- 1The Key Sericultural Laboratory of Agricultural Ministry, College of Biotechnology, Southwest University, Chongqing, China
- 2Research and Development Department, Chongqing Auleon Biological Co., Ltd., Chongqing, China
- 3College of Life Science, Changchun Sci-Tech University, Shuangyang, China
- 4College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 5Animal Health Center, Shandong New Hope Liuhe Group Co., Ltd., Qingdao, China
- 6Animal Health Center, Qingdao Jiazhi Biotechnology Co., Ltd., Qingdao, China
Echinococcosis is a zoonosis caused by the larval stage of cestode species that belong to the genus Echinococcus. The infection of hydatid in sheep is very common in China, especially in the northwestern China. Here, we conducted the first systematic review and meta-analysis of echinococcosis in sheep in China. Six databases (PubMed, ScienceDirect, Baidu Library, CNKI, Wanfang, and VIP Chinese Journal Database) were used to retrieve the literatures on echinococcosis in sheep in China from 1983 to 2020, and 74 studies. The random effects model was used in the “meta” package of the R software and the PFT was chosen for rate conversion. The research data were analyzed through subgroup analysis and univariate meta-regression analysis to reveal the factors that lead to research heterogeneity. The combined prevalence of Echinococcus in the selected period was estimated to be 30.9% (192,094/826,406). In the analysis of sampling year, the lowest positive rate was 13.9% (10,296/177,318) after 2011. The highest prevalence of Echinococcus was 51.1% (278/531) in the southwestern China. The highest infection rate in sheep was 20.1% (58,344/597,815) in the liver. The analysis based on age showed that the infection rate of elderly sheep was significantly higher than that in younger animals (P < 0.05). We also evaluated the effects of different geographic and climatic factors on the prevalence of Echinococcus in sheep. The results showed that the prevalence of Echinococcus was higher in high altitude, cold, humid, and high rainfall areas. It is necessary to carry out long-term monitoring and control of echinococcosis, cut off the infection route, and reduce the risk of infection in the high risk areas.
Introduction
Echinococcosis is a zoonosis caused by the larval stage of cestode species that belong to the genus Echinococcus (Villard et al., 2003). The disease is one of the 17 neglected tropical diseases (NTDs) recognized by the World Health Organization (Agudelo Higuita et al., 2016). Echinococcosis is a chronic infection disease in both animals and humans. This disease can take years before being noticed (Ohiolei et al., 2020b). Detection of hydatid infection is common during postmortem examination of animals and incidentally found in humans (Budke et al., 2013). Echinococcus is sometimes asymptomatic during its development stage except a cysts rupture for releasing antigenic material that causes reaction or active cysts located in certain anatomical regions (e.g., joints and eyes), and then exerts pressure on surrounding tissues, thus resulting in pain or discomfort (Budke, 2002; Kern et al., 2017).
Generally, the “Ingestion of contaminated food and water” and “direct contact/playing with dogs” are classically mentioned as the sources of human infection and are biologically plausible potential risk factors (Tamarozzi et al., 2019). Humans usually get infections from canines, the infection occurred in sheep directly reflects the endemicity degree and levels of human risk (Rashid et al., 2017). Intermediate hosts accidentally ingesting infective eggs that develop into a metacestode stage in different organs (such as liver, lung, and kidney), leading to echinococcosis. The predators can release infective eggs, which could lead to a contamination for the environment, thus threatening human health (Carmena et al., 2008; Assefa et al., 2015). Echinococcosis was identified as a limiting disease in livestock production, and the infected sheep would cause economic losses to some extent (Wahlers et al., 2012). Recent, a report showed that approximately 30 million livestock were affected by echinococcosis in China. An average of increase of echinococcosis was 7 million per year. Among all infected livestock, sheep occupied approximately 70%, and caused a total economic loss of approximately 1 billion Yuan (RMB) (Yu et al., 2008; Yang et al., 2015).
Notably, it was estimated that a total of one million disability-adjusted life years (DALYs) were caused by echinococcosis globally, out of which 0.40 million were in China (Qian et al., 2017). Additionally, echinococcosis could lead to a loss of US$ 1.92 billion globally per year, China was responsible for US$ 0.66 billion. The annual global livestock production losses associated with echinococcosis were also high, reaching US$ 2.19 billion, of which China occupied a great proportion (Budke et al., 2006; Qian et al., 2017). Sheep can provide meat, milk, and wool for human beings, thus becoming an important livestock in the world. With an increase of human population, the needs of sheep by-products have elevated worldwide (Zhu et al., 2018). China as a big sheep-raising country (Zhao and Zhang, 2019). Thus, it is necessary to estimate the prevalence of Echinococcus in sheep in China and identify potential risk factors for providing basic data for researchers. At present, there is no study on the potential risk factors of Echinococcus infection in sheep in China. Therefore, a systematic review and meta-analysis were conducted-required to determine the prevalence of Echinococcus in sheep in China and to assess potential risk factors (sampling site, region, infection organ, season, detection method, age, geographical location and climate factors, etc.).
Materials and Methods
Search Strategy and Selection Criteria
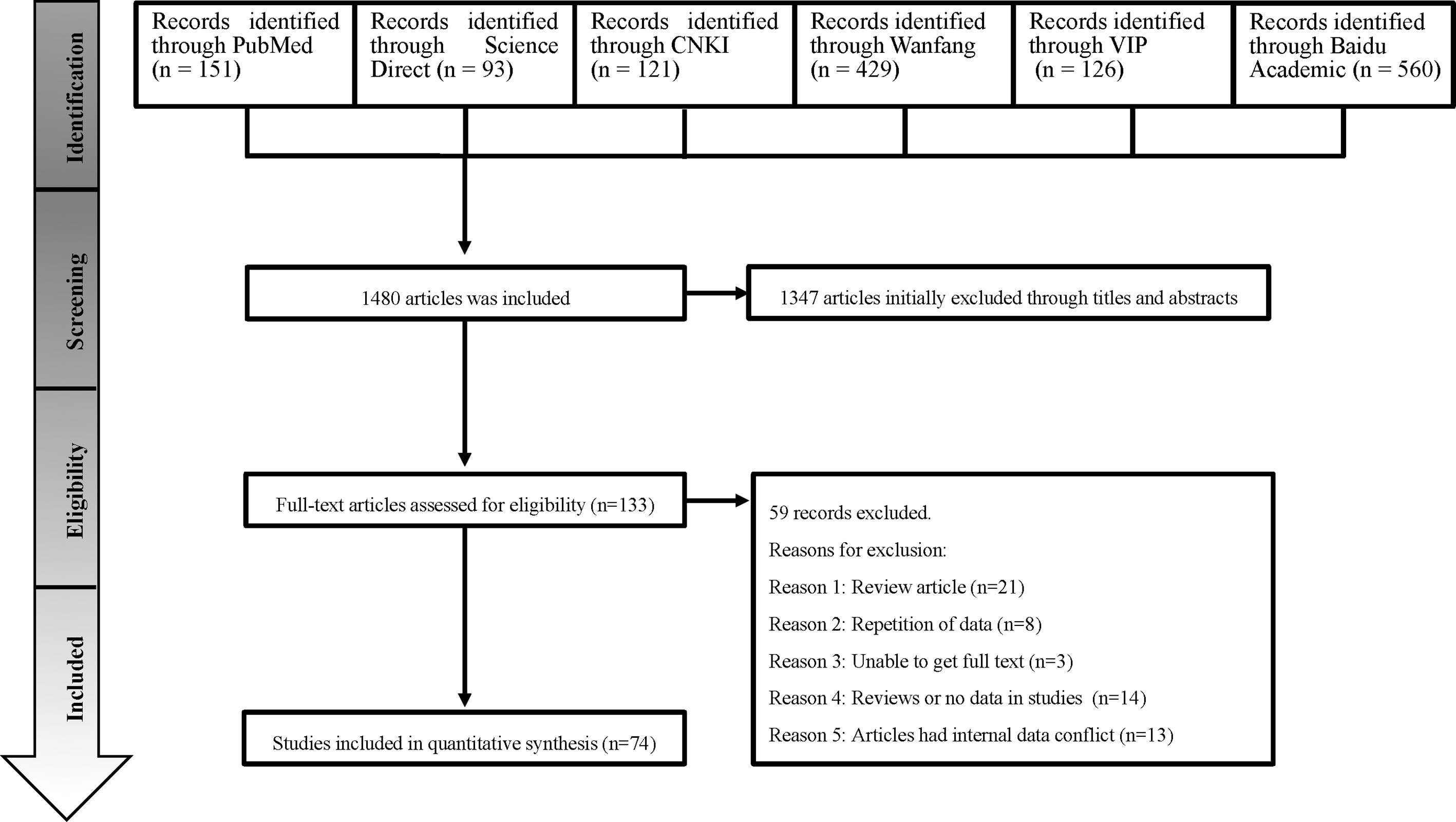
This study has been prepared according to the PRISMA guidelines for the design and analysis of selected qualified studies (Table S1) (Moher et al., 2009; Moher et al., 2015). The web of the six literature databases were employed to search for articles that related to the epidemiology of CE in sheep in China, including the China National Knowledge Infrastructure (CNKI), Baidu Library, PubMed, ScienceDirect, VIP Chinese Journal Database, and Wanfang Data. We searched all published papers with regard to CE in sheep from 1983 to December 20, 2020. We used MeSH terms “Echinococcosis”, “sheep” and “China”, as well as similar terms, such as “Echinococcoses”, “Echinococcus Infection”, “Echinococcus Infections”, “Infection, Echinococcus” “Cystic Echinocccosis”, “Cystic Echinocccoses”, “Echinocccoses, Cystic”, “Echinocccosis, Cystic”, “Hydatidosis”, “Hydatidoses”, “Cysts, Hydatid”, “Cyst, Hydatid”, “Hydatid Cysts”, “Hydatid Cyst”, “Hydatid Disease”, “Hydatid Diseases”, “Echinococcus Granulosus Infection”, “Echinococcus Granulosus Infections”, “Granulosus Infection, Echinococcus”, “Granulosus Infections, Echinococcus”, “Infection, Echinococcus Granulosus”, and “Infections, Echinococcus Granulosus”. Boolean operators “AND” and “OR” were used to connect MESH and entry terms. In PubMed, the search formula was ((((((((((((((((((((((((“Echinococcosis”[Mesh]) OR (Echinococcoses)) OR (Echinococcus Infection)) OR (Echinococcus Infections)) OR (Infection, Echinococcus)) OR (Cystic Echinocccosis)) OR (Cystic Echinocccoses)) OR (Echinocccoses, Cystic)) OR (Echinocccosis, Cystic)) OR (Hydatidosis)) OR (Hydatidoses)) OR (Cysts, Hydatid)) OR (Cyst, Hydatid)) OR (Hydatid Cysts)) OR (Hydatid Cyst)) OR (Hydatid Disease)) OR (Hydatid Diseases)) OR (Echinococcus Granulosus Infection)) OR (Echinococcus Granulosus Infections)) OR (Granulosus Infection, Echinococcus)) OR (Granulosus Infections, Echinococcus)) OR (Infection, Echinococcus Granulosus)) OR (Infections, Echinococcus Granulosus)) AND (((((((“Sheep”[Mesh]) OR (Ovis)) OR (Dall Sheep)) OR (Ovis dalli)) OR (Sheep, Dall)) OR (Sheep, Bighorn)) OR (Sheep, Domestic))) AND ((((((“China”[Mesh]) OR (People’s Republic of China)) OR (Mainland China)) OR (Manchuria)) OR (Sinkiang)) OR (Inner Mongolia)). In the Sciencedirect database, we searched for keywords sheep, Hydatid, Echinococcosis, Epidemiology, prevalence, China and the selected article type was research articles. In the four Chinese databases, “sheep” (in Chinese) and “Echinococcosis” (in Chinese) OR “sheep” (in Chinese) and “Hydatidosis” (in Chinese) were used as keywords for advanced search and were set to use synonym expansion or fuzzy search. We restricted the search to review and research articles and conference abstracts.
