- 1Department of Neurology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 2Department of Interventional Neurology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 3Department of Gastroenterology, Xishan People’s Hospital of Wuxi City, Wuxi, China
- 4Department of Neurology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Radiology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 6Department of Psychology and Sleep Medicine, The Second Hospital of Anhui Medical University, Hefei, China
- 7Center for Scientific Research and Experiment, the Second Affiliated Hospital of Anhui Medical University, Hefei, China
Background: The current study aimed to explore the specific oral microbiota profiles in major depressive disorder (MDD) patients with sleep disturbances, and to evaluate the potential mechanisms by which oral microbiota may be implicated in MDD.
Method: Thirty-eight MDD patients experiencing sleep disturbances and thirty healthy controls (HCs) were included. All MDD patients underwent a 14-day antidepressive treatment regimen. Neuropsychological assessments were conducted, and 16S rRNA sequencing was used to determine the abundance of oral bacteria.
Results: Oral genera Solobacterium, Granulicatella, Campylobacter, and Haemophilus showed significant changes in their relative abundances between the MDD and HC groups. Significant correlations were found between the abundance of Haemophilus and Pittsburgh Sleep Quality Index (PSQI) and 24-item Hamilton Depression Scale (HAMD-24) scores in MDD patients with sleep disturbances. In MDD patients, lower relative abundances of oral Haemophilus prior to treatment were negatively correlated with the changed rates of PSQI and HAMD-24 scores after antidepressive treatment. The glial fibrillary acidic protein as the mediator, affected the relationship between the relative abundance of oral Haemophilus and sleep disturbances in MDD patients.
Conclusion: Oral Haemophilus dysbiosis may drive sleep disturbances in MDD patients, possibly through its impact on neuroinflammation.
Introduction
Major depressive disorder (MDD), a leading global disease burden by 2030 (Cui et al., 2024), is characterized by persistent low mood and anhedonia (Anderson et al., 2024). Notably, 60-90% of MDD patients experience sleep disturbances (Malhi and Mann, 2018; Chen RF. et al., 2022), suggesting shared mechanisms involving HPA axis dysfunction and neuroinflammation (Irwin et al., 2016; Szmyd et al., 2021; Bhat, 2024). Furthermore, there exists a bidirectional relationship between gut microbiota dysbiosis and both sleep disturbances and MDD, wherein inflammation and endocrine hormones play pivotal roles (Li et al., 2018; Chen et al., 2024). Microbial metabolism generates an array of neurotransmitters and metabolites, including serotonin, which is intricately linked to the occurrence of rapid eye movement sleep and the manifestation of MDD (Shirolapov et al., 2024). Concurrently, emotional stress and disruptions in the host’s circadian rhythms can induce intestinal dysbiosis and activate intestinal immunity (Yang et al., 2023; Manosso et al., 2024). However, the exact pathophysiology remains unclear, and current treatments [e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs)] lack reliable efficacy predictors or biomarkers for MDD with sleep disturbances (Wichniak et al., 2017).
The oral microbiome influences brain disorders via neuroinflammation, neuroendocrine regulation, and central nervous system signaling (Bowland and Weyrich, 2022; Zhang et al., 2025). Specific oral bacteria (Spirochaetaceae, Actinomyces, Treponema, Fusobacterium) correlate with depressive symptoms through cortisol and CRP modulation (Simpson et al., 2020). Depression-like mice show oral dysbiosis (increased Pseudomonas/Pasteurellaceae, decreased Streptococcus) linked to blood-brain barrier disruption (Lou et al., 2024). Furthermore, sleep apnea (OSA) patients demonstrate distinct oral microbiota and metabolic pathways, including periodontitis-related species (Bianchi et al., 2023; Gao et al., 2023; Zhang et al., 2023; Ye et al., 2024). However, no studies have examined oral microbiota’s role in sleep disturbances specific to MDD.
The present study aimed to pinpoint the specific oral microbiota in MDD patients with concurrent sleep disturbances, which differ from those present in a healthy condition. Additionally, we further evaluated the association between these specific oral bacteria and various factors, including the severity of sleep disturbances and depression, neuroinflammation marker levels, and the effectiveness of antidepressant therapy.
Materials and methods
Participants
There were 68 participants were included in the present study. Thirty-eight MDD patients with sleep disturbances and 30 healthy controls (HCs) were recruited from the Second Affiliated Hospital at Anhui Medical University. All MDD patients met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria (Uher et al., 2014).
The inclusion criteria for patients with MDD encompassed: (1) those experiencing their first episode, whether as outpatients or inpatients; (2) patients who were either naive to pharmacological treatment or had discontinued all antidepressive treatments for a period exceeding four weeks prior to the study’s commencement; (3) patients exhibiting a definitive sleep disorder, as confirmed through sleep assessments; and (4) individuals without a familial history of psychosis. Meanwhile, HCs had no history of DSM-V Axis I disorders, mental health issues, or significant physical ailments, and demonstrated good sleep quality. Furthermore, participants were excluded if they met any of the following criteria: (1) the presence of organic lesions within the central nervous system or neurodegenerative disorders, such as stroke or Parkinson’s disease; (2) secondary mental disorders arising from severe physical conditions; (3) history of alcohol or drug abuse and dependence; (4) significant physical ailments, encompassing endocrine disorders, autoimmune conditions, or impaired liver or kidney function; (4) any form of neoplasia or cerebral trauma; (5) pregnant and breastfeeding women; or (6) history of OSA.
The ethical approval was obtained from the Ethics Committee of the Second Hospital of Anhui Medical University (approval number: SL-YX2024-022). All participants or their legal guardians provided informed consent.
Follow-up study
All patients diagnosed with MDD underwent a 14-day antidepressive treatment. During this period, only one type of SSRIs or SNRIs was administered as the primary antidepressant. Because of the primary objective of this study was not to assess the efficacy of antidepressive treatment, the study was designed as an observational follow-up rather than a randomized controlled trial.
Neuropsychological assessments
The interviews of each participant were completed by a trained psychiatrist. Neuropsychological assessments were conducted for HCs at baseline, as well as for patients with MDD both prior to and following treatment.
In the present study, Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) was performed to measures sleep quality over the previous month. The questionnaire evaluates seven clinically derived domains of sleep quality, sleep onset latency, sleep duration, sleep efficiency, sleep difficulty, sleep medication, and daytime dysfunction. These domains are collectively scored to determine a single factor representing global sleep quality, with a global score exceeding 11 serving as an indicative marker of significant sleep disturbances.
Furthermore, the 24-item Hamilton Depression Scale (HAMD-24) (Hamilton, 1960) and Self-Rating Depression Scale (SDS) (Zung, 1965) were used to evaluate the depressive symptoms. Meanwhile, each MDD patient also received an individual sleep disturbance score, which was computed by summing the scores of items 4, 5, and 6 from the HAMD-24 (Shi et al., 2020a; Shi et al., 2021a; Shi et al., 2021b).
Collection of oral samples and 16S rRNA gene sequencing
In the current study, oral biospecimens were collected noninvasively from the tongue dorsum using a sterile cotton swab, placed them in a sterile centrifuge tube with 1.5 mL Tris-EDTA buffer solution (Solarbio) and rapidly transported and stored them at -80°C. All participants completed the oral sample collection process after neuropsychological assessment.
