- Japanese Academy of Health and Practice, Tokyo, Japan
The obesity paradox describes a counterintuitive phenomenon where overweight or mildly obese individuals with chronic diseases show better survival compared to those with normal weight. While this paradox has been reported in conditions such as heart failure and type 2 diabetes, its presence in type 1 diabetes (T1D) remains uncertain. This mini review summarizes current evidence from large cohort studies and a meta-analysis examining the association between body mass index (BMI) and clinical outcomes in individuals with T1D. Most findings do not support a protective effect of higher BMI; instead, both underweight and obesity are associated with increased risks of cardiovascular events and all-cause mortality. Notably, some evidence suggests that individuals with advanced diabetic nephropathy or chronic kidney disease (CKD) may show the lowest mortality at mildly elevated BMI levels. However, these observations may reflect the limitations of using BMI alone to evaluate obesity. Given that individuals with T1D often have reduced skeletal muscle mass, and that those with advanced diabetic complications or comorbidities such as CKD or cancer may develop cachexia, body composition analysis is essential. Accurate assessment of fat mass, muscle mass, bone mass, and water content is critical for understanding obesity-related risks. Future research should integrate body composition metrics to improve risk stratification in T1D.
1 Introduction
The obesity paradox refers to a phenomenon in which, contrary to the general understanding that obesity increases the risk of cardiovascular (CV) disease and mortality, individuals with preexisting conditions such as heart failure (1, 2), chronic kidney disease (CKD) (3), or type 2 diabetes (T2D) (4) exhibit higher survival rates when classified as “overweight to mildly obese” (approximately body mass index (BMI) 25 to 30 kg/m²) compared to those with normal or low body weight. Several hypotheses have been proposed to explain this paradox, including (a) bias resulting from the tendency of such patients to become underweight and malnourished due to the effects of the disease itself (5), (b) confounding factors such as smoking (6), (c) limitations of BMI in capturing differences in muscle mass and fat distribution (7–9), (d) reduced sympathetic activation observed in conditions such as obesity and heart failure (10), and (e) increased energy expenditure and muscle catabolism caused by inflammation and elevated metabolic demands in chronic illnesses, with greater fat and muscle reserves potentially acting as protective nutritional buffers (11). However, causal validation through well designed studies including randomized controlled trials remains limited, and it is still unclear whether this paradox represents a universal phenomenon.
The obesity paradox in patients with diabetes remains a subject of ongoing debate. However, emerging evidence suggests that observed outcomes vary depending on patient demographics and the clinical characteristics of the disease. One cohort study reported that a BMI in the overweight range (25 to 30 kg/m²) was associated with the lowest risk of all-cause mortality and CV events, indicating a more favorable prognosis (12). In contrast, another study demonstrated that the obesity paradox was not observed among non-smokers (13). Furthermore, an analytical review concluded that the obesity paradox is largely driven by reverse causation and confounding factors, particularly smoking and severe comorbid conditions, and that obesity is in fact associated with increased mortality (14). These findings raise the question of whether the obesity paradox is also present in individuals with type 1 diabetes (T1D). Although both T1D and T2D are characterized by chronic hyperglycemia, they are fundamentally distinct in terms of their underlying pathophysiological mechanisms. In recent years, the prevalence of obesity has increased among individuals with T1D (15). Nevertheless, obesity remains more prevalent in those with T2D (16), and while it plays a major role in the pathogenesis of T2D, it is not directly implicated in the development of T1D (17). In this mini review, the author summarizes previous clinical studies to evaluate whether the obesity paradox exists in individuals with T1D. The author further discusses its potential underlying mechanisms and the clinical implications that should be considered in patient care.