We adopted the following inclusion criteria: (1) the purpose of the study was to investigate the positive rate of Echinococcus in sheep; (2) the study provided the total number of sheep tested and the number of sheep that tested positive; (3) the study had a clear test method; (4) the research location was in China, and a precise sampling area was provided; (5) each sample was from one sheep and could not be mixed. Articles that did not meet these criteria were excluded. In addition, we did not contact the original authors to obtain more information, and unpublished data were not taken into account.
Data Extraction
Three researchers individually used standardized data collection forms to extract the required data for the research. If the researchers held different views or expressed uncertainty about specific articles, these would be evaluated by a fourth researcher (Y.G., the main reviewer of the meta-analysis). The database was established using Microsoft Excel (version 16.39, Microsoft Corp., Redmond, WA, USA).
The following information was recorded: the first author, the total number of sheep samples examined and the number of positive samples, the year of publication, sampling time and location, the geographic data, the test method, age, gender, season, Echinococcus infection organs, Echinococcus species, and sample type. Statistical geographic factor data were obtained from the National Meteorological Information Center of China Meteorological Administration, including longitude range, latitude range, annual average rainfall, altitude, average yearly temperature, and average yearly humidity.
Quality Assessment
The quality of the included studies was scored based on the GRADE criteria (Guyatt et al., 2008). The adopted criteria included random sampling, a precise sampling time, a clear detection method, a detailed sampling method, and an analysis containing four or more risk factors.
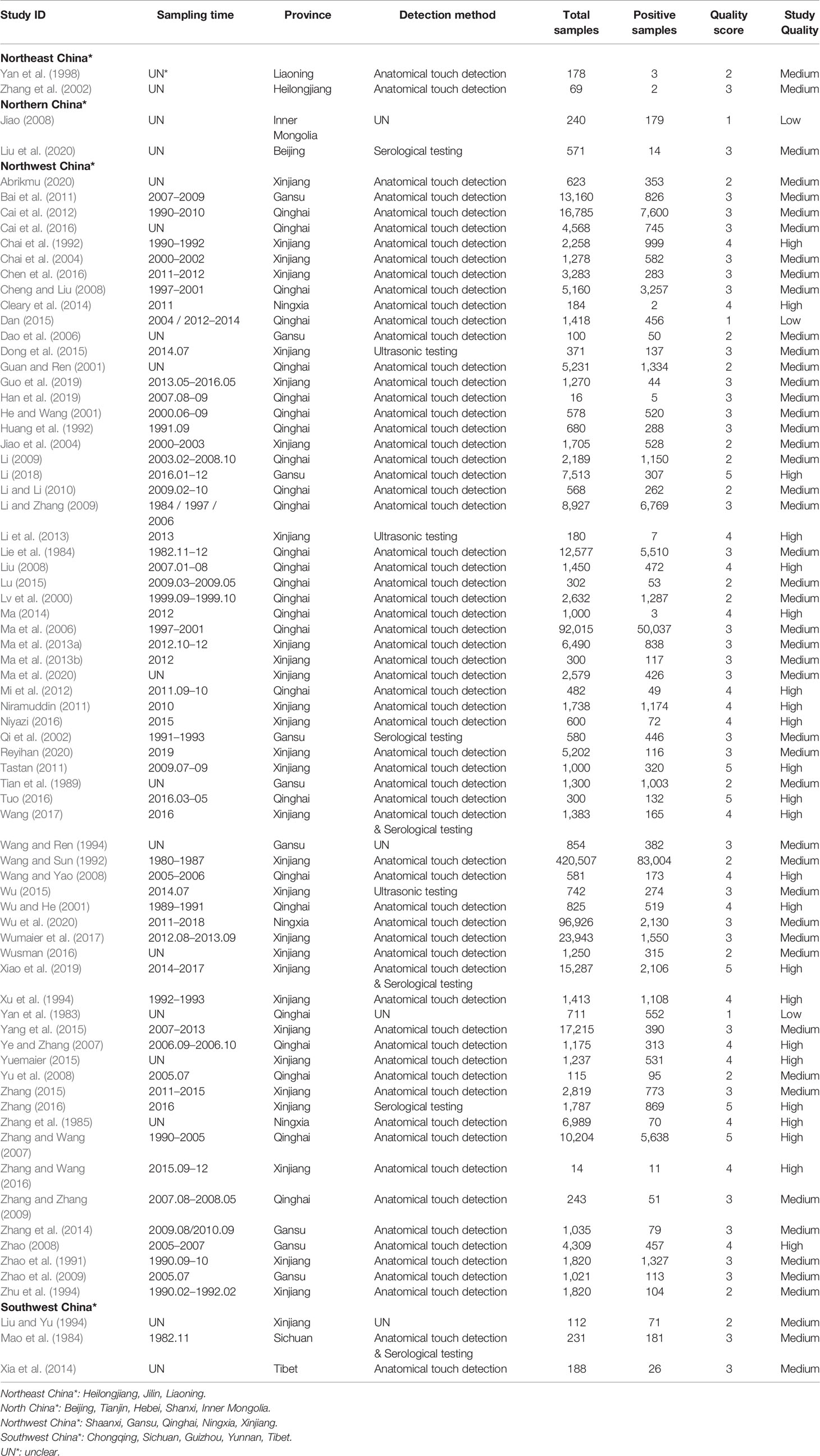
Each criterion was scored as 1 point. The total score was 5 points if a study met all mentioned criteria. Studies with 5 or 4 points were considered as high quality, studies with a score of 3 or 2 were considered as medium quality, and studies with a score of 1 or 0 were marked as low quality.
Statistical Analysis
The R Studio software version 1.2.5019 (“R core team, R: A language and environment for statistical computing” R core team 2018) was used for data analysis (using the meta package). Table S2 showed the code in R for this meta-analysis. Before conducting the meta-analysis, we tested four conversion methods to make the data closer to the Normal distribution, namely logarithmic conversion (PLN), logit transformation (PLOGIT), arcsine transformation (PAS), and double-arcsine transformation (PFT). After referring to the research of Wang et al., we chose PFT for rate conversion (Li et al., 2020; Wang et al., 2020). Due to the apparent heterogeneity of the included studies, we chose a random-effects model for meta-analysis. Forest plots were used for the overall assessment of meta-analysis. The funnel plot, trim and fill analysis, and Egger’s test were used to assess the publication bias of studies. A sensitivity analysis was conducted, and one study was deleted at a time to check whether any study would have a significant impact on the estimated results. Heterogeneity for studies was calculated by Cochran-Q, I2 statistics, and χ² test. A P-value < 0.05 and an I2 statistic with a cut-off of 50% were used to define a statistically significant degree of heterogeneity (Wei et al., 2021).
