- 1Department of Ageing, Orthopedics and Rheumatology, University “Cattolica del Sacro Cuore”, Rome, Italy
- 2National Institute for Cardiovascular Research (INRC), Bologna, Italy
- 3Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 4Azienda Ospedaliera Universitaria Policlinico Umberto I, Geriatric Unit, Rome, Italy
- 5Department of Clinical Medicine, Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy
- 6“Sapienza” University of Rome, Rome, Italy
- 7Department of Neuroscience, Imaging and Clinical Sciences, “G. d’Annunzio” University of Chieti-Pescara, Chieti, Italy
- 8Department of Surgical, Medical and Dental Department of Morphological Sciences related to Transplant, Oncology and Regenerative Medicine, University of Modena and Reggio Emilia, Modena, Italy
- 9Department of Clinical and Internal Medicine, Anesthesiology and Cardiovascular Sciences, “Sapienza” University of Rome, Rome, Italy
- 10Department of Medical and Surgical Sciences Alma Mater Studiorum University-IRCCS AOU S. Orsola-Malpighi, Bologna, Italy
- 11Department of Quality of Life Sciences, Alma Mater Studiorum University, Bologna, Italy
Background: Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality among women with type 2 diabetes (T2DM). The interplay between sex-specific biological factors, social determinants, and environmental exposures amplifies cardiometabolic risk across the female life course.
Objectives: This manuscript explores how socioeconomic disparities, environmental pollution, chronic stress, food insecurity, and climate change synergistically increase the burden of T2DM and cardiovascular complications in women, and reviews potential preventive interventions including dietary strategies.
Methods: A comprehensive narrative review was conducted, synthesizing current evidence on the exposome, social inequities, environmental insults, and evidence-based lifestyle interventions that contribute to or mitigate the development and progression of T2DM and CVD in women.
Results: Lower socioeconomic status, limited education, housing instability, and inadequate access to healthcare and nutritious foods profoundly affect T2DM management and CVD prevention in women. Concurrently, exposure to air pollutants (PM2.5, NO2, O3), climate change-induced food insecurity, and heat-related stress further exacerbate insulin resistance, systemic inflammation, and vascular dysfunction. Life transitions such as gestational diabetes mellitus and menopause further magnify these risks. Current healthcare models insufficiently address these multilayered factors.
Conclusion: Effective cardiovascular prevention in women with T2DM requires a life-course approach that integrates biological transitions with environmental and social determinants to deliver sex-sensitive, stage-specific strategies.
1 Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is rising globally, with women increasingly affected during key life stages such as pregnancy, menopause, and aging (1–3). Among women with T2DM, cardiovascular disease (CVD) risk is markedly higher, due to a combination of metabolic dysfunction, hormonal shifts, and social/environmental vulnerabilities (4, 5). This review specifically focuses on women with T2DM, examining how environmental and social determinants interact with hormonal transitions (puberty, pregnancy, menopause, aging) to outline cardiovascular risk across the lifespan (Figure 1). Cardiovascular risk persists across the lifespan, underscoring the necessity for dynamic, stage-specific strategies that adapt to women’s evolving biological and social contexts (6).
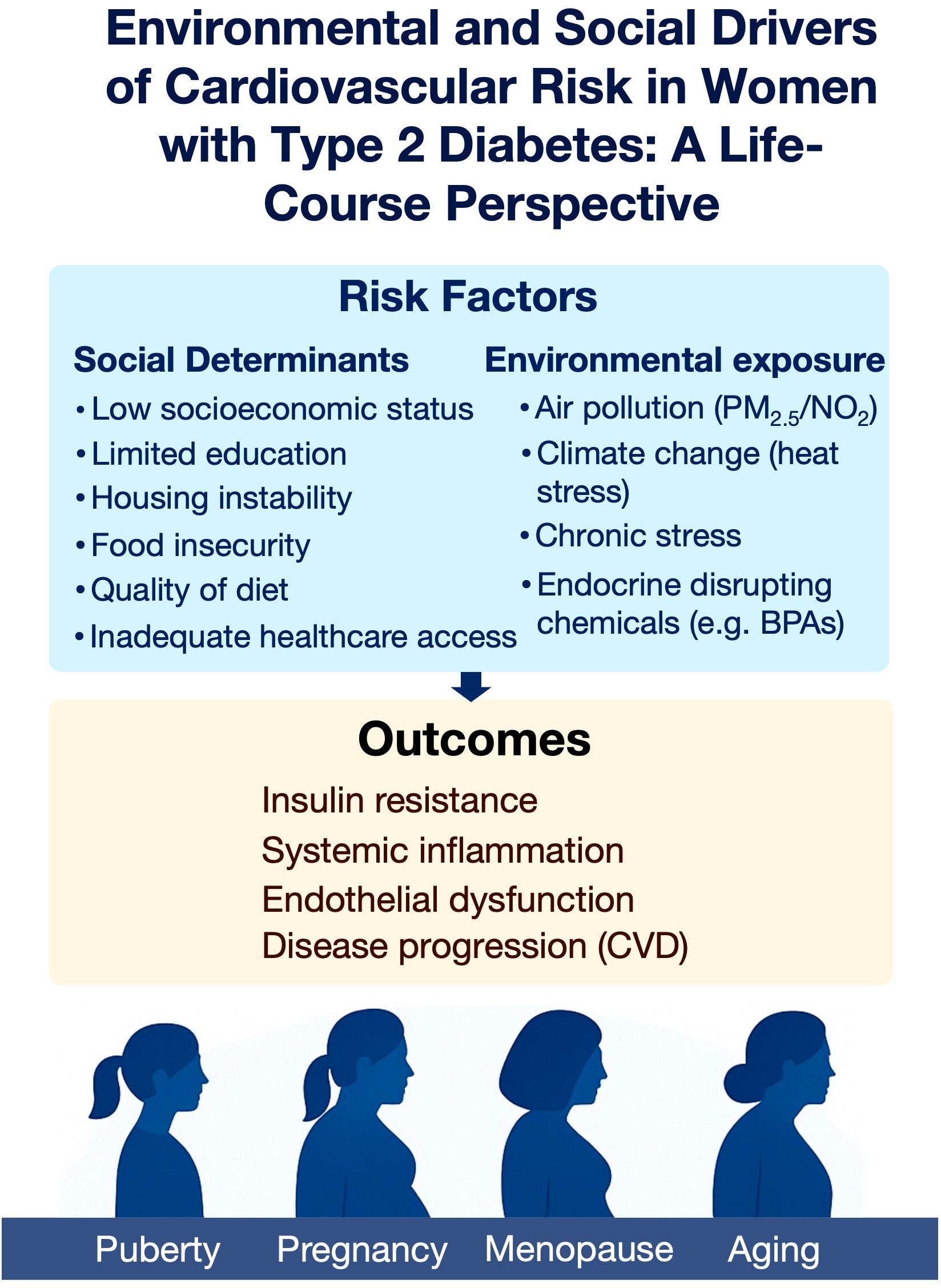
Figure 1. Environmental and social determinants of cardiovascular risk in women with type 2 diabetes across the life course. Social and environmental stressors converge to promote insulin resistance, systemic inflammation, endothelial dysfunction, and cardiovascular disease progression. These risks vary in impact across key life stages, including puberty, pregnancy, menopause, and aging.
2 Methods
This narrative review was informed by a structured search of PubMed, Embase, and Web of Science from January 2005 to March 2025. Search terms included combinations of “women,” “diabetes,” “cardiovascular disease,” “life course,” “hormonal transitions,” “puberty,” “pregnancy,” “menopause,” “aging,” “environmental exposures,” “social determinants,” “pollution,” and “climate change.” Inclusion criteria were peer-reviewed original research articles, systematic reviews, or meta-analyses focusing on cardiovascular risk in women with T2DM, published in English. Exclusion criteria were animal studies, conference abstracts, and papers without sex-disaggregated data. Reference lists of key articles were hand-searched to identify additional relevant publications. A simplified PRISMA flow diagram summarizing study selection is provided as Supplementary Figure S1.
To contextualize the strength of the evidence, we applied elements of the GRADE/WHO framework (7) to evaluate the certainty of evidence and risk of bias for each major exposure–outcome relationship. Factors considered included study design, risk of bias, consistency of results, and directness of evidence. The results of this assessment are presented in Table 1. In addition, to further interpret the strength and consistency of observed associations, we applied the Bradford-Hill criteria for causality, with particular attention to temporality, consistency, biological plausibility, and coherence with existing evidence (8).
