Abstract
Background:
Avian pathogenic Escherichia coli (APEC) infection causes high mortality in chicks and leads to significant economic losses in the poultry industry. During the initial infection, APEC colonizes host cells using type 1 fimbriae and subsequently forms biofilms, resulting in persistent and chronic infections. fimC is a chaperone protein associated with type 1 fimbriae and plays a crucial role in the assembly of fimbriae. However, its regulatory role in agn43-mediated autoaggregation remains unclear.
Methods:
By constructing fimC gene mutant strains, the autoaggregation, motility, biofilm formation, and the adhesion and invasion ability to HD-11 cells were examined. The transcriptome and the electrophoretic mobility shift assay (EMSA) were used to screen and verify the regulation of fimC on downstream genes.
Results:
The results demonstrated that the lack of fimC, but not fimbriae, significantly increased autoaggregation (p < 0.001) while promoting the transcription of agn43 (p < 0.01). Transcriptomic analysis showed that the deletion of fimC caused significant changes in the gene transcription levels in a variety of pathways, such as flagellar synthesis, biofilm formation, quorum sensing, and bis-(3′-5′)-cyclic diguanylic acid (c-di-GMP) metabolism. Further investigation revealed that fimC directly interacted with the promoter region of agn43 and inhibited its transcription. In addition, both fimC and agn43 had regulatory effects on biofilm formation, motility, adhesion, and invasion.
Conclusion:
This study demonstrated that fimC acts as an atypical DNA-binding protein to regulate the transcription of agn43. It also highlights the importance of fimC in the biofilm formation and adhesion ability of APEC, which provides new insights into the functions of the fimbrial chaperone protein FimC.
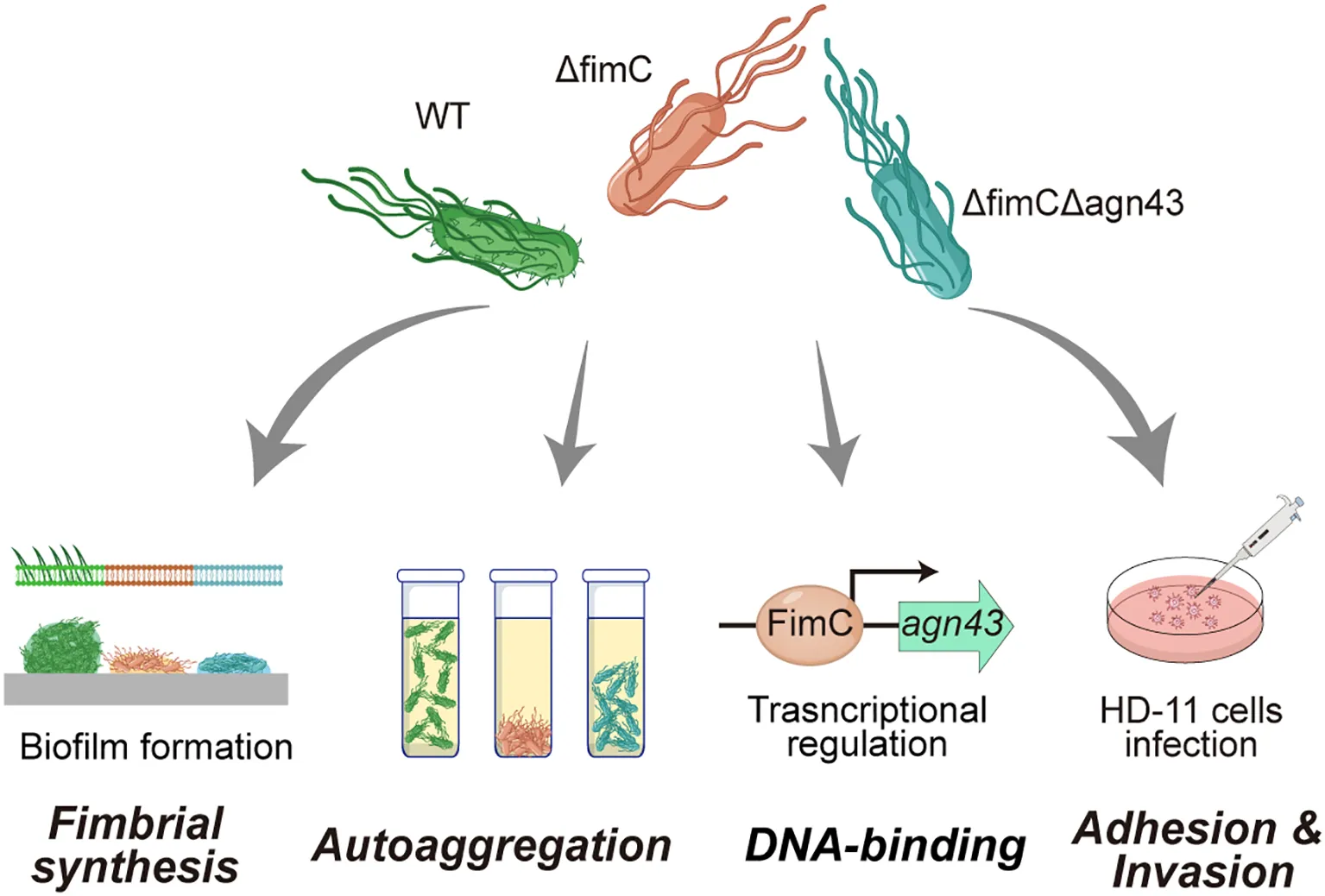
The autoaggregation of fimC mutan strain was significantly decreased. However, deletion of fimC and agn43 resulted in significant restored of autoaggregation. Mechanically, fimC binds to the agn43 promoter region and regulates its transcription. In addition, fimC and agn43 affect the biological characteristics of APEC such as biofilm and adhesion.
