is likely multifactorial involving autonomic and neurohormonal dysregulation (104). Unfortunately, people with T2D who are experiencing symptoms of depression are much less likely to exercise or engage in other self-management behaviors required for ideal diabetes management (105, 106).
3.8 Solution: physical activity improves mental health in T2D
Mental health disorders such as depression and anxiety, which occur in higher rates in people with T2D compared to the general population, are a well-known barrier to exercise (105, 106). Paradoxically, exercise is associated with fewer symptoms of mental health disorders and improved quality of life in people with T2D (107–109). Unfortunately, this solution requires overcoming the inertia of initiating exercise in order to become beneficial. A systematic review evaluating the effect of exercise on mental health in people with T2D demonstrated some improvement in symptoms of depression, anxiety and emotional well-being with exercise, although it acknowledges better randomized controlled trials are needed for corroboration (110). In 2019, 60 participants with T2D were randomized to a 12-week exercise intervention versus no exercise in the control group to evaluate the effect of exercise on mental health. At the end of the 12-week period, the exercise intervention group had significant improvement in self-esteem and mental health as well as reduced anxiety and insomnia compared to the control group (111). Fewer studies are available on the impact of physical activity on anxiety in people with T2D. A meta-analysis of healthy adults without diabetes demonstrated that increasing physical activity reduces symptoms of anxiety (112). Interventions that were most successful to reduce symtpoms of anxiety included those targeting only physical activity (instead of more than one health behavior), supervised exercise, moderate-high intensity exercise (instead of low-intensity) and exercising at a fitness facility (rather than at home). More research is needed to determine if similar reductions in anxiety symptoms in response to physical activity are also present in people with T2D. Mental health benefits from exercise can be particularly helpful in older adults with and without T2D, as evidenced by several systematic reviews demonstrating decreased symptoms of depression in older adults (113–115). One study of older adults with T2D of Puerto Rican heritage, who experience higher rates of diabetes compared to non-Hispanic Whites (38% vs 23%) showed significantly improved mental health via lower geriatric depression scale scores after a 16-week resistance exercise training program (116, 117). Furthermore, multiple regression analysis of people with diabetes, who reported a moderate to low quality of life, found that the only self-management behavior that was predictive of quality of life was level of physical activity (118). This finding further emphasizes the importance of physical activity as a solution for people with T2D to overcome psychological barriers to exercise including mental health disorders. In contrast to depression and anxiety, exercise is not consistently beneficial for diabetes distress as one component of diabetes distress is concern for changes in blood glucose, high or low, in response to exercise. The majority of research on diabetes distress is reported in people with type 1 diabetes. Treatment strategies for diabetes distress include acknowledgment of distress as a normal response to the demands of diabetes, peer-support, cognitive therapy including emotional regulation training, workshops with psychologists, diabetes educators, providers and emotional specialists, as outlined in a recent interventional study (119). More research is warranted in this area for people with all types of diabetes.
4 Social and cultural barriers to exercise in T2D and potential solutions
4.1 Barrier: women with T2D experience social and cultural barriers to exercise
Lack of social support is another common barrier to exercise reported by 9-29% of people with T2D, a barrier more frequently reported by females, closely influenced by cultural norms and expectations (30, 66, 67, 120). A qualitative study investigating barriers to exercise in South Asians residing in the United Kingdom with T2D found people had a well-informed understanding of the importance of exercise, but of the 32 respondents only 7 had attempted to increase their physical activity, and of those 7, only 1 was female (121). Barriers such as childcare and domestic responsibilities, putting others before oneself, language and maintaining cultural and religious standards are among deterrents to exercise in women with T2D and at risk for T2D across the world (32, 67, 121–124). In a systematic review, women with a history of gestational diabetes, and therefore at risk for developing T2D, commonly reported children and domestic responsibilities as a reason why they do not exercise. They also reported guilt associated with “putting themselves first” which expresses cultural norms of the role of wife and mother within a family unit (32). Other qualitative reports have gone even further to suggest women who take time to exercise are “selfish.” (121) South Asian respondents state that “once a woman got married, she was expected to stay indoors, attending to domestic chores and responsibilities: women cannot go out” (121). In Qatar, traditional cultural values impair womens’ ability to exercise. Many families still do not permit women to go outside unaccompanied (124). Additionally, conservative dress can hinder opportunities for exercise. South Asian women with T2D describe that they are unable to go to gyms or swimming pools to exercise because of cultural expectations of conservative dress around the opposite sex (67, 121). Language is an additional barrier. Spanish speaking women with T2D were more likely to report social barriers to exercise than their English-speaking counterparts of similar ethnic background (122). An anecdote from qualitative interviews describes a non-English speaking woman who was injured while walking outside and was unable to ask for help. Since that time, she has been “too frightened to go out alone,” therefore hindering her ability to exercise (121). Inability to communicate is isolating and a vulnerability experienced by women with T2D. Even with a shared language, women cite isolation as a reason they do not exercise. In a study of low-income African Americans with T2D, no childcare and “I do not have anyone to exercise with,” were common social barriers to exercise (125). Gender-specific social and cultural barriers to exercise in T2D are alarming given the known excess morbidity and mortality of women compared to men with T2D (33, 126). Even more concerning is the lack of gender and sex-specific research and cardiovascular guidelines that are desperately needed to provide equitable culturally competent care for those with T2D (127, 128). More research is needed in this area.
