- 1Department of Respiratory and Critical Care Medicine, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Thoracic Surgery, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Checkpoint inhibitor pneumonitis (CIP) and radiation pneumonitis (RP) lead to anti-cancer therapy discontinuation and poor diagnosis. The human microbiome is related to various respiratory diseases. However, the role of the lung microbiome in CIP and RP remains unknown. Our study aimed to explore the lower respiratory tract (LRT) microbiome in CIP/RP patients.
Methods: The study enrolled 61 patients with pneumonitis or pneumonia, including 23 with CIP/RP, and 38 with lung cancer with pneumonia (LC-P). Metagenomic next-generation sequencing (mNGS) was performed to identify the microbiota in bronchoalveolar lavage fluid (BALF), and bioinformatics methods were used to compare the microbial differences between CIP/RP and LC-P groups. Correlation analysis was conducted to explore the relationship between LRT microbiota and clinical features.
Results: The Prevotella was the dominant genus in both groups. The Prevotella melaninogenica, which belongs to the Prevotella genus, was the dominant species in the CIP/RP group and the second most abundant species in the LC-P group. Compared to the LC-P group, the CIP/RP group had significantly high levels of Prevotella melaninogenica species and lymphocyte percentage in BALF but significantly low levels of lymphocytes, eosinophils and albumin in peripheral blood. In addition, the Prevotella melaninogenica species had a negative correlation with peripheral blood lymphocytes.
Conclusion: The enrichment of Prevotella melaninogenica species in LRT and a decreased level of peripheral blood lymphocytes are associated with CIP/RP.
Introduction
Immune checkpoint inhibitors targeting PD-1/PD-L1 have changed the treatment landscape for oncology, but their clinical benefits are tempered by immune-related adverse events (Suresh et al., 2018a; Shankar et al., 2020; Sun et al., 2023). Among these, checkpoint inhibitor pneumonitis (CIP) affects 2.49–19% of patients, often leading to therapy discontinuation and poor prognosis (Naidoo et al., 2017; Suresh et al., 2018b; Suresh et al., 2019; Tiu et al., 2022; Yin et al., 2022). Similarly, radiation pneumonitis (RP) occurs in 1–25% of thoracic radiotherapy recipients, with shared features of dysregulated inflammation and fibrosis complicating differential diagnosis (Hanania et al., 2019). In addition, CIP is more common in patients receiving curative-intent radiotherapy followed by anti-PD-1/PD-L1 agents (Shaverdian et al., 2017; Voong et al., 2019). However, it turns out to be difficult to distinguish CIP from RP (Rahi et al., 2021).
The human microbiome is related to various respiratory diseases (Frayman et al., 2024; Özçam and Lynch, 2024; Song et al., 2024). For instance, the gut microbiota influences chronic obstructive pulmonary disease (COPD) development and fecal microbiota transplantation restores the pathogenesis of COPD (Lai et al., 2022). The gut protist Tritrichomonas musculis induces the migration of gut-derived lymphoid cells to the lung and further promotes steady state eosinophilia, which exacerbates asthma and hinders the systemic dissemination of pulmonary Mycobacterium tuberculosis (Burrows et al., 2025). The gut microbiome shapes the immune system and may play a protective role in respiratory diseases, suggesting that managing the gut microbiome represents a powerful way to prevent and treat respiratory diseases (Perdijk et al., 2024). However, the role of the lung microbiome in cancer treatment-related pneumonitis, particularly CIP and RP, remains unknown. Exploring lung microbial dysbiosis is crucial to understanding the occurrence of CIP/RP on microbial terms and managing the microbial imbalance may be a potential therapy for CIP/RP.
Our study aimed to explore the LRT microbiome in CIP/RP patients, analyze the microbial composition and diversity, compare the microbial differences, and further explore the relationship between LRT microbiome and clinical features.
Methods
Recruitment of patients
This retrospective study was conducted at Renji Hospital, Shanghai Jiao Tong University School of Medicine. A total of 61 patients were enrolled from 20 June 2021 to 20 October 2024. Among them, 23 were classified as CIP/RP group (of whom, 16 had CIP and 7 had RP), and 38 patients were diagnosed with LC-P. Inclusion criteria for CIP or RP included: (1) the cancer patients had received immunotherapy or radiotherapy; (2) imaging studies showed new pulmonary infiltrates (radiologic patterns of CIP include cryptogenic organizing pneumonia, ground glass opacities and interstitial pneumonia, while radiologic features of RP are ground glass opacities or consolidation conforming precisely to the shape of the radiation field.); (3) LRT infection or lung tumor progression was excluded; and (4) the patients were not treated with antibiotics or steroid within 2 weeks.
BALF collection
BALF samples were collected from all 61 patients according to the standard procedures. The lung was lavaged with 100mL of sterile saline solution and the BALF recovery rate was more than 35%. BALF samples for further mNGS were then transported to the hospital laboratory under cold-chain conditions. Clinical information and laboratory results were also collected when the patients were sampled.