1 Introduction
Pathogenic Escherichia coli is a common pathogen that is typically divided into two major categories: intestinal pathogenic E. coli (InPEC) and extraintestinal pathogenic E. coli (ExPEC). Avian pathogenic E. coli (APEC) belongs to the ExPEC category. This disease has caused significant economic losses to the poultry industry due to increased mortality and decreased productivity (Barbieri et al., 2021; Li et al., 2016). APEC is also considered a foodborne pathogen associated with human urinary tract infections and is regarded as a potential reservoir for the transfer of virulence and antimicrobial resistance genes to human ExPEC strains, including those causing urinary tract infections (uropathogenic E. coli, UPEC) (Apostolakos et al., 2021; Kathayat et al., 2021).
APEC primarily induces avian diseases through its virulence factors, which include flagella, adhesins, autotransporters, iron acquisition systems, adhesins and invasins, and toxins (Nawaz et al., 2024). Adhesins, as components of the bacterial cell surface, promote adhesion to other cells or substances, typically within their host environment. During infection, adhesins initially contact host cells and trigger signaling pathways by recognizing specific receptors on the cell surface (Adlerberth et al., 1998; Arciola et al., 2015; Blumer et al., 2005; Hetta et al., 2021; Hoiby et al., 2010). This interaction is crucial for achieving colonization and the subsequent invasion.
Type 1 fimbriae play a vital role in the attachment to host cells and the initiation of biofilm formation, which are important processes in bacterial infections (Costa et al., 2015). These hair-like appendages are present on the outer membrane of APEC and specifically bind to mannose-containing residues. The fim gene cluster of the APEC strain includes fimA, fimI, fimC, fimD, fimF, fimG, and fimH (Spaulding et al., 2017). Under the guidance of the chaperone protein fimC and the usher protein FimD, these fimbrial subunits are assembled and fixed on the outer membrane. The fimA gene encodes the major subunit of type 1 fimbriae, while the fimH gene encodes the adhesin subunit that mediates the binding to α-d-mannosylated receptors, promoting bacterial adhesion to host epithelial cells (Pere et al., 1987). As a chaperone protein, fimC is crucial for the assembly of type 1 fimbriae (Jones et al., 1993). It aids in transporting the fimbrial subunit proteins to the periplasmic and, subsequently, to the outer membrane (Schembri and Klemm, 2001).
Autotransporters are a class of outer membrane proteins in E. coli that play a vital role in adhesion, autoaggregation, and biofilm formation. Antigen 43 (agn43) is a typical autotransporter in E. coli that promotes cell aggregation and enhances biofilm formation. Moreover, agn43 contributes to bacterial survival within macrophages and sustains persistent urinary tract infections in the colorectal region (Court et al., 2003; Hasman et al., 2000; Korea et al., 2011). This multifunctionality underscores the importance of autotransporters in the pathogenesis and adaptability of E. coli. In addition, the AIDA (adhesin involved in diffuse adherence) and TibA (autotransporter adhesin/invasin) proteins appear to belong to an E. coli autotransporter subfamily, which has shown ~25% amino acid homology (Henderson and Nataro, 2001). Although these proteins are also virulent factors of bacteria and are involved in biofilm formation, the mechanisms of autoagglutination and biofilm regulation in E. coli still need to be explored further.
In this study, we elaborated the effects of fimC and agn43 on biological characteristics such as autoagglutination, biofilm formation, and adhesion and explored the regulatory relationship between fimC and agn43, providing a reference for the prevention and control of APEC.
2 Materials and methods
2.1 Strains and plasmids, medium, and growth conditions
APEC81, a clinically isolated APEC O78 serotype strain, was preserved in our laboratory. pKD46, pCP20, and pKD3, which were used for gene knockout, were also preserved in our laboratory. The primers used in this study are shown in Supplementary Table S1. All strains were cultured at 37°C in Luria–Bertani (LB) broth. When necessary, the antibiotics ampicillin (Amp, 100 μg/ml), kanamycin (Kan, 50 μg/ml), streptomycin (Str, 50 μg/ml), and chloramphenicol (Cm, 35 μg/ml) were supplemented.
2.2 Construction of mutant and complementary strains
The ΔfimC strain was constructed based on the phage lambda-derived Red recombination system, with some modifications (Datsenko and Wanner, 2000). In brief, 400-bp DNA sequences upstream and downstream of the fimC gene from the APEC81 genome and the chloramphenicol resistance gene open reading frame (ORF) with the associated promoter and FRT (Flp recognition target) sites from pKD3 were connected using overlapping PCR. The PCR products were used to replace the 1,001-bp DNA within the pdeN gene with the Exo, Beta, and Gam proteins expressed by pKD46. The chloramphenicol resistance gene was eliminated by the Flp recombinase expressed by pCP20. The recombinant plasmid pSTV28–fimC was electro-transformed into the ΔfimC strain to construct the complementary strain ΔfimC–CfimC. The rest of the single gene mutant strains and the ΔfimCΔagn43 (based on the ΔfimC strain) strain were constructed as above.
2.3 Crystal violet staining
The biofilm formation ability was determined using crystal violet staining, with some modifications (Han et al., 2015). Briefly, 20 μl of each strain (OD600 = 1.0) was added in 96-well polystyrene plates (FCP962; Beyotime, Jiangsu, China) with 200 μl fresh LB broth and incubated at 37°C for 12 h. The cultures were gently discarded and the wells washed three times with 250 µl phosphate-buffered saline (PBS). After incubation at 60°C for 20 min, 250 μl crystal violet solution (1%) was added to the plates, which were then incubated at 37°C for 20 min, washed five times with 250 µl ddH2O, and air-dried completely at room temperature for 20 min. Images of crystal violet on the 96-well plates were captured. Crystal violet was resuspended in situ with 200 µl 95% ethanol and measured at an absorbance of 595 nm.