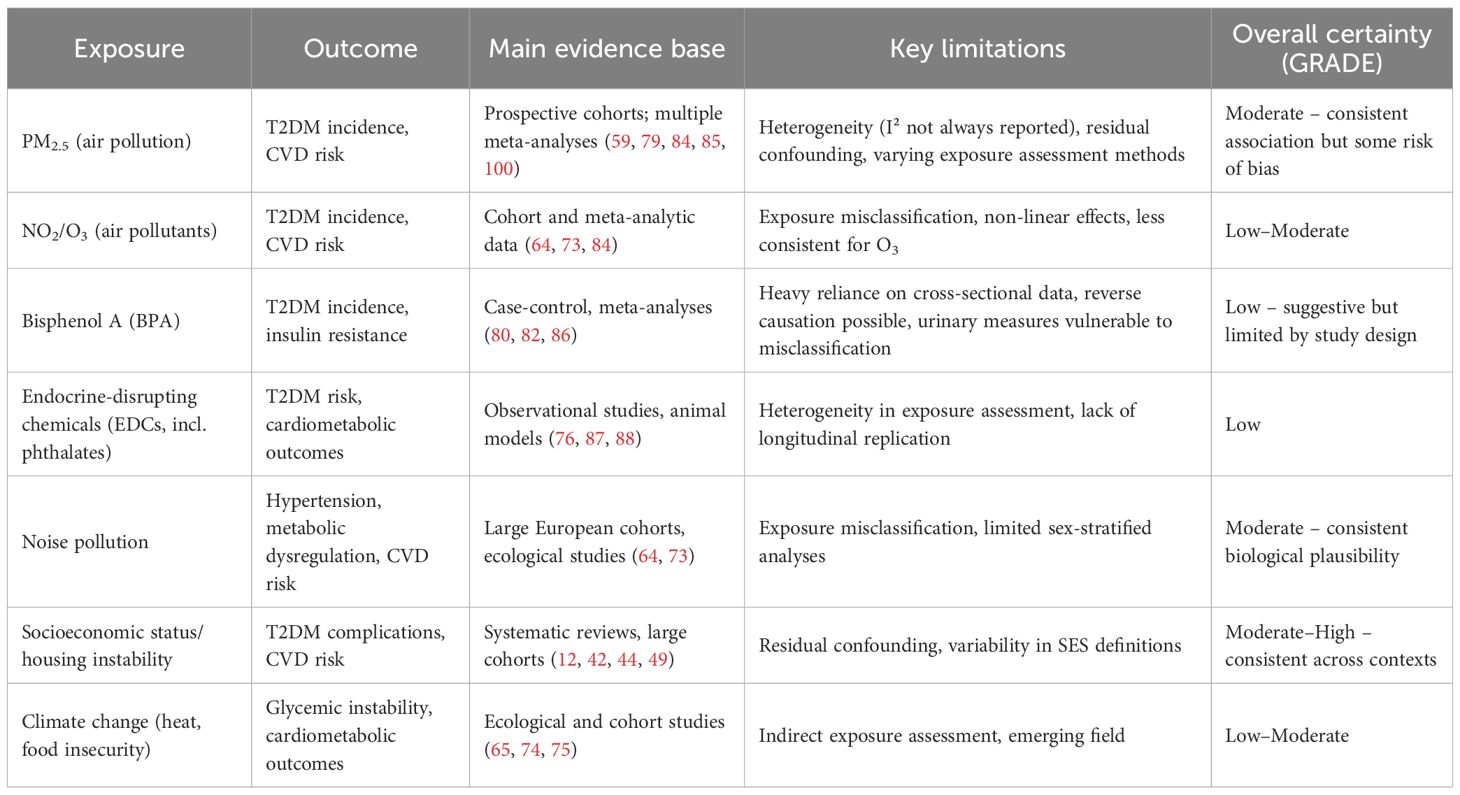
Table 1. Certainty of evidence and risk of bias assessment for environmental exposures and T2DM related cardiovascular risk in women.
Although the literature search was conducted in a structured manner across multiple databases, a formal systematic review could not be undertaken. The available studies were highly heterogeneous with respect to design, populations, and reported outcomes, and many did not provide sex-disaggregated data. These factors limited the possibility of applying standardized quality assessment tools or conducting a meta-analysis. For these reasons, we chose to synthesize the evidence in the form of a narrative review, which allows for a broader and more integrative discussion of the topic.
3 Life stages, hormonal changes, and T2DM in women
Distinct life stages and hormonal transitions exert a profound influence on the pathogenesis and progression of T2DM and CVD in women (9). Critical physiological milestones, such as puberty, pregnancy, menopause, and aging, interact intricately with metabolic pathways, collectively heightening cardiovascular risk (1, 2, 9).
Puberty is characterized by a physiological increase in insulin resistance secondary to hormonal fluctuations, which may unmask subclinical metabolic dysfunction and predispose to early-onset T2DM (10). In adolescent females affected by obesity or polycystic ovary syndrome (PCOS), this risk is markedly exacerbated, yet often remains underdiagnosed and undertreated (11). Adolescents from socioeconomically disadvantaged backgrounds or those living in environments with limited access to healthy foods and safe recreational spaces face an amplified risk of early cardiometabolic dysfunction, highlighting the intersection of hormonal and environmental stressors during this stage. Targeted interventions during adolescence, including early screening for insulin resistance, promotion of healthy dietary habits, and structured physical activity programs, have been shown to yield enduring benefits in the preservation of cardiometabolic health (12, 13).
Pregnancy represents an additional critical window of vulnerability. Gestational diabetes mellitus (GDM), a condition affecting a substantial proportion of pregnancies, constitutes a powerful predictor of subsequent T2DM and CVD risk (14, 15). Women with a history of GDM exhibit a nearly sevenfold increased likelihood of developing T2DM within a decade postpartum and demonstrate elevated incidences of hypertension and metabolic syndrome (14, 16, 17). A cohort study from the Taiwan National Health Insurance Research Database (TNHIRD) analyzed a study population of 2,297,613 pregnant women. Women in the GDM cohort exhibited a significantly higher risk of developing T2DM, hypertension, and metabolic syndrome compared to the normal cohort, with hazard ratios of 7.07, 1.54, and 2.51, respectively. Similarly, the pregnancy-induced hypertension (PIH) cohort demonstrated an increased risk for these conditions, with hazard ratios of 3.41 for T2DM, 7.26 for hypertension, and 2.68 for metabolic syndrome. Notably, individuals with both GDM and PIH had the greatest risk, showing hazard ratios of 21.47 for postpartum T2DM, 8.02 for hypertension, and 5.04 for metabolic syndrome compared to the normal cohort (18). While observational data such as the Taiwan NHIRD cohort suggest possible interaction between GDM and PIH, most studies have not reported formal interaction terms or attributable proportions. Thus, evidence for true synergistic effects remains limited, and further analyses are needed to quantify these relationships. Women with a history of GDM are known to have a significantly elevated risk of developing T2DM (19, 20). A systematic review of 20 studies reported that individuals with prior GDM face an approximately sevenfold greater risk of T2DM compared to women without GDM (16). In a large population-based study, 18.9% of women with previous GDM developed T2DM within nine years after the index pregnancy, whereas only 2.0% of women without GDM were diagnosed with T2DM over the same period (16). Notably, the risk of developing T2DM was highest within the first nine months postpartum, during which 3.7% of women with prior GDM had already progressed to T2DM (21). Postpartum follow-up is often inadequate, representing a missed opportunity for early intervention (15). Structured screening, lifestyle counseling, and glucose monitoring post GDM are essential for long-term cardiovascular prevention (22, 23). Social determinants, including inadequate prenatal care access, food insecurity, and chronic psychosocial stress, may further increase the likelihood of adverse outcomes in women with GMD, thereby compounding future cardiovascular risk (22, 23).
Menopause represents a pivotal transition characterized by a decline in estrogen levels which worsens adiposity, insulin resistance, and dyslipidemia, thereby accelerating cardiovascular risk. Estrogen exerts protective effects on endothelial integrity and metabolic regulation; thus, its diminution is associated with increases in central adiposity, insulin resistance, and dyslipidemia (5, 6, 24). These metabolic disturbances exacerbate pre-existing diabetes-related cardiovascular risk, suggesting that the postmenopausal period can be a critical window for comprehensive risk reassessment and intensification of preventive and therapeutic strategies (25). Echocardiographic markers of hypertensive cardiac damage have been shown to provide valuable insights for such comprehensive assessment (26). The cardiovascular impact of menopause is magnified in women with lower SES or chronic exposure to stress and pollutants, which accelerate vascular aging and worsen postmenopausal metabolic dysfunction. Hormone replacement therapy (HRT), when initiated in selected populations with appropriate risk stratification, has been associated with a 20–30% reduction in new-onset T2DM. Its administration must be carefully individualized, taking into account the timing of initiation, overall cardiovascular risk profile, and the presence of comorbid conditions (27–30).
The aging process complicates T2DM management in women. In older patients, chronic hyperglycemia, oxidative stress, and inflammation accelerate frailty, sarcopenia, cognitive decline, and cardiovascular vulnerability (31–33). Multiple comorbidities, frequent hospitalizations, and heightened drug sensitivity further increase the risk of hypoglycemia, falls, fractures, and mortality (34). Cumulative life-course exposures, including socioeconomic disadvantage, air pollution, and social isolation, exert synergistic effects that worsen frailty, polypharmacy outcomes, and cardiovascular prognosis (6). Frailty is closely associated with both T2DM and hypertension through inflammatory, vascular, and metabolic pathways, with elevated cytokines such as IL-6 and TNF-α contributing to sarcopenia and diminished resilience (35, 36). Hyperglycemia promotes oxidative stress via excess reactive oxygen species and the accumulation of advanced glycation end products (AGEs), leading to cellular damage, tissue stiffening, impaired mobility, and accelerated ageing (35, 37). Endothelial dysfunction and reduced perfusion to muscle and brain further heighten risks of cardiovascular disease, stroke, and functional decline (36, 37). The burden of comorbidities such as cardiovascular and kidney disease compounds this trajectory (35, 37, 38).
To mitigate these outcomes, life stage–specific screening, personalized risk assessment, and culturally competent interventions are needed. Healthcare providers should adopt a proactive, multidisciplinary approach that integrates education, regular follow-up, and lifestyle modification aligned with hormonal and life-course transitions (13).
