- 1Complications Research, Steno Diabetes Center Copenhagen, Herlev, Denmark
- 2Department of Clinical Medicine, Faculty of Health and Medical Sciences, The University of Copenhagen, Copenhagen, Denmark
- 3Novo Nordisk, Bagsvaerd, Denmark
- 4Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Rigshospitalet, Copenhagen, Denmark
- 5Department of Cardiology, Rigshospitalet, Copenhagen, Denmark
Background: The mechanisms linking cardiovascular autonomic neuropathy, diabetic kidney disease and cardiovascular mortality in type 2 diabetes are widely unknown. We investigated the relationship between baseline cardiovascular autonomic function and changes in kidney and myocardial function over six years in patients with type 2 diabetes and healthy controls.
Methods: Post-hoc analysis of a cohort study in 24 patients with type 2 diabetes and 18 healthy controls. Baseline determinants were cardiovascular autonomic reflex tests (heart rate response to: standing (30:15); deep breathing (E:I); and the Valsalva test) and time- and frequency-domain heart rate variability indices. Outcomes were changes in estimated glomerular filtration rate (eGFR), albuminuria, myocardial flow reserve (MFR) measured by cardiac 82Rb Positron emission tomography computed tomography (PET/CT), and coronary artery calcium score (CACS).
Results: Mean age at inclusion was 61 ± 10 years and 36% were female. Mean follow up time was 6 ± 0 years. A lower response in heart rate to the Valsalva test (corresponding to weaker autonomic function) was associated with a larger decline in eGFR (p=0.04), but not significantly after adjustment for sex, baseline age, smoking status, systolic blood pressure, heart rate, HbA1c, body mass index and baseline eGFR (p=0.12). A higher baseline response in heart rate to standing (30:15) was associated with a larger decline in myocardial flow reserve in the unadjusted analysis (p=0.02) and after adjustment (p=0.02). A higher response in heart rate to the Valsalva maneuver was associated with a larger increase in CACS (p = 0.02), but the association became insignificant after adjustment (p = 0.16).
Conclusion: A lower response in heart rate to the Valsalva test was associated with a larger decline in kidney function, indicating that autonomic dysfunction may predict future loss of kidney function. However, we did not find any association between lower values in cardiovascular autonomic function at baseline and a worsening in albuminuria, myocardial function, or atherosclerotic burden.
Introduction
Cardiovascular autonomic neuropathy (CAN) is an overlooked and frequent diabetic complication seen in up to 65% of persons with type 2 diabetes (1). CAN has been suggested to promote the progression of diabetic kidney disease and is an independent risk factor for cardiovascular mortality (1–3). The mechanisms linking CAN, diabetic kidney disease and cardiovascular mortality are widely unknown.
The role of CAN in the progression of kidney and coronary disease in diabetes have been investigated in various studies. An association between presence of CAN and faster progression in diabetic kidney disease has been demonstrated both in type 1 diabetes (3) and in type 2 diabetes (4, 5). A reduced myocardial flow reserve is a risk factor for cardiac death, non-fatal myocardial infarction (6) and independently associated with presence of CAN in type 2 diabetes (7). However, we could not find studies investigating the association between cardiovascular autonomic function and longitudinal changes in myocardial flow reserve in type 2 diabetes. Another cardiovascular measure associated with CAN in diabetes is the coronary artery calcium score, an established marker of coronary atherosclerosis (8–11). Only one study was found investigating the effect of CAN on changes in the coronary artery calcium score in type 1 diabetes, which could not demonstrate an association after two years of follow up (12). We found no studies investigating the association between CAN and changes in kidney and myocardial function concurrently.
We recently demonstrated that the myocardial flow reserve was lower, and the coronary artery calcium score was higher in type 2 diabetes as compared with healthy controls (13). In this post hoc analysis in the same population, we investigated the association between cardiovascular autonomic function at baseline and changes over six years in outcomes reflecting kidney function, myocardial function and atherosclerotic burden. We hypothesized that lower measures of cardiovascular autonomic function at baseline would be associated with a larger decline in kidney function and myocardial flow reserve and a larger increase in coronary artery calcium score.
Methods
Design
Post hoc analysis of a cohort study in type 2 diabetes and healthy controls studying temporal changes in myocardial function in patients with type 2 diabetes and non-diabetic controls (13). The study was performed in compliance with the Declaration of Helsinki. All participants gave informed written consent, and the study protocol was approved by a regional research ethics committee (H-19024534).
Study Population
In 2013-2014, 60 persons with type 2 diabetes and 30 age- and sex-matched healthy controls were recruited at Steno Diabetes Center Copenhagen (7). In the type 2 diabetes group, 30 had normoalbuminuria (<30mg/24h) and 30 had albuminuria (≥30mg/24h). Participants were between 35 and 80 years of age and free of overt cardiovascular disease. In 2019-2020, all living participants (n=82) were invited for a follow up visit. A total of 48 participants from the original cohort participated. Reasons for not participating were severe illness (n=5), no response (n=8) and not interested (n=21). Cardiovascular autonomic function data was missing for six participants (5 with type 2 diabetes and 1 control) due to lack of compliance or malfunction of the equipment at the time of measurement. Thus, the present post hoc analysis includes 42 participants (24 with type 2 diabetes and 18 controls) who had a complete dataset on cardiovascular autonomic function at baseline and on the study outcomes at baseline and follow up (Figure 1).
