- 1Laboratory Animal Research Facility, National Infrastructures for Translational Medicine, Institute of Clinical Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2The State Key Laboratory for Complex, Severe, and Rare Diseases, Peking Union Medical College Hospital, Beijing, China
- 3School of Life Sciences, Henan University, Kaifeng, Henan, China
- 4Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
- 5Biomedical Engineering Facility of National Infrastructures for Translational Medicine, Institute of Clinical Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 6School of Engineering Medicine, Beihang University, Beijing, China
- 7Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Interdisciplinary Innovation Institute of Medicine and Engineering, Beihang University, Beijing, China
Despite numerous studies investigating intratumoral microorganisms and their significant roles in cancer initiation, progression, and treatment efficacy, a systematic understanding of intratumoral microorganisms remains lacking. Herein, we conducted a study using 16S rDNA data on seven types of cancer, comprising a total of 783 samples. It’s worth noting that Pseudomonas, Streptococcus, and Prevotella were found to be shared with the microbial communities of the seven cancers, suggesting that these may be associated with the occurrence and development of cancers. We anticipate establishing a foundation for related research and exploring potential methods for cancer treatment.
Introduction
According to the 2024 World Health Organization report, cancer is listed in the top 10 causes of death (Global health estimates: Leading causes of death). Crosby’s study in 2022 also shows that cancer remains a major global health challenge (Crosby et al., 2022). Microorganisms in tumors were discovered over a hundred years ago (Gagliani et al., 2014). However, due to limitations in technology and potential contamination issues, studies on microorganisms within tumors have yet to be comprehensively developed. It was not until 2014 that Gagliani et al. introduced the concept of intratumoral microbiome in tumor tissue while studying bowel cancer (Gagliani et al., 2014). Subsequently, a study published in Science in 2020 conducted an in-depth analysis of intratumoral microbiomes in up to multiple of cancer tissues, revealing that the intratumoral microbes predominantly reside within tumor cells and immune cells (Nejman et al., 2020). Further studies indicate that intratumoral microbes may play an important role in the initiation and development of cancer as well as its efficacy through DNA mutations, promotion of chronic inflammation, etc (Yang et al., 2023). Therefore, the study of intratumoral microbes in cancer is necessary.
Along with the study of intratumoral microbes, some far-reaching findings have been produced in recent years. For example, Abe et al. revealed a significant positive correlation between a shorter survival time and the presence of anaerobic bacteria such as Bacteroides, Lactobacillus, and Peptoniphilus (Yu et al., 2025). In Chai’s microbial study of cholangiocarcinoma, Paraburkholderia fungorumwas was found to inhibit tumor growth through alanine, aspartate, and glutamate metabolism (Chai et al., 2023). Zhang et al. found that intra-tumoral Fusobacterium nucleatum further promotes the growth and immune infiltration of senescent esophageal squamous cell carcinoma cells to secretion of senescence-associated secretory phenotype, which accelerated tumor growth (Zhang et al., 2023). Li et al. pinpoint intracellular Shewanella algae as a foremost LM risk factor in both AI- and non-AI-treated patients (Li et al., 2024). All these results suggest a close link between intratumoral microorganisms and tumors. However, owing to the diversity of cancer types, there is still a lack of systematic understanding of intratumoral microbes.
In this study, we collected a total of 783 samples from seven cancer types, including breast, esophageal, gastric, liver, lung, pancreatic cancers and oral squamous cell carcinoma(OSCC) to study the intratumoral microbiota. In order to explore the intratumoral microbiota in detail, based on the publicly available samples, we performed various analysis strategies such as Alpha diversity index comparison, LDA analysis, and Co-abundance network analysis et al. Our goal is to provide a holistic understanding of the intratumoral microbial composition of cancer tissues through the above analyses, to advance research related to intratumoral microbiology, and to provide a scientific basis for possible therapeutic options.
Materials and methods
Data accession and sequence processing
For all available samples, we downloaded the pair-end sequencing files of 16S rDNA for each sample from ENA database, and all files were downloaded as FASTQ format. We performed data quality control using FastQC version 0.12.0 for the raw data of each cancer-type sample (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). For raw reads, we performed splice removal using TrimGalore version 0.6.5 with the default splice ‘AGATCGGAAGAGC’ (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Subsequently, the trimmed pair-end reads were merged in EasyAmplicon software using vsearch software version v2.22.1 and the merged sequences were dereplicated (Rognes et al., 2016; Liu et al., 2023). We analyzed these 16S data using uniform standards to ensure that data noise was minimized.
Taxonomic classification and bioinformatic analysis
We denoised the dereplicated sequences to obtain ASVs using usearch software at minsize=10. Taxonomy classification was performed in EasyAmplicon using the rdp_16s_v18.fa database, and then the same types of taxa were summarized at the phylum, class, order, family, and genus levels when the raw counts were normalized to an ASV table of relative abundance groups (Liu et al., 2023). Subsequently, the visualization of the taxonomy classification of microbial communities was performed using the tax_stackplot.R script in EasyAmplicon software (Liu et al., 2023).
Using R package vegan version 2.6-8, we calculated the alpha diversity indices (Shannon index, Simpson index, observed_features index, and pielou_evenness index) of microorganisms from seven different cancer tissues (vegan: Community Ecology Package). The alpha diversity visualization was performed with R package Tidyverse version 2.0.0 (Wickham et al., 2019).The diversity of the bacterial community was assessed by the Shannon and Simpson indices, the richness of the bacterial community was assessed by the observed_features index, and the evenness of the bacterial community was assessed by the pielou_evenness index. To compare the differences in the diversity indices of microbial communities between control and case in six cancer types, we conducted an ANOVA significance test (p < 0.05). In addition, we performed β-diversity analysis based on Bray-Curtis distance using the online analysis platform www.bioincloud.tech (Gao et al., 2024). The identification of abundance-differentiated microorganisms was carried out using linear discriminant analysis (LDA) of effect sizes. LDA scores indicate the effect sizes of each ASV, and ASVs with LDA values >2.0 were defined as ASVs of differential abundance. The results obtained were used for data visualization using ggplot2 version 3.5.1 and RColorBrewer version 1.1–3 packages in R4.3.2. The online analysis platform www.bioincloud.tech was utilized to visualize the co-abundance networks of microbial communities at the genus level for each type of cancer, as well as to create a clustering heatmap of the thirteen most common bacteria, which is selected from many past researches (Eun et al; Farrell et al; Qiao et al; Tsay et al; Zhang et al; Urbaniak et al., 2016; Klann et al., 2020; Nalluri et al., 2021; Gao et al., 2024).
Statistical analysis
We performed statistical analyses on the IBM SPSS Statistics 25 platform. We used ANOVA and LSD tests to compare the differences in each diversity index between groups. The co-abundance network among microbial taxa were calculated using Spearman rank correlation analysis. Only correlation coefficients greater than 0.4 will be showed.
