- 1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou, Guangdong, China
Background: Despite advances in understanding sepsis pathophysiology and extensive research, few treatments effectively target its underlying immune dysfunction. Thymosin α1 (Tα1) shows promise as an immunomodulator, but its impact on sepsis remains unclear.
Methods: A search strategy was designed to include any prospective clinical studies using Tα1 for assessing 28-day mortality in patients with sepsis, excluding combination therapy studies. We conducted trial sequential analysis (TSA) to assess the robustness of meta-analyses findings. Heterogeneity of treatment effects (HTE) was conducted based on individual data from two multicenter randomized clinical trials (RCTs), with result credibility assessed through the instrument to assess the credibility of effect modification analyses (ICEMAN).
Results: Out of 3003 identified studies, 11 RCTs met the inclusion criteria (967 patients in Tα1 group and 960 patients in control group). The comprehensive meta-analysis demonstrated a significant reduction in 28-day mortality associated with Tα1 administration (OR 0.73, 95%CI: 0.59-0.90, P = 0.003). Nonetheless, analyses of high-quality (OR 0.82, 95%CI: 0.65-1.03, P = 0.09) and multi-center (OR 0.86, 95%CI: 0.68-1.08, P = 0.20) subgroups did not reveal a mortality benefit. The HTE analysis of multiple subgroups in two large RCTs (representing 75% of the total patients) showed heterogeneity. Potential benefits were noted in subgroups of cancer (moderate credibility), diabetes (low credibility), and coronary heart disease (low credibility). Furthermore, the trial sequential analysis (TSA) suggests that the current sample size is inadequate.
Conclusion: Tα1 has the potential to decrease 28-day mortality rates in patients with sepsis; however, it is crucial to recognize that its efficacy differs among various subgroups. These observations underscore the significance of personalized immunotherapy strategies in forthcoming clinical trials.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024628937.
Introduction
Sepsis is defined as life-threatening organ dysfunction resulting from a dysregulated host response to infection, accounting for almost 20% of all deaths worldwide (Singer et al., 2016; Rudd et al., 2020; Meyer and Prescott, 2024). Dysregulated immune responses serve as critical intermediary between infection and organ dysfunction (van der Poll et al., 2017; Venet and Monneret, 2018; Hotchkiss and Opal, 2020). Immunomodulatory therapy is regarded as one of the most promising approaches to reduce mortality by regulating immune function in sepsis patients (Pei et al., 2018; Torres et al., 2022; Slim et al., 2024; Zhang et al., 2024).
Over 700 studies have investigated various immunomodulatory treatments, including Thymosin α1 (Tα1), granulocyte-macrophage colony-stimulating factor, and interleukin-7 (Slim et al., 2024). Of these, Tα1 is a promising drug with bidirectional modulatory function, exerting multiple effects in infectious diseases, such as promoting naive T cell maturation (Shehadeh et al., 2022), reversing T cell exhaustion (Liu et al., 2020), alleviating cytokine storms (Matteucci et al., 2021; Tian et al., 2025), and enhancing Th1-dependent antifungal immunity (Romani et al., 2004). A previous meta-analysis of 19 studies involving 1354 adult patients suggested that Tα1 might benefit patients with sepsis (Liu F. et al., 2016), However, the subsequent TESTS trial, the largest multicenter, double-blind RCT to date with 1106 patients with sepsis, found no mortality reduction or clinical improvement with Tα1, though elderly and diabetic subgroups showed potential effects (Wu et al., 2025), While the TESTS findings may influence practice, a comprehensive analysis of all available data is needed to establish definitive clinical guidance.
Traditional meta-analyses have predominantly concentrated on data pooling without adequately assessing statistical power. Trial sequential analysis (TSA) addressed this limitation by identifying type I and type II errors, thereby enhancing the reliability of meta-analytic findings (Shah and Smith, 2020). TSA also determines the necessary sample sizes for achieving meaningful outcomes and assesses the potential value of future trials. Furthermore, existing meta-analyses often neglect patient-centered outcomes, which constrains our understanding of how individual patient characteristics influence the efficacy of immunomodulatory therapy in sepsis, often erroneously implying a “one size fits all” approach.
Given these considerations, there is an urgent need for an updated meta-analysis to achieve the following objectives: (1) to synthesize the evidence regarding the efficacy of Tα1 in patients with sepsis overall, and (2) to evaluate the comparative efficacy of Tα1 across various subgroups of septic patients.
Methods
Study design and registration
This is an updated systematic review and meta-analysis aimed to evaluate the efficacy of adding Tα1 therapy compared to conventional therapy alone in reducing the 28-day mortality in patients with sepsis. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 statement (Page et al., 2021). The protocol was registered at PROSPERO International prospective register of systematic reviews, with registration number CRD42024628937.
Searching strategy
We searched for all studies that investigate whether Tα1 could improve the prognosis of sepsis or septic shock patients while excluding COVID-19 pandemic influences. Search terms “thymosin alpha1” or “thymosin” or “thymus” or “Maipuxin” or “thymalfasin” or “Zadaxin” referred to thymosin alpha1 and “severe infection” or “sepsis” or “septic shock” referred to sepsis were used. The strategy was implemented in both English and Chinese databases including PubMed, all databases of Web of Science, Embase, Cochrane library, China National Knowledge Internet (CNKI), China Science and Technology Journal Database (VIP) and Wanfang Database. Registers, websites, organizations, reference lists, preprints, conference literature and other sources were consulted to identify studies comprehensively. Searching strategies in each database were shown in Supplementary Material-search strategy.
The literature search was performed on December 4, 2024 and repeated on January 17, 2025 before final analysis. All the results were imported into EndNote X9 (Clarivate Analytics) software for further selection.
Selection criteria
Inclusion criteria were as follows: (1) adults patients aged over 18 years; (2) reported the 28-day mortality; (3) according to the latest diagnostic criteria, the patient had to be diagnosed with sepsis, severe sepsis or septic shock; (4) Tα1 was the only different treatment in interference group; (5) patients in control group were treated with conventional therapy according to Surviving Sepsis Campaign (SSC) guidelines. The follows were excluded: (1) a review, case report or only abstract; (2) objects were animals or cells; (3) not provided related outcomes; (4) studies had not been completed. (5) key results cannot be extracted; (6) study about COVID-19.
