- Independent Researcher, Montréal, QC, Canada
For prey species that visually monitor their surroundings for signs of danger, obstruction in the field of view is expected to increase vigilance against predators. However, visual obstruction could also make it more difficult to monitor neighbors, which might increase vigilance on its own and also reduce the widespread ability to synchronize vigilance with neighbors. To address these issues, I performed an experiment in which the ability to monitor neighbors in chickens (n = 14) was prevented by visual obstruction while the ability to monitor predators was maintained for some group members but not for others. Vigilance increased with visual obstruction, but remained at the same level for foragers prevented from monitoring neighbors and predators and those only prevented from monitoring neighbors. This suggests that the increase in vigilance with visual obstruction was caused by the inability to monitor neighbors rather than predators. Synchronization of vigilance between group members also decreased in the visual obstruction treatment, suggesting that visual cues from neighbors rather than external cues are important to synchronize vigilance. The ability to visually monitor neighbors is thus an important determinant of the level of vigilance maintained by a prey species, and also an important factor in the synchronization of vigilance between neighbors.
Introduction
Prey species can increase their chances of detecting predators before it is too late by visually monitoring their surroundings for signs of danger (Beauchamp, 2015a). Many features of the habitat, however, can obstruct the field of view during vigilance. Examples include stubble in a field (Murton and Isaacson, 1962), rocks on the shore (Metcalfe, 1984), and vegetation cover (Underwood, 1982). Visual obstruction can thus interfere with vigilance and increase predation risk (Lima, 1987). Observational studies with naturally-occurring visual obstacles and experimental studies, in which the field of view is altered by the researchers, have typically documented an increase in vigilance with visual obstruction (Lima, 1992; Arenz and Leger, 1997; Lima and Bednekoff, 1999; Blumstein and Daniel, 2003; Whittingham et al., 2004; Butler et al., 2005; Ebensperger and Hurtado, 2005; Fernández-Juricic et al., 2005; Devereux et al., 2006; Bednekoff and Blumstein, 2009; Iribarren and Kotler, 2012) although there are exceptions (Lima, 1987; Foster-McDonald et al., 2006; Hannon et al., 2006; Hall et al., 2013).
Visual obstruction is thought to increase vigilance by decreasing the ability to detect predators. However, other researchers have argued that visual obstruction can also affect the ability to monitor nearby companions (Elgar et al., 1984; Duncan and Jenkins, 1998; Harkin et al., 2000; Fernández-Juricic et al., 2005). For instance, visual obstacles might temporally hide companions from view and also give the impression that neighbors are further away. The perception of a smaller group with more distant neighbors would be sufficient to increase vigilance on its own. In addition, subtle signs of alert, which are important to communicate danger across the group, might also be missed more often with visual obstruction (Harkin et al., 2000). Teasing apart the contribution of these two factors, namely, the ability to monitor predators or neighbors, requires experiments. In one type of experiment, the ability to monitor neighbors can be maintained constant so that visual obstruction only prevents the detection of predators. In another type of experiment, the ability to detect predators can be maintained constant so that visual obstacles only prevent the detection of neighbors. Few such experiments have been carried out thus far for group-foraging prey species (Fernández-Juricic et al., 2005; Pomeroy et al., 2006; Beauchamp, 2015b).
Visual obstruction could also influence other aspects of vigilance. For prey species that live in groups, vigilance was first thought to be carried out independently by each group member (Pulliam, 1973). However, recent models suggest that group members pay attention to the vigilance of their neighbors and might actually copy their vigilance. One model proposed that the level of vigilance maintained by neighbors can be seen as public information about current predation risk (Sirot, 2006). Such information could be used by others to adjust their vigilance according to the prevailing perception of predation risk in the group. It has also been suggested that by synchronizing their vigilance bouts, individuals could reduce the risk of being left behind during an attack, which could be dangerous if predators preferentially target laggards (Sirot and Touzalin, 2009). Vigilance synchronization has been documented in several species of birds and mammals, suggesting that monitoring neighbors is common in prey species (Fernández-Juricic et al., 2004; Pays et al., 2007a,b, 2009, 2012; Beauchamp, 2009; Ge et al., 2011; Michelena and Deneubourg, 2011; Öst and Tierala, 2011; Butler et al., 2016; Podgórski et al., 2016).