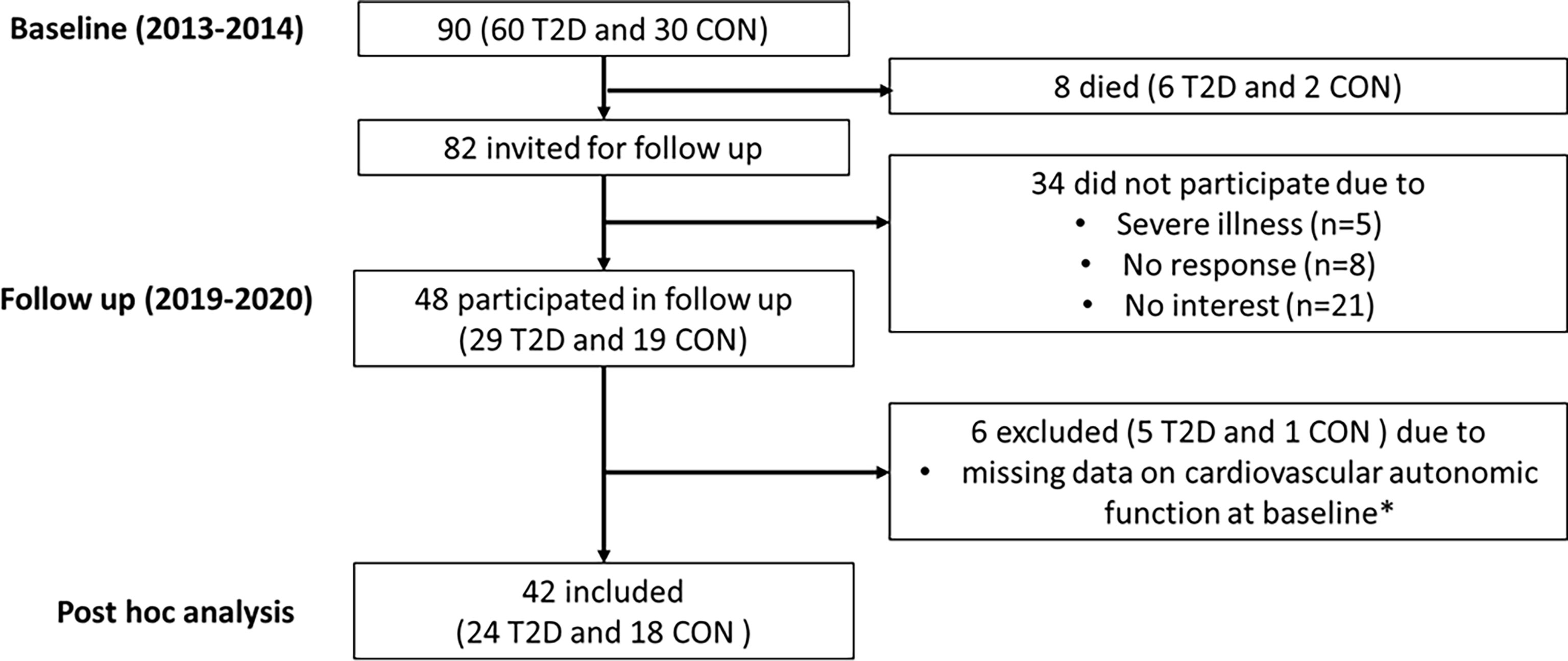
Figure 1 Flowchart for participants. T2D, Type 2 diabetes; CON, Healthy control. *Due to lack of compliance or malfunction of the equipment at the time of measurement.
Baseline Measurements
Laboratory variables included HbA1c, lipid profile and plasma creatinine measured by standard methods. Upper arm blood pressure was measured in sitting position on the right arm after 10-minutes rest with a validated blood pressure monitor and the mean of three consecutive measurements was calculated.
Explanatory Variables at the Baseline Visit
Participants were advised to abstain from hard physical activity 24 hours before the examination. All tests of cardiovascular autonomic function were performed between 8:00 A.M. and 2:00 P.M. in a quiet examination room. Three standard cardiovascular autonomic reflex tests (CARTs) and 5-min resting heart rate recordings for calculating time- and frequency-domain heart rate variability indices were obtained once (3, 14). Parameters of cardiovascular autonomic function were obtained using the Vagus Device (Medicus Engineering, Aarhus, Denmark) (15). The CARTs included the heart rate response to: 1) standing (30:15); 2) deep breathing (E:I); and 3) the Valsalva test. The 30:15 ratio, E:I ratio and Valsalva tests are measures mainly of parasympathetic function (1). The CARTs were evaluated using age-related reference intervals (16). CAN was defined using the American Diabetes Association criteria and participants with two or three pathological CARTs were classified as having CAN (17). From the 5-min resting heart rate recordings, time-domain heart rate variability indices were derived: the standard deviation of normal-to-normal (SDNN) intervals and the root mean square of successive differences (RMSSD). Frequency-domain heart rate variability indices were calculated using fast Fourier transformation and included low frequency (LF) power (0.04–0.15 Hz), high frequency (HF) power (0.15–0.4 Hz), and the total (Total) power (0.4 Hz). The ratio of LF-to-HF (LF/HF Ratio) was also calculated. Where RMSSD and HF power reflects parasympathetic activity, the SDNN, LF power and Total power are measures of combined sympathetic and parasympathetic activity.
