- 1Nursing Department, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Pharmacy, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 3Department of Pharmacy, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China
- 4Department of Pharmacy, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 5Department of Pharmacy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 6Research Center for Clinical Pharmacy, College of Pharmaceutical Science, Zhejiang University, Hangzhou, China
Objective: On time administration of carbapenem is important, but there are few data concerning the prevalence of timeliness and its impact on pharmacokinetic/pharmacodynamic (PK/PD) target achievement in hospitalized patients.
Methods: This was a multicenter retrospective study. Inpatients who received imipenem or meropenem for more than 3 consecutive days were included according to preset criteria. Patient information and dosing information were collected. The actual administration intervals were compared to the scheduled intervals, and those within a 1-hour error interval were defined as standard time window administration (STWA); otherwise, they were defined as noncompliant time window administration (NTWA). The 100% fT>MIC and 100% fT>4×MIC targets were applied for PK/PD target attainment analysis. A multivariable logistic regression model was used to identify independent risk factors associated with timely administration and the PK/PD target attainment rate.
Results: A total of 474 patients and 1,372 actual administration intervals were included in this study. Among these patients, 82 had drug concentration data and were analyzed for PK/PD target attainment. A total of 427 dosing intervals (31.12%) complied with the standard time window and were STWA, whereas 945 (68.88%) were NTWA. Weekend, night shift, and scheduled dosing intervals were found to be independent influencing factors for STWA. STWA was an independent influencing factor for the 100% fT>MIC and 100% fT>4×MIC target attainment rates.
Conclusion: Our results indicate a low rate of on time carbapenem administration. The on time carbapenem administration was a risk factor for PK/PD target attainment and should be well controlled in clinical practice.
1 Introduction
Carbapenems, mainly meropenem and imipenem–cilastatin, exert rapid bactericidal effects by binding to penicillin-binding proteins (PBPs) and inhibiting bacterial cytoderm synthesis. They have good activity against gram-positive bacteria, gram-negative bacteria, and multidrug-resistant (MDR) pathogens (e.g., extended-spectrum β-lactamase-producing gram-negative bacteria) (Mehta et al., 2021; Gerges et al., 2023). According to pharmacokinetic/pharmacodynamic (PK/PD) principles, carbapenems are classified as time-dependent antibiotics, with clinical efficacy strongly correlated with the time fraction of the free drug concentration exceeding the bacterial minimum inhibitory concentration (MIC) during the dosing interval, expressed as fT>MIC (Gatti et al., 2021; Maguigan et al., 2021; Cizmarova et al., 2024). Evidence indicates that achieving fT>MIC ≥ 40% serves as the baseline efficacy threshold, whereas critically ill patients require intensified targets of 100% fT>1-4×MIC to optimize therapeutic outcomes and mitigate resistance development (Heffernan et al., 2018; Guilhaumou et al., 2019; Beganovic et al., 2021; Eslami et al., 2025). Thus, PK/PD target attainment is crucial for clinical effectiveness. Several previous articles have revealed that continuous infusion of carbapenem, a dosing strategy that can increase the PK/PD index, can significantly improve the clinical outcomes of critically ill patients but has no effect on the outcomes of noncritically ill patients (Phe et al., 2020; Liebchen et al., 2021; Wu et al., 2021; Angelini et al., 2023).
Antibiotic dosing intervals are typically designed on the basis of drug half-life and PK/PD models to maintain the required plasma concentrations or drug exposures (Rodríguez-Gascón et al., 2021). Standard time window administration (STWA) is consistent with absolute deviations between actual and scheduled dosing intervals ≤ 1 hour. The administration time being in accordance with the STWA is considered timely (Loput et al., 2022). Non-timely administered drugs may increase fluctuations in the plasma drug concentration and may lower PK/PD target attainment. However, deviations from scheduled dosing intervals frequently occur in clinical practice because of fluctuations in the nursing workload and individual differences (Blignaut et al., 2017; Schutijser et al., 2018; Martyn et al., 2019; Stolic et al., 2022; Allison Rout et al., 2023). However, this issue is often overlooked, and there are few data concerning the prevalence of on time administration of carbapenem, as well as its impact on PK/PD target attainment.
Therefore, we performed this multicenter retrospective study to assess the patterns of administration timeliness for carbapenems, identify risk factors associated with STWA, and further investigate whether on time administration is an independent risk factor for PK/PD target achievement.
2 Methods
2.1 Study design and ethical approval
This multicenter retrospective study was performed in 3 tertiary hospitals in China. The study protocol was approved by the ethics committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (reference number 2025-0136). Informed consent was waived as part of the approval.
2.2 Patient inclusion
Patients who were hospitalized at 3 research centers from August 2021 to March 2025 and used carbapenem drugs were randomly selected for inclusion. The exclusion criteria were as follows: (a) aged under 18 years; (b) received carbapenem therapy for ≤ 3 consecutive days; (c) had fewer than two consecutive records of dosing timestamps; (d) received carbapenem for urinary infections; and (e) incomplete or missing key clinical data.
2.3 Data collection
The following clinical data of the included patients were extracted from the hospital information system: (a) Demographic data, including patient sex, age, weight (kg), Body Mass Index (BMI, kg/m²), and admission department (ICU or non-ICU). (b) Disease severity indicators: vasoactive agent use. (c) Infection information: infection site and pathogen culture results, if available. (d) Carbapenem treatment regimens: drugs (including imipenem–cilastatin and meropenem), dosages, scheduled dosing intervals and treatment durations. (e) Actual administration time: for patients without carbapenem TDM, the actual administration times of carbapenem on the third day of treatment and the time of the last dose of the previous day were recorded. Thus, two to five consecutive dosing intervals can be calculated. For patients with TDM, the dosing intervals of the day TDM was performed were recorded. To analyze the influence of working time on timely administration, weekends and night shifts (as long as the dosing interval included one administration from 10:00 pm to 8:00 am, it was recorded as a night shift) were also recorded.
For patients with carbapenem TDM, additional information was collected: (a) Paired actual administration timestamps. (b) Continuous renal replacement therapy (CRRT) implementation status. (c) Laboratory biomarkers: trough concentration of target drug, red blood corpuscles (RBCs), total protein (TP), albumin (ALB), total bilirubin (TBil), blood urea nitrogen (BUN), creatinine (Cr), high-sensitivity C-reactive protein (hs-CRP), and procalcitonin (PCT).
2.4 Data analysis
The administration time deviation was operationally defined as the absolute deviation between the actual and scheduled dosing intervals. Deviations ≤ 1 hour were categorized as STWA, whereas those exceeding this threshold were classified as nonstandard time window administration (NTWA).
In the analysis of the influence of timely administration on PK/PD target attainment, only patients with delayed administration were classified into the NTWA group, and those with early administration were excluded from the analysis. Through comprehensive data integration of patient demographics, carbapenem regimens, and measured drug concentrations, we evaluated the impact of timely administration on target (100% fT>MIC and 100% fT>4×MIC) attainment rates. For the convenience of statistical analysis, an MIC breakpoint of 2 mg/L was used (Bonnin et al., 2022; Fratoni et al., 2024; Beijer et al., 2025; Luo et al., 2025).
