Abstract
Objectives:
Fecal microbiota transplantation (FMT) is a recommended therapy for recurrent Clostridioides difficile infection and is being investigated as a potential therapy for dozens of microbiota-mediated indications. Stool banks centralize FMT donor screening and FMT material preparation with the goal of expanding access to FMT material while simultaneously improving its safety, quality, and convenience. Although there are published consensuses on donor screening guidelines, there are few reports about the implementation of those guidelines in functioning stool banks.
Methods:
To help inform consensus standards with data gathered from real-world settings and, in turn, to improve patient care, here we describe the general methodology used in 2018 by OpenBiome, a large stool bank, and its outputs in that year.
Results:
In 2018, the stool bank received 7,536 stool donations from 210 donors, a daily average of 20.6 donations, and processed 4,271 of those donations into FMT preparations. The median time a screened and enrolled stool donor actively donated stool was 5.8 months. The median time between the manufacture of an FMT preparation and its shipment to a hospital or physician was 8.9 months. Half of the stool bank’s partner hospitals and physicians ordered an average of 0.75 or fewer FMT preparations per month.
Conclusions:
Further knowledge sharing should help inform refinements of stool banking guidelines and best practices.
Introduction
Fecal microbiota transplantation (FMT), the transfer of minimally manipulated stool and its associated microbiota from a healthy donor into the gastrointestinal tract of the patient (Khoruts and Sadowsky, 2016; Allegretti et al., 2019a), is a recommended therapy for recurrent Clostridioides difficile infection (Surawicz et al., 2013; Debast et al., 2014; McDonald et al., 2018; Mullish et al., 2018; Davidovics et al., 2019). C. difficile infection is the most common healthcare-associated infection in the United States, with 462,000 cases and 20,500 deaths in the US in 2017 (US Centers for Disease Control and Prevention., 2019; Guh et al., 2020). FMT’s reported safety profile and efficacy in preventing recurrence of C. difficile infection, approximately 80-90% (van Nood et al., 2013; Youngster et al., 2014a; Youngster et al., 2014b; Cammarota et al., 2015; Hirsch et al., 2015; Youngster et al., 2016; Allegretti et al., 2016; Kelly et al., 2016; Kao et al., 2017), has inspired research using FMT to treat a wide range of microbiota-mediated indications (Allegretti et al., 2019a; Olesen et al., 2020).
There are two main models supplying stool for FMT: patient-selected donors and stool banking (Smith et al., 2015; Costello et al., 2016; Terveer et al., 2017; Panchal et al., 2018; Kragsnaes et al., 2020; McCune et al., 2020).
Under the patient-selected donor model, the patient or their guardian identifies their own stool donor candidate. The treating physician screens the candidate and processes the donor’s stool into an FMT preparation. The donor typically donates material for only that single patient. This approach places substantial logistical burden on the physician (Bakken et al., 2013) and creates delays between the determination that FMT is indicated and the delivery of therapy. For example, if a patient’s first candidate donor fails the screen, another must be found, who may also fail the screen, all before the patient can be treated (Kim and Gluck, 2019; Kim et al., 2019). Furthermore, the patient-directed model poses certain risks. First, different practitioners may use variable screening standards, potentially exposing the patient to substandard screening. Second, the donor stool may be processed into an FMT treatment in an uncontrolled, ad hoc workspace, like a physician’s office, increasing the risk of contamination. Barriers to prompt access to FMT have also been reported as reasons for patients to seek “do-it-yourself” (DIY) FMT, which comes with significant risk to patient safety (Ekekezie et al., 2018).
Stool banks address many of the logistical limitations inherent to the patient-selected donor model. A stool bank is a centralized facility that screens donors, processes stool, stores FMT preparations, fulfills clinicians’ and researchers’ requests for those preparations, and monitors the safety and efficacy of the material (Panchal et al., 2018; Kim and Gluck, 2019). Centralized donor screening enables more rigorous and consistent safety standards, with donor qualification rates as low as 2.5% (Kassam et al., 2019). Centralized processing is more cost-efficient and controlled: qualified donors provide material that can be used to treat many patients, and a purpose-built facility allows for stringent manufacturing quality standards (Cammarota et al., 2019; Kragsnaes et al., 2020; McCune et al., 2020). Centralized distribution minimizes the delay in treating the patient, as physicians can access a well-screened, quality-assured FMT preparation delivered overnight. Centralized safety reporting may also contribute to improved FMT safety through a better understanding of the risks associated with human-derived microbial therapeutics and subsequent implementation of improved screening and manufacturing processes. Thus, stool banking aims to improve both the safety and accessibility of FMT material.
Although there are differences between stool banks, in part due to variation in FMT regulation between countries (Scheeler, 2019), stool banks generally adhere to a common-six part methodology, shown below (Smith et al., 2015; Terveer et al., 2017; Jørgensen et al., 2018; Mullish et al., 2018; Cammarota et al., 2019; Kragsnaes et al., 2020; Haifer et al., 2020; McCune et al., 2020). In some banks, these six elements are joined together by rigorous quality systems (Kragsnaes et al., 2020; McCune et al., 2020).
Donor recruitment: Encourage candidate donors to undergo evaluation
Donor evaluation: Assess whether candidate donors qualify to donate stool
Manufacturing: Process donated stool into formulations suitable for use in FMT
Health monitoring and release: Confirm donor health before releasing FMT material from quarantine
Fulfillment: Provide FMT material to clinicians and researchers, and track that material
Patient Safety: Evaluate FMT safety and quality, and respond to emerging safety and quality issues
Despite the field’s coalescence around this overall methodology, stool banking continues to evolve, delivering material that is more rigorously screened and more carefully prepared, with greater efficiency and reliability. Because consensus guidelines for stool bank operations have been strongly informed by the practice of existing stool banks (Costello et al., 2016; Cammarota et al., 2017; Cammarota et al., 2019; Haifer et al., 2020), improved sharing of different banks’ methodologies (Woodworth et al., 2017), exemplified by recent reports from English and Danish stool banks that emphasized the importance of quality management systems (Kragsnaes et al., 2020; McCune et al., 2020), should help advance consensus standards and, in turn, to improve patient care. Improved sharing will also support the development of other stool banks and invite broader participation in the improvement of stool banking methods.
Here we report on the methodology used in 2018 by OpenBiome (Cambridge, MA, USA), a large stool bank, to fulfill clinicians’ requests for FMT material to treat recurrent C. difficile infection under enforcement discretion (United States Food and Drug Administration., 2013). Since its founding in 2013, this bank has shipped more than 56,000 FMT preparations to a network of 1,250 healthcare facilities. We describe the bank’s overall methodology in 2018, the most recent complete year when this study began, and the bank’s outputs in that year.
Methods
The stool bank follows phase-appropriate current good manufacturing practices (cGMP) (US Food and Drug Administration., 2008), which includes well-defined procedures, a highly-controlled manufacturing environment, and meticulous record-keeping.
In 2018, the bank used the six-part methodology described above and outlined previously: donor recruitment, donor evaluation, manufacturing, health monitoring and release, fulfillment, and patient safety (Smith et al., 2015). This methodology does not necessarily reflect the bank’s current operation as changes are continuously being made to stool bank operations based on the latest scientific evidence and regulatory guidance. The bank fulfills requests for FMT material to treat C. difficile infection under enforcement discretion as well as requests for material for research purposes. Some procedures differ between enforcement discretion and research. For clarity, only the enforcement discretion procedures are described here.
Donor Recruitment
The bank encourages members of the public between 18 and 50 years old to enroll as stool donors using conventional press and social media campaigns. Because the bank requires that donated stool be passed and donated inside the stool bank’s facility, rather than at home, the campaigns only target the metropolitan area where the stool bank is located. Individuals interested in becoming stool donors complete an initial, online health questionnaire focused on eligibility criteria that commonly lead to a priori exclusion (Supplemental Material). Important categories used for preliminary screening include logistics (e.g., ability to donate at the stool bank at least three times per week), infectious risk (e.g., high risk travel history) (Tangden et al., 2010), and compromised microbial diversity (e.g., recent antibiotic use) (Kassam et al., 2019). Candidates who meet eligibility on the pre-screen questionnaire are invited for an on-site evaluation.
To protect the security and quality of stool donation programs, the bank does not publicize the precise inclusion and exclusion criteria for donors nor the precise methodology for donor recruitment or evaluation, described below. This policy is intended to ensure that donors or donor candidates provide truthful information to the bank, rather than answering questions in a way that ensures that they are permitted to donate stool.
Donor Evaluation
During on-site evaluation, candidates provide informed consent and sign an affidavit attesting that the health information they provide is true and complete (Paramsothy, 2015). Next, candidates complete an in-depth donor health questionnaire, which includes questions about gastrointestinal comorbidities, metabolic conditions, neuro-psychiatric comorbidities, infectious diseases, autoimmune diseases, atopy, asthma and allergies, malignancy (e.g., colorectal cancer), surgeries or other medical history, current symptoms and behaviors (e.g., bowel habit), medications (e.g., antimicrobial therapy), diet, social history, and family history. The screening criteria were previously outlined by the bank in more detail (Kassam et al., 2019). A clinical staff member then meets with the candidate to review and clarify their answers. Next, the clinical staff member measures the candidate’s body temperature, blood pressure, heart rate, respiratory rate, body mass index, and waist circumference. Finally, a supervising clinician reviews the questionnaire and vital signs.
