- Stazione Zoologica Anton Dohrn, Naples, Italy
The common octopus (Octopus vulgaris Cuvier, 1797) is one of the most widely distributed species belonging to the genus Octopus as well as an important commercially harvested species and a model organism for behavioral biology of invertebrates. It has been described for the first time in the Mediterranean Sea but it is considered a cosmopolitan species inhabiting the temperate and tropical seas of the northern and southern hemispheres. In the last few years, several species previously considered as O. vulgaris have been recognized as new species, limiting the distributional range of “vulgaris” and reinforcing the thesis of a species complex. Where it is an important fishery resource, numerous studies have been conducted in order to define its genetic structure with the purpose of managing different stocks. However, many locations are still poorly investigated from this point of view and others are under taxonomic revision to exclude or confirm its occurrence. Here we provide a summary of the current status of knowledge on distribution and genetic structure in this species in the different oceanic regions.
From Populations to Species and Species Complexes
In its simplest form, a population can be defined as “a group of interbreeding individuals that exist together in time and space” (Hedrick, 2000). Several factors, called evolutionary processes, affect the genetic structure of a population leading to phenomena such as genetic divergence, local adaptation or extinction. In presence of high gene flow, populations lack of clear boundaries and form a continuous population, a condition known as panmixy. On the contrary, over a long time, isolated populations tend to diverge genetically up to not being able to interbreed: a new species is raised (Mayr, 1942). When the time of separation between two species is recent or when hybridization occurs among them, they tend to be well differentiated morphologically but not genetically (Shaffer and Thomson, 2007). Conversely, species can be well differentiated genetically, but not morphologically: this is when “cryptic species complexes” can arise (Bickford et al., 2007; Barley et al., 2013).
Within cephalopods, several “cryptic species complexes” are known (Anderson et al., 2011), especially among octopuses (Norman and Finn, 2001; Amor et al., 2014). One of the most investigated is exactly the O. vulgaris species complex. To date, more than 10 species were recognized in this complex (Norman, 2000), and only a few have been validated with molecular markers (Söller et al., 2000; Pérez-Losada et al., 2002). However, Voss et al. (1998) highlight that numerous “forms” or subspecies of O. vulgaris exist worldwide, although most of them lack of a description or a reference. Despite several authors consider the common octopus as a cosmopolitan species (Figure 1), Norman (2000) suggests that several populations, such as the ones from the Caribbean Sea, Japan and South Africa, are likely to be separated species because of the strong isolation and the different environment in which they live. Warnke et al. (2004) rejected this hypothesis and confirmed the presence of O. vulgaris in Japan using mitochondrial genes. More recently, Guerra et al. (2010) showed that the Japanese specimens cluster separately from the others. However, these conclusions deriving from mitochondrial data are not ultimate and need to be integrated with nuclear data too because speciation is not a clockwise process and sometimes recent speciation events have not reached monophyly yet. As outlined by Allcock et al. (2014), more analysis including more specimens and multiple genes should be performed.
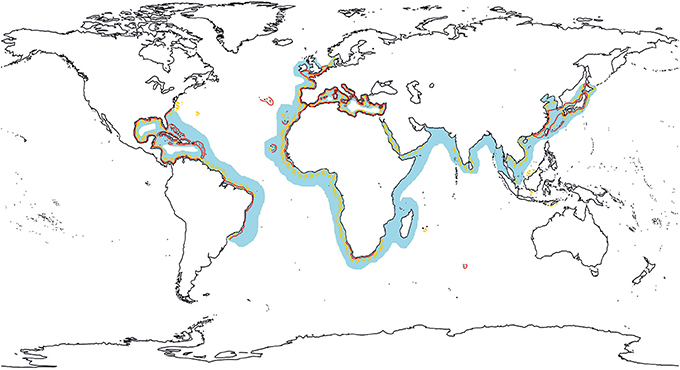
Figure 1. Distribution of O. vulgaris after Mangold (1983), Roper et al. (1984) and Norman et al. (2013), in orange, light blue and red respectively.
Current Knowledge on Distribution and Population Structure
Mediterranean and Black Sea
Together with the Eastern Atlantic Ocean, the Mediterranean region is considered to be one of the areas in the world where more information exist on cephalopods (Mangold, 1998). Here the common octopus is well known by the time of Aristotle, which provided its earliest written observations in the eastern Mediterranean (Mangold, 1983) and it has been intensively studied from the end of the eighteenth century to date. Despite the descriptions of Cuvier (1797) and Lamarck (1798), the holotype is missing and, as far as we know, a neotype has been designated in 1998 from the Catalonian Sea off Banyuls-sur-Mer and the species is being redescribed (Mangold and Hochberg, 1991). It is found in the entire basin, where it finds suitable environmental and ecological conditions, but it is absent in the Marmara and Black Sea, as any other cephalopod species, due to low salinity in the upper waters and reduced gas exchange in the deeper ones (Torchio, 1968; Mangold and Boletzky, 1988).
