Abstract
The escalating cancer burden in Sub-Saharan Africa (SSA), with projected doubling of incidence and mortality by 2040, necessitates innovative, cost-effective strategies for prevention, diagnosis, and treatment. While known infectious triggers like HPV, hepatitis viruses, and H. pylori account for an estimated 28.7% of cancers in SSA, the full scope of microbially-mediated oncogenesis remains underexplored. We examine existing data and formulate plausible hypotheses regarding the potential roles of additional infectious agents in cancer development within SSA. We explore mechanisms through which microbes may directly or indirectly contribute to oncogenesis, including the action of viral oncogenes, induction of chronic inflammation, mutational signatures, and the impact of immunosuppression, particularly in the context of HIV. Potential microbial triggers warrant further investigation, such as viruses (MMTV, CMV, polyomaviruses, SARS-CoV-2), bacteria (Fusobacterium nucleatum, Cutibacterium acnes, Salmonella Typhi), fungi (Candida, Aspergillus), parasites (Schistosoma japonicum and mansoni and Toxoplasma gondii) and the complex interplay with the microbiome. Given the significant challenges in establishing causation for microbial facilitators of cancer, with traditional postulates showing limited utility, we propose a refined set of criteria tailored to microbial oncogenesis, aiming to guide future research efforts. These criteria incorporate elements of both Koch’s postulates and the Bradford Hill framework, adapted to address the unique characteristics of microbial interactions with human hosts. By leveraging existing knowledge and plausible causal relationships, and by implementing advanced experimental tools such as next-generation sequencing and multi-omics analyses, coupled with machine learning approaches and collaborative, multidisciplinary research, we propose to accelerate the identification of novel microbial links to cancer. This knowledge may pave the way for targeted interventions such as new approaches for screening and diagnosis, and strategies for prevention including vaccine development or modification of existing vaccines (or recommendations for immunization timing and population targets). While acknowledging the inherent complexities of studying polymicrobial interactions and the challenges of translating in vitro findings to human populations, this work aims to provide a framework for future research and intervention strategies to reduce the escalating cancer burden and address global inequities in SSA. The ultimate goal is to inform evidence-based public health policies and clinical practices that will improve cancer outcomes in this vulnerable region.
Introduction
Cancer incidence and mortality in Sub Saharan Africa are predicted to double by 2040 (Sharma et al., 2022). In 2020, annual cancer incidence in Sub Saharan Africa (SSA) was estimated at 132 per 100,000 population (age standardized incidence rates) with a mortality rate of 88.9 per 100,000 (Sharma et al., 2022). This equates to 1.1 million new cases and 711,000 deaths from cancer in 2020. The significant prevalence of HIV infection also leads to a higher cancer burden in many parts of SSA with both increased incidence and mortality observed (Casper et al., 2017; Yarchoan and Uldrick, 2018). The rising burden of cancer is coupled with suboptimal access to health care; diagnosis often comes too late for effective treatment or after death (Akuoko et al., 2017; Espina et al., 2017; Mwamba et al., 2023). When cancers are diagnosed early, potentially life-saving therapeutics are frequently not available and access to care, especially in rural and other impoverished, remote areas, is limited with trained oncologists in very short supply (Grover et al., 2015; Ruff et al., 2016; Mallum et al., 2024). Advances in chemotherapy and radiation therapy in high income countries have markedly improved five-year survival for many cancers (Siegel et al., 2021), but new immunotherapies and chemotherapeutic drugs are extremely costly, making them currently out of reach for the vast majority of people living in SSA. Consequently, disparities in cancer burden are increasing. While efforts are underway to bring immunotherapies and advance precision medicine in Africa (Ruff et al., 2016), solutions have not yet been identified to reduce costs and provide access to most Africans. This drives a search for discoveries that could be translated to low-cost, highly effective preventive, diagnostic and therapeutic approaches.
One avenue for advancing cancer prevention, diagnosis and screening, and therapy is to study microbial precipitators for cancer. While not a mainstream cancer research focus, there are dramatic examples of how understanding microbial links to cancers can be transformative. For instance, determining that >90% of cervical cancer cases, the top cause of cancer-related mortality among women in Africa, is precipitated by infection with human papillomavirus (HPV), led to the development and use of highly effective HPV vaccines, which are essentially cancer vaccines (Chan et al., 2019; Lei et al., 2020). It is now possible to prevent cervical cancer in Brazzaville just as effectively as in San Francisco. However, there are still knowledge gaps to fill regarding characteristics and scope of oncogenicity of HPV; for instance, several prevalent oncogenic HPV serotypes (such as 35, 52 and 58) circulating in Africa are not included in the bivalent vaccines currently used for national immunization programs in SSA (Dehlendorff et al., 2021). HIV and HPV are also intrinsically linked, where infection with one, increases infection rates for the other. Moreover, increased cancer progression rates of HPV in the context of HIV infection are significant (Liu et al., 2018; Marima et al., 2021). Vaccine formulations with higher valency will be required to optimize prevention of HPV and subsequently cervical cancer. Optimized screening tests, including self-testing, for detecting HPV in vaginal secretions are also advancing the capacity to save lives through early detection and treatment of cervical cancer (Chan et al., 2019; WHO, 2022). And, importantly, HPV has been shown to cause other cancers, including those that occur in men; yet, currently, the vaccine is routinely given to only girls and not boys, throughout most of Africa.
The effectiveness of prevention of hepatocellular cancer via hepatitis B immunization provides additional evidence for the value of filling knowledge gaps on microbial facilitation of cancers. Vaccines against hepatitis B virus and effective therapeutics against hepatitis C have made attainable the prevention of the vast majority of hepatocellular cancers that are not solely related to chronic alcohol use with attendant cirrhosis (Levrero and Zucman-Rossi, 2016; Virzì et al., 2018; Hwang et al., 2019; Flores et al., 2022).
It is estimated that 28.7% (range: 18-53%; Figure 1) of cancers occurring in sub-Saharan Africa are linked to a known infectious trigger (Plummer et al., 2016; de Martel et al., 2020) with the main contributor in this estimate being HPV-related cancers (15% of cancers ranging from 10-38.3%) (Figure 1), including oral and throat, penile, and anal cancers in addition to cervical cancer, and HBV HCV, contributing to hepatocellular carcinoma. In addition, some lymphomas (Epstein-Barr virus) and head and neck cancers (Epstein-Barr virus and HPV), gastric cancers (Helicobacter pylori), bladder cancers (Schistosoma haemotobium), and Kaposi’s sarcoma (Human Herpesvirus 8—HHV8, also referred to as Kaposi’s Sarcoma Herpesvirus-KSHV), have known infectious mediators; however, interventions have not been developed or are not widely used for these other facilitators for cancer, thus far. Prevalence rates for some of these infectious triggers are extremely high with H pylori prevalence ranging to 50-70% in SSA or HPV prevalence similarly showing reports up to 64% (Mbulawa et al., 2018; de Martel et al., 2020; Asempah, 2021; Emmanuel et al., 2024). However, for many cancers, there is lack of systematic surveillance in SSA; thus, prevalence is likely substantially underestimated (Omotoso et al., 2023). The pathogen attributable proportion of cancers will likely become substantially higher than estimated, as new links between microbes and oncogenesis, the focus of this paper, are still being elucidated (de Martel et al., 2020; Ngwa et al., 2022).
Figure 1
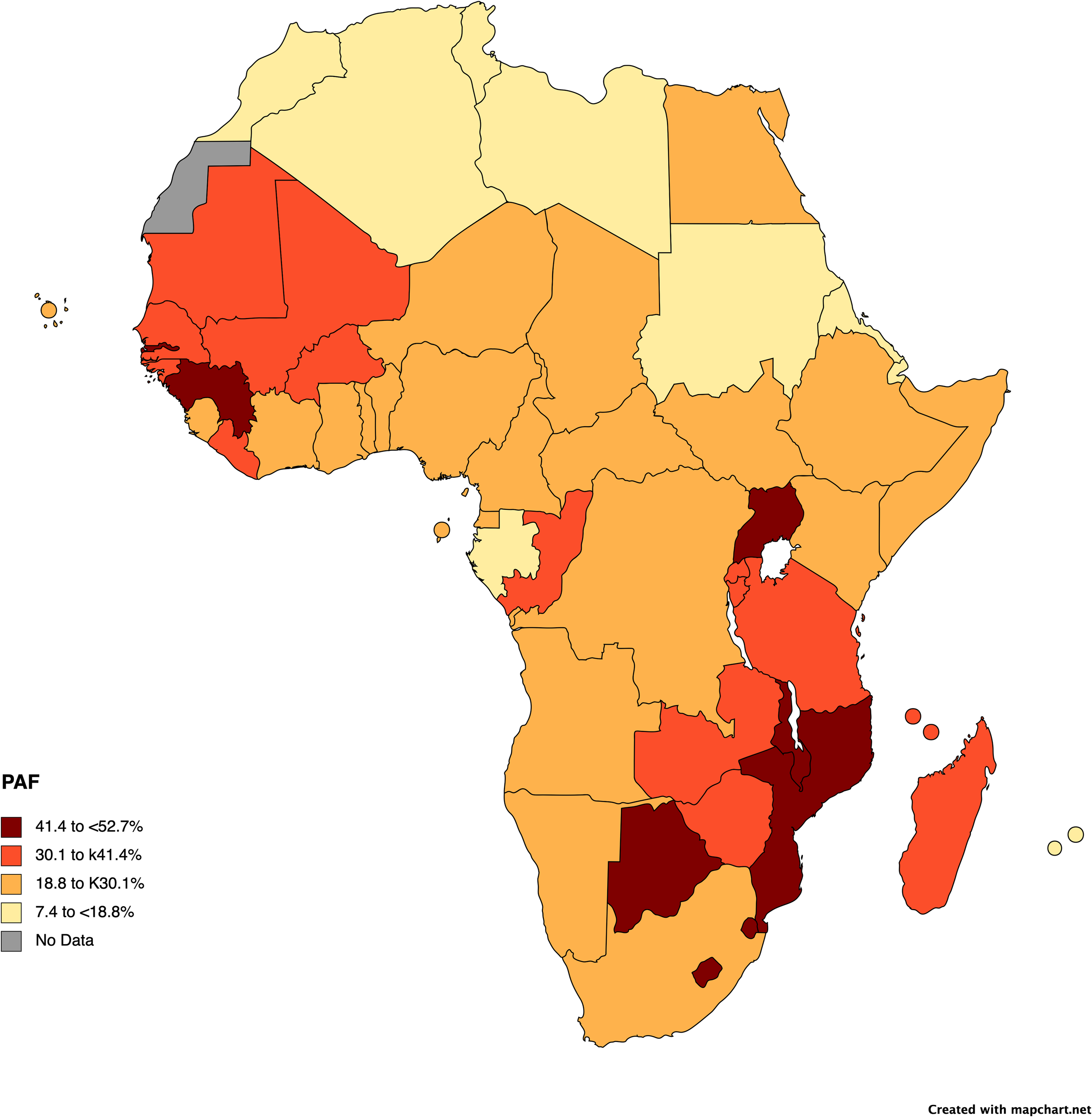
Pathogen attributable fractions of cancer incidence in Africa by country. The map was created with mapchart, using data from the International Agency for Research on Cancer (IARC). Cancers due to HPV, HBV, HCV, and H. pylori in 2018 provide the bases for the calculations. Full details for pathogen attributable fraction calculations and data can be found in the following references and within a tool available (and referenced) on the IARC website (de Martel et al., 2020; IARC, 2020; Ngwa et al., 2022).
In this review, we focus on plausible hypotheses to suggest pathways for additional microbially mediated cancers. Recognizing the immense potential to modify cancer disease burden through the understanding of the role of microbial factors in triggering cancer and/or facilitating its spread and translating that knowledge to new products and/or strategies, we review available data on cancers for which evidence suggests that there may be infectious mediators. Beyond the scope of this paper (but a worthwhile exercise) is detailing the many gaps in using existing knowledge of infection-cancer relationships to develop tools (or optimally use existing tools) or strategies for prevention of cancers; for instance, the demonstrated relationship between H. pylori and gastric cancer (Parsonnet et al., 1991) has not led to routinely available approaches to prevent that cancer, nor has the known association of Epstein-Barr virus with Burkitt and Hodgkin lymphoma, and nasopharyngeal carcinoma (Pathmanathan et al., 1995; Brady et al., 2007; Carbone and Gloghini, 2018; Su et al., 2023). Our intent here is to provide a starting point to identify knowledge gaps and define priorities for research on novel infectious mechanisms for oncogenesis.
How infections cause cancer
Viral oncogenes and proteins
Pathway alterations and expression of viral oncoproteins are observed in multiple viruses that are directly carcinogenic (Burd, 2003; Young and Murray, 2003; Levrero and Zucman-Rossi, 2016; Kuss-Duerkop et al., 2018; Virzì et al., 2018; Afzal et al., 2022; Burton and Gewurz, 2022; Marongiu et al., 2022). While other unidentified processes are likely, understanding known mechanisms may be helpful for discovering previously unrecognized microbial facilitators of oncogenesis. For example, HPV oncogenesis is mediated by viral oncogenes, such as E6 (Oyervides-Muñoz et al., 2018); E6 recruits intracellular E3 ubiquitin ligase (also named E6AP), which targets p53 for proteasomal degradation (Li et al., 2019). P53 is a tumor suppressor gene which plays critical roles in pathways to prevent DNA damage, marking cells for apoptosis or delaying cell cycle progression in the presence of DNA damage. HPV infection, therefore, inactivates p53 and leads to unregulated cell division, cell growth, cell survival, and DNA damage promotion. E7, another HPV oncogene, also interacts with the p53 pathway through retinoblastoma tumor suppressor protein (pRb), resulting in unregulated cell cycle progression. These oncogenes/proteins are also known to drive other oncogenic pathways, including telomerase regulation. The cancer cells depend on constitutive expression of these proteins, making them prime targets for therapeutic vaccines and biomarker detection (Burd, 2003; Ghebre et al., 2017; Marima et al., 2021).
Oncogene expression tends to interfere with important cell regulation or immortalization pathways. These can also be mediated by host-microbe interactions through proteins which mimic host proteins and interact with key signal pathways causing the overexpression of oncogenes and suppression of tumor suppressor proteins (Guven-Maiorov et al., 2019; Tempera and Lieberman, 2021). For example, EBV produces peptides (BHRF1 and BALF-1) and microRNAs which inhibit Bcl-2 and prevent apoptosis (Young and Murray, 2003; Brady et al., 2007; Carbone and Gloghini, 2018; Burton and Gewurz, 2022). Similarly, KSHV (HHV8) (for which seroprevalence ranges between 30-90% in SSA) inhibits ORF16 which is required for apoptosis (Wan et al., 2024). Viral proteins may also act synergistically with existing cancers by inducing the Warburg effect, i.e. where cells switch to glycolysis fermentation to generate energy instead of oxidative phosphorylation, allowing for the usage of alternative metabolites and the switching to anaerobic respiration (Liberti and Locasale, 2016). For example, HPV, KSHV and Merkel cell polyomavirus (MCPyV) affect glycolysis and induce increased glucose utilization in cancer cells, resulting in stress in healthy surrounding cells. EBV infection can modify lipid and cholesterol metabolism to induce anaerobic metabolism (Burton and Gewurz, 2022). Further mechanisms are reviewed elsewhere (Tempera and Lieberman, 2021)
Direct integration of viruses can cause differential expression of key proteins affecting the cell cycle, DNA repair mechanisms, and apoptosis, each important for oncogenesis. For example, HTLV-1 integrates into CD4+ T-cells causing chromosomal instability, mediated by its oncoprotein RNF8 (Zhi et al., 2020). Integration of HBV can both cause genome instability, and also leads to chronic inflammation due to sustained presence of HBV and its antigens (Borgia et al., 2021; Jin et al., 2023). For HPV and MCPyV, integration into epithelial cells (Yang and You, 2022; Karimzadeh et al., 2023), allows for cell immortalization of these cells due to the constant presence of the oncogenic driver (Elkhalifa et al., 2023).
Indirect and undefined mechanisms
Pathophysiology of some infectious diseases overlap with oncogenic mechanisms in ways which could promote cancer initiation and growth. Infection with HBV and HCV, for instance, are associated with chronic infection which may lead to chronic inflammation, thereby causing type 2 carcinogenic effects (Borgia et al., 2021; Yang et al., 2021). These viruses are synergistically carcinogenic with other factors such as aflatoxins and liver cirrhosis due to alcohol usage (Borgia et al., 2021; Jin et al., 2023). The links between HBV and HCV and hepatocellular carcinoma are well established, but chronic HBV and HCV infections also may be associated with other cancers, especially gastric adenocarcinomas. For instance, a metanalysis of ten studies found that patients with HBV infection had a higher risk (hazard risk =1.26;95% CI=1.08-1.47)) for gastric cancer when compared with controls (without HBV infection) (Chen et al., 2019; Yang et al., 2021; Yu et al., 2023b). Hypothesized mechanisms awaiting confirmation include chronic inflammation resulting in carcinogenesis, direct viral integration into gastric epithelial cells, expression of viral proteins (like HBx which interferes with cell signaling pathways, gene expression and apoptosis) and immune response modulation (Yang et al., 2021). Likewise, EBV may be associated with a subset of gastric adenocarcinoma; chronic inflammation is one hypothesized mechanism (Salnikov et al., 2024).