Subgroup Analysis
In order to further study the potential sources of heterogeneity, the research data subjected to subgroup analysis and univariate meta-regression analysis were used to reveal the factors that led to a research heterogeneity. The boundary division in the subgroup was based on our statistical evaluation results to divide the cut-off value. The survey factors included the year of publication (after 2011 vs. before), geographic region (northeastern China vs. other regions), age (lamb vs. other age groups), gender (ewes vs. rams), detection method (ultrasonic test vs. other methods), season (autumn vs. other three seasons), infected organs (other vs. liver, both, lung), Echinococcus species (E. granulosus vs. E. multilocularis), and study quality (low quality vs. other levels of quality).
Besides, we assessed the impact of geographic risk factors on the study, including longitude (91-100° vs. other longitude ranges), latitude (30-35° vs. other longitude ranges), average yearly precipitation (401-1,000 mm vs. other precipitation groups), average yearly temperature (-5-0°C vs. other temperature ranges), average yearly humidity (61–68% vs. other humidity value groups), and altitude (30,001-100,000 dm vs. other altitude value groups).
Results
Search Results and Eligible Studies
According to the inclusion and exclusion criteria, a total of 74 studies were used for meta-analysis by searching on six databases (Figure 1). 70 of them were from the Chinese database and 4 from the English database. Studies with 4 or 5 scores were considered as high-quality (23 studies), 2 or 3 scores as medium-quality (48 studies), and 0 or 1 score as low-quality research (3 studies; Table S3).
Publication Bias and Sensitivity Analysis
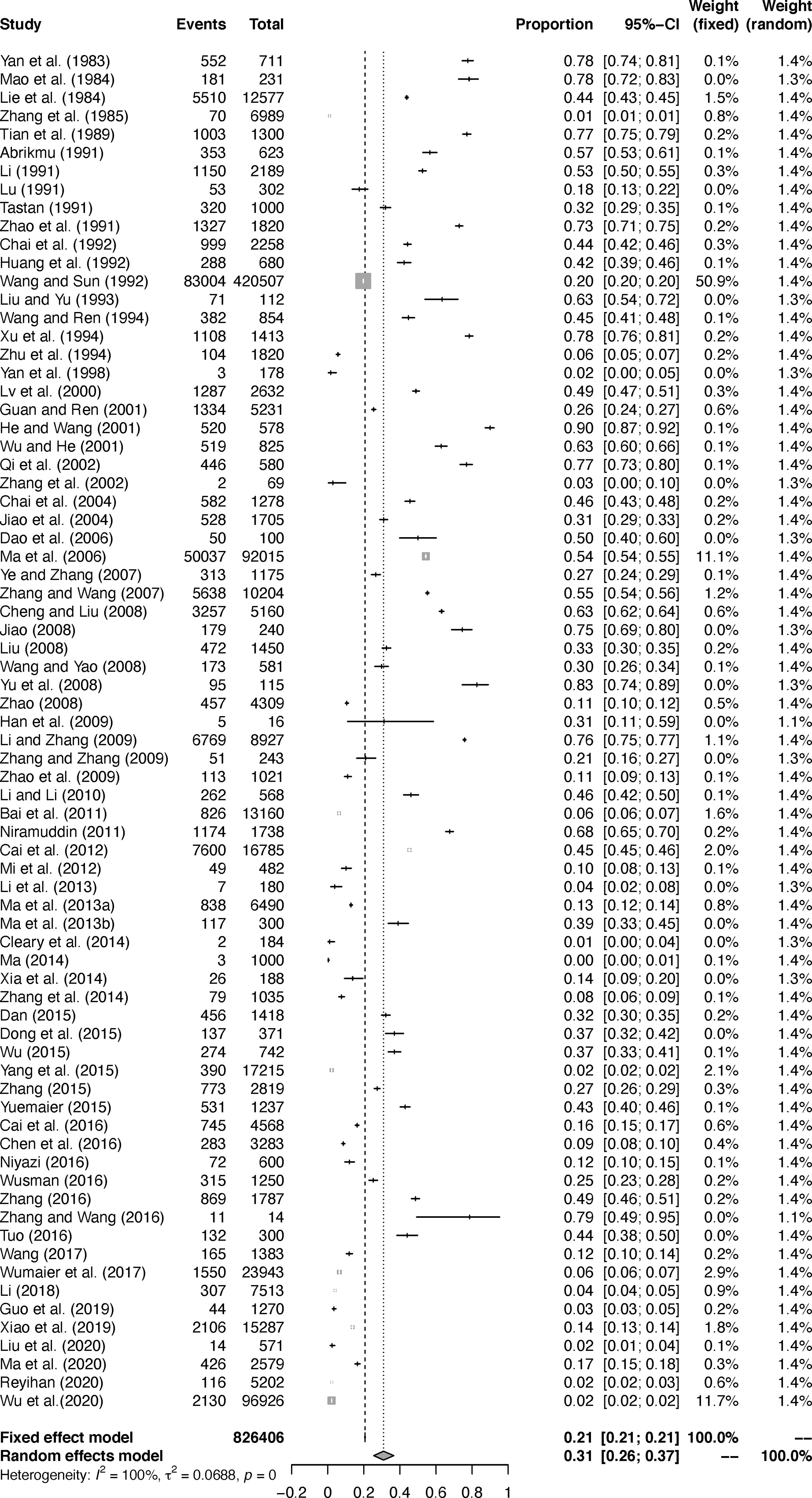
The extent of heterogeneity in the selected studies was measured and demonstrated by a forest plot (Figure 2). The funnel plot showed that the included studies might have publication biases (Figure S1). Meanwhile, the trim and fill analysis indicated a possible publication bias or a small-study effect in our study (Figure S2). However, P < 0.05 was found by an Egger’s test, manifesting that all the included studies may had publication bias (Figure S3; Table S4). According to the sensitivity tests, the combined prevalence was not significantly affected by any study that was omitted (Figure S4). These results validated that our analyses were reasonable and reliable.
Pooling and Heterogeneity Analyses
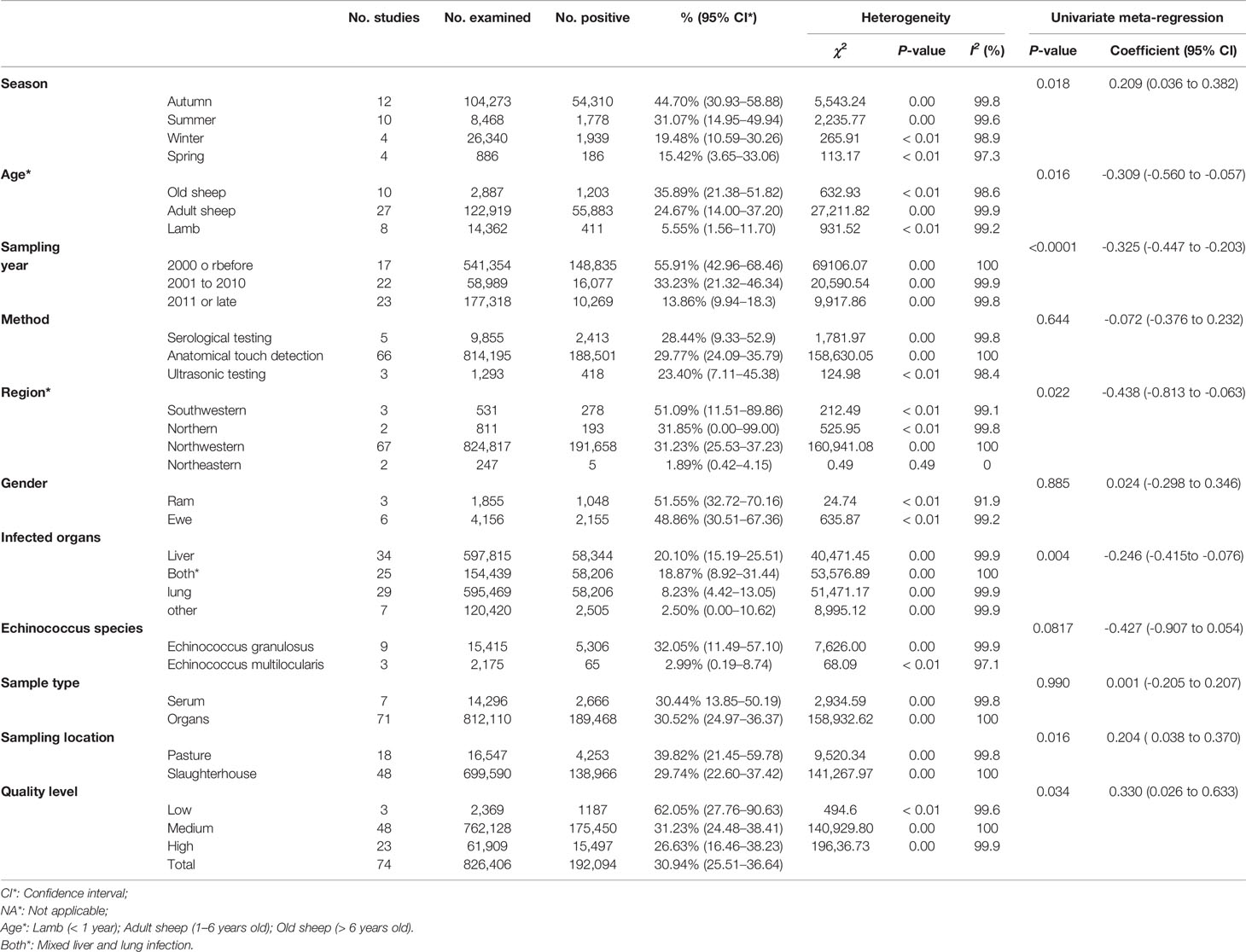
In the selected studies, ten provinces were included (Table 1; Figure 3). In the subgroup analysis, we chose “PFT” for rate conversion data (Table S5), due to the high degree of heterogeneity in most subgroups, all estimated seroprevalence for each subgroup was calculated using random effect models (Table 2).