2.4 Transmission electron microscopy
Transmission electron microscopy (TEM) was performed as previously described (Primo et al., 2020). Each strain was cultured in LB at 37°C (OD600 = 1.0) and washed three times with PBS (pH 7.4). Of the cells, 10 μl was placed on a 200-mesh Formvar-coated copper mesh. After incubation at room temperature for 10 min, the samples were stained with 2% uranyl acetate aqueous solution for 30 s. After drying, the copper mesh was gently placed into a transmission microscope (HitachiHT-7700), as required, and the cell morphology determined.
2.5 Swimming motility assay
Determination of the swimming ability was performed with some modifications (Fredericks et al., 2006). In brief, using Petri dishes with semi-solid tryptone agar plates (1% bacto-tryptone, 0.5% sodium chloride, and 0.2% agar), 5 μl of each strain (OD600 = 1.0) was added into the center of the dishes and incubated for 8–10 h at 37°C. Free-swimming cells were captured.
2.6 Autoaggregation assay
The autoaggregation assay was performed as previously described (Elhenawy et al., 2021). Briefly, the strains (OD600 = 1.0) were collected and washed with PBS three times, then resuspended in PBS at OD600 = 2.0. All strains were incubated in stasis at 37°C. The absorbance at 600 nm of the upper suspension liquid was assessed using the same time interval.
2.7 Adhesion and invasion assay
Chicken macrophage DF-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at 37°C with 5% CO2. For the adhesion and invasion assay (Hu et al., 2011; Han et al., 2014), each strain was infected with DF-1 for 2 h in 24-well plates (multiplicity of infection, MOI = 50), the cells were collected and lysed using 0.5% Triton X-100, and the number of adherent bacteria were counted using the dilution method. After infection for 2 h, uninfected bacteria were discarded and a fresh culture medium supplemented with 100 μg/ml gentamycin sulfate was added. After incubation for 1 h, the subsequent processes were similar to those previously described.
2.8 RNA extraction and quantitative RT-PCR
RNA extraction and quantitative RT-PCR were performed with some modifications (Han et al., 2015). In brief, 15 ml of each strain was statically cultured in LB broth at 37°C and harvested at OD600 = 1.0. RNA was extracted with TRIzol. RNA sequencing was performed in Sangon Biotech (Shanghai, China). For quantitative RT-PCR, 2 ml of each strain (OD600 = 1.0) was harvested and the RNA extracted using TRIzol. The cDNA was reverse-transcribed using a Reverse Transcription Kit (R333; Vazyme Biotechnology, Nanjing, China). Quantitative PCR (qPCR) primers were designed, which are shown in Supplementary Table S1. qPCR was performed with the SYBR qPCR Kit (Q711; Vazyme Biotechnology) in Applied Biosystems QuantStudio 5. The 2−ΔΔCt method was used for the calculation of fold change.
2.9 Protein expression and purification
The fimC gene ORF was amplified and constructed in the pET-28a plasmid. pET28a-fimC was transformed into BL21(DE3). The expression and the purification of the fimC protein were performed as previously described. Briefly, the BL21(DE3) strain was cultured in LB broth at 25°C and supplemented with 50 μg/ml kanamycin. The strains (OD600 = 0.4) were induced with IPTG (final concentration, 0.5 mM) at 28°C for 18 h. Bacterial cells were harvested and resuspended in PBS and then ultrasonically disrupted at 15% power for 3 min. The soluble His-tagged fimC protein retained on the Ni-NTA column was then eluted with increasing concentrations of imidazole buffer. Enriched proteins were analyzed using SDS-PAGE and were concentrated with ultrafiltration.
2.10 Western blot analysis
The promoter DNA of the agn43 gene (from −262 to −1 bp) was fused with the flag-tagged AmCyan ORF (cyan fluorescent protein from Anemonia majano) in the low-cpy plasmid pACYC184. The plasmids were transformed into wild type (WT), ΔfimC, or the ΔfimC–CfimC strain. pACYC184 containing the Flag-tagged amCyan ORF without fusing any promoters was used as a negative control. For the Western blot analysis, 10 ml of each strain (OD600 = 1.0) was harvested and resuspended in PBS and then ultrasonically disrupted at 15% power for 3 min. Soluble proteins were analyzed using SDS-PAGE. After transfer to a PVDF membrane (ISEQ00010; Merck Millipore, Darmstadt, Germany) with the Trans-Blot® Turbo™ Transfer Starter System (1704155), the PVDF membrane was blocked with 5% skimmed milk. An anti-flag monoclonal antibody was used for analysis of the expression of AmCyan (30502ES60; YEASEN, Shanghai, China). An anti-DnaK polyclonal antibody was used as reference (PH3459S; Abmart, Berkeley Heights, NJ, USA).
2.11 Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed according to the protocol in the LightShift® Chemiluminescent EMSA Kit (no. 20148; Thermo, Waltham, MA, USA). Briefly, 2.5 μg fimC protein was incubated at 37°C for 20 min in 20 μl reaction buffer [containing 1× binding buffer, 1 μg Poly (dI-dC), 2.5% glycerol, 0.05% NP-40, 5 mM Mn2+, and 5 mM Zn2+]. Approximately 1 ng cy5.5-labeled agn43 promoter DNA or 100 ng unlabeled agn43 promoter DNA was added and incubated at 37°C for 20 min. All samples were analyzed by electrophoresis in 4% native TBE polyacrylamide gels at constant 100 V for 60 min. The gel was scanned using a laser imager (Odyssey, LI-COR, Lincoln, NE, USA) with focus at 1.5 mm.
