- Laboratory of Apiculture and Social Insects, School of Life Sciences, University of Sussex, Brighton, UK
A honey bee colony has been likened to an oil company. Some members of the company or colony prospect for valuable liquid resources. When these are discovered other group members can be recruited to exploit the resource. The recruitment of nestmates to a specific location where there is a patch of flowers should change the economics of scouting, that is, the search for new resource patches. In particular, communication is predicted to make scouting at longer distances worthwhile because a profitable resource patch, once discovered, will enhance the foraging not only of the discoverer but also of nestmates that can be directed to the patch. By virtue of having large colonies and dance communication, honey bees are predicted to be able to profitably scout, and hence forage, at greater distances from the nest than either solitary bees or social bees without communication. We test this hypothesis by first examining existing data on foraging distance to evaluate whether honey bees do indeed forage at greater distances than other bees given their body size. Second, we present a simple cost-benefit analysis of scouting which indicates that communication causes longer range scouting to be more profitable. Overall, our analyses are supportive, but not conclusive, that honey bees forage further than would be expected given their size and that the waggle dance is a cause of the honey bee's exceptional foraging range.
Introduction
Honey bee workers, Apis mellifera, have a great foraging range. This is known from a variety of types of evidence, including training bees to syrup feeders, observing workers of rare body color on flowers at known distances from their hives, honey production in relation to distance to key forage sources, and the decoding of waggle dances (von Frisch, 1967; Visscher and Seeley, 1982; Beekman and Ratnieks, 2000; Ratnieks, 2007). Overall, it appears that the maximum foraging distance is in the region of 14 km (Beekman and Ratnieks, 2000). However, these long distances are not typical. Mean honey bee foraging distances range from a fraction of a kilometer to several kilometers (Visscher and Seeley, 1982; Beekman and Ratnieks, 2000; Couvillon et al., 2014a). Honey bees are economically sensitive foragers and prefer more rewarding food sources, such as those with higher sugar concentration (Seeley, 1995). Foraging further from the hive will incur greater costs (e.g., time, energy, risk) and distant food sources are likely used when high quality food patches are scarce or unavailable locally (Beekman and Ratnieks, 2000; Couvillon et al., 2014a).
Before a bee can forage on a patch of flowers, that patch must first be discovered. In the honey bee there is division of labor between scout and non-scout foragers (Seeley, 1995). The location of a rewarding flower patch discovered by a scout bee, or being worked by any forager bee, can be communicated to nestmate workers via the waggle dance (von Frisch, 1967). Honey bee foraging has been likened to an oil company in which some members of the company or colony prospect for valuable liquid resources (Ratnieks, 2002). When these are discovered other group members can be recruited to exploit the resource. In solitary bees or social bees that do not communicate the locations of food sources to nestmates, such as bumble bees, each foraging bee must scout for its own flower patches. Bumble bee foragers invest time in “minoring,” visiting a range of flower species, rather than just a single “major” species (Heinrich, 1976, 1979). This is a form of individual scouting and monitoring of foraging opportunities.
The ecological benefits of the waggle dance have been shown to vary with season and habitat. Through experimentally denying honey bees the ability to communicate the directional information in the dance, Sherman and Visscher (2002) showed that dance communication increased foraging success only at certain times of the year. Dance communication has also been shown to increase food collection in some habitats, namely those with clustered resources, higher flower species richness and number of flowers per patch, but not other habitats (Dornhaus and Chittka, 2004; Donaldson-Matasci and Dornhaus, 2012). Models have been slightly less conservative in predicting the benefits of dance communication. Dornhaus et al. (2006) and Schürch and Grüter (2014) both found that dance communication should be beneficial under a wide range of resource densities, but especially when resources were sparsely distributed. Beekman and Lew (2008) meanwhile, found that dancing was beneficial when resources were hard to find independently, i.e., they were small and distant, and dancing became detrimental when resources were both large and nearby. While the precise circumstances under which dance communication improves colony foraging, and the extent of these benefits, may be difficult to determine, what seems clear is that benefits depend on the distribution and abundance of floral resources in the environment.