Candidates who pass the in-person clinical assessment undergo a three-part laboratory screening: blood, stool, and nasal swab. Samples are sent to external laboratories for testing (Table 1), including tests suggested by the European FMT Workgroup guidance and international consensus recommendations (Bakken et al., 2011; Cammarota et al., 2019). Any abnormality is reviewed by the bank’s supervising clinician. Candidates who pass the laboratory screens are accepted into the stool donation program. Candidates who fail the laboratory screens are either temporarily deferred or permanently excluded from the program. For example, if any screened multi-drug resistant organism is detected, the candidate is permanently excluded as a stool donor, but a patient carrying a transient enteric pathogen may be invited to re-screen after a temporary deferral. Candidates and donors are informed of any significant incidental findings observed during the health evaluation and monitoring process and referred to the appropriate healthcare services. To protect the security and quality of the donor program, candidates are generally not informed about the reason for their deferral or exclusion other than to share significant incidental findings.
Table 1
| Clinical Assessment |
|---|
| Infectious risk factors |
| Known HIV or viral hepatitis exposures |
| High risk sexual behaviors |
| Tattoo or body piercing within previous 6 months |
| Known history of infectious disease |
| Travel history to endemic regions with a high risk acquiring infectious pathogens |
| Risk factors for multi-drug resistant organisms (MDROs) including work in clinical environment or long-term care facility, recent hospitalization, or recent discharge from a long term care facility |
| Potentially microbiota-mediated conditions and factors |
| Gastrointestinal conditions (e.g., history of inflammatory bowel disease, irritable bowel syndrome, chronic constipation, chronic diarrhea, celiac disease) |
| Atopic conditions (e.g., asthma, atopic dermatitis, eosinophilic disorders of the gastrointestinal tract) |
| Autoimmune conditions |
| Chronic pain syndromes |
| Metabolic conditions (i.e., clinician assessment of height, weight, and waist circumference) |
| Hypertension |
| Neurological conditions |
| Psychiatric conditions |
| Malignancy history |
| Surgeries/Other medical history |
| Current symptoms |
| Medications including antibiotics, antifungals, antivirals, and immunosuppressants |
| Diet |
| Family history (e.g., family history of inflammatory bowel disease or colon cancer) |
| Laboratory Testing |
| Serological Testing |
| Complete blood count with differential |
| Hepatic function panel (AST, ALT, ALP, bilirubin, albumin) |
| HIV-1/2 antigen and antibodies (fourth-generation test) |
| Hepatitis A (IgM) |
| Hepatitis B panel (HBsAg, HBsAb, HBcAb) |
| Hepatitis C (antibody) |
| Treponema pallidum (cascade with reflex to RPR) |
| Human T-lymphotropic virus I and II (antibody) |
| Strongyloides (IgG) |
| Stool Testing |
| Clostridioides difficile toxin B (PCR) |
| Enteric pathogens including Salmonella, Shigella, Campylobacter, and Vibrio (culture) |
| Shiga toxin (EIA, with reflex to E. coli O157 culture)† |
| Helicobacter pylori (EIA) |
| Ova and parasites |
| Giardia lamblia (EIA and microscopy) |
| Cryptosporidium (EIA) |
| Cyclospora and Isospora (microscopy) |
| Microsporidia (microscopy) |
| Rotavirus (EIA) |
| Norovirus (real-time PCR) |
| Adenovirus (EIA) |
| Vancomycin-resistant Enterococcus (culture) |
| Extended spectrum beta-lactamase producing Enterobacteriaceae (culture) |
| Carbapenem-resistant Enterobacteriaceae (culture) |
| Nasal Swab Culture |
| Methicillin-resistant Staphylococcus aureus (culture) |
Donor evaluation, including clinical assessment and laboratory screenings, used by the bank in 2018. This list does not reflect the bank’s current screening*.
AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; RPR, rapid plasma regain; EIA, enzyme immunoassay; PCR, polymerase chain reaction. *Screening for SARS-CoV-2 via nasopharyngeal swab PCR was implemented in March 2020. Material donated in December 2019 and onward will also be subject to a stool-based PCR test for SARS-CoV-2. †EIA for Shiga toxin was replaced by stx1/2 PCR in March 2020.
Manufacturing
Donors provide donations by visiting the stool bank’s collection facility, where they are given a stool collection kit. After passing the stool on site, donors close the container’s lid and place it in a resealable plastic bag for secondary containment. A staff member labels the donation with a donor identification number and the time of passage. The donor’s health status is re-assessed at each donation as described below. Donors are remunerated for the time and travel required to provide each processed donation.
The donation is transferred to a dedicated biosafety cabinet that is cleaned with a sporicidal agent and ethanol. A trained technician opens the container and evaluates the stool for any visible pathology (e.g., contamination, discoloration, blood, or mucus). Donations with poor consistency (Bristol stool scale outside 3-5) or visual pathology are destroyed, and clinical staff are notified of the abnormality. Donations that do not meet a minimum weight of 55 grams are not cost-effective to process and are destroyed. After visual inspection and weighing, the stool is transferred to a sterile 330 μm filter bag, diluted in a sterile, US Pharmacopeia-grade glycerol-saline solution (12.5% glycerol in 0.90% w/v NaCl in water), and fully homogenized while still in the filter bag using a paddle blender for at least 180 seconds. Fibrous material remains on one side of the filter while bacteria, small molecules, and water are pressed to the other side of the filter.
The filtrate is diluted and aliquoted depending on the final FMT preparation. Each FMT preparation is derived from a single donor; filtrates from different donors are never mixed. All donations are processed within six hours of the donor’s initial passage, and material that cannot be processed in time is destroyed (Chu et al., 2017).
The bank produces two liquid preparations (Figure 1) and one capsule preparation. The first liquid preparation is 250 mL diluted at a 10:1 ratio (i.e., approximately 22.7 g of stool per preparation) and is intended for delivery by colonoscopy, sigmoidoscopy, or enema. The second liquid preparation is 30 mL diluted at a 5:2 ratio (i.e., approximately 8.6 g of stool) and is intended for delivery by esophagogastroduodenoscopy or nasoenteric tube. Liquid FMT preparations are transferred to sterile polyethylene terephthalate bottles using sterile, disposable serological pipettes.
Figure 1
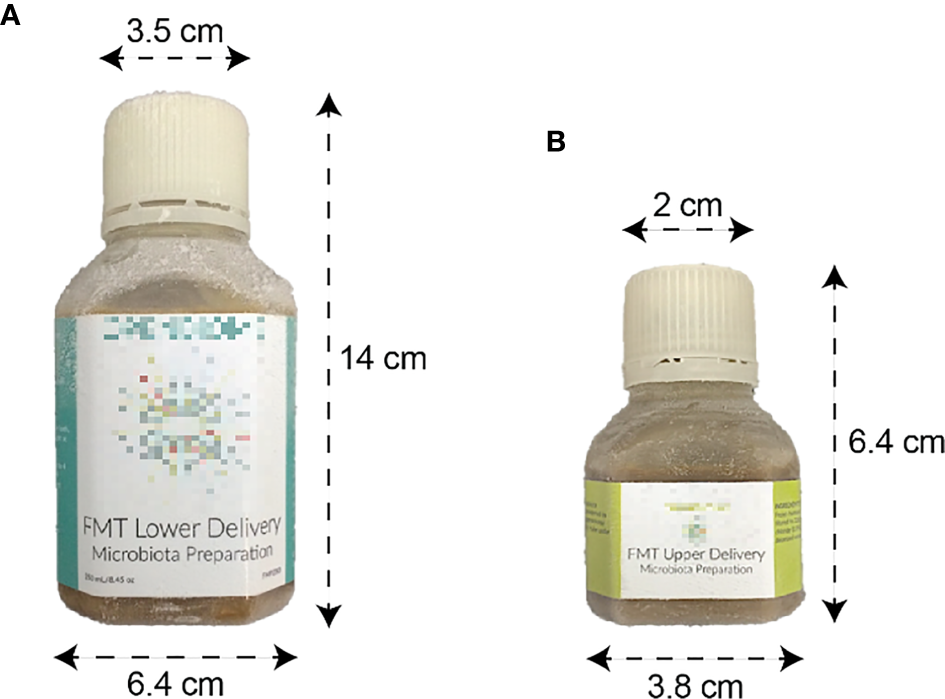
Liquid FMT preparations. (A) 250 mL preparation intended for lower delivery. (B) 30 mL preparation intended for upper delivery.
The capsule formulation is designed to be swallowed and resist degradation from stomach acid until reaching the small intestine (Stollman et al., 2015). Each capsule (size 00) consists of approximately 275 mg of stool. The stool, combined with a lipid buffer and a glycerol buffer, is contained within an inner gelatin capsule and an outer acid-resistant shell. A capsule dose consists of 30 capsules (approximately 8.25 g of stool). From each donation that is processed into an FMT preparation, a 30 mL safety aliquot is set aside for safety testing and quality purposes. Additional aliquots are retained for safety testing or research purposes. All liquid and capsule preparations are sealed with tamper-evident bottle caps and stored at –80° C.
To support traceability, each FMT preparation and aliquot is labeled with a barcode that links it to the donation. All steps in the manufacturing process are monitored and logged according to cGMP standards. Completed FMT preparations are kept in quarantine until they are determined to be free of specific risk factors, as described below.
Health Monitoring and Material Release
A donor’s health and eligibility for continued donation are continually assessed by five mechanisms.
First, donors must report travel or change in health status while actively part of the program, including fever, cough, congestion, change in bowel habit, nausea, vomiting, seasonal allergies, medical or dental procedures, bodily injury, and use of oral or topical over-the-counter medications. If an illness or travel event is reported, the donor is further evaluated by a member of the clinical staff, who determines if the donor should be temporarily deferred or permanently excluded from the stool donation program. For example, travel to certain countries entails a high risk for acquiring antibiotic-resistant bacteria and may lead to temporary deferral or permanent exclusion. Any donor who is temporarily deferred undergoes a partial or complete re-screening before being re-admitted to the program.
