Abstract
Ceropegia species (Apocynaceae) have deceptive pitfall flowers and exploit small flies as pollinators, supposedly by chemical mimicry. Only preliminary data on the composition of flower scents are available for a single species so far, and the mimicry system is not yet understood in any species. We collected data on basic pollination aspects of C. dolichophylla, analyzed floral scent by gas chromatography linked to mass spectrometry (GC/MS), identified electrophysiologically active scent components by gas chromatography coupled with electroantennographic detection (GC/EAD), and determined compounds responsible for pollinator attraction in bioassays. We found that flowers of C. dolichophylla are visited by small flies of several taxa. Only Milichiidae and Chloropidae carried pollinaria and are, thus, pollinators. The pollen transfer efficiency (PTE) at two different sites was 2% and 4%, respectively. The floral scent was dominated by spiroacetals, mainly (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane, n-tridecane, and N-(3-methylbutyl)acetamide. This spiroacetal and the acetamide elicited the most intense electrophysiological responses in fly antennae, and bioassays confirmed the capability of the spiroacetal in eliciting behavioral responses in pollinators. Most flies, determined as pollinators of C. dolichophylla, are kleptoparasites. They exploit insect prey of predatory arthropods as food source to which they are attracted by volatiles. 8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane and N-(3-methylbutyl)acetamide have not been identified before as volatiles of other plants, however, they are known as insect volatiles. Both compounds occur in the venom glands of paper wasps, a potential food source for the pollinators of C. dolichophylla. We propose that C. dolichophylla shows a kleptomyiophilous pollination strategy. It mimics insect related odors to exploit the food-seeking behavior of its kleptoparasitic pollinators.
Introduction
Apart from the showiness of visual cues, flowers often use floral scent to attract their pollinators (Raguso, 2008). Floral scents usually advertise a food source provided by the flowers. In deceptive plants, however, flower scents are a false promise of a reward, such as food, a mating partner or an oviposition site (Salzmann et al., 2007; Jürgens et al., 2013; Bohmann et al., 2014) that these plants do not actually offer. Among these cheaters are plants of the genus Ceropegia L. (Apocynaceae, Asclepiadoideae) with more than 200 described species, characterized by sophisticated pitfall flowers (Vogel, 1961; Masinde, 2004). Despite great morphological diversification, the basic floral structure is similar among these species (see Vogel, 1961), and the functionality of the pollination system is extremely specialized and conservative (Vogel, 1961; Ollerton et al., 2009). Species investigated so far are pollinated by small flies (but see Coombs et al., 2011), which are trapped inside the flowers for a limited time during which they deposit or take up pollinaria/pollinia. The fly pollinators of Ceropegia belong to diverse families, but, typically, only species of a single or a few fly families interact with a single species of Ceropegia (Ollerton et al., 2009). This specificity is likely due to distinct floral scents, which are responsible for pollinator attraction (Vogel, 1961; Heiduk et al., 2010). It was suggested that the flowers mimic rotting plant material, male sex pheromones or animal related odors, leading to the idea that pollinating flies are attracted by chemical deceit (Vogel, 1961; Ollerton et al., 2009). In a preliminary analysis on C. dolichophylla Schltr., Heiduk et al. (2010) proposed that this species mimics a food-source for its pollinators. C. dolichophylla and other Ceropegia species are pollinated by kleptoparasitic flies, which are known to feed on the insect prey of predatory arthropods that they find on the basis of volatile insect secretions (Robinson and Robinson, 1977; Sivinski and Stowe, 1980; Sabrosky, 1983; Sivinski, 1985; Eisner et al., 1991; Sivinski et al., 1999). Heiduk et al. (2010) showed the natural scent of C. dolichophylla to be highly attractive to flies, however, the compounds attracting the pollinators could not be identified. Furthermore, the study was based on greenhouse grown plants of C. dolichophylla and was not conducted in Asia, where it is native. Its natural pollinators were still unknown, and other pollination aspects such as pollination success have not yet been studied in C. dolichophylla. Also, the composition of floral scent of wild plants and its attractiveness to fly pollinators in the natural habitat was not determined.
The aim of the present study was to collect additional data on basic pollination aspects for C. dolichophylla in its native range in China and to identify flower volatiles that mediate the pollination system. We specifically asked: (1) Who are the natural pollinators? (2) What is the pollination success in natural populations? (3) Which compounds characterize the floral scent of wild (two different areas) and greenhouse plants? (4) Is the natural flower bouquet attractive to pollinators? (5) Which scent components can be perceived by the pollinators? (6) Do specific electrophysiologically active volatiles attract pollinators?
Thus, we collected and identified flower visitors in the native range, and determined the pollen transfer efficiency (PTE) of plants in natural habitats. We also investigated the floral scent of wild plants in China using dynamic headspace methods followed by gas chromatography linked to mass spectrometry (GC/MS). We tested the pollinator attractiveness of natural flower scent and identified electrophysiologically and behaviorally active compounds by gas chromatography coupled with electroantennographic detection (GC/EAD) and field bioassays, respectively.
Materials and methods
Plant species
Ceropegia dolichophylla Schtlr. is a climbing herb that grows in forests from 500 to 1500 m a.s.l. Typically, it twines on other vegetation up to 1.5 m in height. Anthesis of individual flowers lasts for 1 day (opening in the morning; Heiduk et al., 2010), and the flowering season spans from July to early September (Zhou and Xie, pers. comm.; eFloras, 2015). Fruits can be found beginning at the end of September (Zhou and Xie, pers. comm.). In Ceropegia, the pollen is packed into discrete packages, the pollinia, two of which are connected via caudicles and the corpusculum to form a pollinarium. Due to the complicated pollination mechanism with fused and highly synorganized reproductive organs (gynostegium), where pollinia of a previously extracted pollinarium need to be inserted between “guide rails” (Vogel, 1961), C. dolichophylla depends on pollinators for successful reproduction.
Study sites
Investigations were conducted in both China and Germany. In July and August 2012 bioassays were performed at the non-native location in Bayreuth, Germany, where previous studies took place on greenhouse plants (Heiduk et al., 2010). In the native range, plants of C. dolichophylla were studied in the Mt. Fanjing area in northeast Guizhou province, China (27°49′N-27°50′N, 108°44′E-108°46′E). This area bears vegetation characterized by broad-leaved evergreen forests of high diversity, in a subtropical, humid monsoon climate. C. dolichophylla plants were studied at two sites (henceforth Area 1 and Area 2) approximately 5 km apart from each other. At both sites, floral scent, flower visitors/pollinators, and data on pollen transfer efficiency (PTE) were collected in September 2013. Bioassays were performed in August 2012 and September 2013 at Area 1 and Area 2 and additionally in South China Botanical Garden (SCBG), Guangzhou (distance to Area 1 and Area 2: ca. 1000 km), where C. dolichophylla does not naturally occur.
Voucher specimens collected in China were deposited in the herbarium of the University of Bayreuth [Voucher/Accession: China: Guizhou, Tongren, Fanjing Mt., 874 m, A. Heiduk, I. Schäffler and Y. Hong, Sep. 2012 (UBT)].
Flower visitors/pollinators and pollen transfer efficiency (PTE)
To obtain information about pollination of C. dolichophylla in its native range in China, ca. 400 flowers from 12 individual plants (Area 1: 5 plants; daily collection from 7 to 13th September 2013; Area 2: 7 plants; collection on 8th September 2013) were picked in the evening and checked for trapped insects. Insects trapped inside the flowers were examined for pollinaria and number of pollinia carried, and only those carrying pollinaria/pollinia were designated as pollinators. To determine pollination success, 267 flowers from Area 1 and 58 flowers from Area 2 were examined in the field using a 10× hand lens, and the gynostegia were checked for pollinaria removal and pollinia insertion. For both areas, the mean number of removed pollinaria as well as the mean number of inserted pollinia was calculated. These data were used to calculate PTE separately for Area 1 and Area 2. PTE was calculated as the percentage of removed pollinia that were inserted between guide rails. Since each pollinarium consists of two pollinia, the mean number of inserted pollinia was divided by twice the mean number of removed pollinaria (Johnson et al., 2005; Coombs et al., 2009, 2011).
Collection of volatiles
Floral volatiles were collected during daytime from newly opened flowers in situ using dynamic headspace methods (Dötterl et al., 2005b). Flowers were enclosed in a polyester oven bag (6 × 5 cm; Toppits®, Germany) for 10 min to allow accumulation of floral scent. Subsequently, volatiles were trapped by pulling the air from the bag through small adsorbent tubes (Varian Inc. ChromatoProbe quartz microvials; length: 15 mm, inner diameter: 2 mm) for 5 min using a membrane pump (G12/01 EB, Rietschle Thomas Inc., Puchheim, Germany; flow rate: 200 ml/min). The tubes contained 1.5 mg Tenax-TA (mesh 60–80) and 1.5 mg Carbotrap B (mesh 20–40; both Supelco) fixed by glass wool plugs.
In Area 1 seven samples were collected from seven different plants. Five of the samples were collected from a single flower, one sample was collected from two flowers, and one from three flowers (in cases where flowers grew closely together, they were enclosed in a single bag to avoid any injury of flowers). In Area 2 seven samples were collected from five plants (from two of these plants, two samples each were collected). Four of the samples were collected from single flowers, two samples from two flowers, and one sample from three flowers. At each location samples of the surrounding air were also collected as controls.
