- 1Department of Endocrinology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 2Metabolic Vascular Disease Key Laboratory of Sichuan Province, Luzhou, China
- 3Sichuan Clinical Research Center for Nephropathy, Luzhou, China
- 4Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, Luzhou, China
- 5Southwest Medical University, Luzhou, China
Objective: Despite previous research that focused on aspartate aminotransferase/alanine aminotransferase ratio (AAR) as predictors of type 2 diabetes mellitus (T2DM) and cardiovascular disease, there has been limited research evaluating the association between AAR and diabetic microvascular complications. This study aimed to investigate the association of AAR with diabetic peripheral neuropathy (DPN).
Methods: A total of 1562 hospitalized patients with T2DM were divided into four groups according to AAR quartiles. The relationship between AAR and DPN and related parameters was explored by the Spearman correlation coefficients, multivariable logistic regression analysis, and receiver operating characteristic (ROC) curves.
Results: Patients with higher AAR quartiles had higher levels of vibration perception threshold (VPT) and presence of DPN, and AAR was positively associated with VPT and presence of DPN independent of sex, age, body mass index, and diabetic duration (P<0.01 or P<0.05). Moreover, AAR remained significantly associated with a higher odds ratio (OR) of DPN (OR 2.413, 95% confidence interval [CI] 1.081-5.386, P<0.05) after multivariate adjustment. Additionally, the risk of presence of DPN increased progressively as AAR quartiles increased (all P for trend <0.01) in both male and female subjects, and the highest quartile of AAR of male and female subjects was respectively associated with 107.3% (95% CI: 1.386-3.101; P<0.01) and 136.8% (95% CI: 1.550-3.618; P<0.01) increased odds of DPN compared with the lower quartiles. Last, the analysis of receiver operating characteristic curves revealed that the best cutoff values for AAR to predict the presence of DPN were 0.906 (sensitivity: 70.3%; specificity: 49.2%; and area under the curve [AUC]: 0.618) and 1.402 (sensitivity: 38%; specificity: 81.9%; and AUC: 0.600) in male and female subjects, respectively.
Conclusions: These findings suggest that the high AAR may be associated with the presence of DPN in Chinese patients with T2DM, and may be used as an additional indicator of risk of DPN.
Introduction
Diabetic peripheral neuropathy (DPN) is the most common but usually underestimated chronic microvascular complication that first present in the distal extremities and can result in either numbness or chronic pain; and is a major risk factor for Charcot joints, diabetic foot ulcers (DFU), and limb amputation in diabetic patients (1, 2). DPN has now been considered an increasing public health problem, owing to its close association with considerable morbidity and mortality, heavy economic burden, and compromised quality of life (1, 3). However, the current treatment for DPN involves only symptomatic relief, and often the results are disappointing. Therefore, it is urgent to find an indicator for screening the high-risk population of DPN, resulting in early identification and, consequently, early intervention.
A number of studies have shown that type 2 diabetes mellitus (T2DM) is an independent risk factor for the development of nonalcoholic fatty liver disease (NAFLD) and progression to liver fibrosis and cirrhosis (4, 5). Also, NAFLD and liver fibrosis have been reported to play an important role in the presence and progression of DPN (6–8). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were the two most common liver enzymes that reflect hepatocellular injury and death, and liver function. The concept of AST/ALT ratio (AAR) that represents the simultaneous alteration of AST and ALT levels was first put forwarded by De Ritis in 1957 (9). Since then, AAR has been reported to be a widely used liver fibrosis marker, and in addition, an established predictive marker of liver fibrosis severity in patients with liver disease and other non-hepatic diseases (10, 11). Besides, AAR was correlated with oxidative stress, systemic inflammation, and insulin resistance (IR) (12, 13), and implicated in the incidence and development of a wider range of cardiometabolic diseases, including metabolic syndrome (MetS) and its components including obesity, hyperglycemia or T2DM, hypertension, and hyperlipidemia, NAFLD as a hepatic manifestation of MetS, peripheral artery disease (PAD), arteriosclerosis, arterial stiffness, stroke, and cardiovascular diseases (CVD) (14–19), all of which have been proved to be closely associated with diabetic microvascular complications (20–22). Considering the strong interrelationship between diabetic microvascular complications and above-mentioned cardiometabolic diseases and the important role of liver fibrosis in diabetic microvascular complications (7, 23, 24), it is reasonable to hypothesize that T2DM individuals with high AAR would have a high risk for diabetic microvascular complications. Indeed, two clinical studies suggested that high AAR was an independent risk factor for diabetic nephropathy (DN), and was associated with more severe renal pathologic lesions and worse renal function (12, 25). As far as we are aware, the relationship of the AAR with DPN, however, has never been determined, and the underpinning mechanisms are less well understood.
Therefore, this cross-sectional study was conducted to investigate the relationship between AAR and risk of presence of DPN in Chinese adults with T2DM. Moreover, the possible mechanisms were explored by analyzing the potential relationships among AAR and metabolic and vascular parameters, and inflammation and oxidative stress markers.
Methods
Study population
A total of 3514 confirmed or newly diagnosed T2DM inpatients aged 18–89 years between August 2012 and September 2015, who were admitted to the Department of Endocrinology at the Affiliated Hospital of Southwest Medical University for screening of diabetic chronic complications and to optimize their anti-diabetic regimen, were initially recruited. T2DM was diagnosed based on the 1999 World Health Organization criteria (26). Subjects were excluded if they had any of the following criteria: 1) other types of diabetes other than T2DM, severe DFU (grades III-V according to the Wagner classification) or previous amputation, recent acute complications of diabetes, including diabetic ketoacidosis, hyperglycemic hyperosmolar state, hyperosmolar coma and hypoglycemia; 2) endocrine diseases other than T2DM, such as thyroid disease, parathyroid disease, adrenal diseases, pituitary diseases; 3) presence of non-diabetes-related neuropathy such as chronic inflammatory demyelinating polyneuropathy, cervical and lumbar diseases, and severe cerebrovascular disease (ischemic and haemorrhagic stroke); 4) severe respiratory disease, congestive heart failure (New York Heart Association functional class IV), severe renal failure (estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2), hematological diseases, thromboembolic disease; 5) autoimmune or viral hepatitis, alcohol-induced or drug-induced liver disease, cholestatic or metabolic/genetic liver disease, liver cirrhosis, and other chronic liver disease, gall bladder and biliary tract diseases; 6) connective tissue, inflammatory and recent active infectious disease, and stress conditions, autoimmune diseases; 7) history of malignancies and mental illness; 8) alcoholism; 9) pregnancy and lactation; 10) use of immunosuppressive agents, antioxidants, anti-inflammatory, antibiotics, analgesics, systemic corticosteroids, multivitamins or vitamin B12 supplements; 11) use of possible or known drugs affecting peripheral nerve function and sympathetic system; 12) missing or incomplete demographic or clinical characteristic data. After applying the exclusion criteria, 1562 participants aged 18-89 years were eligible and finally enrolled in the cross-sectional study.
The study was reviewed and approved by the human research ethics committee of the Affiliated Hospital of Southwest Medical University, and was performed in accordance with the Helsinki Declaration. All patients gave informed consent before participating in this study.
Data collection and measurements
During face-to-face interviews, trained interviewers administered a detailed standardized questionnaire, which consisted of information on their demographic characteristics (sex, age), lifestyle characteristics (physical activity, smoking and drinking status, etc.), personal medical history (hypertension, coronary heart disease (CHD), DFU, PAD, diabetic retinopathy (DR), DN, NAFLD, and other diseases), disease duration, family history, as detailed elsewhere. Then, all patients with T2DM received anthropometric examination, physical examination, laboratory tests, and evaluation of diabetes-related complications.
Body weight and height were measured by trained interviewers under standardized conditions following a standardized protocol, and body mass index (BMI) was calculated as body weight (kg) divided by the square of the height (m). Systolic blood pressures (SBP) and diastolic blood pressures (DBP) were measured in all subjects on the right arm using a standard mercury sphygmomanometer (27).