2.12 Statistical analysis
Each experiment was repeated at least three times. Data are presented as the mean ± standard deviation (SD). Student’s t-tests were performed to compare two groups, while one-way ANOVA and Dunn’s post-hoc tests for statistical analysis were used for comparisons among multiple groups. P-values were calculated using GraphPad Prism software (version 9.0). A p < 0.05 was considered statistically significant (*0.01 < p < 0.05, **0.001 < p < 0.01, ***p < 0.001).
3 Results
3.1 fimC is necessary for the biosynthesis of fimbriae
fimC has been reported as a chaperone protein associated with type 1 fimbrial synthesis (Nishiyama et al., 2003). The fimA, fimC, and fimD mutant strains were constructed for the analysis of the biosynthesis of fimbriae. The results showed that the transcription levels of fimA, fimC, and fimD were significantly decreased in these mutant strains (p < 0.001) (Supplementary Figure S1), while the transcription level of fimC in ΔfimC–CfimC was significantly increased compared with the ΔfimC strain (p < 0.001). All results showed that fimA, fimC, and fimD cannot be detected at the transcription level in their mutant strains, indicating that mutant strains were successfully constructed.
TEM showed that, in the WT strain, the fimbriae appeared thick and densely packed (Figure 1). In contrast, the ΔfimC strain displayed neither intact nor broken fimbriae. Furthermore, fimbriae appeared in the ΔfimC–CfimC strain. Moreover, fimA and fimD were deleted as controls, without showing either intact or broken fimbriae, which is consistent with previous reports (Giese et al., 2023; Nishiyama et al., 2008). The results indicate that the lack of fimC led to complete loss of the ability to synthesize type 1 fimbriae in APEC.
Figure 1
3.2 fimC represses the autoaggregation
The fim gene cluster, as a type 1 fimbrial synthesis gene, determines the integrity of fimbrial synthesis. However, whether fimbriae affect autoaggregation remains unclear. Here, the autoaggregation results showed that, after incubating statically at 37°C for 24 h, the ΔfimC strain showed a significantly increased autoaggregation compared with the WT strain (p < 0.001) (Figure 2A). In addition, autoaggregation in the ΔfimC–CfimC strain was restored to WT levels (p > 0.05). Moreover, to further verify whether the increase in autoaggregation is caused by the inability to produce fimbriae, the effect of the fimA and fimD gene mutants on autoaggregation was established. The results showed that the autoaggregation of the ΔfimA and/or the ΔfimD strain remained no different from that of the WT strain (p > 0.05) (Figure 2A). These results indicate that, although either lacking fimC or fimA/fimD result in the inability to produce fimbriae, but only lacking fimC significantly increased autoaggregation.
Figure 2
3.3 Transcriptomic analysis reveals that deletion of fimC alters the transcription of multiple pathways
To explore the mechanism for the increased autoaggregation caused by the deletion of fimC, as well as other potential related clues, a transcriptomic analysis was performed. The results showed that the transcription levels of 600 genes were upregulated and that 170 genes were downregulated (Figure 3A). All genes were classified using gene function classification (Gene Ontology, GO) into three categories: biological process, cellular component, and molecular function (Figure 3C). GO enrichment analysis demonstrated the top 20 enriched pathways. Half of these pathways are involved in flagellum-dependent cell motility and cell adhesion (Figure 3B). In addition, the pathway of bis-(3′-5′)-cyclic diguanylic acid (c-di-GMP) metabolism was also enriched (Figure 3C). These results indicate that, although fimC is a chaperonin protein associated with type 1 fimbrial synthesis, its potential function may involve multiple regulatory pathways.
Figure 3
To further explore the function of fimC in cell adhesion, motility, and related potential regulatory functions, RT-qPCR was performed. All selected genes were classified according to the categories of transcriptome clustering. The results showed that the transcription level of the fim cluster genes significantly increased, particularly fimB (log2FC = 2.5) and fimH (log2FC = 1.5; p < 0.01). Furthermore, the transcription level of the flagellar primary transcription regulator flhDC was increased (log2FC = 2.0; p < 0.01). It has been reported that quorum sensing and c-di-GMP are important regulatory factors through small molecules associated with the regulation of cell autoaggregation, motility, and biofilm formation, among others. In this study, the transcription levels of the quorum sensing autoinducer-2 kinase lsrK (log2FC = −1.2) and the transcriptional regulator lsrR (log2FC = −1.5) were significantly decreased with the deletion of fimC (p < 0.01). The c-di-GMP metabolic genes such as pdeH were significantly increased (log2FC = 2.4, p < 0.01). Furthermore, the transcription level of the self-recognizing antigen 43 (agn43), a autotransporter, was significantly upregulated ~8.2-fold (p < 0.001) (Figure 3C). Taken together, these results showed that fimC is involved in multiple regulatory pathways in E. coli, demonstrating a complex regulatory network that has not yet been reported.
3.4 The autoaggregation regulated by fimC is mediated by agn43
It has been reported that agn43, as a self-recognizing antigen and autotransporter, mediates the autoaggregation and biofilm formation in E. coli; therefore, a Δagn43 strain was constructed (Sherlock et al., 2006). However, in this study, it was shown that the Δagn43 strain did not significantly influence the autoaggregation compared with the WT strain (p > 0.05) (Figure 2B). These data suggest that species diversity and gene compensatory effects may be the reasons for agn43 deletion not affecting autoagglutination.
However, to explore whether there is a correlation between fimC and agn43, a ΔfimCΔagn43 mutant strain was constructed based on ΔfimC. The results showed that, although the autoaggregation of ΔfimCΔagn43 increased compared with WT (p < 0.05), the autoaggregation of the ΔfimCΔagn43 strain was significantly decreased compared with that of ΔfimC (p < 0.05). Taken together, these results indicate that fimC represses the autoagglutination and is mediated by agn43.