One independent reviewer (BG) evaluated titles and abstracts while two reviewers (YZ and YN) thereafter screened full-text independently. References of the selected studies were also screened by the two reviewers afterwards. Duplicates were removed automatically and then manually using EndNote X9 software. We determined whether the included studies were RCTs or retrospective studies based on the descriptions in the abstracts and methods sections of each article. Disagreement was resolved by discussion with a third reviewer (FP).
Data extraction
Two reviewers independently extracted data into consensual standard table. The following characteristics of included studies were collected: first author, publication year, type of study, implementation period, country and settings, dosage and time of Tα1 use, number of patients, primary and secondary outcomes. Besides, demographic characteristics of patients were also collected including age, gender, inclusion and exclusion criterion, biochemical indicators, 28-day mortality, ICU mortality, length of ICU stay, duration of mechanical ventilation, sequential organ failure assessment (SOFA) score and acute physiology and chronic health evaluation (APACHE) II score.
Any disagreement between the two reviewers was resolved by discussion or consulted with the third reviewer. When encountering self-contradictory data or errors in studies, we also e-mailed the author for more detailed information; if there was no response, the study was excluded.
Quality assessment
The quality of studies included in extracting data process were judged by the Cochrane Collaboration’s tool for assessing risk of bias (Higgins et al., 2011). We used scores to quantify the quality of every study: for each aspect of bias, two scores for low risk of bias, one for unclear risk of bias and zero for high risk of bias (Georgiou et al., 2009; Fan et al., 2012). A total of 7 aspects of bias were judged and summed up. A maximum score of 14 was possible, with 11–14 considered relatively high quality and 0–10 considered relatively low quality. Two reviewers (YZ and BG) independently judged the studies. Disagreement was solved through discussion or consensus meeting with senior investigators. Any study that received a high score was reassessed.
Statistical analysis
For dichotomous variables, odds ratio (OR) and 95% confidence intervals (CI) of every study were calculated. For continuous variables, weighted mean difference was calculated. I2 and chi-squared statistics were applied to estimate heterogeneity. The random-effects model was used if heterogeneity is significant (I2 ≥ 50%), otherwise the fixed-effects model was used (Higgins et al., 2003). Both the Mantel-Haenszel test and inverse-variance (I-V) weighting were applied.
We used Egger’s test to evaluate publication bias and constructed funnel plot if at least ten studies were available for meta-analysis (Sterne et al., 2011). The sensitivity analysis was conducted by taking each single study away from the total and reanalyzing the remaining studies. In addition, we performed the subgroup analyses to identify the source of heterogeneity and the effect of confounding based on the following variables: study quality, study design, dosage and applied SSC guidelines. The significance of the pooled index was determined using the Z test.
