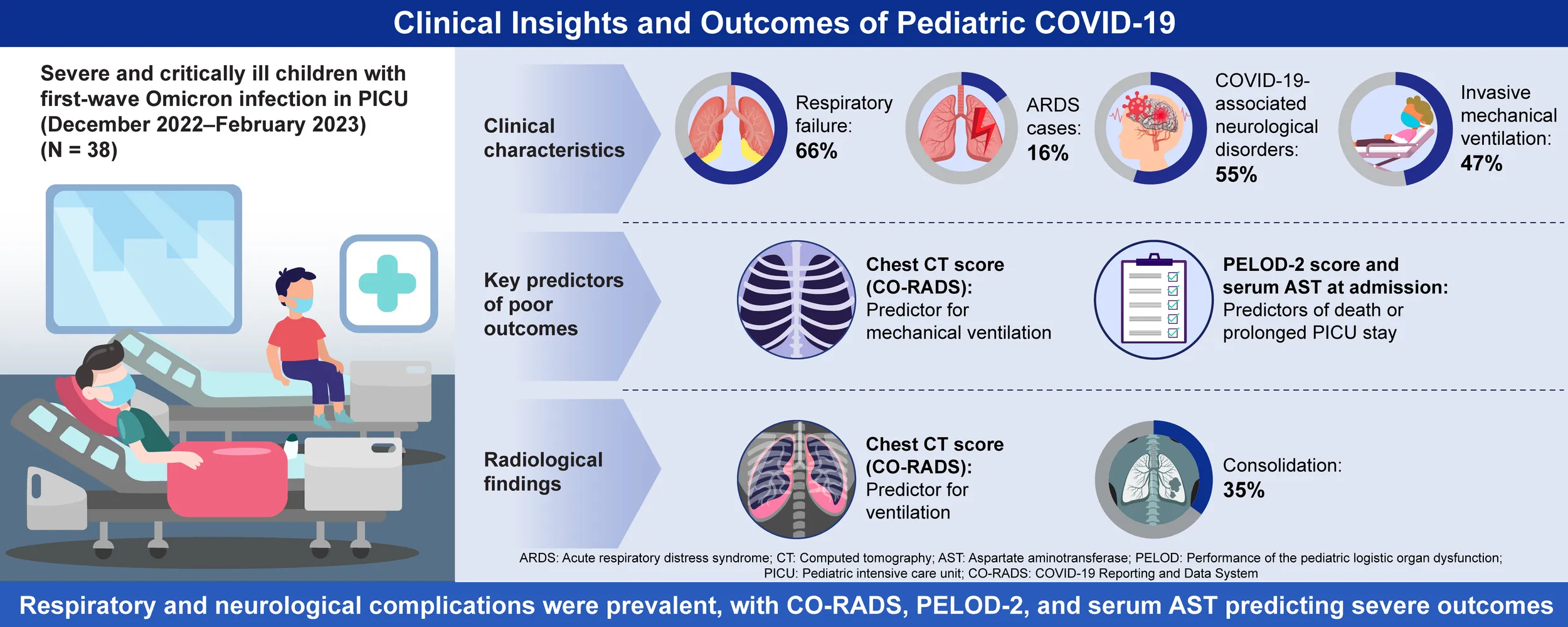
Abstract
Aim:
To describe the characteristics of severe and critically ill children with first-wave SARS-CoV-2 Omicron infection admitted to the pediatric intensive care unit (PICU) at the National Children’s Regional Medical Center in Northeast China and to explore factors associated with poor outcomes.
Methods:
This observational cohort study was conducted in a PICU in northeastern China and included children under 18 years of age who were severely and critically ill due to SARS-CoV-2 Omicron infection between December 2022 and February 2023. Patients were categorized into two groups: the invasive mechanical ventilation (IMV) group and the non-IMV group. The primary outcome measured was the need for IMV, while secondary outcomes included death or prolonged PICU stay. Univariate and multivariate logistic regression analyses were performed to identify risk factors for poor outcomes.
Results:
A total of 38 severe and critically ill children were included in the study. Of these, 25 (66%) were diagnosed with respiratory failure, and four (16%) developed acute respiratory distress syndrome. Additionally, 21 (55%) were diagnosed with COVID-19-associated neurological disorders, and 18 (47%) received IMV. Multivariate logistic regression analysis identified the chest computed tomography (CT) score, based on the COVID-19 Risk Assessment and Diagnosis System (CO-RADS), was statistically significant as an independent predictor for IMV in severe and critically ill children (odds ratio [OR]: 2.781 [95% confidence interval (CI): 1.021–7.571]). Furthermore, the Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score and serum aspartate aminotransferase (AST) levels at admission were found to be independent predictors of death or prolonged PICU stay.
Conclusions:
Respiratory failure and COVID-19-associated neurological disorders were the most common complications among severe and critically ill children with first-wave SARS-CoV-2 Omicron infection. Chest CT score, PELOD-2 score, and serum AST levels may serve as important indicators of poor outcomes in this patient population.
Characteristics and outcomes in severe and critically Ill children with first wave SARS-CoV-2 omicron infection.
1 Introduction
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has spread globally since its emergence in late 2019 (Baker et al., 2022). As the virus continues to mutate, different COVID-19 variants have emerged with varying characteristics. The Omicron variant (B.1.1.529), first identified in South Africa in November 2021, became the predominant strain during the fourth wave of the global COVID-19 pandemic (World Health Organization, 2021). The Chinese government implemented strict epidemic control measures, including the “zero COVID” policy, which remained in effect until December 2022. After the zero-tolerance policy on COVID-19 ended in 2022, China experienced a widespread COVID-19 epidemic driven by the Omicron variant (Chinese Center for Disease Control and Prevention, 2023). Between December 2022 and February 2023, the cumulative number of confirmed cases increased by approximately 89.4 million, bringing the cumulative total to 99 million in China (World Health Organization, 2023a). During this period, all locally transmitted SARS-CoV-2 cases were confirmed to be caused by the Omicron variant according to the Chinese Center for Disease Control and Prevention.
Studies have shown that while the Omicron variant is more transmissible than previous variants, it is generally associated with lower disease severity and a reduced incidence of multisystem inflammatory syndrome in children (MIS-C) (Tian et al., 2022; Wang et al., 2022; Whittaker et al., 2022; Wolter et al., 2022; Choi et al., 2023; Ross et al., 2023; Wang et al., 2023). However, extra-pulmonary organ involvement, the need for invasive mechanical ventilation (IMV), and mortality rates in severe and critical cases have not significantly decreased (Corriero et al., 2022; de Prost et al., 2022). The high transmissibility of Omicron led to an increased medical burden on both adult and pediatric healthcare systems. Studies have reported a rapid rise in pediatric SARS-CoV-2 infections and hospitalizations during the first Omicron wave (Cloete et al., 2022; Menni et al., 2022). Although most pediatric cases are mild to moderate, some children develop severe illness requiring intensive care unit (ICU) admission (Bhalala et al., 2022; Uka et al., 2022; Zhang et al., 2023). There is limited research on children who have experienced severe or critical illness due to first-time infection with the Omicron variant.
In this study, we investigated children with severe and critical COVID-19 who were admitted to the pediatric ICU (PICU) of the National Regional Medical Center in Northeast China during the first Omicron epidemic and explored factors associated with poor outcomes.
2 Methods
2.1 Study design and population
In this retrospective study, we investigated children aged <18 years who were admitted to the largest PICU at Shengjing Hospital of China Medical University, which serves as the National Children’s Regional Medical Center in Northeast China. The study was approved by the institutional ethics committee (Ethics No. 2023PS30K). Medical records from the electronic medical record system were reviewed for patients admitted to the PICU between December 1, 2022, and February 28, 2023. All enrolled children had laboratory-confirmed COVID-19 infection, confirmed by real-time reverse-transcriptase polymerase chain reaction of nasopharyngeal swab samples. The Chinese Center for Disease Control and Prevention tested hospitalized patients to determine the COVID-19 strain during the epidemic, confirming that all cases were caused by the Omicron variant.