4 The exposome and diabetes-related cardiovascular risk in women
The following sections explore how socioeconomic, environmental, and lifestyle factors intersect with critical hormonal transitions across the female lifespan. Rather than acting uniformly, these determinants exert stage-specific effects, for example, socioeconomic status (SES) and stress strongly influence cardiometabolic trajectories during adolescence and pregnancy, while pollutant exposures and social isolation have cumulative impacts during menopause and aging. This life-course approach allows for a better understanding of CVD risk in women with T2DM.
In diabetic patients, the exposome concept helps identify cumulative environmental and social exposures that drive disease progression. For women with T2DM, pollution, poor nutrition, chronic stress, and socioeconomic disadvantages increase the burden of endothelial dysfunction, systemic inflammation, and atherosclerosis (13, 39).
Moreover, individual level socioeconomic status substantially modifies the vulnerability to environmental insults, with disadvantaged populations exhibiting heightened susceptibility. Collectively, these factors underscore the life-course influence of environmental and social determinants on the pathogenesis and progression of T2DM These risks are often compounded by insufficient healthcare access and sex-specific barriers to diagnosis and treatment.
4.1 Socioeconomic status, education, and T2DM
Women with T2DM who come from lower SES backgrounds encounter complex and interrelated challenges that elevate both the incidence and severity of cardiovascular complications (40–42). SES exerts a pervasive influence on multiple aspects of T2DM management and CVD prevention, including access to healthcare services, medication affordability, dietary quality, and opportunities for regular physical activity (43, 44). Individuals with lower SES are disproportionately affected by food insecurity, limited transportation to healthcare facilities, and restricted access to environments conducive to health promoting behaviors (45).
Nutritional quality in socioeconomically disadvantaged communities is frequently compromised due to the elevated cost and reduced availability of fresh fruits, vegetables, whole grains, and lean protein sources (12, 45, 46). Consequently, dietary patterns in these populations are often characterized by high intake of refined carbohydrates, added sugars, and saturated fats, dietary components that exacerbate insulin resistance and glycemic variability (46–48). This suboptimal nutritional profile contributes to impaired T2DM control and increases cardiovascular risk by promoting dyslipidemia and hypertension (12, 48).
Environmental factors such as housing instability, residential crowding, and unsafe neighborhoods reduce opportunities for physical activity, increase psychological stress, and are independently associated with cardiovascular morbidity (49, 50). These exposures contribute to sustained activation of neuroendocrine stress pathways, including the hypothalamic–pituitary–adrenal (HPA) axis, which promotes hypertension, insulin resistance, and vascular inflammation (51, 52). Chronic psychosocial stress, whether acute or cumulative (i.e., “allostatic load”), is now recognized as a key mediator linking social disadvantage to incident CVD events (52, 53). Incorporating housing insecurity into cardiovascular prevention frameworks is thus essential, particularly for diabetic women who already face heightened vulnerability. Chronic exposure to stress adversely affects hormonal homeostasis, impairing insulin sensitivity and elevating blood glucose levels, thereby intensifying cardiovascular risk (54, 55). These structural barriers are particularly detrimental for women, who frequently assume caregiving responsibilities and may prioritize the health needs of family members over their own (56, 57).
Beyond individual socioeconomic resources, the role of social support and community context is increasingly recognized as a determinant of cardiovascular health. Strong social networks buffer psychosocial stress, promote adherence to T2DM management, and enhance resilience against adverse events, whereas social isolation and weak community ties are linked with higher incidence of CVD and all-cause mortality (54, 55). For women with T2DM, the intersection of caregiving roles and limited community support may further exacerbate vulnerability to poor cardiovascular outcomes.
Educational attainment represents another critical determinant of health outcomes. Women with lower levels of education may have limited awareness of the symptoms and risks associated with T2DM and CVD and may face challenges in comprehending complex medical instructions, resulting in suboptimal adherence to treatment regimens and delays in seeking medical care (22, 43, 58, 59). In contrast, higher educational attainment is associated with enhanced problem solving skills, improved self-efficacy, and greater proficiency in navigating healthcare systems, all of which contribute to more favorable disease trajectories (58).
4.2 Environmental pollution and climate change
Environmental pollution and climate change are critical, yet frequently underrecognized, determinants in the development and progression of T2DM and its associated cardiovascular complications (59, 60). An expanding body of evidence links exposure to environmental pollutants, including fine particulate matter (PM2.5), nitrogen dioxide (NO2), and ozone (O3), to key pathophysiological processes such as insulin resistance, systemic inflammation, and oxidative stress, which underlie the onset of T2DM and atherosclerosis.
Air pollution contributes to endothelial dysfunction, inflammation, insulin resistance, and hypertension (61). Chronic exposure to PM2.5 and traffic-related pollutants is also linked to dyslipidemia, compounding risks for T2DM and cardiovascular disease (62). In addition to chemical pollutants, noise pollution, particularly from road traffic, rail, and air transport, has been independently associated with hypertension, metabolic dysregulation, and increased cardiovascular morbidity. Chronic nocturnal noise exposure disrupts circadian rhythms and sleep quality, contributing to sympathetic nervous system activation, insulin resistance, and endothelial dysfunction (59, 62). Women living in high-density urban areas with limited housing stability are especially vulnerable to these overlapping exposures.
The built environment influences opportunities for physical activity, diet, and stress regulation. Access to green spaces and walkable neighborhoods is associated with lower obesity rates, better glycemic control, and reduced cardiovascular risk. In contrast, poorly designed urban areas without safe recreational spaces or transport infrastructure promote sedentary lifestyles and widen health inequities (59, 61). For women with T2DM, supportive built environments can yield direct benefits (greater physical activity) and indirect ones (improved weight and glycemic control), thereby contributing to cardiometabolic health (63).
Environmental determinants such as the extent of green infrastructure (61), transportation modalities, and traffic density (62) modulate both local pollutant concentrations and individual patterns of physical activity. High levels of airborne pollutants may deter outdoor physical activity, while chronic exposure to environmental noise disrupts sleep architecture and adversely affects mental health, both of which are recognized contributors to cardiometabolic dysfunction.
Emerging studies also highlight the role of drinking water quality in cardiometabolic health. Contaminants such as arsenic, nitrates, and heavy metals have been linked to increased incidence of T2DM, endothelial dysfunction, and vascular disease (64). Ensuring equal access to clean, safe drinking water is therefore an essential component of cardiovascular prevention, particularly in socioeconomically disadvantaged communities.
Climate change further exacerbates these environmental threats through both direct and indirect mechanisms affecting metabolic health (65). Ultraviolet (UV) radiation exposure represents another underappreciated environmental factor. While excessive UV exposure contributes to oxidative stress, inflammation, and skin cancer risk, moderate exposure is essential for endogenous vitamin D synthesis, which exerts protective effects on cardiometabolic health (66). Women with T2DM may thus experience dual vulnerability to both vitamin D deficiency and environmental stressors linked with excessive UV exposure (67).
Rising global temperatures, altered precipitation patterns, and an increase in extreme weather events compromise agricultural productivity and disrupt food supply chains, diminishing the availability of fresh, nutrient-dense foods (68). These effects disproportionately impact low-income populations and women, who often face preexisting economic and geographic barriers to accessing healthy food options (66, 69, 70). Consequently, reliance on calorie dense, nutrient-poor processed foods increases, elevating the risk of obesity, T2DM, and cardiovascular disease.
In addition, climate change has been shown to alter the nutritional quality of staple crops (71). Elevated atmospheric carbon dioxide (CO2) concentrations reduce the levels of essential micronutrients such as zinc, iron, and protein in grains and legumes. Micronutrient deficiencies, particularly in individuals with T2DM, can impair insulin sensitivity, compromise immune function, and heighten susceptibility to infections and chronic inflammation (72, 73).
Heatwaves, a prominent manifestation of climate change, impose substantial cardiovascular strain, especially in individuals with T2DM. Heat stress may precipitate dehydration, electrolyte imbalances, and glycemic instability (65, 73). Furthermore, certain pharmacological agents commonly used in T2DM management, including diuretics and beta-adrenergic blockers, impair thermoregulatory responses, thereby increasing vulnerability to heat-related morbidity and mortality (72, 74). Beyond heat stress, climate change also influences cardiovascular risk through increased frequency of wildfires (leading to acute surges in PM2.5 exposure), flooding events (which compromise access to healthcare and medications), and shifting patterns of vector-borne diseases, all of which disproportionately impact vulnerable populations (65). These compounded exposures increase risk for women with T2DM, particularly in resource limited settings.
Women, particularly those who experience systemic inequalities in access to healthcare and disproportionate environmental burdens, are more adversely affected by compounded exposures to environmental and social stressors. Indeed, pregnant women with T2DM are especially vulnerable: environmental toxins and climate related stressors (e.g., heat, pollution) can impair both maternal and fetal health, increasing the risk of preeclampsia, preterm birth, and low birth weight (75–77).
Addressing these intertwined challenges necessitates systemic interventions. Policy initiatives aimed at reducing pollutant emissions, improving urban air quality, and promoting sustainable and resilient food systems are imperative (78). Public health strategies must prioritize environmental balance and the resilience of communities most susceptible to environmental degradation. Furthermore, healthcare providers should receive education regarding environmental risk factors and incorporate environmental health assessments into routine T2DM management.