Second, at each stool donation, donors complete a short heath questionnaire. If the donor reports a change in health status or a clinical staff member’s direct observation of the donor leads them to suspect a change in the donor’s health status, a clinician interviews the donor to gather additional information. Clinical staff may determine that the donation should be destroyed, and the donor may be temporarily deferred or permanently excluded. If a donor is temporarily deferred because of a transient illness, the donor must pass a partial or complete re-screening before being re-admitted to the program.
Third, if a manufacturing technician observes a suspected stool pathology in the donation as described above, the technician takes a photograph of that donation, destroys the donation, and shares the photograph with the clinical staff. Clinical staff review the photograph and follow up with the donor to determine if the abnormal stool pathology is related to a risk of infection or an underlying disease.
Fourth, donors must agree to undergo random health checks. A clinician examines the donor’s vital signs and assesses the donor’s health status, with a focus on bowel habits, infectious risk factors, and new behaviors that may impact the microbiota. If the clinician detects any clinical concerns, the donor may be temporarily deferred or permanently excluded. Any donor who is temporarily deferred undergoes a partial or complete re-screening before being re-admitted to the program.
Finally, every donor repeats the complete set of clinical and laboratory assessments, the same set that they passed when first enrolling as a donor, approximately every 60 days. These re-assessments “bookend” 60-day collection periods. FMT preparations produced from stool donations during a collection period are released from quarantine only after the donor passes the second full assessment at the end of the collection period. It is not the case that every donation is screened. Instead, the assessments of a donor’s health at the beginning and end of a 60-day period and a review of all clinical data collected during the collection window are taken as sufficient evidence that the intervening donations are fit for use as FMT preparations (Kazerouni et al., 2015; Smith et al., 2015).
Individuals exposed to HIV or other pathogens may not test positive until some days after their initial infection (CDC., 2014). To account for the possibility that a donor acquires a pathogen with an extended seroconversion time, FMT material release is offset from reassessments: FMT preparations produced from stool donated less than 21 days before the reassessment are only released if the donor passes the assessment at the end of the following 60-day collection period.
Material is released from quarantine only after a full review of all clinical and laboratory data in each collection period and requires approval from two clinicians and one quality assurance staff member. All material releases are performed in accordance with cGMP standards.
If a donor newly tests positive for any infectious pathogens or other clinically significant abnormalities indicating there may be a potential underlying exclusionary medical condition, all FMT preparations produced from the corresponding collection period are destroyed. Donors who newly test positive for certain pathogens, including C. difficile as well as chronic infections like hepatitis B and HIV, are permanently excluded from the donor program and all material collected since the donor’s last screening is destroyed. In other cases, such as rotavirus, infections are transient, and donors are temporarily deferred. As stated above, temporarily deferred donors must undergo a partial or complete re-screening before being re-admitted to the program.
Fulfillment
The stool bank provides FMT preparations to gastroenterologists and infectious disease physicians treating patients with recurrent C. difficile infection not responsive to standard therapies (United States Food and Drug Administration., 2013). The bank also fulfills requests for FMT material for research purposes, but procedures for fulfillment and patient safety monitoring in that case differ. For clarity, only the enforcement discretion procedures are described here.
The stool bank’s website provides information on FMT regulations in the United States, description of FMT treatment modalities, and clinical guidance, as well as registration forms that interested facilities must complete and submit in order to receive material from the bank. Institutional shipping and billing information, as well as contact information for material control, the overseeing physician, and adverse event reporting, are collected during registration. When registered partners submit a written purchase order, FMT preparations are removed from storage at –80° C and shipped via overnight air on dry ice in Styrofoam containers. Each container is shipped with a temperature indicator verifying that the preparations remained frozen during shipping. Preparations are marked as expiring 6 months after shipping, contingent on being stored at –20° C.
Registered physicians or healthcare facilities who received stool bank material submit three kinds of information back to the stool bank. First, they must complete a log confirming that each FMT preparation was frozen upon arrival and report whether it remains in inventory, was used in treatment, or destroyed due to expiration or other reasons. Second, they are asked to complete a clinical follow-up form for each patient within 8 weeks of the FMT procedure, indicating the severity and subtype of C. difficile infection that was treated and the patient’s outcome following FMT. Finally, they are contractually required to report any serious adverse event (defined as death, life-threatening health event, hospitalization, disability or permanent damage, congenital anomaly, or other serious important medical event) to the stool bank within 24 hours of the event.
Patient Safety
When a clinician reports a serious adverse event to the bank, the bank begins an investigation. Safety staff appraise the event with respect to seriousness, severity, expectedness, and relatedness. Safety staff engage subject matter experts, including the stool bank’s clinical advisory board, which is an independent group of gastroenterologists, infectious diseases specialists, and other subject matter experts.
Barcoding, tracking, and quality assurance systems allow the bank to identify the donation and donor associated with an adverse event. If the adverse event presents a risk to other individuals who would receive material from the same donor, existing material from that donor is immediately placed in quarantine and no more is shipped until safety and quality staff have determined that it is safe to do so. If the event involves an infectious pathogen, safety and quality staff can retrieve the safety aliquot taken from that donation and test it for the presence of the pathogen. If the adverse event is linked to the FMT material, quarantined material from the donor is destroyed, material from the donor that was already shipped may be recalled, and the donor may be permanently excluded from the donation program. If the adverse event is found to be unrelated to FMT, the quarantined material will be released.
Findings from the investigation are entered into a safety database with MedDRA terminology. (MedDRA, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). Data are analyzed through an analysis of similar events to detect safety signals which may indicate a trend or safety risk. Serious adverse events considered related to the FMT are reported to the appropriate regulatory authorities through the Council for International Organizations of Medical Sciences or the MedWatch (Craigle, 2007) system. Regular reviews of safety data inform additional safety practices and risk management strategies in a continuous improvement process.
Results
Between March 2018 and July 2018, 731 candidate stool donors completed a survey asking for their motivations for donating stool. Most donors’ motivations include helping C. difficile infection patients, advancing research, and receiving the per-donation compensation (Figure 2). Donors who pass screenings and enroll in the program remain for varying amounts of time: more than half of donors who donated in 2018 were active donors for less than 6 months, but one donor who donated in 2018 had been active for 3.4 years (Figure 3).
Figure 2
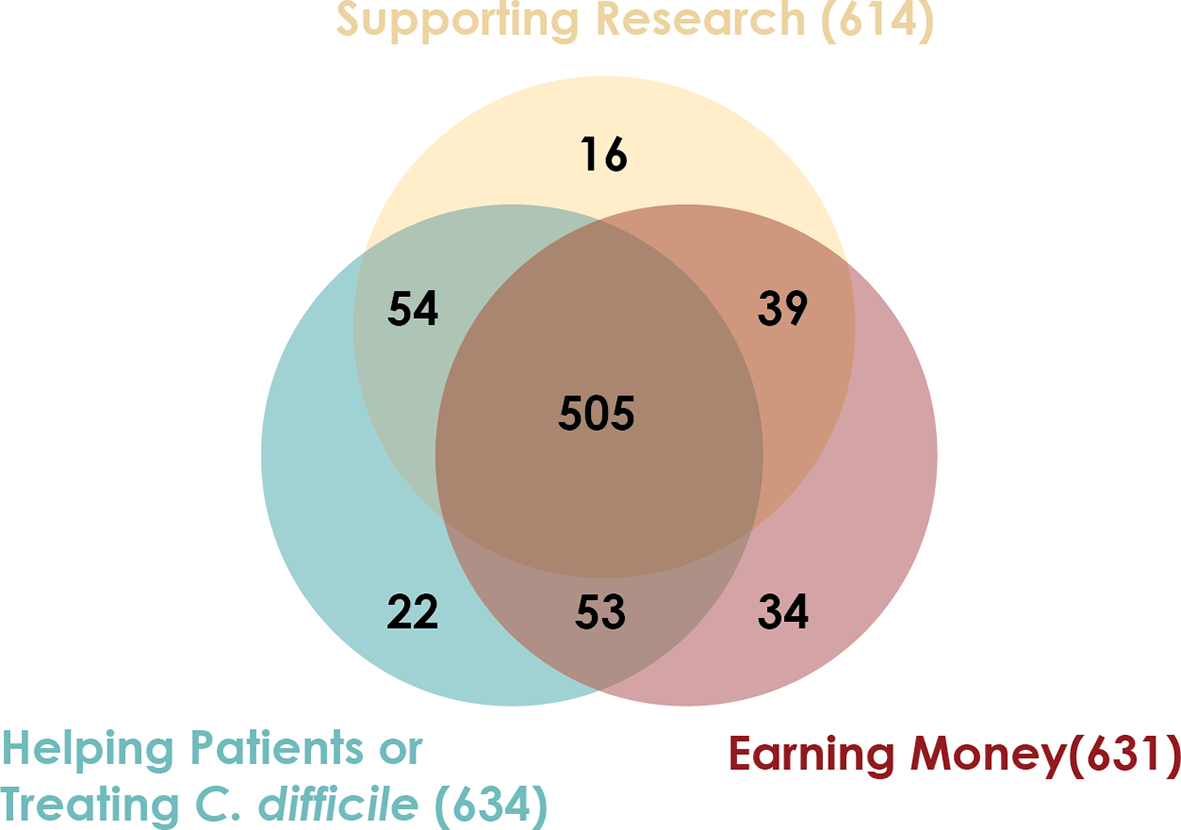
Stool donors have multiple motivations for providing stool. Between March 2018 and July 2018, 731 candidate stool donors completed a survey asking their motivations for donating stool. The survey instructed candidates to indicate all motivating factors that applied to them.
