- 1Engineering Laboratory for Agro Biomass Recycling and Valorizing, College of Engineering, China Agricultural University, Beijing, China
- 2School of Ecology and Environment, Beijing Technology and Business University, Beijing, China
- 3Key Laboratory of Functional Dairy, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing, China
- 4Sino-French Joint Venture Dynasty Winery Ltd., Tianjin, China
Cabernet Sauvignon grape pomace contains carbohydrates and various amino acids that could be used as substrates for lactic acid (LA) production. In this study, a mutant strain of L. plantarum with deletion of the D-lactate synthesis gene was developed and used to produce optical pure L-LA from grape pomace. The highest optical purity of the L-LA produced by this mutant strain was 99.61%. The direct bioconversion of the raw substrate showed a low LA yield. Several pretreatment methods were applied to improve the LA yield, including ball milling, hydrothermal, dilute acid pretreatment, alkaline pretreatment, and combined wet alkaline mechanical pretreatment. Due to the efficient delignification, alkaline pretreatment achieved the highest lactic acid yield of 96.26% at 15% solid loading, with corresponding LA concentration and volumetric productivity of 18.45 g/L and 2.30 g/L·h, respectively.
1 Introduction
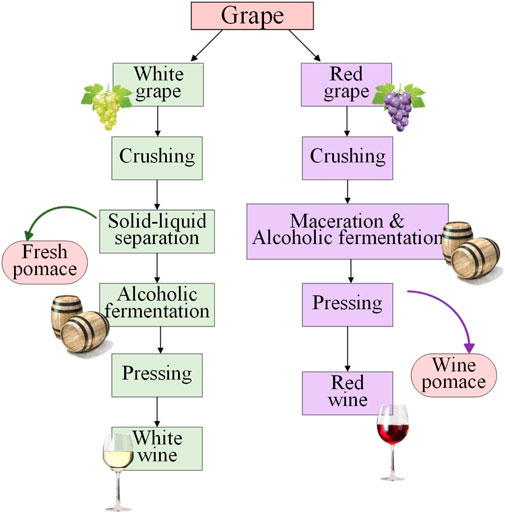
Grape is one of the world’s most widely developed fruit crops with a global production of over 70 million tons in 2021, out of which about 75% is used for the winemaking industry (OIV, 2023; Khan et al., 2020). China is now the biggest grape-production country in the world, the annual grape production in China reached its peak of 14.3 million tons in 2020 (FAO, 2023). A huge amount of waste is generated in the winemaking process, constituting about 25% of the total grape weight. Because winemaking is a seasonal task, the wastes are generated during a short period thus burdening the waste treatment (Ilyas et al., 2021). Grape pomaces are the major solid waste from the wine industry, which is mainly composed of grape seeds and skins. There are two types of grape pomaces, named the “fresh pomace” from the white wine making process (also known as the white grape pomace) and the “wine pomace” from the red wine making process (also known as the red grape pomace) (Figure 1) (Sirohi et al., 2020). To make white wine, the grapes are first crushed, and the juice will be separated from the solid for the subsequent alcoholic fermentation. The remaining solids arecalled “fresh pomace” because they are generated directly from fresh grapes and do not go through the fermentation step. For the red wine process, the whole grape slurry after crushing will be sent to fermentation. The solids are separated from red wine after the fermentation step, so they are called the “wine pomace”. Therefore, the white wine making process produces more pomace than the red wine process (Sirohi et al., 2020). The utilization of fresh pomace has drawn more attention as they retain more bioactive compounds (Calinoiu et al., 2017). The value of wine pomace is lower and they are generally incinerated or subjected to landfill. The disposal of wine pomace showed a negative impact on the environment. The gaseous and particulate pollutants emitted by uncontrolled incineration are major source of air pollution (Deng et al., 2011). Due to the high phenolic content in grape pomace which increases its resistance to biological degradation, the direct landfilling of grape pomace is detrimental to the environment (Northup and Mccoll, 1998; Chen et al., 2018). The large amounts of condensed tannins and other polyphenols on the grape pomace will bring toxicity to soil microorganisms and enzymes (Northup and Mccoll, 1998; Chen et al., 2018). Other environmental problems of grape pomace include surface and groundwater pollution, foul odor, flies, and pests attraction that may spread diseases (Drevelegka and Goula, 2020). Therefore, The China government is implementing more and more strict regulations in waste treatment that call for new strategies in wine pomace recycling and utilization.
Cabernet Sauvignon grape is the most representative red grape and the total carbohydrates present in its pomace are among the highest in different grape varieties, making it a suitable substrate for microbial fermentation (Lin, 2021). Lin, etc., compared the nutritional components of 3 different grape seeds and found the total carbohydrates in Cabernet Sauvignon seed (−3%) is higher than Muscat (−1.2%) and Jufeng (−2.7%) seeds (Lin, 2021). Please note that the low carbohydrate value in Lin’s paper was due to the different measurement methods. Corbin, etc., found the total carbohydrates in Cabernet Sauvignon grape marc was up to 31% (Corbin et al., 2015). The red wine pomace in Pedras’s study contains 26% carbohydrates, but the grape species was not specified (Pedras et al., 2020). A mixed red wine pomace from 6 wineries in Castilla León (Spain) was found to contain 30% total carbohydrates (Guerra-Rivas et al., 2017). Lactic acid (LA) is one of the top value-added building block chemicals listed by the National Renewable Energy Laboratory (NREL) of the United States of America (Werpy and Petersen, 2004). The most recent and promising application of LA is to make biodegradable poly-lactic acid (PLA), an environmentally-friendly plastic. The LA produced from wild strains of lactic acid bacteria (LAB), such as L. plantarum, is generally racemic LA containing equal molar of D- and L-LA (Yi et al., 2016). Due to the superior biocompatibility of poly-L-lactic acid, it has vast potential in the medical device industry. If the D-lactate synthesis gene were selectively removed, a mutant LAB strain with the capability of producing optical pure L-LA could be developed.
In this study, the Cabernet Sauvignon grape pomace was chosen as the substrate for the production of LA. Several pretreatment methods were applied and compared to obtain a satisfactory LA yield. A mutant strain of L. plantarum was developed for the production of high-purity of L-LA.