The ability of honey bees, and also of other social insects including many ants (Wilson, 1971; Czaczkes et al., 2015) to direct nestmates to resource patches should change the economics of scouting, which is the search for new resource patches. In particular, Ratnieks (2002) proposed that communication should make scouting at longer distances from the nest more worthwhile. This is because a profitable resource patch, once discovered, will enhance the foraging not only of the discoverer but also of any nestmates that can be directed to it. This will benefit the colony as a whole, and will also increase the inclusive fitness of the worker who made the communication signal and those that received and acted on that signal. In other words, communication should change the optimal scouting strategy because it changes the cost to benefit ratio of scouting. By virtue of having both an effective means of communicating resource locations via the waggle dance and large colonies with many potential recruits, honey bees are predicted to be able to profitably scout, and hence forage, at greater distances from the nest than expected given their body size.
Here we develop the logic behind this hypothesis by formalizing it as a simple benefit-cost model, in which scouting at greater distance is more costly but can detect more high-quality resource patches because a larger area is surveyed. In support of the hypothesis, we show that there are foraging conditions under which a communicating bee will benefit more from far scouting while a non-communicating bee will benefit more from near scouting. In addition, we examine published data on the foraging range of different bee species to evaluate whether honey bees, Apis spp., have longer foraging ranges than other bees without the waggle dance. Unfortunately, the available data do not allow a conclusive test to be successfully made. In particular, it seems that A. mellifera may be the only bee species in which the existing data are sufficient to determine maximum foraging distance as opposed to actual foraging distances, which, from our knowledge of honey bee foraging distances, will often underestimate the maximum. Nevertheless, the data are compatible, and even suggestive, of greater foraging range in honey bees than expected from their body size.
Comparative Analysis of Bee Foraging Distances
Bee foraging distances have been determined using a wide variety of methods. In homing experiments, bees are captured and then released at various distances from the nest and the proportion of returning individuals is recorded (Janzen, 1971). In feeder-training experiments, foragers are trained to artificial syrup feeders near the nest, which are then incrementally moved to greater distances from the nest until foragers cease to visit (van Nieuwstadt and Iraheta, 1996). Tracking individuals with harmonic radar involves fitting bees with transponders and tracking them in real-time using radar (Osborne et al., 1999). Mark-recapture methods involve marking bees at the nest and locating them again in the field or vice-versa (Dramstad, 1996). In dance decoding, which is only possible with bees in the genus Apis, dances made by foragers when back at the nest are decoded to give the direction and distance vector to the food source from the nest (Visscher and Seeley, 1982; Dyer and Seeley, 1991; Beekman and Ratnieks, 2000; Couvillon et al., 2014b).
Each method has advantages and disadvantages for determining maximum foraging distance. For example, training honey bees to syrup feeders at long distances is difficult, especially when natural forage is abundant (Lindauer, 1948; FR personal experience). Beutler (1951) was able to train bees to 3 km while Lindauer (1948) did so to 12 km, but only under exceptional environmental circumstances: warm, sunny, and settled weather after autumn frosts had killed most flowers (von Frisch, 1967). Lindauer was also an exceptionally patient and talented experimenter (Seeley et al., 2002). Harmonic radar has the advantage of allowing insects to be tracked in real-time, but cannot detect insects behind obstacles such as hedges or buildings (Osborne et al., 1999). In practice, its range is limited to approximately 600 m, which means that it is not well-suited to determining maximum distances if they are greater than this.
Waggle dance decoding can be used to determine the foraging distances of thousands of bees, and can give a picture of how foraging distance changes seasonally (e.g., Couvillon et al., 2014a). If carried out in seasons of nectar dearth, some dances that are close to the maximum will be included, given that the maximum in the honey bee is also known from other methods and concurs with that found from dance decoding (Ratnieks, 2007). However, dance decoding may slightly underestimate the maximum as the bees trained to syrup feeders by Lindauer foraged to 12 km but did not dance beyond 11 km (von Frisch, 1967; Ratnieks, 2007). Decoding the distance information of a dance becomes increasingly imprecise the greater the distance indicated (Schürch et al., 2013), meaning that overestimation can also occur. Perhaps the biggest disadvantage of the waggle dance is that it is only made by the genus Apis, including A. mellifera and approximately eight additional species (Oldroyd and Wongsri, 2006), several of which have been studied using dance decoding (Dyer and Seeley, 1991).