The inclusion criteria were: (a) Compliance with the diagnostic criteria for severe and critical cases as defined by the National Health Commission of the People’s Republic Of China (NHCC) (Sun et al., 2025) (b) Availability of clinical data.
The exclusion criteria were (a) Refusal to participate (b) Discontinuation of therapy, including self-discharge against medical advice.
2.2 Criteria for diagnosis and definition
According to the guidelines on the diagnosis and treatment of new coronavirus pneumonia (version 10), issued by the NHCC on January 5, 2023 (Sun et al., 2025), all cases were classified into four severity groups: mild, moderate, severe, and critical. Severe cases were defined as meeting at least one of the following conditions: Persistent high fever > 3 days, shortness of breath, SpO2 ≤ 93% on room air at rest, alar flapping, the presence of three concave signs or wheezing, unconsciousness, convulsions, or difficulty feeding with signs of dehydration. Critical cases were defined as meeting at least one of the following criteria: Respiratory failure requiring mechanical ventilation, shock, and ICU admission for other organ dysfunctions. Details of the diagnostic criteria and differences from the WHO guidelines (World Health Organization, 2023b) are provided in Supplementary Table 1. Only severe and critical cases were included in this study.
Data extracted from medical records included: Pediatric Critical Illness Score (PCIS) at admission (Zhang et al., 2025), Pediatric Logistic Organ Dysfunction (PELOD-2) score at admission (Leteurtre et al., 2013), and modified Rankin Scale (mRS) score at discharge (Quinn et al., 2009). The diagnostic criteria for pediatric acute respiratory distress syndrome (ARDS) were based on the guidelines established by the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) group in 2023 (Emeriaud et al., 2023). The chest CT score was based on the COVID-19 Risk Assessment and Diagnosis System (CO-RADS) (Prokop et al., 2020), which was employed to evaluate the severity of lung involvement in COVID-19 pneumonia. Furthermore, we assessed the mRS score for several patients 90 days post-discharge.
2.3 Data collection and classification
Age, sex, preexisting medical conditions, clinical data (including symptoms, time of onset, clinical diagnosis, and presence of complications), laboratory data, radiological findings (X-ray, computed tomography [CT], and magnetic resonance imaging [MRI] characteristics), treatment strategies, and patient outcomes were collected. Data were verified directly from the electronic medical records by at least two members of the research group to reduce information and transcription biases.
Patients were categorized into two groups based on the use of IMV. A comprehensive analysis was conducted on demographic information, clinical characteristics, laboratory and imaging results, treatment, outcomes, and risk factors associated with poor outcomes in both groups. Primary outcomes were defined as the need for IMV, while secondary outcomes included death or prolonged PICU stay (defined as ≥ 14 days).
2.4 Statistical analysis
The data in this study did not follow a normal distribution. Therefore, quantitative data were presented as the median and interquartile range (IQR), while categorical variables were reported as numbers (percentages). Cases in which corresponding tests or examinations were not performed were excluded, and the results were expressed as the number of positive outcomes divided by the total number tested. The Wilcoxon signed-rank test was used to compare continuous variables, while the corrected chi-square test or Fisher’s exact test was applied for categorical variables. Bivariate regression analyses were conducted to screen variables for inclusion in multivariate regression models, which aimed to identify factors associated with the need for IMV and factors influencing prolonged PICU stay or death. Backward stepwise regression was applied in the multivariable analysis. Statistical significance was set at p < 0.05. Variables included in the final multivariate model were reported with odds ratios (ORs) and their 95% confidence intervals (CIs). All statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp.).
3 Results
3.1 Demographic data and clinical features
A total of 38 severe and critically ill children admitted to the PICU were included in this study, of whom 26 (68%) were classified as critical cases. Among these patients, 20 (53%) were male. The ages of the patients ranged from 30 days to under 17 years, with a median age of 25.5 (IQR: 12.5, 79.75) months. Children aged < 3 years accounted for 61% of the cases. Among the study population, 15 (39%) had received at least one dose of an inactivated COVID-19 vaccine (provided by Sinopharm CO.Ltd in Beijing or Wuhan, China and Kexing Biotch CO.Ltd in Shenzhen, China). Additionally, nine (24%) patients had pre-existing medical conditions. A total of 18 (47%) patients received IMV. Five of 38 patients (13%) developed cardiopulmonary arrest post-procedure, and four of five cases (80%) were successfully resuscitated.
Baseline characteristics and complications of the study population are summarized in Table 1. Several categorical variables, including fever, dyspnea, respiratory failure, laryngeal obstruction, ARDS, and the number of complications, were significantly associated with IMV. Among continuous variables, the PCIS, the PELOD-2, and the chest CT score (CO-RADS) were all significantly higher in the IMV group.
Table 1
| Item | Total (N=38) | Non-IMV (n=20) | IMV (n=18) | p- value |
|---|---|---|---|---|
| Male n (%) | 20 (53) | 9 (45) | 11 (61) | 0.352 |
| Age Group n (%) | 0.757 | |||
| <1 y | 9 (24) | 6 (30) | 3 (17) | 0.454 |
| 1–2 yr | 14 (37) | 6 (30) | 8 (44) | 0.503 |
| 3–6 yr | 7 (18) | 4 (20) | 3 (17) | 1.0 |
| 7–17 yr | 8 (21) | 4 (20) | 4 (22) | 1.0 |
| Age (months) median (Q1,Q3) | 25.5 (12.5,79.75) | 25 (5,85.25) | 26 (15,84) | 0.988 |
| Disease severity classification n (%) | 0.000 | |||
| Severe disease | 12 (32) | 12 (60) | 0 | |
| Critical disease | 26 (68) | 8 (40) | 18 (100) | |
| Vaccine n (%) | 15 (39) | 8 (40) | 7 (39) | 0.604 |
| Pre-existing medical conditions n (%) | 9 (24) | 4 (15) | 5 (22) | 0.709 |
| BPD | 3 (8) | 2 (10) | 1 (6) | |
| Congenital atrial septal defect | 1 (3) | – | 1 (6) | - |
| Methylmalonic acidemia | 1 (3) | – | 1 (6) | - |
| Aplastic anemia | 1 (3) | – | 1 (6) | - |
| Diabetes | 1 (3) | 1 (5) | – | - |
| Epilepsy | 1 (3) | 1 (5) | – | - |
| Congenital hypothyroidism | 1 (3) | – | 1 (6) | - |
| Symptoms n (%) | ||||
| Fever | 24 (63) | 9 (45) | 15 (83) | 0.02 |
| Cough | 26 (68) | 15 (75) | 11 (61) | 0.489 |
| Dyspnea | 27 (71) | 9 (45) | 18 (100) | 0.000 |
| Altered consciousness | 26 (68) | 15 (75) | 11 (61) | 0.489 |
| Convulsion/seizures | 15 (39) | 10 (50) | 5 (28) | 0.198 |
| Convulsive status | 3 (8) | 2 (10) | 1 (6) | 1.0 |
| Nausea/Vomiting | 6 (16) | 3 (15) | 3 (17) | 1.0 |
| Diarrhea | 5 (13) | 1 (5) | 4 (22) | 0.17 |
| Complications n (%) | ||||
| Respiratory failure | 25 (66) | 7 (35) | 18 (100) | 0.000 |
| Central nervous system | 21 (55) | 12 (60) | 9 (50) | 0.745 |
| Myocardial damage | 9 (24) | 2 (10) | 7 (39) | 0.058 |
| Laryngeal obstruction | 6 (16) | 0 | 6 (33) | 0.007 |
| DIC | 3 (8) | 0 | 3 (17) | 0.097 |
| ARDS | 4 (11) | 0 | 4 (22) | 0.041 |
| Number of complications | 3 (2,5) | 2 (1,4.5) | 5 (3,7.5) | 0.001 |
| Coinfection n (%) | 15 (39) | 5 (25) | 10 (56) | 0.096 |
| Mycoplasma pneumoniae | 11 (29) | 4 (20) | 7 (39) | 0.288 |
| EB virus | 2 (5) | 0 | 2 (11) | 0.218 |
| Herpes simplex virus | 2 (5) | 1 (5) | 1 (6) | 1.0 |
| PCIS median (Q1,Q3) | 92 (88,98) | 96 (92,100) | 90 (81,93) | 0.001 |
| PELOD2 median (Q1,Q3) | 1.5 (0,3) | 0 (0,1) | 2 (2,8.25) | 0.000 |
| P/F median (Q1,Q3) | 268 (158,380) | 456 (224,520) | 260 (154,310) | 0.057 |
Baseline information of 38 children with severe or critical COVID-19.