4.2 Solution: social and culturally competent interventions
Improving physical activity in people with T2D requires thoughtful application of solutions across cultures to address specific cultural barriers. In women with children who have a history of gestational diabetes, and therefore a 7-fold increased risk of developing T2D, a systematic review revealed that interventions which accounted for childcare were most effective, surpassing techniques such as education and wearable fitness devices that have had success in other populations (32). Studies in socially and culturally conservative populations have found remarkable success with walking as a method of exercise for women (123, 129). Although, as previously noted, some families in culturally conservative Qatar still require women to be accompanied when leaving the home, there has been a national cultural shift towards more independence for women. As culture has shifted, more women are able to exercise through walking without being required to be supervised by a family member as this is a more culturally appropriate form of exercise rather than sport (124). In other cultures walking is simply preferred by women as the exercise of choice due to feasibility, safety and avoidance of gyms and swimming pools (123). Similar preferences for walking as physical activity have been shown in middle-aged American women, mostly due to accessibility (129). Children, although sometimes a barrier to exercise, can also be inspirations for exercise. Women with T2D who view themselves as role models for their children are more likely to participate in exercise (32). Additionally older women who have grown children, particularly daughters, who support their exercise habits find their children to be a positive social influence (124). Culturally competent interventions have been studied with variable success. A culturally competent intervention for low-income African Americans with T2D provided culturally tailored education on diet and physical activity in low-income African American communities, from African American educators and leaders. Outcomes included a decrease in HbA1c at 6 months, which was not sustained at 12 and 18 months. Additionally, there was no increase in physical activity with this intervention (130). Similarly, in Qatar, a culturally competent intervention addressing Arabic language, cultural health and food habits and exercise was administered to people with T2D in a randomized controlled trial. The intervention resulted in significant reductions in HbA1c, BMI and albumin/creatinine ratio. Physical activity changes were not measured as an outcome (131). Overall, more culturally competent interventions are needed to improve exercise for people, particularly women with T2D as well as other self-management behaviors required in T2D.
5 Environmental barriers to exercise in T2D and potential solutions
5.1 Barrier: neighborhood deprivation
Neighborhood deprivation defined as the lack of resources in an area influencing socioeconomic status including many social determinants of heath (SDOH) such as income, housing conditions, living environment, education and employment influences risk of developing T2D as well as impact glycemic control, blood pressure control and LDL among other measures of cardiovascular risk (132, 133). Such CVD risk factors are associated with low CRF and increased CVD mortality (15). Higher indices of neighborhood deprivation are also associated with increased risk of all-cause, cardiovascular and cancer related mortality (134). There is emerging evidence that the built environment, considered as referring to man-made conditions including urban planning, landscape, architecture and transportation development, also impacts T2D (135, 136). This interaction primarily occurs through available food environment and physical activity opportunities. Some barriers to physical activity named by people with T2D are directly tied to the built environment including “dangerous roads” and “transportation issues” (30, 137). More data is needed to better understand the influence of the built environment on T2D and exercise.
5.2 Barrier: climate change
“Weather” has been a commonly cited environmental barrier to physical activity by people with T2D (30). Weather and seasonal variations impact physical activity in all-comers. For example, people are less active when it rains and there is typically an abrupt increase in physical activity in the spring after a less active winter (138, 139). While some weather impacts may be reduced by the built environment, there are newer data that extreme weather influenced by climate change has further reduced physical activity levels (140, 141). One article reviewing impacts of climate change on human health and physical activity found consistent negative effects of air pollution, natural disasters and extreme temperatures on physical activity levels (140). These changes were seen the most in people with chronic disease, such as T2D and overweight/obesity and in the aging population (140). Further evidence of this impact was seen while measuring urban trail use in Austin, Texas. As anticipated, extreme temperatures were associated with decreased trail use and therefore reduced physical activity (141).
5.3 Solution: built environment and climate change
Walkability and open space have been associated with lower risk of developing T2D and lower overall prevalence of T2D, most likely through creating safe spaces for sustained increased physical activity (136). Designing built environments to encourage physical activity for all community members would include development of sidewalks, crosswalks, green space, playground equipment, fitness equipment, park renovations and transportation infrastructure (40). One systematic review revealed that improved infrastructure for walking, cycling and public transportation was associated with increased overall and transportation-dependent physical activity, in part owed to improved aesthetics and perceived safety (142). Specifically for older adults, a systematic review of 60 studies showed that those who lived in the top 25% of the greenest neighborhoods had a lower risk of developing T2D (143). Intentional design of the built neighborhood with the intent to maintain higher levels of physical activity for community members can in turn increase CRF, which at sustained levels has been shown to decrease incidence of T2D (144). There may be solutions that positively impact both physical activity and societal contribution to preventing climate change, such as decreasing use of fossil fuels via improving access to walking and cycling as means of transportation. More research on the intersection of climate change, physical activity, and health is needed.