Bees are capable of flying great distances. For example, a female of the large euglossine bee Euplusia surinamensis was recorded returning from 23 km in a homing experiment (Janzen, 1971) which suggests but does not prove great foraging range. Most individual bees however, typically forage much closer to the nest than the recorded maximum for their species (Zurbuchen et al., 2010; Couvillon et al., 2014a,b). This suggests that long distance foraging is only a profitable strategy under certain ecological conditions.
There is a well-established positive relationship between body size and foraging distance in birds and mammals (Haskell et al., 2002). For bees, a meta-analysis of 96 records for 62 species by Greenleaf et al. (2007) showed that larger bees also foraged at greater maximum distances. Separate analyses were made for distances obtained through homing and feeder-training experiments, and the latter included data from four honey bee species: A. cerana, A. dorsata, A. florea, and A. mellifera (Figure 1). However, the authors did not analyze waggle dance data as this was not available from other species. Most data points fell close to the regression line, but two for A. mellifera have large positive residuals (i.e., a greater foraging range that expected given body size). Since A. mellifera is better studied than the other bees analyzed, it is likely that this resulted in more extreme distances being recorded and hence the positive residuals. Indeed, two other data points for A. mellifera lie on the regression line. A suggestion from these data, but not a clear conclusion, is that A. mellifera is able to forage at greater distances than predicted by its body size.
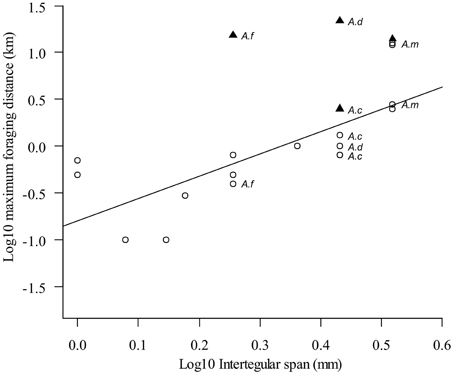
Figure 1. Larger bees are capable of foraging at greater distances, but dance data indicates that honey bees forage further than predicted from their body size. White circles indicate maximum ranges from feeder training experiments analyzed in Greenleaf et al. (2007). Black triangles indicate maximum ranges from dances to natural foraging sites from Dyer and Seeley (1991) for A. cerana, A. dorsata, and A. florea and Beekman and Ratnieks (2000) for A. mellifera. Dance data indicate that all members of the genus Apis forage further than predicted by their body size. Reproduction of Figure 1b from Greenleaf et al. (2007) with permission.
What about the other honey bee species? All have waggle dances and so, if the communication hypothesis is correct, should also have greater than expected foraging distances. However, their data points fall close to the regression line. The feeder-training data for A. cerana, A. dorsata, and A. florea originated from Dyer and Seeley (1991), who studied colonies in a tropical rainforest in Thailand at the time of year when natural forage was at its peak. This will likely underestimate maximum foraging range for two reasons. First, we know from studies in both temperate and tropical ecosystems that honey bees travel furthest when resources in the landscape are most scarce (Schneider and McNally, 1993; Couvillon et al., 2014b,c). Second, training bees to feeders is difficult, especially when natural forage is abundant (Lindauer, 1948; FR personal experience).
Dyer and Seeley (1991) also recorded waggle dances to natural forage locations during their study. These dances indicated that some bees were foraging further than their feeder-trained bees. They were able to train A. dorsata to feeders at a maximum distance of 1 km, and bees danced for feeders only up to 900 m away. However, some dances for natural forage indicated distances of 21 km. In Figure 1, we plotted the foraging distances indicated by the foragers' dances alongside the data from Greenleaf et al. (2007). All four honey bee species now appear as positive residuals. For example, a bee the size of A. mellifera would be predicted to have a maximum foraging range of approximately 2.5 km, but the dance data indicates an actual foraging range of 14 km (from Beekman and Ratnieks, 2000). With the exception of A. cerana, all forage at least 10 km further than predicted by the regression line in Greenleaf et al. (2007). This suggests that all four honey bee species are able to forage further than predicted by their body size.
While there is compelling evidence for impressive maximum foraging distance in honey bees, Apis, we need to be wary about drawing a firm conclusion that honey bees forage at greater distances than expected from their body size. We have compared data gathered using two different methods, feeder-training and dance-decoding, which may not give comparable results for maximum foraging distance unless extensive data are available, as for A. mellifera. Greenleaf et al. (2007) also compared their predicted values from feeder-training data against observed measures gathered using alternate techniques such as mark-recapture and molecular methods. They found that their model using feeder-training data underestimated foraging distance vs. most other techniques.