Data are presented as the median (interquartile range) or number of patients (percentage) unless otherwise indicated. BPD, Bronchopulmonary dysplasia; PCIS, Pediatric Clinical Illness Score; PELOD2, pediatric logistic organ dysfunction-2 score.
P-values < .05 are in bold type.
P/F, PaO2/FiO2.
Figure 1 illustrates the clinical manifestations and complications in both IMV and non-IMV groups. The most common symptom was respiratory distress, followed by cough, altered mental state, and fever. The most significant complication was respiratory failure, followed by acute brain dysfunction.
Figure 1
3.2 Biochemical and radiological findings
Several continuous variables derived from laboratory data and the chest CT score based on CO-RADS were associated with the requirement for IMV support. Patients in the IMV group had lower mean platelet volume (MPV) levels and higher levels of creatine kinase (CK) and CK-MB isoenzyme. Additional laboratory findings are detailed in Table 2.
Table 2
| Item | Total (N=38) | Non-IMV (n=20) | IMV (n=18) | p-value |
|---|---|---|---|---|
| Blood routine test Median (Q1,Q3) | ||||
| WBC counts cell (*109/L) | 6.2 (5.1,8.43) | 6.045 (5.15,8.8) | 6.3 (4.99,8.13) | 0.953 |
| Neutrophil counts cell (*109/L) | 3.55 (1.9,5.63) | 3.5 (1.5,4.6) | 3.95 (2.375,6.275) | 0.365 |
| Lymphocyte counts cell (*109/L) | 1.35 (0.9,3.23) | 1.4 (0.925,3.475) | 1.3 (0.85,3.225) | 0.539 |
| Hemoglobin levels (mg/L) | 117 (101.5,128.5) | 116.6 (99.25,127.25) | 118 (105.5,129) | 0.831 |
| Platelet counts cell (*109/L) | 179.5 (127,276.5) | 174.5 (138,257) | 185.5 (101.25,386.25) | 0.569 |
| Eosinopenia counts cell (*109/L) | 0.15 (0,0.6) | 0.35 (0,0.9) | 0.1 (0,0.525) | 0.367 |
| MPV fL | 8.45 (7.875,9.6) | 9.1 (8.025,9.8) | 8.25 (7.475,8.875) | 0.035 |
| NLR | 2.29 (0.71,5.91) | 1.61 (0.55,4.88) | 3.194 (0.958,9.202) | 0.219 |
| PLR | 123.32 (73.96,218.33) | 88.35 (53.95,207.77) | 149.09 (93.807,225.865) | 0.169 |
| SII | 349.82 (131.68,1168.08) | 247.31 (83.76,737.68) | 559.15 (248,1370.88) | 0.108 |
| Cardiac biomarkers Median (Q1,Q3) | ||||
| CK (U/L) | 188 (90.75,801.75) | 118 (75.25,240.75) | 294.5 (129.5,1117.75) | 0.044 |
| CKMB (U/L) | 38.5 (19.68,51.25) | 41.5 (20.5,47.75) | 33 (16.6,90) | 0.953 |
| Troponin T (ng/L) | 0.0155 (0.005,0.1183) | 0.013 (0.00425,0.0835) | 0.0165 (0.008,0.182) | 0.497 |
| Troponin I (ug/L) | 0.0274 (0.0046,0.1340) | 0.0061 (0.0039,0.1746) | 0.0355 (0.0088,0.2088) | 0.317 |
| pro-BNP (pg/mL) | 749.45 (223.25,1995.5) | 545 (317.25,7502.75) | 925.45 (182.5,1501) | 0.671 |
| CK - MB isoenzyme (ug/L) | 5.3 (1.55,12.65) | 2.6 (1.3,5.9) | 9.45 (2.175,27.375) | 0.045 |
| LDH (U/L) | 382.5 (316.25,837.25) | 401 (308,1215.75) | 365.5 (308.25,2034.25) | 0.792 |
| Liver function markers Median (Q1,Q3) | ||||
| ALT (U/L) | 31.5 (17,81.5) | 34 (17.5,73.25) | 26.5 (17,121) | 0.93 |
| AST (U/L) | 52.5 (42.75,174) | 49 (39.75,174) | 62.5 (43,215.5) | 0.511 |
| Albumin (g/L) | 38.1 (34.3,41.65) | 37.4 (34.2,41.5) | 38.95 (33.825,43.2) | 0.331 |
| Kidney function Median (Q1,Q3) | ||||
| Cr (µmol/L) | 24.7 (19.9,40.35) | 23.45 (18.6,43.35) | 27 (21.35,39.9) | 0.604 |
| Inflammation markers Median (Q1,Q3) | ||||
| CRP (mg/L) | 6.6 (1.19,21) | 6.6 (1.1775,17.4) | 7.2 (1.55,24.98) | 0.538 |
| IL-6 (pg/mL) | 17.15 (6.69,47.76) | 16.17 (7.59,29.95) | 22.99 (3.88,245.93) | 0.233 |
| PCT (ng/mL) | 0.495 (0.195,2.35) | 0.408 (0.231,1.56) | 0.834 (0.15,2.905) | 0.692 |
| Coagulation function Median (Q1,Q3) | ||||
| PT (s) | 12.8 (11.1,14.6) | 12.2 (10.65,14.2) | 13.3 (11.25,15.1) | 0.409 |
| APTT (s) | 34 (30,43) | 34.9 (32,43) | 34 (28,45) | 0.741 |
| INR | 1.2 (1.0,1.3) | 1.1 (0.95,1.25) | 1.25 (1.0,1.425) | 0.157 |
| Fib (g/L) | 2.1 (1.5,2.6) | 2 (1.455,2.515) | 2.3 (1.475,2.725) | 0.457 |
| D-dimer (DDU) | 314 (211,1242) | 287 (208,1173) | 376 (220,2180) | 0.428 |
| Myoglobin (ug/L) Median (Q1,Q3) | 34.65 (16.58,150.68) | 17.25 (14.48,36.35) | 78.65 (22.5,364.38) | 0.012 |
| Lactate (mmol/L) Median (Q1,Q3) | 1.7 (1.1,2.4) | 1.3 (1.0,1.72) | 2.05 (1.1,3.65) | 0.111 |
| LAR | 0.0414 (0.0295,0.0701) | 0.0390 (0.0265,0.0574) | 0.0582 (0.0309,0.0809) | 0.181 |
| FAR | 0.0550 (0.0369,0.0661) | 0.0550 (0.0402,0.0655) | 0.0562 (0.0367,0.0783) | 0.717 |
| CAR | 0.1570 (0.0315,0.6274) | 0.1570 (0.0295,0.5673) | 0.1921 (0.0412,0.6860) | 0.649 |
| LDH/Alb | 9.6062 (8.1433,24.9026) | 9.7608 (8.2740,36.4935) | 9.4517 (6.6339,15.1263) | 0.622 |
| Glu (mmol/L) Median (Q1,Q3) | 5.6 (4.74,7.05) | 5.32 (4.31,6.62) | 6.2 (4.95,8.65) | 0.223 |
| Immunoglobulin (g/l) Median (Q1,Q3) | ||||
| IgA | 0.4285 (0.1525,1.1465) | 0.466 (0.08,1.25) | 0.296 (0.154,1.183) | 0.692 |
| IgM | 0.7935 (0.4953,1.1375) | 0.787 (0.489,1.17) | 0.8 (0.473,1.165) | 0.925 |
| IgG | 6.35 (4.2875,9.0425) | 7.33 (4.97,14.9) | 5.23 (3.475,8.19) | 0.168 |
| T-lymphocyte subsets cell (/ul) Median (Q1,Q3) | ||||
| T-lymphocyte count | 656 (510,1822.5) | 868 (464.5,2159) | 645 (528,1133) | 0.63 |
| Th count | 313 (219,830) | 435 (212.5,1570.5) | 435 (212.5,1570) | 0.324 |
| Ts count | 266 (177.5,518.5) | 266 (154,590.5) | 249.5 (192,465.75) | 0.809 |
| Natural killer cells count | 185.5 (87,273) | 213 (107,389.5) | 145 (67,243) | 0.222 |
| B-lymphocyte count | 480 (287,785.5) | 712 (208.5,1381) | 479 (360,526) | 0.475 |
| Ts/Th | 1.21 (0.945,1.615) | 1.3 (0.955,2.29) | 1.1 (0.708,1.48) | 0.293 |
| Chest CT Score | 2.5 (1,4.25) | 1 (1,3) | 4 (1.75,5) | 0.008 |
Comparison of the laboratory indices between non-IMV and IMV groups.