The first investigation on the genetic structure of Octopus vulgaris in the Mediterranean basin has been conducted by Maltagliati et al. (2002) and Casu et al. (2002) using allozymes and a single microsatellite locus respectively (Table 1). Both studies focused mainly on the Western and central Mediterranean with just one sample in the Eastern and one in the Atlantic (Casu et al., 2002) and found no isolation-by-distance among populations. Furthermore, the allozyme analysis highlighted a breaking point between western and eastern Mediterranean populations which is not found with microsatellites, probably due to the different resolution of the two markers utilized and to the small representativeness of a single microsatellite locus.
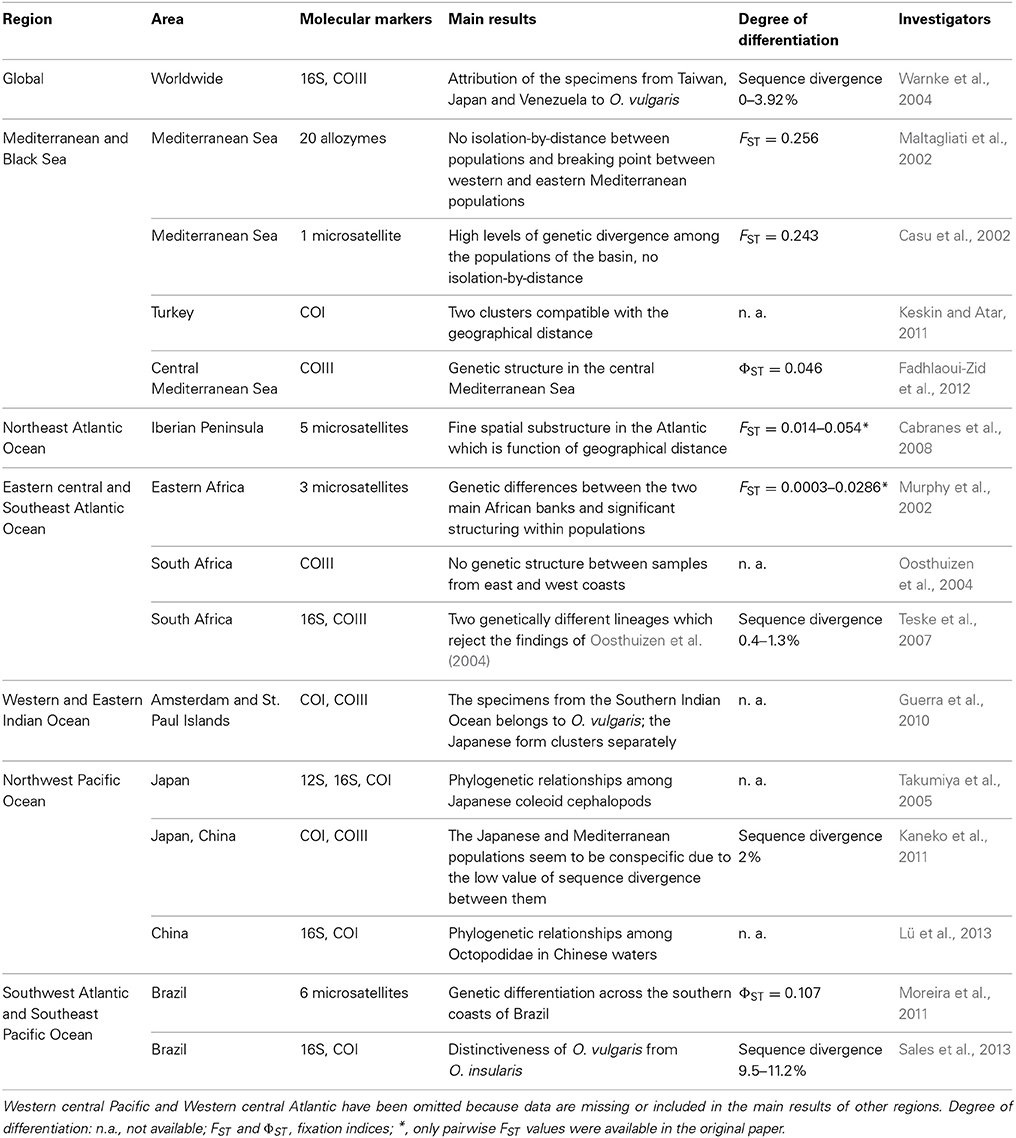
Table 1. Resume of the main genetic studies on population structure, phylogeography and phylogenetic relationships in O. vulgaris on a global scale.
A significant genetic structure has been found in several populations from the central Mediterranean Sea (Strait of Sicily) using mitochondrial markers (Fadhlaoui-Zid et al., 2012; Table 1). The authors also mention a significant genetic divergence between western and eastern samples, which could be interpreted as a breaking point between Western and Eastern Mediterranean basin.