Nucleic acid extraction, library preparation, sequencing, and bioinformatics analysis
The TIANamp Magnetic DNA Kit (Tiangen) was used to extract DNA. Quantity and quality of DNA were assessed using the Qubit and NanoDrop (Thermo Fisher Scientific), respectively. DNA libraries were prepared using the Hieff NGS C130P2 OnePot II DNA Library Prep Kit for MGI (Yeasen Biotechnology) according to the manufacturer’s protocols. Agilent 2,100 was used for quality control and DNA libraries were 50 bp single-end sequenced on MGISEQ-200. Raw sequencing data were split by bc12fastq2 (version 2.20), and high-quality sequencing data were generated using Trimmomatic (version 0.36) by removing low-quality reads, adapter contamination, duplicated and shot (length, 36bp) reads. Human host sequences were subtracted by mapping to human reference genome (hs37d5) using bowtie2 (version 2.2.6). Reads that could not be mapped to the human genome were retained and aligned with the microorganism genome database for microbial identification by Kraken (version 2.0.7), and species abundance estimating by Bracken (version 2.5.0). The microorganism genome database contained genomes or scaffolds of bacteria, fungi, viruses, and parasites (download from GenBank release 238, ftp://ftp.ncbi.nlm.nih.gov/genomes/genbank/).
Statistical analysis
We performed the microbial diversity analysis using R software (version 4.0.1). The alpha-diversity was assessed by taxonomic profiles, and the beta-diversity was estimated by Bray- Curtis distance. PERMANOVA (vegan) was used to analyze beta-diversity differences. Differences of the relative genus abundances were tested by the Kruskal-Wallis test (Kruskal.test package). Only genera with greater than 1% mean abundance and 40% prevalence were compared. Linear discriminant analysis (LDA) effect size (LEfSe) was performed to assess the statistical differences of the relative abundance of microorganisms between CIP/RP and LC-P patients. Spearman’s correlations between clinical indicators and relative genus abundances were determined by R package cor. test and adjusted by false discovery rate. A random forest binary classification model integrating key microbes and significantly clinical indicators was assessed by Receiver Operating Characteristic Curve.
Student’s t-test or Mann-Whitney U test was used to compare the continuous variables. For categorical variables, Chi-square test or Fisher’s exact test was used to explore the association. All significance tests were two-tailed and a P value < 0.05 was considered statistically significant.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (KY2021-102-B). Informed consent was obtained from all patients.
