- Department of Evolution, Ecology and Organismal Biology, The Ohio State University, Columbus, OH, USA
Genome reduction has been widely studied in obligate intracellular bacterial mutualists of insects because they have, in comparison to closely-related, nonhost-associated bacteria, extremely small genomes. Pentatomid stinkbugs also maintain bacterial symbionts, yet they are extracellular, residing within host-derived crypts, and are transmitted to offspring outside of the host's tissues, which exposes them to the external environment. In this review, we explore how the multiphasic lifestyle of stinkbug symbionts (e.g., on the surfaces of eggs in various matrices during transmission and inside host-derived tissues during much of the host's life), in contrast with the solely intracellular lifestyle of many insect endosymbionts, may impact their genome's architecture, size and content. Furthermore, we demonstrate how additional stinkbug symbiont genomes are needed to more fully explore these questions and the potential value of the stinkbug-symbiont system in understanding genome evolution and reduction in the absence of intracellularity.
Introduction
Insects spanning many species-rich groups, including aphids, cicadas, cockroaches, and whiteflies, maintain mutualisms with intracellular bacteria that are essential to their growth and development (Moran et al., 2008). Typical characteristics of these bacterial partners are that they are not cultivable by classical methods, maternally transmitted to offspring (with one reported exception; Watanabe et al., 2014), and have highly reduced, A+T% biased genomes (Moran and Bennett, 2014). Among the most likely contributors to this latter phenotype of obligate endosymbiotic lifestyles are (1) the oft-observed absence of genes encoding DNA repair and recombination functions in endosymbiont genomes (Wernegreen, 2002); (2) relaxed purifying selection due to the stable intracellular conditions (Nikoh et al., 2011); (3) repeated vertical transmission of small, clonal populations with limited-to-no opportunities for gene exchange with other bacteria (Wernegreen, 2002; Batut et al., 2014) and (4) loss of genes not essential for a strictly intracellular lifestyle (Mira et al., 2001), many of which can be >4500 nt in length and their loss can contribute significantly to genome size shrinkage (Kenyon and Sabree, 2014).
The abundance of genomes for bacteria occupying a wide array of habitats facilitates comparative analyses that can reveal habitat-specific genomic characteristics. Given genomic evolutionary trends observed with intracellular insect symbionts, we are interested in examining if similar characteristics of an obligate host-bacterial mutualism would be observed in insects that harbor symbionts in specialized tissues but transmit them to offspring by alternative modes of inheritance (Hosokawa et al., 2005, 2012a,b; Prado and Almeida, 2009a; Bansal et al., 2014; Bistolas et al., 2014). Phytophagous stinkbugs are useful exemplars in that they harbor bacterial symbionts in modified sections of their midgut, called crypts or caeca, and employ a variety of extracellular intergenerational transmission strategies. In this mini-review, we will detail what is known about the genomic architecture and content of stinkbug bacterial symbionts in the context of alternative intergenerational transmission strategies.
Who Are the Stinkbugs?
“Stinkbug” is the common name given to members of the family Pentatomidae (Hemiptera) due to the production of noxious secretions from abdominal glands. However, the name has been applied to members of the Pentatomoidea superfamily (including Cydnidae, Acanthosomatidae, Scutelleridae, Plataspidae, Urostylidae, Parastrachiidae, and Tessaratomidae) and even to members of the more general infraorder Pentatomorpha (including members of the superfamilies Lygaeoidea, Coreoidea, and Pyrrhocoroidea). Symbionts from members of these groups have been demonstrated to undergo genome reduction (Hosokawa et al., 2010; Nikoh et al., 2011; Kenyon et al., 2015). The present mini-review will focus on the primary extracellular gammaproteobacterial gut symbionts of the Pentatomoidea and mention more distant members for comparative purposes.
Transmission Strategies
The means for reliable transgenerational transmission is necessary for the evolution of stable host-symbiont mutualisms. Stinkbug symbionts are not transmitted to offspring during oogenesis, as observed in obligate intracellular symbionts (e.g., aphid-Buchnera, cockroach-Blattabacterium, carpenter ant-Blochmannia), but rather they are deposited either on or proximal to the surfaces of eggs, outside of the insect, and await their consumption by newly emerged nymphs to complete their transmission. While intracellular symbionts have shown to be housed in specialized bacteriomes (Moran et al., 2008) located inside or adjacent to the reproductive system where they have access to nascent oocytes (Matsuura et al., 2012), stinkbug symbionts are restricted to the posterior section of the midgut (Prado and Almeida, 2009a). As the gammaproteobacterial primary symbionts have not been reliably detected in the ovaries (see Matsuura et al., 2014 for suggestive evidence) of female pentatomids, the only way for symbionts to be acquired by each generation is through infection of midgut tissues following their consumption by nymphs.