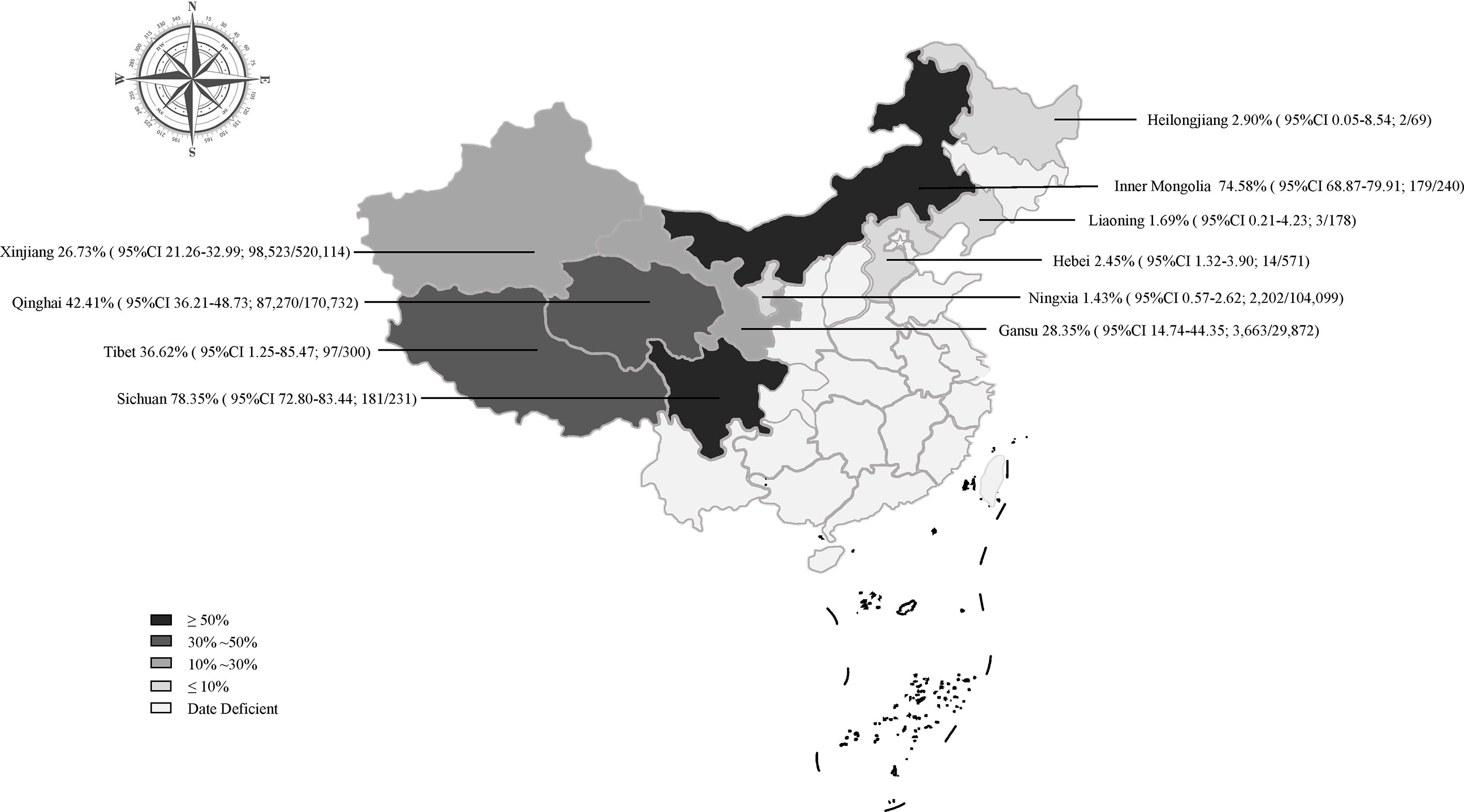
The prevalence was significantly different in different regions. The southwestern China had the highest prevalence (50.1%), and northeastern China had the lowest prevalence (1.9%). The pooled prevalence of Echinococcus in sheep ranged from 1.4% to 78.4% in different provinces (Figure 3). In the provinces, Sichuan kept the highest prevalence of 78.4%, and Ningxia was the lowest (1.4%; Figure 3).
Our findings showed that the prevalence of Echinococcus was higher in studies with sampling site from pasture (39.8%) than slaughterhouse (29.7%). Among these studies, the highest prevalence of Echinococcus based on sampling time was 55.9% in 2000 or before, and the lowest prevalence was 13.9% in 2011 or later. The highest detectable rate of Echinococcus was 20.1% in samples from liver, and the lowest was 2.5% in other. The prevalence of Echinococcus in elderly sheep was the highest (35.9%), and the lowest in young sheep (5.6%; Table 2).
Detailed geographical and climatic factors were further analyzed. The results showed that the prevalence of Echinococcus at altitude range (3,000-100,000m; 50.7%), rainfall range (401-1,000mm; 43.5%); latitude range (30-35°; 46.9%); longitude range (91-100°; 42.7%); minimum annual mean temperature range (< -5°C; 68.8%); average annual maximum temperature range (0-10°C; 53.0%); temperature range (-5-0°C; 58.7%), and humidity range (61-68; 38.5%) were higher than those in other ranges (Table S6).
Discussion
For the first time, we conducted a systematic review and meta-analysis of the infection of Echinococcus sheep in China. The statistical results show that season, age, region, infected organization, sampling location, sampling year, article quality and geographic factors (precipitation, temperature and altitude) may be risk factors for sheep infection.
According to the statistics, the combined prevalence of Echinococcus in sheep in China was 30.9% (Figure 2), which was higher than 12.1% in Africa (Ohiolei et al., 2020a) and 8.8% in Ethiopia (Asmare et al., 2016), in published meta-analysis. Thus, it requires us to pay enough attention and take certain measures to prevent the disease. In regard to the sampling year subgroup, the prevalence of Echinococcus in sheep had a significant downward trend, and the combined prevalence after 2011 was the lowest (13.9%, P < 0.05; Table 2). In 2012, in accordance with the mission objectives of the “Medium and Long-term Animal Disease Prevention and Control Plan (2012-2020)” issued by China, each region formulated a series of comprehensive prevention and control measures based on the actual situation of echinococcosis in local animals (Zhang, 2016). The measures include immunization of newborn lambs in key areas, deworming of dogs in pastoral areas, and harmless treatment of diseased animal organs in slaughterhouses, etc. The implementation of these measures has played a key role in reducing sheep infections.
Among the included articles, most of the epidemiological investigations were concentrated in the northwestern region (91%; Table 2). The northwestern region was the main breeding area for sheep in China and was also a high-epidemic area for echinococcosis (Han et al., 2019), among which Qinghai province has the highest prevalence rate (Figure 3). From the perspective of geographic environment, the altitude subgroup analysis showed a relatively high prevalence of Echinococcus among sheep in high altitude areas, such as Qinghai province (P < 0.05; Table S6). Qinghai province has a plateau continental climate with sufficient sunshine and little precipitation, and the strong wind can provide condition for the spread of insect eggs on the pasture. In addition, the land is vast and rich in animal resources. Many domestic animals and rodents can serve as natural intermediate hosts for echinococcosis, thus providing favorable condition for the spread of the disease (Wen et al., 2019). From the perspective of feeding habits, the local herders are used to raising herding dogs and herding sheep, and often feed the dogs with the organs of dead sheep, causing a large number of infections in dogs (Yang et al., 2015). After an infection, the Echinococcus eggs in the dog feces contaminate the pasture and the sheep are infected. This makes a completion of the life cycle of Echinococcus in livestock. In addition, several surveys showed that the infection rate of Echinococcus in dogs, foxes, and rodents in Qinghai province was relatively high (Cai et al., 2016), indicating that the environment in this area was highly contaminated by Echinococcus eggs. It also indicated that there was a food chain relationship among the infected animals, which forms the cycle chain of life history (Wen et al., 2019). The production tradition and geographical environment of the local herders have caused a high incidence of Echinococcus, bringing a great difficulty to the prevention and control work. It is recommended that the health department in this area strengthen the herders’ awareness of livestock breeding and disease prevention, and regularly feed dogs with anthelmintics and not feed the internal organs of animals in order to cut off the path of infection.