Outcome Variables at the Baseline and Follow Up Visits
The estimated glomerular filtration rate (eGFR) was calculated by the CKD-EPI equation (18). The urine albumin excretion was measured at the baseline visit in two 24 hours urine collections and estimated as the urine albumin excretion rate and measured at the follow up visit in three consecutive morning urine samples and estimated as the urine albumin creatinine ratio. Cardiac 82Rb Positron emission tomography computed tomography (PET/CT) imaging provides a quantitative measurement of the myocardial perfusion (ml/min/g of tissue) during stress and rest. This enables calculation of the myocardial flow reserve (19). The cardiac 82Rb PET/CT scans were performed using a hybrid PET/CT scanner in 3D mode (Siemens Biograph mCT 128, Siemens, Munich, Germany) after administration of 1,100 MBq 82Rb (CardioGen-82, Bracco Diagnostics, Monroe Township, NJ, USA). The scans were performed at rest and at stress (adenosine infusion to induce maximum myocardial hyperemia). The scans were performed with similar equipment, protocol and analyzed using identical software (syngo.via VB20A) by the same single observer at the baseline and follow up visits. The coronary artery calcium score was calculated by the method described by Agatston et al. as the sum of coronary artery calcium content in the three main coronary arteries (20) using semi-automated commercially available software (Syngovia 4.0).
Statistical Analysis
Clinical characteristics of the participants are presented as n (%), mean ± standard deviation (SD) or if skewed distributed, as medians with interquartile range [quartile 1 to quartile 3]. The variables with skewed distributions were log transformed (natural logarithm) before analyses and normal distribution was obtained. A value of 1 was added to the coronary artery calcium score before log transformation, since the skewed distribution included values of zero. We applied the Chi-squared test or Fisher’s exact test for categorical data and unpaired Student’s t-tests for continuous variables to analyze differences between the participants with type 2 diabetes and the healthy controls. Cross-sectional associations in the total population were assessed in the baseline study as already publiched (7). We repeated these analyses in the present sub-population, using unadjusted linear regression models to evaluate the association between baseline cardiovascular autonomic function parameters and baseline levels of outcomes. Next, to control for possible confounders, we adjusted the significant associations for sex, baseline age, smoking, systolic blood pressure, heart