- 1Università di Firenze - Dipartimento di Biologia, Sesto Fiorentino, Italy
- 2Centre for Biodiversity and Environment Research, University College London, London, United Kingdom
Nestmate recognition, i.e., the ability to discriminate nestmates from foreign individuals, is a crucial feature of insect societies, and it has been traditionally considered to be predominantly based on chemical cues. Recent empirical evidence, however, suggests a relevant plasticity in the use of different communication channels according to cue availability and reliability in different contexts. In particular, visual cues have been shown to influence various types of social recognition in several social insects, but their role in nestmate recognition is still under-investigated. We tested the hypothesis of plasticity in the use of visual and chemical recognition cues in the primitively eusocial wasp Polistes dominula, in which the availability and reliability of recognition cues vary across the colony cycle. Indeed, before the emergence of workers, P. dominula colonies are rather small (one to few individuals), and the variability in the facial pattern might allow resident wasps to use visual cues for nestmate recognition. After workers' emergence, the increase in the number of colony members reduces the reliability of visual cues, thus leaving chemical cues as the most reliable nestmate recognition cues. We thus predict a differential use of chemical and visual cues along colony life. We experimentally separated visual and chemical cues of nestmates and non-nestmates and presented them alone or in combination (with coherent or mismatched cues) to resident wasps to test which communication channel was used in the two stages and, in case, how visual and chemical cues interacted. Our results show, for the first time in a social insect, the differential use of visual and chemical cues for nestmate recognition in two different phases of colony, which supports the hypothesis of a plastic, reliability-based use of recognition cues in this species according to the different colonial contexts.
Introduction
Social organization relies upon social recognition, which is the ability of individuals to distinguish among the individuals they encounter and to bias their behavior accordingly, i.e., responding with an adaptive behavior toward the appropriate individual (Ward and Webster, 2016). Social recognition thus plays a crucial role in regulation of social interactions within animal groups, by shaping parent–offspring interactions, competitive aggression, mate choice, and cooperative behaviors (Waldman, 1988; Gherardi et al., 2012; Aquiloni and Tricarico, 2015). Eusocial insects, such as ants, wasps, termites, and bees, live in complex societies that represent pinnacles of social evolution and whose organization relies on sophisticated forms of social recognition, such as the ability to recognize caste, dominance and fertility status, gender, and nestmates from non-nestmates (Wilson, 1971; van Zweden and d'Ettorre, 2010; Cervo et al., 2015).
Nestmate recognition (hereafter NMR), i.e., the ability to discriminate nestmates from non-nestmates, is the quintessential form of social recognition that occurs in insect societies (d'Ettorre and Lenoir, 2009). Social insect colonies are rich in resources that conspecific and heterospecific individuals may exploit: nests are costly to produce and advantageous in the protection they provide, colonies are full of harmless and meaty brood, and workers efficiently provide alloparental care that might be selfishly exploited. Many species across the whole range of the animal kingdom indeed benefit from exploiting social insect colonies at various extents, from predation to social parasitism (Fürst et al., 2011; Cini et al., 2019). NMR evolved to allow colony members to recognize and accept each other while strongly repelling potentially dangerous intruders, thus allowing the protection of the colony and directing altruistic acts toward related recipients (Hamilton, 1987).
NMR occurs through a process of phenotype matching that involves the perception of a label carried by encountered individual and the comparison of this label with an internal reference (template), i.e., a neural representation of the trait stored within the evaluator peripheral and central nervous system (Crozier and Pamilo, 1996; Leonhardt et al., 2007; d'Ettorre and Lenoir, 2009; Signorotti et al., 2015). The response of the evaluator depends on how well the label matches the template (van Zweden and d'Ettorre, 2010), with the aggressive response triggered when the mismatch exceeds a certain threshold (Reeve, 1989). Decades of research convincingly demonstrated that colony identity is mainly encoded in the blend of cuticular hydrocarbons (CHCs) (Howard and Blomquist, 2005; Blomquist and Bagnères, 2010). Typically, colonies of a given species have a qualitatively similar CHC profile, which differs in the relative amounts of each compound (Lorenzi et al., 1996; Dani, 2006; Bruschini et al., 2010; van Zweden and d'Ettorre, 2010).
CHC blends have several advantages as NMR cues compared to other potential cues pertaining to different sensory modalities. First, the CHC blend usually entails several dozens of compounds, which vary in their relative abundance across colonies, so that the signal arising from such a complex mixture can be informative about colony membership (van Zweden and d'Ettorre, 2010; Sturgis and Gordon, 2012). Then, CHC blend profile is highly influenced by the environment (e.g., by diet, Liang and Silverman, 2000; Buczkowski et al., 2005) and CHCs can be exchanged through social contact, which makes the CHC signal highly flexible, thus enabling to keep the colony signature updated in a continuously changing environment (Richard and Hunt, 2013).
While it has been repeatedly shown that CHCs are the main cues used in NMR (reviewed in Blomquist and Bagnères, 2010; van Zweden and d'Ettorre, 2010), recent experimental evidence revealed that olfaction might be coupled with, or even overcame by, other sensory modalities, such as vision (Cervo et al., 2015). Indeed, in a tropical hover wasp species characterized by small and flexible societies, Parischnogaster flavolineata, colony members are able to perform NMR using individual facial patterns in addition to chemical cues, and in case of contrasting information, visual cues are preferred over chemical ones (Baracchi et al., 2013, 2015).
