- 1Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 2Institute of Zoology, Zoological Society of London, London, United Kingdom
- 3Hihi Conservation Charitable Trust, Wellington, New Zealand
- 4Department of Animal and Plant Sciences, University of Sheffield, Sheffield, United Kingdom
- 5Helsinki Institute of Life Sciences, University of Helsinki, Helsinki, Finland
- 6Research Programme in Organismal and Evolutionary Biology, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Many young birds die soon after fledging, as they lack the skills to find food and avoid predation. Post-fledging parental care is assumed to assist acquisition of these vital skills. However, we still lack empirical examples examining the length of time fledglings spend with parents, how they associate during this critical time, or whether such variation in the fledgling dependency period has consequences for the survival and behaviour of young as they navigate their first year of independent life. Here, we make use of observations and radio frequency identity (RFID) logs of visits to supplementary feeding stations to investigate how condition of fledgling hihi (stitchbird, Notiomystis cincta), a New Zealand passerine, predicts dispersal behaviour and tendency to follow parents during their 2 week post-fledging dependence period. We find that thinner fledglings followed their parents more closely in time when visiting feeding stations, compared to fatter siblings (all following ranged from 3 s to 10 min). However, broods in poorer condition tended to disperse from the natal territory up to 6.5 days earlier than broods of fatter fledglings (all dispersed within 14 days). Our results did not find that sociality or survival during the first year of life differed depending on variation in fledgling behaviour; neither following parents closely nor dispersing later predicted each bird's number of associates (degree), or survival over winter. These results suggest that fledglings may be able to compensate for early differences in condition with behaviour, either during the post-fledging dependence period or when independent.
Introduction
Once altricial birds fledge the nest, life becomes challenging. Fledglings lack foraging and anti-predator skills, and so mortality is often high. In short-lived passerines, for example, a quarter of fledglings on average do not survive their first month outside the nest (Anders et al., 1997; Ringsby et al., 1998; Naef-Daenzer et al., 2001; Low and Pärt, 2009). Remaining with parents is thought to enhance survival (Clutton-Brock, 1991), although the length of this period of post-fledging dependency is highly variable across species [between 5 and 200 days in passerines, see Russell (2000)]. During this time, parents may provide their offspring with food (Davies, 1976), may defend them from predators (Balda and Balda, 1978; Le Bohec et al., 2005) and can limit competition by preventing other animals from accessing the natal territory (Ekman et al., 2000; Ekman and Griesser, 2002). Parents can also provide opportunities for independent learning (Heinsohn, 1991), or social learning (Griesser and Suzuki, 2016) through direct teaching (Thornton, 2006; Thornton and Raihani, 2008) and observing parents whilst in spatiotemporal proximity (Griesser and Suzuki, 2017). However, it remains unclear how early-life condition mediates fledgling behaviour or the length of time spent with parents (e.g., Kouba et al., 2013), despite this being a key factor explaining fledgling survival (Tinbergen and Boerlijst, 1990; Naef-Daenzer et al., 2001; Monrós et al., 2002; Naef-Daenzer and Grüebler, 2016). In part, this may be because studying the post-fledge stage is challenging: it requires detailed observation of individuals and, unlike previous stages of family life, is not localised at a nest. As a result, and despite extensive work on parental care and offspring behaviour before fledging (Royle et al., 2012), we still know relatively little about how conditions in the nest affect the period of post-fledging dependency, or how this sets up fledglings for later life.
Within the nest, it is well-known that parents influence their nestlings' condition (Price, 1998) because of genetic effects, maternal effects, or environmental conditions such as timing reproduction and/or placing nests in an optimal territory (Kirkpatrick and Lande, 1989; Monaghan, 2008). Nestling condition can affect siblings' effectiveness at competing for parental attention; nestlings in good condition jostle and position themselves to extract more care than parents may wish to provide (Kacelnik et al., 1995). Once young fledge, nestling condition continues to provide benefits, because fatter fledglings have more of a buffer to avoid starvation (Tinbergen and Boerlijst, 1990; Naef-Daenzer et al., 2001; Monrós et al., 2002; Naef-Daenzer and Grüebler, 2016). However, we rarely consider if condition might also affect how fledglings extract care from their parents, such as the extent to which they associate. In the few species where fledgling behaviour has been studied in detail, fledglings in poor condition may attempt to compensate for their worse start by begging more intensely (including vocalisation, wing-fluttering, and actively following parents) to obtain more food (Middleton et al., 2007). Alternatively, better-condition individuals may outcompete poorer-condition siblings, meaning the more dominant young then remain with parents for longer and monopolise parental attention (e.g., Siberian jays, Perisoreus infaustus, Ekman et al., 2002). Thus, good-condition fledglings may attain even better condition and self-feeding efficiency through receiving care for longer (Ridley and Raihani, 2007).
How fledgling condition affects time with parents could also have consequences for later life, but this is not yet well-understood. The social environment experienced during parental care can affect the extent to which juveniles associate with or rely on other individuals once independent (Riebel et al., 2012; Boogert et al., 2013; Farine et al., 2015). Thus, if individual sociality is consistent (Aplin et al., 2015), young that associate with parents might also associate closely with others later in life and build more social connections based on such propensity for spatiotemporal proximity (Psorakis et al., 2015). As there is evidence sociality correlates with how readily individuals find food (Ward and Zahavi, 2008; Aplin et al., 2012) and the types of food they select (Slagsvold and Wiebe, 2011), how they avoid predation (Croft et al., 2006), or how readily they contract disease (VanderWaal et al., 2014), early-life sociality may have lifelong consequences for survival and behaviour.