2 Current evidence
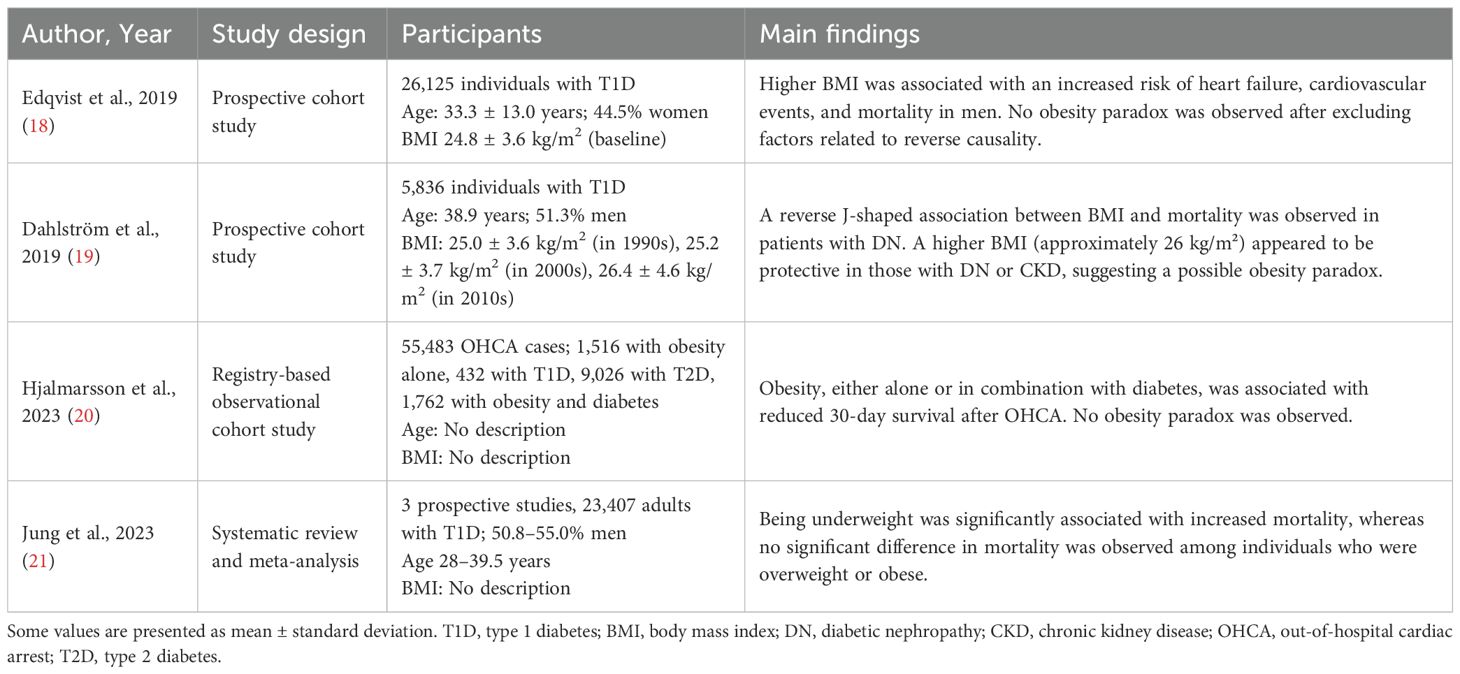
The study by Edqvist et al. (18) evaluated the relationship between BMI and adverse outcomes in individuals with T1D, utilizing data from the Swedish National Diabetes Register. A total of 26,125 patients without established CV disease were followed for a median duration of 10.9 years. For all-cause mortality, the overall incidence rate (per 1,000 person years) was 3.92 (95% confidence interval (CI), 3.68 to 4.16). Among patients with normal BMI (18.5 to less than 25 kg/m²), the rate was 3.83, increasing to 3.95 in the overweight group (25 to less than 30 kg/m²), and further to 4.39 in the obese group (30 kg/m² or higher). CV mortality followed a similar trend. The overall incidence was 1.30 (95% CI, 1.16 to 1.44); within the normal BMI group, the rate was 1.18, rising to 1.42 in the overweight group and to 1.67 in the obese group. For major CV events, the overall incidence was 5.69 (95% CI, 5.40 to 5.99). Individuals with normal BMI had a rate of 4.99, compared with 6.58 and 6.94 in the overweight and obese groups, respectively. Regarding hospitalization for heart failure, the overall incidence was 2.22 (95% CI, 2.04 to 2.41), with corresponding rates of 1.84 in the normal weight group, 2.72 in the overweight group, and 2.85 in the obese group. These findings demonstrate a consistent increase in adverse outcomes with higher BMI, thereby challenging the concept of an obesity paradox in individuals with T1D. Rather than conferring protective effects, higher BMI was independently associated with elevated risks of CV events, heart failure, and mortality. The authors emphasized that excess adiposity in T1D negatively affects long term outcomes and that weight management should be prioritized as an important clinical target.
A longitudinal cohort study from the Finnish Diabetic Nephropathy study examined the association between BMI and mortality in individuals with T1D (19). Among 6,957 adults initially identified, a final sample of 5,836 individuals was included in the mortality analysis after excluding those with unknown renal status, age at diagnosis greater than 40 years, and inadequate follow-up. Over a median follow-up of 13.7 years, 876 deaths occurred. Using World Health Organization BMI categories, underweight individuals exhibited a markedly increased risk of mortality compared with those of normal weight (hazard ratio (HR) = 4.26; 95% CI, 2.84 to 6.39), whereas obese individuals had a 25% higher mortality risk (HR = 1.25; 95% CI, 1.01 to 1.54), and overweight individuals demonstrated a modest reduction in mortality risk (HR = 0.86; 95% CI, 0.74 to 0.99). A reverse J-shaped relationship was observed between BMI and all-cause mortality, while a U-shaped association was seen for CV mortality. The BMI associated with the lowest risk of mortality was 24.3 kg/m² for all-cause mortality and 24.8 kg/m² for CV mortality. These nonlinear associations remained significant after adjustment for diabetic nephropathy (DN) status, chronic kidney disease (CKD) status, systolic blood pressure, use of antihypertensive agents, and hemoglobin A1c (HbA1c). The relationship between BMI and mortality differed significantly by DN status, but not by CKD status as defined by estimated glomerular filtration rate. Among individuals without DN or CKD, the nadir BMI associated with the lowest mortality was 24.0 kg/m², whereas in those with DN or CKD, the nadirs were 26.1 and 25.9 kg/m², respectively. Moreover, the association between BMI and mortality varied significantly by sex and age at diabetes onset, but not by chronological age, HbA1c, or smoking history. In summary, this study provides evidence that both low and high BMI are associated with increased mortality in individuals with T1D, with optimal survival observed at BMI levels within the normal to mildly elevated range. Although the study does not provide a definitive conclusion regarding the presence or absence of the obesity paradox, it underscores the importance of diabetes-related complications, such as renal function and DN, along with demographic factors, in discussions of BMI-related mortality.