Venous blood samples were gathered from each participant in the morning after an overnight fast (at least 8 h) for measurement of fasting blood glucose (FBG), glycated hemoglobin A1C (HbA1c), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (apoA), apolipoprotein B (apoB), AST, ALT, total bilirubin (TBIL), glutamyl transpeptidase (GGT), serum albumin (ALB), creatinine (Cr), uric acid (UA), white blood cell (WBC), neutrophil, and lymphocyte counts, red blood cell distribution width (RDW), and fibrinogen according to relevant protocols and guidelines at the registered central laboratory located at the Affiliated Hospital of Southwestern Medical University, which is accredited in line with the international organization for standardization (ISO) 15189 standard for quality management specific to medical laboratories.
Triglyceride-glucose (TyG) index was calculated using the following equation: ln (fasting TG [mg/dL] × FBG [mg/dL]/2) (28). The atherogenic index of plasma (AIP) was calculated as ln (TG/HDL-C) and the atherogenic coefficient (AC) was calculated as (TC-HDL-C/HDL-C) (29). Hepatic steatosis index (HSI) was defined as follows: HSI = 8 × ALT/AST ratio + BMI (+2, if diabetes; +2, if female) (30). The AAR was calculated as AST/ALT ratio. Neutrophil to lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by lymphocyte count. The eGFR was evaluated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations modified by a Japanese coefficient (31, 32). Urinary albumin and Cr were measured from three fresh morning spot urine sample on three separate occasions within 6 months. Urinary albumin was measured with immunoturbidimetric tests. Urinary Cr was measured enzymatically. The urinary albumin-to- Cr ratio (ACR; mg/g creatinine) was calculated by dividing urinary albumin by urinary Cr (33, 34). Patients were then classified as having DN if they had an eGFR < 60 mL/min/1.73m2 and/or an ACR > 30 mg/g in two out of three random voided urine samples (32–34).
Foot examination and definition of DPN, PAD, and DFU
All patients with T2DM were asked whether they had numbness, pain (prickling or stabbing, shooting, burning or aching pain), and paresthesia (abnormal cold or heat sensation, allodynia and hyperalgesia) in the toes, feet, legs or upper-limb. Then, an experienced physician performed the neurologic examination which included vibration, light touch, and achilles tendon reflexes on both sides in the knee standing position (as being either presence or weakening or loss). Vibration perception threshold (VPT) was assessed at the metatarsophalangeal joint dig I using a neurothesiometer (Bio- Thesiometer; Bio-Medical Instrument Co., Newbury, OH, USA). First, the patients were informed how to know the vibration sensation is felt by gradually turning the amplitude from zero to maximum, then the test began again from zero and they were asked to say the moment that they first felt it. Measurements were made on the planter aspect of the big toe bilaterally, three times consecutively for each big toe. The median of three readings is accepted as the VPT value of that measurement (35). Sensitivity to touch was also tested using a 5.07/10-g Semmes-Weinstein monofilament (SWM) at four points on each foot: three on the plantar and one on the dorsal side. The 10-g SWM was placed perpendicular to the skin and pressure was applied until the filament just buckled with a contact time of 2 s. Inability to perceive the sensation at any one site was considered abnormal (36, 37). DPN was defined as VPT ≥25 V and/or inability to feel the monofilament (35), and then participants were divided into DPN group and no DPN group.
Ankle brachial index (ABI) was measured noninvasively by a continuous-wave Doppler ultrasound probe (Vista AVS, Summit Co., USA) with participants in the supine position after at least 5 min of rest. Leg-specific ABI was calculated by dividing the higher SBP in the posterior tibial or dorsalis pedis by the higher of the right or left brachial SBP (33, 38). Patients were diagnosed as having PAD if an ABI value <0.9 on either limb (33, 38).
DFU was defined as ulceration of the foot (distally from the ankle and including the ankle) associated with neuropathy and different grades of ischemia and infection (39).
Other classifications and definitions
A Canon CR-2 Digital Retinal Camera was performed to obtain two-field fundus photography of patient’s eyes (Canon Inc., Kanagawa, Japan). The presence of DR was assessed by high-quality fundus photographs and an ophthalmologist. NAFLD diagnosis was based on the detection of hepatic steatosis by abdominal ultrasound while excluding drugs, viruses, or alcohol as the cause (40). MetS was defined according to Chinese Diabetes Society (CDS) criteria (41) if they have three or more of the following risk factors (1): overweight or obese (BMI ≥ 25.0 kg/m2) (2); hyperglycemia (FBG ≥ 6.1 mmol/l and/or 2-hour postprandial plasma glucose ≥ 7.8 mmol/l, or under treatment for diabetes) (3); hypertension (SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or on antihypertensive medication); and (4) dyslipidemia, defined as TG ≥ 1.7 mmol/l and/or HDL-C < 0.9 mmol/l (men) or <1.0 mmol/l (women). CHD was defined as a positive history of myocardial infarction, bypass operation, a diagnostic finding in angiography or positive exercise test (42).
Statistical analysis
Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) (version 20.0; IBM, Chicago, IL). All data were first analyzed for normality of distribution using the Kolmogorov–Smirnov test of normality, and homogeneity of variance using the Levene homogeneity of variance test. Continuous data are presented as mean ± standard deviation (SD), and categorical data are presented as absolute and relative frequencies (n, %).
All patients with T2DM were placed into four groups according to AAR quartiles: quartile (Q) 1 group, 0.32–0.80; Q2 group, 0.81–1.00; Q3 group, 1.01–1.27; and Q4 group, 1.28-5.26. Meanwhile, male and female patients were divided into four quartile groups by AAR level, respectively: Q1 group (male: 0.32-0.75; female: 0.36–0.88), Q2 group (male: 0.76–0.94; female: 0.89–1.08), Q3 group (male: 0.95–1.19; female: 1.09–1.34), and Q4 group (male: 1.20– 3.98; female: 1.35– 5.26). Continuous variables were compared by Student’s t test and one-way analysis of variance (ANOVA), whereas skewed distribution variables were compared by Mann-Whitney U and Kruskal-Wallis tests. Categorical variables were compared across groups using χ2 tests. As AAR was non-normally distributed, Spearman correlation coefficients were performed to assess whether there was an association between AAR and other variables, and the partial correlation coefficient was also used to control for the effects of age, sex, BMI, and diabetic duration. The collinearity diagnostics analysis in linear regression models was also performed to assess whether multiple collinearity exists in these independent variables. The associations of AAR and other variables with the risk of presence of DPN in all T2DM patients were explored by the univariable logistic regression analysis, and then determined using a multivariable logistic regression analysis with those variables achieving P ≤ 0.20 in our univariable analysis entered into this model. Further, binary logistic regression analyses were conducted to investigate the association of AAR quartiles with the risk of presence of DPN in all subjects, male subjects, and female subjects, and odd ratio (OR) and 95% confidence interval (CI) were estimated. Possible dose-response relationships between AAR and DPN were examined by the trend test. Last, the predictive validity of AAR for the presence of DPN was determined using receiver operating characteristic (ROC) curves and area under the curve (AUC) in all subjects, male subjects, and female subjects.
Results were considered to be statistically significant at a P value <0.05.