Total microbial genomic DNA was extracted using the Bacterial DNA Extraction Mini Kit (Mabio, Guangzhou, China) according to manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Inc., USA). The hypervariable region V3-V4 of the bacterial 16S rRNA gene were amplified using specific primers (338F and 806R) by an T100 Thermal Cycler (BIO-RAD, USA). Using the NEXTFLEX Rapid DNA-Seq Kit (Bioo Scientific, USA) to generate the sequencing libraries. Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina NextSeq 2000 PE300 platform (Illumina, San Diego,USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Raw FASTQ files were de-multiplexed using an in-house perl script, and then quality-filtered by fastp version 0.19.6 and merged by FLASH version 1.2.11. The optimized sequences were clustered into operational taxonomic units (OTUs) using Usearch 11 with 97% sequence similarity level. The metagenomic function was predicted by PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) based on OTU representative sequences. More details could be found in the Supplementary Materials.
Serum samples collection and detection of serum indicators
Following an overnight fast, peripheral venous blood was collected into a vacutainer tube without anticoagulant. Within 30 minutes after collection, the blood samples were centrifuged at 3500 revolutions per minute at a temperature of 4°C for a duration of 10 minutes, subsequently serum was aspirated and stored at -80°C until needed for analysis. Notably, all MDD patients provided blood samples prior to initiating treatment.
The serum levels of glial fibrillary acidic protein (GFAP) and S100beta protein (S100β) were assayed in triplicate, using the commercial Enzyme-linked Immunosorbent Assay kits (FineTest, Wuhan, China; Catalog Number: EH0410 for GFAP and EH0543 for S100β) in accordance with the manufacturer’s protocols. The protein concentration in each plate was determined based on the standard curves. Both the inter-assay and intra-assay coefficients of variation were less than 5%.
Statistical analysis
The data were analyzed utilizing SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) and R software package (version 4.2.1).
Alpha and beta diversity analyses were conducted for the diversity analysis of oral microbiota. In the alpha diversity analysis, Observed species, Chao, and ACE indices were utilized to evaluate the richness of the microbial community, whereas Shannon, Simpson, and Coverage indices were harnessed to assess the diversity of the community. To investigate the variations in the composition of oral microbiota, beta diversity analysis was performed using Partial Least Squares-Discriminant Analysis (PLS-DA). Meanwhile, the microbial dysbiosis index (MDI) acted as an indicator to assess the extent of microbial imbalance within the study population, with a higher MDI value signifying a more pronounced degree of bacterial disturbance (Gunathilake et al., 2020). It was calculated using the formula MDI=log10[(total abundance in genera increased in disease group)/(total abundance in genera decreased in disease group)]. Furthermore, MetagenomeSeq analysis was conducted to compare the relative abundances of oral microbiota between the MDD and HC groups (Barot et al., 2024; He et al., 2024).
To ascertain the normal distribution of the data, the Kolmogorov-Smirnov test was performed. For categorical variables, a chi-squared test was employed for analysis. As for continuous variables, the independent-samples t test was utilized when the data were normally distributed; otherwise, the Mann-Whitney U test was applied. The paired t-test was employed to compare the changes in variables before and after treatment. In MDD patients, partial correlation analysis was conducted to ascertain the associations between the two variables, adjusting for factors such as age, sex, years of education, duration of the disease, and body mass index (BMI). Additionally, mediation analysis in MDD patients, controlling for age, sex, education years, duration of the disease, and BMI, was conducted to determine whether oral bacteria mediated the relationship between neuropsychological assessments and serum molecular indicators, based on a standard three-variable mediation model (Baron and Kenny, 1986; He et al., 2019). A detailed description of the method can be seen in Supplementary Materials. Receiver operating characteristic (ROC) curves were utilized to calculate the area under the curve (AUC), thereby assessing the diagnostic accuracy of oral bacteria in identifying MDD. The Youden index (Youden, 1950) was used to assess optimal values of sensitivity and specificity. Notable, changed rate of scores=(before treatment score - after treatment score)/before treatment score. Statistical significance was established at a p-value of less than 0.05 (two-tailed).
Results
Characteristics of participants
The demographic and clinical characteristics of the participants in the two groups are succinctly summarized in Table 1. No significant differences were observed between the two groups in terms of age, sex, education years, and body mass index. When compared to HCs, patients with MDD exhibited significantly elevated scores on the PSQI, HAMD-24, SDS, as well as increased serum levels of GFAP and S100β (Table 1). Additionally, there were significant differences in seven factor scores of the PSQI scale and the somnipathy factor scores of HAMD-24 scale between the two groups, as highlighted in Table 1.
Following a 14-day antidepressive intervention, MDD patients displayed significant reductions in their PSQI, HAMD-24, and SDS scores when compared to their pretreatment assessment scores (Table 1). Additionally, there were notable decreases in seven factor scores of the PSQI scale and the somnipathy factor scores of the HAMD-24 scale (Table 1).
Compositional analysis of oral microbiota
A total 4,024,824 sequences (SRA accession number: PRJNA1279610) were obtained from 68 samples using QIIME software (version 1.91; URL link: https://qiime.org/). The operational taxonomic units were assigned based on a threshold of 97% sequence similarity. The MDD group demonstrated a greater abundance of OTUs compared to the HC group, with a count of 1106 versus 1063, respectively, encompassing 750 shared OTUs (Supplementary Figure S1). The rarefaction curves for the samples reached a saturation plateau at a sequencing depth of 42,952 reads, indicating that the majority of microbial species were adequately covered by the sequencing depths employed, and that the sample size was appropriately sized (Supplementary Figure S2).
Diversity analysis
In the alpha diversity analysis, six alpha-diversity indices, i.e., Observed species, Chao1, ACE, Shannon, Simpson and Coverage, were analyzed in the present study. The levels of these six indices showed no significant difference between MDD and HC groups (Supplementary Figure S3A). Furthermore, in the beta diversity analysis, PLS-DA analysis was conducted for the reduction of the impact of intergroup differences (Supplementary Figure S3B).
MDI analysis
In the current study, MDD patients exhibited significantly elevated levels of MDI compared to HCs (Figure 1A). Furthermore, within the MDD patient group, there were positive correlations observed between MDI levels and scores on the PSQI and HAMD-24 scales, as well as with factor scores pertaining to sleep efficiency and daytime dysfunction, as depicted in Figures 1B-D.