All the non-Apis species shown in Figure 1 are stingless bees (Apidae: Meliponinae), which also live in eusocial colonies but do not perform the waggle dance. However, stingless bees do have mechanisms for recruiting nestmates to specific locations (Lindauer and Kerr, 1958; Nieh and Roubik, 1995; Jarau et al., 2000; Nieh, 2004). These mechanisms, while less well-understood and perhaps less sophisticated than the waggle dance, could enhance the ability of these species to forage profitably at longer distances.
One of the methods used by stingless bees, trail pheromone marking (Lindauer and Kerr, 1958; Nieh et al., 2004), is unlikely to work at long distance as it would be energetically expensive and difficult to implement due to the volatile nature of pheromones. However, sound pulses, which are made by some Melipona (Nieh and Roubik, 1998), may function similarly to the waggle dance (Nieh et al., 2003). Unfortunately, compared with the wealth of studies measuring foraging distance in honey bees, the literature is less comprehensive for stingless bees. In one study, M. mandacaia foragers were trained to feeders up to 2.1 km away (Kuhn-Neto et al., 2009). This is almost the exact distance predicted by its body size, but the distance was based on visits to feeders, not natural forage.
Cost-benefit Analysis of Scouting Distance
Our argument is shown in Figure 2, which shows the benefit minus the cost for the maximum scouting distance for bees in a colony. Scouting at greater distances from the nest should have benefits as the chance to locate a high quality food patch is increased, although additional costs are incurred through increased time, energy expenditure and mortality risk. The benefit for the communicating bee is multiplied however, as scouts can recruit nestmates to the flower patches discovered. More importantly, the distance at which the benefit minus the cost is maximized is greater in the communicating bee than the non-communicating bee as is the maximum distance at which foraging remains profitable. This is because longer distance scouting will result in the discovery of more high quality patches to which recruits can be directed.
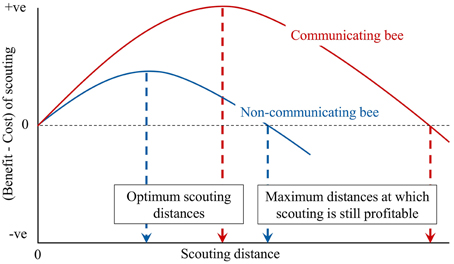
Figure 2. Benefit minus cost for scouting distance for scout bees in a colony. In a communicating bee, such as a honey bee, the benefit is increased as scouts can recruit nestmates to high quality flower patches. More importantly, the distance at which benefit minus cost is maximized is greater in a communicating bee because longer distance scouting results in the discovery of more high quality patches to which recruits can be directed. In a non-communicating species, all bees are scouts as they have to find their own foraging patch. In the honey bee approximately 10% of the foragers are scouts.
Figure 3 shows scenarios that make this argument clearer. Here, two maximum scouting distances are considered, in which the “far” maximum is arbitrarily set at twice the “near” maximum. There are two types of flower patches in the environment, “high” and “low” quality. In Figure 3B, the rarer high quality resources (solid flowers) are quite common and so will be easy to find. In this situation near scouting will discover enough high quality patches to allow all or most of the colony's foragers to be directed to high quality patches. However, in Figure 3A the high quality patches are scarce. In this situation far scouting will enable more high quality patches to be discovered so that a greater proportion of the colony's foragers can be directed to high quality patches.
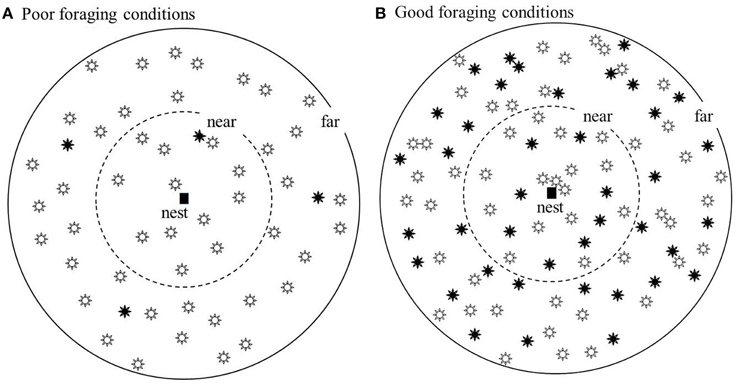
Figure 3. Showing conditions where high quality resource patches, filled flowers, are scarce (A) or common (B); low quality resource patches are shown as open flowers. In (B), high quality patches are sufficiently common that enough to satisfy the colony can be found within the “near” scouting distance. In (A) but not (B) scouting to the “far” distance is needed to find enough to satisfy the colony's foragers.
