Abstract
Pseudomonas aeruginosa is known as an opportunistic pathogen that often causes persistent infections associated with high level of antibiotic-resistance and biofilms formation. Chemical interference with bacterial cell-to-cell communication, termed quorum sensing (QS), has been recognized as an attractive approach to control infections and address the drug resistance problems currently observed worldwide. Instead of imposing direct selective pressure on bacterial growth, the right bioactive compounds can preferentially block QS-based communication and attenuate cascades of bacterial gene expression and production of virulence factors, thus leading to reduced pathogenicity. Herein, we report on the potential of itaconimides as quorum sensing inhibitors (QSI) of P. aeruginosa. An initial hit was discovered in a screening program of an in-house compound collection, and subsequent structure-activity relationship (SAR) studies provided analogs that could reduce expression of central QS-regulated virulence factors (elastase, rhamnolipid, and pyocyanin), and also successfully lead to the eradication of P. aeruginosa biofilms in combination with tobramycin. Further studies on the cytotoxicity of compounds using murine macrophages indicated no toxicity at common working concentrations, thereby pointing to the potential of these small molecules as promising entities for antimicrobial drug development.
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen and a major cause of nosocomical infections in patients with pneumonia, chronic wounds, urinary tract infections, and intensive care units (ICUs) (Vincent et al., 1995). As an opportunistic pathogen, this organism is highly adaptive, versatile and exhibits remarkable resistance toward many antimicrobial agents. Resistance is a distinctive characteristic of P. aeruginosa, due to its ability to express multiple resistance mechanisms, including enzymes and efflux pumps (Poole, 2001; Lister et al., 2009). U.S. Centers for Disease Control and Prevention (CDC) (2013) estimated that more than 20,000 deaths per year are attributed to antibiotic resistance cases. It has become a global issue, and we are threatened with the slow progress of new antibiotics development and lack of preventive measure for the spread of resistance.
In addition, P. aeruginosa is well-known to form biofilms, which have been identified as a major underlying cause of persistent infections in immunocompromised patients, chronic wounds as well as on medical devices like implants, catheters, tubes, artificial hip, and many more (Costerton et al., 1999). Biofilms infections are often characterized by their broad range resistance toward host defense mechanisms and antibiotic therapy. This results in prolonged treatment, complications in clinical outcomes, and additional socio-economic burdens. In cystic fibrosis patients, chronic biofilm infections of P. aeruginosa can cause premature death despite intensive antibiotic therapy care (Bjarnsholt et al., 2009).
The bacteria in biofilms often exhibit different phenotypic and genetic variants as compared to their planktonic counterparts. In the biofilm mode of life, bacterial cells are enclosed within a matrix of extracellular polymeric substances (EPS) comprises of exopolysaccharides, proteins, deoxyribonucleic acid (DNA), lipids or surfactants, and macromolecules that are self-produced by the cells (Flemming and Wingender, 2010). All of these could render antibiotics impenetrable, chelated or sequestered, and diminish the efficacy of the treatment. The presence of persister cells in biofilms also contributes to multidrug resistance property of biofilms (Lewis, 2007). Overall, the complex biology of biofilms represents a tremendous challenge to develop therapeutic agents that could successfully prevent or eradicate biofilms-associated infections.
The cell-to-cell communication system called quorum sensing (QS) has been reported to play major roles for establishing persistent, biofilm based infections (Hentzer et al., 2003; Alhede et al., 2009; Van Gennip et al., 2009; Chiang et al., 2013). The QS system in P. aeruginosa utilizes acyl homoserine lactones (AHLs) as signal molecules and comprises the Lux homologs LasRI and RhlRI. LasI synthase is responsible for the synthesis of N-(3-oxododecanoyl) homoserine lactone (3-oxo-C12-HSL), which will bind to its receptor LasR and activate transcription of genes responsible for virulence such as lasB, apr, and toxA (Gambello et al., 1993; Passador et al., 1993). The las system also positively regulates rhl system, where RhlI directs the synthesis of N-butanoylhomoserine lactone (C4-HSL) that would bind to its receptor RhlR and subsequently activate gene expression of QS target genes (Brint and Ohman, 1995; Pearson et al., 1995; Pesci et al., 1997). In addition, there is also a third signaling molecule “pseudomonas quinolone signal” (PQS) which is intertwined between the las and rhl systems (Schertzer et al., 2009). Recently, a fourth signal molecules called Integrative Quorum Sensing Signal (IQS) has been reported, which could overtake the central las system under phosphate depletion condition (Lee et al., 2013). QS defective P. aeruginosa mutants are attenuated as compared to the wild-type strain, and their biofilms are more susceptible toward antibiotics treatment and host immune system as compared to the wild-type (Pearson et al., 2000; Hentzer et al., 2002, 2003).
As QS governs various patterns of genes expression to control virulence and biofilm formation, it has been proposed that interfering with the communication system could be a promising strategy for the control and prevention of bacterial infections (Hentzer et al., 2003). Quorum sensing inhibitors (QSI) are compounds that interfere with QS pathways, reduce expression of QS-controlled genes and attenuate infecting bacteria. As such compounds do not affect the growth of bacteria, these molecular entities pose lower selective pressure on bacteria and lower the risk of resistance development. Recent exploration of new classes of QSI comprises natural products, synthetic molecules, and enzymes that may quench or inactivate QS signals (Dong et al., 2001; Hentzer et al., 2002; Jakobsen et al., 2012; Fong et al., 2017). Unfortunately, no clinical candidates have yet been developed for therapy.
In the present study, we report a new class of small molecules that disrupt QS pathways in P. aeruginosa. The structurally related itaconimides and citraconimides have previously been reported to inhibit growth of mycobacteria (Balganesh et al., 1999). We synthesized a range of small molecules by an iterative structure-activity relationship (SAR) study and found two promising candidates for further biological investigation. The efficacies of these compounds were tested on P. aeruginosa QS bioreporter strains (lasB-gfp; rhlA-gfp; pqsA-gfp) and also on QS-controlled virulence phenotypes, such as elastase, pyocyanin, and rhamnolipid production.
Materials and Methods
General Information
All chemicals were purchased from Sigma Aldrich and used without further purification. For biological studies, synthesized compounds were prepared in DMSO as 10 mM stock solution and stored at −20°C until further usage. Overnight culture of bacteria was grown in Lysogeny broth (LB) which consisted of 1% tryptone, 0.5% yeast extract, 0.5% NaCl. For bioreporter assay, strains were grown in ABTGC (AB minimal medium supplemented with 0.2% glucose and 0.2% casamino acids) (Clark and Maaloe, 1967) to minimize fluorescence interference. ABTG (with no casamino acid) medium was used for biofilms study in flow chambers. Strains used in this study can be found in Table 1. Summary of chemical synthesis and spectroscopy data can be found in Supplementary Material.
Table 1
| Strains or plasmids | Relevant genotype and/or characteristicsa |
|---|---|
| Strains | |
| PAO1 | ATCC Pseudomonas aeruginosa (Hentzer et al., 2002) |
| PAO1-gfp | GFP-tagged wild-type Pseudomonas aeruginosa (Yang et al., 2007) |
| PAO1-lasB-gfp | PAO1 containing lasB-gfp(ASV) reporter fusion (Hentzer et al., 2002) |
| PAO1-rhlA-gfp | PAO1 containing rhlA-gfp(ASV) reporter fusion (Yang et al., 2007) |
| PAO1-pqsA-gfp | PAO1 containing pqsA-gfp(ASV) reporter fusion (Yang et al., 2009) |
| PAO1 ΔlasIΔrhlI | Gma; PAO1 lasI and rhlI mutant (Hentzer et al., 2003) |
| PAO1 ΔlasR | PAO1 lasR mutant (Hentzer et al., 2003) |
| ΔlasR-rhlA-gfp | PAO1 lasR mutant containing rhlA-gfp(ASV) reporter fusion (Tan et al., 2013) |
| ΔlasR-pqsA-gfp | PAO1 lasR mutant containing pqsA-gfp(ASV) reporter fusion (Tan et al., 2013) |
Bacterial strains used in this study.