rate, HbA1c and body mass index. Change from baseline to follow up was analyzed using paired Student’s t-tests. Changes in the outcomes were expressed per year as the change from the baseline to the follow up visit, divided by the individual follow up time in years. Unadjusted and adjusted linear regression models were applied to assess associations between the baseline cardiovascular autonomic function parameters (30:15, E:I, Valsalva, log (SDNN), log (RMSSD), log (LF), log (HF), log (Total) and log (LF/HF Ratio)) and yearly changes in the outcomes. Adjusment included sex, baseline age, smoking, systolic blood pressure, heart rate, HbA1c, body mass index and the baseline value of the given outcome. All participants were pooled in the linear regression analyses and standardized regression coefficients are reported. We used SAS Enterprise Guide, version 7.15 for analyses. Two-sided p values < 0.05 were considered statistically significant. Due to the exploratory nature of this post hoc analysis, we did not perform a sample size calculation for this study.
Results
Baseline Characteristics
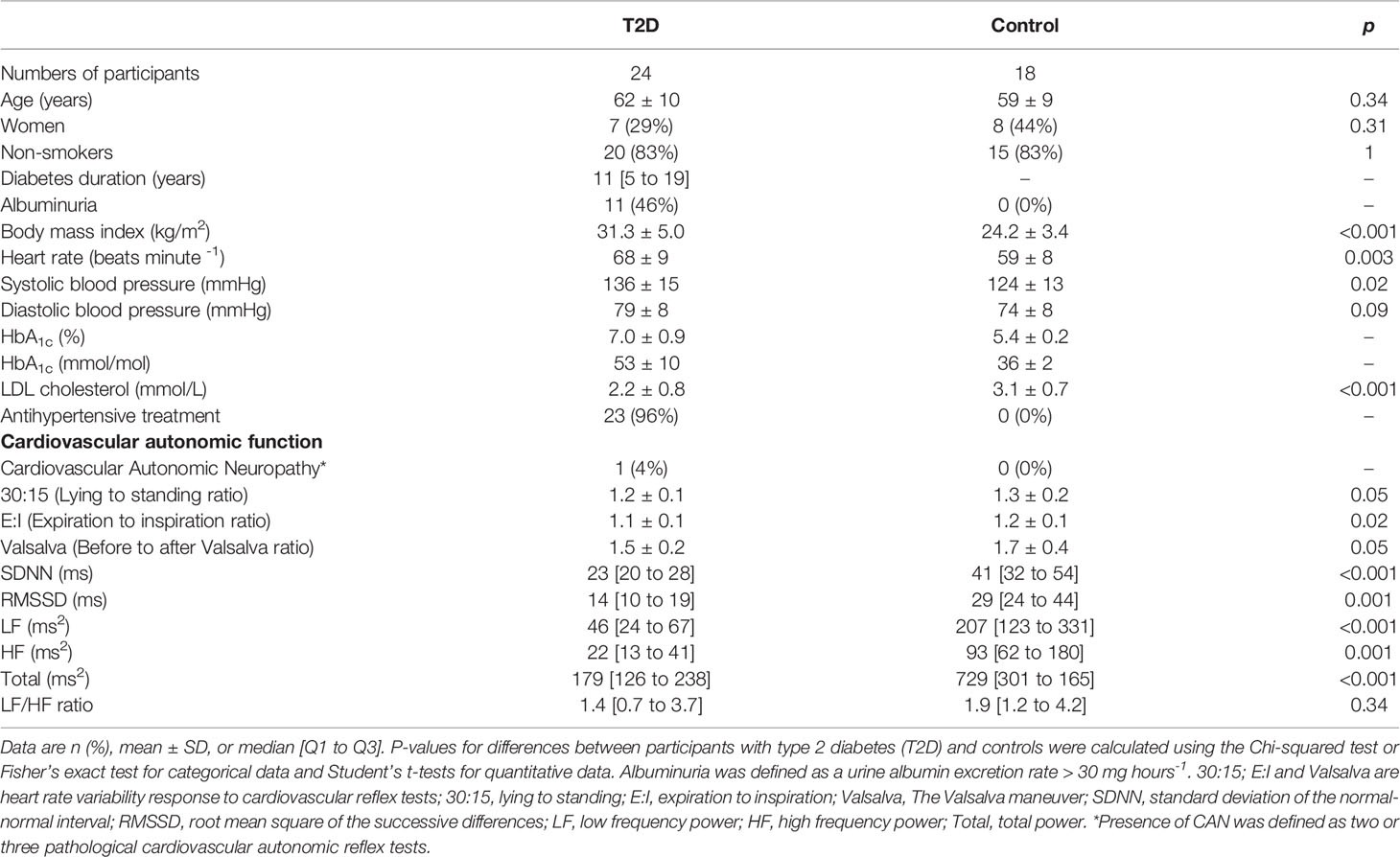
Age and sex distributions were equal in the 24 persons with type 2 diabetes and the 18 healthy controls. The persons with type 2 diabetes had a higher body mass index and heart rate and lower LDL and total cholesterol compared with controls. The participants with type 2 diabetes had a median [Q1 to Q3] diabetes duration of 11 [5 to 19] years, mean HbA1c of 53 ± 10 mmol/mol, eGFR of 79 ± 22 ml min−1 1.73 m−2 and 11 (46%) had albuminuria (Table 1).
Cardiovascular Autonomic Function at Baseline
All measures of cardiovascular autonomic function at baseline, except the 30:15, the Valsalva and the LF/HF ratio, were lower in the participants with type 2 diabetes compared with the healthy controls. Only one participant (with type 2 diabetes) had CAN (Table 1). Baseline associations between cardiovascular autonomic function and measures of heart and kidney function are shown in Table S1. A higher (more beneficial) 30:15 ratio was associated with a higher eGFR. Higher values of all measures of cardiovascular autonomic function, except the E:I ratio, RMSSD, high frequency power and the LF/HF ratio, were associated with lower urine albumin excretion rate. Higher values of all measures of cardiovascular autonomic function, except the Valsalva ratio, High Frequency power and LF/HF ratio, correlated with higher baseline myocardial flow reserve. Finally, higher values of all cardiovascular autonomic function parameters, except the LF/HF ratio, were associated with lower baseline coronary artery calcium score. None of the associations at baseline remained significant after confounder adjustment (Table S1).