To obtain solutions of natural scent (19 in total) for bioassays and electrophysiological analyses (see below), floral scent from 17 individual flowers (Area 1, Fanjing Mt., China), and two individual flowers (Bayreuth, Germany) was collected for at least 4 h into large adsorbent tubes (glass capillaries; length: 8 cm, inner diameter: 2.5 mm) containing 15 mg Tenax-TA (mesh 60–80) and 15 mg Carbotrap B (mesh 20–40). The trapped volatiles were eluted with 70 μl of acetone (SupraSolv, Merck KgaA, Germany; following Dötterl et al., 2005a) per adsorbent tube. Subsequently, 2 × 5 and 1 × 7 samples collected in China, and both samples collected in Bayreuth, were combined to provide three samples (2 × 350 μl, 1 × 490 μl) from field plants (China) and one sample (140 μl) from greenhouse plants (Bayreuth), for further experiments (see below).
Chemical analysis
The volatiles trapped in small adsorbent tubes were analyzed by GC/MS using an automatic thermal desorption (TD) system (TD-20, Shimadzu, Japan) coupled to a Shimadzu GC/MS-QP2010 Ultra equipped with a ZB-5 fused silica column (5% phenyl polysiloxane; 60 m, i.d. 0.25 mm, film thickness 0.25 μm, Phenomenex). The samples were run with a split ratio of 1:1 and a constant helium carrier gas flow of 1.5 ml/min. The GC oven temperature started at 40°C, then increased by 6°C/min to 250°C and held for 1 min. The MS interface worked at 250°C. Mass spectra were taken at 70 eV (EI mode) from m/z 30 to 350. GC/MS data were processed using the GCMSolution package, Version 2.72 (Shimadzu Corporation 2012).
The solvated scent samples were analyzed by GC/MS using a Shimadzu GCMS-QP2010 Ultra equipped with an AOC-20i auto injector (Shimadzu, Tokyo, Japan) and again a ZB-5 fused silica column (5% phenyl polysiloxane; 30 m long, inner diameter 0.32 mm, film thickness 0.25 μm, Phenomenex). One μl of the samples was injected (injection temperature: 220°C; split ratio: 1:1), and the column flow (carrier gas: helium) was set at 3 ml/min. The GC oven temperature was held at 40°C for 1 min, then increased by 10°C/min to 220°C and held for 2 min. The MS interface worked at 220°C. Mass spectra were again taken at 70 eV (in EI mode) from m/z 30 to 350 and data processed as described above.
Identification of the compounds was carried out using the NIST 11, Wiley 9, FFNSC 2, Adams (2007) databases, the database available in MassFinder 3, and published plotted spectra (Francke et al., 1981; Bergström et al., 1982; Francke and Kitching, 2001). Structures of several compounds were confirmed by comparing mass spectra and retention times with those of synthetic reference samples. The assignment of 8-methyl-2-propyl-1,7-dioxaspiro[5.5]undec-3-ene, was based on the mass spectrum of the natural product and the general fragmentation pattern of spiroacetals (Francke and Kitching, 2001).
Double bond positions of alkenes were determined by reaction with dimethyl disulfide (DMDS) (Buser et al., 1983) and subsequent separation of the adducts on a 30 m × 0.25 mm i.d. 0.25 μm film thickness HP5-MS fused silica capillary column (Agilent Technologies, Santa Clara, CA, USA), starting at 60°C for 3 min, increased at a rate of 3°C/min to 300°C, held for 70 min.
Total scent emission was estimated by injecting known amounts of monoterpenoids, aromatics, and aliphatics (added to small adsorbent tubes). The mean response of these compounds (mean peak area) was used to determine the total amount of each compound extracted from the small adsorbent tubes (Dötterl et al., 2005b).
Statistical analysis
To screen for quantitative differences in absolute amounts of scent between Area 1 and Area 2, the total amount of scent per sample and flower was compared between areas using a t-test (StatSoft Inc., 2005). Normality was tested by Shapiro-Wilk test and homogeneity of variances by Hartley's test (StatSoft Inc., 2005).
To screen for semi-quantitative (percentage amount contributed per compound) differences in scent among samples of plants from Area 1 and plants from Area 2, the Bray-Curtis (BC) similarity index was calculated using Primer 6.1.11, including the add-on package Permanova + 1.0.1 (Clarke and Gorley, 2006; Anderson et al., 2008). If more than one sample was taken from the same individual plant, the mean scent composition was calculated and used for analyses. Based on the BC matrix a PERMANOVA (Factor: Area; 10,000 permutations) was performed using the same software package to test for an Area effect.
Electrophysiological analysis
The scent components from C. dolichophylla flowers that were perceived by flower visitors/pollinators, were identified by gas chromatography coupled to electroantennographic detection (GC/EAD) and GC/MS (see above). Altogether 34 GC/EAD measurements with 14 flies from China and seven measurements with five flies from Bayreuth, Germany, were performed. The five flies (female Desmometopa sordida) from Bayreuth were collected from flowers of C. dolichophylla greenhouse plants. Four (one Oscinella frit, one Desmometopa varipalpis, two Neophyllomyza sp.) of the 14 Chinese flies were collected at SCBG while feeding on dead honey bees. The other 10 flies were attracted during bioassays performed in China with synthetic compounds of the C. dolichophylla flower scent (see bioassays). All flies were kept separately in Eppendorf® tubes (1.5 ml) with a piece of humid paper towel and stored in the dark at 4°C until electrophysiological measurements were performed.
For measurements, the head of a fly was cut off at the base of the thorax, mounted between two electrodes filled with insect Ringer's solution (8.0 g/l NaCl, 0.4 g/l KCl, 0.4 g/l CaCl2) and connected to silver wires. The reference electrode was placed in contact with the cutting surface of the head while the recording electrode was brought into contact with the tip of the funiculus (cf. first flagellomere) of an antenna.
For measurements we either used a Carlo Erba Vega 6000 Series 2 (Rodano, Italy) or an Agilent 7890A (Santa Clara, California, USA) gas chromatograph, both equipped with a flame ionization detector (FID) and an EAD setup (heated transfer line, 2-channel USB acquisition controller) provided by Syntech (Kirchzarten, Germany). For each measurement 1 μl of an acetone solution of the C. dolichophylla scent was injected (injector temperature at 250°C) in splitless mode at 40°C oven temperature. The oven of both systems was heated at a rate of 10°C/min to 220°C, and the split vent was opened 0.5 min after injection. A Zebron ZB-5 column was used for analysis (5% phenyl polysiloxane; 30 m × 0.32 mm i.d. film thickness 0.25 μm, Phenomenex) in both GCs. The column of the Carlo Erba GC was split at the end by the four-arm flow splitter GRAPHPACK 3D/2 (Gerstel, Mühlheim, Germany) into two deactivated capillaries (length 50 cm × 0.32 mm i.d.) leading to the FID and to the EAD setup. Nitrogen was introduced as a make-up gas through the fourth arm of the splitter. The column of the Agilent GC was split at the end by a μFlow splitter (Gerstel, Mühlheim, Germany) into two deactivated capillaries leading to the FID (2 m × 0.15 mm i.d.) and EAD (1 m × 0.2 mm i.d.) setup. In both systems the outlet of the EAD was placed in a cleaned and humidified airflow directed over the fly antenna. Acetone solutions of the scent of C. dolichophylla were tested on antennae of five female D. sordida (3 × 1 and 2 × 2 runs per specimen), one female D. sp. nr. sordida (1 × 3 runs), two female D. varipalpis (5 and 3 runs), six female Neophyllomyza sp. (2 × 1, 2 × 2, and 2 × 3 runs), three female N. leanderi (1, 2, and 3 runs), one female Conioscinella sp. (2 runs), and one female Oscinella frit (3 runs). After the measurements, head and body of each fly were stored in a 4% solution of glycerin in ethanol (99.8%) for identification of genus and/or species.
Synthesis of EAD-active compounds
Racemic spiroacetals were synthesized according to established methods (Phillips et al., 1980; Jacobsen et al., 1982; Doubský et al., 2004). To prepare (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) (Figure 1), commercially available 2-ethoxycarbonylcyclopentanone (a) was alkylated to yield the disubstituted cyclopentanone b, which after acidic hydrolysis was decarboxylated to produce 2-propylcyclopentanone (c). Baeyer-Villiger oxidation of c produced the racemic lactone d. Alkynylation of (2S)-2-methyloxirane (e) using lithium acetylide yielded (2S)-4-pentyne-2-ol (f) which was benzylated to g. Racemic d and the anion of g were linked to form the intermediate h (not isolated) which upon hydrogenation furnished the (8S)-configured spiroacetal S4 as a mixture of the three stereoisomers S4a-c (see Figure 2A).