Data are presented as the median (interquartile range) or number of patients (percentage) unless otherwise indicated. ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BNP, Brain natriuretic peptide; IL-6, Interleukin-6; CRP, C-reactive protein; LDH, Lactate dehydrogenase; LAR, Lactate/Albumin; FAR, Fibrinogen/Albumin; CAR, CRP/Albumin; NLR, neutrophil-lymphocyte ratio; PLR, Platelet-Lymphocyte Ratio; SII, systemic immune inflammation index; MLR, monocyte-lymphocyte ratio.
P-values < .05 are in bold type.
Chest CT was performed in 34 (89%) patients, all of whom exhibited inflammatory lesions, with 27 (79%) showing bilateral lung involvement. Consolidation was present in 12 (35%) cases (Supplementary Table 2). The chest CT scans of four children with ARDS with Omicron infections are shown in Figure 2. As depicted in the figure, the image revealed ground-glass opacity and large inflammation areas with partial consolidation, with two cases showing pneumothorax. Additional chest CT scans are provided in Supplementary Figure 1.
Figure 2
Eighteen of 21 children (86%) with COVID-19-linked neurological disorders received head MRI, of whom 12 (67%) showed abnormalities. The most frequent clinical manifestations in these patients were unconsciousness and convulsions. MRI findings indicated that multiple brain regions were affected, either focally or diffusely. Figure 3 displays typical MRI findings from five critically ill children with neurological disorders. Nine of the 12 (75%) patients with MRI abnormalities had bilateral brain involvement, with the most commonly affected regions being the paraventricular white matter and the frontal and occipital lobes. These abnormalities were associated with higher mRS scores at discharge. Detailed descriptions of MRI findings are available in Supplementary Table 3. Additionally, 17 (81%) patients received lumbar puncture for cerebrospinal fluid (CSF) analysis, but none tested positive for the new coronavirus in the CSF. Details are described in Supplementary Table 2.
Figure 3
3.3 Therapeutic and outcome data
Details on medications, respiratory support, and advanced therapies administered to patients, along with their outcomes, are summarized in Table 3. Children in the IMV group had significantly longer PICU stays (p < 0.001). In this group, two patients (5%) died: one patient succumbed to acute necrotizing encephalopathy, while the other died due to ARDS, lung air leak, and cutaneous emphysema. Meanwhile, eight patients had mRS scores ≥ 3 at discharge, including the two deceased patients. At 90-day follow-up, mRS scores ≥ 3 persisted in two patients.
Table 3
| Item | Total (N=38) | Non-IMV (n=20) | IMV (n=18) | p-value |
|---|---|---|---|---|
| Treatment n (%) | ||||
| Antibiotics | 34 (89) | 16 (80) | 18 (100) | 0.107 |
| Corticosteroids | 31 (82) | 13 (65) | 18 (100) | 0.009 |
| Dexamethasone | 16 (42) | 4 (20) | 12 (67) | 0.008 |
| Methylprednisolone | 17 (45) | 9 (45) | 8 (44) | 1.0 |
| IVIG | 15 (39) | 7 (35) | 8 (44) | 0.741 |
| Mannitol | 13 (34) | 7 (35) | 6 (33) | 1.0 |
| Albumin | 7 (18) | 1 (5) | 6 (33) | 0.038 |
| Antiviral drugs | 3 (8) | 0 | 3 (17) | 0.097 |
| Azvudine | 1 (3) | 0 | 1 (6) | - |
| Remdesivir | 1 (3) | 0 | 1 (6) | - |
| Monoclonal antibodies | 1 (3) | 0 | 1 (6) | - |
| Alprostadil | 10 (26) | 4 (20) | 6 (33) | 0.468 |
| Adrenaline | 3 (8) | 0 | 3 (17) | - |
| Breathing support n (%) | ||||
| IMV≥96 h | 16 (42) | 0 | 16 (89) | 0.000 |
| IMV<96 h | 2 (5) | 0 | 2 (11) | - |
| Duration of IMV (h) | 103.5 (0,170) | 0 | 103.5 (0,170) | <0.001 |
| High frequency ventilation | 1 (3) | 0 | 1 (6) | 0.474 |
| Inhaled nitric oxide therapy | 2 (5) | 0 | 2 (11) | 0.218 |
| Non-invasive assisted ventilation | 11 (29) | 5 (25) | 6 (33) | 0.724 |
| Nasal cannula oxygen therapy | 7 (18) | 3 (15) | 4 (22) | 0.687 |
| Advanced therapies n (%) | ||||
| Plasma exchange | 7 (18) | 4 (20) | 3 (17) | 1.0 |
| CRRT | 1 (3) | 0 | 1 (6) | – |
| Tracheotomy | 1 (3) | 0 | 1 (6) | – |
| LOS (days) Median (Q1,Q3) | 11.5 (7,18.25) | 7.5 (5,11.75) | 17 (12.25,22.5) | 0.000 |
| Outcomes n (%) | ||||
| LOS < 14 days and discharge | 22 (58) | 17 (85) | 5 (28) | 0.001 |
| LOS ≥ 14 days and discharge | 14 (37) | 3 (15) | 11 (61) | 0.006 |
| Death | 2 (5) | 0 | 2 (11) | 0.218 |
| mRS score at discharge Median (Q1,Q3) | 0 (0.1.25) | 0 (0,0) | 0 (0,4) | 0.298 |
Treatment and outcomes of IMV and non-IMV groups with Omicron variant infection.
Data are presented as the median (interquartile range) or number of patients (percentage). IVIG, Intravenous immunoglobulin; IMV, Invasive Mechanical Ventilation; CRRT, Continuous Renal Replacement Therapy; LOS, Length of hospital stays; IQR, interquartile range; mRS score, Modified Rankin Scale.
P-values < .05 are in bold type.
3.4 Analysis of factors associated with poor outcomes
Based on univariate analysis, sex and age were excluded from the subsequent multivariate analysis. In the multivariable model, the results showed that the chest CT score was the only independent predictor of IMV requirement in severe and critically ill children (OR 2.781 [95% CI 1.021–7.571]) (Table 4).