An observational study investigated whether obesity and diabetes influence short-term survival following out-of-hospital cardiac arrest (20). A total of 55,483 adult cases were identified in the Swedish Registry of Cardiopulmonary Resuscitation between January 1, 2010, and December 31, 2020. Among these, 12,700 individuals (22.9%) had a history of obesity, diabetes, or both conditions. Individuals with obesity alone (n = 1,516) were younger, with a mean age of 62.0 years. Patients with T1D (n = 432) had a mean age of 64.7 years, whereas those with T2D (n = 9,026) had the highest mean age, at 75.1 years. The prevalence of CV comorbidities such as hypertension and heart failure was notably higher in groups with diabetes or obesity compared to those without these conditions. Thirty-day survival rates were 12.7% in the reference group without obesity or diabetes (n = 43,467), 9.6% in the obesity alone group, 10.6% in the T1D group, 7.3% in the T2D group, and 6.9% in the group with both obesity and diabetes (n = 1,762). After adjustment for age and sex, the odds ratio (OR) for thirty-day survival was 0.69 (95% CI, 0.57 to 0.82) for individuals with obesity alone, 0.78 (95% CI, 0.56 to 1.05) for those with T1D, 0.65 (95% CI, 0.59 to 0.71) for those with T2D, and 0.55 (95% CI, 0.45 to 0.66) for individuals with both obesity and diabetes. Further adjustment for location of arrest, time to resuscitation, and initial cardiac rhythm did not substantially alter these associations. These findings do not support the presence of an obesity paradox in individuals with diabetes. The presence of obesity did not improve survival, and among individuals with both obesity and diabetes, survival was significantly reduced. However, whether an obesity paradox exists in individuals with T1D remains unclear, as the analysis did not separately assess individuals with both obesity and T1D or those with both obesity and T2D.
Although only three studies were included in the analysis, Jung and colleagues (21) conducted a systematic review and meta-analysis to investigate the risk of all-cause mortality across different BMI categories among individuals with T1D. The study by Dahlström (19), mentioned above, was one of the three studies included. This systematic review and meta-analysis incorporated data from 23,047 patients drawn from three longitudinal cohort studies (19, 22, 23). BMI categories were defined according to the World Health Organization classification, with the reference group consisting of individuals with normal weight, defined as a BMI between 18.5 and less than 25 kg/m². The pooled HR for the underweight group (BMI less than 18.5 kg/m²) was 3.38 (95% CI, 1.67 to 6.85). In contrast, individuals in the overweight category (BMI 25 to 30 kg/m²) had an HR of 0.90 (95% CI, 0.66 to 1.22), indicating no significant difference in mortality. For individuals classified as obese (BMI 30 kg/m² or higher), the pooled HR was 1.36 (95% CI, 0.86 to 2.15). Heterogeneity across the three studies was moderate to high for all non-reference categories; however, the directional trends were consistent. In summary, the authors found no evidence supporting the existence of the so-called obesity paradox in individuals with T1D. However, the findings suggest a U-shaped relationship between BMI and mortality, with the lowest risk observed among individuals in the normal BMI range. Both underweight and, to a lesser extent, obesity were associated with increased mortality risk. These results show the importance of maintaining an appropriate weight range in the management of T1D and highlight the need for future research on the role of body composition and metabolic health.
Table 1 summarizes the results of the aforementioned studies.
3 Discussion
Recent investigations have explored the validity of the obesity paradox in individuals with T1D. Large prospective cohort studies, including those conducted in Sweden and Finland, have consistently demonstrated that a higher BMI is not associated with improved survival in this population. Instead, a nonlinear relationship has been observed, whereby both low and high BMI are linked to increased risks of all-cause and CV mortality. Collectively, these findings suggest that the optimal BMI range for favorable long-term outcomes lies within the normal to mildly elevated category. Notably, the Swedish National Diabetes Register study reported a graded increase in adverse CV events and mortality with increasing adiposity, underscoring the detrimental impact of excess body weight even in individuals without baseline CV disease. Similarly, findings from the Finnish Diabetic Nephropathy cohort indicated that the relationship between BMI and mortality is further modified by diabetes-related complications, such as nephropathy and impaired kidney function. In the context of acute events, such as out-of-hospital cardiac arrest, national registry data likewise failed to demonstrate any survival advantage associated with obesity or its coexistence with diabetes. These results further challenge the hypothesis that excess body weight confers cardiometabolic resilience in individuals with T1D. Complementing these findings, a recent systematic review and meta-analysis synthesizing data from multiple cohorts concluded that the obesity paradox is not supported in this population. However, maintaining a healthy body composition appears to be a critical goal for mitigating long-term mortality risk. Future research should investigate the roles of fat distribution, skeletal muscle mass, and overall metabolic health to refine individualized management strategies.