The two environmental scenarios shown in Figure 3 reflect differences in foraging availability that may occur at a single location in different seasons, with different overall flower abundance (Couvillon et al., 2014a,b). In some seasons longer range foraging may be more important than in others. Indeed, in some seasons waggle dance communication does not enhance overall colony foraging performance, but seems to be most valuable in seasons when resources are scarce (Sherman and Visscher, 2002).
Discussion
Overall, our cost-benefit analysis shows how communication could increase the maximum distance at which bees should scout for flower patches and our comparative analysis provides empirical support for this. However, in carrying out this research we have been struck by the difficulty in making more than modest progress in either area. In particular, the data needed to go further seem not to be available, whether this is to build a detailed cost-benefit model of optimal scouting (and hence foraging) distances or to make a fair analysis of maximum foraging distance as a function of body size and communication ability.
Honey bee colonies simultaneously exploit multiple resources in the landscape (Seeley, 1995; Beekman et al., 2004). Locating nectar sources in the landscape is probably not that hard. However, locating high quality patches is likely far more difficult especially in habitats or during times of year when total available forage is low (Figure 3A). Between them, scouts will locate and advertise many resource patches. Scouts are more likely to dance for high quality patches (Seeley, 1995), and nestmates are more likely to be recruited by dances to the more profitable resources (Seeley et al., 1991). Recruits can thus bring back greater rewards on average than scouts (Seeley and Visscher, 1988). A colony whose workers scout at greater distances will discover more flower patches, including more high quality patches, than a colony in which scouting is at lesser distances. The colony can therefore, exploit the best resources in the landscape while ignoring the poor ones (Beekman and Lew, 2008). This will especially be the case in species with large colonies, as scouting at shorter distances will result in the same resource being discovered multiple times and so will not provide additional opportunities to other workers in the colony via communication.
Our cost-benefit analysis follows the advice of the late Maynard Smith (1998, 2002), of our Department, who was of the opinion that “any theory to explain the complicated activities of organisms must always be simple.” The cost-benefit analysis provides a scenario where communication will favor longer distance scouting and supports the previous conjecture to this effect (Ratnieks, 2002). This conclusion is based on what would appear to be sound assumption, namely that scouting at longer distance increases the chance of locating high quality resources.
This conclusion will not satisfy biologists who would like to be told the distances honey bees would scout at, were they unable to communicate via the waggle dance vs. able to do this. However, to make a more complete cost-benefit analysis of this would require data that would be hard to obtain. Among other things, we would need to know the sizes and distributions of resource patches and the costs of locating them. Indeed, there are many additional parameters involved, including the probability that a recruit will find the advertized resource patch as a function of distance to the nest (e.g., Schürch and Grüter, 2014). Our model also treats foragers in a binary fashion as either scouts or recruits. The situation in nature is more complex with foragers existing in several more dynamic states (Biesmeijer and de Vries, 2001; Biesmeijer and Seeley, 2005; Beekman et al., 2007).
The large numbers of workers in honey bee colonies probably has an influence on the benefits of the waggle dance to colony foraging. All things being equal, larger colonies will make long distance foraging more profitable, as there are a greater number of potential recruits. There is some evidence for this, as Beekman et al. (2004) found that while both small and large colonies of honey bees foraged further in the summer than spring, large colonies traveled further in the summer than did small ones. Large and small colonies exploited a similar number of patches, so rather than a large colony exploiting more patches, the data suggest that they may only exploit the best ones but must travel further to do so.