Results
Demographic information of participants
The study included 61 patients. Among these patients, 23 were classified as grade1–2 CIP/RP (of whom, 16 had CIP and 7 had RP), while 38 were confirmed to have LC-P. The demographic and clinical characteristics of the patients are detailed in Table 1. The patients in the CIP/RP group were younger than those in the LC-P group. Comorbidities comprised chronic obstructive pulmonary disease (COPD, 17.4%), hypertension (13%) and diabetes (13%) in the CIP/RP group. The CIP/RP group had lower levels of lymphocytes (P = 0.001), EOS (P = 0.008), and ALB (P = 0.006) in peripheral blood than those in the LC-P group. In BALF, the percentage of lymphocyte was significantly high in the CIP/RP group (P = 0.010). The CIP/RP group also had higher levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Krebs Von den Lungen-6 (KL-6), and D-dimer, but no significant differences were observed in these clinical indicators.
Lung microbial diversity and composition
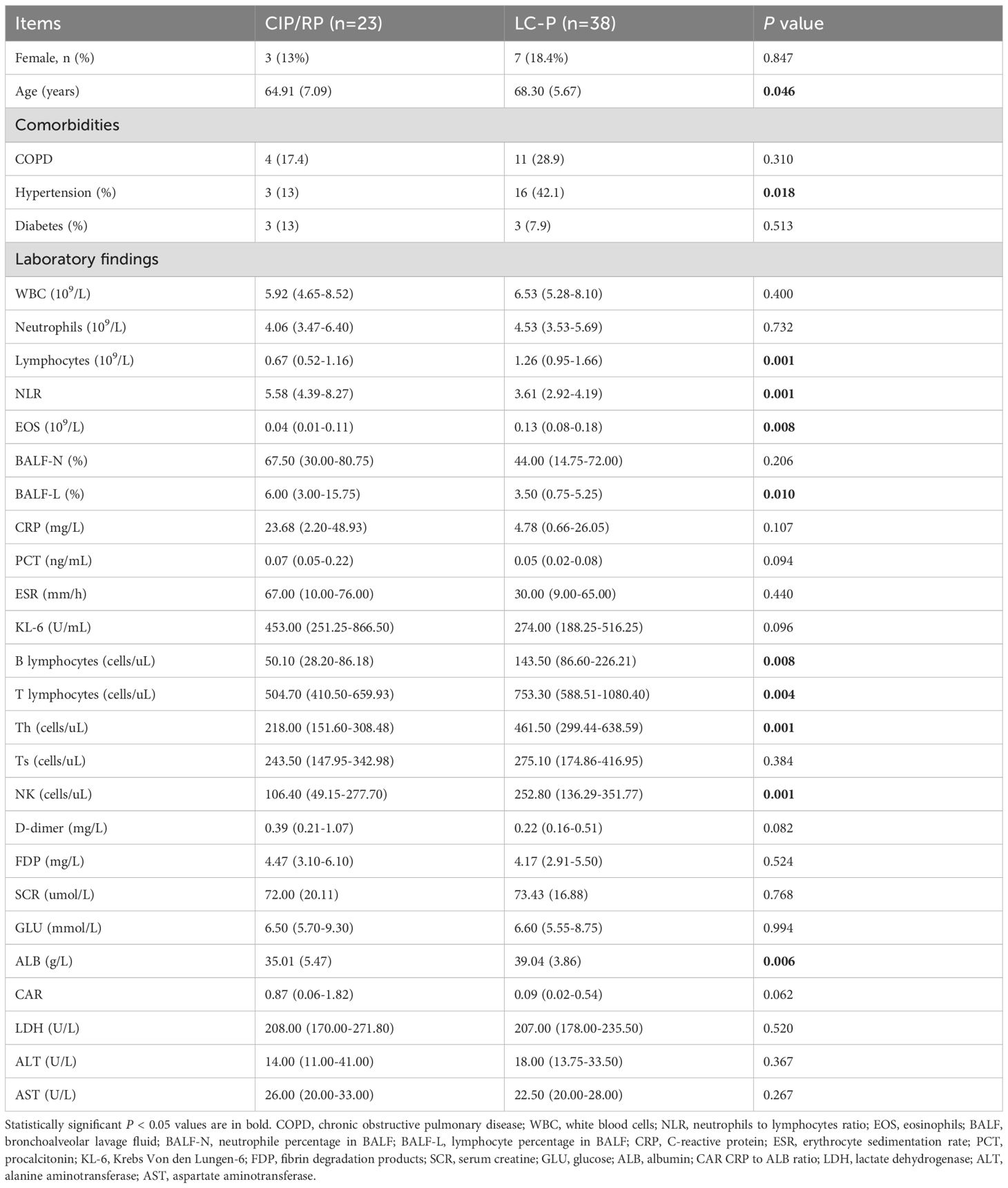
At species level, alpha-diversity was based on ACE, Chao1, Shannon, and Simpson indexes (Supplementary Figure 1A). However, there were no significant differences in ACE (P = 0.63), Chao1 (P = 0.63), Shannon (P = 0.8), and Simpson (P = 0.92) indexes. Additionally, PCoA and PC analysis based on the Bray-Curtis distances also showed that no difference was observed in beta-diversity (P = 0.481 and P = 0.477, respectively) (Supplementary Figure 1B). We then analyzed the lung microbial composition in CIP/RP and LC-P groups. In the CIP/RP group, the top five phyla were Pseudomonadota (27.0%), Bacteroidota (26.1%), Bacillota (19.4%), Actinomycetota(19.0%) and Peploviricota (3.0%) (Figure 1A). The top five genera included Prevotella (22.7%), Rothia (8.7%), Pseudomonas (7.8%), Streptococcus (7.7%), and Veillonella (6.7%) (Figure 1B). The top five species were Prevotella melaninogenica (11.6%), Rothia mucilaginosa (9.7%), Pseudomonas aeruginosa (6.0%), Prevotella jejuni (5.6%) and Haemophilus parainfluenzae (4.1%) (Figure 1C). In the LC-P group, the top five phyla included Bacteroidota (27.2%), Pseudomonadota (22.2%), Bacillota (21.6%), Actinomycetota (17.4%) and Ascomycota (4.2%) (Figure 1A). The top five genera were Prevotella (19.4%), Rothia (9.2%), Streptococcus (6.9%), Veillonella (6.6%) and Haemophilus (4.8%) (Figure 1B). The top five species included Rothia mucilaginosa (6.9%), Prevotella melaninogenica (5.4%), Prevotella jejuni (4.7%), Prevotella pallens (4.1%) and Haemophilus parainfluenzae (3.7%) (Figure 1C).
