- 1Department of Biomedical Sciences, Institute of Tropical Medicine, Antwerp, Belgium
- 2Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium
- 3Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium
Leishmania aethiopica is a zoonotic Old World parasite transmitted by Phlebotomine sand flies and causing cutaneous leishmaniasis in Ethiopia and Kenya. Despite a range of clinical manifestations and a high prevalence of treatment failure, L. aethiopica is one of the most neglected species of the Leishmania genus in terms of scientific attention. Here, we explored the genome diversity of L. aethiopica by analyzing the genomes of twenty isolates from Ethiopia. Phylogenomic analyses identified two strains as interspecific hybrids involving L. aethiopica as one parent and L. donovani and L. tropica respectively as the other parent. High levels of genome-wide heterozygosity suggest that these two hybrids are equivalent to F1 progeny that propagated mitotically since the initial hybridization event. Analyses of allelic read depths further revealed that the L. aethiopica - L. tropica hybrid was diploid and the L. aethiopica - L. donovani hybrid was triploid, as has been described for other interspecific Leishmania hybrids. When focusing on L. aethiopica, we show that this species is genetically highly diverse and consists of both asexually evolving strains and groups of recombining parasites. A remarkable observation is that some L. aethiopica strains showed an extensive loss of heterozygosity across large regions of the nuclear genome, which likely arose from gene conversion/mitotic recombination. Hence, our prospection of L. aethiopica genomics revealed new insights into the genomic consequences of both meiotic and mitotic recombination in Leishmania.
1 Introduction
Leishmania is a vector-borne parasite causing leishmaniasis in humans and animals. Depending on the Leishmania species, the disease can present itself in various clinical representations, ranging from cutaneous to visceral leishmaniasis. Leishmania aethiopica is an Old World species transmitted by sand flies of the Phlebotomus genus, mainly P. longipes (Lemma et al., 1969) and P. pedifer (Ashford et al., 1973), and is known to cause local (LCL), diffuse (DCL) and the occasional mucocutaneous leishmaniasis (MCL). Where LCL will present as self-healing lesions at the place of inoculation, DCL appears as non-self-healing lesions widespread over the whole body and MCL at the mucosal membranes (i.e. nose, mouth and throat) (Reithinger et al., 2007; Steverding, 2017; Assefa, 2018; van Henten et al., 2018).
Leishmania aethiopica is endemic in Ethiopia and the highlands of Kenya, with respectively 1,402 and 398 reported cases of cutaneous leishmaniasis in 2020 (van Henten et al., 2018). The species is considered to be zoonotic and transmitted mainly by Procavia capensis and Heterohyrax brucei (rock hyraxes) that mostly reside in rocky cliffs (van Henten et al., 2018). Prevalence of active CL ranges from 0.01 to 10.8%, while prevalence including past CL (suspected scar lesions) ranged from 14.0 to 65.4% (van Henten et al., 2018). However, the total burden of L. aethiopica-associated disease is difficult to estimate since most of the infections will not result in disease, and if they do, they often remain unreported due to the risk of stigmatization (Reithinger et al., 2007). In addition to being a species that clinically presents three forms of CL, L. aethiopica has a high prevalence of treatment failure that remains poorly understood (van Henten et al., 2018). Field isolates of L. aethiopica also bear the endosymbiotic and immunogenic double-stranded Leishmania RNA virus, which may have potential implications for disease severity (Zangger et al., 2014). Despite these observations of biomedical and epidemiological relevance, research on L. aethiopica is almost non-existent, leaving large gaps in the knowledge on the biology of this neglected Leishmania species.
Genome diversity studies allow understanding the population dynamics and biology of Leishmania parasites, revealing insights into e.g. the epidemic history of the deadly L. donovani in the Indian subcontinent (Downing et al., 2011; Imamura et al., 2016), the population structure of L. panamensis within its endemic range (Patino et al., 2020; Llanes et al., 2022), the colonization history of L. infantum in the Americas (Schwabl et al., 2021), the Pleistocene origin of Andean Leishmania parasites (Van den Broeck et al., 2020) and the genetic consequences of hybridization (Rogers et al., 2014; Inbar et al., 2019; Cotton et al., 2020; Glans et al., 2021). Unfortunately, studies on the genetic diversity of L. aethiopica are scanty and limited to analyses of microsatellite genotyping, isoenzyme analysis, fragment length polymorphism analyses and/or single gene sequencing (Schönian et al., 2000; Pratlong et al., 2009; Odiwuor et al., 2011a; Krayter et al., 2015). These molecular studies demonstrated that the species L. aethiopica is genetically very heterogeneous despite its restricted geographic distribution (Schönian et al., 2000; Pratlong et al., 2009; Krayter et al., 2015) and suggested the existence of a L. aethiopica/L. donovani hybrid (MHOM/ET/94/ABAUY) (Odiwuor et al., 2011a). Such findings indicate that the L. aethiopica-related disease may be caused by a genetically diverse and recombining species, which may have consequences towards the clinical management and epidemiology of CL in East Africa.
Here, we present the first genome diversity study of L. aethiopica to gain a better understanding of the evolutionary history and population biology of this species. We generated a total of 28 Leishmania genomes and complemented our dataset with publicly available genomes from L. donovani, L. infantum, L. tropica and L. major for comparative purposes. Population genomic and phylogenomic analyses provide genomic confirmation of interspecific hybridization including L. aethiopica as one of the two parental species and confirm that L. aethiopica is genetically highly diverse. Our prospection of L. aethiopica genomics may promote future studies on the genomic basis of treatment failure and clinical outcome.
2 Methods
2.1 Dataset of genomic sequences
Throughout this manuscript, we use the term ‘strain’ when referring to an isolate that has been characterized (here through whole genome sequencing), ‘clone’ when referring to a strain that has been cloned and ‘genome’ when referring to strains/clones that have been sequenced (Proposals for the nomenclature of salivarian trypanosomes and for the maintenance of reference collections, 1978).
We have sequenced a total of 28 Leishmania genomes from Ethiopia within the context of this study (Supplementary Table 1), including (i) 20 genomes originating from 19 L. aethiopica strains collected between 1972 and 1994, and previously typed as L. aethiopica by the Centre National de Référence des Leishmania (Montpellier, France) or the WHO International Leishmania Reference Center (London School of Hygiene and Tropical Medicine, London, United Kingdom). For two strains (GERE and KASSAYE), only the derived clone was sequenced. For one strain (L100), both the strain and a derived clone were sequenced. The L100 strain is the WHO reference strain that represents the same strain as the one used to create the reference genome (https://tritrypdb.org/tritrypdb/app/record/dataset/NCBITAXON_1206056#description). (ii) One genome of the L. donovani strain HUSSEN. (iii) One genome of the putative L. aethiopica/L. tropica strain L86. (iv) Six genomes of the putative L. aethiopica/L. donovani strain ABAUY (hereafter referred to as LEM3469) and its derived clones (LEM3469cl1, LEM3469cl5, LEM3469cl7, LEM3469cl8, LEM3469cl9). All genomes were sequenced (150bp paired-end) on the Illumina sequencing platform of GenomeScan, Leiden, The Netherlands.
For strains L100 and LEM3469, for which we sequenced both the strain and derived clone(s), we here provide information about the number of passages that occurred at ITM before the in vitro culturing done within the context of this study. The numbers below reflect the minimum number of passages, as we don’t know how many passages occurred between isolation from patients and shipment from the reference center to ITM. The L100 strain has undergone 29 passages; its derived clone L100cl1 was generated at passage 27 and has undergone a total of 31 passages. Hence, L100 and L100cl1 differ by only 2 passages. The LEM3469 strain has undergone 3 passages; its derived clones were generated after 26 (clones 5, 7 and 8) or 28 (clone 1 and 9) passages, and have undergone a total of 30 (clones 5, 7 and 8) or 34 (clone 1 and 9) passages. Hence, LEM3469 and derived clones differ by about 30 passages, and the clones themselves by either 0 or 4 passages.