Horizontal (e.g., between nest mates) or vertical (e.g., mother to offspring) transmission of gut symbionts has been observed in insect orders including the Blattodea (Ohkuma, 2008) and Hymenoptera (Anderson et al., 2012) in the form of trophallaxis (exchange of gastric contents) between individuals (Sabree et al., 2012). This method can minimize exposure of symbionts, namely strict anaerobes, to the environment and ensure receipt of viable microbes. However, some or all elements of sociality (e.g., gregariousness, multigenerational colonies, maternal care, brood care) are also necessary but not present in many insect groups. While some stinkbug species are regarded as sub-social for their display of behaviors such as gregariousness and alarm pheromones (Eurydema rugosa, E. pulchra, and Nezara viridula Megalotomus quinquespinosus, Alydus eurinus, A. pilosulus, Dysdercus intermedius; Bell and Cardé, 1984), maternal egg guarding and food provisioning (Adomerus triguttulus, Sehirus cinctus, Canthophorus niveimarginatus, Parastrachia japonensis; Filippi et al., 2009) and paternal egg guarding (Lopadusa augur and Edessa nigropunctata; Requena et al., 2010), gut symbiont transmission through trophallaxis has been recorded only in Brachypelta atterima (Hosokawa et al., 2012a), and Pyrrhocoris apterus (Kaltenpoth et al., 2009).
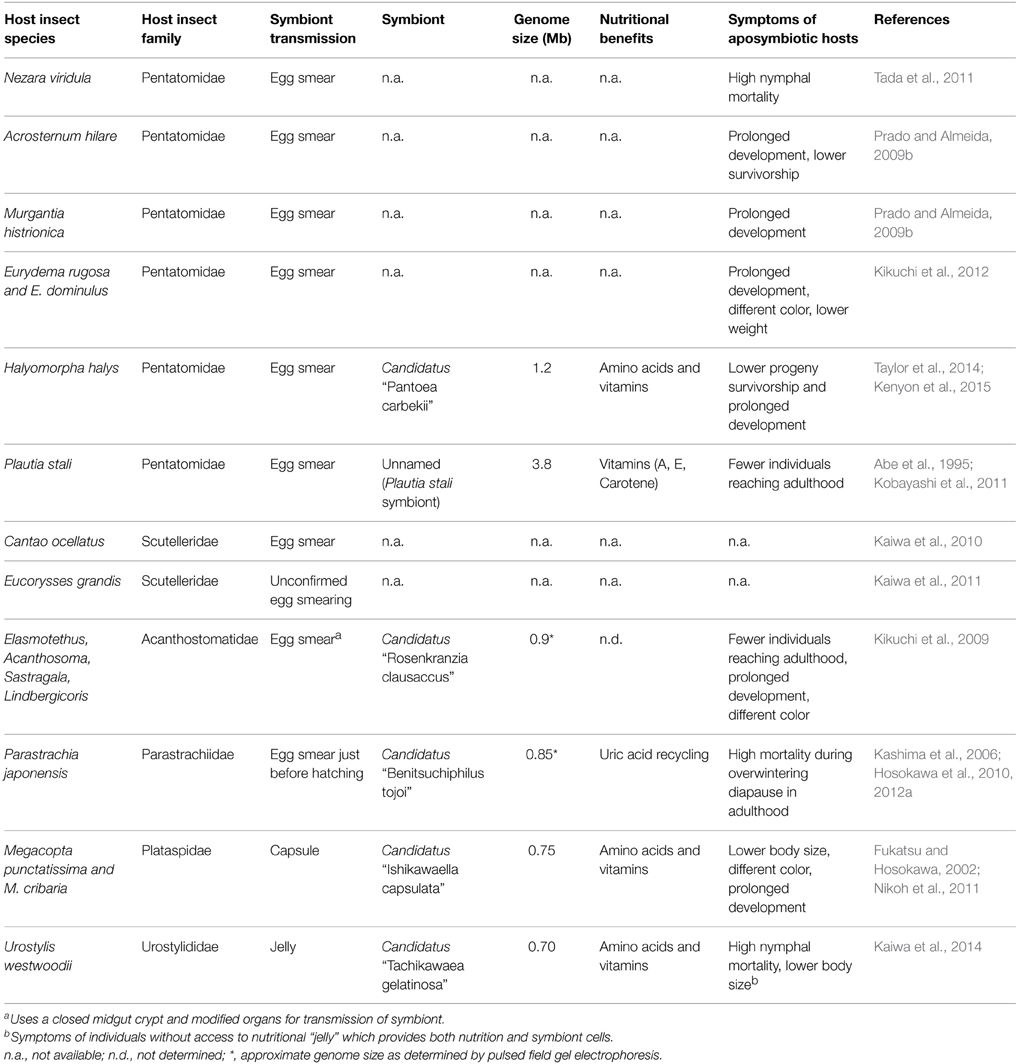
The most common method for stinkbug symbiont transmission, even in species exhibiting maternal guarding of the nymphs, is through egg smearing, which consists of females smearing egg masses with symbiont-containing secretions immediately following egg deposition. Upon hatching, nymphs consume the maternal secretions and the symbiont traverses the gastric tract and colonizes rows of tissues comprised of crypts in the distal midgut (Prado et al., 2006; Kikuchi et al., 2009, 2012; Prado and Almeida, 2009b; Kaiwa et al., 2010; Kobayashi et al., 2011; Hosokawa et al., 2013; Bauer et al., 2014). In this case, the symbiont is exposed to the environment outside the insect for ~1–3 weeks with only the maternal secretions to protect it. After the nymphs hatch, the symbiont must also be able to survive the passage through the immature nymph's digestive system up to the last section of the midgut where it colonizes the crypts. Through this strategy, vertically transmitted symbionts experience distinct habitats (e.g., maternal secretions-egg surface-gastric tract-distal midgut) that vertically transmitted intracellular symbionts do not. It is expected that this multiphasic lifestyle would select for genes that would not be essential for intracellular symbionts (e.g., cell wall biosynthesis) (Moran and Bennett, 2014). This has been evidenced for Candidatus “Pantoea carbekii” and for the symbiont of Plautia stali, which are transmitted by this means, whose sequenced genomes reveal the presence of genes involved peptidoglycan and cell wall biosynthesis that are not usually present in intracellular mutualists (Kenyon et