How animals synchronize their vigilance is not well known. The above models suggest that visual cues are important although auditory cues, such as calls or sounds from neighbors, could also be used (Radford and Ridley, 2007; Pereira et al., 2012). Visual obstruction would thus be predicted to alter the ability to synchronize vigilance if visual cues are important when copying vigilance. Synchronization could also be achieved by independent responses by each group member to external cues such as noises or movement in the vegetation, in which case synchronization serves no purpose (Ruxton and Roberts, 1999; Hoppitt et al., 2007). If such external cues rather than those provided by neighbors are predominant, visual obstruction is not expected to have a strong impact on the synchronization of vigilance.
To address these issues, I performed an experiment in which the ability to monitor neighbors was prevented by visual obstruction while the ability to monitor predators was maintained for some group members but not for others. I predicted that the level of vigilance would increase with visual obstruction. If visual obstruction restricts the ability to monitor neighbors as well as predators, I predicted that the increase in vigilance with visual obstruction would be higher for individuals prevented from monitoring both neighbors and predators rather than neighbors only. If visual cues generated by the behavior of neighbors drive synchronization of vigilance, I predicted less synchronization with visual obstruction.
Methods
Study Animals
I used 14 subadult Bovan Brown (a layer breed) female chickens obtained from a commercial hatchery, which I raised together from day old in the same indoor pen. Birds were tagged with unique colored spiral rings for identification. The aviary housing the chickens consisted of a 3 × 3 × 3 m indoor pen that connected through a small trap door to a 3 × 3 × 3 m covered outdoor pen. The indoor pen, under a 13L:11D photoperiod regime, included several perches for roosting and a sandy patch for dust-bathing. Wire mesh covered the sides of the outdoor pen allowing visual and auditory contact with the outside. Water and a commercial chicken grower feed were available at all times inside and outside. I am not aware of other vigilance studies with Bovan Brown chickens. However, other layer breeds of chickens show the same type of vigilance as their wild ancestors albeit at a lower level (Schütz et al., 2001).
Setup and Experimental Procedure
I randomly formed seven pairs of birds. Trials with each pair were conducted in the outdoor pen. To minimize foraging interference during trials, I kept each bird in adjacent but separate 1 × 1 × 1 m cages covered with wire mesh to allow visual and auditory contact (Figure 1). To familiarize birds with the cages, I placed the two cages along with the wood panel used in the visual obstruction treatment (see below) in the outdoor pen 1 week prior to the beginning of the trials.
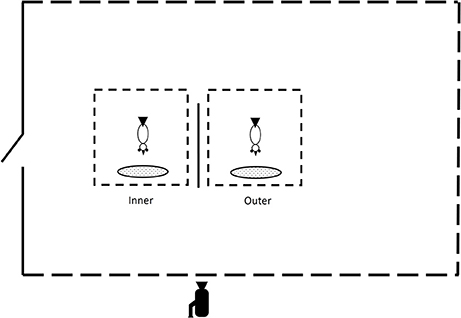
Figure 1. Schematic representation of the experimental setup. Pairs of chickens were held in separate cages open on all sides in a large outdoor pen open on three sides (dashed lines). A trap door on the dividing wall to the left allowed access to an indoor pen (not shown). A wood panel inserted between the two cages obstructed the field of view of the two birds in each pair. Both birds could not visually monitor one another. The outer bird could monitor all three open sides of the outdoor pen. By contrast, the inner bird could not visually monitor much of the area outside the pen. A large amount of food was provided in front of the cages. All trials were videotaped from the outside of the outdoor pen.
The experiment consisted of two treatments applied in cross-over fashion. In the unobstructed treatment, no wood panel was used to separate the cages holding the two birds of each pair, and the two birds could see each other at all times and also monitor the area surrounding the outdoor pen for lurking threats. In the obstructed treatment, a wood panel inserted between the two cages prevented birds from visually monitoring one another (Figure 1). From the point of view of the bird closest to the dividing wall (the inner bird), the panel blocked the view of the nearby cage along with much of the area surrounding the outdoor pen thus restricting the ability to detect lurking predators. By contrast, the bird in the outer cage benefitted from an unobstructed view of the three open sides of the outdoor pen, which would allow a quicker detection of any approaching threat.