Results
Intratumoral microbial data collection and statistics
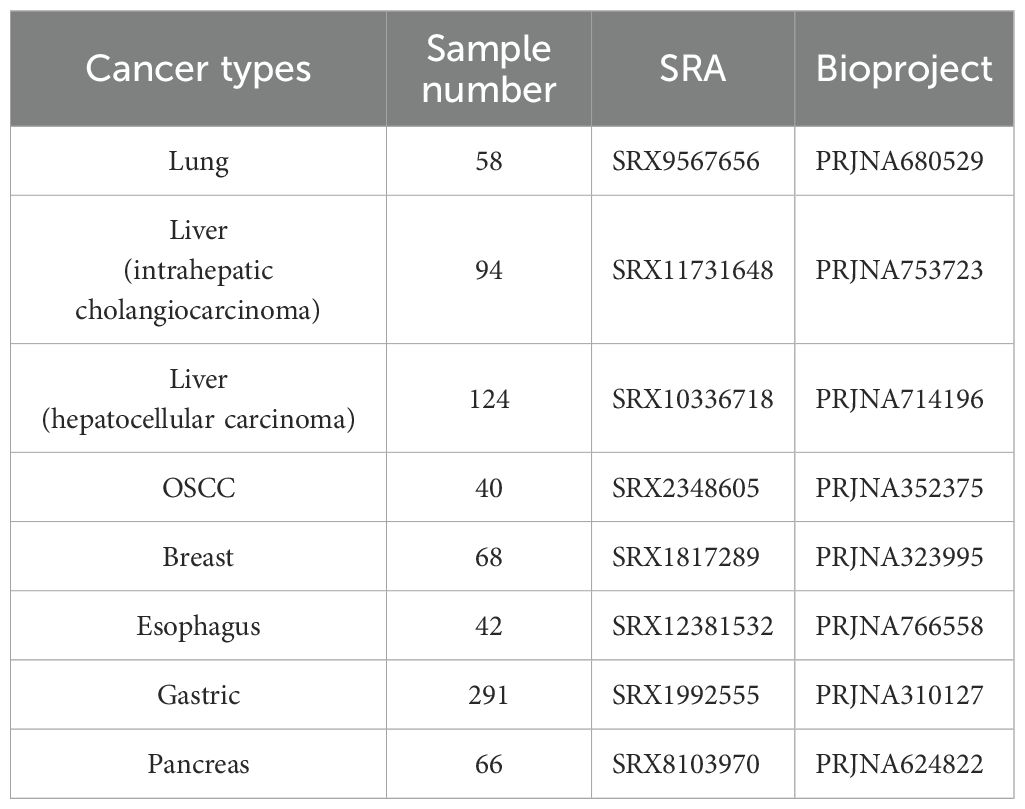
A total of 783 samples (31.7 GB in Genome size) of seven cancer types (Supplementary Table S1) were selected from eight Bioprojects for this study, and 43689 ± 41918 reads (mean ± SD) were obtained from each sample after quality control (Table 1; Supplementary Table S1). There are lung cancer (29 cancerous samples; 29 healthy tissue samples), intrahepatic cholangiocarcinoma (45 cancerous samples; 49 paracancerous samples), hepatocellular carcinoma (63 cancerous samples; 61 paracancerous samples), OSCC(20 cancerous samples; 20 healthy tissue samples), breast cancer(45 cancerous samples; 23 healthy tissue samples), esophageal cancer (21 cancerous samples; 21 healthy tissue samples), gastric cancer (134 cancerous samples; 157 healthy samples), and pancreatic cancer (66 cancerous samples). Among them, we combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma into liver cancer tissues for further analysis.
α diversity and β diversity of intratumoral microorganism in pan-cancer analysis
In the α-diversity analysis, we found that gastric cancer and liver cancer were extremely significant difference (p < 0.001) in mean Shannon index and mean microbial Simpson’s index between healthy tissue samples and cancerous samples. There was no significance (p > 0.05) difference of mean microbial Shannon index and mean microbial Simpson’s index between healthy tissue samples and cancerous samples in esophageal cancer, OSCC and pancreatic cancer. The mean microbial Shannon index and mean microbial Simpson’s index difference in breast cancer and liver cancer were also significant difference, p – value < 0.01 and p – value < 0.05, respectively (Figures 1A, B; Supplementary Table S2). The mean value of the microbial observed_features index between control and tumor of liver cancer samples and gastric cancer samples was significantly difference(p<0.001) (Figure 1C; Supplementary Table S2). There is no significant difference in the mean observed_features index of microbial diversity in remaining cancer types between control and case (Figure 1C; Supplementary Table S2). The mean microbial evenness index between control and case was greatly significant (p < 001) difference in breast tumor, gastric tumor and lung tumor (Figure 1D; Supplementary Table S2). There was significant difference in the mean microbial evenness index between control and case in liver cancer and OSCC with a different degree, p – value < 0.01 and p – value < 0.05, respectively (Figure 1D; Supplementary Table S2). There was no significance difference in the mean microbial evenness index between control and case of esophageal cancer and pancreatic cancer (Figure 1D; Supplementary Table S2).
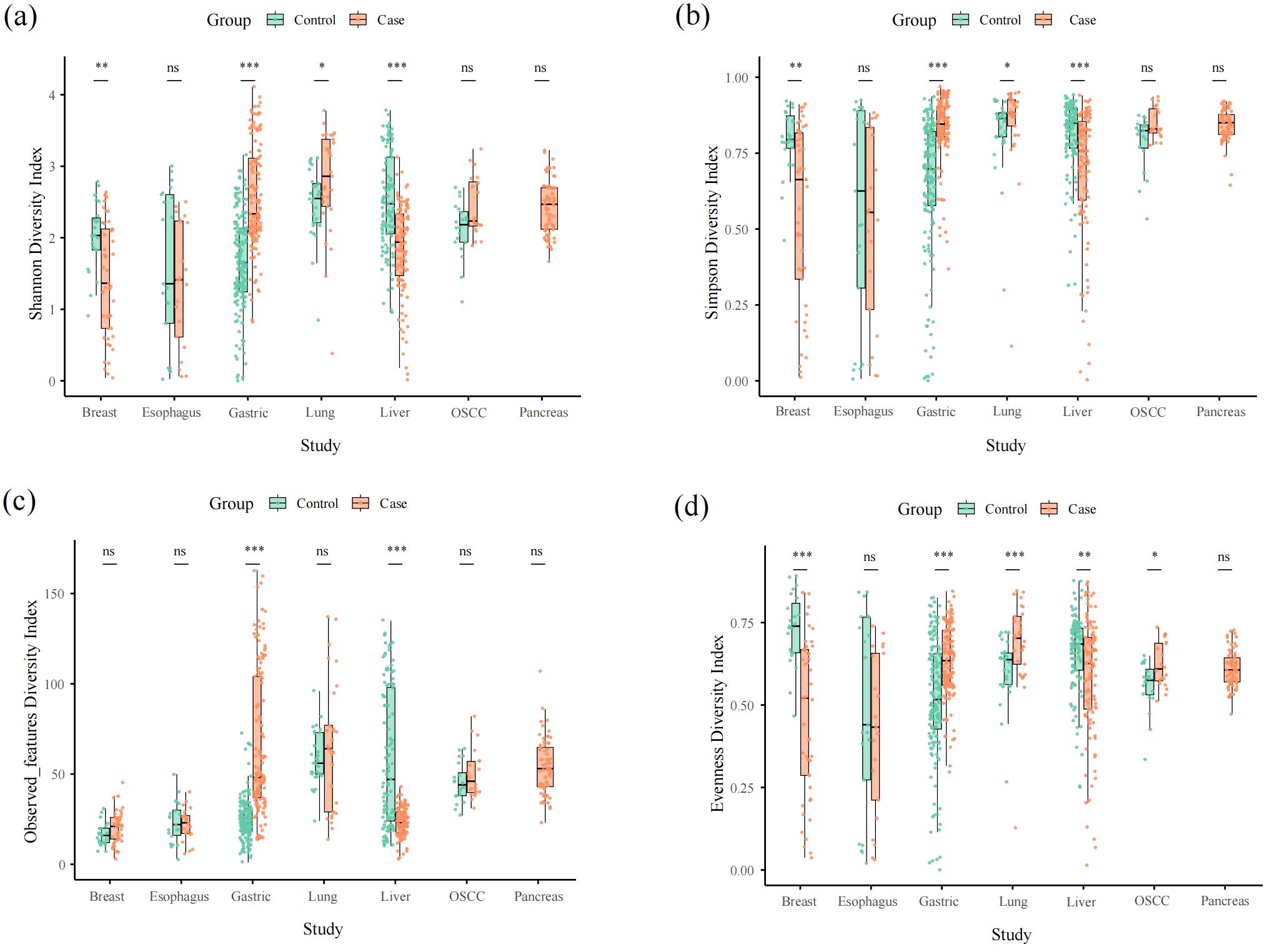
Figure 1. Alpha diversity box plot (Shannon, Simpson, observed_features and pielou_evenness) among seven cancer types. (a–d) represent shannon, simpson, observed_features and evenness aplha diversity, respectively.