The importance of visual cues in social insect recognition remained overlooked for many decades. The last 15 years of researches, especially in paper and stenogastrine wasps, provided strong empirical evidence about the use of visual cues in several forms of social recognition inside and outside social insect colonies, both in the intraspecific and interspecific context (Cervo et al., 2015). Wasps do indeed show remarkable variation in the color patterning of faces and abdomen and the use of such cues in social recognition has been shown for almost all species (even if few) investigated so far. This suggests that this ability could be widespread in social wasps, especially in those that live in nests without envelopes, where communication by using reflected light to produce visual signals is possible (reviewed in Cervo et al., 2015).
Despite the potentially smaller informative content of visual cues compared to chemical ones (but see Baracchi et al., 2016) and their static nature (individual color patterning remains stable after emergence, while CHC blend is continuously updated), visual cues might be advantageous over chemical ones to enable NMR as they can be quickly processed and do not require contact or really close distance (contrary to CHCs), thus enabling a faster NMR decision. Indeed, when assessing a potential intruder, colony members are faced with a trade-off between speed and accuracy of recognition and, depending on the context, speed might be prioritized over accuracy (Chittka et al., 2009; Baracchi et al., 2015). We can thus predict visual cues to be mostly used in species characterized by small colonies, where repeated encounters with a low number of colony members might allow learning their visual pattern through a familiarization process. Given that small societies are indeed common in many social insect groups, the use of visual cues in NMR potentially involves many species, especially in the primitively eusocial taxa that represent an interesting experimental window on the evolution of sociality (Rehan and Toth, 2015).
Our current understanding of the cues underlying NMR in insect societies thus suggests an association between the sensory channel used for NMR and colony size. Chemicals might be preponderant in large societies, such as in honeybees and many ant species, where visual cues could not clearly be reliable, while visual cues might be involved (together with or replacing chemicals) in small societies with variable visual cues (such as those of paper wasps). In some insect species, however, such as independent founding wasps, colony size dramatically changes throughout the colonial development, passing from few to hundreds of colony members (Reeve, 1991). A compelling question, so far unanswered, is therefore to what extent a species can plastically shift from using cues of one sensory modality (e.g., visual) to those of another one (e.g., chemical) during the colony development. In other words, we wonder if the sensory modality used for NMR is hardwired within the species behavioral repertoire or can change according to the availability and reliability that it assumes in different colonial phases. By answering this question, we aim to unveil an unexpected and yet undocumented level of plasticity in insect communication and to provide an experimental model system for future studies regarding cognitive abilities of social insect mini-brains.
Here, we tested the hypothesis of plasticity in the use of visual and chemical recognition cues according to their reliability as NMR cues in the primitively eusocial wasp Polistes dominula, i.e., that NMR is based on different cues in different phases of the colony cycle (Figure 1).
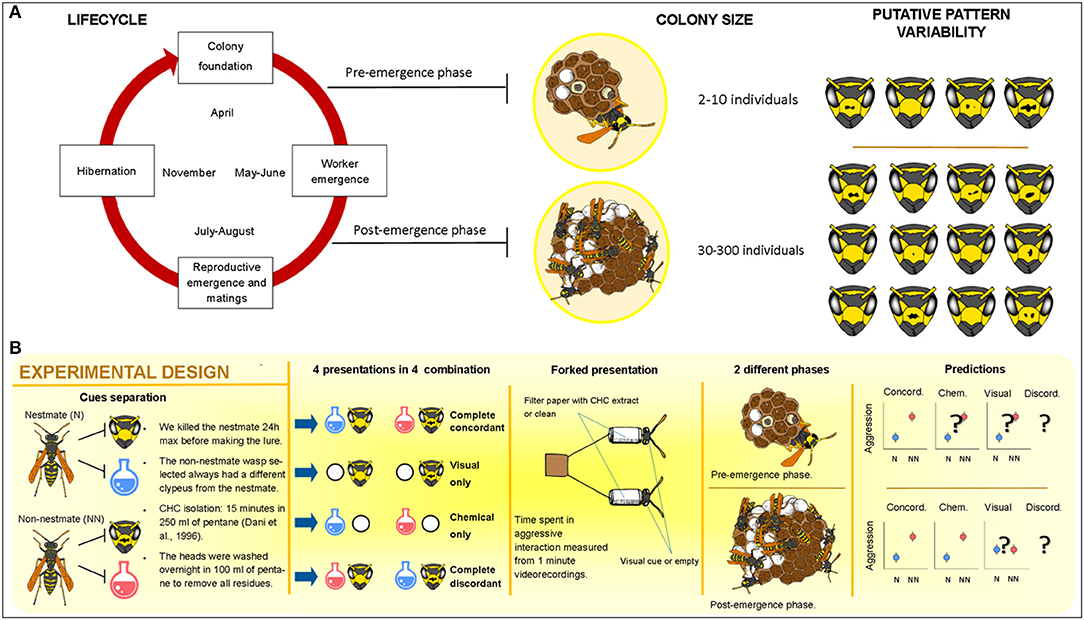
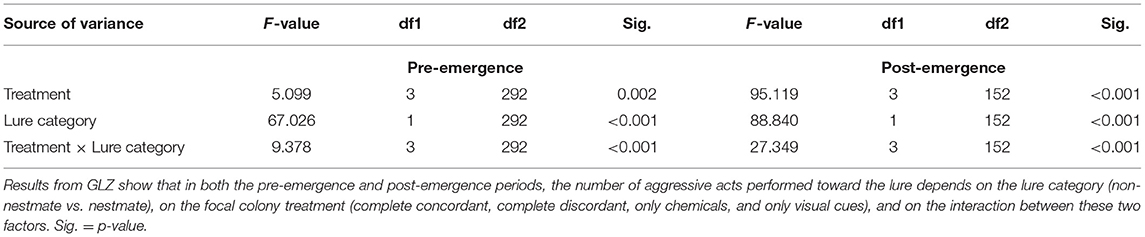
Figure 1. (A) Life cycle of Polistes dominula: the two phases considered in this work are shown together with their variation in colony size and the putative facial pattern variability; (B) the experimental design used in the study. Our prediction was that a differential use of chemical and visual cues occurs along colony life. When both stimuli (chemical and visual) are presented together (concordant lure), proper nestmate recognition is expected [i.e., Non-nestmates (NN) are attacked more than Nestmates (N)]. When stimuli are presented alone (i.e., only chemical or only visual), the presence of proper recognition will depend on the reliability of cues. We predict that in the pre-emergence stage, both single-cue lure (visual and chemical) will elicit proper NMR. On the contrary, we predict that in the post-emergence stage, proper NMR only occurs when chemicals are presented, while no NMR will occur when only visual cues are presented. No a priori prediction about discordant lures (conflict) can be made without knowing the results of single-cue lures. Question marks indicates that no previous experimental evidence has ever been produced for such a comparison (Drawing: Leonardo Platania).