Figure 1
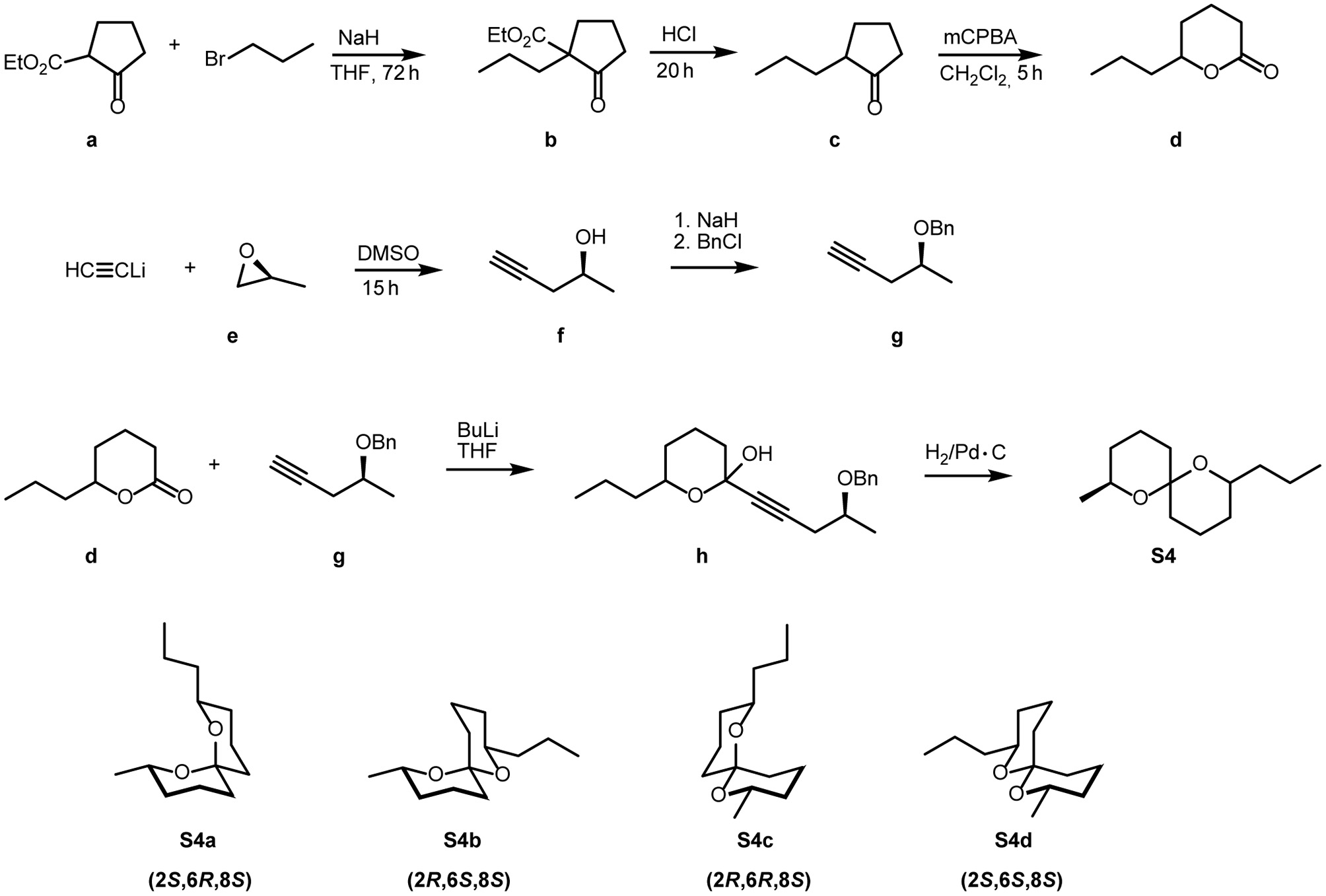
Synthesis of (8 S )-8-methyl-2-propyldioxaspiro[5.5]undecanes S4a–d.
Figure 2

(A) Gas chromatogram of crude S4a containing small amounts of the three stereoisomers; (B) Gas chromatogram of purified S4a.
To a solution of 2.67 g (15.4 mmol) (S)-benzyloxypent-4-yne (g) in 30 mL abs. THF, cooled to −78°C, were dropwise added 7.50 mL (18.8 mmol) of a 2.5 M-solution of n-BuLi in hexane. After stirring for 90 min at −78°C, 2.00 mL (16.2 mmol) BF3•Et2O, dissolved in 20 mL abs. THF, were slowly added, followed by a solution of 2.39 g (16.8 mmol) 6-propyltetrahydro-2H-pyran-2-one (d) in 10 mL THF. Over a period of 3 h, the mixture was warmed to room temperature, and the reaction was quenched by the addition of a mixture of 20 ml water, 20 mL diethyl ether, and ammonium chloride/ammonia (2:1). After separation of the layers, the aqueous phase was extracted 4 times with 20 mL portions of diethyl ether. The combined organic solutions were washed with brine and dried over magnesium sulfate. Filtration over silica and removal of the solvent in vacuo yielded 4.35 g of crude h. This was dissolved in 10 mL methanol and hydrogenated for 21 h at 20 bar, using 5% Pd-C catalyst. After removal of the catalyst by filtration over silica, the crude product (see Figure 2A) was purified by chromatography on silica using a 50:1-mixture of pentane and diethyl ether. A further chromatographic step using benzene as the eluent yielded 113 mg (0.53 mmol, 3.5%) highly pure S4a (see Figure 2B).
NMR-Spectra were run on a Bruker (Billerica, MA, USA) AMX-400 instrument. For the numbering of structural elements see Figure 2B.
1H-NMR, based on 1H-1H-COSY, HSQC, HMBC (400 MHz, CDCl3): δ [ppm] = 0.92 (t, 3JH14−H13 = 7.1 Hz, 3H, CH3 C14), 1.10–1.20/1.51–1.59 (2m, 2H, CH2 C3 ax/eq), 1.12–1.23/1.54–1.61 (2m, 2H, CH2 C9 ax/eq), 1.13 (d, 3JH15−H8 = 6.3 Hz, 3H, CH3 C15), 1.30–1.39/1.46–1.55 (2m, 2H, CH2 C13), 1.33–1.43/1.56–1.64 (2m, 4H, CH2 C5 C11 ax/eq), 1.31–1.40/1.43–1.51 (2m, 2H, CH2 C12), 1.50–1.58/1.88 (m/ddddd, 3JH−H = 13.9, 13.2, 13.2, 4.0, 4.0 Hz, 4H, 2 × CH2 C4 C10 eq/ax), 3.54 (dddd, 3JH−H = 11.0, 8.7, 4.0, 2.0 Hz, 1H, CH C2ax), 3.70 (dqd, 3JH−H = 11.4, 6.3, 2.0 Hz, 1H, CH C8ax).
13 C-NMR, based on HSQC, HMBC (126 MHz, CDCl3): δ [ppm] = 14.39 (q, C14), 19.09 (t, C10), 19.16 (t, C13), 19.28 (t, C4), 21.99 (q, C15), 31.55 (t, C3), 33.01 (t, C9), 35.50/35.69 (2t, C5 C11), 38.89 (t, C12), 65.21 (d, C8), 68.85 (d, C2), 96.12 (s, C6).
The 70 eV mass spectrum of S4a was identical to the plotted one published earlier (Francke et al., 1981).
Due to the double anomeric effect (Deslongchamps et al., 1981) and the equatorial orientation of both alkyl substituents (Francke et al., 1980), S4a was the highly dominating stereoisomer. The two thermodynamically less stable (E,Z)-isomers S4b and S4c were formed as by-products, whereas the diequatorially linked highly unstable 4d was not obtained in detectable amounts. The same synthetic approach, but using racemic 2-methyloxirane yielded a mixture of all eight possible stereoisomers of 8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (rac-S4), dominated by the racemate of the (2E,8E)-isomer. Enantioselective gas chromatography, employing a home-made 30 m × 0.25 mm i.d. fused silica capillary coated with a 1:1-mixture of OV-1701 and heptakis-[2,3-di-O-methyl-6-O-(tert-butyldimethylsilyl)]-β-cyclodextrin as the stationary phase, separated the enantiomers well. Hydrogen as the carrier gas at a constant oven temperature of 90°C produced an α-value of 1.22 = (ret. time 2S,6R,8S):(ret. time 2R,6S,8R).
Bioassays
The attractiveness of acetone solutions of the scent of C. dolichophylla (see above) was tested in China (for experiments in Germany see Heiduk et al., 2010). Samples were assayed on six different days (4× Fanjing Mt. Area 1, 2× SCBG). Each time a glass vial containing an acetone solution of natural scent (see before) was offered against a similar glass vial filled with pure acetone (control). Within a distance of 30 cm to each other the vials were tucked into the ground and offered for at least 30 min and up to 60 min. The amount of scent available in a sample was sufficient for two assays. The attractiveness of single EAD-active compounds was tested both in China (Fanjing Mt., SCBG) and in Germany (Bayreuth). The compounds were chosen based on preliminary assays with fractions of the complete flower scent. We used the major EAD-active (see below) volatile compounds N-(3-methylbutyl)acetamide (1), stereochemically pure (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) and its racemate (including ca. 5% of the three other stereoisomers, which slightly differs from the natural proportions, see 2.8). Racemic (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane was tested in addition. Since this compound eluted shortly after S4a, it was potentially considered also EAD-active. (E,E)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane was also used as a racemic mixture. The substances were diluted (dilution: 10−3; v/v; final volume: 400–600 μl) in acetone (SupraSolv, Merck KgaA, Germany) and offered in glass vials similar to those used for tests with natural samples. As determined by dynamic headspace and GC/MS for S4a, the amount of scent released from the vials resembled the amount of scent released from single flowers as described by Heiduk et al. (2010).
We tested (a) the single components, 1, S5a, and S4/S4a, (b) the three possible two-component mixtures, (c) the three component mixture, and (d) all three components against each other. When using mixtures, proportions were adapted to the ratios found in C. dolichophylla flowers as indicated by dynamic headspace and GC/MS analysis. Vials containing the samples were tucked into the ground with a distance of 20 cm to each other and offered for at least 40 min and up to 60 min. In each bioassay a glass vial with pure acetone was offered as the control. Approaching flies showed a characteristic zig-zag flight with abrupt landing. They were caught when arriving within a maximum distance of 10 cm to the vial containing the sample. Due to their fast and frantic behavior, not all approaching flies could be caught.
Results
Flower visitors/pollinators and PTE
The flowers of C. dolichophylla collected in China altogether contained 119 dipteran individuals, 107 thereof were collected in Area 1 and the other 12 in Area 2. The flies belonged to the families Milichiidae, Chloropidae, Phoridae, and to taxa of lower Diptera (Table 1). Chloropidae were only present in flowers of Area 1.