al., 2015). However, it cannot be conclusively determined that taxa exposed to these distinct habitats will experience habitat-specific selection of loci, given that few complete genomes of extracellular symbionts are currently available. Specifically, as extracellular mutualists have a different transmission strategy, exactly how this exposure to the environment affects genome reduction is currently unknown. Sampling more genomes from symbionts that use this strategy can show if the retention of these genes is a common trend and if the genome reduction for these extracellular mutualists reaches a limit because of this exposure.
Plataspid stinkbugs employ a variation on external symbiont transmission that involves production of symbiont capsules that consist of a cuticle-like envelope surrounding a resin-like matrix that contains symbiont cells (Hosokawa et al., 2005). The envelope and matrix protect symbiont cells from exposure. Capsules are placed on the apex of each egg, compelling nymphs to consume the capsule and its symbiont infusion as it emerges. This strategy is highly specialized in that the host organism has had to develop a specialized system for the protection of the symbiont during the vertical transmission phase. The different secretions involved in the capsule are produced in different regions of the midgut and are stored until oviposition, and capsule-based transmission is unique to members of the Plataspidae family (Hosokawa et al., 2005). While this is similar to trophic egg production, which is where infertile eggs are laid along with fertile eggs to be eaten by the nymphs upon hatching for nutritional purposes, capsules are produced by different mechanisms. Urostylid stinkbugs use a variation on capsule-based symbiont transmission where the symbiont is embedded in a voluminous gel like substance or “jelly” that covers the eggs and provides a food source for nymphs during the winter (Kaiwa et al., 2014).
Evolution of the symbiont capsule and jelly structures suggests that reduced environmental exposure provides higher fitness for the host-symbiont association, highlighting that transmission is critical for the symbiotic relationship. These protective structures may also protect the symbionts from contamination by environmental bacteria that could jeopardize the fidelity of the transmission if competing environmental microorganisms are allowed to replace the symbiont. Also, exposure to UV radiation or desiccation may affect the symbionts before they are able to reach their new hosts or reduce their titers below infective levels. On the other hand, horizontal symbiont transfer among stinkbugs has been speculated to happen due to symbiont cross contamination between eggs of different species in clades where clear host-symbiont coevolution is not evident (Kikuchi et al., 2009). This is especially important when considering that symbiont transfers may grant their new hosts the ability to exploit different plant resources (Hosokawa et al., 2007).