Based on the Bray-Curtis distance, principal coordinate analysis (PCoA) revealed that the similarity of microbial communities between breast cancer samples and pancreatic cancer samples is high (Supplementary Figure S1). A distinct trend of dispersion was observed in gastric group, suggesting a high variability of bacterial composition (Supplementary Figure S1).
Taxonomy classification of intratumoral microorganism in pan-cancer analysis
A total of 32 phyla, 62 classes, 109 orders, 246 families, and 721 genera were detected in Pan-cancer analysis. Overall, the intramural microorganisms of the seven different cancers were mainly composed of Proteobacteria, Firmicutes, and Actinobacteria at the phylum level, and the proportion of Proteobacteria in the seven different cancer was as follows in descending order: pancreatic cancer (79.88%), hepatocellular carcinoma (48.71%), breast cancer (47.06%), lung cancer (44.31%), OSCC (27.14%), gastric cancer (26.89%) and esophageal cancer (26.31%) (Figure 2A). Proteobacteria had the highest proportion in pancreatic cancer. The proportion of Firmicutes in the seven cancer samples in order of highest to lowest is as follows: OSCC (29.54%), breast cancer (29.47%), esophageal cancer (27.63%), gastric cancer (27.15%), pancreatic cancer (13.41%), lung cancer (10.35%), and liver cancer (5.60%). We found that Firmicutes had a high and approximately equal percentage in OSCC, breast, esophageal, and gastric cancer samples, and the lowest percentage in liver cancer. Actinobacteria had a high proportion of 24.74% and 23.48% in liver and lung cancer, respectively, while the lowest percentage is 1.76% in OSCC samples. Bacteroidetes was higher in OSCC, gastric, and esophageal cancer samples with 17.11%, 15.77%, and 15.41%, respectively. The lowest percentage was found in pancreatic cancer with 1.38%. Campilobacterota had the highest percentage of 19.99% in gastric cancer samples and was also present in OSCC, esophageal, lung, and breast cancer samples, but with a smaller proportion. In addition, Parcubacteria had a higher percentage of 14.69% in liver cancer. Fusobacteria and Spirochaetes had higher percentages of 15.50%, 11.39%, 5.23%, and 3.06%, 3.24%, 1.68% in OSCC, esophageal, and gastric cancer samples, respectively. Planctomycetes and Verrucomicrobia were only present and relatively high in lung cancer with 5.81% and 3.83%, respectively. In healthy tissue microbial composition, Proteobacteria, Firmicutes, Actinobacteria was also predominant (Supplementary Figure S2). However, the relative abundance of these microorganisms was different form tumor tissue samples (Supplementary Figure S2). For example, in healthy tissues of OSCC, Firmicutes had a higher relative abundance (Supplementary Figure S2). Compared with esophageal cancer, the relative abundance of Proteobacteria was higher in healthy esophageal tissues (Supplementary Figure S2).

Figure 2. Bar plots of the phylum and genus taxonomic levels among seven cancer types. (a, b) represent the composition of bacteria in phylum and genus, respectively.
At the genus level, the microbial composition of different cancer samples varied considerably, with Pseudomonas (21.26%) and Citrobacter (4.92%) being more prevalent in breast cancer (Figure 2B). In esophageal cancer, Fusobacterium (8.71%), Streptococcus (7.39%), and Prevotella (4.87%) accounted for the top three in descending order. In gastric cancer, the top three genera were Helicobacter (19.74%), Streptococcus (8.24%), and Prevotella (8.01%). Rhodococcus (22.69%), Ralstonia (20.76%), and Parcubacteria_genera_incertae_sedis (14.69%) had the highest percentage in hepatocellular carcinoma. Ralstonia (13.61%) had the highest percentage in lung cancer. In OSCC cancer, Streptococcus (13.18%), Fusobacterium (9.41%) and Prevotella (6.58%) had the highest proportion. Finally, in pancreatic cancer, the top three genera in descending order were dominated by Halomonas (26.12%), Pseudomonas (12.99%) and Citrobacter (7.53%). Also, we found that Pseudomonas, Streptococcus and Prevotella were present in seven cancer samples, while Rhodococcus and Acinetobacter were present in the remaining six cancers except for OSCC. Fusobacterium was present in all remaining cancers except liver and lung cancer. The microbial composition of healthy tissues was great difference between groups (Supplementary Figure S2). It was evident that pseudomonas which had the most relative abundance in tumor tissues was less prevalent in healthy tissues (Supplementary Figure S2).
Identification of abundance differential intratumoral microorganisms in pan-cancer analysis
We performed abundance differential microorganisms analysis by Lefse (LDA>2). There were 13, 19, 10, 2, 3, 19 significant abundance differential microorganisms in tumor group from breast cancer, esophageal cancer, gastric cancer, liver cancer, lung cancer and OSCC, respectively (Supplementary Figure S3). Then, the number of microorganisms with significant differences among different cancers, from high to low, was esophageal cancer (9), OSCC (8), breast cancer (7), hepatocellular carcinoma (5), gastric cancer (2), lung cancer (1), and pancreatic cancer (2) (Figure 3). Pseudomonas (LDA>5), Bacillus (LDA>4), Cutibacterium (LDA>4), etc. were significantly enriched in breast cancer Treponema (LDA>4), Peptostreptococcus (LDA>4), Peptoanaerobacter (LDA>3), etc. were enriched in esophageal cancer. Streptococcus (LDA>5), Prevotella (LDA>4) were significantly enriched in gastric cancer. Acinetobacter (LDA>5), Pelomonas (LDA>4), Sediminibacterium (LDA>4), etc. were significantly enriched in liver cancer. Fusobacterium (LDA>5), Campylobacter (LDA>4), Aggregatibacter (LDA>4), etc. were significantly enriched in OSCC. Dialister (LDA>4) and Shewanella (LDA > 5) were significantly enriched in lung cancer and pancreatic cancer, respectively.