P. dominula is a temperate paper wasp species whose small colony size and phenotypic plasticity have made it a model organism for social evolution and communication studies (Pardi, 1948, 1996; Dani, 2006; Jandt et al., 2014; Cervo et al., 2015) and, thanks to the recent release of its sequenced genome, also for omic studies (Standage et al., 2016). P. dominula species also represents a good model to test the existence of plasticity in the use of visual and chemical cues according to their availability and reliability, as (i) both chemical and visual cues are known to be used in several forms of social recognition, and (ii) availability and reliability of recognition cues vary across the season (Dani, 2006; Cervo et al., 2015) (Figure 1A).
NMR in P. dominula is behaviorally evident, with non-nestmates that are highly repelled through aggressive reactions by resident wasps (Dani et al., 2001) and it is based on chemical cues, in particular in the variation in CHC signature among different colonies (Bruschini et al., 2011). On the contrary, facial color patterns, which are widely variable in this species and consists of one or more black spots, with variable size and shape, or no black spots at all on the yellow clypeus, are used for different social recognition forms, such as signaling of dominance status and agonistic abilities (e.g., Tibbetts and Dale, 2004; Tibbetts and Lindsay, 2008, but see Cervo et al., 2008), gender recognition (Cappa et al., 2016) and possibly species recognition (Cervo et al., 2015; Cini et al., 2015), but they have never been shown to allow NMR (Cervo et al., 2015).
Here, we experimentally separated visual and chemical cues of nestmate and non-nestmate P. dominula wasps and presented the cues alone or in combination (with coherent or mismatched cues) to resident wasps in NMR behavioral trials (Figure 1B). We aimed to test which communication channel is used in the two different stages of the colony cycle (at the beginning, when colonies are inhabited by only a few individuals and, after the emergence of workers, when the number of colony members dramatically increases, Figure 1A) and, if so, how visual and chemical cues interacted. Our prediction was that a differential use of chemical and visual cues occurs along colony life, with visual cues used only, if ever, in the pre-emergence period, while chemical cues would be used in both periods (Figure 1B). To our knowledge, our results show, for the first time in a social insect, a differential use of chemical and visual cues across the colony cycle, and provide the first experimental proof that, in this species, visual cues, in addition to chemical cues, are used to recognize nestmates from non-nestmates.
Materials and Methods
Animal Collection and Laboratory Rearing
P. dominula colonies are founded in early spring, when one or more females build a new colony and take care of the immature brood (pre-emergence phase). At the end of May, the first brood emerges: these females are workers that do not reproduce but rather take care of the nest and of the immature brood (post-emergence phase). Reproductive individuals, males and gynes, emerge only later in the season, from the end of July (reproductive phase) (Reeve, 1991). Mating occurs outside of the colony at the end of summer (Beani, 1996); mated females overwinter in large groups and then start new colonies in the following spring (Dapporto and Palagi, 2006; Cini and Dapporto, 2009).
For the first experiment (pre-emergence phase), 36 bigynic colonies (i.e., colonies founded by two foundresses) were collected, during the first half of May 2015, before worker emergence, from three different sites throughout Tuscany (Central Italy). In the same period, foundresses from a different population were collected to be used as non-nestmate lures (see below).
For the second experiment (post-emergence phase), 10 colonies in workers' phase (with at least 5 workers) were collected in the same sites at the beginning of July 2016. Non-nestmate workers used as lures were collected on colonies belonging to different populations located in the same area (Tuscany, Central Italy). In both experiments the wasps used as non-nestmates were collected in populations at least 3 km apart from the populations where focal experimental colonies were collected, in order to minimize the likelihood of high relatedness and prior encounter among tested individuals.
Colonies were brought to the laboratory, and each colony was transferred to a 15 cm × 15 cm × 15 cm glass cage provided with ad libitum sugar, water, fly maggots, and paper as nest-building material. Colonies were maintained under natural light cycle and temperature conditions with additional illumination from neon lighting with a daily rhythm (L:D 10:14).