Coevolution
In most reported cases, stinkbug symbionts are well-integrated into the growth and development their host. Aposymbiotic individuals commonly display lower fitness parameters such as longer periods between molts (Kikuchi et al., 2009), reduced size (Hosokawa et al., 2006), and lower progeny survivorship (Taylor et al., 2014) (see Table 1). While there are examples in which aposymbiotic insects do not display reduced fitness in comparison to their symbiotic counterparts (Prado et al., 2006; Prado and Almeida, 2009b), it is possible the measured parameters or laboratory conditions, such as a particular diet, would not detect the impact of the loss. Nevertheless, host physiological and behavioral investments in the maintenance of the symbiont support the general hypothesis that the relationship is mutually beneficial. This is further evidenced by a range of traits developed specifically to harbor and stably inherit these symbionts, including organs such as the midgut crypts in most pentatomids, the lubricating organ and further modified isolated midgut crypt in Acanthosomatidae (Kikuchi et al., 2009) and certain pentatomids (Hayashi et al., 2015); secretions such as the symbiont capsule in Plataspidae (Nikoh et al., 2011), and the nutritional symbiont jelly in Urostylidae (Kaiwa et al., 2014); and even behaviors such as pre-hatching mucus secretion in Parastrachiidae (Hosokawa et al., 2012a) and nymphal aggregation or wandering in relation to symbiont acquisition (Hosokawa et al., 2008). Additionally, benefits for the symbiont likely include a regulated and isolated environment, free from host defenses (Futahashi et al., 2013) with a continued provision of nutrients and protection from invasion of competing bacteria.
On a smaller scale, the coevolution of insect symbiont can be evidenced through examples where vertical transmission is stricter. In most cases of simple egg smearing, symbiont and host are consistently grouped as corresponding monophyletic groups at the genus level (Bansal et al., 2014; Matsuura et al., 2014), or even subfamily level (Bistolas et al., 2014) even when sampled from distinct geographic locations. However, at the family level this tendency is not always maintained (Hosokawa et al., 2012b). When symbionts are not inherited but are reacquired from the environment, no such tendencies are evidenced (Kikuchi et al., 2011). However, stricter methods of transmission show a more consistent relationship between host and symbiont phylogeny in the form of co-cladogenesis (Hosokawa et al., 2006; Kikuchi et al., 2009).
Genome Reduction
The <1 Mb genome is commonly observed for intracellular obligate endosymbionts (Moran and Bennett, 2014). Intracellular residence under stable environmental conditions results in relaxed purifying selection that subsequently facilitates mutation fixation and accumulation. Additionally, the loss of DNA repair and recombination mechanisms (as evidenced by a survey of complete genomes), erroneous DNA replication and successive genetic bottlenecks with each generation also contribute to dramatic losses of genes nonessential for the maintenance of the mutualism (Kenyon and Sabree, 2014). The abundance of genomes for endosymbionts of insect for which life history information is available, clear habitat conditions (e.g., consistent intracellular localization) and host demands (e.g., phytophagy and low assimilable nitrogen) are strongly correlated with endosymbiont gene content (e.g., maintenance of essential amino acid biosynthesis pathways). Drawing similar conclusions for the crypt-dwelling, extracellular symbionts of stinkbugs is limited by the paucity of available genomes.