4.3 Environmental exposures, endocrine disruptors, and T2DM risk: quantitative evidence and policy implications
Emerging scientific data supports a potential association between environmental exposures and the development of T2DM, particularly among women. A meta-analysis examining the impact of fine particulate matter (PM2.5) on metabolic health reported a pooled hazard ratio (HR) of approximately 1.11 per 10 µg/m³ increment (95% CI: 1.03–1.20), indicating a significant increase in the risk of T2DM with rising pollution exposure (79). These findings are supported by mechanistic studies that implicate PM2.5 in systemic inflammation, oxidative stress, and endothelial dysfunction, hallmarks of insulin resistance and cardiometabolic disruption (22, 23, 30).
To improve clinical interpretability, we translated relative risks into absolute risks. For example, the reported hazard ratio of 1.11 per 10 µg/m³ of PM2.5 corresponds to a meaningful difference in real-world settings. A woman with T2DM living in a moderately polluted European city (annual mean PM2.5 ≈ 20 µg/m³) would have an absolute 10-year risk of cardiovascular events that is approximately 2–3% higher than that of a woman in a rural area with PM2.5 levels around 10 µg/m³. This equates to a number-needed-to-harm of roughly 35–50 over a decade, depending on baseline cardiovascular risk.
Endocrine-disrupting chemicals (EDCs), such as bisphenol A (BPA), represent an additional and growing concern. A meta-analysis including over 40,000 individuals demonstrated that urinary BPA concentrations were associated with a 28% increased odds of T2DM (OR: 1.28; 95% CI: 1.14–1.44) (80). Notably, even short-term BPA exposure at the US Environmental Protection Agency’s “safe” dose of 50 µg/kg/day has been shown to impair insulin sensitivity within four days, as confirmed by euglycemic-hyperinsulinemic clamp studies in healthy adults (81).
Emerging data also point to a non-linear, U-shaped dose–response curve for BPA. A 2024 population study revealed increased metabolic and mortality risk at both low and high urinary BPA concentrations, with the greatest inflection observed at around 1.99 ng/mL (82). These findings challenge the notion of a clear “safe threshold” and underscore the potential for endocrine interference even at low doses.
Mechanistically, air pollutants such as PM2.5 and NO2 provoke systemic oxidative stress, disrupt vascular homeostasis, and activate inflammatory cascades, which impair insulin signaling and β-cell function (30, 51). In contrast, BPA acts primarily through estrogen and thyroid hormone receptor pathways, affecting pancreatic insulin secretion and promoting insulin resistance. Preclinical models have also demonstrated that BPA exposure in pregnancy can result in epigenetic modifications and β-cell abnormalities in offspring, suggesting a transgenerational diabetogenic effect (83).
These environmental threats are not equally distributed. Women from socioeconomically disadvantaged backgrounds and racial minorities are disproportionately exposed to both air pollution and EDCs (12, 30, 44). This inequity is compounded by barriers to healthcare access, healthy food, and safe physical environments. The intersection of environmental injustice with social determinants of health contributes to persistent disparities in T2DM incidence and cardiovascular outcomes (12, 48). Table 1 provides a summary of the certainty of evidence and risk of bias for the environmental exposures most frequently reported in relation to T2DM and cardiovascular risk in women.
Furthermore, transgenerational evidence suggests that environmental exposures during critical life stages such as puberty or pregnancy can affect future generations through epigenetic inheritance. Studies in both humans and rodents have linked ancestral exposure to obesogenic or diabetogenic environments with metabolic dysfunction in descendants (83, 87). This finding reinforces the need for early-life and preconception interventions, especially in women of childbearing age.
While the literature on environmental pollutants and endocrine disruptors is extensive, other determinants such as housing instability and chronic stress also exert substantial and well-documented effects on cardiometabolic health.
Strengthening the evidence synthesis across these domains ensures a balanced representation of both established and emerging risk factors.
Targeted environmental policy reforms and public education campaigns will be essential to reduce the burden of T2DM in women and ensure equitable cardiometabolic health across generations.
Data are summarized in Table 2.
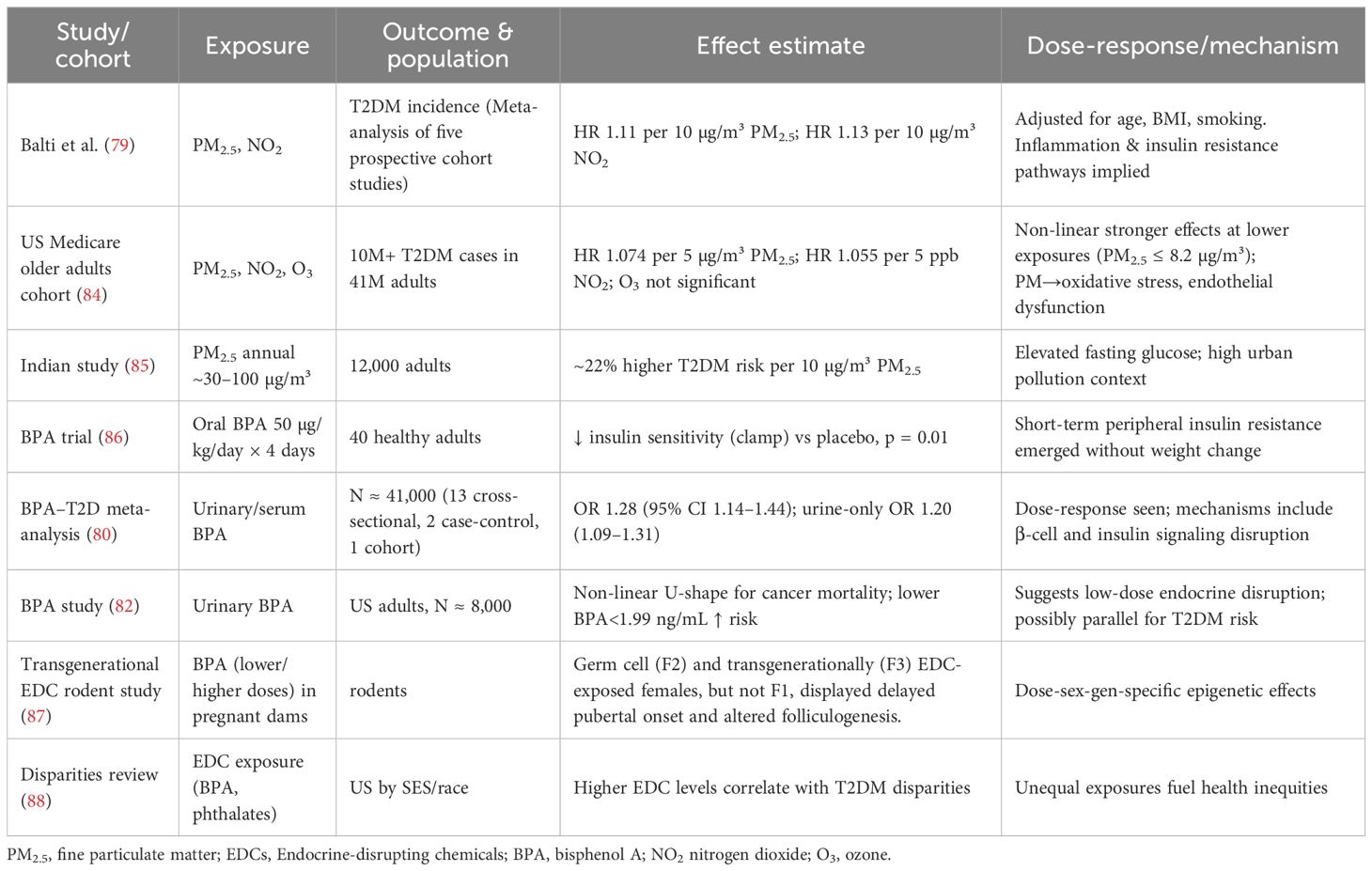
Table 2. Clinical and epidemiological studies on T2DM and environmental exposures, now including effect sizes, dose–response details, and mechanisms.
4.4 The Mediterranean diet and prevention of metabolic and vascular complications in women with T2DM
Many studies support the role of the Mediterranean Diet (MedD) in mitigating the metabolic and cardiovascular consequences of T2DM. Several high-quality studies have demonstrated that adherence to the MedD reduces the risk of T2DM, enhances insulin sensitivity, and protects against diabetes-related vascular complications.
An umbrella review by Dinu et al. found that greater adherence to the MedD significantly reduced the risk of T2DM and several chronic diseases, reinforcing the importance of dietary interventions in cardiometabolic prevention (89). The PREDIMED-Reus study showed a lower risk of T2DM, particularly among female participants adhering to a MedD, emphasizing the need for sex-sensitive dietary research (90).
Multiple findings from the CORDIOPREV trial strengthen these conclusions. Boughanem et al. reported that women who adhered to a MedD showed a significant increase in insulin sensitivity and Disposition Index, especially in those with reduced neutrophil counts—a marker of improved inflammatory status (91). Another substudy of the CORDIOPREV study, authors demonstrated that improved diet quality through MedD adherence was associated with a lower risk of developing T2DM in patients with coronary heart disease (92). Jiménez-Torres et al. further confirmed the protective vascular effects of a long-term MedD enriched with extra-virgin olive oil, which was associated with reduced atherosclerosis progression (93). A recent meta-analytic review revealed that adherence to the MedD was correlated with a reduced incidence of diabetic complications, particularly nephropathy and retinopathy, underscoring the diet’s systemic protective effects (94). In the United States, the Keto-Med crossover trial by Gardner et al. compared a well-formulated ketogenic diet with a Mediterranean-Plus diet in patients with prediabetes or T2DM. Both interventions improved HbA1c, but LDL cholesterol improved more under the MedD, highlighting its cardioprotective metabolic profile (95).
