- 1Centre for Heart Lung Innovation, University of British Columbia and St. Paul's Hospital, Vancouver, BC, Canada
- 2Department of Graduate Studies, Experimental Medicine, University of British Columbia, Vancouver, BC, Canada
- 3Divison of Respiratory Medicine, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
- 4Divison of Critical Care Medicine, Department of Medicine, University of British Columbia, Vancouver, BC, Canada
- 5Department of Biological Sciences, Simon Fraser University, Burnaby, BC, Canada
- 6Prevention of Organ Failure Centre of Excellence, Vancouver, BC, Canada
Aspergillus fumigatus is an opportunistic fungal pathogen capable of causing severe infection in humans. One of the limitations in our understanding of how A. fumigatus causes infection concerns the initial stages of infection, notably the initial interaction between inhaled spores or conidia and the human airway. Using publicly-available datasets, we identified the Arp2/3 complex and the WAS-Interacting Protein Family Member 2 WIPF2 as being potentially responsible for internalization of conidia by airway epithelial cells. Using a cell culture model, we demonstrate that RNAi-mediated knockdown of WIPF2 significantly reduces internalization of conidia into airway epithelial cells. Furthermore, we demonstrate that inhibition of Arp2/3 by a small molecule inhibitor causes similar effects. Using super-resolution fluorescence microscopy, we demonstrate that WIPF2 is transiently localized to the site of bound conidia. Overall, we demonstrate the active role of the Arp2/3 complex and WIPF2 in mediating the internalization of A. fumigatus conidia into human airway epithelial cells.
Introduction
Aspergillus fumigatus is a saprophytic filamentous fungus present throughout the world. The spores or conidia of A. fumigatus are a potentially infectious agent and are inhaled by most people every day (Latgé, 1999). A. fumigatus is known to be capable of behaving as an opportunistic fungal pathogen in immunocompromised individuals, causing a variety of diseases such as allergic bronchopulmonary aspergillosis (ABPA) and invasive aspergillosis (IA). Understanding the mechanisms of interaction between airway epithelial cells (AECs) and the conidia of this organism is vital to develop an understanding of the overall mechanism of infection.
Most infections caused by A. fumigatus conidia occur once they have been inhaled by the host, however further knowledge regarding the mechanism of pathogenesis is poorly understood. One hypothesis is that conidia may be internalized by the local airway epithelial cells, whereupon the conidia may germinate and lead to infection (Wasylnka and Moore, 2002; Croft et al., 2016). Specifically, the internalization process occurs via phagocytosis, the process by which cells uptake particulate matter such as pathogens and air pollutants (Gordon, 2016). Since conidia have been demonstrated to survive phagocytosis by non-professional phagocytes and germinate, it is possible that phagocytosis by airway epithelial cells allows them to escape the immune response mediated by macrophages patrolling the airway epithelium. It has been demonstrated that internalization of conidia by airway epithelial cells is dependant on actin polymerization and reorganization, although more detailed mechanistic insights are not yet available (Wasylnka and Moore, 2002; Chen et al., 2015; Toor et al., 2018). One protein complex that is responsible for mediating actin polymerization is the actin reorganization complex 2 and 3 (Arp2/3), which mediates actin reorganization by adding branches to actin filaments (Goley and Welch, 2006). There exist a number of proteins responsible for mediating the activity of Arp2/3, such as Wiskott-Aldrich Syndrome Protein (WASP) and its associated WAS-interacting proteins such as WAS-interacting protein family member 1 and 2 (WIPF1, WIPF2). To address the lack of mechanistic knowledge surrounding the phagocytosis and internalization of A. fumigatus conidia, we have employed a data mining approach coupled with in vitro cell biology to identify and assess a potential mechanism by which A. fumigatus conidia are internalized into airway epithelial cells.
Methods
Detailed methods have been described in the Supplementary Presentation.
Data Mining
Statistical analysis was performed using R. Microarray data accessed from the Gene Expression Omnibus was tested for differential expression using the limma package (Smith, 2005). For the RNA-seq data, limma-voom was used (Law et al., 2014). Sparse partial least squares was performed using the spls function from mixOMICS (Lê Cao et al., 2009).
Culture and Growth Conditions
1HAEo- cells, SV40-transformed normal human airway epithelial cells (Cozens et al., 1992) were routinely grown at 37° C until 80% confluency in Dulbecco's Modified Eagle's Media, 10% fetal bovine serum and 1% Penicillin-Streptomycin. A. fumigatus conidia were grown at 30° C as described (Wasylnka and Moore, 2002).