6 Conclusion
The global prevalence of T2D is increasing and is associated with increased mortality and morbidity compared to the general population, mostly due to CVD. Low CRF is a strong modifiable risk factor for CVD that can be improved with exercise, making it a cornerstone of diabetes treatment. People with T2D exercise less than the general population and do not exercise at recommended levels. In this narrative review we explore traditional and novel barriers to exercise in those with T2D, including impaired peak exercise performance (an impairment more pronounced in women than men), greater perceived exertion during exercise, self-reported low perceptions of self-efficacy and motivation, mental health disorders, and lack of social and cultural support. Environmental barriers include neighborhood deprivation, lack of safe walkable space, and climate change. Potential unique evidence-based solutions to overcome these barriers include self-regulatory behavior strategies utilizing wearable fitness trackers, CGMs as a real-time feedback tool, and promoting thoughtfully built environments to increase green space for physical activity. We discussed the benefits of exercise to increase CRF and potentially reduce uncomfortable physical symptoms that are often barriers to exercise. Additionally, we discussed how exercise can decrease symptoms of depression to help promote sustained exercise behaviors. Continued research to augment physical activity in T2D is needed, especially in women who have unique barriers to exercise and a higher mortality rate than men with T2D. Interventions should focus on creating sustainable increases in exercise that target improvements in CRF to ultimately improve global mortality from T2D.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This manuscript is supported by RO1DK124344 to JEBR and JGR, K12ARO84226 to JGR, P30DK116073 to JEBR, UL1TR001082 to JEBR, R01AG066562 to JEBR and the Department of Veterans Affairs (BX002046 to JEBR and CX001532 to JEBR) the Ludeman Family Center for Women’s Health Research(JEBR and JGR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health (2020) 10(1):107–11. doi: 10.2991/jegh.k.191028.001
3. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia (2001) 44(Suppl 2):S14–21. doi: 10.1007/PL00002934
4. VIrani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
5. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation (2019) 139(10):e56–e528. doi: 10.1161/CIR.0000000000000659
6. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep (1985) 100(2):126–31.
7. Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J, et al. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis (2017) 60(1):11–20. doi: 10.1016/j.pcad.2017.03.001
8. Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care (2004) 27(1):83–8. doi: 10.2337/diacare.27.1.83
9. Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc (2009) 84(9):780–6. doi: 10.4065/84.9.780
10. Seyoum B, Estacio RO, Berhanu P, Schrier RW. Exercise capacity is a predictor of cardiovascular events in patients with type 2 diabetes mellitus. Diabetes Vasc Dis Res (2006) 3(3):197–201. doi: 10.3132/dvdr.2006.030
11. Jiménez-Pavón D, Lavie CJ, Blair SN. The role of cardiorespiratory fitness on the risk of sudden cardiac death at the population level: A systematic review and meta-analysis of the available evidence. Prog Cardiovasc Dis (2019) 62(3):279–87. doi: 10.1016/j.pcad.2019.05.003
12. Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) (2011) 111(5):1497–504. doi: 10.1152/japplphysiol.00420.2011
13. Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med (2000) 132(8):605–11. doi: 10.7326/0003-4819-132-8-200004180-00002
14. Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care (1999) 22(10):1640–6. doi: 10.2337/diacare.22.10.1640
15. Grundy SM, Barlow CE, Farrell SW, Vega GL, Haskell WL. Cardiorespiratory fitness and metabolic risk. Am J Cardiol (2012) 109(7):988–93. doi: 10.1016/j.amjcard.2011.11.031
16. Mobasseri M, Yavari A, Najafipoor F, Aliasgarzadeh A, Niafar M. Effect of a long-term regular physical activity on hypertension and body mass index in type 2 diabetes patients. J Sports Med Phys Fitness (2015) 55(1-2):84–90.
17. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med (2013) 369(2):145–54. doi: 10.1056/NEJMoa1212914
18. Momma H, Sawada SS, Sloan RA, Gando Y, Kawakami R, Terada S, et al. Importance of achieving a "Fit" Cardiorespiratory fitness level for several years on the incidence of type 2 diabetes mellitus: A Japanese cohort study. J Epidemiol (2018) 28(5):230–6. doi: 10.2188/jea.JE20160199
19. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med (2002) 346(6):393–403. doi: 10.1056/NEJMoa012512
20. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Circulation (2019) 140(11):e563–e95. doi: 10.1161/cir.0000000000000677
21. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 1. Improving care and promoting health in populations: standards of care in diabetes-2023. Diabetes Care (2023) 46(Supple 1):S10–s8. doi: 10.2337/dc23-S001
22. Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American college of sports medicine. Med Sci Sports Exerc (2022) 54(2):353–68. doi: 10.1249/MSS.0000000000002800
23. Lanhers C, Duclos M, Guttmann A, Coudeyre E, Pereira B, Ouchchane L. General practitioners' Barriers to prescribe physical activity: the dark side of the cluster effects on the physical activity of their type 2 diabetes patients. PloS One (2015) 10(10):e0140429. doi: 10.1371/journal.pone.0140429
24. Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc (2011) 59(1):132–7. doi: 10.1111/j.1532-5415.2010.03236.x
25. Zhao G, Ford ES, Li C, Mokdad AH. Compliance with physical activity recommendations in US adults with diabetes. Diabetes Med (2008) 25(2):221–7. doi: 10.1111/j.1464-5491.2007.02332.x
26. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care (2002) 25(10):1722–8. doi: 10.2337/diacare.25.10.1722
27. Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care (2007) 30(2):203–9. doi: 10.2337/dc06-1128
28. Hanefeld M, Fischer S, Schmechel H, Rothe G, Schulze J, Dude H, et al. Diabetes Intervention Study. Multi-intervention trial in newly diagnosed NIDDM. Diabetes Care (1991) 14(4):308–17. doi: 10.2337/diacare.14.4.308
29. Ménard J, Payette H, Baillargeon JP, Maheux P, Lepage S, Tessier D, et al. Efficacy of intensive multitherapy for patients with type 2 diabetes mellitus: a randomized controlled trial. CMAJ (2005) 173(12):1457–66. doi: 10.1503/cmaj.050054
30. Egan AM, Mahmood WA, Fenton R, Redziniak N, Kyaw Tun T, Sreenan S, et al. Barriers to exercise in obese patients with type 2 diabetes. QJM (2013) 106(7):635–8. doi: 10.1093/qjmed/hct075
31. Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J (2004) 80(943):287–91. doi: 10.1136/pgmj.2003.010553
32. Buelo AK, Kirk A, Lindsay RS, Jepson RG. Exploring the effectiveness of physical activity interventions in women with previous gestational diabetes: A systematic review of quantitative and qualitative studies. Prev Med Rep (2019) 14:100877. doi: 10.1016/j.pmedr.2019.100877
33. Regensteiner JG, Bauer TA, Huebschmann AG, Herlache L, Weinberger HD, Wolfel EE, et al. Sex differences in the effects of type 2 diabetes on exercise performance. Med Sci Sports Exerc (2015) 47(1):58–65. doi: 10.1249/MSS.0000000000000371
34. Kobayashi Y, Christle JW, Contrepois K, Nishi T, Moneghetti K, Cauwenberghs N, et al. Peripheral oxygen extraction and exercise limitation in asymptomatic patients with diabetes mellitus. Am J Cardiol (2021) 149:132–9. doi: 10.1016/j.amjcard.2021.03.011
35. Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc (1995) 27(6):875–81.
36. Abushamat LA, McClatchey PM, Scalzo RL, Schauer I, Huebschmann AG, Nadeau KJ, et al. Mechanistic causes of reduced cardiorespiratory fitness in type 2 diabetes. J Endocr Soc (2020) 4(7):bvaa063. doi: 10.1210/jendso/bvaa063
37. Huebschmann AG, Reis EN, Emsermann C, Dickinson LM, Reusch JE, Bauer TA, et al. Women with type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl Physiol Nutr Metab (2009) 34(5):851–7. doi: 10.1139/H09-074
38. Unick JL, Gaussoin S, Bahnson J, Crow R, Curtis J, Killean T, et al. Validity of ratings of perceived exertion in patients with type 2 diabetes. J Nov Physiother Phys Rehabil (2014) 1(1). doi: 10.17352/2455-5487.000002
39. Johansen MY, MacDonald CS, Hansen KB, Karstoft K, Christensen R, Pedersen M, et al. Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA (2017) 318(7):637–46. doi: 10.1001/jama.2017.10169
40. Schmidt SK, Hemmestad L, MacDonald CS, Langberg H, Valentiner LS. Motivation and barriers to maintaining lifestyle changes in patients with type 2 diabetes after an intensive lifestyle intervention (The U-TURN trial): A longitudinal qualitative study. Int J Environ Res Public Health (2020) 17(20). doi: 10.3390/ijerph17207454
41. Schopfer DW, Forman DE. Cardiac rehabilitation in older adults. Can J Cardiol (2016) 32(9):1088–96. doi: 10.1016/j.cjca.2016.03.003
42. Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care (2005) 28(12):2877–83. doi: 10.2337/diacare.28.12.2877
43. Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Liapis CD, Alevizos M. Beneficial effects of rosiglitazone on novel cardiovascular risk factors in patients with Type 2 diabetes mellitus. Diabetes Med (2008) 25(3):333–40. doi: 10.1111/j.1464-5491.2007.02375.x
44. Kadoglou NP, Iliadis F, Sailer N, Athanasiadou Z, Vitta I, Kapelouzou A, et al. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism (2010) 59(4):599–607. doi: 10.1016/j.metabol.2009.09.002
45. Yokota T, Kinugawa S, Hirabayashi K, Suga T, Takada S, Omokawa M, et al. Pioglitazone improves whole-body aerobic capacity and skeletal muscle energy metabolism in patients with metabolic syndrome. J Diabetes Investig (2017) 8(4):535–41. doi: 10.1111/jdi.12606
46. Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, et al. Molecular transducers of physical activity consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell (2020) 181(7):1464–74. doi: 10.1016/j.cell.2020.06.004
47. Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell (2008) 134(3):405–15. doi: 10.1016/j.cell.2008.06.051
48. Wall CE, Yu RT, Atkins AR, Downes M, Evans RM. Nuclear receptors and AMPK: can exercise mimetics cure diabetes? J Mol Endocrinol (2016) 57(1):R49–58. doi: 10.1530/JME-16-0073
49. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest (2001) 108(8):1167–74. doi: 10.1172/JCI13505
50. Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci (2008) 65(23):3737–55. doi: 10.1007/s00018-008-8244-6
51. Wang N, Tan AWK, Jahn LA, Hartline L, Patrie JT, Lin S, et al. Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care (2020) 43(3):634–42. doi: 10.2337/dc19-1465
52. Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes (2012) 61(4):888–96. doi: 10.2337/db11-1073
53. Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab (2016) 311(3):E640–8. doi: 10.1152/ajpendo.00205.2016
54. Scalzo RL, Knaub LA, Hull SE, Keller AC, Hunter K, Walker LA, et al. Glucagon-like peptide-1 receptor antagonism impairs basal exercise capacity and vascular adaptation to aerobic exercise training in rats. Physiol Rep (2018) 6(13):e13754. doi: 10.14814/phy2.13754
55. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation (2008) 117(18):2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938
56. Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol (2015) 65(2):137–47. doi: 10.1097/FJC.0000000000000170
57. Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, et al. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol (2002) 13(10):2478–87. doi: 10.1097/01.ASN.0000032418.67267.F2
58. Matsui T, Nishino Y, Maeda S, Takeuchi M, Yamagishi S. Irbesartan inhibits advanced glycation end product (AGE)-induced up-regulation of vascular cell adhesion molecule-1 (VCAM-1) mRNA levels in glomerular endothelial cells. Microvasc Res (2011) 81(3):269–73. doi: 10.1016/j.mvr.2011.01.001
59. Yamagishi S, Matsui T, Nakamura K, Inoue H, Takeuchi M, Ueda S, et al. Olmesartan blocks inflammatory reactions in endothelial cells evoked by advanced glycation end products by suppressing generation of reactive oxygen species. Ophthalmic Res (2008) 40(1):10–5. doi: 10.1159/000111152
60. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med (2001) 345(12):870–8. doi: 10.1056/NEJMoa011489
61. Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk C, Tarnow L, et al. Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes (2006) 55(12):3550–5. doi: 10.2337/db06-0827
62. Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, et al. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest (1997) 27(8):690–5. doi: 10.1046/j.1365-2362.1997.1730718.x
63. Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, et al. Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med (2003) 8(3):169–75. doi: 10.1191/1358863x03vm489oa
64. Bandura A. Self-efficacy: the exercise of control. J Cognit Psychother (1997) 13(2). doi: 10.1891/0889-8391.13.2.158
65. Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care (1995) 18(6):754–60. doi: 10.2337/diacare.18.6.754
66. Martin CG, Pomares ML, Muratore CM, Avila PJ, Apoloni SB, Rodríguez M, et al. Level of physical activity and barriers to exercise in adults with type 2 diabetes. AIMS Public Health (2021) 8(2):229–39. doi: 10.3934/publichealth.2021018
67. Alghafri T, Alharthi SM, Al Farsi YM, Bannerman E, Craigie AM, Anderson AS. Perceived barriers to leisure time physical activity in adults with type 2 diabetes attending primary healthcare in Oman: a cross-sectional survey. BMJ Open (2017) 7(11):e016946. doi: 10.1136/bmjopen-2017-016946
68. Misra R, Adelman MM, Kirk B, Sambamoorthi U. Relationship among diabetes distress, health literacy, diabetes education, patient-provider communication and diabetes self-care. Am J Health Behav (2022) 46(5):528–40. doi: 10.5993/AJHB.46.5.4
69. Perrin NE, Davies MJ, Robertson N, Snoek FJ, Khunti K. The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Med (2017) 34(11):1508–20. doi: 10.1111/dme.13448
70. Al-Khawaldeh OA, Al-Hassan MA, Froelicher ES. Self-efficacy, self-management, and glycemic control in adults with type 2 diabetes mellitus. J Diabetes Complications (2012) 26(1):10–6. doi: 10.1016/j.jdiacomp.2011.11.002
71. Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process (1991) 50(2):248–87. doi: 10.1016/0749-5978(91)90022-L
72. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol (2009) 28(6):690–701. doi: 10.1037/a0016136
73. Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health (2011) 11:119. doi: 10.1186/1471-2458-11-119
74. Williams DM, Dunsiger S, Davy BM, Kelleher SA, Marinik EL, Winett RA. Psychosocial mediators of a theory-based resistance training maintenance intervention for prediabetic adults. Psychol Health (2016) 31(9):1108–24. doi: 10.1080/08870446.2016.1179740