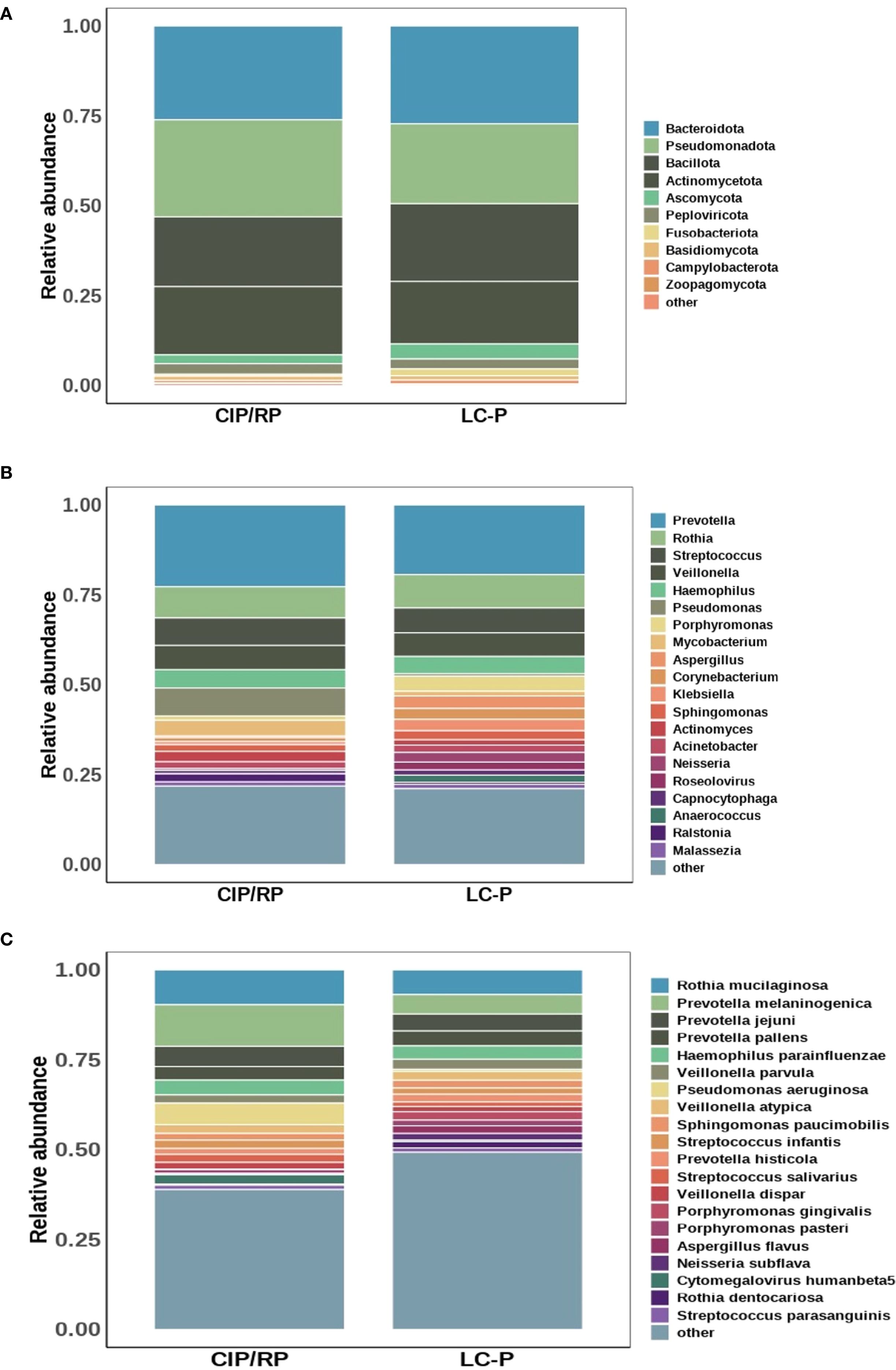
Figure 1. Comparison of the lung microbial profile between CIP/RP and LC-P groups. (A) Dominant phyla. (B) Dominant genera. (C) Dominant species.
Differential microbiota analysis
We further analyzed the differential relative abundance of top 10 phyla, top 20 genera, and top 20 species between CIP/RP and LC-P groups. At the phyla level, no significant differences were observed in the relative abundance of the top 10 phyla. At the genera level (Figure 2A), the relative abundance of Porphyromonas (P = 0.028) and Neisseria (P = 0.010) were significantly lower in the CIP/RP group than those in the LC-P group. No significant differences were observed in the relative abundance of the other genera between the two groups. At the species level (Figure 2B), the relative abundance of Prevotella melaninogenica (P = 0.018) and Cytomegalovirus humanbeta5 (P = 0.010) were significantly higher in the CIP/RP group than those in the LC-P group. However, the relative abundance of Neisseria subflava (P = 0.024) and Porphyromonas pasteri (P = 0.045) were significantly lower in the CIP/RP group than those in the LC-P group. No significant differences were observed in the relative abundance of the other species between the two groups. 22 discriminative features were identified by LEfSe. Among them, 13 taxa were discriminative for the CIP/RP group and 9 taxa were discriminative for the LC-P group (Figure 2C). At the genera level, the Cytomegalovirus (LDA scores >4, P = 0.010) was significantly higher in the CIP/RP group while Porphyromonas (LDA scores >4, P = 0.028) and Neisseria (LDA scores >4, P = 0.010) were abundant in the LC-P group. At the species level, Prevotella melaninogenica (LDA scores >4, P = 0.018) and Cytomegalovirus_humanbeta5 (LDA scores >4, P = 0.010) were significantly higher in the CIP/RP group while Neisseria_subflava (LDA scores >2, P = 0.024) and Porphyromonas pasteri (LDA scores >2, P = 0.045) were significantly more abundant in the LC-P group.