Longitudinal Changes in Outcomes
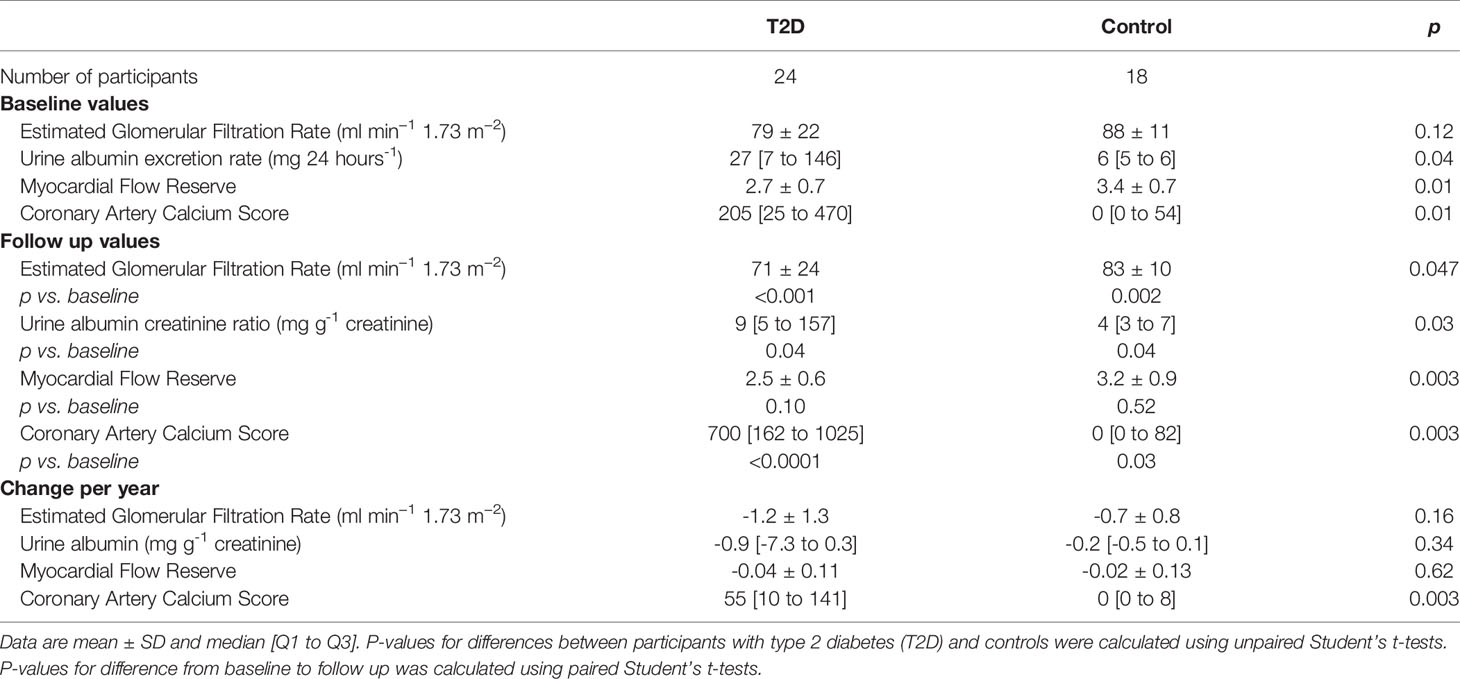
Baseline and follow-up values of outcomes are shown in Table 2. The mean ± SD follow up time was 6.2 ± 0.2 years for participants with type 2 diabetes and 6.3 ± 0.2 years for the healthy controls. The mean change per year in eGFR was -1.2 ± 1.3 ml min−1 1.73 m−2 for participants with type 2 diabetes and -0.7 ± 0.8 ml min−1 1.73 m−2 for healthy controls, with no significant difference between groups (p=0.16). The median change per year in urine albumin was -0.9 [-7.3 to 0.3] mg g-1 for the type 2 diabetes group and -0.2 [-0.5 to 0.1] mg g-1 for the healthy controls, with no significant difference between groups (p=0.34). There was no difference between groups in the yearly change in myocardial flow reserve. The median change per year in coronary artery calcium score was significantly larger in the type 2 diabetes group (p=0.003) (Table 2).
Associations Between Cardiovascular Autonomic Function at Baseline and Changes in Outcomes
Associations between cardiovascular autonomic function at baseline and changes in heart and kidney function are shown in Table 3. A lower response in heart rate to the Valsalva test, reflecting impaired cardiovascular autonomic function, was associated with a larger decline in eGFR in the unadjusted model (β per 1 SD = 0.37 ml min−1 1.73 m−2 increase per year; p=0.04), but not after adjustment (p=0.12). No other associations between cardiovascular autonomic function at baseline and changes in eGFR were found. No associations with changes in urine albumin were found. A higher response in heart rate to standing (30:15), reflecting better cardiovascular autonomic function, was associated with a larger decline in myocardial flow reserve in the unadjusted model (β per 1 SD = 0.04 decrease per year; p=0.02) and after adjustment (p=0.02). No other associations between cardiovascular autonomic function at baseline and changes in myocardial flow reserve were demonstrated. A higher response in heart rate to the Valsalva maneuver was associated with a larger increase in coronary artery calcium score (β per 1 SD = 0.34 increase per year; p = 0.02), but the association became insignificant after adjustment (p = 0.16). There were no other associations between cardiovascular autonomic function at baseline and changes in coronary artery calcium score (Table 3).
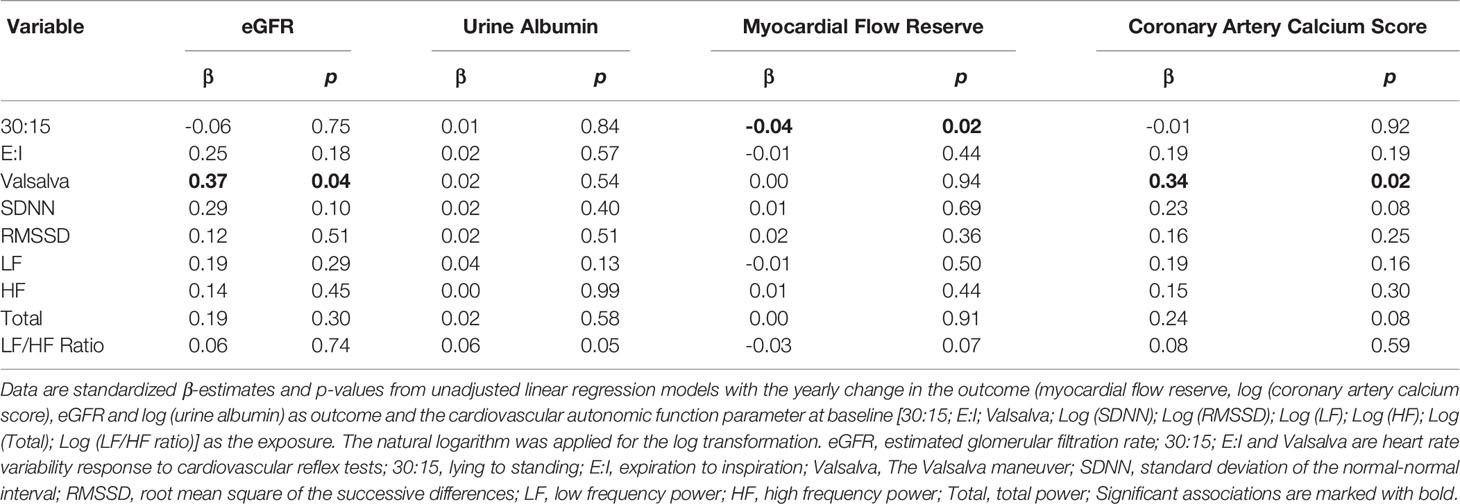
Table 3 Associations between cardiovascular autonomic function at baseline and changes in heart and kidney function.