The number of individuals with T1D who are also obese is increasing, and debate continues regarding both the impact of obesity on the pathophysiology of T1D and the reasons why those with T1D are prone to obesity. For example, individuals with T1D who experience frequent hypoglycemic episodes may overconsume high-carbohydrate foods, and it has been reported that an additional intake of only 15 g of carbohydrates per day can result in approximately 2.7 kg of annual weight gain. Moreover, endocrine alterations such as abnormal glucagon or amylin secretion in the absence of endogenous insulin, together with intensive insulin therapy, have been shown to promote weight gain (24). Although these observations do not indicate the presence of an obesity paradox, a noteworthy case report from Japan described fulminant T1D with severe obesity and positive anti-GAD antibodies, in which pronounced obesity appeared to enhance ketone body production and aggravate acidosis (25). Thus, while it remains unclear whether obesity precedes the onset of T1D or develops subsequently, progression of obesity is likely to have adverse effects on health in the context of T1D. At the same time, as previously noted, underweight individuals with T1D have a 3.4-fold higher mortality risk compared with those of normal weight, whereas those who are overweight or obese do not show a significant increase in mortality risk (21). Furthermore, although based on a small cross-sectional analysis, an investigation of the relationship between glycemic indices monitored by continuous glucose monitoring, body weight, and BMI in T1D found that both weight and BMI were positively associated with improved glycemic control (26). From the perspective of the obesity paradox, these findings suggest that maintaining a certain level of body weight and BMI may help stabilize glycemic variability and support better overall health. Nevertheless, the optimal weight and BMI remain undefined in the current literature.
In individuals with T1D, skeletal muscle fat content has been reported to be higher than in those with T2D. Conversely, individuals with T2D exhibit more pronounced visceral fat accumulation, which is strongly associated with increased insulin resistance (27). Importantly, even T1D patients with a normal or mildly elevated BMI demonstrate significantly higher CV risk scores when visceral fat levels are elevated. This finding suggests that “hidden visceral obesity,” which is not adequately captured by conventional metrics such as BMI or waist circumference, may contribute to the underestimation of CV risk in this population (28). Ectopic fat depots have been shown to secrete bioactive substances that influence insulin resistance, glucose and lipid metabolism, coagulation, and inflammatory pathways, thereby heightening the risk of CV disease and atherosclerosis (29, 30).
On the other hand, accumulating evidence indicates that greater muscle mass is associated with lower mortality (31–33). A study by Wei et al. (34) showed that a higher appendicular skeletal muscle mass to visceral fat area ratio, which reflects a relatively greater muscle mass compared to visceral adiposity, is associated with lower CV and cancer mortality in individuals with diabetes. Skeletal muscle mass tends to decline in individuals with diabetes due to multiple mechanisms, including impaired muscle protein synthesis resulting from insulin deficiency, activation of proteolytic pathways, increased protein breakdown driven by oxidative stress and inflammation, mitochondrial dysfunction, and reduced levels of insulin like growth factor 1 (35). Individuals with T1D, in particular, have been shown to exhibit decreased skeletal muscle mass (36, 37), which represents a significant health concern in addition to increases in fat mass. Adults with long-term T1D (more than 20 years) treated with insulin therapy exhibited higher adiposity and lower lean mass compared with healthy individuals (38). If T1D is regarded as one of the autoimmune diseases, the impact of autoimmunity on skeletal muscle mass must also be considered. Autoimmune diseases exert profound effects on skeletal muscle, primarily through chronic inflammation that drives sarcopenia. Proinflammatory cytokines such as interleukin-1β, interleukin-6, and tumor necrosis factor-α activate catabolic pathways including NF-κB and p38 MAPK, leading to proteolysis via ubiquitin ligases such as atrogin-1 and MuRF-1 (39). In rheumatoid arthritis, the prevalence of sarcopenia ranges from 10% to 45% and is associated with bone loss, frailty, and CV risk. In T1D, hyperglycemia promotes intramyocellular lipid accumulation, advanced glycation end-product deposition, and mitochondrial dysfunction, all of which impair muscle quality. These mechanisms highlight that autoimmunity accelerates muscle atrophy (39). Although obesity has been associated with a 34% lower risk of sarcopenia in older adults (OR = 0.66; 95% CI, 0.48 to 0.91), this association appears to depend on the preservation of muscle mass and strength (40). These observations emphasize the limitations of evaluating obesity and its health consequences solely through traditional metrics such as body weight, BMI, or waist to hip ratio, as well as measurements of visceral or total body fat.
In the author’s view, the most significant limitation of the obesity paradox lies in the way obesity is evaluated. Obesity should be evaluated not solely by body weight or BMI, but rather by body composition, which encompasses the relative proportions of muscle mass, fat mass, and body water. In the study by Dahlström and colleagues (19), individuals with T1D who had advanced DN or CKD exhibited the lowest mortality at a BMI of approximately 26, suggesting the potential presence of the obesity paradox. This suggests that the obesity paradox may manifest particularly in patients with diabetic complications.