Categorical variables are described as counts and percentages and were tested by the Pearson χ2 test or Fisher’s exact test. Normally distributed variables are described as the means with SDs and were compared via the independent t-test or 1-way analysis of variance, whereas other continuous variables are described as medians with quartiles and were tested via the Wilcoxon Mann–Whitney U test or the Kruskal–Wallis test. To determine factors independently associated with the timeliness of drug administration, variables with P < 0.1 in the univariate analysis were analyzed by multivariable logistic regression, and a P value < 0.05 was considered significant. Relative risk was estimated via odds ratios (ORs) with corresponding 95% confidence intervals. All the statistical tests were performed via SPSS software (version 23.0).
3 Results
3.1 Clinical characteristics
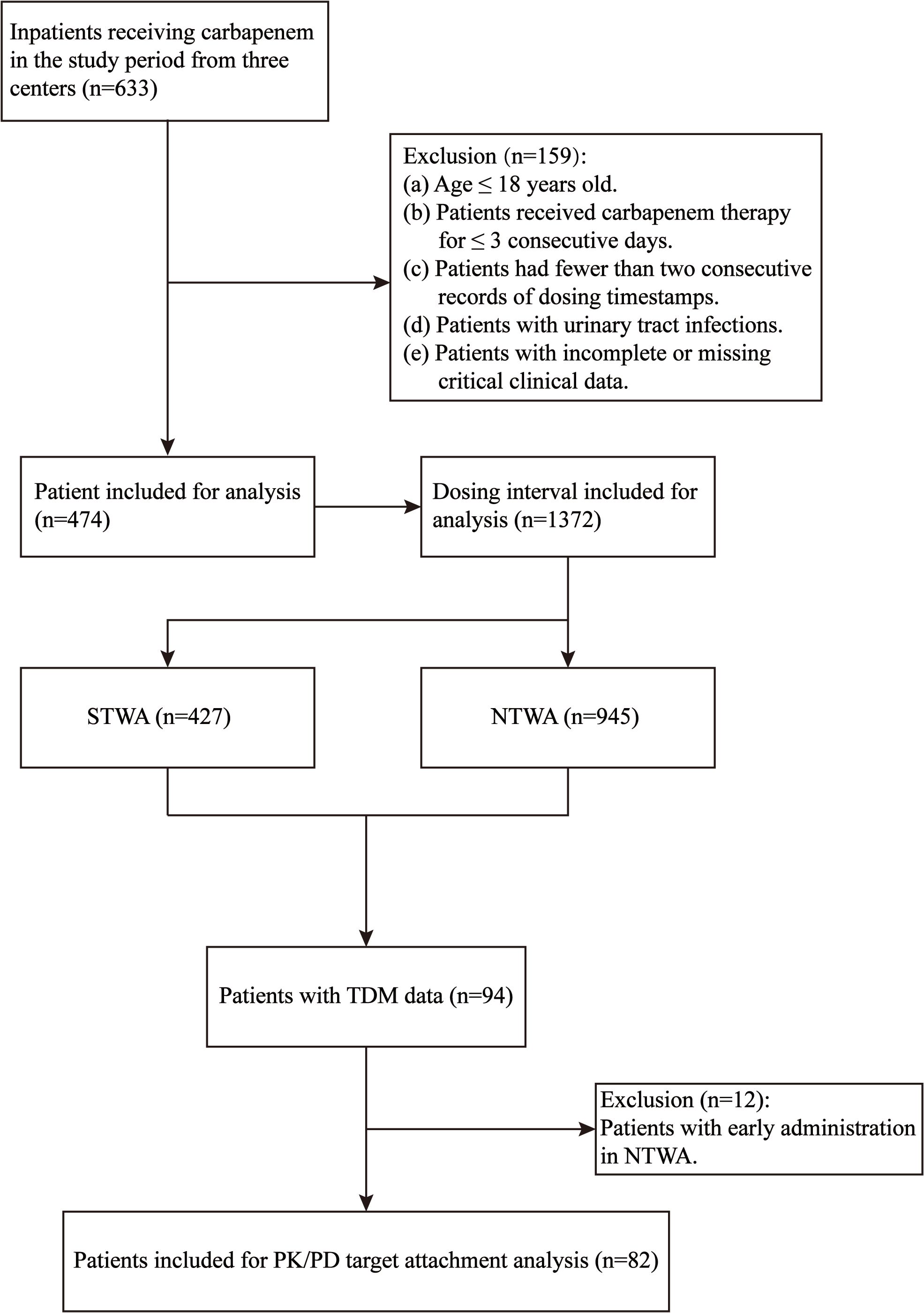
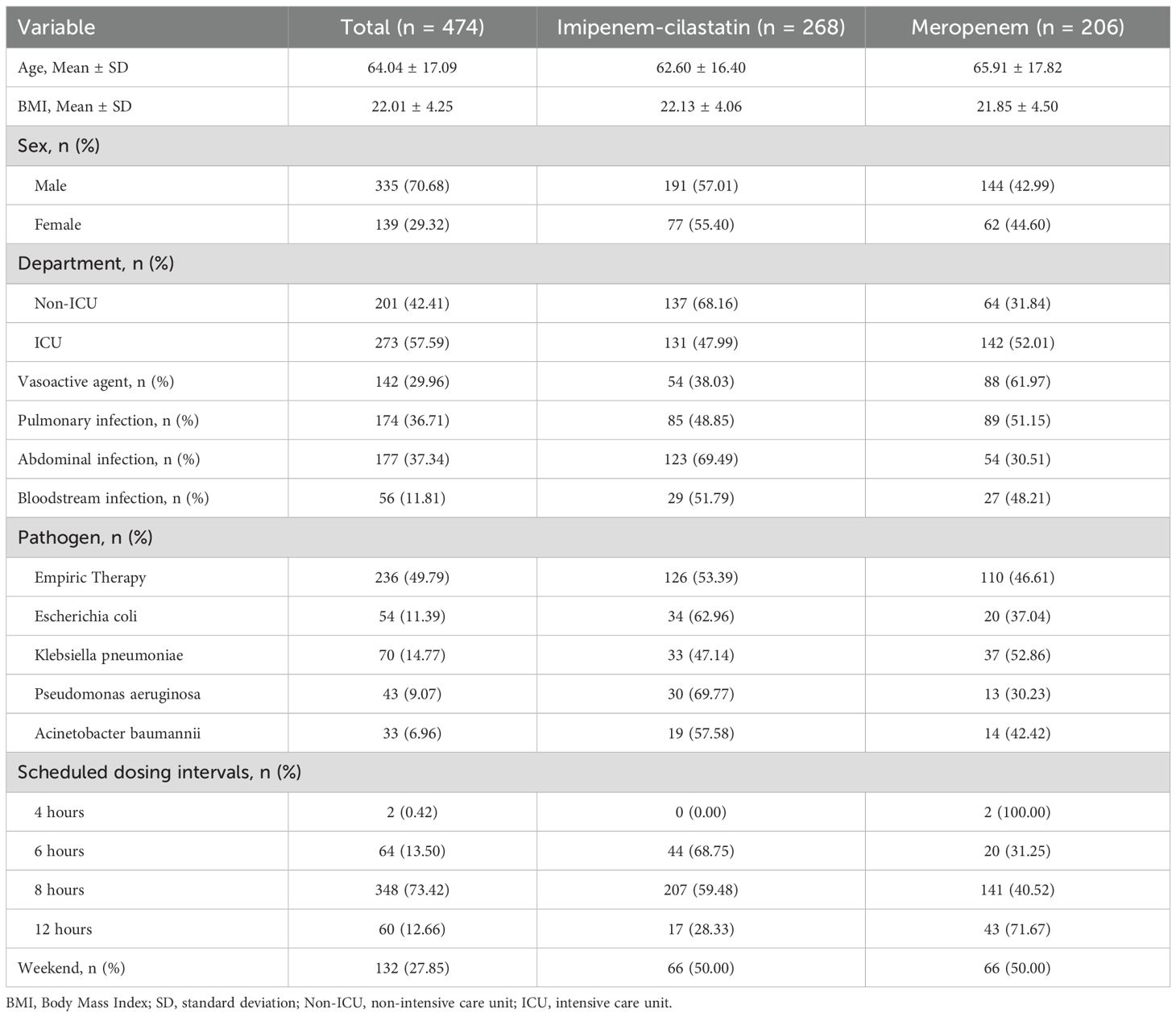
A total of 633 patients who received carbapenem drug treatment were identified for screening. The inclusion and exclusion processes are shown in Figure 1. Finally, 474 patients were eligible for the final analysis and included. The demographic characteristics of the included patients are shown in Table 1.