Here we took advantage of existing data from an experiment focused on juvenile flocking (Franks et al., 2018b) to explore how condition of young passerines relates to their post-fledge dispersal timing, attentiveness to parents, and survival and sociality during the first year of life. Our study species was the hihi (stitchbird, Notiomystis cincta), a forest-dwelling New Zealand passerine that feeds on nectar, fruits, and insects (Craig, 1985; Rasch and Craig, 1988). Hihi are easy to observe compared to many passerine species, as their evolutionary history means they do not fear mammals (including humans), and their breeding biology has been studied intensively (Thorogood et al., 2013). We know that parent hihi feed nestlings more if they only have one annual clutch vs. two, suggesting parental investment in offspring care varies depending on its current and future payoffs (Thorogood et al., 2011). Variation in chick provisioning may be associated with long-term consequences for offspring fitness, for example the extent of expression of secondary sexual traits (Walker et al., 2013), indicating an importance of early-life condition. We also know that juveniles aggregate together in groups after dispersing from their natal territory (Franks et al., 2018c) and that they have lower survival if they suddenly lose these associates (Franks et al., 2018a), indicating social relationships may be important. However, we still know little about the post-fledging period, or how this shapes survival later in life. Therefore, here we explored: (1) if fledglings in better condition follow their parents more closely while in the natal territory, and disperse later; and (2) if more attentive fledglings go on to gain more associates and/or show higher survival once independent.
Methods
We investigated patterns in fledgling condition, time between fledging and dispersing, and how closely fledglings followed their parents while in the natal territory during one breeding season (October 2015–April 2016) in a population of hihi on Tiritiri Matangi Island, New Zealand (36°36′01″S, 174°53′22″E), which is a 2.5 km2 open scientific reserve characterised by patches of remnant and regenerating native flora. Hihi were reintroduced to the site in 1995 and at the time of our study the population numbered approximately 88 adults and 132 fledglings (raw minimum counts) (McCready and Ewen, 2016). As part of conservation management, hihi are habituated to six feeding stations across the island where they are provided with supplementary sugar water ad-libitum; this means we can introduce new temporary feeders which hihi will readily use.
Data Collection
Chick Condition Data
Hihi on Tiritiri Matangi are monitored intensively during the breeding season following an established protocol (see Ewen et al., 2018). Nest-boxes allow accurate records of all nesting attempts, and daily checks of nests allowed us to estimate fledging age as the day the last chick left each nest (mean fledging age during this study = 30 ± 0.27 days post-hatch). All nestlings are ringed 21 days after hatching (hatch day = day 0) with a unique combination of coloured leg rings. In our study year, parent hihi and their offspring also carried Passive Integrated Transponder (PIT) tags in one colour ring (IB Technology), approved by the Auckland Zoo Ethics Committee (New Zealand). During ringing, asymptotic morphometrics [mass (g) and full tarsus length (mm)] were also taken.
Feeder Use
As part of a separate experiment investigating the effects of social experiences on juvenile behaviour (see Franks et al., 2018b), 12 nests (40 fledglings; out of a total of 36 successful first-clutches) were provided with temporary supplementary feeders. Feeders were placed approximately 10 m from the nestbox 14 days after chicks hatched to ensure parental use, and remained in place until fledglings dispersed from the natal territory. Entryways were fitted with PIT tag data-loggers (IB Technology model EM4102) to record time-stamped visits of individual hihi; these data allowed us to ascertain which fledglings used feeders, and how closely they followed their parents when accessing this food source. The study design received ethical approval from the Zoological Society of London Ethics Committee (UK).
Dispersal Times
After chicks fledged, we observed nest territories for 45 min every 2 days and recorded if fledglings were present by listening for their distinctive, repeated, begging calls. If we heard calls in the territory, this indicated at least one fledgling was present from that brood; if no calls were heard then we recorded fledglings as absent. As nests were separated in space and time, calls were unlikely to be confused between adjacent nests. We used this binary measure as it was not always possible to assess the number of fledglings (for example, if they were secluded high up in trees). We determined dispersal as occurring when no fledglings were heard for two consecutive observation bouts, but used the first day no fledglings were heard as the dispersal time. For 11/12 broods, at least one fledgling was observed alive in the following months (the remaining brood dispersed 8 days after fledging); thus, an absence of calls most likely reflected dispersal rather than mortality.
Post-dispersal Sociality and Survival
Following dispersal from their natal territory, juvenile hihi formed groups at three consistent sites (Franks et al., 2018c), each located at the bottom of separate gullies containing mature flora and a permanent water source (distances between group sites ranged from 200 to 1,000 m). To measure sociality of juveniles, we placed temporary supplementary feeders with PIT tag data-loggers (IB Technology) at each of the three sites for 6 weeks and collected 11,928 records of time-stamped visits from 64 individuals, including the 19 juveniles used to answer question (2). No new individuals were recorded after 6 weeks, suggesting that the majority of hihi that used group sites were included in our dataset. See Franks et al. (2018c) for more details on group behaviour and feeder use.