Table 4
| Item | Non-IMV (n=20) | IMV (n=18) | OR (95%CI) | p-value |
|---|---|---|---|---|
| Univariable logistic regression analyses | ||||
| Disease severity classification (Critical) | 8 (40) | 18 (100) | – | 0.998 |
| PCIS ≤ 80 | 1 (5) | 4 (22) | - | 0.149 |
| Number of complications≥3 | 8 (40) | 16 (89) | 12 (2.147-67.067) | 0.005 |
| Chest CT Score based on CO-RADS | 1 (1,3) | 4 (1.75,5) | 2.028 (1.135,3.624) | 0.017 |
| CK≥188 (U/L) | 4 (20) | 9 (50) | 4.0 (0.954,16.769) | 0.058 |
| CK-MB isoenzyme (ug/L) ≥10 | 1 (5) | 9 (50) | 14 (1.507,130.099) | 0.02 |
| Myoglobin (ug/L)≥34.65 | 1 (5) | 6 (33.3) | 6.75 (0.662-68.779) | 0.107 |
| IL-6≥20 pg/mL | 4 (20) | 10 (56) | 5 (1.188-21.039) | 0.028 |
| Multivariable logistic regression analysis | ||||
| Number of complications≥3 | 8 (40) | 16 (89) | 4.919 (0.307-78.74) | 0.26 |
| CK-MB isoenzyme (ug/L) ≥10 | 1 (5) | 9 (50) | - | 0.999 |
| Chest CT Score based on CO-RADS | 1 (1,3) | 4 (1.75,5) | 2.781 (1.021-7.571) | 0.045 |
| IL-6≥20 pg/mL | 4 (20) | 10 (56) | 4.045 (0.212-77.011) | 0.353 |
Predictors of invasive mechanical ventilation.
Data are presented as the median (interquartile range) or number of patients (percentage). P-values < .05 are in bold type.
For secondary outcomes, multivariate analysis identified PELOD-2 (OR 2.717 [95% CI 1.011–7.299]) and serum AST levels (OR 14.766 [95% CI 1.395–156.308]) at admission as predictors of poor outcomes (death or prolonged PICU stay) in all severe and critically ill children with SARS-CoV-2 Omicron infection (Table 5).
Table 5
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| Univariable logistic regression analyses | OR (95%CI) | p-value | OR (95%CI) | p-value |
| Number of complications≥3 | 21.667 (2.412,194.649) | 0.006 | – | – |
| mRS score | 2.01 (1.173,3.445) | 0.011 | - | – |
| PELOD2≥3 | 23.8 (3.99,141.963) | 0.001 | 2.717 (1.011,7.299) | 0.047 |
| Neutrophil counts cell ≥3.55 × 109/L | 4.714 (1.178,18.861) | 0.028 | – | - |
| CK≥188 (U/L) | 4.714 (1.178,18.861) | 0.028 | – | - |
| Troponin I (ug/L) ≥0.0274 | 4.4 (1.041,18.599) | 0.044 | – | – |
| AST≥52.5 (U/L) | 11.556 (2.411,55.392) | 0.002 | 14.766 (1.395,156.308) | 0.025 |
| IL-6≥20 pg/mL | 8.25 (1.65,41.247) | 0.008 | – | – |
| PCT (ng/mL)≥0.5 | 6.5 (1.467,28.804) | 0.014 | – | - |
| PT≥12.8 (s) | 12.133 (2.405,61.202) | 0.003 | – | – |
| INR≥1.2 | 12.133 (2.405,61.202) | 0.003 | – | – |
| D-dimer (DDU)≥314 | 12.133 (2.405,61.202) | 0.003 | 4.51 (0.494,41.169) | 0.182 |
| Lactate≥1.7 (mmol/l) | 5.28 (1.196,23.317) | 0.028 | – | – |
Predictors of death or PICU stay ≥ 14 days.
P-values < .05 are in bold type.
4 Discussion
In this study, we described the clinical characteristics of severe and critically ill children diagnosed with the first wave of SARS-CoV-2 Omicron infection who were admitted to the PICU of the National Regional Children’s Medical Center in Northeast China. Our findings indicate that chest CT score based on CO-RADS, serum AST levels at admission, and the PELOD-2 score may predict adverse outcomes in this specific population.
Our data showed that 61% of severely and critically ill children were younger than 3 years old. A statistical analysis of children in France with acute COVID-19 infections in the PICU found that 45.1% of patients were aged <2 years (excluding those under 1 month), while 60.7% were aged <5 years (Recher et al., 2023). Similarly, a British study reported that 61% (36/59) of PICU patients were aged <5 years (Ward et al., 2023). With the emergence of the Omicron variant, mathematical modeling and quantitative analyses of empirical data predict that first-time infections may occur at younger ages due to rising population immunity from prior Omicron infections and vaccination efforts (Koelle et al., 2022). A plausible explanation for this trend is that children aged <3 years remain unvaccinated (Tachikawa et al., 2025), making them more vulnerable to severe initial infections and increasing likelihood of requiring PICU admission.
Comorbidities have been consistently associated with severe SARS-CoV-2 infection in pediatric populations, with studies reporting that 15.6% to 83% of pediatric patients have at least one pre-existing condition across various cohorts (Ungar et al., 2023; Kulkarni et al., 2024). Our study aligns with this range, revealing that 24% of patients had comorbidities, with 13% requiring IMV and 8% receiving non-IMV. Although the clinical symptoms of Omicron infection are mild in the vast majority of healthy children, those with comorbidities may experience further deterioration of underlying conditions and a decline in quality of life (Dryden et al., 2022; Jassat et al., 2023; Calcaterra et al., 2024). Compared to the Alpha variant, the Omicron group had a higher prevalence of comorbidities, worse initial laboratory data, and higher in-hospital mortality rates (40.6% vs 15.2%, p = 0.004). The Charlson Comorbidity Index was identified as an independent risk factor for in-hospital mortality (Cheng et al., 2025). These findings highlight the heightened risk of adverse outcomes in pediatric patients with comorbidities, emphasizing the need for closer monitoring and targeted interventions.
At admission, 25 children (66%) were diagnosed with respiratory failure, and four (16%) developed ARDS. A previous study in southern China (Guangdong) supports our findings (Lin et al., 2024b). Similarly, research on critically ill adults and children in the northeastern United States (New York) found that 90% of patients who developed ARDS initially presented with dyspnea (Derespina et al., 2020). Accurate identification of these presentations, combined with the early administration of respiratory support, may improve patient outcomes by minimizing disease progression and alleviating stress in healthcare facilities (Mizuno et al., 2023). Initial studies during the pandemic linked respiratory system impairment at hospital admission to adverse outcomes with ARDS, serving as an independent predictor of disease severity (Ni et al., 2024). Some research suggests that COVID-19 comorbidities increase with latitude, but we found no evidence to confirm an association between geographic location and complications or comorbidities in children.
COVID-19 is known to cause neurological complications (Bhalala et al., 2022). Studies report that approximately one-third of hospitalized children with Omicron infection experience neurological disorders, such as convulsions (Choi et al., 2023; Tang L. et al., 2024), with higher incidence rates in severe cases (LaRovere et al., 2023). A study by Lin et al. found that 70% of critically ill children with SARS-CoV-2 Omicron infection (Lin et al., 2024b) developed encephalopathy. Among the cohort, 21 (55%) had neurological complications, with convulsion being the most common manifestation (39%). However, we could not determine whether SARS-CoV-2 infection was the primary cause of these neurological symptoms. There are some potential pathogenic mechanisms of SARS-CoV-2 infection affecting the central nervous system (CNS): direct invasion of the CNS, excessive release of pro-inflammatory cytokines, and the immune escape effect of the virus (Viviani et al., 2003; Hautala et al., 2021; Kurd et al., 2021; Shi et al., 2023).
In our study, 12 patients had abnormal head MRI findings. A single-center study conducted in the United States found that the most prevalent imaging findings among adults were nonspecific white matter microangiopathy (55.4%), chronic infarcts (19.4%), acute or subacute ischemic infarcts (5.4%), and acute hemorrhage (4.5%) (Radmanesh et al., 2020). Additionally, a study focusing on ICU patients in France reported that 23% (6/26) of patients experienced cerebrovascular events (Cleret de Langavant et al., 2021). Research in South China involving children supports our findings, indicating that all cases exhibited bilateral involvement, particularly in the thalamus and basal ganglia (Lin et al., 2024a). Possible reasons for these differences in brain involvement or clinical manifestations may relate to age and cardiovascular risk factors (Lu et al., 2024), or the likelihood of children developing acute necrotizing encephalopathy may be higher (Karami et al., 2023). Multicenter cohort studies on the adverse neurological functional outcomes and exploring factors are needed.