Currently, complete sequences for four primary bacterial symbionts of stinkbug are available: the unnamed symbiont of P. stali (Kobayashi et al., 2011); Megacopta punctatissima symbiont, Candidatus “Ishikawaella capsulata” (Nikoh et al., 2011); H. halys symbiont, Candidatus “Pantoea carbekii” (Kenyon et al., 2015); and Urostylis westwoodii symbiont, Candidatus “Tachikawaea gelatinosa” (Kaiwa et al., 2014). Both I. capsulata and T. gelatinosa have considerably reduced genomes (0.7 and 0.75 Mb, respectively) that largely reflect genic repertoires observed in intracellular symbionts. During intergenerational transmission, these symbionts are ensconced in host-derived structures (capsules and jelly, respectively) that may significantly reduce the symbiont's exposure to the external environment, and thus these bacteria may require fewer genome-encoded products to maintain their viability while awaiting nymphal uptake. Conversely, P. carbekii and P. stali's show great differences in genome size despite their identical transmission strategy and their host's phylogenetic closeness, which may indicate different ages of their symbiotic relationships with their hosts. With the exception of the P. stali symbiont, the genomes from all of the stinkbug symbionts lack genes involved in DNA replication and repair, which is a characteristic shared with intracellular symbiont genomes. It is common in both intra- and extra-cellular symbionts that amino-acid and vitamin synthesis pathways are selectively lost or retained according to the hosts diet (phytophagy) (Kaiwa et al., 2014; Kenyon et al., 2015), which suggests a similar condition in which the host exerts a positive selection for symbiont genes involved in the production of nutrients they cannot obtain from their diets. Extreme genome reduction (genomes smaller than <0.25 Mb) has only been observed in obligate intracellular symbionts (McCutcheon and Moran, 2011; Moran and Bennett, 2014), yet moderately reduced genomes (0.7-1.2 Mb) are observed in their extracellular counterparts when compared to free-living relatives, namely Pantoea ananatis and E. coli (Table 1; Kenyon et al., 2015). Additional characteristics stinkbug symbionts share with intracellular symbionts are A+T%-bias (with symbiont genomes between 69 and 75%, excluding P. stali's symbiont) and high rates of sequence evolution (Hosokawa et al., 2013; Kenyon and Sabree, 2014; Moran and Bennett, 2014).
While some intracellular symbionts have lost genes involved in canonical ATP synthesis, the available extracellular symbiont genomes have retained them. Host-generated ATP present within bacteriocyte cytoplasm is likely sourced by intracellular symbionts for their energetic needs (Moran and Bennett, 2014) while crypt-dwelling symbionts may not have access to and therefore must retain these pathways. Additionally, genes involved in lipid and cell wall biosynthesis tend to be missing in intracellular symbionts, and loss of these is probably enabled by the presence of the host cell membrane that provides the missing protective and transport functions (McCutcheon and Moran, 2011). However, these genes remain present in P.carbekii and P. stali's symbiont and absent in I. capsulata, which might correspond to the ex vivo experience of these symbionts on egg surfaces during intergenerational transmission while I. capsulata may receive sufficient ex vivo protection by the capsule that alleviates the need for de novo production of these cell wall components (Nikoh et al., 2011). Although extracellular stinkbug symbionts do not reside within the host cells, they are often the sole occupants of elaborate specialized host-derived structures, which reflect a significant adaptation and investment of physiological resources for supporting the mutualism.
Given the dynamic life histories of stinkbug symbionts and relatively minimal amount of time needed to generate complete bacterial genomes, these organisms provide an exemplary opportunity to study the effects of genome reduction in extracellular symbionts. While the vertical transmission method is reliable enough for strict mutualisms to develop, it is possible that new, competing microorganisms can occasionally replace the symbiont and start the association anew. As a result, different stinkbug symbionts display different degrees of association and reliance on their hosts (Prado and Almeida, 2009b). This is evidenced by the different degrees of dependence from the host on their symbiont, as shown by the differential survivorship and multiple symptoms of aposymbiotic hosts (see Table 1), and the varying levels of genome reduction among the symbionts. Because of this, studying different symbionts could give us detailed information on the different stages of genome reduction as a symbiotic relationship strengthens. On the other hand, they occupy very similar niches (the midgut crypts), they must undergo exposure to the environment while they are transmitted, and are pressured by their hosts for certain nutritional benefits (see Table 1). This translates to a relatively consistent set of evolutionary pressures that grants reproducibility for the comparison of genomes.
Conclusion
The last few years have greatly increased the amount of knowledge on primary extracellular gut symbionts of pentatomid stinkbugs. While genome reduction of extracellular symbionts has not achieved the extremes it has on intracellular counterparts, evidence shows intracellularity is not necessary for genome reduction, while placing greater importance on transmission systems. The life history of this particular mutualistic association has shown several factors that allow an unparalleled study on the coevolution of insect-microbe mutualism, and an integrative analysis of this group seems very promising. The few genomes available, while incredibly informative, come from radically different backgrounds that do not allow certain hypotheses to be tested. Obtaining more complete genomes from stinkbug symbionts and comparing them among similar or different life stories, as well as with the several already published genomes from intracellular symbionts facilitates a greater understanding of the requirements of a non-intracellular symbiont. In conjunction with studies on host diet, physiology, and metabolism, the effects of particular pressures from the host on the symbiont can be elucidated such as the requisite for diet supplementing pathways and mechanisms for host-symbiont communication and nutrient transport between cells.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Ohio Agricultural Research Development Center SEEDS Research Enhancement-Interdisciplinary Grant and The Ohio State University for funding to AO and ZS, respectively.
