Abstract
Adaptive learning systems (ALSs), powered by artificial intelligence (AI), represent a transformative approach to biotechnological and pharmaceutical education that addresses the critical limitations of traditional standardized pedagogy. This review highlights empirical evidence demonstrating how ALS dynamically personalizes learning through knowledge state modeling (KSM) and the synergistic integration of knowledge level (KL) and knowledge structure (KS) dimensions. This framework enables mastery-based progression in sequential domains (e.g., genetic engineering and pharmacodynamics), ensuring foundational competency before advancement. In addition, key applications of adaptive learning (AL) in the field of biological and pharmaceutical education are also detailed, including scaffolding complex foundational sciences (e.g., real-time misconception detection in Clustered Regularly Interspaced Short Palindromic Repeats—CRISPR-associated protein 9 [CRISPR-Cas9]), enhancing technical skills via AI-driven virtual labs simulating industry workflows (e.g., High-Performance Liquid Chromatography [HPLC] and bioreactors), and navigating regulatory compliance through contextual simulations. The documented benefits include significant cost reduction, accelerated skill acquisition, and strengthened industry alignment. Nevertheless, challenges persist in terms of technical fragmentation, algorithmic bias, and equitable resource access. Finally, it is suggested that future research priorities should involve developing integrated architectures with blockchain-secured micro-credentials, human-AI synergy frameworks for ethical oversight, and equity-driven deployment via federated edge learning. The strategic implementation of ALS promises to cultivate a globally competitive, interdisciplinary workforce for next-generation biopharmaceutical innovation while establishing rigorous, regulatory-grade training.
1 Introduction
AI has profoundly accelerated global development across multiple domains and has served as a catalyst for innovation and efficiency. In environmental protection, AI aids climate modeling and resource management, enabling smarter strategies to combat ecological challenges (Chutcheva et al., 2022; Al-Sharafi et al., 2023). Healthcare has also seen revolutionary advancements through AI-powered diagnostics and personalized treatment plans (Alowais et al., 2023), exemplified by systems such as IBM Watson Oncology (Zou et al., 2020; Park et al., 2023), which improves cancer care accuracy. Education benefits from adaptive learning (AL) platforms that tailor content to individual student needs, thus democratizing access to quality education (Knopp et al., 2023). Importantly, AI has also profoundly revolutionized the biotechnology and pharmaceutical industries, and the current applications of AI in pharmacy practice have further emphasized its role in improving workflow efficiency and patient outcomes (Jessica et al., 2025). While AI has been extensively utilized in medical disciplines such as diagnostics and treatment protocols since the 1970s, its adoption in pharmacy remains limited, primarily focusing on operational tasks (e.g., stock management) rather than direct patient care. Nevertheless, recent technological innovations have precipitated a significant shift in research focus toward integrating artificial intelligence into pivotal aspects of pharmacy practice, particularly in refining clinical decision-making for pharmacist interventions (Alowais et al., 2023)(Jamrat et al., 2023), optimizing medication adherence through intelligent monitoring systems, and advancing precision medicine frameworks for tailored therapeutic strategies (Figure 1). Moreover, the inevitability of AI in medicine and the need to prepare future physicians to critically engage with AI tools have been highlighted (Ngo et al., 2022). These emerging demands call for a strategic restructuring of tertiary education systems to nurture multifaceted talent capable of synthesizing expertise from diverse academic domains.
Figure 1
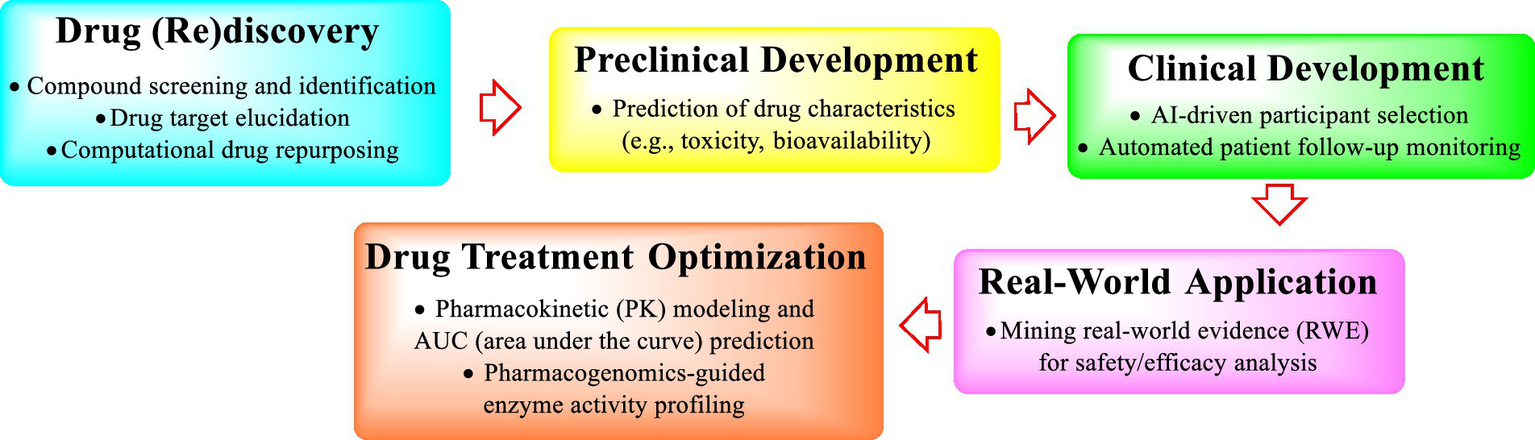
AI applications spanning the drug development continuum.
The appropriate integration of AI into educational ecosystems represents a paradigm shift in addressing the systemic limitations inherent to traditional pedagogical frameworks (Costa et al., 2025; Wang and Zhang, 2025). Historically constrained by mass standardization constraints, personalized instruction barriers, and inequitable resource distribution, modern education systems have undergone a transformative evolution through strategic AI adoption. Drawing parallels with pharmaceutical applications, where machine learning optimizes drug-related problem detection (Corny et al., 2020) and clinical decision support (Levivien et al., 2022), educational technologies employ similar computational architectures to achieve precise interventions (Lu et al., 2023; Singh et al., 2025). The integration of three core AI modalities—adaptive machine learning (Peng and Fu, 2022), natural language processing (Yang, 2022), and multimodal computer vision systems (Gao et al., 2025)—enables sophisticated predictive analytics and intelligent systems within contemporary educational ecosystems. Adaptive machine learning algorithms could personalize learning pathways by analyzing individual performance and errors and dynamically adjusting content difficulty (Balestra et al., 2021). This process is similar to pharmaceutical prioritization models. Meanwhile, advanced natural language processing (NLP) engines are capable of providing real-time diagnostics of comprehension and writing, identifying knowledge gaps, and supporting metacognitive growth. Multimodal vision systems, leveraging techniques from medical imaging, could be used to track behavior and micro-expressions to quantify engagement and affect, enabling timely interventions (Oren et al., 2020). These technological convergences facilitate data-driven personalization at scale, mirroring AI successes in domains such as medication adherence monitoring, where intelligent tutoring systems autonomously refine instructional strategies based on real-time learner interactions and competency benchmarks.
During the past few years, AL has shown great potential in reshaping education, particularly in the dynamic and demanding fields of biotechnology and pharmaceutical science. Its application in biotech/pharmaceutical education addresses critical challenges and unlocks unprecedented opportunities for effective, efficient, and engaging learning. Based on these findings, this review examines the AI-powered ALS for biopharmaceutical education. It draws on a curated selection of publications from major databases such as PubMed, Web of Science, and IEEE Xplore, focusing on key terms including “adaptive learning,” “AI in education,” “biopharmaceutical education,” and “knowledge state modeling.” The selection prioritizes recent and high-impact studies (primarily from 2010 to 2025) that illustrate the core concepts, applications, and challenges in the field. In particular, this review details how ALS uses KSM and integrates knowledge level/structure to enable mastery-based progression, scaffold complex sciences, and enhance technical/regulatory training, which is believed to be capable of accelerating competency development while addressing challenges such as technical fragmentation and equity.
2 Key methodologies utilized in adaptive learning
AL represents a paradigm shift from static, one-size-fits-all instruction to dynamic, data-driven personalization, leveraging AI to optimize learning efficacy. Its application in high-stakes fields such as biopharmaceutical education underscores its transformative potential.
2.1 Review methodology
This review was conducted following a structured approach to ensure a comprehensive and representative analysis of the current landscape of AI-powered ALS in biopharmaceutical education. A literature search was performed across major academic databases, including PubMed, Web of Science, IEEE Xplore, and Scopus, to capture interdisciplinary perspectives from the life sciences, education technology, and computational fields.
The search strategy employed key terms and their combinations, such as “adaptive learning,” “AI in education,” “biopharmaceutical education,” “knowledge state modeling,” “intelligent tutoring systems,” and “competency-based education.” The primary inclusion criteria were peer-reviewed articles, conference proceedings, and seminal reviews published between 2010 and 2025. We also prioritized studies that presented empirical evidence, conceptual frameworks, or clear applications of ALS in biomedical or pharmaceutical contexts. The exclusion criteria included articles not available in English, those lacking a direct focus on education or AI methodology, and publications without a clear description of the adaptive learning mechanism.
The analytical framework was centered on synthesizing evidence around core themes, which include the foundational mechanisms of ALS (e.g., knowledge state modeling), architectural components, specific applications in biotech and pharma education, documented advantages, and prevailing challenges. The selected publications were systematically categorized and analyzed to identify emerging trends, technological convergence, and critical research gaps, which were used to form the basis for the structured discussion and future directions presented in this review.
2.2 Foundational mechanisms of AL
This section outlines the core principles and operational mechanics of ALS. It begins by explaining the basic closed-loop architecture of AL, followed by a description of its key iterative cycles. Central to this discussion is the role of KSM in inferring learner proficiency and the synergistic integration of knowledge level (KL) and knowledge structure (KS) to enable personalized and pedagogically coherent learning pathways. It is believed to be essential to understand these foundational mechanisms for appreciating how AL systems deliver tailored educational experiences.
2.2.1 Basic principles
ALS leverages AI, machine learning (ML), and data analytics (Table 1) to personalize educational experiences by dynamically adjusting content, pace, and instructional strategies based on individual learner needs (Alawneh et al., 2024; Naseer et al., 2024; Tan et al., 2025). ALS operates as a closed-loop system, which is usually comprised of three core components (Figure 2): the learner, learner model, and educator (Tan et al., 2025). The learner interacts with dynamically generated content, receiving personalized instruction and real-time feedback while simultaneously producing behavioral data (e.g., responses and engagement metrics) that fuel system adaptation. These data will be further processed by the learner model, the AI engine of ALS, which utilizes an adaptation model (powered by algorithms such as Bayesian knowledge tracing (Xu et al., 2023) or reinforcement learning (RL) (Ma et al., 2025)) to interpret learner states, predict needs, and make real-time pedagogical decisions about content sequencing, difficulty adjustment, and feedback delivery. Concurrently, the learner model generates learner analytics reports and transforms raw data into actionable insights about knowledge gaps, progress, and engagement. Finally, the educator utilized these reports to refine the teaching strategies and iteratively improve the system. This involves authoring educational content and defining the domain model, a structured knowledge ontology specifying concepts, skill prerequisites, and learning objectives, which critically informs decision-making from the adaptation model. Thus, ALS creates a continuous feedback loop, and this includes learner interactions that drive AI personalization, educator interventions that optimize content and domain structure, and system refinements that enhance future learning through increasingly precise adaptation.