The records of O. vulgaris in the Levantine Basin (east of 23°E) are less common in the literature compared to the ones from the Western and Central Basin and generally come from Turkish or Israeli waters (Adam, 1967; Ruby and Knudsen, 1972). A recent work by Keskin and Atar (2011) investigated the genetic structure of the common octopus along the Turkish coasts using mitochondrial markers (Table 1) and found two clusters compatible with geographical distance, one in the eastern side and the other one in the southern side of the country.
In summary, although it is evident that the use of different molecular markers with different resolution power leads to different scenarios about population structure, the topographic fragmentation of the Mediterranean Basin and the different ecological conditions which occur in the western, central and eastern part should account for a certain degree of population structure (Mona et al., 2014).
Northeast Atlantic Ocean
The Northeast Atlantic region stretches from the coast of Greenland eastward to the North Sea, and from the North Pole southward to the Straits of Gibraltar, including open ocean islands such as the Azores. In this region, O. vulgaris reaches its northern distributional limit, being very common (e.g., along the Iberian Peninsula), rare (English Channel) or even absent (North Sea) in different regions. Interestingly, Hoyle (1886) during the “Challenger Expedition” reports this species from the Scandinavian Region and not from the Lusitanian region. On the contrary, Rees (1950) considers it as “a Lusitanian member of our fauna” and reports its occurrence in the English Channel both on British and French coasts up to the German coasts. He also discusses about its abundance during the 1899–1900 years due to a warmer climate in the previous years and hypothesizes that the octopus is probably not able to maintain a breeding population on the English side of the Channel, and so its occurrence is due to an immigrant population from the south. Several records are also reported from Helgoland, in the German part of the North Sea (Hertling, 1936; Rees and Lumby, 1954 both in Jaeckel, 1957) but they are not corroborated by more recent data and might constitute sporadic individuals carried beyond their normal range.
Quite different is, however, the situation in the Iberian Peninsula. Here the occurrence of O. vulgaris is unquestioned and information about the population structure is available. Analyzing six populations around the Iberian Peninsula and Canary Islands, Cabranes et al. (2008) found high levels of microsatellite genetic variability and a fine spatial substructure in the Atlantic, which is function of geographical distance (Table 1). Furthermore, genetic divergence was also observed between Atlantic and nearby Mediterranean populations, stressing the role of the Gibraltar strait as a genetic break in octopus, as previously showed for many marine taxa (Patarnello et al., 2007). The analysis conducted by Casu et al. (2002) mentioned earlier did not record such a break, probably as a consequence of the use of a single microsatellite locus.
Several reports confirm the presence of the species in the oceanic islands of Azores (Joubin, 1920; Schmidt, 1939), but no genetic studies exist to assess the connectivity between islands and coastal populations.
Eastern Central and Southeast Atlantic Ocean
Ranging from the Strait of Gibraltar to the South African coasts, this region sustains one of the most productive O. vulgaris fishery stock, the Sahara Bank, and studies performed here provided substantial contributions to our knowledge of the biology of the species (Hatanaka, 1979; Mangold, 1983). Its occurrence along the coasts of this region appears in several expeditions' report (Hoyle, 1886; Adam, 1952, 1962; Voss, 1962) and is confirmed in some recent studies which allowed to define the genetic structure in this area. In north-western Africa, two fishery banks occur and they are genetically distinct (Murphy et al., 2002). Furthermore, the authors also hypothesize the existence of a fine spatial structure in this area because samples collected from a research cruise in the same region did not cluster with any of the two banks.
In South Africa the situation is more complex. A first study by Oosthuizen et al. (2004) showed no distinction between the samples collected on the eastern and western coasts using the COIII region. On the other hand, reanalyzing these samples with different molecular markers (16S and COI), Teske et al. (2007) found two different lineages: one containing all the analyzed populations from South Africa and another one characterized by samples from Durban (see Table 1). This divergent lineage is interpreted by the authors either as a recent introduction by ships' ballast water or as a long-established lineage disappearing in most of its southern African distribution, but only a larger sampling plan can resolve this controversy.
Western and Eastern Indian Ocean
Our knowledge about the occurrence of O. vulgaris in this region is limited to the Red Sea and the St. Paul and Amsterdam Islands, because the specimens from the Andaman's and Sri Lanka analyzed by Goodrich (1896) actually belongs to O. cyanea according to Adam (1939). Anyway, also in the Red Sea the situation is not controversy free. Despite numerous expeditions and reports, O. vulgaris is specifically reported in the area only by Hoyle (1886); other authors such as Wülker (1920) and Adam (1942) just list it based on previous reports, and it was not found in following expeditions (Adam, 1955, 1960). Torchio (1968) considers the species absent in the Red Sea and questions about its occurrence in the Indo-Pacific region. The most recent record from the Red Sea refers to the comparative study between specimens from the Mediterranean (Alexandria) and the Red Sea (Suez) based on the assessment of morphological characters (Riad and Gabr, 2007). In general, due to the scarcity of records, it is possible to assume that the species is rare in the Red Sea, where it could have migrated from the Mediterranean Sea (i.e., anti-Lessepsian migrant).