Sensitivity Analyses
Changes in medical therapy from baseline to follow up are shown in Table S2. In the type 2 diabetes group, a total of 21 (88%) had changes in their diabetes therapy from baseline to follow up (insulin; sodium-glucose cotransporter 2 inhibitor (SGLT2i); glucagon-like peptide-1 receptor agonist (GLP1-RA); metformin) which might have influenced our results. To take this into account, we adjusted our analyses further for change in diabetes therapy from baseline to follow up (yes vs. no). Results were confirmatory. To account for the influence on beta-blockers on the cardiovascular autonomic function tests, we performed a sensitivity analysis, excluding the two participants treated with a beta-blocker at baseline. Results were confirmatory.
Discussion
We observed that a lower response in heart rate to the Valsalva test was associated with a larger decline in kidney function over six years. We could not demonstrate any associations between lower cardiovascular autonomic function and a larger decline over six years in myocardial flow reserve as hypothesized, but we did observe an association in the opposite direction with a higher response in heart rate to standing (30:15). Finally, there was no association between cardiovascular autonomic function at baseline and changes in coronary artery calcium score.
The presence of cardiovascular autonomic dysfunction has been associated with faster progression of kidney disease in type 2 diabetes. This was shown by Yun and colleagues in a longitudinal study including 755 persons with type 2 diabetes and 9.6 years of follow up, and by Tahrani et al. in a longitudinal study including 204 persons with type 2 diabetes followed for 2.5 years (4, 5). We observed an association between a lower response in heart rate to the Valsalva test and a larger decline in eGFR, which was in line with our hypothesis and with these studies. The mechanisms are largely unknown, but renal hemodynamic changes associated with autonomic dysfunction (e.g. higher blood pressure during the night) could play a role (21). Another possibility is that parasympathetic dysfunction and a sympathetic overdrive promotes renal destruction, as already suggested in type 1 diabetes (22).
Measures of cardiovascular autonomic function were lower at baseline in the type 2 diabetes group than in the healthy controls, indicating some degree of autonomic dysfunction, but only one participant (4%) was diagnosed with CAN based on pathologic response in CARTs. We expected a prevalence in the range of 44% to 65% in a type 2 diabetes population with this mean age and diabetes duration (1, 3). This very low prevalence of cardiovascular autonomic dysfunction could have influenced our results. Lower values in the response in heart rate to standing (30:15), deep breathing (E:I) and the Valsalva test and lower values of the time- and frequency-domain heart rate variability indices (SDNN, RMSSD, LF power, HF power and Total power) were all associated with a lower myocardial flow reserve in the previously published cross-sectional baseline study (7) and similar associations were demonstrated at baseline in this subpopulation. Thus, we expected an association between lower cardiovascular autonomic function at baseline and a larger decline in myocardial flow reserve, but the only association we found was between a higher response in heart rate to standing (30:15) and a larger decline in myocardial flow reserve. The estimated decline in myocardial flow reserve of 0.04 per year per SD increase in the response in heart rate was however quite small compared to the mean difference in myocardial flow reserve of 0.7 (95%CI 0.2-1.1) between the groups at baseline (considered as a clinically relevant difference). Regression to the mean could in part explain this association. Finally, survival bias might be an important limitation to this study and might have influenced our results as discussed below. We are not aware of previous studies investigating associations between cardiovascular autonomic function and changes in myocardial flow reserve and further studies in larger populations are warranted.
The coronary artery calcium score increased during follow up in both groups and significantly more in the type 2 diabetes group. Lower autonomic cardiovascular function was associated with a higher atherosclerotic burden at baseline (7), but not with faster progression during six years of follow up. This is in line with a previous study in type 1 diabetes (12).
Limitations
The investigated population was small, and this introduces a risk of false negative findings due to lack of power. Only 42 out of the original 90 persons participated in the follow up. The participants in the follow-up examination were younger, had a higher myocardial flow reserve, better kidney function and a lower coronary artery calcium score at the baseline examination compared with non-participants (13). The baseline characteristics of the full original cohort of 90 subjects and the follow up cohort of 42 subjects are presented in Table S3. This introduces a considerable risk of selection- and survival bias which weakens our chance to find associations. Thus, our results should be interpreted as exploratory and with this limitation in mind. When conducting a large number of tests as we have done there is a risk of type 1 errors. The myocardial flow reserve did not change in neither of the groups during follow up which makes it difficult to study associations between autonomic function and change in myocardial flow reserve. It is a limitation that participants were not abstinent from medication before measurements of cardiovascular autonomic function at baseline. Moreover, we did not evaluate the cardiovascular autonomic function at follow up with the Vagus device as in the baseline examination and are therefore not able to relate changes in cardiovascular autonomic function to the outcomes.