Table 1
| Location | # assays (positive) | # caught flies | Desmometopa microps (Lamb, 1914) | Desmometopa singaporensis (Kertész, 1899) | sordida (Fallén, 1820) Desmometopa | Desmometopa sp. nr. sordida | Desmometopa varipalpis (Malloch, 1927) | Desmometopa Loew, 1866 sp. | Neophyllomyza leanderi (Hendel, 1924) | Neophyllomyza Melander, 1913 sp. | Aphanotrigonum trilineatum (Meigen, 1830) | Conioscinella Duda, 1929 sp. | Polyodaspis Duda, 1933 sp. | Tricimba Lioy, 1864 sp.1 | Tricimba sp.2 | Phoridae | other Brachycera | lower Diptera | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly families | Milichiidae | Chloropidae | Others | ||||||||||||||||
| Collected from flowers | Fanjing Mt. | 13f10m | 1f | 10f | 9f | 2f | 2f1m | 1f | 1f | 3 | 1 | 65 | |||||||
| TWO-CHOICE ASSAY: SUBSTANCE/MIXTURE VS. ACETONE | |||||||||||||||||||
| Natural scent sample (in acetone) | Fanjing Mt. | 4 (0) | 0 | ||||||||||||||||
| SCBG | 2 (2) | 12 | 5f | 7f | |||||||||||||||
| N-(3-methylbutyl)acetamide (1) | Fanjing Mt. | 5 (1) | 1 | 1 | |||||||||||||||
| SCBG | 5 (0) | 0 | |||||||||||||||||
| Bayreuth | 3 (2) | 2 | 1f | 1f | |||||||||||||||
| (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane (S5a) | Fanjing Mt. | 3 (0) | 0 | ||||||||||||||||
| SCBG | 1 (0) | 0 | |||||||||||||||||
| Bayreuth | 1 (0) | 0 | |||||||||||||||||
| rac-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane as a mixture of all 8 stereoisomers (rac-S4) | Fanjing Mt. | 3 (2) | 4 | 1m | 1f | 1 | 1 | ||||||||||||
| SCBG | 4 (0) | 0 | |||||||||||||||||
| Bayreuth | 1 (1) | 7 | 7f | ||||||||||||||||
| (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) | Fanjing Mt. | 5 (2) | 4 | 3 | 1 | ||||||||||||||
| SCBG | 5 (4) | 8 | 4f | 4f | |||||||||||||||
| Bayreuth | 5 (5) | 62 | 57f2m | 1m | 2 | ||||||||||||||
| mixture of S4a+1 (ratio 8:1) | Fanjing Mt. | 4 (2) | 4 | 1m | 3 | ||||||||||||||
| SCBG | 4 (2) | 26 | 12f | 3f | 10f | 1f | |||||||||||||
| mixture of S5a+1 (ratio 3:1) | Fanjing Mt. | 3 (0) | 0 | ||||||||||||||||
| SCBG | 1 (1) | 2 | 2f | ||||||||||||||||
| mixture of S4a+S5a (ratio 8:3) | Fanjing Mt. | 3 (0) | 0 | ||||||||||||||||
| SCBG | 1 (1) | 0 | |||||||||||||||||
| mixture of 1+S4a+S5a (ratio 1:8:3) | Fanjing Mt. | 4 (0) | 0 | ||||||||||||||||
| FOUR-CHOICE ASSAY: | |||||||||||||||||||
| 1 vs. S4a vs. S5a vs. acetone | SCBG | 1 (1) | 17a | 1f | 2f | 6f | 7f | 1f | |||||||||||
Flies collected from flowers of Ceropegia dolichophylla and attracted to natural flower scent or synthetic scent samples during bioassays in China (Fanjing Mt., SCBG) and Germany (Bayreuth). The locations and the number of (successful) bioassays performed are also given. The sex of flies is indicated if known (f, female; m, male). Bold, numbers of collected/attracted individuals of pollinating species.
All individuals attracted to S4a.
Different taxa from lower Diptera were the most abundant visitors, however, they did not carry pollinaria - nor did the phorid flies. Milichiids were the second most abundant group, and many of these flies carried pollinaria (60.5%). They were determined as Desmometopa microps (13 females, 10 males; 12 with pollinaria), D. varipalpis (one female), Neophyllomyza sp. (9 females, 7 with pollinaria) and N. leanderi (10 females, 7 with pollinaria). With seven individuals, chloropid flies were not very abundant, however, four (57%) of them carried pollinaria. Chloropids were determined as Conioscinella sp. (2 females, 1 with pollinarium), Polyodaspis sp. (1 male, 2 females, all with pollinaria), and Tricimba spp. (2 females of different species, both with pollinaria).
Among the 267 flowers analyzed in Area 1, 51 pollinaria were found to be removed and 4 pollinia inserted, resulting in a PTE of 4%. The percentage of flowers with removed pollinaria was 13%, and 1% of flowers had pollinia inserted. On average and per flower, 0.19 pollinaria were removed and 0.02 pollinia inserted. Of the 58 flowers collected in Area 2 altogether 47 pollinaria were removed and 2 pollinia inserted, yielding a PTE of 2%. The percentage of flowers with removed pollinaria was 28%, and 3% of flowers had pollinia inserted. On average 0.81 pollinaria were removed per flower, whereas 0.03 pollinia were inserted.
Flower scent
In the floral scent of C. dolichophylla 53 different components were detected: 14 spiroacetals (40.3%), 6 alkanes (16.8%), 4 alkenes (9.4%), 4 other aliphatics (0.1%), one nitrogen containing compound (7.4%), and 23 unknown compounds (1.4%) (Table 2, Figure 3). The most abundant scent components were (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) (27%), tridecane (15%) and N-(3-methylbutyl)acetamide (1) (7,4%), contributing 49% to the total scent. Apart from (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane (S5a) (8%) all other compounds did not exceed 5%.
Table 2
| KRI | Area 1 | Area 2 | Bayreuth* | ||
|---|---|---|---|---|---|
| Median (Min–Max) (N = 7 plants) | Median (Min–Max) (N = 5 plants) | Plant 1 | Plant 2 | ||
| Total amount of scent (ng/15 min*flower) | 32.2 (14.6–66.7) | 79.8 (16.1–117.0) | 311.3 | 195.1 | |
| ALIPHATICS | |||||
| Alkanes | |||||
| Undecane#1 | 1100 | 0.3 (0–12.8) | 1.5 (0–18.0) | 0 | 0 |
| Tridecane#1 | 1302 | 7.0 (1.4–54.0) | 22.2 (13.3–40.2) | 19.7 | 22.3 |
| 2-Acetoxyundecane | 1432 | tr (0–1.0) | 0.3 (0–1.8) | 0 | 0 |
| Pentadecane#1 | 1500 | 0.3 (0–0.6) | 0.4 (0.2–1.1) | 0.7 | 0.8 |
| 2-Acetoxytridecane#2 | 1629 | 0.7 (0.2–5.1) | 0.8 (0.3–2.6) | 2.4 | 2.1 |
| Alkenes | |||||
| 6-Tridecene 3EAD | 1289 | 0.1 (0–0.7) | 1.1 (0.1–2.2) | 0.9 | 2.8 |
| 5-Tridecene 4EAD | 1291 | 0.4 (0–1.7) | 1.2 (0.3–2.9) | 0.9 | 1.6 |
| 6,9-Pentadecadiene 8EAD | 1481 | 1.9 (0.1–10.7) | 3.8 (1.1–9.5) | 4.6 | 5.4 |
| 6- + 7-Pentadecene 9EAD | 1485 | 1.8 (0.5–14.6) | 3.6 (1.4–18.1) | 10.6 | 12.4 |
| 5-Pentadecene | 1491 | 0.1 (0–1.0) | 2.1 (0–18.1) | 0.1 | 0.3 |
| SPIROACETALS | |||||
| (E,E)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane S1a#3,EAD | 1149 | 2.8 (0.7–14.3) | 3.1 (0.1–11.8) | 7.8 | 10.6 |
| 2-Ethyl-7-methyl-1,6-dioxaspiro[4.5]decane S2#3,EAD | 1163 | 0 (0–0.4) | 0 (0–0.6) | 0 | 0 |
| (E,Z)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane S1b#3,EAD | 1223 | 0.1 (0.1–0.4) | 0.1 (0–0.7) | 0.6 | 0.7 |
| (E,E)-2-Ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane S3#3,EAD | 1239 | 0.2 (0.1–0.1) | 0.7 (0.1–3.2) | 0.4 | 0.8 |
| (2S,6R,8S)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane S4a#3,EAD | 1325 | 33.0 (9.6–59.3) | 21.8 (14.5–43.6) | 36.1 | 28.4 |
| (E,E)-2,8-Diethyl-1,7-dioxaspiro[5.5]undecane S5a#3,EAD | 1330 | 9.0 (3.2–16.0) | 6.5 (4.0–12.1) | 10.9 | 7.8 |
| 7-Ethyl-2-propyl-1,6-dioxaspiro[4.5]decane S6EAD | 1333 | 0.3 (0–0.4) | 0.2 (0.1–0.4) | 0 | 0 |
| 8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undec-3-ene S7EAD | 1346 | 0.2 (0.1–0.4) | 0.2 (0–0.3) | 0.2 | 0.1 |
| 2-Ethyl-7-propyl-1,6-dioxaspiro[4.5]decane S8EAD | 1354 | 0.1 (0–0.2) | 0.1 (0–0.2) | 0 | 0 |
| 2-Ethyl-8-methyl-1,7-dioxaspiro[5.6]dodecane S9 | 1377 | 0.1 (0.1–0.3) | 0.1 (0.1–0.2) | 0.3 | 0 |
| (E,Z)-2,8,Diethyl-1,7-dioxaspiro[5.5]undecane S5b#3,EAD | 1389 | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.4 | 0.3 |
| (E,Z)/(Z,E)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane S4b/c#3,EAD | 1392 | 0.3 (0.1–0.7) | 0.3 (0.1–0.9) | 0.5 | 0.4 |
| (E,Z)/(Z,E)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane S4b/c#3 | 1397 | 0.3 (0.1–0.7) | 0.3 (0.2–0.7) | 0.6 | 0.6 |
| (Z,Z)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane S4d#3 | 1449 | 0.1 (0.1–0.3) | 0.1 (0.1–0.2) | 0.1 | 0.1 |
| OTHERS | |||||
| Undecan-2-one#1 | 1296 | 0 (0–0.1) | 0.1 (0–0.3) | 0 | 0 |
| α-Ionone | 1444 | 0 | 0 | 0.1 | 0.2 |
| Tridecan-2-one 10#1,EAD | 1499 | 0 (0–0.4) | tr (0–0.2) | 0 | 0 |
| NITROGEN CONTAINING COMPOUNDS | |||||
| N-(3-Methylbutyl)acetamide 1#3,EAD | 1135 | 10.3 (7.8–52.1) | 4.5 (1.2–11.1) | 1.7 | 0.8 |
| UNKNOWNSa | 0.9 (0.3–2.3)21 | 1.2 (0.4–7.8)21 | 0.11 | 0.11 | |
| m/z: 55,97,115 5EAD | 1332 | 0.3 (0–0.7) | 0.1 (0–0.3) | 0 | 0 |
| m/z: 45,83,97,126,154 11EAD | 1504 | 0 (0–0.3) | tr (0–tr) | 0 | 0 |
Volatiles of Ceropegia dolichophylla flowers collected from field plants in China ( Area 1 and Area 2 ) and from greenhouse plants in Germany (Heiduk et al., 2010).