To determine juveniles' survival during their first winter we used presence/absence of individuals from a 40-h constant-effort population survey conducted at the start of the next breeding season (September 2016) as part of standard monitoring of our study population. For any birds not observed in the September survey, we cross-checked whether they were observed in the following routine survey (February 2017), to limit false-positive records of mortality. Thus, each juvenile was assigned either a “yes” or “no” for whether it survived over winter.
Data Analysis
Dispersal Timing and Following With Parents
Analyses were conducted in R (version 3.5.0) (R Core Team, 2018). We considered whether time to disperse from the natal territory was predicted by two measures of nestling condition. Using mass and tarsus length we calculated each individuals' (N = 40) residual mass (“condition”; how much leaner or fatter it was than expected, given its size) using a linear model (Supplementary Figure 1). Then, as we had measured days to disperse at the level of the brood, we calculated the average residual per brood (11/12 broods contained 2–5 fledglings). Second, we calculated the range of condition scores within a nest, assuming that a smaller range reflected more equal allocation of parental care among offspring.
To analyse the relationship between condition and dispersal, we used Poisson-distributed Generalised Linear Models (GLMs) where days to dispersal was the response, and average chick condition and range in condition were predictors. We also included if juveniles had used their nest feeder, in case this delayed dispersal (yes/no; fledglings from 5/12 nests used feeders), and days between collecting nestling data (day 21) and fledging to explore whether chicks that were slower to fledge also took longer to disperse. We used a model selection and averaging approach with the R package AICcmodavg (Mazerolle, 2019) to explore effects of different potential explanatory variables (Burnham and Anderson, 2002; Symonds and Moussalli, 2011; Harrison et al., 2018). Candidate models including each predictor were ranked by their corrected Akaike Information Criterion (AICc); for all models within 2 AICc units of the top-ranked model, we calculated averaged effect sizes (±95% confidence intervals) of predictors (Burnham and Anderson, 2002; Nakagawa and Cuthill, 2007). Any effects where confidence intervals did not span 0.00 were considered significant. We assessed overdispersion using the value of ĉ (R package AICcmodavg), but no correction was needed. Finally, we ensured fledgling behaviour was not due to the mother beginning another reproductive attempt, by comparing dispersal days with how quickly (in days) the mother re-laid; however, as only 10/12 females re-nested we ran a separate Spearman's rank correlation for this analysis.
Using PIT-tag recorded visits of the subset of fledglings that used their nest feeder, we investigated how closely they followed their parents (9 juveniles from 4 broods; at a 5th nest site, failed PIT tags meant we could not reliably infer parental visits). The number of visits each fledgling made varied (range = 1–34, all but one <8 visits) so we only included the first 10 visits (N = 25 visits in total). We calculated the length of time (s) between each fledgling's visit and the preceding visit of a parent (“following time”; seconds were log-10-transformed to account for a large range in following times: 1–1941s). We then used Linear Mixed Effects Models (LMMs) implemented using the lme4 package (Bates et al., 2015), with following time as the response. Our predictors included chick condition before fledging, plus age at visit and visit number (to assess if feeder use changed depending on personal experience). We included individual identity as a random effect to account for repeated visits by individuals. Again, we used model selection and averaging to explore the relationships between our predictors and following.
Post-dispersal Sociality and Survival
Using data of all visits to the temporary feeders set up at juvenile group sites, we built a weighted social network using the R package asnipe (Farine, 2013). This estimated likely associations among individuals based on spatiotemporal proximity of visits (Psorakis et al., 2015). For each juvenile that was later recorded visiting these group site feeders (N = 19) we calculated its number and strength of associates from the entire recorded population (weighted degree), then ranked juveniles from least to most social. We used Cumulative Link Models (CLMs) implemented from the ordinal package (Christensen, 2019) to analyse change in degree rank depending on each bird's condition at fledging and their dispersal timing (days). Finally, for the subset of juveniles that also used their nest feeders (N = 9), we used Cumulative Link Mixed Models (CLMMs) to investigate the relationship between following parents and sociality in groups by testing how degree ranks depended on following time using all juvenile visits to nest feeders (N = 25); we included a random effect of individual identity as some individuals made multiple visits. As analyses using network measures violate assumptions of many statistical tests (Farine and Whitehead, 2015), to calculate significance for both analyses including degree, we permuted the data-stream of the entire network 1,000 times to shuffle associations then re-calculated randomised degree scores for each individual. We then compared our observed model coefficients to coefficients from models using randomised degree ranks as covariates to generate p-values (Prand) (Farine and Whitehead, 2015; Farine, 2017).
We analysed whether fledglings from nests that dispersed later had higher overwinter survival using a binomial GLMM (package lme4) with overwinter survival as the response (1 = yes, 0 = no) and days to dispersal for each fledgling's nest as the predictor. As most broods (11/12) had multiple fledglings, we included nest as a random effect to control for pseudoreplication. Models were not over-dispersed according to the value of ĉ so no correction was needed. Again, we used model selection to compare against a null model. We did not analyse effects of following at nest feeders on later survival, because all but one juvenile in this dataset was recorded in the population the following breeding season.