3.2 Overall distribution of administration timeliness and independent risk factors
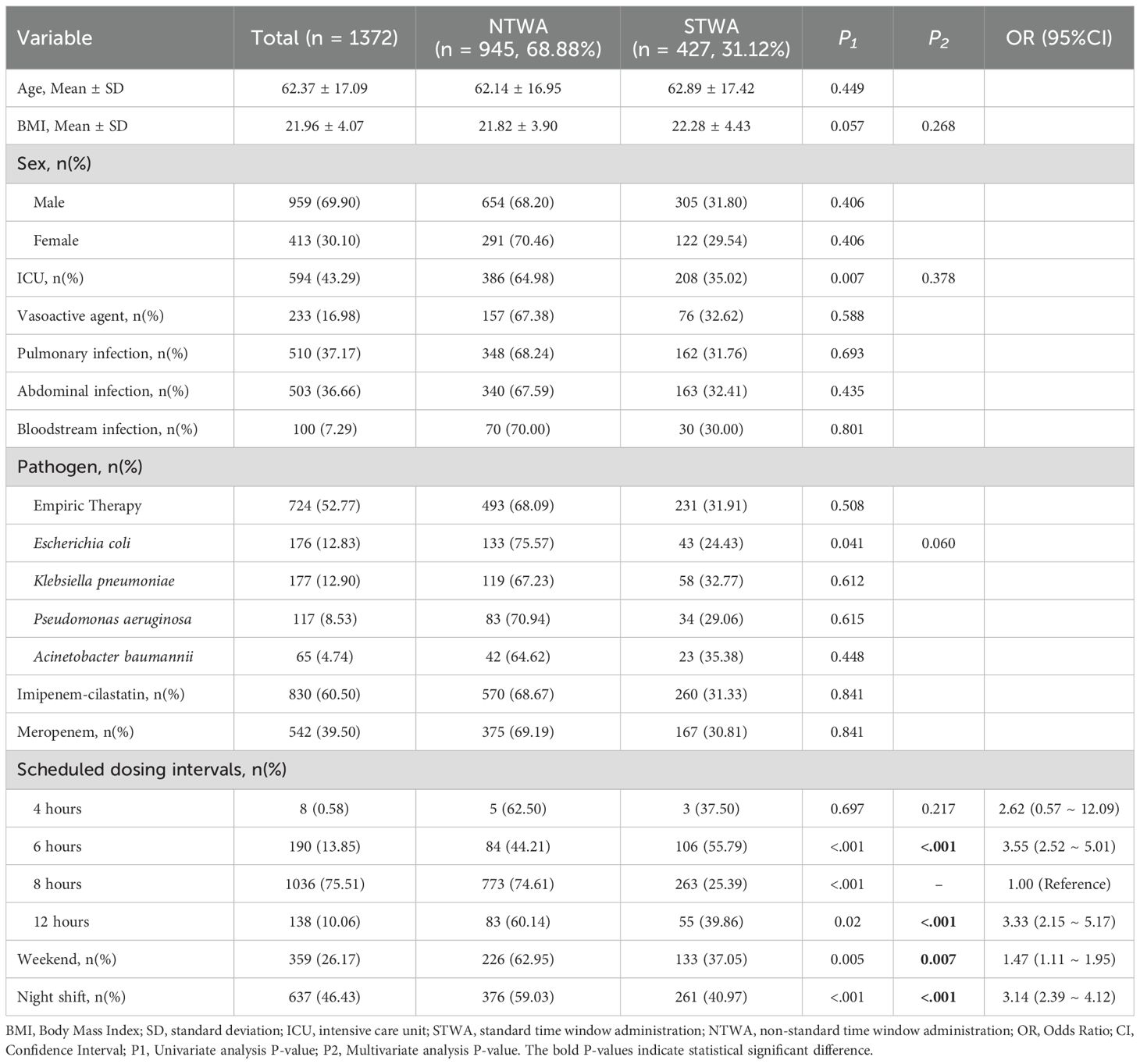
As delineated in Table 2, the timestamp analysis of 474 patients yielded 1,372 actual administration interval records. The overall rate of on time carbapenem administration was 31.12%. Among all the dosing intervals, 427 intervals (31.12%) complied with the STWA, whereas 945 (68.88%) were noncompliant (NTWA). The details of the STWA and NTWA groups are also shown in Table 2. The ICU patients demonstrated a significantly higher STWA compliance rate (35.02%) than did the NTWA patients (P = 0.007). Conversely, Escherichia coli-infected patients exhibited markedly lower compliance, with compliance rates of only 24.43% in STWA patients and 75.57% in NTWA patients. The 6-hour dosing regimen demonstrated optimal compliance (55.79%), followed by the 12-hour (39.86%) and 4-hour (37.5%) schedules. The 8-hour regimen showed minimal compliance (25.39%). Interestingly, weekend administrations accounted for 37.05% of STWA events versus 62.95% of NTWA events. The same trends were also found for night shifts, which were also different from expectations.