Description of the strains' antibiotic resistance. Gm, gentamicin.
QS Inhibition Assay
From its frozen stock, compounds were diluted appropriately to their working concentration in ABTGC medium. Experiments were done as previously reported (Fong et al., 2017). Briefly, 200 μL of compounds was pipetted into the first rows of 96-well plates (Nunc, Denmark), followed by two-fold serial dilution to the rest of the rows. The last two rows were allocated to solvent control (DMSO 0.1%) and blank (media control). Overnight cultures of P. aeruginosa bioreporter strains were diluted to optical density at 600 nm (OD600) of 0.02 (~2.5 × 108 CFU/mL). Next, 100 μL of the bacteria culture was added into each well to make final OD600 of 0.01. The plate was incubated at 37°C for 16 h, with time-point measurement of GFP fluorescence (excitation 485 nm, emission 535 nm) and OD600 recorded at every 15 min using Tecan Infinite 200 Pro plate reader (Tecan Group Ltd, Männedorf, Switzerland). The data were exported into excel files, and IC50 value calculation was determined using GraphPad Prism 6 software. For IC50 values determination, the GFP/OD600 values were taken at the time point between 4 and 6 h, where inhibition started to occur. All experiments were done in triplicate manner and repeated at least twice to confirm the results.
QS-Regulated Virulence Factor Assays
Elastase activity was measured using EnzChekElastase kit (Invitrogen, USA), following the manufacturer's instruction. Rhamnolipid was extracted and quantified using method reported by Koch et al. with modifications (Koch et al., 1991). Briefly, overnight cultures of P. aeruginosa were diluted into ABTGC medium (OD600 = 0.01), with and without the presence of compounds (DMSO as control). Cultures were grown overnight at 37°C, 200 rpm. Rhamnolipid was extracted from the supernatant with diethyl ether (twice), and organic fractions were concentrated to yield yellowish-white solids. The solids were re-suspended in deionized water and added with 0.19% (w/v) orcinol in 50% H2SO4. It was then heated at 80°C for 20–30 min to give dark orange color. Absorbance was measured at 421 nm and the values were normalized with cell density at OD600. Pyocyanin was extracted from overnight culture of P. aeruginosa grown in Kings Medium A Base [MilliQ water supplemented with proteose peptone (20 g/L), potassium sulfate (10 g/L), magnesium chloride, anhydrous (1.640 g/L), and glycerol (10% v/v)]. Supernatants were collected and extracted with chloroform and 0.2 M HCl. The presence of pyocyanin would turn the HCl solution into pinkish color. Absorbance was measured at 520 nm and normalized with cell density OD600 values. Experiments were done in triplicate manner and repeated at least twice to confirm the results.
Biofilm Experiments
GFP-tagged P. aeruginosa were grown in ABTG medium and flowed through flow chambers as previously described (Sternberg and Tolker-Nielsen, 2006). Each flow chamber is consisted of three-channel flow cells that were supplied with a flow of medium and oxygen, while waste medium would be directed into a waste flask. Briefly, overnight cultures were diluted 1,000 times in ABTG medium and injected into each channel for 1 h incubation time without flow. Next, the medium was allowed to flow into the flow cells and the velocity was maintained at 0.2 mm/s using Cole-Palmer peristaltic pump. Biofilms were grown for 72 h before treatment with compounds for further 48 h. To visualize dead cells, 300 μL of propidium iodide (PI) stain was injected into each flow cells. Biofilm images were taken with LSM confocal laser scanning microscope (Carl Zeiss, Germany) at 20x objective lens. Microscopy images were processed with IMARIS software (Bitplane AG, Zurich, Switzerland). Experiments were done in triplicate manner and repeated at least twice to confirm the results.
Cytotoxicity Assay
Toxicity assay was done as previously reported (Fong et al., 2017). Murine macrophage RAW264.7 cell lines were grown in Dulbecco's Modified Eagle's Medium (DMEM, Life Technologies), supplemented with 10% fetal bovine serum (Gibco) at 37°C and 5% CO2. The cells were passaged into 96-well microplates, with each well containing 1 × 104 macrophages. After 16 h, the cells were washed with phosphate-buffered saline (PBS) and treated with compounds at varying concentration in 100 μL DMEM. The plate was incubated at 37°C and 5% CO2 for further 16 h. Resazurin was then added into each well to reach final concentration of 10 μM for cell viability measurement. The live cells would be able to convert the dye to red color, whereby dead cells would remain as blue color. Absorbance was taken at 595 nm using Tecan Infinite 200 Pro plate reader (Tecan Group Ltd, Männedorf, Switzerland). Experiments were done in triplicate manner and repeated at least twice to confirm the results.
Results
Synthesis and SAR Study
Recently, we discovered that 3-methylene-1-tetradecylpyrrolidine-2,5-dione (Table 2, 1a) displayed QSI activity against P. aeruginosa QS reporter strain (carrying a lasB-gfp fusion). Different variation on the left-hand side of 1a proved that the exo-cyclic double bond was essential for its biological activity. This led us to focus on the synthesis and biological evaluation of N-substituted itaconimide analogs against P. aeruginosa (Scheme 1). The procedure for the synthesis of the itaconimides have been described by Cava et al. (1961) and Leow et al. (2008). The commercially available itaconic anhydride was treated with anilines (1.0 equiv.) in CHCl3 to afford the corresponding α-itaconamic acids (Kyung et al., 1974). The resulting acids were subsequently treated with Ac2O (3.5 equiv.) and NaOAc (0.5 equiv.) at elevated temperature to afford a mixture of itaconimide and the isomerized product citraconimide. Generally, the yield for the aniline derivatives was higher than that of the aliphatic amines. An overview of the synthesized compounds is provided below (Table 2).
Table 2
| Compound | R | a (%)a | b (%)a | Compound | R | a (%)a | b (%)a |
|---|---|---|---|---|---|---|---|
| 1 | 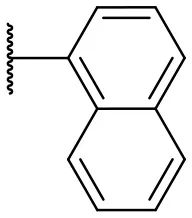 | 50 | 14 | 10 | 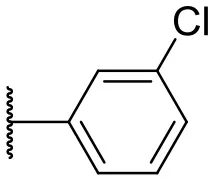 | 35 | 15 |
| 2 | 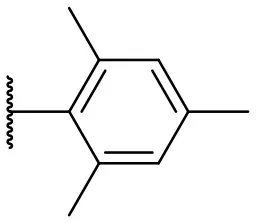 | 37 | –b | 11 | 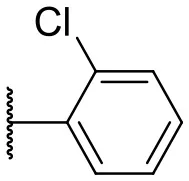 | 61 | 9 |
| 3 | 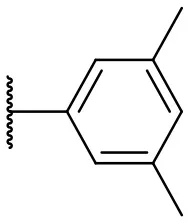 | 17 | –b | 12 | 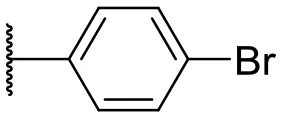 | 37 | 20 |
| 4 | 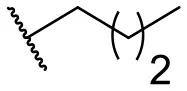 | 10 | –b | 13 | 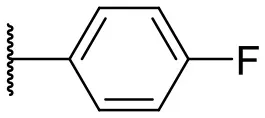 | 40 | 19 |
| 5 | 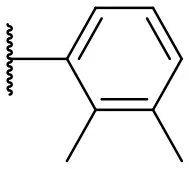 | 47 | –b | 14 | 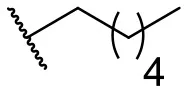 | 8 | 14 |
| 6 | 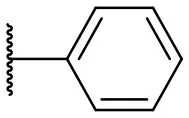 | 49 | 29 | 15 | 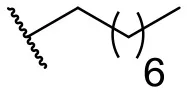 | 16 | –b |
| 7 | 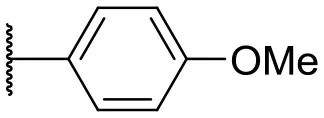 | –c | 16 | 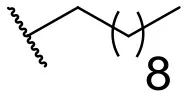 | 13 | 10 | |
| 8 | 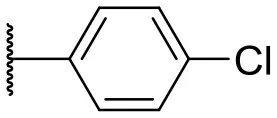 | 31 | 40 | 17 | 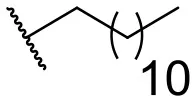 | 4 | 12 |
| 9 | 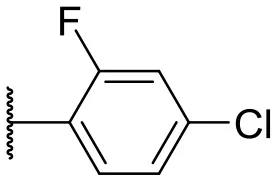 | 24 | 14 | 18 | 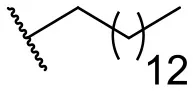 | 2 | 11 |
Summary of the synthesized compounds and the corresponding yields.