I evenly split the 14 trials (two trials per pair) over two different days with similar environmental conditions. On the first day, I performed four unobstructed trials and three obstructed trials, and completed the cross-over on the second day to balance the two types of trials as much as possible over the two experimental days. I randomly allocated a pair to each trial on the first day. I also randomly allocated one bird of each pair to occupy the inner or the outer cage, and reversed their position on the second day. On a given day, trials of the two types were conducted one after the other in random order.
Prior to a trial day, food was removed 1 h before lights out. Trials were conducted the following morning 1 h after lights on. For each trial, birds were caught in the indoor pen and placed in the individual cages. The birds were given 2 min to settle down before a trial started. A large amount of food was placed in front of each cage, and the cages were simply moved over the food patches to signal the start of a trial. A trial started when the birds started to feed and ended 3 min later. I videotaped all trials from the outside of the outdoor pen.
Data Collection
From the videotapes, I measured the % of time spent vigilant by each bird in each trial. All food handling of the crumbly feed occurred in the head down position. Therefore, I assumed that any interruption from feeding by the hungry birds was related to monitoring the surroundings (vigilance). I timed the duration of each vigilance bout by playing videos frame by frame (1 frame = 33 ms). A vigilance bout started when the bird raised its head from the food patch and maintained the bill at the horizontal level, and ended when the bird lowered its head and started to peck at food. Chickens often closed their eyes prior to pecking, and the start of pecking seemed a good point to end the current vigilance bout.
To measure synchronization of vigilance, I isolated all overlapping bouts of vigilance by the two birds of each pair during a trial. I ignored cases where one bird in a pair initiated vigilance after the other resumed feeding following its own scan. In such cases, I considered that the previous scan by the other bird was ignored. This made is easier to determine which bird initiated vigilance during an overlapping bout and which one responded in turn. I thus determined which bird became vigilant first in the overlapping bout and calculated the time it took for the other bird to become vigilant in turn (latency to respond). The number of overlapping bouts varied from pair to pair depending on the overall level of vigilance maintained by each bird (range: 3–11). To calculate the latencies to respond expected under the null hypothesis that vigilance bouts are initiated at random by the two birds of a pair, I first generated a series of randomly selected times falling anywhere during the duration of a trial. If the random time fell during the vigilance bout of one of the birds, I calculated the interval between the initiation of the vigilance bout by this bird and the random time, which served as a random response to the initiation of the vigilance bout. This selection process was repeated as many times as necessary to get the same number of empirical latencies to respond obtained for each pair.
Statistical Analyses
Two trials ended before the set limit due to external disturbances but lasted long enough to be retained for analysis. The large amount of food in each cage was not depleted during one trial, and birds fed intently during the whole trial.
The effect of treatment on the % of time spent vigilant was analyzed using a mixed linear model with pair id and bird id nested with the pair id as random factors and treatment (obstructed vs. unobstructed) as a fixed factor. I used the arcsine square-root transformation to normalize the distribution of time spent vigilant. To compare time spent vigilant by the inner and outer birds of the same pair during obstructed trials (n = 7), I used a mixed linear model with pair id as a random factor and position (inner vs. outer) as a fixed factor. I compared the distribution of expected and observed latencies to respond using a mixed linear model with pair id as a random factor and type of latencies (observed vs. expected) and treatment as fixed factors followed by a priori contrasts to compare observed and expected latencies in each treatment. The unit of analysis here is the pair because synchronization can only be measured within a pair (n = 7 pairs for each type of trials). Shorter latencies than predicted under the null hypothesis would be evidence that the initiation of a vigilance bout by one bird increases the chances that the other bird becomes vigilant thereby synchronizing vigilance within the pair. Latencies were log10 transformed to normalize the distributions.
Ethics Statement
This study was approved by the Ethics Committee of the Veterinary College of the University of Montréal, Canada.
Results
Obstruction of the field of view by the wood panel increased time spent vigilant [F(1, 13) = 6.9, p = 0.02; Figure 2]. Time spent vigilant by the inner bird of a pair did not differ from that of the outer bird during obstructed trials [back-transformed means (95% C.I.); inner: 32.4% (19.2%, 47.1%), outer: 31.1% (18.2%, 45.8%); F(1, 6) = 0.04, p = 0.85].