Different is the situation for the specimens from the St. Paul and Amsterdam Islands in the southern Indian Ocean (Guerra et al., 2010; Table 1). According to morphological and genetic analysis, these animals match O. vulgaris sensu stricto (from the Mediterranean), even if molecular data rely only on two mitochondrial genes. Anyway, up to now and to new findings, it can be considered the only effective evidence for this region.
Western Central Pacific Ocean
In this region, which extends from the south of Vietnam up to the northern coasts of Australia including the Malay Archipelago, just historical data of the “Challenger Expedition” exist (Hoyle, 1886). The author reports O. vulgaris specimens from what he calls “the Indo-Malayan region” but since such region was intended to extend from the Red Sea eastward up to the Malay Archipelago, it is possible that the specimens where collected in the present western and eastern Indian Ocean region (see paragraph above). If so, the occurrence of the species in this Western central Pacific Ocean region is questioned.
Northwest Pacific Ocean
In this area, the common octopus is reported from the Chinese waters northwards up to Tsugaru Strait, even if it is more common in central and southern Japan (Nesis, 1987). In respect to the populations from China and Korea, only the Japanese ones have been studied for a long time under several aspects of their biology (Sasaki, 1929; Tanaka, 1958), probably because of their commercial value. Despite Norman (2000) argues about this Japanese form as the most likely to be a valid species due to its geographical isolation with the Atlantic and South African ones, Kaneko et al. (2011) consider it conspecific with the Mediterranean populations on the basis of the low value of sequence divergence of mitochondrial markers. Other studies in this area focus on the phylogenetic relationships between coleoid cephalopods (Takumiya et al., 2005) or within the Octopodidae (Lü et al., 2013) but just at a local scale, providing no information about the degree of connectivity between different populations (Table 1). The development of a new set of microsatellite loci by Zuo et al. (2012) from samples in Chinese waters might be a starting point for this kind of investigations.
Western Central Atlantic Ocean
The western-central Atlantic Ocean region embraces the Atlantic Ocean section from Cape Hatteras to the regions of South America within the Northern Hemisphere, including the Caribbean Sea and the oceanic islands. Despite the numerous contributions of some of the major cephalopod workers such as d'Orbigny and Verrill in the nineteenth century and Adam, Pickford and Voss in the twentieth century systematic problems remain. Here this species (or similar species) is distributed along the coasts of United States (Vecchione et al., 1989; Whitaker et al., 1991) and Bermuda (Voss, 1960), in the Gulf of Mexico and Caribbean Sea (Pickford, 1945; Voss, 1955; Judkins, 2009), in Central America (Hochberg and Camacho-García, 2009) and in Venezuelan waters (Arocha and Urosa, 1982). In some regions of Central and northern South America it is known just from few specimens (Pickford, 1945). One of the most evident problems in this geographic region is the abundance of synonymous and uncertain species due to the resemblance of many specimens collected there with the Atlantic-Mediterranean “form” or to the lack of a holotype to be used as reference. Consequently, the western Atlantic “form” of O. vulgaris is referred to as O. americanus despite no holotype exists for this entity, as Octopus cf. vulgaris, or just as O. vulgaris. Pickford (1945) raised the issue if the American octopus is conspecific with O. vulgaris “Lam.” and, after a morphological examination, she concluded that “even in respect to the hectocotylus, the American vulgaris is identical with its European counterpart.” She also reported geographical variations in specimens from Bermuda and coastal waters of United States and little concrete differences with museum specimens labeled as Octopus rugosus.
Up to date, no genetic studies have been conducted in this area to clarify the relationships among the different forms of O. vulgaris. Moreover no genetic structure studies exist. The development of microsatellite loci in O. maya (Juárez et al., 2013), one of the most harvested octopus species in the Gulf of Mexico, and the following analysis of population structure could stimulate similar analysis also in the common octopus.
Southwest Atlantic and Southeast Pacific Ocean
The knowledge of O. vulgaris in the southwest Atlantic is limited to Brazil, where it constitutes the most important fishery resource. After the description of a new species (O. insularis) from the northeastern coasts of Brazil by morphological and genetic characters (Leite et al., 2008), new genetic data limit the distributional range of O. vulgaris to southern Brazil (downstream of Rio de Janeiro) and several localities in the northern and western part (Sales et al., 2013). In southern Brazil, Moreira et al. (2011), using microsatellite loci, highlighted the occurrence of four genetic populations with no significant evidence for isolation by distance, although several bordering populations were the less divergent (Table 1).
No records exist about the occurrence of O. vulgaris in Argentina and the southeast Pacific Ocean, where it is probably replaced by O. mimus, but a deeper investigation in countries such as Peru, Ecuador and Colombia is still needed.