Yields based on starting itatonic anhydride and purified after cyclization by flash column chromatography.
Not isolated.
Commercial sample.
Scheme 1
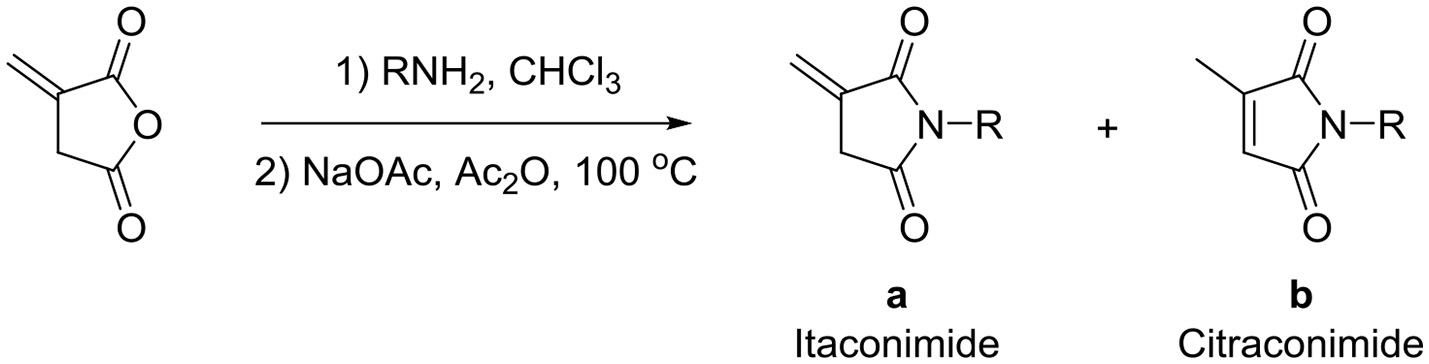
Synthesis of itaconimides and citraconimides.
QSI Activities of Itaconimides
The synthesized compounds were tested for their QS inhibitory activity against the P. aeruginosa lasB-gfp reporter strain. None of our synthesized compounds showed antibiotic properties (Supplementary Figures 1, 2). We found that both the p-bromophenyl and tetradecyl-substituted itaconimide (12a and 18a) showed strong QS inhibition activity against the lasB-gfp reporter strain (Figure 1A). Both compounds inhibit the expression of lasB-gfp in a dose-dependent manner (Figure 1B). 18a was proved to be the most active compound synthesized in this series, with almost 25-fold higher activity (IC50) compared to our hit compound (Figures 1C,D).
Figure 1
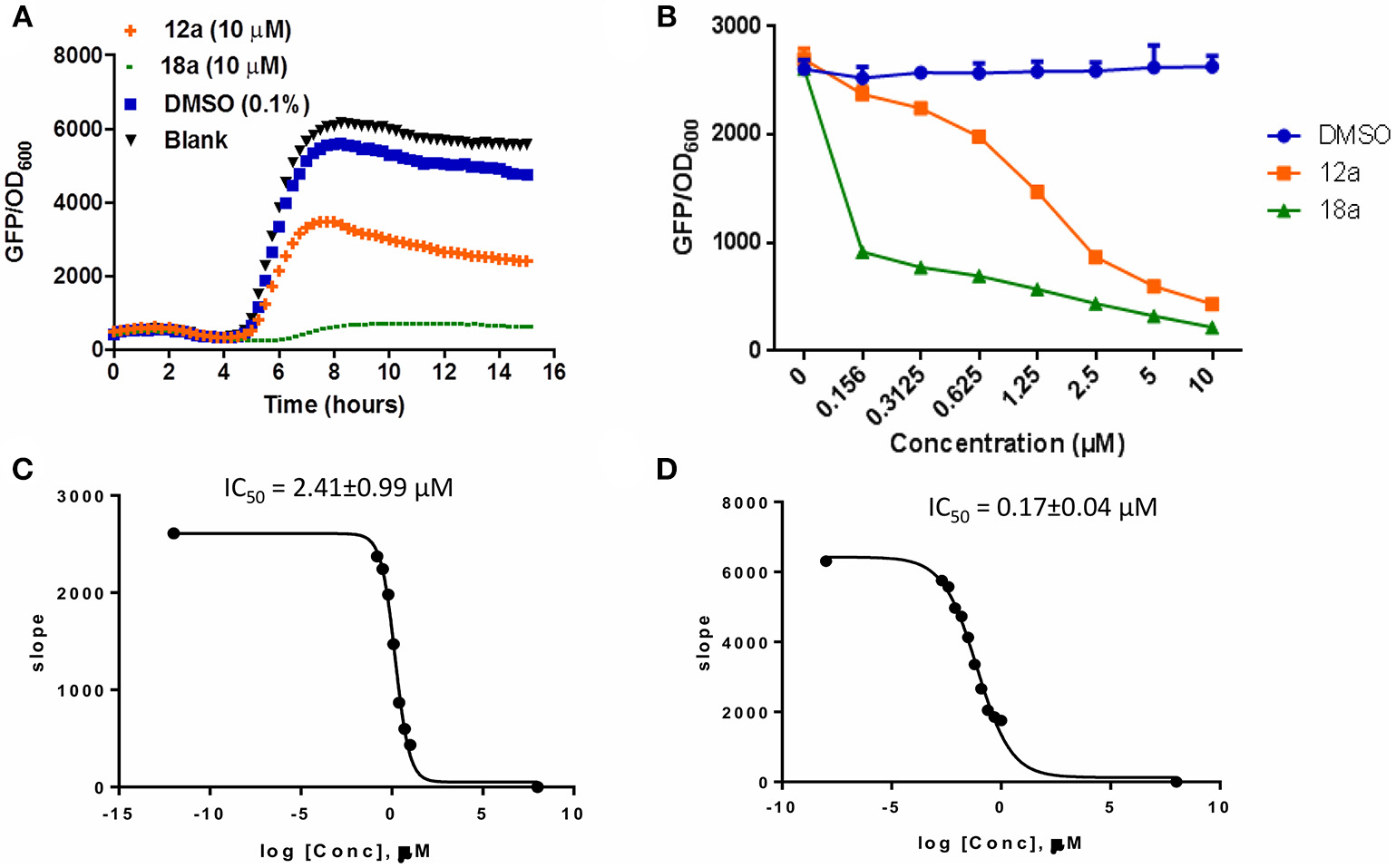
(A) Effects of compounds 12a and 18a on PAO1-lasB-gfp reporter strain. DMSO 0.1% was used as solvent control. (B) Dose-response effects of compounds on P. aeruginosa lasB-gfp. (C) IC50 values of 12a and 18a (D) on lasB-gfp. Calculation was done using GraphPad Prism 6 software, taken at time point between 4 and 6 h when QS inhibition started to occur. All experiments were done in triplicate manner (technical and biological replicates), only representative data are shown.