In the bigynic nests, each foundress was individually marked with a different combination of enamel colors (Humbrol, UK) on the wings for individual identification. Behavioral observations were carried out before NMR experiments in order to establish the dominant individual for each colony, on the basis of well-established dominant rank-related behaviors such as ritualized dominance behaviors, egg-laying, and low foraging effort (Pardi, 1948; Pratte, 1989).
General Experimental Procedure
Each colony was subjected to four NMR trials, which consisted in the simultaneous presentation of two lures carrying NMR cues related to one or both sensory modalities—visual (i.e., an odorless wasp head) and chemical (i.e., CHCs) cues—in a concordant (both from the same individual, which could be a nestmate or a non-nestmate) or discordant (one from a nestmate and the other from a non-nestmate individual) combination (Figure 1B). Lures were presented to colonies in a random order and behavioral response was video-recorded for 1 min after the first interaction between lure and resident wasps. An aggressive response index was computed as the total number of aggressive acts (bites and stings) performed toward the lure (see Data Analysis below). The aggressive response of the colony as a whole was measured. This corresponds to the aggressive reaction of the alpha female, the only wasp present in the colony, in the pre-emergence phase, and to the aggressive reaction of all the workers that responded in the post-emergence phase. Both pre-emergence and post-emergence colonies were tested once with the same protocol.
Lure Selection
In the pre-emergence experiment, for each colony, two wasp lures were selected: (i) the beta female of the tested nest as nestmate lure and (ii) a foundress belonging to a different population as non-nestmate lure. Lures were coupled based on a clear different color pattern on the clypeus, i.e., the non-nestmate lure was chosen randomly within a pool of wasps with a clypeus patterning different from that of the nestmate wasp. Three categories of clypeus pattern were selected: 1 = totally yellow clypeus, 2 = one spot, and 3 = two or more spots on the clypeus (Tibbetts and Lindsay, 2008).
Analogously, also in the post-emergence one, two wasp lures with a different color pattern on clypeus (see above) were selected for each colony: (i) a worker of the tested nest as nestmate lure and (ii) a worker belonging to a different population as non-nestmate lure. The frequency distribution of clypeus pattern was not different between treatments in either experiment (χ2 = 2.19, df = 2, p = 0.335; χ2 = 5.01, df = 2, p = 0.082). Apart from the clypeus pattern, all lure wasps were randomly chosen. Lure size (estimated by measuring head width, Cini et al., 2011a) was not different among treatments or in the pre-emergence experiment (Wilcoxon test, W: 369 p = 0.802, n = 37) or in the post-emergence one (Wilcoxon test, W: 369 p = 0.349, n = 20).
We used alpha females as resident focal females (and thus we used the removed beta females as nestmate lures) for the pre-emergence phase experiment for both biological and experimental reasons. From the biological point of view, the reaction of alpha females is expected to be more uniform than that of beta females. Indeed, while the alpha female must defend her nest against any kind of individual, the beta female might have divergent interests according to the identity and strength of the opponent. While beta females usually show colony defense and NMR, in some cases, they might accept (or attack to a different degree) a very dominant individual. This might occur as beta females are defending a resource (the colony and the brood therein) that represents a smaller fitness gain to them than to alpha females (only the alpha females reproduce and alpha females and beta females are often unrelated, Queller et al., 2000). From the experimental point of view, in order to do the test and present a nestmate together with a non-nestmate as lures, we needed to kill the nestmate (the beta female in our case). If it was the alpha female to be killed and used as a lure, we would have created an orphan colony, even if for just a few hours. This would have been different from the post-emergence phase, where we would have removed a worker (to be matched with a non-nestmate worker), so we would have another (and bigger) difference.
Lure Preparation
Chemical Cues
All wasps selected to be used as a lure were killed by freezing 1 day before the bioassay. For obtaining CHC extract, the entire body of each wasp lure was individually placed in a glass vial with 250 μl of an apolar solvent (pentane) for 15 min (Dani et al., 1996). After wasp body removal, vials with pentane extracts were left to dry overnight. The following day, before NMR bioassays, extracts were resuspended in 100 μl of pentane and transferred on pentane-washed filter paper sheets (2.7 × 1 cm). Filter paper sheets were then fixed on an inert support (half filter tip, ultraslim, Rizla) to obtain lures bearing the sole chemical cues (Figure 1B).
Visual Cues
After washing the wasp body in pentane, the head of each lure was separated from the rest of the body and kept in 1 ml of pentane overnight (Cini et al., 2015) to totally remove the residual CHC fraction. The following day, before NMR bioassays, the heads were mounted on entomological pins over the inert support (see above).
Lure Presentation
During each of the four NMR experiments, two lures were simultaneously presented to each colony (following a procedure already tested for both visual and chemical stimuli; Ortolani et al., 2010; Bruschini et al., 2011; Cini et al., 2011b, 2015). Each lure was composed of one out of four possible combinations of stimuli obtained by nestmate and non-nestmate wasps (Figure 1B): (i) “only visual” lures, i.e., odorless heads of nestmate (see above) and non-nestmate wasps mounted on pentane washed paper filters; (ii) “only chemical” lures, i.e., filter tip with filter paper sheet loaded with CHC extracts of nestmate and non-nestmate; (iii) concordant lures, bearing together chemical and visual cues of each wasp, recreating the natural coupling of visual and chemical cues where each individual presents its own array of stimuli; and (iv) discordant lures, composed of nestmate visual cues (head) and non-nestmate chemical cues (scent) on one lure and non-nestmate visual cues and nestmate chemical cues on the other, creating an artificial combination of mixed visual and chemical stimuli. In both pre-emergence and post-emergence experiments in the discordant treatment, the lure with the visual stimulus of the non-nestmate (and thus the chemical stimulus of the nestmate) was considered as the “non-nestmate” lure and the lure with the visual stimulus of the nestmate (and thus the chemical stimulus of the non-nestmate) was considered as the “nestmate” lure (Figure 1B).