KRI, Kovats retention index; tr, amount <0.05%; in bold, values >5.0%;
Compound verified through authentic standard, which were purchased from Sigma-Aldrich (#1) or available in the collections of TT (#2) and WF (#3);
, electrophysiologically active;
Samples collected by Heiduk et al. (2010) and reanalyzed for present work;
Upper script digits indicate the number of compounds pooled.
Figure 3
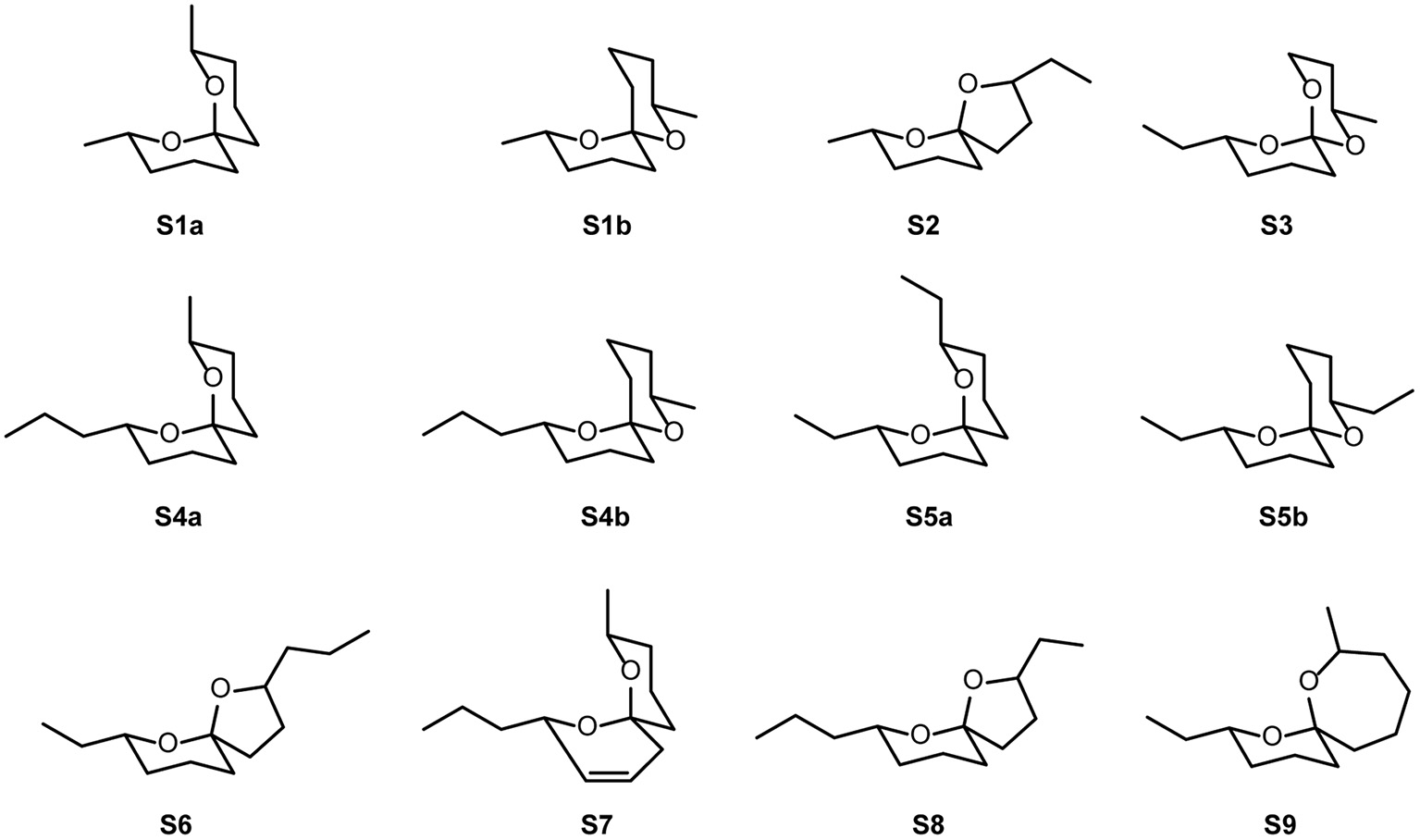
Structures of spiroacetals S1–S9 without stereochemical assignments. For stereochemically correct structures of naturally occurring S4a–d see Figure 1.
The total amount of scent per flower (ng/15 min; 10 min accumulation + 5 min sampling) was highly variable and ranged from 15 to 67 ng in Area 1 (median: 32 ng), and 16 to 117 ng in Area 2 (median: 80 ng), respectively. There was no significant difference in total amounts of scent between Area 1 and Area 2 [t(10) = −2.0, p = 0.074]. Furthermore, no significant differences in scent profiles (relative scent composition) were found [PERMANOVA: Pseudo-F(1, 10): 1.4, p = 0.198] between plants of Area 1 and Area 2.
A comparison of scent from field and greenhouse (Heiduk et al., 2010) plants revealed eight compounds exclusively present in field plants and two compounds only present in greenhouse plants (Table 2).
Electrophysiological analysis
Only two (Desmometopa sp. nr. sordida and D. varipalpis) of the 14 flies from China and three of the five female D. sordida from Bayreuth gave obvious antennal signals. Antennae of the other flies had too much noise in their signals and, thus, were not included in the analysis.
Of the 53 components found in scent samples of C. dolichophylla collected in the field, 22 compounds (Table 2) were electrophysiologically active in the two species tested. The antenna of D. varipalpis responded to 19 compounds, most strongly to N-(3-methylbutyl)acetamide (1), (2S,6R,8S)-8-methy-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) + (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane (S5a), (E,Z)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane (S5b), and an unknown compound (Figure 4A). Of the 19 components with EAD-activity in D. varipalpis, the antenna of D. sp. nr. sordida responded only to 1 and to 6,9-pentadecadiene (8) as well as to 6- + 7-pentadecene (9) (Figure 4A).