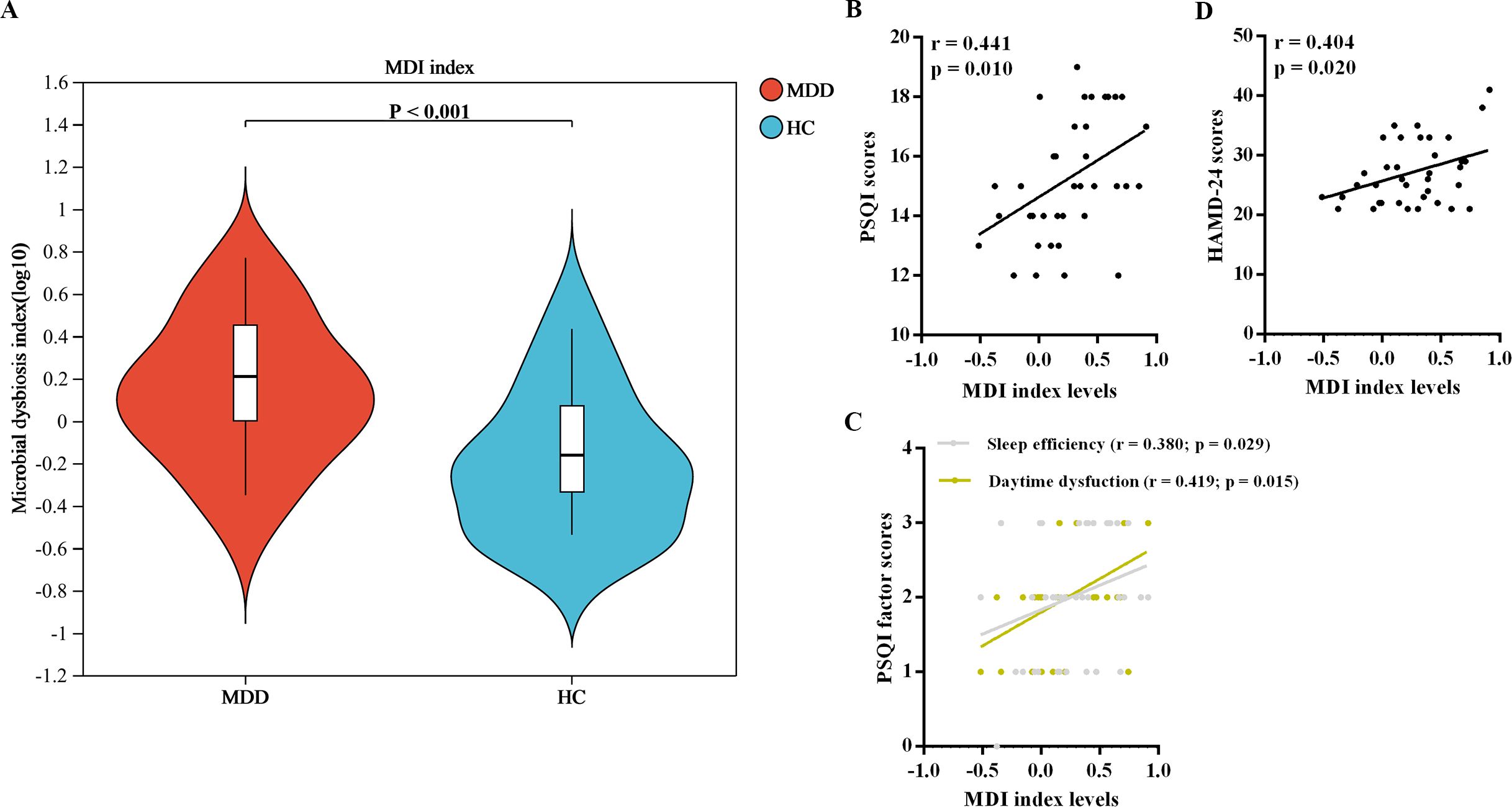
Figure 1. MDI analysis of oral microbiota. (A) Comparision of MDI levels between the two groups. (B) Correlations between MDI levels and neuropsychological assessments. MDI, microbial dysbiosis index; MDD, major depressive disorder; HC, healthy control.
Compositional analysis of oral microbiota at genus level levels between two groups
Figure 2A illustrates the top 50 most prevalent bacterial genera, characterized by their highest relative abundances in both groups. These genera belong to ten different phyla, including Firmicutes, Fusobacteriota, Actinobacteriota, Bacteroidota, Proteobacteria, Cyanobacteria, etc. The MDD patients displayed 36 exclusive genera, while 140 identical genera were identified in both the MDD and HC groups (Figure 2B). Among the overlapping bacterial genera shared by the two groups, Streptococcus, Prevotella, Neisseria, Veillonella, Leptotrichia, and Haemophilus exhibited the six highest relative abundances (Figure 2C).
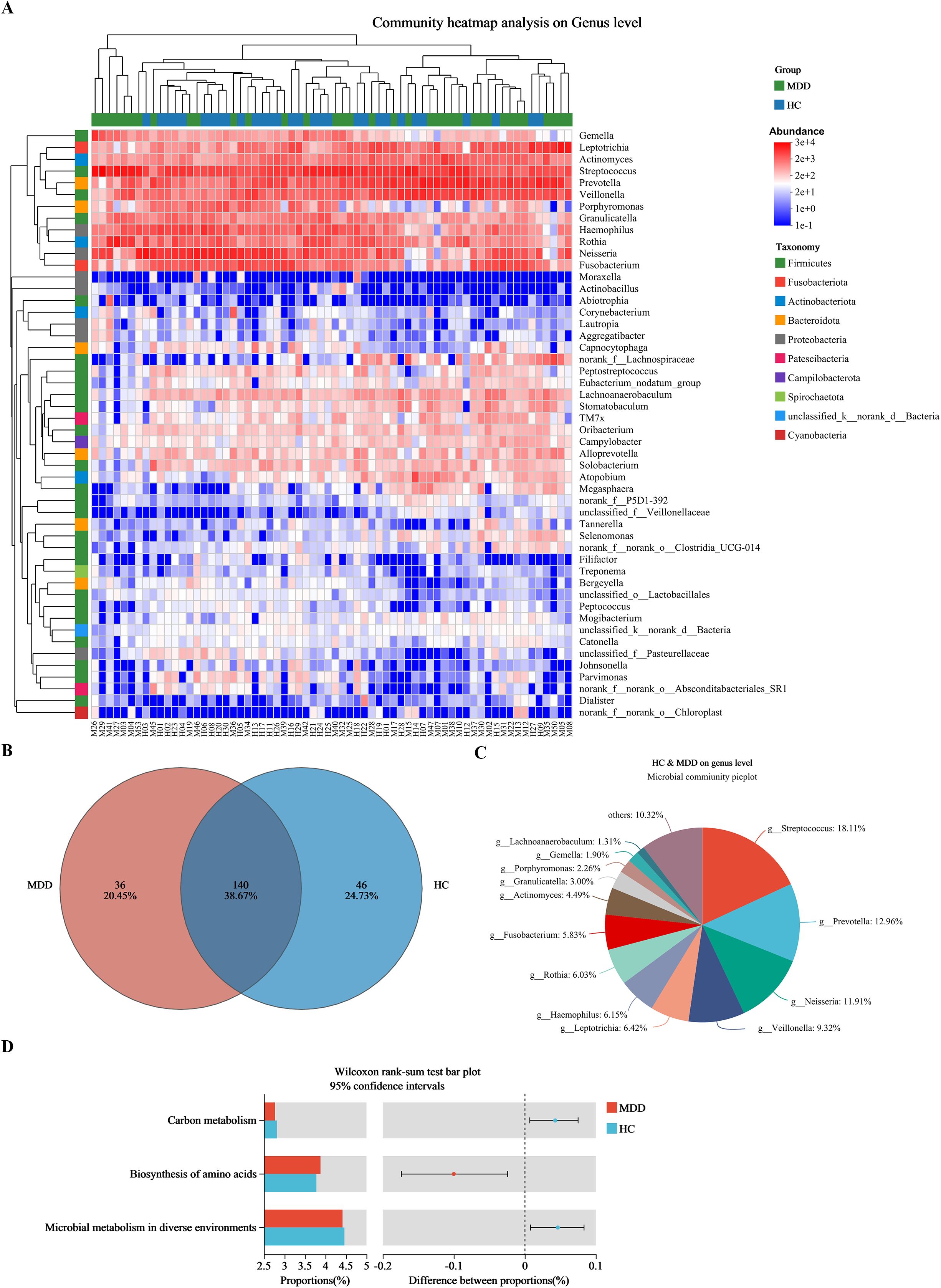
Figure 2. Relative abundances at genus level between MDD and HC groups. (A) Heat-map analysis at genus (top 50). Abscissa is the sample and ordinate is the taxa at genus level. The colors in heat-map represent the species abundance. (B) The distribution of genus level. (C) Composition of the overlapping bacterial genera. (D) Predicted functional pathways with significant differences. MDD (M), major depressive disorder; HC (H), healthy control.