Results
Dispersal Timing and Following With Parents
Broods of fatter chicks showed a tendency to disperse later (Figure 1; effect of increasing residual average mass per nest on days to disperse = 0.12 ± 0.05, 95% CI = 0.01–0.22; Supplementary Table 1). However, this effect was statistically weak (our confidence intervals approached 0.00), and disappeared if we excluded one brood that was never heard in the natal territory but were seen when independent (effect estimate = 0.03 ± 0.06, 95% CI = −0.09–0.14). We did not find evidence that dispersal time changed between nests depending on the difference between the fattest and thinnest chicks within each brood (model including within-brood range of condition ranked with ΔAICc > 2; Supplementary Table 1). There was limited support that using a feeder at the nest delayed fledgling dispersal; while this predictor was included in the top model set, its confidence interval overlapped 0.00 (effect size = 0.35 ± 0.19, 95% CI = −0.01–0.72; Supplementary Table 1). Finally, there was no support for an effect of dispersal timing on how long it took chicks to fledge (model containing time to fledging ranked with ΔAICc > 2; Supplementary Table 1). Dispersal was unlikely to be linked to future reproductive behaviour of the mother: in 10/12 broods where the mother later re-nested, there was no significant correlation between the number of days from fledging to dispersal, and until the mother laid the first egg of her next clutch (Spearman's rank correlation: r = −0.18; S = 195.14; P = 0.61).
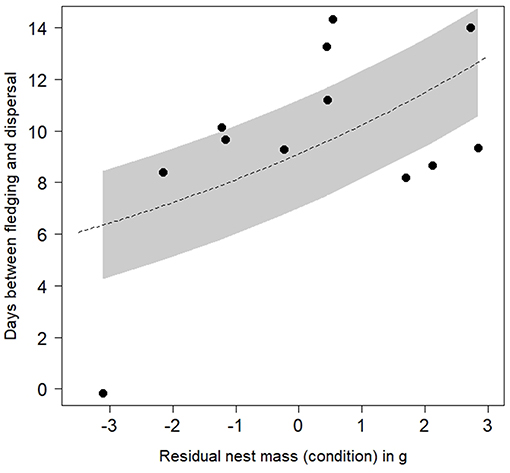
Figure 1. Variation in the time it took for fledglings of N = 12 nests to disperse from their nest territories, depending on the average condition of nestmates (average of residual mass against tarsus length when 21 days old). Line of best fit and 95% confidence interval are predicted from a GLM exploring effects on dispersal timing (see Supplementary Table 1).
There was some evidence that condition was linked to following behaviour: fledglings in better condition as nestlings visited supplementary feeders with longer intervals between their visits and their parents (Figure 2; effect of condition on following time = 0.27 ± 0.10, 95% CI = 0.08–0.46; Supplementary Table 2). However, the null model exploring following times also ranked with ΔAICc < 2 (Supplementary Table 2), suggesting the significance of the effect of condition was weak. Time between visits by fledglings and their parents did not change with increasing personal experience as fledglings aged or made more visits (Supplementary Table 2).
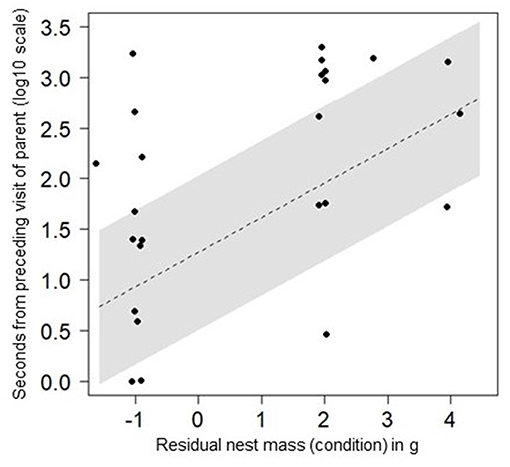
Figure 2. How closely N = 9 fledglings followed their parents to supplementary feeders on N = 25 visits, depending on their condition as nestlings. Line of best fit and 95% confidence intervals are predicted from an LMM exploring effects of condition on follow timings, which included a random effect to account for repeated visits by some individuals (see Supplementary Table 2).
Post-dispersal Sociality and Survival
There was no evidence for a link between early and later life sociality: we did not find that juveniles with higher degree scores in groups had been in better condition as nestlings (N = 19 juveniles; effect size for condition on degree = −0.01 ± 0.23; z = −0.01; Prand = 0.14) or were from broods with later dispersal (effect size from dispersal time on degree = −0.04 ± 0.20; z = −0.20; Prand = 0.11). Fledglings that followed parents more closely when using nest feeders also did not have higher-ranked degree scores than expected at random (N = 9; effect of following time on later degree = 0.03 ± 1.58; z = 0.02; Prand = 0.68). Thus, early-life condition or social experiences did not appear to correlate with how connected an individual was later in life.
Half of the 40 fledglings from our 12 experimental nests survived their first winter. However, overwintering likelihood did not differ depending on when fledglings dispersed from their natal territories (Figure 3): the model containing days to dispersal as a predictor was ranked lower than the null model, with an ΔAICc value > 2 (Supplementary Table 3).