Table 1
| Technology | Role in ALS |
|---|---|
| Machine learning | Knowledge tracing (BKT), risk prediction, and clustering learners |
| Knowledge representation | Domain model ontologies and competency graphs |
| Data mining and learning analytics | Extracts patterns for analytics reports |
| Rule-based systems and decision engines | Implements pedagogical logic in adaptation |
| Natural language processing | Analyzes open-ended responses or generates feedback |
Key AI technologies applied in the process of ALS.
Figure 2
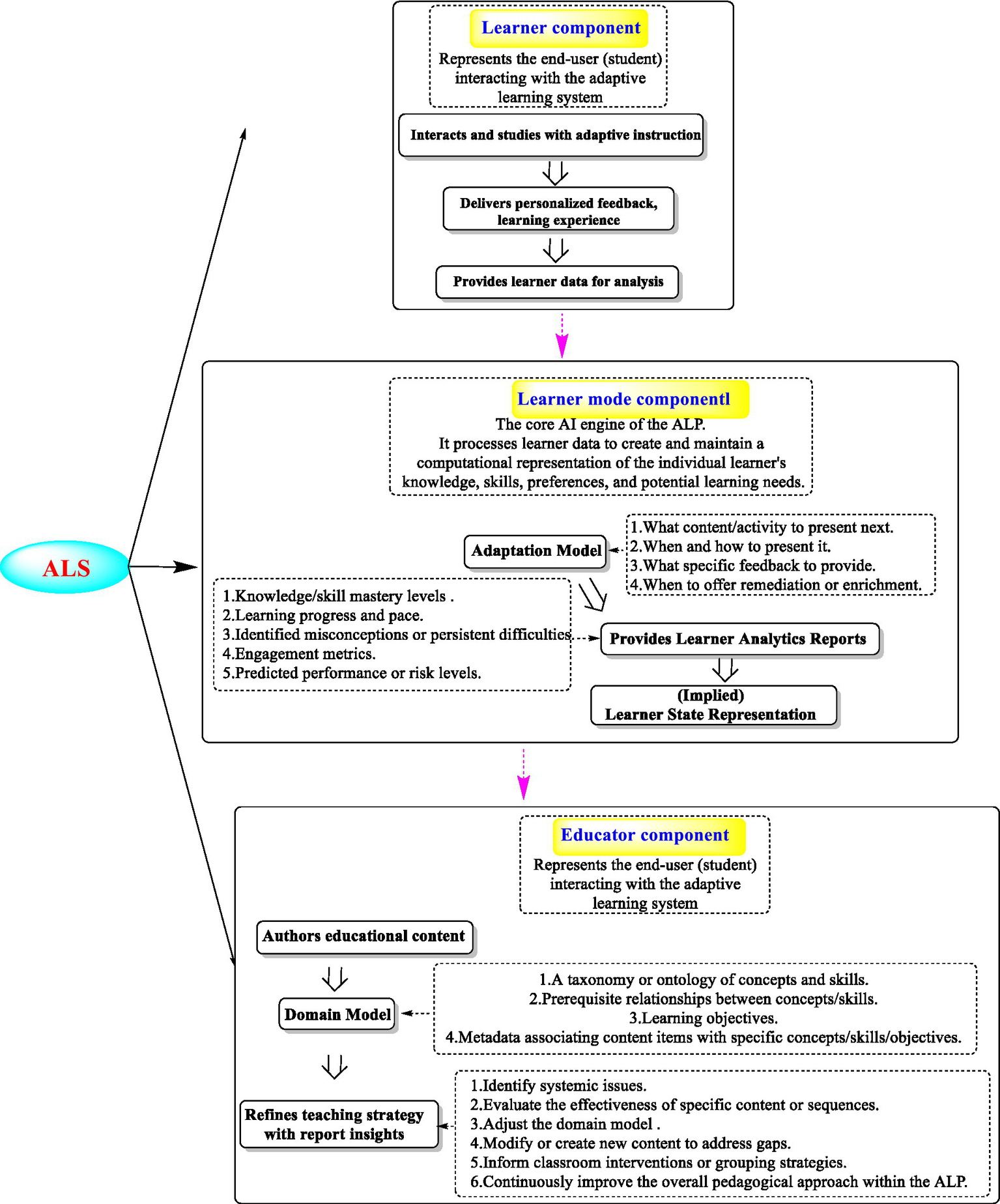
Component architecture of an AI-driven adaptive learning platform.
2.2.2 Iterative operational cycle of ALS
Typically, these systems operate through five iterative cycles: data collection, learner profiling, content delivery, performance evaluation, and system optimization. (1) For data collection, multisource data collection incorporates direct assessments [quiz scores (Dorri et al., 2025)], engagement metrics [clickstream patterns (Rizwan et al., 2025)], and contextual inference (Dubey et al., 2025). Advanced implementations can incorporate environmental sensor data. Various open-source tools facilitate this process, such as Bboss-Datatran (a high-performance ETL tool for multisource synchronization and custom processing)1 and DataPipeline (an enterprise platform for real-time heterogeneous data integration).2 (2) Learner profiling is the process by which ML techniques [supervised/unsupervised learning (Huang et al., 2024)] construct models classifying learners by competency, pacing, or preferences. (3) In the phase of content delivery, AI-curated resources (e.g., targeted practice modules and adaptive quizzes) are deployed via rule-based or neural network-driven logic (Ali et al., 2025). (4) During the state of performance evaluation, learning outcomes are compared against predefined benchmarks to refine future interventions. This mirrors AI applications in medicine, such as the Human Dx project,3 an open-source platform aggregating global clinical reasoning data to develop dynamic diagnostic tools through continuous metric analysis. (5) Finally, for system optimization, iterative feedback mechanisms continuously refine the algorithmic models and enhance predictive accuracy via adaptive learning processes.
2.3 Key component of AL
This section focuses on KSM as the central mechanism for assessing learner proficiency and details the critical integration of KL and KS. This synergy enables the creation of personalized and logically sequenced learning paths that form the basis of an effective AL.
2.3.1 Knowledge state modeling
KSM constitutes a foundational mechanism within AL systems (Kou et al., 2023; Alatrash et al., 2024), which serves to infer and maintain a dynamic, latent representation of the evolving mastery across specific knowledge components (KCs) or concepts (e.g., CRISPR/Cas9 mechanisms, affinity chromatography techniques). The key principle underpinning KSM is that the true understanding of a learner is a hidden state that continuously evolves through learning interactions and is subject to uncertainty, which necessitates probabilistic inference from observable evidence such as quiz responses, simulation outcomes, and problem-solving attempts. This state is inherently multidimensional and captures probabilistic estimates of proficiency levels (e.g., unfamiliar to master) for numerous interconnected KCs. Methodologically, KSM usually employs diverse computational techniques, and its evolution of KSM usually spans from traditional to modern deep learning methods. Traditional approaches include Bayesian knowledge tracing (BKT) (Sun et al., 2022), performance factor analysis (PFA), and item response theory (IRT). For more complex sequential data, deep knowledge tracing (DKT) (Ma et al., 2024; Zhang et al., 2025), which leverages recurrent neural networks (RNNs) or long short-term memory networks (LSTMs) to represent the knowledge state as a dense vector for predicting future performance, has become prominent. Subsequent enhancements to DKT integrate mechanisms such as attention and prerequisite structures using graph neural networks (GNNs) or transformers. Separately, factor models such as knowledge tracing machines (KTM) approach the problem through a collaborative filtering lens.
The inferred knowledge state further facilitates AL personalization through five key mechanisms. First, personalized content sequencing selects optimal KCs based on prerequisite status, delivers items targeting the zone of proximal development (ZPD), and triggers remediation for decaying knowledge. Second, dynamic scaffolding provides targeted hints and adaptive explanations to address struggling KCs while identifying potential misconceptions. Third, mastery-based progression and pacing utilize probabilistic thresholds to determine concept mastery and enable individualized learning speeds. In addition, predictive intervention forecasts learner performance and identifies at-risk students through a prerequisite gap analysis. Finally, personalized learning path generation dynamically constructs customized curriculum sequences. Nevertheless, significant challenges persist, such as the cold-start problem, limited interpretability of complex models (particularly deep-learning-based KSM), dependencies on high-quality interaction data with accurate KC mapping, difficulties in modeling, forgetting, simulating knowledge transfer, and scalability constraints. Despite these limitations, KSM remains the fundamental mechanism enabling AL systems to adapt instruction effectively, thereby optimizing learning efficiency and outcomes for individual learners.
2.3.2 Synergistic integration of cognitive dimensions in AL
The efficacy of advanced AL systems fundamentally hinges on the synergistic integration of two indispensable and complementary cognitive dimensions: knowledge level and knowledge structure. The knowledge level dimension dynamically quantifies the current proficiency of a learner in specific KCs, which can be modeled as latent variables such as the probability of mastery [e.g., P(mastery of ‘PCR primer design’) = 0.85] using techniques such as Bayesian knowledge tracing or item response theory. This continuous assessment enables personalized interventions by identifying the learner’s zone of ZPD, which allows for the delivery of content precisely tailored to their current readiness. Conversely, the knowledge structure dimension encodes the semantic prerequisite relationships and conceptual dependencies within the domain ontology (e.g., mastery of “enzyme kinetics” as a prerequisite for “pharmacodynamics”). This structure is computationally implemented through explicit prerequisite graphs or implicitly implemented through embeddings in Q-matrices. Therefore, it could provide a pedagogically valid roadmap that governs the logical sequencing and progression of learning content. It should be noted that neglecting either cognitive dimension can induce significant inefficiencies within adaptive learning systems. For instance, an isolated focus on knowledge level might risk recommending KCs that fall technically within the learner’s estimated zone of ZPD, yet lack necessary prerequisites. This mismatch, exemplified by suggesting complex pharmacodynamic modeling without foundational mastery of enzyme kinetics, could result in cognitive overload and learner frustration. Conversely, over-reliance on knowledge structure forces unnecessary review of already mastered KCs (e.g., revisiting enzyme kinetics fundamentals), which might lead to redundancy, disengagement, and inefficient use of learning time. Therefore, AL systems must employ sophisticated KSM to concurrently evaluate real-time proficiency (KL) and conceptual dependencies (KS). KSM is able to integrate dynamic probabilistic estimates of KC mastery with static or inferred ontological constraints and then generate a comprehensive view of the learner’s state. This integrated model empowers the system to dynamically select the next best instructional action, whether introducing new KCs, providing practice, or offering remediation, which is both appropriately challenging (leveraging the ZPD via KL) and logically sequenced (respecting prerequisite dependencies via KS). The obtained result is the generation of cognitively optimal and efficient learning trajectories, which could maximize learning effectiveness by adapting to both what the learner knows and how that knowledge is conceptually organized.