Figure 3
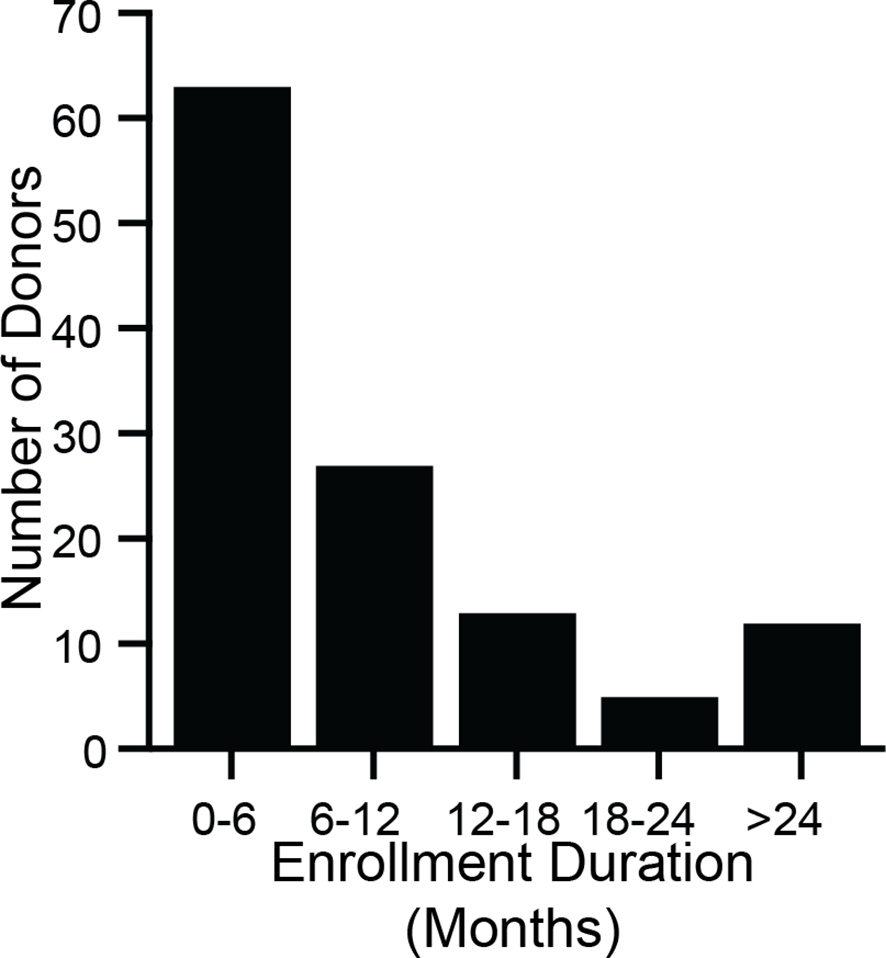
Stool donors are active over a wide range of time. During 2018, 120 donors made at least 1 donation that was processed into an FMT preparation. For each of those donors, enrollment duration was calculated as the time between a donor’s first donation (which may have taken place before 2018) and their last donation in 2018. The minimum enrollment duration was 21 days, and the maximum was 3.4 years. The median enrollment duration was 5.8 months (interquartile range 3.2 months to 12.1 months).
In 2018, the stool bank received 7,536 donations (Figure 4A) from 210 donors (Figure 4B), an average of 20.6 donations per day. 7% of donations (516/7,536) were used for stool screening purposes, to assess the donors’ health. 36% of donations (2,749/7,536) were rejected due to the donation’s low weight, poor Bristol stool score, visual stool pathology, or because the donation could not be processed within 6 hours of passage. The remaining 57% of donations (4,271/7,536) were processed into 22,068 liquid and 2,755 capsule FMT preparations (Figure 4A). Donors varied in their productivity and the proportion of their samples that were rejected or processed (Figure 4B). The time between a donation’s passage and when it was processed into an FMT formulation varied but was always less than 6 hours (Figure 4C).
Figure 4
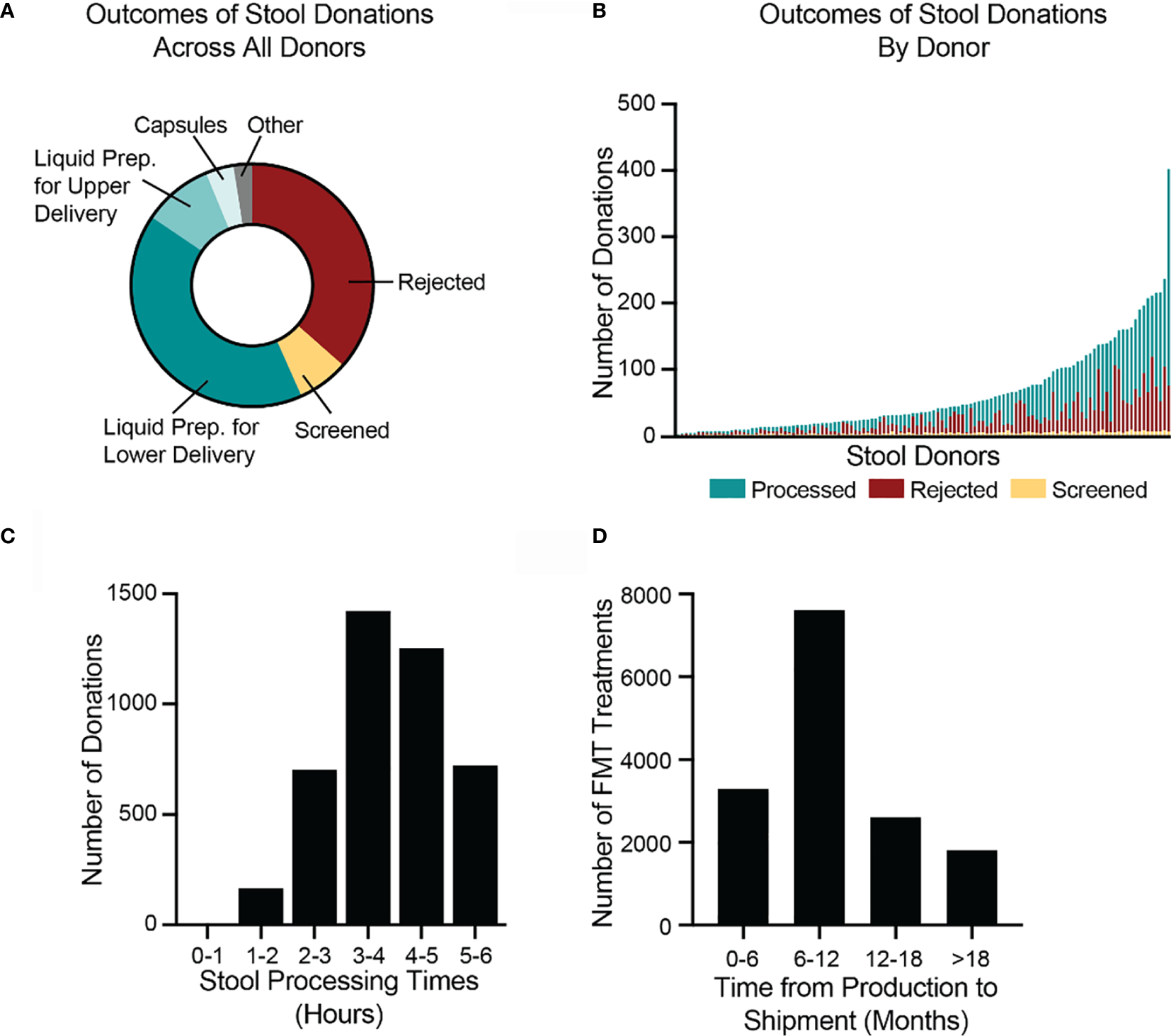
Donated stool is inspected for quality, processed into FMT treatments, and shipped to hospitals and physicians. (A) In 2018, the bank received 7,536 donations. 2,749 donations (36%) were rejected, due to visual detection of potentially pathological morphology, low weights, or failure to process the stool within six hours of passage. 516 donations (7%) were used for screening purposes to assess the health of donors. Of the remaining 4,271 donations (57%), 3,099 were processed into lower-delivery liquid preparations (e.g., for colonoscopy), 701 into upper-delivery liquid preparations (e.g., for nasoenteric delivery), 285 into capsule preparations, and 186 into other preparations. (B) During 2018, 120 stool donors provided at least 1 stool donation that was processed into an FMT preparation. Donors varied in the number of donations as well as proportion of donations that were processed, rejected, and screened. The most productive donor made 402 donations in 2018. (C) In 2018, the bank processed 4,271 stool donations into FMT preparations. All donations were processed within six hours of passage. The fastest time to processing was 47 minutes. The longest time was 5.99 hours. The median processing time was 3.9 hours (interquartile range 3.2 to 4.7 hours). (D) By 1 July 2020, the bank had shipped 15,323 of the FMT preparations produced in 2018. Preparations were shipped between 2.2 and 28 months after production. The median time to shipment was 8.9 months (interquartile range 6.3 to 13.3 months). Because not all material produced in 2018 had been shipped as of 1 July 2020, the data are skewed toward earlier shipment.