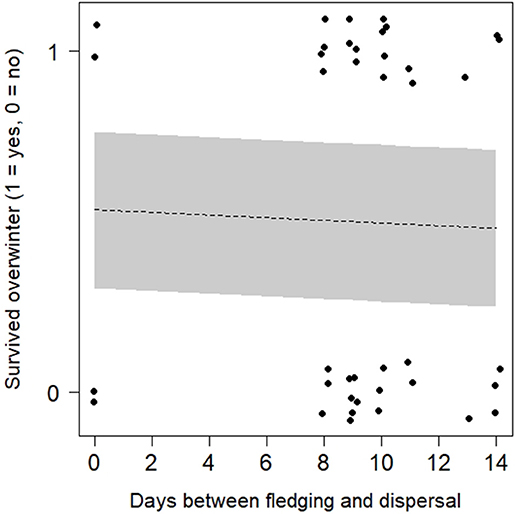
Figure 3. Likelihood of surviving to the following breeding season for fledglings of N = 12 experiment nests, depending on how quickly they dispersed from the natal territory. Points are jittered (by 0.8 on x-axis, 0.5 on y-axis) to improve visibility. Line of best fit (dotted line) and 95% confidence intervals (grey polygon) are calculated from a binomial GLMM exploring variation in survival depending on dispersal timing (Supplementary Table 3).
Discussion
Despite intense effort to understand avian parental care within the nest, how parents and young interact after fledging remains poorly understood. Here we attempted to address this knowledge gap by exploring the relationship between condition and behaviour in fledglings, and whether this predicts their sociality and survival once independent. In the small number of hihi broods we studied, we found some evidence that fledgling condition correlated with both the length of time spent with parents, and attentiveness to parents, albeit in opposite directions. Fledglings from thinner broods tended to disperse earlier than broods in better condition, although further data is needed to strengthen this finding. Within broods, however, fledglings in poorer condition tended to follow their parents more closely during their time in the natal territory. From our dataset, we were unable to detect any effects of fledgling condition or how they spent time with parents on later sociality, or juvenile survival to recruitment, which indicated that young hihi (at least in our study population) may compensate for differences in condition once they leave the nest.
Becoming independent can be considered a manifestation of parent-offspring conflict (Trivers, 1974) where young would like to extend their stay while parents prefer to defend their resources for future reproduction (Davies, 1976, 1978). Fledglings should therefore disperse once feeding themselves becomes more profitable than relying on parental provisioning, either because parents become “mean” and withhold provisioning (Davies, 1978; Ekman and Rosander, 1992) or they no longer prevent competition (Ekman and Griesser, 2002), or because the young themselves are in suboptimal natal habitats where fewer resources are available (Edwards, 1985; some studies suggest parental effects on dispersal override habitat quality effects, see Ekman et al., 2000). Our finding that fledglings from broods in poorer condition tended to shorten their period of post-fledging dependency fits this model and suggests that either the parents, fledglings, or both had an incentive for them to leave the breeding territory. Unfortunately, we did not have any measures of habitat quality or parental provisioning, so we are unable to tease apart whether parents intentionally withheld food to encourage fledglings to disperse vs. fledglings learning to self-feed more quickly in response to poor environmental conditions. Our sample size was restricted by concurrent experiments and limited deployment of PIT tags. Nevertheless, the statistically weak difference that we detected in dispersal time between the fattest and thinnest broods suggests that further exploration of how condition impacts dispersal timing is warranted at both the level of the individual and the brood.
While broods of poorer-condition chicks dispersed earlier, at the individual level chicks that fledged in poorer condition showed a tendency to be more attentive to parents and follow them more closely. It is possible that remaining in closer spatiotemporal proximity to parents was an attempt to use location to maximise the chance of being fed (Thompson et al., 2013). In American dippers (Cinclus mexicanus), begging intensity has been shown to increase when food abundance is low and fledglings are in poorer condition (Middleton et al., 2007), and similar to many passerines, hihi fledglings beg while following parents (Franks, pers. obs.). If following corresponded to begging in our study, this might suggest individuals within-broods adjusted their attentiveness to parents depending on their condition. Additionally, skill acquisition can depend on attention from parents: for example, juvenile Eurasian dippers (Cinclus cinclus) with higher rates of intake during parental care then became capable of independent feeding more quickly (Yoerg, 1998). Perhaps, by paying close attention to parents and accruing benefits quickly, fledglings in poorer condition were better able to cope with dispersing earlier due to poor quality habitat (Przybylo et al., 2001), parents being unwilling to provide more care (Davies, 1978), or from being driven out by more dominant siblings (Ekman et al., 2002). However, as our data on following behaviour were based on temporal visits to feeders recorded using PIT tags, further observational data would need to be collected to investigate the links between following, begging, and learning, and explore this hypothesis in detail.