Results
Clinical and laboratory characteristics of study participants
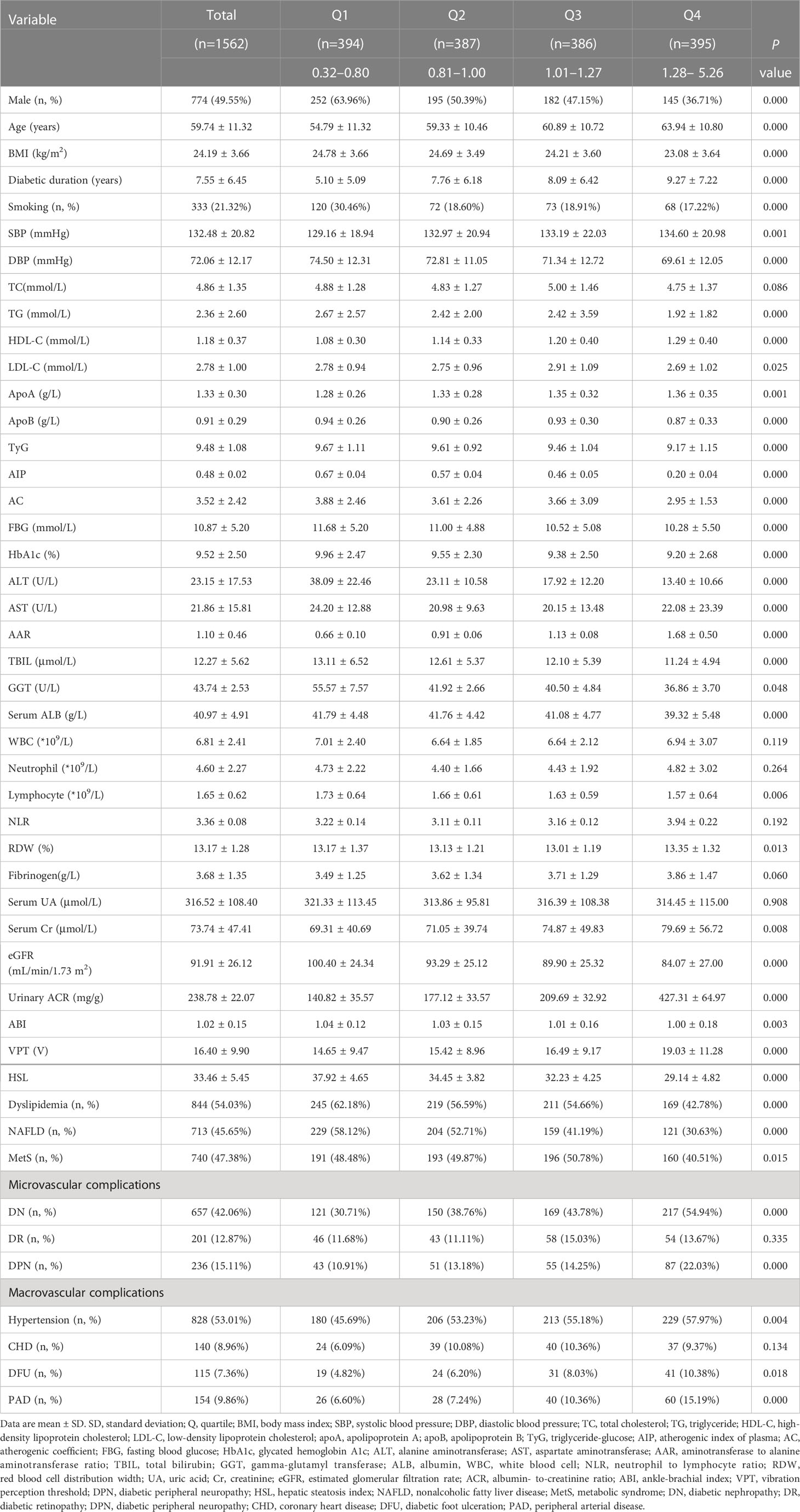
The clinical and laboratory characteristics of 1562 patients with T2DM (774 male, 49.55%, and 788 female, 50.45%) according to AAR quartiles were summarized in Table 1. Overall, mean age was 59.74 years, BMI was 24.19 kg/m2, diabetic duration was 7.55 years, and AAR was 1.10. Patients with higher AAR quartiles tended to be female and relatively older, less user of smoking, and have longer diabetic duration, higher levels of SBP, HDL-C, apoA, AST, AAR, RDW, serum Cr, urinary ACR, VPT, presence of DPN, DN, hypertension, DFU, PAD, and lower BMI, DBP, TG, LDL-C, apoB, TyG, FBG, HbA1c, ALT, TBIL, GGT, serum ALB, lymphocyte counts, eGFR, ABI, HSL, prevalence of dyslipidemia, NAFLD, and MetS compared to those with lower quartiles (P<0.01 or P<0.05). Supplementary Table 1 reported characteristics of all T2DM patients by DPN. Patients with DPN had significantly older age, longer diabetic duration, higher SBP, FBG, HbA1c, AAR, WBC, neutrophil counts, NLR, fibrinogen, serum Cr, urinary ACR, VPT, prevalence of DN, DR, hypertension, CHD, DFU, PAD, and lower BMI, DBP, TC, TG, apoA, ALT, AST, TBIL, GGT, serum ALB, lymphocyte counts, eGFR, ABI, HSL, and prevalence of NAFLD than those without DPN (P<0.01 or P<0.05).
Association of AAR with clinical and laboratory characteristics in study subjects
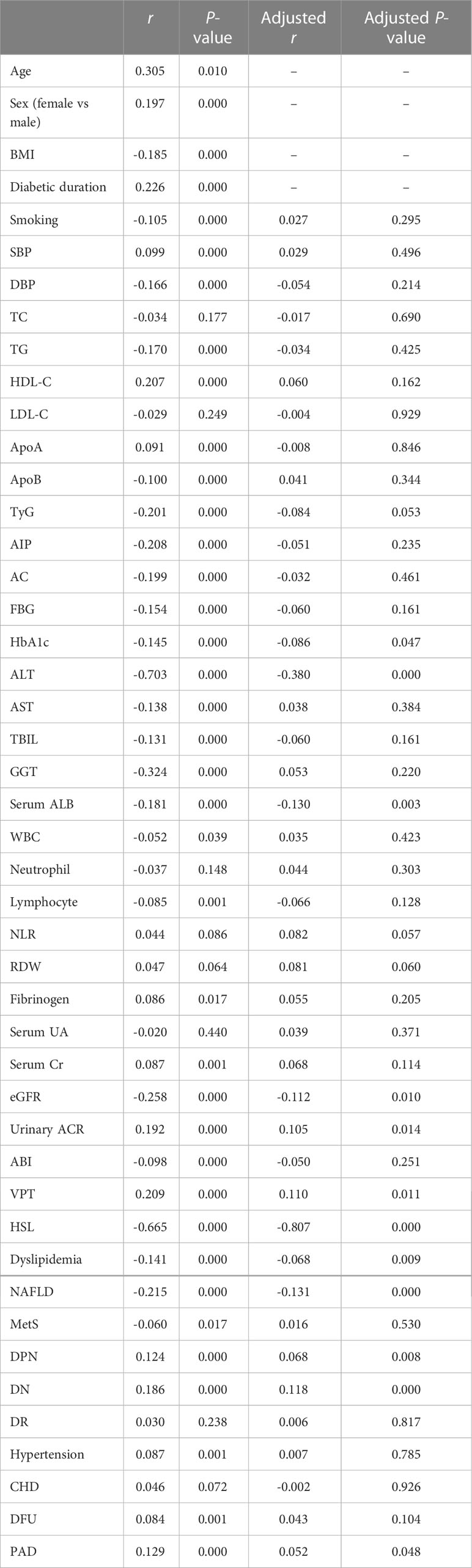
Table 2 showed the association of AAR with clinical and laboratory characteristics in all patients with T2DM performed by Spearman and partial correlation coefficient. The results revealed that AAR was positively associated with age, sex distribution, diabetic duration, SBP, HDL-C, apoA, fibrinogen, serum Cr, urinary ACR, VPT and prevalence of DPN, DN, hypertension, DFU, PAD, and negatively with BMI, smoking, DBP, TG, apoB, TyG, FBG, HbA1c, ALT, AST, TBIL, GGT, serum ALB, WBC, lymphocyte counts, eGFR, ABI, HSL, and prevalence of dyslipidemia, NAFLD and MetS (P<0.01 or P<0.05). After adjustments for sex, age, BMI, and diabetic duration, the associations among HbA1c, ALT, serum ALB, eGFR, urinary ACR, VPT, HSL, presence of dyslipidemia, NAFLD, DPN, DN, PAD and AAR were attenuated but remained statistically significant (P<0.01 or P<0.05).