Figure 3. LDA analysis implied differentially abundant microorganisms among seven cancer types (LDA Score > 2).
Co-abundance networks of intratumoral microorganisms in pan-cancer analysis
We performed Co-abundance networks analysis of seven microorganisms with different cancer types (correlation coefficient >0.4, p-value<0.05) (Figure 4). We found that Pseudomonas (relative abundance =20.82, degree=14), Fusobacterium (relative abundance =8.56, degree=17), Streptococcus (relative abundance =8.53, degree=2), and Ralstonia (relative abundance =13.43,degree=5) all had high abundance and high degrees, which were predominant in breast, esophageal, gastric, and lung cancers, respectively (Figures 4A,B,D,E; Supplementary Tables S3, S4). In addition, in breast cancer network, there was a positive correlation (correlation index >0.6) between Pseudomonas and Staphylococcus significantly enriched bacteria(LDA > 4). In esophageal cancer network, there was a negative correlation (correlation index< -0.6) between Fusobacterium and significantly enriched genera (Peptostreptococcus, LDA>3). There were more connections among esophageal cancer network in genera. In liver cancer network, Ralstonia (relative abundance =20.66,degree=5), Rhodococcus(relative abundance = 22.69, degree = 5) and Lysobacter (relative abundance =1.19,degree=15) played an important role, with first two having the higher abundance and lower degree and the latter had lower abundance and highest degree (Figure 4C). In the OSCC network, Streptococcus (relative abundance =13.22, degree=6) had the highest abundance and medium degree and Fusobacterium (relative abundance =9.56, degree=11) had the highest degree and lower abundance (Figure 4F). Leptotrichia (relative abundance =6.02, degree=9) had higher abundance, all three played a key role in OSCC microbiological network. Among pancreatic and esophageal cancer microorganisms, Halomonas and Shewanella played key roles, both of which had higher abundance and higher degrees (Figure 4G). We found mostly positive correlations in breast, gastric, liver, and pancreatic cancers, whereas there was no distinct patterns of correlation among esophagus, lung, and OSCC microbiological network.
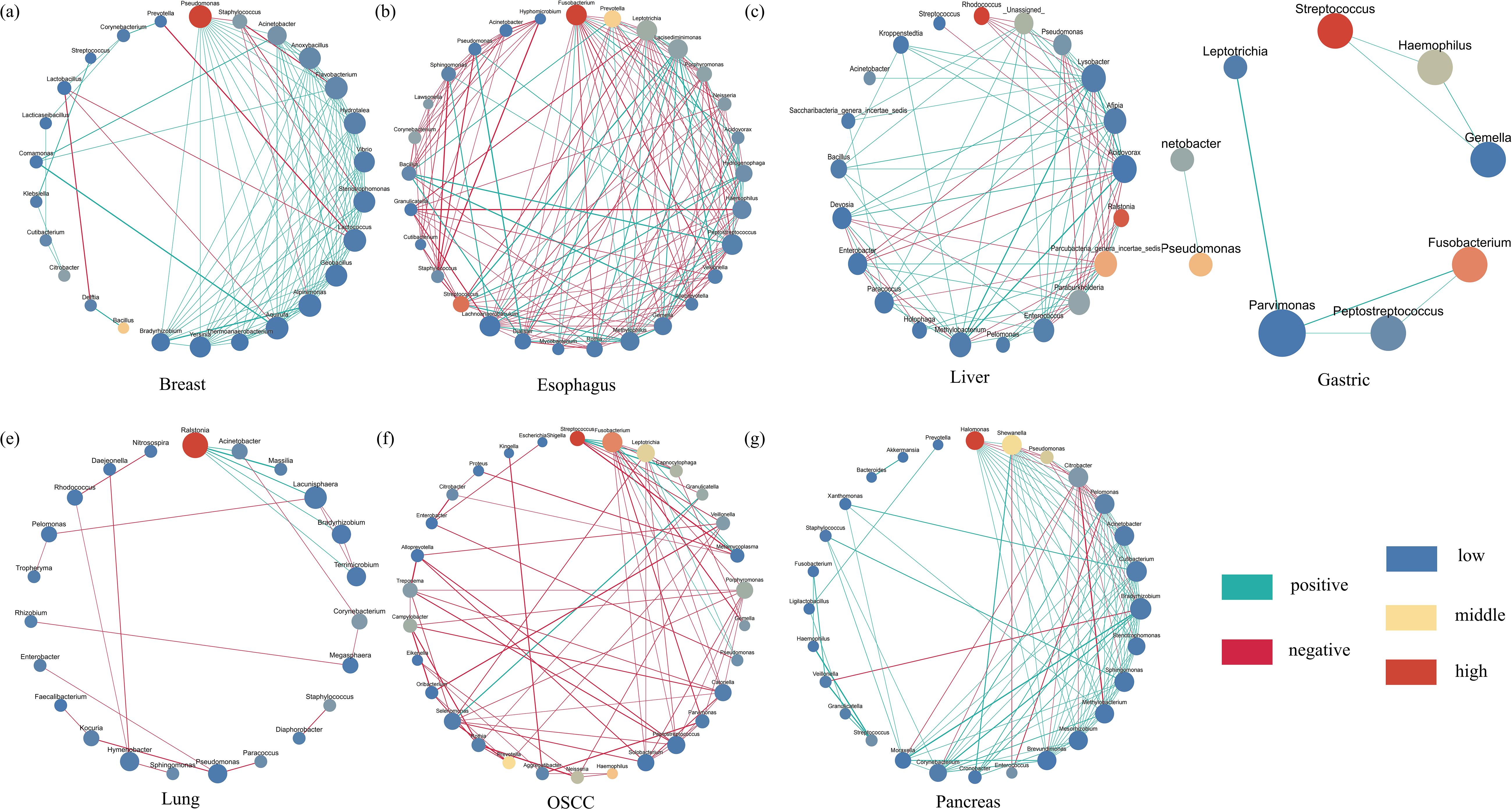
Figure 4. The Co–abundance network of six cancer types. The blue color represents least abundant, yellow color represents intermediate abundance and red represents the most abundant. The light green color of connecting lines represents negative correlation and red represents positive correlation. The size of the nodes indicates degree number and set from 30 to 60. The biggest one means that it has most degrees, and the smallest one means least degrees. Only correlation coefficients greater than 0.4 will be showed. The correlation coefficients p – value threshold is 0.05. (a–g) represent the co-abundance network of bacteria in breast, esophagus, liver, gastric, lung, oscc and pancreas, respectively.