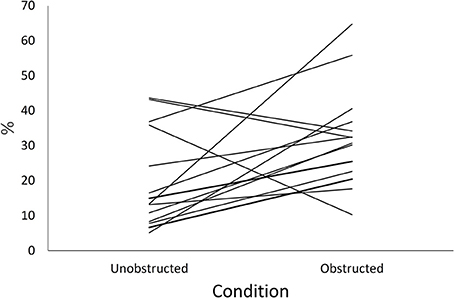
Figure 2. The overall amount of time allocated to vigilance (%) during trials. Time allocated to vigilance in trials with (Obstructed) and without (Unobstructed) visual obstruction. Separate lines join data for each bird (n = 14). Back-transformed means are shown.
With respect to the synchronization of vigilance, mean latency to become vigilant following the initiation of a vigilance bout by the companion was significantly shorter than predicted by the null hypothesis of random vigilance by each bird in unobstructed trials (t = 2.5, p = 0.015; Figure 3) but not in obstructed trials (t = 1.3, p = 0.19; Figure 3).
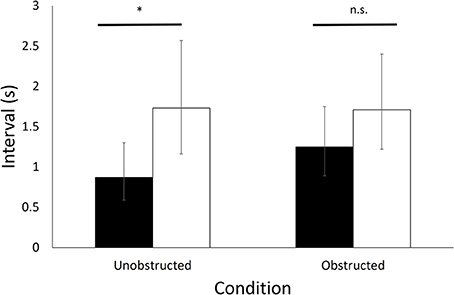
Figure 3. Synchronization of vigilance in pairs of birds. The latency (s) between the initiation of a vigilance bout by one bird in a pair and the subsequent initiation of a vigilance bout by the other bird during trials with (Obstructed) and without (Unobstructed) visual obstruction. Expected latencies were obtained from randomly initiated vigilance bouts (see text for details). Back-transformed means (95% C.I.) are shown for the empirical data (black bars) and means (95% C.I.) for the expected values (white bars). *Indicates a statistically significant difference.
Discussion
Visual obstruction caused an increase in vigilance. In previous experiments with birds and mammals, visual obstruction also typically increased the allocation of time to vigilance as noted earlier. Why visual obstruction causes an increase in vigilance, however, is less well known. Vigilance can increase because visual obstruction reduces the ability to monitor the surroundings and/or because neighbors are more difficult to monitor. In obstructed trials, birds in the inner position could not monitor their neighbors and much of the surroundings for lurking predators. By contrast, the outer birds were only prevented from monitoring their neighbors. Nevertheless, time spent vigilant did not differ for inner and outer birds, which suggests that the main reason why vigilance increased with visual obstruction here was related to the inability to monitor neighbors. Earlier work proposed that social monitoring might be hindered by visual obstruction (Elgar et al., 1984; Duncan and Jenkins, 1998; Harkin et al., 2000; Fernández-Juricic et al., 2005), and this experiment provides experimental evidence in support of this hypothesis.
The finding that vigilance of experimental birds did not appear to respond to threats in the surrounding environment does not mean that visual monitoring of the surroundings is not important. In a natural experiment in which visual obstruction prevented visual monitoring of the surroundings but not visual monitoring of neighbors, vigilance was indeed higher with visual obstruction (Beauchamp, 2015b). Other studies with solitary foragers also suggest that visual obstruction can increase predation risk by blocking the view of the surroundings (Arenz and Leger, 1997; Bednekoff and Blumstein, 2009; Iribarren and Kotler, 2012; Wheeler and Hik, 2014). For social species, my results indicate that visual monitoring of neighbors can be an important component of the increase in vigilance with visual obstruction.