The procedure for all the experiments consisted of the simultaneous presentation of two stimulus lures. Following a protocol reported for similar bioassays carried out on the same species (Ortolani et al., 2010; Bruschini et al., 2011; Cini et al., 2015), we used a 30 cm-long stick with a fork at one end. The two different lures (belonging to same combination of stimuli) were mounted on the tips of the fork, 1.5 cm apart, and were randomly placed on the left or right. The fork device was slowly introduced into the colony box while the alpha female (pre-emergence experiment) or the workers (post-emergence experiment) were on the nest, and held at a distance of 1 cm from the comb for 1 min after the first interaction between the alpha female/workers and the presented lures. In the post-emergence experiment, for each of 10 colonies, we performed two set of tests, each one presenting the lures to a group of different workers (i.e., in total, we tested 20 groups of 5–10 wasps in the whole post-emergence experiment). As in post-emergence colonies, the queen only rarely participates in NMR, and we temporary removed it from the colony for the duration of the test. Colonies were presented with all four combinations of stimuli in a random order at 1 h interval between successive trials (a trial is the simultaneous presentation of the two lures). Presentations were video-recorded. Experiments were carried out from 11:00 AM to 3:00 PM, on sunny days. All experiments were performed blindly by a first experimenter and video-recorded by a second experimenter. A total of 144 trials on 36 colonies were carried out in the first experiment (pre-emergence phase) and 80 trials on 20 different groups of workers from 10 colonies in the second experiment (post-emergence phase).
Data Analysis
Video recordings were watched blindly by two observers to avoid biases in counting the interactions between the wasps and the lures during presentations. We measured the number of aggressive acts performed (i.e., wasps open their mandibles and attack the presented lure biting and, more rarely, stinging the lure), as this is the typical behavioral response that allows the evaluation of NMR (Dani et al., 2001) toward each of the two presented lures.
In order to evaluate NMR, we assessed whether, as expected, aggressive response was significantly greater toward non-nestmate lures than toward nestmate ones, and whether this depended on the kind of stimuli presented, i.e., visual, chemical, and their combination (see above, Figure 1B). For each experiment, we separately used a generalized mixed model, with Poisson distribution and log-link function, followed by post-hoc pairwise comparison with sequential Sidak correction. We set the aggressive reaction (i.e., time spent in aggressive acts toward the lure) as the dependent variable. We used as predictors lure category (i.e., nestmate or non-nestmate) and treatment (i.e., only visual, only chemicals, concordant, and discordant stimuli) as fixed factors together with their interaction (lure × treatment). We considered colony id as random factor (each colony performed multiple trials). Under our predictions (Figure 1B), we expect to find an effect of lure (nestmate vs. non-nestmate, with non-nestmate eliciting a greater aggression than nestmate), an effect of treatment (with some cues and/or a combination of cues eliciting a greater reaction than others), and an effect of the interaction (the difference in aggression toward nestmates and non-nestmates depends on the kind of treatment, i.e., cues, presented).
This model could not include the variable “pattern of clypeus” of the lures as the lures of the “only chemical” treatment were represented by only filter papers with chemicals, and they were both bearing any information about the clypeal pattern. Thus, in order to assess the influence of clypeus pattern on the aggressive response of resident wasps, in the case that NMR was found in the visual treatment in the post-hoc comparisons of the first model, a second model was run. This model had the same settings of the first one, but it has been run by excluding the chemical only treatment and including the lure clypeus pattern (1 = totally yellow clypeus, 2 = one spot, 3 = two or more spots on the clypeus) together with lure category, treatment, and their interactions (lure category × treatment, lure category × lure clypeus pattern, and treatment × lure clypeus pattern).
Results
In both experiments, aggressive response was significantly influenced by treatment, lure category, and their interaction (Table 1).
Treatment influenced aggressive response in a similar way in both experiments, with treatments involving both chemical and visual stimuli together (concordant and discordant treatments), which overall evoked more aggression, and the treatment presenting only chemical stimuli evoking the lowest levels of aggression. In particular, in the pre-emergence experiment, the highest levels of aggression were evoked by the concordant treatment, followed by discordant, only visual, and then only chemical. Post-hoc comparisons were significant for the only chemical vs. concordant treatment comparison (p < 0.001) and close to significance threshold for the only chemical vs. only visual (p = 0.057), for the only chemical vs. discordant (p = 0.060) and for the only visual vs. discordant (p = 0.061) treatment comparison, while non-significant for the concordant vs. discordant treatment comparisons (p = 0.410). In the post-emergence experiment, the highest level of aggression was found in the discordant treatment, followed by the concordant one, then the only visual, and then the only chemical treatment. All pairwise comparisons were statistically significant (concordant vs. only chemical p < 0.001; concordant vs. visual p = 0.043; concordant vs. discordant p = 0.017; only chemical vs. only visual p < 0.001; only chemical vs. discordant p < 0.001; only visual vs. discordant p < 0.001).