The Problem of O. rugosus
An important step for the definition of the distributional range in O. vulgaris is the assessment of the taxonomic status of O. rugosus Bosc (1792). Robson (1929) considers it as a distinct species based on the rough, finely granular skin and shorter arms and hectocotylus compared with O. vulgaris but Pickford (1945) and Adam (1952) refer to it as synonymous of O. vulgaris. Anyway, its occurrence is recorded from the Red Sea (Adam, 1942), the western and eastern Indian region (Goodrich, 1896; Adam, 1939, 1942), the Caribbean island of Bonaire (Adam, 1937) and along the African, Japanese, Australian and Atlantic coasts (Adam, 1942). If subsequent analysis will prove that this species is actually a synonymous of O. vulgaris, all the localities in which it has been reported might be included in the distributional range of the common octopus.
Conclusions
This review aimed to provide a general picture of the distribution and genetic structure in Octopus vulgaris on a global scale, highlighting pitfalls and clues, which could represent the basis for following investigations. The amount of data available in literature is huge and often incomplete, so here we just selected the main and most useful information. In general, few data support the occurrence of O. vulgaris in several regions and they are quite doubtful and controversial, making the range hypothesized by Mangold closer to the reality in respect to the one by Roper et al. (Figure 1). Regarding the genetic structure, some regions have been investigated more than others, but almost all analysis are concordant in finding genetic structure among populations (Table 1), which could derive from low dispersal and enhanced homing of adults, although the potential dispersal of larvae remains to be addressed. Hence, several questions are at the moment unsolved: (i) is O. vulgaris a real cosmopolitan species or the hypothesis of species complex is correct? (ii) is there a fine population structure as consequence of the limited adult dispersal or do paralarval meso-scale migrations connect nearby populations? (iii) are these migrations affected by water mass circulation? The answers to all of these questions will contribute to a major comprehension of the ecology of this species and of its biogeographical patterns, with strong impact in fishery and biodiversity management. The FAO statistics reveal that there are real problems in the identification of the cephalopod species caught by the fisheries, with O. vulgaris being the only octopus identified to species level (Boyle and Rodhouse, 2005). We know that this can be not always correct. In this context, genetic approaches will constitute a useful tool to investigate biodiversity, assign the catches to the species level and define the stocks in order to prevent their overexploitation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors thank the Stazione Zoologica Anton Dohrn of Naples and the MIUR Italian Flagship project RITMARE for partially funding the research. Daniele De Luca is PhD student in Environmental and Evolutionary Biology (curriculum Animal Biology) at Sapienza University of Rome.
References
Adam, W. (1952). Résultats scientifiques des expéditions océanographiques belges dans les eaux cotieres africaines de l'Atlantique Sud (1948–1949), Céphalopodes. Bull. Mus. R. Hist. Nat. Belgique 3, 1–142.
Adam, W. (1955). Cephalopodes. Résultats scientifiques des Campagnes de la Calypso, I. Campagnes 1951–1952 en Mer Rouge. Ann. Inst. Océan. 30, 185–194.
Adam, W. (1962). Céphalopodes de l'archipel du Cap-Vert, de l'Angola et du Mozambique. Mem. Junta Invest. Ultram. 33, 8–64.
Allcock, A. L., Lindgren, A., and Strugnell, J. M. (2014). The contribution of molecular data to our understanding of cephalopod evolution and systematics: a review. J. Nat. Hist. doi: 10.1080/00222933.2013.825342. [Epub ahead of print].
Amor, M. D., Norman, M. D., Cameron, H. E., and Strugnell, J. M. (2014). Allopatric speciation within a cryptic species complex of Australasian octopuses. PLoS ONE 9:e98982. doi: 10.1371/journal.pone.0098982
Anderson, F. E., Engelke, R., Jarrett, K., Valinassab, T., Mohamed, K. S., Asokan, P. K., et al. (2011). Phylogeny of the Sepia pharaonis species complex (Cephalopoda: Sepiida) based on analyses of mitochondrial and nuclear DNA sequence data. J. Mollus. Stud. 77, 65–75. doi: 10.1093/mollus/eyq034
Arocha, F., and Urosa, L. J. (1982). Cefalópodos del género Octopus en el área nororiental de Venezuela. Bol. Inst. Oceanogr. Venez. Univ. Oriente 21, 167–189.