Visual obstruction decreased the synchronization of vigilance in pairs of birds feeding close to one another. Synchronization of vigilance was documented in unobstructed trials using the timing of vigilance bouts between pairs of birds in visual and auditory contact. Vigilance synchronization has been documented in several species of birds and mammals as noted earlier. Nevertheless, how such synchronization is achieved is less well known. My results indicate that visual cues are important in triggering synchronization because the level of synchronization decreased in trials with visual obstruction. Alternatively, synchronization could occur through independent responses to external events (such as noises or movement in the vegetation) (Ruxton and Roberts, 1999; Hoppitt et al., 2007). This hypothesis can be ruled out because synchronization did not persist to the same level when birds could not see one another but could still respond to the same external stimuli. Use of auditory cues from a neighbor could also induce synchronization. Work with rats suggests that lack of feeding noises from a neighbor, which can be indicative of higher vigilance, is sufficient to induce vigilance in a visually isolated companion (Pereira et al., 2012). Vocal cues such as calls can also trigger vigilance adjustments in group members that are visually isolated (Radford and Ridley, 2007). In this experiment, I rarely recorded vocalizations during trials. Other auditory cues, if any, were apparently insufficient to trigger synchronization of vigilance in obstructed trials.
Synchronization of vigilance can be used to get information from neighbors about current predation risk and also to reduce the chances of being targeted during an attack (Sirot, 2006; Sirot and Touzalin, 2009). Visual obstruction could thus decrease the amount of public information about predation risk and the risk of being targeted by predators. It has long been thought that visual obstruction can decrease the ability to detect predators. In species where public information about predation risk is important and/or where predators can target laggards, visual obstruction could also increase predation risk even if visual obstruction did not prevent the detection of predators. This could be further tested in prey species that can detect predators easily because, say, they tend to attack from below or above, but where visual obstacles prevent monitoring of neighbors.
This experiment shows that the ability to visually monitor neighbors is an important determinant of the level vigilance maintained by a prey species, and also an important factor in the synchronization of vigilance between neighbors. Future work with other species, especially in the field where animals are exposed to direct threats from predators, could determine the extent to which visual cues from neighbors influence vigilance patterns.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arenz, C. L., and Leger, D. W. (1997). Artificial visual obstruction, antipredator vigilance, and predator detection in the thirteen-lined ground squirrel (Spermophilus tridecemlineatus). Behaviour 134, 1101–1114. doi: 10.1163/156853997X00421
Beauchamp, G. (2009). Sleeping gulls monitor the vigilance behaviour of their neighbours. Biol. Lett. 5, 9–11. doi: 10.1098/rsbl.2008.0490
Beauchamp, G. (2015a). Animal Vigilance: Monitoring Predators and Competitors. Oxford: Academic Press.
Beauchamp, G. (2015b). Visual obstruction and vigilance: a natural experiment. J. Avian Biol. 46, 476–481. doi: 10.1111/jav.00612
Bednekoff, P. A., and Blumstein, D. T. (2009). Peripheral obstructions influence marmot vigilance: integrating observational and experimental results. Behav. Ecol. 20, 1111–1117. doi: 10.1093/beheco/arp104
Blumstein, D. T., and Daniel, J. C. (2003). Foraging behavior of three Tasmanian macropodid marsupials in response to present and historical predation threat. Ecography 26, 585–594. doi: 10.1034/j.1600-0587.2003.03516.x
