- 1UMR 7221, Muséum National d'Histoire Naturelle, Paris, France
- 2Behavioural Ecology and Ecophysiology Group, Department of Biology, University of Antwerp, Antwerp, Belgium
- 3Laboratory of Evolutionary Ecophysiology, Institute of Biology, University of Neuchâtel, Neuchâtel, Switzerland
Editorial on the Research Topic
Oxidative Stress and Signal Honesty
Scientists have long been fascinated by the huge diversity among species and among individuals in how animals look like or emit sounds and odors. From the extravagant colors and parades of paradise birds to the horns of ungulates, scientists have been looking at the causes and consequences of such variation. It is now well established that the production of these visual, acoustic or odor signals is a key component of social communication and reproduction (Darwin, 1871; Andersson, 1994). Except for deceptive signals (e.g., Batesian mimicry), these signals are used by the receivers (either conspecifics or heterospecifics) because they convey reliable information about their emitters. The theory of honest signaling states that, in order to be reliable, signals need to be either produced or maintained at a cost that only high quality individuals are able to afford (i.e., production and maintenance cost paradigms). In absence of a mechanism ensuring its honesty, such as a production cost, a signal is quickly counter selected because it is amenable to cheating, and therefore becomes unreliable. A major question in evolutionary ecology is thus the identification of those production costs. In the last ten years, evolutionary ecologists have recognized that the need to achieve an optimal oxidative balance may be an important constraint driving the outcome of many life-history trade-offs, including optimal resource investment between the production of honest signals and other competing life-history traits (e.g., immunity, survival). Much recent research has focused on whether the production of signals induces oxidative stress, and on whether oxidative stress itself may constrain investment in signaling. In a survey using the Web of Science, we found that more than 90% of the papers indexed with the keywords “oxidative stress,” “oxidative damage” or “antioxidant*” and “honest signaling” or “handicap,” excluding those articles not dealing with natural environmental issues, have appeared in the literature since 2007 (Figure 1). This clearly shows how flourishing this area of research is. In preparing this research topic, our aim was to capture the spirit of this emerging field and the dedicated efforts to marry mechanistic and functional perspectives to fundamental questions in sexual selection and evolution of animal communication. Opportunities for studying truly natural processes are diminishing because pristine or near-pristine environments are rapidly shrinking and becoming more difficult to reach. This should remind us that we need to know more about how changing and emerging environments are influencing natural animal populations. Therefore, our aim was also to show how basic research on honest signaling can be translated into applied work because honest signals may be sensitive to environmental changes, making them possible indicators of environmental quality.
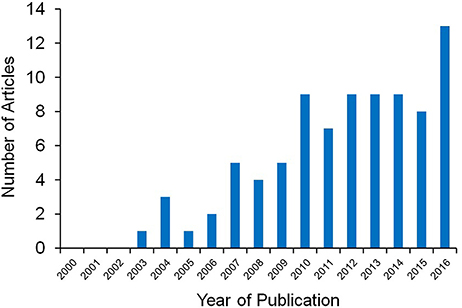
Figure 1. Since the seminal work by von Schantz et al. (1999), most of the articles on the role of oxidative stress as a cost of and constraint on honest signaling have been published in the last decade.
First, Rojas Mora et al. show that the size of the badge in house sparrows (Passer domesticus) might reflect male investment into the antioxidant protection of their sperm relative to a given social environment, suggesting that females may accrue both direct and indirect benefits by mating with large-badged males producing less oxidized (hence more fertile) ejaculates. Then, Vitousek et al. suggest that there may be divergence among co-specific populations in the relationship between signals and relevant physiological parameters, and changes in this relationship may degrade the reliability of a signal, or even change its underlying information content. Friesen et al. discuss how the connection between production of signals and oxidative stress is, however, more complex than generally thought, with factors like body condition or aggressiveness possibly involved as mediators for such connection. Casagrande et al. remind us that signals are not all visual, but can also be acoustic. The authors point out that the link between acoustic communication and oxidative stress has been largely overlooked so far. Finally, Marasco and Costantini and Hutton and McGraw review the mechanistic underpinnings and functional consequences of how exposure to contaminants and urbanization affects the oxidative balance in animals and how this in turn affects signal expression and use.
In conclusion, it has been a fruitful first decade of life for the marriage between oxidative stress and honest signaling. We hope that readers find this Research Topic in Frontiers in Ecology and Evolution both timely and useful, in stimulating new directions and research collaborations for years to come.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
FH was supported by a grant from the Swiss National Science Foundation n° PP00P3_139011.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Keywords: honest signals, oxidative stress, color vision, sexual selection, life-history
Citation: Costantini D and Helfenstein F (2017) Editorial: Oxidative Stress and Signal Honesty. Front. Ecol. Evol. 5:32. doi: 10.3389/fevo.2017.00032
Received: 02 March 2017; Accepted: 31 March 2017;
Published: 24 April 2017.
Edited and reviewed by: Jordi Figuerola, Estación Biológica de Doñana (CSIC), Spain
Copyright © 2017 Costantini and Helfenstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Costantini, ZGF2aWRjb3N0YW50aW5pQGxpYmVyby5pdA==
 David Costantini
David Costantini Fabrice Helfenstein
Fabrice Helfenstein