For comparative purposes, we also included publicly available sequences from four Old World Leishmania species (Supplementary Table 1): L. donovani from Ethiopia (n=5), L. infantum (n=1), L. major (n=1) and L. tropica (n=1) (González-de la Fuente et al., 2017; Zackay et al., 2018; Warren et al., 2021). We only included one or a few genomes per species with the aim to reveal the parental species of two putative interspecific hybrid strains (LEM3469 and L86) that were included in this study. While we acknowledge that there are many genomes available for the L. donovani species complex that would allow us to refine the ancestry analyses of the putative L. donovani/L. aethiopica strain LEM3469, a deep phylogenomic study goes beyond the scope of this our study and would not be possible for the putative L. tropica/L. aethiopica hybrid strain L86 as there are not many L. tropica genomes available.
Altogether, this provided us with a total of 36 genomes for downstream analyses.
2.2 Bioinformatic analyses
All sequences were mapped against the L. aethiopica’s reference genome L147 (available on tritrypdb.org as TriTrypDB-54_LaethiopicaL14710) using BWA (Li and Durbin, 2010). Resulting SAM-files were converted to BAM-files using SAMtools (Li et al., 2009). All duplicates were marked using the GATK (Genome Analysis ToolKit) software (McKenna et al., 2010). BAM-files were checked for mapping quality by examining flagstat files and coverage was calculated with SAMtools depth to determine the average mapped read depth across the whole genome. Single Nucleotide Polymorphisms (SNPs) and small insertions/deletions (INDELs) were called twice with GATK HaplotypeCaller: once including all 36 Leishmania genomes and once including 20 L. aethiopica genomes.
SNPs and INDELs were separated with the GATK SelectVariants command. SNPs were filtered based on the GATK hard filter recommendations, including a mapping quality larger than 40 and a quality by depth larger than 2, in combination with a filter that excludes SNPs within SNP clusters (defined by 3 SNPs in windows of 10bp) (Van der Auwera et al., 2013). In addition, SNPs were retained only when the allelic depth per genotype was larger than 5 and the genotype quality was larger than 20 (when genotyping was done across all 35 Leishmania genomes) or larger than 40 (when genotyping was done on the 20 L. aethiopica genomes). SNPs were annotated using the L. braziliensis M2904 annotation file with SNPEFF v4.5 (Cingolani et al., 2012). SNPs were counted per genome for the whole genome and per chromosome in Rstudio (RCoreTeam). Alternate allele read depth frequencies were counted using the vcf2freq.py script (available at github.com/FreBio/mytools/blob/master/vcf2freq.py).
2.3 Chromosomal and local copy number variation
Chromosomal and local copy number variations were studied for the 20 L. aethiopica genomes and the two interspecific hybrids L86 and LEM3469 based on the per-site read depth as obtained with SAMTools depth. To obtain haploid copy numbers (HCN) for each chromosome, the median chromosomal read depths were divided by the median genome-wide read depth. Somy variation was then obtained by multiplying HCN by two (assuming diploidy for all L. aethiopica genomes and L86; see results) or three (assuming trisomy for LEM3469 and its derived clones; see results). To obtain local HCN, the median read depth in non-overlapping 2kb windows was divided by the median genome-wide read depth. These calculations were done using the depth2window.py script (available at github.com/FreBio/mytools/blob/master/depth2window.py).
Windows of reduced or increased HCN were identified by deducting the median HCN across 20 L. aethiopica genomes from the HCN as estimated per Leishmania genome. This results in a distribution of HCN centered around zero, allowing us to identify 2kb windows with increased (z-score > 5) or decreased (z-score < -5) HCN in each of the 20 L. aethiopica genomes and the two interspecific hybrids (LEM3469 and L86). Consecutive 2kb windows showing a significant increase/decrease in HCN were joined by averaging HCN across the consecutive windows, allowing us to detect larger copy number variants. Small deletions/amplifications (i.e. <= 6kb) that do not cover protein coding genes were ignored.
2.4 Population genomic and phylogenomic analyses
The quality-filtered SNP VCF files were converted to the fasta format using the vcf2fasta.py script (available at github.com/FreBio/mytools/blob/master/vcf2fasta.py). To get an initial idea on the phylogenetic relationships between the genomes, a phylogenetic network was reconstructed with SplitsTree version 4.17.2 (Huson, 1998; Huson and Bryant, 2006) based on concatenated bi-allelic SNPs. Uncorrected p-distances (proportion of positions at which two sequences differ from each other) were calculated from the SNP genotypes. For the calculated distances, the NeighborNet method (Bryant and Moulton, 2002) was used to generate a split network and the EqualAngle algorithm (Dress and Huson, 2004) was used for final network visualization. For the chromosomal networks, the chromosomal SNPs were selected using BCFtools view and converted into the fasta format for network analyses.
The population structure of L. aethiopica was investigated after excluding putative near-identical genomes (see results), i.e. genomes showing fixed differences at <3 sites. In addition, sites exhibiting high linkage disequilibrium (LD) were removed in a pairwise manner (–indep-pairwise) with plink v1.9 (Purcell et al., 2007) within 50 bp windows with a 10 bp step size for three different squared correlation coefficients (r2 = 0.3, 47,244 SNPs retained; r2 = 0.5, 85,725 SNPs retained; r2 = 0.7, 112,241 SNPs retained). ADMIXTURE v1.3 was run for K equals 1 to 5 along with a five-fold cross validation [23]. Principal component analysis (PCA) was done using the glPCA function within the Adegenet R-package [25] (RCoreTeam R, 2021). Nucleotide diversity (π), Tajima’s D and Weir and Cockerham’s pairwise FST (mean and weighted), were calculated in non-overlapping windows of 50kb for the populations as inferred by ADMIXTURE using VCFtools v0.1.13 (Danecek et al., 2011).
Loss-of-Heterozygosity (LOH) regions were identified by analyzing the distribution of heterozygous sites in non-overlapping 10kb windows as described elsewhere (Weir et al., 2016), using the following criteria: min number of SNPs = 1, max number of heterozygous sites allowed per 10kb window = 0, minimum number of contiguous 10kb windows = 4, maximum ⅓ of all 10 kb windows within a LOH region can be gap, max number of heterozygous sites allowed within gap = 2.
The recombination history of L. aethiopica was investigated by calculating the LD decay with PopLDdecay v3.41 (Zhang et al., 2019) and the inbreeding coefficient FIS after taking into account Wahlund effects. To this end, we considered five strains (117-82, 1561-80, 169-83, 32-83 and 68-83) belonging to the same genetic population as inferred by ADMIXTURE and sampled over a period of four years. FIS was calculated as 1-Ho/He, with Ho the observed heterozygosity and He the expected heterozygosity.
3 Results
3.1 Genomic confirmation of interspecific hybridization including L. aethiopica as parent
Sequences were mapped against the L. aethiopica L147 reference genome, resulting in a median coverage of 27x to 70x for the publicly available sequence data, and 44x to 119x for the L. aethiopica genomes (Supplementary Table 2). At least 80% of the positions in the reference genome were covered with at least 20 reads, and 72% of the paired reads aligned in proper pairs (Supplementary Table 2). These results show that the coverage of the L. aethiopica reference genome was sufficiently large for variant calling, despite the diversity of species included in this study. Genotyping was performed with GATK HaplotypeCaller across all 36 Leishmania genomes, revealing a total of 988,363 high-quality bi-allelic SNPs.