3.5 fimC inhibits the transcription of agn43
Although the lack of fimC significantly increased the transcription of agn43, its regulatory function at the protein level remains unknown. To further confirm that the promoter activity of agn43 was enhanced by the deletion of fimC, a plasmid that contained the agn43 promoter and fused with the flag-tagged AmCyan ORF was constructed. Using Western blot, it was shown that the lack of fimC increased the expression of the flag-tagged AmCyan (Figure 4A).
Figure 4
To further confirm the regulatory relationship between fimC and agn43, a pBAD plasmid for the overexpression of fimC was used to detect the agn43 promoter activity. Before detection, it was noted that the agn43 promoter is predicted to have two regions—p1 (from −262 to −213 bp) and p2 (from −213 to −164 bp) (Figure 4C)—using an online promoter prediction tool (https://www.fruitfly.org/seq_tools/promoter.html). The promoter patterns are illustrated in Figure 4B. The promoters were then fused into a plasmid containing the lacZ reporter gene through detection of β-galactosidase (LacZ) activity. The aim was to establish that p1 or p2 is the primary promoter of agn43. Through the deletion of p1 or p2, the activity of the promoter was verified to determine whether the activity of p1 or p2 is affected. The results showed that the lack of p3 (from −262 to −164 bp) completely inhibited the promoter activity (Figure 4D). The same results were shown with the lack of p1. However, the lack of p2 showed significant difference compared with WT (p < 0.01) (Figure 4D), indicating that p1, and not p2, is the main promoter for the transcription of the agn43 gene. By overexpression of fimC, it showed that the activity of Δp1 or p2 showed not significantly changed (p > 0.05), while a significant difference was found in p3 (p < 0.01) (Figure 4E). Taken together, these results showed that promoter p1 is crucial for the transcription of agn43 and that fimC inhibits the transcription of agn43 by inhibiting the promoter activity of p1.
3.6 fimC binds to the promoter region of agn43
It has been shown that fimC regulates the promoter activity and represses the transcription of agn43. Therefore, we speculate that fimC binds to the p1 promoter region of agn43. As the EMSA results showed, with increasing concentration of the fimC protein, the shift bands increased, while the free DNA decreased (Figure 4D). However, the p2 promoter DNA was used as a control, which showed no shift bands with increasing concentrations of fimC. In addition, 200 μM (~100-fold) p1 DNA without the cy5.5 tag was used as the negative control, which showed no shift bands. Taken together, fimC binds to the p1 promoter region of agn43.
3.7 fimC and agn43 affect motility and the early stage of biofilm formation
As the transcriptomic data demonstrated the enrichment of the flagellum-mediated motility-related genes in the ΔfimC strains (Figure 3B), the flagellum-mediated motility of ΔfimC, Δagn43, and ΔfimCΔagn43 was therefore determined. As shown, ΔfimC exerted significantly increased motility compared with the WT strain (p < 0.001) (Figure 5A), while the lack of agn43 significantly decreased the motility (p < 0.01). In the ΔfimCΔagn43 strain, the motility was significantly decreased compared with that in ΔfimC, but it still showed high motility compared with Δagn43 (p < 0.01). These results indicate that fimC and agn43 affect bacterial motility. However, it cannot be elaborated whether the regulation of motility by fimC is mediated by agn43.
Figure 5
Biofilm formation is an important biological characteristic regulated by several factors, such as adhesion, autoaggregation, motility, and c-di-GMP, among others. In order to explore whether the regulation of fimC and agn43 on biofilm formation is related, the biofilm formation ability was examined. The results showed that, in the earlier (12 h) or the later stage (24 h) of biofilm formation, the deletion of fimC led to complete loss of the ability to form a biofilm (p < 0.001) (Figure 5B). However, the lack of agn43 significantly increased the biofilm formation at 12 h (p < 0.01), but showed no significant difference at 24 h (p > 0.05) compared with the WT strain. In addition, the ΔfimCΔagn43 strain demonstrated complete inability to form a biofilm at either 12 or 24 h. In conclusion, these results indicate that fimC and agn43 affect bacterial motility and biofilm formation and that, more importantly, agn43 mediates the early stage of biofilm formation.
3.8 fimC and agn43 coordinate to increase the adhesion of APEC to HD11 cells
Adhesion and invasion are pathogenic processes mediated by bacterial fimbrial proteins such as type 1 fimbriae and other adhesins. To determine whether agn43 is regulated by fimC during the adhesion to and invasion of avian macrophage HD-11 cells, the adhesion and invasion abilities were determined. The results showed that the lack of fimC significantly decreased the adhesion ability of APEC to HD-11 cells (p < 0.001). The Δagn43 strain also exhibited a significantly reduced adhesion ability to HD-11 cells compared with the WT (p < 0.01) (Figure 6A). Furthermore, the adhesion of ΔfimCΔagn43 was significantly decreased compared with either the ΔfimC or the Δagn43 strain (p < 0.01). These findings suggest that fimC and agn43 synergistically enhance the adhesion of APEC to HD11 cells.
Figure 6
For the invasion ability, the results showed that the lack of fimC significantly increased the invasion ability (p < 0.01) (Figure 6B), while the deletion of agn43 significantly decreased the invasion ability (p < 0.01). However, in ΔfimCΔagn43, the invasion ability was significantly decreased and showed no difference compared with Δagn43 (p > 0.05). Taken together, these findings demonstrate that fimC and agn43 coordinate to increase the adhesion of APEC to HD11 cells, but that fimC may suppress the invasive ability by negatively modulating agn43.
4 Discussion
Gram-negative pathogens utilize adhesins, filamentous protein complexes anchored to the outer membrane, known as fimbriae, to bind to the surface glycans of host cells and initiate infection (Hospenthal and Waksman, 2019; Jones et al., 1995; Lindberg et al., 1987; Martinez et al., 2000; Roberts et al., 1994). Type 1 fimbriae and the related P pili are among the most well-characterized pilus systems found in E. coli (Werneburg and Thanassi, 2018).