2 Materials and methods
2.1 Grape pomace characterization
The Cabernet Sauvignon grape pomace was kindly provided by Sino-French Joint Venture Dynasty Winery Ltd. The major components of this pomace sample were seed and skin. The wet weight of seed and skin in the raw sample is about 70% and 30%, respectively. The pomace was air-dried to moisture below 10% upon receiving and then ground by a coffee grinder to the size of 20–80 mesh for further use. The carbohydrates, lignin, and ash content in the pomace were determined according to NREL’s standard procedure (Sluiter, 2008). The active compounds analysis was performed at Sino-French Joint Venture Dynasty Winery Ltd. Amino acids analysis and elemental analysis was performed at the Analytical Laboratory in the Zhongkebaice Technology Service Co., Ltd. (Beijing, China). The elemental contents (C, H, N, O, and S) analysis was performed using an elemental analyzer (Elementar UNICUBE, Germany). The phosphorus analysis was performed with an Inductively Coupled Plasma-Optical Emission Spectrometer (Agilent ICP-OES 730, United States). The detailed procedure of elemental analysis has been described earlier (Li et al., 2020; Anastasiou et al., 2021). The amino acids were measured by an HPLC system (Shimazu 20AD) equipped with a C18 column. The method of amino acids analysis was the same as Baranenko’s work (Baranenko et al., 2014).
2.2 Pretreatment of grape pomace
The most common pretreatment methods used in the bioconversion of lignocellulosic biomass, including ball milling (BM), hydrothermal (HT), dilute acid (DA), alkaline (AP), and combined wet alkaline mechanical (ABM) pretreatment, were applied in this study to break down the dense structure of grape pomace and reduce its recalcitrance in the LA fermentation. The detail for each pretreatment is listed below:
Ball milling (BM) pretreatment was performed with a ball mill machine CJM-SY-B (Qinhuangdao Taiji Ring Nano Co., China). Grape pomace was mixed with zirconia balls (6–10 mm diameter) at a mass ratio of 1:30 and then ground for 30 min. The temperature of the chamber was maintained at around 20°C during the ball milling process by circulating water in its cooling jacket. The BM experiment was performed in duplicate.
Hydrothermal (HT) pretreatment was performed in a high-pressure reactor (Parr 4532, United States). DI water was used as the solvent at a solid-to-liquid ratio of 1:15. The temperature was 175°C and the reaction time was 20 min. After the HT treatment, the pomace solids were separated from the liquid stream and washed till neutral with tap water. The treated pomace solids were then dried at 60°C to constant weight for further use. The HT experiment was performed in duplicate.
Dilute acid (DA) pretreatment was conducted in glass pressure tubes (Ace Glass 8684-88, United States) with 1.5wt% sulfuric acid as solvent. The solid-to-liquid ratio was 1:15 and the temperature was maintained at 121°C for 1 h in an autoclave (Sanyo MLS-3750, Japan). The solids collection after acid pretreatment was the same as HT pretreatment. The DA experiment was performed in duplicate.
Alkaline (AL) pretreatment was also conducted in the pressure glass tubes. Sodium hydroxide solution (2.0 wt%) was used as the solvent. The temperature, reaction time, and solid-to-liquid ratio for the AL experiment, as well as the solids collection, were the same as the DA treatment. The AL experiment was performed in duplicate.
Combined wet alkaline mechanical (ABM) pretreatment was the combination of the BM and AL treatment methods. The pomace was thoroughly mixed with 5 wt% NaOH solution at a 1:3 (w/w) ratio (condition was determined by our preliminary optimization experiments) and then subjected to BM for 15 min. The solids after the wet ball milling was washed and dried as previously described.
2.3 Scanning electron microscopy analysis of grape pomace samples
The surface morphology of the grape pomace before and after pretreatments were analyzed with Hitachi SU3500 (Hitachi, Japan). Each sample was evenly attached to the carbon tape and gold sprayed for 2 min before SEM observation.
2.4 Fourier transform infrared spectroscopy analysis of grape pomace samples
The PerkinElmer spectrometer 400 (PerkinElmer, United States) was used to scan and collect spectral information. The scanning wavenumber range was 4000–400 cm−1, the resolution was 4 cm−1 and the number of scans was 64. Each sample was measured twice.
2.5 Enzyme and microorganism
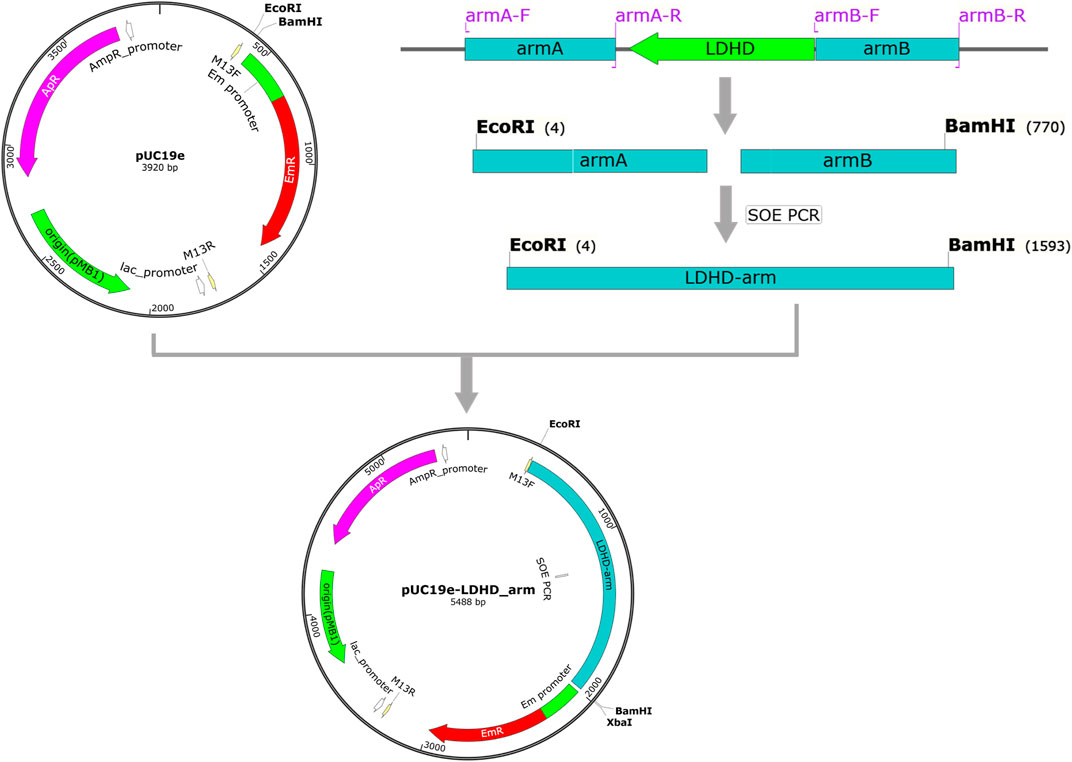
Cellulase enzyme (Novozymes Cellic CTec2, 116 FPU/mL) was purchased from Sigma-Aldrich (Shanghai, China). Lactobacillus plantarum (CAUH2-1), a wild strain of LAB, was screened and provided by the Key Laboratory of Functional Dairy at the College of Food Science and Nutritional Engineering, China Agricultural University. MRS Broth (Acumedia) was used as the growth medium. Q5 DNA polymerase (New England Biolabs, America) was used for PCR amplification. The genome DNA of L. plantarum was used as a template. The deletion of the ldhD and larA genes was done successively through a two-step homologous recombination process (Okano et al., 2018). The suicide plasmid for ldhD (pUCldhD) was first constructed by amplifying the upstream and downstream flanking regions of ldhD with two primers as shown in Figure 2. The upstream and downstream flanking regions of ldhD were ligated by SOE PCR. Then, the ligated fragment digested with EcoRⅠ and BamHⅠ was cloned into pUChlyl. Similarly, the suicide plasmid for larA (pUClarA) was constructed using the oligonucleotide primers.