In the seasonal subgroup, the prevalence of Echinococcus in sheep was higher in summer and autumn, but the size of data in the subgroup was relatively small. Therefore, we combined geographical factors (temperature, humidity and precipitation subgroups) to specifically analyze the suitable living condition for Echinococcus eggs and the impact on the prevalence of Echinococcus in sheep. According to the results, the prevalence of sheep was higher in the range of 91-100° longitude and 30-35° latitude and in cold, wet, and rainy areas (Table S6). Echinococcus eggs were extremely resistant to cold and can maintain vigor in ice and snow. A Swiss study showed that a low temperature may be positively correlated with the infection rate of the intermediate hosts of Echinococcus (Burlet et al., 2011). Consistent with our research results. In winter, herders have the habit of using melted ice and snow as drinking water, making ice and snow contaminated by insect eggs the main sources of echinococcosis in humans and animals (Zhao, 2008). The eggs of Echinococcus were very sensitive to dryness and high temperature. The infection rate of multilocular echinococcosis in Slovakia was obviously positively correlated with the amount of precipitation. in addition, the seasonal changes can cause stress responses to the animal body. It will affect the prevalence of hydatid disease. For example, in Zurich, the infection rate of E. multilocularis in young foxes was highest in winter, 56.75%, and lowest in spring, 13.20% (Li, 2018). We speculate that the geographical and climatic factors may be the risk factors for hydatid infection in sheep, and it is recommended that herders in high-cold and humid areas should pay more attention to the safety of water sources.
Some studies showed that the infection of Echinococcus might be related to the age and immunity of livestock (Yang et al., 2015). Therefore, we conducted a subgroup analysis to investigate whether there was a correlation between age and sex of host and Echinococcus infection. The subgroup data showed that the highest prevalence rate of Echinococcus in elderly sheep was 35.89%, which was much higher than 5.55% of young sheep, and the infection rate was positively correlated with an increase of age (P < 0.05; Table 2). Similar results have been found in other studies, showing the prevalence of Echinococcus in animals over 5 years old was higher (Cabrera et al., 2003; Azlaf et al., 2006). It is generally believed that with the increase of age, the chance of exposure to pathogens is increased, which makes the infection rate of elderly sheep higher than that of lambs and adult sheep. In the gender subgroup, the prevalence of Echinococcus in rams was slightly higher than that of ewes, but no significant difference was observed (P = 0.88; Table 2). In the subgroup of infected organs in sheep, it was shown that the Echinococcus infection was involved in different organs, with the liver being the most susceptible organ, the highest infection rate was 21.10%. A meta-analysis in Iran showed the same results, with the highest infection rate in the liver of 55% (Mahmoudi et al., 2019). A systematic review of the literature of human cystic Echinococcus (CE) indicated that E. granulosus sensu stricto metacestodes preferentially developed in the liver (73.4%), and secondly in the lungs (19.6%), with the remainder organs including the brain, spleen, kidney, and heart (Kern et al., 2017). This result can be explained by the fact that the liver and lung were the most important body filters and were the first sites to encounter the migrating parasite larvae, and a few parasites can escape from them and gain access to other organs (Ahmadi and Badi, 2011). From the perspective of the types of hydatid, the infection rate of E. granulosus was higher than that of E. multilocularis, but only a few articles recorded the types of Echinococcus. This may not reflect the true situation.
At present, the investigation of sheep Echinococcus is still mainly based on the on-site inspection of the slaughterhouse recommended by OIE. Most of the samples (90%) tested in the study were derived from organs, and a small part (10%) of the samples were serum (Table 2). Among them, the anatomical touch method has the highest detection rate, and a small number of them used serology and ultrasound methods. Visceral hydatid cyst inspection can only be performed at the time of livestock slaughter, which has a great limitation. In contrast, the application of serological antibody detection methods is superior to traditional detection methods in sensitivity, specificity, and practicability. Commercial ELISA kits were widely used in a large-scale epidemiological investigation (Siles Lucas et al., 2017), but studies have also shown false negatives and false positives, in addition to low repetition rates (Paul and Stefaniak, 2001; Auer et al., 2009), whereas western blot results showed a better sensitivity (Liance et al., 2000). Imaging techniques are essential for diagnosis, with benefits of relatively inexpensive cost. The portable ultrasound was widely used to diagnose CE liver lesions; X-ray was used for lung cysts (Solomon et al., 2018; Tamarozzi et al., 2018). Ultrasound diagnosis of liver echinococcosis has been employed in China since 1950s. Ultrasound examination is a non-invasive, painless, reproducible, and highly accurate examination method. The diagnostic accuracy rate of ultrasound is as high as 97.2%, and it can be used for an early diagnosis and differential diagnosis of echinococcosis (Zhao, 2008). Therefore, the combination of serological, clinical, and imaging methods is the most suitable diagnostic approach for echinococcosis.
The 74 investigated studies overall were of high quality, among which 23 high-quality studies accounted for 31%, and 48 medium-quality studies accounted for 65% (Table S3). The main reason for the loss of scores in low and medium-quality research was that the sampling method was not described in detail or a random sampling. Thus, it is recommended that researchers should record and analyze the actual situation in detail when conducting epidemiological investigations and in-depth excavation, and analysis of the specific causes of sheep infection, in order to provide accurate data for the study of echinococcosis.
This study conducted a comprehensive and detailed analysis of the risk factors for the epidemiology of Echinococcus in sheep in China. However, some limitations were also present in this study. First, although we have established a comprehensive search method, omissions may still exist. Secondly, lack of data in some regions, heterogeneity among studies, and insufficient research on certain subgroups (such as the species infected with Echinococcus and the gender subgroup of sheep) may affect the results of the analysis. Despite these limitations, this report has reflected an actual prevalence of echinococcosis in sheep in China.
Conclusions
In the past three decades, the prevalence of Echinococcus in sheep in China has declined. However, the infection of Echinococcus in sheep in China is still severe, according to the published data. We comprehensively analyzed various risk factors affecting the prevalence of hydatid cysts and found that the prevalence rate was higher in high-altitude, cold, humid and rainy areas. More attention should be paid to the prevention and control of echinococcosis in the northwestern region that meets the conditions for oocyst survival and is dominated by animal husbandry. Due to a serious effect of echinococcosis on the livestock and poultry breeding industry, and a threat for human health, it is necessary to implement long-term monitoring and control measures for echinococcosis, cut off the path of infection to reduce the risk of human infection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
Y-QX, Z-GR, and QZ were responsible for the idea and concept of the paper. WW, X-YW, and YC built the database. WW and YG analyzed the data. YG wrote the manuscript. CL critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Scientific and Technological Achievements Transformation Project of Jilin Province (20170307016NY). The authors declare that this study received funding from Key Scientific and Technological Achievements Transformation Project of Jilin Province (20170307016NY). The funder had the following involvement in the study: Quan Zhao. Quan Zhao was responsible for the idea and concept of the paper.
Conflict of Interest
YG and Z-GR were employed by Chongqing Auleon Biological Co., Ltd. CL was employed by Shandong New Hope Liuhe Group Co., Ltd., and Qingdao Jiazhi Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.711332/full#supplementary-material
References
Abrikmu, Y. (2000). Investigation of Hydatid Disease Infection in Sheep Slaughtered in Zhonghuan Road Slaughterhouse in Urumqi. Rural. Sci. Technol. 7, 23. doi: 10.19777/j.cnki.issn1002-6193.2000.04.028 (In Chinese).
Agudelo Higuita, N. I., Brunetti, E., McCloskey, C. (2016). Cystic Echinococcosis. J. Clin. Microbiol. 54, 518–523. doi: 10.1128/JCM.02420-15
Ahmadi, N. A., Badi, F. (2011). Human Hydatidosis in Tehran, Iran: A Retrospective Epidemiological Study of Surgical Cases Between 1999 and 2009 at Two University Medical Centers. Trop. Biomed. 28, 450–456.
Asmare, K., Sibhat, B., Abera, M., Haile, A., Degefu, H., Fentie, T., et al. (2016). Systematic Review and Meta-Analysis of Metacestodes Prevalence in Small Ruminants in Ethiopia. Prev. Vet. Med. 129, 99–107. doi: 10.1016/j.prevetmed.2016.05.006
Assefa, H., Mulate, B., Nazir, S., Alemayehu, A. (2015). Cystic Echinococcosis Amongst Small Ruminants and Humans in Central Ethiopia. Onderstepoort J. Vet. Res. 82, E1–E7. doi: 10.4102/ojvr.v82i1.949
Auer, H., Stöckl, C., Suhendra, S., Schneider, R. (2009). Sensitivity and Specificity of New Commercial Tests for the Detection of Specific Echinococcus Antibodies. Wien. Klin. Wochenschr. 121 (Suppl), 37–41. doi: 10.1007/s00508-009-1233-4
Azlaf, R., Dakkak, A. (2006). Epidemiological Study of the Cystic Echinococcosis in Morocco. Vet. Parasitol. 137, 83–93. doi: 10.1016/j.vetpar.2006.01.003
Bai, T. J., Yang, W. D., Bai, T. X., Ma, Y. S. (2011). Investigation and Control Measures of Hydatid Infection in Sheep in Tianzhu County. Anim. Husb. Vet. Med. 61, 73–75. (In Chinese).