Why did we find no correlation between sociality during post-fledging dependence and sociality or survival in later life? Slower-dispersing pied babblers (Turdoides bicolor) and Siberian jays have been shown to be more likely to survive (Ekman et al., 2000; Griesser et al., 2006; Ridley and Raihani, 2007), and experiments with captive zebra finches (Taeniopygia guttata) suggest that stressful rearing conditions can alter who juveniles associate with, as well as what they learn (Boogert et al., 2014; Farine et al., 2015). However, here we found no link between condition, or close association with parents, and sociality or survival once young became independent. This could reflect alternative strategies by fledglings depending on their condition (Yoerg, 1998), with fatter fledglings spending longer being fed by parents vs. poorer-condition fledglings that favoured independent feeding, and then both mixing in juvenile groups where any social learning was not influenced by natal condition. Alternatively, our study species is short-lived (average lifespan is approximately 4 years), and thus there may be fewer long-term effects from early life than in longer-lived species. Finally, it is also possible that survival is elevated in our population of hihi due to the presence of a reliable, non-depleting food source (permanent supplementary feeders), and this could have masked any effects from fledging on survival (Jansson et al., 1981). However, overall our study provides some insight into how the behaviour of young passerines varies during their first few weeks of life outside the nest, and that there appeared to be few consequences of these differences during their first year of life. This suggests that juveniles may be able to adjust their behaviour to either maximise, or limit, the long-term consequences of time they spend with parents.
Data Availability
Datasets used for this study are included as Supplementary Files.
Ethics Statement
This study was carried out in accordance with the recommendations of the Zoological Society of London ethics committee, who also approved the protocol.
Author Contributions
VF, JS, and RT: conceptualisation, analysis, writing (original draft), and writing (review and editing). VF, MM, JS, and RT: methodology. VF and MM: investigation and data curation.
Funding
VF was supported by a Balfour Studentship from the Department of Zoology, University of Cambridge; RT was supported by an Independent Research Fellowship from the Natural Environment Research Council UK (NE/K00929X/1); and a start-up grant from the Helsinki Institute of Life Science (HiLIFE), University of Helsinki.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Department of Conservation and Supporters of Tiritiri Matangi for their help in supporting this research. We are extremely grateful to our reviewers for their insightful comments, which greatly improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00322/full#supplementary-material
References
Anders, A. D., Dearborn, D. C., Faaborg, J., and Iii, F. R. T. (1997). Juvenile survival in a population of neotropical migrant birds. Conserv. Biol. 11, 698–707. doi: 10.1046/j.1523-1739.1997.95526.x
Aplin, L. M., Farine, D. R., Morand-Ferron, J., and Sheldon, B. C. (2012). Social networks predict patch discovery in a wild population of songbirds. Proc. R. Soc. 279, 4199–4205. doi: 10.1098/rspb.2012.1591
Aplin, L. M., Firth, J. A., Farine, D. R., Voelkl, B., Crates, R. A., Culina, A., et al. (2015). Consistent individual differences in the social phenotypes of wild great tits. Parus major. Anim. Behav. 108, 117–127. doi: 10.1016/j.anbehav.2015.07.016
Balda, R. P., and Balda, J. H. (1978). The care of young Piñon Jays (Gymnorhinus cyanocephalus) and their integration into the flock. J. Ornithol. 119, 146–171. doi: 10.1007/BF01644586
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–91. doi: 10.18637/jss.v067.i01
Boogert, N. J., Farine, D. R., and Spencer, K. A. (2014). Developmental stress predicts social network position. Biol. Lett. 10:20121088. doi: 10.1098/rsbl.2014.0561
Boogert, N. J., Zimmer, C., and Spencer, K. A. (2013). Pre- and post-natal stress have opposing effects on social information use. Biol. Lett. 9:20121088. doi: 10.1098/rsbl.2012.1088
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer. doi: 10.1016/j.ecolmodel.2003.11.004
Christensen, R. H. B. (2019). ordinal - Regression Models for Ordinal Data, R package version 2019. 4–25. Available online at: https://cran.r-project.org/web/packages/ordinal/index.html
Clutton-Brock, T. H. (1991). The Evolution of Parental Care. New Jersey, NJ: Princeton University Press. doi: 10.1046/j.1420-9101.1992.5040719.x
Craig, J. L. (1985). Status and foraging in New Zealand honeyeaters. New Zeal. J. Zool. 12, 589–597. doi: 10.1080/03014223.1985.10428308
Croft, D. P., James, R., Thomas, P. O. R., Hathaway, C., Mawdsley, D., Laland, K. N., et al. (2006). Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata). Behav. Ecol. Sociobiol. 59, 644–650. doi: 10.1007/s00265-005-0091-y
Davies, N. B. (1976). Parental care and the transition to independent feeding in the young spotted flycatcher (Muscicapa striata). Behaviour 59, 280–294. doi: 10.1163/156853976X00415
Davies, N. B. (1978). Parental meanness and offspring independence: an experiment with hand-reared great tits Parus major. Ibis 120, 509–514. doi: 10.1111/j.1474-919X.1978.tb06815.x
Edwards, P. J. (1985). Brood division and transition to independence in Blackbirds Turdus merula. Ibis 127, 42–59. doi: 10.1111/j.1474-919X.1985.tb05036.x
Ekman, J., Bylin, A., and Tegelström, H. (2000). Parental nepotism enhances survival of retained offspring in the Siberian jay. Behav. Ecol. 11, 416–420. doi: 10.1093/beheco/11.4.416
Ekman, J., Eggers, S., and Griesser, M. (2002). Fighting to stay: the role of sibling rivalry for delayed dispersal. Anim. Behav. 64, 453–459. doi: 10.1006/anbe.2002.3075
Ekman, J., and Griesser, M. (2002). Why offspring delay dispersal: experimental evidence for a role of parental tolerance. Proc. R. Soc. 269, 1709–1713. doi: 10.1098/rspb.2002.2082
Ekman, J., and Rosander, B. (1992). Survival enhancement through food sharing: a means for parental control of natal dispersal. Theor. Popul. Biol. 42, 117–129. doi: 10.1016/0040-5809(92)90008-H
Ewen, J. G., Armstrong, D. P., McInnes, K., Parker, K. A., Richardson, K. M., Walker, L. K., et al. (2018). Hihi Best Practice Guide. Wellington: Department of Conservation.