The concept of “mastery-based progression” is a direct application of the synergistic integration of the knowledge level and knowledge structure. It is capable of mandating that learners advance to subsequent KCs only upon empirically demonstrating their competency in prerequisite KCs. This progression mechanism is intrinsically governed by the KSM framework, which is capable of generating real-time proficiency metrics (KL) with ontological dependencies (KS). Within sequential domains, such as genetic engineering (e.g., from PCR amplification to recombinant vector construction) or GMP compliance (e.g., from equipment calibration to aseptic technique validation), the system enforces strict prerequisite verification through adaptive assessments modeled through IRT or BKT. Failure thresholds (e.g., mastery probability <0.8) could trigger targeted remediation, while success unlocks ZPD-aligned advanced content. By structurally prohibiting progression without validated mastery, this approach mitigates cascading deficits that would otherwise arise from unresolved foundational gaps, such as attempting plasmid transfection without DNA ligase proficiency or performing sterility testing without understanding cleanroom protocols. Consequently, it ensures pedagogical integrity and reduces cognitive load in inherently hierarchical domains.
2.4 Core architectural framework
AL systems operate through a tightly integrated architecture comprising four functionally distinct yet interdependent computational models, as systematically outlined in Table 2. This framework dynamically orchestrates the personalization process by continuously exchanging data across models, thereby actualizing the synergistic integration of the knowledge level and knowledge structure dimensions previously established. Each model plays a critical role in the adaptive cycle.
Table 2
| Model | Primary input | Core function | Output to |
|---|---|---|---|
| Learner model | Interaction data (Interface) | Estimate knowledge state via KSM | Instructional model |
| Domain model | Curricular expertise | Encode KC relationships & constraints | Learner/instructional models |
| Instructional model | Learner state + Domain rules | Generate pedagogical actions per ZPD/sequencing rules | Interface model |
| Interface model | Instructional prescriptions | Present content & capture behavioral evidence | Learner model |
Core components of an adaptive learning system.
2.4.1 The learner model (cognitive state engine)
The functions of the learner model are to continuously estimate and update the learner’s knowledge state across all KCs using probabilistic frameworks (e.g., BKT and deep knowledge tracing). Crucially, it could produce real-time proficiency metrics [KL, e.g., mastery probability P(KC_x)] with ontological dependencies (KS, sourced from the domain model) through KSM and generate a multidimensional proficiency profile.
2.4.2 The domain model (structural ontology)
The role of the domain model is to encode the subject matter’s semantic architecture as a computational ontology, which explicitly defines KC interdependencies (e.g., from “enzyme kinetics” to “pharmacodynamics” prerequisites) and KC metadata (e.g., complexity, type). Typically, this is implemented as a prerequisite for graphs or Q-matrices. Therefore, this model could enforce pedagogically valid learning pathways and enable mastery-based progression logic (e.g., blocking plasmid transfection in genetic engineering without DNA ligase mastery).
2.4.3 Instructional model (pedagogical agent)
The instructional model is used to translate the state of the learner model and the constraints of the domain model into pedagogical actions using rule-based systems or reinforcement learning policies. It dynamically selects interventions aligned with ZPD principles, where it will remediate below-threshold KCs (e.g., mastery_prob <0.8), advance to subsequent KCs upon mastery validation, or adjust content granularity. Based on these, these strategies collectively enable four critical instructional functionalities: prerequisite-compliant content sequencing (governed by domain ontologies), adaptive scaffold selection (contextual hints/procedural workflows), performance-contingent feedback specificity, and dynamic challenge calibration through difficulty scaling.
2.4.4 Interface model (experience mediator)
The interface model is capable of mediating learner-system interactions and collecting granular behavioral data (response latency and error patterns) while rendering personalized content. It transforms interactions (e.g., drag-and-drop plasmid construction simulations and GMP checklist completions) into evidence for learner model updates and adapts presentations based on cognitive load heuristics (e.g., segmenting complex pharmacodynamic models for struggling learners).
This synergistic data flow fundamentally underpins system efficacy. The interaction data captured by the interface model updates the learner model. The updated parameters of the learner model, integrated with the domain model rules, then inform the adaptation decisions generated by the instructional model. These decisions are executed through the interface model, and the resulting performance data could complete the loop by triggering learner model recalibration. As detailed in Table 2, this cohesive architecture enables AL systems to deliver cognitively optimized and efficient learning trajectories, thereby transforming static content into dynamically personalized educational experiences.
2.5 The implementation workflow
The implementation of the adaptive learning system follows a structured three-phase workflow (Figure 3).
Figure 3
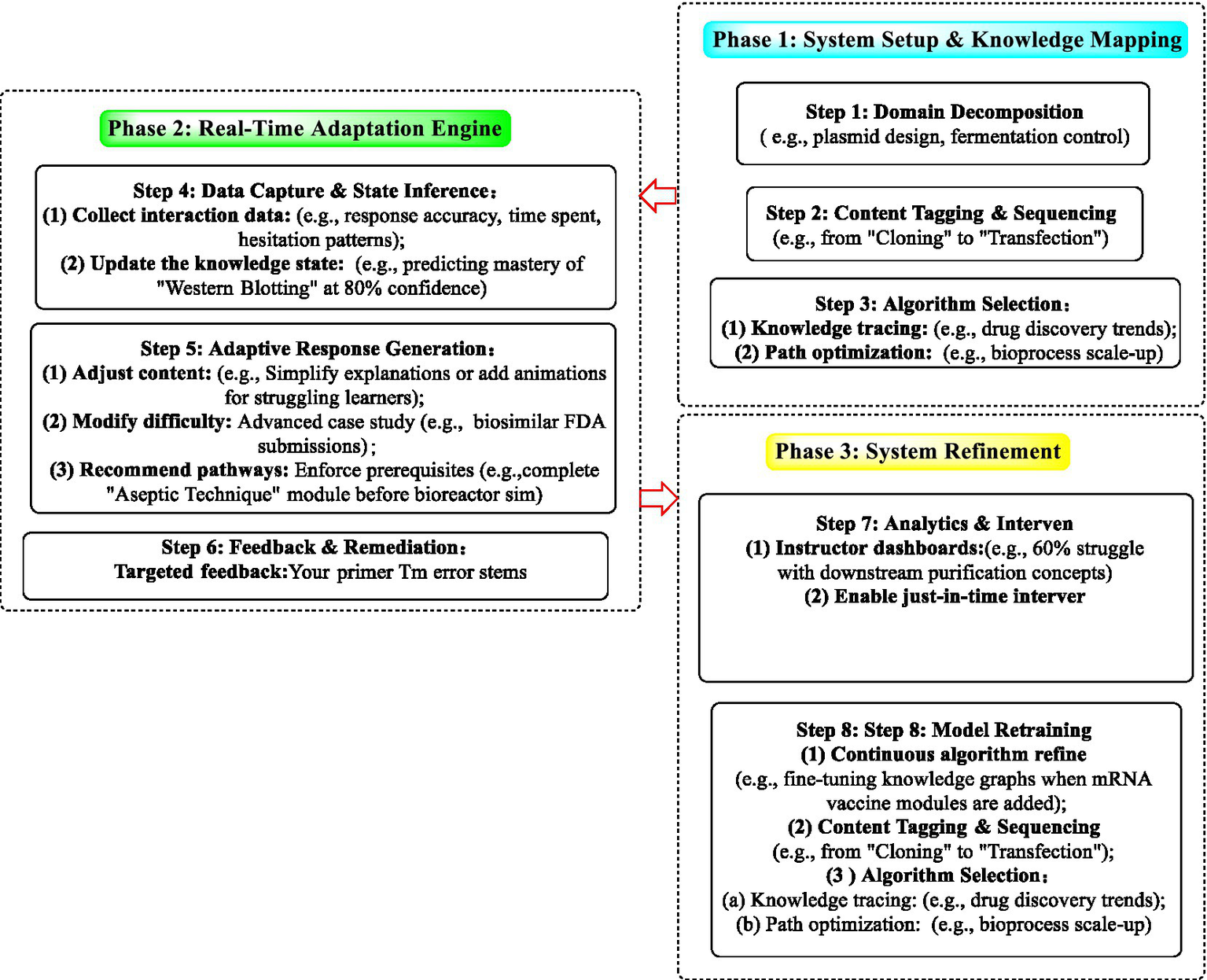
The tri-phase adaptive learning framework for biopharmaceutical training.
Phase 1: system setup and knowledge mapping.
This initial stage involves structuring the educational domain into granular knowledge nodes (e.g., plasmid design, fermentation control) with explicit prerequisite links (e.g., from “Cloning” to “Transfection”). The learning resources are then tagged and sequenced according to these nodes. Finally, computational models are selected to align with learning objectives, such as RNNs for tracking knowledge progression in dynamic topics or reinforcement learning (RL) for optimizing complex skill pathways such as bioprocess scale-up.
Phase 2 real-time adaptation engine.
During this operational phase, learner interactions (e.g., response accuracy and time-on-task) are continuously captured to infer and update individual knowledge states using probabilistic models. Using these inferences, the system dynamically personalizes instructions by adjusting the content complexity, modulating the task difficulty, and enforcing prerequisite learning sequences. Concurrently, targeted feedback is provided based on error analysis (e.g., correcting miscalculations in primer Tm due to omitted Mg2+ concentration).
Phase 3 system refinement.
The final phase focuses on continuous improvement through analytics and model retraining. Instructor dashboards highlight cohort-wide and individual risk patterns (e.g., widespread difficulty with downstream purification), enabling timely interventions. The core algorithms are iteratively refined with new data to maintain relevance, particularly when integrating emerging content (e.g., mRNA vaccine modules), thereby sustaining an adaptive and responsive learning environment.
2.6 Applications of AL in curriculum design and pedagogy
This section examines how ALS transforms educational practices. It analyzes the key application domains in educational research, presents a concrete implementation example for medical education, and concludes with strategic recommendations for teaching reform. This is believed to be capable of offering a comprehensive view of ALS integration in modern curricula.