Budke, C. M., Carabin, H., Ndimubanzi, P. C., Nguyen, H., Rainwater, E., Dickey, M., et al. (2013). A Systematic Review of the Literature on Cystic Echinococcosis Frequency Worldwide and its Associated Clinical Manifestations. Am. J. Trop. Med. Hyg. 88, 1011–1027. doi: 10.4269/ajtmh.12-0692
Budke, C. M. (2002). WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Eckert, J., Gemmell, M.A., Meslin, F.-X., Pawlowski, Z.S. (Eds.); Office International des Epizooties, Paris, 265 pages, ISBN 92-9044-522-X (Euros 40). Vet. Parasitol. 104, 357. doi: 10.1016/S0304-4017(01)00631-8
Budke, C. M., Deplazes, P., Torgerson, P. R. (2006). Global Socioeconomic Impact of Cystic Echinococcosis. Emerg. Infect. Dis. 12, 296–303. doi: 10.3201/eid1202.050499
Burlet, P., Deplazes, P., Hegglin, D. (2011). Age, Season and Spatio-Temporal Factors Affecting the Prevalence of Echinococcus Multilocularis and Taenia Taeniaeformis in Arvicola Terrestris. Parasitol. Vectors. 4, 6. doi: 10.1186/1756-3305-4-6
Cabrera, P. A., Irabedra, P., Orlando, D., Rista, L., Harán, G., Viñals, G., et al. (2003). National Prevalence of Larval Echinococcosis in Sheep in Slaughtering Plants Ovis Aries as an Indicator in Control Programmes in Uruguay. Acta Trop. 85, 281–285. doi: 10.1016/s0001-706x(02)00214-0
Cai, H. X., Wang, H., Han, X. M., Ma, X., Liu, Y. F., Liu, P. Y., et al. (2012). Study on the Infection Status and Significance of Different Hosts of Echinococcus in Qinghai Plateau. Chin. J. Endemiol. 31, 296–300. (In Chinese).
Cai, H. X., Wang, H., Han, X. M., Ma, X., Zhang, J. X., Liu, Y. F., et al. (2016). Investigation on the Prevalence of Hydatidosis in Hainan Tibetan Autonomous Prefecture, Qinghai Province. J. Pathog. Biol. 11, 1022–1025. doi: 10.13350/j.cjpb.161115 (In Chinese).
Carmena, D., Sánchez Serrano, L. P., Barbero Martínez, I. (2008). Echinococcus Granulosus Infection in Spain. Zoonoses. Public Health 55, 156–165. doi: 10.1111/j.1863-2378.2007.01100.x
Chai, J. J., Jiao, W., Yi, S. L. Y., Chang, Q., Meng, H. B. T., Fu, C., et al. (2004). Epidemic Status of Cystic Echinococcosis in Northern Xinjiang. Trop. Dis. Parasitol. 2, 139–143. (In Chinese).
Chai, J. J., Jiao, W., Zhang, W. L., Yi, S. L. Y., Qu, Q., Li, X. L., et al. (1992). Comparative Observation on Infection Characteristics of Echinococcus Granulosus in Cattle and Sheep in Different Areas of Xinjiang. Endemic. Dis. Bull. 7, 1–7. doi: 10.13215/j.cnki.jbyfkztb.1992.s1.001 (In Chinese).
Cheng, H. P., Liu, X. R. (2008). Investigation on Infection of Two Types of Hydatid Disease in Plateau Animals in Qingnan Area. J. High Altitude Med. 18 (002), 56–58. (In Chinese).
Chen, X. Y., Setiwaldi, Y., Osman, Y. (2016). Epidemiological Studies on Echinococcosis in Kizilsu Kirgiz Autonomous Prefecture of Xinjiang. Chin. J. Parasitol. Parasitic. Dis. 34, 409–413. (In Chinese).
Cleary, E., Barnes, T. S., Xu, Y., Zhao, H., Clements, A. C., Gray, D. J., et al. (2014). Impact of “Grain to Green” Programme on Echinococcosis Infection in Ningxia Hui Autonomous Region of China. Vet. Parasitol. 205, 523–531. doi: 10.1016/j.vetpar.2014.08.023
Dan, Z. C. (2015). Analysis on Control Effect of Echinococcosis Among Livestock in Guinan County of Qinghai Province. Anim. Husb. Feed Sci. 36, 125–126. doi: 10.16003/j.cnki.issn1672-5190.2015.05.056 (In Chinese).
Dao, J. C., Ge, D., Wan, D. C. (2006). Epidemiological Investigation of Hydatid Disease in Maqu County, Gannan Prefecture. China Anim. Health 9, 34–36. (In Chinese).
Dong, J., Yang, L. F., Zhang, W. B., Li, H. T., Jiang, T., Qi, X. W., et al. (2015). Prevalence Rate of Ovine Hepatic Cystic Echinococcosis in Quaker Wusu Area of Bayinbuluke of Xinjiang 2014. Chin. J. Epidemiol. 36, 136–138. (In Chinese).
Guan, G., Ren, G. J. (2001). Investigation on the Infection of Chickenwood in Cattle and Sheep Slaughtered in Geonangbenbu. Anim. Inspect. China. 18, 33–34. (In Chinese).
Guo, B. P., Zhang, Z. Z., Zheng, X. T., Guo, Y. Z., Guo, G., Zhao, L., et al. (2019). Prevalence and Molecular Characterization of Echinococcus Granulosus Sensu Stricto in Northern Xinjiang, China. Korean. J. Parasitol. 57, 153–159. doi: 10.3347/kjp.2019.57.2.153
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck Ytter, Y., Alonso Coello, P., et al. (2008). GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336, 924–926. doi: 10.1136/bmj.39489.470347.AD
Han, X., Jian, Y., Zhang, X., Ma, L., Zhu, W., Cai, Q., et al. (2019). Genetic Characterization of Echinococcus Isolates From Various Intermediate Hosts in the Qinghai-Tibetan Plateau Area, China. Parasitology 146, 1305–1312. doi: 10.1017/S0031182019000544
Han, X. M., Wang, H., Cai, H. X., Ma, X., Liu, Y. F., Wei, B. H., et al. (2009). Epidemiological Survey on Echinococcosis in Darlag County of Qinghai Province. Chin. J. Parasitol. Parasitic. Dis. 27, 22–26. (In Chinese).
He, D. L., Wang, H. (2001). Epidemiological Evaluation Report of Hydatid Disease in Zeku County, Qinghai Province. Bull. Dis. Control. Prev. (China) 16, 36–38. doi: 10.13215/j.cnki.jbyfkztb.2001.04.015 (In Chinese).
Huang, C. G., Chao, J. T. (1992). About Heimahe and Five Other Villages’ Cattle Sheep Echinococcus Infections and Hazard Investigation. Qinghai Husb. 3, 25–26. (In Chinese).
Jiao, F. R. (2008). Investigation on Epidemic Status of Echinococcosis in Xilinguole League. Bull. Dis. Control. Prev. 2, 46–48. doi: 10.13215/j.cnki.jbyfkztb.2008.02.034 (In Chinese).
Jiao, W., Fu, C., Qu, Q., Nu, E. B. K., Xu, S. D., Sun, L. F., et al. (2004). Epidemiological Evaluation on the Efficacy of the Slowly Released Bar of Parziquantel for Dog Use in the Prevention of Cystic Echinococcosis in Human and Sheep. Chin. J. Zoonoses. 20, 557–560. (In Chinese).
Kern, P., Menezes da Silva, A., Akhan, O., Müllhaupt, B., Vizcaychipi, K. A., Budke, C., et al. (2017). The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv. Parasitol. 96, 259–369. doi: 10.1016/bs.apar.2016.09.006
Li, Q. Q. (2009). Investigation on Echinococcus Infection in Cattle and Sheep in Ulan County. Chin. Qinghai J. Anim. Vet. Sci. 39, 3. (In Chinese).
Li, J. F. (2018). Epidemiological Investigation of Sheep Echinococcosis in Yumen City. Gansu Agr. Univ. 50, 1–50. (In Chinese).
Li, Y. F., Li, D. S. (2010). Investigation on Epidemic Situation of Echinococcosis in Haiyan Area. Anim. Quarantine China 27, 38. (In Chinese).
Li, X., Ni, H. B., Ren, W. X., Jiang, J., Gong, Q. L., Zhang, X. X. (2020). Seroprevalence of Toxoplasma Gondii in Horses: A Global Systematic Review and Meta-Analysis. Acta Trop. 201, 105222. doi: 10.1016/j.actatropica.2019.105222
Li, H. T., Song, T., Duan, X. Y., Qi, X. W., Feng, X. H., Wang, Y. H., et al. (2013). Liver Echinococcosis Screening Report of Population and Flock in Xinjiang and Buxaer Mongolian Autonomous County. Chin. J. Epidemiol. 34, 1176–1178. (In Chinese).
Liance, M., Janin, V., Bresson-Hadni, S., Vuitton, D. A., Houin, R., Piarroux, R. (2000). Immunodiagnosis of Echinococcus Infections: Confirmatory Testing and Species Differentiation by a New Commercial Western Blot. J. Clin. Microbiol. 38, 3718–3721. doi: 10.1128/JCM.38.10.3718-3721.2000
Lie, T. F., Li, Q. C., Zhou, J. H., Gongbao, D. J. (1984). Investigation Report on Echinococcus Infection in Cattle and Sheep in Gande Area. Qinghai. J. Anim. Husb. Vet. Med. 19–21. (In Chinese).
Liu, Y. M. (2008). Investigation of Sheep Hydatid Infection in Chaka District. Chin. Qinghai J. Anim. Vet. Sci. 39, 34. (In Chinese).
Liu, K. Y., Guan, C., Liu, Y. H., Fang, S. F., Cui, P. (2020). Investigation on Sheep Hydatid Infection in Zhangjiakou City. Chin. Herbiv. Sci. 40, 89–90. (In Chinese).