Immunofluorescence Microscopy
1HAEo- cells were grown to 80% confluency on eight-well chamber slides (Thermo-Fisher). Cells were infected with conidia at a multiplicity of infection of 10 conidia per cell for the indicated periods of time. After infection, samples were washed with PBS, permeabilized with 0.5% Triton X-100 and fixed with 4% paraformaldehyde. Samples were subsequently immunolabeled and prepared for microscopy. Slides were scanned using a Zeiss LSM-880 Inverted Confocal Microscopy with Airyscan technology.
RNAi of WIPF2
1HAEo- cells were transfected with TriFECTa double stranded dicer-substrate siRNA (DsiRNA) targeting either WIPF2, no known transcript in the human genome (NC-1) (Integrated DNA Technologies) or media as control. Cells were transfected using Lipofectamine RNAiMAX (Thermo-Fisher). Three different siRNA sequences were transfected simultaneously at 3.3 nM concentration each. Transfected cells were grown for 72 h before usage.
Nystatin Protection Assay
Cells were transfected as described above and infected with A. fumigatus conidia for 3 h. After 3 h, unbound conidia were aspirated and media was replaced with media containing nystatin for a further 3 h. Nystatin-containing media was removed and cells were lysed using 0.05% Triton X-100 in water. Lysates were diluted, plated in duplicate onto rich media and colony forming units were counted.
Arp2/3 Chemical Inhibition and Two-Color Immunofluorescence
Cells were pre-treated with 200 μM CK-666 (Sigma-Aldrich), an Arp2/3 inhibitor in media or equivalent volume of DMSO for 30 min prior to infection with A. fumigatus conidia for 3 h under the same treatment conditions. Two-color immunofluorescence was used as described previously (Wasylnka and Moore, 2002) to assess the degree of internalization.
Results
Data Mining Identifies Candidate Genes
In order to identify candidate genes for analysis, we first assessed data obtained in a previous experiment analyzing the interaction of A. fumigatus conidia and human airway epithelial cells (Gomez et al., 2010; Oosthuizen et al., 2011). We identified 652 and 118 genes as being differentially expressed post-infection in the human and the A. fumigatus datasets, respectively (FDR < 0.15) (Supplementary Tables 1, 2). To further filter our dataset, we used sparse partial least squares (sPLS) (Rohart et al., 2017) to identify highly correlated differentially expressed gene pairs between the human and A. fumigatus datasets. We selected the top 20 gene pairs from both datasets for further analysis.
We hypothesized that the airway epithelial cells may internalize conidia without regard for their identity. In an attempt to lend strength to this hypothesis, we accessed publicly-available RNA-seq datasets relevant to this interaction. Specifically we selected experiments which used primary airway epithelial cells that were infected in vitro by a pathogen for under 8 h. We identified three datasets matching these criteria (Table 1). The data were aligned and analyzed for differential expression post-infection (FDR < 0.15) (Supplementary Table 3). Two genes identified within the RNA-seq dataset were replicated in the sPLS dataset: RNA Pseudouridylate Synthase Domain Containing 4 (RPUSD4) and WAS-interacting protein family member 2 (WIPF2). We elected to specifically evaluate WIPF2's impact on the internalization of A. fumigatus conidia further, as the potential mechanism of action of WIPF2, a gene involved in actin polymerization was much more clear than RPUSD4, an RNA-binding protein.
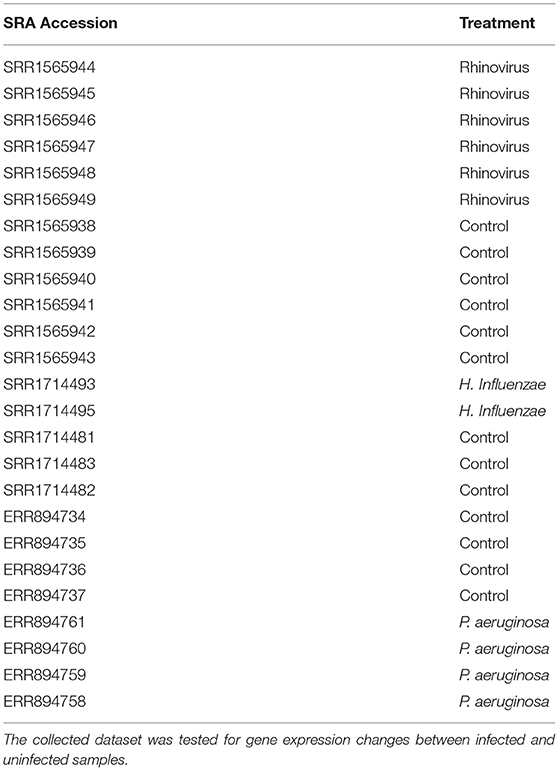
Table 1. Indicated RNA-seq datasets corresponding to bronchial epithelial cells infected by various pathogens were downloaded from the NCBI SRA.