Lure category affected the aggressive response in a similar way in both experiments: non-nestmate lures were attacked more than nestmates (Table 1). A significant interaction lure category × treatment highlighted that the kind of treatment influenced the differential aggressive response toward nestmate and non-nestmate lures in both experiments (Table 1; Figure 2). Post-hoc comparisons showed that the kind of stimuli allowing efficient NMR was different in the two experiments. In the pre-emergence experiment, non-nestmate lures were attacked more than nestmate ones (thus highlighting a proper NMR) when complete concordant stimuli (chemical and visual) and only visual stimuli were presented (p < 0.001 in both cases; Figure 2A). On the contrary, no significant difference was found in the aggressive response toward nestmate and non-nestmate lures or when only chemical stimuli or when discordant stimuli were presented (p = 0.174 and p = 0.142, respectively; Figure 2A). In the post-emergence period, non-nestmate lures were attacked more than nestmate ones (thus highlighting a proper NMR) when complete concordant stimuli (chemical and visual) and only chemical stimuli were presented (p < 0.001 in both cases; Figure 2B). On the contrary, no significant difference was found in the aggressive response toward nestmate and non-nestmate lures, neither when only visual stimuli nor when discordant stimuli were presented (p = 0.318 and p = 0.341, respectively; Figure 2B).
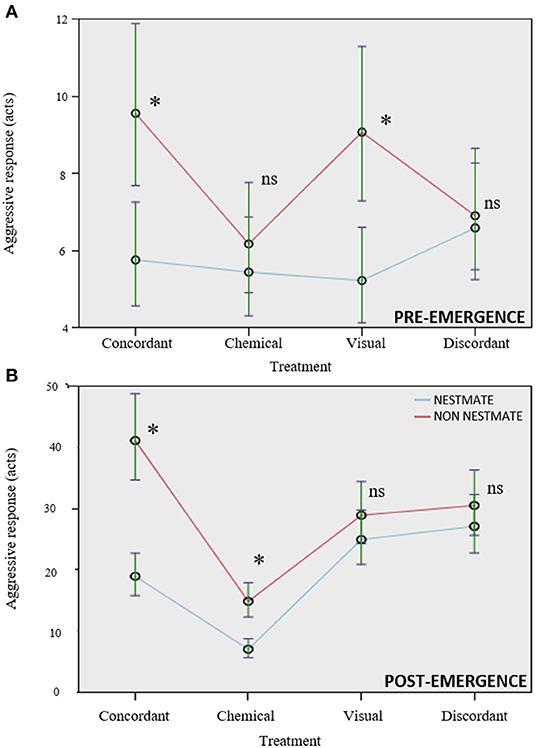
Figure 2. NMR is expressed when complete concordant lures are presented, and not when complete discordant lures are presented. Visual cues alone allow NMR only in the pre-emergence period (A) while chemical cues alone allow NMR only in the post-emergence period (B). Circle and bars, respectively, represent mean and standard error of the mean; non-nestmate (in red), nestmates (in blue). ns, not significant pairwise comparisons, *significant pairwise comparisons (p < 0.05), which means effective NMR.
The effect of clypeus pattern on aggressive response was investigated only for the pre-emergence experiment, as in the post-emergence phase, a significant NMR was not found in the visual treatment. In addition to confirming the significant effects of lure category, treatment, and their interaction, as in the first model, the main result of this second model is that clypeus pattern had a significant influence on aggressive response, with the pattern with two or more spots being less attacked than the two other patterns (no spot and one spot, p = 0.025 and 0.004, respectively) (Table 2). The significant interaction between treatment and clypeus pattern also revealed that the treatment affected how lures with different clypeus patterns were treated. In particular, the clypeus pattern showed a significant effect only in the “only visual” experiment (F = 16.171, df = 2.214, p < 0.001), while no differences exist in the concordant and discordant treatment (F = 2.216, df = 2.214, p = 0.112; F = 1.284, df = 2.214, p = 0.279, respectively) (Table 2; Figure 3). Wasps with 0 spot and 1 spot pattern were significantly more attacked than those with 2 or more spots (p < 0.001), while no significant difference existed between no spot and 1 spot patterns (p = 0.124). The significant interaction between lure category and clypeus pattern (Table 2) showed that, while in all cases non-nestmates were significantly more attacked than nestmates, this difference was reduced and lost significance for lures with 1 spot pattern.
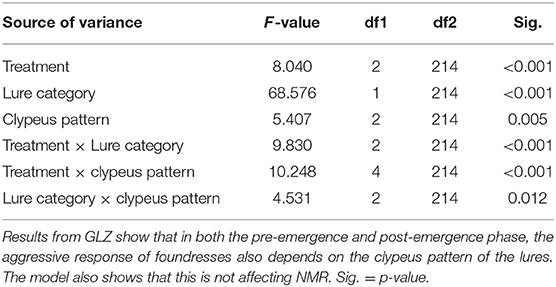
Table 2. The clypeus pattern of the lures significantly affects aggressiveness, but only in the “only visual” treatment.