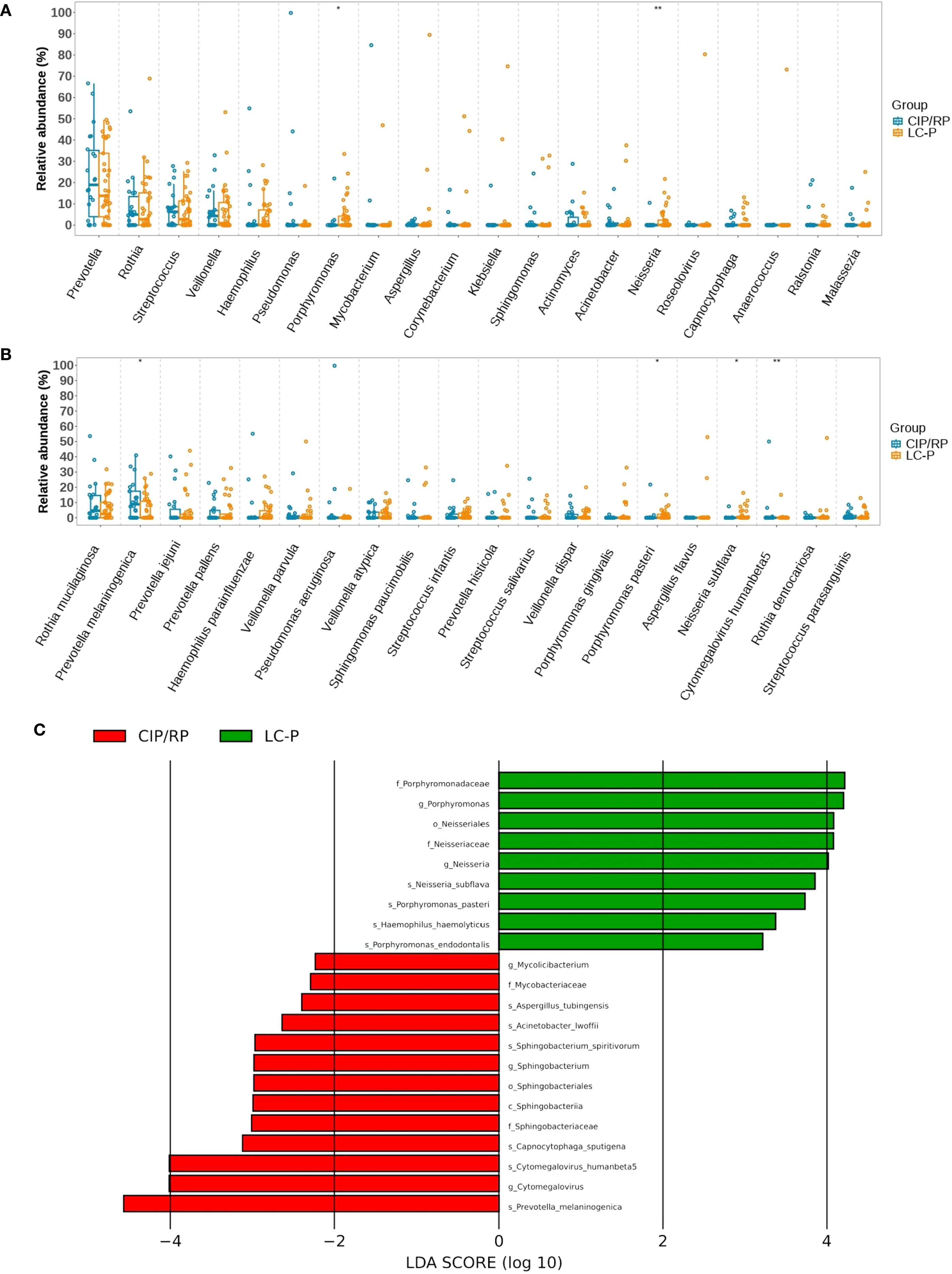
Figure 2. Differential relative abundances between CIP/RP and LC-P groups. (A) Differential relative abundance (log10) of top 20 genera. (B) Differential relative abundance (log10) of top 20 species. (C). Linear discriminant analysis (LDA) effect size (LEfSe) analysis of the differential microbiota between CIP/RP and LC-P groups.
Correlation between differential microbial taxa and clinical indicators
A Spearman correlation analysis was used to further explore the relationship between top 20 genera (or species) and clinical indicators. A two-dimensional heatmap showed the results (Figures 3A, C). We mainly focused on differential microbiota and clinical indicators with significant difference. At the genera level, the Porphyromonas had no correlation with all clinical indicators. The Neisseria showed a positive correlation with ALB. At the species level, the Prevotella melaninogenica had a negative correlation with B lymphocytes, NK, and lymphocytes in peripheral blood. The Cytomegalovirus humanbeta5 showed a negative correlation with B lymphocytes and ALB in peripheral blood. The Neisseria subflava and Porphyromonas pasteri had no correlation with significantly different clinical indicators. Furthermore, a CCA analysis showed that the levels of peripheral blood EOS, ALB, and BALF-L were found to have a strong relation with the top 20 genera or species (Figures 3B, D). In addition, a random forest binary classification model was constructed. The model integrated Prevotella melaninogenica and significantly clinical indicators including peripheral blood lymphocyte, EOS, ALB and BALF-L, yielding an AUC of 0.755 (Figure 3E).