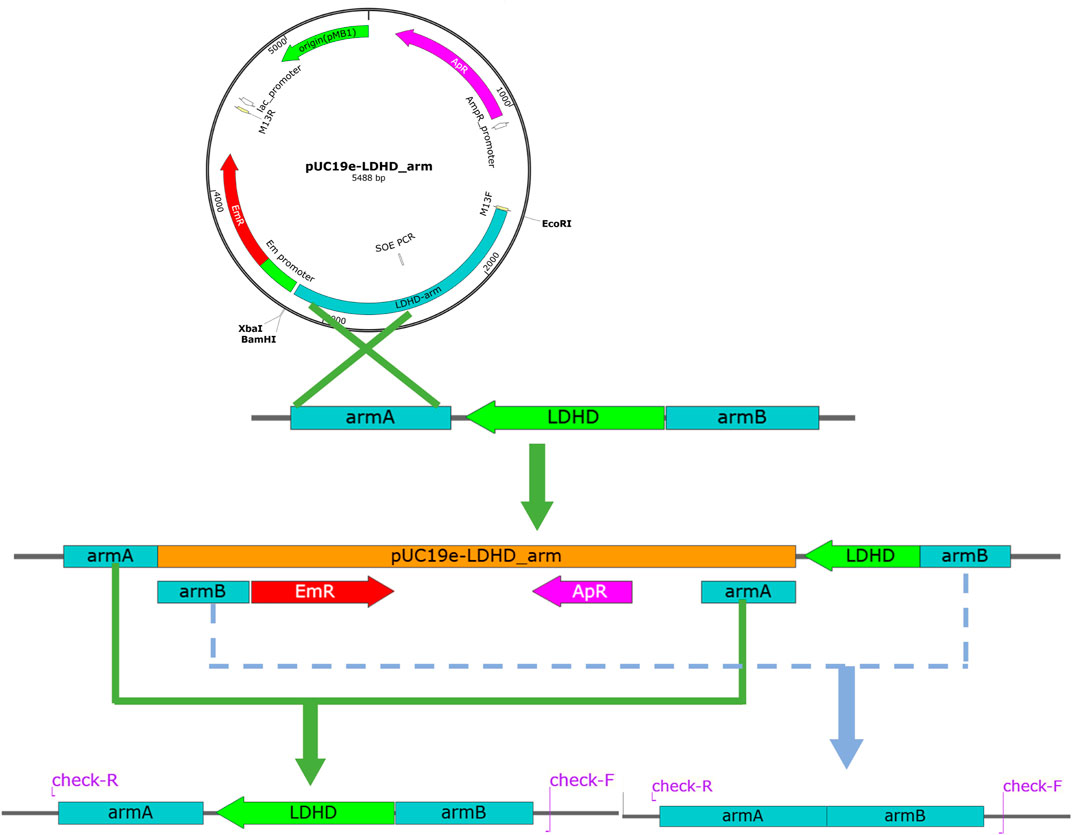
The ΔldhD and ΔldhDΔlarA mutants of L. plantarum were generated successively through a two-step homologous recombination process as shown in Figure 3. The suicide plasmid pUCldhD was transferred into CAUH2-1 cells by electromycin. Campbell-type integration of pUCldhD into the L. plantarum chromosome via the armAl or armBl region results in tandem of wild-type and deleted copies of the ldhD gene. Two reorganizations of double-crossover homologous recombination occur on the upstream and downstream of the ldhD gene respectively, which will lead to the lack of ldhD genes. Both re-organization occur on the same homologous arm, which will lead to the recovery of wild strains. To distinguish between these two possibilities, a pair of primers (ldhD-check_F/ldhD-check_R) was designed on both sides of the deletion site. Then, plasmid pUClarA was used to knock out the larA gene of the ΔldhD mutants of L. plantarum with the same procedure. One of the transformants isolated on selective MRS plates was further cultivated for about 50 generations in a nonselective medium to obtain mutants.