In vivo, the assembly of type 1 fimbriae follows the chaperone–usher pathway (Crespo et al., 2012; Werneburg and Thanassi, 2018) and relies on the periplasmic chaperone fimC. The fimC chaperone selectively recognizes the disulfide forms of the unfolded subunits and catalyzes their folding (Choudhury et al., 1999; Vetsch et al., 2004).
Aggregation is considered an important characteristic for bacterial survival during host infection (Klemm et al., 2006; Charbonneau and Mourez, 2008). Evidence accumulated in the literature suggests that fimC regulates autoaggregation. As expected, it was observed that the lack of fimC significantly increased the autoaggregation of APEC (Figure 2A). In addition, initially, it was believed that the deletion of fimC resulted in failure to synthesize the fimbriae, which further led to autoaggregation. However, the ΔfimA or the ΔfimD strain was completely unable to form fimbriae while having no effect on autoaggregation (Figure 2A). This provided proof that fimC, but not fimbriae, is crucial for the regulation of aggregation.
With further evidence, we showed the DNA-binding ability of fimC. As an atypical DNA-binding protein, fimC, which codes the PapD_N-PapD_C domain, did not bear any DNA-binding domain. The PapD_N or the PapD_C domain is a pilus and flagellar assembly chaperone for pilus and flagellar assembly. In all native fimC–subunit complexes, the periplasmic chaperone fimC significantly accelerates the folding of pilus subunits into a defined tertiary structure, increasing the process up to ~104-fold (Choudhury et al., 1999; Vetsch et al., 2004). These are notably faster chaperonin-bound subunits in archaic and alternative chaperone–usher pilus systems, where the subunits may only be partially folded (Pakharukova et al., 2018). This demonstrates the importance of fimC for type 1 fimbrial synthesis, as well as bacteria. Although there is no typical DNA-binding domain in fimC, such as the helix–turn–helix (HTH) DNA-binding domain that is widely spread in prokaryotic transcription regulatory proteins, we speculate that fimC may only bind to the promoter DNA and occupy the binding sites, leading to loss of the transcription regulatory modulation by other DNA-binding proteins. However, the premise of this hypothesis is that fimC has a higher affinity with DNA compared with the processes of typical DNA-binding domain proteins. However, in this study, although the binding and regulatory effects of fimC on the agn43 promoter have been proven, the regulation of other genes remains unknown. Although the transcription levels of several genes were altered after fimC deletion (Figure 3A), these changes may not be directly caused by fimC.
In addition, it has been proven that the aggregation mediated by agn43 was directly regulated by fimC. However, there was no evidence illustrating that the effects on motility, biofilm formation, and adhesion and invasion were directly regulated by fimC (Figures 5A, B). Although fimC and agn43 have antagonistic effects on the regulation of motility and synergistic effects on the regulation of adhesion, fimC or agn43 plays a regulatory role in the later stage of biofilm formation and invasion (Figures 5C, 6B), forming the complex regulatory relationship and making it difficult to elucidate the regulatory network. The effects on the biological characteristic regulation between fimC and agn43 need to be explored further.
It is worth noting that the flagellum-mediated motility and the c-di-GMP metabolism were enriched in transcriptomics, and both have significant regulatory effects on biofilm formation. c-di-GMP, as a ubiquitous secondary messenger in bacteria, affects multiple biological characteristics including biofilm formation and motility. However, due to the complexity of the regulatory network of c-d-GMP, with ~30 genes involved in c-di-GMP metabolism (Povolotsky and Hengge, 2016), it is difficult to establish whether there is a regulatory relationship between fimC and c-di-GMP. Nevertheless, there is still a potential that fimC could affect the biological characteristics of bacteria such as autoagglutination, biofilm formation, and motility by regulating the transcription of the c-di-GMP metabolic genes.
In conclusion, in this study, we demonstrated the regulatory relationship of fimC and agn43 and confirmed that the effect of the lack of fimC, but not fimbriae, on autoaggregation is mediated by agn43 (Figure 7). Both fimC and agn43 have regulatory effects on biofilm formation, motility, and adhesion and invasion; however, the specific mechanism still needs to be explored further. This study provides new insights into the prevention and control of APEC and a novel idea for the development of fimbrial-based vaccines.
Figure 7
5 Conclusion
fimC is an essential component for the synthesis of type 1 fimbriae. However, in this study, it was demonstrated that fimC not only regulated fimbrial synthesis but also affected bacterial autoaggregation by regulating agn43. The results showed that fimC, rather than fimbriae, affected bacterial autoaggregation. Further investigation showed that fimC inhibited the transcription of agn43 by directly binding to its promoter region. Finally, the effects of fimC and agn43 on biofilm formation, motility, and the adhesion and invasion ability for HD-11 cells were also demonstrated. In conclusion, this study broadens the regulatory role of proteins without a DNA-binding domain on gene transcription, providing a new perspective for further understanding the function of fimC.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
ZW: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. XN: Investigation, Methodology, Writing – review & editing, Writing – original draft. NZ: Data curation, Formal Analysis, Writing – review & editing. LK: Formal Analysis, Investigation, Writing – review & editing. SN: Data curation, Formal Analysis, Writing – review & editing. HZ: Data curation, Investigation, Writing – review & editing. WJ: Data curation, Supervision, Writing – review & editing. YL: Formal Analysis, Supervision, Writing – review & editing. JT: Data curation, Supervision, Writing – review & editing. XH: Data curation, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Shanghai (Grant No. 22ZR1475800), the National Natural Science Foundation of China (Grant Nos. U22A20518), and Shanghai Municipal Science and Technology Commission (Grant No. 22N31900400).