75. Direito A, Carraça E, Rawstorn J, Whittaker R, Maddison R. mHealth technologies to influence physical activity and sedentary behaviors: behavior change techniques, systematic review and meta-analysis of randomized controlled trials. Ann Behav Med (2017) 51(2):226–39. doi: 10.1007/s12160-016-9846-0
76. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc. Nurs (2013) 28(4):320–9. doi: 10.1097/JCN.0b013e318250a3e7
77. Bort-Roig J, Gilson ND, Puig-Ribera A, Contreras RS, Trost SG. Measuring and influencing physical activity with smartphone technology: a systematic review. Sports Med (2014) 44(5):671–86. doi: 10.1007/s40279-014-0142-5
78. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: A review of the evidence for increasing physical activity. Front Public Health (2016) 4:289. doi: 10.3389/fpubh.2016.00289
79. Hsia J, Guthrie NL, Lupinacci P, Gubbi A, Denham D, Berman MA, et al. Randomized, controlled trial of a digital behavioral therapeutic application to improve glycemic control in adults with type 2 diabetes. Diabetes Care (2022) 45(12):2976–81. doi: 10.2337/dc22-1099
80. Brickwood KJ, Watson G, O'Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth (2019) 7(4):e11819. doi: 10.2196/11819
81. Kooiman TJM, de Groot M, Hoogenberg K, Krijnen WP, van der Schans CP, Kooy A. Self-tracking of physical activity in people with type 2 diabetes: A randomized controlled trial. Comput Inform Nurs (2018) 36(7):340–9. doi: 10.1097/CIN.0000000000000443
82. Hodgson W, Kirk A, Lennon M, Paxton G. Exploring the use of fitbit consumer activity trackers to support active lifestyles in adults with type 2 diabetes: A mixed-methods study. Int J Environ Res Public Health (2021) 18(21). doi: 10.3390/ijerph182111598
83. Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, et al. Diabetes self-management education and support in adults with type 2 diabetes: A consensus report of the american diabetes association, the association of diabetes care & Education specialists, the academy of nutrition and dietetics, the american academy of family physicians, the American academy of PAs, the american association of nurse practitioners, and the American pharmacists association. Diabetes Care (2020) 43(7):1636–49. doi: 10.2337/dci20-0023
84. Dickinson JK, Guzman SJ, Maryniuk MD, O'Brian CA, Kadohiro JK, Jackson RA, et al. The use of language in diabetes care and education. Diabetes Care (2017) 40(12):1790–9. doi: 10.2337/dci17-0041
85. Barker K, Eickmeyer S. Therapeutic exercise. Med Clin North Am (2020) 104(2):189–98. doi: 10.1016/j.mcna.2019.10.003
86. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 randomized clinical trial. JAMA (2019) 321(9):880–90. doi: 10.1001/jama.2019.0922
87. Huebschmann AG, Glasgow RE, Leavitt IM, Chapman K, Rice JD, Lockhart S, et al. Integrating a physical activity coaching intervention into diabetes care: a mixed-methods evaluation of a pilot pragmatic trial. Transl Behav Med (2022) 12(4):601–10. doi: 10.1093/tbm/ibac014
88. Fogg B. A Behavior Model for Persuasive Design. New York, NY, USA: Association for Computing Machinery (2009).
89. Kang HJ, Wang JCK, Burns SF, Leow MK. Is self-determined motivation a useful agent to overcome perceived exercise barriers in patients with type 2 diabetes mellitus? Front Psychol (2021) 12:627815. doi: 10.3389/fpsyg.2021.627815
90. Sporrel K, Wang S, Ettema DDF, Nibbeling N, Krose BJA, Deutekom M, et al. Just-in-time prompts for running, walking, and performing strength exercises in the built environment: 4-week randomized feasibility study. JMIR Form Res (2022) 6(8):e35268. doi: 10.2196/35268
91. Tang MY, Smith DM, Mc Sharry J, Hann M, French DP. Behavior change techniques associated with changes in postintervention and maintained changes in self-efficacy for physical activity: A systematic review with meta-analysis. Ann Behav Med (2019) 53(9):801–15. doi: 10.1093/abm/kay090
92. Ehrhardt N, Al Zaghal E. Behavior modification in prediabetes and diabetes: potential use of real-time continuous glucose monitoring. J Diabetes Sci Technol (2019) 13(2):271–5. doi: 10.1177/1932296818790994
93. Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: A randomized clinical trial. Diabetes Res Clin Pract (2008) 80(3):371–9. doi: 10.1016/j.diabres.2008.01.006
94. Allen N, Whittemore R, Melkus G. A continuous glucose monitoring and problem-solving intervention to change physical activity behavior in women with type 2 diabetes: a pilot study. Diabetes Technol Ther (2011) 13(11):1091–9. doi: 10.1089/dia.2011.0088
95. Bailey KJ, Little JP, Jung ME. Self-monitoring using continuous glucose monitors with real-time feedback improves exercise adherence in individuals with impaired blood glucose: A pilot study. Diabetes Technol Ther (2016) 18(3):185–93. doi: 10.1089/dia.2015.0285
96. Yoo HJ, An HG, Park SY, Ryu OH, Kim HY, Seo JA, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract (2008) 82(1):73–9. doi: 10.1016/j.diabres.2008.06.015