Butler, S. J., Whittingham, M. J., Quinn, J. L., and Cresswell, W. (2005). Quantifying the interaction between food density and habitat structure in determining patch selection. Anim. Behav. 69, 337–343. doi: 10.1016/j.anbehav.2004.06.006
Butler, S. R., Hosinski, E. C., Lucas, J. R., and Fernández-Juricic, E. (2016). Social birds copy each other's lateral scans while monitoring group mates with low-acuity vision. Anim. Behav. 121, 21–31. doi: 10.1016/j.anbehav.2016.08.002
Devereux, C. L., Whittingham, M. J., Fernández-Juricic, E., Vickery, J. A., and Krebs, J. R. (2006). Predator detection and avoidance by starlings under differing scenarios of predation risk. Behav. Ecol. 17, 303–309. doi: 10.1093/beheco/arj032
Duncan, R. D., and Jenkins, S. H. (1998). Use of visual cues in foraging by a diurnal herbivore, Belding's ground squirrel. Can. J. Zool. 76, 1766–1770. doi: 10.1139/z98-119
Ebensperger, L. A., and Hurtado, M. J. (2005). On the relationship between herbaceous cover and vigilance activity of degus (Octodon degus). Ethology 111, 593–608. doi: 10.1111/j.1439-0310.2005.01084.x
Elgar, M. A., Burren, P. J., and Posen, M. (1984). Vigilance and perception of flock size in foraging house sparrows Passer domesticus L. Behaviour 90, 215–223. doi: 10.1163/156853984X00146
Fernández-Juricic, E., Siller, S., and Kacelnik, A. (2004). Flock density, social foraging, and scanning: an experiment with starlings. Behav. Ecol. 15, 371–379. doi: 10.1093/beheco/arh017
Fernández-Juricic, E., Smith, R., and Kacelnik, A. (2005). Increasing the costs of conspecific scanning in socially foraging starlings affects vigilance and foraging behaviour. Anim. Behav. 69, 73–81. doi: 10.1016/j.anbehav.2004.01.019
Foster-McDonald, N. S., Hygnstrom, S. E., and Korte, S. P. (2006). Effects of a visual barrier fence on behaviour and movements of black-tailed prairie dogs. Wildl. Soc. Bull. 34, 1169–1174. doi: 10.2193/0091-7648(2006)34[1169:EOAVBF]2.0.CO;2
Ge, C., Beauchamp, G., and Li, Z. (2011). Coordination and synchronisation of anti-predation vigilance in two crane species. PLoS ONE 6:e26447. doi: 10.1371/journal.pone.0026447
Hall, L. K., Day, C. C., Westover, M. D., Edgel, R. J., Larsen, R. T., Knight, R. N., et al. (2013). Vigilance of kit foxes at water sources: a test of competing hypotheses for a solitary carnivore subject to predation. Behav. Processes 94, 76–82. doi: 10.1016/j.beproc.2012.12.007
Hannon, M. J., Jenkins, S. H., Crabtree, R. L., and Swanson, A. K. (2006). Visibility and vigilance: behavior and population ecology of uinta ground squirrels (Spermophilus armatus) in different habitats. J. Mammal. 87, 287–295. doi: 10.1644/05-MAMM-A-081R2.1
Harkin, E. L., van Dongen, W. F. D., Herberstein, M. E., and Elgar, M. A. (2000). The influence of visual obstructions on the vigilance and escape behaviour of house sparrows, Passer domesticus. Aust. J. Zool. 48, 259–263. doi: 10.1071/ZO00003
Hoppitt, W., Blackburn, L., and Laland, K. N. (2007). Response facilitation in the domestic fowl. Anim. Behav. 73, 229–238. doi: 10.1016/j.anbehav.2006.05.013
Iribarren, C., and Kotler, B. P. (2012). Patch use and vigilance behaviour by Nubian ibex: the role of the effectiveness of vigilance. Evol. Ecol. Res. 14, 223–234.
Lima, S. L. (1987). Distance to cover, visual obstructions, and vigilance in house sparrows. Behaviour 102, 231–238. doi: 10.1163/156853986X00144
Lima, S. L. (1992). Vigilance and foraging substrate: anti-predatory considerations in a non-standard environment. Behav. Ecol. Sociobiol. 30, 283–289. doi: 10.1007/BF00166714
Lima, S. L., and Bednekoff, P. A. (1999). Back to the basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim. Behav. 58, 537–543. doi: 10.1006/anbe.1999.1182
Metcalfe, N. B. (1984). The effects of visibility on the vigilance of shorebirds: is visibility important? Anim. Behav. 32, 981–985. doi: 10.1016/S0003-3472(84)80210-9
Michelena, P., and Deneubourg, J.-L. (2011). How group size affects vigilance dynamics and time allocation patterns: the key role of imitation and tempo. PLoS ONE 6:e18631. doi: 10.1371/journal.pone.0018631