As of 1 July 2020, 23% (5,049/22,068) of the liquid FMT preparations manufactured in 2018 had been destroyed before leaving quarantine. The most frequent reasons for destruction were a failed bookend laboratory screening (47%; 2,367/5,049), a change in donor’s clinical status, such as the onset of an illness (32%; 1,616/5,049), a donor not completing a re-screen within the required window (7%; 378/5,049), and deviations during the manufacturing process (5%; 273/5,049). By 1 July 2020, the bank had shipped 62% (15,323/24,823) of the FMT preparations produced in 2018 to physicians and hospitals for clinical use to treat recurrent C. difficile infection. Due to the complex logistics of health screening and quarantine releases, very few FMT preparations are shipped less than 4 months after they are produced. The median time between production and fulfillment was 8.9 months (Figure 4D).
To quantify how different hospitals and physicians used different amounts of the bank’s material, we measured the number of FMT preparations shipped to each recipient in 2018, regardless of when those preparations were manufactured. In 2018, 12,453 preparations were shipped to 968 recipients (Figure 5). One recipient received 141 preparations, an average of 11.75 per month, but the median number of preparations was 9, or 0.75 per month. Thus, half of hospitals and physicians who received material from the bank treated an average of less than 1 patient per month with FMT using that material.
Figure 5
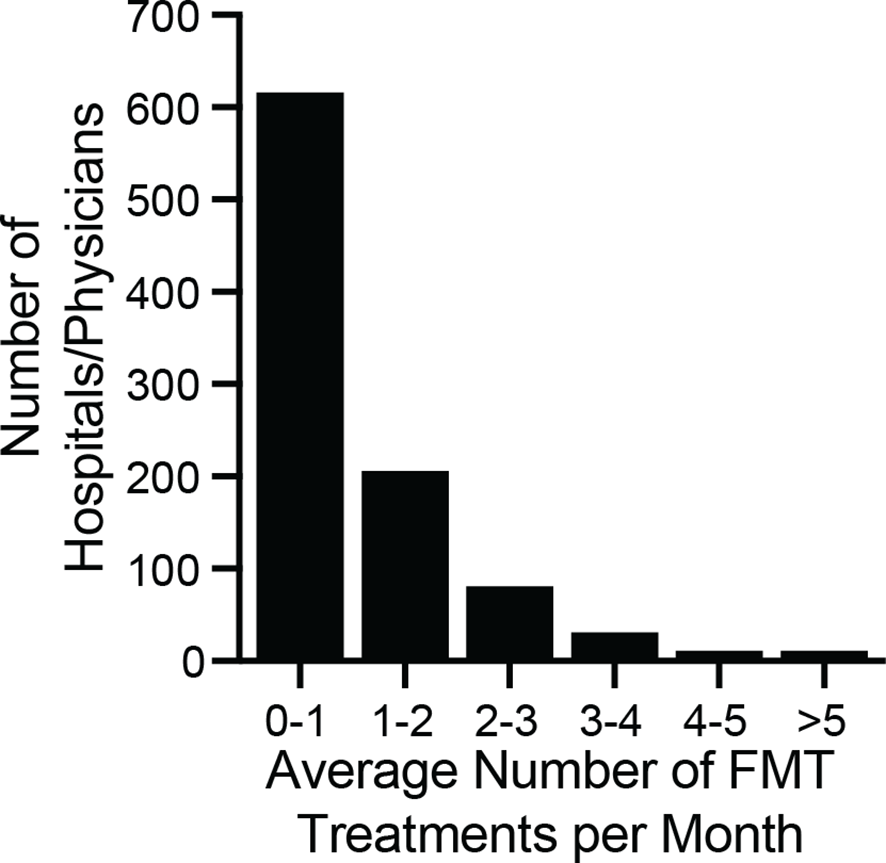
Monthly volume of preparations by physician/hospital during 2018. The number of preparations per month was computed by dividing the total number of shipped units by 12. The median number of ordered preparations across physicians/hospitals was 9 per year, or 0.75 treatments per month (interquartile range 0.25 months to 1.4 months). The maximum number of preparations ordered was 11.75 per month. The majority of physicians/hospitals ordered an average of less than 1 FMT preparation per month.
Discussion
In 2018, the stool bank’s protocol was designed principally to improve the safety and accessibility of FMT for treating C. difficile infection in a specific regulatory environment (United States Food and Drug Administration., 2013), using donors from a limited geographic location and guided by the scientific and medical understanding of FMT at the time. The protocol described here would require modification if implemented in a different regulatory environment (Scheeler, 2019; McCune et al., 2020) or geography (Paramsothy et al., 2015), or with different resource requirements. Furthermore, as the field’s understanding of the safety profile and molecular mechanisms of FMT improves, there will be further opportunities to optimize human-derived microbial therapies in general and stool banking protocols in particular. Even if the use of FMT to treat C. difficile infection ceases to fall under enforcement discretion and is instead regulated as a biological drug product, the overall methodology described here still informs best practice for stool banking.
We shared these methods to help inform consensus standards with data gathered from real-world settings, to support the advancement of similar operations, and to invite broader participation in the improvement of these methods. To help explore the thematic areas in which stool banking protocols could be improved and adapted, we lay out and discuss five scenarios about a hypothetical donor’s lifecycle.
Scenario 1: A Healthy Donor
In this scenario, the hypothetical donor passes initial screening and subsequent health checks. Donated stool is used to prepare FMT preparations, which successfully resolve patients’ C. difficile infection. No adverse events related to this donor’s material are reported, and the donor eventually leaves the program for their own reasons. Even this scenario presents multiple questions about optimal stool banking protocols.
First, the stool bank compensates donors $40 USD to remunerate them for the time and effort associated with making a usable on-site donation. Compensation should be high enough to fairly remunerate donors for their efforts. However, compensation should also avoid perverse incentivization or coercing candidate donors into sharing private health information and human biospecimens. The optimal compensation for stool donors is an open question: while whole blood donors and organ donors in the US are not compensated, plasma donors, sperm donors, and egg donors are (National Organ Transplant Act of 1984; Rodriguez del Pozo, 2008; Glauser, 2014; Farrugia et al., 2015; American Association of Blood Banks., 2019; Cammarota et al., 2019).
Second, the requirement that donations be passed on-site limits the bank’s donor pool to one metropolitan area. Although stool from any donor passing the stool bank’s screens appears equally efficacious for treating C. difficile infection (Budree et al., 2017a; Budree et al., 2017b; Budree et al., 2018; Osman et al., 2018; Olesen, 2020), the same may not hold true for other diseases. For example, it may or may not be important for donors and recipients to be geographically “matched” to ensure maximally safe and effective FMT (Yatsunenko et al., 2012; Gaulke and Sharpton, 2018; Pasolli et al., 2019).
Third, because stool from any donor in the bank’s program appears nearly equally efficacious (Olesen, 2020), the stool bank does not direct material from particular donors to particular patients except as part of specific research protocols. However, it may at some point become clear that individual donors (“super donors”) (Wilson et al., 2019) or donations with particular characteristics (“superstool”) (Olesen et al., 2018a) yield more effective FMT preparations for treating C. difficile infection or other indications. As the field’s understanding of FMT grows, the universal donor approach may require adjustment.
Fourth, the active ingredient of human-derived microbial therapeutics like FMT, although suspected to be live or viable bacteria, is not definitively known (Ott et al., 2017), which makes the optimal manufacturing protocol unclear. Current manufacturing processes, such as aerobic preparation and freezing FMT preparations, are supported by clinical evidence (Lee et al., 2016; Mendolia et al., 2019; Allegretti et al., 2020) but may require adjustment as clinical and scientific understanding of FMT grows. In particular, the optimal dosing of FMT to treat C. difficile is not well understood (Ianiro et al., 2018; Allegretti et al., 2019b). FMT preparations with smaller or larger volumes, or with proportionally more or less donor stool, may be more optimal. Furthermore, a human-derived therapeutics active ingredient may not be the same for all theorized indications (Olesen et al., 2018b). Current testing for FMT potency focuses on donations’ viable bacterial density (Carlson, 2020), but there is the possibility that future research into the variability of human stool and the correlation between those variations and the efficacy of FMT for treating various indications may reveal a more accurate predictor of FMT potency (Olesen et al., 2018a).
Fifth, even with rigorous health screenings, human-derived microbial therapeutics present risks that patients should be counseled on during the informed consent process. Screening standards used by the bank for FMT material are informed by criteria set forth by regulatory bodies, blood banks, an independent clinical advisory board, national and international stool banking consensus guidelines (Bakken et al., 2013; Cammarota et al., 2017; Mullish et al., 2018; Cammarota et al., 2019; United States Food and Drug Administration., 2019), and patient outcomes reported by partner hospitals and physicians. However, not all known pathogens are screened for, and previously unknown pathogens or other risk factors can arise in human-derived material (Ianiro et al., 2020). Thus, screening standards for human-derived microbial therapeutics require continuous re-evaluation and updating.
Finally, even if human-derived microbial therapeutic material is screened for a pathogen or condition, different test modalities may lead to different results. Ideally, a screening assay reliably determines whether a donor’s material carries the minimum infective dose of a pathogen. In practice, the fact that a donor or their material screens negative for a pathogen on an available assay does not fully negate the risk that their donated material carries that pathogen, and patients should be counseled on the risk of acquiring pathogens during the informed consent process.
Scenario 2: A Donor Fails the Initial Screen
In this scenario, the potential donor is excluded during the pre-screen survey, on-site evaluation by a clinical staff, or laboratory testing. The candidate never provides a stool sample. This scenario highlights two major areas of ongoing development.