Figure 3. Correlation analysis between microbial taxa and clinical indicators. (A) Heatmap showing the correlation between top 20 genera and clinical indicators. (B) CCA analysis showing the relationship between genera and clinical indicators. The acute angle shows a positive correlation, and an obtuse angle shows a negative correlation. The longer arrow means a greater influence of the indicator.(C) Heatmap showing the correlation between top 20 species and clinical indicators. (D) CCA analysis showing the relationship between species and clinical indicators. (E) Receiver Operating Characteristic Curve for random forest binary classification model. *, p < 0.05; **, p < 0.01; ***, p < 0.001. WBC, white blood cells; NLR, neutrophils to lymphocytes ratio; EOS, eosinophils; BALF, bronchoalveolar lavage fluid; BALF-N, neutrophil percentage in BALF; BALF-L, lymphocyte percentage in BALF; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; KL-6, Krebs Von den Lungen-6; FDP, fibrin degradation products; SCR, serum creatine; GLU, glucose; ALB, albumin; CAR CRP to ALB ratio; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
In this study, we explored the LRT microbiome in the CIP/RP patients, analyzed microbial composition and diversity, compared the differences between CIP/RP and LC-P groups, and further explored the relationship between LRT microbiome and clinical features. We found that the CIP/RP group had higher levels of Prevotella melaninogenica species and BALF-L but lower levels of lymphocytes, EOS, and ALB in peripheral blood. In addition, the Prevotella melaninogenica species had a negative correlation with the peripheral blood lymphocytes.
The prevotella is one of the most common genera among the bacteriome of healthy lung microbiome (Li et al., 2024). As the commensal bacterial microbiota colonized in healthy human airway, the gram-negative prevotella spp. are found to have weak inflammatory properties and be intrinsically tolerated by the respiratory immune system (Larsen et al., 2015). However, the alteration of the prevotella is related to occurrence and development of various diseases (Rofael et al., 2019; De Martin et al., 2021; Wang et al., 2022). De Martin et al. found that the relative abundance of Prevotella melaninogenica was increased in tonsil cancer (De Martin et al., 2021). Sylvia A.D. Rofael et al. used 16S rRNA gene sequencing to detect the respiratory pathogen in induced sputum collected from young adults born extremely preterm and found that the relative abundance of prevotella, particularly prevotella melaninogenica was significantly decreased (Rofael et al., 2019). Once colonizing the stomach, the Prevotella melaninogenica was associated with gastric inflammation or carcinogenesis (Xia et al., 2025). The relative abundance of Prevotella melaninogenica showed a significantly high level in the gastric juice of patients with gastric cancer and bile reflux gastritis and the Prevotella melaninogenica was found to induce gastric inflammation in mice, suggesting that the Prevotella melaninogenica may be associated with the gastric carcinogenesis (Wang et al., 2022).
The role of Prevotella in respiratory diseases is intricate (Larsen et al., 2015; Horn et al., 2022; Lu et al., 2024). Lung dysbiosis with decreased prevotella spp. and increased pathogenic proteobacteria in chronic airway diseases suggests that prevotella spp. play a protective role in chronic airway diseases (Larsen et al., 2015). Kadi J. Horn et al. showed that the Prevotella melaninogenica induced an innate immune response and reinforced protection against bacterial pathogen Streptococcus pneumoniae in a mouse lung co-infection model, highlighting airway Prevotella as a protective role in respiratory tract health (Horn et al., 2022). However, Fan Lu et al. reported that the Prevotella melaninogenica as an opportunistic pathogen may lead to immune dysregulation in immunocompromised patients with sepsis-induced acute lung injury (Lu et al., 2024). In this study, the Prevotella was the dominant genus in both groups, but no significant difference was observed between the two groups. The Prevotella melaninogenica, which belongs to the Prevotella genus, was the dominant species in the CIP/RP group and the second most abundant species in the LC-P group. Compared to the LC-P group, the CIP/RP group had significantly high levels of the Prevotella melaninogenica species. The enrichment of Prevotella melaninogenica may represent its pathogenicity in CIP/RP.
The CIP/RP patients have unique clinical features that consist of an increased lymphocyte percentage in BALF and a decreased level of lymphocytes in peripheral blood (Roberts et al., 1993; Zhou et al., 2020; Lin et al., 2021; Chen et al., 2024). In addition, the reduction of EOS and ALB in peripheral blood is related to the occurrence of CIP (Lin et al., 2021; Li et al., 2022). In this study, the CIP/RP group had a significantly high level of BALF-L but low levels of lymphocytes, EOS, and ALB in peripheral blood, which is consistent with previous studies. We also found that the Prevotella melaninogenica species had a negative correlation with peripheral blood lymphocytes, suggesting the interplay between LRT microbiota and clinical features.
This study has several limitations. First, the number of CIP/RP was small. A future study with large samples would be valuable to validate these findings. Second, the role of the other species, which were not abundant but significantly different, was not well understood. Finally, this study didn’t collect BALF and clinical features from healthy group and patients after the recovery of CIP/RP. Future research is needed to elucidate the interplay between LRT microbiota and clinical features in CIP/RP.