Univariate and multivariate analysis of determinants of DPN in study subjects
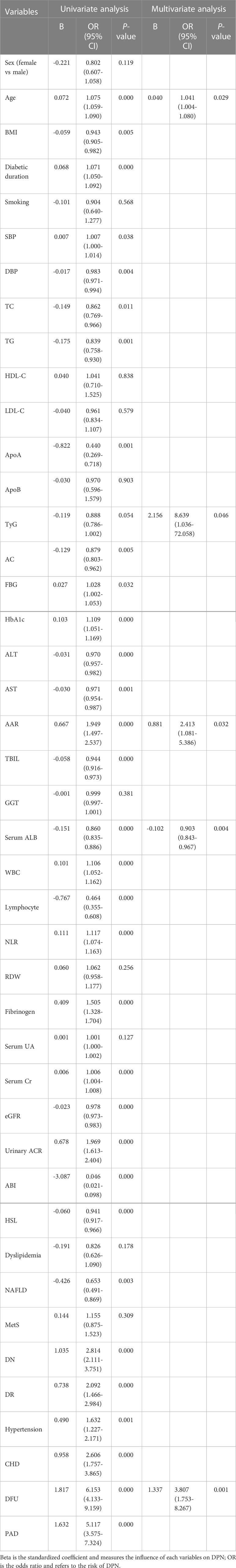
Table 3 displayed the associations of AAR and other variables with the risk of presence of DPN. The univariate logistic regression analysis revealed that age, BMI, diabetic duration, SBP, DBP, TC, TG, apoA, FBG, HbA1c, ALT, AST, AAR, TBIL, serum ALB, WBC, neutrophil and lymphocyte counts, NLR, fibrinogen, serum Cr, eGFR, urinary ACR, ABI, HSL, and prevalence of NAFLD, DN, DR, hypertension, CHD, DFU, PAD were significantly associated with the presence of DPN (P<0.01 or P<0.05).Multivariable logistic regression analysis showed that age, TyG, AAR, serum ALB, and DFU were significantly and independently associated with the presence of DPN (P<0.01 or P<0.05). Notably, each SD increase in AAR was associated with a significant 2.413-fold increased odds of DPN (95% CI, 1.081-5.386, P<0.05).
Association of AAR quartiles with the risk of presence of DPN in study subjects
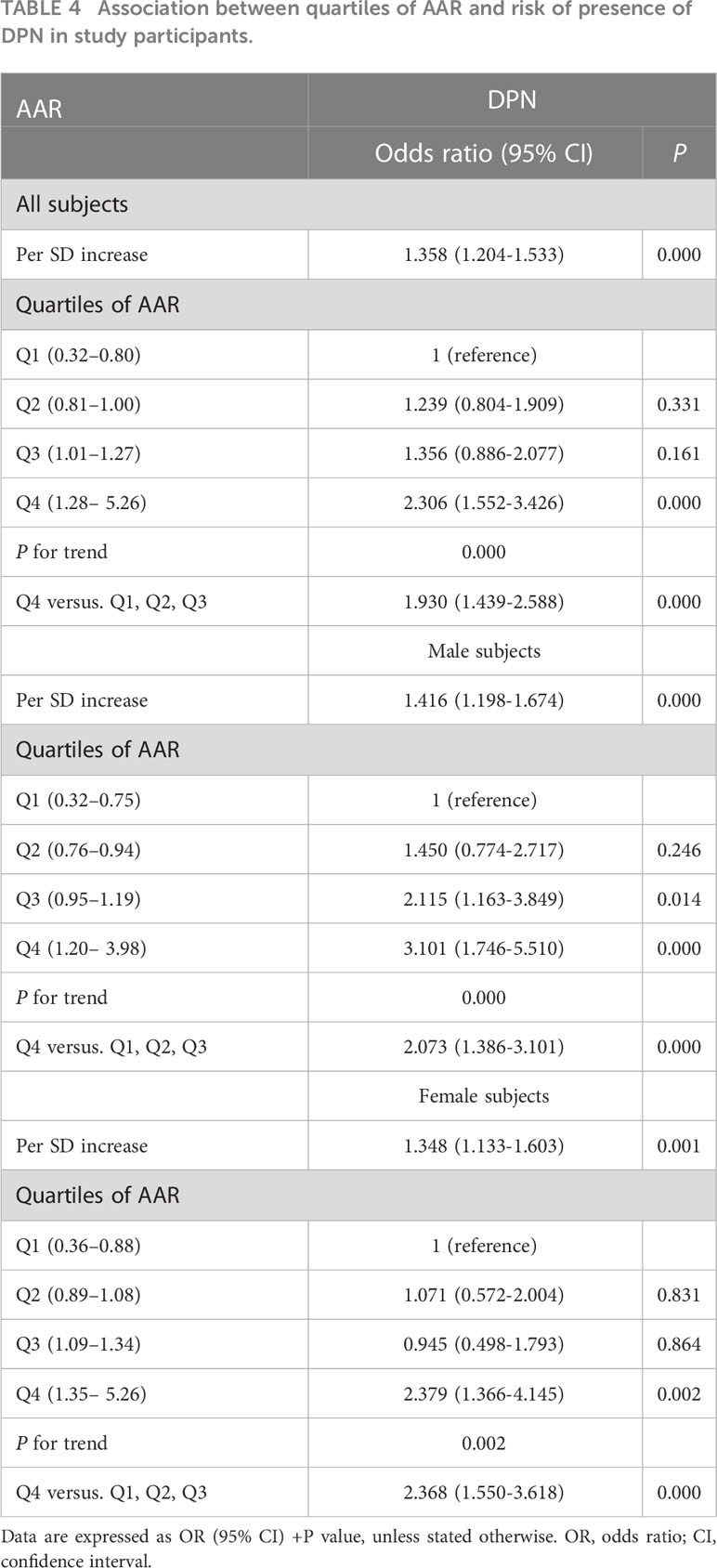
As shown in Table 4, the risk of presence of DPN also increased progressively as AAR quartiles increased in all subjects, male subjects, and female subjects, respectively (all P for trend <0.01). When compared to the lower quartiles (Q1, Q2, and Q3), the highest quartile of AAR (Q4) of all subjects, male subjects, and female subjects were significantly associated with increased odds for DPN (OR = 1.930, 2.073, and 2.368, respectively). Even per SD increase in AAR of all subjects, male subjects, and female subjects were respectively associated with were more likely to have DPN (OR = 1.358, 1.416, and 1.348, respectively).
Predictive value of AAR in screening for the presence of DPN in T2DM patients
To explore the predictive value of AAR for DPN, we analyzed the ROC curves of AAR. The results revealed that the best cutoff value for AAR to predict the presence of DPN was 1.40 (sensitivity: 30.90%; specificity: 85.50%; and AUC: 0.600; Figure 1A) in all subjects, and the best cutoff values for AAR to predict the presence of DPN were 0.906 (sensitivity: 70.3%; specificity: 49.2%; and AUC: 0.618; Figure 1B) and 1.402 (sensitivity: 38%; specificity: 81.9%; and AUC: 0.600; Figure 1C) in male and female subjects, respectively.