To address the specificity of our compounds, we also tested our compounds against PAO1 wild-type (WT) and ΔlasR harboring either rhlA-gfp or pqsA-gfp fusions. In this case, we would be able to determine if the compounds could affect other QS pathways in las-dependent or independent manner. Both rhlA and pqsA are the first genes of rhl operon and pqs operon that code for the production of the rhamnolipid and PQS precursor molecules (Ochsner et al., 1994; Gallagher et al., 2002). To eliminate false positive, we also tested both compounds with a gfp-tagged P. aeruginosa (expresses GFP constitutively) as control and did not observe any reduction in the fluorescence signals (Supplementary Figure 3).
Compounds 12a and 18a were found to inhibit expression of rhlA-gfp and pqsA-gfp in PAO1 WT. IC50 values calculated for 12a are 6.67 ± 0.27 μM for rhlA-gfp and 2.51 ± 0.19 μM for pqsA-gfp, whereby compound 18a provides IC50 values of 0.61 ± 0.04 μM for rhlA-gfp and 0.143 ± 0.13 for pqsA-gfp (Figure 2). At 10 μM, compound 12a was observed to inhibit rhlA-gfp more strongly in ΔlasR (78% inhibition) as compared to the wild-type (58% inhibition). In ΔlasR pqsA-gfp reporter strain, the inhibition only happened in the later stage. Meanwhile, compound 18a was found to inhibit both rhlA-gfp and pqsA-gfp efficiently in ΔlasR (Figure 3). Therefore, it is likely that two compounds have different mechanisms, and compound 18a affect multiple QS pathways in las-independent pathway.
Figure 2
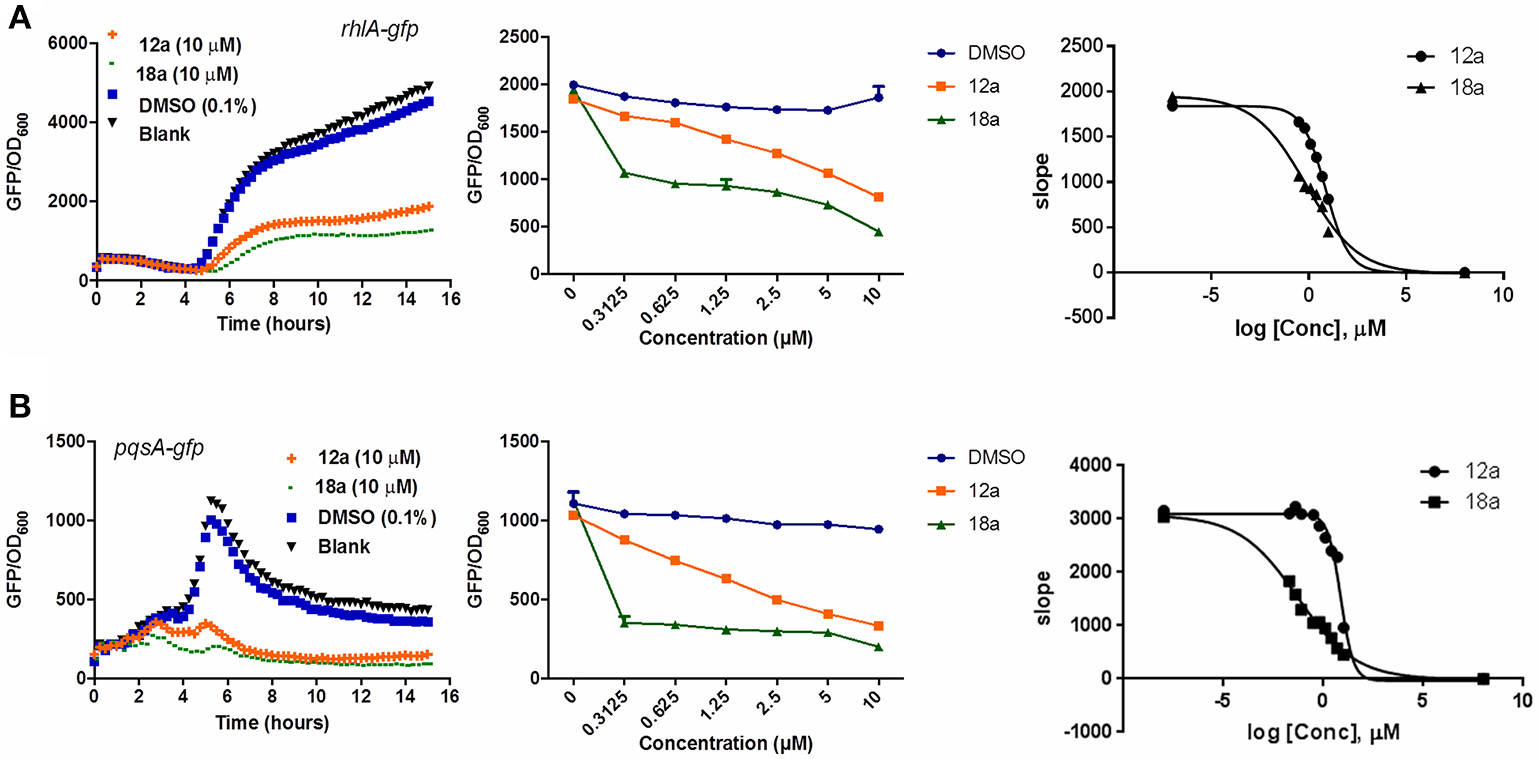
QSI inhibition, dose-response and IC50 values of compounds 12a and 18a on P. aeruginosa rhlA-gfp(A) and pqsA-gfp(B). DMSO 0.1% was used as solvent control. For the IC50 values, calculation was done using GraphPad Prism 6 software, taken at time point between 4 and 6 h when QS inhibition started to occur. All experiments were done in triplicate manner (technical and biological replicates), only representative data are shown.
Figure 3
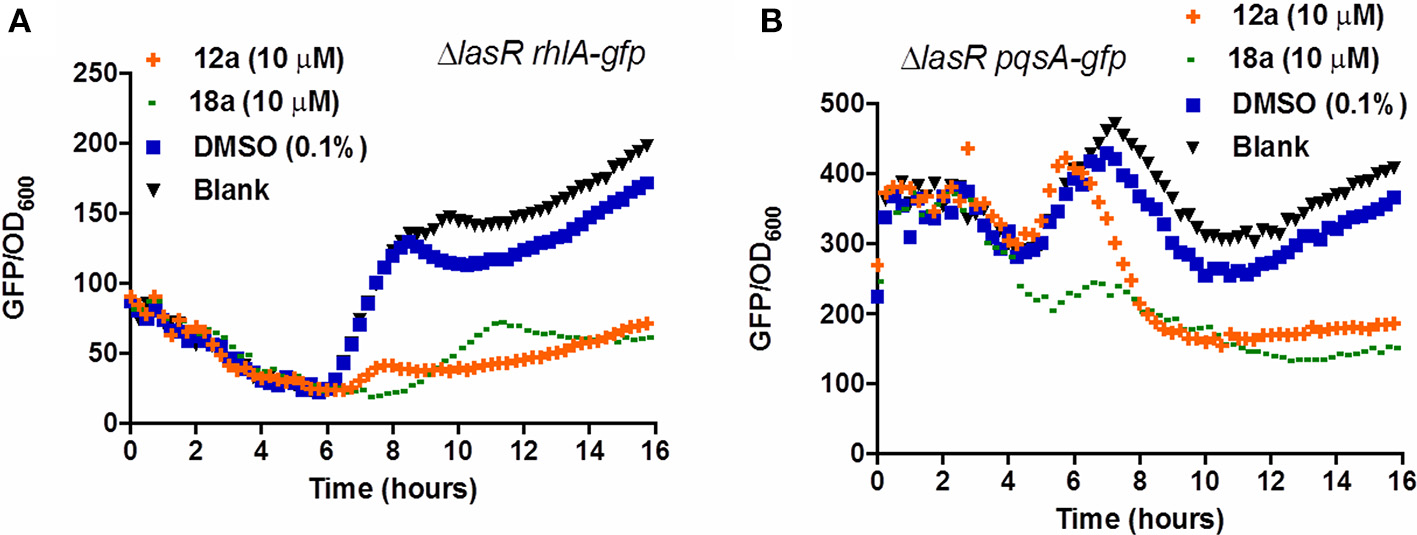
QSI inhibition of compounds 12a and 18a on PAO1 ΔlasR rhlA-gfp(A) and pqsA-gfp(B). DMSO 0.1% was used as solvent control. All experiments were done in triplicate manner (technical and biological replicates), only representative data are shown.