Immunosuppression
HIV causes cancer both by its integration into oncogenes, and, also through an indirect mechanism of immune suppression, which allows existing cancers to progress or oncoviruses to establish infection. HIV/AIDS progression is accompanied by AIDS-defining cancers, such as Non-Hodgkin lymphoma (NHL), Kaposi sarcoma, and cervical cancer) (Beral et al., 1990; Martínez-Maza and Breen, 2002; De Martel et al., 2015; Bohlius et al., 2016; Yarchoan and Uldrick, 2018), with cancer risk in people living with HIV (PLWHIV) significantly elevated, ranging between 25-40%, despite widely accessed antiretroviral therapy (De Martel et al., 2015). Lung cancer, Hodgkin lymphoma, hepatocellular cancer, and anal cancers are associated with HIV infection despite effective ART treatment, suggesting a possible direct oncogenic effect of HIV beyond immune suppression (Khandwala et al., 2021; Navarro et al., 2021; Haas et al., 2022; McGee-Avila et al., 2024). For Burkitt lymphoma, HIV may directly drive oncogenesis through its immunomodulation and engagement of C-C motif chemokine receptor 5 (CCR5) (Samson et al., 1996; Silverberg et al., 2007; Bohlius et al., 2009; Martorelli et al., 2015; Shindiapina et al., 2020). HIV appears to potentiate the oncogenic effect of some viruses to increase risk for cancer. For example, the attributable fraction of EBV-associated Hodgkin lymphoma in the general population is 20-50%, but in HIV-infected patients, 75%-100% of Hodgkin lymphoma is attributable to EBV, possibly due to aberrant CD4 T-cell responses to EBV infection (Carbone and Gloghini, 2018; Shindiapina et al., 2020; Navarro et al., 2021).
Other types of immunosuppression are similarly linked to cancer progression (Haas, 2019; Herrera et al., 2019); for example, advanced age leading to immune senescence and immune suppression drugs are both highly associated with cancer and cancer progression (Haas, 2019; Fu et al., 2023). In addition, tumors facilitate their growth and metastasis by actively suppressing the immune system in their direct microenvironment (Arner and Rathmell, 2023; Tie et al., 2022).
Criteria for causation
Proving that a microbe facilitates cancer is not straightforward and may vary by pathogen. The Bradford Hill criteria for causation provide epidemiologic evidence for a causal relationship between an exposure and cancer (Bradford Hill, 1965; Table 1). The criteria were originally developed to examine environmental exposure (like tobacco smoke, dyes, and other chemicals), but they fall short when assessing potential microbial facilitators of cancer, since microbes, as living organisms, interact with humans in dynamic ways that are in contrast with human interactions with static substances (Table 1B). Likewise, Koch’s postulates can be useful for establishing the cause of a novel acute illness due to an infectious disease, but is less relevant for diseases for which there are long delays between exposure and disease expression
Table 1
| 1A. Bradford Hill criteria for causation Designed to assess whether environmental exposures or ingestions are associated with specific diseases |
|---|
| 1. Effect size (strength of association) |
| 2. Reproducibility (consistency) |
| 3. Specificity (between a factor and effect) |
| 4. Temporality (effect has to occur after the exposure and after an expected delay between cause and effect for cancer) |
| 5. Dose-response relationship (biological gradient) |
| 6. Plausibility (there should be a plausible mechanism for cause and effect although this might be affected by state of current knowledge) |
| 7. Coherence between epidemiologic and laboratory findings |
| 8. Experimental evidence |
| 9. Analogy (between observed association and other associations) |
| 10. Deletion or reversibility effect (if the exposure is deleted then the effect should be reduced or deleted) |
| 1B Koch’s postulates Designed to identify microbial causes for acute infectious diseases of unknown cause |
|---|
| 1. Microbe should be found in abundance in all organisms suffering from the disease but not found in healthy organisms (not accounted for in chronic infection or carriage) |
| 2. Microorganism must be isolated from a diseased organism and grown in pure culture (not relevant for microbes that do not grow in pure culture and not relevant for viruses that utilize host cells for growth) |
| 3. The cultured microorganism should cause disease when introduced into a healthy organism |
| 4. The microorganism must be re-isolated from the inoculated, diseased experimental host and identified as identical with the original specific causative agent. |
Existing approaches for establishing causation of disease.
Criteria as per Bradford-Hill’s and Koch’s original criteria (Bradford Hill, 1965; Fedak et al., 2015; Opal, 2017).
Given that study of microbial facilitators for cancer is an emerging discipline, the absence of relevant causation criteria impedes research focus and advances. Adapting and building upon Bradford Hill criteria and Koch’s postulates, we propose a set of criteria for hypothesis generation, to guide study, and to confirm specific microbial oncogenesis (Table 2).
Table 2
| Criteria | Definition/details | Tools | ||
|---|---|---|---|---|
| 1. Epidemiologic Association (**) | Consistent association of a microbe (or a combination of microbes) with a specific cancer type within a population (considering demographics and host factors) of humans (or across all populations), especially when compared to people within the same population(s) without cancer (Mason et al., 2021) | Case-control and Cohort (retrospective and longitudinal) studies Histopathology, immunochemistry, PCR, Genomics and mass spectrometry Strong association when consistent findings across different geographical regions and consistent risk ratios in case-control or cohort studies |
||
| 2. Histopathologic association (**) | Consistent detection of the microbe (virus, bacterium, fungus, or parasite) in cancer tissues compared to healthy controls ( Ziegler et al., 2021 ) |
In Vitro assays (PCR, FACS, imaging) Histopathology immunochemistry, PCR, Genomics and mass spectrometry |
||
| 3. Temporal association (**) | Evidence that infection precedes onset of cancer ( Bosch et al., 1995 ; Walboomers et al., 1999 ) | Longitudinal (long term) studies | ||
| 4. Experimental evidence of facilitation of oncogenesis (**/***) + | Induction of cancer/precancerous changes upon introduction of the isolated microbe into appropriate models. Does oncogenic cellular transformation occur when a microbe is introduced into an animal (ideally a primate or other human-representative) model or tissue mode ( Ziegler et al., 2021 ). | 3D biosystems (organoids) or animal models | ||
| 5. Molecular and Multi-omics evidence for interaction (***) | •Epigenetics (e.g. DNA methylation changes in H. pylori or HPV E6 oncogene resulting in P53 degradation), as well as mutational signatures •Mutation effects (stimulating cellular mutations promoting cancer) •Stimulating immune evasion mechanisms reducing immunosurveillance for emerging cancer cells Integration of microbial DNA into host genome |
in vitro assays, organoids, multi-omics assessments including: | ||
| a. Molecular Evidence |
For bacteria: Identification of toxins, effector proteins, or metabolites that alter cellular signaling For viruses: Characterization of viral oncoproteins or insertional mutagenesis For fungi: Demonstration of mycotoxins or immune-modulating molecules with carcinogenic potential For parasites: Identification of chronic inflammatory responses or direct tissue damage mechanisms Evidence of microbial interference with DNA repair, cell cycle regulation, apoptosis, or immune surveillance (Dyson et al., 1989; Franco et al., 2005; Botelho et al., 2009; Zhu et al., 2021) |
|||
| b. Genomic evidence: c. Transcriptomic evidence d. Proteomic evidence e. Metabolomic evidence f. Microbiome analyses |
• Microbial DNA sequences or integration sites in tumor genome; • Host genetic susceptibility factors that enhance oncogenic potential of specific microbes, including demonstration of how microbial exposure can alter (or promote) known host genetic risk factors for cancer. • Demonstratable facilitation of cancer-associated mutational signatures (Péneau et al., 2022) Expression of microbial genes or altered host gene expression profiles (Ego et al., 2005) Detection of microbial proteins or altered host protein responses (Dyson et al., 1989; Celegato et al., 2022) Microbial metabolites or altered host metabolic pathways (Pérez Escriva et al., 2025) Consistent dysbiosis patterns associated with specific cancers (Guo et al., 2021; Pourali et al., 2024) |
|||
| 6. Prevention (***) | Does preventing the infection (or removing exposure to the microbe) reduce cancer or evidence of oncogenesis ( Fukase et al., 2008 ; Yoon et al., 2014 ) | Clinical trials Relevant animal models |
||
|
For viruses: Reduced cancer incidence following vaccination (e.g., HPV, HBV) For bacteria: Cancer prevention through antibiotic treatment or bacterial elimination For fungi: Antifungal intervention effects on precancerous lesions For parasites: Impact of antiparasitic treatment on cancer development Prevention trials showing reduced cancer incidence after targeting the microbe |
||||
| 7. Dose Response relationship (**) | Relationship of severity or chronicity of the presumed offending infection with cancer initiation and severity in human longitudinal studies or in experimental models demonstrate the association of infectious dose with development of cancer ( Chen et al., 2006 ; Yang et al., 2016 ) | Organoids, animal models, or clinico-epidemiologic longitudinal studies | ||
| 8. Plausibility (*) | Are there plausible mechanisms for considering a potential role for a microbe | Knowledge-based; i.e. understanding the physiology of microbial interaction with humans may suggest or support a role in carcinogenesis | ||
| 9. Impact of co-Factors (*) | Microbial carcinogenesis occurs (or is accelerated) in an environment with promotive host factors, such as genetic risk, immunodeficiencies, nutritional status, and/or with environmental factors (including but not limited to known carcinogens) ( Kim et al., 2012 ; Gargiulo Isacco et al., 2023 ) | Epidemiologic and laboratory studies in humans and animal models | ||
Proposed microbial oncogenesis criteria incorporating Koch’s postulates and Bradford-Hill criteria.
Importance or strength the evidence provides is given in stars, with:
***Gold standard evidence. If one of these criteria is met, it highly suggests that the microbial agent is causative,
**Highly suggestive or supportive information for association
*Further investigation required.
+For criterion 4, primate models or non-human primate models are rated *** while mouse models, or in vitro models are rated **. Organoid models are an evolving field and need further assessment as to their confirmatory strength.
Context applies in all these criteria as new discoveries are being made, and new technologies are developed. This table is unable to provide all the nuances that may apply. For example, criteria such as epidemiological data are associated with a level of significance.
Potential microbial triggers for cancer needing further investigation
A variety of microbes are hypothesized to trigger oncogenesis with a number of criteria for causation met (including the plausibility criterion) (Table 3), but each needs further investigation. We recognize that our list of hypothesized and potential microbial facilitators is likely incomplete—our intent is to describe those microbial cancer pairs for which research has provided some compelling clues. A few examples are provided below.
Table 3
| Pathogen | Cancer associations | Level of evidence | Criteria met | References |
|---|---|---|---|---|
| Virus | ||||
| Epstein-Barr virus | Lymphomas, nasopharyngeal, head and neck cancer | Confirmed | 1, 2, 3, 4, 5, 7, 8, 9 | (Young and Murray, 2003; Brady et al., 2007; Carbone and Gloghini, 2018; Burton and Gewurz, 2022; Su et al., 2023) |
| Stomach cancer | Medium | 1, 2, 3, 5, (7, 8) may be dependent on cofactors |
(Young and Murray, 2003; Tavakoli et al., 2020; Jeong et al., 2022; Salnikov et al., 2024) | |
| Breast cancer, squamous cell carcinomas (oral, conjunctiva) | Hypothesized | (1) | (Núñez-Acurio et al., 2020; Tavakoli et al., 2020; Jeong et al., 2022; Julius et al., 2022; Heawchaiyaphum et al., 2023; Salnikov et al., 2024) | |
| Hepatitis B Virus | Hepatocellular carcinoma | Confirmed | 1, 2, 3, 4, 5, 6, 7, 8, 9 | (Levrero and Zucman-Rossi, 2016; Zeisel et al., 2021; Flores et al., 2022; Péneau et al., 2022; Cui et al., 2023) |
| Hepatitis C Virus | Hepatocellular carcinoma | Confirmed | 1, 2, 3, 4, (5), 6, 7, 9 mechanism partially understood |
(Nash et al., 2010; Hwang et al., 2019; Koike and Tsutsumi, 2021) |
| Non-Hodgkin lymphoma | Strong | 1, (2, 3, 4, 5), 6, (7), 8 | (Tasleem and Sood, 2015; Alkrekshi et al., 2021) | |
| HHV8 | Kaposi sarcoma | Confirmed | 1, 2, 3, 4, 5, 8, 9 | (Akula et al., 2001; Dollard et al., 2010; Dow et al., 2013; Newton et al., 2018; Wan et al., 2024) |
| HIV** | Cofactor (Kaposi sarcoma, cervical cancer) |
Confirmed (as a co-factor) | 1, 3, 6, 7, 8, 9 | (Liu et al., 2018; Looker et al., 2018; Yarchoan and Uldrick, 2018; Atallah-Yunes et al., 2020) |
| Non-AIDS defining (NHL, Hodgkin, liver, lung, anal, head and neck) | Medium (as a co-factor) | 1, likely indirect | (Brune et al., 2016; Sigel et al., 2017; Yarchoan and Uldrick, 2018; Khandwala et al., 2021; Navarro et al., 2021; Haas et al., 2022; McGee-Avila et al., 2024) | |
| HPV | Cervical, head and neck, oral, throat, cervical, penile, anal | Confirmed | 1, 2, 3, 4, 5, 6, 7, 8, 9 | (Williams et al., 2010; Picard et al., 2016; Hoppe-Seyler et al., 2018; Oyervides-Muñoz et al., 2018; ICO/IARC Information Centre on HPV and Cancer, 2023; Jensen et al., 2024) |
| Prostate, esophageal, colorectal and breast cancer |
Hypothesized | (1, 2, 3), 8 | (Ludmir et al., 2015; Hsu et al., 2022; Kudela et al., 2022; Tsydenova et al., 2023) | |
| Merkel cell polyoma virus | Merkel cell carcinoma | Confirmed | 1, 2, 4, 5, 9, | (Arora et al., 2012; Yang and You, 2022) |
| HTLV-1 | Leukemia, Lymphomas Acute T-cell Lymphoma |
Strong | 1, 2, 3, 4, 5, 8 ATL is rare in endemic areas, despite high HTLV-1 prevalence. Potentially cofactor dependent |
(Bangham, 2023; Zhi et al., 2020) |
| Polyoma viruses | Colon cancer | Hypothesized | (1, 2), 8 | (Shoraka et al., 2020; Zheng et al., 2022) |
| Cytomegalovirus | Glioblastoma | Medium | (1, 2) (4, 5) (8, 9) | (Scheurer et al., 2008; Yang et al., 2022a; Guyon et al., 2024; Mercado et al., 2024) |
| Mouse Mammary Tumor Virus/ Human Mammary Tumor Virus |
Breast cancer | Hypothesized | (2), (8) 4 (in mouse models only; not HMTV) |
(Lawson and Glenn, 2022; Parisi et al., 2022) |
| SV40 | Mesothelioma, glioblastoma | Hypothesized | (1, 2), (8, 9) 4 (mice) |
(Rotondo et al., 2019) |
| Human Herpes Virus 6 | Lymphomas/ glioblastoma |
Hypothesized | (1, 2), 8/ 1, (2, 3), 8 ± |
(Kiani et al., 2016; Gu et al., 2019; Wells et al., 2019; Chen et al., 2023) |
| SARS-CoV2 | – | Hypothesized | 8 | (Jahankhani et al., 2023) |
| Bacteria | ||||
| Helicobactor pylori | Gastric | Confirmed | 1, 2, 3, 4, 5, 6, 7, 8, 9 | (Parsonnet et al., 1991; Franco et al., 2005; Salvatori et al., 2023) |
| Lung/ Esophageal |
Under investigation | (1)/ 1, 6, |
(Islami and Kamangar, 2008; Mounika, 2013) | |
| Fusobacterium nucleatum | Colorectal | Strong | 1, 2, 4, 5, 8, (9) | (Kostic et al., 2013; Ou et al., 2022; Wang and Fang, 2022; Zepeda-Rivera et al., 2024) |
| Streptococcus gallolyticus (S. bovis) | Colon cancer | Strong | 1, 2, 4, 5, 8, (9) | (Abdulamir et al., 2011; Kreikemeyer et al., 2018; Eldegla et al., 2021; Şahin et al., 2023; Taylor et al., 2023) |
| Salmonella Typhi | Gall bladder | Medium | 1, (4, 5), 8 | (Nagaraja and Eslick, 2014; Sepe et al., 2020; Upadhayay et al., 2022) |
| Chlamydia trachomatis | Cervical cancer | Medium (co-factor) | 1, (2), (4, 5), 8, 9 | (Zhu et al., 2016; Challagundla et al., 2023; Gargiulo Isacco et al., 2023) |
| Escherichia coli | Colorectal cancer, UTI leading to bladder cancer, | Medium | 1, 4, (5), (8, 9) | (Abd-El-Raouf et al., 2020; Lichtenstern and Lamichhane-Khadka, 2023) |
| Peptostreptococcus anaerobius | Breast cancer, colorectal | Medium (new) | (1, 2), (4) ± | (Tsoi et al., 2017; Long et al., 2019; Gu et al., 2023; Hurst et al., 2024; Liu et al., 2024) |
| Microbiome (dysbiosis) | Colorectal, breast, pancreatic, cervical, blood cancers | Co-factor, therapy modulating | 1, 2, (4) many confounders |
(Francescone et al., 2014; Koay et al., 2018; Kadosh et al., 2020; Ciernikova et al., 2021; Guo et al., 2021; Akbar et al., 2022; Sohail and Burns, 2023; Pourali et al., 2024) |
| Cutibacterium acnes | Prostate | Hypothesized | (1, 2), 8 | (Davidsson et al., 2016, Davidsson et al., 2021; Sayanjali et al., 2016; Zhang et al., 2018) |
| Bacteroides fragilis | Colorectal | Hypothesized | (1, 2), 4, 5, (8) | (Cheng et al., 2020; Scott et al., 2022; Dadgar-Zankbar et al., 2023) |
| Mycoplasma | Lung, breast, ovarian | Hypothesized, co-factor | (1, 2, 5, 8) | (Tantengco et al., 2021; Yacoub et al., 2021; Pang et al., 2023; Benedetti et al., 2024) |
| Parasites | ||||
| Schistosoma haematobium | Bladder cancer | Confirmed | 1, (2), 3, (4, 5), 6, 7, 8 | (Botelho et al., 2009; Zaghloul et al., 2020) |
| Clonorchis sinensis | Cholangiocarcinoma | Confirmed | 1, (2), 3, 4, 5, 6, 7, 8 | (Shin et al., 2010; Wonid et al., 2019; Chang et al., 2021; Ren et al., 2025) |
| Opisthorchis viverrin | Cholangiocarcinoma | Confirmed | 1, (2), 3, 4, 5, 6, 7, 8 | (Sripa et al., 2007, Sripa et al., 2012; Perakanya et al., 2022) |
| Schistosoma mansoni | Hepatocellular | Medium (co-factor) | (1, 2, 3, 4, 5, 6), 7, 8 | (Toda et al., 2015; Roderfeld et al., 2020; von Bülow et al., 2021) |
| Schistosoma japonicum | Hepatocellular | Medium (co-factor) | (1, 2, 3, 4, 5, 6), 7, 8 | (Liu et al., 2022; Kato et al., 2024; Sheng et al., 2024) |
| Entamoeba histolytica | Colorectal cancer | Hypothesized | (1, 2), 8 | (Ankri, 2015; Goel et al., 2018; Haghighi et al., 2022) |
| Trichomonas vaginalis | Cervical, prostate | Hypothesized | (1, 2), 8 | (Zhang et al., 2023) |
| Toxoplasma gondii | Glioblastoma | Hypothesized | (1), (5), 8 | (Zhou et al., 2019; Hodge et al., 2021; Abdollahi et al., 2022; Jung et al., 2022) |
| Fasciola hepatica | Liver cirrhosis/cholangiocarcinoma | Hypothesized | (1), 8 | (Machicado et al., 2016; Tanabe et al., 2024) |
| Helminths | (chronic inflammation), leukemia, liver cancer | Hypothesized | 8 | (Oikonomopoulou et al., 2016; Pastille et al., 2017; Sava et al., 2020; Berriel et al., 2021) |
| Fungi | ||||
| Aspergillus flavus, Aspergillus parasiticus (aflatoxin) | Liver | Confirmed | 1, (2), 3, 4, 5, 6, 7, 8, 9 | (Zhu et al., 2021; Jin et al., 2023; Khan et al., 2024) |
| Lung | Hypothesized | (1), (5), 8 | (Cui et al., 2015; Kang et al., 2020) | |
| Candida albicans | Oral, esophageal, anogenital cancer |
Hypothesized | (1, 2), 8 | (Di Cosola et al., 2021; Wang et al., 2023a, Wang et al., 2023b; Malavika et al., 2025; Guo et al., 2025) |
| Fusarium species (mycotoxin) | Esophageal, renal, liver, testicular, lung cancer | Hypothesized | 1 for esophageal, | (Dragan et al., 2001; Ekwomadu et al., 2022; Khan et al., 2024) |
Known and hypothesized links between infectious disease and cancer based on the proposed causation criteria.