2.6 Simultaneous saccharification and fermentation of grape pomace
The LA fermentation was conducted in 125 mL serum bottles with a total working volume of 50 mL and a solid loading level of 15%. To neutralize the LA produced during the fermentation and maintain the pH near optimal, CaCO3 was added (0.5 g/g sugar) as a pH buffer. Cellulase enzyme loading was chosen to be 10 FPU/g-solid to enable the SSF process. Yeast extract was applied at 15 g/L to ensure optimal growth of LAB. After loading all fermentation materials, the serum bottles were flushed with nitrogen and then crimp-sealed with a rubber stopper to maintain anaerobic conditions during the fermentation. The bottles were steam-sterilized at 121°C for 15 min in an autoclave (Sanyo MLS-3750, Japan). When bottles were cooled to room temperature after the sterilization, the microbe inoculum (3 mL) and enzyme were injected to start the SSF process. Samples were collected at a 2-h interval for the first 12 h of fermentation, and then at a 12-h interval for fermentation up to 72 h. Fermentation was performed at 37°C in a rotary incubator with an agitation speed of 150rpm. The SSF experiments were carried out in duplicate.
2.7 Analytical methods
Sugars and LA concentrations of samples were determined by the HPLC system (Waters, United States) with a BioRad-HPX-87H column. The conditions for HPLC analysis have been described in our previous report (Li et al., 2021). The D- and L-LA were determined by an assay kit (Megazyme International Ireland Ltd.). The volumetric productivity (P) was used to show the LA production rate, and it was calculated as the maximum concentration of LA divided by the corresponding fermentation time. LA yield was defined as LA produced divided by the total amount of available glucose.
3 Results and discussion
3.1 Characteristics of Cabernet Sauvignon grape pomace
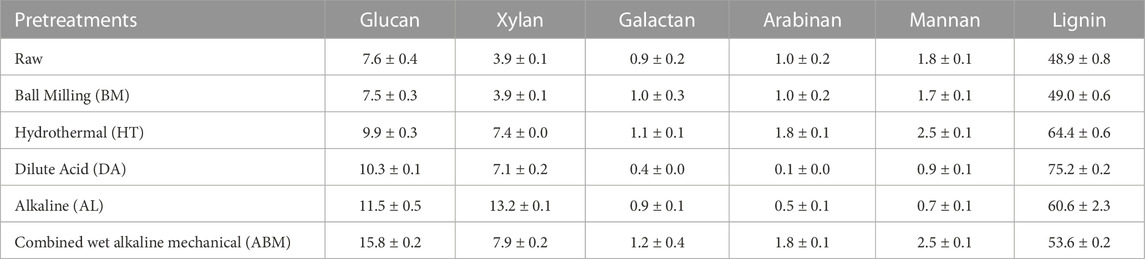
The composition of grape pomace varies greatly depending on the type of grape and the winemaking process. Fresh pomace tends to contain more carbohydrates than wine pomace. Heuze and Tran summarized that the sugar content in red pomace was in the range of 4%–9%, while the sugar in white pomace can be as high as 28%-31% (Ilyas et al., 2021). A better knowledge of the source of the pomace and compositional analysis is critical for the recycling and utilization of these residues. The Cabernet Sauvignon grape pomace used in this study was found to contain 7.6% glucan, 3.9% xylan, 0.9% galactan, 1.0% arabinan, and 1.8% mannan. The total sugars constitute 15.2% of the dry weight of the pomace, making it a possible carbohydrate source for LA fermentation. The sugar values are in agreement with the literature’s values, Bordiga et al., 2019 reported an average sugar content of 15%–33% in grape pomaces. Bayrak and Büyükkileci, 2018 found a very high soluble sugar content of over 35% in fresh white grape pomace, but the structural polysaccharides in their sample were 14.01%, which was very close to our result. The lignin value in this pomace sample was 48.9% which might impede the SSF of LA. The elemental analysis results were typical for grape pomace and in good agreement with literature values (Ilyas et al., 2021), the most abundant elements in the pomace sample were carbon (50.37%), oxygen (37.20%), and hydrogen (5.88%). The amount of nitrogen was 2.34%, and a trace amount of phosphorus (0.23%) and sulfur (0.21%) were also found.
Grape pomace has some unique advantages for LA fermentation, such as its high protein content and variable amino acids. LAB is incapable of biosynthesis of some essential amino acids for their growth (Hugenholtz and Kleerebezem, 1999). Therefore, specific peptides, vitamins, and some other nutrients are needed to ensure their optimal growth (Arasaratnam et al., 1996). The protein content was determined to be 9.02% in the Cabernet Sauvignon grape pomace, which is slightly lower than the average protein value reported by Ilyas et al., 2021. The amino acid content and bioactive compounds were summarized in Table 1. The impact of these bioactive compounds on LA fermentation needs further investigation.
3.2 Carbohydrate composition change of grape pomace after pretreatment
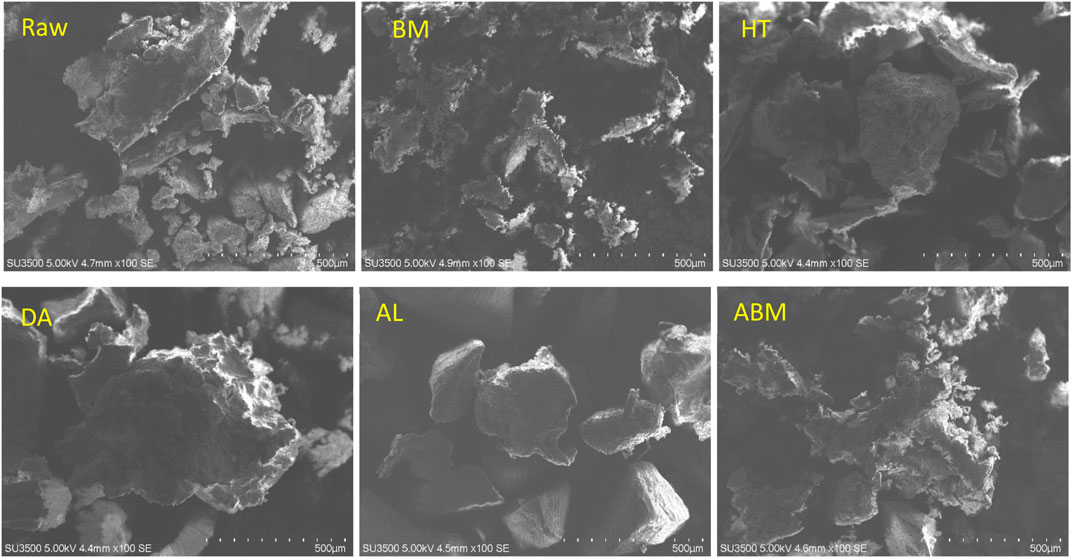
Physical and chemical pretreatment could reduce the recalcitrance of biomass and thus improve the subsequent enzymatic hydrolysis and fermentation step. Pretreatments will cause physiochemical property changes in the material (Table 2; Figure 4). For the fermentative production of LA, the carbohydrate contents are the most concerned parameter. The changes in the sugar content and the surface morphology of the grape pomace after different pretreatments were summarized in Table 2 and Figure 4, respectively. The lignin content in this grape pomace sample took up almost half of its total dry weight. The other task of the pretreatments was to selectively remove the lignin from the pomace sample.