To mitigate the risk of misinterpreting random error in meta-analysis, trial sequential analysis (TSA) was performed for 28-day mortality using the TSA software (0.9.5.10 Beta, The Copenhagen Trial Unit, Denmark) (Shah and Smith, 2020). We set conventional test boundary at type I error 5% (two-sided), and dichotomous alpha-spending boundary using O’Brien-Fleming function at the same type I error. Information size was estimated through power of 90%, low bias based relative risk reduction (RRR) and calculated incidence in control group of all included randomized clinical trials (RCTs). Heterogeneity correction was model variance based.
We acquired detailed information to investigate hazard ratio (HR) of subgroups classified by age, gender, cancer, hypertension, diabetes, coronary heart disease and chronic obstructive pulmonary disease of two multi-center, high quality studies (Wu et al., 2013; Wu et al., 2025). Cox regression was used to calculate HR adjusted by different study with 95% CI as well as reporting test of interaction (Wallach et al., 2017). Results of subgroups with P < 0.1 for interaction were graded by Instrument for the Credibility of Effect Modification Analyses (ICEMAN) (Schandelmaier et al., 2020).
A two-tailed P < 0.05 was considered statistically significant. The meta-analysis was done using Stata/MP statistical software (version 14.0), Review Manager software (version 5.3) and SPSS software (version 25.0).
Results
Characteristics of eligible studies
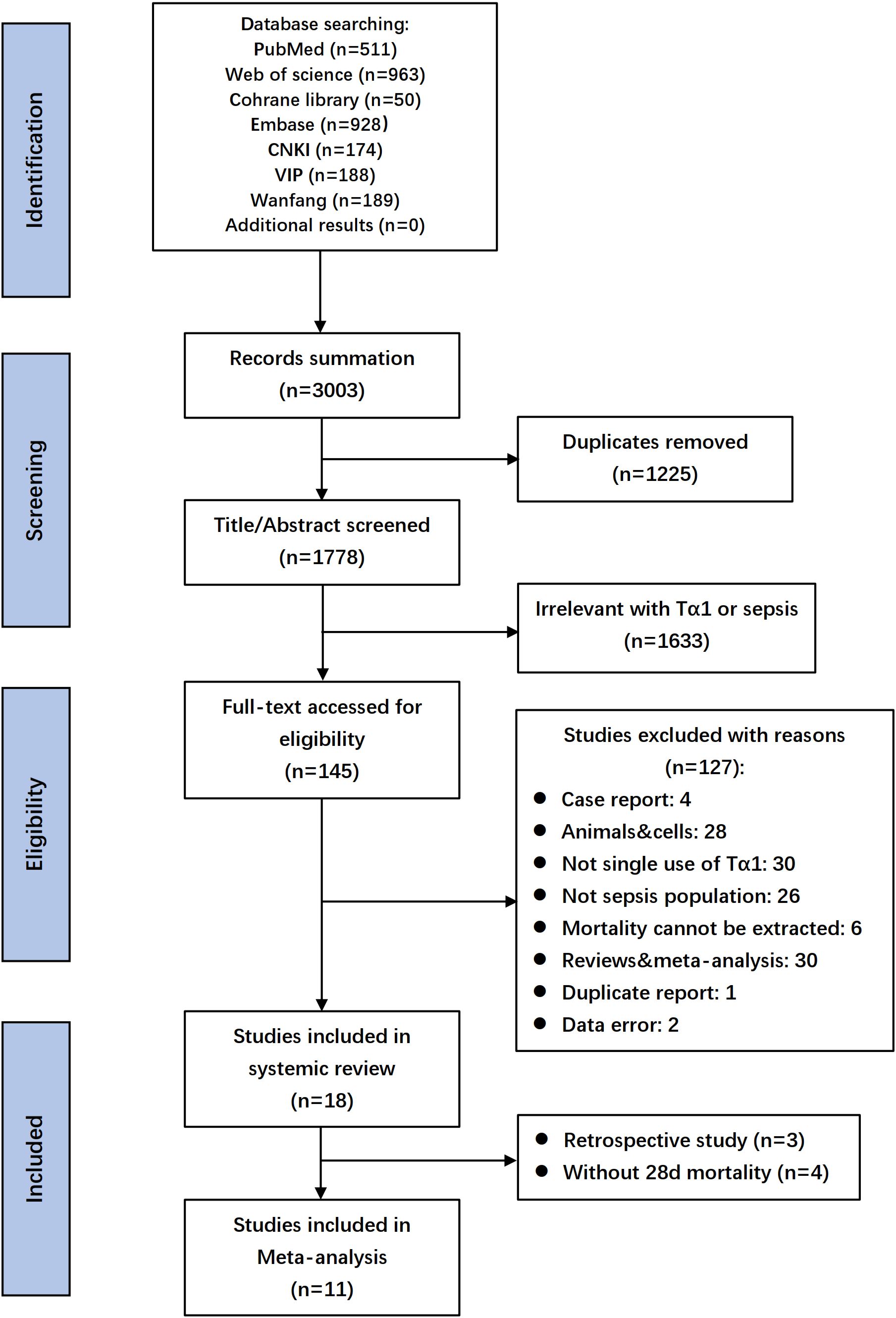
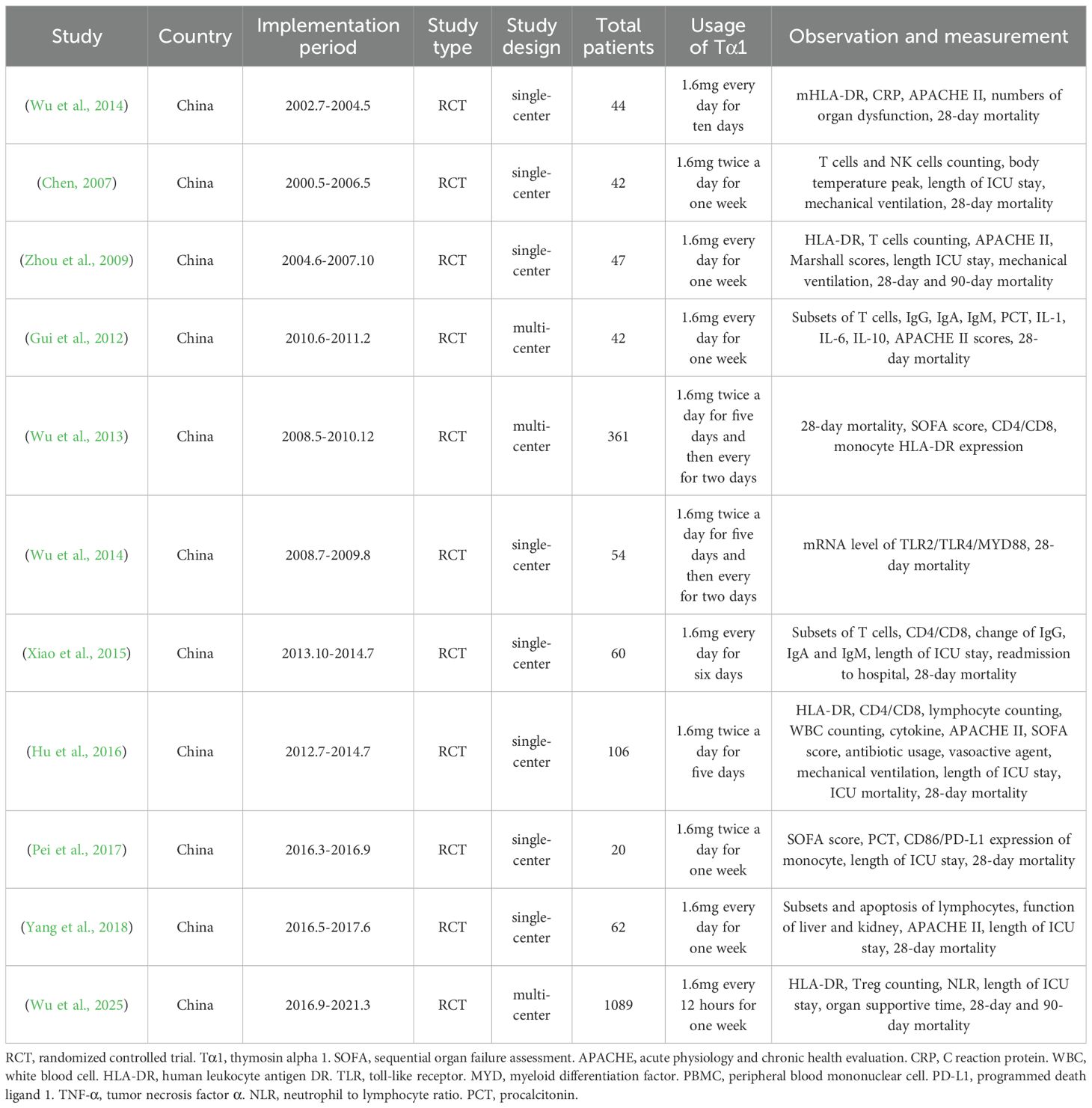
A database search identified 3003 records, with 18 selected after full-text screening. Excluding three retrospective studies and four RCTs lacking 28-day mortality data, 11 RCTs with 1927 patients remained for evaluating Tα1 efficacy in the meta-analysis (Wu and Fang, 2004; Chen, 2007; Zhou et al., 2009; Gui et al., 2012; Wu et al., 2013; Wu et al., 2014; Xiao et al., 2015; Hu et al., 2016; Pei et al., 2017; Yang et al., 2018; Wu et al., 2025). Flow diagram shown the details of screening process (Figure 1), and the characteristics of the included studies were listed in Table 1 and Supplementary Table S1. Summary and details of quality assessment of each RCT were shown in the Supplementary Figure S1. Five studies scored 11 and above were regarded as relatively high quality.
Primary outcome for the 28-day mortality
A total of 1927 septic patients from 11 RCTs, including 967 in Tα1 group and 960 in control group, were evaluated for the 28-day mortality, with 218 and 274 deaths in the Tα1 and control groups, respectively. The results showed that Tα1 therapy significantly reduced 28-day mortality compared to controls (OR 0.73, 95%CI: 0.59-0.90, P = 0.003, Figure 2A). A meta-analysis including four RCTs without 28-day mortality data, showed similar results (Supplementary Figure S2).