The number of individuals with T1D who develop CKD is increasing, and there are reports indicating that, after adjusting for age, CKD is more prevalent among individuals with T1D than among those with T2D (41, 42). Diabetic complications can have a significant impact on the prognosis of diabetes patients. Individuals with diabetic neuropathy, one of the major complications of diabetes, often experience reductions in skeletal muscle mass (43, 44), while those with advanced CKD commonly develop fluid imbalances, deteriorating nutritional status, and muscle wasting, frequently presenting as cachexia or protein energy wasting (45). These pathophysiological changes may compromise the validity of using BMI alone to assess obesity. In addition, bone mass, which constitutes approximately 14% of total body weight, represents another critical factor (46). Bone mass declines with age, and this decline has been reported to progress more rapidly in individuals with obesity (47). Fat free mass and muscle strength are closely associated with bone mass, and visceral adiposity has been suggested to exert a detrimental effect on skeletal integrity (48).
Therefore, in order to accurately assess obesity and determine the presence or absence of the obesity paradox, it is essential to precisely quantify body composition components that contribute to body weight and BMI, including muscle mass, fat mass, bone mass, and body water. Furthermore, careful consideration must be given to factors such as age and disease status, which influence each of these components. For instance, in patients with cancer, nutritional and metabolic disturbances induced by malignancy can lead to pathological loss of muscle mass, resulting in a condition known as cancer cachexia (49). In such cases, evaluating obesity based solely on body weight or BMI becomes clearly inadequate. In the context of type 1 diabetes, which is the focus of this review, the presence of complications such as DN and CKD plays a critical role in the interpretation of BMI and its association with clinical outcomes.
In conclusion, the current evidence does not support the presence of an obesity paradox in individuals with T1D (Figure 1). This mini review includes only three cohort studies and a single meta-analysis, which may limit the generalizability of the findings regarding the obesity paradox in T1D. The small number of included studies represents a major limitation. However, one study has reported that among those with advanced DN and CKD, individuals classified as overweight have the lowest mortality. Although the limited number of studies precludes definitive conclusions, this observation likely reflects the limitations of assessing obesity using BMI alone. In recent years, the average BMI among individuals with T1D has been increasing, highlighting the need for accurate evaluation of obesity. It is essential to assess body composition precisely, including muscle mass, fat mass, visceral fat, bone mass, and body water, rather than relying on body weight or BMI alone. Particular attention should be paid to imbalances, such as reduced muscle or bone mass accompanied by increased fat mass. Moreover, the evaluation must account for comorbid conditions, including diabetic complications and cancer. Continued improvement in measurement methodologies and further research are warranted.
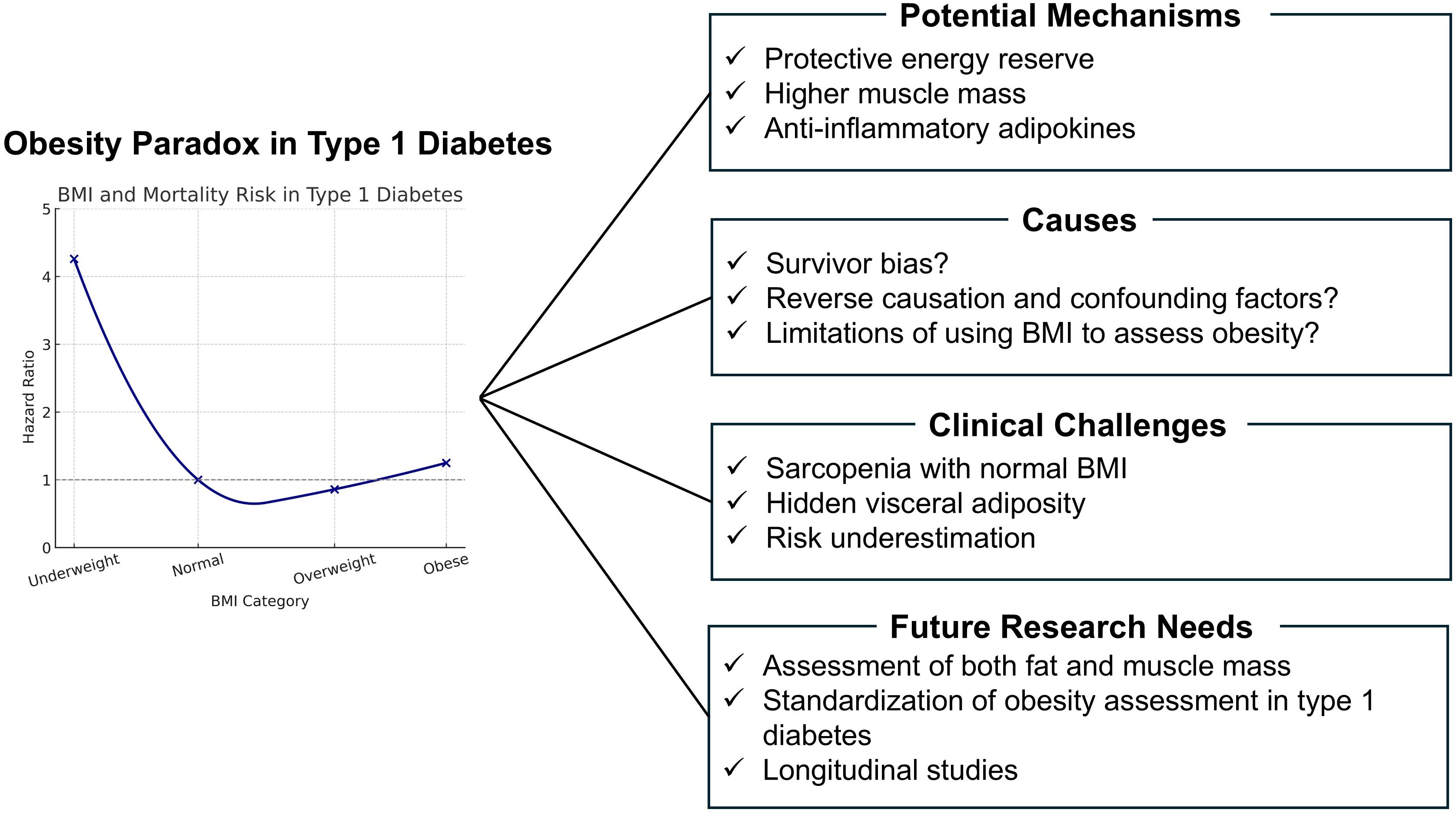
Figure 1. Obesity paradox in type 1 diabetes: Potential mechanisms, challenges, and future directions.
Author contributions