The ball milling pretreatment is a purely physical process and its major effect was reducing the particle size of the sample (Figure 4). However, the chemical composition of grape pomace, such as sugar content, remained unchanged after BM pretreatment as shown in Table 2. For the HT pretreatment, although no acid or base was involved, there was a group of chemical reactions that happened during the HT process due to the high temperature. The acetyl group in the pomace will release into the water and lower the pH of the liquid, which will in turn accelerate the hydrolysis and degradation reactions. So the prehydrolysate after the HT process is normally an acidic solution (Sun et al., 2022). The condition is considered to be mild for the HT process, so the compositional change after the HT pretreatment is not significant compared to dilute acid pretreatment. The percentage of all sugars and lignin increased a little after HT, indicating some more susceptible components were hydrolyzed and dissolved into the liquid, such as protein and extractives.
Chemical pretreatments such as dilute acid treatment and alkaline treatment could result in clear compositional changes. But the effect of acid and alkali for biomass pretreatment was quite different. The DA process is very effective in hydrolyzing hemicellulose but has little effect on delignification. The DA pretreatment is now considered one of the most promising pretreatment methods for biomass. The bioethanol roadmap reports from U.S. National Renewable Energy Laboratory (NREL) often use the DA process as the model pretreatment method (Sheehan, 2001; Zhao et al., 2015; Houghton et al.). The composition of Cabernet Sauvignon grape pomace after DA pretreatment showed more change than HT pretreatment. The lignin content increased to 75.2% in the DA-treated sample. This is due to the removal of hemicellulose, such as galactan, arabinan, and mannan as shown in Table 2, and other components. One interesting result observed for this pomace sample was that the xylan content after the DA treatment increased a little, which was not usually seen for DA pretreatment (Saha et al., 2005; Hsu et al., 2010). Possible reasons were a) the xylan content in this pomace sample was relatively more resistant than those in crop residues; b) more susceptible components were hydrolyzed and dissolved into the liquid during the DA process. Compared to the DA process, the AL process is good at removing lignin from substrates. In this study, for the AL and combined ABM pretreatments, a high level of solid loss was observed (data not shown). Although the lignin content in the treated pomace seemed to increase from 48.9% to 60.6% and 53.6% for AL and ABM processes, respectively, a higher percentage of the lignin was removed considering the total solid loss. The AL-treated sample showed a very smooth surface compared to other pretreatment (Figure 4). The ABM process brought in higher delignification which was in agreement with literature reports (Moset et al., 2018; Araujo et al., 2019; Yang et al., 2022). The highest cellulose content of 15.8% was obtained by ABM pretreatment, while the highest xylan content of 13.2% was achieved by AL pretreatment.
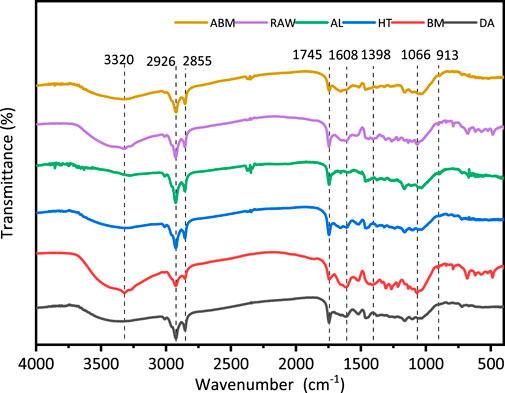
The infrared spectrum absorption peak shift and absorption intensity of a substance is related to its original vibrational frequency, and its chemical composition and chemical bond type are closely related. Therefore, the functional group changes of grape pomace before and after pretreatment were detected by FTIR (Figure 5). The absorption peak at 3,320 cm−1 of untreated grape pomace and BM-pretreated grape pomace samples was related to the stretching vibration of O-H, which was attributed to the intramolecular and intermolecular hydrogen bonding of cellulose chains. The absorption peaks of all samples appeared at 2,926 cm−1 and 2,855 cm−1, which were caused by saturated C-H stretching vibration and bending vibration (Ping et al., 2012). There was an obvious absorption peak at 1745 cm−1 in untreated grape pomace and BM pretreated grape pomace, which was the absorption peak generated by the C=O stretching vibration (Dong et al., 2021). This peak decreased in the spectra of other pretreated grape pomace samples, among which AL and ABM pretreatment decreased significantly, indicating that the pretreatment could remove part of the hemicellulose and lignin. The characteristic absorption peak of the benzene ring was close to 1,608 cm−1, and the absorption peaks of ABM and AL pretreatment samples almost disappeared, indicating that the lignin content decreased significantly. While the absorption peak at 1,608 cm−1 for HT and DA pretreated samples did not decrease significantly, indicating that these two pretreatment methods had little effect on delignification. The peaks at 1,398 cm−1 and 1,066 cm−1 are representative of CH2 bond and C-O bond, respectively. It can be seen that the samples treated with ABM and AL were significantly weaker than the untreated grape pomace, indicating that the crystallinity of the grape pomace treated with these two methods was reduced. There was no significant shift in the absorption peak of β-glycosidic linkage near 913 cm−1. The results of the infrared spectrum analysis are consistent with the results of the above component analysis.