The multivariate regression results are also shown in Table 2. The analysis revealed that both weekends (OR = 1.47, 95% CI = 1.11–1.95, P = 0.007) and night shifts (OR = 3.14, 95% CI = 2.39–4.12, P < 0.001) were significantly associated with an increased likelihood of STWA. Furthermore, compared with the 8-hour dosing interval, both the 6-hour dosing interval (OR = 3.55, 95% CI = 2.52–5.01, P < 0.001) and the 12-hour dosing interval (OR = 3.33, 95% CI = 2.15–5.17, P < 0.001) were also significantly associated with a greater likelihood of STWA (Table 3).
3.3 PK/PD target attainment rate and independent risk factors
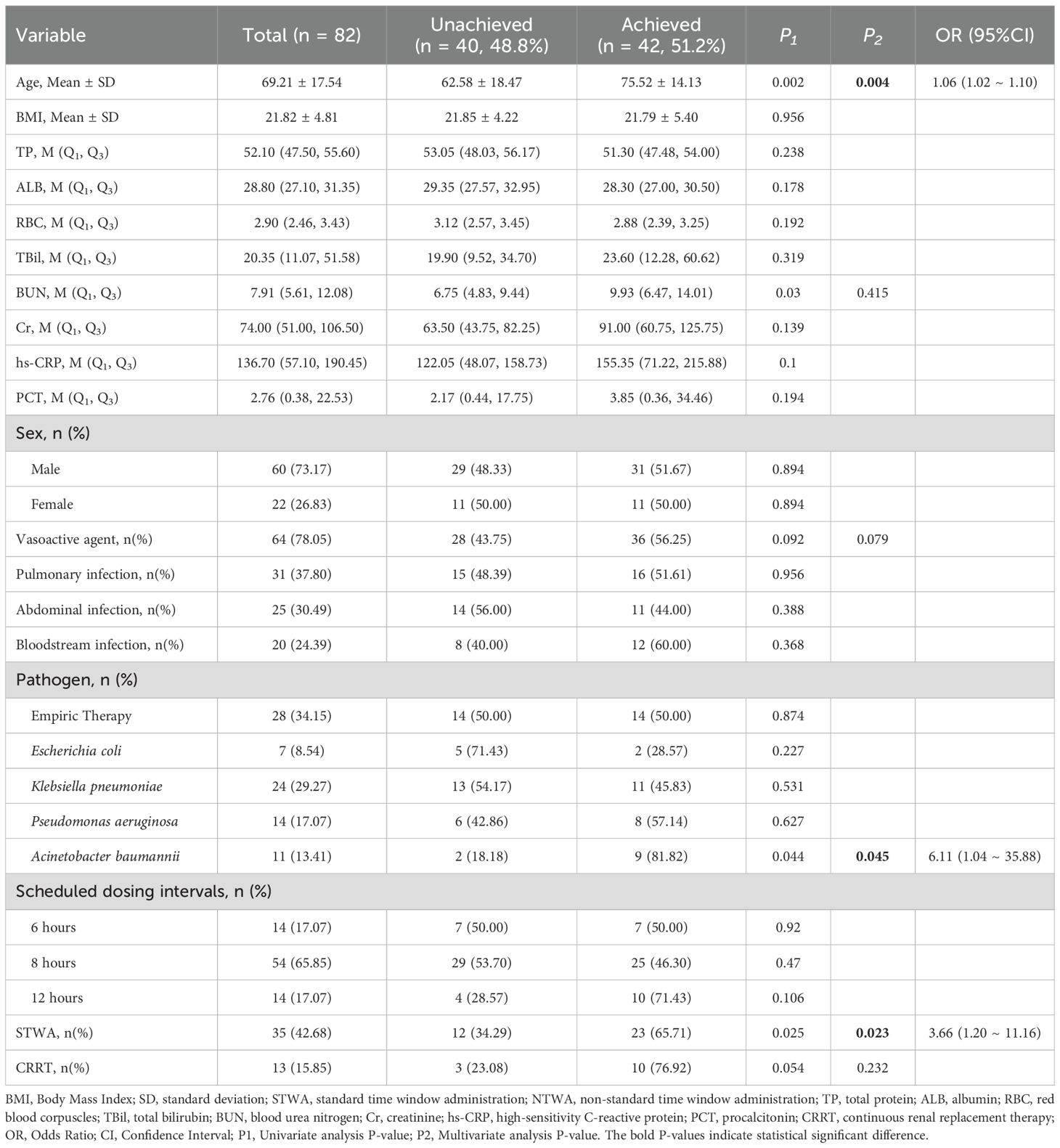
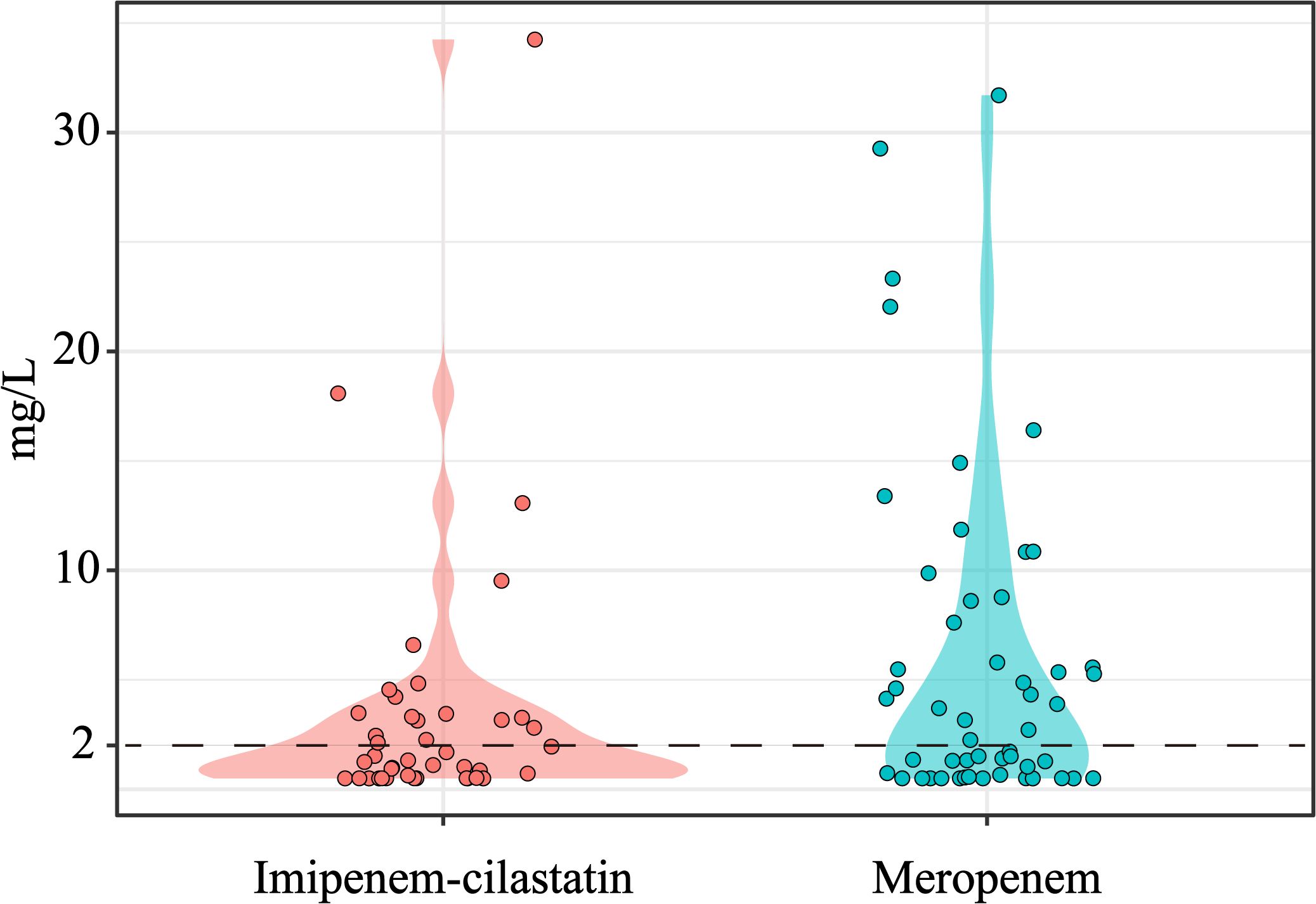
Finally, 82 patients with TDM results were analyzed for PK/PD target attainment. The distributions of meropenem and imipenem concentrations are shown in Figure 2. Among these patients, 42 (51.2%) achieved the PK/PD target of 100% fT>MIC, whereas 16 (19.5%) attained the stricter target of 100% fT>4×MIC (Table 3; Supplementary Table S1). Univariate analysis revealed that patients who achieved 100% fT>MIC were significantly older than unachievers (75.52 ± 14.13 vs. 62.58 ± 18.47 years, P < 0.05) and presented higher median BUN levels [9.93 (IQR 6.47–14.01) vs. 6.75 (IQR 4.83–9.44), P < 0.01]. Notably, compliance with the STWA was significantly greater in the 100% fT>MIC group than in the unachieved group (65.71% vs. 34.29%, P = 0.025). However, the STWA compliance rates are markedly lower for the 100% fT>4×MIC target (34.29% vs. 65.71%, P = 0.006).
Multivariate logistic regression incorporating age, BUN, Acinetobacter baumannii infection, vasoactive agent, STWA, and CRRT. The results indicated that age (OR = 1.06, 95% CI = 1.02–1.10, P = 0.004), Acinetobacter baumannii infection (OR = 6.11, 95% CI = 1.04–35.88, P = 0.045), and STWA (OR = 3.66, 95% CI = 1.20–11.16, P = 0.023) were independent risk factors associated with 100% fT>MIC target attainment (Table 3). Notably, STWA was also positively correlated with 100% fT>4×MIC attainment (Supplementary Table S1).
4 Discussion
To the best of our knowledge, this is the first multicenter study that evaluated the prevalence of on time administration of carbapenems and its impact on PK/PD target attainment in hospitalized patients. A total of 474 patients and 1,372 dosing intervals from three tertiary hospitals were included in this study, which made the results representative. Notably, the prevalence of STWA is low. Scheduled dosing intervals were identified as independent risk factors for STWA, which should be well controlled in clinical practice. Furthermore, STWA was an independent risk factor associated with PK/PD target attainment, which emphasized the importance of on time administration.
Our study evaluated the timeliness of carbapenem administration in hospitalized patients. Notably, only 31.12% of the carbapenems were administered within the STWA, which was lower than the available prevalence rates. Tolley and Poon reported that the probabilities of timing errors occurring in hospitals were 11.5% and 4.77%, respectively (Poon et al., 2010; Tolley et al., 2022). For time-critical medications, the incidence of timing errors in administration is greater. Craig et al. reported a 68.88% adherence rate to the STWA for time-critical medications (Furnish et al., 2021). Our study demonstrated a significantly lower rate of timely