2.6.1 Potential applications of ALS in educational research
In educational research, ALS demonstrates the transformative potential for optimizing traditional pedagogical models through several strategies (Table 3). (1) Dynamic personalization of learning pathways creates individualized trajectories for students (Mejeh and Rehm, 2024). ALS is able to leverage AI-driven analytics to assess individual learner profiles (knowledge levels, cognitive styles, and engagement patterns) and generate tailored curricula. This replaces static “one-size-fits-all” approaches with adaptive content sequencing, ensuring optimal challenge levels and minimizing knowledge gaps. (2) Real-time feedback loops and interventions detect struggling learners early (Naseer and Khawaja, 2025), which is similar to AI-driven prescription-checking systems in pharmacy practice. By continuously monitoring learner performance (e.g., quiz responses and interaction frequency), ALS can provide immediate corrective feedback and automatically adjust instructional strategies. This contrasts with the delayed feedback in conventional models, enhancing metacognitive awareness and retention. (3) ALS also enables data-driven instructional optimization by aggregating granular learning analytics (e.g., time-on-task and error patterns). These insights facilitate evidence-based refinements of both the system architecture and pedagogical practices. Educators gain actionable insights to modify content delivery and address cohort-level deficiencies. (4) Ethical-AI frameworks maintain educational governance. ALS integrates human oversight to preserve educators’ authority over critical decisions (curricula/interventions). This ethical safeguard confines AI to its assistive roles, thereby preventing autonomous overreaching. (5) Competency-based progression embodies educational paradigms aligned with the Accreditation Council for Graduate Medical Education (ACGME) core competencies, particularly practice-based learning and improvement. By prioritizing skill mastery over time-based benchmarks, it ensures learners achieve predefined proficiency levels before advancing—a principle validated in competency-based medical education (CBME) frameworks. (6) Hybrid instructional models, exemplified by immersive technologies such as VR surgery simulators in residency training (Mariani et al., 2021), synthesize adaptive digital modules with traditional didactic methods. Such integration enhances procedural skill development through risk-free, repeatable simulations, which have been successfully implemented in Canadian neurosurgery programs (Ryu et al., 2017). The hybrid model also incorporates AI-driven adaptive learning systems, which tailor content delivery based on individual performance metrics, as seen in competency frameworks for healthcare AI integration. (7) As for AI literacy development, modern medical education frameworks systematically cultivate the capacity of learners to critically evaluate AI tools (Paranjape et al., 2019). This involves staged training in the development of fundamental AI principles (e.g., algorithm bias detection), ethical implications of clinical AI deployment, and hands-on experimentation with diagnostic support systems. As outlined in the scaffolded AI literacy framework (LaFlamme, 2025), progression occurs through four tiers, from basic understanding to advanced evaluation, which ensures that clinicians can responsibly leverage AI while maintaining human oversight in decision-making.
Table 3
| Application domain | Core mechanism | Educational impact | Contrast with traditional models | Implementation examples |
|---|---|---|---|---|
| 1. Dynamic personalization | AI-driven analytics assess learner profiles (knowledge levels, cognitive styles, and engagement patterns) | • Generates individualized learning trajectories • Ensures optimal challenge levels • Minimizes knowledge gaps | Replaces static “one-size-fits-all” curricula | Adaptive content sequencing based on real-time diagnostics |
| 2. Real-time intervention | Continuous monitoring of performance metrics (quiz responses, interaction frequency) | • Early detection of struggling learners • Enhanced metacognitive awareness • Improved knowledge retention | Eliminates delayed feedback cycles | AI-driven prescription checking is analogous to pharmacy systems |
| 3. Instructional optimization | Aggregation of granular learning analytics (time-on-task, error patterns) | • Evidence-based refinement of pedagogy • Identification of cohort-level deficiencies • Actionable educator insights | Transcends subjective teaching adjustments | Analytics dashboards informing content delivery modifications |
| 4. Ethical-AI governance | Embedded human oversight mechanisms | • Preserves educator authority on curriculum/ intervention • Prevents autonomous AI overreach | Confines AI to assistive roles | Framework requiring educator approval for critical decisions |
| 5. Competency-based progression | Mastery verification prior to advancement | • Aligns with ACGME core competencies (e.g., practice-based learning) • Ensures predefined proficiency attainment | Shifts focus from time-based to skill-based benchmarks | Validation through Competency-Based Medical Education (CBME) frameworks |
| 6. Hybrid instructional models | Synthesis of adaptive digital modules with didactic methods | • Enhances procedural skill development • Enables risk-free repetitive practice | Integrates immersive technologies with conventional teaching | • VR surgery simulators in residency training • Canadian neurosurgery programs’ implementation • Healthcare AI competency frameworks |
| 7. AI literacy development | Scaffolded training curriculum (4-tier progression) | • Critical evaluation of clinical AI tools • Responsible deployment with human oversight | Systematically cultivates missing technical competencies | • Algorithmic bias detection training • Ethical analysis of clinical AI • Hands-on diagnostic system experimentation |
Potential applications of ALS in educational research.
These enhancements collectively address the longstanding limitations of standardized education models while preserving essential human-centric pedagogical values.
2.6.2 Example workflow of ALS in medical education reform
Figure 4 explores the integration of AI literacy skills within a third-year clinical diagnostics curriculum. This comprehensive framework for neurology education (e.g., ALS diagnosis) operates across four integrated phases. Phase 1 (Pre-class) involves faculty-AI collaboration to build dynamic knowledge graphs linking symptoms to pathophysiology and treatments, while AI personalizes pre-class tasks using student performance data. Phase 2 (In-class) deploys adaptive virtual patient simulations, where AI adjusts case complexity in real time and provides tiered scaffolding for high performers. Phase 3 (Post-class) uses AI-generated quizzes targeting individual weaknesses, integrates ethics training on algorithmic bias, identifies at-risk students via error analysis, and enables faculty to refine teaching based on analytics. Phase 4 (Longitudinal) ensures continuous evolution through cross-institutional resource sharing and system updates with real-world data. Key outcomes include improved student diagnostic accuracy and critical AI evaluation skills alongside institutional cost reductions via shared AI ecosystems.
Figure 4
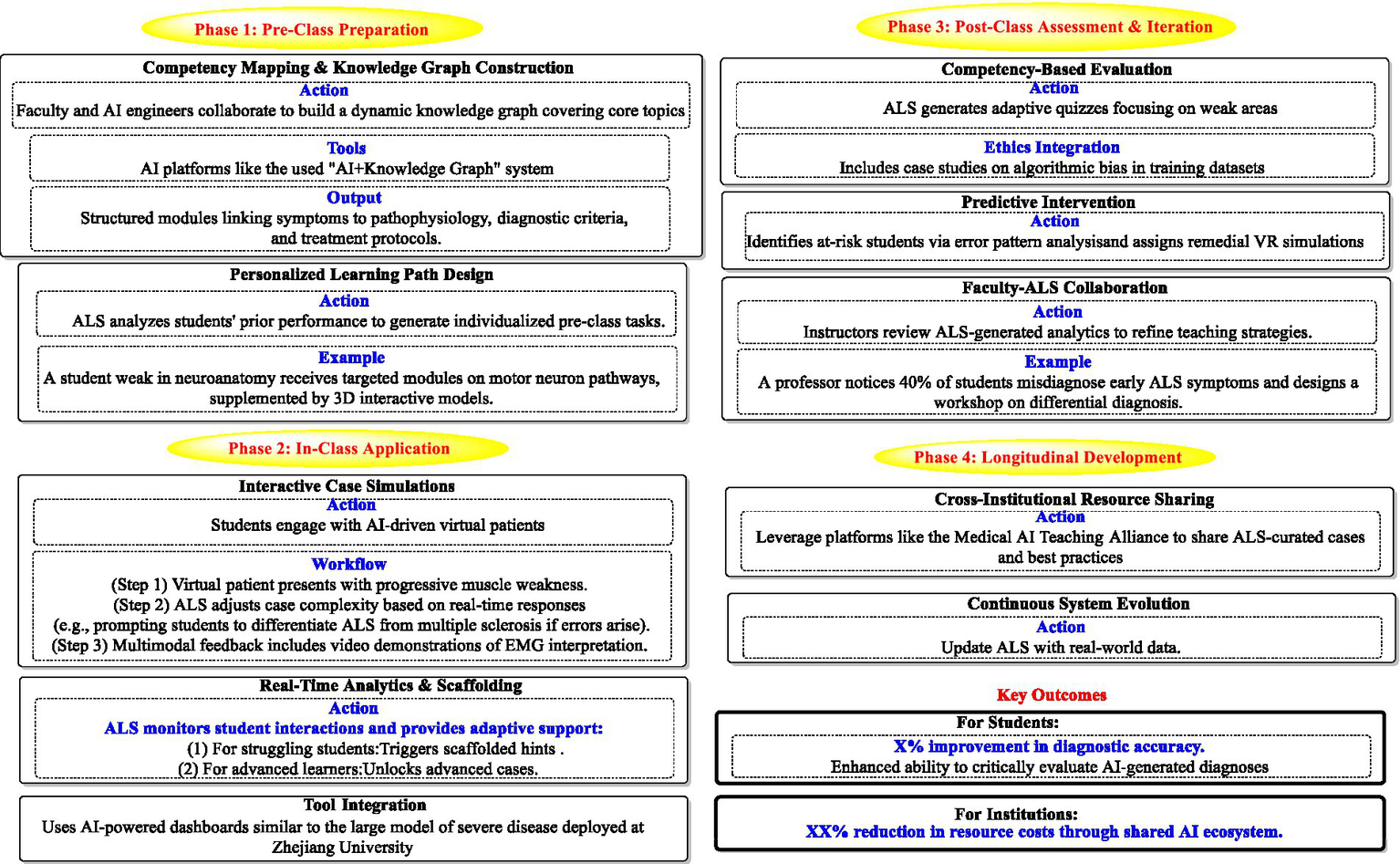
Proposed integration of AI literacy skills within a third-year clinical diagnostics curriculum.
2.6.3 Strategic recommendations for teaching reform
As shown in Table 4, strategic teaching reform requires a dual-pronged transformation, including evolving teacher training and implementing outcome-driven evaluation. Faculty members must be equipped to curate AI-generated case studies (e.g., optimizing mRNA vaccine stability) and anchor ALS feedback within core scientific principles. Parallel to this, adopting “AI co-teaching” frameworks will refocus educators on higher-order mentoring, such as critically evaluating drug formulation strategies proposed by ALS. Concurrently, assessment should transition from static exams to ALS-facilitated adaptive simulations of real-world scenarios, such as managing time-pressured bioprocess contamination events. Finally, learning analytics should track longitudinal competence development and correlate adaptive engagement with professional outcomes, including residency performance.
Table 4
| Domain | Short-term actions | Long-term vision |
|---|---|---|
| Curriculum design | Map ALS modules to ACPE competency standards | Industry-codeveloped adaptive micro-credentials |
| Faculty roles | Train educators as “AI interpreters” for ALS outputs | Shift to learning experience designers |
| Assessment | Embed ALS-driven analytics in longitudinal competence tracking | Real-time adaptive OSCEs with AI proctoring |
| Infrastructure | Hybrid cloud solutions for computational loads | Federated learning networks across institutions |
Implementation priorities for ALS integration.
2.7 Specific applications in biotech and pharmaceutical education
As shown in Figure 5, adaptive learning could significantly streamline biopharmaceutical education through domain-specific strategies, including molecular biology scaffolding that enables complex concept mastery, reinforcement learning that optimizes precision dosing protocols, and hybrid AI biotech models that enhance bioprocessing efficiency. All of these are capable of collectively accelerating competency development across drug discovery-to-manufacturing pipelines. These critical functions are detailed in the subsequent sections.