Liu, X. T., Yu, D. J. (1994). Preliminary Investigation of Animal Echinococcosis Infection in Naqu Area. Chin. J. Parasitol. Parasiti. Dis. 77. (In Chinese).
Li, L. Z., Zhang, L. C. (2009). Investigation and Control of Echinococcosis in Tibetan Sheep. Chin. Anim. Husb. Vet. 36, 149–150. (In Chinese).
Lu, Y. (2015). Investigation and Control of Sheep Hydatid Disease in Gangcha County. Contemp. Anim. Husb. 33, 61–62. (In Chinese).
Lv, C. F., Li, X. Y., Li, S. Y., Li, X. (2000). Investigation of Hydatid Disease in Sheep in Republic Area. Chin. Qinghai J. Anim. Vet. Sci. (In Chinese) 28, 48.
Ma, Y. L. (2014). Screening and Analysis of Hydatid Disease in Hualong County of Qinghai Province in 2012. Med. Anim. Control. 30, 577. doi: 10.7629/yxdwfz201405041 (In Chinese).
Ma, L. K., Lin, H. L., Nu, S. L. T., Zuli, H. M. E., Yan, H., Ba, T. L., et al. (2013b). Comprehensive Prevention and Control Measures for Livestock Hydatid Disease in Burqin County. China Anim. Husb. Vet. Abstracts. 29, 85–86. (In Chinese).
Ma, L., Shen, X., He, G. M. (2013a). Investigation and Analysis of Echinococcosis in Sheep in Slaughterhouse in Wusu City, Xinjiang. Beijing Agr. 32, 181–182. (In Chinese).
Ma, B., Su, X. Y., Batai, M., Joe, G. (2020). Epidemiological Investigation of Sheep Hydatid Disease in Some Areas of Bazhou. Mod. J. Anim. Husb. Vet. Med. (In Chinese) 49, 45–48.
Ma, S. M., Wang, H., Li, W. M. (2006). Analysis of Hydatid Disease Data in Qingnan Area From 1997 to 2001. J. Trop. Med. 28, 55–57. (In Chinese).
Mahmoudi, S., Mamishi, S., Banar, M., Pourakbari, B., Keshavarz, H. (2019). Epidemiology of Echinococcosis in Iran: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 19 (1), 929. doi: 10.1186/s12879-019-4458-5
Mao, G. H., Weng, J. Y., Cao, Y. Q. (1984). Investigation on Echinococcosis of Yak and Tibetan Sheep in Shiqu County, Sichuan Province. J. Vet. Sci. Technol., (In Chinese) 31–32. doi: 10.16656/j.issn.1673-4696.1984.01.012
Mi, X. L., Li, D. M., Feng, S. Q., Zhou, Y. Q., Gao, J. M. (2012). Investigation of Echinococcosis in Cattle and Sheep in Menyuan County. Qinghai J. Anim. Husb. Vet. Med. 42, 31–32. (In Chinese).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS. Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 4, 1. doi: 10.1186/2046-4053-4-1
Niramuddin, A. (2011). Comparative Observation on the Infection Characteristics of Echinococcosis in Sheep and Cattle in Northern and Southern Xinjiang. Chin. J. Vet. Med. (In Chinese) 47, 81–82.
Niyazi, A. (2016). Investigation on Echinococcosis Infection of Livestock in Kuqa County, Xinjiang. Vet. Orienta. 10, 115–115. (In Chinese).
Ohiolei, J. A., Li, L., Ebhodaghe, F., Yan, H. B., Isaac, C., Bo, X. W., et al. (2020a). Prevalence and Distribution of Echinococcus Spp. In Wild and Domestic Animals Across Africa: A Systematic Review and Meta-Analysis. Transbound Emerg. Dis. 67, 2345–2364. doi: 10.1111/tbed.13571
Ohiolei, J. A., Yan, H. B., Li, L., Zhu, G. Q., Muku, R. J., Wu, Y. T., et al. (2020b). Review of Cystic Echinococcosis in Nigeria: A Story of Neglect. Acta Parasitol. 65, 1–10. doi: 10.2478/s11686-019-00124-x
Paul, M., Stefaniak, J. (2001). Comparison of the Dot Immunobinding Assay and Two Enzyme-Linked Immunosorbent Assay Kits for the Diagnosis of Liver Cystic Echinococcosis. Hepatol. Res. 21, 14–26. doi: 10.1016/s1386-6346(00)00149-2
Qian, M. B., Abela-Ridder, B., Wu, W. P., Zhou, X. N. (2017). Combating Echinococcosis in China: Strengthening the Research and Development. Infect. Dis. Poverty. 6, 161. doi: 10.1186/s40249-017-0374-3
Qi, Y. Z., Wen, Z. Q., Song, T., Wang, Y. S. (2002). Epidemiological Investigation of Hydatidosis in Tianzhu Tibetan Autonomous County, Gansu Province. Endemic. Dis. Bull. 18, 51–53. (In Chinese).
Rashid, A., Darzi, M. M., Mir, M. S., Dar, L. M., Mir, A., Kashani, S. B., et al. (2017). Prevalence of Ovine Cystic Echinococcosis in Kashmir Valley, North India. Vet. Parasitol. Reg. Stud. Rep. 10, 85–89. doi: 10.1016/j.vprsr.2017.08.007
Reyihan, Y. S. F. (2020). Molecular Epidemiological Investigation of Cattle and Sheep Echinococcosis in Parts of Xinjiang. Tarim. Univ. 1–51. (In Chinese).
Siles Lucas, M., Casulli, A., Conraths, F. J., Müller, N. (2017). Laboratory Diagnosis of Echinococcus Spp. In Human Patients and Infected Animals. Adv. Parasitol. 96, 159–257. doi: 10.1016/bs.apar.2016.09.003
Solomon, N., Zeyhle, E., Subramanian, K., Fields, P. J., Romig, T., Kern, P., et al. (2018). Cystic Echinococcosis in Turkana, Kenya: 30 Years of Imaging in an Endemic Region. Acta Trop. 178, 182–189. doi: 10.1016/j.actatropica.2017.11.006
Tamarozzi, F., Akhan, O., Cretu, C. M., Vutova, K., Akinci, D., Chipeva, R., et al. (2018). Prevalence of Abdominal Cystic Echinococcosis in Rural Bulgaria, Romania, and Turkey: A Cross-Sectional, Ultrasound-Based, Population Study From the HERACLES Project. Lancet Infect. Dis. 18, 769–778. doi: 10.1016/S1473-3099(18)30221-4
Tamarozzi, F., Akhan, O., Cretu, C. M., Vutova, K., Fabiani, M., Orsten, S., et al. (2019). Epidemiological Factors Associated With Human Cystic Echinococcosis: A Semi-Structured Questionnaire From a Large Population-Based Ultrasound Cross-Sectional Study in Eastern Europe and Turkey. Parasitol. Vectors. 12, 371. doi: 10.1186/s13071-019-3634-1
Tastan, A. (2011). Investigation and Research on the Infection Situation of Cystic Echinococcosis in Sheep in the Surrounding Area of Yining. Xinjiang Agr. Univ. 1–40. (In Chinese).
Tian, G. F., Li, Z. H., Liu, J. F., Jia, W. Z., Ye, P. Z., Ming, R. J., et al. (1989). Epidemiological Investigation of Hydatid Disease in Huangcheng Sheep Farm in Gansu Province. Chin. Vet. Sci., 13–16. doi: 10.16656/j.issn.1673-4696.1989.11.008 (In Chinese).
Tuo, J. Z. (2016). Investigation and Control of Hydatid Disease in Sheep. Chin. Anim. Husb. Vet. Digest. 32, 121. (In Chinese).
Villard, O., Filisetti, D., Roch-Deries, F., Garweg, J., Flament, J., Candolfi, E. (2003). Comparison of Enzyme-Linked Immunosorbent Assay, Immunoblotting, and PCR for Diagnosis of Toxoplasmic Chorioretinitis. J. Clin. Microbiol. 41, 3537–3541. doi: 10.1128/jcm.41.8.3537-3541.2003
Wahlers, K., Menezes, C. N., Wong, M. L., Zeyhle, E., Ahmed, M. E., Ocaido, M., et al. (2012). Cystic Echinococcosis in Sub-Saharan Africa. Lancet Infect. Dis. 12, 871–880. doi: 10.1016/S1473-3099(12)70155-X
Wang, L. M. (2017). Epidemiological Investigation of Echinococcus Infection and It’s Influences on Sheep in Different Terrains in Akesu Prefecture. Tarim. Univ. 1–43. (In Chinese).
Wang, W., Gong, Q. L., Zeng, A., Li, M. H., Zhao, Q., Ni, H. B. (2020). Prevalence of Cryptosporidium in Pigs in China: A Systematic Review and Meta-Analysis. Transbound Emerg. Dis. 68 (3), 1400–1413. doi: 10.1111/tbed.13806
Wang, J. G., Ren, D. Y. (1994). Investigation on Hydatid Infection of Cattle and Sheep in Xiahe County, Gansu Province. Chin. Vet. Sci. Technol., 17–18. doi: 10.16656/j.issn.1673-4696.1994.12.008 (In Chinese).
Wang, F. Y., Sun, H. S. (1992). Investigation and Analysis of Sheep Echinococcosis in Hami Area. Meat. Hyg. 9, 12–13. (In Chinese).