carbapenem administration. This discrepancy likely reflects the confluence of several factors, notably the inherently time-consuming preparation process specific to carbapenems and potential inefficiencies within the hospital’s medication management workflow (encompassing physician order entry, pharmacy dispensing, and nursing execution) (Yu et al., 2023). Multivariate regression analysis revealed that administration during weekends and night shifts was positively correlated with STWA attainment. This counterintuitive observation may be attributed to reduced competing clinical demands (e.g., fewer scheduled surgeries or physician rounds) during these periods, potentially enabling nursing staff to prioritize medication administration tasks. Compared with the 8-hour dosing interval, both the 6-hour and 12-hour intervals were significantly associated with a greater likelihood of STWA. This association may be attributed to the better alignment of the 6-hour dosing interval with routine nursing workflows and shift handovers, facilitating integration into planned care activities. The 12-hour intervals, typically administered at fixed times (e.g., 8:00 am and 8:00 pm), benefit from a simpler, twice-daily regimen that is less prone to omission or confusion. Importantly, the 8-hour dosing interval constituted the predominant group in this study cohort, whereas data for the 6-hour dosing interval and 12-hour dosing interval were comparatively limited. This imbalance in sample sizes may introduce potential bias into the observed associations.
These findings highlight systemic inefficiencies in routine clinical workflows and emphasize the urgent need for staffing optimization during weekdays and daytime shifts. In addition, previous studies indicate that communication between medical teams may be an important factor in patient care, and the assessment ability of healthcare workers and awareness of time-critical drugs should be improved (Vijayan et al., 2021; Amano et al., 2023; Wu et al., 2025). To increase carbapenem stewardship, hospitals should implement targeted interventions: (1) standardize workflow protocols to minimize interruptions during peak hours; (2) integrate automated alerts for high-priority antimicrobials into electronic health records; and (3) conduct regular staff training to reinforce the clinical significance of timely carbapenem administration.
While pre-clinical investigations have historically informed PK/PD targets for beta-lactam antibiotics, the applicability of these thresholds requires careful contextualization. This value originated largely from animal infection models, where carbapenems demonstrated efficacy at 40% fT>MIC owing to their prolonged post-antibiotic effect (Assefa et al., 2024). However, such thresholds may not directly translate to clinical practice, especially in critically ill patients who exhibit altered pharmacokinetics due to underlying illness, comorbidities, or life-saving treatment modalities. In these populations, targeting higher thresholds such as 100% fT>MIC or even 100% fT>4×MIC is increasingly adopted (De Waele et al., 2013; Abdulla et al., 2020; Peng et al., 2025; Tseng et al., 2025). These more aggressive targets aim to compensate for pharmacokinetic variability and ensure adequate drug exposure throughout the dosing interval, particularly when treating pathogens with elevated MICs or in immunocompromised hosts.