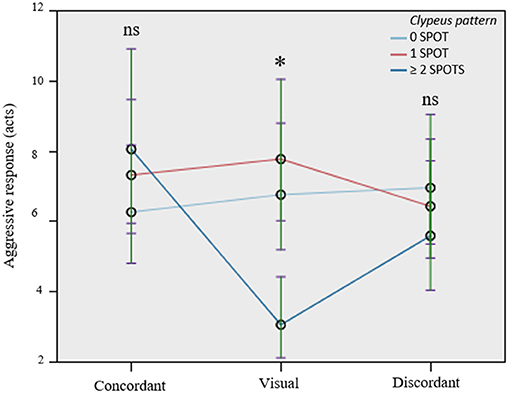
Figure 3. The clypeus pattern of the lures significantly affects aggressiveness, but only in the “only visual” treatment. Circle and bars, represent mean and standard error of the mean, respectively; the clypeus pattern of the lure is depicted by lines of different colors (no spot = light blue, one spot = red, two or more spots = dark blue). ns, not significant pairwise comparisons, *significant pairwise comparisons (p < 0.05).
Discussion
Our results show that the relative importance of NMR cues of the two sensory modalities, visual and chemical, changes according to colony phase in P. dominula wasps. In the early phase of the colony cycle, before workers' emergence, foundresses favor visual over chemical cues in the NMR recognition process. Conversely, in a more advanced colony stage, when many individuals are on the nest, workers rely on the chemical cues rather than on the visual ones to discriminate among nestmates and foreign individuals.
This difference in the importance of visual and chemical cues for NMR between the two conditions can be explained by the interplay between the features of the two sensory modalities and the different colony contexts across the season, which translates into different reliability of NMR cues in the two different colonial phases. Before emergence of workers, P. dominula colonies are composed only by foundresses and colony size is thus relatively small (ranging from 1 to 10 individuals, usually around 2–4; Reeve, 1991). After the emergence of workers, colony size rapidly increases up to dozens of wasps. It is thus conceivable that visual cues might be sufficiently variable and easier to be used in the first but not in the second phase. Indeed, the variation in the color patterning of the clypeus, the only visual cue so far shown to be perceived and used in intraspecific communication outside the sexual context (reviewed in Cervo et al., 2015), is limited. Actually, in many populations, a significant percentage of wasps show very similar facial pattern (Cervo et al., 2008; Zanette and Field, 2009; Green and Field, 2011), so that they can be categorized in a few classes (Cervo et al., 2008, 2015; Tibbetts and Lindsay, 2008). This suggests that the reliability of visual cues for NMR rapidly decreases as colony size increases. Moreover, reliability might also decrease because of an intrinsic cognitive difficulty for wasp brain to remember many visual patterns. The clypeal color patterning is, indeed, only partially genetically determined and seems to be affected by environmental factors, such as food and climate, during larval development (Tibbetts and Curtis, 2007; Green et al., 2012). This eventually results in large colonies having many kinds of facial patterns (personal observation), which might make the visual-cue-based NMR less effective and reliable.
Reliability of chemical cues might instead follow an opposite path. Despite the fact that a proper comparison of reliability of CHCs as NMR cues in different phases of colony life has never been done, it is conceivable that early season colonies (and thus pre-emergence ones) have a less marked colonial chemical signature than advanced stage colonies. This is suggested by the following: (i) in the pre-emergence phase, CHC profiles of foundresses are strongly influenced by individual social rank (Sledge et al., 2001); and (ii) the more homogeneous composition of advanced colonies, in terms of both physiology (for example in terms of fertility) and relatedness, compared to pre-emergence colonies. Indeed, few weeks after workers' emergence, the colony is consists of the dozens of sister workers, which share the genotype and many physiological features (above all, they are almost all unfertile or poorly fertile) (Queller et al., 2000). On the contrary, small pre-emergence colonies show a higher heterogeneity, with wider variation in the physiological status of foundresses (Pardi, 1946, 1948; Röseler et al., 1980; Röseler, 1991) and relatedness (Queller et al., 2000; Leadbeater et al., 2011), all factors that are known to affect CHC individual profile (Bonavita-Cougourdan et al., 1991; Sledge et al., 2001; Dapporto et al., 2004b, 2005). The colonial chemical signature is the product of a template shared by all individuals thanks to social interactions (contacts, trophallaxis) and through the nest material (Signorotti et al., 2015). It is likely that the more homogeneous conditions of late-season colonies allow the production of a more marked and reliable colonial chemical signature, while in pre-emergence colonies, individual level heterogeneity might somehow reduce inter-colony differences in the chemical profile. Moreover, the internal reference template might also be weaker in foundresses than workers. This is reasonable, as foundresses start to create their templates on their natal colonies (months before colony founding) and then update it during their life (Dapporto et al., 2004a), so that several months separate template formation and its use in NMR, while for workers, only a few days separate template formation from its use in NMR.
The finding that chemical or visual cues were not sufficient to allow NMR in pre-emergence and post-emergence colony phases, respectively, does not mean that they had no influence in the decisional process of wasps. Indeed, when both visual and chemical stimuli were coupled on the sample lure in a discordant combination (with both nestmate and non-nestmate cues on the same lure), wasps were not able to distinguish nestmates from non-nestmates, even if the relevant set of cues (i.e., visual in pre-emergence experiment, chemical in the post-emergence experiment) was still present. This suggests that the discordance in provided cues weakened the NMR process, highlighting a possible cross-modality sensory integration.