Murton, R. K., and Isaacson, A. J. (1962). The functional basis of some behaviour in the wood pigeon, Columba palumba. Ibis 104, 503–521. doi: 10.1111/j.1474-919X.1962.tb08683.x
Öst, M., and Tierala, T. (2011). Synchronized vigilance while feeding in common eider brood-rearing coalitions. Behav. Ecol. 22, 378–384. doi: 10.1093/beheco/arq223
Pays, O., Dubot, A.-L., Jarman, P. J., Loisel, P., and Goldizen, A. W. (2009). Vigilance and its complex synchrony in the red-necked pademelon, Thylogale thetis. Behav. Ecol. 20, 22–29. doi: 10.1093/beheco/arn110
Pays, O., Jarman, P. J., Loisel, P., and Gerard, J.-F. (2007a). Coordination, independence or synchronization of individual vigilance in the eastern grey kangaroo? Anim. Behav. 73, 595–604. doi: 10.1016/j.anbehav.2006.06.007
Pays, O., Renaud, P., Loisel, P., Petit, M., Gerard, J., and Jarman, P. (2007b). Prey synchronize their vigilant behaviour with other group members. Proc. R. Soc. B Biol. Sci. 274, 1287–1291. doi: 10.1098/rspb.2006.0204
Pays, O., Sirot, E., and Fritz, H. (2012). Collective vigilance in the Greater Kudu: towards a better understanding of synchronization patterns. Ethology 118, 1–9. doi: 10.1111/j.1439-0310.2011.01974.x
Pereira, A. G., Cruz, A., Lima, S. Q., and Moita, M. A. (2012). Silence resulting from the cessation of movement signals danger. Curr. Biol. 22, 627–628. doi: 10.1016/j.cub.2012.06.015
Podgórski, T., de Jong, S., Bubnicki, J. W., Kuijper, D. P. J., Churski, M., and Jędrzejewska, B. (2016). Drivers of synchronized vigilance in wild boar groups. Behav. Ecol. 27, 1097–1103. doi: 10.1093/beheco/arw016
Pomeroy, A. C., Butler, R. W., and Ydenberg, R. (2006). Experimental evidence that migrants adjust usage at a stopover site to trade off food and danger. Behav. Ecol. 17, 1041–1045. doi: 10.1093/beheco/arl043
Pulliam, H. R. (1973). On the advantages of flocking. J. Theor. Biol. 38, 419–422. doi: 10.1016/0022-5193(73)90184-7
Radford, A. N., and Ridley, A. R. (2007). Individuals in foraging groups may use vocal cues when assessing their need for anti-predator vigilance. Biol. Lett. 3, 249–252. doi: 10.1098/rsbl.2007.0110
Ruxton, G. D., and Roberts, G. (1999). Are vigilance sequences a consequence of intrinsic chaos or external changes? Anim. Behav. 57, 493–495. doi: 10.1006/anbe.1998.0965
Schütz, K. E., Forkman, B., and Jensen, P. (2001). Domestication effects on foraging strategies, social behaviour and different fear responses: a comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl. Anim. Behav. Sci. 74, 1–14. doi: 10.1016/S0168-1591(01)00156-3
Sirot, E. (2006). Social information, antipredatory vigilance and flight in bird flocks. Anim. Behav. 72, 373–382. doi: 10.1016/j.anbehav.2005.10.028
Sirot, E., and Touzalin, F. (2009). Coordination and synchronization of vigilance in groups of prey: the role of collective detection and predators' preference for stragglers. Am. Nat. 173, 47–59. doi: 10.1086/593358
Underwood, R. (1982). Vigilance behaviour in grazing African antelopes. Behaviour 79, 81–107. doi: 10.1163/156853982X00193
Wheeler, H. C., and Hik, D. S. (2014). Giving-up densities and foraging behaviour indicate possible effects of shrub encroachment on arctic ground squirrels. Anim. Behav. 95, 1–8. doi: 10.1016/j.anbehav.2014.06.005
Keywords: antipredator vigilance, chicken, group, synchronization, visual obstruction
Citation: Beauchamp G (2017) Difficulties in Monitoring Conspecifics Mediate the Effects of Visual Obstruction on the Level and Synchronization of Vigilance. Front. Ecol. Evol. 5:12. doi: 10.3389/fevo.2017.00012
Received: 07 December 2016; Accepted: 20 February 2017;
Published: 03 March 2017.
Edited by:
Michaela Hau, Max Planck Institute for Ornithology, GermanyReviewed by:
Markus Öst, Åbo Akademi University, FinlandCarsten Schradin, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2017 Beauchamp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guy Beauchamp, Z3V5Z2lsbGVzYmVhdWNoYW1wQGdtYWlsLmNvbQ==
 Guy Beauchamp
Guy Beauchamp