3.3 Simultaneous saccharification and fermentation of grape pomace
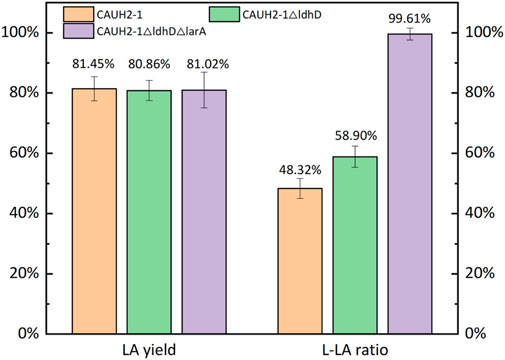
The wild type of L. Plantarum (CAUH2-1) used in this study was initially screened from Chinese pickled cabbage. This strain has a glucose-to-LA conversion yield of over 80%. But the LA produced was a racemic product containing equal molar of D- and L-LA. There are two genes in charge of the formation of D-LA in L. Plantarum, Lactate Dehydrogenase D (ldhD gene) and Lactic Acid Racemase (larA gene). The lactate dehydrogenase D enzyme catalyzes the reaction of pyruvate to D-LA. The ldhD gene was first knocked out and the obtained strain was named CAUH2-1
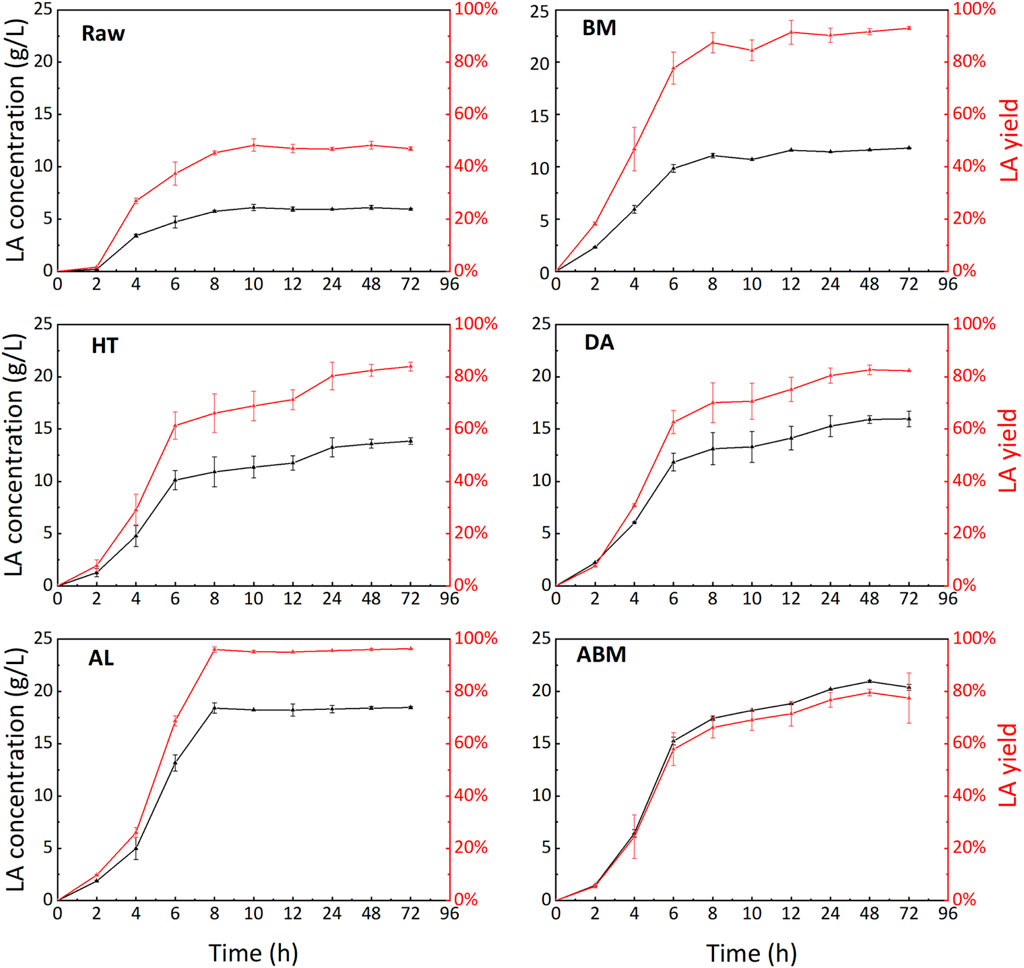
The engineering strain, CAUH2-1∆ldhD∆larA, was used in the SSF of grape pomace. The L-LA ratio in all fermentation broth from SSF of pomace was tested to be over 99%. The time progress of LA production for each substrate were shown in Figure 7. The Cabernet Sauvignon grape pomace was one type of wine pomace. It has gone through the alcoholic fermentation process (Figure 1), so its direct conversion to LA without any pretreatment was much higher than normal lignocellulosic materials, such as wood chips, and corn stover. The 72-h SSF of the raw pomace sample achieved a LA yield of 46.93% with a final LA concentration of 5.94 g/L. Although this yield was higher than that of lignocellulosic biomass which was normally in the range of 5%–20%, the LA concentration was far from satisfactory. The non-carbohydrate components of the pomace need to be removed to increase the available sugar per gram of pomace and to further improve the LA yield.
BM pretreatment was first employed since this process does not require the use of chemicals or high temperatures. Although the carbohydrate composition was not changed after BM treatment (Table 2), the LA concentration and yield from BM-treated pomace almost doubled to 11.80 g/L and 93.16%, respectively. BM pretreatment could significantly alter the physical property of feedstock, such as particle size, crystallinity, surface area, and porosity (Li et al., 2020). These changes could greatly improve the enzymatic hydrolysis and thus increase the LA yield.
The HT pretreatment removed some easily hydrolyzed components and made the sugar content higher than the raw pomace. The final LA concentration after 72-h SSF of the HT-treated sample was 13.85 g/L, and the corresponding LA yield was 83.93%. The high lignin content in the HT-treated sample (Table 2) impeded the LA yield. Similar results were observed for the DA pretreatment. The LA concentration and yield of DA-treated pomace were 14.13 g/L and 82.31%, respectively. No discernible improvement was observed from HT to DA pretreatment, suggesting that DA pretreatment was not a suitable pretreatment method for Cabernet Sauvignon grape pomace.