Criteria in parenthesis (-) are inconsistent across studies or show contradicting evidence.
± relatively new findings, or new link between pathogen and cancer
**HIV is often synergistic and required; however, many of these cancers have separate primary pathogenic causes (such as cervical cancer and HPV). Under non-AIDS defining cancers, we mention those, which may be dependent on HIV associated inflammatory processes, and not necessarily immune suppression. Exposure to similar risk factors (as with HBV infection and Hepatocellular cancer) may contribute to the increases a confounder in epidemiological risk; therefore we have classified HIV as a co-factor. confirmed even though it is not implicated directly in these
Scientific judgment is required to evaluate the strength of the evidence for each criterion, which cannot be captured in categorizing these cancers. However, we propose to use these criteria to determine which infectious disease may be investigated further and is likely to show an associative or causal link. We have classified as follows.
Confirmed= Meets most criteria including one (***) and shows reproducibility across labs, and trials. The confirmed diseases described fit all but 1 or 2 criteria. These also meet consistent reproducibility: i.e. confirmation across independent laboratories, optimally using varied methodologies or models, replication in diverse human populations and geographic settings, concordance between in vitro, animal, and human studies.
Strong evidence = meets >4 criteria among proposed microbial oncogenesis criteria (including one ***).
Medium evidence = 2 or 3 criteria met with other criteria not evaluated or not evaluated optimally, inconsistent results or not conclusive results. For example, CMV, as a trigger for glioblastoma, has inconsistent results for criteria 1,2, and for criteria 4,5,8,9, the experimental evidence is not conclusive.
Hypothesized = meets plausibility criterion or one other criterion with either conflicting or suboptimal data within other criteria.
A virus originally found in mice
A retrovirus known as mouse mammary tumor virus (MMTV) has been shown to cause mammary tumors in rodents; multiple studies have shown that human mammary tumor virus (HMTV), which is 90-95% homologous to MMTV is present more often in human breast cancer tissue when compared with healthy breast tissue (Lawson and Glenn, 2022; Parisi et al., 2022). Using our proposed criteria for causation, HMTV/MMTV meets criterion 4 (with oncogenesis demonstrated in an animal model), and partially meets criterion 2 (Consistent detection of the microbe - virus, bacterium, fungus, or parasite- in cancer tissues compared to healthy controls) with consistent findings in some laboratories, but not across all geographies. When detected, it is not clear whether the virus is part of the causation pathway or whether breast cancer tissue is simply conducive to colonization with the virus (Lawson and Glenn, 2022). Infections with viruses like MMTV may induce signaling alterations which causes negative effects only under certain conditions. Microbiome characteristics might determine such a conducive environment. Recent studies have correlated gut microbiome features with breast cancer stages and progression, although a causative link has also not been established (Lee et al., 2023; Sohail and Burns, 2023; Viswanathan et al., 2023).
Cytomegalovirus
CMV is highly prevalent in SSA with a pooled prevalence of 81.9% (55–97%) (Bates and Brantsaeter, 2016). The potential oncogenicity of CMV has long been a focus for study. CMV proteins and nucleic acids have been found (meeting Criteria 5d and 5e [Tables 2, 3] in some, but not all, studies) within glioblastoma multiforme tumors suggesting that the virus may play a role in facilitating oncogenic transformation or progression of glioblastomas (Scheurer et al., 2008; Yang et al., 2022a; Mercado et al., 2024). Human astrocytes infected with CMV have formed glioblastoma-like cancers in mice models (Guyon et al., 2024) (Criterion 2) and infection with CMV leads to poorer prognosis for glioblastoma (Criterion 6); targeting CMV infected cells has shown potential for glioblastoma therapeutics (Yang et al., 2022a; Mercado et al., 2024). While the association has not been conclusively confirmed, potential mechanisms include chronic inflammation, immune evasion or modulation, and viral gene expression. A variety of characteristics of CMV infection might contribute to cellular transformation to cancer: induction of expression of pro-angiogenic factors, interaction with oncogenic signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is often dysregulated in cancers, and epigenetic changes like DNA methylation and histone changes, which can contribute to tumorigenesis (Chan et al., 2010). Among the cancers contributing to substantial burden in Africa, some studies have suggested that CMV may have a role in breast, prostate and colorectal cancers, among others, presumably with similar mechanisms that have been hypothesized for a putative role with glioblastomas (Yu et al., 2023a).
Polyomavirus
Polyomaviruses have been linked to colon cancers, with JCPyV and BK polyoma viruses showing the strongest causal links (Gorish et al., 2019; Shoraka et al., 2020). Although the majority of humans are latent carriers for these viruses and no comprehensive SSA data was found, immunosuppression either through disease or natural immune senescence can lead to reactivation. Higher levels of JCPyV and BK are found in colon cancer tissues (Criterion 2), as well as in solid B cell leukemia, when compared with non-cancerous tissue, and have been identified in many other cancers (Gorish et al., 2019; Loutfy et al., 2017). Injection of T antigen derived from JCPyV in mice was shown to cause a variety of cancers including neural and breast and hepatocellular cancers (Criterion 4) (Zheng et al., 2022). Similarly, Merkel Cell polyoma virus shows high prevalence in skin cancer tissues (Klufah et al., 2021).
Fusobacterium nucleatum
A variety of studies have suggested that the oral anaerobic bacterium F. nucleatum may have a role in initiating oncogenic transformation in colonic and rectal cells (Kostic et al., 2013). While more study is needed to confirm this potential association, especially within African settings, there is evidence that colorectal cancer cells when colonized with specific subclades of F. nucleatum, may increase the potential for local tumor spread and metastasis (Kostic et al., 2013; Rubinstein et al., 2013; Bullman et al., 2017; Ou et al., 2022; Wang and Fang, 2022; Zepeda-Rivera et al., 2024). We further discuss this possibility within the section on microbiomes.
Cutibacterium acnes
C. acnes (a skin commensal, implicated in superficial skin infections, especially acne vulgaris) has been shown to colonize the prostate, resulting in chronic inflammation, which appears to be a pivotal factor for prostate cancer (Davidsson et al., 2021; Goldstein and Mascitelli, 2024). Studies have demonstrated the presence of C. acne in prostate cancer specimens in much higher proportions than in non-cancerous prostate tissue (Criterion 2) (Kakegawa et al., 2017). Furthermore, cohort epidemiologic studies have shown that severe acne during adolescence is associated with a higher likelihood of prostate cancer later in life (Criterion 1) (Zhang et al., 2018). To demonstrate causation, future studies must evaluate and reproducibly confirm other criteria for causation, as well as validate the epidemiologic evidence. Investigations carried out thus far, have yielded inconsistent results (Drott et al., 2010; Sayanjali et al., 2016). Furthermore, existing data are from European studies with a lack of data from Africa, where prostate cancer appears to occur at an earlier age and can be more aggressive (Davidsson et al., 2016; Janivara et al., 2024). That gap needs to be filled to drive further research that could lead to preventive approaches, assuming a triggering role was established.
Salmonella Typhi
S Typhi can colonize the gall bladder following symptomatic typhoid fever or asymptomatic systemic infection, resulting in chronic carriage in 1-4% of patients acutely infected. In SSA there are 1.2 million acute typhoid fever cases annually; however, it is unclear how many of these infections lead to chronic carriage (Kim et al., 2024). Moreover, there are distinct genotypes in SSA that have a higher propensity for invasive disease and antibiotic resistance — both factors could influence potential for chronic infection (Kingsley et al., 2009). Gall bladder colonization results in chronic inflammation directly and by stimulated the formation of gallstones. A meta-analysis of 17 studies suggested that chronic S Typhi infection of the gall bladder is associated with gallbladder cancer (Criterion 1) (Nagaraja and Eslick, 2014; Upadhayay et al., 2022). S Typhi has a high prevalence in sub-Saharan Africa with a substantial proportion of infections being undiagnosed or untreated. This is coupled with the increasing incidence of multidrug resistance S Typhi, and low vaccination levels (Kariuki and Onsare, 2024; Khan et al., 2022).
SARS-CoV-2
While SARS-CoV-2 has not yet been linked to specific cancers (Jahankhani et al., 2023), there has not been sufficient follow-up period to observe an effect. However, this virus has distinct infection-associated patterns which may contribute to being an oncogenic virus. For instance, SARS-CoV-2 causes RAAS (renin-angiotensin-aldosterone system) pathway dysregulation, induces degradation of the tumor repressor retinoblastoma protein, via nsp15 and P53 via nsp3, affects cell cycle through among others nsp7, interferes with DNA methylation through NSP8, and generates reactive oxygen species (ROS), all common pathways involved in oncogenesis, making a link to cancer plausible (Criterion 9) (Jahankhani et al., 2023). Evidence of prolonged, persistence of replicating SARS CoV2 in tissues (Yang et al., 2024) raises a potential for chronic inflammation which may also increase a risk of cancer formation. Longitudinal cohorts, such as the Rotterdam study, maintained over time may provide insight into the role of infectious triggers including SARS-CoV2 in oncogenesis (Ikram et al., 2020; Sijtsma et al., 2022).
Considering a role for fungi
Candida has been observed in colorectal cancer tissue samples and is associated with decreased survival and metastatic disease in colon cancer. Similarly, Blastomyces is has been detected in lung cancer tumor tissues (Dohlman et al., 2022). However, for these and other examples of fungal colonization, association but no direct causal relationships have been established. Fungal colonization has been suggested to drive carcinogenesis through immune recruitment of TH2 cells in pancreatic and esophageal cancer. Pathogenic infections of Candida species correlate with a higher oral cancer incidence (Chung et al., 2017; Di Cosola et al., 2021). These associations may partially be a consequence of the lower levels of immunity in these individuals (increasing risk for colonization) or could indicate synergistic relationships that promote both cancer and fungal colonization.
Fungi play a demonstrable role in hepatocellular cancer, however in a more indirect way, where they (Aspergillus flavus and Aspergillus parasiticus mainly) infect food sources such as maize and grains, imparting high levels of hepatotoxic aflatoxins which in turn directly contribute to oncogenesis (Cui et al., 2015; Jin et al., 2023; Yu et al., 2023b). This is of particular importance in some regions of SSA (especially west Africa and parts of east Africa) where food storage conditions combined with high heat and humidity contribute to a high aflatoxin burden in staple foods (Falade et al., 2022). Other mycotoxins have been studied such as Ochratoxin A, produced by aspergillus or T-2 and Zearalenone, both fusarium toxins; these link to nephropathies and potentially neurological disorders such as Parkinsons and dementia (Khan et al., 2024). However, these toxins were linked to various cancers (esophageal, kidney, colon, urinary tract, gastrointestinal, uterine, breast) in animal and in in vitro models (Claeys et al., 2020; Ekwomadu et al., 2021, Ekwomadu et al., 2022; Khan et al., 2024). For example, fumonisins have been linked to esophageal cancer with recent studies suggesting that it affects PI3K/Akt pathway in human esophageal cells (Yu et al., 2021). Nonetheless, clear epidemiological evidence for these links has not been established (Claeys et al., 2020; Ekwomadu et al., 2022).
The microbiome
In healthy individuals the gut microbiota, consisting of bacteria, bacteriophages, viruses, archaea, and fungi, play a role in immune regulation through presentation of short chain fatty amino acids (SCFAs) (Mann et al., 2024). Firmicutes and Bifidobacteriaceae species present these SCFA’s which are taken up by the intestinal cells and regulate the pro-inflammatory cytokines, TNFa IL12 and IL6. Moreover, the microbiome also trains the immune system and inhibits the growth of pathogenic biota (such as Enterobacteriaceae) and the development of pathobionts. Pathobionts are microbes that under normal circumstances do not cause disease; however, in the context of cancer or microbiome dysregulation, they become pathogenic (Jochum and Stecher, 2020).
One such pathobiont is F. nucleatum, an oral commensal anaerobic bacterium, which as mentioned above, may play an important role in facilitating colorectal cancer incidence and metastasis. F. nucleatum colonizes colorectal cancer cells through Fap2, a galactose adhesion hemagglutinin. It produces virulence factors such as FadA, which provides a scaffold for colonization with other bacteria, contributing to dysbiosis, potentially inducing oncogenesis in host cells. FadA and other virulence factors (e.g. AvrA in Salmonella) bind to the E cadherin receptor, inducing the Wnt signaling pathway, one of the major pathways implicated in colorectal cancer oncogenesis and progression (Kostic et al., 2013; Rubinstein et al., 2013; Bullman et al., 2017; Silva-García et al., 2019). It may enhance colorectal cancer proliferation by upregulation of the wnt signaling pathways and metastasis by inducing the expression of CXCL1 and IL-8 which promotes migration and upregulating CCL20 (Ou et al., 2022). F nucleatum also induces immune evasion through binding of FapA to immune cells. It is similarly potentially implicated in oral cancers where it enhances proliferation and inhibits cell cycle control mechanisms through p27 (Chen et al., 2022; Wang and Fang, 2022). Lastly it was shown induce metastases by modulating mitogen-activated protein kinase p38, which is involved in mesenchymal transition (Lin et al., 2016).