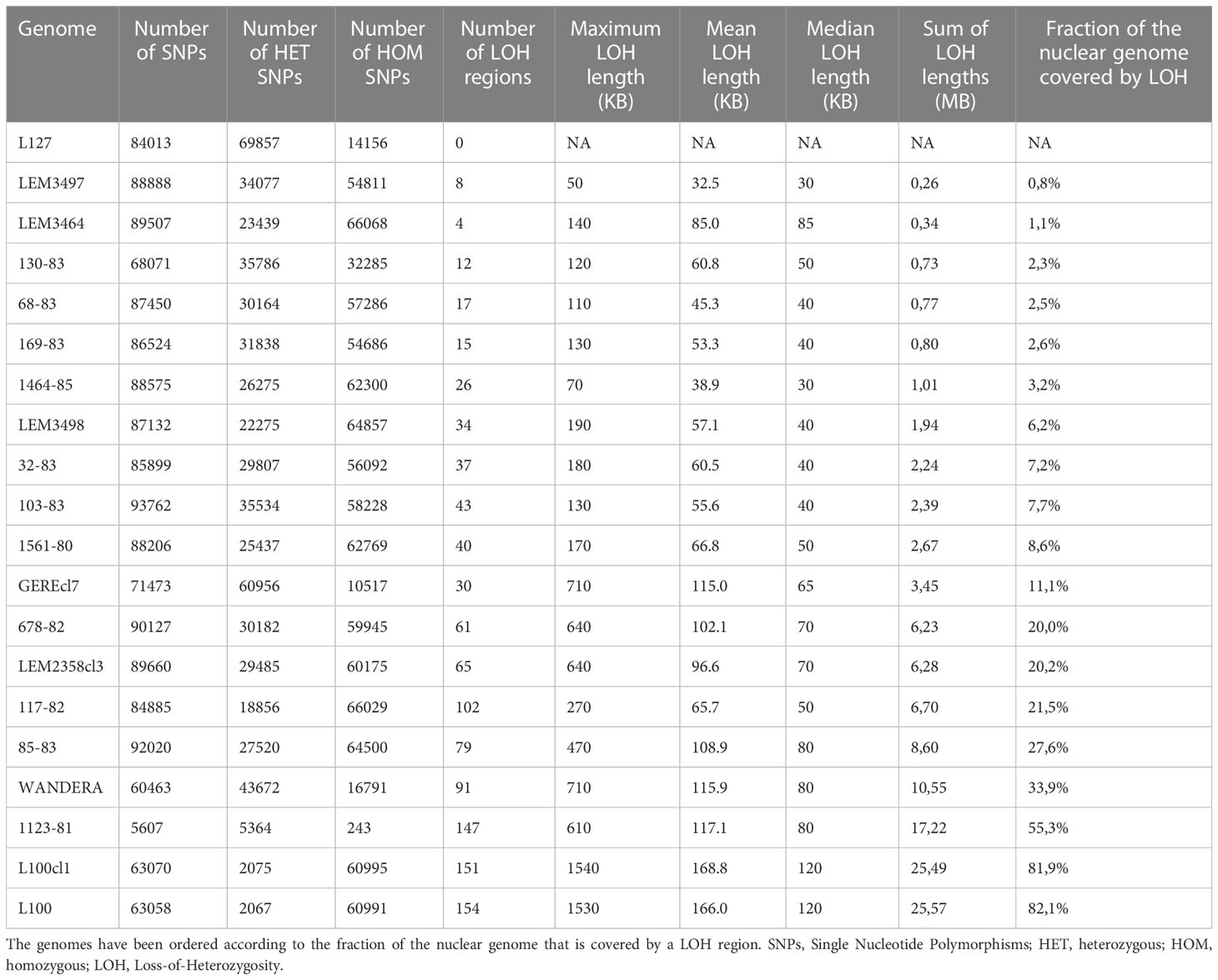
A phylogenetic network revealed a total of four groups of genomes that corresponded to the four species included in this study (Figure 1A): L. aethiopica (20 genomes), L. donovani species complex including L. donovani (11 genomes) and L. infantum (1 genome), L. major (1 genome) and L. tropica (1 genome). Two strains did not cluster with any of these species: LEM3469 and its derived clones are positioned in between L. aethiopica and the L. donovani species complex, and L86 is positioned in between L. aethiopica and L. tropica (Figure 1A). The network shows reticulation and a net-like pattern along the branches of these two strains, suggesting that LEM3469 and L86 are interspecific hybrid parasites or the result of a mixed infection. The observation that the five clones of LEM3469 are also positioned in between L. aethiopica and the L. donovani species complex indicates that they are hybrids, rather than a mixed infection. Below, we have done additional analyses to confirm that both LEM3469 and L86 are hybrid parasites.
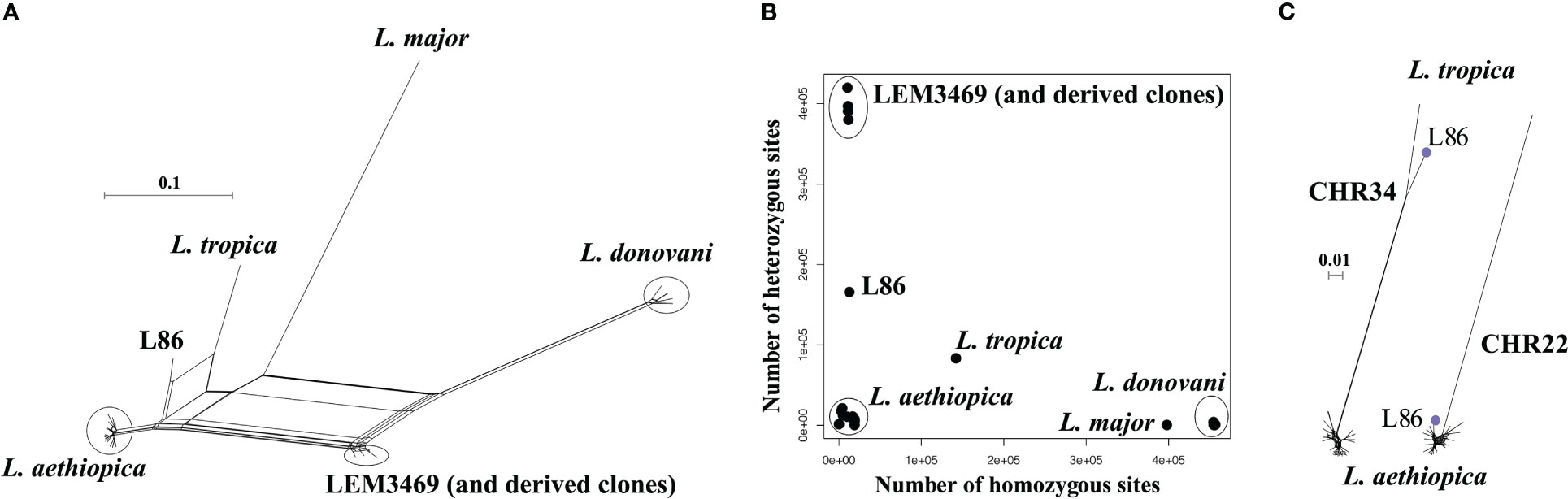
Figure 1 (A) Phylogenetic network based on SNPs called across 36 genomes of L. aethiopica, the L. donovani species complex, L. major and L. tropica. (B) Number of heterozygous sites versus number of homozygous sites for each of the 36 Leishmania genomes. (C) Phylogenetic network based on SNPs called in the last 700kb of chromosome 34 and the first 300kb in chromosome 22. Blue dot indicates the position of the interspecific L. aethiopica - L. tropica hybrid L86.
For each genome, we counted the number of homozygous SNPs (where both haplotypes are different from the L147 reference) and heterozygous SNPs (where one haplotype is similar to the L147 reference and the other different). This showed that the L. donovani species complex (median 455,265 SNPs), L. major (397,822 SNPs) and L. tropica (142,059 SNPs) genomes contain a large amount of homozygous SNPs, and are thus genetically distant from L. aethiopica L147 (Figure 1B). In contrast, L86, LEM3469 (and derived clones) and all L. aethiopica genomes contained respectively 12,507, a median 16,941, and a median 11,125 homozygous SNPs (Figure 1B). The genomes of uncertain ancestry (L86 and LEM3469) displayed a much larger amount of heterozygous SNPs (165,703 in L87 and a median 390,606 in LEM3469 and derived clones) compared to the L. aethiopica genomes (422-20,783 SNPs) (Figure 1B). Such high levels of heterozygosity in L87 and LEM3469 further indicate the presence of divergent homologous chromosomes, either as the result of hybridization or because of a mixed infection.