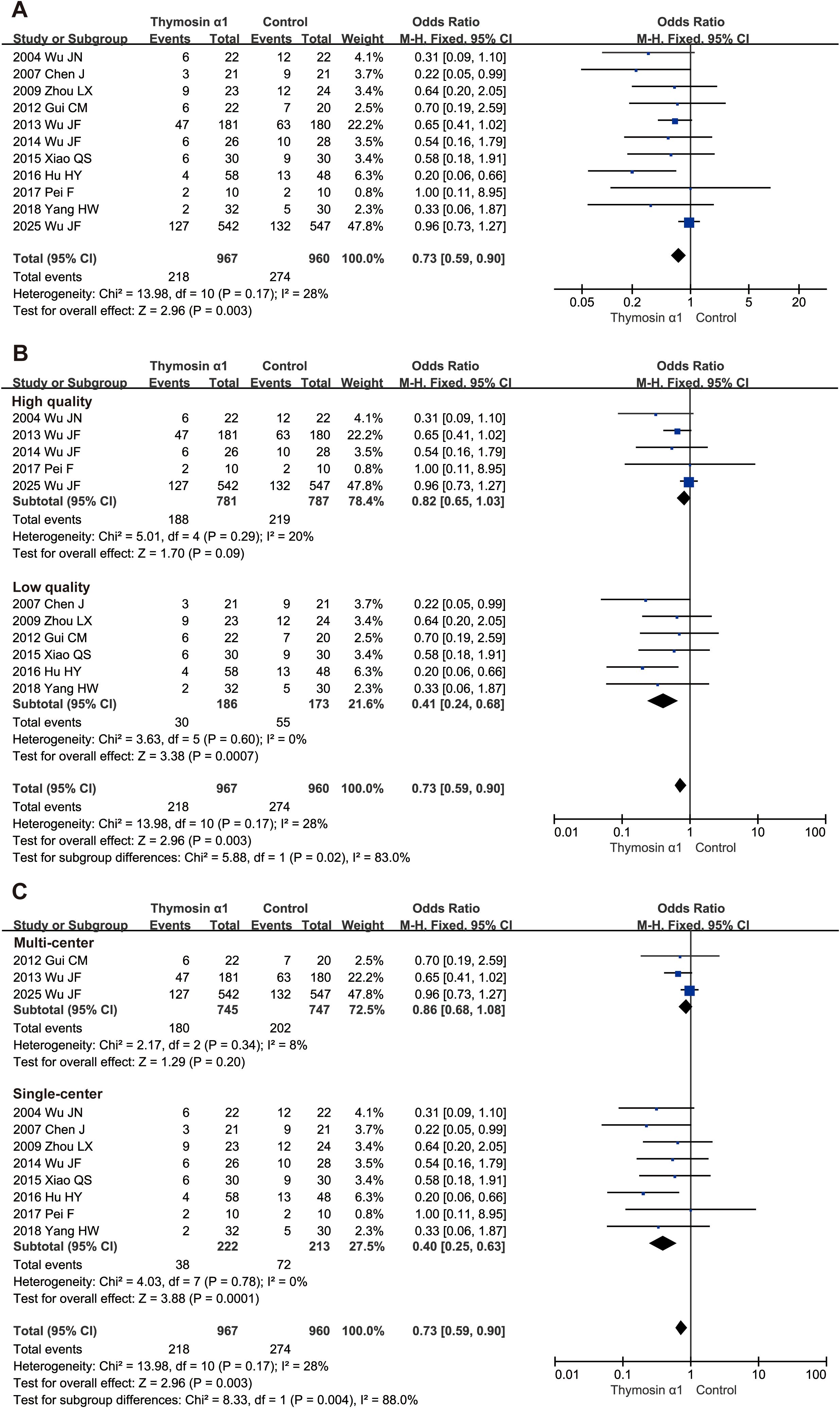
Figure 2. Forest plot of thymosin α1 on 28-day mortality. (A) pooled analysis of 11 RCTs, (B) subgroup analysis of relatively high- and low- quality studies, (C) subgroup analysis of multi-center and single-center studies.
Sensitivity analysis indicated this result was robust although one study had huge impact (Supplementary Figure S3). However, publication bias was evident as shown in the funnel plot (Supplementary Figure S4) and confirmed by Egger’s test (P = 0.01).
Subgroup analysis for primary outcome
We conducted several subgroup analyses at the study level to further examine the primary outcome. First, we categorized studies by quality. Five higher-quality studies (1568 patients with sepsis) showed no significant benefit of Tα1 therapy (OR 0.82, 95% CI: 0.65–1.03, P = 0.09), while six lower-quality studies (359 patients) demonstrated a significant reduction in 28-day mortality (OR 0.41, 95% CI: 0.24–0.68, P = 0.0007, Figure 2B). Subgroup differences were significant (P = 0.02), with low heterogeneity in each subgroup. Second, we grouped studies by design into single-center and multi-center subgroups. Tα1 therapy significantly reduced 28-day mortality in single-center studies (OR 0.40, 95% CI: 0.25–0.63, P = 0.0001) but not in multi-center studies (OR 0.86, 95% CI: 0.68–1.08, P = 0.20, Figure 2C). Subgroup differences were significant (P = 0.004), with low heterogeneity in both subgroups. Third, regardless of whether the drug dosage was twice a day or once a day, the subgroup results both favored Tα1 treatment (OR 0.60, 95% CI: 0.37–0.96, P = 0.03 and OR 0.51, 95% CI: 0.29–0.91, P = 0.02 respectively, Supplementary Figure S5). In addition, two studies which followed sepsis 3.0 diagnosis criterion showed no significant 28-day survival benefit, while sum of the others suggested Tα1 treatment (Supplementary Figure S6).
Then, we also conducted subgroup analyses at the patient level. The heterogeneity of treatment effects (HTE) was analyzed using individual data from two high-quality, multicenter RCTs (Wu et al., 2013; Wu et al., 2025), which including 75% patients of total. By day 28 post-randomization, 174 of 723 patients (24.1%) in the Tα1 group and 195 of 727 patients (26.8%) in the control group had died (HR 0.88, 95% CI: 0.72–1.08, Figure 3). HTE analysis across seven subgroups showed Tα1 improved 28-day survival in septic patients with cancer (HR 0.59, 95% CI: 0.37–0.94, Pinteraction = 0.04; moderate credibility), diabetes (HR 0.64, 95% CI: 0.41–0.98; low credibility) and coronary heart disease (HR 0.56, 95% CI: 0.31–0.99; low credibility) (Figure 3). Detailed HTE analyses for three subgroups are provided in Supplementary Material-ICEMAN reports.