Figure 5
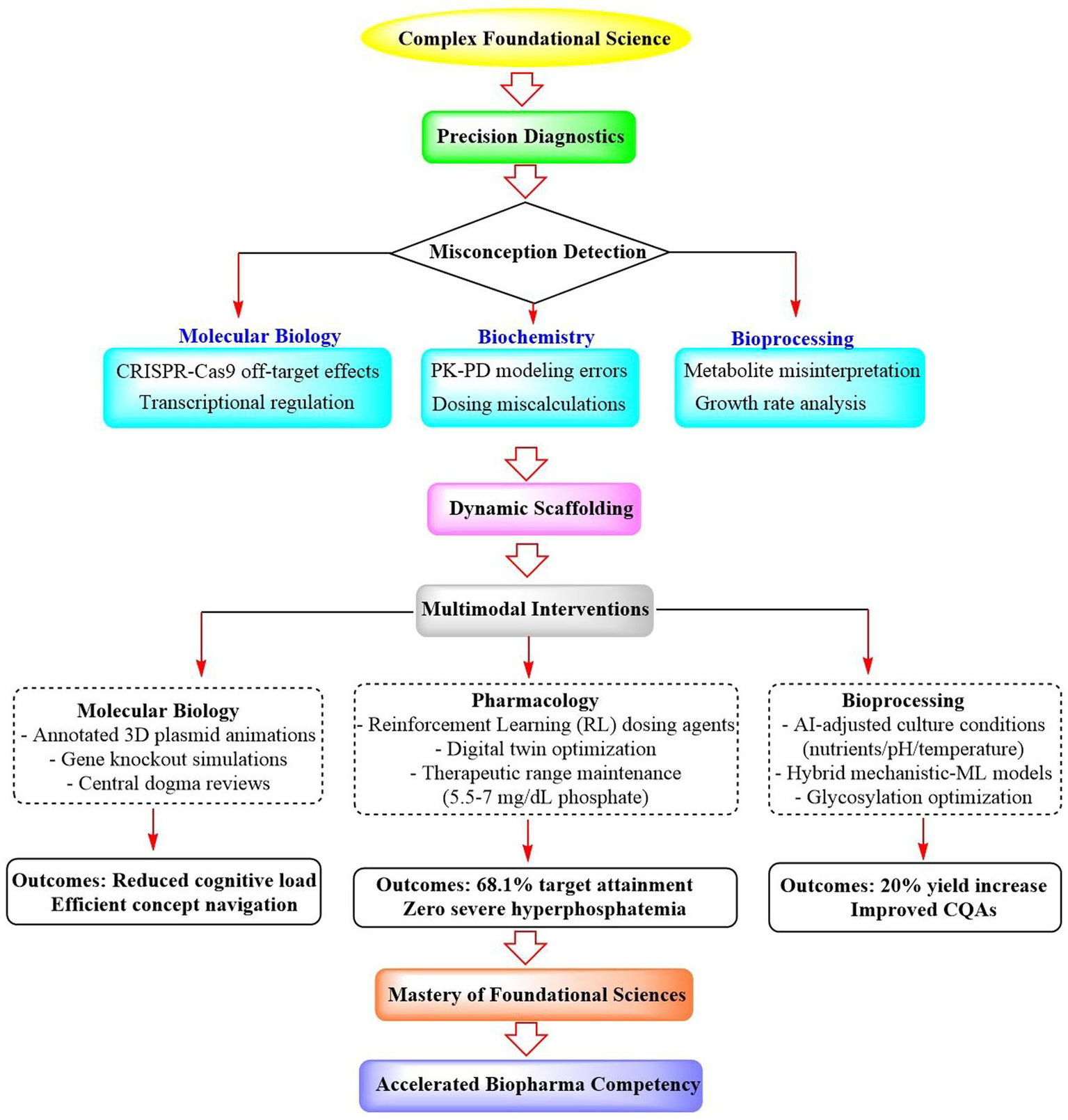
Precision-to-mastery framework: adaptive scaffolding of complex biopharmaceutical sciences.
2.7.1 Mastering complex foundational sciences
ALS demonstrates significant efficacy in the scaffolding mastery of core biotechnology and pharmaceutical sciences through precision diagnostics and dynamic scaffolding.
In the fields of molecular biology and genetics, ALS deploys knowledge tracing algorithms to detect misconceptions in real time, such as misinterpretations of CRISPR-Cas9 off-target effects (Luo et al., 2024) or transcriptional regulation dynamics (Josephs-Spaulding et al., 2024). Upon identifying conceptual gaps, the system activates multimodal interventions. For instance, annotated 3D animations elucidate plasmid vector assembly, interactive simulations guide gene knockout experimental design, and automated prerequisite reviews reinforce central dogma principles. In this way, it could reduce cognitive load and enable efficient navigation of complex topics.
In biochemistry and pharmacology education, ALS implements competency-based progression architectures, wherein mastery of foundational concepts controls advancement. For example, in a study, an RL framework integrated with PK-PD modeling was developed to personalize erdafitinib dosing for metastatic urothelial carcinoma (De Carlo et al., 2024). Each patient had a dedicated Q-learning agent trained on their digital twin (PK-PD model) to optimize adaptive dosing rules, aiming to maintain serum phosphate levels within the therapeutic range while minimizing toxicity. The results showed that the RL approach outperformed the FDA-approved protocol; it increased the percentage of patients within the target phosphate range from 56.7 to 68.1% at the end of treatment and eliminated severe hyperphosphatemia events. This method enables tailored initial doses and dynamic adjustments, thereby enhancing efficacy and safety. This demonstrates the potential of RL for precision dosing in oncology, leveraging digital twins to individualize therapy beyond population-based protocols and improving outcomes for drugs with narrow therapeutic windows.
In cell culture and bioprocessing training, it could leverage AI algorithms to analyze real-time data (e.g., metabolites and growth rates) and dynamically adjust culture conditions (e.g., nutrient feed, pH, and temperature)(Qian et al., 2025; Ranpura et al., 2025). This enables personalized process optimization for specific cell lines or products, improving titer yields (e.g., up to 20%) and critical quality attributes, such as glycosylation patterns. Hybrid models combining mechanistic knowledge with machine learning further enhance predictive accuracy and reduce experimental costs. Overall, adaptive learning accelerates bioprocess development, ensures consistency, and supports Industry 4.0 goals in biomanufacturing.
2.7.2 Enhancing laboratory and technical skill development
ALS revolutionizes experimental training through intelligent simulation scaffolding and precision skill remediation, effectively bridging the theory-practice gap in biotechnology education. Virtual laboratories integrated with industry-standard platforms (e.g., Bio-Rad ELISA workflows, Agilent HPLC systems, and Sartorius bioreactors) deploy dynamic complexity modulation driven by real-time performance analytics. For instance, adaptive learning was proposed to be capable of enhancing laboratory and technical skill development through dynamic knowledge graphs that integrate resources across organizations (Bai et al., 2024). Autonomous agents execute workflows, enabling real-time collaborative optimization (e.g., linking labs in Cambridge and Singapore for pharmaceutical reaction optimization). This approach automates design-make-test-analyze cycles, records data provenance for reproducibility, and dynamically adjusts experiments based on performance. It accelerates discovery (e.g., generating a Pareto front in 3 days) and supports scalable, high-throughput experimentation while overcoming geographical and technical barriers.
ALS utilizes AI to personalize skill development in biopharma and dynamically tailor training content (e.g., complex techniques, regulatory compliance, and computational biology) to individual knowledge gaps and learning pace. Recently, AIxFuse was developed, which integrates pharmacophore combinations and molecular docking via collaborative RL and AL (Chen et al., 2024). It contains two key steps: two self-play MCTS agents capable of optimizing pharmacophore fusion and an AL-trained critic used for evaluating dual-target binding. It generated molecules satisfying dual-target structural constraints, achieving 32.3% higher success rates than those of state-of-the-art methods (e.g., GSK3β/JNK3 and RORγt/DHODH). This is believed to be capable of accelerating therapies for complex diseases by overcoming structural constraints in a multitarget design. Similarly, AMVL was designed by integrating chemical-induced transcriptional profiles (CTPs), knowledge graph embeddings, and large language model (LLM) representations (Yan et al., 2025). This process is mainly achieved through multiview learning, matrix factorization, and ensemble optimization. It outperformed state-of-the-art methods in predicting drug-disease associations across benchmark datasets, with 7 of the top 10 predictions validated by the post-2011 literature. This framework provides a robust and scalable solution for accelerating drug repurposing by unifying multisource data and enhancing translational medicine research. Therefore, it is essential to integrate these strategies into modern higher education curricula and training programs to accelerate the development of the biopharmaceutical industry.
2.7.3 Navigating regulatory affairs and quality systems
ALS fundamentally reconfigures compliance training through contextually embedded simulation architectures and failure-driven remediation protocols, directly addressing escalating global regulatory demands in biopharmaceutical development. The American Society of Mechanical Engineers (ASME) and Verification and Validation 40 (V&V 40) were proposed for risk-based model credibility assessment and the FDA’s AI/ML lifecycle management framework to verify, validate, and manage computational models (including statistical, mechanistic, and ML) in biopharmaceutical manufacturing (Bideault et al., 2021). Using curated hypothetical examples, this study demonstrates the utility of these frameworks for ensuring model credibility and argues for standardized approaches to facilitate adoption and alignment with existing good practices. Specifically, for adaptive learning models, the FDA framework provides critical value by enabling structured lifecycle management. It allows models to safely learn from new data post-deployment within GxP environments through pre-approved change protocols, rigorous monitoring, and controlled retraining/adaptation, thereby maintaining credibility as the conditions evolve.
In a recent study, three novel machine learning models (APMLR, AIOM, and IAMRF) were developed for predicting critical nephrology laboratory results (eGFR, creatinine, and urea)(Pawus et al., 2025). These models leverage adaptive learning to personalize predictions based on individual patient profiles and dynamically adjust them to temporal data and unique characteristics. APMLR achieved 96.97% accuracy using linear SVR, whereas gradient boosting yielded ~95% accuracy for AIOM/IAMRF. This highlights that AL could provide key value by enabling continuous patient-specific refinement, handling non-stationary clinical data, and improving long-term monitoring accuracy for personalized renal care.
2.7.4 Competency-based assessment and certification
AL serves as a key enabler for competency-based pharmacy education (CBPE) by personalizing educational pathways, allowing students to progress at their own pace, and dynamically adjusting content to address individual strengths and weaknesses. Therefore, this finding supports the shift from time-bound to competency-focused outcomes.
In one study, entrustable professional activities (EPAs) were integrated into a competency-based clinical assessment tool for a Family Nurse Practitioner (FNP) program (Anthamatten and Pitts, 2024). This system defines four performance levels (Novice to Proficient) linked to preceptor support needs. In addition, it requires students to document EPA performance frequency during clinical experience to track competency development toward practice readiness. In this system, AL holds a significant value by analyzing individual student EPA performance data to automatically identify specific competency gaps. Then, it dynamically delivers personalized simulations or learning modules targeting those weaknesses, thereby enabling efficient remediation and optimized progression toward clinical competence.