Effects of Itaconimides on Virulence Production
Next, we investigated the effects of synthesized compounds on virulence factors produced by P. aeruginosa, notably elastase, rhamnolipid, and pyocyanin. The three virulence factors are under QS-regulation, therefore they could be a good indicator for evaluating antivirulence activities of our compounds. We also included QS mutants as control strains, where they are defective in producing quorum sensing signals hence lower level of virulence production. At 10 μM, both compounds were able to reduce all three virulence factors production. Elastase level was reduced almost half, and rhamnolipid and pyocyanin productions were abolished almost to the same level as mutant strains (Figure 4). The results showed that both compounds could indeed lower the production of virulence factors.
Figure 4
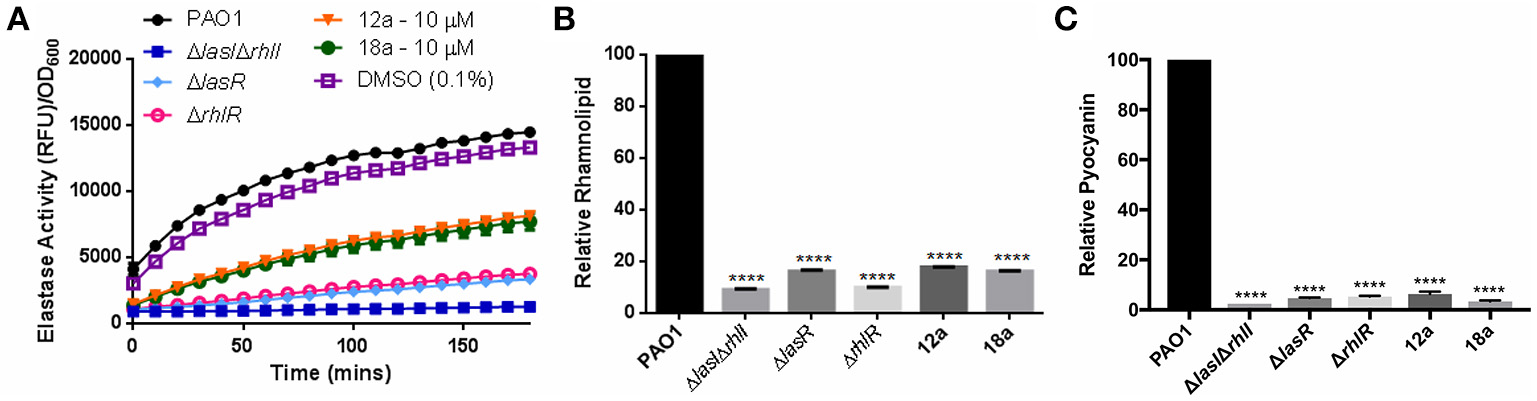
Effects of compounds 12a and 18a on elastase (A), rhamnolipid (B), and pyocyanin production (C). Compounds were tested at final concentration of 10 μM. All experiments were done in triplicate manner (technical and biological replicates). Error bars are means ± SDs. ****p < 0.0001, Student's t-test.
Effects of Itaconimides on P. aeruginosa Biofilms
QS has been shown to play important roles in biofilms matrix formation (Davey et al., 2003; Sakuragi and Kolter, 2007). Mutants lacking QS often form flat and undifferentiated biofilms that could be easily cleared by antimicrobial agents and host immune system (Wu et al., 2001; Christensen et al., 2007). Here, we investigated if the itaconimides could eradicate biofilms formation when used in combination with tobramycin antibiotic.
From Figure 5, it can be seen that treatment with tobramycin alone (10 μg/ml) couldn't clear the whole population of P. aeruginosa biofilms. The dead cells only appeared on the surface of the biofilms, which indicated that the antibiotic could not penetrate into the biofilms. In addition, biofilms often contain extracellular DNA (eDNA) which could chelate aminoglycoside antibiotics and render it inactive (Chiang et al., 2013). At 10 μM, itaconimide 12a could remarkably kill the base population of the biofilms, but not the surface. When added together with tobramycin, the combination treatment successfully eradicated the whole population of biofilms, which appeared red. Compound 18a did not show any synergistic effect when used together with tobramycin, which could be due to poor solubility in the aqueous media.
Figure 5
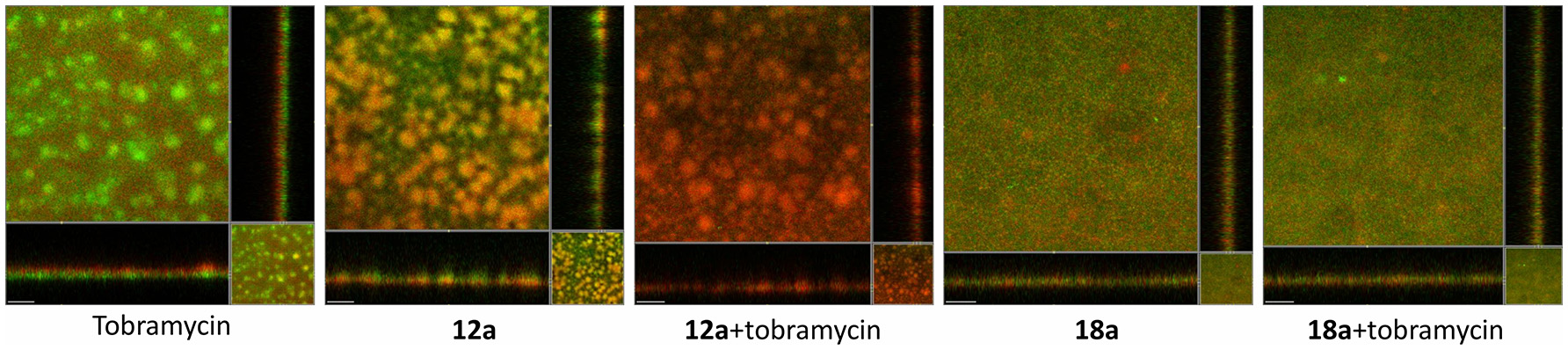
P. aeruginosa biofilms formed on flow cells for 72 h, followed by treatment with medium containing antibiotic (tobramycin 10 μg/ml) and compounds (10 μM) for further 48 h. Live cells are P. aeruginosa tagged with GFP which appeared as green, and dead cells appeared as red. Scale bars, 50 μm. Experiments were done in triplicate manner (technical and biological replicates), only representative images were shown.
Cytotoxic Effects of Itaconimides on Macrophages
We next evaluated the potential application of compounds 12a and 18a for therapeutic application. To test the cytotoxic effects, both compounds were added into murine macrophage RAW2647 cell lines, and the cell viability was measured after 16 h incubation time. Both compounds were observed to be toxic at 40 μM, but not cytotoxic at their working concentration (10 μM and lower, Figure 6). This indicates that they could be further used for subsequent in vivo experiments at their relevant concentration.