Our research shows that chest CT score is associated with the need for IMV in severe and critically ill children. Li et al. did not calculate chest CT scores but described CT findings of consolidation, linear opacities, crazy-paving pattern, bronchial wall thickening, high CT scores, and extrapulmonary lesions as features of severe or critical COVID-19 pneumonia (Li et al., 2020). A study conducted in Brazil on non-invasive respiratory support and IMV concluded that alternating non-invasive respiratory support with HFNO increased IMV rates, regardless of comorbidities and chest CT scores among patients with COVID-19. The chest CT scores were significantly different among all groups (da Cruz et al., 2024). For immunocompromised patients, the total chest CT score was associated with longer hospitalization and ICU admission (Ghadery et al., 2024). Compared with prior studies, the results in our cohort accurately predicted the need for IMV in severe and critically ill children with first-time Omicron infection. Our findings further support the predictive value of chest CT scores for IMV requirements, which could be enhanced by combining CT analysis with other clinical parameters (Orlandi et al., 2021). The results above suggest that the chest CT score based on CO-RADS could be an important factor in predicting poor outcomes for severe and critically ill children.
The study showed that PELOD-2 and serum AST levels ≥52.5 U/L at admission were associated with increased mortality or prolonged PICU stays. A multi-center survey on prolonged mechanical ventilation (MV) showed that the use of vasoactive agents and higher PELOD-2 scores at the time of the prolonged MV diagnosis were significantly associated with an increased risk of prolonged MV-related death. Meanwhile, early rehabilitation intervention was identified as crucial for improving patient outcomes (Zhang et al., 2024). Several studies used the PELOD-2 to predict mortality and prognosis (El-Nawawy et al., 2017; Schlapbach et al., 2018; Lee et al., 2022). These findings suggest that the predictive power of PELOD-2 may vary across specific subpopulations.
The results of this study indicated that abnormalities in various laboratory indicators were more pronounced in critically ill children within the IMV group. Notably, serum AST levels were a predictor of PICU duration. Other indicators included CK-MB isoenzyme (µg/L) ≥10, IL-6 ≥20 pg/mL, and coagulation indices (PT, INR, D-dimer). Early Omicron infection may induce subclinical cholangiocyte damage through a multifactorial and complex pathogenic process, differing from earlier strains of SARS-CoV-2 (Iheanacho and Enechukwu, 2022). Cao et al. (2024) observed that patients with abnormal liver enzyme levels exhibited significantly elevated inflammatory markers, including PCT, IL-6, and CRP. IL-6, as an inflammatory factor, may be attributed to stress responses in the liver, characterized by the release of major acute-phase cytokines in response to Omicron infection (Dufour et al., 2022). A study on adenovirus (ADV) infection found that AST levels were also associated with longer hospital stays in children (Tang S. et al., 2024). Based on these findings, serum AST levels can be considered a reliable indicator of prolonged PICU stays.
This study has some limitations. First, the relatively small sample size might limit the generalizability of our conclusions to patients in other regions. Second, since this was a retrospective study, potential biases and confounding factors might have influenced the results. Additionally, long-term follow-up data were not collected. Finally, standardized scores were not used to assess disease severity in patients, which should be considered in future research.
5 Conclusions
Respiratory failure and COVID-19-associated neurological disorders are major complications in severe and critically ill children with first-wave SARS-CoV-2 Omicron infection. Chest CT score based on CO-RADS may predict severe and critically ill children with SARS-CoV-2 Omicron infection requiring intensive mechanical ventilation. Additionally, serum AST levels at admission and the PELOD-2 score may predict prolonged PICU stays and mortality, helping to avert the development of potentially life-threatening complications.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The ethics committee of Shengjing Hospital(Ethics No. 2023PS30K). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
TS: Validation, Writing – original draft, Writing – review & editing. YH: Validation, Writing – original draft, Writing – review & editing. LW: Supervision, Validation, Conceptualization, Methodology, Writing – review & editing. ZW: Data curation, Methodology, Writing – review & editing. CL: Supervision, Validation, Data curation, Methodology, Writing – review & editing. WX: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing – review & editing. KY: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China Youth Fund (81501292), China Postdoctoral Fund Surface Project (2017M611285), and the Basic Research Program of Liaoning Province (2022JH2/101500053).
Acknowledgments
The authors would like to express their gratitude to the research group at the Department of Pediatrics of Shengjing Hospital, which is affiliated to China Medical University. Additionally, the authors would like to thank Editage (www.editage.cn) for their assistance with English language editing. Finally, the authors thank editors and reviewers for their valuable comments in the improvement of the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1495783/full#supplementary-material
Abbreviations
PELOD-2, Pediatric Logistic Organ Dysfunction 2 score; AST, Aspartate Aminotransferase; IMV, Invasive Mechanical Ventilation; CT, Computed Tomography; CO-RADS, COVID-19 Reporting and Data System; ARDS, Acute Respiratory Distress Syndrome; mRS, Modified Rankin Scale; PICU, Pediatric Intensive Care Unit; CRP, C-Reactive Protein; IL-6, Interleukin 6; CK-MB, Creatine Kinase-MB isoenzyme; PCT, Procalcitonin; NIV, Non-invasive Ventilation; HFNO, High-Flow Nasal Oxygen; INR, International Normalized Ratio; D-dimer, Fragment of fibrinogen produced when blood clots break down; ADV, Adenovirus; MV, Mechanical Ventilation.
References
1
BakerM. A.SandsK. E.HuangS. S.KleinmanK.SeptimusE. J.VarmaN.et al. (2022). The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections. Clin. Infect. Dis.74, 1748–1754. doi: 10.1093/cid/ciab688
2
BhalalaU. S.GistK. M.TripathiS.BomanK.KumarV. K.RetfordL.et al. (2022). Characterization and outcomes of hospitalized children with coronavirus disease 2019: A report from a multicenter, viral infection and respiratory illness universal study (Coronavirus Disease 2019) Registry. Crit. Care Med.50, e40–e51. doi: 10.1097/CCM.0000000000005232
3
CalcaterraV.TagiV. M.D’AuriaE.LaiA.ZanelliS.MontanariC.et al. (2024). Long-term effects of SARS-CoV-2 infection in hospitalized children: findings from an Italian single-center study. Ital. J. Pediatr.50, 27. doi: 10.1186/s13052-024-01596-y
4
CaoX.XieY. L.YiJ. Y.LiuZ. L.HanM.DuanJ. H.et al. (2024). Altered liver enzyme markers in patients with asymptomatic, and mild Omicron infection: A retrospective study. J. Inflamm. Res.17, 6875–6885. doi: 10.2147/JIR.S478812
5
ChengH. I.ChangK. W.WuB. C.TeoM. Y.HungW. S.WuH. M.et al. (2025). Comparison of clinical characteristics and mortality outcome in critical COVID-19 patients infected with alpha and omicron variants. Infect. Drug Resist.18, 151–160. doi: 10.2147/IDR.S479896
6
Chinese Center for Disease Control and Prevention (2023). Epidemic situation of 2019 novel corona virus infection in China. Available online at: https://www.Chinacdc.cn/jkyj/crb2/yl/xxgzbdgr/xggrqk/202409/t20240906_297057.htm (Accessed March 11, 2023).