Farine, D. R. (2013). Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol. Evol. 4, 1187–1194. doi: 10.1111/2041-210X.12121
Farine, D. R. (2017). A guide to null models for animal social network analysis. Methods Ecol. Evol. 8, 1309–1320. doi: 10.1111/2041-210X.12772
Farine, D. R., Spencer, K. A., and Boogert, N. J. (2015). Early-life stress triggers juvenile zebra finches to switch social learning strategies. Curr. Biol. 25, 2184–2188. doi: 10.1016/j.cub.2015.06.071
Farine, D. R., and Whitehead, H. (2015). Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. doi: 10.1111/1365-2656.12418
Franks, V. R., Andrews, C. E., Ewen, J. G., McCready, M., Parker, K. A., and Thorogood, R. (2018a). Changes in social groups across reintroductions and effects on post-release survival. bioRxiv 430280. doi: 10.1101/430280
Franks, V. R., Ewen, J. G., McCready, M., Rowcliffe, M., Smith, D., and Thorogood, R. (2018c). One of the gang: social group dynamics in a juvenile passerine bird. bioRxiv 456376. doi: 10.1101/456376
Franks, V. R., Ewen, J. G., McCready, M., and Thorogood, R. (2018b). Copy parents or follow friends? Juvenile foraging behaviour changes with social environment. bioRxiv 429688. doi: 10.1101/429688
Griesser, M., Nystrand, M., and Ekman, J. (2006). Reduced mortality selects for family cohesion in a social species. Proc. R. Soc. 273, 1881–1886. doi: 10.1098/rspb.2006.3527
Griesser, M., and Suzuki, T. N. (2016). Kinship modulates the attention of naïve individuals to the mobbing behaviour of role models. Anim. Behav. 112, 83–91. doi: 10.1016/j.anbehav.2015.11.020
Griesser, M., and Suzuki, T. N. (2017). Naive juveniles are more likely to become breeders after witnessing predator mobbing. Am. Nat. 189, 58–66. doi: 10.1086/689477
Harrison, X. A., Donaldson, L., Eugenia Correa-Cano, M., Evans, J., Fisher, D. N., Goodwin, C., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. doi: 10.7717/peerj.4794
Heinsohn, R. G. (1991). Slow learning of foraging skills and extended parental care in cooperatively breeding white-winged choughs. Am. Nat. 137, 864–881. doi: 10.1086/285198
Jansson, C., Ekman, J., and Brömssen, A. V. (1981). Winter mortality and food supply in tits Parus spp. Oikos 37, 317–322. doi: 10.2307/3544122
Kacelnik, A., Cotton, P. A., Stirling, L., and Wright, J. (1995). Food allocation among nestling starlings: sibling competition and the scope of parental choice. Proc. R. Soc. 259,259–263. doi: 10.1098/rspb.1995.0038
Kirkpatrick, M., and Lande, R. (1989). The evolution of maternal characters. Evolution. 43, 485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x
Kouba, M., Barto,š, L., and Štastný, K. (2013). Differential movement patterns of juvenile Tengmalms owls (Aegolius funereus) during the post-fledging dependence period in two years with contrasting prey abundance. PLoS ONE 8:e67034. doi: 10.1371/journal.pone.0067034
Le Bohec, C., Gauthier-Clerc, M., and Le Maho, Y. (2005). The adaptive significance of crèches in the king penguin. Anim. Behav. 70, 527–538. doi: 10.1016/j.anbehav.2004.11.012
Low, M., and Pärt, T. (2009). Patterns of mortality for each life-history stage in a population of the endangered New Zealand stitchbird. J. Anim. Ecol. 78, 761–771. doi: 10.1111/j.1365-2656.2009.01543.x
Mazerolle, M. J. (2019). AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). Available online at: https://cran.r-project.org/package=AICcmodavg
McCready, M., and Ewen, J. G. (2016). Hihi (Stitchbird) Breeding on Tiritiri Matangi Island: 2015-2016 Breeding Season. Internal Report.
Middleton, H. A., Green, D. J., and Krebs, E. A. (2007). Fledgling begging and parental responsiveness in American dippers (Cinclus mexicanus). Behaviour 144, 485–501. doi: 10.1163/156853907780713064
Monaghan, P. (2008). Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. B Biol. Sci. 363, 1635–1645. doi: 10.1098/rstb.2007.0011
Monrós, J. S., Belda, E. J., and Barba, E. (2002). Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos 99, 481–488. doi: 10.1034/j.1600-0706.2002.11909.x
Naef-Daenzer, B., and Grüebler, M. U. (2016). Post-fledging survival of altricial birds: ecological determinants and adaptation. J. F. Ornithol. 87, 227–250. doi: 10.1111/jofo.12157
Naef-Daenzer, B., Widmer, F., and Nuber, M. (2001). Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 70, 730–738. doi: 10.1046/j.0021-8790.2001.00533.x
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. doi: 10.1111/j.1469-185X.2007.00027.x
Price, T. (1998). “Maternal and paternal effects in birds: effects on offspring fitness,” in Maternal Effects as Adaptations, eds T. A. Mousseau and C. W. Fox (New York, NY: Oxford University Press, 202–226.