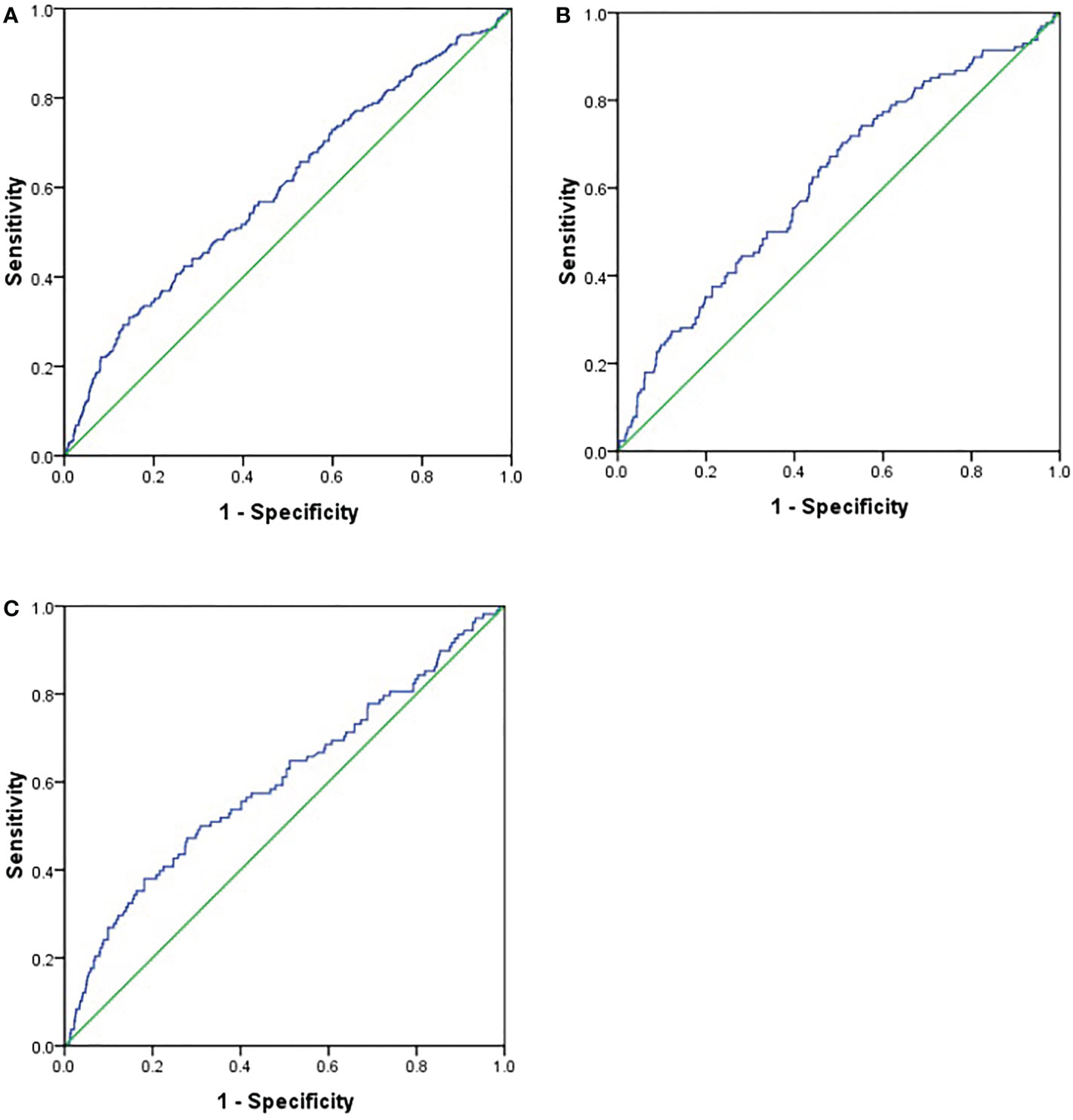
Figure 1 Receiver operating characteristics (ROC) curve analysis of aminotransferase to alanine aminotransferase ratio (AAR) to inidicate DPN. (A). all subjects; (B). male subjects; (C). female subjects.
Discussion
To our knowledge, this was the first study to investigate the relationship between AAR and risk of presence of DPN. We found that patients with higher AAR quartiles had higher presence of DPN, and AAR was an independent determinant of presence of DPN after multivariate adjustment. Additionally, the risk of presence of DPN increased progressively as AAR quartiles increased in both sexes. Last, the analysis of ROC curves revealed that AAR could predict the presence of DPN in both sexes. These findings suggest that high AAR may be associated with the presence of DPN in hospitalized Chinese T2DM patients, and may be used as an additional indicator of risk of DPN.
As mentioned earlier, AAR, an emerging indicator of liver function, has been reported to effectively predict the severity of liver fibrosis in patients with various liver disease including NAFLD (10, 11). There is now growing evidence that NAFLD is more common and often advanced in patients with T2DM, easily progressing to nonalcoholic steatohepatitis and advanced liver fibrosis, than in the general population (6, 43–46). Considering a certain intrinsic correlation among AAR, NAFLD and liver fibrosis, and diabetic vascular complication (6, 10, 11, 15–19, 43–46), it is plausible that AAR may be associated with the presence of DPN, and high AAR may be an early signal for being at risk for DPN. In the present study, we found that patients with higher AAR quartiles tended to have higher VPT, a widely recommended indicator of the presence and severity of confirmed clinical neuropathy (47), and similarly, patients with DPN had significantly higher AAR than those without. Moreover, AAR was positively associated with VPT and presence of DPN. Altogether, these data preliminarily argue that there was a potential relationship between AAR and the presence and severity of DPN. Besides, AAR was significantly and independently associated with the presence of DPN after multivariate adjustment. Additionally, the risk of presence of DPN increased progressively as AAR quartiles increased in both sexes. More importantly, AAR could predict the presence of DPN in both sexes. These data were broadly similar to the findings of previous studies showing that noninvasive biomarkers of liver fibrosis, such as NAFLD fibrosis score and fibrosis-4 score were independently associated with DPN (6, 8, 48, 49), further suggesting that higher AAR, another novel liver fibrosis marker, could be linked to an increased risk of the presence and severity of DPN, and AAR may be a novel and reliable marker for identifying subjects at high risk for DPN in patients with T2DM, however, the underlying mechanisms potentially responsible for the association remain unclear.
Growing evidence suggests that NAFLD is closely associated with the presence of DPN (50–52), while IR has been suggested to play a central role in the development and progression of NAFLD (53). In the present study, we found that patients with DPN had significantly lower HSL, which is a accurate proxy of NAFLD that can assess liver steatosis in predominantly Asian populations (30), and prevalence of NAFLD than those without DPN. Moreover, the logistic regression analysis revealed that HSL, TyG, a biochemical marker of IR (28), and prevalence of NAFLD were significantly associated with the presence of DPN. Our findings are largely in line with results from prior studies (50, 51, 54, 55). Yan et al. reported that patients with NAFLD diagnosed earlier than T2DM had a lower prevalence of DPN compared with those with T2DM diagnosed earlier than NAFLD or those with T2DM only (51). Another cross-sectional study demonstrated that the prevalence of NAFLD in Chinese T2DM patients with DPN was significantly lower than those without DPN, and NAFLD was negatively correlated with the prevalence of DPN (50). Recently, Zhao and colleagues revealed that a higher level of AUC of C-peptide was inversely associated with prevalence of diabetic neuropathy, and positively associated with homeostasis model assessment of IR index and NAFLD in 885 patients with T2DM (54). Similar results were also obtained by Guo et al. in T2DM patients (55). Together, these lines of evidence, combined with our results, suggest that NAFLD and its key component IR may protect against the development and progression of DPN in T2DM patients. Moreover, we demonstrated that patients with higher AAR quartiles tended to have longer diabetic duration and lower TyG, HSL, and prevalence of NAFLD compared to those with lower quartiles. Additionally, the Spearman correlation analysis revealed that AAR was negatively associated with HSL, TyG, and prevalence of NAFLD. Qiao et al. found that C-peptide and insulin levels progressively decreased (inadequate insulin secretion) and IR was relatively low because of weakened or even deterioration of pancreatic islet β cell function induced by long-term hyperglycemia along with increased diabetic duration, leading to increased prevalence of DPN (56). Combined, these data suggest that there might be a negative correlation between AAR and IR and NAFLD, and higher AAR might contribute to the development of DPN through a complex mechanism associated with IR and NAFLD; however, the mechanism of action needs to be further investigated.