Figure 6
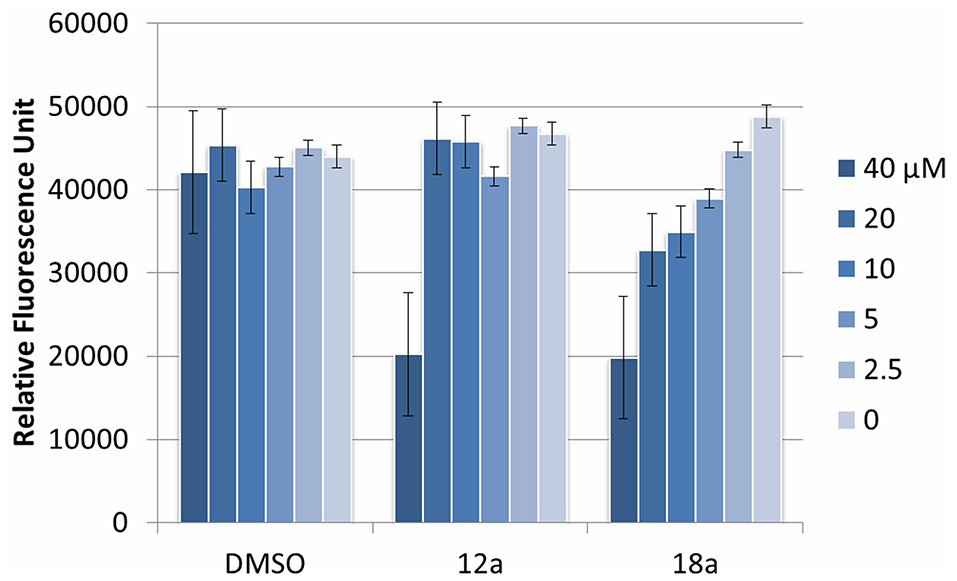
Cytotoxic effect of compounds 12a and 18a on murine macrophages. DMSO was used as vehicle control. Compounds were tested at various concentrations (40 μM and subsequent 2x dilution). Experiments were done in triplicate manner (technical and biological replicates). Error bars are means ± SDs.
Discussion
In this study, we present the investigation of itaconimides as a novel QSI against P. aeruginosa. The approach of using QSI molecules to attenuate the virulence of pathogenic bacteria has advantages over conventional antibiotics therapy. Instead of targeting the growth of bacteria (bactericidal or bacteriostatic strategies), QSI compounds pose lesser selective pressure for the development of resistant mutants. In several studies, it has been shown that mice treated with QSI afford better survival profile and lower bacteria count loads when compared to the control group (Hentzer et al., 2002, 2003; Jakobsen et al., 2013; Fong et al., 2017). With limited options of treatment available, antivirulence therapy could be a viable approach to mitigate the future crisis of antibiotic resistance.
In this study, a structure-activity relationship (SAR) study was performed to investigate the QSI activities of itaconimides and citraconimide analogs. We screened the compounds against QS reporter strain lasB-gfp, where gene expression is under control of the QS las system. We first investigated the inhibitory properties of the two isomers with respect to lasB-gfp expression. Interestingly, the itaconimide scaffold was found to be more active than the citaconimide. Next, we investigated the influence of electron donating groups (EDGs) and electron withdrawing groups (EWGs) on the phenyl group. Whereas, p-anisidine moiety failed to improve activity, the more electron-withdrawing 4-chloro derivative induced a 1.4-fold inhibition of PAO1-lasB-gfp. This guided us to explore anilines with different EWGs. The most potent compound in this series was 12a from 4-bromoaniline that results in a 4.4-fold increase over the parent aniline analog. To our surprise, the 4-fluoroaniline analog 13a did not afford a more potent inhibitor. Alongside the findings that 4-bromoaniline provided a more potent analog, we also found that the octylamine analog 14a was more active than our hit compound 1a. Lastly, we also included aliphatic chains to mimic the long alkyl chain of C4-HSL and 3-oxo-C12-HSL. On this note, a second series was synthesized from commercially available aliphatic amines with variating carbon lengths (n = 4, 6, 8, 10, 12, 14). A clear trend was observed, where longer alkyl chain resulted in lower IC50 values. The outcome was quite interesting, as IC50 values of the citraconimide substituents of these analogs (16b, 17b, and 18b) were significantly lowered. The most potent compound was found to be the tetradecylamine analog, 18a, being the most active of both series. By substituting the naphthalene group with either 4-bromoaniline or long chain alkyl amines, IC50 values against PAO1-lasB-gfp were significantly reduced to the low micromolar range (2.41 ± 0.99 μM for 12a and 0.17 ± 0.04 μM for 18a). In summary, our SAR studies revealed two structurally important variations for the itaconimides that are highly important for QSI activity (Table 3).
Table 3
| Compound | a | b |
|---|---|---|
| 1 | 7.37 ± 0.71 | – |
| 2 | 23.52 ± 0.81 | – |
| 3 | 10.27 ± 0.05 | – |
| 4 | – | – |
| 5 | 17.95 ± 0.49 | – |
| 6 | 10.67 ± 0.49 | – |
| 7 | – | NA |
| 8 | 7.45 ± 0.65 | – |
| 9 | 7.65 ± 0.37 | – |
| 10 | 6.53 ± 0.61 | – |
| 11 | 17.68 ± 0.16 | – |
| 12 | 2.41 ± 0.99 | – |
| 13 | 11.89 ± 0.34 | – |
| 14 | 26.66 ± 0.98 | – |
| 15 | 1.71 ± 0.34 | – |
| 16 | 0.30 ± 0.05 | 2.01 ± 0.39 |
| 17 | 0.30 ± 0.17 | 0.91 ± 0.20 |
| 18 | 0.17 ± 0.04 | 0.53 ± 0.12 |
Summary of IC50 values of itaconimide (a) and citraconimide (b) analogs against PAO1-lasB-gfp.
All IC50 values were reported as μM. Experiments were done in triplicate manner and repeated at least twice to confirm the results. NA, Not available.
Previous studies have emphasized the advantage of therapeutics that suppress multiple QS pathways (Fong et al., 2018). Indeed, lasR mutants are commonly found in patients suffering from cystic fibrosis and other clinical setting (Hamood et al., 1996; Cabrol et al., 2003; Marvig et al., 2014). Nevertheless, the loss-of-function lasR mutants continuously express virulence traits so P. aeruginosa could use other pathways to bypass LasR in controlling pathogenicity (Dekimpe and Déziel, 2009; Lee et al., 2013). Using different QS reporter strains in both PAO1 WT and ΔlasR, it can be deduced that our compounds do not specifically inhibit one QS pathway. Both compounds could inhibit expression of rhlA-gfp and pqsA-gfp in lasR mutant. The inhibition of pqsA-gfp in ΔlasR was less apparent for compound 12a, which could indicate that the compound inhibits PQS system through las-dependent manner. The itaconimide analogs presented here hold unique potential as broad target QSIs to control infections via an anti-virulence strategy. Future work will aim to elucidate the mechanism on how both compounds inhibit QS in P. aeruginosa.
Next, we also tested the effects of our compounds on the production of various virulence factors, such as elastase, rhamnolipid, and pyocyanin production. Elastase is one of the major proteases produced by P. aeruginosa, involved in host tissue damage and host immune responses (Kamath et al., 2002). Rhamnolipid is also an essential virulence factor and plays several key roles in biofilms formation, swarming, and in particular host immune evasion. It promotes rapid necrotic killing of polymorphonuclear (PMNs) leukocytes and also infiltration of respiratory epithelial cells (Zulianello et al., 2006; Jensen et al., 2007). P. aeruginosa also secretes pyocyanin, blue redox-active secondary metabolite that has several deleterious effects on mammalian cells (Lau et al., 2004), which is also regulated by QS under pqs system. When tested at 10 μM, the compounds could reduce production of various virulence factors controlled by QS. This shows potential of our compounds as antivirulence agent of P. aeruginosa.