97. Cowart K, Updike WH, Franks R. Continuous glucose monitoring in persons with type 2 diabetes not using insulin. Expert Rev Med Devices (2021) 18(11):1049–55. doi: 10.1080/17434440.2021.1992274
98. Krittanawong C, Kumar A, Wang Z, Jneid H, VIrani SS, Levine GN. Pet ownership and cardiovascular health in the US general population. Am J Cardiol (2020) 125(8):1158–61. doi: 10.1016/j.amjcard.2020.01.030
99. Levine GN, Allen K, Braun LT, Christian HE, Friedmann E, Taubert KA, et al. Pet ownership and cardiovascular risk: a scientific statement from the American Heart Association. Circulation (2013) 127(23):2353–63. doi: 10.1161/CIR.0b013e31829201e1
100. Reeves MJ, Rafferty AP, Miller CE, Lyon-Callo SK. The impact of dog walking on leisure-time physical activity: results from a population-based survey of Michigan adults. J Phys Act Health (2011) 8(3):436–44. doi: 10.1123/jpah.8.3.436
101. Hielscher-Zdzieblik B, Gansloßer U, Serpell J, Froboese I. The long-term influence of puppy acquisition on physical activity: results of a 3-year, longitudinal, pilot study. Healthcare (Basel) (2022) 10(9). doi: 10.3390/healthcare10091687
102. Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia (2010) 53(12):2480–6. doi: 10.1007/s00125-010-1874-x
103. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Med (2006) 23(11):1165–73. doi: 10.1111/j.1464-5491.2006.01943.x
104. Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs (2015) 75(6):577–87. doi: 10.1007/s40265-015-0347-4
105. Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care (2004) 27(9):2154–60. doi: 10.2337/diacare.27.9.2154
106. Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care (2007) 30(9):2222–7. doi: 10.2337/dc07-0158
107. Lysy Z, Da Costa D, Dasgupta K. The association of physical activity and depression in Type 2 diabetes. Diabetes Med (2008) 25(10):1133–41. doi: 10.1111/j.1464-5491.2008.02545.x
108. Green AJ, Fox KM, Grandy S. Impact of Regular Exercise and Attempted Weight Loss on Quality of Life among Adults with and without Type 2 Diabetes Mellitus. J Obes (2011) 2011. doi: 10.1155/2011/172073
109. Imayama I, Plotnikoff RC, Courneya KS, Johnson JA. Determinants of quality of life in type 2 diabetes population: the inclusion of personality. Qual Life Res (2011) 20(4):551–8. doi: 10.1007/s11136-010-9772-8
110. van der Heijden MM, van Dooren FE, Pop VJ, Pouwer F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia (2013) 56(6):1210–25. doi: 10.1007/s00125-013-2871-7
111. Gilani SRM, Feizabad AK. The effects of aerobic exercise training on mental health and self-esteem of type 2 diabetes mellitus patients. Health Psychol Res (2019) 7(1):6576. doi: 10.4081/hpr.2019.6576
112. Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nurs Res (2010) 59(3):224–31. doi: 10.1097/NNR.0b013e3181dbb2f8
113. Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil (2009) 23(10):873–87. doi: 10.1177/0269215509337449
114. Sjösten N, Kivelä SL. The effects of physical exercise on depressive symptoms among the aged: a systematic review. Int J Geriatr Psychiatry (2006) 21(5):410–8. doi: 10.1002/gps.1494
115. Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry (2012) 201(3):180–5. doi: 10.1192/bjp.bp.111.095174
116. Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health (2000) 90(8):1288–93. doi: 10.2105/ajph.90.8.1288
117. Lincoln AK, Shepherd A, Johnson PL, Castaneda-Sceppa C. The impact of resistance exercise training on the mental health of older Puerto Rican adults with type 2 diabetes. J Gerontol B Psychol Sci Soc Sci (2011) 66(5):567–70. doi: 10.1093/geronb/gbr034
118. Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care (1997) 20(4):562–7. doi: 10.2337/diacare.20.4.562
119. Stenov V, Due-Christensen M, Cleal BR, Tapager IW. Significant reduction in diabetes-distress and improvements in psychosocial outcomes: A pilot-test of an intervention to reduce diabetes distress in adults with type 1 diabetes and moderate to severe diabetes distress (REDUCE). Diabetes Med (2023):e15187. doi: 10.1111/dme.15187
120. Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int (2009) 24(4):416–27. doi: 10.1093/heapro/dap031
121. Lawton J, Ahmad N, Hanna L, Douglas M, Hallowell N. 'I can't do any serious exercise': barriers to physical activity amongst people of Pakistani and Indian origin with Type 2 diabetes. Health Educ Res (2006) 21(1):43–54. doi: 10.1093/her/cyh042
122. Chang C, Khurana S, Strodel R, Camp A, Magenheimer E, Hawley N. Perceived barriers to physical activity among low-income latina women at risk for type 2 diabetes. Diabetes Educ (2018) 44(5):444–53. doi: 10.1177/0145721718787782
123. Tariq O, Rosten C, Huber J. Experiences of living with type 2 diabetes in Pakistan: the role of culture and family in physical activity. Int J Equity Health (2022) 21(1):103. doi: 10.1186/s12939-022-01706-4
124. Donnelly TT, Al Suwaidi J, Al Enazi NR, Idris Z, Albulushi AM, Yassin K, et al. Qatari women living with cardiovascular diseases-challenges and opportunities to engage in healthy lifestyles. Health Care Women Int (2012) 33(12):1114–34. doi: 10.1080/07399332.2012.712172