Wang, H. G., Yao, H. R. (2008). Investigation on Echinococcus Infection of Sheep in Zhaba Town of Hualong County. Chin. Qinghai J. Anima. Vet. Sci. 38, 44. (In Chinese).
Wei, X. Y., Gong, Q. L., Zeng, A., Wang, W., Wang, Q., Zhang, X. X. (2021). Seroprevalence and Risk Factors of Toxoplasma Gondii Infection in Goats in China From 2010 to 2020: A Systematic Review and Meta-Analysis. Prev. Vet. Med. 186:105230. doi: 10.1016/j.prevetmed.2020.105230
Wen, H., Vuitton, L., Tuxun, T., Li, J., Vuitton, D. A., Zhang, W., et al. (2019). Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 32, e00075–e00018. doi: 10.1128/CMR.00075-18
Wu, A. M. (2015). Survey on the Prevalence of Hepatic Cystic Hydatid Disease in Sheep in Emin County, Xinjiang in 2014. Psychol. Doctor. 21, 240–241. (In Chinese).
Wu, X. L., Duan, H. J., Qi, R. T., Yan, F., Fu, Y. R., Ma, T. B. (2020). Evaluation of the Effect of the Integrated Echinococcosis Control Program in Ningxia Hui Autonomous Region From 2011 to 2018. Chin. J. Schistosomiasis. Control. 32, 598–604. doi: 10.16250/j.32.1374.2020227 (In Chinese).
Wu, X. H., He, D. L. (2001). An Epidemiological Investigation on Hydatid Disease in Gonghe County, Qinghai Province. Bull. Dis. Control. Prev. 32, 29–31. doi: 10.13215/j.cnki.jbyfkztb.2001.01.012(InChinese
Wumaier, M., Usman, I., Simayi, A., Hou, Y. Y., Xiao, N. (2017). Investigation and Analysis of Animal Echinococcus Infection in Xinjiang Uygur Autonomous Region. Chin. J. Parasitol. Parasitic. Dis. 35, 145–149.
Wusman, A. (2016). Detection and Epidemiological Analysis of Aspidistra Spinosa in Livestock Slaughterhouse of Kuqa County. Chin. Anim. Husb. Vet. Abstracts. 111. (In Chinese).
Xia, C. Y., Liu, J. Z., Tsering, D., Yuan, Z. J., Gesang, D., Ban, D., et al. (2014). Investigation of Three Finds of Tapeworm Larvae Infections in Tibetan Domestic Animals. Chin. Vet. Sci. 44, 1205–1209. doi: 10.16656/j.issn.1673-4696.2014.11.017 (In Chinese).
Xiao, G. L., Zhong, Q., Xie, W. H., Wang, X. H. (2019). Epidemiological Survey of Sheep Hydatidosis in Kashi Area of Xinjiang From 2014 to 2017. Chin. Anim. Health Inspection. 36, 1–5. (In Chinese).
Xu, X. P., Li, X. J., Zhu, H. C., Tao, Y., Li, Y. X. (1994). Investigation on Current Situation of Hydatid Disease in Shihezi Area. Shihezi Technol., 64–65. (In Chinese).
Yang, S., Wu, W., Tian, T., Zhao, J., Chen, K., Wang, Q., et al. (2015). Prevalence of Cystic Echinococcosis in Slaughtered Sheep as an Indicator to Assess Control Progress in Emin County, Xinjiang, China. Korean. J. Parasitol. 53, 355–359. doi: 10.3347/kjp.2015.53.3.355
Yan, Q. L., Wei, M. L., Fan, C. B., Cai, D., Shi, Y. (1983). Investigation Report on Echinococcosis Infection of Cattle and Sheep in Huangnan Prefecture of Qinghai Province. Chin. Qinghai J. Anim. Vet. Sci. 4, 34–36. (In Chinese).
Yan, H. C., Zhou, Z. S., Liu, X. G., Du, H. (1998). Investigation on Parasite Infection in Sheep Lungs. Meat. Hyg. 21, 6. (In Chinese).
Ye, W. X., Zhang, X. G. (2007). Investigation of Hydatid Infection in Sheep in Minhe County. Chin. Qinghai J. Anim. Vet. Sci. 27. (In Chinese).
Yuemaier, T. (2015). Investigation on the Infection Status of Domestic Animal Hydatid Disease in Wushi County, Xinjiang. Vet. Guide. 000, 153–153. (In Chinese).
Yu, S. H., Wang, H., Wu, X. H., Ma, X., Liu, P. Y., Liu, Y. F., et al. (2008). Cystic and Alveolar Echinococcosis: An Epidemiological Survey in a Tibetan Population in Southeast Qinghai, China. Jpn. J. Infect. Dis. 61, 242–246.
Zhang, X. (2015). Demonstration and Application of Hydatid Detection and Comprehensive Prevention and Control Measures for Hydatid Disease. Xinjiang Agr. Univ. 1–49. (In Chinese).
Zhang, R. (2016). Detection of Individual Prevalence of Sheep Hydatid Disease in Wusu City, Xinjiang. Xinjiang. Agr. Univ. 1–41. (In Chinese).
Zhang, H. W., Ding, Y. L., Qiao, T. S. (2002). A Survey on the Parasite System of Sheep in Shuangcheng City, Heilongjiang Province. Chin. Vet. Sci. 32, 15–16. (In Chinese).
Zhang, J. X., Wang, H. (2007). Epidemiological Survey on Echinococcus Infection in Animals in Qinghai Province. Chin. J. Parasitol. Parasitic. Dis. 24, 350–352. doi: 10.16795/j.cnki.xjxmy.2016.07.008 (In Chinese).
Zhang, L. Y., Wang, X. F. (2016). Investigation and Research on Endoparasites of Cattle and Sheep in Tuergate Port Area. Xinjiang Anim. Husb. 7, 28–30. (In Chinese).
Zhang, K. F., Wu, T. J., Zhao, Z. Y. (2014). Analysis of Surveillance Results of Hydatid Disease in Minle County From 2009 to 2010. Fifth Cross-Strait (Qinghai) Featured Agr. Industrialization Forum. 18, 258–260. (In Chinese).
Zhang, F., Xu, W. P., Wang, H. P., Zhang, Q. H., Yan, R. L. (1985). Preliminary Investigation on the Epidemiology of Polycephalytaeniasis in Ningxia. Ningxia Agri Sci. Technol., 35–36. (In Chinese).
Zhang, H. C., Zhang, Y. Q. (2009). Investigation of Hydatid Infection in Dogs and Sheep in Zhugu Township, Menyuan County. Anim. Husbandry. Vet. Med. 41, 109. (In Chinese).
Zhao, Y. M. (2008). Epidemiological Study on Hydatid Disease in the Eastern Section of the Qinghai-Tibet Plateau (Gannan Tibetan Autonomous Prefecture). Gansu. Agr. Univ. 1–98. (In Chinese).
Zhao, H. J., An, H. H., Wu, D. X., Ro, Z. G. L., Ka, H., Shen, Y., et al. (1991). Investigation of Sheep Hydatid Disease in Yiwu County. Xinjiang Husb. 7, 15–17. doi: 10.16795/j.cnki.xjxmy.1991.05.004 (In Chinese).
Zhao, Y. M., Tong, S. X., Jing, T., Zhong, S. G., Cai, X. P., Jing, Z. Z., et al. (2009). Investigation on Echinococcosis in Animals in Gannan Tibetan Autonomous Prefecture. Chin. J. Parasitol. Parasitic. Dis. 27, 27–30. (In Chinese).
Zhao, Z. D., Zhang, L. (2019). Applications of Genome Selection in Sheep Breeding. Yi Chuan. 41, 293–303. doi: 10.16288/j.yczz.18-251
Zhu, G. L., Guo, X. H., Liu, Z. Q., Wu, Q. W., Li, L. F. (1994). Investigation on Hydatid Disease of Sheep in Southern Xinjiang Reclamation Area. Heilongjiang. Anim. Husb. Vet., 26–27. (In Chinese).
Keywords: Echinococcus, sheep, Echinococcosis, meta-analysis, China
Citation: Gao Y, Wang W, Lyu C, Wei X-Y, Chen Y, Zhao Q, Ran Z-G and Xia Y-Q (2021) Meta-Analysis of the Prevalence of Echinococcus in Sheep in China From 1983 to 2020. Front. Cell. Infect. Microbiol. 11:711332. doi: 10.3389/fcimb.2021.711332
Received: 18 May 2021; Accepted: 05 July 2021;
Published: 26 July 2021.
Edited by:
Wei Cong, Shandong University, Weihai, ChinaReviewed by:
Jin Lei Wang, Lanzhou Veterinary Institute (CAAS), ChinaMohammad Amin Ghatee, Yasuj University of Medical Sciences, Iran
Copyright © 2021 Gao, Wang, Lyu, Wei, Chen, Zhao, Ran and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Zhao, emhhb3F1YW4wODI1QDE2My5jb20=; Zhi-Guang Ran, emhncmFuQHNpbmEuY29t; You-Qing Xia, eGlhcXlAc3dhdS5jcS5jbg==
†These authors have contributed equally to this work
 Yang Gao
Yang Gao Wei Wang
Wei Wang Chuang Lyu5,6†
Chuang Lyu5,6† Quan Zhao
Quan Zhao