Barley, A. J., White, J., Diesmos, A. C., and Brown, R. M. (2013). The challenge of species delimitation at the extremes: diversification without morphological change in Philippine sun skinks. Evolution 67, 3556–3572. doi: 10.1111/evo.12219
Bickford, D., Lohman, D. J., Sodhi, N. S., Ng, P. K., Meier, R., Winker, K., et al. (2007). Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22, 148–155. doi: 10.1016/j.tree.2006.11.004
Boyle, P., and Rodhouse, P. (2005). Cephalopods: Ecology and Fisheries. Oxford: Blackwell Science. doi: 10.1002/9780470995310
Cabranes, C., Fernandez-Rueda, P., and Martínez, J. L. (2008). Genetic structure of Octopus vulgaris around the Iberian Peninsula and Canary Islands as indicated by microsatellite DNA variation. ICES J. Mar. Sci. 65, 12–16. doi: 10.1093/icesjms/fsm178
Casu, M., Maltagliati, F., Meloni, M., Casu, D., Cossu, P., Binelli, G., et al. (2002). Genetic structure of Octopus vulgaris (Mollusca, Cephalopoda) from the Mediterranean Sea as revealed by a microsatellite locus. Ital. J. Zool. 69, 295–300. doi: 10.1080/11250000209356472
Fadhlaoui-Zid, K., Knottweis, L., Aurelle, D., Nafkha, C., Ezzedine, S., Fiorentino, F., et al. (2012). Genetic structure of Octopus vulgaris (Cephalopoda, Octopodidae) in the central Mediterranean Sea inferred from the mitochondrial COIII gene. C.R. Biol. 335, 625–636. doi: 10.1016/j.crvi.2012.10.004
Goodrich, E. S. (1896). Report on a collection of Cephalopoda from the Calcutta Museum. Trans. Linn. Soc. Lond. 2nd Ser. Zool. 7, 1–24. doi: 10.1111/j.1096-3642.1896.tb00399a.x
Guerra, Á., Roura, Á., González, Á. F., Pascual, S., Cherel, Y., and Pérez-Losada, M. (2010). Morphological and genetic evidence that Octopus vulgaris Cuvier, 1797 inhabits Amsterdam and Saint Paul Islands (southern Indian Ocean). ICES J. Mar. Sci. 67, 1401–1407. doi: 10.1093/icesjms/fsq040
Hatanaka, H. (1979). Studies on the fisheries biology of common octopus off the northwest coast of Africa. Bull. Far Seas Fish. Res. Lab. 17, 13–124.
Hertling, H. (1936). Mitteilungen über Todaropsis eblanae (Ball), Octopus vulgaris L. und Eledone cirrosa (Lam.) aus der Nordsee. Zool. Anz. 114, 289–296.
Hochberg, F. G., and Camacho-García, Y. E. (2009). “Squids and Octopuses,” in Marine Biodiversity of Costa Rica, Central America. Netherlands: Springer.
Hoyle, W. E. (1886). Report on the Cephalopoda collected by H. M. S. Challenger during the years 1873–76. Zool. Chall. Exp. 16, 1–245.
Jaeckel, S. H. (1957). Kopffüsser (Tintenfische). Die Neue Brehm-Bücherei, Vol. 190. Lutherstadt Wittenberg: A. Ziemsen Verlag.
Joubin, L. (1920). Céphalopodes provenant des campagnes de la Princesse-Alice (1898-1910) (3e Série). Résult. Camp. Scient. Prince Albert I 54, 1–95.
Juárez, O. E., Rosas, C., Arena, L., Enríquez, L., Camarena, F., McKeown, N., et al. (2013). Characterization of microsatellite loci developed for the Mexican four-eyed octopus Octopus maya. Conserv. Genet. Resour. 5, 803–805. doi: 10.1007/s12686-013-9912-x
Judkins, H. L. (2009). Cephalopods of the Broad Caribbean: Distribution, Abundance, and Ecological Importance. St. Petersburg: Dissertation, College of Marine Science.
Kaneko, N., Kubodera, T., and Iguchis, A. (2011). Taxonomic study of shallow-water octopuses (Cephalopoda: Octopodidae) in Japan and adjacent waters using mitochondrial genes with perspectives on octopus DNA Barcoding. Malacologia 54, 97–108. doi: 10.4002/040.054.0102
Keskin, M., and Atar, H. H. (2011). Genetic divergence of Octopus vulgaris species in the eastern Mediterranean. Biochem. Syst. Ecol. 39, 277–282. doi: 10.1016/j.bse.2011.08.015
Leite, T. S., Haimovici, M., Molina, W., and Warnke, K. (2008). Morphological and genetic description of Octopus insularis, a new cryptic species in the Octopus vulgaris complex (Cephalopoda: Octopodidae) from the tropical southwestern Atlantic. J. Mollus. Stud. 74, 63–74. doi: 10.1093/mollus/eym050
Lü, Z. M., Cui, W. T., Liu, L. Q., Li, H. M., and Wu, C. W. (2013). Phylogenetic relationships among Octopodidae species in coastal waters of China inferred from two mitochondrial DNA gene sequences. Genet. Mol. Res. 12, 3755–3765. doi: 10.4238/2013.September.19.7
Maltagliati, F., Belcari, P., Casu, D., Casu, M., Sartor, P., Vargiu, G., et al. (2002). Allozyme genetic variability and gene flow in Octopus vulgaris (Cephalopoda, Octopodidae) from the Mediterranean Sea. Bull. Mar. Sci. 71, 473–486.