In addition, the Mann-Whitney U test revealed significant differences in 30 predicted functional pathways between the two groups (Supplementary Figure S4). Among these pathways, “Carbon metabolism”, “Biosynthesis of amino acids”, and “Microbial metabolism in diverse environments” demonstrated the highest relative abundances within the oral microbiota (Figure 2D).
Furthermore, the MetagenomeSeq analysis represented 25 genera with significant differences in their relative abundances between the two groups (Figure 3A). To ensure the precision of the analysis exploring group disparities, four oral bacteria at the genus level were retained, as the test values for these bacteria could reliably be obtained from each subject (Figure 3A). Consequently, MDD patients exhibited a significant decline in the relative abundance of Solobacterium and Granulicatella, whereas Campylobacter and Haemophilus demonstrated a significant increase, in comparison to the HC group (Figure 3B). However, only Granulicatella and Haemophilus demonstrated statistically significant differences following multiple comparison correction (adjusted p < 0.0125, Bonferroni method).
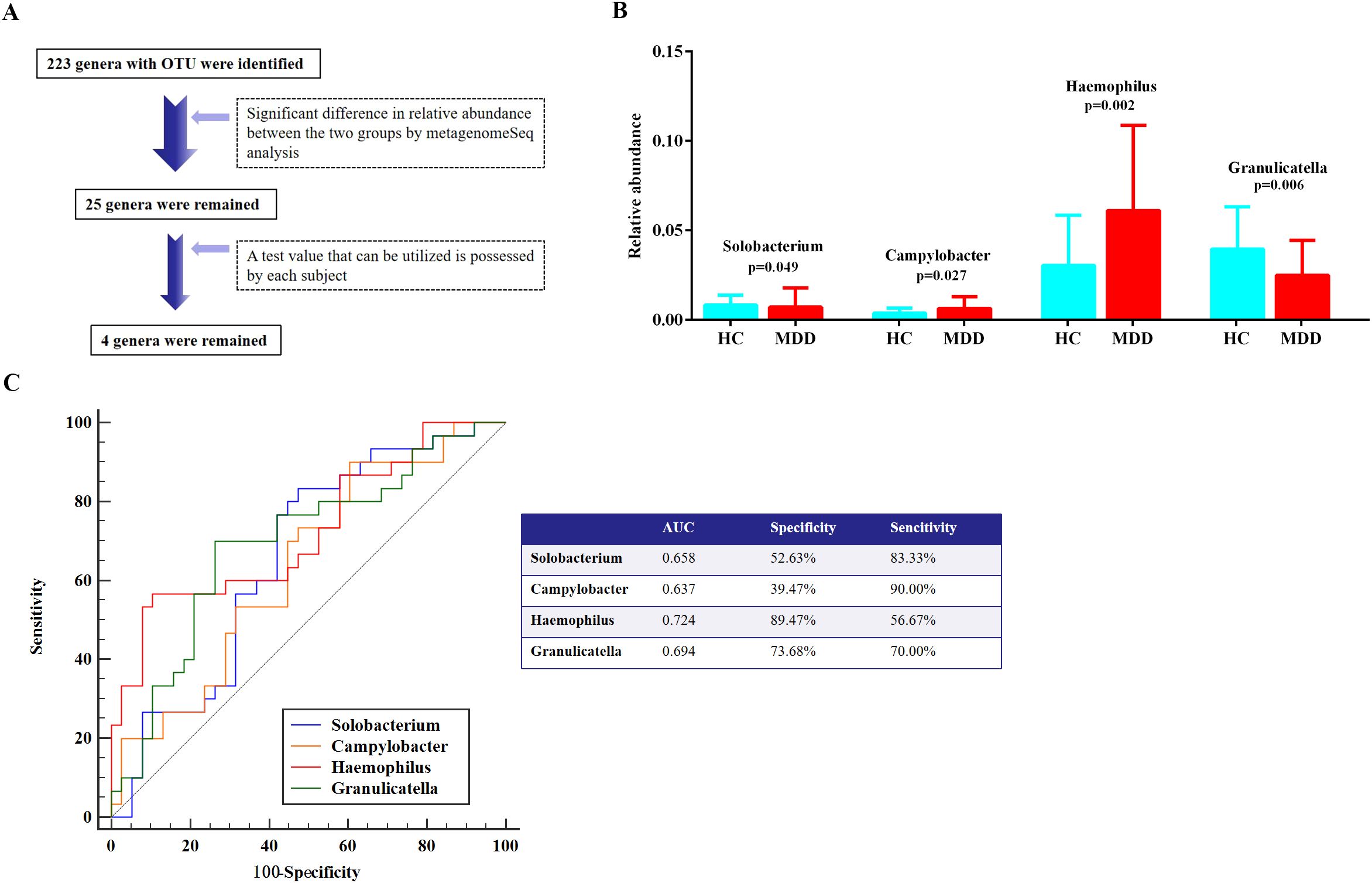
Figure 3. Differentiation of oral microbiota at the genus level in comparative expression analysis between MDD and HC groups. (A) Oral microbial screening process. (B) Taxa with significant differences at genus level between the MDD and HC groups. (C) ROC curve analysis. MDD, major depressive disorder; HC, healthy control; ROC, receiver operating characteristic; AUC, area under the curve.
ROC curve analysis
To assess the diagnostic efficacy of these four oral bacteria in identifying MDD with sleep disturbances, the ROC curve analysis revealed that Haemophilus showed an optimal AUC value of 0.724, accompanied by a sensitivity of 56.67% and a specificity of 89.47% (Figure 3C). Additionally, the remaining three oral bacteria demonstrated tolerable diagnostic performance, each possessing an AUC value exceeding 0.6 (Figure 3C).
Association analysis of oral bacteria with environmental factors in MDD patients
In Figure 4A, a correlation matrix was constructed utilizing partial correlation analysis, taking into account environmental factors in MDD patients prior to treatment, while adjusting for variables such as age, sex, years of education, duration of the disease, and BMI. This analysis incorporated four oral microbes at the genus level that exhibited specific alterations in MDD patients. The relative abundance of the genus Haemophilus showed significant positive correlations with PSQI and HAMD-24 scores, as well as serum levels of GFAP and S100β (Figure 4A). Meanwhile, there were significant correlations between relative abundance of the genus Haemophilus and sleeping quality and sleep onset latency factor scores in MDD patients (Supplementary Table S1). However, no significant correlations were observed between the other three oral microbes and the environmental factors in MDD patients.