Figure 4
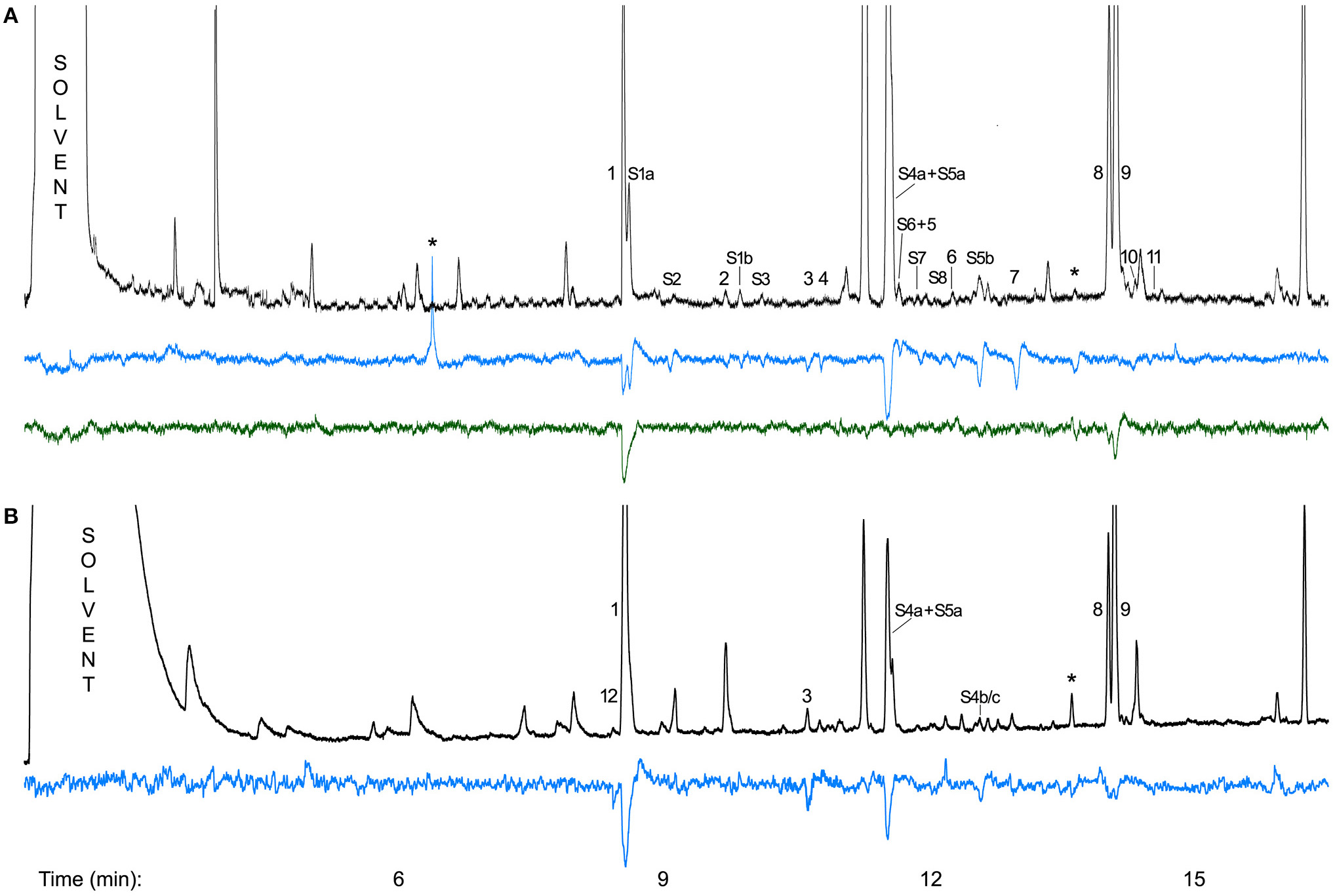
(A) Antennal responses of a female Desmometopa varipalpis (blue) and a female D. sp. nr. sordida (green) to components of the flower scent of Ceropegia dolichophylla collected in Fanjing Mt., China; (B) Antennal responses of a female Desmometopa sordida to a flower scent sample of C. dolichophylla collected in Bayreuth, Germany. 1, N-(3-methylbutyl)acetamide;
S1a, (E,E)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane;and Figure S2, 2-Ethyl-7-methyl-1,6-dioxaspiro[4.5]decane; 2, unknown compound not detected in TD-samples;
S1b, (E,Z)-2,8-Dimethyl-1,7-dioxaspiro[5.5]undecane;
S3, (E,E)-2-Ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane; 3, 6-Tridecene; 4, 5-Tridecene;
S4a + S5a, (2S,6R,8S)-8-Methy-2-propyl-1,7-dioxaspiro[5.5]undecane + (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane;
S6 + 5, 7-Ethyl-2-propyl-1,6-dioxaspiro[4.5]decane + KI 1332;
S7, 8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undec-3-ene;
S8, 2-Ethyl-7-propyl-1,6-dioxaspiro[4.5]decane; 6, unknown compound not detected in TD-samples;
S5b, (E,Z)-2,8-Diethyl-1,7-dioxaspiro[5.5]undecane; 7, unknown compound not detected in TD-samples; 8, 6,9-Pentadecadiene; 9, 6- + 7-Pentadecene; 10, 2-Tridecanone; 11, KI 1504; 12, unknown compound not detected in TD-samples;
S4b/c, (E,Z)/(Z,E)-8-Methyl-2-propyl-1,7-dioxaspiro[5.5]undecane. *, artifact/response to contamination; KI, Kovats retention index. All compounds except S3 elicited signals in at least two of the three species and/or were consistently active in repeated measurements with single individuals.
All three female D. sordida from Bayreuth responded to the same seven compounds (Figure 4B). Five of them were also active on flies from China and scent samples from plants collected in the field. In each run, 1 and/or S4a + S5a elicited the strongest antennal responses (Figure 4).
Bioassays
During bioassays in China (Fanjing Mt. and SCBG) and Germany (Bayreuth) only Diptera were attracted, and no fly responded to the negative controls (Table 1).
Samples of natural flower scent were tested in China (for experiments in Germany see Heiduk et al., 2010) at Fanjing Mt. Area 1 (4 replicates), and at SCBG (2 replicates), and only at SCBG were flies attracted. The first flies approached in zig-zag flight within a minute after opening the sample vial. Altogether, 12 of the approaching flies (seven female Neophyllomyza sp., five female N. leanderi) were caught, all of them from taxa that occur as natural pollinators of C. dolichophylla.
In bioassays with synthetic samples (China and Germany), altogether 137 attracted flies were collected, the majority of them in Bayreuth (N = 71) and SCBG (N = 53), and a few (N = 13) in Fanjing Mt. Their behavior in approaching the sample vials was identical to that elicited by samples of natural flower scent. Milichiidae were the most numerous attracted flies (97%). They represented eight different species, among them three species pollinating C. dolichophylla. Two different species of Chloropidae were represented by four flies, and one of these species was identified as a pollinator of C. dolichophylla. The remaining flies were from non-pollinating taxa.
Flies were attracted mostly by (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a) and mixtures containing this spiroacetal. Pollinating species were attracted to S4a, N-(3-methylbutyl)acetamide (1), to the mixture of S4a + 1, and to the mixture of (E,E)-2,8-diethyl-1,7-dioxaspiro[5.5]undecane (S5a) + 1. Non-pollinating species responded to the same lures except the mixture of S5a + 1.
In the four-choice assays which offered 1, S5a, S4a, and acetone, all flies responded to S4a. Overall, most flies were attracted to S4a, and the majority of them were the pollinating milichiid D. microps. The majority of non-pollinating species were attracted to the mixture of S4a + 1.
Discussion
This study specifies milichiid and chloropid flies as pollinators of C. dolichophylla, shows that the pollination rate is low, and identifies an uncommon spiroacetal, (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a), as the main scent component and as a compound capable of attracting fly pollinators.
Identification of flies trapped in flowers revealed natural pollinators of C. dolichophylla. They belong to several milichiid and chloropid genera, only two of which (Milichiidae: Desmometopa, Neophyllomyza) were previously described as flower visitors of C. dolichophylla (Heiduk et al., 2010). All species found to act as pollinators in China (Table 1, printed in bold) did not occur as pollinators of C. dolichophylla in Germany (Heiduk et al., 2010), and the milichiid fly D. sordida, pollinator of C. dolichophylla in Germany (Heiduk et al., 2010) does not pollinate the flowers in China. This discrepancy can only partly be explained by the distribution range of the flies, because several of the Chinese pollinators (e.g., N. leanderi, D. microps) occur in Germany as well. D. sordida has been found in Mongolia (Papp, 1976) and Japan (Iwasa, 1996) and is likely to occur in China, though possibly not as far south as our study site. Chloropid flies were not present in flowers of Area 2. However, all flowers from this area were sampled on a single day and due to local population dynamics chloropid flies might just have been absent in Area 2 at that point of time. Furthermore, floral scent as the attractive cue did not differ among the sites and should thus not have been responsible for observed differences in the presence of Chloropidae.
Though the abundance of pollen carrying flies was quite high in flowers, the pollination success was found to be very low in the investigated species. This finding is consistent with data published for C. ampliata (Coombs et al., 2011), the only other Ceropegia studied in this context.
The pollinating taxa of C. dolichophylla identified to species level are not yet known as visitors/pollinators of other Ceropegia species, however, all genera except Polyodaspis are already known from Ceropegia (Knuth, 1898--1905; Vogel, 1961, 1993; Masinde, 2004; Ollerton et al., 2009; Heiduk et al., 2010). Milichiidae and Chloropidae have rarely been described as pollinators in other angiosperms, but are known as pollinators from other Apocynaceae (Raspi et al., 2009; Pisciotta et al., 2011), rewarding and non-rewarding orchid species (Borba and Semir, 2001; Chase et al., 2014; Nunes et al., 2014), and several species of Aristolochia (Brantjes, 1980; Wolda and Sabrosky, 1986; Oelschlägel et al., 2015). Lower Diptera were the most abundant flower visitors but did not carry pollinia and, therefore, are no pollinators of C. dolichophylla. However, different taxa of lower Diptera are described as pollinators for several other Ceropegia species (Ollerton et al., 2009). Lower Diptera are small enough to enter the flowers of C. dolichophylla but they fail as pollinators probably due to morphological features. Successful removal of pollinaria requires an optimal fit of the fly headfirst into the coronal cavities below and around the guide rail entrances. After insertion of the proboscis (or parts of it) the fly has to be strong enough to pull the pollinarium off the style-head. Possibly, the proboscides of lower Diptera are too short for successful guide rail insertion or pollinarium attachment, or the flies are too weak to remove the pollinarium. Selection against flies that are either too big or too small through morphological features is also described in Aristolochia, another plant group with pitfall flowers pollinated by flies (Berjano et al., 2009; Oelschlägel et al., 2009).
As shown already by Heiduk et al. (2010) and confirmed in the present study, flower visiting/pollinating flies are attracted to extracts of natural scent samples. We additionally identified corresponding biologically active compounds.
Our electrophysiological studies show that only a subset of the volatiles, including most of the spiroacetals, is perceived by the flies (Table 2, Figures 4A,B). Furthermore, we found that there are differences in perception among different pollinating fly species. Nevertheless, all tested species perceive at least one of the main compounds N-(3-methylbutyl)acetamide (1) or (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a). Furthermore, in field bioassays S4a was especially attractive to flies of several taxa, including pollinators. This spiroacetal as well as the other spiroacetals identified in the present study are unknown plant volatiles (cf. Knudsen et al., 2006). Generally, spiroacetals are rare constituents of floral scent. Just recently spiroacetals were shown to have a function in attracting pollinators, as they are key signals for host plant recognition of a solitary bee that specializes on Campanula flowers (Milet-Pinheiro et al., 2013).