The common intratumoral microorganisms profiling in pan-pancer analysis
We investigated 13 common intratumoral microorganisms (genus level) abundance profiling of seven cancers. The abundance of the thirteen microorganisms varied widely among different cancers (Figure 5). We found that the Neisseria, Porphyromonas, and Fusobacterium exhibited higher abundances in OSCC samples. In esophageal cancer samples, Fusobacterium, Porphyromonas, and Corynebacterium were found to be more abundant. In gastric cancer tissues, Prevotella and Streptococcus showed higher abundances, while in breast cancer tissues, Bacillus, Pseudomonas, and Staphylococcus were more enriched. Brevundimonas and Corynebacterium were found to have higher abundances in lung cancer. Notably, there were no high-abundance genera identified in liver and pancreatic cancer samples. We found the types and abundance of common bacteria were relatively high in OSCC samples. In summary, the microbial profiling pattern of esophageal and gastric cancer samples are relatively similar, as so as liver and pancreatic cancer samples.
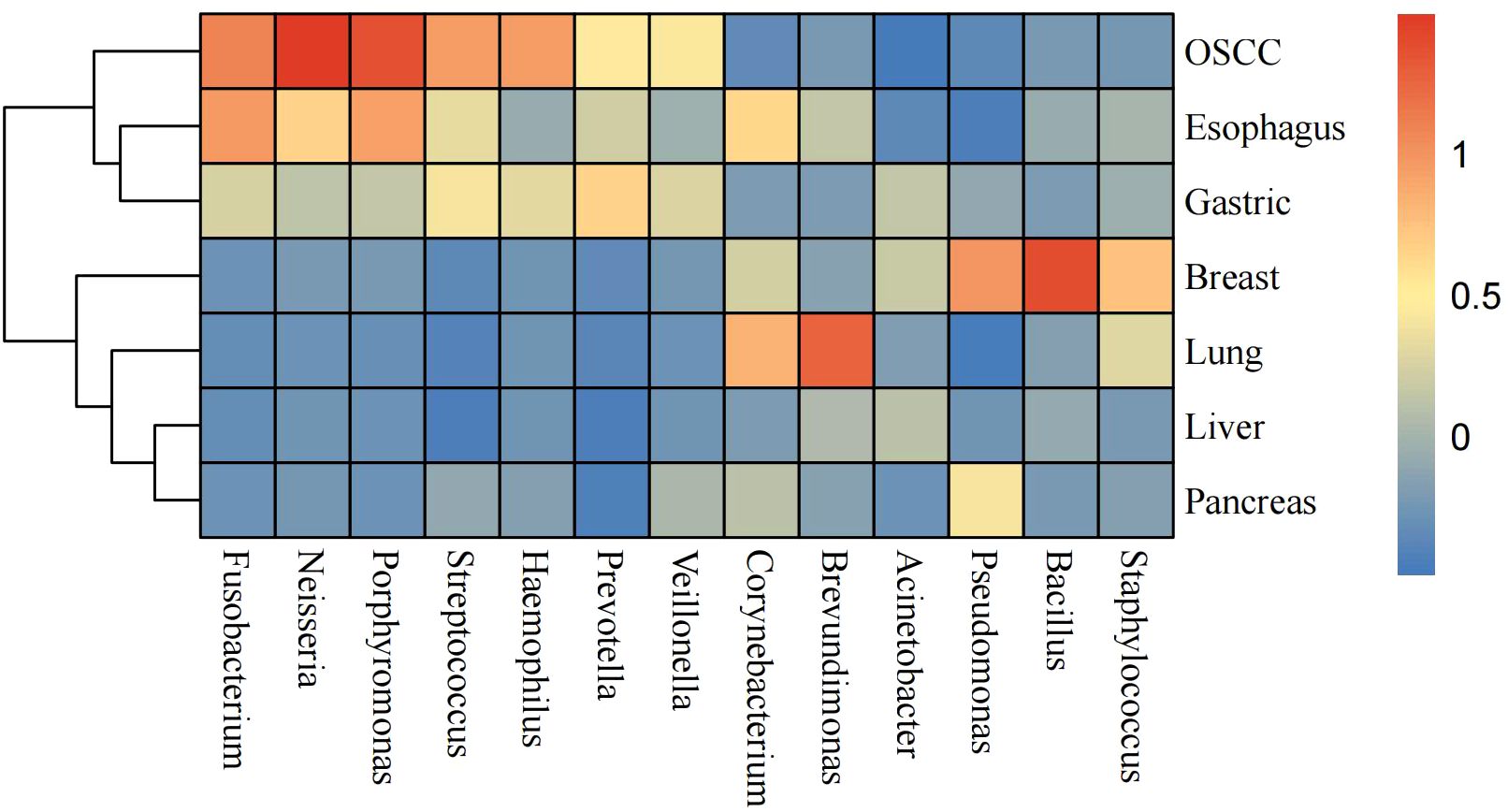
Figure 5. Heatmap of 13 selected most common intratumoral microorganisms at the genus level. The blue color represents less abundant, yellow color represents intermediate abundance and red represents the most abundant.
Discussion
Currently, intratumoral microbiology research is hot but controversial. Nejman et al. conducted the first more comprehensive and systematic study of intratumoral microbiology in 2020 (Nejman et al., 2020). This has sparked a boom in intratumoral microbial research. In contrast to the study by Nejman et al., we analyze five different cancer types to provide a more comprehensive situation for more cancer types. Similarly, we all collect a large number of samples to diminish the error made by samples. Uniquely, we offer refined co-abundance network for each cancer type (Nejman et al., 2020). Sepich-Poore et al. in 2021 explored intratumoral microbes in cancer therapy by discovering that intratumoral bacteria produce a new tumor antigen (Sepich–Poore et al., 2021). Qu et al. in 2022 explored the microbial composition of hepatocellular carcinoma tissues and identified new diagnostic markers for primary liver tumors (Qu et al., 2022). Research articles on intratumoral microbes have been climbing in recent years. At the same time, some have raised concerns; for example, Ge et al. inferred in 2024 that the presence of microorganisms within tumors is a result of technical errors (Ge et al., 2024). In our study, we utilized the concept of pan-cancer to conduct a meta-analysis of intratumoral microorganisms across 783 samples of 16S rDNA from up to seven different cancer types, thereby maximizing the potential evidence for the presence of intratumoral microorganisms.