When investigating the distribution of SNPs across the 35 chromosomes in LEM3469 and L86, we found that the majority of chromosomes consists almost entirely of heterozygous SNPs (Supplementary Table 3). Exception was one genomic region in L86 (the last 700kb of chromosome 34) that was entirely homozygous for the alternate allele (both haplotypes are thus different from L147). In addition, the first 300kb of chromosome 22 in L86 and chromosomes 9, 10 and 15 in LEM3469 (Supplementary Table 3) were virtually devoid of SNPs (both haplotypes are thus similar to L147). This observation of a largely heterozygous genome that is interrupted by homozygous stretches suggests that L86 and LEM3469 are hybrid parasites, rather than the result of a mixed infection.
Similarly to LEM3469, all five derived clones of LEM3469 were SNP-poor in chromosomes 9, 10 and 15 (Supplementary Figure 1; Supplementary Table 3). All five clones were also SNP-poor in chromosomes 11 and 24, LEM3469 clones 1 and 9 were SNP-poor in chromosome 20, LEM3469 clone 7 was SNP poor in chromosome 1 and LEM3469 clone 8 was SNP poor in chromosome 33 (Supplementary Figure 1; Supplementary Table 3). Given that LEM3469 clones 1 and 9 were generated after 28 passages and LEM3469 clones 5, 7 and 8 after 26 passages, the loss of SNP diversity in chromosome 20 for clones 1 and 9 must have occurred between passage 26 and 28, while the loss of SNP diversity in chromosome 1 for clone 7 and chromosome 33 for clone 8 must have occurred after they were cloned at passage 26. Our results thus imply a loss of heterozygosity through the process of cloning and culturing of LEM3469.
Phylogenies based on SNPs in genomic regions that were either largely homozygous or SNP-poor in L86 revealed that this strain clustered with either L. tropica or L. aethiopica, respectively (Figure 1C). This clearly points to L. aethiopica and L. tropica as the two parental species for L86. A phylogeny based on SNP-poor genomic regions showed that LEM3469 and derived clones clustered within the L. aethiopica group (see Supplementary Figure 2 for an example), confirming that L. aethiopica is one of the parental species of this hybrid. The absence of largely homozygous genomic regions in LEM3469 and its derived clones precluded us from confirming the other parental species based on phylogenetic analyses alone.
In order to identify and confirm the other parental species of the hybrid parasites, we identified fixed SNPs specific to L. tropica (53,477 SNPs), L. donovani species complex (297,070 SNPS) and L. major (255,443 SNPs). This revealed that 85% of the fixed SNPs specific to L. tropica were heterozygous in L86, compared to 0.3% of the L. donovani species complex or L. major-specific SNPs. Similarly, we found that 91% of the fixed SNPs specific to L. donovani species complex were heterozygous in LEM3469, compared to 0.01% of the L. major and L. tropica-specific SNPs. We next focused on the fixed SNPs that were specific to the six L. donovani strains (3,389 SNPs) and the L. infantum strain (8,965 SNPs). This revealed that 1% of the L. infantum-specific SNPs and 88% of the L. donovani-specific SNPs were heterozygous in LEM3469, suggesting L. donovani rather than L. infantum was the parental species.
Altogether, our results demonstrate that L86 and LEM3469 are the result of hybridization, rather than the result of a mixed infection, between L. aethiopica and L. tropica (in case of L86) or between L. aethiopica and L. donovani (in case of LEM3469).
3.2 Population genomic structure and diversity of L. aethiopica
Variant calling was repeated on a dataset including solely the 20 L. aethiopica genomes, i.e. excluding the other Old World Leishmania species and the interspecific hybrids LEM3469 and L86. This resulted in a total of 94,581 high-quality INDELs and 284,776 high-quality SNPs called across 20 L. aethiopica genomes. Despite the high genome-wide density of SNPs (median 89 SNPs and average 91 SNPs per 10 kb window), we found a low number of heterozygous sites (median 6 SNPs and average 7 SNPs per 10 kb window). The allele frequency spectrum was dominated by low-frequency variants, with 58% of SNPs (170,163 SNPs) occurring at <0.1% of the population. A total of 116,140 SNPs (39.5% of the total) are found within the coding region of the genome, with an approximately equal number of synonymous (56,225 SNPs) and non-synonymous (59,579 SNPs) mutations. A total of 267 mutations had a high impact on protein coding genes, such as the gain of a stop codon (176 SNPs) or the loss of a start codon (41 SNPs). About two third of the high impact mutations (183 SNPs) occurred at low frequency (<= 0.1%) in our panel of 20 genomes. Of the remaining 84 mutations, 66 mutations occurred within hypothetical proteins and only 18 mutations occurred within coding regions with predicted function (Supplementary Table 4).
Our panel of L. aethiopica genomes displayed substantial differences in terms of the number of SNPs (5,607 - 93,762) and the number of homozygous (243 - 66,068) and heterozygous (2,067 - 69,857) SNP counts (Table 1). Most remarkable were i) 1123-81 showing a low number of SNPs (5.607) compared to the other genomes (mean = 82,251; median = 87,132) and ii) L100 and L100cl1 that were almost devoid of heterozygous SNPs (2,071) compared to the other genomes (mean = 32,251; median = 29,985). In addition, the number of fixed SNP differences between genomes ranged between 1 and 76,477 (average = 32,998 SNPs, median = 28,688 SNPs, sd = 20,955 SNPs) (Supplementary Table 5). Exceptions were two groups that each contained two near-identical genomes, each pair showing fixed nucleotide differences at only 1 or two sites (Supplementary Table 5): i) L100 and L100cl1 and ii) 678-82 and LEM2358cl3, which were sampled nine years from each other and were probably the result of clonal propagation in nature. The sequence similarity (in terms of fixed nucleotide differences) of 678-82 and LEM2358cl3 does not originate from a mix-up because we find the following two differences between both genomes: i) 678-82 is trisomic for chromosomes 30 and 32 while LEM2358cl3 is nearly euploid (see below), and ii) we find single point mutations at 230 sites (i.e. sites where both genomes differ by 1 nucleotide only). These minor differences suggest that both genomes do not originate from cross-contamination. Altogether, our results suggest that the L. aethiopica species is highly diverse and consists of both genetically similar and divergent parasites.
The large number of fixed SNP differences prompted us to investigate the genome-wide distribution of LOH regions. This effort revealed major differences in the number and length of LOH regions between L. aethiopica genomes (Table 1) (Figure 2). The median length of LOH regions ranged between 30 kb in LEM3497 and 1464-85 to 120 kb in L100 and L100cl1 (Table 1). In particular 1123-81, L100 and L100cl1 showed a high number of LOH regions (147 for 1123-81, 151 for L100 and 154 for L100cl1) covering a substantial proportion of their nuclear genome (55.3% for 1123-81, 81.9% for L100 and 82.1% for L100cl1) (Table 1) (Figure 2). The largest LOH regions were at least 1.5 Mb long (Table 1) and were found in chromosome 36 of L100 and L100cl1.
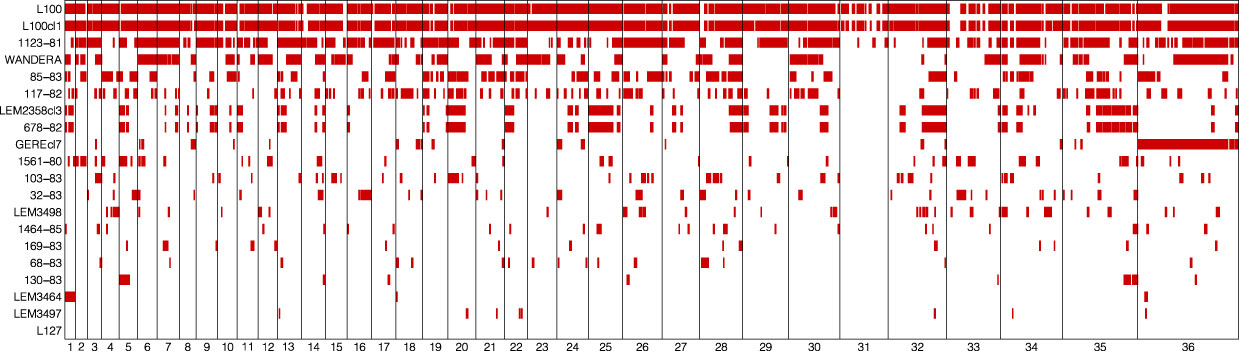
Figure 2 Loss-Of-Heterozygosity regions (red bars) in each of the 20 L. aethiopica genomes across the 36 chromosomes.