Conclusion
The CIP/RP patients had an increased relative abundance of Prevotella melaninogenica species that showed a negative correlation with peripheral blood lymphocytes, suggesting that the enrichment of Prevotella melaninogenica species in LRT associated with a decreased level of peripheral blood lymphocytes may be a potential biomarker of diagnosis and treatment for CIP/RP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (KY2021-102-B). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Writing – original draft, Formal Analysis, Conceptualization, Data curation, Software. QX: Methodology, Validation, Writing – original draft, Writing – review & editing, Investigation. LZ: Writing – review & editing, Investigation, Methodology. DZ: Methodology, Investigation, Writing – review & editing. XW: Conceptualization, Writing – review & editing, Supervision, Funding acquisition, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Science and Technology Commission of Shanghai Municipality (23Y31900103, 20Z11901003).
Acknowledgments
We sincerely thank Dinfectome Inc., Nanjing, China for providing help in mNGS and results interpretation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1594460/full#supplementary-material
Supplementary Figure 1 | Diversity of LRT microbial flora between CIP/RP and LC-P groups. (A) Alpha-diversity based on ACE, Chao1, Shannon, and Simpson indexes. (B) Beta-diversity based on PCoA and PC algorithm.
Abbreviations
CIP, Checkpoint inhibitor pneumonitis; RP, radiation pneumonitis; LC-P, lung cancer with pneumonia; LRT, lower respiratory tract; mNGS, metagenomic next-generation sequencing; EOS, eosinophils; BALF, bronchoalveolar lavage fluid; BALF-N, neutrophile percentage in BALF; BALF-L, lymphocyte percentage in BALF; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; KL-6, Krebs Von den Lungen-6; ALB, albumin.
References
Burrows, K., Ngai, L., Chiaranunt, P., Watt, J., Popple, S., Forde, B., et al. (2025). A gut commensal protozoan determines respiratory disease outcomes by shaping pulmonary immunity. Cell 188, 316–330. doi: 10.1016/j.cell.2024.11.020
Chen, R., Shi, Y., Fang, N., Shao, C., Huang, H., Pan, R., et al. (2024). Bronchoalveolar lavage fluid analysis in patients with checkpoint inhibitor pneumonitis. Cancer immunology immunotherapy 73, 235. doi: 10.1007/s00262-024-03834-y
De Martin, A., Lutge, M., Stanossek, Y., Engetschwiler, C., Cupovic, J., Brown, K., et al. (2021). Distinct microbial communities colonize tonsillar squamous cell carcinoma. Oncoimmunology 10, 1945202. doi: 10.1080/2162402X.2021.1945202
Frayman, K. B., Macowan, M., Caparros-Martin, J., Ranganathan, S. C., Marsland, B. J., and SYNERGY, C. F. (2024). The Longitudinal Microbial and Metabolic Landscape of infant Cystic Fibrosis: The gut-lung axis. Eur. Respir. J. 63, 2302290. doi: 10.1183/13993003.02290-2023
Hanania, A. N., Mainwaring, W., Ghebre, Y. T., Hanania, N. A., and Ludwig, M. (2019). Radiation-induced lung injury: assessment and management. Chest 156, 150–162. doi: 10.1016/j.chest.2019.03.033
Horn, K. J., Schopper, M. A., Drigot, Z. G., and Clark, S. E. (2022). Airway Prevotella promote TLR2-dependent neutrophil activation and rapid clearance of Streptococcus pneumoniae from the lung. Nat. Commun. 13, 3321. doi: 10.1038/s41467-022-31074-0
Lai, H. C., Lin, T. L., Chen, T. W., Kuo, Y. L., Chang, C. J., Wu, T. R., et al. (2022). Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 71, 309–321. doi: 10.1136/gutjnl-2020-322599
Larsen, J. M., Musavian, H. S., Butt, T. M., Ingvorsen, C., Thysen, A. H., and Brix, S. (2015). Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144, 333–342. doi: 10.1111/imm.12376
Li, Y., Jia, X., Du, Y., Mao, Z., Zhang, Y., Shen, Y., et al. (2022). Eosinophil as a biomarker for diagnosis, prediction, and prognosis evaluation of severe checkpoint inhibitor pneumonitis. Front. Oncol. 12. doi: 10.3389/fonc.2022.827199
Li, R., Li, J., and Zhou, X. (2024). Lung microbiome: new insights into the pathogenesis of respiratory diseases. Signal Transduct Target Ther. 9, 19. doi: 10.1038/s41392-023-01722-y
Lin, X., Deng, H., Yang, Y., Wu, J., Qiu, G., Li, S., et al. (2021). Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front. Oncol. 11. doi: 10.3389/fonc.2021.698832
Lu, F., Huang, T., Chen, R., and Yin, H. (2024). Multi-omics analysis reveals the interplay between pulmonary microbiome and host in immunocompromised patients with sepsis-induced acute lung injury. Microbiol. Spectr. 12, e142424. doi: 10.1128/spectrum.01424-24
Naidoo, J., Wang, X., Woo, K. M., Iyriboz, T., Halpenny, D., Cunningham, J., et al. (2017). Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. 35, 709–717. doi: 10.1200/JCO.2016.68.2005