In addition, an adaptive learning implementation framework (ALIF) was developed (Mirata and Bergamin, 2023), which identifies empirical relationships between determinants (e.g., technological barriers and leadership commitment), implementation strategies (e.g., institutional investment and stakeholder training), and outcomes (e.g., stakeholder acceptability and scaled implementation) in higher education. Derived from a Delphi study across Swiss and South African universities, ALIF emphasizes organizational readiness and stakeholder acceptance as critical for successful deployment. Within this framework, adaptive learning serves as the core technological innovation requiring systemic institutional support, pedagogical redesign, and contextual adaptation to overcome implementation barriers and achieve personalized, scalable education.
2.7.5 Continuous professional development (CPD)
Recently, a 3-year longitudinal personal-professional development (LPPD) program was developed for biomedical students to cultivate adaptive competencies such as self-awareness, resilience, and lifelong learning through coached group sessions and individual consultations (van Ede et al., 2023). Adaptive learning serves as the core pedagogical framework, which is capable of enabling personalized feedback, self-directed goal setting, and iterative skill refinement in response to real-world challenges, ultimately fostering professional identity formation amid uncertainty. Similarly, the Master Adaptive Clinician Educator (MACE) framework was introduced, which extends the master adaptive learner (MAL) model to clinician-educators (Snydman et al., 2025). This emphasizes continuous self-directed growth, innovation in teaching, and adaptation to evolving educational needs. Adaptive learning underpins the MACE model by enabling educators to iteratively plan, implement, assess, and adjust teaching strategies using metacognitive reflection and feedback, thus fostering expertise in curriculum design, mentorship, and leadership beyond traditional approaches.
3 Advantages, challenges, and future directions
The integration of ALS into biopharmaceutical education represents a paradigm shift from standardized instruction to personalized competency-driven training. By leveraging AI algorithms, AL tailors content delivery based on individual learner profiles, real-time performance, and contextual demands. This analysis synthesizes empirical insights and identifies critical pathways for innovation.
3.1 Key advantages of AL in biopharmaceutical education
This section highlights the principal benefits of ALS in biopharmaceutical education and details its transformative impacts through three key dimensions: personalized competency development that addresses individual learning gaps, realistic simulation of industry workflows that bridges theory and practice, and scalable integration of academic training with industry requirements.
3.1.1 Personalized competency development
AL systems are capable of diagnosing gaps in core competencies (e.g., AI literacy, drug analysis, and clinical reasoning) and delivering customized modules. For instance, ChatGPT-assisted multiadvisor systems enable students to tackle complex cases (e.g., therapeutic drug monitoring and gene testing) with scaffolded guidance, significantly improving clinical decision-making skills. Student-centric data revealed that AL could bridge disparities between undergraduates (prioritizing foundational knowledge) and postgraduates (emphasizing project-based learning) by aligning training with diverse career trajectories.
3.1.2 Simulation of real-world workflows
AL transforms simulations into intelligent training ecosystems by dynamically adjusting scenarios based on individual performance, enabling risk-free mastery of complex workflows (e.g., drug analysis or bioprocessing) while bridging skill gaps between theory and practice. Its core value lies in accelerating competency development through personalized context-sensitive repetition, which can prepare learners for real-world challenges with precision and efficiency. For example, drug analysis software (e.g., virtual HPLC-MS simulators) could provide risk-free environments for mastering instrumentation, data interpretation, and GLP compliance. These platforms reduce reagent costs by 30–40% while enabling iterative skill refinement. In the “HVS model” (horizontal cross-discipline fusion, vertical full-cycle coverage, sentimental value internalization), it integrates AL with virtual labs (e.g., GLP safety evaluation simulations), which enables students to navigate drug R&D pipelines from synthesis to pharmacovigilance.
3.1.3 Scalable industry-academia integration
AL platforms could efficiently embed industry standards and real-time data from biomanufacturing facilities. For example, courses on AI-Driven Drug Synthesis (Das, 2025) or Biopharma Digital Twins (Shahab et al., 2025) incorporate live production anomalies, which are capable of training learners to troubleshoot deviations in monoclonal antibody purification. Intellia Therapeutics and MIT co-developed the CRISPR equipment predictive maintenance course using real device logs (e.g., electroporator voltage fluctuations), with Boston/San Francisco campuses specializing in gene editing/AAV production equipment to serve regional bioclusters.
3.2 Critical challenges and limitations
Despite its promising advantages, the implementation of AL systems faces several significant barriers. This section discusses these critical challenges, including technical fragmentation and interoperability issues, algorithmic bias in educational pathways, faculty readiness gaps, computational resource constraints, and broader ethical concerns regarding equity, data privacy, and algorithmic transparency.
3.2.1 Technical and pedagogical fragmentation
A major barrier to scalable ALS implementation is technical fragmentation, primarily because of a lack of interoperability. Isolated tools (e.g., standalone AI tutors, virtual labs, and generative AI chatbots) often operate outside core learning management systems (LMS), which might create data silos that prevent a unified view of learner progress. For example, it was found that 37% of student competency profiles were incomplete due to such disconnected data in the UCSF Pharmacy School, which hinders holistic competency tracking and complicates accreditation. Furthermore, this isolation amplifies the risk of generative AI hallucinations. Unanchored from authoritative databases (e.g., DrugBank, EMBL-EBI), tools such as ChatGPT may propagate dangerous inaccuracies in complex domains (e.g., misstating drug mechanisms), undermining educational integrity. Addressing these issues requires integrated architectures with two key components. The first is LTI 1.3-compliant LMS embedding to maintain data continuity, and the second is retrieval-augmented generation (RAG) frameworks to ground AI outputs in verified knowledge. Thereby, it could ensure both seamless data integration and content validity.
3.2.2 Algorithmic bias in personalized pathways
Another critical challenge in ALS implementation is algorithmic bias, which threatens educational equity. Bias can originate not only from unrepresentative training data but also from pedagogical designs that prioritize technical skill acquisition while overlooking essential non-cognitive competencies such as empathy, ethical reasoning, and communication. An ALS that optimizes solely for procedural mastery risks producing graduates who are technically proficient yet deficient in the humanistic skills vital for patient-centered care.
To mitigate these risks, proactive and continuous bias detection frameworks are essential. Techniques such as SHAP value-monitored demographic parity audits can quantify the influence of input features on model outcomes and identify disparate impacts on demographic subgroups. Furthermore, a holistic evaluation strategy should be adopted that incorporates multimodal metrics, for instance, using natural language processing to assess communication skills in simulated patient interactions. In addition, regular model retraining on ethically curated datasets is also capable of ensuring ongoing fairness. Ultimately, a vigilant approach to bias mitigation could be indispensable for developing ALS that is not only adaptive but also equitable and professionally holistic.
3.2.3 Faculty readiness and computational resource constraints
The effective implementation of ALS may also confront two interconnected barriers: faculty readiness and computational resource limitations. Educators must transition from traditional instructors to “AI interpreters,” who are capable of curating AI-generated content and critically evaluating algorithmic outputs. Without targeted upskilling through initiatives such as AI literacy bootcamps, faculty members might struggle to mediate ALS recommendations effectively, thus limiting their pedagogical value. Concurrently, high-fidelity simulations essential for biopharmaceutical training (e.g., CRISPR workflows or bioreactor operations) demand substantial computational resources, which might exclude many institutions, particularly in resource-limited settings.
To efficiently deal with these challenges, integrated strategies are also required. Faculty development must be coupled with technical solutions, such as edge-cloud hybrid systems and federated learning, which distribute computational loads across institutions while preserving data privacy. Blockchain-based resource consortia could further enable shared access to high-performance computing. This dual approach of empowering educators and optimizing infrastructure may be necessary for equitable and scalable ALS adoption in biopharmaceutical education.
3.2.4 Equity and ethical risks
The implementation of ALS might exacerbate the existing educational inequities, particularly through infrastructural disparities that widen the urban–rural skill gap. Resource-limited institutions may face prohibitive challenges, including inadequate cloud infrastructure and disproportionately high computational costs relative to their budget. This digital divide is compounded by the substantial bandwidth requirements of advanced simulations, which systematically exclude approximately 83% of rural institutions from high-fidelity training in essential techniques, such as CRISPR workflows or bioreactor operations.
To bridge this divide, bandwidth-adaptive content delivery protocols can be utilized to optimize resource allocation for constrained connectivity environments, whereas federated edge learning systems enable offline-capable simulations and privacy-preserving model refinement on local devices. These approaches should also be reinforced through public-private infrastructure partnerships, potentially incorporating sustainable solutions such as solar-powered hardware for operational resilience in remote areas. Collectively, these measures would help transform ALS into a more inclusive educational infrastructure and ensure equitable access to competency development across geographical and socioeconomic boundaries.
3.2.5 Data privacy, consent, and algorithmic transparency
Beyond the issues of algorithmic bias, the ethical deployment of ALS in biopharmaceutical education necessitates a rigorous framework for data governance, informed consent, and algorithmic transparency. The extensive data collection required for effective personalization (e.g., detailed interaction logs, performance metrics, and behavioral biometrics) raises significant privacy concerns. To address this, future ALS architectures must implement privacy-by-design principles and use techniques such as federated learning (where model training occurs locally on user devices without raw data leaving the institution) and differential privacy (which adds statistical noise to the aggregated data) to minimize privacy risks.
Furthermore, the current model of blanket consent obtained at course onset is inadequate, given the dynamic and often intrusive nature of ALS data collection. We therefore propose a shift toward granular, tiered consent models. Such frameworks empower learners by granting them control over how their data are used for personalization, analytics, and research, thereby actively fostering trust.
The lack of transparency in complex models could also undermine their accountability and educational integrity. To build trust and uphold educational standards, it is crucial to develop explainable AI (XAI) techniques. These systems can generate interpretable rationales, such as explaining to a student that their performance on specific prerequisite items necessitates a review of a “Pharmacokinetics” module. This capability is key to making AI’s pedagogical logic clear and actionable for learners. This transparency is crucial because it enables educators to trust the guidance from these systems and encourages students to accept feedback as legitimate. This fundamental shift transforms ALS from an opaque arbitrator into a comprehensible pedagogical partner.
3.3 Future research directions
To address the existing limitations and advance the field, this section outlines priority research avenues for enhancing AL in biopharmaceutical education. These include developing integrated AL architectures with blockchain-secured credentials, establishing human-AI synergy frameworks, implementing equity-driven deployment models, validating AL efficacy through rigorous trials, and expanding immersive technology applications.
3.3.1 Developing integrated AL architectures
Future research must advance beyond the current landscape of fragmented AL tools by developing a truly integrated architecture. Although the use of APIs for achieving technical interoperability is an established concept in computer science, its specific application to create unified and data-fluid ALS ecosystems within biopharmaceutical education remains a novel and critical pursuit. The proposed innovation involves architecting seamless data exchanges between core educational modules (e.g., virtual labs, AI tutoring systems, and dynamic knowledge maps) to enable cross-platform analytics and holistic skill trajectory mapping.
A key example of this integrated approach is the novel proposal of blockchain-secured microcredentialing systems. This extends beyond simple digital badges. It constitutes an innovation for providing tamper-proof and verifiable certifications for specialized pharmaceutical competencies (e.g., aseptic processing and pharmacogenomics analysis). The cryptographic immutability of blockchain would eliminate credential fraud while enabling seamless skill portability across industry-academia boundaries. This directly addresses significant economic losses from critical skill gaps by ensuring that workforce competencies are transparent, trusted, and rapidly recognized. Thereby, it would accelerate talent deployment and reduce onboarding burdens in the global biopharmaceutical sector.