The population genomic diversity and structure of L. aethiopica was investigated after removing one genome from the near-identical pairs and excluding sites with high LD. As explained above, we identified two groups of near-identical genomes where each group showed fixed nucleotide differences at only 1 (678-82 and LEM2358cl3) or two (L100 and L100cl1) sites. Here, we randomly excluded 1 genome per group (678-82 and L100cl1) for inference of population structure with ADMIXTURE. This is because the program ADMIXTURE assumes populations are in Hardy-Weinberg Equilibrium and Linkage Equilibrium, an assumption that may be violated when including near-identical genomes that are likely the result of clonal propagation. ADMIXTURE analyses suggested the possible presence of two (K=2) to three (K=3) populations within our panel of L. aethiopica genomes, although the 5-fold cross validation was approximately similar for K=1 (Supplementary Figure 3). The ancestry estimation for the different values of K were consistent over the different SNP-pruning thresholds (Supplementary Figure 2). Assuming K=3 populations, all but two genomes (GEREcl7 and WANDERA) were assigned to one of the three ancestry components with >99% probability (Figure 3A). Principal Component Analysis (PCA) (Supplementary Figure 4), a phylogenetic network based on genome-wide SNPs (Figure 3B) and phylogenetic networks based on SNPs in LOH-poor chromosomes (Supplementary Figure 4) showed a clear separation among groups of individuals corresponding to the clusters inferred by ADMIXTURE. These results show congruence in L. aethiopica population structure among various inference approaches and suggest that the presence of LOH regions has little impact on the inference process (as shown in Supplementary Figure 5). Mean pairwise FST values among these three populations ranged between 0.175 and 0.235, suggesting the presence of population structure (Supplementary Table 6).
Figure 3 Population genomic diversity and structure of L. aethiopica. (A) Bar plots depicting ancestral components as inferred by ADMIXTURE for K = 2 and K = 3 populations, based on 85,725 SNPs. (B) Phylogenetic network based on uncorrected p-distances among 18 L. aethiopica genomes genotyped at 277,156 bi-allelic SNPs. Colored branches and tip labels correspond to the inferred populations by ADMIXTURE at K=3. (C) Linkage decay plot for 1561-80, 68-83, 169-83, 32-83 and 117-82 controlling for spatio-temporal Wahlund effects (see methods). (D) Fis distribution after correction for spatio-temporal Wahlund effects for 1561-80, 68-83, 169-83, 32-83 and 117-82. The solid line represents the mean Fis values (0.027) while the dashed lines represent the standard deviation (± 0.47).
The phylogenetic network also revealed a reticulated pattern and long terminal branches, indicative of recombination (Figure 3B). Estimates of relative recombination rates in L. aethiopica were calculated by FIS per site and LD decay controlling for spatio-temporal Wahlund effects (see methods) (Figures 3C, D). The per-site FIS was unimodally distributed and close to zero (mean FIS = 0.027 ± 0.47). In addition, LD levels were low with r2 descending to 0.2 at 21.9kb. These results suggest frequent genetic exchange in L. aethiopica.
3.3 Chromosomal and local copy number variations in L. aethiopica
The ploidy of all genomes was investigated using the genome-wide distribution of alternate allele read depth frequencies at heterozygous sites (ARDF), which should be centered around 0.5 in diploid organisms. The genome-wide distribution of ARDF was unimodal and centered around 0.5 for all L. aethiopica genomes and the L. aethiopica - L. tropica hybrid L86, suggesting that the baseline ploidy of these parasites is diploid. However, the distribution of ARDF was bimodal with modes 0.33 and 0.67 for the L. aethiopica - L. donovani hybrid LEM3469 and its derived clones, suggesting that the baseline ploidy of this hybrid is triploid.
Variation in chromosomal copy numbers was further investigated using normalized chromosomal read depths (RD). The RD estimates revealed that chromosome 31 was at least tetrasomic in all genomes (Figure 4). Little variation was detected for six L. aethiopica genomes that were largely diploid (box 1 in Figure 4). The rest of the L. aethiopica genomes were trisomic at 1 to 6 chromosomes, including a group of 8 genomes that was trisomic for chromosome 1 (box 2 in Figure 4). The largest variability was found in L. aethiopica genome LEM3498, the L. aethiopica - L. donovani hybrid LEM3469 and its derived clones and the L. aethiopica - L. tropica hybrid L87 (box 3 in Figure 4). Altogether, our results demonstrate that L. aethiopica genomes are aneuploid, as shown for other Leishmania species. Moreover, note that we also observed non-integer values of somy for some chromosomes (Figure 4), suggesting the presence of mosaic aneuploidy (Negreira et al., 2022).
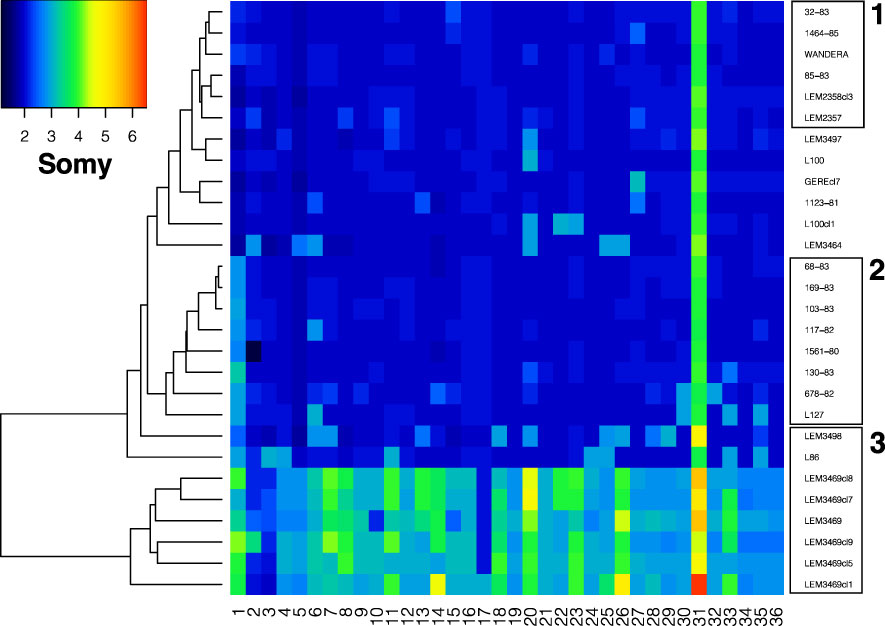
Figure 4 Somy variation across 36 chromosomes for the 28 Leishmania genomes sequenced in this study. Box 1 highlights genomes that are nearly diploid, box 2 highlights genomes with a trisomic chromosome 1 and box 3 highlights genomes showing high somy variability (see text).
Local copy number variations were investigated using normalized haploid copy numbers (HCN) as estimated in non-overlapping 2kb windows. We identified a total of 379 genomic regions with decreased or amplified HCN across our panel of L. aethiopica genomes. The majority of these windows were only 2kb long (N = 266; 70%) and the mean (1.1 HCN) and median (0.79 HCN) differences in HCN across our panel were minor, suggesting that our sample of L. aethiopica displays little local copy number variation. Apart from this observation, most notable was a 46 kb telomeric genomic region on chromosome 29 in strain 1561-80 showing a 10-fold increase in HCN compared to the other L. aethiopica genomes. This region covers a total of 18 protein coding genes, including a ribonuclease inhibitor-like protein (LAEL147_000545200), an actin-like protein (LAEL147_000544400) and a putative inosine-adenosine-guanosine-nucleoside hydrolase (LAEL147_000545100) (Supplementary Table 7). Four other strains (L127, GEREcl7, 169-83 and 32-83) displayed intrachromosomal amplifications across genomic regions of 10 kb to 20 kb long, but these involved only 0.3 to 1.2 increase in HCN (Supplementary Table 7). Finally, small (<= 4kb) intrachromosomal amplifications were identified across the genomes of L100 and L100cl1 (Supplementary Table 7).