In the comparative analysis the main problem is that maximum foraging distances are imperfectly known. Indeed, it may well be the case that they are only well-known in A. mellifera. In this species several lines of evidence all give foraging maximum foraging distances of >10 km. In other Apis species, the maximum distances determined by training foragers to syrup feeders are markedly less than in A. mellifera. This almost certainly reflects the fact that A. mellifera is well-studied and more researchers have trained it to feeders than other Apis species. Furthermore, the A. mellifera distances were determined under environmental conditions that were suitable for determining the maximum distance, and by a highly skilled researcher. In our lab, we have trained honey bees to syrup feeders numerous times and have never been able to get them to visit feeders at great distances. In August, which is a month when foraging conditions are relatively poor (Couvillon et al., 2014c), the maximum was 1.28 km (Schürch et al., 2013), one tenth of what Lindauer achieved but close to what Dyer and Seeley (1991) achieved with A. dorsata.
On balance, we must accept the fact that the data for a full comparative analysis of bee foraging ranges in relation to the waggle dance are lacking. Ideally we would have actual maximum foraging ranges to natural food sources for several Apis species, non-Apis social bees known to communicate food locations (e.g., several stingless bees), social bees which do not communicate food locations (e.g., bumble bees), and solitary bees. There would be problems however, with collecting this data. As detailed here and in Greenleaf et al. (2007), there is no perfect method applicable to all bee species. If such a method did exist, the effort in collecting the data would still be huge. Additionally, if such a data set did exist it would have phylogenetic constraints, as there are no non-Apis species which make waggle dances (although similar mechanisms exist in stingless bees), and no Apis species which do not.
Another option is an experimental approach. Honey bee colonies can be manipulated so that they cannot communicate the direction element of the dance (e.g., Sherman and Visscher, 2002; Donaldson-Matasci and Dornhaus, 2012). This involves removing gravity and other factors (such as a directional light source) which might be used as a directional cue. This causes the now disorientated bees to dance in random directions. Under these conditions, the colony would forage at the locations found by bees scouting individually, without the quality filtering provided by the waggle dance in which only the best patches are advertised (Grüter et al., 2010). We predict that when waggle dance information is used to find flower patches, the average foraging distance will be greater in seasons of forage dearth due to the increased use of higher quality but more distant patches.
Despite the gaps which exist in the data, we can be sure that honey bees do forage at very great distances. The data are also suggestive that this is more than expected from their body size when compared to other bees. Our model indicates that the waggle dance should permit longer distance foraging under environmental conditions that can readily exist. However, our progress is modest and we cannot be conclusive in stating that the waggle dance is what makes long range foraging possible. Since von Frisch's early work, research on the waggle dance has continued to give insights into the behavior and ecology of the honey bee. The dance is now being studied with applications to environmental management, such as using honey bees as indicators of forage quality in the landscape (Couvillon et al., 2014b). Our analysis adds a small piece to this picture. However, we remain hopeful that the hypothesis will be more thoroughly tested when additional data allow a more complete comparative analysis and perhaps, also, through experiments that manipulate dance communication. Not only is the waggle dance an extraordinary behavior in itself, but it allows the honey bee to accomplish extraordinary things.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Christoph Grüter for advice on feeder-training distances, and Claire Kremen for allowing us to reproduce Figure 1. We also thank two anonymous reviewers for their constructive comments. KS's Ph.D. is funded by the National Environment Research Council (Grant Number: NE/K501347/1) and School of Life Sciences at the University of Sussex.
References
Beekman, M., and Lew, J. B. (2008). Foraging in honeybees—when does it pay to dance? Behav. Ecol. 19, 255–261. doi: 10.1093/beheco/arm117