Mangold, K. (1983). “Octopus vulgaris,” in Cephalopod Life Cycles, Vol. I, ed P. R. Boyle (London: Academic Press), 335–364.
Mangold, K. (1998). “The Octopodinae from the Eastern Atlantic Ocean and the Mediterranean Sea,” in Systematics and Biogeography of Cephalopods, Vol. II, eds N. A. Voss, M. Vecchione, R. B. Toll, and M. J. Sweeney (Washington: Smithsonian Institution Press), 521–528.
Mangold, K., and Boletzky, S. V. (1988). “Mediterranean cephalopod fauna,” in The Mollusca Paleontology and Neontology of Cephalopods, Vol. XII, eds K. M. Wilbur, M. R. Clarke, and E. R. Trueman (London: Academic Press), 315–330. doi: 10.1016/B978-0-12-751412-3.50025-5
Mangold, K., and Hochberg, F. G. (1991). Defining the genus Octopus: redescription of Octopus vulgaris. Bull. Mar. Sci. 49, 665.
Mona, S., Ray, N., Arenas, M., and Excoffier, L. (2014). Genetic consequences of habitat fragmentation during a range expansion. Heredity 112, 291–299. doi: 10.1038/hdy.2013.105
Moreira, A. A., Tomás, A. R. G., and Hilsdorf, A. W. S. (2011). Evidence for genetic differentiation of Octopus vulgaris (Mollusca, Cephalopoda) fishery populations from the southern coast of Brazil as revealed by microsatellites. J. Exp. Mar. Biol. Ecol. 407, 34–40. doi: 10.1016/j.jembe.2011.06.029
Murphy, J. M., Balguerías, E., Key, L. N., and Boyle, P. R. (2002). Microsatellite DNA markers discriminate between two Octopus vulgaris (Cephalopoda: Octopoda) fisheries along the northwest African coast. Bull. Mar. Sci. 71, 545–553.
Nesis, K. (1987). Cephalopods of the World: Squids, Cuttlefishes, Octopuses and Allies. Neptune City, NJ: TFH Publications.
Norman, M. D., Finn, J. K., and Hochberg, F. G. (2013). “Family Octopodidae,” in Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Volume 3. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes, Vol. 3, eds P. Jereb, C. F. E. Roper, M. D. Norman, and J. K. Finn (Rome: FAO), 36–215.
Norman, M. D., and Finn, J. (2001). Revision of the Octopus horridus species-group, including erection of a new subgenus and description of two member species from the Great Barrier Reef, Australia. Invertebr. Syst. 15, 13–35. doi: 10.1071/IT99018
Oosthuizen, A., Jiwaji, M., and Shaw, P. (2004). Genetic analysis of the Octopus vulgaris population on the coast of South Africa. S. Afr. J. Sci. 100, 603–607.
Patarnello, T., Volckaert, F. A. M. J., and Castilho, R. (2007). Pillars of Hercules: is the Atlantic-Mediterranean transition a phylogeographical break? Mol. Ecol. 16, 4426–4444. doi: 10.1111/j.1365-294X.2007.03477.x
Pérez-Losada, M., Guerra, A., Carvalho, G. R., Sanjuan, A., and Shaw, P. W. (2002). Extensive population subdivision of the cuttlefish Sepia officinalis (Mollusca: Cephalopoda) around the Iberian Peninsula indicated by microsatellite DNA variation. Heredity 89, 417–424. doi: 10.1038/sj.hdy.6800160
Pickford, G. E. (1945). Le poulpe Américain: a study of the littoral Octopoda of the Western Atlantic. Trans. Conn. Acad. Arts Sci. 36, 701–811.
Rees, W. J. (1950). The distribution of Octopus vulgaris Lamarck in British waters. J. Mar. Biol. Assoc. U.K. 29, 361–378. doi: 10.1017/S0025315400055417
Rees, W. J., and Lumby, J. R. (1954). The abundance of Octopus in the English Channel. J. Mar. Biol. Assoc. U.K. 33, 515–536. doi: 10.1017/S0025315400008511
Riad, R., and Gabr, H. R. (2007). Comparative study on Octopus vulgaris (Cuvier, 1797) from the Mediterranean and Red Sea coasts of Egypt. Egypt. J. Aquat. Res. 33, 140–146.
Robson, G. C. (1929). A Monograph of the Recent Cephalopoda Based on the Collections in the British Museum (Natural History). Part I, Octopodinae. London: The Trustees of the British museum.
Roper, C. F. E., Sweeney, M. J., and Nauen, C. E. (1984). FAO Species Catalogue. Vol. 3. Cephalopods of the World. An Annotated and Illustrated Catalogue of Species of Interest to Fisheries. Rome: FAO Fisheries Synopsis 125.
Ruby, G., and Knudsen, J. (1972). Cephalopoda from the eastern Mediterranean. Israel J. Zool. 21, 83–97.