Within the L. aethiopica - L. donovani hybrid LEM3469 and its derived clones, we detected a 6kb genomic region in chromosome 29 showing a 2-fold increase in HCN and covering a Leucine Rich Repeat gene (LAEL147_000538100) (Supplementary Table 8). Within the L. aethiopica - L. tropica hybrid L87, we found a 130 kb genomic region in chromosome 35 with a 1.6 fold increase in HCN and covering a total of 46 protein coding genes (Supplementary Table 9). Within both hybrids, we detected a 14 kb - 16 kb genomic region in chromosome 30 showing a ~1-fold increase in HCN and covering protein coding genes such as a the putative ferric reductase gene (LAEL147_000563600) (Supplementary Tables 8, 9).
4 Discussion
We present the first comprehensive genome diversity study of L. aethiopica by analyzing high-resolution WGS data. This revealed insights into the genetic consequences of recombination in Leishmania at both the species- and population-level.
At the species level, we provide genomic evidence of hybridization involving L. aethiopica as one of the parental species and L. tropica (in case of the L86 hybrid strain) or L. donovani (in case of the LEM3469 hybrid strain) as the other parental species. Strain LEM3469 has already been described as a potential L. aethiopica/L. donovani hybrid based on microsatellite data and single-gene sequences (Odiwuor et al., 2011a). Strain L86 has been flagged as a potential hybrid, although it remained classified as L. aethiopica due to lack of molecular evidence (Odiwuor et al., 2011b). Here, our data provide genomic evidence that these two Leishmania strains are interspecific hybrids. Our finding of genome-wide heterozygosity that is only occasionally interrupted by patches of homozygosity suggest that these two hybrids are equivalent to F1 progeny that propagated mitotically since the initial hybridization event. Similar genomic descriptions of naturally circulating F1-like hybrids were advanced previously for L. braziliensis x L. peruviana in Peru (Van den Broeck et al., 2020) and for L. braziliensis x L. guyanensis in Costa Rica (Van den Broeck et al., 2022). Our results thus support a growing body of genomic evidence for extensive genetic exchange in protozoan parasites in the wild (Ramírez and Llewellyn, 2014; Rogers et al., 2014; Tihon et al., 2017; Van den Broeck et al., 2018; Inbar et al., 2019; Schwabl et al., 2019; Van den Broeck et al., 2020; Glans et al., 2021; Van den Broeck et al., 2022).
The L. tropica/L aethiopica (L86) hybrid strain was near diploid, being disomic at 27/36 chromosomes, suggesting balanced segregation of the chromosomes during hybridization. Trisomic chromosomes in L86 contained either one or two copies of the L. tropica parent, which may suggest that these trisomies arose after the hybridization event through multiplication of one of the parental chromosomes, possibly due to culturing in vitro (Dumetz et al., 2017). These observations seem unlikely to have arisen by a random parasexual process, and suggest that the L86 hybrid is the result of meiotic recombination. The L. donovani/L aethiopica (LEM3469) hybrid strain was near triploid, being trisomic at 21/36 chromosomes. Triploid hybrids have been routinely recovered from experimental matings in Leishmania (Inbar et al., 2019) and from clinical samples of cutaneous leishmaniasis patients (Van den Broeck et al., 2022), and are observed across a variety of organisms capable of sexual reproduction (Schultz and Dobzhansky, 1933; Hegarty and Hiscock, 2008; Stöck et al., 2010). Results from experimental crosses in Leishmania suggested that interspecific hybrids with close to 3n DNA content were likely due to an asymmetric meiosis between a parental 2n cell that failed to undergo meiosis and 1n gamete from the other parent (Inbar et al., 2019), similar to what has been suggested for T. brucei (Gibson and Stevens, 1999). One observation that requires further investigation is that triploid hybrids may occur more frequently when the two parental species are genetically divergent (in the case of L. donovani/L aethiopica, L. guyanensis/L. braziliensis (Van den Broeck et al., 2022) and L. infantum/L. major (Inbar et al., 2019) hybrids), while diploid hybrids seem to occur when the parental species are more closely related (in the case of L. tropica/L. aethiopica and L. braziliensis/L. peruviana (Van den Broeck et al., 2020) hybrids).
At the population level, our analyses of sequence variation confirmed previous observations that the L. aethiopica species is genetically diverse, despite its restricted geographic distribution (Schönian et al., 2000; Pratlong et al., 2009; Krayter et al., 2015). Indeed, we detected on average 91 SNPs per 10kb in L. aethiopica, which is comparable to the genetically diverse L. braziliensis (106 SNPs per 10kb) but twice the number observed in the demographically bottlenecked L. peruviana (41 SNPs per 10kb) (Van den Broeck et al., 2020). By comparison, the human genome contains ~8 SNPs per 10kb (Zhao et al., 2003). Also, the total number of SNPs (284,776) across our set of 20 L. aethiopica genomes from Ethiopia is of the same magnitude as the number of SNPs (395,624) observed in a set of 151 globally sampled genomes of the L. donovani species complex (Franssen et al., 2020). In contrast, we detected little variation in local copy numbers: the majority of genomes contained only up to 20 genomic regions with increased/decreased read depths. Hence, adaptation in our sample of L. aethiopica genomes may depend on sequence variation rather than gene dosage. Future studies involving direct sequencing of biopsy samples (Domagalska et al., 2019) should allow deciphering the relative contribution of different genomic variants in clinical outcome or treatment failure.
Population genomic analyses revealed that the L. aethiopica species consists of both asexually evolving strains (as indicated by the existence of near-identical genomes) and groups of parasites that show signatures of recombination (as indicated by linkage decay). In addition, parasite populations were genetically structured, suggesting that L. aethiopica consists of divergent populations, although lack of geographic/ecological data precluded us from studying its evolutionary history in greater detail. A remarkable observation was the extensive LOH in some L. aethiopica strains. Such LOH likely arose from gene conversion/mitotic recombination and indicates that L. aethiopica strains may evolve asexually over long time periods. This is exemplified by two near-identical genomes (678-82 and LEM2358cl3) that were sampled nine years from each other. Interestingly, we observed four additional LOH regions in LEM2358cl3 (sampled in 1991) impacting an additional 50 kb of the nuclear genome compared to 678-82 (sampled in 1982), suggesting that nine years of clonal evolution has resulted in LOH across 50kb of the genome.
Extensive LOH regions have been described previously for obligate asexual eukaryotes, such as the water flea Daphnia pulex (Tucker et al., 2013) and the protozoan parasite Trypanosoma brucei gambiense (Weir et al., 2016; Geerts et al., 2022). In T. b. gambiense, most LOH regions are ancestral and thus present in all strains, or at least within sets of strains that share a common ancestry (Weir et al., 2016). In contrast, we observed large differences in the number and length of LOH regions between L. aethiopica strains, irrespective of their ancestral relationships. For instance, LOH regions were absent in the L127 strain while a total of 154 LOH regions were found in the L100 strain, impacting at least 82% of its nuclear genome. The L100 strain (also known as LEM144) was isolated in 1972 and has - to the best of our knowledge - only been cultured in vitro for about 30 passages before sequencing. Hence, the extensive loss of heterozygosity cannot be explained by maintenance in vitro, and is probably due to long-term mitotic recombination in the wild, as described for the asexually evolving T. b. gambiense (Weir et al., 2016). Alternatively, the genome-wide loss-of-heterozygosity in L100 may be explained by genome-wide DNA haploidization, possibly due to the production of haploid gametes as seen in trypanosomes (Peacock et al., 2021), followed by whole-genome diploidization (as our analyses demonstrated that L100 is diploid). However, there is currently no evidence in literature for the occurrence of whole-genome haploidization/diploidization in Leishmania parasites.
In conclusion, our study has shown that L. aethiopica is genetically diverse and divided into structured populations, suggesting that strains may be ecologically/geographically confined. While linkage decay suggests the occurrence of genetic exchange, the discovery of extensive loss of heterozygosity also suggests that some L. aethiopica strains may evolve asexually for long periods of time. Hence, L. aethiopica presents an ideal model to understand the impact of parasite population structure and hybridization on genome evolution in protozoan parasites. Our preliminary observations should thus be investigated in further detail using larger sets of samples for which detailed eco-epidemiological data are available, preferably using direct-sequencing approaches that would also allow understanding the genomic basis of clinical and treatment outcome.