While there has been a paucity of data characterizing microbiomes in SSA, recent studies have revealed unique taxa and diversity in both South African and Tanzanian samples (Nobels et al., 2025). Click or tap here to enter text. Some investigations have examined the role of the microbiome in cancer development in SSA, linking cervical microbiome characteristics and cervical cancer, as well as suggesting that changes to the gut microbiome after urbanization may correlate with development of colon cancer (Klein et al., 2020; Come et al., 2021; Yang et al., 2022b; Ramaboli et al., 2024), However, most microbiome research in SSA relies on time-intensive culture-based experiments, compared to the more rapid sequence-based technology applied in higher income settings (Paulo et al., 2023; Ayeni et al., 2024). Substantial knowledge gaps remain regarding links between microbiome characteristics and cancers in SSA, opening the door for prioritizing support for pivotal research on the topic
Complex interplay between the microbiome and cancer
Disturbances in microbiota may alter metabolic pathways and disrupt homeostasis, leaving vulnerabilities to disease (Francescone et al., 2014). The microbiome’s role in cancer development is complex; both protective and pro-cancer effects, which may be dependent on other external factors, have been demonstrated (Kadosh et al., 2020; Akbar et al., 2022).
Cancer associated mutations can also have different outcomes depending on the microbiotic background. This has been shown in p53 mutations, which can cause either oncogenesis or tumor repression depending on the microenvironment. The presence of gallic-acid-producing bacteria in the distal gut induced cancer in mice, while its presence in the proximal gut provided protective effects due to wnt signaling inhibition (Kadosh et al., 2020). Similar supporting roles have been found in Kras and p53 mouse models, which in the absence of microbiota in the lung could not cause lung cancer (Jin et al., 2019).
Similarly, bacteria can acquire additional proteins which change them into pathobionts, such as, E coli, when expressing colibactin. This mutagen is found to trigger mutational signatures related to oral squamous cell carcinoma (Boot et al., 2020). Similar mutational signatures which are oncogenic have also been found in colorectal cancer and could indicate a common mutagen in these groups of cancers (Boot et al., 2020; Koh et al., 2021; Cornish et al., 2023).
Dysregulation of the microbiome may also impact treatment as outcomes after hematopoietic stem cell transplantation depend strongly on regulation of inflammation and barrier integrity. The microbiome can modulate the effects of radiotherapy where treatment of dysbiosis with vancomycin can enhance radiotherapy efficacy in melanoma and lung cancer mice models (Ciernikova et al., 2021; Viswanathan et al., 2023; Zhao et al., 2023). The latter may be a cause of complacency which is discussed below. The role of the microbiome in cancer was recently reviewed (Nobels et al., 2025).
The microbiome as an inconsequential bystander
While some bacteria may play a causative role in cancer, contributing to immune evasion and cancer progression, dysregulation is often a consequence of opportunistic infections, indicating inconsequential presence of bacteria within the microbiome, rather than causation. There is a host of studies where non-commensal or dysbiotic bacteria such as Salmonella or Helicobacter bacteria are found in tumor tissue (Zhao et al., 2022; Zhu et al., 2022; Schorr et al., 2023). An analysis determined that for most solid tumors, 105 to 106 bacteria are present per palpable 1-cm3 tumor, which represents 34 bacterial cells per 5000 cancer cells. The levels of these bacteria are therefore generally low, which complicates analyses. Even when presence is established, presence is not sufficient to indicate causative or synergistic relationships. Indeed, studying the interaction between microbiota and cancer needs careful consideration as demonstrated by a recent re-analysis of links between pancreatic cancer and the microbiome, initially suggesting, then refuting an oncogenic role for microbiota (Guo et al., 2021; Fletcher et al., 2023; Eckhoff et al., 2024; Pourali et al., 2024). The authors argue that the low-biomass of human tissue specimens increases the risk for errors, including distinguishing between low-biomass microbial communities and contamination introduced during sample collection, and errors made during processing, and sequencing. Therefore, PCR confirmed presence and characterizations of the microbiome, cannot not indicate that these organisms were viable in this tissue, nor can determine whether they were causative or bystanders. To form a better understanding of this complex interplay, standardized methods are needed for generating and analyzing microbiome sequencing data to enhance the reproducibility of results across different studies (Aykut et al., 2019; Fletcher et al., 2023).
HIV has also been associated with significant changes in the microbiome. Upon infection, there is rapid spread throughout the lymph system of the gut through mechanisms of cell-to-cell transmission. HIV uses virological synapses to spread throughout the entire CD4 T cell network causing massive cell death and local immune dysregulation. These also result in permanent disruptions in the epithelium of the gut. Even when anti-retroviral therapy (ART) is given early during the course of HIV infection, the gut based immune system is not fully restored and the damage to the epithelial damage causes long lasting dysbiosis and microbial translocation (Zicari et al., 2019; Govindaraj et al., 2023). Microbiota associated with HIV infection are similar to those associated with other inflammatory diseases, such as inflammatory bowel disease. The dysbiosis in HIV may contribute to the continued systemic inflammation (with corollary impacts on cancer risks) observed in HIV, which persists in patients on ART (Serrano-Villar et al., 2017; Herrera et al., 2019).
Utilizing new approaches to identify novel microbial links with cancer
As opposed to the 1980s, when the associations between HPV and cervical cancer and H. pylori and gastritis and ulcers, initially, and ultimately with gastric cancer (Warren and Marshall, 1983; Parsonnet et al., 1991) were suggested using histopathology and other relatively primitive (by current standards) tools available at the time, new instruments and techniques will likely accelerate the process for identifying previously unrecognized associations For instance, next-generation sequencing (NGS) allows for comprehensive analysis of microbial communities and the identification of novel pathogens in cancer tissues. Assessing genetic material (metagenomics) recovered directly from such as tumor tissue can potentially identify microbes associated with cancers. Proteomics and metabolomics can identify microbial proteins and metabolites in cancer tissues, providing insights into triggers for carcinogenesis. Applying such experimental approaches to longitudinal cohorts, overlying infection status and cancer incidence over time in large populations can yield hypotheses generation to be applied to more focused studies to identify new microbial facilitators and potentiators of cancer.
In addition, prompting artificial intelligence (AI)/large language models trained on all published literature can provide a systematic approach for prioritizing the most likely carcinogenic mediators and mechanisms for study, especially when financial resources are limited. AI models could suggest novel microbial cancer linkages that have not yet been studied or hypothesized, based on aligning oncogenic pathways with microbial pathophysiologies. With such approaches, we propose strict criteria such as ours to steer AI findings. While some models, have advanced beyond pattern recognition to critical thinking, not all have this capacity. In addition, findings models can be limited by CPU power and availability. Thus, caution is needed in applying AI to the complexities of microbial oncogenicity. AI may yield biased conclusions, as it relies on currently available data, which is often sourced from developed nations. Consequently, infectious triggers for cancer, which are much more common in SSA than in areas where most of the data currently exists (i.e. the “global north”) might be overlooked. AI tools may massively accelerate discovery in this field, but will need careful training and coding to correct for biases, and until this is done, such models should be carefully validated (Estiri et al., 2022; Mbunge and Batani, 2023; Mittermaier et al., 2023; Lawsen, 2025; Shojaee et al., 2025).
Priority areas for discovery
Many of the microbes that have been shown to be oncogenic for specific cancers may cause additional cancers beyond what has already been demonstrated. HPV, implicated in a host of cancers, including cervical, head and neck, anal and penile cancers, may also be associated with prostate (Tsydenova et al., 2023), breast (Kudela et al., 2022), and colorectal (Hsu et al., 2022) cancers. For example, studies have found a predilection for immunohistochemistry-associated HPV presence in prostate cancer tissue when compared with healthy tissue (Zambrano et al., 2002; Lawson and Glenn, 2020). Further research to determine whether there is a facilitative role for HPV in prostate cancer could be considered a priority since, existing tools to prevent HPV infection would be used differently (in boys, perhaps with boosters later in adulthood) and could have dramatic public health benefits, should it be confirmed that a proportion of prostate cancer is triggered by HPV infection and persistence.
Likewise, there are data suggesting that EBV may be associated with breast cancer and gastric adenocarcinoma (Tavakoli et al., 2020; Jeong et al., 2022; Agolli et al., 2023). Determining such relationships could be pivotal for prioritizing EBV vaccine development. In addition to its role as an established trigger for gastric cancer, H. pylori, has been hypothesized to be linked to lower esophageal adenocarcinoma (Islami and Kamangar, 2008). Finding further cancer associations for H pylori, could increase the application of resources to utilize the knowledge to develop diagnostic and prevention tools.
Oncogenesis theories must consider “hit and run” cancer mechanisms, where the oncogenic driver may have initiated processes many years ago and now be undetectable; radiation and known mutagen exposure years prior to cancer detection are classic examples, but the concept also applies to microbial oncogenesis (Smith and Saveria Campo, 1988;Tommasino, 2017) This is the case for HPV and head and neck cancers, as well as β-HPV and cutaneous cancers, and may also be implicated in the other oncogenic infectious diseases mentioned in this paper (Ferreira et al., 2021, Ferreira et al., 2023). Other causal criteria may be fulfilled, but it may not be possible to find histopathological presence in tumor tissue nor persistence of the viral genome (Ferreira et al., 2021). The determinant for causation, in a “hit and run” circumstance, may be a pattern of dysregulation, as discussed in this review, that if present, suggest an infectious trigger. Alternatively, microbes producing similar disruption as observed with known microbial cancer pairs (as with HPV and cervical cancer) may provide an indication despite the offending microbe not being present (Irrazábal et al., 2014; Muhammad et al., 2019).
Concluding vision
Discovery of novel microbial-based triggers for oncogenesis and cancer severity will shine a light on feasible pathways to prevent cancer incidence and mortality globally with greatest impact in low-income settings. Such pathways could include vaccine development or modification in use of existing vaccines, as well as new approaches for screening and diagnosis, and other strategies for prevention, and innovative therapies. Machine learning, combined with advances in experimental tools, and multi-disciplinary global collaborations, bringing expertise together across multiple disparate fields of study for innovative approaches, provides the potential a new era for scientific advances in the field of microbial oncogenesis (Breiman et al., 2023). This opportunity should be prioritized because of its consequential potential to lead to products and strategies that will address the massive growing impact and global inequities in cancer burden.
Statements
Author contributions
RD: Writing – original draft, Writing – review & editing. RB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We acknowledge the use of Google Gemini pro to confirm no major microbial drivers were overlooked during the writing of this manuscript. Similarly we used Google Gemini pro to confirm if our proposed criteria were correctly applied and if any factor was overlooked during the review process.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abd-El-Raouf R. Ouf S. A. Gabr M. M. Zakaria M. M. El-Yasergy K. F. Ali-El-Dein B. (2020). Escherichia coli foster bladder cancer cell line progression via epithelial mesenchymal transition, stemness and metabolic reprogramming. Sci. Rep.10, 1 10, 1–11. doi: 10.1038/s41598-020-74390-5
2
Abdollahi A. Razavian I. Razavian E. Ghodsian S. Almukhtar M. Marhoommirzabak E. et al . (2022). Toxoplasma gondii infection/exposure and the risk of brain tumors: A systematic review and meta-analysis. Cancer Epidemiol.77, 102119. doi: 10.1016/J.CANEP.2022.102119
3
Abdulamir A. S. Hafidh R. R. Bakar F. A. (2011). The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res.30, 11. doi: 10.1186/1756-9966-30-11
4
Afzal S. Fiaz K. Noor A. Sindhu A. S. Hanif A. Bibi A. et al . (2022). Interrelated oncogenic viruses and breast cancer. Front. Mol. Biosci.9. doi: 10.3389/FMOLB.2022.781111/BIBTEX
5
Akbar N. Khan N. A. Muhammad J. S. Siddiqui R. (2022). The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev.2, 100010. doi: 10.1016/J.HSR.2021.100010
6
Akula S. M. Pramod N. P. Wang F. Z. Chandran B. (2001). Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology284, 235–249. doi: 10.1006/VIRO.2001.0921
7
Akuoko C. P. Armah E. Sarpong T. Quansah D. Y. Amankwaa I. Boateng D. (2017). Barriers to early presentation and diagnosis of breast cancer among African women living in sub-Saharan Africa. PloS One12, e0171024. doi: 10.1371/JOURNAL.PONE.0171024
8
Alkrekshi A. Kassem A. Park C. Tse W. (2021). Risk of non-hodgkin’s lymphoma in HCV patients in the United States between 2013 and 2020: A population-based study. Clin. Lymphoma Myeloma Leuk21, e832–e838. doi: 10.1016/j.clml.2021.06.014
9
Ankri S. (2015). Entamoeba histolytica – tumor necrosis factor: a fatal attraction. Microbial Cell2, 216. doi: 10.15698/MIC2015.07.216
10
Arner E. N. Rathmell J. C. (2023). Metabolic programming and immune suppression in the tumor microenvironment. Cancer Cell41, 421–433. doi: 10.1016/J.CCELL.2023.01.009
11
Arora R. Chang Y. Moore P. S. (2012). MCV and merkel cell carcinoma: A molecular success story. Curr. Opin. Virol.2, 489. doi: 10.1016/J.COVIRO.2012.05.007
12
Asempah E. (2021). Cervical cancer prevalence in sub-saharan africa and HPV vaccination policy: A public health grand challenge? J. Cancer Immunol.3, 87–97. doi: 10.33696/CANCERIMMUNOL.3.043
13
Atallah-Yunes S. A. Murphy D. J. Noy A. (2020). HIV-associated burkitt lymphoma. Lancet Haematol7, e594–e600. doi: 10.1016/S2352-3026(20)30126-5
14
Ayeni K. I. Berry D. Ezekiel C. N. Warth B. (2024). Enhancing microbiome research in sub-Saharan Africa. rends Microbiol.32 (2), 111–115. doi: 10.1016/j.tim.2023.11.003
15
Aykut B. Pushalkar S. Chen R. Li Q. Abengozar R. Kim J. I. et al . (2019). The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature574, 264–267. doi: 10.1038/s41586-019-1608-2
16
Bangham C. R. M. (2023) HTLV-1 persistence and the oncogenesis of adult T-cell leukemia/lymphoma. Blood141 (19), 2299–2306. doi: 10.1182/blood.2022019332
17
Bates M. Brantsaeter A. B. (2016). Human cytomegalovirus (CMV) in Africa: a neglected but important pathogen. J. Virus Erad2 (3), 136–142. doi: 10.1016/s2055-6640(20)30456-8
18
Benedetti F. Silvestri G. Denaro F. Finesso G. Contreras-Galindo R. Munawwar A. et al . (2024). Mycoplasma DnaK expression increases cancer development in vivo upon DNA damage. Proc. Natl. Acad. Sci. U.S.A.121, e2320859121. doi: 10.1073/PNAS.2320859121/SUPPL_FILE/PNAS.2320859121.SAPP.PDF
19
Beral V. Peterman T. A. Berkelman R. L. Jaffe H. W. (1990). Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet335, 123–128. doi: 10.1016/0140-6736(90)90001-L
20
Berriel E. Freire T. Chiale C. Rodríguez E. Morón G. Fernández-Graña G. et al . (2021). Human hydatid cyst fluid-induced therapeutic anti-cancer immune responses via NK1.1+ cell activation in mice. Cancer Immunol. Immunother.70, 3617. doi: 10.1007/S00262-021-02948-X
21
Bohlius J. Maxwell N. Spoerri A. Wainwright R. Sawry S. Poole J. et al . (2016). Incidence of AIDS-defining and other cancers in HIV-positive children in South Africa: record linkage study. Pediatr. Infect. Dis. J.35, e164. doi: 10.1097/INF.0000000000001117
22
Bohlius J. Schmidlin K. Costagliola D. Fätkenheuer G. May M. Caro-Murillo A. M. et al . (2009). Incidence and risk factors of HIV-related non-Hodgkin’s lymphoma in the era of combination antiretroviral therapy: a European multicohort study. Antivir Ther.14, 1065. doi: 10.3851/IMP1462
23
Boot A. Ng A. W. T. Chong F. T. Ho S. C. Yu W. Tan D. S. W. et al . (2020). Characterization of colibactin-associated mutational signature in an Asian oral squamous cell carcinoma and in other mucosal tumor types. Genome Res.30 (6), 803–813. doi: 10.1101/gr.255620.119
24
Borgia M. Dal Bo M. Toffoli G. (2021). Role of virus-related chronic inflammation and mechanisms of cancer immune-suppression in pathogenesis and progression of hepatocellular carcinoma. Cancers (Basel)13 (17), 4387. doi: 10.3390/CANCERS13174387
25