WIPF2 Is Transiently Localized to the Site of A. fumigatus Adhesion
Previously, actin polymerisation has been demonstrated to occur at the site of adhesion of A. fumigatus conidia to AECs (Wasylnka and Moore, 2002). The Arp2/3 complex is known to be responsible for extending actin filaments and mediate the formation of filopodia (Goley and Welch, 2006). WAS-interacting protein family member 2 (WIPF2), a gene whose protein is involved in mediating the activity of the Arp2/3 complex via activity of the Wiskott-Aldrich syndrome protein (WASP) (Takenawa and Miki, 2001) was selected as a surrogate for the Arp2/3 pathway in this experiment. 1HAEo- cells were infected with GFP-expressing A. fumigatus conidia for 15 or 60 min before immunolabeling with anti-WIPF2 antibody and imaging with super-resolution airyscan microscopy. DAPI was used as a nuclear counter-stain. Localization of WIPF2 fluorescent signal with GFP signal from the conidia was observed to be localized as a “ring” around the conidia 15 min post-infection (Figure 1A). Furthermore, 60 min post-infection the WIPF2 signal was observed to be diffused throughout the cells (Figure 1B). This indicated that WIPF2 was involved in the immediate early stages of interaction between 1HAEo- cells and conidia. Orthographic projection of Z-stacks indicated that the conidia were not internalized at either time point (Figures 1A,B).
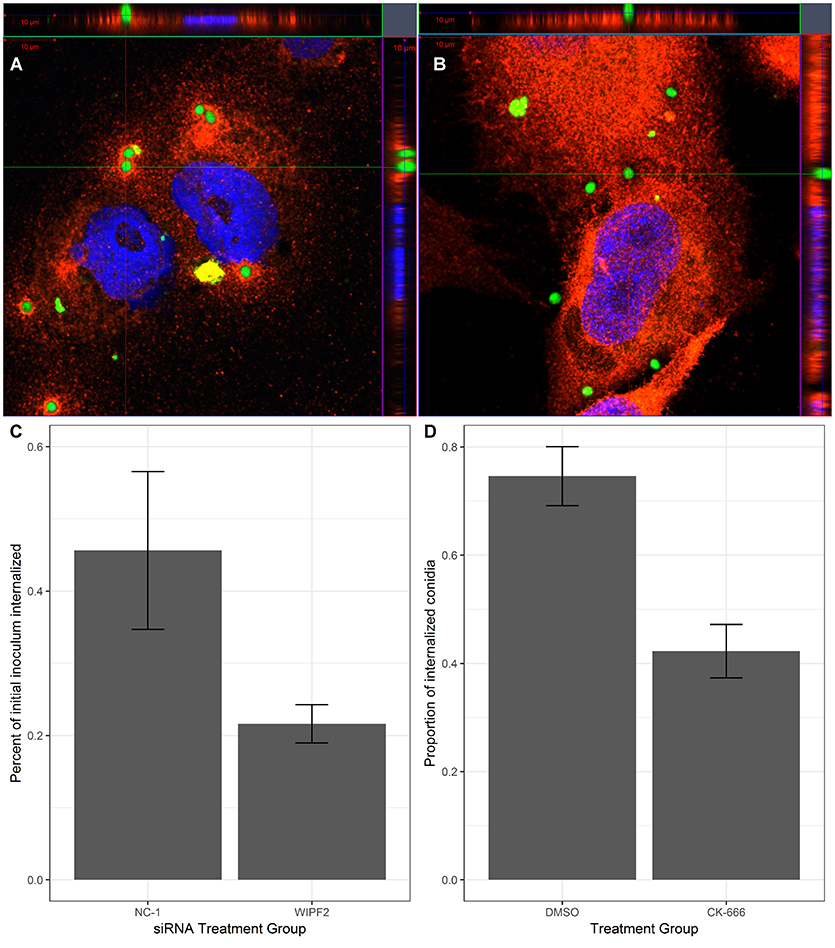
Figure 1. WIPF2 and Arp2/3 mediate internalization of conidia into 1HAEo- cells. (A,B) 1HAEo- cells were infected with GFP-expressing A. fumigatus conidia for 15 min (A) or 1 h (B). After infection, samples were immunolabeled using anti-WIPF2 antibody and DAPI and imaged using super-resolution confocal microscopy. (C) 1HAEo-cells were transfected with siRNA targeting no known gene in the genome (NC-1) or WIPF2. The nystatin protection assay was used to assess the extent of internalization. P < 0.05 (Student's T-test). (D) 1HAEo-cells were treated with CK-666, an Arp2/3 inhibitor or DMSO vehicle before infection by GFP-expressing A. fumigatus conidia. Extracellular conidia were labeled by immunolabeling without permeabilization followed by manual counting. The proportion of observed conidia that were internalized is shown. P < 0.05 (Student's T-test).