First, just as blood donation regulations aim to reduce risk “to the lowest level reasonably achievable without unduly decreasing the availability of [blood]” (United States Food and Drug Administration., 2021), so stool donor screening procedures aim to limit the risk of FMT without unduly restricting access to FMT. However, the optimal screening battery remains an area of active development, and screening for every possible pathogen may not be the best approach. For example, given the high prevalence of prior exposure to Epstein-Barr virus (EBV) and cytomegalovirus (CMV) as well as the lack of reported patient events related to transmission of these viruses via FMT, international guidelines do not recommend that donors be excluded based on their exposure to these viruses (Cammarota et al., 2019). Instead, patients at risk of CMV or EBV infection should therefore be appropriately counseled on the risks, and alternatives to FMT should be considered. The label on the bank’s shipped material includes a disclaimer to this effect.
Second, the optimal screening battery may be different for different patient populations or geographies. As a hypothetical example, it may become clear that testing for some pathogen or risk factor is important for FMT safety, but only when used in a certain patient population, such as immunocompromised patients, thus requiring a tailored match between donor screening and patient population. This tailoring is practiced in blood banks, which provide, for example, CMV-negative blood to CMV-naïve patients. Similarly, stool banks in different geographies should determine their screening criteria based on the local burden of disease to design locally appropriate screening programs.
Scenario 3: A Donor Is Permanently Excluded From the Program
In this scenario, an active donor contracts an exclusionary infection, such as C. difficile or HIV, or is diagnosed with a disqualifying, potentially microbiota-related condition, such as hypertension or rheumatoid arthritis, for the first time. The donor is permanently excluded from donation program, and FMT preparations made from their donations since their last battery of negative screens are destroyed. The stool bank may also destroy material made before the previous negative screening.
Similar to Scenario 2, the optimal procedures for determining criteria for permanent donor exclusion are an area of active development. While HIV, hepatitis B, and hepatitis C are obvious criteria for permanent exclusion, the need to permanently exclude donors because of other infectious diseases or because of potentially microbiota-mediated conditions will continue to be refined. As the field’s understanding of the microbiota’s role in disease grows and the potential risk for transmission of microbiota-mediated diseases becomes clearer, human-derived microbial therapeutics, including FMT and stool banking procedures, will likely adapt to improve patient safety (Bibbò et al., 2017; Giles et al., 2019; Kassam et al., 2019).
Scenario 4: A Donor Is Temporarily Deferred After Contracting an Acute Infectious Disease
In this scenario, a donor self-reports, tests positive for, or is diagnosed with an acute infection, such as a viral upper respiratory infection or acute gastrointestinal illness. The donor is temporarily deferred and any material from the current stool collection period is destroyed. The deferral is maintained at least until the donor is asymptomatic, usual stool patterns have returned, and the donor passes a partial or complete re-screening, which includes tests for infectious diseases. Stool bank clinical staff use their clinical judgment to determine if the deferral should be extended to account for pathogen shedding that can continue after resolution of symptoms. Although the fecal shedding periods for some pathogens have been studied, there is no published clinical guidance about the appropriate intervals for stool donor deferrals after acute infection, making this an aspect of stool banking that requires further discussion and development.
Scenario 5: Patient Experiences an Adverse Event Following FMT
In this scenario, a clinician treating a patient with FMT material from the stool bank under enforcement discretion reports that the patient experienced an adverse event. Centralized safety reporting and surveillance may contribute to improved FMT safety through a better understanding of the risks associated with human-derived microbial therapeutics and subsequent implementation of improved screening and manufacturing processes, but there are important areas of active development with respect to FMT safety.
First, improving methods for collection, integration, and operationalization of safety data for human-derived microbial therapeutics is an important area of ongoing development. Safety data about FMT is obtained from three main sources. Clinical trials carefully track adverse events from a relatively small number of enrolled patients. Registries like the National Pediatric FMT Registry (Nicholson et al., 2020) and the American Gastroenterological Society’s FMT National Registry (Kelly et al., 2017), which aims to track 4,000 FMT patients for ten years, are critical tools in identifying and informing the mitigation of any long-term risks of FMT, an area of ongoing clinical interest (Agrawal et al., 2016). Stool banks can collect large amounts of real-world data (Osman et al., 2016) that may be more representative of relevant patient populations than data collected from clinical trials (Kelly et al., 2019), but improved reporting of adverse events to stool banks remains an important challenge for the field. Data from each of these 3 sources are valuable and complementary, and they should be used in concert to improve donor screening and manufacturing protocols for all human-derived microbial therapeutics.
Second, assessment of any potential causal relationship between FMT material and a single reported adverse event is often complicated by the patient’s comorbidities. For example, although several reports describe worsening inflammatory bowel disease flares after FMT, it has not yet been determined whether or not FMT contributed to those flares: patients may have experienced those flares even if they had not undergone FMT (Qazi et al., 2017). Controlled trials and registries remain key tools to address these questions.
Third, in the case of an adverse event caused by an infection, linking pathogens in donated stool to the infective pathogen in the patient is an area of active development. Whole genome sequencing has emerged as the leading tool to assess whether FMT transferred a pathogen (DeFilipp et al., 2019), but definitive proof of transmission or non-transmission via human-derived material is not always possible. In particular, retained stool samples from the donor and the patient are essential for definitively investigating a possible pathogen transmission but collecting patient samples, to support comparison with the donor samples retained by stool banks, may not yet be common practice at most facilities (Kragsnaes et al., 2020).
Conclusion
Optimal stool banking methods likely depend on the target patient population, evolving regulations, and emerging scientific understanding about the microbiome. Here we described and discussed the stool banking protocol used by a large stool bank in 2018 to illustrate current practice and to highlight areas for potential improvement. We hope this report is a step toward analyses that can help inform evidence-based stool banking protocols.
OpenBiome Team Members
(The “OpenBiome Team” includes current and former OpenBiome employees, members of OpenBiome’s Board of Directors, and members of OpenBiome’s independent, unpaid Clinical Advisory Board.) Jessica R. Allegretti, Kanchana Amaratunga, Daniel Blackler, Michael Bougas, Shrish Budree, Laura Burns, Jessica W. Crothers, Michael Dickens, Jacob Dixon, Nancy DuBois, Carolyn Edelstein, Michael Edmond, Ryan J. Elliott, Monika Fischer, Dinara Gabdrakhmanova, Richard Hunt, Stacy Kahn, Daelyn Kauffman, Deberly Kauffman, Colleen Kelly, Ciaran Kelly, Clara Kerwin, Kelly Ling, Daniel Martin, Gina Mendolia, Rodrigo Munoz, Kelsey O’Brien, Majdi Osman, Allison Perrotta, Sonny Qazi, Alexandra Scheeler, Monica Seng, Madeline Sovie, Neil Stollman, Zac Stoltzner, Elaine Vo, Kurt Warren, Karl Yoder, Charles Young.