In the present study, TDM results were available for 82 patients. The target of 100% fT>MIC was achieved in 51.2% of patients, whereas 19.5% attained 100% fT>4×MIC. In a study investigating the relationship between % fT>MIC and the clinical efficacy of meropenem, the number of sepsis patients treated with meropenem reached 40% fT>MIC and 100% fT>MIC, which were 92.9% and 71.4%, respectively (Vijayan et al., 2021). Kim et al. achieved a 30% compliance rate of 100% fT> 4×MIC by administering 2 g of meropenem every 6 hours as a 3 h extended infusion (Kim et al., 2024). This discrepancy may be attributable to methodological differences between the studies. Specifically, Kim et al. applied a lower MIC threshold (1 mg/L for meropenem), in contrast to the 2 mg/L threshold used in our study. Areskog et al. demonstrated that 88% of meropenem-treated patients achieved 100% fT>MIC and that 53% reached 100% fT>4×MIC (Areskog Lejbman et al., 2024). Notably, their protocol employed a standardized regimen of 1 g every 8 hours, whereas our study included individualized dosing regimens (0.5 g or 1 g administered every 6, 8, or 12 hours), which likely contributed to the observed pharmacokinetic variability.
Timely administration critically influences the PK profiles of time-dependent antimicrobial agents. A systematic analysis of ICU patients receiving carbapenems revealed PK/PD target attainment rates, with 100% fT>MIC achieved in 65.71% of cases and 100% fT>4×MIC achieved in 34.29% of cases. Multivariate regression analysis demonstrated that timely administration significantly improved both the 100% fT>MIC and 100% fT>4×MIC attainment rates. Carbapenems exhibit time-dependent bactericidal activity, where clinical efficacy is maximized when fT>MIC exceeds a certain threshold. For carbapenems, maintaining fT>MIC ≥ 40% of the dosing interval is critical; achieving 100% fT>MIC further optimizes bacterial eradication and suppresses resistance (Tilanus and Drusano, 2023; Pokorná et al., 2024). For short-half-life β-lactams (e.g., meropenem, imipenem–cilastatin), delays cause a rapid decline in serum concentrations, which would be below the MIC threshold. Timely administration ensures consistent concentration–time curve (AUC) overlap, sustaining drug levels above the MIC throughout the interval. Pathogens such as Pseudomonas aeruginosa exhibit adaptive resistance when exposed to subinhibitory antibiotic concentrations. Delayed dosing creates sub-MIC windows, allowing bacterial regrowth and increasing the risk of resistance mutations (Chen et al., 2021; Sanz-García et al., 2022). Most previous studies have focused on the rate of attaining PK/PD targets for carbapenems with continuous and intermittent infusions (Wunderink et al., 2021; Monti et al., 2023; Johnson et al., 2024). Our findings underscore that strict compliance with scheduled dosing intervals ensures sustained drug concentrations above the MIC and 4×MIC thresholds, thereby maximizing bactericidal efficacy and mitigating resistance selection.
The clinical imperative for on time administration is magnified in critically ill patients. ICU patients frequently exhibit altered pharmacokinetics due to pathophysiological changes or extracorporeal therapies, leading to unpredictable drug exposure (Morales Castro et al., 2023). Timely administration mitigates concentration fluctuations, preventing transient trough concentrations below the MIC (even for 1–2 hours), so that it blocks the selective proliferation of drug-resistant mutants. To operationalize precision dosing for time-critical medications, hospitals must implement workflow innovations. For carbapenems, strategies such as premixed formulations, smart infusion pumps, and rapid bedside administration systems can minimize delays between prescription and drug delivery.
This study has several limitations inherent to its retrospective design, which may introduce selection bias and unmeasured confounding factors. The analysis did not directly assess the associations between administration timeliness and clinical outcomes such as mortality or resistance emergence. Future prospective cohort studies are warranted to establish causality between timely administration and therapeutic efficacy. Despite the overall large sample size of this study, only 82 patients (17.3%) had TDM data available for PK/PD analysis. This may introduce selection bias, as patients with more complex conditions are more likely to undergo TDM monitoring. Therefore, the findings still require further validation with larger datasets. An international survey revealed considerable variation in TDM practices for β-lactam antibiotics in intensive care units (Wong et al., 2014). Although carbapenem TDM has been reported in clinical settings, most studies focus on meropenem (Joynt et al., 2023). Moreover, a literature search using the keywords “China” AND “carbapenem” AND “TDM” yielded very few results, indicating that carbapenem TDM has not yet been routinely implemented in clinical practice in China. This limitation underscores the need for more prospective studies on carbapenem TDM in the future.
Additionally, further research should address critical knowledge gaps: (1) the development of dose-compensation protocols for delayed administration and (2) the clinical validation of novel delivery technologies such as nanoparticle-based controlled-release systems to bridge the gap between theoretical PK/PD targets and real-world effectiveness.
5 Conclusions
This multicenter retrospective study investigated the prevalence of on time carbapenem administration, and the results indicated that the timeliness of carbapenem administration was poor. Several factors, including scheduled dosing intervals, weekend days and night shifts, were significantly associated with improved timely administration. Furthermore, on time administration was found to be associated with PK/PD target attainment rates (100% fT>MIC and 100% fT>4×MIC), which indicated that timely administration might be important in optimizing the efficacy of antibiotics. In the future, systematic improvement and interdisciplinary collaboration are needed to improve the timeliness of drug administration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by ethics committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived due to the retrospective nature of the study.
Author contributions
JS: Data curation, Formal Analysis, Writing – original draft. XP: Data curation, Formal Analysis, Methodology, Writing – original draft. HC: Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – review & editing. PZ: Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. MY: Data curation, Formal Analysis, Writing – review & editing. RX: Conceptualization, Resources, Supervision, Writing – review & editing. ZY: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Public welfare technology application research projects of Jinhua, Zhejiang (2023-4-223) and Zhejiang Pharmaceutical Association (2017ZYY036). The funders played no role in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1690269/full#supplementary-material
Supplementary Table 1 | Distribution of 100% fT>4×MIC achievement and multivariate logistic analysis.