Data availability statement
Genomic sequence reads of all sequenced genomes are available on the European Nucleotide Archive (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA924694) under accession number PRJNA924694.
Author contributions
J-CD and MD conceived the original idea. IM performed the lab experiments. FB and SH supervised the work of AH. AH, SH, and FB performed the computational analyses and wrote the manuscript. All authors discussed the results. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Dioraphte foundation (Spatial-CL, project number: CFP-RD2020 20020401) and the Belgian Directorate General for Development Cooperation (fourth framework agreement, project 907004). FB and SH acknowledge support from the Research Foundation Flanders (Grants 1226120N and G092921N).
Acknowledgments
From ITM Antwerp, we thank Pieter Monsieurs for help with generating whole genome sequence data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1147998/full#supplementary-material
References
Ashford, R. W., Bray, M. A., Hutchinson, M. P., Bray, R. S. (1973). The epidemiology of cutaneous leishmaniasis in Ethiopia. Trans. R. Soc Trop. Med. Hyg. 67, 568–601. doi: 10.1016/0035-9203(73)90088-6
Assefa, A. (2018). Leishmaniasis in Ethiopia: A systematic review and meta-analysis of prevalence in animals and humans. Heliyon 4, e00723. doi: 10.1016/j.heliyon.2018.e00723
Bryant, D., Moulton, V. (2022). NeighborNet: An Agglomerative method for the construction of planar phylogenetic networks. Algorithms in Bioinformatics. Conference Paper. 375–91.
Cingolani, P., Platts, A., Wang, L. L., Coon, M., Nguyen, T., Wang, L., et al. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of drosophila melanogaster strain w 1118; iso-2; iso-3. Fly 6, 80–92. doi: 10.4161/fly.19695
Cotton, J. A., Durrant, C., Franssen, S. U., Gelanew, T., Hailu, A., Mateus, D., et al. (2020). Genomic analysis of natural intra-specific hybrids among Ethiopian isolates of leishmania donovani. PloS Negl. Trop. Dis. 14, e0007143. doi: 10.1371/journal.pntd.0007143
Danecek, P., Auton, A., Abecasis, G., Albers, C. A., Banks, E., DePristo, M. A., et al. (2011). The variant call format and VCFtools. Bioinformatics 27, 2156–2158. doi: 10.1093/bioinformatics/btr330
Domagalska, M. A., Imamura, H., Sanders, M., Van den Broeck, F., Bhattarai, N. R., Vanaerschot, M., et al. (2019). Genomes of leishmania parasites directly sequenced from patients with visceral leishmaniasis in the Indian subcontinent. PloS Negl. Trop. Dis. 13, e0007900. doi: 10.1371/journal.pntd.0007900
Downing, T., Imamura, H., Decuypere, S., Clark, T. G., Coombs, G. H., Cotton, J. A., et al. (2011). Whole genome sequencing of multiple leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 21, 2143–2156. doi: 10.1101/gr.123430.111
Dress, A. W., Huson, D. H. (2004). Constructing splits graphs. IEEE/ACM Trans. Comput. Biol. Bioinform. 1 (3), 109–115.
Dumetz, F., Imamura, H., Sanders, M., Seblova, V., Myskova, J., Pescher, P., et al. (2017). Modulation of aneuploidy in leishmania donovani during adaptation to different In vitro and In vivo environments and its impact on gene expression. MBio 8, e00599–17. doi: 10.1128/mBio.00599-17
Franssen, S. U., Durrant, C., Stark, O., Moser, B., Downing, T., Imamura, H., et al. (2020). Global genome diversity of the leishmania donovani complex. Elife 9, e51243. doi: 10.7554/eLife.51243.sa2
Geerts, M., Chen, Z., Bebronne, N., Savill, N. J., Schnaufer, A., Büscher, P., et al. (2022). Deep kinetoplast genome analyses result in a novel molecular assay for detecting trypanosoma brucei gambiense-specific minicircles. NAR Genomics Bioinf. 4, lqac081. doi: 10.1093/nargab/lqac081
Gibson, W., Stevens, J. (1999). Genetic exchange in the trypanosomatidae. Adv. Parasitol. 43, 1–46. doi: 10.1016/S0065-308X(08)60240-7
Glans, H., Lind Karlberg, M., Advani, R., Bradley, M., Alm, E., Andersson, B., et al. (2021). High genome plasticity and frequent genetic exchange in leishmania tropica isolates from Afghanistan, Iran and Syria. PloS Negl. Trop. Dis. 15, e0010110. doi: 10.1371/journal.pntd.0010110
González-de la Fuente, S., Peiró-Pastor, R., Rastrojo, A., et al. (2017). Resequencing of the Leishmania infantum (strain JPCM5) genome and de novo assembly into 36 contigs. Sci. Rep. 7, 18050. doi: 10.1038/s41598-017-18374-y
Hegarty, M. J., Hiscock, S. J. (2008). Genomic clues to the evolutionary success of polyploid plants. Curr. Biol. 18, R435–R444. doi: 10.1016/j.cub.2008.03.043
Huson, D. H. (1998). SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14, 68–73. doi: 10.1093/bioinformatics/14.1.68
Huson, D. H., Bryant, D. (2006). Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23 (2), 254–67.
Imamura, H., Downing, T., Van den Broeck, F., Sanders, M. J., Rijal, S., Sundar, S., et al. (2016). Evolutionary genomics of epidemic visceral leishmaniasis in the Indian subcontinent. Elife 5, e12613. doi: 10.7554/eLife.12613
Inbar, E., Shaik, J., Iantorno, S. A., Romano, A., Nzelu, C. O., Owens, K., et al. (2019). Whole genome sequencing of experimental hybrids supports meiosis-like sexual recombination in leishmania. PloS Genet. 15, 1–28. doi: 10.1371/journal.pgen.1008042
Krayter, L., Schnur, L. F., Schönian, G. (2015). The genetic relationship between leishmania aethiopica and leishmania tropica revealed by comparing microsatellite profiles. PloS One 10, e0131227. doi: 10.1371/journal.pone.0131227
Lemma, A., Foster, W. A., Gemetchu, T., Preston, P. M., Bryceson, A., Minter, D. M. (1969). Studies on leishmaniasis in ethiopia. i. preliminary investigations into the epidemiology of cutaneous leishmaniasis in the highlands. Ann. Trop. Med. Parasitol. 63, 455–472. doi: 10.1080/00034983.1969.11686649
Li, H., Durbin, R. (2010). Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics 26, 589–595. doi: 10.1093/bioinformatics/btp698
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Llanes, A., Cruz, G., Morán, M., Vega, C., Pineda, V. J., Ríos, M., et al. (2022). Genomic diversity and genetic variation of leishmania panamensis within its endemic range. Infect. Genet. Evol. 103, 105342. doi: 10.1016/j.meegid.2022.105342
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Negreira, G. H., Monsieurs, P., Imamura, H., Maes, I., Kuk, N., Yagoubat, A., et al. (2022). High throughput single-cell genome sequencing gives insights into the generation and evolution of mosaic aneuploidy in leishmania donovani. Nucleic Acids Res. 50, 293–305. doi: 10.1093/nar/gkab1203
Odiwuor, S., De Doncker, S., Maes, I., Dujardin, J.-C., van der Auwera, G. (2011a). Natural leishmania donovani/Leishmania aethiopica hybrids identified from Ethiopia. Infect. Genet. Evol. 11, 2113–2118. doi: 10.1016/j.meegid.2011.04.026
Odiwuor, S. O. C., Saad, A. A., De Doncker, S., Maes, I., Laurent, T., El Safi, S., et al. (2011b). Universal PCR assays for the differential detection of all old world leishmania species. Eur. J. Clin. Microbiol. Infect. Dis. 30, 209–218. doi: 10.1007/s10096-010-1071-3
Patino, L. H., Muñoz, M., Muskus, C., Méndez, C., Ramírez, J. D. (2020). Intraspecific genomic divergence and minor structural variations in leishmania (Viannia) panamensis. Genes 11, 252. doi: 10.3390/genes11030252
Peacock, L., Kay, C., Farren, C., Bailey, M., Carrington, M., Gibson, W. (2021). Sequential production of gametes during meiosis in trypanosomes. Commun. Biol. 4, 555. doi: 10.1038/s42003-021-02058-5
Pratlong, F., Dereure, J., Ravel, C., Lami, P., Balard, Y., Serres, G., et al. (2009). Geographical distribution and epidemiological features of old world cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop. Med. Int. Health 14, 1071–1085. doi: 10.1111/j.1365-3156.2009.02336.x
Proposals for the nomenclature of salivarian trypanosomes and for the maintenance of reference collections. (1978). Bull. World Health Organ 56, 467–480. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395593/.