When alkaline was used as the pretreatment agent, either by itself or combined with physical treatment, the SSF performance of treated pomace showed significant improvement (Figure 7). The AL-pretreated pomace resulted in the highest LA yield of 96.26% among all pomace samples tested in this study. The final LA concentration was 18.45 g/L which was more than 3 times of the raw grape pomace sample. Besides the satisfactory LA yield and concentration, it took only 8 h to reach the maximum LA concentration for AL-pretreated pomace, bringing in a high LA productivity of 2.30 g/L·h. The delignification showed a prominent effect on LA production.
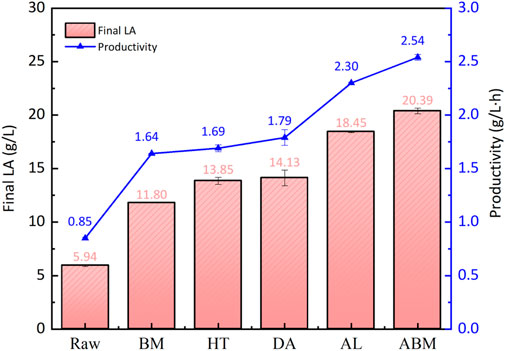
When the alkaline process was combined with ball milling treatment, the highest LA concentration of 20.40 g/L was obtained. This high titer of LA came from the high content of glucan of 15.8% in the ABM-treated pomace (Table 2), the lignin content for this sample was also the lowest among the five treated pomace samples. But the LA yield of 77.42% for the ABM-treated sample was remarkably lower than the AL-treated sample. We don’t have a clear explanation for this result now. Figure 8 summarized the final LA concentration and the LA productivity for all 6 pomace samples investigated in this study. If the final LA concentration and productivity were considered as the key parameters for pomace utilization, alkaline pretreatment with the effect of delignification showed clear superiority over other methods for this high-lignin pomace sample.
4 Conclusion
The recycling and utilization of grape pomace not only help to protect the environment but also provide an opportunity for the wine industry to generate extra revenue. The most commonly used pretreatment methods were systematically compared in this study. A mutant strain of L. plantarum with the capability of producing optical pure L-LA was successfully developed in this study, and it was applied in the microbial fermentation of grape pomace. The Cabernet Sauvignon grape pomace contains a relatively low amount of carbohydrates and a very high level of lignin. The alkaline pretreatment was more suitable for this grape pomace sample which gave a final LA concentration of 18.45 g/L and an LA yield of 96.26%. The LA concentration obtained in this study was relatively low for industrial purposes, high solid fermentation is suggested for further study with fed-batch fermentation as a possible process to increase the final titer of LA. If new routes were developed to selectively remove and collect the high lignin content in this pomace sample and convert it into value-added products, such as biochar, the overall recycling scheme would be more feasible.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YS and ZZ designed and performed experiments, and analyzed samples; BK, YL, XD, and CQ took part in the performance of experiments; SS wrote the manuscript; JL, RY, and LH joined in the discussion of experimental plans and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key R&D Program of China (2022YFD1300602, 2022YFC2104600); the National Natural Science Foundation of China (No. 32001422); the Innovative Research Team in University of Education and the Ministry of China (IRT_17R105); and the Earmarked Fund for CARS36; and the Chinese Universities Scientific Fund.
Conflict of interest
Author RY is employed by the Sino-French Joint Venture Dynasty Winery Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anastasiou, I., Ferlin, F., Viteritti, O., Santoro, S., and Vaccaro, L. (2021). Pd/C-catalyzed aerobic oxidative C–H alkenylation of arenes in γ-valerolactone (GVL). Mol. Catal.513, 111787. doi:10.1016/j.mcat.2021.111787
Arasaratnam, V., Senthuran, A., and Balasubramaniam, K. (1996). Supplementation of whey with glucose and different nitrogen sources for lactic acid production by Lactobacillus delbrueckii. Enzyme Microb. Technol.19 (7), 482–486. doi:10.1016/s0141-0229(95)00147-6
Araujo, D., Vilarinho, M., and Machado, A. (2019). Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Industrial Crops Prod.141, 111785. doi:10.1016/j.indcrop.2019.111785
Baranenko, D., Kolodyaznaya, V., and Broyko, Y. (2014). Effect of cold treatment on the amino acid composition of veal. Agron. Res.12 (3), 705–716.
Bayrak, E., and Büyükkileci, A. O. (2018). Utilization of white grape pomace for lactic acid production. GIDA/The J. FOOD43 (1), 129–138. doi:10.15237/gida.gd17088
Bordiga, M., Travaglia, F., and Locatelli, M. (2019). Valorisation of grape pomace: An approach that is increasingly reaching its maturity–a review. Int. J. Food Sci. Technol.54 (4), 933–942. doi:10.1111/ijfs.14118
Calinoiu, L.-F., Mitrea, L., Precup, G., Bindea, M., Rusu, B., Dulf, F. V., et al. (2017). Characterization of grape and apple peel wastes’ bioactive compounds and their increased bioavailability after exposure to thermal process. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol.74, 80–89. doi:10.15835/buasvmcn-fst:0028
Chen, L.-C., Guan, X., Wang, Q. K., Yang, Q. P., Zhang, W. D., and Wang, S. L. (2018). Effects of phenolic acids on soil nitrogen mineralization over successive rotations in Chinese fir plantations. J. For. Res.31 (1), 303–311. doi:10.1007/s11676-018-0842-z
Corbin, K. R., Hsieh, Y. S., Betts, N. S., Byrt, C. S., Henderson, M., Stork, J., et al. (2015). Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol.193, 76–83. doi:10.1016/j.biortech.2015.06.030
Deng, Q., Penner, M. H., and Zhao, Y. (2011). Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int.44 (9), 2712–2720. doi:10.1016/j.foodres.2011.05.026
Dong, X., Zhu, C. P., Huang, G. Q., and Xiao, J. X. (2021). Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochem.109, 37–45. doi:10.1016/j.procbio.2021.06.022
Drevelegka, I., and Goula, A. M. (2020). Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Processing-Process Intensif.149, 107845. doi:10.1016/j.cep.2020.107845
Guerra-Rivas, C., Gallardo, B., Mantecón, Á. R., del Álamo-Sanza, M., and Manso, T. (2017). Evaluation of grape pomace from red wine by-product as feed for sheep. J. Sci. Food Agric.97 (6), 1885–1893. doi:10.1002/jsfa.7991
Houghton, J., Weatherwax, S., and Ferrell, J. (2006). A research roadmap resulting from the biomass to biofuels workshop December 7–9 2005: Breaking the biological barriers to cellulosic ethanol. DOE SC/0095. Washington, DC: USDOE.