These studies collectively suggest that the MedD as a clinically validated, sustainable strategy for improving metabolic control, preventing T2DM progression, and reducing the burden of vascular complications in women with T2DM. Although evidence generally supports the Mediterranean diet, it is important to acknowledge that the PREDIMED trial was retracted and subsequently republished with corrected analyses, and that the Women’s Health Initiative Dietary Modification trial did not demonstrate cardiovascular benefit. These controversies highlight that not all large-scale studies have shown consistent positive effects.
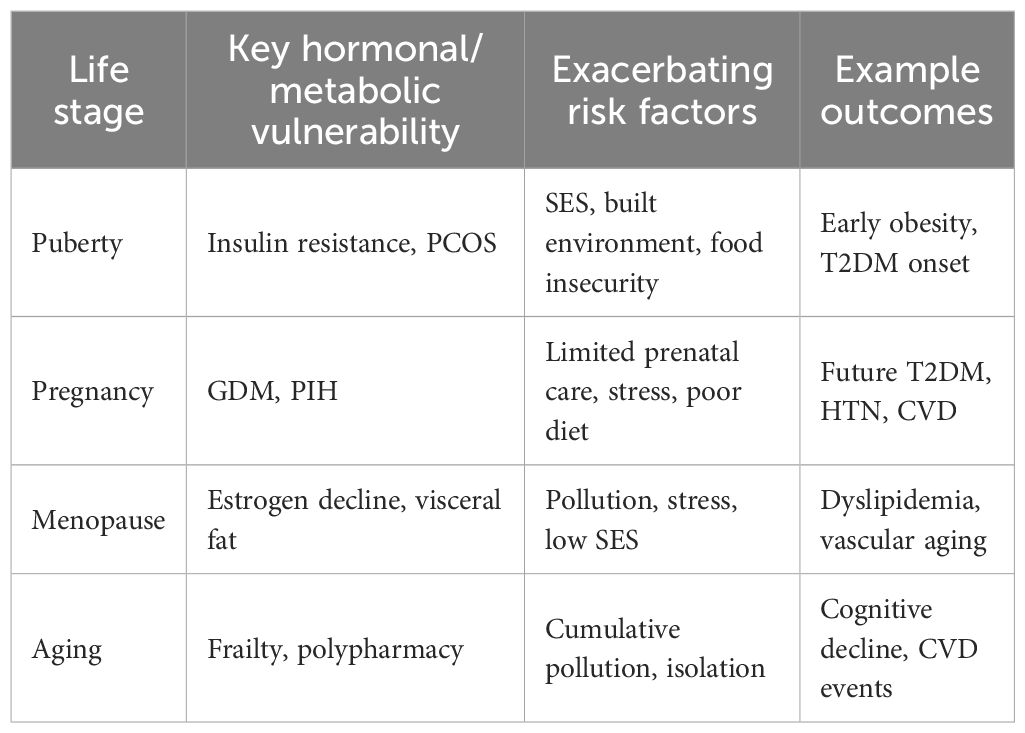
Ultimately, a holistic approach that recognizes environmental exposures as integral components of T2DM and cardiovascular disease prevention and management is essential for safeguarding the health of women living with T2DM in the context of a rapidly changing global environment. To further illustrate the dynamic interplay between life-course transitions and environmental or social determinants, we provide a synthesis linking hormonal and metabolic vulnerabilities with stage-specific risk factors and outcomes (Table 3). This integrated framework highlights how puberty, pregnancy, menopause, and aging each represent critical windows during which socioeconomic disadvantage, chronic stress, pollution, and other exposures exert differential effects on cardiometabolic health in women with T2DM. Such an approach underscores the necessity for prevention strategies that are both life-stage–sensitive and contextually tailored to women’s lived environments.
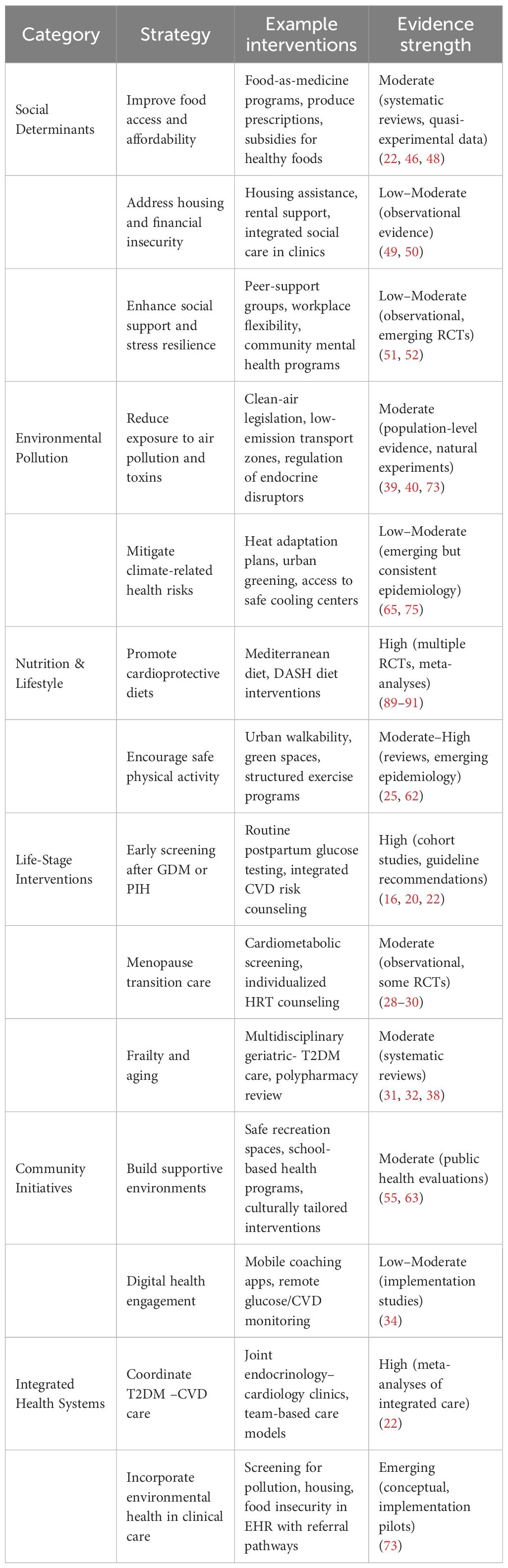
To mitigate cardiovascular risk in diabetic women, comprehensive and multilevel interventions are essential. Table 4 provides a structured overview of multilevel prevention strategies for women with T2DM, organized across social, environmental, lifestyle, life-stage, community, and health-system domains. By presenting specific examples alongside a qualitative grading of evidence strength, this framework illustrates both established interventions (e.g., Mediterranean diet, integrated care models) and emerging areas requiring further research (e.g., housing stability, endocrine disruptor regulation, climate resilience strategies). The table highlights how prevention must be tailored across the life course, from postpartum follow-up after GDM to frailty management in older age. While some strategies are supported by high-quality randomized evidence, others remain hypotheses for translational trials. Together, these strategies emphasize that effective cardiovascular prevention in women with T2DM will require coordinated action across clinical, community, and policy levels.
This review has some limitations, particularly the reliance on observational studies and variability in reporting. The certainty of evidence varied across exposures: associations with PM2.5 and other air pollutants are supported by prospective cohorts and graded as moderate certainty, whereas evidence for BPA and other endocrine-disrupting chemicals remains low due to reliance on cross-sectional biomarker data, exposure misclassification, and vulnerability to reverse causation. Notably, substantial heterogeneity (I²) across air pollution studies limits the precision of pooled estimates. To interpret these findings, we applied the Bradford-Hill criteria for causality (96). While exposures such as PM2.5 meet several criteria including consistency, temporality, and biological plausibility, others such as BPA remain supported largely by associative data with limited experimental evidence and should not be overinterpreted as causal.
5 Future perspectives
Looking ahead, reducing cardiovascular risk in women with T2DM requires integrated strategies that bridge biology, environment, and social policy. Clean air and tobacco control legislation remain foundational, with well-documented benefits for population level cardiovascular outcomes. Expanding these approaches to include climate adaptation policies (e.g., urban greening, heat mitigation strategies, reduction of traffic related pollutants) is crucial, given the disproportionate impact of climate change on women’s cardiometabolic health. Nutrition focused policies such as “food-as-medicine” programs, produce prescriptions, and food bank partnerships have already shown benefits for diet quality, food security, and in some cases glycemic outcomes (97, 98). Embedding these programs within health systems and linking them to insurance reimbursement could enhance scalability and sustainability. At the clinical level, integrated care models, where endocrinology, cardiology, gynecology, and primary care coordinate, have been associated with reduced mortality and hospitalization in people with T2DM (99). Future innovations could incorporate digital health platforms (e.g., continuous glucose monitoring linked with cardiovascular monitoring, mobile health coaching) to personalize interventions across life stages. A promising frontier is the incorporation of environmental and social determinants into routine risk assessment. Screening tools for housing insecurity, food access, and environmental exposures could be embedded into electronic health records, enabling providers to identify high-risk women and connect them with tailored resources. Similarly, community level interventions, such as safe green spaces, walkable neighborhoods, and culturally tailored physical activity programs, can provide synergistic benefits.