3.3.2 Human-AI synergy frameworks
While task partitioning between human and artificial intelligence represents an established research domain, a novel “multiadvisor” system specifically designed for pharmacy education could be introduced. This framework is designed to be capable of strategically delegating computational tasks, including ADMET property prediction via molecular dynamics, to AI components. Conversely, context-dependent challenges, such as designing ethical trials for vulnerable populations and navigating regulatory ambiguities, are reserved for human mentors.
The innovation of this study lies in its detailed three-phase technical roadmap. This involves fine-tuning models such as Llama-3 on ethics transcripts, integrating electronic lab notebooks, and ultimately deploying a “Regulatory Copilot” in clinical platforms. This structured approach, enhanced by BioBERT (Lee et al., 2020)-powered dialogue understanding, is able to transform AI from merely assistive tools into collaborative teammates that significantly accelerate data processing while maintaining crucial human oversight in complex decision-making processes.
3.3.3 Equity-driven AL deployment
Equity-driven adaptive learning deployment could bridge global biopharmaceutical education gaps through federated edge learning systems. It enables offline simulations on accessible devices that are capable of overcoming bandwidth constraints in resource-limited regions while ensuring data sovereignty through privacy-preserving architectures. Public-private infrastructure partnerships can amplify this approach by establishing shared computing resources for computationally intensive training. These partnerships could be further strengthened through solar-powered hardware and mesh networking capabilities, which ensure operational resilience in challenging environments. Culturally responsive design integrates localized knowledge systems and multilingual support governed by inclusive policy frameworks mandating gender equity and disability access. Collectively, these strategies would transform AL into a universally accessible infrastructure and enable remote learners to achieve parity with leading institutions through government-subsidized technologies. Moreover, it could systematically address connectivity barriers, economic limitations, and culturally relevant gaps in global pharmaceutical education.
3.3.4 Validation and standardization
Validation and standardization of AL in pharmaceutical education require robust efficacy benchmarking through controlled comparative trials to systematically address the prevalent methodological gaps in current studies. This entails evaluating competency outcomes across diverse instructional modalities while integrating longitudinal performance-tracking mechanisms. Global regulatory frameworks must establish comprehensive bias-auditing protocols that cover the verification of dataset representativeness and runtime fairness monitoring through quantifiable equity metrics. These protocols should be reinforced by internationally harmonized certification standards aligned with pharmaceutical quality systems. This dual approach could elevate AL to clinically validated training tools and subject it to GMP-grade verification standards. It establishes rigorous performance thresholds, ensuring regulatory-grade safety and equitable outcomes across learning environments.
3.3.5 Expanding immersive technologies
The advancement of immersive technologies in pharmaceutical training has been revolutionized through two interconnected paradigms. On the one hand, augmented reality systems provide hands-free sterile manufacturing guidance via integrated sensing and biomechanical monitoring, where empirical validation demonstrates a significant reduction in procedural errors and enhanced aseptic success rates in industrial settings. Meanwhile, adaptive bioreactor digital twins dynamically modulate instructions through real-time sensor networks, which trigger context-specific learning modules when the critical process parameters deviate. The convergence of these approaches establishes a cross-reality validation framework demonstrating accelerated competency development and improved fault diagnosis capabilities. In addition, machine learning algorithms could further enable predictive contamination risk management. These systems now satisfy key regulatory requirements for process validation through integration with the industrial IoT infrastructure and effectively transform immersive tools into cognitive extensions that actively prevent contamination during production operations. Concurrently, they continuously develop engineering expertise while enabling the digital transformation of manufacturing documentation and substantial resource optimization via AI-driven process refinement.
4 Conclusion
This review demonstrates that AI-powered ALS constitutes a transformative lever for biopharmaceutical education, fundamentally shifting the paradigm from standardized time-based instruction to precision mastery-oriented training. By integrating probabilistic KSM with real-time analytics of knowledge level and knowledge structure, ALS ensures that learners achieve validated competency before advancing through inherently sequential domains such as genetic engineering or pharmacodynamics. This review presents empirical evidence from diverse applications, including molecular biology scaffolding, AI-driven virtual labs, regulatory affairs simulations, and competency-based microcredentialing. This body of evidence demonstrates tangible benefits such as shortened time-to-competence, significant cost reduction, and strengthened alignment with industry workflows.
Nevertheless, the path to widespread adoption is contingent on overcoming three critical and interconnected bottlenecks. The first is the technical fragmentation that isolates specialized tools from institutional learning-management ecosystems. The second is the algorithmic and infrastructural inequities that risk exacerbating urban–rural skill gaps. Finally, there is prevailing faculty unpreparedness for new and AI-augmented pedagogical roles. Addressing these challenges necessitates a coordinated research and policy agenda, which is prioritized as follows: First, the development of open and API-driven interoperability standards, which could be fortified by blockchain-secured micro-credentials to ensure verifiable skill portability. Second, the implementation of equity-driven deployment models that leverage federated edge learning and bandwidth-adaptive content delivery to guarantee inclusive access. Third, the execution of longitudinal validation trials, benchmarked against pharmaceutical-quality regulatory frameworks, to establish ALS as a rigorously validated and regulatory-grade training infrastructure.
By implementing this strategic roadmap, educational institutions and industry stakeholders can co-create a resilient and globally inclusive ecosystem. Such an ecosystem will not only accelerate the cultivation of a versatile, interdisciplinary biopharmaceutical workforce but also firmly establish ALS as a cornerstone of next-generation training infrastructure. This is believed to be ready to meet the demands of the coming decade in drug discovery and biomanufacturing innovation.
Statements
Author contributions
TW: Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. NX: Methodology, Project administration, Writing – original draft, Writing – review & editing. FL: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors express their gratitude to the Natural Science Foundation of RiZhao (no. RZ2024ZR07), the Shandong Provincial University Youth Innovation Team, China (no. 2022KJ102), the National Natural Science Foundation of China (no. 32000194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AlatrashR.ChattiM. A.AinQ. U.JoarderS. (2024). "transparent learner knowledge state Modeling using personal knowledge graphs and graph neural networks", in: Adjunct proceedings of the 32nd ACM conference on user Modeling, adaptation and personalization. Cagliari, Italy: Association for Computing Machinery.
2
AlawnehY. J. J.SleemaH.SalmanF. N.AlshammatM. F.OteerR. S.AlrashidiN. K. N. (2024). "Adaptive learning systems: revolutionizing higher education through AI-driven curricula", in 2024 International Conference on Knowledge Engineering and Communication Systems (ICKECS), 1–5.
3
AliW.FangC.KhanA. (2025). A survey on the state-of-the-art CDN architectures and future directions. J. Netw. Comput. Appl.236:104106. doi: 10.1016/j.jnca.2025.104106
4
AlowaisS. A.AlghamdiS. S.AlsuhebanyN.AlqahtaniT.AlshayaA. I.AlmoharebS. N.et al. (2023). Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med. Educ.23:689. doi: 10.1186/s12909-023-04698-z
5
Al-SharafiM. A.Al-EmranM.ArpaciI.IahadN. A.AlqudahA. A.IranmaneshM.et al. (2023). Generation Z use of artificial intelligence products and its impact on environmental sustainability: a cross-cultural comparison. Comput. Hum. Behav.143:107708. doi: 10.1016/j.chb.2023.107708
6
AnthamattenA.PittsC. (2024). Integration of Entrustable professional activities in a competency-based clinical assessment tool in a nurse practitioner program. Nurse Educ.49, 241–245. doi: 10.1097/NNE.0000000000001697
7
BaiJ.MosbachS.TaylorC. J.KaranD.LeeK. F.RihmS. D.et al. (2024). A dynamic knowledge graph approach to distributed self-driving laboratories. Nat. Commun.15:462. doi: 10.1038/s41467-023-44599-9
8
BalestraN.SharmaG.RiekL. M.BuszaA. (2021). Automatic identification of upper extremity rehabilitation exercise type and dose using body-worn sensors and machine learning: a pilot study. Digit Biomark5, 158–166. doi: 10.1159/000516619
9
BideaultG.ScacciaA.ZahelT.LandertingerR. W.DaluwatteC. (2021). Verification and validation of computational models used in biopharmaceutical manufacturing: potential application of the ASME verification and validation 40 standard and FDA proposed AI/ML model life cycle management framework. J. Pharm. Sci.110, 1540–1544. doi: 10.1016/j.xphs.2021.01.016
10
ChenS.XieJ.YeR.XuD. D.YangY. (2024). Structure-aware dual-target drug design through collaborative learning of pharmacophore combination and molecular simulation. Chem. Sci.15, 10366–10380. doi: 10.1039/D4SC00094C
11
ChutchevaY. V.KuprianovaL. M.SereginaA. A.KukushkinS. N. (2022). Environmental management of companies in the oil and gas markets based on AI for sustainable development: an international review. Front. Environ. Sci.10:952102. doi: 10.3389/fenvs.2022.952102
12
CornyJ.RajkumarA.MartinO.DodeX.LajonchèreJ. P.BilluartO.et al. (2020). A machine learning-based clinical decision support system to identify prescriptions with a high risk of medication error. J. Am. Med. Inform. Assoc.27, 1688–1694. doi: 10.1093/jamia/ocaa154
13
CostaC.Husain-HabibN.ReiterA. (2025). Integrating AI into education: successful strategies, ideas, and tools from psychology instructors. Teach. Psychol.52, 330–338. doi: 10.1177/00986283241297635
14
DasU. (2025). Generative AI for drug discovery and protein design: the next frontier in AI-driven molecular science. Med. Drug Discov.27:100213. doi: 10.1016/j.medidd.2025.100213
15
De CarloA.ToscaE. M.FantozziM.MagniP. (2024). Reinforcement learning and PK-PD models integration to personalize the adaptive dosing protocol of erdafitinib in patients with metastatic urothelial carcinoma. Clin. Pharmacol. Ther.115, 825–838. doi: 10.1002/cpt.3176
16
DorriR.Al-OmariE.Al-HassanM.MohamedS.AnsarS. (2025). Global integration of adaptive quizzing in nursing education: enhancing student performance and bridging educational gaps: a narrative review. Nurse Educ. Pract.84:104334. doi: 10.1016/j.nepr.2025.104334
17
DubeyG.DubeyA. K.KaurK.RajG.KumarP. (2025). Adaptive contextual memory graph transformer with domain-adaptive knowledge graph for aspect-based sentiment analysis. Expert Syst. Appl.278:127300. doi: 10.1016/j.eswa.2025.127300
18
GaoY. F.MengD.LiX. Y.WangS. H.ChenJ. X.CaoR. Y.et al. (2025). Integrating computer vision into the SiO2 precipitation synthesis experiment: digital innovation enhancing chemistry education. J. Chem. Educ.102, 2436–2442. doi: 10.1021/acs.jchemed.4c01418
19
HuangL.ZhangC.ZhangH. Y. (2024). Self-adaptive training: bridging supervised and self-supervised learning. IEEE Trans. Pattern Anal. Mach. Intell.46, 1362–1377. doi: 10.1109/TPAMI.2022.3217792
20
JamratS.SukasemC.SratthaphutL.HongkaewY.SamanchuenT. (2023). A precision medicine approach to personalized prescribing using genetic and nongenetic factors for clinical decision-making. Comput. Biol. Med.165:107329. doi: 10.1016/j.compbiomed.2023.107329
21
JessicaH.BritneyR.SariraE. D.ParisaA.JoeZ.BettyB. C. (2025). Applications of artificial intelligence in current pharmacy practice: a scoping review. Res. Social Adm. Pharm.21, 134–141. doi: 10.1016/j.sapharm.2024.12.007
22
Josephs-SpauldingJ.RajputA.HefnerY.SzubinR.BalasubramanianA.LiG. Y.et al. (2024). Reconstructing the transcriptional regulatory network of probiotic L. reuteri is enabled by transcriptomics and machine learning. mSystems9:e0125723. doi: 10.1128/msystems.01257-23
23
KnoppM. I.WarmE. J.WeberD.KelleherM.KinnearB.SchumacherD. J.et al. (2023). AI-enabled medical education: threads of change, promising futures, and risky realities across four potential future worlds. JMIR Med Educ9:e50373. doi: 10.2196/50373
24
KouY.ZhangX.YangY.HuY.WuB.LiuJ. (2023). "A learner knowledge state Modeling method based on the improved DKVMN", in 2023 IEEE smart world congress (SWC), 1–4.