HH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This study is dedicated to the memory of my father, Yasuteru Hamasaki, who devoted his life to caring for patients and always encouraged me to pursue research.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author used generative AI to correct English grammar and spelling and to format the references according to the style of Frontiers in Clinical Diabetes and Healthcare.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alebna PL, Mehta A, Yehya A, daSilva-deAbreu A, Lavie CJ, and Carbone S. Update on obesity, the obesity paradox, and obesity management in heart failure. Prog. Cardiovasc. Dis. (2024) 82:34–42. doi: 10.1016/j.pcad.2024.01.003
2. Merkel ED, Behon A, and Masszi R. Obesity paradox in patients with reduced ejection fraction eligible for device implantation: an observational study. ESC Heart Fail. (2024) 11:3616–25. doi: 10.1002/ehf2.14961
3. Naderi N, Kleine CE, Park C, Hsiung JT, Nguyen DV, Ku E, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog. Cardiovasc. Dis. (2018) 61:168–81. doi: 10.1016/j.pcad.2018.07.001
4. Han SJ and Boyko EJ. The evidence for an obesity paradox in type 2 diabetes mellitus. Diabetes Metab. J. (2018) 42:179–87. doi: 10.4093/dmj.2018.0055
5. Preston SH and Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. (2014) 25:454–61. doi: 10.1097/EDE.0000000000000075
6. Badrick E, Sperrin M, Buchan IE, and Renehan AG. Obesity paradox and mortality in adults with and without incident type 2 diabetes: a matched population level cohort study. BMJ Open Diabetes Res. Care. (2017) 5:e000369. doi: 10.1136/bmjdrc-2016-000369
7. Hainer V and Aldhoon Hainerová I. Obesity paradox does exist. Diabetes Care. (2013) 36 Suppl 2:S276–81. doi: 10.2337/dcS13-2023
8. Bozorgmanesh M, Arshi B, Sheikholeslami F, Azizi F, and Hadaegh F. No obesity paradox BMI incapable of adequately capturing the relation of obesity with all cause mortality an inception diabetes cohort study. Int. J. Endocrinol. (2014) 2014:282089. doi: 10.1155/2014/282089
9. Donini LM, Pinto A, Giusti AM, Lenzi A, and Poggiogalle E. Obesity or BMI paradox beneath the tip of the iceberg. Front. Nutr. (2020) 7:53. doi: 10.3389/fnut.2020.00053
10. Farré N, Aranyó J, Enjuanes C, Verdú JM, Molina L, Domingo M, et al. Differences in neurohormonal activity partially explain the obesity paradox in patients with heart failure the role of sympathetic activation. Int. J. Cardiol. (2015) 181:120–6. doi: 10.1016/j.ijcard.2014.12.025
11. Webster JM, Kempen LJAP, Hardy RS, and Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. (2020) 11:597675. doi: 10.3389/fphys.2020.597675
12. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, et al. The obesity paradox in type 2 diabetes mellitus relationship of body mass index to prognosis a cohort study. Ann. Intern. Med. (2015) 162:610–8. doi: 10.7326/M14-1551
13. Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body mass index and mortality among adults with incident type 2 diabetes. N Engl. J. Med. (2014) 370:233–44. doi: 10.1056/NEJMoa1304501
14. Tobias DK and Manson JE. The obesity paradox in type 2 diabetes and mortality. Am. J. Lifestyle Med. (2016) 12:244–51. doi: 10.1177/1559827616650415
15. Kueh MTW, Chew NWS, Al Ozairi E, and Le Roux CW. The emergence of obesity in type 1 diabetes. Int. J. Obes. (Lond). (2024) 48:289–301. doi: 10.1038/s41366-023-01429-8
16. Xu G, Liu B, Sun Y, Snetselaar LG, Hu FB, Bao W, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017 population based study. BMJ. (2018) 362:k1497. doi: 10.1136/bmj.k1497
17. Abbasi A, Juszczyk D, van Jaarsveld CHM, and Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: A retrospective cohort study. J. Endocr. Soc. (2017) 1:524–37. doi: 10.1210/js.2017-00044
18. Edqvist J, Rawshani A, Adiels M, Björnson E, Gudbjörnsdottir S, Svensson AM, et al. BMI, mortality, and cardiovascular outcomes in type 1 diabetes findings against an obesity paradox. Diabetes Care. (2019) 42:1297–304. doi: 10.2337/dc18-1446