Conflict of interest
Author YL was employed by the company Qingdao Orisess Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1591206/full#supplementary-material
References
1
AdlerberthI.SvanborgC.CarlssonB.MellanderL.HansonL. A.JalilF.et al. (1998). P fimbriae and other adhesins enhance intestinal persistence of Escherichia coli in early infancy. Epidemiol. Infect.121, 599:608. doi: 10.1017/s0950268898001137
2
ApostolakosI.LaconiA.Mughini-GrasL.YapicierO. S.PiccirilloA. (2021). Occurrence of colibacillosis in broilers and its relationship with avian pathogenic Escherichia coli (APEC) population structure and molecular characteristics. Front. Vet. Sci.8. doi: 10.3389/fvets.2021.737720
3
ArciolaC. R.CampocciaD.RavaioliS.MontanaroL. (2015). Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front. Cell. Infect. Microbiol.5. doi: 10.3389/fcimb.2015.00007
4
BarbieriN. L.PimentaR. L.de MeloD. A.NolanL. K.de SouzaM.LogueC. M. (2021). Mcr-1 identified in fecal Escherichia coli and avian pathogenic E. coli (APEC) from Brazil. Front. Microbiol.12. doi: 10.3389/fmicb.2021.659613
5
BlumerC.KleefeldA.LehnenD.HeintzM.DobrindtU.NagyG.et al. (2005). Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology. (Reading).151, 3287–3298. doi: 10.1099/mic.0.28098-0
6
CharbonneauM. E.MourezM. (2008). The Escherichia coli AIDA-I autotransporter undergoes cytoplasmic glycosylation independently of export. Res. Microbiol.159, 537–544. doi: 10.1016/j.resmic.2008.06.009
7
ChoudhuryD.ThompsonA.StojanoffV.LangermannS.PinknerJ.HultgrenS. J.et al. (1999). X-ray structure of the fimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science285, 1061–1066. doi: 10.1126/science.285.5430.1061
8
CostaT. R.Felisberto-RodriguesC.MeirA.PrevostM. S.RedzejA.TrokterM.et al. (2015). Secretion systems in gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol.13, 343–359. doi: 10.1038/nrmicro3456
9
CourtD. L.SwaminathanS.YuD.WilsonH.BakerT.BubunenkoM.et al. (2003). Mini-lambda: a tractable system for chromosome and BAC engineering. Gene315, 63–69. doi: 10.1016/s0378-1119(03)00728-5
10
CrespoM. D.PuorgerC.ScharerM. A.EidamO.GrutterM. G.CapitaniG.et al. (2012). Quality control of disulfide bond formation in pilus subunits by the chaperone fimC. Nat. Chem. Biol.8, 707–713. doi: 10.1038/nchembio.1019
11
DatsenkoK. A.WannerB. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A.97, 6640–6645. doi: 10.1073/pnas.120163297
12
ElhenawyW.HordienkoS.GouldS.ObercA. M.TsaiC. N.HubbardT. P.et al. (2021). High-throughput fitness screening and transcriptomics identify a role for a type IV secretion system in the pathogenesis of Crohn’s disease-associated Escherichia coli. Nat. Commun.12, 2032. doi: 10.1038/s41467-021-22306-w
13
FredericksC. E.ShibataS.AizawaS.ReimannS. A.WolfeA. J. (2006). Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol. Microbiol.61, 734–747. doi: 10.1111/j.1365-2958.2006.05260.x
14
GieseC.PuorgerC.IgnatovO.BecarovaZ.WeberM. E.ScharerM. A.et al. (2023). Stochastic chain termination in bacterial pilus assembly. Nat. Commun.14, 7718. doi: 10.1038/s41467-023-43449-y
15
HanX.LiuL.FanG.ZhangY.XuD.ZuoJ.et al. (2015). Riemerella anatipestifer lacks luxS, but can uptake exogenous autoinducer-2 to regulate biofilm formation. Res. Microbiol.166, 486–493. doi: 10.1016/j.resmic.2015.06.004
16
HanY.HanX.WangS.MengQ.ZhangY.DingC.et al. (2014). The waaL gene is involved in lipopolysaccharide synthesis and plays a role on the bacterial pathogenesis of avian pathogenic Escherichia coli. Vet. Microbiol.172, 486–491. doi: 10.1016/j.vetmic.2014.05.029
17
HasmanH.SchembriM. A.KlemmP. (2000). Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol.182, 1089–1095. doi: 10.1128/JB.182.4.1089-1095.2000
18
HendersonI. R.NataroJ. P. (2001). Virulence functions of autotransporter proteins. Infect. Immun.69, 1231–1243. doi: 10.1128/IAI.69.3.1231-1243.2001
19
HettaH. F.Al-KadmyI.KhazaalS. S.AbbasS.SuhailA.El-MokhtarM. A.et al. (2021). Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep.11, 10751. doi: 10.1038/s41598-021-90208-4
20
HoibyN.BjarnsholtT.GivskovM.MolinS.CiofuO. (2010). Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents.35, 322–332. doi: 10.1016/j.ijantimicag.2009.12.011
21
HospenthalM. K.WaksmanG. (2019). The remarkable biomechanical properties of the type 1 chaperone-usher pilus: a structural and molecular perspective. Microbiol. Spectr.7, 1. doi: 10.1128/microbiolspec.PSIB-0010-2018
22
HuQ.HanX.ZhouX.DingC.ZhuY.YuS. (2011). OmpA is a virulence factor of Riemerella anatipestifer. Vet. Microbiol.150, 278–283. doi: 10.1016/j.vetmic.2011.01.022
23
JonesC. H.PinknerJ. S.NicholesA. V.SlonimL. N.AbrahamS. N.HultgrenS. J. (1993). fimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc. Natl. Acad. Sci. U. S. A.90, 8397–8401. doi: 10.1073/pnas.90.18.8397
24