References
Abdulla, A., Dijkstra, A., Hunfeld, N. G. M., Endeman, H., Bahmany, S., Ewoldt, T. M.J., et al. (2020). Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: a two-center prospective study (EXPAT). Crit. Care (London England) 24, 558. doi: 10.1186/s13054-020-03272-z
Allison Rout, J., Yusuf Essack, S., and Brysiewicz, P. (2023). Evaluation of intermittent antimicrobial infusion documentation practices in intensive care units: A cross-sectional study. Intensive Crit. Care Nurs. 79, 103527. doi: 10.1016/j.iccn.2023.103527
Amano, A., Brown-Johnson, C. G., Winget, M., Sinha, A., Shah, S., Sinsky, C. A., et al. (2023). Perspectives on the intersection of electronic health records and health care team communication, function, and well-being. JAMA Network Open 6, e2313178. doi: 10.1001/jamanetworkopen.2023.13178
Angelini, J., Giuliano, S., Flammini, S., Pagotto, A., Lo Re, F., Tascini, C., et al. (2023). Meropenem PK/PD variability and renal function: “We go together. Pharmaceutics 15, 2238. doi: 10.3390/pharmaceutics15092238
Areskog Lejbman, I., Torisson, G., Resman, F., and Sjövall, F. (2024). Beta-lactam antibiotic concentrations in critically ill patients with standard and adjusted dosages: A prospective observational study. Acta Anaesthesiologica Scandinavica 68, 530–537. doi: 10.1111/aas.14382
Assefa, G. M., Roberts, J. A., Mohammed, S. A., and Sime, F. B. (2024). What are the optimal pharmacokinetic/pharmacodynamic targets for β-lactamase inhibitors? A systematic review. J. Antimicrobial Chemotherapy 79, 946–958. doi: 10.1093/jac/dkae058
Beganovic, M., Daffinee, K. E., Luther, M. K., and LaPlante, K. L. (2021). Minocycline Alone and in Combination with Polymyxin B, Meropenem, and Sulbactam against Carbapenem-Susceptible and -Resistant Acinetobacter baumannii in an In Vitro Pharmacodynamic Model. Antimicrobial Agents Chemotherapy 65, e01680–e01620. doi: 10.1128/AAC.01680-20
Beijer, G., Swartling, M., Nielsen, E. I., Breuer, O., Giske, C. G., Eliasson, E., et al. (2025). First dose target attainment with extended infusion regimens of piperacillin and meropenem. Crit. Care (London England) 29, 208. doi: 10.1186/s13054-025-05445-0
Blignaut, A. J., Coetzee, S. K., Klopper, H. C., and Ellis, S. M. (2017). Medication administration errors and related deviations from safe practice: an observational study. J. Clin. Nurs. 26, 3610–3623. doi: 10.1111/jocn.13732
Bonnin, R. A., Bernabeu, S., Emeraud, C., Creton, E., Vanparis, O., and Naas, T. (2022). Susceptibility of OXA-48-producing Enterobacterales to imipenem/relebactam, meropenem/vaborbactam and ceftazidime/avibactam. Int. J. Antimicrobial Agents 60, 106660. doi: 10.1016/j.ijantimicag.2022.106660
Chen, J., Zhou, H., Huang, J., and Rao, X. (2021). Virulence alterations in staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J. Advanced Res. 31, 165–175. doi: 10.1016/j.jare.2021.01.008
Cizmarova, I., Mikus, P., Svidrnoch, M., and Piestansky, J. (2024). Development and validation of a capillary zone electrophoresis-tandem mass spectrometry method for simultaneous quantification of eight β-lactam antibiotics and two β-lactamase inhibitors in plasma samples. Pharm. (Basel Switzerland) 17, 562. doi: 10.3390/ph17040526
De Waele, J., Carrette, S., Carlier, M., Stove, V., Boelens, J., Claeys, G., et al. (2013). Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med. 40, 380–387. doi: 10.1007/s00134-013-3187-2
Eslami, M., Safaripour, A., Banihashemian, S. Z., Nikjoo Niaragh, S., Hemmati, M. A., Shojaeian, A., et al. (2025). Innovative antibiotic therapies for carbapenem-resistant gram-negative bacterial infections: clinical efficacy, safety, and comparative studies. Microorganisms 13, 295. doi: 10.3390/microorganisms13020295
Fratoni, A. J., Kois, A. K., Gluck, J. A., Nicolau, D. P., and Kuti, J. L. (2024). Imipenem/relebactam pharmacokinetics in critically ill patients supported on extracorporeal membrane oxygenation. J. Antimicrobial Chemotherapy 79, 1118–1125. doi: 10.1093/jac/dkae079
Furnish, C., Wagner, S., Dangler, A., Schwarz, K., Trujillo, T., Stolpman, N., et al. (2021). Evaluation of medication administration timing-are we meeting our goals? J. Pharm. Pract. 34, 750–754. doi: 10.1177/0897190020905456
Gatti, M., Cojutti, P. G., Pascale, R., Tonetti, T., Laici, C., Dell'Olio, A., et al. (2021). Assessment of a PK/PD target of continuous infusion beta-lactams useful for preventing microbiological failure and/or resistance development in critically ill patients affected by documented gram-negative infections. Antibiotics (Basel Switzerland) 10, 1311. doi: 10.3390/antibiotics10111311
Gerges, B., Rosenblatt, J., Shellburne, S. A., Chaftari, A.-M., Hachem, R., and Raad, I. (2023). In vitro activity of tebipenem and comparator agents against bacterial pathogens isolated from patients with cancer. JAC-antimicrobial Resistance 5, dlad132. doi: 10.1093/jacamr/dlad132
Guilhaumou, R., Benaboud, S., Bennis, Y., Dahyot-Fizelier, C., Dailly, E., Gandia, P., et al. (2019). Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care (London England) 23, 104. doi: 10.1186/s13054-019-2378-9
Heffernan, A. J., Sime, F. B., Lipman, J., and Roberts, J. A. (2018). Individualising therapy to minimize bacterial multidrug resistance. Drugs 78, 621–641. doi: 10.1007/s40265-018-0891-9
Johnson, E. G., Maki Ortiz, K., Adams, D. T., Kaur, S., Faust, A. C., Yang, H., et al. (2024). A retrospective analysis of intravenous push versus extended infusion meropenem in critically ill patients. Antibiotics (Basel Switzerland) 13, 835. doi: 10.3390/antibiotics13090835
Joynt, G. M., Ling, L., Wong, W. T., and Lipman, J. (2023). Therapeutic drug monitoring of carbapenem antibiotics in critically ill patients: an overview of principles, recommended dosing regimens, and clinical outcomes. Expert Rev. Clin. Pharmacol. 16, 703–714. doi: 10.1080/17512433.2023.2194629
Kim, Y. K., Kang, G., Zang, D. Y., and Lee, D. H. (2024). Precision dosing of meropenem in adults with normal renal function: insights from a population pharmacokinetic and monte carlo simulation study. Antibiotics (Basel Switzerland) 13, 849. doi: 10.3390/antibiotics13090849
Liebchen, U., Salletmeier, H., Kallee, S., Scharf, C., Huebner, L., Weber, A., et al. (2021). Optimal loading dose of meropenem before continuous infusion in critically ill patients: a simulation study. Sci. Rep. 11, 17211. doi: 10.1038/s41598-021-96744-3
Loput, C. M., Saltsman, C. L., Rahm, R. C., Roberts, W. D., Sharma, S., Borum, C., et al. (2022). Evaluation of medication administration timing variance using information from a large health system’s clinical data warehouse. Am. J. Health-system Pharm. 79, S1–S7. doi: 10.1093/ajhp/zxab378