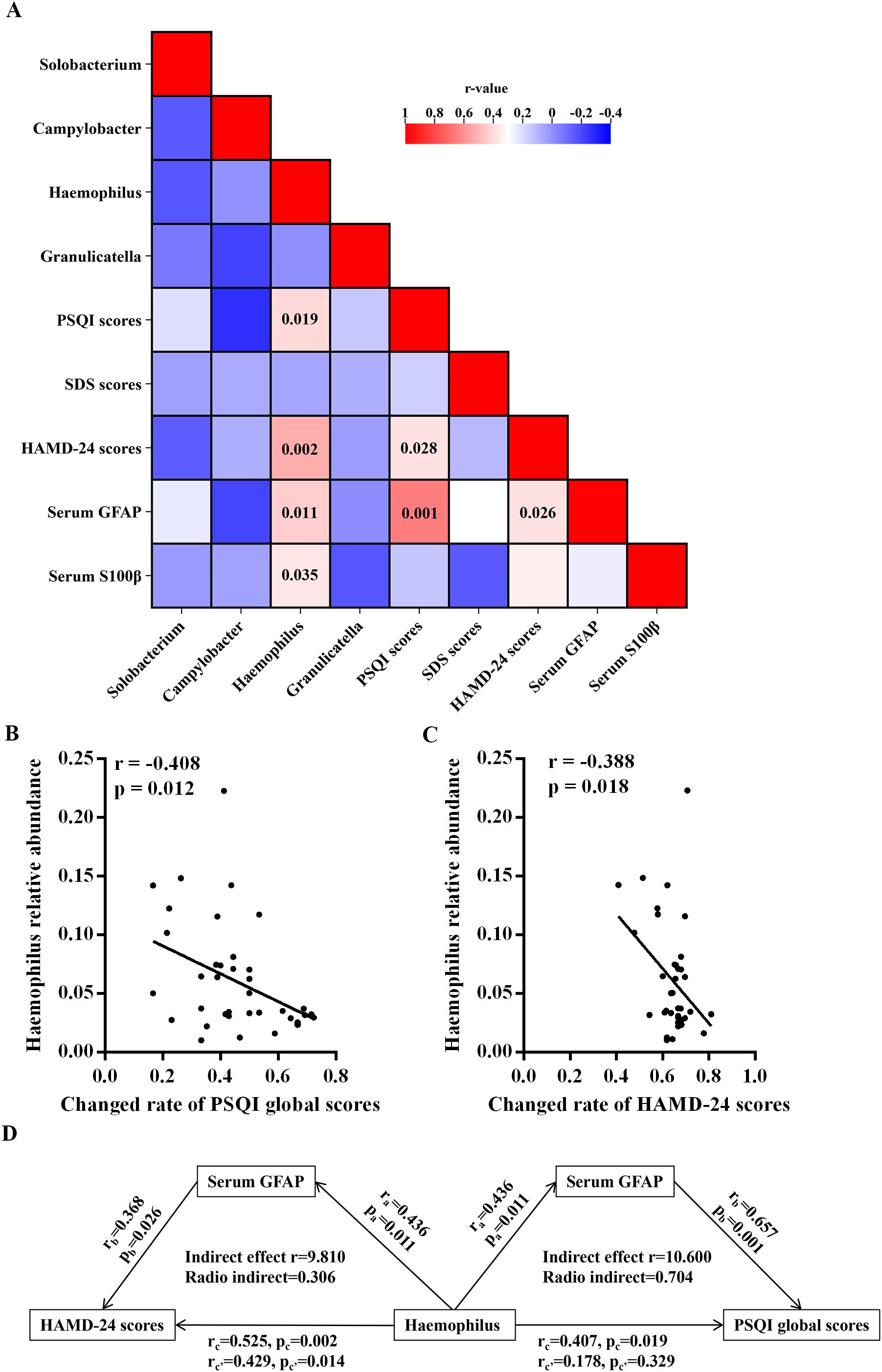
Figure 4. Associations of neuropsychological assessments and serum levels of indicators with oral microbiota at genus level in the MDD group. (A) Heatmap shows the correlation coefficient between neuropsychological assessments and serum levels of indicators and oral microbiota at genus level. The numbers in the box are p-values. (B) Correlation analyses of the abundance of oral Haemophilus prior to treatment with the changed rates of PSQI and HAMD-24 scores after antidepressive treatment. (C) Mediation analyses for the association between the relative abundance of oral Haemophilus and the assessments of sleep disturbances and depression. MDD, major depressive disorder; HC, healthy control; BMI, body mass index; HAMD-24, 24-item Hamilton Depression Scale; SDS, Self-Rating Depression Scale; PSQI, Pittsburgh Sleep Quality Index; GFAP, glial fibrillary acidic protein; S100β, S100beta protein.
After undergoing a 14-day antidepressive treatment, MDD patients exhibited significant reductions in PSQI, HAMD-24, and SDS scores compared to their pretreatment levels (Table 1 and Supplementary Figure S5). Additionally, correlation analyses were performed for the environmental factors in MDD patients after treatment, with controlling age, sex, years of education, duration of the disease, BMI, and antidepressant medicines. In the MDD group, there were significant negative correlations between the relative abundance of the genus Haemophilus and the changed rates of PSQI and HAMD-24 scores (Figures 4B, C).
Further mediation analyses conducted in MDD patients revealed that environmental factors played a significant role in affecting the relationship between the relative abundance of oral Haemophilus and the severity of sleep disturbances and depression. These analyses accounted for covariates such as age, sex, years of education, duration of the disease, and BMI. Additionally, the serum levels of GFAP were found to influence the relative abundance of Haemophilus genera in relation to PSQI and HAMD-24 scores (Figure 4D). Notably, serum GFAP exhibited a direct mediating effect on the association between the relative abundance of Haemophilus and PSQI scores, as depicted in Figure 4D.
Sample-size estimation
The sample size calculation was performed using an online calculator available at https://sample-size.net/. Based on the relative abundance of Haemophilus, with parameters set at α = 0.05 and β = 0.2, the analysis indicated that 27 subjects per group would achieve a statistical power of 0.8082. Consequently, the current sample sizes of 38 and 30 subjects in the respective groups are adequate, as they provide a power value exceeding the conventional threshold of 0.8. Nevertheless, a larger sample size remains necessary to validate the current findings and improve their reliability.
Discussion
The main findings of this study are summarized below. (1) MDD patients showed significantly higher MDI levels than HCs, and these levels correlated strongly with worse sleep quality and more severe depressive symptoms. (2) At the genus level, oral Granulicatella and Haemophilus demonstrated significant alterations in their relative abundances between MDD patients and HCs. Notably, Haemophilus showed the highest diagnostic accuracy for identifying MDD patients who experience sleep disturbances. (3) In MDD patients, higher abundance of oral Haemophilus were associated with poorer sleep quality, more severe depressive states, and increased levels of neuroinflammation. (4) MDD patients who exhibited lower relative abundances of oral Haemophilus prior to treatment were more likely to obtain significant improvements in sleep disturbances and depressive symptoms after antidepressant treatment. (5) GFAP-mediated neuroinflammation might be a key mediator in the relationship between Haemophilus abundance and sleep disturbance severity. Therefore, Haemophilus-driven oral dysbiosis may significantly contribute to the onset and worsening of sleep disturbances in MDD patients through its neuroinflammatory effects. These findings suggest potential clinical strategies for individualized treatment, including targeted Haemophilus antimicrobial or probiotic interventions, which may help alleviate depressive and sleep disorder symptoms while enhancing antidepressant treatment efficacy.
In the current study, a distinct difference in oral microbiota was observed between the two groups, with MDD patients experiencing sleep disturbances demonstrating elevated MDI levels, indicative of more pronounced oral dysbiosis (Wei et al., 2021). Additionally, significant correlations were found between MDI levels and both sleep and depressive assessments, further suggesting that these alterations in oral microbiota expression may may influence sleep disturbances and depressive symptoms. At the genus level, Solobacterium, Granulicatella, and Campylobacter showed marked abundance differences in MDD patients versus HCs, although results of Solobacterium and Campylobacter could not meet multiple comparison correction. Previous studies have linked these bacteria (Solobacterium, Granulicatella, Campylobacter) to sleep disorders. An increase in the relative abundance of the genus Solobacterium was observed in children who exhibit mouth breathing, potentially suggesting a correlation with pediatric OSA (Marincak Vrankova et al., 2024). Concurrently, Peizeng Jia et al. discovered that individuals suffering from OSA syndrome exhibited a significantly decreased relative abundance of salivary Granulicatella compared to HCs (Jia et al., 2020). Furthermore, a prior study has suggested that individuals with MDD and OSA exhibited an increased abundance of Campylobacter, which positively correlated with elevated plasma interleukin-6 levels (Ye et al., 2024). This finding supported the hypothesis that an inflammatory response serves as a potential underlying mechanism linking oral microbial dysbiosis to MDD patients experiencing sleep disturbances. Given the similarity between these prior findings and our current results, our findings possess sufficient credibility. However, in the current study, these three oral bacteria did not demonstrate a strong association with sleep quality or depressive symptoms. This observation may be attributed to the limited sample size or differences in study populations, which could have reduced the statistical power to detect significant relationships. Nevertheless, given their potential clinical relevance, these microbial taxa remain important candidates for future investigation.