P. aeruginosa biofilms are highly resistant to most antibiotics, including the last-resort polymyxin antibiotic available, colistin (Chua et al., 2016). As QS has definite role in biofilms development, it has been proposed that QSI compounds could be used as prophylactic treatment for biofilms infections. One such case is using azithromycin, which inhibits QS and biofilm formation at sub-MIC concentration. Promising results were observed in pulmonary infections and CF patients in many clinical trials data upon treatment of low-dose of AZM (2 μg/ml) (Hansen et al., 2005; Fleet et al., 2013). In this study, we utilized a combination of our compounds with the aminoglycoside tobramycin to treat P. aeruginosa biofilms grown on flow chambers for 3 days. We chose to study tobramycin because of its clinical relevance to cystic fibrosis (CF) patients. The clinical isolates from lung patients often confer resistance to aminoglycoside antibiotics (Hurley et al., 1995; Saiman et al., 1996). Our data also shows that treatment with tobramycin alone only kills the upper layer of biofilms. Combined with 12a, we observed that the whole population of biofilms was eradicated. The results offer promising application of QSI in combination with antibiotics as a control for biofilm-associated infections.
It has been reported that stringent response, which provides rapid adaptation to environmental stresses, regulates QS network and also bacteria's survival in biofilms of P. aeruginosa (van Delden et al., 2001; Nguyen et al., 2011; Schafhauser et al., 2014). QS-deficient mutants also have lower catalase and superoxide dismutase activities, and therefore more sensitive to oxidative stress (Hassett et al., 1999). Through our biofilms experiment, we could observe some cells death upon treatment with compound 12a in the base of the biofilms. This raises the possibility that our compound could also target stringent response and other stress-response genes, which resulted in the observed killing effect. For compound 18a, it contains long alkyl chain, which may render its solubility and penetration into the biofilms matrix. Future study will investigate how our compounds could synergize with other antibiotics to treat biofilms from other clinical isolates.
Lastly, we investigated the cytotoxic profile of our compounds on macrophages. The cytotoxicity of the compounds is important if they are to be used in animal studies for subsequent drug development. Results indicated that compound 12a and 18a are not toxic up to 40 μM concentration. Still, further studies are needed to qualify the efficacy of the compounds in mice models for their potential as anti-biofilm agents, as well as their pharmacodynamic and pharmacokinetic profiles.
In conclusion, we report the novel use of itaconimides as antivirulence compounds for P. aeruginosa. These compounds suppress the las, rhl, and pqs QS systems of P. aeruginosa, and effectively abolish virulence expression activities. Compounds 12a and 18a showed low micromolar IC50 values against all three QS reporter strains with only little toxicity against macrophages at the administrated concentration. Moreover, a synergistic effect with tobramycin was observed for the killing of P. aeruginosa biofilms, including otherwise the tolerant and hard to target sub-population cells. Overall, our findings point to a new class of hit compounds of relevance to the development of new drugs against the superbug P. aeruginosa.
Statements
Author contributions
LY, TN, and MG designed methods and experiments. JF and KM performed the experiments and analyzed the data. AN, KQ, CT, and TN were in charge of the chemical synthesis. JF and KM wrote the paper and carefully revised by LY, TN, and MG. All authors have contributed to read and approved the manuscript.
Funding
This research was supported by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Program and AcRF Tier 2 (MOE2016-T2-1-010) from Ministry of Education, Singapore. KM, KQ, and TN are grateful to The Danish Council for Independent Research (FTP grant 40616) for supporting this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00443/full#supplementary-material
References
1
AlhedeM.BjarnsholtT.JensenP. Ø.PhippsR. K.MoserC.ChristophersenL.et al. (2009). Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology155, 3500–3508. 10.1099/mic.0.031443-0
2
BalganeshM.EthirajuluK.GangulyB. S.JanakiramanR.KaurP.KajipalyaR.et al. (1999). Mycobacterial inhibitors. WO1999065483A1. Available online at: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1999065483&tab=PCTBIBLIO&maxRec=1000
3
BjarnsholtT.JensenP. Ø.FiandacaM. J.PedersenJ.HansenC. R.AndersenC. B.et al. (2009). Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol.44, 547–558. 10.1002/ppul.21011
4
BrintJ. M.OhmanD. E. (1995). Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol.177, 7155–7163. 10.1128/jb.177.24.7155-7163.1995
5
CabrolS.OlliverA.PierG. B.AndremontA.RuimyR. (2003). Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J. Bacteriol.185, 7222–7230. 10.1128/JB.185.24.7222-7230.2003
6
CavaM. P.DeanaA. A.MuthK.MitchellM. J. (1961). N-Phenylmaleimide. Org. Synth.41:93. 10.15227/orgsyn.041.0093
7
ChiangW.-C.NilssonM.JensenP. Ø.HøibyN.NielsenT. E.GivskovM.et al. (2013). Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother.57, 2352–2361. 10.1128/AAC.00001-13
8
ChristensenL. D.MoserC.JensenP. Ø.RasmussenT. B.ChristophersenL.KjellebergS.et al. (2007). Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology153, 2312–2320. 10.1099/mic.0.2007/006122-0
9
ChuaS. L.YamJ. K. H.HaoP.AdavS. S.SalidoM. M.LiuY.et al. (2016). Selective labelling and eradication of antibiotictolerant bacterial populations in Pseudomonas aeruginosa biofilms. Nat. Commun.7:10750. 10.1038/ncomms10750
10
ClarkD. J.MaaloeO. (1967). DNA replication and the division cycle in Escherichia coli. J. Mol. Biol.23, 99–112. 10.1016/S0022-2836(67)80070-6
11
CostertonJ. W.StewartP. S.GreenbergE. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science284, 1318–1322. 10.1126/science.284.5418.1318
12
DaveyM. E.CaiazzaN. C.O'tooleG. A. (2003). Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol.185, 1027–1036. 10.1128/JB.185.3.1027-1036.2003
13
DekimpeV.DézielE. (2009). Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology155, 712–723. 10.1099/mic.0.022764-0
14
DongY.-H.WangL.-H.XuJ.-L.ZhangH.-B.ZhangX.-F.ZhangL.-H. (2001). Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature144, 813–817. 10.1038/35081101
15
FleetJ. E.GuhaK.PiperS.BanyaW.BiltonD.HodsonM. E. (2013). A retrospective analysis of the impact of azithromycin maintenance therapy on adults attending a UK cystic fibrosis clinic. J. Cyst. Fibros.2013, 49–53. 10.1016/j.jcf.2012.05.010
16
FlemmingH.-C.WingenderJ. (2010). The biofilm matrix. Nat. Rev. Microbiol.8, 623–633. 10.1038/nrmicro2415
17
FongJ.YuanM.JakobsenT. H.MortensenK. T.SantosM. M. S. D.ChuaS. L.et al. (2017). Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem.60, 215–227. 10.1021/acs.jmedchem.6b01025
18
FongJ.ZhangC.YangR.BooZ. Z.TanS. K.NielsenT. E.et al. (2018). Combination therapy strategy of quorum quenching enzyme and quorum sensing inhibitor in suppressing multiple quorum sensing pathways of P. aeruginosa. Sci. Rep.8:1155. 10.1038/s41598-018-19504-w
19
GallagherL. A.McknightS. L.KuznetsovaM. S.PesciE. C.ManoilC. (2002). Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol.184, 6472–6480. 10.1128/JB.184.23.6472-6480.2002
20
GambelloM. J.KayeS.IglewskiB. H. (1993). LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin a expression. Infect. Immun.61, 1180–1184.
21
HamoodA. N.GriswoldJ.ColmerJ. (1996). Characterization of elastase-deficient clinical isolates of Pseudomonas aeruginosa. Infect. Immun.64, 3154–3160.