WIPF2 Controls Internalization of Conidia by 1HAEo- Cells
The role of actin polymerization in the interaction of AECs and conidia is the mediation of internalization of the conidia by the AECs (Wasylnka and Moore, 2002; Croft et al., 2016). We hypothesized that knockdown of WIPF2 in AECs would lead to a reduction in internalization rates. We demonstrated effective depletion of WIPF2 protein product in 1HAEo- cells by RNAi 72 h post-transfection using three siRNA targeting WIPF2 (RNAi-WIPF2 cells) compared to non-targeting siRNA (RNAi-NC-1 cells) (Supplementary Figure 1). In order to quantify differential internalization between RNAi-WIPF2 cells and RNAi-NC-1 cells, we used the nystatin protection assay, a previously published method adapted from the gentamicin protection assay (Wasylnka and Moore, 2002; Chen et al., 2015). Briefly, 1HAEo- cells were incubated with conidia for a period of 3 h, after which time the inoculum was aspirated and replaced with media containing nystatin. Nystatin is a fungicide which kills conidia but cannot penetrate the cell membrane. After a further 3 h, the nystatin-containing media was removed and cells were gently lysed to release internalized conidia. Lysates were subsequently diluted and plated onto rich media and colony-forming units were counted to approximate total internalized conidia. We demonstrated that RNAi-WIPF2 cells internalized 52% fewer conidia compared RNAi-NC-1 cells (Figure 1C) (Supplementary Table 4).
The Arp2/3 Complex Mediates Internalization of Conidia by 1HAEo- Cells
Although we have specifically interrogated the role of WIPF2 on the internalization of conidia, it is an upstream regulator of the actual complex responsible for actin reorganization: Arp2/3. Using CK-666, a small molecule inhibitor of Arp2/3 (Hetrick et al., 2013) we performed small molecule inhibition of Arp2/3 and measured internalization of conidia via fluorescence microscopy. In the cultures treated with CK-666, we observed a 43% reduction in internalization when compared with DMSO vehicle (Figure 1D).
Discussion
The human Arp2/3 complex is responsible for actin rearrangement and is regulated by a number of proteins. Particularly, WASP and associated WAS-interacting proteins are known to mediate this process. One notable WAS-interacting protein is WIPF2, which binds cooperatively with WASP to mediate actin filopodia formation by Arp2/3 (Takenawa and Miki, 2001). Filopodia, or actin microspikes have been described as the “phagocytic tentacles” of macrophages (Goley and Welch, 2006; Kress et al., 2007). Our data suggest that the internalization of A. fumigatus conidia by AECs occurs through this mechanism.
The early stages of interaction between A. fumigatus conidia and AECs is characterized by adhesion of conidia and the initiation of actin polymerization (Croft et al., 2016). Our data indicate that WIPF2 may have an activating role in this process, as we observed localization of WIPF2 to conidia only during the earliest stages of this interaction. Since WIPF2 mediates activity of WASP, which in turn mediates activity of Arp2/3, this indicates that WIPF2 may play a regulatory role in activating WASP at the site of adhesion and subsequently dissociating once the process of actin polymerization has been initiated. WIPF2 is predicted to be a highly disordered protein, which are known to have regulatory roles and is consistent with this model (Berman et al., 2000; Oldfield and Dunker, 2014).