Numerous studies have demonstrated that low-grade inflammation and oxidative stress are also contributing factors in the development and progression of DPN (7, 21, 49). Serum ALB is the most abundant circulating protein in blood synthesized and secreted from liver cells. It has been reported that serum ALB is the major source of extracellular reduced sulfhydryl groups, which act as potent scavengers of reactive oxygen and nitrogen species, thus constituting the dominant antioxidant in the circulatory system (57, 58). In addition, some substances such as nitric oxide and bilirubin are carried by serum albumin and provide additional protection against oxidative stress (57). Also, serum ALB can bind various inflammatory mediators and inhibit the secretion of pro-inflammatory cytokines, thus involving in regulating the inflammatory immune response and endothelial stabilization (58, 59). It has been suggested that elderly people are susceptible to oxidative stress due to a decline in the inefficiency of their endogenous antioxidant systems (60), and oxidative stress and inflammatory mediators increase with aging (61). In the present study, we found that patients with DPN had significantly older age and lower serum ALB than those without DPN. The logistic regression analysis revealed that age and serum ALB were significantly and independently associated with the presence of DPN after multivariate adjustment. Our findings were in agreement with previous studies (62–65) showing that serum ALB has neuroprotective effects via its antioxidant/anti-inflammatory activity, and its lower level was related to abnormal peripheral nerve function and a significantly increased risk of DPN, and older age is a risk factor for DPN, providing further evidence that inflammation and oxidative stress induced by lower serum ALB and older age may be closely associated with the presence of DPN. Moreover, patients with higher AAR quartiles tended to be relatively older and had significantly lower serum ALB compared to those with lower quartiles. Additionally, AAR was positively associated with age, and negatively with serum ALB. Our findings were in agreement with most previous studies (15, 66–69). Liu et al. reported that Chinese hypertensive patients with higher AAR had significantly lower levels of serum ALB and other endogenous antioxidant substances compared with those with lower AAR (15). Evidence from an animal study has also suggested that mice with elevated AAR had a reduced ability to carry oxygen, which was accompanied by significantly elevated levels of markers of oxidative stress (66). Several studies also have announced that hepatic steatosis assessed by AAR is associated with increased production of interleukin-6 and other pro-inflammatory cytokines by hepatozytes and nonperynchymal cells (67–69). Together, these lines of evidence, combined with our results, suggest that higher AAR may be closely associated with increased inflammation and oxidative stress, and inflammation and oxidative stress induced by lower serum ALB and older age might at least partially mediate the potential relationship between AAR and DPN, but larger, well-characterized, prospective research is still needed to validate these findings.
Experimental and epidemiological studies have shown that atherosclerotic vascular disease plays a critical role in the development and progression of DPN (23, 64). In the present study, we found that patients with DPN had significantly higher prevalence of DFU and PAD, two major diabetic macrovascular complications associated with atherosclerosis, than those without DPN. Moreover, the logistic regression analysis revealed that the prevalence of PAD was significantly positively associated with the presence of DPN, while DFU was an independent risk factor for DPN. Our findings further provided evidence that atherosclerotic vascular disease, especially DFU, and DPN are closely interconnected, and nerve ischemia associated with vascular dysfunction may contribute to nerve damage, eventually leading to the development of DPN. Moreover, patients with higher AAR quartiles tended to have higher prevalence of DFU and PAD and lower ABI, and AAR was significantly positively associated with prevalence of PAD and DFU, which was in general agreement with two previous studies (15, 70). A cross-sectional study that included 10900 Chinese adults with hypertension discovered that a high AAR was independently and positively associated with associated with PAD risk (15). Similarly, another cross-sectional study conducted by Rief and his colleagues reported that an elevation in AAR is significantly associated with an increased risk of occurrence of critical limb ischemia, independently of well-established risk factors, in patients with peripheral arterial occlusive disease (70). Together, these results indicate that high AAR might be linked to PAD and critical limb ischemia, an important risk factor for DFU, and vascular damage, especially DFU, might be associated with the presence of DPN. It is well-known that AST is abundantly present in many different types of tissue in addition to the liver, such as skeletal, cardiac, smooth muscle, kidney, and brain, whereas ALT is low in cells other than hepatocytes (71). Thus, an increased vulnerability of the liver and several other tissue associated with AST distribution to ischemia due to vascular damage caused by DFU would lead to an higher AAR in T2DM patients with DPN (6, 71, 72). However, the exact mechanism responsible for the relationship between AAR and DPN is still obscure and required further investigation.
Some potential limitations of our study should be noted. First, the causality of the relationship between AAR and DPN could not be established because this design of the present study is cross-sectional. Second, individuals with T2DM are at high risk for both microvascular complications and macrovascular complications, and thus may usually needs to take multiple medications at the same time, of which might affect liver transaminase due to potential drug-drug interactions. However, detailed medication history, such as statins, for these subjects was unavailable. Third, the present study population consisted of T2DM inpatients of Chinese Han ancestry, who generally had more serious illness than diabetic outpatients, and thus, our findings cannot to be extrapolated to diabetic outpatients and other types of diabetes with different ethnic back grounds. Finally, it has been reported that a sedentary lifestyle and unhealthy dietary habits are associated with elevated liver enzyme levels (73), however, insufficient data were available for the information about their diet and lifestyle, which might have influenced the results. Despite these limitations, this study has several strengths such as a relatively large sample size, use of a standardized method at a single center, and thorough adjustment for possible confounding variables.
In conclusion, the present study demonstrated that AAR was significantly increased in T2DM patients with DPN, and was independently associated with increased risk of presence of DPN in Chinese patients with T2DM, thereby suggesting that AAR may serve as an useful and reliable biomarker of DPN, and highlighting that it is crucial to pay more attention to T2DM patients with high AAR to further prevent and reduce the development of DPN and related unfavorable health outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the human research ethics committee of the Affiliated Hospital of Southwest Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All the authors contributed significantly to the manuscript. PY conducted the population study, analysed and interpreted the data, and drafted the manuscript. QW significantly revised the draft, interpreted the data, and involved in data analyses. YW, XD, XW, and QT conducted the study, collected the information and participated in data interpretation. XC, YX, JZ, and YM involved in the sample test, data management and draft revision. QW is the PI of project, who designed the study and critically revised the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the grants from the Ministry Science and Technology of China (2016YFC0901200, 2016YFC0901205), and research grants from Sichuan Clinical Research Center for Nephropathy (2019YFS0537-15).