Figure 3. Heterogeneity of treatment effects analysis. Hazard ratio (HR) was adjusted by the studies. COPD, chronic obstructive pulmonary disease.
Furthermore, TSA graphs were presented in Figure 4, which revealed that the current systematic review did not achieve the required information size (RIS) to determine the effect on 28-day mortality. While the cumulative Z-curve from all 11 RCTs crossed conventional meta-analysis boundaries, it did not cross trial sequential boundaries, indicating a risk of false positives and the need for cautious interpretation. For the five higher-quality RCTs, the cumulative Z-curve did not cross conventional meta-analysis, trial sequential, or futility boundaries, underscoring the need for more rigorously designed studies to confirm the efficacy of Tα1 in patient with sepsis.

Figure 4. Trial sequential analysis for 28-day mortality. (A) all included 11 RCTs, (B) five relatively high-quality RCTs. The blue Z-curve represents the pooled odds ratio, with yellow dotted lines indicating conventional meta-analysis boundaries (5% alpha level). Trial sequential boundaries are shown by the symmetric red line above the Z-curve. Between the yellow dotted lines, the triangular futility zone indicates conclusive evidence that treatment effects fail to reach significance. RIS, required information size.
The severity of sepsis
To assess the value of Tα1 in attenuating the disease severity in patients with sepsis, SOFA score and APACHE II score were compared. The pooled results indicated no significant difference in SOFA between Tα1 and control groups (mean difference: -0.38, 95%CI: -1.35 to 0.60, P = 0.45, Supplementary Figure S5). We found Tα1 therapy reduced APACHE II score more significantly compared to control group (mean difference: -2.81, 95%CI: -4.39 to -1.22, P = 0.0005, Supplementary Figure S6).
Discussion
In this updated meta-analysis, we found that thymosin alpha 1 (Tα1) may reduce 28-day mortality in patients with sepsis compared to the control group. However, the reliability of the current evidence remains uncertain because the analysis did not reach the required sample size. Furthermore, both study-level and patient-level subgroup analyses exhibited high heterogeneity, indicating that future clinical trials should adopt personalized treatment strategies instead of a one-size-fits-all approach.
Tα1, as an immunomodulator, has the potential to improve prognosis by reestablishing immune homeostasis (Serafino et al., 2012; Pei et al., 2018; Aynekulu Mersha et al., 2025). In other acute diseases, its anti-inflammatory effects had been studied (Liu et al., 2025; Tian et al., 2025). Previous meta-analyses had suggested that Tα1, whether administrated as monotherapy or in conjunction with anti-inflammatory agents, could reduce mortality among septic patients (Li et al., 2015; Feng et al., 2016; Liu D. et al., 2016; Liu F. et al., 2016; Wang et al., 2016). Approximately a decade ago, Liu and colleagues (Liu F. et al., 2016) conducted an analysis of 10 randomized controlled trials encompassing 530 sepsis patients, proposing that Tα1 treatment might reduce mortality, although this conclusion was constrained by small sample sizes and low quality of evidence. Concurrently, a meta-analysis by Wang et al. (2016) investigated 944 sepsis patients across 6 randomized controlled trials, demonstrating that Tα1, when combined with ulinastatin, could improve short-term survival. Despite these early findings, a recent high-quality, multi-center randomized clinical trial failed to corroborate these results (Wu et al., 2025). This inconsistency prompted us to undertake an updated meta-analysis. Consistent with previous results, the findings of this study endorse the hypothesis that Tα1 reduces mortality in patients with sepsis. However, further TSA analysis reveals that the assertion regarding Tα1’s effect on reducing sepsis mortality remains inconclusive due to an inadequate sample size.
Sepsis presents as a highly heterogeneous clinical syndrome, wherein single therapeutic interventions often exhibit variable efficacy across different patient populations (Seymour et al., 2019; Shah et al., 2021; Pei et al., 2024). In this study, we assessed heterogeneity at two different levels: the study level and the patient level. At the study level, subgroups derived from multi-center studies and those with higher quality did not yield results consistent with the overall findings. This underscores the critical importance of conducting high-quality, multi-center studies in sepsis immunology research. At the patient level, multiple subgroups with chronic diseases also exhibited significant treatment heterogeneity. Although these findings considerably undermine our primary results, it may also indicate potential target populations for Tα1 treatment. As Kalil et al. (2025) stated, stratifying sepsis patients into subphenotypes may aid in elucidating the complexities of sepsis.
Interestingly, our subgroup analysis suggests that patients with chronic conditions may exhibit heightened responsiveness to Tα1 therapy. This observation is in consistent with the immunomodulatory effects of the drug observed in elderly COVID-19 patients (Liu et al., 2020; Yu et al., 2020). Recent studies have showed that Tα1 therapy can benefit elderly COVID-19 patients by modulating T lymphocyte responses, specifically by increasing CD4+ and CD8+ T cell populations while preventing excessive activation of CD8+ T cells (Liu et al., 2020; Yu et al., 2020). The increasing prevalence of chronic diseases introduces additional complexity to sepsis treatment (Zheng et al., 2018; Zhou et al., 2021). These conditions can cause sustained damage to the immune system and may exacerbate the already compromised immune function in sepsis patients, potentially leading to immunosuppression (Mian et al., 2014; Pene et al., 2016; Frydrych et al., 2017; Trevelin et al., 2017; Mikolajczyk and Guzik, 2019). Although our findings suggest that patients with chronic conditions might be an appropriate target population for Tα1 treatment, this hypothesis requires validation through high-quality clinical trials before definitive conclusions can be established.
While we have compared different administration methods of Tα1, the optimal dosage for sepsis treatment remains undetermined. Lin (2007) identified a dose-dependent effect of Tα1, with administration twice daily resulting a prognostic improvement. A subgroup analysis within this study revealed that both once-daily and twice-daily dosing reduced 28-day mortality, suggesting that twice-daily administration was safe. In addition, similarly to the therapeutic dose, there is insufficient evidence regarding the optimal therapeutic duration of Tα1. Current clinical studies have set the therapeutic course at seven days, which may facilitate study implementation. However, the restoration of immune function in sepsis patients is a prolonged process, with some patients experiencing immune imbalance for three weeks or longer (Inoue et al., 2013; Stortz et al., 2018). Consequently, real-world studies are necessary to further ascertain the appropriate therapeutic duration.