In addition, numerous prior studies conducted on diverse cohorts with OSA have reported a significant increase in the abundance of oral Haemophilus, potentially implicating them in autoimmune processes (Johnston et al., 2018; Chen X. et al., 2022; Zhang et al., 2023; Zhu and Teng, 2024). Our study provides the first evidence of Haemophilus abundance variations specifically in MDD-associated sleep disturbances, which differ from OSA patterns. When compared to HCs, MDD patients with sleep disturbances exhibited a notably heightened relative abundance of oral Haemophilus. This oral microorganism holds promise as a potential biomarker for distinguishing individuals with sleep disturbances from those in the HC group. Furthermore, in MDD patients with sleep disturbances, we observed a positive correlation between the relative abundance of oral Haemophilus and assessments of both sleep quality and depressive symptoms. While prior research has tentatively indicated that patients with MDD exhibit an elevated expression of oral Haemophilus and that antidepressive therapy can diminish this expression (Hoisington et al., 2024), the current findings offer additional insights, suggesting a plausible role for the dysregulation of oral Haemophilus in the sleep disturbances experienced by MDD patients. Meanwhile, in the current study, the abundances of oral Haemophilus in MDD patients prior to antidepressive treatment exhibited a negative correlation with the rate of change in assessments pertaining to sleep disturbances and depressive symptoms after antidepressant administration. These findings suggest that the elevated expression of oral Haemophilus among MDD patients with sleep disturbances may contribute to a reduced efficacy in alleviating these sleep disturbances and depressive symptoms. Previous studies have demonstrated that tricyclic antidepressants (e.g., desipramine) can alter oral microbiota by affecting salivary gland function (Koller et al., 2000a; Koller et al., 2000b). However, the effects of SSRI or SNRI antidepressants on oral microbiota remain unexplored. Further research is needed to determine whether the observed changes in Haemophilus abundance following antidepressant treatment result from pharmacological effects or the disease state itself. Therefore, our preliminary study revealed oral Haemophilus might be implicated in the onset of sleep disturbances in patients with MDD and affect the prognosis of sleep disturbances following antidepressive treatment. In this preliminary exploratory study, we used 16S rRNA sequencing to characterize oral microbiota composition and identify potential microbial signatures associated with MDD-related sleep disturbances. However, due to the inherent taxonomic resolution limitations of 16S rRNA sequencing, we plan to conduct follow-up investigations using shotgun metagenomics or species-level identification of Haemophilus strains and to elucidate their functional pathways more comprehensively.
Peripheral GFAP and S100β are crucial biomarkers indicative of the extent of neuroinflammation and neuronal damage (Steinacker et al., 2021; Zozulya et al., 2021), and they have been established as integral factors in the pathophysiology of MDD (Shi et al., 2020b; Levchuk et al., 2023; Zhao et al., 2024). In this study, significant correlations were identified between the relative abundances of oral Haemophilus and serum levels of GFAP and S100β in MDD patients with sleep disturbances, indicating that an increased expression of oral Haemophilus may be linked to heightened neuroinflammation. Alba Troci et al. also observed an underlying association between oral Haemophilus, a Gram-negative, proinflammatory bacterium, and neuroinflammation (Troci et al., 2024), providing additional support for the present finding. Previous studies have demonstrated a correlation between serum levels of GFAP and objective sleep quality in individuals diagnosed with chronic insomnia disorder (Kong et al., 2021), and serum GFAP levels may serve as a biomarker for assessing disease severity in patients with OSA (Guzel and Salış, 2024). In our study, we observed that serum GFAP exerted an indirect mediated influence on the relationship between the relative abundance of Haemophilus genus and HAMD-24 scores. Moreover, serum GFAP had a direct mediated effect on the association between the expression of oral Haemophilus and PSQI scores. The current data suggest neuroinflammatory involvement in oral Haemophilus-associated sleep disturbances. However, whether neuroinflammation serves as the principal mechanism linking oral Haemophilus to MDD requires further investigation through fundamental research beyond the scope of this preliminary study.
There were some limitations in the present study. (1) Measurement of the oral microbiota in MDD patients after antidepressive treatment was not completed due to some patients declining to provide oral and blood samples a second time at the end of their treatment. This preliminary study reveals distinct oral microbiota signatures in MDD with sleep disturbances. Future work will employ larger cohorts to track microbiome changes longitudinally before and after antidepressant treatment. (2) Due to the nature of the current study being purely observational, not all MDD patients received the same antidepressant medication during their treatment course. To address this limitation, we will conduct a subsequent randomized controlled trial employing a standardized monotherapy regimen with [e.g., sertraline] to systematically evaluate its effects on oral microbiota composition and clinical outcomes. (3) Our study lacks a comparison group of MDD patients without sleep disturbances. Including such a group would help determine whether the observed bacteria differences are linked to MDD in general or are specific to sleep disturbances in MDD patients. In future research, we plan to validate present findings by comparing depressed patients with and without sleep disorders. (4) This study did not account for potential geographic and dietary influences on oral microbiota composition. Future investigations will account for potential geographic and dietary confounders. We will implement standardized monthly questionnaires to collect these demographic and nutritional data, ensuring comprehensive adjustment in our analytical models. To enhance generalizability, future studies should replicate these findings across diverse racial and ethnic populations. (5) Animal experiments are lacking. It is necessary to conclusively determine whether the artificial introduction of oral Haemophilus can expedite the onset of sleep disturbances in animal exhibiting depressive-like behaviors. Furthermore, it is essential to conduct additional fundamental researches to elucidate the underlying mechanism linking oral Haemophilus with sleep disturbances in MDD.
Conclusion
The current study revealed significant dysregulation of oral microbiota in patients with MDD and sleep disturbances compared to HCs. Among the examined oral bacteria, the relative abundance of Haemophilus was markedly elevated in MDD patients with sleep disturbances. This increase in oral Haemophilus was associated with both the onset and progression of sleep disturbances in MDD patients. Furthermore, GFAP-related neuroinflammation may mediate the relationship between oral Haemophilus and sleep disturbances in MDD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Formal Analysis, Writing – original draft, Funding acquisition, Software, Conceptualization, Project administration. EZ: Funding acquisition, Software, Writing – original draft, Visualization. WG: Formal Analysis, Writing – original draft, Funding acquisition. QG: Writing – original draft, Visualization, Methodology. YL: Methodology, Writing – original draft, Data curation. GX: Writing – review & editing, Methodology, Formal Analysis. YH: Validation, Data curation, Writing – original draft. HW: Validation, Writing – original draft. FW: Supervision, Writing – original draft, Investigation. FG: Conceptualization, Resources, Writing – review & editing. GZ: Resources, Conceptualization, Funding acquisition, Formal Analysis, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (No. 82301715 and 82201457), Wuxi Association for Science and Technology Soft Science Research Project (No. KX-24-C141), Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession (2020THRC-DJ-SNW), and research grants from Health Research Program of Anhui (AHWJ2023A10080).