Sales, J. B. D. L., Rego, P. S. D., Hilsdorf, A. W. S., Moreira, A. A., Haimovici, M., Tomás, A. R., et al. (2013). Phylogeographical features of Octopus vulgaris and Octopus insularis in the Southeastern Atlantic Based on the Analysis of Mitochondrial Markers. J. Shellfish Res. 32, 325–339. doi: 10.2983/035.032.0211
Sasaki, M. (1929). A monograph of the dibranchiate cephalopods of the Japanese and adjacent waters. J. College Agriculture 20, 1–357.
Schmidt, J. (1939). Report on the Danish Oceanographical Expeditions 1908-1910 to the Mediterranean and Adjacent Seas. Copenhagen: A.F. Høst.
Shaffer, H. B., and Thomson, R. C. (2007). Delimiting species in recent radiations. Syst. Biol. 56, 896–906. doi: 10.1080/10635150701772563
Söller, R., Warnke, K., Saint-Paul, U., and Blohm, D. (2000). Sequence divergence of mitochondrial DNA indicates cryptic biodiversity in Octopus vulgaris and supports the taxonomic distinctiveness of Octopus mimus (Cephalopoda: Octopodidae). Mar. Biol. 136, 29–35. doi: 10.1007/s002270050004
Takumiya, M., Kobayashi, M., Tsuneki, K., and Furuya, H. (2005). Phylogenetic relationships among Octopodidae species in coastal waters of China inferred from two mitochondrial DNA gene sequences. Zool. Sci. 22, 147–155. doi: 10.2108/zsj.22.147
Tanaka, J. (1958). On the stock of Octopus (Octopus) vulgaris Lamarck, on the East Coast of Boso Peninsula, Japan. Bull. Jap. Soc. Sci. Fish 24, 601–607. doi: 10.2331/suisan.24.601
Teske, P. R., Oosthuizen, A., Papadopoulos, I., and Barker, N. P. (2007). Phylogeographic structure of Octopus vulgaris in South Africa revisited: identification of a second lineage near Durban harbour. Mar. Biol. 151, 2119–2122. doi: 10.1007/s00227-007-0644-x
Torchio, M. (1968). Elenco dei cefalopodi del Mediterraneo con considerazioni biogeografiche ed ecologiche. Ann. Mus. Civ. St. Nat. Genova 77, 257–269.
Vecchione, M., Roper, C. F. E., and Sweeney, M. J. (1989). Marine flora and fauna of the eastern United States Mollusca: Cephalopoda. NOAA Tech. Rep. NMFS 73, 1–23.
Voss, G. L. (1955). The cephalopoda obtained by the Harvard-Havana expedition off the coast of Cuba in 1938-39. Bull. Mar. Sci. 5, 81–115.
Voss, G. L. (1962). South African cephalopods. Trans. Roy. Soc. S. Afr. 36, 245–272. doi: 10.1080/00359196209519051
Voss, N. A., Vecchione, M., Toll, R. B., and Sweeney, M. J. (1998). Systematics and Biogeography of Cephalopods. Vol. II. Washington: Smithsonian Institution Press.
Warnke, K., Söller, R., Blohm, D., and Saint-Paul, U. (2004). A new look at geographic and phylogenetic relationships within the species group surrounding Octopus vulgaris (Mollusca, Cephalopoda): indications of very wide distribution from mitochondrial DNA sequences. J. Zool. Syst. Evol. Res. 42, 306–312. doi: 10.1111/j.1439-0469.2004.00277.x
Whitaker, J. D., DeLancey, L. B., and Jenkins, J. E. (1991). Aspects of the biology and fishery potential for Octopus vulgaris off the coast of South Carolina. Bull. Mar. Sci. 49, 482–493.
Keywords: Octopus vulgaris, Cephalopoda, genetic structure, species complex, phylogenetics
Citation: De Luca D, Catanese G, Procaccini G and Fiorito G (2014) An integration of historical records and genetic data to the assessment of global distribution and population structure in Octopus vulgaris. Front. Ecol. Evol. 2:55. doi: 10.3389/fevo.2014.00055
Received: 10 April 2014; Accepted: 13 August 2014;
Published online: 02 September 2014.
Edited by:
Melanie April Murphy, University of Wyoming, USAReviewed by:
Miguel Arenas, Consejo Superior de Investigaciones Científicas, SpainAthanasios Exadactylos, University of Thessaly, Greece
Octavio Salgueiro Paulo, Universidade de Lisboa, Portugal
Copyright © 2014 De Luca, Catanese, Procaccini and Fiorito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele De Luca, Stazione Zoologica Anton Dohrn, Villa Comunale, Naples 80121, Italy e-mail:ZGFuaWVsZS5kZWx1Y2FAc3puLml0
 Daniele De Luca
Daniele De Luca Gaetano Catanese
Gaetano Catanese Gabriele Procaccini
Gabriele Procaccini Graziano Fiorito
Graziano Fiorito