125. Dutton GR, Johnson J, Whitehead D, Bodenlos JS, Brantley PJ. Barriers to physical activity among predominantly low-income African-American patients with type 2 diabetes. Diabetes Care (2005) 28(5):1209–10. doi: 10.2337/diacare.28.5.1209
126. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet (2020) 396(10250):565–82. doi: 10.1016/S0140-6736(20)31561-0
127. Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, et al. Sex differences in the cardiovascular consequences of diabetes mellitus: A scientific statement from the American heart association. Circulation (2015) 132(25):2424–47. doi: 10.1161/CIR.0000000000000343
128. Regensteiner JG, Reusch JEB. Sex differences in cardiovascular consequences of hypertension, obesity, and diabetes: JACC focus seminar 4/7. J Am Coll Cardiol (2022) 79(15):1492–505. doi: 10.1016/j.jacc.2022.02.010
129. Vanden Bosch M, Wesley E, Strouse S. Perceptions of physical activity in middle-aged women with type 2 diabetes. West J Nurs Res (2021) 43(7):640–8. doi: 10.1177/0193945920973151
130. Lynch EB, Mack L, Avery E, Wang Y, Dawar R, Richardson D, et al. Randomized trial of a lifestyle intervention for urban low-income african americans with type 2 diabetes. J Gen Intern Med (2019) 34(7):1174–83. doi: 10.1007/s11606-019-04894-y
131. Mohamed H, Al-Lenjawi B, Amuna P, Zotor F, Elmahdi H. Culturally sensitive patient-centred educational programme for self-management of type 2 diabetes: a randomized controlled trial. Prim Care Diab (2013) 7(3):199–206. doi: 10.1016/j.pcd.2013.05.002
132. Walker RJ, Smalls BL, Campbell JA, Strom Williams JL, Egede LE. Impact of social determinants of health on outcomes for type 2 diabetes: a systematic review. Endocrine (2014) 47(1):29–48. doi: 10.1007/s12020-014-0195-0
133. Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Soc Sci Med (2012) 74(7):1082–90. doi: 10.1016/j.socscimed.2011.11.036
134. Major JM, Doubeni CA, Freedman ND, Park Y, Lian M, Hollenbeck AR, et al. Neighborhood socioeconomic deprivation and mortality: NIH-AARP diet and health study. PloS One (2010) 5(11):e15538. doi: 10.1371/journal.pone.0015538
135. Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, et al. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA (2016) 315(20):2211–20. doi: 10.1001/jama.2016.5898
136. Amuda AT, Berkowitz SA. Diabetes and the built environment: evidence and policies. Curr Diabetes Rep (2019) 19(7):35. doi: 10.1007/s11892-019-1162-1
137. Collins KA, Huffman KM, Wolever RQ, Smith PJ, Siegler IC, Ross LM, et al. Determinants of dropout from and variation in adherence to an exercise intervention: the STRRIDE randomized trials. Transl J Am Coll Sports Med (2022) 7(1). doi: 10.1249/TJX.0000000000000190
138. Atkinson G, Drust B. Seasonal rhythms and exercise. Clin Sports Med (2005) 24(2):e25–34, xii-xiii. doi: 10.1016/j.csm.2004.11.001
139. Aspvik NP, Viken H, Ingebrigtsen JE, Zisko N, Mehus I, Wisløff U, et al. Do weather changes influence physical activity level among older adults? - The Generation 100 study. PloS One (2018) 13(7):e0199463. doi: 10.1371/journal.pone.0199463
140. Bernard P, Chevance G, Kingsbury C, Baillot A, Romain AJ, Molinier V, et al. Climate change, physical activity and sport: A systematic review. Sports Med (2021) 51(5):1041–59. doi: 10.1007/s40279-021-01439-4
141. Lanza K, Gohlke J, Wang S, Sheffield PE, Wilhelmi O. Climate change and physical activity: ambient temperature and urban trail use in Texas. Int J Biometeorol (2022) 66(8):1575–88. doi: 10.1007/s00484-022-02302-5
142. Kärmeniemi M, Lankila T, Ikäheimo T, Koivumaa-Honkanen H, Korpelainen R. The built environment as a determinant of physical activity: A systematic review of longitudinal studies and natural experiments. Ann. Behav Med (2018) 52(3):239–51. doi: 10.1093/abm/kax043
143. Dendup T, Feng X, Clingan S, Astell-Burt T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int J Environ Res Public Health (2018) 15(1). doi: 10.3390/ijerph15010078
Keywords: type 2 diabetes, exercise, barriers, cardiorespiratory fitness, prediabetes, solutions
Citation: Thielen SC, Reusch JEB and Regensteiner JG (2023) A narrative review of exercise participation among adults with prediabetes or type 2 diabetes: barriers and solutions. Front. Clin. Diabetes Healthc. 4:1218692. doi: 10.3389/fcdhc.2023.1218692
Received: 07 May 2023; Accepted: 02 August 2023;
Published: 30 August 2023.
Edited by:
Steven K. Malin, The State University of New Jersey, United StatesReviewed by:
Roberto Codella, University of Milan, ItalyMartha Funnell, University of Michigan, United States
Leanna M. Ross, Duke University, United States
Copyright © 2023 Thielen, Reusch and Regensteiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha C. Thielen, U2FtYW50aGEudGhpZWxlbkBjdWFuc2NodXR6LmVkdQ==
†These authors share senior authorship
 Samantha C. Thielen
Samantha C. Thielen Jane E. B. Reusch1,2,3,4†
Jane E. B. Reusch1,2,3,4†