Acknowledgments
The authors acknowledge all the participants who participated in this study and all the research assistants who collected data in this study. Meanwhile, we are extremely grateful to Mr. Bingyou Chen and Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China) for their help in 16S rRNA sequencing analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1617553/full#supplementary-material
References
Anderson, E., Crawford, C. M., Fava, M., Ingelfinger, J., Nikayin, S., Sanacora, G., et al. (2024). Depression - understanding, identifying, and diagnosing. N Engl. J. Med. 390, e41. doi: 10.1056/NEJMp2310179
Baron, R. M. and Kenny, D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. doi: 10.1037/0022-3514.51.6.1173
Barot, S. V., Sangwan, N., Nair, K. G., Schmit, S. L., Xiang, S., Kamath, S., et al. (2024). Distinct intratumoral microbiome of young-onset and average-onset colorectal cancer. EBioMedicine 100, 104980. doi: 10.1016/j.ebiom.2024.104980
Bhat, M. A. (2024). Indoor microplastics and microfibers sources and impacts on human health. Immun. Inflammation Dis. 12, e70046. doi: 10.1002/iid3.70046
Bianchi, G., de'Angelis, N., Gavriilidis, P., Sobhani, I., de'Angelis, G. L., and Carra, M. C.. (2023). Oral microbiota in obstructive sleep apnea patients: a systematic review. Sleep Breath 27, 1203–1216. doi: 10.1007/s11325-022-02718-8
Bowland, G. B. and Weyrich, L. S. (2022). The oral-microbiome-brain axis and neuropsychiatric disorders: an anthropological perspective. Front. Psychiatry 13, 810008. doi: 10.3389/fpsyt.2022.810008
Buysse, D. J., Reynolds, C. F., 3rd, Monk, T. H., Berman, S. R., and Kupfer, D. J.. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Chen, R. F., Cai, Y., Zhu, Z. H., Hou, W. L., Chen, P., Wang, J., et al. (2022). Sleep disorder as a clinical risk factor of major depression: associated with cognitive impairment. Asian J. Psychiatr. 76, 103228. doi: 10.1016/j.ajp.2022.103228
Chen, X., Chen, Y., Feng, M., Huang, X., Li, C., Han, F., et al. (2022). Altered salivary microbiota in patients with obstructive sleep apnea comorbid hypertension. Nat. Sci. Sleep 14, 593–607. doi: 10.2147/NSS.S347630
Chen, G., Du, X., Cui, J., Song, J., Xiong, M., Zeng, X., et al. (2024). Role of gut microbiota in ischemic stroke: A narrative review of human and animal studies. Neuroprotection 2, 120–136. doi: 10.1002/nep3.v2.2
Cui, L., Li, S., Wang, S., Wu, X., Liu, Y., Yu, W., et al. (2024). Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 9, 30. doi: 10.1038/s41392-024-01738-y
Gao, Y., Wang, H., Hu, Y., Li, J., Xu, W., Zhao, L., et al. (2023). Whole-genome metagenomic analysis of the oral microbiota in patients with obstructive sleep apnea. Sleep Breath 27, 1383–1398. doi: 10.1007/s11325-022-02732-w
Gunathilake, M., Lee, J., Choi, I. J., Kim, Y. I., Yoon, J., Sul, W. J., et al. (2020). Alterations in gastric microbial communities are associated with risk of gastric cancer in a korean population: A case-control study. Cancers (Basel) 12:2619. doi: 10.3390/cancers12092619
Guzel, A. and Salış, O. (2024). The role of serum C-Fos and glial fibriller acidic protein levels in detecting the severity of obstructive sleep apnea. Sleep Breath 28, 2295–2302. doi: 10.1007/s11325-024-03069-2
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
He, C., Gong, L., Yin, Y., Yuan, Y., Zhang, H., Lv, L., et al. (2019). Amygdala connectivity mediates the association between anxiety and depression in patients with major depressive disorder. Brain Imaging Behav. 13, 1146–1159. doi: 10.1007/s11682-018-9923-z
He, P., Jiang, C., Ni, J., Zhang, X., Wu, Z., Chen, G., et al. (2024). Identifying gut microbiota with high specificity for ischemic stroke with large vessel occlusion. Sci. Rep. 14, 14086. doi: 10.1038/s41598-024-64819-6
Hoisington, A. J., Stearns-Yoder, K. A., Stamper, C. E., Simonetti, J. A., Oslin, D. W., and Brenner, L. A.. (2024). Longitudinal influence of prescribed antidepressants on fecal and oral microbiomes among veterans with major depressive disorder. J. Neuropsychiatry Clin. Neurosci. 36, 151–159. doi: 10.1176/appi.neuropsych.20220221
Irwin, M. R., Olmstead, R., and Carroll, J. E. (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52. doi: 10.1016/j.biopsych.2015.05.014
Jia, P., Zou, J., Yin, S., Chen, F., Yi, H., and Zhang, Q.. (2020). Analysis of the salivary microbiome in obstructive sleep apnea syndrome patients. Can. J. Infect. Dis. Med. Microbiol. 2020, 6682020. doi: 10.1155/2020/6682020
Johnston, J., Hoggard, M., Biswas, K., Astudillo-García, C., Waldvogel-Thurlow, S., Radcliff, F. J., et al. (2018). The bacterial community and local lymphocyte response are markedly different in patients with recurrent tonsillitis compared to obstructive sleep apnoea. Int. J. Pediatr. Otorhinolaryngol 113, 281–288. doi: 10.1016/j.ijporl.2018.07.041
Koller, M. M., Maeda, N., Scarpace, P. J., and Humphreys-Beher, M. G.. (2000a). Desipramine changes salivary gland function, oral microbiota, and oral health in rats. Eur. J. Pharmacol. 408, 91–98. doi: 10.1016/S0014-2999(00)00770-6
Koller, M. M., Purushotham, K. R., Maeda, N., Scarpace, P. J., and Humphreys-Beher, M. G.. (2000b). Desipramine induced changes in salivary proteins, cultivable oral microbiota and gingival health in aging female NIA Fischer 344 rats. Life Sci. 68, 445–455. doi: 10.1016/s0024-3205(00)00951-6
Kong, X. Y., Hu, T., Ge, Y. J., Zhang, P., Li, X. Y., Song, X., et al. (2021). Correlations of diurnal brain functional variations with serum biomarkers and objective sleep quality in patients with chronic insomnia disorder. Zhonghua Yi Xue Za Zhi 101, 249–253. doi: 10.3760/cma.j.cn112137-20200430-01386
Levchuk, L. A., Roschina, O. V., Mikhalitskaya, E. V., Epimakhova, E. V., Simutkin, G. G., Bokhan, N. A., et al. (2023). Serum levels of S100B protein and myelin basic protein as a potential biomarkers of recurrent depressive disorders. J. Pers. Med. 13:1423. doi: 10.3390/jpm13091423
Li, Y., Hao, Y., Fan, F., and Zhang, B.. (2018). The role of microbiome in insomnia, circadian disturbance and depression. Front. Psychiatry 9, 669. doi: 10.3389/fpsyt.2018.00669
Lou, F., Luo, S., Kang, N., Yan, L., Long, H., Yang, L., et al. (2024). Oral microbiota dysbiosis alters chronic restraint stress-induced depression-like behaviors by modulating host metabolism. Pharmacol. Res. 204, 107214. doi: 10.1016/j.phrs.2024.107214