Acknowledgments
The authors would like to thank all the colleagues in clinical laboratory center and endocrine laboratory, and all the nurses in our department for their hard work and valuable assistance with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1064125/full#supplementary-material
References
1. Tesfaye S, Sloan G. Diabetic polyneuropathy - advances in diagnosis and intervention strategies. Eur Endocrinol (2020) 16(1):15–20. doi: 10.17925/EE.2020.16.1.15
2. Dalla Paola L, Faglia E. Treatment of diabetic foot ulcer: An overview strategies for clinical approach. Curr Diabetes Rev (2006) 2(4):431–47. doi: 10.2174/1573399810602040431
3. Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: Clinical and quality-of-life issues. Mayo Clin Proc (2006) 81(4 Suppl):S3–11. doi: 10.1016/s0025-6196(11)61474-2
4. Tuong TTK, Tran DK, Phu PQT, Hong TND, Dinh TC, Chu DT. Non-alcoholic fatty liver disease in patients with type 2 diabetes: Evaluation of hepatic fibrosis and steatosis using fibroscan. Diagnostics (Basel) (2020) 10(3):159. doi: 10.3390/diagnostics10030159
5. Chen K, Sng WK, Quah JH, Liu J, Chong BY, Lee HK, et al. Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PloS One (2020) 15(8):e0236977. doi: 10.1371/journal.pone.0236977
6. Kim K, Oh TJ, Cho HC, Lee YK, Ahn CH, Koo BK, et al. Liver fibrosis indices are related to diabetic peripheral neuropathy in individuals with type 2 diabetes. Sci Rep (2021) 11(1):24372. doi: 10.1038/s41598-021-03870-z
7. Huang J, Li R, Liu N, Yi N, Zheng H, Zhang Q, et al. Liver fibrosis is independently associated with diabetic peripheral neuropathy in type 2 diabetes mellitus. J Diabetes Investig (2021) 12(11):2019–27. doi: 10.1111/jdi.13562
8. Williams KH, Burns K, Constantino M, Shackel NA, Prakoso E, Wong J, et al. An association of large-fibre peripheral nerve dysfunction with non-invasive measures of liver fibrosis secondary to non-alcoholic fatty liver disease in diabetes. J Diabetes Complications (2015) 29(8):1240–7. doi: 10.1016/j.jdiacomp.2015.06.015
9. De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta (1957) 2(1):70–4. doi: 10.1016/0009-8981(57)90027-x
10. Åberg F, Danford CJ, Thiele M, Talbäck M, Rasmussen DN, Jiang ZG, et al. A dynamic aspartate-to-Alanine aminotransferase ratio provides valid predictions of incident severe liver disease. Hepatol Commun (2021) 5(6):1021–35. doi: 10.1002/hep4.1700
11. Long MT, Pedley A, Massaro JM, Hoffmann U, Fox CS. The association between non-invasive hepatic fibrosis markers and cardiometabolic risk factors in the framingham heart study. PloS One (2016) 11(6):e0157517. doi: 10.1371/journal
12. Xu J, Shi X, Pan Y. The association of aspartate Aminotransferase/Alanine aminotransferase ratio with diabetic nephropathy in patients with type 2 diabetes. Diabetes Metab Syndr Obes (2021) 14:3831–7. doi: 10.2147/DMSO.S330741
13. Simental-Mendía LE, Rodríguez-Morán M, Gómez-Díaz R, Wacher NH, Rodríguez-Hernández H, Guerrero-Romero F. Insulin resistance is associated with elevated transaminases and low aspartate aminotransferase/alanine aminotransferase ratio in young adults with normal weight. Eur J Gastroenterol Hepatol (2017) 29(4):435–40. doi: 10.1097/MEG.0000000000000811
14. Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: The insulin resistance atherosclerosis study. Diabetes (2005) 54(11):3140–7. doi: 10.2337/diabetes.54.11.3140
15. Liu H, Zha X, Ding C, Hu L, Li M, Yu Y, et al. AST/ALT ratio and peripheral artery disease in a Chinese hypertensive population: A cross-sectional study. Angiology (2021) 72(10):916–22. doi: 10.1177/00033197211004410
16. Mori T, Yoshioka K, Tanno Y. Non-alcoholic fatty liver disease frequency and associated factors at admission of acute stroke. Hepatol Int (2022) 16(1):81–8. doi: 10.1007/s12072-021-10253-z
17. Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP, et al. Non- alcoholic fatty liver disease, liver biomarkers and stroke risk: The reasons for geographic and racial differences in stroke cohort. PloS One (2018) 13(3):e0194153. doi: 10.1371/journal.pone.0194153
18. Weng SF, Kai J, Guha IN, Qureshi N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart (2015) 2(1):e000272. doi: 10.1136/openhrt-2015-000272
19. Liu Y, Zhao P, Cheng M, Yu L, Cheng Z, Fan L, et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population: A secondary analysis based on a cross-sectional study. Lipids Health Dis (2018) 17(1):275. doi: 10.1186/s12944-018-0920-4
20. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol (2019) 7(12):938–48. doi: 10.1016/S2213-8587(19)30081-6
21. Su JB, Zhao LH, Zhang XL, Cai HL, Huang HY, Xu F, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol (2018) 17(1):47. doi: 10.1186/s12933-018-0693-0
22. Hägg S, Thorn LM, Putaala J, Liebkind R, Harjutsalo V, Forsblom CM, et al. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care (2013) 36(12):4140–6. doi: 10.2337/dc13-0669
23. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med (2005) 352(4):341–50. doi: 10.1056/NEJMoa032782
24. Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int (2020) 40(2):347–54. doi: 10.1111/liv.14274
25. Wu Y, Liu F. Aspartate aminotransferase to alanine aminotransferase ratio and the risk of diabetic nephropathy progression in patients with type 2 diabetes mellitus: A biopsy-based study. J Diabetes Complications (2022) 36(8):108235. doi: 10.1016/j
26. Mostafa SA, Davies MJ, Webb D, Gray LJ, Srinivasan BT, Jarvis J, et al. The potential impact of using glycated haemoglobin as the preferred diagnostic tool for detecting type 2 diabetes mellitus. Diabetes Med (2010) 27(7):762–9. doi: 10.1111/j
27. Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol (2020) 19(1):118. doi: 10.1186/s12933-020-01095-4
28. Liu L, Xia R, Song X, Zhang B, He W, Zhou X, et al. Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: A cross-sectional study. J Diabetes Investig (2021) 12(4):557–65. doi: 10.1111/jdi
29. Yan PJ, Xu Y, Wan Q, Feng J, Li H, Gao CL, et al. Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity c-reactive protein in newly diagnosed type 2 diabetes mellitus patients: A cross-sectional study. Acta Diabetol (2017) 54(12):1091–9. doi: 10.1007/s00592-017-1044-4
30. Damba T, Bourgonje AR, Abdulle AE, Pasch A, Sydor S, van den Berg EH, et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int (2020) 40(9):2148–59. doi: 10.1111/liv.14562
31. Levey AS, Inker LA, Coresh J. GFR estimation: From physiology to public health. Am J Kidney Dis (2014) 63(5):820–34. doi: 10.1053/j.ajkd.2013.12.006
32. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
33. Yan P, Zhang Z, Wan Q, Zhu J, Li H, Gao C, et al. Association of serum uric acid with bone mineral density and clinical fractures in Chinese type 2 diabetes mellitus patients: A cross-sectional study. Clin Chim Acta (2018) 486:76–85. doi: 10.1016/j.cca.2018.07.033
34. Amatruda JG, Katz R, Peralta CA, Estrella MM, Sarathy H, Fried LF, et al. Association of non-steroidal anti-inflammatory drugs with kidney health in ambulatory older adults. J Am Geriatr Soc (2021) 69(3):726–34. doi: 10.1111/jgs.16961
35. Lu B, Yang Z, Wang M, Yang Z, Gong W, Yang Y, et al. High prevalence of diabetic neuropathy in population-based patients diagnosed with type 2 diabetes in the shanghai downtown. Diabetes Res Clin Pract (2010) 88(3):289–94. doi: 10.1016/j.diabres.2010.02.002
36. Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig (2014) 5(6):714–21. doi: 10.1111/jdi.12223
37. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American diabetes association, with endorsement by the American association of clinical endocrinologists. Diabetes Care (2008) 31(8):1679–85. doi: 10.2337/dc08-9021