7
ChoiS. H.ChoiJ. H.LeeJ. K.EunB. W.SongS. H.AhnB.et al. (2023). Clinical characteristics and outcomes of children with SARS-CoV-2 infection during the Delta and Omicron variant-dominant periods in Korea. J. Korean. Med. Sci.38, e65. doi: 10.3346/jkms.2023.38.e65
8
Cleret de LangavantL.PetitA.NguyenQ. T. R.GendreT.AbdelhediJ.DjellaouiA.et al. (2021). Clinical description of the broad range of neurological presentations of COVID-19: A retrospective case series. Rev. Neurol. (Paris).177, 275–282. doi: 10.1016/j.neurol.2021.01.004
9
CloeteJ.KrugerA.MashaM.du PlessisN. M.MawelaD.TshukuduM.et al. (2022). Pediatric hospitalizations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicenter observational study. Lancet Child Adolesc. Health6, 294–302. doi: 10.1016/S2352-4642(22)00027-X
10
CorrieroA.RibezziM.MeleF.AngrisaniC.RomanielloF.DalenoA.et al. (2022). COVID-19 variants in critically ill patients: A comparison of the Delta and Omicron variant profiles. Infect. Dis. Rep.14, 492–500. doi: 10.3390/idr14030052
11
da CruzA. P.MartinsG.MartinsC. M.MarquesV.ChristovamS.BattagliniD.et al. (2024). Comparison between high-flow nasal oxygen (HFNO) alternated with non-invasive ventilation (NIV) and HFNO and NIV alone in patients with COVID-19: a retrospective cohort study. Eur. J. Med. Res.29, 248. doi: 10.1186/s40001-024-01826-3
12
de ProstN.AudureauE.HemingN.GaultE.PhamT.ChaghouriA.et al. (2022). Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat. Commun.13, 6025. doi: 10.1038/s41467-022-33801-z
13
DerespinaK. R.KaushikS.PlichtaA.ConwayE. E.BercowA.ChoiJ.et al. (2020). Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J. Pediatr.226, 55–63.e2. doi: 10.1016/j.jpeds.2020.07.039
14
DrydenM.MudaraC.VikaC.BlumbergL.MayetN.CohenC.et al. (2022). Post-COVID-19 condition 3 months after hospitalization with SARS-CoV-2 in South Africa: a prospective cohort study. Lancet Glob. Health10, e1247–e1256. doi: 10.1016/S2214-109X(22)00286-8
15
DufourJ. F.MarjotT.BecchettiC.TilgH. (2022). COVID-19 and liver disease. Gut71, 2350–2362. doi: 10.1136/gutjnl-2021-326792
16
El-NawawyA.MohsenA. A.Abdel-MalikM.TamanS. O. (2017). Performance of the pediatric logistic organ dysfunction (PELOD) and (PELOD-2) scores in a pediatric intensive care unit of a developing country. Eur. J. Pediatr.176, 849–855. doi: 10.1007/s00431-017-2916-x
17
EmeriaudG.López-FernándezY. M.IyerN. P.BembeaM. M.AgulnikA.BarbaroR. P.et al. (2023). Second pediatric acute lung injury consensus conference (PALICC-2) group on behalf of the pediatric acute lung injury and sepsis investigators (PALISI) network. Executive summary of the second international guidelines for the diagnosis and management of pediatric acute respiratory distress syndrome (PALICC-2). Pediatr. Crit. Care Med.24, 143–168. doi: 10.1097/PCC.0000000000003147
18
GhaderyA. H.AbbasianL.JafariF.YazdiN. A.AhmadinejadZ. (2024). Correlation of clinical, laboratory, and short-term outcomes of immunocompromised and immunocompetent COVID-19 patients with semi-quantitative chest CT score findings: A case-control study. Immun. Inflamm. Dis.12, e1239. doi: 10.1002/iid3.1239
19
HautalaM.ArvilaJ.PokkaT.MikkonenK.KoskelaU.HelanderH.et al. (2021). Respiratory viruses and febrile response in children with febrile seizures: A cohort study and embedded case-control study. Seizure84, 69–77. doi: 10.1016/j.seizure.2020.11.007
20
IheanachoC. O.EnechukwuO. H. (2022). COVID-19-associated liver injury, role of drug therapy and management: a review. Egypt. Liver. J.12, 66. doi: 10.1186/s43066-022-00230-y
21
JassatW.MudaraC.VikaC.WelchR.ArendseT.DrydenM.et al. (2023). A cohort study of post-COVID-19 condition across the Beta, Delta, and Omicron waves in South Africa: 6-month follow-up of hospitalized and nonhospitalized participants. Int. J. Infect. Dis.128, 102–111. doi: 10.1016/j.ijid.2022.12.036
22
KaramiS.KhalajF.SotoudehH.TajabadiZ.ShahidiR.HabibiM. A.et al. (2023). Acute necrotizing encephalopathy in adult patients with COVID-19: A systematic review of case reports and case series. J. Clin. Neurol.19, 597–611. doi: 10.3988/jcn.2022.0431
23
KoelleK.MartinM. A.AntiaR.LopmanB.DeanN. E. (2022). The changing epidemiology of SARS-CoV-2. Science375, 1116–1121. doi: 10.1126/science.abm4915
24
KulkarniD.IsmailN. F.ZhuF.WangX.Del Carmen MoralesG.SrivastavaA.et al. (2024). Epidemiology and clinical features of SARS-CoV-2 infection in children and adolescents in the pre-Omicron era: A global systematic review and meta-analysis. J. Glob. Health14, 5003. doi: 10.7189/jogh.14.05003
25
KurdM.HashavyaS.BenensonS.GilboaT. (2021). Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure92, 89–93. doi: 10.1016/j.seizure.2021.08.017
26
LaRovereK. L.PoussaintT. Y.YoungC. C.NewhamsM. M.KucukakS.IrbyK.et al. (2023). Changes in distribution of severe neurologic involvement in US pediatric inpatients With COVID-19 or multisystem inflammatory syndrome in children in 2021 vs 2020. JAMA Neurol.80, 91–98. doi: 10.1001/jamaneurol.2022.3881
27
LeeE. J.LeeB.KimY. S.ChoiY. H.KwakY. H.ParkJ. D. (2022). Clinical implications of discrepancies in predicting pediatric mortality between Pediatric Index of Mortality 3 and Pediatric Logistic Organ Dysfunction-2. Acute. Crit. Care37, 454–461. doi: 10.4266/acc.2021.01480
28
LeteurtreS.DuhamelA.SalleronJ.GrandbastienB.LacroixJ.LeclercF.et al. (2013). PELOD-2: An update of the Pediatric Logistic Organ Dysfunction score. Crit. Care Med.41, 1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd
29
LiK.WuJ.WuF.GuoD.ChenL.FangZ.et al. (2020). The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol.55, 327–331. doi: 10.1097/RLI.0000000000000672
30
LinF.ChenM. T.ZhangL.XieH.YangZ.HuangB.et al. (2024a). Hospitalized children with COVID-19 infection during large outbreak of SARS-CoV-2 Omicron strain: a retrospective study in Chaozhou, Guangdong, China. Ann. Med.56, 2389301. doi: 10.1080/07853890.2024.2389301
31
LinF.JiangD. J.ZhangS.YangZ.ZengH. S.LiuZ. P.et al. (2024b). Critically ill children with SARS-COV-2 Omicron infection at a national children medical center, Guangdong, China. BMC Pediatr.24, 254. doi: 10.1186/s12887-024-04735-w
32
LuJ.ZuoX.CaiA.XiaoF.XuZ.WangR.et al. (2024). Cerebral small vessel injury in mice with damage to ACE2-expressing cerebral vascular endothelial cells and post COVID-19 patients. Alzheimers Dement.20, 7971–7988. doi: 10.1002/alz.14279
33
MenniC.ValdesA. M.PolidoriL.AntonelliM.PenamakuriS.NogalA.et al. (2022). Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet399, 1618–1624. doi: 10.1016/S0140-6736(22)00327-0
34
MizunoS.OgawaE.NozakiM.ChoY.KasaiM. (2023). Hospital burden and characteristics of pediatric COVID-19 based on a multicenter collaborative retrospective study in Japan. IJID. Reg.6, 108–112. doi: 10.1016/j.ijregi.2023.01.006
35
NiR.ZhongM.XieM. (2024). Comparative analysis of prognostic scoring systems in predicting severity and outcomes of Omicron variant COVID-19 pneumonia. Front. Med. (Lausanne).11. doi: 10.3389/fmed.2024.1419690