In our result, we found gastric cancer and liver cancer were all significant difference (p < 0.001) of the four diversity indices except evenness diversity index of liver cancer (p < 0.001). Noticeably, there was a great difference. The mean value of four diversity indices in case were significant higher than control in gastric cancer and it was opposite in liver cancer. In taxonomy classification at the phylum level, we found that the top three phyla in OSCC cancer were Firmicutes, Proteobacteria, and Bacteroidetes, respectively. In different OSCC studies, it coincided with Su’s findings (Su et al., 2021). In taxonomy classification at the genus level, the most prevalent genus in our study was Streptococcus, which is consistent with Su’s research (Su et al., 2021). Furthermore, in our study, Fusobacterium and Prevotella exhibited higher relative abundances, a conclusion that aligns with the findings of Michikawa et al. (2022). As a bridge between different colonizers in oral cavity, Fusobacterium will increase uncertainty of biofilms with an abnormal abundance (Ye et al., 2024). In breast cancer tissues, we found that Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes were the most abundant phyla. This finding is consistent with the results of Klann et al. in various breast cancer studies (Klann et al., 2020), but it differs from the results of Urbaniak et al. (2014), where Firmicutes was the most abundant phylum (Urbaniak et al., 2014). At the genus level, Pseudomonas was overwhelmingly dominant. However, Urbaniak et al. (2016) found that Staphylococcus, Enterobacteriaceae, and Bacillus were more highly represented in breast cancer (Urbaniak et al., 2016). Meanwhile, German et al. found that Ralstonia and Staphylococcus were also higher in breast cancer (German et al., 2023). There is a big difference between our study and two other different studies. This may be related to the fact that the microbial composition of breast cancer may vary with stages (Smith et al., 2019). The abundance of Fusobacterium, which had been proved to worsen cancer in breast through lectin Fap2, is low (Parhi et al., 2020). Regarding the microbial composition in esophageal cancer, we found that in various esophageal cancer studies, both at the phylum and genus levels, our results were similar to those of Liu et al. and Wang et al (Liu et al., 2018; Wang et al., 2021). The only difference is that Campylobacterota exhibited a relatively higher abundance in our study. In gastric cancer, we found that compared to other gastric cancer studies, Campylobacterota exhibited a higher abundance at the phylum level in our research compared to Shao’s study, which aligns with its characteristic extensive presence in the digestive system (Shao et al., 2019). Further, Liu et al. found the infiltration level of Tregs was negatively correlated with microbial abundance, which indicates that the higher abundance of gastric tumor microbiome may pose an adverse impact on therapy (Liu et al). The higher abundance of Fusobacterium may induce organelle dysfunction by up-regulating the expression of related genes and promote cancer metastasis (Zhou et al., 2022). In various studies of microbial composition in liver cancer, we observed that our results were similar to those of Qu et al. at the phylum level (Qu et al., 2022). However, there were significant differences at the genus level. Acinetobacter was the dominant genus in Qu’s research, whereas it constituted a relatively lower proportion in our study (Qu et al., 2022). Maybe it is not the sequencing depth effects, because we normalized the raw data and took relative abundance to analysis. Subsequent plans are to validate the distribution of this microbiota in liver cancer by RNA single-cell sequencing, microbial single-cell sequencing, and microbial culture. What’s more, Xue et al. discovered the metabolism influences made by liver tumor microbiome, presenting that the metabolites of 13Z, 16Z-docosadienoic acid is overexpressed in liver tumor (Xue et al., 2024). In the microbial composition of lung cancer, our study identified Proteobacteria as the dominant phylum, in contrast to Lee’s research, where Bacteroidetes was the prevalent phylum and represented a smaller proportion in our findings (Lee et al., 2016). Additionally, Ralstonia showed a higher relative abundance in our study compared to its lower proportion in Lee’s research (Lee et al., 2016). Regarding the microbial composition in pancreatic cancer, we found that our results were highly similar to those of Nejman et al. at the phylum level across different studies (Nejman et al., 2020). The high abundance of Proteobacteria will lead to T cell anergy in a Toll-like receptor-dependent manner, accelerating tumor progression (Pushalkar et al., 2018). Noticeably, the presence of Fusobacterium will mediate tumorigenesis and metastasis by promoting the synthesis of GMCSF and CXCL1 in pancreas (Nejman et al., 2020). Interestingly, Josie et al. innovated Electro-antibacterial therapy to enhance intracellular bacteria clearance in pancreatic cancer cells, which is a potential method to cure pancreatic cancer (Duncan et al., 2024). It is noteworthy that we detected the presence of Pseudomonas, Streptococcus and Prevotella in the microbial compositions of the seven cancer types we studied, suggesting a potential close association between these genera and cancer.
From our results, we observed that there are significant abundant differential microorganisms in cancers such as breast cancer, esophageal cancer and OSCC, while pancreatic cancer and lung cancer exhibited fewer abundant differential microorganisms. Additionally, in the comparison between the microbial composition of normal tissue and cancer tissue, we found that Treponema was significantly enriched in three types of cancer tissue, which indicated that it was likely consistent with cancer.
Based on the existing researches of intratumoral microorganisms, we selected thirteen genera that are frequently found in various cancer tissues (according to Xue et al.) (Xue et al., 2023; Xuan et al., 2024). Of these thirteen genera, most are parthenogenetic anaerobes or anaerobes, and only Neisseria is aerobic. We can see that Neisseria is only found in OSCC, esophageal cancer, and gastric cancer, which may be related to human physiology. Fusobacterium is an anaerobic bacterium commonly associated with various infections and inflammations. We found its presence in OSCC, esophageal cancer, and gastric cancer, suggesting that this genus may possess potential pathogenicity. Additionally, due to its reported association with multiple cancers, it is also referred to as “oncobacterium” (Brennan and Garrett, 2019; McIlvanna et al., 2021; Alon–Maimon et al., 2022). According to the results, Brevundimonas was found to be abundantly present in lung cancer, while it was almost absent in other cancers. Furthermore, this bacterium is often associated with the occurrence of pneumonia, indicating that it may have a greater tendency to inhabit lung tissue and exhibit pathogenic potential (Hassan et al., 2023). We found Bacillus and Staphylococcus to be present in large numbers in breast cancers. Noticeably, Liu et al. found that there are positive correlations between tumoral Staphylococcus and CD8+ TIL activity exclusively in triple-negative breast cancer, demonstrating the effect of Staphylococcus on immunity (Liu et al., 2024). In general, Bacillus is only present in the environment and is not pathogenic, but certain species such as Bacillus anthracis can sometimes cause serious illness (Atlas, 1998; Watson et al., 2017). Since our samples could only be localized to the genus level, we were unable to determine which species of Bacillus were enriched in breast cancer. Finally, we found that these thirteen bacteria had low abundance or were absent in liver cancer and pancreatic cancer, suggesting that the microbial communities in these cancers may be dominated by other specific taxa.
However, there are some shortcomings in our study. Firstly, our study is based on online public data and 16S rDNA data, which cannot be precisely localized to the species level. Secondly, this study lacks the validation of wet experiments, which will be added in the future. We have provide a more comprehensive understanding of intratumoral microbiome. However, We hope to further investigate the composition of intratumoral microorganisms systematically through more pan-cancer samples, which will lay the foundation for related research and provide a scientific basis for possible cancer treatment options. In the future, more researches on microbiome metabolomics may further elucidate the influences of microbiome posed to tumor.
Conclusion
In summary, we conducted a more systematic study of intratumoral microbial communities in seven different cancer types based on 16S data in pan-cancer analysis. The microbial communities of different cancers were found to have differences but also commonalities in diversity and species composition. Four genera, Pseudomonas, Streptococcus, and Prevotella were found to be common to the microbial communities of the seven cancers, suggesting that these microorganisms may be associated with the occurrence and development of cancer. In addition, it is noteworthy that we identified the core microorganisms in the intratumoral microbial interactions network of different cancers, which may help us to study the tumor microenvironment in depth. We hope that our results will lay the foundation for further research in the field of intratumoral microbes and provide new ideas for cancer diagnosis and treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because this study uses strains obtained from the European Nucleotide Archive (ENA) database. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because the PUMCH Institutional Review Board did not require the study to be reviewed or approved by an ethics committee because the samples were deidentified human samples.