Hsu, T.-C., Guo, G. L., Chen, W. H., and Hwang, W. S. (2010). Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol.101 (13), 4907–4913. doi:10.1016/j.biortech.2009.10.009
Hugenholtz, J., and Kleerebezem, M. (1999). Metabolic engineering of lactic acid bacteria: Overview of the approaches and results of pathway rerouting involved in food fermentations. Curr. Opin. Biotechnol.10 (5), 492–497. doi:10.1016/s0958-1669(99)00016-6
Ilyas, T., Chowdhary, P., Chaurasia, D., Gnansounou, E., Pandey, A., and Chaturvedi, P. (2021). Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innovation23, 101592. doi:10.1016/j.eti.2021.101592
Khan, N., Fahad, S., Naushad, M., and Faisal, S. (2020). Grape production critical review in the world. SSRN 3595842, Available at: https://ssrn.com/abstract=3595842.
Li, J., Lai, Y., Zhu, X., Liao, Q., Xia, A., Huang, Y., et al. (2020). Pyrolysis kinetics and reaction mechanism of the electrode materials during the spent LiCoO2 batteries recovery process. J. Hazard. Mater.398, 122955. doi:10.1016/j.jhazmat.2020.122955
Li, J., Shi, S., Wang, Y., and Jiang, Z. (2021). Integrated production of optically pure L-lactic acid from paper mill sludge by simultaneous saccharification and co-fermentation (SSCF). Waste Manag.129, 35–46. doi:10.1016/j.wasman.2021.05.008
Lin, T. (2021). Analysis of comprehensive nutritional components of different grape seeds. J. Chin. Cereals Oils Assoc.36 (3), 59–65.
López-Gómez, J. P., Alexandri, M., Schneider, R., Latorre-Sánchez, M., Coll Lozano, C., and Venus, J. (2020). Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod.247, 119165. doi:10.1016/j.jclepro.2019.119165
Moset, V., Xavier, C. d. A. N., Feng, L., Wahid, R., and Møller, H. B. (2018). Combined low thermal alkali addition and mechanical pre-treatment to improve biogas yield from wheat straw. J. Clean. Prod.172, 1391–1398. doi:10.1016/j.jclepro.2017.10.173
Northup, R. R., and Mccoll, D. J. G. (1998). Polyphenols as regulators of plant-litter-soil interactions in northern California's pygmy forest: A positive feedback?Biogeochemistry42 (1-2), 189–220. doi:10.1023/a:1005991908504
Okano, K., Uematsu, G., Hama, S., Tanaka, T., Noda, H., Kondo, A., et al. (2018). Metabolic engineering of Lactobacillus plantarum for direct l-lactic acid production from raw corn starch. Biotechnol. J.13 (5), 1700517. doi:10.1002/biot.201700517
Pedras, B. M., Regalin, G., Sá-Nogueira, I., Simões, P., Paiva, A., and Barreiros, S. (2020). Fractionation of red wine grape pomace by subcritical water extraction/hydrolysis. J. Supercrit. Fluids160, 104793. doi:10.1016/j.supflu.2020.104793
Ping, L., Pizzi, A., Guo, Z. D., and Brosse, N. (2012). Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Industrial Crops Prod.40, 13–20. doi:10.1016/j.indcrop.2012.02.039
Saha, B. C., Iten, L. B., Cotta, M. A., and Wu, Y. V. (2005). Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem.40 (12), 3693–3700. doi:10.1016/j.procbio.2005.04.006
Sheehan, J. (2001). The road to bioethanol: A strategic perspective of the US department of energy's national ethanol program. ACS Publications.
Sirohi, R., Tarafdar, A., Singh, S., Negi, T., Gaur, V. K., Gnansounou, E., et al. (2020). Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol.314, 123771. doi:10.1016/j.biortech.2020.123771
Sluiter, A. (2008). Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced.1617, 1–16.
Sun, D., Lv, Z. W., Rao, J., Tian, R., Sun, S. N., and Peng, F. (2022). Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr. Polym.281, 119050. doi:10.1016/j.carbpol.2021.119050
Werpy, T., and Petersen, G. (2004). Top value added chemicals from biomass: Volume I--results of screening for potential candidates from sugars and synthesis gas. Golden, CO (US): National Renewable Energy Lab.
Yang, J., Gao, C., Yang, X., Su, Y., Shi, S., and Han, L. (2022). Effect of combined wet alkaline mechanical pretreatment on enzymatic hydrolysis of corn stover and its mechanism. Biotechnol. biofuels Bioprod.15 (1), 31–11. doi:10.1186/s13068-022-02130-0
Yi, X., Zhang, P., Sun, J., Tu, Y., Gao, Q., Zhang, J., et al. (2016). Engineering wild-type robust Pediococcus acidilactici strain for high titer L-and D-lactic acid production from corn stover feedstock. J. Biotechnol.217, 112–121. doi:10.1016/j.jbiotec.2015.11.014
Keywords: grape pomace, pretreatment, lactic acid, delignification, Cabernet Sauvignon
Citation: Shen Y, Kang B, Lu Y, Du X, Qin C, Li J, Zhao Z, Yu R, Shi S and Han L (2023) Production of optical pure L-lactic acid from Cabernet Sauvignon grape pomace by engineered Lactiplantibacillus plantarum. Front. Energy Res. 11:1228827. doi: 10.3389/fenrg.2023.1228827
Received: 25 May 2023; Accepted: 03 July 2023;
Published: 14 July 2023.
Edited by:
Jitendra Kumar Saini, Central University of Haryana, IndiaReviewed by:
Senthilkumar Sivaprakasam, Indian Institute of Technology Guwahati, IndiaLaura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2023 Shen, Kang, Lu, Du, Qin, Li, Zhao, Yu, Shi and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, amluZ2xpQGJ0YnUuZWR1LmNu; Suan Shi, c3VhbnNoaUBjYXUuZWR1LmNu
 Yuli Shen1
Yuli Shen1 Suan Shi
Suan Shi