Malhi, G. S. and Mann, J. J. (2018). Depression. Lancet 392, 2299–2312. doi: 10.1016/S0140-6736(18)31948-2
Manosso, L. M., Duarte, L. A., Martinello, N. S., Mathia, G. B., and éus, G. Z.. (2024). Circadian rhythms and sleep disorders associated to major depressive disorder: pathophysiology and therapeutic opportunities. CNS Neurol. Disord. Drug Targets 23, 1085–1100. doi: 10.2174/0118715273254093231020052002
Marincak Vrankova, Z., Brenerova, P., Bodokyova, L., Bohm, J., Ruzicka, F., and Borilova Linhartova, P.. (2024). Tongue microbiota in relation to the breathing preference in children undergoing orthodontic treatment. BMC Oral. Health 24, 1259. doi: 10.21203/rs.3.rs-4653787/v1
Shi, Y., Song, R., Wang, L., Qi, Y., Zhang, H., Zhu, J., et al. (2020a). Identifying Plasma Biomarkers with high specificity for major depressive disorder: A multi-level proteomics study. J. Affect. Disord. 277, 620–630. doi: 10.1016/j.jad.2020.08.078
Shi, Y., Luan, D., Song, R., and Zhang, Z.. (2020b). Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 41, 40–51. doi: 10.1016/j.euroneuro.2020.09.633
Shi, Y., Zhang, L., He, C., Yin, Y., Song, R., Chen, S., et al. (2021a). Sleep disturbance-related neuroimaging features as potential biomarkers for the diagnosis of major depressive disorder: A multicenter study based on machine learning. J. Affect. Disord. 295, 148–155. doi: 10.1016/j.jad.2021.08.027
Shi, Y., Song, R., Wang, Z., Zhang, H., Zhu, J., Yue, Y., et al. (2021b). Potential clinical value of circular RNAs as peripheral biomarkers for the diagnosis and treatment of major depressive disorder. EBioMedicine 66, 103337. doi: 10.1016/j.ebiom.2021.103337
Shirolapov, I. V., Gribkova, O. V., Kovalev, A. M., Shafigullina, L. R., Ulivanova, V. A., Kozlov, A. V., et al. (2024). The interactions along the microbiota-gut-brain axis in the regulation of circadian rhythms, sleep mechanisms and disorders. Zh Nevrol Psikhiatr Im S S Korsakova 124, 79–86. doi: 10.17116/jnevro202412405279
Simpson, C. A., Adler, C., du Plessis, M. R., Landau, E. R., Dashper, S. G., Reynolds, E. C., et al. (2020). Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol. Behav. 226, 113126. doi: 10.1016/j.physbeh.2020.113126
Steinacker, P., Al Shweiki, M. R., Oeckl, P., Graf, H., Ludolph, A. C., Schönfeldt-Lecuona, C., et al. (2021). Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J. Psychiatr. Res. 144, 54–58. doi: 10.1016/j.jpsychires.2021.09.012
Szmyd, B., Rogut, M., Białasiewicz, P., and Gabryelska, A.. (2021). The impact of glucocorticoids and statins on sleep quality. Sleep Med. Rev. 55, 101380. doi: 10.1016/j.smrv.2020.101380
Troci, A., Philippen, S., Rausch, P., Rave, J., Weyland, G., Niemann, K., et al. (2024). Disease- and stage-specific alterations of the oral and fecal microbiota in Alzheimer’s disease. PNAS Nexus 3, pgad427. doi: 1093/pnasnexus/pgad427
Uher, R., Payne, J. L., Pavlova, B., and Perlis, R. H.. (2014). Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety 31, 459–471. doi: 10.1002/da.2014.31.issue-6
Wei, S., Bahl, M. I., Baunwall, S. M. D., Hvas, C. L., and Licht, T. R.. (2021). Determining gut microbial dysbiosis: a review of applied indexes for assessment of intestinal microbiota imbalances. Appl. Environ. Microbiol. 87:e00395–21. doi: 10.1128/AEM.00395-21
Wichniak, A., Wierzbicka, A., Walęcka, M., and Jernajczyk, W.. (2017). Effects of antidepressants on sleep. Curr. Psychiatry Rep. 19, 63. doi: 10.1007/s11920-017-0816-4
Yang, D. F., Huang, W. C., Wu, C. W., Huang, C. Y., Yang, Y. S. H., and Tung, Y. T.. (2023). Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 268, 127292. doi: 10.1016/j.micres.2022.127292
Ye, J., Lv, Y., Xie, H., Lian, K., and Xu, X.. (2024). Whole-genome metagenomic analysis of the oral microbiota in patients with obstructive sleep apnea comorbid with major depressive disorder. Nat. Sci. Sleep 16, 1091–1108. doi: 10.2147/NSS.S474052
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer 3, 32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
Zhang, X., Li, X., Xu, H., Fu, Z., Wang, F., Huang, W., et al. (2023). Changes in the oral and nasal microbiota in pediatric obstructive sleep apnea. J. Oral. Microbiol. 15, 2182571. doi: 10.1080/20002297.2023.2182571
Zhang, X., Zhong, M., Li, Y., Wang, H., Xi, G., Wang, F., et al. (2025). Oral microbiota and central nervous system diseases: A review. Neuroprotection 3, 79–94. doi: 10.1002/nep3.v3.1
Zhao, E., Yu, Q., Wang, M., Wang, Z., Jiang, B., Ma, X., et al. (2024). Value of serum brain-derived neurotrophic factor and glial fibrillary acidic protein for detecting depression in patients with Helicobacter pylori infection. Neurosci. Lett. 825, 137687. doi: 10.1016/j.neulet.2024.137687
Zhu, S. and Teng, Z. (2024). The characteristics of pharyngea microbiological in children with obstructive sleep apnea. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 38, 1178–1182. doi: 10.13201/j.issn.2096-7993.2024.12.017
Zozulya, S. A., Tikhonov, D. V., Kaleda, V. G., and Klyushnik, T. P.. (2021). Immune-inflammatory markers in remission after a first-episode psychosis in young patients. Zh Nevrol Psikhiatr Im S S Korsakova 121, 59–66. doi: 10.17116/jnevro202112106159
Keywords: sleep disturbances, major depressive disorder, oral microbiota, haemophilus, glial fibrillary acidic protein
Citation: Shi Y, Zhao E, Gong W, Gao Q, Li Y, Xi G, Han Y, Weng H, Wang F, Geng F and Zhang G (2025) Exploratory analysis of potential association between oral Haemophilus and sleep disturbances in major depressive disorder patients. Front. Cell. Infect. Microbiol. 15:1617553. doi: 10.3389/fcimb.2025.1617553
Received: 24 April 2025; Accepted: 24 June 2025;
Published: 11 July 2025.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Nicole Pesantes, Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO), SpainAmeer Luqman, Chongqing University, China
Copyright © 2025 Shi, Zhao, Gong, Gao, Li, Xi, Han, Weng, Wang, Geng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yachen Shi, eWFjaGVuX3NoaUAxNjMuY29t; Feng Geng, cHN5Z2VuZ2ZlbmdAMTI2LmNvbQ==; Gaojia Zhang, Z2ppYXpoYW5nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yachen Shi
Yachen Shi En Zhao3†
En Zhao3† Weigang Gong
Weigang Gong Qianqian Gao
Qianqian Gao Feng Wang
Feng Wang Feng Geng
Feng Geng Gaojia Zhang
Gaojia Zhang