Bosch F. X. Manos M. M. Muñoz N. Sherman M. Jansen A. M. Peto J. et al . (1995). Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. J. Natl. Cancer Inst87 (11), 796–802. doi: 10.1093/jnci/87.11.796
26
Botelho M. Ferreira A. C. Oliveira M. J. Domingues A. MaChado J. C. da Costa J. M. C. (2009). Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int. J. Parasitol.39, 1083–1091. doi: 10.1016/J.IJPARA.2009.02.016
27
Bradford Hill A. S. (1965). The environment and disease: association or causation? Proc. R Soc. Med.58, 295–300. doi: 10.1177/003591576505800503
28
Brady G. MacArthur G. J. Farrell P. J. (2007). Epstein–Barr virus and Burkitt lymphoma. J. Clin. Pathol.60, 1397. doi: 10.1136/JCP.2007.047977
29
Breiman R. F. Demetriou G. Naidu G. Papathanasopoulos M. A. Ruff P. Madhi S. A. (2023). Shifting the center of gravity for addressing the rising cancer disease burden in Africa: A rationale for African-based integrative infectious diseases and oncology research. PloS Global Public Health3, e0001970. doi: 10.1371/JOURNAL.PGPH.0001970
30
Brune K. A. Ferreira F. Mandke P. Chau E. Aggarwal N. R. D’alessio F. R. et al . (2016). HIV impairs lung epithelial integrity and enters the epithelium to promote chronic lung inflammation. PloS One11, e0149679. doi: 10.1371/JOURNAL.PONE.0149679
31
Bullman S. Pedamallu C. S. Sicinska E. Clancy T. E. Zhang X. Cai D. et al . (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science358, 1443–1448. doi: 10.1126/SCIENCE.AAL5240
32
Burd E. M. (2003). Human papillomavirus and cervical cancer. Clin. Microbiol. Rev.16, 1–17. doi: 10.1128/CMR.16.1.1-17.2003
33
Burton E. M. Gewurz B. E. (2022). Epstein–Barr virus oncoprotein–driven B cell metabolism remodeling. PloS Pathog.18, e1010254. doi: 10.1371/JOURNAL.PPAT.1010254
34
Carbone A. Gloghini A. (2018). Epstein barr virus-associated hodgkin lymphoma. Cancers (Basel)10, 163. doi: 10.3390/CANCERS10060163
35
Casper C. Crane H. Menon M. Money D. (2017). “HIV/AIDS comorbidities: impact on cancer, noncommunicable diseases, and reproductive health,” in Disease control priorities, third edition (Volume 6): major infectious diseases. (Washington, DC: World Bank), 45–66. doi: 10.1596/978-1-4648-0524-0_CH3
36
Celegato M. Messa L. Bertagnin C. Mercorelli B. Loregian A. (2022). Targeted disruption of e6/p53 binding exerts broad activity and synergism with paclitaxel and topotecan against hpv-transformed cancer cells. Cancers (Basel)14. doi: 10.3390/cancers14010193
37
Challagundla N. Chrisophe-Bourdon J. Agrawal-Rajput R. (2023). Chlamydia trachomatis infection co-operatively enhances HPV E6-E7 oncogenes mediated tumorigenesis and immunosuppression. Microb. Pathog.175, 105929. doi: 10.1016/J.MICPATH.2022.105929
38
Chan C. K. Aimagambetova G. Ukybassova T. Kongrtay K. Azizan A. (2019). Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination—Review of current perspectives. J. Oncol., 1–11. doi: 10.1155/2019/3257939
39
Chan G. Nogalski M. T. Bentz G. L. Smith M. S. Parmater A. Yurochko A. D. (2010). PI3K-dependent upregulation of mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J. Immunol.184, 3213–3222. doi: 10.4049/JIMMUNOL.0903025
40
Chang J. I. Lee K. Kim D. Yang J. I. Park J. K. Choi K. et al . (2021). Clinical characteristics of clonorchis sinensis-associated cholangiocarcinoma: A large-scale, single-center study. Front. Med. (Lausanne)8. doi: 10.3389/FMED.2021.675207/FULL
41
Chen C. W. Cheng J. S. Chen T. Le P. H. Ku H. P. Chang M. L. (2019). The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts. Therap Adv. Gastroenterol.12. doi: 10.1177/1756284819855732
42
Chen Y. Huang Z. Tang Z. Huang Y. Huang M. Liu H. et al . (2022). More than just a periodontal pathogen –the research progress on fusobacterium nucleatum. Front. Cell Infect. Microbiol.12. doi: 10.3389/FCIMB.2022.815318/BIBTEX
43
Chen C. J. Yang H. I. Su J. Jen C. L. You S. L. Lu S. N. et al . (2006). Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA Level. J. Am. Med. Assoc.295. doi: 10.1001/jama.295.1.65
44
Chen L. Zhao X. Liu Y. Wu M. Li S. Xu C. et al . (2023). Comprehensive analysis of HHV-6 and HHV-7-related gene signature in prognosis and response to temozolomide of glioma. J. Med. Virol.95. doi: 10.1002/JMV.28285
45
Cheng W. T. Kantilal H. K. Davamani F. (2020). The mechanism of bacteroides fragilis toxin contributes to colon cancer formation. Malays J. Med. Sci.27, 9. doi: 10.21315/MJMS2020.27.4.2
46
Chung L. M. Liang J. A. Lin C. L. Sun L. M. Kao C. H. (2017). Cancer risk in patients with candidiasis: a nationwide population-based cohort study. Oncotarget8, 63562. doi: 10.18632/ONCOTARGET.18855
47
Ciernikova S. Kasperova B. Drgona L. Smolkova B. Stevurkova V. Mego M. (2021). Targeting the gut microbiome: An emerging trend in hematopoietic stem cell transplantation. Blood Rev.48, 100790. doi: 10.1016/J.BLRE.2020.100790
48
Claeys L. Romano C. De Ruyck K. Wilson H. Fervers B. Korenjak M. et al . (2020). Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf.19, 1449–1464. doi: 10.1111/1541-4337.12567
49
Come J. Pereira J. B. Pinto R. Carrilho C. Pereira L. Lara Santos L. (2021). The upper digestive tract microbiome and oesophageal squamous cell carcinoma: epidemiology, pathogenesis, and clinical implications in africa. Pathobiology88 (2), 141–155. doi: 10.1159/000511422
50
Cornish A. J. Gruber A. J. Kinnersley B. Chubb D. Frangou A. Caravagna G. et al (2024). The genomic landscape of 2,023 colorectal cancers. Nature633, 127–136. doi: 10.1038/s41586-024-07747-9
51
Cui A. Hua H. Shao T. Song P. Kong Q. Luo T. et al . (2015). Aflatoxin B1 induces Src phosphorylation and stimulates lung cancer cell migration. Tumour Biol.36, 6507–6513. doi: 10.1007/S13277-015-3341-2
52
Cui X. Li Y. Xu H. Sun Y. Jiang S. Li W. (2023). Characteristics of Hepatitis B virus integration and mechanism of inducing chromosome translocation. NPJ Genom Med.8 (1), 11. doi: 10.1038/s41525-023-00355-y
53
Dadgar-Zankbar L. Shariati A. Bostanghadiri N. Elahi Z. Mirkalantari S. Razavi S. et al . (2023). Evaluation of enterotoxigenic Bacteroides fragilis correlation with the expression of cellular signaling pathway genes in Iranian patients with colorectal cancer. Infect. Agent Cancer18, 1–10. doi: 10.1186/S13027-023-00523-W/TABLES/3
54
Davidsson S. Carlsson J. Greenberg L. Wijkander J. Söderquist B. Erlandsson A. (2021). Cutibacterium acnes induces the expression of immunosuppressive genes in macrophages and is associated with an increase of regulatory T-cells in prostate cancer. Microbiol. Spectr.9, e01497-21. doi: 10.1128/SPECTRUM.01497-21
55
Davidsson S. Mölling P. Rider J. R. Unemo M. Karlsson M. G. Carlsson J. et al . (2016). Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect. Agent Cancer11, 1–10. doi: 10.1186/S13027-016-0074-9/FIGURES/3
56
Dehlendorff C. Baandrup L. Kjaer S. K. (2021). Real-world effectiveness of human papillomavirus vaccination against vulvovaginal high-grade precancerous lesions and cancers. J. Natl. Cancer Inst113, 869–874. doi: 10.1093/JNCI/DJAA209
57
de Martel C. Georges D. Bray F. Ferlay J. Clifford G. M. (2020). Global burden of cancer attributa ble to infections in 2018: a worldwide incidence analysis. Lancet Glob Health8, e180–e190. doi: 10.1016/S2214-109X(19)30488-7
58
De Martel C. Shiels M. S. Franceschi S. Simard E. P. Vignat J. Hall H. I. et al . (2015). Cancers attributa ble to infections among adults with HIV in the United States. AIDS29, 2173–2181. doi: 10.1097/QAD.0000000000000808
59
Di Cosola M. Cazzolla A. P. Charitos I. A. Ballini A. Inchingolo F. Santacroce L. (2021). Candida albicans and oral carcinogenesis. A brief review. J. Fungi7, 476. doi: 10.3390/JOF7060476
60
Dohlman A. B. Klug J. Mesko M. Gao I. H. Lipkin S. M. Shen X. et al . (2022). A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell185, 3807–3822.e12. doi: 10.1016/j.cell.2022.09.015
61
Dollard S. C. Butler L. M. Jones A. M. G. Mermin J. H. Chidzonga M. Chipato T. et al . (2010). Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: Insights on the origin of the “kaposi’s sarcoma belt. Int. J. Cancer127. doi: 10.1002/ijc.25235
62
Dow D. E. Cunningham C. K. Buchanan A. M. (2013). A review of human herpesvirus 8, the kaposi’s sarcoma-associated herpesvirus, in the pediatric population. J. Pediatr. Infect. Dis. Soc.3, 66. doi: 10.1093/JPIDS/PIT051
63
Dragan Y. P. Bidlack W. R. Cohen S. M. Goldsworthy T. L. Hard G. C. Howard P. C. et al . (2001). Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: fumonisin B(1) as an example. Toxicol. Sci.61, 6–17. doi: 10.1093/TOXSCI/61.1.6
64
Drott J. B. Alexeyev O. Bergström P. Elgh F. Olsson J. (2010). Propionibacterium acnes infection induces upregulation of inflammatory genes and cytokine secretion in prostate epithelial cells. BMC Microbiol.10, 126. doi: 10.1186/1471-2180-10-126/TABLES/2
65
Dyson N. Howley P. M. Münger K. Harlow E. (1989). The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science243. doi: 10.1126/science.2537532
66
Eckhoff A. M. Fletcher A. A. Kelly M. S. Dohlman A. McIntyre C. A. Shen X. et al . (2024). Comprehensive assessment of the intrinsic pancreatic microbiome. Ann. Surg. doi: 10.1097/SLA.0000000000006299
67
Ego T. Tanaka Y. Shimotohno K. (2005). Interaction of HTLV-1 Tax and methyl-CpG-binding domain 2 positively regulates the gene expression from the hypermethylated LTR. Oncogene24. doi: 10.1038/sj.onc.1208394
68
Ekwomadu T. I. Akinola S. A. Mwanza M. (2021). Fusarium mycotoxins, their metabolites (Free, emerging, and masked), food safety concerns, and health impacts. Int. J. Environ. Res. Public Health18, 11741. doi: 10.3390/IJERPH182211741
69
Ekwomadu T. Mwanza M. Musekiwa A. (2022). Mycotoxin-linked mutations and cancer risk: A global health issue. Int. J. Environ. Res. Public Health19 (13), 7754. doi: 10.3390/IJERPH19137754
70
Eldegla H. E. Abdel-Wahhab M. Moemen D. (2021). Association between Streptococcus gallolyticus and colorectal cancer in Mansoura University hospitals. Egyptian J. Basic Appl. Sci.8, 397–406. doi: 10.1080/2314808X.2021.2001618
71
Elkhalifa A. M. E. Nabi S. U. Shah O. S. Bashir S. M. Muzaffer U. Ali S. I. et al . (2023). Insight into oncogenic viral pathways as drivers of viral cancers: implication for effective therapy. Curr. Oncol.30, 1924. doi: 10.3390/CURRONCOL30020150
72
Emmanuel B. N. Peter D. A. Peter M. O. Adedayo I. S. Olaifa K. (2024). Helicobacter pylori infection in Africa: comprehensive insight into its pathogenesis, management, and future perspectives. J. Umm Al-Qura Univ. Appl. Sci.11, 378–401. doi: 10.1007/s43994-024-00166-6
73
Espina C. McKenzie F. dos-Santos-Silva I. (2017). Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann. Epidemiol.27, 659–671.e7. doi: 10.1016/J.ANNEPIDEM.2017.09.007
74
Estiri H. Strasser Z. H. Rashidian S. Klann J. G. Wagholikar K. B. McCoy T. H. et al . (2022). An objective framework for evaluating unrecognized bias in medical AI models predicting COVID-19 outcomes. J. Am. Med. Inf. Assoc.29 (8), 1334–1341. doi: 10.1093/jamia/ocac070
75
Falade T. D. O. Neya A. Bonkoungou S. Dagno K. Basso A. Senghor A. L. et al . (2022). Aflatoxin contamination of maize, groundnut, and sorghum grown in Burkina Faso, Mali, and Niger and aflatoxin exposure assessment. Toxins (Basel)14. doi: 10.3390/toxins14100700
76
Fedak K. M. Bernal A. Capshaw Z. A. Gross S. (2015). Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol.12 (1), 14. doi: 10.1186/s12982-015-0037-4
77
Ferreira D. A. Idris A. McMillan N. A. J. (2023). Analysis of a hit-and-run tumor model by HPV in oropharyngeal cancers. J. Med. Virol.95, e28260. doi: 10.1002/jmv.28260
78
Ferreira D. A. Tayyar Y. Idris A. McMillan N. A. J. (2021). A “hit-and-run” affair – A possible link for cancer progression in virally driven cancers. Biochim. Biophys. Acta Rev. Cancer1875. doi: 10.1016/j.bbcan.2020.188476
79
Fletcher A. A. Kelly M. S. Eckhoff A. M. Allen P. J. (2023). Revisiting the intrinsic mycobiome in pancreatic cancer. Nature620, E1–E6. doi: 10.1038/s41586-023-06292-1
80
Flores J. E. Thompson A. J. Ryan M. Howell J. (2022). The global impact of hepatitis B vaccination on hepatocellular carcinoma. Vaccines (Basel)10, 793. doi: 10.3390/VACCINES10050793/S1
81
Francescone R. Hou V. Grivennikov S. I. (2014). Microbiome, inflammation, and cancer. Cancer J. (United States)20, 181–189. doi: 10.1097/PPO.0000000000000048
82
Franco A. T. Israel D. A. Washington M. K. Krishna U. Fox J. G. Rogers A. B. et al . (2005). Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A.102. doi: 10.1073/pnas.0504927102
83
Fu Z. Xu H. Yue L. Zheng W. Pan L. Gao F. et al . (2023). Immunosenescence and cancer: Opportunities and challenges. Medicine102, e36045. doi: 10.1097/MD.0000000000036045
84
Fukase K. Kato M. Kikuchi S. Inoue K. Uemura N. Okamoto S. et al . (2008). Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet372. doi: 10.1016/S0140-6736(08)61159-9
85
Gargiulo Isacco C. Balzanelli M. G. Garzone S. Lorusso M. Inchingolo F. Nguyen K. C. D. et al . (2023). Alterations of vaginal microbiota and chlamydia trachomatis as crucial co-causative factors in cervical cancer genesis procured by HPV. Microorganisms662 11, 662. doi: 10.3390/MICROORGANISMS11030662
86
Ghebre R. G. Grover S. Xu M. J. Chuang L. T. Simonds H. (2017). Cervical cancer control in HIV-infected women: Past, present and future. Gynecol Oncol. Rep.21, 101–108. doi: 10.1016/j.gore.2017.07.009
87
Goel P. Tyagi R. Kaur G. Garg B. Selhi P. K. Kaur H. et al . (2018). Entamoeba histolytica: A surprising coexistence with adenocarcinoma – Never brush aside brushings for biopsy. J. Lab. Physicians10, 251. doi: 10.4103/JLP.JLP_129_17
88
Goldstein M. R. Mascitelli L. (2024). How might acne and prostate cancer be connected? Med. Hypotheses192, 111495. doi: 10.1016/J.MEHY.2024.111495
89
Gorish B. M. T. Ournasseir M. E. H. Shammat I. M. (2019). A correlation study of BK Polyoma Virus infection and prostate Cancer among Sudanese patients - immunofluorescence and molecular based case-control study. Infect Agents Cancer14, 25. doi: 10.1186/s13027-019-0244-7
90
Govindaraj S. Babu H. Kannanganat S. Vaccari M. Petrovas C. Velu V. (2023). Editorial: CD4+ T cells in HIV: A friend or a foe? Front. Immunol.14. doi: 10.3389/FIMMU.2023.1203531/BIBTEX
91
Grover S. Xu M. J. Yeager A. Rosman L. Groen R. S. Chackungal S. et al . (2015). A systematic review of radiotherapy capacity in low- and middle-income countries. Front. Oncol.4. doi: 10.3389/FONC.2014.00380
92
Gu B. Li M. Zhang Y. Li L. Yao K. Wang S. (2019). DR7 encoded by human herpesvirus 6 promotes glioma development and progression. Cancer Manag Res.11, 2109. doi: 10.2147/CMAR.S179762
93
Gu J. Lv X. Li W. Li G. He X. Zhang Y. et al . (2023). Deciphering the mechanism of Peptostreptococcus anaerobius-induced chemoresistance in colorectal cancer: the important roles of MDSC recruitment and EMT activation. Front. Immunol.14. doi: 10.3389/FIMMU.2023.1230681/BIBTEX
94
Guo X. Lam S. Y. Janmaat V. T. de Jonge P. J. F. Hansen B. E. Leeuwenburgh I. et al . (2025). Esophageal candida infection and esophageal cancer risk in patients with achalasia. JAMA Netw. Open8, e2454685. doi: 10.1001/jamanetworkopen.2024.54685
95
Guo W. Zhang Y. Guo S. Mei Z. Liao H. Dong H. et al . (2021). Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol.4, 1 4, 1–1 4,13. doi: 10.1038/s42003-021-02557-5
96
Guven-Maiorov E. Tsai C. J. Nussinov R. (2019). Oncoviruses can drive cancer by rewiring signaling pathways through interface mimicry. Front. Oncol.9. doi: 10.3389/fonc.2019.01236
97
Guyon J. Haidar Ahmad S. El Baba R. Le Quang M. Bikfalvi A. Daubon T. et al . (2024). Generation of glioblastoma in mice engrafted with human cytomegalovirus-infected astrocytes. Cancer Gene Ther.31, 7 31, 1070–1080. doi: 10.1038/s41417-024-00767-7
98
Haas O. A. (2019). Primary Immunodeficiency and cancer predisposition revisted: Embedding two closely related concepts into an intergrative conceptual framework. Front. Immunol.10. doi: 10.3389/FIMMU.2018.03136/BIBTEX
99
Haas C. B. Engels E. A. Horner M. J. Freedman N. D. Luo Q. Gershman S. et al . (2022). Trends and risk of lung cancer among people living with HIV in the USA: a population-based registry linkage study. Lancet HIV9, e700–e708. doi: 10.1016/S2352-3018(22)00219-3
100
Haghighi L. Razmjou E. Rafiei-Sefiddashti R. Meamar A. R. Akhlaghi L. (2022). Entamoeba histolytica and probable effect on production microsatellite instability in colorectal cancer. Curr. Microbiol.79, 1–7. doi: 10.1007/S00284-022-02782-Z/METRICS
101
Heawchaiyaphum C. Yoshiyama H. Iizasa H. Burassakarn A. Tumurgan Z. Ekalaksananan T. et al (2023). Epstein–barr virus promotes oral squamous cell carcinoma stemness through the warburg effect. Int. J. Mol. Sci.24. doi: 10.3390/ijms241814072
102
Herrera S. Martínez-Sanz J. Serrano-Villar S. (2019). HIV, cancer, and the microbiota: common pathways influencing different diseases. Front. Immunol.10. doi: 10.3389/FIMMU.2019.01466
103
Hodge J. M. Coghill A. E. Kim Y. Bender N. Smith-Warner S. A. Gapstur S. et al . (2021). Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. Int. J. Cancer148, 2449–2456. doi: 10.1002/IJC.33443
104
Hoppe-Seyler K. Bossler F. Braun J. A. Herrmann A. L. Hoppe-Seyler F. (2018). The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol.26. doi: 10.1016/j.tim.2017.07.007
105
Hsu C. H. Lin Y. J. Chen Y. C. Liu I. L. You S. L. Hu J. M. et al . (2022). Human papillomavirus and risk of colorectal cancer: an analysis of nationwide claims data. Medicina (B Aires)58, 1461. doi: 10.3390/MEDICINA58101461
106
Hurst R. Brewer D. S. Gihawi A. Wain J. Cooper C. S. (2024). Cancer invasion and anaerobic bacteria: new insights into mechanisms. J. Med. Microbiol.73, 1817. doi: 10.1099/JMM.0.001817
107
Hwang J. P. LoConte N. K. Rice J. P. Foxhall L. E. Sturgis E. M. Merrill J. K. et al . (2019). Oncologic implications of chronic hepatitis C virus infection. J. Oncol. Pract.15, 629–637. doi: 10.1200/JOP.19.00370
108
IARC (2020). Cancers Attributab le to Infections. Available online at: https://gco.iarc.fr/causes/infections/tools-map?mode=3&sex=0&continent=1&agent=0&cancer=0&key=asr&scale=threshold (Accessed August 23, 2023).