Conclusion
With this post hoc analysis we are the first to investigate the longitudinal associations between cardiovascular autonomic function at baseline and changes in kidney function, myocardial function, and atherosclerotic burden over six years of follow up. A lower response in heart rate to the Valsalva test was associated with a larger decline in kidney function, indicating that autonomic dysfunction may be a risk factor for future loss of kidney function. We unexpectedly found no association between lower cardiovascular autonomic function at baseline and larger decline in the myocardial flow reserve. Furthermore, we found no association between baseline cardiovascular autonomic function measures and changes in the atherosclerotic burden. Further studies investigating the relation between cardiovascular autonomic function and changes in kidney and myocardial function in diabetes are needed.
Data Availability Statement
Anonymized data can be obtained from the corresponding author upon reasonable request. Necessary data protection agency and ethical committee approvals must be provided in compliance with relevant legislation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Regional Research Ethics Committee (Central Region) (H-19024534). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL drafted the manuscript. JL, IR, EZ, PH, BS, LH, RR, CH, MF-M, AK, PR, and TH analyzed and interpreted the research. JL, IR, EZ, RR, AK, PR, and TH conceived and designed the research. JL and TH performed the statistical analysis. JL, IR, EZ, PH, BS, RR, and AK did measurements. TH supervised the study. EZ, RR, AK, PR, and TH obtained the funding. JL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the interpretation of the results, reviewed and edited the manuscript and approved the final version of the manuscript.
Funding
The authors acknowledge the support from the Novo Nordisk Foundation (grant number NNFOC0013659): PROTON: Personalizing Treatment of Diabetic Nephropathy; The Novo Nordisk Foundation (grant number NNF19OC0054674) ‘Deep phenotyping of the heart with advanced imaging modalities in Type 2 diabetes implications for pathophysiology and prognosis (DIA-HEART study)’, Skibsreder Per Henriksen, R. og Hustrus Fond and internal funding from the Steno Diabetes Center Copenhagen, Denmark and Rigshospitalet, Copenhagen, Denmark.
Conflict of Interest
PR reports having received research grants from Astra Zeneca and Novo Nordisk and given lectures for Astra Zeneca, Mundipharma and Boehringer Ingelheim and has served as a consultant for Astra Zeneca, Bayer, Eli Lilly, Boehringer Ingelheim, Astellas, Gilead, Sanofi Aventis Vifor, and Novo Nordisk, all fees given to Steno Diabetes Center.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all participants and acknowledge the work of laboratory technicians at Steno Diabetes Center Copenhagen. A special thank you to study nurse L. Jelstrup and laboratory technicians M.L.D. Halkjaer, T. Nielsen, D. Riis, T. R. Juhl, and J. A. Hermann.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.780679/full#supplementary-material
References
1. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular Autonomic Neuropathy in Diabetes: Clinical Impact, Assessment, Diagnosis, and Management. Diabetes/Metabolism Res Rev (2011) 27(7):639–53. doi: 10.1002/dmrr.1239
2. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care (2017) 40(1):136–54. doi: 10.2337/dc16-2042
3. Spallone V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab J (2019) 43(1):3–30. doi: 10.4093/dmj.2018.0259
4. Tahrani AA, Dubb K, Raymond NT, Begum S, Altaf QA, Sadiqi H, et al. Cardiac Autonomic Neuropathy Predicts Renal Function Decline in Patients With Type 2 Diabetes: A Cohort Study. Diabetologia (2014) 57(6):1249–56. doi: 10.1007/s00125-014-3211-2
5. Yun JS, Ahn YB, Song KH, Yoo KD, Kim HW, Park YM, et al. The Association Between Abnormal Heart Rate Variability and New Onset of Chronic Kidney Disease in Patients With Type 2 Diabetes: A Ten-Year Follow-Up Study. Diabetes Res Clin Pract (2015) 108(1):31–7. doi: 10.1016/j.diabres.2015.01.031
6. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients With and Without Diabetes Mellitus. Circulation (2012) 126(15):1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402
7. von Scholten BJ, Hansen CS, Hasbak P, Kjaer A, Rossing P, Hansen TW. Cardiac Autonomic Function Is Associated With the Coronary Microcirculatory Function in Patients With Type 2 Diabetes. Diabetes (2016) 65(10):3129–38. doi: 10.2337/db16-0437
8. Moon SS, Choi YK, Seo HA, Jeon JH, Lee JE, Jeong JY, et al. Relationship Between Cardiovascular Autonomic Neuropathy and Coronary Artery Calcification in Patients With Type 2 Diabetes. Endocr J (2010) 57(5):445–54. doi: 10.1507/endocrj.K09E-299