WIPF2 plays a key role in mediating the activity of Arp2/3. Despite being two proteins upstream of the actual mediator of actin polymerization, Arp2/3, inhibition of WIPF2 protein expression resulted in a large decrease in the internalization of conidia. This decrease was comparable to the reduction in internalization we observed after directly inhibiting Arp2/3 with a chemical inhibitor, indicating that WIPF2 plays a central and possibly rate-limiting role in this process.
In healthy individuals, conidia are removed from the airway by mucociliary action or are phagocytosed and destroyed by macrophages (Bhatia et al., 2011; Lee et al., 2016). It has been demonstrated that while macrophages are capable of killing conidia after phagocytosis and formation of the phagolysosome, conidia are able to survive and germinate within non-professional phagocytes (Wasylnka and Moore, 2003). Within immunocompromized individuals, macrophages may be less abundant or incompetent, allowing time for internalization of conidia by AECs. This would lead to germination of conidia and potentially development of disease. Further research into the role of internalization of conidia on pathogenesis will be necessary in order to test this hypothesis.
In the present study, we propose a model governing the initial stages of interaction between AECs and conidia. The role of other agents in the Arp2/3 pathway in this dual-organism interaction are unknown. Since WIPF2 was identified in our data screen which was not specific to A. fumigatus or even fungi, it is possible that this mechanism is not specific to the internalization of A. fumigatus conidia, or even any organism. Further research will be required in a number of areas, such as the kinetics and regulation of this biomechanical process, as well as determination of the generalizability of our model. Overall we provide evidence implicating a specific mechanistic pathway, the Arp2/3 pathway as being responsible for a key process in this host-pathogen system.
Data Availability Statement
The datasets analyzed for this study can be found in the NCBI SRA (https://www.ncbi.nlm.nih.gov/sra). Dual-organism microarray data may be found in the NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) under accession GSE16637.
Author Contributions
LC: performed experiments and wrote the manuscript; CC, GS, SY, and AT: provided substantial contributions to study design and acquisition of data; MM, DD, and ST: provided substantial contributions to study design. All authors contributed in the revision of the manuscript and approve of submission.
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2015-05043.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the laboratory of Dr. Dana V. Devine (UBC) for providing laboratory space and materials for SDS-PAGE and western blots. We would also like to thank Dr. Amrit Singh (UBC), Alison Hadwin (SFU), Cole Schonhofer (SFU) and Dr. Aaron Barlow (St. Paul's Hospital) for valuable discussions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00016/full#supplementary-material
Supplementary Table 1. Human genes differentially expressed in AECs after A. fumigatus exposure. Microarray data were accessed from the gene expression omnibus and analyzed for differential expression (FDR < 0.15).
Supplementary Table 2. A. fumigatus genes differentially expressed after co-incubation with AECs. Microarray data were accessed from the gene expression omnibus and analyzed for differential expression (FDR < 0.15).
Supplementary Table 3. Human genes differentially expressed in primary AECs after infection with a variety of pathogens. RNA-seq data were accessed from the NCBI SRA and processed for differential expression (FDR < 0.15).
Supplementary Table 4. Raw counts of A. fumigatus colonies after nystatin protection assay.