Author Contributions
JC and SO conceived the project and performed the analysis. JC, AZ, BR, and SO wrote the original draft. All authors contributed to the article and approved the submitted version.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflict of interest
BR is a former employee of Finch Therapeutics. MedDRA® trademark is registered by IFPMA on behalf of ICH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.622949/full#supplementary-material
References
1
AgrawalM.AroniadisO. C.BrandtL. J.KellyC.FreemanS.SurawiczC.et al. (2016). The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J. Clin. Gastroenterol.50, 403–407. doi: 10.1097/MCG.0000000000000410
2
AllegrettiJ. R.FischerM.PapaE.ElliottR. J.KlankJ.MendoliaG.et al. (2016). Su1738 Fecal Microbiota Transplantation Delivered via Oral Capsules Achieves Microbial Engraftment Similar to Traditional Delivery Modalities: Safety, Efficacy and Engraftment Results From a Multi-Center Cluster Randomized Dose-Finding Study. Gastroenterology150, S540 doi: 10.1016/S0016-5085(16)31855-8
3
AllegrettiJ. R.MullishB. H.KellyC.FischerM. (2019a). The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet (London England)394, 420–431. doi: 10.1016/S0140-6736(19)31266-8
4
AllegrettiJ. R.FischerM.SagiS. V.BohmM. E.FaddaH. M.RanmalS. R.et al. (2019b). Fecal Microbiota Transplantation Capsules with Targeted Colonic Versus Gastric Delivery in Recurrent Clostridium difficile Infection: A Comparative Cohort Analysis of High and Lose Dose. Digest. Dis. Sci.64, 1672–1678. doi: 10.1007/s10620-018-5396-6
5
AllegrettiJ. R.ElliottR. J.LadhaA.NjengaM.WarrenK.O’BrienK.et al. (2020). Stool processing speed and storage duration do not impact the clinical effectiveness of fecal microbiota transplantation. Gut Microbes11, 1806–1808. doi: 10.1080/19490976.2020.1768777
6
American Association of Blood Banks. (2019). FAQs about blood and blood donation. Available at: https://www.aabb.org/for-donors-patients/faqs-about-blood-and-blood-donation [Accessed March 30, 2021]
7
BakkenJ. S.BorodyT.BrandtL. J.BrillJ. V.DemarcoD. C.FranzosM. A.et al. (2011). Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol.: Off. Clin. Pract. J. Am. Gastroenterol. Assoc.9, 1044–1049. doi: 10.1016/j.cgh.2011.08.014
8
BakkenJ. S.PolgreenP. M.BeekmannS. E.RiedoF. X.StreitJ. A. (2013). Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe.24, 20–24. doi: 10.1016/j.anaerobe.2013.08.007
9
BibbòS.IaniroG.GasbarriniA.CammarotaG. (2017). Fecal microbiota transplantation: Past, present and future perspectives. Minerva Gastroenterol. e Dietol.63. doi: 10.23736/S1121-421X.17.02374-1
10
BudreeS.TuE.LeithT.AllegrettiJ. R.RaoS.DayR.et al. (2017a). The Association of Stool Donor Diet on Microbial Profile and Clinical Outcomes of Fecal Microbiota Transplantation in Clostridium Difficile Infection. Gastroenterology152, S630–S631. doi: 10.1016/S0016-5085(17)32234-5
11
BudreeS.WongW. F.TuE.RaoS.AllegrettiJ. R.FischerM.et al. (2017b). Do Specific Bacteria Drive Clinical Cure in Fecal Microbiota Transplantation for Clostridium Difficile Infection?: Clinical, Microbial and Metabolomic Characterization of Universal FMT Donors. Gastroenterology152, S349. doi: 10.1016/S0016-5085(17)31428-2
12
BudreeS.OsmanM.PanchalP.ShuE.CarrellasM.KassamZ.et al. (2018). 618. Do Clinical Factors Affect Microbial Engraftment After Fecal Microbiota Transplantation in Recurrent Clostridium difficile Infection? Open Forum Infect. Dis.5, S225–6 doi: 10.1093/ofid/ofy210.625
13
CammarotaG.MasucciL.IaniroG.BibbòS.DinoiG.CostamagnaG.et al. (2015). Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther.41, 835–843 doi: 10.1111/apt.13144
14
CammarotaG.IaniroG.TilgH.Rajilić-StojanovićM.KumpP.SatokariR.et al. (2017). European consensus conference on faecal microbiota transplantation in clinical practice. Gut.66, 569–580 doi: 10.1136/gutjnl-2016-313017
15
CammarotaG.IaniroG.KellyC. R.MullishB. H.AllegrettiJ. R.KassamZ.et al. (2019). International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut68, 2111–2121. doi: 10.1136/gutjnl-2019-319548
16
CarlsonP. E. (2020). Regulatory Considerations for Fecal Microbiota Transplantation Products. Cell Host Microbe27, 173–175. doi: 10.1016/j.chom.2020.01.018
17
ChuN. D.SmithM. B.PerrottaA. R.KassamZ.AlmE. J. (2017). Profiling living bacteria informs preparation of fecal microbiota transplantations. PloS ONE.12, e0170922 doi: 10.1371/journal.pone.0170922
18
CostelloS. P.TuckerE. C.La BrooyJ.SchoemanM. N.AndrewsJ. M. (2016). Establishing a Fecal Microbiota Transplant Service for the Treatment of Clostridium difficile Infection. Clin. Infect. Dis.62, 908–914. doi: 10.1093/cid/civ994
19
CraigleV. (2007). MedWatch: The FDA Safety Information and Adverse Event Reporting Program. J. Med. Library Assoc.95, 224–225. doi: 10.3163/1536-5050.95.2.224
20
DavidovicsZ. H.MichailS.NicholsonM. R.KociolekL. K.PaiN.HansenR.et al. (2019). Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection and Other Conditions in Children. J. Pediatr. Gastroenterol. Nutr.68, 130–143. doi: 10.1097/MPG.0000000000002205
21
DebastS. B.BauerM. P.KuijperE. J. (2014). European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect.20, 1–26. doi: 10.1111/1469-0691.12418
22
DeFilippZ.BloomP. P.Torres SotoM.MansourM. K.SaterM. R. A.HuntleyM. H.et al. (2019). Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. New Engl. J. Med.381, 2043–2050. doi: 10.1056/NEJMoa1910437
23
EkekezieC.SimmonsS.PerlerB.BurhkeT.DuffC.HottB.et al. (2018). Understanding the Scope of Do-It-Yourself (DIY) Fecal Microbiota Transplant (FMT). Am. J. Gastroenterol.113, S102. doi: 10.14309/00000434-201810001-00180
24
FarrugiaA.PenrodJ.BultJ. M. (2015). The Ethics of Paid Plasma Donation: A Plea for Patient Centeredness. HEC Forum.27, 417–429 doi: 10.1007/s10730-014-9253-5
25
GaulkeC. A.SharptonT. J. (2018). The influence of ethnicity and geography on human gut microbiome composition. Nat. Med.24, 1495–6 doi: 10.1038/s41591-018-0210-8
26
GilesE. M.D’AdamoG. L.ForsterS. C. (2019). The future of faecal transplants. Nat. Rev. Microbiol.17, 719 doi: 10.1038/s41579-019-0271-9
27
GlauserW. (2014). Payment for plasma raises ethical issues. CMAJ : Can. Med. Assoc.186, E446. doi: 10.1503/cmaj.109-4855
28
GuhA. Y.MuY.WinstonL. G.JohnstonH.OlsonD.FarleyM. M.et al. (2020). Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. New Engl. J. Med.382, 1320–1330. doi: 10.1056/NEJMoa1910215
29
HaiferC.KellyC. R.ParamsothyS.AndresenD.PapanicolasL. E.McKewG. L.et al. (2020). Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut69, 801–810. doi: 10.1136/gutjnl-2019-320260
30
HirschB. E.SaraiyaN.PoethK.SchwartzR. M.EpsteinM. E.HonigG.et al. (2015). Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect. Dis.15. doi: 10.1186/s12879-015-0930-z
31
IaniroG.MaidaM.BurischJ.SimonelliC.HoldG.VentimigliaM.et al. (2018). Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. U. Eur. Gastroenterol. J.6, 1232–1244. doi: 10.1177/2050640618780762
32
IaniroG.MullishB. H.KellyC. R.SokolH.KassamZ.NgS. C.et al. (2020). Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol. Hepatol.5, 430–432. doi: 10.1016/S2468-1253(20)30082-0
33
JørgensenS. M. D.ErikstrupC.DinhK. M.LemmingL. E.DahlerupJ. F.HvasC. L.et al. (2018). Recruitment of feces donors among blood donors: Results from an observational cohort study. Gut Microbes.1–11 doi: 10.1080/19490976.2018.1458179
34
KaoD.RoachB.SilvaM.BeckP.RiouxK.KaplanG. G.et al. (2017). Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: A randomized clinical trial. JAMA318, 1985. doi: 10.1001/jama.2017.17077
35
KassamZ.DuboisN.RamakrishnaB.LingK.QaziT.SmithM.et al. (2019). Donor screening for fecal microbiota transplantation. New Engl. J. Med.381, 2070–2072. doi: 10.1056/NEJMc1913670
36
KazerouniA.BurgessJ.BurnsL. J.WeinL. M. (2015). Optimal screening and donor management in a public stool bank. Microbiome.3. doi: 10.1186/s40168-015-0140-3
37
KellyC. R.KhorutsA.StaleyC.SadowskyM. J.AbdM.AlaniM.et al. (2016). Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection a randomized trial. Ann. Internal Med.165, 609. doi: 10.7326/M16-0271
38
KellyC. R.KimA. M.LaineL.WuG. D. (2017). The AGA’s Fecal Microbiota Transplantation National Registry: An Important Step Toward Understanding Risks and Benefits of Microbiota Therapeutics. Gastroenterology.152, 681–684 doi: 10.1053/j.gastro.2017.01.028
39
KellyC. R.FischerM.GrinspanA.AllegrettiJ. R. (2019). Patients Eligible for Trials of Microbe-Based Therapeutics Do Not Represent the Population With Recurrent Clostridioides difficile Infection. Clin. Gastroenterol. Hepatol.18, 1099–1101. doi: 10.1016/j.cgh.2019.06.034
40
KhorutsA.SadowskyM. J. (2016). Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol.13, 508–516. doi: 10.1038/nrgastro.2016.98
41
KimK. O.GluckM. (2019). Fecal microbiota transplantation: An update on clinical practice. Clin. Endoscopy.53, 137–143. doi: 10.5946/CE.2019.009
42
KimK. O.SchwartzM. A.LinO. S. T.ChioreanM. V.GluckM. (2019). Reducing Cost and Complexity of Fecal Microbiota Transplantation Using Universal Donors for Recurrent Clostridium difficile Infection. Adv. Ther.36, 2052–2061. doi: 10.1007/s12325-019-00974-x
43
KragsnaesM. S.NilssonA. C.KjeldsenJ.HoltH. M.RasmussenK. F.GeorgsenJ.et al. (2020). How do I establish a stool bank for fecal microbiota transplantation within the blood- and tissue transplant service? Transfusion60, 1135–1141. doi: 10.1111/trf.15816
44
LeeC. H.SteinerT.PetrofE. O.SmiejaM.RoscoeD.NematallahA.et al. (2016). Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection. JAMA315, 142. doi: 10.1001/jama.2015.18098
45
McCuneV. L.QuraishiM. N.ManzoorS.MoranC. E.BanavathiK.SteedH.et al. (2020). Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile infection. EClinicalMedicine20, 100301. doi: 10.1016/j.eclinm.2020.100301
46
McDonaldL. C.GerdingD. N.JohnsonS.BakkenJ. S.CarrollK. C.CoffinS. E.et al. (2018). Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis.66, e1–e48. doi: 10.1093/cid/ciy149
47
MendoliaG. M.BiH. S.KassamZ.ChuN.SmithM.AlmE.et al. (2019). P1036 - anaerobic versus aerobically prepared fecal microbiota transplantation capsules: a pilot comparative cohort analysis using a novel sequencing approach. ACG Annu. Sci. Meeting Abstracts.