Luo, J., Liu, J., Lin, H., Yang, Y., Chen, C., Chen, J., et al. (2025). Optimization of meropenem dosing regimens in critically ill patients with augmented renal clearance. Front. In Med. 12, 1550053. doi: 10.3389/fmed.2025.1550053
Maguigan, K. L., Al-Shaer, M. H., and Peloquin, C. A. (2021). Beta-lactams dosing in critically ill patients with gram-negative bacterial infections: A PK/PD approach. Antibiotics (Basel Switzerland) 10, 1154. doi: 10.3390/antibiotics10101154
Martyn, J.-A., Paliadelis, P., and Perry, C. (2019). The safe administration of medication: Nursing behaviours beyond the five-rights. Nurse Educ. In Pract. 37, 109–114. doi: 10.1016/j.nepr.2019.05.006
Mehta, S. C., Furey, I. M., Pemberton, O. A., Boragine, D. M., Chen, Y., and Palzkill, T. (2021). KPC-2 β-lactamase enables carbapenem antibiotic resistance through fast deacylation of the covalent intermediate. J. Biol. Chem. 296, 100155. doi: 10.1074/jbc.RA120.015050
Monti, G., Bradic, N., Marzaroli, M., Konkayev, A., Fominskiy, E., Kotani, Y., et al. (2023). Continuous vs intermittent meropenem administration in critically ill patients with sepsis: the MERCY randomized clinical trial. JAMA 330, 141–151. doi: 10.1001/jama.2023.10598
Morales Castro, D., Dresser, L., Granton, J., and Fan, E. (2023). Pharmacokinetic alterations associated with critical illness. Clin. Pharmacokinet. 62, 209–220. doi: 10.1007/s40262-023-01213-x
Peng, Y., Liu, Y., Cheng, Z., Zhang, Q., Xie, F., Zhu, S., et al. (2025). Population pharmacokinetics of prolonged infusion for meropenem: tailoring dosing recommendations for chinese critically ill patients on continuous renal replacement therapy with consideration for renal function. Drug Design Dev. Ther. 19, 1105–1117. doi: 10.2147/DDDT.S489603
Phe, K., Heil, E. L., and Tam, V. H. (2020). Optimizing pharmacokinetics-pharmacodynamics of antimicrobial management in patients with sepsis: A review. J. Infect. Dis. 222, S132–S141. doi: 10.1093/infdis/jiaa118
Pokorná, P., Michaličková, D., Tibboel, D., and Berner, J. (2024). Meropenem disposition in neonatal and pediatric extracorporeal membrane oxygenation and continuous renal replacement therapy. Antibiotics (Basel Switzerland) 13, 419. doi: 10.3390/antibiotics13050419
Poon, E. G., Keohane, C. A., Yoon, C. S., Ditmore, M., Bane, A., Levtzion-Korach, O., et al. (2010). Effect of bar-code technology on the safety of medication administration. New Engl. J. Med. 362, 1698–1707. doi: 10.1056/NEJMsa0907115
Rodríguez-Gascón, A., Solinís, M.Á, and Isla, A. (2021). The role of PK/PD analysis in the development and evaluation of antimicrobials. Pharmaceutics 13, 833. doi: 10.3390/pharmaceutics13060833
Sanz-García, F., Hernando-Amado, S., and Martínez, J. L. (2022). Evolution under low antibiotic concentrations: a risk for the selection of Pseudomonas aeruginosa multidrug-resistant mutants in nature. Environ. Microbiol. 24, 1279–1293. doi: 10.1111/1462-2920.15806
Schutijser, B., Klopotowska, J. E., Jongerden, I., Spreeuwenberg, P., Wagner, C., and de Bruijne, M. (2018). Nurse compliance with a protocol for safe injectable medication administration: comparison of two multicentre observational studies. BMJ Open 8, e019648. doi: 10.1136/bmjopen-2017-019648
Stolic, S., Ng, L., Southern, J., and Sheridan, G. (2022). Medication errors by nursing students on clinical practice: An integrative review. Nurse Educ. Today 112, 105325. doi: 10.1016/j.nedt.2022.105325
Tilanus, A. and Drusano, G. (2023). Optimizing the use of beta-lactam antibiotics in clinical practice: A test of time. Open Forum Infect. Dis. 10, ofad305. doi: 10.1093/ofid/ofad305
Tolley, C. L., Watson, N. W., Heed, A., Einbeck, J., Medows, S., Wood, L., et al. (2022). The impact of a novel medication scanner on administration errors in the hospital setting: a before and after feasibility study. BMC Med. Inf. Decision Making 22, 86. doi: 10.1186/s12911-022-01828-3
Tseng, Y.-J., Tai, C.-H., Chen, G.-Y., Chen, Y.-L., Ku, S.-C., Pai, T.-Y., et al. (2025). Navigating pharmacokinetic and pharmacodynamics challenges of β-lactam antibiotics in patients with low body weight: efficacy, toxicity, and dosage optimization. Ther. Adv. In Drug Saf. 16, 20420986251320414. doi: 10.1177/20420986251320414
Vijayan, A., Abdel-Rahman, E. M., Liu, K. D., Goldstein, S. L., Agarwal, A., Okusa, M. D., et al. (2021). Recovery after critical illness and acute kidney injury. Clin. J. Am. Soc. Nephrology: CJASN 16, 1601–1609. doi: 10.2215/CJN.19601220
Wong, G., Brinkman, A., Benefield, R. J., Carlier, M., De Waele, J. J., El Helali, N., et al. (2014). An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrobial Chemotherapy 69, 1416–1423. doi: 10.1093/jac/dkt523
Wu, C.-C., Su, Y.-C., Wu, K.-S., Wu, T.-H., and Yang, C.-S. (2021). Loading dose and efficacy of continuous or extended infusion of beta-lactams compared with intermittent administration in patients with critical illnesses: A subgroup meta-analysis and meta-regression analysis. J. Clin. Pharm. Ther. 46, 424–432. doi: 10.1111/jcpt.13301
Wu, J., Xue, E., Liu, C., Shao, J., Fu, Y., Zhao, B., et al. (2025). Classifying and characterising unmet integrated care needs of older adults with multimorbidity: A latent profile analysis. J. Clin. Nurs. doi: 10.1111/jocn.17520
Wunderink, R. G., Matsunaga, Y., Ariyasu, M., Clevenbergh, P., Echols, R., Kaye, K. S., et al. (2021). Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 21, 213–225. doi: 10.1016/S1473-3099(20)30731-3
Keywords: meropenem, imipenem, administration time, therapeutic drug monitoring, PK/PD
Citation: Song J, Pang X, Chen H, Zhang P, Ying M, Xu R and Yu Z (2025) Prevalence of on time administration of carbapenem and its impact on PK/PD target attainment in hospitalized patients: a multicenter retrospective study. Front. Cell. Infect. Microbiol. 15:1690269. doi: 10.3389/fcimb.2025.1690269
Received: 21 August 2025; Accepted: 26 September 2025;
Published: 17 October 2025.
Edited by:
Lorena V. N. Oliveira, University of Massachusetts Medical School, United StatesReviewed by:
Changcheng Shi, Westlake University, ChinaWentong Fang, Nanjing Medical University, China
Copyright © 2025 Song, Pang, Chen, Zhang, Ying, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renai Xu, eXN4dXJlbmFpQGhvdG1haWwuY29t; Zhenwei Yu, eXp3X3NycnNoQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Jin Song1†
Jin Song1† Huadong Chen
Huadong Chen Renai Xu
Renai Xu Zhenwei Yu
Zhenwei Yu