36
OrlandiD.BattagliniD.RobbaC.ViganòM.BergamaschiG.MignattiT.et al. (2021). Coronavirus disease 2019 phenotypes, lung ultrasound, chest computed tomography and clinical features in critically ill mechanically ventilated patients. Ultrasound. Med. Biol.47, 3323–3332. doi: 10.1016/j.ultrasmedbio
37
ProkopM.van EverdingenW.van Rees VellingaT.Quarles van UffordH.StogerL.BeenenL.et al. (2020). Co-rads: a categorical ct assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology296, E97–E104. doi: 10.1148/radiol.2020201473
38
QuinnT. J.DawsonJ.WaltersM. R.LeesK. R. (2009). Reliability of the modified rankin scale: a systematic review. Stroke40, 3393–3395. doi: 10.1161/STROKEAHA.109.557256
39
RadmaneshA.RazE.ZanE.DermanA.KaminetzkyM. (2020). Brain imaging use and findings in COVID-19: A single academic center experience in the epicenter of disease in the United States. AJNR. Am. J. Neuroradiol.41, 1179–1183. doi: 10.3174/ajnr.A6610
40
RecherM.LeteurtreS.JavouheyE.MorinL.BaudinF.RambaudJ.et al. (2023). Risk of admission to the pediatric intensive care unit for SARS-CoV-2 Delta and Omicron infections. J. Pediatr. Infect. Dis. Soc12, 189–197. doi: 10.1093/jpids/piad010
41
RossC. E.BurnsJ. P.GrossestreuerA. V.BhattaraiP.McKiernanC. A.FranksJ. D.et al. (2023). Trends in disease severity among critically ill children with severe acute respiratory syndrome coronavirus 2: A retrospective multicenter cohort study in the United States. Pediatr. Crit. Care Med.24, 25–33. doi: 10.1097/PCC.0000000000003105
42
SchlapbachL. J.StraneyL.BellomoR.MacLarenG.PilcherD. (2018). Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med.44, 179–188. doi: 10.1007/s00134-017-5021-8
43
ShiT.BianY.WuJ.LiX.DengJ.FengT.et al. (2023). Decreased NK cell count is a high-risk factor for convulsion in children with COVID-19. BMC Infect. Dis.23, 856. doi: 10.1186/s12879-023-08556-7
44
SunR.WangH.SunJ.YangM.ZhangS.HuX.et al. (2025). Effectiveness and safety of oral azvudine for elderly hospitalized patients with COVID-19: A multicenter, retrospective, real-world study. Adv. Sci. (Weinh)., e2404450. doi: 10.1002/advs.202404450
45
TachikawaJ.AizawaY.IkuseT.YamanakaT.HasegawaS.SaitohA. (2025). Clinical characteristics of hospitalized children with coronavirus disease 2019 after the spread of the BA.5 Omicron variants in Japan. Pediatr. Infect. Dis. J. doi: 10.1097/INF.0000000000004729
46
TangL.GuoY.ShuC.PengX.QiuS.LiR.et al. (2024). Neurological manifestations and risk factors associated with poor prognosis in hospitalized children with Omicron variant infection. Eur. J. Pediatr.183, 2353–2363. doi: 10.1007/s00431-024-05495-6
47
TangS.QinR.ZhangD.HeX.YuC.ChenD.et al. (2024). Liver injury and prolonged hospitalization as indicators of severity in patients with adenovirus infections. BMC Infect. Dis.24, 430. doi: 10.1186/s12879-024-09324-x
48
TianD.SunY.XuH.YeQ. (2022). The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol.94, 2376–2383. doi: 10.1002/jmv.27643
49
UkaA.BuettcherM.Bernhard-StirnemannS.FougèreY.MoussaouiD.KottanattuL.et al. (2022). Factors associated with hospital and intensive care admission in pediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur. J. Pediatr.181, 1257. doi: 10.1007/s00431-021-04359-7
50
UngarS. P.SolomonS.StachelA.ShustG. F.ClouserK. N.BhavsarS. M.et al. (2023). Hospital and ICU admission risk associated with comorbidities among children with COVID-19 ancestral strains. Clin. Pediatr. (Phila).62, 1048–1058. doi: 10.1177/00099228221150605
51
VivianiB.BartesaghiS.GardoniF.VezzaniA.BehrensM. M.BartfaiT.et al. (2003). Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci.23, 8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003
52
WangL.BergerN. A.KaelberD. C.DavisP. B.VolkowN. D.XuR. (2022). COVID infection severity in children under 5 years old before and after Omicron emergence in the US. MedRxiv. doi: 10.1101/2022.01.12.22269179
53
WangB.YuY.YuY.WangN.ChenF.JiangB.et al. (2023). Clinical features and outcomes of hospitalized patients with COVID-19 during the Omicron wave in Shanghai. China. J. Infect.86, e27–e29. doi: 10.1016/j.jinf.2022.08.001
54
WardJ. L.HarwoodR.KennyS.CruzJ.ClarkM.DavisP. J.et al. (2023). Pediatric hospitalizations and ICU admissions due to COVID-19 and pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 in England. JAMA Pediatr. 177, 947–955. doi: 10.1001/jamapediatrics.2023.2357
55
WhittakerR.Greve-IsdahlM.BøåsH.SurenP.BuanesE. A.VenetiL. (2022). COVID-19 hospitalization among children <18 years by variant wave in Norway. Pediatrics150, e2022057564. doi: 10.1542/peds.2022-057564
56
WolterN.JassatW.WalazaS.WelchR.MoultrieH.GroomeM.et al. (2022). Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet399, 437–446. doi: 10.1016/S0140-6736(22)00017-4
57
World Health Organization (2021). Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern (Accessed November 26,2021).
58
World Health Organization (2023a). Coronavirus (COVID-19) dashboard. Available online at: https://data.who.int/dashboards/covid19/data?n=c (Accessed March 16, 2023).
59
World Health Organization (2023b). Statement on the update of WHO’s working definitions and tracking system for SARS-CoV-2 variants of concern and variants of interest. Available online at: https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest (Accessed March 16,2023).
60
ZhangZ.CaiX.MingM.HuangL.LiuC.RenH.et al. (2024). Incidence, outcome, and prognostic factors of prolonged mechanical ventilation among children in Chinese mainland: a multi-center survey. Front. Pediatr.12. doi: 10.3389/fped.2024.1413094
61
ZhangJ. J.DongX.LiuG. H.GaoY. D. (2023). Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol.64, 90–107. doi: 10.1007/s12016-022-08921-5
62
ZhangC.LiuX.YanR.NieX.PengY.ZhouN.et al. (2025). The development and validation of a prediction model for post-AKI outcomes of pediatric inpatients. Clin. Kidney J.18, sfaf007. doi: 10.1093/ckj/sfaf007
Summary
Keywords
COVID-19, Omicron variant, PICU, respiratory failure, neurological disorder
Citation
Sun T, He Y, Wang Z, Wang L, Liu C, Xu W and You K (2025) Characteristics and outcomes in severe and critically ill children with first wave SARS-CoV-2 Omicron infection in Northeast China. Front. Cell. Infect. Microbiol. 15:1495783. doi: 10.3389/fcimb.2025.1495783
Received
13 September 2024
Accepted
17 March 2025
Published
15 April 2025
Volume
15 - 2025
Edited by
Zhongjie Shi, Wayne State University, United States
Reviewed by
Praveen Neeli, Baylor College of Medicine, United States
Chuanke Zhao, Peking University, China
Ying Meng, Chongqing Medical University, China
Updates
Copyright
© 2025 Sun, He, Wang, Wang, Liu, Xu and You.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai You, youk@sj-hospital.org; Wei Xu, tomxu.123@163.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.