Strengths and limitations
The present study has several notable strengths. First, our comprehensive systematic search and rigorous quality assessment, alongside the exclusive inclusion of RCTs, have enhanced the reliability of our findings. Second, we have innovatively applied TSA to evaluate the efficacy of Tα1, thereby providing valuable insights for future trial design. Third, our heterogeneity analyses, which utilizes individual patient data from two high-quality multicenter RCTs (accounting for 75% of the total study population) and is validated by ICEMAN, offers a rigorous evaluation of potential effect modifications. These methodological strengths collectively render our study the most rigorous evaluation of Tα1 treatment in sepsis to date.
However, this meta-analysis also has several limitations that should be acknowledged. First, all included studies were conducted in China, raising concerns about the generalizability of the findings to other racial or ethnic populations. Second, the potential for publication bias cannot be ruled out, particularly due to the influence of the recent TESTS study (Wu et al., 2025). Third, a significant limitation of the included studies lies in their small sample sizes. Ten out of fourteen studies enrolled fewer than 100 participants, potentially diminishing the statistical power and reliability of the results. Fourth, this analysis exclusively considered 28-day mortality as the primary outcome measure, due to incomplete or inconsistently reported data on ICU and in-hospital across many studies. We strongly recommended that future randomized controlled trials implement standardized and comprehensive outcome reporting. Fifth, to specifically assess the effect of Tα1, combination therapies (e.g., ulinastatin or continuous renal replacement therapy) were excluded, which may limit the applicability of our findings to real-world clinical settings where such combination therapies are prevalent. Sixth, the diagnostic criteria for sepsis varied across the included studies. Over the past two decades, sepsis definitions have been undergone multiple refinements, leading to inconsistencies in diagnosis and treatment strategies, which may contribute to heterogeneity in the results.
Conclusion
Recent evidence suggests that Tα1 may reduce 28-day mortality in patients with sepsis. However, it is important to emphasize that the current sample size is insufficient to confirm this conclusion. Furthermore, the efficacy of Tα1 varies significantly among different subgroups, suggesting that uniform clinical trials across the entire sepsis population may not be appropriate. Future research in immunotherapy should focus on developing personalized treatment strategies to improve therapeutic efficacy and patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
BG: Resources, Funding acquisition, Writing – review & editing, Writing – original draft, Conceptualization, Validation, Project administration, Data curation, Supervision, Investigation. YZ: Formal Analysis, Methodology, Visualization, Writing – review & editing, Writing – original draft, Data curation, Software. YN: Writing – review & editing, Supervision, Methodology, Validation, Data curation, Visualization, Project administration, Formal Analysis. LW: Supervision, Software, Writing – review & editing, Methodology, Resources, Project administration. LL: Investigation, Writing – original draft, Software, Data curation, Methodology, Project administration, Formal Analysis. ZL: Writing – review & editing, Investigation, Resources, Validation. JYW: Writing – review & editing, Formal Analysis, Project administration, Data curation. XG: Supervision, Conceptualization, Funding acquisition, Writing – review & editing, Resources. MC: Writing – review & editing, Supervision, Validation, Conceptualization, Resources. JFW: Funding acquisition, Resources, Conceptualization, Validation, Writing – review & editing, Supervision. FP: Conceptualization, Investigation, Resources, Funding acquisition, Formal Analysis, Methodology, Supervision, Data curation, Writing – original draft, Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This manuscript was supported by the National Natural Science Foundation of China (Grant No. 82302415 to FP, No. 82472221 to JW, No. 82002076 to BG and No.82272186 to XG), the Sun Yat-sen University Clinical Research Program 5010 (Grant No. 2024006 to JW and No. 2019002 to XG), Guangdong Provincial Enterprise Joint Foundation for Basic and Applied Basic Research Key program Project (Grant No. 2023B1515230005 to JW and No. 2024B1515230008 to XG) and the Guangdong Clinical Research Center for Critical Care Medicine (Grant No.2020B1111170005 to XG).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1673959/full#supplementary-material
References
Aynekulu Mersha, D. G., Fromme, S. E., van Boven, F., Arteaga-Henriquez, G., Wijkhuijs, A., van der Ent, M., et al. (2025). Indications for an antidepressive effect of thymosin alpha-1 in a small open-label proof of concept study in common variable immune deficiency patients with depression. Brain Behav. Immun. Health 43, 100934. doi: 10.1016/j.bbih.2024.100934
Chen, J. (2007). Effects of thymosin-alpha1 on cell immunity function in patients with septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 19, 153–155. doi: 10.3760/j.issn:1003-0603.2007.03.008
Fan, X., Lin, T., Xu, K., Yin, Z., Huang, H., Dong, W., et al. (2012). Laparoendoscopic single-site nephrectomy compared with conventional laparoscopic nephrectomy: a systematic review and meta-analysis of comparative studies. Eur. Urol 62, 601–612. doi: 10.1016/j.eururo.2012.05.055