References
Abe, Y., Mishiro, K., and Takanashi, M. (1995). Symbiont of brown-winged green bug, Plautia stali Scott. Jpn. J. Appl. Entomol. Zool. 39, 109–115. doi: 10.1303/jjaez.39.109
Anderson, K. E., Russell, J. A., Moreau, C. S., Kautz, S., Sullam, K. E., Hu, Y., et al. (2012). Highly similar microbial communities are shared among related and trophically similar ant species. Mol. Ecol. 21, 2282–2296. doi: 10.1111/j.1365-294X.2011.05464.x
Bansal, R., Michel, A., and Sabree, Z. (2014). The crypt-dwelling primary bacterial symbiont of the polyphagous pentatomid pest Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 43, 617–625. doi: 10.1603/EN13341
Batut, B., Knibbe, C., Marais, G., and Daubin, V. (2014). Reductive genome evolution at both ends of the bacterial population size spectrum. Nature 12, 841–850. doi: 10.1038/nrmicro3331
Bauer, E., Salem, H., Marz, M., Vogel, H., and Kaltenpoth, M. (2014). Transcriptomic immune response of the cotton stainer Dysdercus fasciatus to experimental elimination of vitamin-supplementing intestinal symbionts. PLoS ONE 9:e114865. doi: 10.1371/journal.pone.0114865
Bell, W. J., and Cardé, R. T. (1984). Chemical Ecology of Insects. London: Springer. doi: 10.1007/978-1-4899-3368-3
Bistolas, K. S. I., Sakamoto, R. I., Fernandes, J. A. M., and Goffredi, S. K. (2014). Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from Costa Rica. Front. Microbiol. 5:349. doi: 10.3389/fmicb.2014.00349
Filippi, L., Baba, N., Inadomi, K., Yanagi, T., Hironaka, M., and Nomakuchi, S. (2009). Pre- and post-hatch trophic egg production in the subsocial burrower bug, Canthophorus niveimarginatus (Heteroptera: Cydnidae). Naturwissenschaften 96, 201–211. doi: 10.1007/s00114-008-0463-z
Fukatsu, T., and Hosokawa, T. (2002). Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 68, 389–396. doi: 10.1128/AEM.68.1.389-396.2002
Futahashi, R., Tanaka, K., Tanahashi, M., Nikoh, N., Kikuchi, Y., Lee, B. L., et al. (2013). Gene expression in gut symbiotic organ of stinkbug affected by extracellular bacterial symbiont. PLoS ONE 8:e64557. doi: 10.1371/journal.pone.0064557
Hayashi, T., Hosokawa, T., Meng, X.-Y., Koga, R., and Fukatsu, T. (2015). Female-specific specialization of a posterior end region of the midgut symbiotic organ in Plautia splendens and allied stinkbugs. Appl. Environ. Microbiol. 81, 2063–2611. doi: 10.1128/aem.04057-14
Hosokawa, T., Hironaka, M., Inadomi, K., Mukai, H., Nikoh, N., and Fukatsu, T. (2013). Diverse strategies for vertical symbiont transmission among subsocial stinkbugs. PLoS ONE 8:e65081. doi: 10.1371/journal.pone.0065081
Hosokawa, T., Hironaka, M., Mukai, H., Inadomi, K., Suzuki, N., and Fukatsu, T. (2012a). Mothers never miss the moment: a fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim. Behav. 83, 293–300. doi: 10.1016/j.anbehav.2011.11.006
Hosokawa, T., Kikuchi, Y., Nikoh, N., and Fukatsu, T. (2012b). Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl. Environ. Microbiol. 78, 4758–4761. doi: 10.1128/AEM.00867-12
Hosokawa, T., Kikuchi, Y., Nikoh, N., Shimada, M., and Fukatsu, T. (2006). Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4:e337. doi: 10.1371/journal.pbio.0040337
Hosokawa, T., Kikuchi, Y., Nikon, N., Meng, X. Y., Hironaka, M., and Fukatsu, T. (2010). Phylogenetic position and peculiar genetic traits of a midgut bacterial symbiont of the stinkbug Parastrachia japonensis. Appl. Environ. Microbiol. 76, 4130–4135. doi: 10.1128/AEM.00616-10