109
ICO/IARC Information Centre on HPV and Cancer (2023). Human papillomavirus and related cancers, fact sheet 2023. Available online at: www.hpvcentre.net (Accessed March 28, 2024).
110
Ikram M. A. Brusselle G. Ghanbari M. Goedegebure A. Ikram M. K. Kavousi M. et al . (2020). Objectives, design and main findings until 2020 from the Rotterdam Study. Eur. J. Epidemiol.35. doi: 10.1007/s10654-020-00640-5
111
Irrazábal T. Belcheva A. Girardin S. E. Martin A. Philpott D. J. (2014). The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell54. doi: 10.1016/j.molcel.2014.03.039
112
Islami F. Kamangar F. (2008). Helicobacter pylori and esophageal cancer risk – A meta-analysis. Cancer Prev. Res. (Phila)1, 329. doi: 10.1158/1940-6207.CAPR-08-0109
113
Jahankhani K. Ahangari F. Adcock I. M. Mortaz E. (2023). Possible cancer-causing capacity of COVID-19: Is SARS-CoV-2 an oncogenic agent? Biochimie213, 130. doi: 10.1016/J.BIOCHI.2023.05.014
114
Janivara R. Chen W. C. Hazra U. Baichoo S. Agalliu I. Kachambwa P. et al . (2024). ). Heterogeneous genetic architectures of prostate cancer susceptibility in sub-Saharan Africa. Nat. Genet.56, 10 56, 2093–2103. doi: 10.1038/s41588-024-01931-3
115
Jensen J. N. E. Becker G. L. Jackson J. B. Rysavy M. B. (2024). Human Papillomavirus and Associated Cancers: A Review. Viruses16, 680. doi: 10.3390/V16050680
116
Jeong Y. Cho C. E. Kim J. E. Lee J. Kim N. Jung W. Y. et al . (2022). Deep learning model to predict Epstein–Barr virus associated gastric cancer in histology. Sci. Rep.12, 1–10. doi: 10.1038/s41598-022-22731-x
117
Jin J. Kouznetsova V. L. Kesari S. Tsigelny I. F. (2023). Synergism in actions of HBV with aflatoxin in cancer development. Toxicology499, 153652. doi: 10.1016/J.TOX.2023.153652
118
Jin C. Lagoudas G. K. Zhao C. Bullman S. Bhutkar A. Hu B. et al . (2019). Commensal microbiota promote lung cancer development via γδ T cells. Cell176, 998–1013.e16. doi: 10.1016/J.CELL.2018.12.040
119
Jochum L. Stecher B. (2020). Label or concept – what is a pathobiont? Trends Microbiol.28, 789–792. doi: 10.1016/J.TIM.2020.04.011
120
Julius P. Siyumbwa S. N. Moonga P. Maate F. Kaile T. Haynatski G. et al . (2022). Epstein–barr virus, but not human papillomavirus, is associated with preinvasive and invasive ocular surface squamous neoplasias in Zambian patients. Front. Oncol.12. doi: 10.3389/fonc.2022.864066
121
Jung B. K. Song H. Shin H. Chai J. Y. (2022). Exosomal miRNA-21 from Toxoplasma gondii-infected microglial cells induces the growth of U87 glioma cells by inhibiting tumor suppressor genes. Sci. Rep.12, 1 12, 1–11. doi: 10.1038/s41598-022-20281-w
122
Kadosh E. Snir-Alkalay I. Venkatachalam A. May S. Lasry A. Elyada E. et al . (2020). The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature586, 133–138. doi: 10.1038/S41586-020-2541-0
123
Kakegawa T. Bae Y. Ito T. Uchida K. Sekine M. Nakajima Y. et al . (2017). Frequency of propionibacterium acnes infection in prostate glands with negative biopsy results is an independent risk factor for prostate cancer in patients with increased serum PSA titers. PloS One12, e0169984. doi: 10.1371/JOURNAL.PONE.0169984
124
Kang L. Guo N. Liu X. Wang X. Guo W. Xie S. M. et al . (2020). High mobility group box-1 protects against Aflatoxin G1-induced pulmonary epithelial cell damage in the lung inflammatory environment. Toxicol. Lett.331, 92–101. doi: 10.1016/J.TOXLET.2020.05.013
125
Karimzadeh M. Arlidge C. Rostami A. Lupien M. Bratman S. V. Hoffman M. M. (2023). ). Human papillomavirus integration transforms chromatin to drive oncogenesis. Genome Biol.24, 1–27. doi: 10.1186/S13059-023-02926-9/FIGURES/7
126
Kariuki S. Onsare R. S. (2024). High burden of typhoid disease in sub-Saharan Africa calls for urgent roll-out of typhoid conjugate vaccines. Lancet Glob Health12, e537–e538. doi: 10.1016/S2214-109X(24)00079-2
127
Kato S. Shigematsu Y. Saito R. Ito H. Inamura K. (2024). Schistosoma japonicum-related hepatitis: potential contributor to hepatocellular carcinoma. QJM: Int. J. Med.117, 737–738. doi: 10.1093/QJMED/HCAE116
128
Khan R. Anwar F. Ghazali F. M. (2024). A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon10, e28361. doi: 10.1016/J.HELIYON.2024.E28361
129
Khan Z. A. Khan M. U. Brand M. (2022). Gallbladder cancer in Africa: A higher than expected rate in a “low-risk” population. Surgery171, 855–858. doi: 10.1016/J.SURG.2021.09.016
130
Khandwala P. Singhal S. Desai D. Parsi M. Potdar R. (2021). HIV-associated anal cancer. Cureus13, e14834. doi: 10.7759/CUREUS.14834
131
Kiani H. Makvandi M. Samarbafzadeh A. Teimoori A. Nisi N. Mehravaran H. et al . (2016). Association of HHV-6 with Hodgkin and non Hodgkin lymphoma. Iran J. Microbiol.8, 153.
132
Kim J. Kim B. K. Lee C. H. Seo S. S. Park S. Y. Roh J. W. (2012). Human papillomavirus genotypes and cofactors causing cervical intraepithelial neoplasia and cervical cancer in Korean women. Int. J. Gynecological Cancer22. doi: 10.1097/IGC.0b013e31826aa5f9
133
Kim J.-H. Parajulee P. Nguyen T. T. Wasunkar S. Mogasale V. Park S. E. et al . (2024). Occurrence of human infection with Salmonella Typhi in sub-Saharan Africa. Sci. Data11, 1089. doi: 10.1038/s41597-024-03912-x
134
Kingsley R. A. Msefula C. L. Thomson N. R. Kariuki S. Holt K. E. Gordon M. A. et al . (2009). Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res.19. doi: 10.1101/gr.091017.109
135
Klein C. Kahesa C. Mwaiselage J. West J. T. Wood C. Angeletti P. C. (2020). How the cervical microbiota contributes to cervical cancer risk in sub-saharan africa. Front. Cell Infect. Microbiol.10. doi: 10.3389/fcimb.2020.00023
136
Klufah F. Mobaraki G. Liu D. Alharbi R. A. Kurz A. K. Speel E. J. M. et al . (2021). Emerging role of human polyomaviruses 6 and 7 in human cancers. Infect. Agents Cancer16, 1 16, 1–12. doi: 10.1186/S13027-021-00374-3
137
Koay W. L. A. Siems L. V. Persaud D. (2018). The microbiome and HIV persistence: Implications for viral remission and cure. Curr. Opin. HIV AIDS13, 61–68. doi: 10.1097/COH.0000000000000434
138
Koh G. Degasperi A. Zou X. Momen S. Nik-Zainal S. (2021). Mutational signatures: emerging concepts, caveats and clinical applications. Nat. Rev. Cancer21. doi: 10.1038/s41568-021-00377-7
139
Koike K. Tsutsumi T. (2021). The oncogenic role of hepatitis C virus. Recent Results Cancer Res.217, 91–105. doi: 10.1007/978-3-030-57362-1_5/COVER
140
Kostic A. D. Chun E. Robertson L. Glickman J. N. Gallini C. A. Michaud M. et al . (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe14, 207–215. doi: 10.1016/J.CHOM.2013.07.007
141
Kreikemeyer B. Xu Y. Meile L. Zürich E. Aymeric L. Pasquereau-Kotula E. et al . (2018). Significance of Streptococcus gallolyticus subsp. gallolyticus Association With Colorectal Cancer. Front. Microbiol.9. doi: 10.3389/FMICB.2018.00614
142
Kudela E. Kudelova E. Kozubík E. Rokos T. Pribulova T. Holubekova V. et al . (2022). HPV-associated breast cancer: myth or fact? Pathogens11, 1510. doi: 10.3390/PATHOGENS11121510
143
Kuss-Duerkop S. K. Pyeon D. Westrich J. A. (2018). DNA tumor virus regulation of host DNA methylation and its implications for immune evasion and oncogenesis. Viruses82 10, 82. doi: 10.3390/V10020082
144
Lawsen A. (2025). Comment on The Illusion of Thinking: Understanding the Strengths and Limitations of Reasoning Models via the Lens of Problem Complexity. Available online at: http://arxiv.org/abs/2506.09250 (Accessed June 17, 2025).