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A. R., Bender, D., et al. (2007). PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Ramírez, J. D., Llewellyn, M. S. (2014). Reproductive clonality in protozoan pathogens-truth or artefact? Mol. Ecol. 23, 4195–4202. doi: 10.1111/mec.12872
RCoreTeam R. (2021). A language and environment for statistical computing (Accessed 11 February 2021).
Reithinger, R., Dujardin, J.-C., Louzir, H., Pirmez, C., Alexander, B., Brooker, S. (2007). Cutaneous leishmaniasis. Lancet Infect. Dis. 7, 581–596. doi: 10.1016/S1473-3099(07)70209-8
Rogers, M. B., Downing, T., Smith, B. A., Imamura, H., Sanders, M., Svobodova, M., et al. (2014). Genomic confirmation of hybridisation and recent inbreeding in a vector-isolated leishmania population. PloS Genet. 10, e1004092. doi: 10.1371/journal.pgen.1004092
Schönian, G., Akuffo, H., Lewin, S., Maasho, K., Nylén, S., Pratlong, F., et al. (2000). Genetic variability within the species leishmania aethiopica does not correlate with clinical variations of cutaneous leishmaniasis. Mol. Biochem. Parasitol. 106, 239–248. doi: 10.1016/S0166-6851(99)00216-9
Schultz, J., Dobzhansky, T. (1933). Triploid hybrids between drosophila melanogaster and drosophila simulans. J. Exp. Zool. 65, 73–82. doi: 10.1002/jez.1400650105
Schwabl, P., Boité, M. C., Bussotti, G., Jacobs, A., Andersson, B., Moreira, O., et al. (2021). Colonization and genetic diversification processes of leishmania infantum in the americas. Commun. Biol. 4, 139. doi: 10.1038/s42003-021-01658-5
Schwabl, P., Imamura, H., Van den Broeck, F., Costales, J. A., Maiguashca-Sánchez, J., Miles, M. A., et al. (2019). Meiotic sex in chagas disease parasite trypanosoma cruzi. Nat. Commun. 10, 3972. doi: 10.1038/s41467-019-11771-z
Steverding, D. (2017). The history of leishmaniasis. Parasitol. Vectors 10, 82. doi: 10.1186/s13071-017-2028-5
Stöck, M., Ustinova, J., Lamatsch, D. K., Schartl, M., Perrin, N., Moritz, C. (2010). A vertebrate reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup). Evolution 64, 944–959. doi: 10.1111/j.1558-5646.2009.00876.x
Tihon, E., Imamura, H., Dujardin, J.-C., Van Den Abbeele, J., Van den Broeck, F. (2017). Discovery and genomic analyses of hybridization between divergent lineages of trypanosoma congolense, causative agent of animal African trypanosomiasis. Mol. Ecol. 26, 1–15. doi: 10.1111/mec.14271
Tucker, A. E., Ackerman, M. S., Eads, B. D., Xu, S., Lynch, M. (2013). Population-genomic insights into the evolutionary origin and fate of obligately asexual daphnia pulex. Proc. Natl. Acad. Sci. U.S.A. 110, 15740–15745. doi: 10.1073/pnas.1313388110
Van den Broeck, F., Heeren, S., Maes, I., Sanders, M., Cupolillo, E., Cotton, J., et al. (2022). Genome analysis of a triploid hybrid Leishmania parasite from the Neotropics. Emerg. Infect. Dis. In press.
Van den Broeck, F., Savill, N. J., Imamura, H., Sanders, M., Maes, I., Cooper, S., et al. (2020). Ecological divergence and hybridization of Neotropical leishmania parasites. Proc. Natl. Acad. Sci. U.S.A. 117, 1–10. doi: 10.1073/pnas.1920136117
Van den Broeck, F., Tavernier, L. J. M., Vermeiren, L., Dujardin, J. C., Van Den Abbeele, J. (2018). Mitonuclear genomics challenges the theory of clonality in trypanosoma congolense: Reply to tibayrenc and ayala. Mol. Ecol 27 (17), 3425–3431. doi: 10.1111/mec.14809
Van der Auwera, G. A., Carneiro, M. O., Hartl, C., Poplin, R., Del Angel, G., Levy-Moonshine, A., et al. (2013). From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinf. 43, 11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43
van Henten, S., Adriaensen, W., Fikre, H., Akuffo, H., Diro, E., Hailu, A., et al. (2018). Cutaneous leishmaniasis due to leishmania aethiopica. EClinicalMedicine 6, 69–81. doi: 10.1016/j.eclinm.2018.12.009
Weir, W., Capewell, P., Foth, B., Clucas, C., Pountain, A., Steketee, P., et al. (2016). Population genomics reveals the origin and asexual evolution of human infective trypanosomes. Elife 5, e11473. doi: 10.7554/eLife.11473
Warren, W. C., Akopyants, N. S., Dobson, D. E., Hertz-Fowler, C., Lye, L.-F., Myler, P. J., et al. (2021). Genome assemblies across the diverse evolutionary spectrum of leishmania protozoan parasites. Microbiol. Resour. Announc. 10 (35), e0054521.
Zackay, A., Cotton, J. A., Sanders, M., Hailu, A., Nasereddin, A., Warburg, A., et al. (2018). Genome wide comparison of Ethiopian Leishmania donovani strains reveals differences potentially related to parasite survival. Plos Genetics 14 (1), e1007133.
Zangger, H., Hailu, A., Desponds, C., Lye, L. F., Akopyants, N. S., Dobson, D. E., et al. (2014). Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PloS Negl. Trop. Dis 8 (4), e2836. doi: 10.1371/journal.pntd.0002836
Zhang, C., Dong, S.-S., Xu, J.-Y., He, W.-M., Yang, T.-L. (2019). PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 35, 1786–1788. doi: 10.1093/bioinformatics/bty875
Keywords: population genomics, hybridization, loss-of-heterozygosity (LOH), asexual evolution, interspecific hybrid, triploid hybrid
Citation: Hadermann A, Heeren S, Maes I, Dujardin J-C, Domagalska MA and Van den Broeck F (2023) Genome diversity of Leishmania aethiopica. Front. Cell. Infect. Microbiol. 13:1147998. doi: 10.3389/fcimb.2023.1147998
Received: 19 January 2023; Accepted: 27 March 2023;
Published: 20 April 2023.
Edited by:
André Luiz Rodrigues Roque, Laboratory of Tripanosomatid Biology (FIOCRUZ), BrazilReviewed by:
Juan David Ramírez, Icahn School of Medicine at Mount Sinai, United StatesIsabel Mauricio, New University of Lisbon, Portugal
Gabriel Eduardo Ferreira, Fiocruz Rondônia, Brazil
Copyright © 2023 Hadermann, Heeren, Maes, Dujardin, Domagalska and Van den Broeck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frederik Van den Broeck, ZnZhbmRlbmJyb2Vja0BnbWFpbC5jb20=; Malgorzata Anna Domagalska, bWRvbWFnYWxza2FAaXRnLmJl
†Present address: Amber Hadermann, Global Health Institute, University of Antwerp, Antwerp, Belgium
 Amber Hadermann1†
Amber Hadermann1† Senne Heeren
Senne Heeren Jean-Claude Dujardin
Jean-Claude Dujardin Malgorzata Anna Domagalska
Malgorzata Anna Domagalska Frederik Van den Broeck
Frederik Van den Broeck