Beekman, M., and Ratnieks, F. L. W. (2000). Long range foraging in the honey bee. Funct. Ecol. 14, 490–496 doi: 10.1046/j.1365-2435.2000.00443.x
Beekman, M., Gilchrist, A. L., Duncan, M., and Sumpter, D. J. T. (2007). What makes a honeybee scout? Behav. Ecol. Sociobiol. 61, 985–995. doi: 10.1007/s00265-006-0331-9
Beekman, M., Sumpter, D. J. T., Seraphides, N., and Ratnieks, F. L. W. (2004). Comparing foraging behaviour of small and large honey-bee colonies by decoding waggle dances made by foragers. Funct. Ecol. 18, 829–835. doi: 10.1111/j.0269-8463.2004.00924.x
Biesmeijer, J. C., and de Vries, H. (2001). Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 49, 89–99. doi: 10.1007/s002650000289
Biesmeijer, J. C., and Seeley, T. D. (2005). The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59, 133–142. doi: 10.1007/s00265-005-0019-6
Couvillon, M. J., Fensome, K. A., Quah, S. K., and Schürch, R. (2014c). Summertime blues: August foraging leaves honey bees empty-handed. Commun. Integr. Biol. 7:e28821. doi: 10.4161/cib.28821
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Couvillon, M. J., Schürch, R., and Ratnieks, F. L. W. (2014a). Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS ONE 9:e93495. doi: 10.1371/journal.pone.0093495
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Couvillon, M. J., Schürch, R., and Ratnieks, F. L. W. (2014b). Dancing bees communicate a foraging preference for rural lands in high-level agri-environment schemes. Curr. Biol. 24, 1212–1215. doi: 10.1016/j.cub.2014.03.072
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Czaczkes, T. J., Grüter, C., and Ratnieks, F. L. W. (2015). Pheromone trails: an integrative view of their role in colony organization. Annu. Rev. Entomol. 60, 30.1–30.19. doi: 10.1146/annurev-ento-010814-020627
Donaldson-Matasci, M. C., and Dornhaus, A. (2012). How habitat affects the benefits of communication in collectively foraging honey bees. Behav. Ecol. Sociobiol. 66, 583–592. doi: 10.1007/s00265-011-1306-z
Dornhaus, A., and Chittka, L. (2004). Why do honey bees dance? Behav. Ecol. Sociobiol. 55, 395–401. doi: 10.1007/s00265-003-0726-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dornhaus, A., Klügl, F., Oechslein, C., Puppe, F., and Chittka, L. (2006). Benefits of recruitment in honey bees: effects of ecology and colony size in an individual-based model. Behav. Ecol. 17, 336–344. doi: 10.1093/beheco/arj036
Dramstad, W. E. (1996). Do bumblebees (Hymenoptera: Apidae) really forage close to their nests? J. Insect Behav. 9, 163–182. doi: 10.1007/BF02213863
Dyer, F. C., and Seeley, T. D. (1991). Dance dialects and foraging range in three Asian honey bee species. Behav. Ecol. Sociobiol. 28, 227–233. doi: 10.1007/BF00175094
Greenleaf, S. S., Williams, N. M., Winfree, R., and Kremen, C. (2007). Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596 doi: 10.1007/s00442-007-0752-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grüter, C., Leadbeater, E., and Ratnieks, F. L. W. (2010). Social learning: the importance of copying others. Curr. Biol. 20, R683–R685. doi: 10.1016/j.cub.2010.06.052
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haskell, J. P., Ritchie, M. E., and Olff, H. (2002). Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 418, 527–530. doi: 10.1038/nature00840
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heinrich, B. (1976). The foraging specializations of individual bumblebees. Ecol. Monogr. 46, 105–128. doi: 10.2307/1942246
Heinrich, B. (1979). “Majoring” and “minoring” by foraging bumblebees, Bombus vagans: an experimental analysis. Ecology 60, 245–255. doi: 10.2307/1937652
Janzen, D. H. (1971). Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205. doi: 10.1126/science.171.3967.203
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jarau, S., Hrncir, M., Zucchi, R., and Barth, F. G. (2000). Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31, 12. doi: 10.1051/apido:2000108
Kuhn-Neto, B., Contrera, F. A. L., Castro, M. S., and Nieh, J. C. (2009). Long distance foraging and recruitment by a stingless bee, Melipona mandacaia. Apidologie 40, 472–480. doi: 10.1051/apido/2009007
Lindauer, M. (1948). Über die einwirkung von duft- und geschmacksstoffen sowie anderer faktoren auf die tänze der bienen. Z. Vergl. Physiol. 31, 348–412. doi: 10.1007/BF00297951
Lindauer, M., and Kerr, W. E. (1958). Die gegenseitige verständigung bei den stachellosen Bienen, Z. Vergl. Physiol. 41, 405–434. doi: 10.1007/BF00344263
Maynard Smith, J. (2002). “Equations of life,” in It must be Beautiful: Great Equations in Modern Science, eds G. Farmelo (London: Granta books), 161–179.