Feng, Z., Shi, Q., Fan, Y., Wang, Q., and Yin, W. (2016). Ulinastatin and/or thymosin alpha1 for severe sepsis: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 80, 335–340. doi: 10.1016/j.intimp.2015.10.026
Frydrych, L. M., Fattahi, F., He, K., Ward, P. A., and Delano, M. J. (2017). Diabetes and sepsis: risk, recurrence, and ruination. Front. Endocrinol. (Lausanne) 8, 271. doi: 10.3389/fendo.2017.00271
Georgiou, P., Tan, E., Gouvas, N., Antoniou, A., Brown, G., Nicholls, R. J., et al. (2009). Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 10, 1053–1062. doi: 10.1016/s1470-2045(09)70224-4
Gui, C., Ai, Y., and Zhang, L. (2012). Effectiveness of thymosin α1 therapy and its effects on immune function in the patients with sepsis. Chin. J. Crit. Care Med. 32, 255–258. doi: 10.3969/j.issn.1002-1949.2012.03.018
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi: 10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hotchkiss, R. S. and Opal, S. M. (2020). Activating immunity to fight a foe - A new path. N Engl. J. Med. 382, 1270–1272. doi: 10.1056/NEJMcibr1917242
Hu, H., Huang, S., Zhu, Z., Xiong, F., and Liu, W. (2016). The clinical efficiency analysis of thymosin-α1 in treating sepsis. Chin. J. Crit. Care Med. 36, 397–400. doi: 10.3969/j.issn.1002-1949.2016.05.004
Inoue, S., Suzuki-Utsunomiya, K., Okada, Y., Taira, T., Iida, Y., Miura, N., et al. (2013). Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit. Care Med. 41, 810–819. doi: 10.1097/CCM.0b013e318274645f
Kalil, A. C., Povoa, P., and Leone, M. (2025). Subphenotypes and phenotypes to resolve sepsis heterogeneity: hype or hope? Intensive Care Med. 51, 582–584. doi: 10.1007/s00134-025-07828-x
Li, C., Bo, L., Liu, Q., and Jin, F. (2015). Thymosin alpha1 based immunomodulatory therapy for sepsis: a systematic review and meta-analysis. Int. J. Infect. Dis. 33, 90–96. doi: 10.1016/j.ijid.2014.12.032
Lin, H. Y. (2007). Clinical trial with a new immunomodulatory strategy: treatment of severe sepsis with Ulinastatin and Maipuxin. Natl. Med. J. China 87, 451–457. doi: 10.3760/j:issn:0376-2491.2007.07.005
Liu, Y., Pang, Y., Hu, Z., Wu, M., Wang, C., Feng, Z., et al. (2020). Thymosin alpha 1 (Talpha1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin. Infect. Dis. 71 (16), 2150–2157. doi: 10.1093/cid/ciaa630
Liu, H., Qian, S. C., Zhang, Y. Y., Tang, C. B., Yue, H. H., Fan, G. L., et al. (2025). Effect of thymosin alpha1 on Immune response and organ function in acute aortic dissection surgery: PANDA II trial protocol. Future Cardiol. 21, 447–454. doi: 10.1080/14796678.2025.2505401
Liu, F., Wang, H. M., Wang, T., Zhang, Y. M., and Zhu, X. (2016). The efficacy of thymosin alpha1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials. BMC Infect. Dis. 16, 488. doi: 10.1186/s12879-016-1823-5
Liu, D., Yu, Z., Yin, J., Chen, Y., Zhang, H., Xin, F., et al. (2016). Effect of ulinastatin combined with thymosin alpha1 on sepsis: A systematic review and meta-analysis of Chinese and Indian patients. J. Crit. Care 39, 259–266. doi: 10.1016/j.jcrc.2016.12.013
Matteucci, C., Minutolo, A., Balestrieri, E., Petrone, V., Fanelli, M., Malagnino, V., et al. (2021). Thymosin alpha 1 mitigates cytokine storm in blood cells from coronavirus disease 2019 patients. Open Forum Infect. Dis. 8, ofaa588. doi: 10.1093/ofid/ofaa588
Meyer, N. J. and Prescott, H. C. (2024). Sepsis and septic shock. N Engl. J. Med. 391, 2133–2146. doi: 10.1056/NEJMra2403213
Mian, M. O., Paradis, P., and Schiffrin, E. L. (2014). Innate immunity in hypertension. Curr. Hypertens. Rep. 16, 413. doi: 10.1007/s11906-013-0413-9
Mikolajczyk, T. P. and Guzik, T. J. (2019). Adaptive immunity in hypertension. Curr. Hypertens. Rep. 21, 68. doi: 10.1007/s11906-019-0971-6
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi: 10.1136/bmj.n71
Pei, F., Gu, B., Miao, S. M., Guan, X. D., and Wu, J. F. (2024). Clinical practice of sepsis-induced immunosuppression: Current immunotherapy and future options. Chin. J. Traumatol 27, 63–70. doi: 10.1016/j.cjtee.2023.11.001
Pei, F., Guan, X., and Wu, J. (2018). Thymosin alpha 1 treatment for patients with sepsis. Expert Opin. Biol. Ther. 18, 71–76. doi: 10.1080/14712598.2018.1484104
Pei, F., Nie, Y., Si, X., Jiang, Z., Guan, X., Chen, J., et al. (2017). Thymosin alpha 1 reduces the expression of PD-L1 on monocyte of septic patients. J. Trop. Med. 17, 289–292+310. doi: 10.3969/j.issn.1672-3619.2017.03.003
Pene, F., Pickkers, P., and Hotchkiss, R. S. (2016). Is this critically ill patient immunocompromised? Intensive Care Med. 42, 1051–1054. doi: 10.1007/s00134-015-4161-y
Romani, L., Bistoni, F., Gaziano, R., Bozza, S., Montagnoli, C., Perruccio, K., et al. (2004). Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood 103, 4232–4239. doi: 10.1182/blood-2003-11-4036
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211. doi: 10.1016/s0140-6736(19)32989-7
Schandelmaier, S., Briel, M., Varadhan, R., Schmid, C. H., Devasenapathy, N., Hayward, R. A., et al. (2020). Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. Cmaj 192, E901–e906. doi: 10.1503/cmaj.200077
Serafino, A., Pierimarchi, P., Pica, F., Andreola, F., Gaziano, R., Moroni, N., et al. (2012). Thymosin α1 as a stimulatory agent of innate cell-mediated immune response. Ann. N Y Acad. Sci. 1270, 13–20. doi: 10.1111/j.1749-6632.2012.06707.x
Seymour, C. W., Kennedy, J. N., Wang, S., Chang, C. H., Elliott, C. F., Xu, Z., et al. (2019). Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. Jama 321, 2003–2017. doi: 10.1001/jama.2019.5791
Shah, F. A., Meyer, N. J., Angus, D. C., Awdish, R., Azoulay, É., Calfee, C. S., et al. (2021). A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: an official American thoracic society research statement. Am. J. Respir. Crit. Care Med. 204, 891–901. doi: 10.1164/rccm.202108-1908ST