KathayatD.ClossG. J.HelmyY. A.LokeshD.RanjitS.RajashekaraG. (2021). Peptides affecting the outer membrane lipid asymmetry system (MlaA-OmpC/f) reduce avian pathogenic Escherichia coli (APEC) colonization in chickens. Appl. Environ. Microbiol.87, e0056721. doi: 10.1128/AEM.00567-21
25
KlemmP.VejborgR. M.SherlockO. (2006). Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol.296, 187–195. doi: 10.1016/j.ijmm.2005.10.002
26
KoreaC. G.GhigoJ. M.BeloinC. (2011). The sweet connection: solving the riddle of multiple sugar-binding fimbrial adhesins in Escherichia coli: multiple E. coli fimbriae form a versatile arsenal of sugar-binding lectins potentially involved in surface-colonisation and tissue tropism. Bioessays33, 300–311. doi: 10.1002/bies.201000121
27
LiR.LiN.ZhangJ.WangY.LiuJ.CaiY.et al. (2016). Expression of immune-related genes of ducks infected with avian pathogenic Escherichia coli (APEC). Front. Microbiol.7. doi: 10.3389/fmicb.2016.00637
28
LindbergF.LundB.JohanssonL.NormarkS. (1987). Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature328, 84–87. doi: 10.1038/328084a0
29
MartinezJ. J.MulveyM. A.SchillingJ. D.PinknerJ. S.HultgrenS. J. (2000). Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO. J.19, 2803–2812. doi: 10.1093/emboj/19.12.2803
30
NawazS.WangZ.ZhangY.JiaY.JiangW.ChenZ.et al. (2024). Avian pathogenic Escherichia coli (APEC): current insights and future challenges. Poult. Sci.103, 104359. doi: 10.1016/j.psj.2024.104359
31
NishiyamaM.IshikawaT.RechsteinerH.GlockshuberR. (2008). Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science320, 376–379. doi: 10.1126/science.1154994
32
NishiyamaM.VetschM.PuorgerC.JelesarovI.GlockshuberR. (2003). Identification and characterization of the chaperone-subunit complex-binding domain from the type 1 pilus assembly platform FimD. J. Mol. Biol.330, 513–525. doi: 10.1016/s0022-2836(03)00591-6
33
PakharukovaN.McKennaS.TuittilaM.PaavilainenS.MalmiH.XuY.et al. (2018). Archaic and alternative chaperones preserve pilin folding energy by providing incomplete structural information. J. Biol. Chem.293, 17070–17080. doi: 10.1074/jbc.RA118.004170
34
PereA.NowickiB.SaxenH.SiitonenA.KorhonenT. K. (1987). Expression of p, type-1, and type-1c fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis.156, 567–574. doi: 10.1093/infdis/156.4.567
35
PovolotskyT. L.HenggeR. (2016). Genome-based comparison of cyclic di-GMP signaling in pathogenic and commensal Escherichia coli strains. J. Bacteriol.198, 111–126. doi: 10.1128/JB.00520-15
36
PrimoE.BoginoP.CossovichS.ForestoE.NievasF.GiordanoW. (2020). Exopolysaccharide II is relevant for the survival of Sinorhizobium meliloti under water deficiency and salinity stress. Molecules25, 21. doi: 10.3390/molecules25214876
37
RobertsJ. A.MarklundB. I.IlverD.HaslamD.KaackM. B.BaskinG.et al. (1994). The gal (alpha 1-4) gal-specific tip adhesin of Escherichia coli p-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. U. S. A.91, 11889–11893. doi: 10.1073/pnas.91.25.11889
38
SchembriM. A.KlemmP. (2001). Coordinate gene regulation by fimbriae-induced signal transduction. EMBO. J.20, 3074–3081. doi: 10.1093/emboj/20.12.3074
39
SherlockO.DobrindtU.JensenJ. B.MunkV. R.KlemmP. (2006). Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein. J. Bacteriol.188, 1798–1807. doi: 10.1128/JB.188.5.1798-1807.2006
40
SpauldingC. N.KleinR. D.RuerS.KauA. L.SchreiberH. L.CusumanoZ. T.et al. (2017). Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature546, 528–532. doi: 10.1038/nature22972
41
VetschM.PuorgerC.SpirigT.GrauschopfU.Weber-BanE. U.GlockshuberR. (2004). Pilus chaperones represent a new type of protein-folding catalyst. Nature431, 329–333. doi: 10.1038/nature02891
42
WerneburgG. T.ThanassiD. G. (2018). Pili assembled by the chaperone/usher pathway in Escherichia coli and Salmonella. EcoSal Plus.8, 1. doi: 10.1128/ecosalplus
Summary
Keywords
avian pathogenic E. coli (APEC), type 1 fimbriae chaperone protein fimC, autoaggregation, DNA-binding, biofilm, c-di-GMP
Citation
Wang Z, Niu X, Zhong N, Kong L, Nawaz S, Zhang H, Jiang W, Liu Y, Tu J and Han X (2025) FimC binds to the promoter region of agn43 to modulate autoaggregation. Front. Cell. Infect. Microbiol. 15:1591206. doi: 10.3389/fcimb.2025.1591206
Received
10 March 2025
Accepted
23 April 2025
Published
30 May 2025
Volume
15 - 2025
Edited by
He Zhang, Chinese Academy of Agricultural Sciences, China
Reviewed by
Kurni Kurniyati, Virginia Commonwealth University, United States
Fu Chen, Qingdao Agricultural University, China
Hongying Chen, Henan Agricultural University, China
Updates
Copyright
© 2025 Wang, Niu, Zhong, Kong, Nawaz, Zhang, Jiang, Liu, Tu and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangan Han, hanxgan@163.com; Jian Tu, tujian1980@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.