145
Lawson J. S. Glenn W. K. (2020). Evidence for a causal role by human papillomaviruses in prostate cancer - A systematic review. Infect. Agent Cancer15, 1–11. doi: 10.1186/S13027-020-00305-8/TABLES/3
146
Lawson J. S. Glenn W. K. (2022). Mouse mammary tumour virus (MMTV) in human breast cancer-the value of bradford hill criteria. Viruses14. doi: 10.3390/V14040721
147
Lee C. C. Yang H. W. Liu C. J. Lee F. Ko W. C. Chang Y. C. et al . (2023). Unraveling the connections between gut microbiota, stress, and quality of life for holistic care in newly diagnosed breast cancer patients. Sci. Rep.13, 1–11. doi: 10.1038/s41598-023-45123-1
148
Lei J. Ploner A. Elfström K. M. Wang J. Roth A. Fang F. et al . (2020). HPV vaccination and the risk of invasive cervical cancer. New Engl. J. Med.383, 1340–1348. doi: 10.1056/NEJMOA1917338
149
Levrero M. Zucman-Rossi J. (2016). Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol.64, S84–S101. doi: 10.1016/J.JHEP.2016.02.021
150
Li S. Hong X. Wei Z. Xie M. Li W. Liu G. et al . (2019). Ubiquitination of the HPV oncoprotein E6 is critical for E6/E6AP-mediated p53 degradation. Front. Microbiol.10. doi: 10.3389/FMICB.2019.02483/BIBTEX
151
Liberti M. V. Locasale J. W. (2016). The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci.41, 211. doi: 10.1016/J.TIBS.2015.12.001
152
Lichtenstern C. R. Lamichhane-Khadka R. (2023). A tale of two bacteria – Bacteroides fragilis, Escherichia coli, and colorectal cancer. Front. Bacteriology2. doi: 10.3389/FBRIO.2023.1229077
153
Lin Y. Mallen-St. Clair J. Wang G. Luo J. Palma-Diaz F. Lai C. et al . (2016). p38 MAPK mediates epithelial-mesenchymal transition by regulating p38IP and Snail in head and neck squamous cell carcinoma. Oral. Oncol.60, 81–89. doi: 10.1016/J.ORALONCOLOGY.2016.06.010
154
Liu G. Sharma M. Tan N. Barnabas R. V. (2018). HIV-positive women have higher risk of HPV infection, precancerous lesions, and cervical cancer: A systematic review and meta-analysis. AIDS32, 795. doi: 10.1097/QAD.0000000000001765
155
Liu Y. Wong C. C. Ding Y. Gao M. Wen J. Lau H. C. H. et al . (2024). Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nat. Microbiol.9, 6 9, 1467–1482. doi: 10.1038/s41564-024-01695-w
156
Liu Z. Zhang L. Liang Y. Lu L. (2022). Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis. Front. Cell Infect. Microbiol.12. doi: 10.3389/FCIMB.2022.1035765/PDF
157
Long X. Wong C. C. Tong L. Chu E. S. H. Ho Szeto C. Go M. Y. Y. et al . (2019). Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol.4, 2319–2330. doi: 10.1038/S41564-019-0541-3
158
Looker K. J. Rönn M. M. Brock P. M. Brisson M. Drolet M. Mayaud P. et al . (2018). Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J. Int. AIDS Soc.21. doi: 10.1002/JIA2.25110
159
Loutfy S. A. Moneer M. M. Salem S. E. El-Moniem Abada E. A. El-Moniem Ahmed E. A. Ibrahim L. H. et al . (2017). Polyomavirus infections and its clinical relevance in cancer patients: A Prospective Study. J. Infect. Public Health10, 22–30. doi: 10.1016/J.JIPH.2016.01.008
160
Ludmir E. B. Stephens S. J. Palta M. Willett C. G. Czito B. G. (2015). Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J. Gastrointest Oncol.6, 287. doi: 10.3978/J.ISSN.2078-6891.2015.001
161
Malavika G. Ravi S. S. S. Maheswary D. Leela K. V. Lathakumari R. H. K S L. P. (2025). Role of Candida albicans in chronic inflammation and the development of oral squamous cell carcinoma. Cancer Pathog. Ther. doi: 10.1016/j.cpt.2025.03.002
162
Machicado C. Machicado J. D. Maco V. Terashima A. Marcos L. A. (2016). Association of fasciola hepatica infection with liver fibrosis, cirrhosis, and cancer: A systematic review. PloS Negl. Trop. Dis.10, e0004962. doi: 10.1371/JOURNAL.PNTD.0004962
163
Mallum A. Patel S. Olatunji E. Nnko G. Alabi A. Akudugu J. et al . (2024). Treatment delays for cancer patients in Sub-Saharan Africa: South Africa as a microcosm. Ecancermedicalscience18. doi: 10.3332/ECANCER.2024.1747
164
Mann E. R. Lam Y. K. Uhlig H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol.24, 8 24, 577–595. doi: 10.1038/s41577-024-01014-8
165
Marima R. Hull R. Lolas G. Syrigos K. N. Kgoebane-Maseko M. Kaufmann A. M. et al . (2021). The catastrophic HPV/HIV dual viral oncogenomics in concert with dysregulated alternative splicing in cervical cancer. Int. J. Mol. Sci.22 (18), 10115. doi: 10.3390/ijms221810115
166
Marongiu L. Allgayer H. Allgayer C. H. (2022). Viruses in colorectal cancer. Mol. Oncol.16, 1423–1450. doi: 10.1002/1878-0261.13100
167
Martínez-Maza O. Breen E. C. (2002). B-cell activation and lymphoma in patients with HIV. Curr. Opin. Oncol.14, 528–532. doi: 10.1097/00001622-200209000-00009
168
Martorelli D. Muraro E. Mastorci K. Dal Col J. Faè D. A. Furlan C. et al . (2015). A natural HIV p17 protein variant up-regulates the LMP-1 EBV oncoprotein and promotes the growth of EBV-infected B-lymphocytes: implications for EBV-driven lymphomagenesis in the HIV setting. Int. J. Cancer137, 1374–1385. doi: 10.1002/IJC.29494
169
Mason W. S. Jilbert A. R. Litwin S. (2021). Hepatitis b virus dna integration and clonal expansion of hepatocytes in the chronically infected liver. Viruses13. doi: 10.3390/v13020210
170
Mbulawa Z. Z. A. Van Schalkwyk C. Hu N. C. Meiring T. L. Barnabas S. Dabee S. et al . (2018). High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PloS One13. doi: 10.1371/JOURNAL.PONE.0190166
171
Mbunge E. Batani J. (2023). Application of deep learning and machine learning models to improve healthcare in sub-Saharan Africa: Emerging opportunities, trends and implications. Telematics Inf. Rep.11. doi: 10.1016/j.teler.2023.100097
172
McGee-Avila J. K. Argirion I. Engels E. A. O’Brien T. R. Horner M. J. Qiao B. et al . (2024). Risk of hepatocellular carcinoma in people with HIV in the United States-2019. J. Natl. Cancer Inst116, 61–68. doi: 10.1093/JNCI/DJAD172
173
Mercado N. B. Real J. N. Kaiserman J. Panagioti E. Cook C. H. Lawler S. E. (2024). Clinical implications of cytomegalovirus in glioblastoma progression and therapy. NPJ Precis. Oncol.8, 1 8, 1–1 8,11. doi: 10.1038/s41698-024-00709-4
174
Mittermaier M. Raza M. M. Kvedar J. C. (2023). Bias in AI-based models for medical applications: challenges and mitigation strategies. NPJ Digit Med.6. doi: 10.1038/s41746-023-00858-z
175
Mounika P. (2013). Helicobacter pylori infection and risk of lung cancer: A meta-analysis. Lung Cancer Int.2013, 1–6. doi: 10.1155/2013/131869
176
Muhammad J. S. Eladl M. A. Khoder G. (2019). Helicobacter pylori-induced dna methylation as an epigenetic modulator of gastric cancer: Recent outcomes and future direction. Pathogens8 (1), 23. doi: 10.3390/pathogens8010023
177
Mwamba M. Lombe D. C. Msadabwe S. Bond V. Simwinga M. Sentoogo Ssemata A. et al . (2023). A narrative synthesis of literature on the barriers to timely diagnosis and treatment of cancer in sub-saharan africa. Clin. Oncol. (R Coll. Radiol)35, e537–e548. doi: 10.1016/J.CLON.2023.05.011
178
Nagaraja V. Eslick G. D. (2014). Systematic review with meta-analysis: the relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol. Ther.39, 745–750. doi: 10.1111/APT.12655
179
Nash K. L. Woodall T. Brown A. S. M. Davies S. E. Alexander G. J. M. (2010). Hepatocellular carcinoma in patients with chronic hepatitis C virus infection without cirrhosis. World J. Gastroenterology: WJG16, 4061. doi: 10.3748/WJG.V16.I32.4061
180
Navarro J. T. Moltó J. Tapia G. Ribera J. M. (2021). Hodgkin lymphoma in people living with HIV. Cancers (Basel)13, 4366. doi: 10.3390/CANCERS13174366
181
Newton R. Labo N. Wakeham K. Miley W. Asiki G. Johnston W. T. et al . (2018). Kaposi sarcoma-associated herpesvirus in a rural Ugandan cohor-2008. J. Infect. Dis.217, 263–269. doi: 10.1093/INFDIS/JIX569
182
Ngwa W. Brawley O. W. Ngwa W. Addai B. W. Adewole I. Ainsworth V. et al . (2022). The lancet oncology commission in sub-saharan africa: a lancet oncology commission. Lancet Oncol.23, e251–e312. doi: 10.1016/S1470-2045(21)00720-8
183
Nobels A. van Marcke C. Jordan B. F. Van Hul M. Cani P. D. (2025). The gut microbiome and cancer: from tumorigenesis to therapy. Nat. Metab. doi: 10.1038/s42255-025-01287-w
184
Núñez-Acurio D. Bravo D. Aguayo F. (2020). Epstein–barr virus—oral bacterial link in the development of oral squamous cell carcinoma. Pathogens9 (12), 1059. doi: 10.3390/pathogens9121059
185
Oikonomopoulou K. Yu H. Wang Z. Vasiliou S. K. Brinc D. Christofi G. et al . (2016). Association between Echinococcus granulosus infection and cancer risk - a pilot study in Cyprus. Clin. Chem. Lab. Med.54, 1955–1961. doi: 10.1515/CCLM-2016-0125
186
Omotoso O. Teibo J. O. Atiba F. A. Oladimeji T. Paimo O. K. Ataya F. S. et al . (2023). Addressing cancer care inequities in sub-Saharan Africa: current challenges and proposed solutions. Int. J. Equity Health22 (1), 189. doi: 10.1186/s12939-023-01962-y
187
Opal S. M. (2017). The potential role of infectious agents in diseases of unknown etiology. Infect. Dis.2, 625–630.e1. doi: 10.1016/B978-0-7020-6285-8.00069-1
188
Ou S. Wang H. Tao Y. Luo K. Ye J. Ran S. et al . (2022). Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell Infect. Microbiol.12. doi: 10.3389/FCIMB.2022.1020583
189
Oyervides-Muñoz M. A. Pérez-Maya A. A. Rodríguez-Gutiérrez H. F. Gómez-Macias G. S. Fajardo-Ramírez O. R. Treviño V. et al . (2018). Understanding the HPV integration and its progression to cervical cancer. Infection Genet. Evol.61, 134–144. doi: 10.1016/J.MEEGID.2018.03.003
190
Pang G. Z. Zhao Y. H. Wang Y. X. Rong S. Q. Gao H. Zhou D. (2023). Mycoplasma infection of cancer cells enhances anti-tumor effect of oxidized methylcytidines. Biochem. Biophys. Res. Commun.672, 193–200. doi: 10.1016/J.BBRC.2023.06.052
191
Parisi F. Freer G. Mazzanti C. M. Pistello M. Poli A. (2022). Mouse mammary tumor virus (MMTV) and MMTV-like viruses: an in-depth look at a controversial issue. Viruses14, 977. doi: 10.3390/V14050977
192
Parsonnet J. Friedman G. D. Vandersteen D. P. Chang Y. Vogelman J. H. Orentreich N. et al . (1991). Helicobacter pylori infection and the risk of gastric carcinoma. N Engl. J. Med.325, 1127–1131. doi: 10.1056/NEJM199110173251603
193
Pastille E. Frede A. McSorley H. J. Gräb J. Adamczyk A. Kollenda S. et al . (2017). Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PloS Pathog.13 (9), e1006649. doi: 10.1371/JOURNAL.PPAT.1006649
194
Pathmanathan R. Prasad U. Sadler R. Flynn K. Raab-Traub N. (1995). Clonal proliferations of cells infected with epstein–barr virus in preinvasive lesions related to nasopharyngeal carcinoma. New Engl. J. Med.333, 693–698. doi: 10.1056/NEJM199509143331103
195
Paulo L. S. Msema Bwire G. Klipstein-Grobusch K. Kamuhabwa A. Kwesigabo G. Chillo P. et al . (2023). Urbanization gradient, diet, and gut microbiota in Sub-Saharan Africa: a systematic review. Front. Microbiomes2. doi: 10.3389/frmbi.2023.1208166
196
Péneau C. Imbeaud S. La Bella T. Hirsch T. Z. Caruso S. Calderaro J. et al . (2022). Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut71 (3), 616–626. doi: 10.1136/gutjnl-2020-323153
197
Perakanya P. Ungcharoen R. Worrabannakorn S. Ongarj P. Artchayasawat A. Boonmars T. et al . (2022). Prevalence and risk factors of opisthorchis viverrini infection in sakon nakhon province, Thailand. Trop. Med. Infect. Dis.7 (10), 313. doi: 10.3390/TROPICALMED7100313
198
Pérez Escriva P. Correia T. Bernardino C. Letellier E. (2025). De-coding the complex role of microbial metabolites in cancer. Cell Rep.44 (3), 115358. doi: 10.1016/j.celrep.2025.115358
199
Picard A. Badoual C. Hourseau M. Halimi C. Pere H. Dib F. et al . (2016). Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS30, 1257–1266. doi: 10.1097/QAD.0000000000001030
200
Plummer M. de Martel C. Vignat J. Ferlay J. Bray F. Franceschi S. (2016). Global burden of cancers attributa ble to infections in 2012: a synthetic analysis. Lancet Glob Health4, e609–e616. doi: 10.1016/S2214-109X(16)30143-7
201
Pourali G. Kazemi D. Chadeganipour A. S. Arastonejad M. Kashani S. N. Pourali R. et al . (2024). Microbiome as a biomarker and therapeutic target in pancreatic cancer. BMC Microbiol.24, 1–24. doi: 10.1186/S12866-023-03166-4
202
Ramaboli M. C. Ocvirk S. Khan Mirzaei M. Eberhart B. L. Valdivia-Garcia M. Metwaly A. et al . (2024). Diet changes due to urbanization in South Africa are linked to microbiome and metabolome signatures of Westernization and colorectal cancer. Nat. Commun.15 (1), 3379. doi: 10.1038/s41467-024-46265-0
203
Ren X. Wu Y. Song T. Yang Q. Zhou Q. Lin J. et al . (2025). Clonorchis sinensis promotes intrahepatic cholangiocarcinoma progression by activating FASN-mediated fatty acid metabolism. J. Gastroenterol. Hepatol40, 1004–1015. doi: 10.1111/JGH.16879
204
Roderfeld M. Padem S. Lichtenberger J. Quack T. Weiskirchen R. Longerich T. et al . (2020). Schistosoma mansoni Egg–Secreted Antigens Activate Hepatocellular Carcinoma–Associated Transcription Factors c-Jun and STAT3 in Hamster and Human Hepatocytes. Hepatology72, 626–641. doi: 10.1002/HEP.30192
205
Rotondo J. C. Mazzoni E. Bononi I. Tognon M. Martini F. (2019). Association between simian virus 40 and human tumors. Front. Oncol.9. doi: 10.3389/FONC.2019.00670/PDF
206
Rubinstein M. R. Wang X. Liu W. Hao Y. Cai G. Han Y. W. (2013). Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe14, 195–206. doi: 10.1016/j.chom.2013.07.012
207
Ruff P. Al-Sukhun S. Blanchard C. Shulman L. N. (2016). Access to cancer therapeutics in low- and middle-income countries. Am. Soc. Clin. Oncol. Educ. Book35, 58–65. doi: 10.1200/EDBK_155975
208
Şahin T. Kiliç Ö. Acar A. G. Severoğlu Z. (2023). A review the role of Streptococcus bovis in colorectal cancer. Arts Humanities Open Access J. Volume5, 165–173. doi: 10.15406/AHOAJ.2021.05.00203
209
Salnikov M. Y. MacNeil K. M. Mymryk J. S. (2024). The viral etiology of EBV-associated gastric cancers contributes to their unique pathology, clinical outcomes, treatment responses and immune landscape. Front. Immunol.15. doi: 10.3389/FIMMU.2024.1358511/XML/NLM
210
Salvatori S. Marafini I. Laudisi F. Monteleone G. Stolfi C. (2023). Helicobacter pylori and gastric cancer: pathogenetic mechanisms. Int. J. Mol. Sci.24, 2895. doi: 10.3390/IJMS24032895
211
Samson M. Libert F. Doranz B. J. Rucker J. Liesnard C. Farber M. et al . (1996). Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature382, 722–726. doi: 10.1038/382722A0
212
Sava M. Huynh T. Frugoli A. Kong L. Salehpour M. Barrows B. (2020). Colorectal cancer related to chronic strongyloides stercoralis infection. Case Rep. Gastrointest Med.2020, 8886460. doi: 10.1155/2020/8886460
213
Sayanjali B. Christensen G. J. M. Al-Zeer M. A. Mollenkopf H. J. Meyer T. F. Brüggemann H. (2016). Propionibacterium acnes inhibits FOXM1 and induces cell cycle alterations in human primary prostate cells. Int. J. Med. Microbiol.306, 517–528. doi: 10.1016/J.IJMM.2016.06.006
214
Scheurer M. E. Bondy M. L. Aldape K. D. Albrecht T. El-Zein R. (2008). Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol.116, 79–86. doi: 10.1007/S00401-008-0359-1
215
Schorr L. Mathies M. Elinav E. Puschhof J. (2023). Intracellular bacteria in cancer—prospects and debates. NPJ Biofilms Microbiomes9, 1 9, 1–1 9,13. doi: 10.1038/s41522-023-00446-9
216
Scott N. Whittle E. Jeraldo P. Chia N. (2022). A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia29, 100797. doi: 10.1016/J.NEO.2022.100797
217
Sepe L. P. Hartl K. Iftekhar A. Berger H. Kumar N. Goosmann C. et al . (2020). Genotoxic effect of salmonella paratyphi a infection on human primary gallbladder cells. mBio11, 1–39. doi: 10.1128/MBIO.01911-20/SUPPL_FILE/MBIO.01911-20-SF003.TIF
218
Serrano-Villar S. Vázquez-Castellanos J. F. Vallejo A. Latorre A. Sainz T. Ferrando-Martínez S. et al . (2017). The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol.10, 1279–1293. doi: 10.1038/MI.2016.122
219
Sharma R. Nanda M. Fronterre C. Sewagudde P. Ssentongo A. E. et al . (2022). Mapping cancer in africa: A comprehensive and comparable characterization of 34 cancer types using estimates from GLOBOCAN 2020. Front. Public Health10, 839835. doi: 10.3389/FPUBH.2022.839835/BIBTEX
220
Sheng S. Chen B. Xu R. Han Y. Mao D. Chen Y. et al . (2024). A prognostic model for Schistosoma japonicum infection-associated liver hepatocellular carcinoma: strengthening the connection through initial biological experiments. Infect. Agent Cancer19, 1–19. doi: 10.1186/S13027-024-00569-4/FIGURES/8
221
Shin H. R. Oh J. K. Lim M. K. Shin A. Kong H. J. Jung K. W. et al . (2010). Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in korea. J. Korean Med. Sci.25, 1011. doi: 10.3346/JKMS.2010.25.7.1011
222
Shindiapina P. Ahmed E. H. Mozhenkova A. Abebe T. Baiocchi R. A. (2020). Immunology of EBV-related lymphoproliferative disease in HIV-positive individuals. Front. Oncol.10. doi: 10.3389/FONC.2020.01723
223
Shojaee P. Mirzadeh I. Alizadeh Maxwell Horton Samy Bengio Mehrdad Farajtabar Apple K. (2025). The Illusion of Thinking: Understanding the Strengths and Limitations of Reasoning Models via the Lens of Problem Complexity. Available online at: https://ml-site.cdn-apple.com/papers/the-illusion-of-thinking.pdf (Accessed June 10, 2025).