Despite being rare in floral scents, spiroacetals are very widespread in nature and also produced by microorganisms and animals, including mammals. However, the biological significance of these compounds is known only in a few cases (Francke and Kitching, 2001). Apart from a few exceptions, the carbon skeletons are unbranched and show an uneven number of carbon atoms.
Consistent with Heiduk et al. (2010), we found in bioassays that flies respond very quickly, mostly within the first minute after being offered the test sample. This underlines the outstanding importance of the C. dolichophylla floral scent in attracting fly pollinators. The quick response of the flies could also explain why within the natural population of C. dolichophylla only low numbers of flies were attracted. C. dolichophylla flowers open in the morning shortly before sunrise (Heiduk et al., 2010), and bioassays were performed only after sunrise. Thus, most flies available in the habitat may already have been trapped by newly opened flowers before bioassays took place.
Several of the flies attracted by flowers of C. dolichophylla (e.g., Milichiidae: Desmometopa, Neophyllomyza; Chloropidae: Conioscinella, Tricimba) are kleptoparasites which feed on preyed-upon insects (Frost, 1913; Robinson and Robinson, 1977; Sivinski and Stowe, 1980; Landau and Gaylor, 1987; Eisner et al., 1991; Sivinski et al., 1999; Zhang and Aldrich, 2004; Marshall, 2012; Von Tschirnhaus et al., 2014), such as wasps, bees, lacewings, and true bugs. Interestingly, secretions of such insects contain compounds identified as biologically active scent compounds of C. dolichophylla in the present study. Among them are several spiroacetals, N-(3-methylbutyl)acetamide (1), 6-tridecene (3), 7-pentadecene (9), and 2-tridecanone (10) (Dani et al., 2000; Francke and Kitching, 2001; Bruschini et al., 2006; El-Sayed, 2014). Venom glands of paper wasps (Polistes), for example, contain 8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4), (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane (S1a), 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane (S2), and N-(3-methylbutyl)acetamide (1) (see Bruschini et al., 2006). Both spiroacetals S1a and S2 have also been identified in the cephalic secretions of Andrena bees (Francke et al., 1981; Bergström et al., 1982). Interestingly, (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane keeps (2S,6R,8S)-configuration in A. wilkella (Tengö et al., 1990)—the same stereochemistry as in the major spiroacetal of C. dolichophylla. Thus, volatile signals and constituents of defense glands of bees and/or wasps could well be mimicked by C. dolichophylla. Indeed, preyed upon wasps fighting against an arthropod predator (e.g., praying mantis, spider) are a food source for kleptoparasitic flies (Micallef, 2010). Moreover, wasps are predators themselves, and the flies may seek for the wasps' prey item. Wasps stun and/or kill their prey using their venom, and kleptoparasitic flies might use these venom volatiles as key signals to locate a wasp with fresh prey, on which they could feed. Therefore, C. dolichophylla probably makes use of compounds which indicate the presence of prey items for food-seeking kleptoparasitic flies. Thus, the flowers are kleptomyiophilous and fool kleptoparasitic flies into pollinating them.
Kleptomyiophily was unknown until recently, when it was discovered in Aristolochia and Ceropegia in parallel. Oelschlägel et al. (2015) described it for the first time for a deceptive Aristolochia species pollinated by kleptoparasitic Chloropidae. Independently from each other, the early diverged lineage Aristolochia (39.5 million years ago; Naumann et al., 2013) and the much younger group Ceropegia (10 million years ago; Rapini et al., 2007) evolved both, the trap flowers and the kleptomyiophilous pollination strategy.
To conclude, we show that deceptive C. dolichophylla fools its kleptoparasitic fly pollinators by a kleptomyiophilous pollination strategy using exceptional floral scent. Flowers emitted several spiroacetals, many of which were known from insect secretions, but unknown in floral scents before this study. Additional compounds released were N-(3-methylbutyl)acetamide (1) and aliphatic alkenes. Several of the compounds elicited electrophysiological responses in antennae of fly pollinators, among them (2S,6R,8S)-8-methyl-2-propyl-1,7-dioxaspiro[5.5]undecane (S4a). This spiroacetal was proven to be highly attractive for pollinators in behavioral assays. Further studies will show whether other Ceropegia species also evolved a kleptomyiophilous pollination strategy and if so, which compounds they use to trick their pollinators.
Statements
Author contributions
AH, SD, and UM designed the study. HK performed bioassays in China in 2012. AH collected all other field data. SD performed the electrophysiological measurements. IB and MV identified fly pollinators. WF, AT, EW, and TT identified and/or synthesized compounds. AH analyzed the data and wrote the first draft of the manuscript. WF wrote the part of the chemical synthesis. All authors contributed to interpretation of the findings and edited and approved the manuscript.
Acknowledgments
The authors thank Irmgard Schäffler, Yu Hong, Yun Zhou, and Xiaolin Xie for help during field trips, and Irmgard Schäffler additionally for help with preparation of Figures. We also thank Frank Menzel for assistance with the identification of flies, and Kjirsten Wayman for valuable comments on the manuscript. This research was supported by a grant for PhD candidates according to Bavarian elite promotion law (BayEFG) and Natural Science Foundation of China (Project No. 31470319, 30900088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- PTE
pollen transfer efficiency
- GC/MS
gas chromatography linked to mass spectrometry
- GC/EAD
gas chromatography coupled with electroantennographic detection.
Abbreviations
References
1
Adams R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL: Allured Publishing Corporation.
2
Anderson M. J. Gorley R. N. Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E.
3
Bergström G. Tengö J. Reith W. Francke W. (1982). Multicomponent mandibular gland secretions in three species of Andrena bees (Hym., Apoidea). Z. Naturforsch. 37c, 1124–1129.
4
Berjano R. Ortiz P. L. Arista M. Talavera S. (2009). Pollinators, flowering phenology and floral longevity in two Mediterranean Aristolochia species, with a review of flower visitor records for the genus. Plant Biol. 11, 6–16. 10.1111/j.1438-8677.2008.00131.x
5
Bohmann B. Phillips R. D. Menz M. H. M. Berntsson B. W. Flematti G. R. Barrow R. A. et al . (2014). Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: implications for the evolution of sexual deception. New Phytol. 203, 939–952. 10.1111/nph.12800
6
Borba E. L. Semir J. (2001). Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Ann. Bot. 88, 75–88. 10.1006/anbo.2001.1434
7
Brantjes N. B. M. (1980). Flower morphology of Aristolochia species and the consequences for pollination. Acta Bot. Neerl. 29, 212–213.
8
Bruschini C. Dani F. R. Pieraccini G. Guarna F. Turillazzi S. (2006). Volatiles from the venom of five species of paper wasps (Polistes dominulus, P. gallicus, P. nimphus, P. sulcifer and P. olivaceus).Toxicon47, 812–825. 10.1016/j.toxicon.2006.03.002
9
Buser H. R. Arn H. Guerin P. Rauscher S. (1983). Determination of double-bond position in monounsaturated acetates by mass-spectrometry of dimethyl disulfide adducts. Anal. Chem. 55, 818–822. 10.1021/ac00257a003
10
Chase M. W. Cribb P. J. Pridgeon A. M. Rasmussen F. N. (2014). Genera Orchidacearum, Vol. 6: Epidendroideae (Part 3).Oxford: Oxford University Press.
11
Clarke K. R. Gorley R. N. (2006). Primer v6: User Manual/Tutorial. Plymouth: Primer-E.
12
Coombs G. Dold A. P. Peter C. I. (2011). Generalized fly-pollination in Ceropegia ampliata (Apocynaceae-Asclepiadoideae): the role of trapping hairs in pollen export and receipt. Plant Syst. Evol. 296, 137–148. 10.1007/s00606-011-0483-6
13
Coombs G. Peter C. I. Johnson S. D. (2009). A test for allee effects in the self-incompatible wasp-pollinated milkweed Gomphocarpus physocarpus. Aust. Ecol. 34, 688–697. 10.1111/j.1442-9993.2009.01976.x
14
Dani F. R. Jeanne R. L. Clarke S. R. Jones G. R. Morgan E. D. Francke W. et al . (2000). Chemical characterization of the alarm pheromone in the venom of Polybia occidentalis and of volatiles from the venom of P. sericea.Physiol. Entomol. 25, 363–369. 10.1046/j.1365-3032.2000.00205.x
15
Deslongchamps P. Rowan D. D. Pothier N. Sauvé G. Saunders J. K. (1981). 1,7-Dioxaspiro[5.5]undecanes. An excellent system fort the study of stereoelectronic effects (anomeric and exo-anomeric effects) in acetals. Can. J. Chem. 59, 1105–1121.
16
Dötterl S. Füssel U. Jürgens A. Aas G. (2005a). 1,4-Dimethoxybenzene, a floral scent compound in willows that attracts an oligolectic bee. J. Chem. Ecol. 31, 2993–2998. 10.1007/s10886-005-9152-y
17
Dötterl S. Wolfe L. M. Jürgens A. (2005b). Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry66, 203–213. 10.1016/j.phytochem.2004.12.002
18
Doubský J. Streinz L. Šaman D. Zedník J. Koutek B. (2004). Alkynyltrifluoroborates as versatile tools in organic synthesis: a new route to spiroketals. Org. Lett. 6, 4909–4911. 10.1021/ol047987k
19
eFloras. (2015). St. Louis; Cambridge: Missouri Botanical Garden; Harvard University Herbaria. Available online at: http://www.efloras.org (Accessed February 28, 2015).
20
Eisner T. Eisner M. Deyrup M. (1991). Chemical attraction of kleptoparasitic flies to heteropteran insects caught by orb-weaving spiders. Proc. Natl. Acad. Sci. U.S.A. 88, 8194–8197. 10.1073/pnas.88.18.8194
21
El-Sayed A. M. (2014). The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online at: http://www.pherobase.com (Accessed February 28, 2014).