48
MullishB. H.QuraishiM. N.SegalJ. P.McCuneV. L.BaxterM.MarsdenG. L.et al. (2018). The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut67, 1920–1941. doi: 10.1136/gutjnl-2018-316818
49
National Organ Transplant Act, 42 U.S.C. 274e (1984).
50
NicholsonM. R.MitchellP. D.AlexanderE.BallalS.BartlettM.BeckerP.et al. (2020). Efficacy of Fecal Microbiota Transplantation for Clostridium difficile Infection in Children. Clin. Gastroenterol. Hepatol. : Off. Clin. Pract. J. Am. Gastroenterol. Assoc.18, 612–619.e1. doi: 10.1016/j.cgh.2019.04.037
51
OlesenS. W.GurryT.AlmE. J. (2018a). Designing fecal microbiota transplant trials that account for differences in donor stool efficacy. Stat. Methods Med. Res.27, 2906–2917. doi: 10.1177/0962280216688502
52
OlesenS. W.LeierM. M.AlmE. J.KahnS. A. (2018b). Searching for superstool: Maximizing the therapeutic potential of FMT. Nat. Rev. Gastroenterol. Hepatol.15, 387–388. doi: 10.1038/s41575-018-0019-4
53
OlesenS. W.PanchalP.ChenJ.BudreeS.OsmanM. (2020). Global disparities in faecal microbiota transplantation research. Lancet Gastroenterol. Hepatol.5, 241. doi: 10.1016/S2468-1253(19)30452-2
54
OlesenS. W. (2020). Fecal microbiota transplantation “donor effects” are not clinically relevant for Clostridioides difficile infection. Gastroenterology. doi: 10.1053/j.gastro.2020.12.057
55
OsmanM.O'BrienK.StoltznerZ.LingK.KoelschE.DuboisN.et al. (2016). Safety and Efficacy of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection From An International Public Stool Bank: Results From a 2050-Patient Multicenter Cohort. Open Forum Infect. Dis.3. doi: 10.1093/ofid/ofw172.1668
56
OsmanM.AbendA.PanchalP.KassamZ.BudreeS. (2018). Does the Donor Matter? Microbiome Sequencing to Evaluate Lower Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. Gastroenterology154, S–25-S-26. doi: 10.1016/S0016-5085(18)30564-X
57
OttS. J.WaetzigG. H.RehmanA.Moltzau-AndersonJ.BhartiR.GrasisJ. A.et al. (2017). Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology.152, 799–811.e7 doi: 10.1053/j.gastro.2016.11.010
58
PanchalP.BudreeS.ScheelerA.MedinaG.SengM.WongW. F.et al. (2018). Scaling Safe Access to Fecal Microbiota Transplantation: Past, Present, and Future. Curr. Gastroenterol. Rep.20, 14. doi: 10.1007/s11894-018-0619-8
59
ParamsothyS. (2015). Gastroenterologist perceptions of faecal microbiota transplantation. World J. Gastroenterol.21, 10907–10914. doi: 10.3748/wjg.v21.i38.10907
60
ParamsothyS.BorodyT. J.LinE.FinlaysonS.WalshA. J.SamuelD.et al. (2015). Donor Recruitment for Fecal Microbiota Transplantation. Inflamm. Bowel Dis.21, 1600–1606. doi: 10.1097/MIB.0000000000000405
61
PasolliE.AsnicarF.ManaraS.ZolfoM.KarcherN.ArmaniniF.et al. (2019). Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell.176, 649–662.e20 doi: 10.1016/j.cell.2019.01.001
62
QaziT.AmaratungaT.BarnesE. L.FischerM.KassamZ.AllegrettiJ. R. (2017). The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes.8, 574–588. doi: 10.1080/19490976.2017.1353848
63
Rodriguez del PozoP. (2008). Paying donors and the ethics of blood supply. J. Med. Ethics.20, 31–35. doi: 10.1136/jme.20.1.31
64
ScheelerA. (2019). Where Stool is a Drug: International Approaches to Regulating the use of Fecal Microbiota for Transplantation. J. Law Med. Ethics47, 524–540. doi: 10.1177/1073110519897729
65
SmithM. B.KassamZ.BurgessJ.PerrottaA. R.BurnsL. J.MendoliaG. M.et al. (2015). The International Public Stool Bank: A Scalable Model for Standardized Screening and Processing of Donor Stool for Fecal Microbiota Transplantation. Gastroenterology148, S–211. doi: 10.1016/S0016-5085(15)30704-6
66
StollmanN.SmithM.GiovanelliA.MendoliaG.BurnsL.DidykE.et al. (2015). Frozen Encapsulated Stool in Recurrent Clostridium difficile: Exploring the Role of Pills in the Treatment Hierarchy of Fecal Microbiota Transplant Nonresponders. Am. J. Gastroenterol.110, 600–601. doi: 10.1038/ajg.2015.81
67
SurawiczC. M.BrandtL. J.BinionD. G.AnanthakrishnanA. N.CurryS. R.GilliganP. H.et al. (2013). Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol.108, 478–498. doi: 10.1038/ajg.2013.4
68
TangdenT.CarsO.MelhusA.LowdinE. (2010). Foreign Travel Is a Major Risk Factor for Colonization with Escherichia coli Producing CTX-M-Type Extended-Spectrum -Lactamases: a Prospective Study with Swedish Volunteers. Antimicrob. Agents Chemother.54, 3564–3568. doi: 10.1128/AAC.00220-10
69
TerveerE. M.van BeurdenY. H.GoorhuisA.SeegersJ. F. M. L.BauerM. P.van NoodE.et al. (2017). How to: Establish and run a stool bank. Clin. Microbiol. Infect.23, 924–930. doi: 10.1016/j.cmi.2017.05.015
70
United States Centers for Disease Control and Prevention and Association of Public Health Laboratories. (2014). Laboratory Testing for the Diagnosis of HIV Infection Updated Recommendations. doi: 10.15620/cdc.23447
71
United States Food and Drug Administration. (2013). Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. 78(FR) 42965. Available at: https://www.federalregister.gov/d/2013-17223
72
United States Food and Drug Administration. (2021). Blood & Blood Products. Available at: https://www.fda.gov/vaccines-blood-biologics/blood-blood-products [Accessed 30 March 2021].
73
United States Food and Drug Administration. (2019). Information Pertaining to Additional Safety Protections Regarding Use of Fecal Microbiota for Transplantation – Screening and Testing of Stool Donors for Multi-drug Resistant Organisms. Available at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-pertaining-additional-safety-protections-regarding-use-fecal-microbiota-transplantation [Accessed 30 March 2021].
74
United States Food and Drug Administration. (2008). Current Good Manufacturing Practice for Phase 1 Investigational Drugs. Available at: https://www.fda.gov/media/70975/download [Accessed 30 March 2021].
75
van NoodE.VriezeA.NieuwdorpM.FuentesS.ZoetendalE. G.de VosW. M.et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. New Engl. J. Med.368, 407–415. doi: 10.1056/NEJMoa1205037
76
WilsonB. C.VatanenT.CutfieldW. S.O’SullivanJ. M. (2019). The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol.9, 2. doi: 10.3389/fcimb.2019.00002
77
WoodworthM. H.NeishE. M.MillerN. S.DhereT.BurdE. M.CarpentieriC.et al. (2017). Laboratory Testing of Donors and Stool Samples for Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. J. Clin. Microbiol.55, 1002–1010. doi: 10.1128/JCM.02327-16
78
YatsunenkoT.ReyF. E.ManaryM. J.TrehanI.Dominguez-BelloM. G.ContrerasM.et al. (2012). Human gut microbiome viewed across age and geography. Nature486, 222–227. doi: 10.1038/nature11053
79
YoungsterI.RussellG. H.PindarC.Ziv-BaranT.SaukJ.HohmannE. L.et al. (2014a). Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA312, 1772. doi: 10.1001/jama.2014.13875
80
YoungsterI.SaukJ.PindarC.WilsonR. G.KaplanJ. L.SmithM. B.et al. (2014b). Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: A randomized, open-label, controlled pilot study. Clin. Infect. Dis.58, 1515–1522. doi: 10.1093/cid/ciu135
81
YoungsterI.MahabamunugeJ.SystromH. K.SaukJ.KhaliliH.LevinJ.et al. (2016). Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med.14. doi: 10.1186/s12916-016-0680-9
Summary
Keywords
Clostridioides (Clostridium) difficile infection, microbiome, stool banks, stool donor screening, fecal microbiota transplantation
Citation
Chen J, Zaman A, Ramakrishna B and Olesen SW (2021) Stool Banking for Fecal Microbiota Transplantation: Methods and Operations at a Large Stool Bank. Front. Cell. Infect. Microbiol. 11:622949. doi: 10.3389/fcimb.2021.622949
Received
29 October 2020
Accepted
22 March 2021
Published
15 April 2021
Volume
11 - 2021
Edited by
Gianluca Ianiro, Catholic University of the Sacred Heart, Italy
Reviewed by
Stefano Bibbò, Catholic University of the Sacred Heart, Italy; Simon Mark Dahl Baunwall, Aarhus University Hospital, Denmark
Updates
Copyright
© 2021 Chen, Zaman, Ramakrishna and Olesen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott W. Olesen, solesen@openbiome.org
This article was submitted to Microbiome in Health and Disease, a section of the journal Frontiers in Cellular and Infection Microbiology
†ORCID: Justin Chen, orcid.org/0000-0003-4924-106X; Amanda Zaman, orcid.org/0000-0002-0743-1159; Bharat Ramakrishna, orcid.org/0000-0003-3395-9077; Scott W. Olesen, orcid.org/0000-0001-5400-4945
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.