Przybylo, R., Wiggins, D. A., and Merilä, J. (2001). Breeding success in Blue Tits: good territories or good parents? J. Avian Biol. 32, 214–218. doi: 10.1111/j.0908-8857.2001.320302.x
Psorakis, I., Voelkl, B., Garroway, C. J., Radersma, R., Aplin, L. M., Crates, R. A., et al. (2015). Inferring social structure from temporal data. Behav. Ecol. Sociobiol. 69, 857–866. doi: 10.1007/s00265-015-1906-0
Rasch, G., and Craig, J. L. (1988). Partitioning of nectar resources by New Zealand honeyeaters. New Zeal. J. Zool. 15, 185–190. doi: 10.1080/03014223.1988.10422613
Ridley, A. R., and Raihani, N. J. (2007). Variable postfledging care in a cooperative bird: causes and consequences. Behav. Ecol. 18, 994–1000. doi: 10.1093/beheco/arm074
Riebel, K., Spierings, M. J., Holveck, M. J., and Verhulst, S. (2012). Phenotypic plasticity of avian social-learning strategies. Anim. Behav. 84, 1533–1539. doi: 10.1016/j.anbehav.2012.09.029
Ringsby, T. H., Sæther, B.-E., and Solberg, E. J. (1998). Factors affecting juvenile survival in house sparrow Passer domesticus. J. Avian Biol. 29, 241–247. doi: 10.2307/3677106
Royle, N. J., Smiseth, P. T., and Kölliker, M. (Eds.). (2012). The Evolution of Parental Care. New York, NY: Oxford University Press.
Russell, E. M. (2000). Avian life histories: is extended parental care the southern secret? Emu Austral Ornithol. 100, 377–399. doi: 10.1071/MU0005S
Slagsvold, T., and Wiebe, K. L. (2011). Social learning in birds and its role in shaping a foraging niche. Philos. Trans. R. Soc. B Biol. Sci. 366, 969–977. doi: 10.1098/rstb.2010.0343
Symonds, M. R., and Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21. doi: 10.1007/s00265-010-1037-6
Thompson, A. M., Raihani, N. J., Hockey, P. A. R., Britton, A., Finch, F. M., and Ridley, A. R. (2013). The influence of fledgling location on adult provisioning: a test of the blackmail hypothesis. Proc. R. Soc. 280:20130558. doi: 10.1098/rspb.2013.0558
Thornton, A., and Raihani, N. J. (2008). The evolution of teaching. Anim. Behav. 75, 1823–1836. doi: 10.1016/j.anbehav.2007.12.014
Thorogood, R., Armstrong, D. P., Low, M., Brekke, P., and Ewen, J. G. (2013). The value of long-term ecological research: integrating knowledge for conservation of hihi on Tiritiri Matangi Island. New Zeal. J. Zool. 37, 298–306.
Thorogood, R., Ewen, J. G., and Kilner, R. M. (2011). Sense and sensitivity: responsiveness to offspring signals varies with the parents' potential to breed again. Proc. R. Soc. 278, 2638–2645. doi: 10.1098/rspb.2010.2594
Tinbergen, J. M., and Boerlijst, M. C. (1990). Nestling weight and survival in individual great tits (Parus major). J. Anim. Ecol. 59, 1113. doi: 10.2307/5035
VanderWaal, K. L., Atwill, E. R., Isbell, L. A., and McCowan, B. (2014). Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis). J. Anim. Ecol. 83, 406–414. doi: 10.1111/1365-2656.12137
Walker, L. K., Stevens, M., Karadas, F., Kilner, R. M., and Ewen, J. G. (2013). A window on the past: male ornamental plumage reveals the quality of their early-life environment. Proc. R. Soc. 280, 20122852–20122852. doi: 10.1098/rspb.2012.2852
Ward, P., and Zahavi, A. (2008). The importance of certain assemblages of birds as “information centres” for food finding. Ibis 115, 517–534. doi: 10.1111/j.1474-919X.1973.tb01990.x
Keywords: post-fledging parental care, social network, dispersal, nestling condition, passerine, Notiomystis cincta
Citation: Franks VR, McCready M, Savage JL and Thorogood R (2019) Time Spent With Parents Varies With Early-Life Condition, but Does Not Predict Survival or Sociality of Juvenile Hihi. Front. Ecol. Evol. 7:322. doi: 10.3389/fevo.2019.00322
Received: 30 April 2019; Accepted: 09 August 2019;
Published: 27 August 2019.
Edited by:
Rita Covas, University of Porto, PortugalReviewed by:
Michael Griesser, University of Zurich, SwitzerlandMatthieu Paquet, Swedish University of Agricultural Sciences, Sweden
Copyright © 2019 Franks, McCready, Savage and Thorogood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria R. Franks, dml4X2ZyYW5rc0BsaXZlLmNvbQ==
 Victoria R. Franks
Victoria R. Franks Mhairi McCready2,3
Mhairi McCready2,3 James L. Savage
James L. Savage Rose Thorogood
Rose Thorogood