9. Mogensen UM, Jensen T, Køber L, Kelbæk H, Mathiesen AS, Dixen U, et al. Cardiovascular Autonomic Neuropathy and Subclinical Cardiovascular Disease in Normoalbuminuric Type 1 Diabetic Patients. Diabetes (2012) 61(7):1822–30. doi: 10.2337/db11-1235
10. Dayem SM, Battah AA, Bohy Ael M. Cardiovascular Autonomic Neuropathy and Early Atherosclerosis in Adolescent Type 1 Diabetic Patient. Open Access Maced J Med Sci (2015) 3(4):681–8. doi: 10.3889/oamjms.2015.131
11. Hjortkjær H, Jensen T, Hilsted J, Mogensen UM, Rossing P, Køber L, et al. Generalised Arterial Calcification in Normoalbuminuric Patients With Type 1 Diabetes With and Without Cardiovascular Autonomic Neuropathy. Diabetes Vasc Dis Res (2019) 16(1):98–102. doi: 10.1177/1479164118805904
12. Hjortkjær H, Jensen T, Hilsted J, Corinth H, Mogensen UM, Køber L, et al. Possible Early Detection of Coronary Artery Calcium Progression in Type 1 Diabetes: A Case-Control Study of Normoalbuminuric Type 1 Diabetes Patients and Matched Controls. Diabetes Res Clin Pract (2018) 141:18–25. doi: 10.1016/j.diabres.2018.04.027
13. Rasmussen IKB, Hasbak P, von Scholten BJ, Laursen JC, Zobel EH, Jorge Diaz L, et al. Non-Invasive Assessment of Temporal Changes in Myocardial Microvascular Function in Persons With Type 2 Diabetes and Healthy Controls. Diabetic Med (2021) 38(6):e14517. doi: 10.1111/dme.14517
14. Spallone V, Bellavere F, Scionti L, Maule S, Quadri R, Bax G, et al. Recommendations for the Use of Cardiovascular Tests in Diagnosing Diabetic Autonomic Neuropathy. Nutr Metab Cardiovasc Dis (2011) 21(1):69–78. doi: 10.1016/j.numecd.2010.07.005
15. Andersen ST, Witte DR, Fleischer J, Andersen H, Fleischer T, Jørgensen ME, et al. Risk Factors for the Presence and Progression of Cardiovascular Autonomic Neuropathy in Type 2 Diabetes: ADDITION-Denmark. Diabetes Care (2018) 41(12):2586–94. doi: 10.2337/dc18-1411
16. Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, et al. Impact of Early Detection and Treatment of Diabetes on the 6-Year Prevalence of Cardiac Autonomic Neuropathy in People With Screen-Detected Diabetes: ADDITION-Denmark, A Cluster-Randomised Study. Diabetologia (2013) 56(1):101–8. doi: 10.1007/s00125-012-2744-5
17. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic Neuropathies: A Statement by the American Diabetes Association. Diabetes Care (2005) 28(4):956–62. doi: 10.2337/diacare.28.4.956
18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Internal Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
19. Qayyum AA, Hasbak P, Larsson HBW, Christensen TE, Ghotbi AA, Mathiasen AB, et al. Quantification of Myocardial Perfusion Using Cardiac Magnetic Resonance Imaging Correlates Significantly to Rubidium-82 Positron Emission Tomography in Patients With Severe Coronary Artery Disease: A Preliminary Study. Eur J Radiol (2014) 83(7):1120–8. doi: 10.1016/j.ejrad.2014.04.004
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J Am Coll Cardiol (1990) 15(4):827–32. doi: 10.1016/0735-1097(90)90282-T
21. Sundkvist G, Lilja B. Autonomic Neuropathy Predicts Deterioration in Glomerular Filtration Rate in Patients With IDDM. Diabetes Care (1993) 16(5):773. doi: 10.2337/diacare.16.5.773
22. Weinrauch LA, Kennedy FP, Gleason RE, Keough J, D’Elia JA. Relationship Between Autonomic Function and Progression of Renal Disease in Diabetic Proteinuria: Clinical Correlations and Implications for Blood Pressure Control. Am J Hypertension (1998) 11(3):302–8. doi: 10.1016/S0895-7061(97)00472-X
Keywords: type 2 diabetes, kidney function, myocardial function, atheroclerosis, PET-CT, cardiovascular autonomic diabetic neuropathy (CADN)
Citation: Laursen JC, Rasmussen IKB, Zobel EH, Hasbak P, von Scholten BJ, Holmvang L, Ripa RS, Hansen CS, Frimodt-Moeller M, Kjaer A, Rossing P and Hansen TW (2021) The Association Between Cardiovascular Autonomic Function and Changes in Kidney and Myocardial Function in Type 2 Diabetes and Healthy Controls. Front. Endocrinol. 12:780679. doi: 10.3389/fendo.2021.780679
Received: 21 September 2021; Accepted: 15 November 2021;
Published: 13 December 2021.
Edited by:
Gaetano Santulli, Columbia University, United StatesReviewed by:
Tomris Erbas, Hacettepe University, TurkeyCarolina M. Casellini, Eastern Virginia Medical School, United States
Copyright © 2021 Laursen, Rasmussen, Zobel, Hasbak, von Scholten, Holmvang, Ripa, Hansen, Frimodt-Moeller, Kjaer, Rossing and Hansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens Christian Laursen, amVucy5jaHJpc3RpYW4ubGF1cnNlbi4wMUByZWdpb25oLmRr
 Jens Christian Laursen
Jens Christian Laursen Ida Kirstine B. Rasmussen1,2
Ida Kirstine B. Rasmussen1,2 Bernt Johan von Scholten
Bernt Johan von Scholten Rasmus S. Ripa
Rasmus S. Ripa Christian S. Hansen
Christian S. Hansen Marie Frimodt-Moeller
Marie Frimodt-Moeller Peter Rossing
Peter Rossing Tine W. Hansen
Tine W. Hansen