Future research should prioritize sex-specific and life-course–specific trials that test whether targeting environmental exposures, social determinants, and hormonal transitions improves not only glycemic control but also cardiovascular outcomes and quality of life. Implementation science will be critical to scale up successful programs while ensuring equity. Ultimately, the next decade should focus on multisectoral interventions that simultaneously address medical, behavioral, environmental, and policy drivers of cardiometabolic risk. Such approaches hold the potential to transform care for women with T2DM from reactive disease management to proactive, prevention-oriented health promotion across the life course.
6 Conclusion
Women with T2DM face a complex interplay of cardiovascular risks shaped by biological transitions and by environmental and social determinants. This review adopts a life-course perspective, covering puberty, pregnancy, menopause, and aging, while highlighting cross-cutting factors such as socioeconomic status, chronic stress, pollution, and housing instability. As summarized in Table 3, some determinants (e.g., stress, food insecurity) exert their greatest impact in early life stages, whereas others (e.g., pollution, social isolation) accumulate and become more pronounced with aging. Recognizing these dynamic, stage-specific influences is essential for developing prevention and management strategies that are both sex-sensitive and life-stage specific. While pollutant exposure and dietary interventions such as the Mediterranean diet have been extensively studied, the effects of housing instability, chronic stress, and other social determinants remain comparatively underexplored. A comprehensive framework that integrates environmental hazards with modifiable lifestyle factors is needed to strengthen cardiovascular prevention in women with T2DM. Future translational research should prioritize evaluating how such systemic interventions can be embedded into clinical practice.
Author contributions
CC: Writing – original draft, Writing – review & editing, Software, Methodology. VS: Writing – review & editing, Methodology, Writing – original draft, Investigation. GZ: Methodology, Formal Analysis, Writing – review & editing, Software, Writing – original draft. FM: Methodology, Writing – original draft, Writing – review & editing. SS: Validation, Writing – review & editing, Writing – original draft. SG: Writing – review & editing, Supervision, Writing – original draft. MN: Writing – original draft, Supervision, Writing – review & editing. GD: Supervision, Writing – review & editing, Writing – original draft. MP: Writing – review & editing, Conceptualization, Supervision, Writing – original draft. CB: Supervision, Writing – review & editing, Writing – original draft. AM: Conceptualization, Writing – review & editing, Validation, Writing – original draft, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research leading to these results has received funding from the European Union - NextGenerationEU through the Italian Ministry of University and Research under PNRR - M4C2-I1.3 Project PE_00000019 “HEAL ITALIA” to Marcello Pinti, CUP of INSTITUTION UNIMORE E93C22001860006.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1667222/full#supplementary-material
Supplementary Figure 1 | Created in BioRender. Pinti, M. (2025) https://BioRender.com/1bnfxvg”. Footnote to supplemental material Flow chart. ”A formal systematic review was not feasible due to heterogeneity of study designs, outcomes, and lack of gender-disaggregated data; therefore, the evidence was synthesized narratively”.
References
1. Mohile AA, Hedaoo RP, Jadhav SJ, Ainapure AS, Patil MV, and Khatwani NR. Unraveling the link: A comprehensive literature review of type 2 diabetes and menopause onset. Cureus. (2023) 15:e50743. doi: 10.7759/cureus.50743
2. Bucciarelli V, Moscucci F, Dei Cas A, Coppi F, Angeli F, Pizzi C, et al. Maternal-fetal dyad beyond the phenomenology of pregnancy: from primordial cardiovascular prevention on out, do not miss this boat! Curr Probl Cardiol. (2024) 49:102695. doi: 10.1016/j.cpcardiol.2024.102695
3. Ciarambino T, Crispino P, Leto G, Mastrolorenzo E, Para O, and Giordano M. Influence of gender in diabetes mellitus and its complication. Int J Mol Sci. (2022) 23:8850. doi: 10.3390/ijms23168850
4. Garcia M, Mulvagh SL, Merz CN, Buring JE, and Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. (2016) 118:1273–93. doi: 10.1161/CIRCRESAHA.116.307547
5. Mattioli AV, Moscucci F, Sciomer S, Maffei S, Nasi M, Pinti M, et al. Cardiovascular prevention in women: un update By the Italian Society of Cardiology Working Group On “Prevention, Hypertension and peripheral disease. J Cardiovasc Med (Hagerstown). (2023) 24:e147–55. doi: 10.2459/JCM.0000000000001423
6. Global Cardiovascular Risk Consortium, Magnussen C, Alegre-Diaz J, et al. Global effect of cardiovascular risk factors on lifetime estimates. N Engl J Med. (2025). doi: 10.1056/NEJMoa2415879
7. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
8. Hill AB. The environment and disease: association or causation? Proc R Soc Med. (1965) 58:295–300. doi: 10.1177/003591576505800503
9. Ryczkowska K, Adach W, Janikowski K, Banach M, and Bielecka-Dabrowa A. Menopause and women’s cardiovascular health: is it really an obvious relationship? Arch Med Sci. (2022) 19:458–66. doi: 10.5114/aoms/157308
10. Kelsey MM and Zeitler PS. Insulin resistance of puberty. Curr Diabetes Rep. (2016) 16:64. doi: 10.1007/s11892-016-0751-5
11. Osibogun O, Ogunmoroti O, and Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. (2020) 30:399–404. doi: 10.1016/j.tcm.2019.08.010
12. Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: A scientific review. Diabetes Care. (2020) 44:258–79. doi: 10.2337/dci20-0053
13. Mattioli AV and Gallina S. Early cardiovascular prevention: the crucial role of nurse-led intervention. BMC Nurs. (2023) 22:347. doi: 10.1186/s12912-023-01511-6
14. Burlina S, Dalfrà MG, Chilelli NC, and Lapolla A. Gestational diabetes mellitus and future cardiovascular risk: an update. Int J Endocrinol. (2016) 2016:2070926. doi: 10.1155/2016/2070926
15. Mattioli AV, Coppi F, Bucciarelli V, and Gallina S. Cardiovascular risk stratification in young women: the pivotal role of pregnancy. J Cardiovasc Med (Hagerstown). (2023) 24:793–7. doi: 10.2459/JCM.0000000000001557
16. Bellamy L, Casas JP, Hingorani AD, and Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
17. Martini C, Saeed Z, Simeone P, Palma S, Ricci M, Arata A, et al. Preeclampsia: Insights into pathophysiological mechanisms and preventive strategies. Am J Prev Cardiol. (2025) 23:101054. doi: 10.1016/j.ajpc.2025.101054
18. Kek HP, Su YT, Tey SJ, Yang MC, Chang LC, Hung YH, et al. The joint effect of gestational diabetes mellitus and hypertension contribute to higher risk of diabetes mellitus after delivery: a nationwide population-based study. BMC Pregnancy Childbirth. (2023) 23:539. doi: 10.1186/s12884-023-05829-6
19. Laine MK, Kautiainen H, Anttila P, Gissler M, Pennanen P, and Eriksson JG. Early pregnancy particulate matter exposure, pre-pregnancy adiposity and risk of gestational diabetes mellitus in Finnish primiparous women: An observational cohort study. Prim Care Diabetes. (2023) 17:79–84. doi: 10.1016/j.pcd.2022.11.012
20. Kim C, Newton KM, and Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. (2002) 25:1862–8. doi: 10.2337/diacare.25.10.1862
21. Feig DS, Zinman B, Wang X, and Hux MD JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ: Can Med Assoc J. (2008) 179:229–34. doi: 10.1503/cmaj.08001
22. Lewey J, Beckie TM, Brown HL, Brown SD, Garovic VD, Khan SS, et al. Opportunities in the postpartum period to reduce cardiovascular disease risk after adverse pregnancy outcomes: A scientific statement from the american heart association. Circulation. (2024) 149:e330–46. doi: 10.1161/CIR.0000000000001212
23. Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Meheran R, et al. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association [published correction appears in Circulation. 2020 Jun 9;141(23):e904. doi: 10.1161/CIR.0000000000000845.] [published correction appears in Circulation. 2021 Mar 23;143(12):e792-e793. doi: 10.1161/CIR.0000000000000970. Circulation. (2020) 141:e884–903. doi: 10.1161/CIR.0000000000000772
24. Lizcano F and Guzmán G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res Int. (2014) 2014:757461. doi: 10.1155/2014/757461
25. Bucciarelli V, Mattioli AV, Sciomer S, Moscucci F, Renda G, and Gallina S. The impact of physical activity and inactivity on cardiovascular risk across women’s lifespan: an updated review. J Clin Med. (2023) 12:4347. doi: 10.3390/jcm12134347