Author contributions
XL: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft. YY: Formal Analysis, Investigation, Resources, Software, Writing – original draft. AX: Data curation, Investigation, Resources, Software, Writing – review & editing. DC: Formal Analysis, Funding acquisition, Software, Writing – original draft. JZ: Methodology, Resources, Writing – review & editing. YS: Data curation, Formal Analysis, Writing – original draft. QZ: Investigation, Methodology, Writing – original draft. ZH: Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. WW: Funding acquisition, Investigation, Resources, Software, Supervision, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National High Level Hospital Clinical Research Funding (Grant Number 2022-PUMCH-D-001), the National Nature Science Foundation of China (Grant Number 82141102), Shandong Provincial Natural Science Foundation (Grant Number ZR2021LSW004) and CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Number 2021-I2M-1-002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1549319/full#supplementary-material
References
Alon–Maimon, T., Mandelboim, O., and Bachrach, G. (2022). Fusobacterium nucleatum and cancer. Periodontol. 2000. 89, 166–180. doi: 10.1111/prd.12426
Atlas, R. M. (1998). The medical threat of biological weapons. Crit. Rev. Microbiol. 24, 157–168. doi: 10.1080/10408419891294280
Global health estimates: Leading causes of death. Available online at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (Accessed August 16, 2024).
Available online at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (Accessed April 2, 2024).
Available online at: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (Accessed April 2, 2024).
vegan: Community Ecology Package. Available online at: https://vegandevs.github.io/vegan/ (Accessed June 13, 2025).
Brennan, C. A. and Garrett, W. S. (2019). Fusobacterium nucleatum – symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166. doi: 10.1038/s41579-018-0129-6
Chai, X., Wang, J., Li, H., Gao, C., Li, S., Wei, C., et al. (2023). Intratumor microbiome features reveal antitumor potentials of intrahepatic cholangiocarcinoma. Gut. Microbes 15, 2156255. doi: 10.1080/19490976.2022.2156255
Crosby, D., Bhatia, S., Brindle, K. M., Coussens, L. M., Dive, C., Emberton, M., et al. (2022). Early detection of cancer. Science 375, eaay9040. doi: 10.1126/science.aay9040
Duncan, J. L., Ahmad, R. N., Danesi, H., Slade, D. J., Davalos, R. V., and Verbridge, S. S. (2024). Electro–antibacterial therapy (EAT) to enhance intracellular bacteria clearance in pancreatic cancer cells. Bioelectrochemistry 157, 1–-18. doi: 10.1016/j.bioelechem.2024.108669
Eun, Y. G., Lee, J. W., Kim, S. W., Hyun, D. W., Bae, J. W., and Lee, Y. C. (2021). Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Scientific Report 11, 23176, 1-10. doi: 10.1038/s41598-021-02638-9
Farrell, J. J., Zhang, L., Zhou, H., Chia, D., Elashoff, D., Akin, D., et al. (2021). Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61 (4), 582–588, 1-17. doi: 10.1136/gutjnl-2011-300784
Gagliani, N., Hu, B., Huber, S., Elinav, E., and Flavell, R. A. (2014). The fire within: microbes inflame tumors. Cell 157, 776–783. doi: 10.1016/j.cell.2014.03.006
Gao, Y. Y., Zhang, G. X., Jiang, S. Y., and Liu, Y. X. (2024). Wekemo Bioincloud: A user–friendly platform for meta–omics data analyses. iMeta 3, 12. doi: 10.1002/imt2.175
Ge, Y., Lu, J., Puiu, D., Revsine, M., and Salzberg, S. L. (2024). Comprehensive analysis of microbial content in whole–genome sequencing samples from The Cancer Genome Atlas project. bioRxiv. doi: 10.1101/2024.05.24.595788
German, R., Marino, N., Hemmerich, C., Podicheti, R., Rusch, D. B., Stiemsma, L. T., et al. (2023). Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 25, 82. doi: 10.1186/s13058-023-01677-6
Hassan, M. A., Syed, F., Singh, G. P., Pakala, R., and Gasmelseed, H. (2023). Brevundimonas diminuta–induced lung abscess in an immunocompetent adult: A rare case report. Cureus 15, e42371. doi: 10.7759/cureus.42371
Klann, E., Williamson, J. M., Tagliamonte, M. S., Ukhanova, M., Asirvatham, J. R., Chim, H., et al. (2020). Microbiota composition in bilateral healthy breast tissue and breast tumors. Cancer Causes. Control. 31, 1027–1038. doi: 10.1007/s10552-020-01338-5
Lee, S. H., Sung, J. Y., Yong, D., Chun, J., Kim, S. Y., Song, J. H., et al. (2016). Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 102, 89–95. doi: 10.1016/j.lungcan.2016.10.016
Li, Y., Zhang, R., Fu, C., Jiang, Q., Zhang, P., Zhang, Y., et al. (2024). Intratumoral microbiome promotes liver metastasis and dampens adjuvant imatinib treatment in gastrointestinal stromal tumor. Cancer Lett. 601, 1–12. doi: 10.1016/j.canlet.2024.217149
Liu, Y. X., Chen, L., Ma, T. F., Li, X. F., Zheng, M. S., Zhou, X., et al. (2023). EasyAmplicon: An easy–to–use, open–source, reproducible, and community–based pipeline for amplicon data analysis in microbiome research. iMeta 2, 13. doi: 10.1002/imt2.83
Liu, Y., Lin, Z., Lin, Y., Chen, Y., Peng, X. E., He, F., et al. (2018). Streptococcus and Prevotella are associated with the prognosis of esophageal squamous cell carcinoma. J. Med. Microbiol. 67, 1058–1068. doi: 10.1099/jmm.0.000754
Liu, SA-O., Qi, L., Dong, L., Sun, W., Liu, S., Li, PA-O., et al. (2025). Prognostic implications of the interaction between intratumoral microbiome and immune response in gastric cancer. Microbiology Spectrum, 13 (5), e0283024, 1-22. doi: 10.1128/spectrum.02830-24
McIlvanna, E., Linden, G. J., Craig, S. G., Lundy, F. T., and James, J. A. (2021). Fusobacterium nucleatum and oral cancer: a critical review. BMC Cancer 21, 1212. doi: 10.1186/s12885-021-08903-4
Michikawa, C., Gopalakrishnan, V., Harrandah, A. M., Karpinets, T. V., Garg, R. R., Chu, R. A., et al. (2022). Fusobacterium is enriched in oral cancer and promotes induction of programmed death–ligand 1 (PD–L1). Neoplasia 31, 100813. doi: 10.1016/j.neo.2022.100813
Nalluri, H., Jensen, E., and Staley, C. (2021). Role of biliary stent and neoadjuvant chemotherapy in the pancreatic tumor microbiome. BMC Microbiol. 21, 1–8. doi: 10.1186/s12866-021-02339-3