Hosokawa, T., Kikuchi, Y., Shimada, M., and Fukatsu, T. (2007). Obligate symbiont involved in pest status of host insect. Proc. Biol. Sci. 274, 1979–1984. doi: 10.1098/rspb.2007.0620
Hosokawa, T., Kikuchi, Y., Shimada, M., and Fukatsu, T. (2008). Symbiont acquisition alters behaviour of stinkbug nymphs. Biol. Lett. 4, 45–48. doi: 10.1098/rsbl.2007.0510
Hosokawa, T., Kikuchi, Y., Xien, Y. M., and Fukatsu, T. (2005). The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol. Ecol. 54, 471–477. doi: 10.1016/j.femsec.2005.06.002
Kaiwa, N., Hosokawa, T., Kikuchi, Y., Nikoh, N., Meng, X. Y., Kimura, N., et al. (2010). Primary gut symbiont and secondary, sodalis-allied symbiont of the scutellerid stinkbug Cantao ocellatus. Appl. Environ. Microbiol. 76, 3486–3494. doi: 10.1128/AEM.00421-10
Kaiwa, N., Hosokawa, T., Kikuchi, Y., Nikoh, N., Meng, X. Y., Kimura, N., et al. (2011). Bacterial symbionts of the giant jewel stinkbug Eucorysses grandis (Hemiptera: Scutelleridae). Zool. Sci. 28, 169–174. doi: 10.2108/zsj.28.169
Kaiwa, N., Hosokawa, T., Nikoh, N., Tanahashi, M., Moriyama, M., Meng, X., et al. (2014). Symbiont-supplemented maternal investment underpinning host's ecological adaptation. Curr. Biol. 24, 2465–2470. doi: 10.1016/j.cub.2014.08.065
Kaltenpoth, M., Winter, S. A., and Kleinhammer, A. (2009). Localization and transmission route of Coriobacterium glomerans, the endosymbiont of pyrrhocorid bugs. FEMS Microbiol. Ecol. 69, 373–383. doi: 10.1111/j.1574-6941.2009.00722.x
Kashima, T., Nakamura, T., and Tojo, S. (2006). Uric acid recycling in the shield bug, Parastrachia japonensis (Hemiptera: Parastrachiidae), during diapause. J. Insect Physiol. 52, 816–825. doi: 10.1016/j.jinsphys.2006.05.003
Kenyon, L. J., Meulia, T., and Sabree, Z. L. (2015). Habitat visualization and genomic analysis of “Candidatus Pantoea carbekii,” the primary symbiont of the brown marmorated stink bug. Genome Biol. Evol. 7, 620–635. doi: 10.1093/gbe/evv006
Kenyon, L. J., and Sabree, Z. L. (2014). Obligate insect endosymbionts exhibit increased ortholog length variation and loss of large accessory proteins concurrent with genome shrinkage. Genome Biol. Evol. 6, 763–75. doi: 10.1093/gbe/evu055
Kikuchi, Y., Hosokawa, T., and Fukatsu, T. (2011). An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 5, 446–460. doi: 10.1038/ismej.2010.150
Kikuchi, Y., Hosokawa, T., Nikoh, N., and Fukatsu, T. (2012). Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 47, 1–8. doi: 10.1007/s13355-011-0081-7
Kikuchi, Y., Hosokawa, T., Nikoh, N., Meng, X.-Y., Kamagata, Y., and Fukatsu, T. (2009). Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7:2. doi: 10.1186/1741-7007-7-2
Kobayashi, H., Kawasaki, K., Takeishi, K., and Noda, H. (2011). Symbiont of the stink bug Plautia stali synthesizes rough-type lipopolysaccharide. Microbiol. Res. 167, 48–54. doi: 10.1016/j.micres.2011.03.001
Matsuura, Y., Hosokawa, T., Serracin, M., Tulgetske, G. M., Miller, T. A., and Fukatsu, T. (2014). Bacterial symbionts of a devastating coffee plant pest, the stinkbug Antestiopsis thunbergii (Hemiptera: Pentatomidae). Appl. Environ. Microbiol. 80, 3769–3775. doi: 10.1128/AEM.00554-14
Matsuura, Y., Kikuchi, Y., Hosokawa, T., Koga, R., Meng, X.-Y., Kamagata, Y., et al. (2012). Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J. 6, 397–409. doi: 10.1038/ismej.2011.103