26. Cameli M, Lembo M, Sciaccaluga C, Bandera F, Ciccone MM, D'andrea A, et al. Identification of cardiac organ damage in arterial hypertension: insights by echocardiography for a comprehensive assessment. J Hypertens. (2020) 38:588–98. doi: 10.1097/HJH.0000000000002323
27. Ouyang Q, Dong Y, Li R, Hu Y, Xue Q, Yu X, et al. Associations of hysterectomy, oophorectomy, and hormone replacement therapy with the risk of type 2 diabetes mellitus in postmenopausal women. Clin Endocrinol (Oxf). (2025) 103:167–176. doi: 10.1111/cen.15253
28. Mauricio D, Gratacòs M, and Franch-Nadal J. Managing diabetes across female reproductive stages. Trends Endocrinol Metab. (2025) 36:403–417. doi: 10.1016/j.tem.2025.02.003
29. Tang Y, Ma R, Zhang L, Sun X, and Wang Y. Effectiveness and safety of hormone replacement therapy in the treatment of menopausal syndrome: a meta-analysis. Am J Transl Res. (2025) 17:1–15. doi: 10.62347/UGLT3830
30. Hodis HN and Mack WJ. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: it is about time and timing. Cancer J. (2022) 28:208–23. doi: 10.1097/PPO.0000000000000591
31. Dinarvand D, Panthakey J, Heidari A, Hassan A, and Ahmed MH. The intersection between frailty, diabetes, and hypertension: the critical role of community geriatricians and pharmacists in deprescribing. J Pers Med. (2024) 14:924. doi: 10.3390/jpm14090924
32. Umegaki H. Management of older adults with diabetes mellitus: Perspective from geriatric medicine. J Diabetes Investig. (2024) 15:1347–54. doi: 10.1111/jdi.14283
33. Mattioli AV, Selleri V, Zanini G, Nasi M, Pinti M, Stefanelli C, et al. Physical activity and diet in older women: A narrative review. J Clin Med. (2023) 12:81. doi: 10.3390/jcm12010081
34. O’Neil H, Todd A, Pearce M, and Husband A. What are the consequences of over and undertreatment of type 2 diabetes mellitus in a frail population? A systematic review. Endocrinol Diabetes Metab. (2024) 7:e00470. doi: 10.1002/edm2.470
35. Pan Y and Ma L. Inflammatory markers and physical frailty: Towards clinical application. Immun Ageing. (2024) 21:4. doi: 10.1186/s12979-023-00410-3
36. Zanini G, Selleri V, Lopez Domenech S, Malerba M, Nasi M, Mattioli AV, et al. Mitochondrial DNA as inflammatory DAMP: a warning of an aging immune system? Biochem Soc Trans. (2023) 51:735–45. doi: 10.1042/BST20221010
37. Dzięgielewska-Gęsiak S and Muc-Wierzgoń M. Inflammation and oxidative stress in frailty and metabolic syndromes—Two sides of the same coin. Metabolites. (2023) 13:475. doi: 10.3390/metabo13040475
38. Sinclair AJ, Abdelhafiz A, Dunning T, Izquierdo M, Manas LR, Bourdell-Marchasson I, et al. An international position statement on the management of frailty in diabetes mellitus: summary of recommendations. J Frailty Aging. (2017) 7:10–20. doi: 10.14283/jfa.2017.39
39. Dendup T, Feng X, Clingan S, and Astell-Burt T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int J Environ Res Public Health. (2018) 15:78. doi: 10.3390/ijerph15010078
40. Rajagopalan S and Brook RD. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes. (2012) 61:3037–45. doi: 10.2337/db12-0190
41. Khan A, Ullah W, Ikram M, Quasim M, Alam U, Sheraz M, et al. Mortality trends and disparities in cerebrovascular disease among diabetic population in the United States from 1999 to 2020: A CDC WONDER analysis. Endocrinol Diabetes Metab. (2025) 8:e70091. doi: 10.1002/edm2.70091
42. Tatulashvili S, Fagherazzi G, Dow C, Cohen R, Fosse S, and Bihan H. Socioeconomic inequalities and type 2 diabetes complications: A systematic review. Diabetes Metab. (2020) 46:89–99. doi: 10.1016/j.diabet.2019.11.001
43. Cocchi C, Coppi F, Farinetti A, and Mattioli AV. Cardiovascular disease prevention and therapy in women with Type 2 diabetes. Future Cardiol. (2021) 17:487–96. doi: 10.2217/fca-2021-0011
44. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. (2018) 137:2166–78. doi: 10.1161/CIRCULATIONAHA.117.029652
45. Pampel FC, Krueger PM, and Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. (2010) 36:349–70. doi: 10.1146/annurev.soc.012809.102529
46. Darmon N and Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. (2015) 73:643–60. doi: 10.1093/nutrit/nuv027
47. Mattioli AV, Coppi F, Migaldi M, and Farinetti A. Fruit and vegetables in hypertensive women with asymptomatic peripheral arterial disease. Clin Nutr ESPEN. (2018) 27:110–2. doi: 10.1016/j.clnesp.2018.05.010
48. Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, Nugent R, et al. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: A report from the workshop convened by the world heart federation. J Am Coll Cardiol. (2015) 66:1590–614. doi: 10.1016/j.jacc.2015.07.050
49. Sims M, Kershaw KN, Breathett K, Jackson EA, Lewis LM, Mujahid MS, et al. Importance of housing and cardiovascular health and well-being: A scientific statement from the american heart association. Circ Cardiovasc Qual Outcomes. (2020) 13:e000089. doi: 10.1161/HCQ.0000000000000089
50. Hock ES, Blank L, Fairbrother H, Clowes M, Cuevas DC, Booth A, et al. Exploring the impact of housing insecurity on the health and wellbeing of children and young people in the United Kingdom: a qualitative systematic review. BMC Public Health. (2024) 24:2453. doi: 10.1186/s12889-024-19735-9
51. Mattioli AV, Coppi F, Nasi M, and Gallina S. Stress and cardiovascular risk burden after the pandemic: current status and future prospects. Expert Rev Cardiovasc Ther. (2022) 20:507–13. doi: 10.1080/14779072.2022.2092097
52. Evans E, Jacobs M, Fuller D, Hegland K, and Ellis C. Allostatic load and cardiovascular disease: A systematic review. Am J Prev Med. (2025) 68:1072–9. doi: 10.1016/j.amepre.2025.02.016
53. Crump AA, Bather JR, Villalonga-Olives E, Kranz EO, and Cuevas AG. Place-based opportunities and physiological stress: understanding neighborhood-level disparities in allostatic load. Health Place. (2025). doi: 10.1016/j.healthplace.2025.103532
54. Johnson PA, Brindis CD, Donelan K, Goodwin M, Harris L, Kozhimannil KB, et al. New directions for women’s health: expanding understanding, improving research, addressing workforce limitations. Health Aff (Millwood). (2025) 44:156–62. doi: 10.1377/hlthaff.2024.01004
55. Schulz R, Beach SR, Czaja SJ, Martire LM, and Monin JK. Family caregiving for older adults. Annu Rev Psychol. (2020) 71:635–59. doi: 10.1146/annurev-psych-010419-050754
56. Coughlin SS, Vernon M, Hatzigeorgiou C, and George V. Health literacy, social determinants of health, and disease prevention and control. J Environ Health Sci. (2020) 6:3061.
57. Sharkey CM, Cooke F, Dattilo TM, DeLone AM, and Mullins LL. The role of social problem-solving in emerging adult healthcare transition. Health Care Transit. (2025) 3:100099. doi: 10.1016/j.hctj.2025.100099
58. Moscucci F, Baratta F, Mattioli AV, Lavalle F, Desideri G, and Sciomer S. Environmental pollution: a cardiovascular, life-threatening risk factor: is there a different impact between sexes? J Sex- Gender-Specific Med. (2025) 11:102–107. doi: 10.1723/4533.45358
59. Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, and O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. (2016) 119:1204–14. doi: 10.1161/CIRCRESAHA.116.309279
60. Poursafa P, Mansourian M, Motlagh ME, Ardalan G, and Kelishadi R. Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Environ Res. (2014) 134:105–9. doi: 10.1016/j.envres.2014.07.010
61. Nowak DJ, Crane DE, and Stevens JC. Air pollution removal by urban trees and shrubs in the United States. Urban For Urban Green. (2006) 4:115–23. doi: 10.1016/j.ufug.2006.01.007
62. Brownson RC, Baker EA, Housemann RA, Brennan LK, and Bacak SJ. Environmental and policy determinants of physical activity in the United States am. J Public Health. (2001) 91:1995–2003. doi: 10.2105/AJPH.91.12.1995
63. Howell NA and Booth GL. The weight of place: built environment correlates of obesity and diabetes. Endocr Rev. (2022) 43:966–83. doi: 10.1210/endrev/bnac005
64. Gochfeld M and Burger J. Disproportionate exposures in environmental justice and other populations: the importance of outliers. Am J Public Health. (2011) 101 Suppl 1:S53–63. doi: 10.2105/AJPH.2011.300121
65. Bucciarelli V, Moscucci F, Cocchi C, Nodari S, Sciomer S, Gallina S, et al. Climate change versus Mediterranean diet: A hazardous struggle for the women’s heart. Am Heart J Plus. (2024) 45:100431. doi: 10.1016/j.ahjo.2024.100431
66. Hu Y, Wang X, Huan J, Zhang L, Lin L, Li Y, et al. Effect of dietary inflammatory potential on the aging acceleration for cardiometabolic disease: A population-based study. Front Nutr. (2022) 9:1048448. doi: 10.3389/fnut.2022.1048448