Shah, A. and Smith, A. F. (2020). Trial sequential analysis: adding a new dimension to meta-analysis. Anaesthesia 75, 15–20. doi: 10.1111/anae.14705
Shehadeh, F., Benitez, G., Mylona, E. K., Tran, Q. L., Tsikala-Vafea, M., Atalla, E., et al. (2022). A pilot trial of thymalfasin (Tα1) to treat hospitalized patients with hypoxemia and lymphocytopenia due to COVID-19 infection. J. Infect. Dis. 227 (2), 226–235. doi: 10.1093/infdis/jiac362
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810. doi: 10.1001/jama.2016.0287
Slim, M. A., van Mourik, N., Bakkerus, L., Fuller, K., Acharya, L., Giannidis, T., et al. (2024). Towards personalized medicine: a scoping review of immunotherapy in sepsis. Crit. Care 28, 183. doi: 10.1186/s13054-024-04964-6
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343, d4002. doi: 10.1136/bmj.d4002
Stortz, J. A., Murphy, T. J., Raymond, S. L., Mira, J. C., Ungaro, R., Dirain, M. L., et al. (2018). Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock 49, 249–258. doi: 10.1097/SHK.0000000000000981
Tian, Y., Yao, J., Ma, Y., Zhang, P., Zhou, X., Xie, W., et al. (2025). Thymosin alpha 1 alleviates inflammation and prevents infection in patients with severe acute pancreatitis through immune regulation: a systematic review and meta-analysis. Front. Immunol. 16. doi: 10.3389/fimmu.2025.1571456
Torres, L. K., Pickkers, P., and van der Poll, T. (2022). Sepsis-induced immunosuppression. Annu. Rev. Physiol. 84, 157–181. doi: 10.1146/annurev-physiol-061121-040214
Trevelin, S. C., Carlos, D., Beretta, M., da Silva, J. S., and Cunha, F. Q. (2017). Diabetes mellitus and sepsis: A challenging association. Shock 47, 276–287. doi: 10.1097/shk.0000000000000778
van der Poll, T., van de Veerdonk, F. L., Scicluna, B. P., and Netea, M. G. (2017). The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17, 407–420. doi: 10.1038/nri.2017.36
Venet, F. and Monneret, G. (2018). Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14, 121–137. doi: 10.1038/nrneph.2017.165
Wallach, J. D., Sullivan, P. G., Trepanowski, J. F., Sainani, K. L., Steyerberg, E. W., and Ioannidis, J. P. (2017). Evaluation of evidence of statistical support and corroboration of subgroup claims in randomized clinical trials. JAMA Intern. Med. 177, 554–560. doi: 10.1001/jamainternmed.2016.9125
Wang, F. Y., Fang, B., Qiang, X. H., Yu, T. O., Zhong, J. R., Cao, J., et al. (2016). The efficacy and immunomodulatory effects of ulinastatin and thymosin α1 for sepsis: A systematic review and meta-analysis. BioMed. Res. Int. 2016, 9508493. doi: 10.1155/2016/9508493
Wu, J. and Fang, Q. (2004). Immune regulation mechanism of thymosin - α1 on severe sepsis patients. Chin. J. Crit. Care Med. 11), 39–40. doi: 10.3969/j.issn.1002-1949.2004.11.015
Wu, J., Pei, F., Zhou, L., Li, W., Sun, R., Li, Y., et al. (2025). The efficacy and safety of thymosin α1 for sepsis (TESTS): multicentre, double blinded, randomised, placebo controlled, phase 3 trial. Bmj 388, e082583. doi: 10.1136/bmj-2024-082583
Wu, J., Tang, Z., Chen, J., Ouyang, B., Chen, M., and Guan, X. (2014). Changes of TLR2, TLR4 and MyD88 mRNA expressions on peripheral blood mononuclear cell in severe sepsis patients during treatment with Thymosin α1. Chin. Arch. Gen. Surg (Electronic Edition) 8, 270–274. doi: 10.3877/cma.j.issn.1674-0793.2014.04.004
Wu, J., Zhou, L., Liu, J., Ma, G., Kou, Q., He, Z., et al. (2013). The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit. Care 17, R8. doi: 10.1186/cc11932
Xiao, Q., Ma, M., Zhang, X., Deng, M., and Yang, Y. (2015). Effect of acupuncture on prognosis and immune function of sepsis patients. Chin. J. Integrated Tradit Western Med. 35, 783–786. doi: 10.7661/CJIM.2015.07.0783
Yang, H., Yu, Q., and Ye, X. (2018). Effect of thymosin on apoptosis and clinical efficacy of peripheral blood lymphocytes in patients with sepsis. Jiangxi Med. J. 53, 670–673. doi: 10.3969/j.issn.1006-2238.2018.7.006
Yu, K., He, J., Wu, Y., Xie, B., Liu, X., Wei, B., et al. (2020). Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 30, 814–816. doi: 10.1038/s41422-020-0391-9
Zhang, H., Dong, N., and Yao, Y. (2024). Optimal strategy for treatment of sepsis based on the host inflammatory reaction and immune response. J. Intensive Med. 4, 175–180. doi: 10.1016/j.jointm.2023.10.002
Zheng, Y., Ley, S. H., and Hu, F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98. doi: 10.1038/nrendo.2017.151
Zhou, B., Perel, P., Mensah, G. A., and Ezzati, M. (2021). Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 18, 785–802. doi: 10.1038/s41569-021-00559-8
Keywords: sepsis, thymosin α1, personalized immunotherapy, heterogeneity of treatment effects, trial sequential analysis
Citation: Gu B, Zhou Y, Nie Y, Wang L, Liang L, Liao Z, Wen J, Guan X, Chen M, Wu J and Pei F (2025) Efficacy of thymosin α1 for sepsis: a systematic review and meta-analysis of randomized controlled trials. Front. Cell. Infect. Microbiol. 15:1673959. doi: 10.3389/fcimb.2025.1673959
Received: 30 July 2025; Accepted: 22 August 2025;
Published: 03 September 2025.
Edited by:
Georgia Damoraki, National and Kapodistrian University of Athens, GreeceReviewed by:
Marcos Edgar Herkenhoff, Santa Catarina State University, BrazilGawel Solowski, Bingöl University, Türkiye
Copyright © 2025 Gu, Zhou, Nie, Wang, Liang, Liao, Wen, Guan, Chen, Wu and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minying Chen, Y2hlbm1pbnlAbWFpbC5zeXN1LmVkdS5jbg==; Jianfeng Wu, d3VqaWFuZkBtYWlsLnN5c3UuZWR1LmNu; Fei Pei, cGVpZjNAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Bin Gu1,2†
Bin Gu1,2† Yu Zhou
Yu Zhou Xiangdong Guan
Xiangdong Guan Jianfeng Wu
Jianfeng Wu Fei Pei
Fei Pei