Özçam, M. and Lynch, S. V. (2024). The gut–airway microbiome axis in health and respiratory diseases. Nat. Rev. Microbiol. 22, 492–506. doi: 10.1038/s41579-024-01048-8
Perdijk, O., Azzoni, R., and Marsland, B. J. (2024). The microbiome: an integral player in immune homeostasis and inflammation in the respiratory tract. Physiol. Rev. 104, 835–879. doi: 10.1152/physrev.00020.2023
Rahi, M. S., Parekh, J., Pednekar, P., Parmar, G., Abraham, S., Nasir, S., et al. (2021). Radiation-induced lung injury-current perspectives and management. Clin. Pract. 11, 410–429. doi: 10.3390/clinpract11030056
Roberts, C. M., Foulcher, E., Zaunders, J. J., Bryant, D. H., Freund, J., Cairns, D., et al. (1993). Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann. Intern. Med. 118, 696–700. doi: 10.7326/0003-4819-118-9-199305010-00006
Rofael, S., McHugh, T. D., Troughton, R., Beckmann, J., Spratt, D., Marlow, N., et al. (2019). Airway microbiome in adult survivors of extremely preterm birth: the EPICure study. Eur. Respir. J. 53, 1801225. doi: 10.1183/13993003.01225-2018
Shankar, B., Zhang, J., Naqash, A. R., Forde, P. M., Feliciano, J. L., Marrone, K. A., et al. (2020). Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non–small cell lung cancer. JAMA Oncol. 6, 1952–1956. doi: 10.1001/jamaoncol.2020.5012
Shaverdian, N., Lisberg, A. E., Bornazyan, K., Veruttipong, D., Goldman, J. W., Formenti, S. C., et al. (2017). Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet. Oncol. 18 (7), 895–903. doi: 10.1016/S1470-2045(17)30380-7
Song, X., Dou, X., Chang, J., Zeng, X., Xu, Q., and Xu, C. (2024). The role and mechanism of gut-lung axis mediated bidirectional communication in the occurrence and development of chronic obstructive pulmonary disease. Gut Microbes 16, 2414805. doi: 10.1080/19490976.2024.2414805
Sun, Q., Hong, Z., Zhang, C., Wang, L., Han, Z., and Ma, D. (2023). Immune checkpoint therapy for solid tumors: clinical dilemmas and future trends. Signal transduction targeted Ther. 8, 320. doi: 10.1038/s41392-023-01522-4
Suresh, K., Naidoo, J., Lin, C. T., and Danoff, S. (2018a). Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 154, 1416–1423. doi: 10.1016/j.chest.2018.08.1048
Suresh, K., Psoter, K. J., Voong, K. R., Shankar, B., Forde, P. M., Ettinger, D. S., et al. (2019). Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J. Thorac. Oncol. 14, 494–502. doi: 10.1016/j.jtho.2018.11.016
Suresh, K., Voong, K. R., Shankar, B., Forde, P. M., Ettinger, D. S., Marrone, K. A., et al. (2018b). Pneumonitis in non–small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J. Thorac. Oncol. 13, 1930–1939. doi: 10.1016/j.jtho.2018.08.2035
Tiu, B. C., Zubiri, L., Iheke, J., Pahalyants, V., Theodosakis, N., Ugwu-Dike, P., et al. (2022). Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J. immunotherapy Cancer 10, e4670. doi: 10.1136/jitc-2022-004670
Voong, K. R., Hazell, S. Z., Fu, W., Hu, C., Lin, C. T., Ding, K., et al. (2019). Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin. Lung Cancer 20, e470–e479. doi: 10.1016/j.cllc.2019.02.018
Wang, S., Kuang, J., Zhang, H., Chen, W., Zheng, X., Wang, J., et al. (2022). Bile acid-microbiome interaction promotes gastric carcinogenesis. Adv. Sci. (Weinh) 9, e2200263. doi: 10.1002/advs.202200263
Xia, M., Lei, L., Zhao, L., Xu, W., Zhang, H., Li, M., et al. (2025). The dynamic oral-gastric microbial axis connects oral and gastric health: current evidence and disputes. NPJ Biofilms Microbiomes 11, 1. doi: 10.1038/s41522-024-00623-4
Yin, J., Wu, Y., Yang, X., Gan, L., and Xue, J. (2022). Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front. Immunol. 13. doi: 10.3389/fimmu.2022.830631
Keywords: lower respiratory tract, microbiome, checkpoint inhibitor pneumonitis, radiation pneumonitis, metagenomic next-generation sequencing
Citation: Chen J, Xu Q, Zhang L, Zhang D and Wu X (2025) Enrichment of prevotella melaninogenica in the lower respiratory tract links to checkpoint inhibitor pneumonitis and radiation pneumonitis. Front. Cell. Infect. Microbiol. 15:1594460. doi: 10.3389/fcimb.2025.1594460
Received: 16 March 2025; Accepted: 08 September 2025;
Published: 03 October 2025.
Edited by:
Yuseok Moon, Pusan National University, Republic of KoreaReviewed by:
Brahmchetna Bedi, Emory University, United StatesRuotong Ren, BioIntelliDx Corp., China
Copyright © 2025 Chen, Xu, Zhang, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueling Wu, d3V4dWVsaW5nNzZAMTI2LmNvbQ==
 Jiajun Chen
Jiajun Chen Qiong Xu
Qiong Xu Liyan Zhang1
Liyan Zhang1 Xueling Wu
Xueling Wu