67. Mattioli AV, Coppi F, Severino P, Penna C, Pagliaro P, Dei Cas A, et al. A personalized approach to vitamin D supplementation in cardiovascular health beyond the bone: an expert consensus by the italian national institute for cardiovascular research. Nutrients. (2024) 17:115. doi: 10.3390/nu17010115
68. Micheloni G, Cocchi C, Sinigaglia G, Coppi F, Zanini G, Moscucci F, et al. Sustainability of the mediterranean diet: A nutritional and environmental imperative. J Sustain Res. (2025) 7:e250036. doi: 10.20900/jsr20250036
69. Bavaro AR, Tarantini A, Bruno A, Logrieco AF, Gallo A, Mita G, et al. Functional foods in Mediterranean diet: exploring the functional features of vegetable case-studies obtained also by biotechnological approaches. Aging Clin Exp Res. (2024) 36:208. doi: 10.1007/s40520-024-02860-1
70. Truzzi ML, Ballerini Puviani M, Tripodi A, Toni S, Farinetti A, Nasi M, et al. Mediterranean Diet as a model of sustainable, resilient and healthy diet. Prog Nutr. (2020) 22:388–94. doi: 10.23751/pn.v22i2.8632
71. Drewnowski A and Conrad Z. Pulse crops: nutrient density, affordability, and environmental impact. Front Nutr. (2024) 11:1438369. doi: 10.3389/fnut.2024.1438369
72. Yu XL, Zhou LY, Huang X, Li XY, Wang MK, and Yang JS. Role of nutrition in diabetes mellitus and infections. World J Clin Cases. (2025) 13:94389. doi: 10.12998/wjcc.v13.i3.94389
73. Münzel T, Sørensen M, Lelieveld J, Landrigna PJ, Kuntic M, Nieuwenhuijsen M, et al. A comprehensive review/expert statement on environmental risk factors of cardiovascular disease. Cardiovasc Res. (2025). doi: 10.1093/cvr/cvaf119
74. Kenny GP, Sigal RJ, and McGinn R. Body temperature regulation in diabetes. Temperature (Austin). (2016) 3:119–45. doi: 10.1080/23328940.2015.1131506
75. Fan W and Zlatnik MG. Climate change and pregnancy: risks, mitigation, adaptation, and resilience. Obstet Gynecol Surv. (2023) 78:223–36. doi: 10.1097/OGX.0000000000001116
76. Puche-Juarez M, Toledano JM, Moreno-Fernandez J, Gálvez-Ontiveros Y, Rivas A, Diaz Castro J, et al. The role of endocrine disrupting chemicals in gestation and pregnancy outcomes. Nutrients. (2023) 15:4657. doi: 10.3390/nu15214657
77. Lin Y, Chen R, Ge Y, Brunner J, Hopke PK, Miller RK, et al. Exposure to low-level air pollution and hyperglycemia markers during pregnancy: A repeated measure analysis. Environ Sci Technol. (2024) 58:15997–6005. doi: 10.1021/acs.est.4c05612
78. Dong F, Zhang X, Liu Y, Pan Y, Zhang Y, Long R, et al. Economic policy choice of governing haze pollution: evidence from global 74 countries. Environ Sci pollut Res Int. (2021) 28:9430–47. doi: 10.1007/s11356-020-11350-6
79. Balti EV, Echouffo-Tcheugui JB, Yako YY, and Kengne AP. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. (2014) 6:49. doi: 10.1016/j.diabres.2014.08.010
80. Liu B, Hwang S, Lim JE, Choi Y, and Jee SH. Bisphenol A and the risk of metabolic disorders: a meta-analysis. BMC Endocr Disord. (2018) 18:81. doi: 10.1186/s12902-018-0310-y
81. Wade M, Delawder V, Reneau P, and Dos Santos JM. The effect of BPA exposure on insulin resistance and type 2 diabetes - The impact of muscle contraction. Med Hypotheses. (2020) 140:109675. doi: 10.1016/j.mehy.2020.109675
82. Zhu Y, Yuan Y, Chen Q, Ding X, Zhong Q, and Zhong X. Urinary BPA and non-monotonic associations with mortality: evidence from NHANES 2013–2018. Front Public Health. (2024) 12:1341789. doi: 10.3389/fpubh.2024.1341789
83. Sivakumar S, Lama D, and Rabhi N. Childhood obesity from the genes to the epigenome. Front Endocrinol (Lausanne). (2024) 15:1393250. doi: 10.3389/fendo.2024.1393250
84. Hao H, Wang Y, Zhu Q, Zhang H, Rosenberg A, Schwartz J, et al. National cohort study of long-term exposure to PM2.5 components and mortality in medicare american older adults. Environ Sci Technol. (2023) 57:6835–43. doi: 10.1021/acs.est.2c07064
85. Vasishta S and Adiga U. Air pollution and its role in the rising burden of type 2 diabetes in India: urgent call for action. Environ Sci pollut Res. (2025) 32:13527–38. doi: 10.1007/s11356-025-36508-y
86. Hagobian TA, Brunner-Gaydos H, Seal A, Schaffner A, Kitts C, Hubbard R, et al. Rationale and design of a randomized controlled trial examining oral administration of bisphenol A on hepatic glucose production and skeletal muscle insulin sensitivity in adults. Contemp Clin Trials Commun. (2020) 17:100549. doi: 10.1016/j.conctc.2020.100549. Erratum in: Contemp Clin Trials Commun. 2020 Dec 10;20:100691. doi: 10.1016/j.conctc.2020.100691.
87. López-Rodríguez D, Aylwin CF, Delli V, Sevrin E, Campanile M, Martin M, et al. Multi- and transgenerational outcomes of an exposure to a mixture of endocrine-disrupting chemicals (EDCs) on puberty and maternal behavior in the female rat. Environ Health Perspect. (2021) 129:87003. doi: 10.1289/EHP8795
88. Ruiz D, Becerra M, Jagai JS, Ard K, and Sargis RM. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. (2018) 41:193–205. doi: 10.2337/dc16-2765
89. Dinu M, Pagliai G, Casini A, and Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. (2018) 72:30–43. doi: 10.1038/ejcn.2017.58
90. Salas-Salvadó J, Bulló M, Babio N, Martínez-González M, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. (2011) 34:14–9. doi: 10.2337/dc10-1288
91. Boughanem H, Gutierrez-Mariscal FM, Arenas-de Larriva AP, Torres Pena JP, Romero Cabrera JL, Rongel-Zunga OA, et al. Effect of long-term Mediterranean versus low-fat diet on neutrophil count, and type 2 diabetes mellitus remission in patients with coronary heart disease: results from the CORDIOPREV study. Nutr Diabetes. (2025) 15:11. doi: 10.1038/s41387-025-00360-3
92. Rivas-Garcia L, Quintana-Navarro GM, Alcala-Díaz JF, Torres-Pena JD, Arenas-de Larriva JP, Rangel-Zunga OA, et al. Association between diet quality and risk of type 2 diabetes mellitus in patients with coronary heart disease: findings from the CORDIOPREV study. Nutrients. (2024) 16:1249. doi: 10.3390/nu16081249
93. Jimenez-Torres J, Alcalá-Diaz JF, Torres-Peña JD, Gutierrez Maniscal FR, Leon Acuna A, Gomez-Luna P, et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial [published correction appears in Stroke. Stroke. (2021) 52:3440–3449. doi: 10.1161/STR.0000000000000393
94. Zooravar D, Soltani P, and Khezri S. Mediterranean diet and diabetic microvascular complications: a systematic review and meta-analysis. BMC Nutr. (2025) 11:66. doi: 10.1186/s40795-025-01038-w
95. Gardner CD, Landry MJ, Perelman D, Petlura C, Durand LR, Aronica L, et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: the interventional keto-med randomized crossover trial. Am J Clin Nutr. (2022) 116:640–52. doi: 10.1093/ajcn/nqac154
96. Fedak KM, Bernal A, Capshaw ZA, and Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. (2015) :12:14. doi: 10.1186/s12982-015-0037-4
97. Needham BL and Dokshina DA. The impact of federal and state laws on cardiovascular risk. Curr Cardiol Rep. (2025) 27:121. doi: 10.1007/s11886-025-02277-w
98. Doyle J, Alsan M, Skelley N, Lu Y, and Cawley J. Effect of an intensive food-as-medicine program on health and health care use: A randomized clinical trial. JAMA Intern Med. (2024) 184:154–63. doi: 10.1001/jamainternmed.2023.6670
99. Ballotari P, Venturelli F, Manicardi V, Ferrari F, Vicentini M, Greci M, et al. Effectiveness of integrated care model for type 2 diabetes: A population-based study in Reggio Emilia (Italy). PloS One. (2018) 13:e0194784. doi: 10.1371/journal.pone.0194784
Keywords: women, cardiovascular health, diabetes mellitus, socioeconomic determinants, environmental pollution, chronic stress, climate change
Citation: Cocchi C, Selleri V, Zanini G, Moscucci F, Sciomer S, Gallina S, Nasi M, Desideri G, Pinti M, Borghi C and Mattioli AV (2025) Environmental and social determinants of cardiovascular risk in women with type 2 diabetes: a life-course perspective. Front. Endocrinol. 16:1667222. doi: 10.3389/fendo.2025.1667222
Received: 16 July 2025; Accepted: 18 September 2025;
Published: 08 October 2025.
Edited by:
Shihori Tanabe, National Institute of Health Sciences (NIHS), JapanReviewed by:
Elizabeth Katherine Wood, Oregon Health and Science University, United StatesRidwan Abiodun Alimi, University of Ilorin, Nigeria
Copyright © 2025 Cocchi, Selleri, Zanini, Moscucci, Sciomer, Gallina, Nasi, Desideri, Pinti, Borghi and Mattioli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Vittoria Mattioli, YW5uYXZpdHRvcmlhLm1hdHRpb2xpQHVuaWJvLml0
†These authors have contributed equally to this work and share first authorship
 Camilla Cocchi1,2†
Camilla Cocchi1,2† Valentina Selleri
Valentina Selleri Giada Zanini
Giada Zanini Federica Moscucci
Federica Moscucci Susanna Sciomer
Susanna Sciomer Sabina Gallina
Sabina Gallina Milena Nasi
Milena Nasi Marcello Pinti
Marcello Pinti Claudio Borghi
Claudio Borghi Anna Vittoria Mattioli
Anna Vittoria Mattioli