19. Dahlström EH, Sandholm N, Forsblom CM, Thorn LM, Jansson FJ, Harjutsalo V, et al. Body mass index and mortality in individuals with type 1 diabetes. J. Clin. Endocrinol. Metab. (2019) 104:5195–204. doi: 10.1210/jc.2019-00042
20. Hjalmarsson A, Rawshani A, Råmunddal T, Nordberg P, Svensson L, Djärv T, et al. No obesity paradox in out of hospital cardiac arrest data from the Swedish registry of cardiopulmonary resuscitation. Resusc Plus. (2023) 15:100446. doi: 10.1016/j.resplu.2023.100446
21. Jung HN, Kim S, Jung CH, and Cho YK. Association between body mass index and mortality in type 1 diabetes mellitus a systematic review and meta analysis. J. Obes. Metab. Syndr. (2023) 32:151–62. doi: 10.7570/jomes22061
22. Conway B, Miller RG, Costacou T, Fried L, Dorman JS, Orchard TJ, et al. Adiposity and mortality in type 1 diabetes. Int. J. Obes. (Lond). (2009) 33:796–805. doi: 10.1038/ijo.2009.75
23. Vestberg D, Rosengren A, Eeg Olofsson K, Franzén S, Svensson AM, Gudbjörnsdottir S, et al. Body mass index as a risk factor for coronary events and mortality in patients with type 1 diabetes. Open Heart. (2018) 5:e000727. doi: 10.1136/openhrt-2017-000727
24. Corbin KD, Driscoll KA, Pratley RE, Egan AM, Seaquist ER, and Bergenstal RM. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr. Rev. (2018) 39:629–63. doi: 10.1210/er.2017-00191
25. Okubo Y, Shimamoto M, Watanabe K, and Oshima Y. A case of anti-GAD antibody-positive fulminant type 1 diabetes with severe obesity. J. Japan Diabetes Soc. (2022) 65:444–50. doi: 10.11213/tonyobyo.65.444
26. Christou MA, Christou PA, Katsarou DN, Papatheodorou E, Papanas N, Tentolouris N, et al. Effect of body weight on glycaemic indices in people with type 1 diabetes using continuous glucose monitoring. J. Clin. Med. (2024) 13:5303. doi: 10.3390/jcm13175303
27. Dubé MC, Joanisse DR, Prud'homme D, Hancox G, White MD, Tremblay A, et al. Muscle adiposity and body fat distribution in type 1 and type 2 diabetes varying relationships according to diabetes type. Int. J. Obes. (Lond). (2006) 30:1721–8. doi: 10.1038/sj.ijo.0803337
28. Salle L, Julla JB, Fagherazzi G, Pinto G, Renuy A, Amadou C, et al. Beyond overweight visceral adiposity is associated with estimation of cardiovascular risk in patients living with type 1 diabetes findings from the SFDT1 cohort. Cardiovasc. Diabetol. (2025) 24:256. doi: 10.1186/s12933-025-02789-3
29. Lim S and Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler. Thromb. Vasc. Biol. (2014) 34:1820–6. doi: 10.1161/ATVBAHA.114.303035
30. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat atherosclerosis and cardiometabolic disease a position statement. Lancet Diabetes Endocrinol. (2019) 7:715–25. doi: 10.1016/S2213-8587(19)30084-1
31. Srikanthan P and Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. (2014) 127:547–53. doi: 10.1016/j.amjmed.2014.02.007
32. Koon Yee Lee G, Chun Ming Au P, Hoi Yee Li G, Wong SY, Kwok T, Woo J, et al. Sarcopenia and mortality in different clinical conditions a meta analysis. Osteoporos Sarcopenia. (2021) 7:S19–27. doi: 10.1016/j.afos.2021.02.001
33. Wang Y, Luo D, Liu J, Song Y, Jiang B, and Jiang H. Low skeletal muscle mass index and all cause mortality risk in adults a systematic review and meta analysis of prospective cohort studies. PloS One. (2023) 18:e0286745. doi: 10.1371/journal.pone.0286745
34. Wei S, Zhang J, Zheng H, Liu C, Wang Y, Chen Y, et al. Association of the appendicular skeletal muscle mass to visceral fat area ratio with cause specific mortality in diabetes. Calcif Tissue Int. (2025) 116:85. doi: 10.1007/s00223-025-01389-3
35. Shen Y, Li M, Wang K, Zhang W, Liu Y, Chen J, et al. Diabetic muscular atrophy molecular mechanisms and promising therapies. Front. Endocrinol. (Lausanne). (2022) 13:917113. doi: 10.3389/fendo.2022.917113