McCutcheon, J. P., and Moran, N. A. (2011). Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 10, 13–26. doi: 10.1038/nrmicro2670
Mira, A., Ochman, H., and Moran, N. A. (2001). Deletional bias and the evolution of bacterial genomes. Trends Genet. 17, 589–596. doi: 10.1016/S0168-9525(01)02447-7
Moran, N. A., and Bennett, G. M., (2014). The tiniest tiny genomes. Annu. Rev. Microbiol. 68, 195–215. doi: 10.1146/annurev-micro-091213-112901
Moran, N. A., McCutcheon, J. P., and Nakabachi, A. (2008). Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190. doi: 10.1146/annurev.genet.41.110306.130119
Nikoh, N., Hosokawa, T., Oshima, K., Hattori, M., and Fukatsu, T. (2011). Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 3, 702–714. doi: 10.1093/gbe/evr064
Ohkuma, M. (2008). Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends Microbiol. 16, 345–352. doi: 10.1016/j.tim.2008.04.004
Prado, S. S., and Almeida, R. P. P. (2009a). Phylogenetic placement of pentatomid stink bug gut symbionts. Curr. Microbiol. 58, 64–69. doi: 10.1007/s00284-008-9267-9
Prado, S. S., and Almeida, R. P. P. (2009b). Role of symbiotic gut bacteria in the development of Acrosternum hilare and Murgantia histrionica. Entomol. Exp. Appl. 132, 21–29. doi: 10.1111/j.1570-7458.2009.00863.x
Prado, S. S., Rubinoff, D., and Almeida, R. P. P. (2006). Vertical transmission of a pentatomid caeca-associated symbiont. Ann. Entomol. Soc. Am. 99, 577–585. doi: 10.1603/0013-8746(2006)99[577:VTOAPC]2.0.CO;2
Requena, G. S., Nazareth, T. M., Schwertner, C. F., and Machado, G. (2010). First cases of exclusive paternal care in stink bugs (Hemiptera: Pentatomidae). Zool. (Curitiba) 27, 1018–1021. doi: 10.1590/S1984-46702010000600026
Sabree, Z. L., Huang, Y. C., Arakawa, G., Tokuda, G., Lo, N., Watanabe, H., et al. (2012). Genome shrinkage and loss of nutrient-providing potential in the obligate symbiont of the primitive termite Mastotermes darwiniensis. Appl. Environ. Microbiol. 78, 204–210. doi: 10.1128/AEM.06540-11
Tada, A., Kikuchi, Y., Hosokawa, T., Musolin, D. L., Fujisaki, K., and Fukatsu, T. (2011). Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 46, 483–488. doi: 10.1007/s13355-011-0066-6
Taylor, C. M., Coffey, P. L., DeLay, B. D., and Dively, G. P. (2014). The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS ONE 9:e90312. doi: 10.1371/journal.pone.0090312
Watanabe, K., Yukuhiro, F., Matsuura, Y., Fukatsu, T., and Noda, H. (2014). Intrasperm vertical symbiont transmission. Proc. Natl. Acad. Sci. U.S.A. 111, 7433–747. doi: 10.1073/pnas.1402476111
Keywords: extracellular symbionts, Pantoea carbekii, symbiont transmission, stink bugs, mutualisms
Citation: Otero-Bravo A and Sabree ZL (2015) Inside or out? Possible genomic consequences of extracellular transmission of crypt-dwelling stinkbug mutualists. Front. Ecol. Evol. 3:64. doi: 10.3389/fevo.2015.00064
Received: 20 April 2015; Accepted: 08 June 2015;
Published: 23 June 2015.
Edited by:
Luis Delaye, CINVESTAV Irapuato, MexicoCopyright © 2015 Otero-Bravo and Sabree. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zakee L. Sabree, Department of Evolution, Ecology and Organismal Biology, The Ohio State University, 318 W. 12th Avenue Room 300, Columbus, OH 43201, USA,c2FicmVlLjhAb3N1LmVkdQ==
 Alejandro Otero-Bravo
Alejandro Otero-Bravo Zakee L. Sabree
Zakee L. Sabree