22
Francke W. Kitching W. (2001). Spiroacetals in insects. Curr. Org. Chem. 5, 233–251. 10.2174/1385272013375652
23
Francke W. Reith W. Bergström G. Tengp J. (1981). Pheromone bouquet of the mandibular glands in Andrena haemorrhoa F. (Hym., Apoidea). Z. Naturforsch. 36c, 928–932.
24
Francke W. Reith W. Sinnwell V. (1980). Bestimmung der relativen Konfiguration bei Spiroacetalen durch 1H- und 13C-NMR-Spektroskopie. Chem. Ber. 113, 2686–2693. 10.1002/cber.19801130812
25
Frost C. A. (1913). Peculiar habits of small Diptera, Desmometopa latipes Meig. Psyche20, 37. 10.1155/1913/82506
26
Heiduk A. Brake I. Tolasch T. Frank J. Jürgens A. Meve U. et al . (2010). Scent chemistry and pollinator attraction in the deceptive trap flowers of Ceropegia dolichophylla. S. Afr. J. Bot. 76, 762–769. 10.1016/j.sajb.2010.07.022
27
Iwasa M. (1996). The genus Desmometopa Loew (Diptera, Milichiidae) of Japan. Med. Entomol. Zool. 47, 347–353.
28
Jacobsen R. Taylor R. J. Williams H. J. Smith L. R. (1982). Naturally occurring spirocyclic ketals from lactones. J. Org. Chem. 47, 3140–3142. 10.1021/jo00137a020
29
Johnson S. D. Neal P. R. Harder L. D. (2005). Pollen fates and the limits on male reproductive success in an orchid population. Biol. J. Linn. Soc. 86, 175–190. 10.1111/j.1095-8312.2005.00541.x
30
Jürgens A. Wee S.-L. Shuttleoworth A. Johnson S. D. (2013). Chemical mimicry of insect oviposition sites: a global analysis of convergence in angiosperms. Ecol. Lett. 16, 1157–1167. 10.1111/ele.12152
31
Knudsen J. T. Eriksson R. Gershenzon J. Ståhl B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2
32
Knuth P. (1898–1905). Handbuch der Blütenbiologie. Leipzig: Engelmann Verlag.
33
Landau G. D. Gaylor M. J. (1987). Observations on commensal Diptera (Milichiidae and Chloropidae) associated with spiders in Alabama. J. Arachn. 15, 270–272.
34
Marshall S. A. (2012). Flies: The Natural History and Diversity of Diptera. Buffalo, NY: Firefly Books.
35
Masinde P. S. (2004). Trap-flower fly pollination in East African Ceropegia L. (Apocynaceae). Int. J. Trop. Insect Sci. 24, 55–72. 10.1079/ijt20044
36
Micallef C. (2010). Available online at: http://bugguide.net/node/view/512989/bgimage (Accessed November 21, 2014).
37
Milet-Pinheiro P. Ayasse M. Dobson H. E. M. Schlindwein C. Francke W. Dötterl S. (2013). The chemical basis of host-plant recognition in a specialized bee pollinator. J. Chem. Ecol. 39, 1347–1360. 10.1007/s10886-013-0363-3
38
Naumann J. Salomo K. Der J. P. Wafula E. K. Bolin J. F. Maass E. et al . (2013). Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a cretaceous origin of multiple parasitic angiosperm lineages. PLoS ONE8:e79204. 10.1371/journal.pone.0079204
39
Nunes E. L. P. Smidt E. C. Stuetzel T. Coan A. I. (2014). What do floral anatomy and micromorphology tell us about Neotropical Bulbophyllum section Didactyle (Orchidaceae: Bulbophyllinae)?Bot. J. Linn. Soc. 175, 438–452. 10.1111/boj.12176
40
Oelschlägel B. Gorb S. Wanke S. Neinhuis C. (2009). Structure and biomechanics of trapping flower trichomes and their role in the pollination biology of Aristolochia plants (Aristolochiaceae). New Phytol. 184, 988–1002. 10.1111/j.1469-8137.2009.03013.x
41
Oelschlägel B. Nuss M. Von Tschirnhaus M. Pätzold C. Neinhuis C. Dötterl S. et al . (2015). The betrayed thief: the extraordinary strategy of Aristolochia rotunda to deceive its pollinators. New Phytol. 206, 342–351. 10.1111/nph.13210
42
Ollerton J. Masinde S. Meve U. Picker M. Whittington A. (2009). Fly pollination in Ceropegia (Apocynaceae: Asclepiadoideae): biogeographic and phylogenetic perspectives. Ann. Bot. 103, 1501–1514. 10.1093/aob/mcp072
43
Papp L. (1976). Milichiidae and Carnidae (Diptera) from Mongolia. Acta Zool. Acad. Sci. Hung. 22, 369–387.
44
Phillips C. Jacobson R. Abrahams B. Williams H. J. Smith L. R. (1980). Useful route to 1,6-dioxaspiro[4.4]nonane and 1,6-dioxaspiro[4.5]decane derivatives. J. Org. Chem. 45, 1920–1924. 10.1021/jo01298a033
45
Pisciotta S. Raspi A. Sajeva M. (2011). First records of pollinators of two co-occurring Mediterranean Apocynaceae. Plant Biosyst. 145, 141–149. 10.1080/11263504.2010.540779
46
Raguso R. A. (2008). Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 39, 549–569. 10.1146/annurev.ecolsys.38.091206.095601
47
Rapini A. van den Berg C. Liede-Schumann S. (2007). Diversification of Asclepiadoideae (Apocynaceae) in the New World. Ann. Mo. Bot. Gard. 94, 407–422. 10.3417/0026-6493(2007)94[407:DOAAIT]2.0.CO;2
48
Raspi A. Pisciotta S. Sajeva M. (2009). Milichiella lacteipennis: new record for Lampedusa Island (Italy). Bull. Insectol. 62, 133–135.
49
Robinson M. H. Robinson B. (1977). Associations between flies and spiders: bibiocommensalism and dipsoparasitism. Psyche84, 150–157. 10.1155/1977/26019
50
Sabrosky C. W. (1983). A synopsis of the world species of Desmometopa Loew (Diptera, Milichiidae). Contrib. Am. Entomol. Inst. 19, 1–69.
51
Salzmann C. C. Cozzolino S. Schiestl F. P. (2007). Floral scent in food-deceptive orchids: species specificity and sources of variability. Plant Biol. 9, 720–729. 10.1055/s-2007-965614
52
Sivinski J. (1985). Mating by kleptoparasitic flies (Diptera: Chloropidae) on a spider host. Fla. Entomol. 68, 216–222. 10.2307/3494346
53
Sivinski J. Marshall S. Petersson E. (1999). Kleptoparasitism and phoresy in the Diptera. Fla. Entomol. 82, 179–197. 10.2307/3496570
54
Sivinski J. Stowe S. (1980). A kleptoparasitic cecidomyiid and other flies associated with spiders. Psyche87, 337–348. 10.1155/1980/27685
55
StatSoft Inc. (2005). STATISTICA (Data Analysis Software System), Version 7.1. Available online at: www.statsoft.com.
56
Tengö J. Ågren L. Baur B. Isaksson R. Liljefors T. Mori K. et al . (1990). Andrena wilkella male bees discriminate between enantiomers of cephalic secretion components. J. Chem. Ecol. 16, 429–441. 10.1007/BF01021775
57
Vogel S. (1961). Die Bestäubung der Kesselfallen-Blüten von Ceropegia. Beitr. Biol. Pflanzen36, 159–237.
58
Vogel S. (1993). Betrug bei Pflanzen: die Täuschblumen. Akad. Wiss. Mainz, Abh. Math.-Naturwiss. Kl. 1993. Stuttgart: Franz Steiner Verlag GmbH.
59
Von Tschirnhaus M. Borkenstein A. Jödicke R. (2014). Lestes dryas and commensalic flies (Odonata: Lestidae; Diptera: Chloropidae), with an overwiew on kleptoparasitism of frit flies. Mercuriale14, 1–12.
60
Wolda H. Sabrosky C. W. (1986). Insect visitors to two forms of Aristolochia pilosa in Las Cumbres, Panama. Biotropica18, 295–299. 10.2307/2388572
61
Zhang Q. H. Aldrich J. R. (2004). Attraction of scavenging chloropid and milichiid flies (Diptera) to metathoracic scent gland compounds of plant bugs (Heteroptera: Miridae). Environ. Entomol. 33, 12–20. 10.1603/0046-225X-33.1.12
Summary
Keywords
fly pollination, kleptomyiophily, kleptoparasites, spiroacetals, food deception
Citation
Heiduk A, Kong H, Brake I, von Tschirnhaus M, Tolasch T, Tröger AG, Wittenberg E, Francke W, Meve U and Dötterl S (2015) Deceptive Ceropegia dolichophylla fools its kleptoparasitic fly pollinators with exceptional floral scent. Front. Ecol. Evol. 3:66. doi: 10.3389/fevo.2015.00066
Received
30 March 2015
Accepted
10 June 2015
Published
03 July 2015
Volume
3 - 2015
Edited by
Florian Paul Schiestl, University of Zürich, Switzerland
Reviewed by
Cesar Rodriguez-Saona, Rutgers University, USA; Michael Birkett, Rothamsted Research, UK; Marcus Carl Stensmyr, Lund University, Sweden
Copyright
© 2015 Heiduk, Kong, Brake, von Tschirnhaus, Tolasch, Tröger, Wittenberg, Francke, Meve and Dötterl.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Dötterl, Department of Ecology and Evolution, Plant Ecology, University of Salzburg, Hellbrunnerstr. 34, 5020 Salzburg, Austria stefan.doetterl@sbg.ac.at
This article was submitted to Chemical Ecology, a section of the journal Frontiers in Ecology and Evolution
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.