References
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., et al. (2000). The protein data bank. Nucleic Acids Res. 28, 235–242. doi: 10.1093/nar/28.1.235
Bhatia, S., Fei, M., Yarlagadda, M., Qi, Z., Akira, S., Saijo, S., et al. (2011). Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS ONE 6:e15943. doi: 10.1371/journal.pone.0015943
Chen, F., Zhang, C., Jia, X., Wang, S., Wang, J., Chen, Y., et al. (2015). Transcriptome profiles of human lung epithelial cells A549 interacting with Aspergillus fumigatus by RNA-Seq. PLoS ONE 10:e0135720. doi: 10.1371/journal.pone.0135720
Cozens, A. L., Yezzi, M. J., Yamaya, M., Steiger, D., Wagner, J. A., Garber, S. S., et al. (1992). A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell Dev. Biol. 28A, 735–744. doi: 10.1007/BF02631062
Croft, C. A., Culibrk, L., Moore, M., and Tebbutt, S. (2016). Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol. 7:472. doi: 10.3389/fmicb.2016.00472
Goley, E. D., and Welch, M. D. (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726. doi: 10.1038/nrm2026
Gomez, P., Hackett, T. L., Moore, M. M., Knight, D. A., and Tebbutt, S. J. (2010). Functional genomics of human bronchial epithelial cells directly interacting with conidia of Aspergillus fumigatus. BMC Genomics 11:358. doi: 10.1186/1471-2164-11-358
Gordon, S. (2016). Phagocytosis: an immunobiologic process. Immunity 44, 463–475. doi: 10.1016/j.immuni.2016.02.026
Hetrick, B., Han, M. S., Helgeson, L. A., and Nolen, B. J. (2013). Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol. 20, 701–712. doi: 10.1016/j.chembiol.2013.03.019
Kress, H., Stelzer, E. H., Holzer, D., Buss, F., Griffiths, G., and Rohrbach, A. (2007). Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. U.S.A. 104, 11633–11638. doi: 10.1073/pnas.0702449104
Lê Cao, K.-A., González, I., and Déjean, S. (2009). integrOmics: an R package to unravel relationships between two omics data sets. Bioinformatics 25, 2855–2856. doi: 10.1093/bioinformatics/btp515
Latgé, J. P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12, 310–350. doi: 10.1128/CMR.12.2.310
Law, C. W., Chen, Y., Shi, W., and Smyth, G. K. (2014). voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15:R29. doi: 10.1186/gb-2014-15-2-r29
Lee, R. J., Workman, A. D., Carey, R. M., Chen, B., Rosen, P. L., Doghramji, L., et al. (2016). Fungal aflatoxins reduce respiratory mucosal ciliary function. Sci. Rep. 6:33221. doi: 10.1038/srep33221
Oldfield, C. J., and Dunker, A. K. (2014). Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem. 83, 553–584. doi: 10.1146/annurev-biochem-072711-164947
Oosthuizen, J. L., Gomez, P., Ruan, J., Hackett, T. L., Moore, M. M., Knight, D. A., et al. (2011). Dual organism transcriptomics of airway epithelial cells interacting with conidia of Aspergillus fumigatus. PLoS ONE 6:e20527. doi: 10.1371/journal.pone.0020527
Rohart, F., Gautier, B., Singh, A., and Lê Cao, K.-A. (2017). mixOmics: an r package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13:e1005752. doi: 10.1371/journal.pcbi.1005752
Smith, G. K. (2005). “Limma: linear models for microarray data,” Bioinformatics and Computational Biology Solutions Using R and Bioconductor, eds R. Gentleman, V. J. Carey, W. Huber, R. A. Irizarry, and S. Dudoit (New York, NY: Springer), 397–420.
Takenawa, T., and Miki, H. (2001). WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell. Sci. 114(Pt 10):1801–1809.
Toor, A., Culibrk, L., Singhera, G. K., Moon, K.-M., Prudova, A., Foster, L. J., et al. (2018). Transcriptomic and proteomic host response to Aspergillus fumigatus conidia in an air-liquid interface model of human bronchial epithelium. PLoS ONE 13:e0209652. doi: 10.1371/journal.pone.0209652
Wasylnka, J. A., and Moore, M. M. (2002). Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect. Immun. 70, 3156–3163. doi: 10.1128/IAI.70.6.3156-3163.2002
Keywords: phagocytosis, fungi, aspergillus, cytoskeleton, invasion, host, airway, epithelium
Citation: Culibrk L, Croft CA, Toor A, Yang SJ, Singhera GK, Dorscheid DR, Moore MM and Tebbutt SJ (2019) Phagocytosis of Aspergillus fumigatus by Human Bronchial Epithelial Cells Is Mediated by the Arp2/3 Complex and WIPF2. Front. Cell. Infect. Microbiol. 9:16. doi: 10.3389/fcimb.2019.00016
Received: 29 October 2018; Accepted: 17 January 2019;
Published: 07 February 2019.
Edited by:
Yong-Sun Bahn, Yonsei University, South KoreaReviewed by:
Soo Chan Lee, University of Texas at San Antonio, United StatesHee-Soo Park, Kyungpook National University, South Korea
Copyright © 2019 Culibrk, Croft, Toor, Yang, Singhera, Dorscheid, Moore and Tebbutt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott J. Tebbutt, c2NvdHQudGViYnV0dEBobGkudWJjLmNh
 Luka Culibrk
Luka Culibrk Carys A. Croft
Carys A. Croft Amreen Toor
Amreen Toor S. Jasemine Yang1,2
S. Jasemine Yang1,2 Margo M. Moore
Margo M. Moore Scott J. Tebbutt
Scott J. Tebbutt