25
LaFlammeK. A. (2025). Scaffolding AI literacy: an instructional model for academic librarianship. J. Acad. Librariansh.51:103041. doi: 10.1016/j.acalib.2025.103041
26
LeeJ.YoonW.KimS.KimD.KimS.SoC. H.et al. (2020). BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics36, 1234–1240. doi: 10.1093/bioinformatics/btz682
27
LevivienC.CavagnaP.GrahA.BuronfosseA.CourseauR.BézieY.et al. (2022). Assessment of a hybrid decision support system using machine learning with artificial intelligence to safely rule out prescriptions from medication review in daily practice. Int. J. Clin. Pharm.44, 459–465. doi: 10.1007/s11096-021-01366-4
28
LuM. K.YinJ. Y.ZhuQ.LinG. L.MouM. J.LiuF. Y.et al. (2023). Artificial intelligence in pharmaceutical sciences. Engineering27, 37–69. doi: 10.1016/j.eng.2023.01.014
29
LuoY.ChenY. W.XieH. Z.ZhuW. T.ZhangG. S. (2024). Interpretable CRISPR/Cas9 off-target activities with mismatches and indels prediction using BERT. Comput. Biol. Med.169:107932. doi: 10.1016/j.compbiomed.2024.107932
30
MaJ.ZhaoZ. L.LiuY. W.LiT. F.ZhangR. S. (2025). Designing an adaptive learning framework for predicting drug-target affinity using Y learning and graph neural networks. Eng. Appl. Artif. Intell.139:12. doi: 10.1016/j.engappai.2024.109472
31
MaF. L.ZhuC. S.LiuD. K. (2024). A deeper knowledge tracking model integrating cognitive theory and learning behavior. J. Intell. Fuzzy Syst.46, 6607–6617. doi: 10.3233/JIFS-235723
32
MarianiA.PellegriniE.De MomiE. (2021). Skill-oriented and performance-driven adaptive curricula for training in robot-assisted surgery using simulators: a feasibility study. IEEE Trans. Biomed. Eng.68, 685–694. doi: 10.1109/TBME.2020.3011867
33
MejehM.RehmM. (2024). Taking adaptive learning in educational settings to the next level: leveraging natural language processing for improved personalization. ETR&D72, 1597–1621. doi: 10.1007/s11423-024-10345-1
34
MirataV.BergaminP. (2023). Role of organisational readiness and stakeholder acceptance: an implementation framework of adaptive learning for higher education. ETR&D71, 1567–1593. doi: 10.1007/s11423-023-10248-7
35
NaseerF.KhanM. N.TahirM.AddasA.AejazS. M. H. (2024). Integrating deep learning techniques for personalized learning pathways in higher education. Heliyon10:e32628. doi: 10.1016/j.heliyon.2024.e32628
36
NaseerF.KhawajaS. (2025). Mitigating conceptual learning gaps in mixed-ability classrooms: a learning analytics-based evaluation of AI-driven adaptive feedback for struggling learners. Applied Sci.15:473. doi: 10.3390/app15084473
37
NgoB.NguyenD.VansonnenbergE. (2022). The cases for and against artificial intelligence in the medical school curriculum. Radiol Artif Intell4:e220074. doi: 10.1148/ryai.220074
38
OrenO.GershB. J.BhattD. L. (2020). Artificial intelligence in medical imaging: switching from radiographic pathological data to clinically meaningful endpoints. Lancet Digit Health2, e486–e488. doi: 10.1016/S2589-7500(20)30160-6
39
ParanjapeK.SchinkelM.Nannan PandayR.CarJ.NanayakkaraP. (2019). Introducing artificial intelligence training in medical education. JMIR Med Educ5:e16048. doi: 10.2196/16048
40
ParkT.GuP.KimC. H.KimK. T.ChungK. J.KimT. B.et al. (2023). Artificial intelligence in urologic oncology: the actual clinical practice results of IBM Watson for oncology in South Korea. Prostate Int.11, 218–221. doi: 10.1016/j.prnil.2023.09.001
41
PawusD.PorazkoT.PaszkielS. (2025). Automated biomedical measurements analysis: innovative models based on machine learning for predicting laboratory results in nephrology. Expert Syst. Appl.270:126568. doi: 10.1016/j.eswa.2025.126568
42
PengP.FuW. N. (2022). A pattern recognition method of personalized adaptive learning in online education. Mob. Netw. Appl.27, 1186–1198. doi: 10.1007/s11036-022-01942-6
43
QianL.DongZ.GuoT. (2025). Grow AI virtual cells: three data pillars and closed-loop learning. Cell Res.35, 319–321. doi: 10.1038/s41422-025-01101-y
44
RanpuraS.MaralingannavarV.GheorgheA. G.MaE.MorrisseyJ.BetenbaughM. J.et al. (2025). Wheels turning: CHO cell modeling moves into a digital biomanufacturing era: subtitle: CHO metabolic Modeling. Comput. Struct. Biotechnol. J.27, 2796–2813. doi: 10.1016/j.csbj.2025.06.035
45
RizwanS.NeeC. K.GarfanS. (2025). Identifying the factors affecting student academic performance and engagement prediction in MOOC using deep learning: a systematic literature review. IEEE Access13, 18952–18982. doi: 10.1109/ACCESS.2025.3533915
46
RyuW. H. A.ChanS.SutherlandG. R. (2017). Supplementary educational models in Canadian neurosurgery residency programs. Can. J. Neurol. Sci.44, 177–183. doi: 10.1017/cjn.2016.315
47
ShahabM. A.DestroF.BraatzR. D. (2025). Digital twins in biopharmaceutical manufacturing: review and perspective on human-machine collaborative intelligence. ArXiv. abs/2504.00286. doi: 10.48550/arXiv.2504.00286
48
SinghS.KumarS.QuadirS. S.BhandariS.BaniyaB.JoshiG.et al. (2025). Artificial intelligence: preface, applications and future perspective in relation to pharmaceutical sector. J. Pharm. Innov.20:940. doi: 10.1007/s12247-025-09940-3
49
SnydmanL. K.MemariM.PuriA.SottileE. M.KillianK.CallenderD.et al. (2025). The master adaptive clinician educator: a framework for future educational leaders in academic medicine. J. Gen. Intern. Med.40, 927–932. doi: 10.1007/s11606-024-09199-3
50
SunS.HuX. G.BuC. Y.LiuF.ZhangY. H.LuoW. J. (2022). "Genetic algorithm for Bayesian knowledge tracing: A practical application," in Advances in Swarm Intelligence, ICSI 2022, PT I)
51
TanL. Y.HuS.YeoD. J.CheongK. H. (2025). Artificial intelligence-enabled adaptive learning platforms: a review. Comput. Educ. Artif. Int.9:100429. doi: 10.1016/j.caeai.2025.100429
52
Van EdeA. E.ClaessenR. J. M.Van GilsM.GorgelsW. J. M. J.ReuzelR. P. B.SmeetsA. G. J. M.et al. (2023). How to coach student professional development during times of challenges and uncertainties. BMC Med. Educ.23:600. doi: 10.1186/s12909-023-04588-4
53
WangJ. B.ZhangC. B. (2025). Aiming to enhance higher education practice through generative AI integration: a theoretical exploration of critical success factors. SAGE Open15:464. doi: 10.1177/21582440251342464
54
XuS.SunM. F.FangW. L.ChenK.LuoH. B.ZouP. X. W. (2023). A bayesian-based knowledge tracing model for improving safety training outcomes in construction: an adaptive learning framework. Dev. Built Environ.13:10011. doi: 10.1016/j.dibe.2022.100111
55
YanY.YangY.TongZ.WangY.YangF.PanZ.et al. (2025). Adaptive multi-view learning method for enhanced drug repurposing using chemical-induced transcriptional profiles, knowledge graphs, and large language models. J Pharm Anal15:101275. doi: 10.1016/j.jpha.2025.101275
56
YangZ. L. (2022). Natural language enhancement for English teaching using character-level recurrent neural network with back propagation neural network based classification by deep learning architectures. J. Universal Comput. Sci.28, 984–1000. doi: 10.3897/jucs.94162
57
ZhangW.SongL. L.LiuJ. F.LuoP. H.LiZ. X.GongZ. W. (2025). A novel framework for deep knowledge tracing via a dual-state joint interaction mechanism. Inf. Process. Manag.62:104210. doi: 10.1016/j.ipm.2025.104210
58
ZouF. W.TangY. F.LiuC. Y.MaJ. A.HuC. H. (2020). Concordance study between IBM Watson for oncology and real clinical practice for cervical Cancer patients in China: a retrospective analysis. Front. Genet.11:200. doi: 10.3389/fgene.2020.00200
Summary
Keywords
adaptive learning systems, knowledge state modeling, competency-based learning, AI in pharmaceutical education, biopharmaceutical training, interdisciplinary education
Citation
Wang T, Xu N and Liu F (2025) Revolutionizing biotech and pharmaceutical education with adaptive learning. Front. Educ. 10:1679222. doi: 10.3389/feduc.2025.1679222
Received
06 August 2025
Accepted
31 October 2025
Published
18 November 2025
Volume
10 - 2025
Edited by
Ashim Malhotra, California Northstate University, United States
Reviewed by
Francisco Rafael Trejo-Macotela, Universidad Politécnica de Pachuca, Mexico
Pongkit Ekvitayavetchanukul, Khon Kaen University, Thailand
Updates
Copyright
© 2025 Wang, Xu and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Xu, xuna828@cdutcm.edu.cn; Fei Liu liufei092531@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.