224
Shoraka H. R. Aboubakri O. Naghibzadeh-Tahami A. Mollaei H. R. Bagherinezhad Z. Afshar R. M. et al . (2020). Prevalence of JC and BK viruses in patients with colorectal cancer: A systematic review and meta- analysis. Asian Pac J. Cancer Prev.21, 1499. doi: 10.31557/APJCP.2020.21.6.1499
225
Siegel R. L. Miller K. D. Fuchs H. E. Jemal A. (2021). Cancer statistic. CA Cancer J. Clin.71, 7–33. doi: 10.3322/CAAC.21654
226
Sigel K. Makinson A. Thaler J. (2017). Lung cancer in persons with HIV. Curr. Opin. HIV AIDS12, 31. doi: 10.1097/COH.0000000000000326
227
Sijtsma A. Rienks J. van der Harst P. Navis G. Rosmalen J. G. M. Dotinga A. (2022). Cohort Profile Update: Lifelines, a three-generation cohort study and biobank. Int. J. Epidemiol.51 (5), e295–e302. doi: 10.1093/ije/dyab257
228
Silva-García O. Valdez-Alarcón J. J. Baizabal-Aguirre V. M. (2019). Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front. Immunol.10. doi: 10.3389/FIMMU.2019.02135
229
Silverberg M. J. Neuhaus J. Bower M. Gey D. Hatzakis A. Henry K. et al . (2007). Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS21, 1957–1963. doi: 10.1097/QAD.0B013E3282ED6338
230
Smith K. T. Saveria Campo M. (1988). Hit and run” transformation of mouse c127 cells by Bovine Papillomavirus type 4: The viral DNA is required for the initiation but not for maintenance of the transformed phenotype. Virology164 (1), 39–47. doi: 10.1016/0042-6822(88)90617-4
231
Sohail S. Burns M. B. (2023). Integrating current analyses of the breast cancer microbiome. PloS One18, e0291320. doi: 10.1371/JOURNAL.PONE.0291320
232
Sripa B. Brindley P. J. Mulvenna J. Laha T. Smout M. J. Mairiang E. et al . (2012). The tumorigenic liver fluke Opisthorchis viverrini –multiple pathways to cancer. Trends Parasitol.28, 395. doi: 10.1016/J.PT.2012.07.006
233
Sripa B. Kaewkes S. Sithithaworn P. Mairiang E. Laha T. Smout M. et al . (2007). Liver fluke induces cholangiocarcinoma. PloS Med.4, e201. doi: 10.1371/JOURNAL.PMED.0040201
234
Su Z. Y. Siak P. Y. Leong C. O. Cheah S. C. (2023). The role of Epstein–Barr virus in nasopharyngeal carcinoma. Front. Microbiol.14. doi: 10.3389/FMICB.2023.1116143
235
Tanabe M. B. Caravedo M. A. Cabada M. M. (2024). An update on the pathogenesis of fascioliasis: what do we know? Res. Rep. Trop. Med.15, 13–24. doi: 10.2147/RRTM.S397138
236
Tantengco O. A. G. Aquino I. M. C. de Castro Silva M. Rojo R. D. Abad C. L. R. (2021). Association of mycoplasma with prostate cancer: A systematic review and meta-analysis. Cancer Epidemiol.75, 102021. doi: 10.1016/J.CANEP.2021.102021
237
Tasleem S. Sood G. K. (2015). Hepatitis C associated B-cell non-hodgkin lymphoma: clinical features and the role of antiviral therapy. J. Clin. Transl. Hepatol.3, 134. doi: 10.14218/JCTH.2015.00011
238
Tavakoli A. Monavari S. H. Solaymani Mohammadi F. Kiani S. J. Armat S. Farahmand M. (2020). Association between Epstein-Barr virus infection and gastric cancer: A systematic review and meta-analysis. BMC Cancer20, 1–14. doi: 10.1186/S12885-020-07013-X/FIGURES/5
239
Taylor J. C. Kumar R. Xu J. Xu Y. (2023). A pathogenicity locus of Streptococcus gallolyticus subspecies gallolyticus. Sci. Rep.13, 1 13, 1–12. doi: 10.1038/s41598-023-33178-z
240
Tempera I. Lieberman P. M. (2021). Oncogenic viruses as entropic drivers of cancer evolution. Front. Virol.1. doi: 10.3389/FVIRO.2021.753366/PDF
241
Tie Y. Tang F. Wei Y. Wei X. (2022). Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J. Hematol. Oncol.15, 61. doi: 10.1186/S13045-022-01282-8
242
Toda K. S. Kikuchi L. Chagas A. L. Tanigawa R. Y. Paranaguá-Vezozzo D. C. Pfiffer T. et al . (2015). Hepatocellular carcinoma related to schistosoma mansoni infection: case series and literature review. J. Clin. Transl. Hepatol.3, 260. doi: 10.14218/JCTH.2015.00027
243
Tommasino M. (2017). The biology of beta human papillomaviruses. Virus Res.231, 128–138. doi: 10.1016/j.virusres.2016.11.013
244
Tsoi H. Chu E. S. H. Zhang X. Sheng J. Nakatsu G. Ng S. C. et al . (2017). Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology152, 1419–1433.e5. doi: 10.1053/J.GASTRO.2017.01.009
245
Tsydenova I. A. Ibragimova M. K. Tsyganov M. M. Litviakov N. V. (2023). Human papillomavirus and prostate cancer: systematic review and meta-analysis. Sci. Rep.13, 1–8. doi: 10.1038/s41598-023-43767-7
246
Upadhayay A. Pal D. Kumar A. (2022). Salmonella typhi induced oncogenesis in gallbladder cancer: Co-relation and progression. Adv. Cancer Biol. - Metastasis4, 100032. doi: 10.1016/J.ADCANC.2022.100032
247
Virzì A. Suarez A. A. R. Baumert T. F. Lupberger J. (2018). Oncogenic signaling induced by HCV infection. Viruses10 (10), 538. doi: 10.3390/V10100538
248
Viswanathan S. Parida S. Lingipilli B. T. Krishnan R. Podipireddy D. R. Muniraj N. (2023). Role of gut microbiota in breast cancer and drug resistance. Pathogens12 (3), 468. doi: 10.3390/PATHOGENS12030468
249
von Bülow V. Lichtenberger J. Grevelding C. G. Falcone F. H. Roeb E. Roderfeld M. (2021). Does schistosoma mansoni facilitate carcinogenesis? Cells10, 1982. doi: 10.3390/CELLS10081982
250
Walboomers J. M. M. Jacobs M. V. Manos M. M. Bosch F. X. Kummer J. A. Shah K. V. et al . (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol.189 (1), 12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
251
Wan Q. Tavakoli L. Wang T. Y. Tucker A. J. Zhou R. Liu Q. et al . (2024). Hijacking of nucleotide biosynthesis and deamidation-mediated glycolysis by an oncogenic herpesvirus. Nat. Commun.15, 1–19. doi: 10.1038/s41467-024-45852-5
252
Wang N. Fang J.-Y. (2022). Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol.31 (2), 159–172. doi: 10.1016/j.tim.2022.08.010
253
Wang X. Wu S. Wu W. Zhang W. Li L. Liu Q. et al . (2023a). Candida albicans Promotes Oral Cancer via IL-17A/IL-17RAMacrophage Axis. mBio14, e00447-23. doi: 10.1128/mbio.00447-23
254
Wang X. Zhang W. Wu W. Wu S. Young A. Yan Z. (2023b). Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol. Res.272, 127370. doi: 10.1016/j.micres.2023.127370
255
Warren J. R. Marshall B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet321, 1273–1275. doi: 10.1016/S0140-6736(83)92719-8
256
Wells M. J. Jacobson S. Levine P. H. (2019). An evaluation of HHV-6 as an etiologic agent in Hodgkin lymphoma and brain cancer using IARC criteria for oncogenicity. Infect. Agent Cancer14, 1–12. doi: 10.1186/S13027-019-0248-3/TABLES/1
257
WHO (2022). Cervical cancer. Available online at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed August 21, 2023).
258
Williams V. M. Filippova M. Soto U. Duerksen-Hughes P. J. (2010).). HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Available online at: http://dx.doi.org/10.2217/fvl.10.73.
259
Wonid J. Cho Y. Lee D. Jeon B. Y. Ju J. W. Chung S. et al . (2019). Clonorchis sinensis excretory-secretory products increase Malignant characteristics of cholangiocarcinoma cells in three-dimensional co-culture with biliary ductal plates. PloS Pathog.15, e1007818. doi: 10.1371/JOURNAL.PPAT.1007818
260
Yacoub E. Saed Abdul-Wahab O. M. Al-Shyarba M. H. Ben Abdelmoumen Mardassi B. (2021). The relationship between mycoplasmas and cancer: is it fact or fiction? Narrative review and update on the situation. J. Oncol.2021, 9986550. doi: 10.1155/2021/9986550
261
Yang Y. Gao J. Li H. L. Zheng W. Yang G. Zhang W. et al . (2016). Dose-response association between hepatitis B surface antigen levels and liver cancer risk in Chinese men and women. Int. J. Cancer139 (2), 355–362. doi: 10.1002/ijc.30086
262
Yang Y. Jiang Z. Wu W. Ruan L. Yu C. Xi Y. et al . (2021). Chronic hepatitis virus infection are associated with high risk of gastric cancer: A systematic review and cumulative analysis. Front. Oncol.11. doi: 10.3389/FONC.2021.703558/BIBTEX
263
Yang T. Liu D. Fang S. Ma W. Wang Y. (2022a). Cytomegalovirus and glioblastoma: A review of the biological associations and therapeutic strategies. J. Clin. Med.11, 5221. doi: 10.3390/JCM11175221
264
Yang Y. Long J. Wang C. Blot W. J. Pei Z. Shu X. et al . (2022b). Prospective study of oral microbiome and gastric cancer risk among Asian, African American and European American populations. Int. J. Cancer150 (6), 916–927. doi: 10.1002/ijc.33847
265
Yang X. Shi F. Zhang H. Giang W. A. Kaur A. Chen H. et al . (2024). Long COVID among people with HIV: A systematic review and meta-analysis. HIV Med.26, 6. doi: 10.1111/HIV.13708
266
Yang J. F. You J. (2022). Merkel cell polyomavirus and associated Merkel cell carcinoma. Tumour Virus Res.13, 200232. doi: 10.1016/J.TVR.2021.200232
267
Yarchoan R. Uldrick T. S. (2018). HIV-associated cancers and related diseases. N Engl. J. Med.378, 1029. doi: 10.1056/NEJMRA1615896
268
Yoon S. B. Park J. M. Lim C. H. Cho Y. K. Choi M. G. (2014). Effect of Helicobacter pylori Eradication on Metachronous Gastric Cancer after Endoscopic Resection of Gastric Tumors: A Meta-Analysis. Helicobacter19, 243–248. doi: 10.1111/hel.12146
269
Young L. S. Murray P. G. (2003). Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene22, 5108–5121. doi: 10.1038/SJ.ONC.1206556
270
Yu C. He S. Zhu W. Ru P. Ge X. Govindasamy K. (2023a). Human cytomegalovirus in cancer: the mechanism of HCMV-induced carcinogenesis and its therapeutic potential. Front. Cell Infect. Microbiol.13. doi: 10.3389/FCIMB.2023.1202138
271
Yu R. Huang J. Peng H. Yin S. Xie W. Ren S. et al . (2023b). Association between Hepatitis B virus and gastric cancer: A systematic review and meta-analysis. Infect. Med.2, 67–73. doi: 10.1016/j.imj.2023.04.003
272
Yu S. Jia B. Liu N. Yu D. Zhang S. Wu A. (2021). Fumonisin B1 triggers carcinogenesis via HDAC/PI3K/Akt signalling pathway in human esophageal epithelial cells. Sci. Total Environ.787, 147405. doi: 10.1016/J.SCITOTENV.2021.147405
273
Zaghloul M. S. Zaghloul T. M. Bishr M. K. Baumann B. C. (2020). Urinary schistosomiasis and the associated bladder cancer: update. J. Egypt Natl. Canc Inst32, 1–10. doi: 10.1186/S43046-020-00055-Z/TABLES/1
274
Zambrano A. Kalantari M. Simoneau A. Jensen J. L. Villarreal L. P. (2002). Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. Prostate53, 263–276. doi: 10.1002/pros.10157
275
Zeisel M. B. Guerrieri F. Levrero M. (2021). Host epigenetic alterations and hepatitis B virus-associated hepatocellular carcinoma. J. Clin. Med.10 (8), 1715. doi: 10.3390/JCM10081715
276
Zepeda-Rivera M. Minot S. S. Bouzek H. Wu H. Blanco-Míguez A. Manghi P. et al . (2024). A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature628, 424–432. doi: 10.1038/s41586-024-07182-w
277
Zhang Z. Li D. Li Y. Zhang R. Xie X. Yao Y. et al . (2023). The correlation between Trichomonas vaginalis infection and reproductive system cancer: a systematic review and meta-analysis. Infect. Agent Cancer18 (1), 15. doi: 10.1186/s13027-023-00490-2
278
Zhang X. Lin Y. Xie X. Shen M. Huang G. Yang Y. (2018). Is acne in adolescence associated with prostate cancer risk? Evidence from a meta-analysis. PloS One13 (11), e0206249. doi: 10.1371/JOURNAL.PONE.0206249
279
Zhao M. Chen X. Yang Z. Yang X. Peng Q. (2022). Bacteria and tumor: Understanding the roles of bacteria in tumor genesis and immunology. Microbiol. Res.261, 127082. doi: 10.1016/J.MICRES.2022.127082
280
Zhao L. Y. Mei J. X. Yu G. Lei L. Zhang W. H. Liu K. et al . (2023). Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal Transduction Targeted Ther.8, 1–27. doi: 10.1038/s41392-023-01406-7
281
Zheng H. C. Xue H. Zhang C. Y. (2022). The oncogenic roles of JC polyomavirus in cancer. Front. Oncol.12. doi: 10.3389/FONC.2022.976577/PDF
282
Zhi H. Guo X. Ho Y. K. Pasupala N. Engstrom H. A. A. Semmes O. J. et al . (2020). RNF8 dysregulation and down-regulation during HTLV-1 infection promote genomic instability in adult T-cell leukemia. PloS Pathog.16, e1008618. doi: 10.1371/JOURNAL.PPAT.1008618
283
Zhou L. J. Chen M. Puthiyakunnon S. He C. Xia J. He C. Y. et al . (2019). Toxoplasma gondii ROP18 inhibits human glioblastoma cell apoptosis through a mitochondrial pathway by targeting host cell P2X1. Parasit Vectors12, 1–18. doi: 10.1186/S13071-019-3529-1/FIGURES/10
284
Zhu Q. Ma Y. Liang J. Wei Z. Li M. Zhang Y. et al . (2021). AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther.6 (1), 299. doi: 10.1038/s41392-021-00713-1
285
Zhu H. Shen Z. Luo H. Zhang W. Zhu X. (2016). Chlamydia trachomatis infection-associated risk of cervical cancer: A meta-analysis. Medicine95, e3077. doi: 10.1097/MD.0000000000003077
286
Zhu W. Wang J. Z. Liu Z. Wei J. F. (2022). The bacteria inside human cancer cells: Mainly as cancer promoters. Front. Oncol.12. doi: 10.3389/FONC.2022.897330
287
Zicari S. Sessa L. Cotugno N. Ruggiero A. Morrocchi E. Concato C. et al . (2019). Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART11 (3), 200. doi: 10.3390/V11030200
288
Ziegler P. Tian Y. Bai Y. Abrahamsson S. Bäckerholm A. Reznik A. S. et al . (2021). A primary nasopharyngeal three-dimensional air-liquid interface cell culture model of the pseudostratified epithelium reveals differential donor- And cell type-specific susceptibility to Epstein-Barr virus infection. PloS Pathog.17. doi: 10.1371/journal.ppat.1009041
Summary
Keywords
infectious disease, criteria for microbial oncogenesis, cancer causation, sub-Saharan Africa (SSA), oncovirus and cancer, infectious disease and cancer, microbiome and cancer
Citation
van Dorsten RT and Breiman RF (2025) A landscape review with novel criteria to evaluate microbial drivers for cancer: priorities for innovative research targeting excessive cancer mortality in sub-Saharan Africa. Front. Cell. Infect. Microbiol. 15:1625818. doi: 10.3389/fcimb.2025.1625818
Received
09 May 2025
Accepted
21 July 2025
Published
20 August 2025
Volume
15 - 2025
Edited by
Biagio Barone, ASL Napoli 1 Centro, Italy
Reviewed by
Racheal Shamiso Mandishora, Moffitt Cancer Center, United States
Yun Feng, Shanghai Jiao Tong University, China
Updates
Copyright
© 2025 van Dorsten and Breiman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca Toumi van Dorsten, rebeccavd@nicd.ac.za; Robert F. Breiman, robert.breiman@wits.ac.za
‡ORCID: Rebecca Toumi van Dorsten, orcid.org/0000-0002-1808-1588; Robert F. Breiman, orcid.org/0000-0002-7099-2936
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.