Nejman, D., Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L. T., et al. (2020). The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 368, 973–980. doi: 10.1126/science.aay9189
Parhi, L., Alon–Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod–Levi, T., et al. (2020). Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 11, 1–12. doi: 10.1038/s41467-020-16967-2
Pushalkar, S., Hundeyin, M., Daley, D., Zambirinis, C. P., Kurz, E., Mishra, A., et al. (2018). The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416. doi: 10.1158/2159-8290.CD-17-1134
Qiao, H., Tan, X. R., Li, H., Li, J. Y., Chen, X. Z., Li, Y. Q., et al. (2022). Association of intratumoral microbiota with prognosis in patients with nasopharyngeal carcinoma from 2 hospitals in China. JAMA Oncology 8 (9), 1301–1309, 1-9. doi: 10.1001/jamaoncol.2022.2810
Qu, D., Wang, Y., Xia, Q., Chang, J., Jiang, X., and Zhang, H. (2022). Intratumoral microbiome of human primary liver cancer. Hepatol. Commun. 6, 1741–1752. doi: 10.1002/hep4.1908
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahe, F. (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584. doi: 10.7717/peerj.2584
Sepich–Poore, G. D., Carter, H., and Knight, R. (2021). Intratumoral bacteria generate a new class of therapeutically relevant tumor antigens in melanoma. Cancer Cell 39, 601–603. doi: 10.1016/j.ccell.2021.04.008
Shao, D., Vogtmann, E., Liu, A., Qin, J., Chen, W., Abnet, C. C., et al. (2019). Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high–risk region of China. Cancer 125, 3993–4002. doi: 10.1002/cncr.32403
Smith, A., Pierre, J. F., Makowski, L., Tolley, E., Lyn–Cook, B., Lu, L., et al. (2019). Distinct microbial communities that differ by race, stage, or breast–tumor subtype in breast tissues of non–Hispanic Black and non–Hispanic White women. Sci. Rep. 9, 11940. doi: 10.1038/s41598-019-48348-1
Su, S. C., Chang, L. C., Huang, H. D., Peng, C. Y., Chuang, C. Y., Chen, Y. T., et al. (2021). Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 42, 127–135. doi: 10.1093/carcin/bgaa062
Tsay, JA-O., Wu, B. G., Badri, M. H., Clemente, J. C., Shen, N., Meyn, P., et al. (2018). Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. American Journal of Respiratory and Critical Care Medicine, 198 (9), 1188–1198, 1-11. doi: 10.1164/rccm.201710-2118OC
Urbaniak, C., Cummins, J., Brackstone, M., Macklaim, J. M., Gloor, G. B., Baban, C. K., et al. (2014). Microbiota of human breast tissue. Appl. Environ. Microbiol. 80, 3007–3014. doi: 10.1128/AEM.00242-14
Urbaniak, C., Gloor, G. B., Brackstone, M., Scott, L., Tangney, M., Reid, G., et al. (2016). The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 82, 5039–5048. doi: 10.1128/AEM.01235-16
Wang, Y., Guo, H., Gao, X., and Wang, J. (2021). The intratumor microbiota signatures associate with subtype, tumor stage, and survival status of esophageal carcinoma. Front. Oncol. 11, 754788. doi: 10.3389/fonc.2021.754788
Watson, A. K., Ellington, S., Nelson, C., Treadwell, T., Jamieson, D. J., and Meaney–Delman, D. M. (2017). Preparing for biological threats: Addressing the needs of pregnant women. Birth. Defects. Res. 109, 391–398. doi: 10.1002/bdr2.1016
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., et al. (2019). Hester J et al: Welcome to the Tidyverse. J. Open Source Software. 4. 1–6. doi: 10.21105/joss.01686
Xuan, M., Gu, X., Liu, Y., Yang, L., Li, Y., Huang, D., et al. (2024). Intratumoral microorganisms in tumors of the digestive system. Cell Commun. Signal 22, 69. doi: 10.1186/s12964-023-01425-5
Xue, C., Chu, Q., Zheng, Q., Yuan, X., Su, Y., Bao, Z., et al. (2023). Current understanding of the intratumoral microbiome in various tumors. Cell Rep. Med. 4, 100884. doi: 10.1016/j.xcrm.2022.100884
Xue, C., Gu, X., Shi, Q., Ma, X., Jia, J., Su, Y., et al. (2024). The interaction between intratumoral bacteria and metabolic distortion in hepatocellular carcinoma. J. Trans. Med. 22, 1–13. doi: 10.1186/s12967-024-05036-7
Yang, L., Li, A., Wang, Y., and Zhang, Y. (2023). Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 8, 35. doi: 10.1038/s41392-022-01304-4
Ye, C., Liu, X., Liu, Z., Pan, C., Zhang, X., Zhao, Z., et al. (2024). Fusobacterium nucleatum in tumors: from tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 25, 2306676. doi: 10.1080/15384047.2024.2306676
Yu, X., Shao, Y., Dong, H., Yan, J., Zhang, X., and Ye, G. (2025). Molecular subtype of gastric cancer based on apoptosis–related genes reveals differential immune microenvironment and intratumoral microorganisms distribution. BMC Cancer 25, 1–22. doi: 10.1186/s12885-024-13411-2
Zhang, M., Zhang, YA-O., Sun, Y., Wang, S., Liang, H., and Han, Y. (2002). Intratumoral microbiota impacts the first-line treatment efficacy and survival in non–small cell lung cancer patients free of lung infection. Journal of Healthcare Engineering 2022 (1), 1–7. doi: 10.1155/2022/5466853
Zhang, J. W., Zhang, D., Yin, H. S., Zhang, H., Hong, K. Q., Yuan, J. P., et al. (2023). Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression and chemoresistance by enhancing the secretion of chemotherapy–induced senescence–associated secretory phenotype via activation of DNA damage response pathway. Gut. Microbes 15, 2197836. doi: 10.1080/19490976.2023.2197836
Keywords: pan-cancer, intratumoral microbial, 16S rDNA, pseudomonas, streptococcus, prevotella
Citation: Liu X, Yao Y, Xiao A, Cao D, Zhang J, Shi Y, Zhong Q, He Z and Wu W (2025) Pan-cancer analysis reveals intratumoral microbial diversity in multiple cancers by amplicon technology. Front. Cell. Infect. Microbiol. 15:1549319. doi: 10.3389/fcimb.2025.1549319
Received: 09 January 2025; Accepted: 05 August 2025;
Published: 02 September 2025.
Edited by:
Somya Aggarwal, Washington University in St. Louis, United StatesReviewed by:
Yilong Li, First Affiliated Hospital of Harbin Medical University, ChinaTong Qiao, Jinan University, China
Copyright © 2025 Liu, Yao, Xiao, Cao, Zhang, Shi, Zhong, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenming Wu, ZG9jdG9yd3V1QDEyNi5jb20=; Zilong He, aGV6aWxvbmdAYnVhYS5lZHUuY24=
†These authors have contributed equally to this work
 Xudong Liu
Xudong Liu Yuteng Yao
Yuteng Yao Aoyi Xiao4†
Aoyi Xiao4† Zilong He
Zilong He Wenming Wu
Wenming Wu