22
HansenC. R.PresslerT.KochC.HøibyN. (2005). Long-term azitromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. J. Cyst. Fibros4, 35–40. 10.1016/j.jcf.2004.09.001
23
HassettD. J.MaJ. F.ElkinsJ. G.McdermottT. R.OchsnerU. A.WestS. E. H.et al. (1999). Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol.34, 1082–1093. 10.1046/j.1365-2958.1999.01672.x
24
HentzerM.RiedelK.RasmussenT. B.HeydornA.AndersenJ. B.ParsekM. R.et al. (2002). Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology148, 87–102. 10.1099/00221287-148-1-87
25
HentzerM.WuH.AndersenJ. B.RiedelK.RasmussenT. B.BaggeN.et al. (2003). Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J.22, 3803–3815. 10.1093/emboj/cdg366
26
HurleyJ. C.MillerG. H.SmithA. L. (1995). Mechanism of amikacin resistance in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Diagn. Microbiol. Infect. Dis.22, 331–336. 10.1016/0732-8893(95)00138-6
27
JakobsenT. H.BjarnsholtT.JensenP. Ø.GivskovM.HøibyN. (2013). Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol.8, 901–921. 10.2217/fmb.13.57
28
JakobsenT. H.GennipM. V.PhippsR. K.ShanmughamM. S.ChristensenL. D.AlhedeM.et al. (2012). Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother.56:2314. 10.1128/AAC.05919-11
29
JensenP. Ø.BjarnsholtT.PhippsR.RasmussenT. B.CalumH.ChristoffersenL.et al. (2007). Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology153, 1329–1338. 10.1099/mic.0.2006/003863-0
30
KamathS.KapatralV.ChakrabartyA. M. (2002). Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol. Microbiol.30, 933–941. 10.1046/j.1365-2958.1998.01121.x
31
KochA. K.KappeliO.FiechterA.ReiserJ. (1991). Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol.173, 4212–4219. 10.1128/jb.173.13.4212-4219.1991
32
KyungJ. H.ChaS.ClappL. B. (1974). Identification of isomeric amides of itaconic acid by proton magnetic resonance spectroscopy. Organ. Magnet. Res.6, 466–468. 10.1002/mrc.1270060816
33
LauG. W.HassettD. J.RanH.KongF. (2004). The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med.10, 599–606. 10.1016/j.molmed.2004.10.002
34
LeeJ.WuJ.DengY.WangJ.WangC.WangJ.et al. (2013). A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol.9, 339–343. 10.1038/nchembio.1225
35
LeowD.LinS.ChittimallaS. K.FuX.TanC. H. (2008). Enantioselective protonation catalyzed by a chiral bicyclic guanidine derivative. Angew. Chem. Int. Ed.47, 5641–5645. 10.1002/anie.200801378
36
LewisK. (2007). Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol.5, 48–56. 10.1038/nrmicro1557
37
ListerP. D.WolterD. J.HansonN. D. (2009). Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev.22, 582–610. 10.1128/CMR.00040-09
38
MarvigR. L.SommerL. M.MolinS.JohansenH. K. (2014). Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet.47, 1–9. 10.1038/ng.3148
39
NguyenD.Joshi-DatarA.LepineF.BauerleE.OlakanmiO.BeerK.et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science334, 982–986. 10.1126/science.1211037
40
OchsnerU. A.FiechterA.ReiserJ. (1994). Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J. Biol. Chem.269, 19787–19795.
41
PassadorL.CookJ. M.GambelloM. J.RustL.IglewskiB. H. (1993). Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science260, 1127–1130. 10.1126/science.8493556
42
PearsonJ. P.FeldmanM.IglewskiB. H.PrinceA. (2000). Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun.68, 4331–4334. 10.1128/IAI.68.7.4331-4334.2000
43
PearsonJ. P.PassadoriL.IglewskiB. H.GreenbergE. P. (1995). A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A.92, 1490–1494. 10.1073/pnas.92.5.1490
44
PesciE. C.PearsonJ. P.SeedP. C.IglewskiB. H. (1997). Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol.179, 3127–3132. 10.1128/jb.179.10.3127-3132.1997
45
PooleK. (2001). Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol.3, 255–264. Available online at: https://www.karger.com/Journal/Home/22839
46
SaimanL.MeharF.NiuW. W.NeuH. C.ShawK. J.MillerG.et al. (1996). Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin. Infect. Dis.23, 532–537. 10.1093/clinids/23.3.532
47
SakuragiY.KolterR. (2007). Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol.189, 5383–5386. 10.1128/JB.00137-07
48
SchafhauserJ.LepineF.MckayG.AhlgrenH. G.KhakimovaM.NguyenD. (2014). The stringent response modulates 4-hydroxy-2-alkylquinoline biosynthesis and quorum-sensing hierarchy in Pseudomonas aeruginosa. J. Bacteriol.196, 1641–1650. 10.1128/JB.01086-13
49
SchertzerJ. W.BouletteM. L.WhiteleyM. (2009). More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol.17, 189–195. 10.1016/j.tim.2009.02.001
50
SternbergC.Tolker-NielsenT. (2006). Growing and analyzing biofilms in flow cells. Curr. Protoc. Microbiol. Chapter 1, Unit 1B 2. 10.1002/9780471729259.mc01b02s00
51
TanS. Y.ChuaS.-L.ChenY.RiceS. A.KjellebergS.NielsenT. E.et al. (2013). Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother.57, 5629–5641. 10.1128/AAC.00955-13
52
U.S. Centers for Disease Control and Prevention (CDC) (2013). Antibiotic Resistance Threats in the United States.
53
van DeldenC.ComteR.BallyM. (2001). Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol.183, 5376–5384. 10.1128/JB.183.18.5376-5384.2001
54
Van GennipM.ChristensenL. D.AlhedeM.PhippsR.JensenP. Ø.ChristophersenL.et al. (2009). Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS117, 537–546. 10.1111/j.1600-0463.2009.02466.x
55
VincentJ.-L.BihariD. J.SuterP. M.BruiningH. A.WhiteJ.Nicolas-ChanoinM. H.et al. (1995). The prevalence of nosocomial infection in intensive care units in Europe. results of the european prevalence of infection in intensive care (epic) study. epic international advisory committee. J. Am. Med. Assoc.274, 639–644. 10.1001/jama.1995.03530080055041
56
WuH.SongZ.GivskovM.DoringG.WorlitzschD.MatheeK.et al. (2001). Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology147, 1105–1113. 10.1099/00221287-147-5-1105
57
YangL.BarkenK. B.SkindersoeM. E.ChristensenA. B.GivskovM.Tolker-NielsenT. (2007). Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology153, 1318–1328. 10.1099/mic.0.2006/004911-0
58
YangL.RybtkeM. T.JakobsenT. H.HentzerM.BjarnsholtT.GivskovM.et al. (2009). Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob. Agents Chemother. 53, 2432–2443. 10.1128/AAC.01283-08
59
ZulianelloL.CanardC.KohlerT.CailleD.LacroixJ.-S.MedaP. (2006). Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun.74, 3134–3147. 10.1128/IAI.01772-05
Summary
Keywords
quorum sensing, biofilm, itaconimides, antivirulence, chemical biology
Citation
Fong J, Mortensen KT, Nørskov A, Qvortrup K, Yang L, Tan CH, Nielsen TE and Givskov M (2019) Itaconimides as Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 8:443. doi: 10.3389/fcimb.2018.00443
Received
06 October 2018
Accepted
11 December 2018
Published
07 January 2019
Volume
8 - 2018
Edited by
You-Hee Cho, CHA University, South Korea
Reviewed by
Eric Déziel, Institut National de la Recherche Scientifique (INRS), Canada; Daniel Angel Ortiz, Laboratory Corporation of America Holdings (LabCorp), United States
Updates
Copyright
© 2019 Fong, Mortensen, Nørskov, Qvortrup, Yang, Tan, Nielsen and Givskov.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Yang yangl@sustc.edu.cnMichael Givskov mgivskov@sund.ku.dk
This article was submitted to Clinical Microbiology, a section of the journal Frontiers in Cellular and Infection Microbiology
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.