Nieh, J. C. (2004). Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35, 24. doi: 10.1051/apido:2004007
Nieh, J. C., and Roubik, D. W. (1995). A stingless bee (Melipona panamica) indicates food location without using a scent trail. Behav. Ecol. Sociobiol. 37, 63–70. doi: 10.1007/BF00173900
Nieh, J. C., and Roubik, D. W. (1998). Potential mechanisms for the communication of height and distance by a stingless bee, Melipona panamica. Behav. Ecol. Sociobiol. 43, 387–399. doi: 10.1007/s002650050506
Nieh, J. C., Contrera, F. A. L., Rangel, J., and Imperatriz-Fonseca, V. L. (2003). Effect of food location and quality on recruitment sounds and success in two stingless bees, Melipona mandacaia and Melipona bicolor. Behav. Ecol. Sociobiol. 55, 87–94. doi: 10.1007/s00265-003-0680-6
Nieh, J. C., Contrera, F. A. L., Yoon, R. R., Barreto, L. S., and Imperatriz-Fonseca, V. L. (2004). Polarized short odor-trail recruitment communication by a stingless bee, Trigona spinipes. Behav. Ecol. Sociobiol. 56, 435–448. doi: 10.1007/s00265-004-0804-7
Oldroyd, B. P., and Wongsri, S. (2006). Asian Honey Bees: Biology, Conservation and Human Interactions. Cambridge, MA: Harvard University Press, 14.
Osborne, J. L., Clark, S. J., Morris, R. J., Williams, I. H., Riley, J. R., Smith, A. D., et al. (1999). A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J. Appl. Ecol. 36, 519–533. doi: 10.1046/j.1365-2664.1999.00428.x
Ratnieks, F. L. W. (2002). Big businesses: honey bee colonies and oil companies. Beekeepers Q. 69, 22–24.
Schneider, S. S., and McNally, L. C. (1993). Spatial foraging patterns and colony energy status in the African honey bee, Apis mellifera scutellata. J. Insect Behav. 6, 195–210. doi: 10.1007/BF01051504
Schürch, R., Couvillon, M. J., Burns, D. D., Tasman, K., Waxman, D., and Ratnieks, F. L. (2013). Incorporating variability in honey bee waggle dance decoding improves the mapping of communicated resource locations. J. Comp. Physiol. A 199, 1143–1152. doi: 10.1007/s00359-013-0860-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schürch, R., and Grüter, C. (2014). Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS ONE 9:e104660. doi: 10.1371/journal.pone.0104660
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Seeley, T. D. (1995). The Wisdom of the Hive. Cambridge, MA: Harvard University Press, 34–36, 85–88.
Seeley, T. D., Camazine, S., and Sneyd, J. (1991). Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 28, 277–290. doi: 10.1007/BF00175101
Seeley, T. D., Kühnholz, S., and Seeley, R. H. (2002). An early chapter in behavioral physiology and sociobiology: the science of Martin Lindauer. J. Comp. Physiol. A 188, 439–453. doi: 10.1007/s00359-002-0318-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Seeley, T. D., and Visscher, P. K. (1988). Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav. Ecol. Sociobiol. 22, 229–237. doi: 10.1007/BF00299837
Sherman, G., and Visscher, P. K. (2002). Honeybee colonies achieve fitness through dancing. Nature 419, 920–922. doi: 10.1038/nature01127
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Nieuwstadt, M. G. L., and Iraheta, C. E. R. (1996). Relation between size and foraging range in stingless bees (Apidae, Meliponinae). Apidologie 27, 10. doi: 10.1051/apido:19960404
Visscher, P. K., and Seeley, T. D. (1982). Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 63, 1790–1801. doi: 10.2307/1940121
von Frisch, K. (1967). The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press, 17–18.
Keywords: waggle dance, foraging distance, honey bee, central-place forager, foraging ecology
Citation: Ratnieks FLW and Shackleton K (2015) Does the waggle dance help honey bees to forage at greater distances than expected for their body size? Front. Ecol. Evol. 3:31. doi: 10.3389/fevo.2015.00031
Received: 07 January 2015; Accepted: 09 March 2015;
Published: 01 April 2015.
Edited by:
Madeleine Beekman, University of Sydney, AustraliaReviewed by:
Ben Oldroyd, University of Sydney, AustraliaMichael Lee Smith, Cornell University, USA
Copyright © 2015 Ratnieks and Shackleton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle Shackleton, Laboratory of Apiculture and Social Insects, School of Life Sciences, University of Sussex, Biology Road, Brighton, East Sussex BN1 9QG, UKay5zaGFja2xldG9uQHN1c3NleC5hYy51aw==
 Francis L. W. Ratnieks
Francis L. W. Ratnieks Kyle Shackleton
Kyle Shackleton