38. Chen J, Mohler ER 3rd, Garimella PS, Hamm LL, Xie D, Kimmel S, et al. Ankle brachial index and subsequent cardiovascular disease risk in patients with chronic kidney disease. J Am Heart Assoc (2016) 5(6):e003339. doi: 10.1161/JAHA.116.003339
39. Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabetes Med (1993) 10(7):676–9. doi: 10.1111/j.1464-5491
40. American Gastroenterological Association. American Gastroenterological association medical position statement: Nonalcoholic fatty liver disease. Gastroenterology (2002) 123(5):1702–4. doi: 10.1053/gast.2002.36569
41. Wei D, Chen T, Li J, Gao Y, Ren Y, Zhang X, et al. Association of serum gamma- glutamyl transferase and ferritin with the metabolic syndrome. J Diabetes Res (2015) 2015:741731. doi: 10.1155/2015/741731
42. Gordin D, Hiilesmaa V, Fagerudd J, Rönnback M, Forsblom C, Kaaja R, et al. Pre-eclampsia but not pregnancy-induced hypertension is a risk factor for diabetic nephropathy in type 1 diabetic women. Diabetologia (2007) 50(3):516–22. doi: 10.1007/s00125-006-0544-5
43. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism (2016) 65(8):1096–108. doi: 10.1016/j.metabol.2016.01.001
44. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology (2019) 70(2):711–24. doi: 10.1002/hep.30429
45. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol (2017) 23(47):8263–76. doi: 10.3748/wjg.v23.i47.8263
46. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PloS Med (2020) 17(4):e1003100. doi: 10.1371/journal.pmed.1003100
47. Martin CL, Waberski BH, Pop-Busui R, Cleary PA, Catton S, Albers JW, et al. Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: Results from the DCCT/EDIC study. Diabetes Care (2010) 33(12):2635–41. doi: 10.2337/dc10-0616
48. Williams KH, Burns K, Twigg SM. Differing clinical phenotype for higher alanine-aminotransferase (ALT) compared with high-risk NAFLD fibrosis score in type 2 diabetes mellitus. J Diabetes Complications (2018) 32(3):321–4. doi: 10.1016/j
49. Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J Diabetes Complications (2020) 34(11):107684. doi: 10.1016/j.jdiacomp.2020.107684
50. Lv WS, Sun RX, Gao YY, Wen JP, Pan RF, Li L, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol (2013) 19(20):3134–42. doi: 10.3748/wjg.v19.i20.3134
51. Yan LH, Mu B, Guan Y, Liu X, Zhao N, Pan D, et al. Assessment of the relationship between non-alcoholic fatty liver disease and diabetic complications. J Diabetes Investig (2016) 7(6):889–94. doi: 10.1111/jdi.12518
52. Mantovani A, Rigolon R, Mingolla L, Pichiri I, Cavalieri V, Salvotelli L, et al. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J Diabetes Complications (2017) 31(6):1021–6. doi: 10.1016/j.jdiacomp.2017.01.024
53. Ikegami T, Hyogo H, Honda A, Miyazaki T, Tokushige K, Hashimoto E, et al. Increased serum liver X receptor ligand oxysterols in patients with non-alcoholic fatty liver disease. J Gastroenterol (2012) 47(11):1257–66. doi: 10.1007/s00535-012-0585-0
54. Zhao L, Ma J, Wang S, Xie Y. Relationship between β-cell function, metabolic control, and microvascular complications in type 2 diabetes mellitus. Diabetes Technol Ther (2015) 17(1):29–34. doi: 10.1089/dia.2014.0214
55. Guo Y, Zhao X, Liu CQ, Huang ZP, Zou DJ. A novel refined classification system for type 2 diabetes in adults: A Chinese retrospective cohort study. Diabetes Metab Res Rev (2022) 38(8):e3577. doi: 10.1002/dmrr.3577
56. Qiao X, Zheng H, Zhang S, Liu S, Xiong Q, Mao F, et al. C-peptide is independent associated with diabetic peripheral neuropathy: A community-based study. Diabetol Metab Syndr (2017) 9:12. doi: 10.1186/s13098-017-0208-2
57. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014
58. Caraceni P, Domenicali M, Tovoli A, Napoli L, Ricci CS, Tufoni M, et al. Clinical indications for the albumin use: Still a controversial issue. Eur J Intern Med (2013) 24(8):721–8. doi: 10.1016/j.ejim.2013.05.015
59. Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med (2019) 51(7-8):333–44. doi: 10.1080/07853890.2019.1693056
60. Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front Pharmacol (2018) 9:1162. doi: 10.3389/fphar.2018.01162
61. Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci (2007) 62(4):427–33. doi: 10.1093/gerona/62.4.427
62. Li L, Liu B, Lu J, Jiang L, Zhang Y, Shen Y, et al. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine (2015) 50(2):397–404. doi: 10.1007/s12020-015-0588-8
63. Hu Y, Wang J, Zeng S, Chen M, Zou G, Li Y, et al. Association between serum albumin levels and diabetic peripheral neuropathy among patients with type 2 diabetes: Effect modification of body mass index. Diabetes Metab Syndr Obes (2022) 15:527–34. doi: 10.2147/DMSO.S347349
64. Yan P, Tang Q, Wu Y, Wan Q, Zhang Z, Xu Y, et al. Serum albumin was negatively associated with diabetic peripheral neuropathy in Chinese population: A cross- sectional study. Diabetol Metab Syndr (2021) 13(1):100. doi: 10.1186/s13098-021-00718-4
65. Aleidan FAS, Ahmad BA, Alotaibi FA, Aleesa DH, Alhefdhi NA, Badri M, et al. Prevalence and risk factors for diabetic peripheral neuropathy among Saudi hospitalized diabetic patients: A nested case-control study. Int J Gen Med (2020) 13:881–9. doi: 10.2147/IJGM.S273807
66. Liu H, Ding C, Hu L, Li M, Zhou W, Wang T, et al. The association between AST/ ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Med (Baltimore) (2021) 100(31):e26693. doi: 10.1097/MD.0000000000026693
67. Day CP. From fat to inflammation. Gastroenterology (2006) 130(1):207–10. doi: 10.1053/j.gastro.2005.11.017
68. Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev (2008) 29(7):939–60. doi: 10.1210/er.2008-0009
69. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab (2008) 19(10):371–9. doi: 10.1016/j.tem.2008.08.005
70. Rief P, Pichler M, Raggam R, Hafner F, Gerger A, Eller P, et al. The AST/ALT (De- ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Med (Baltimore) (2016) 95(24):e3843. doi: 10.1097/MD.0000000000003843
71. Goessling W, Friedman LS. Increased liver chemistry in an asymptomatic patient. Clin Gastroenterol Hepatol (2005) 3(9):852–8. doi: 10.1016/S1542-3565(05)00416-7
72. Chen SL, Li JP, Li LF, Zeng T, He X. Elevated preoperative serum alanine Aminotransferase/Aspartate aminotransferase (ALT/AST) ratio is associated with better prognosis in patients undergoing curative treatment for gastric adenocarcinoma. Int J Mol Sci (2016) 17(6):911. doi: 10.3390/ijms17060911
Keywords: aspartate aminotransferase/alanine aminotransferase ratio, diabetic peripheral neuropathy, vibration perception threshold, type 2 diabetes mellitus, Chinese population
Citation: Yan P, Wu Y, Dan X, Wu X, Tang Q, Chen X, Xu Y, Zhu J, Miao Y and Wan Q (2023) Aspartate aminotransferase/alanine aminotransferase ratio was associated with type 2 diabetic peripheral neuropathy in a Chinese population: A cross-sectional study. Front. Endocrinol. 14:1064125. doi: 10.3389/fendo.2023.1064125
Received: 07 October 2022; Accepted: 15 February 2023;
Published: 27 February 2023.
Edited by:
Zoltan Kender, Heidelberg University, GermanyReviewed by:
Elettra Mancuso, University of Magna Graecia, ItalyRizaldy Taslim Pinzon, Duta Wacana Christian University, Indonesia
YuCheng Cheng, Taichung Veteran General Hospital, Taiwan
Copyright © 2023 Yan, Wu, Dan, Wu, Tang, Chen, Xu, Zhu, Miao and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Wan, d2FucWluMjMxQDEyNi5jb20=
 Pijun Yan
Pijun Yan Yuru Wu
Yuru Wu Xiaofang Dan1,2,3,4
Xiaofang Dan1,2,3,4 Xiping Chen
Xiping Chen Yong Xu
Yong Xu