36. Pollakova D, Tubili C, Di Folco U, De Giuseppe R, Battino M, and Giampieri F. Muscular involvement in long term type 1 diabetes does it represent an underestimated complication. Nutrition. (2023) 112:112060. doi: 10.1016/j.nut.2023.112060
37. Shimura K, Miura J, Shen Z, Yamaguchi Y, Sasaki H, Takahashi T, et al. Prevalence and risk factors for skeletal muscle mass loss in individuals with type 1 diabetes. Diabetol. Int. (2024) 16:92–9. doi: 10.1007/s13340-024-00770-1
38. Fenner-Pena N, Fajardo VC, Froes L, Carvalho PAM, Comim FV, Sahade V, et al. Phase angle and body composition in long-term type 1 diabetes in adults: a comparative study in a Brazilian public reference outpatient clinic. Diabetol. Metab. Syndr. (2024) 16:269. doi: 10.1186/s13098-024-01485-8
39. An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int. J. Mol. Sci. (2020) 21:5678. doi: 10.3390/ijms21165678
40. Liu C, Wong PY, Chung YL, Zhang H, Lee JS, Chan R, et al. Deciphering the "obesity paradox" in the elderly a systematic review and meta analysis of sarcopenic obesity. Obes. Rev. (2023) 24:e13534. doi: 10.1111/obr.13534
41. Tuttle KR, Reynolds CL, Kornowske LM, Jones CR, Alicic RZ, Daratha KB, et al. Prevalence and severity of chronic kidney disease in a population with type 1 diabetes from a United States health system: a real-world cohort study. Lancet Reg. Health Am. (2025) 47:101130. doi: 10.1016/j.lana.2025.101130
42. Wallace AS, Chang AR, Shin JI, Reider J, Echouffo-Tcheugui JB, Grams ME, et al. Obesity and chronic kidney disease in US adults with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. (2022) 107:1247–56. doi: 10.1210/clinem/dgab927
43. Andersen H, Gadeberg PC, Brock B, and Jakobsen J. Muscular atrophy in diabetic neuropathy a stereological magnetic resonance imaging study. Diabetologia. (1997) 40:1062–9. doi: 10.1007/s001250050788
44. Espino Gonzalez E, Dalbram E, Mounier R, Nguyen Q, Lee S, Patel R, et al. Impaired skeletal muscle regeneration in diabetes from cellular and molecular mechanisms to novel treatments. Cell Metab. (2024) 36:1204–36. doi: 10.1016/j.cmet.2024.02.014
45. Koppe L, Fouque D, and Kalantar Zadeh K. Kidney cachexia or protein energy wasting in chronic kidney disease facts and numbers. J. Cachexia Sarcopenia Muscle. (2019) 10:479–84. doi: 10.1002/jcsm.12421
46. Carretero JM, Rodríguez L, García González R, Quam RM, and Arsuaga JL. Exploring bone volume and skeletal weight in the Middle Pleistocene humans from the Sima de los Huesos site (Sierra de Atapuerca Spain). J. Anat. (2018) 233:740–54. doi: 10.1111/joa.12886
47. Lloyd JT, Alley DE, Hochberg MC, Shardell M, Walston JD, Ferrucci L, et al. Changes in bone mineral density over time by body mass index in the Health ABC study. Osteoporos Int. (2016) 27:2109–16. doi: 10.1007/s00198-016-3506-x
48. Walowski CO, Herpich C, Enderle J, Braun W, Both M, Hasler M, et al. Determinants of bone mass in older adults with normal- and overweight derived from the crosstalk with muscle and adipose tissue. Sci. Rep. (2023) 13:5030. doi: 10.1038/s41598-023-31642-4
Keywords: obesity, type 1 diabetes, body mass index, body composition, skeletal muscle, fat, mortality
Citation: Hamasaki H (2025) Obesity paradox in individuals with type 1 diabetes. Front. Clin. Diabetes Healthc. 6:1670312. doi: 10.3389/fcdhc.2025.1670312
Received: 21 July 2025; Accepted: 17 September 2025;
Published: 01 October 2025.
Edited by:
Arrigo Francesco Giuseppe Cicero, University of Bologna, ItalyReviewed by:
Eperke Dora Merkel, Semmelweis University, HungaryCopyright © 2025 Hamasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidetaka Hamasaki, aC1oYW1hc2FraUB1bWluLmFjLmpw
 Hidetaka Hamasaki
Hidetaka Hamasaki