Overall, our results show, for the first time, a dynamic change in the cues used for NMR by P. dominula colonies. We highlight a few possible limitations of our study. Our study compared two very different periods, to cover the wide variation in contexts that colonies experience. This means that colonies differed under several aspects. First, to respect the natural conditions, focal wasps subjected to NMR trials were foundresses in the first experiment and workers in the second. This implies that age (foundresses are several months old, while workers are only days/weeks old) or caste-related differences (foundresses are reproductive individuals while workers are not) could have played a role. While we believe that age is unlikely to have an influence, as Polistes wasps are able to perform NMR within a few hours after emergence (thus well before the time at which they were tested) (reviewed in Signorotti et al., 2015), we cannot discard the hypothesis of differences between castes in the NMR system as it has been shown in a social bee (Wittwer and Elgar, 2018). Previous studies offered mixed evidence for the related species Polistes fuscatus: one study documented differences in recognition between queens and workers (with queens having a more restrictive acceptance threshold than workers against unrelated conspecific intruders; Fishwild and Gamboa, 1992), while a more recent experiment found no evidence of such a queen–worker variation in recognition (with workers showing similar ability in familiar recognition compared to queens; Injaian and Tibbetts, 2014). As P. dominula shows a very weak caste differentiation, which is mainly behavioral rather than physiological (Pardi, 1948; Reeve, 1991), we believe that the hypothesis of hard-wired castal differences in recognition system is unlikely. However, we believe that this needs to be tested by simultaneously evaluating the cues used for NMR by queens and workers in the same colony stage.
Second, in pre-emergence colonies, we recorded the response of the only individual present on the nest (colonies were founded by two females, one of which became the nestmate lure), while in post-emergence experiments, the response of many workers present was recorded. While analytically this does not represent a problem, as comparisons were internal to colony phase and colony id, one might speculate that group dynamics influence more the use of one sensory modality than the other. Future studies should thus investigate the use of visual and chemical cues for NMR focusing on the same individual (thus having the same phenotype, i.e., foundress or worker) through their entire life cycle in contexts where reliability of cues differ (i.e., the same queen before and after worker emergence, or the same wasps in nest by altering experimentally phenotypic variation in NMR cues). Similarly, it would be interesting to evaluate whether colony size (number of wasps) alone affects the sensory modality used in NMR. Our experiment, which compared two extreme opposite situations (small pre-emergence colonies vs. bigger post-emergence colonies), did not allow the disentanglement of the two factors, colony size and colony stage, which are usually correlated; future experimental work should test colony of the same stage with a different colony size, i.e., pre-emergence colonies with variable number of foundresses or post-emergence colonies with variable number of workers.
Our results indicate that the treatment influenced in a similar way the overall level of aggressive response in both experiments, with treatments involving both chemical and visual stimuli together (concordant and discordant treatments), which overall evoked more aggression, and the treatment presenting only chemical stimuli, which evoked the lowest levels of aggression. Moreover, visual cues alone elicited more aggression (toward both nestmates and non-nestmates) than chemical cues alone (significant result in the post-emergence phase and close to significance in the pre-emergence phase). These results are not surprising, as concordant and discordant treatments had lures that represented more biologically significant stimuli, as they had both chemical cues and visual cues (wasp heads). For the same reason, also the “only visual” treatment, which had wasp heads as lures, represented a more biologically relevant stimulus than the chemical only lures, in which a filter paper was covered with chemical cues extracted from the wasp body surface. It is not surprising thus that resident wasps were more aggressive toward what has a greater resemblance with a potential intruder, as already shown and discussed in this species (Cappa et al., 2016).
Our study also highlighted a significant effect of clypeus pattern of wasps in mediating aggressive behavior. The role of clypeus pattern in shaping aggressive interaction in P. dominula is highly debated. Several studies, performed in the non-native range of distribution (North America), showed that facial markers convey information about the competitive ability of an individual to potential opponents, which would thus use these visual cues to assess the agonistic abilities of potential rivals and minimize the time and costs of interactions, especially during the nest founding stage contests (Tibbetts and Dale, 2004; Tibbetts and Lindsay, 2008; Tibbetts et al., 2010). In particular, wasps having two or more spots are supposed to advertise a higher agonistic ability, and should thus be less challenged than wasps advertising lower agonistic ability (Tibbetts and Lindsay, 2008). However, this hypothesis has been repeatedly tested in the native range populations (Spain and Italy, for example) and no evidence has been found: facial patterns do not correlate with social dominance or other indicators of strength or health (Cervo et al., 2008), nor do they seem to be used in aggressive interactions (Branconi et al., 2018). Finally, it seems that facial pattern has no adaptive value in the wild (Green et al., 2013). Intriguingly, in this study, we report, for the first time in a population of the native range (Italy), that the kind of clypeus pattern of opponents influences, to a certain extent, the aggressive reaction of resident females of P. dominula. In particular, when chemical cues are ruled out, wasps with two spots are less attacked than wasps with one or no spot. This is in accordance with what was suggested by Tibbetts and Lindsay (2008), as wasps with two spots might advertise their greater competitive ability.
Our findings also highlight that visual cues mediate two different facets of social recognition in P. dominula wasps. First, they allow to recognize nestmates from non-nestmates, likely through a process of familiar recognition (i.e., wasps do recognize certain patterns as familiar, see below). Second, they might allow a mutual assessment during aggressive interactions. Our results also show that the two processes coexist, as in the only visual experiment foundresses were able to recognize non-nestmates from nestmates and, at the same time, their aggressive response was also influenced by the kind of clypeus pattern.
Overall, our results shed light on a possible involvement of facial pattern in shaping aggressive encounters also in the population of the native range. However, we also show that these effects are superimposed by chemical cues since they are evident when only visual cues are presented (only visual treatment). This suggests that the importance of clypeus pattern as advertisers of wasp agonistic ability, at least in this population, might come into play only under specific circumstances, as when information provided through other sensorial channels is unreliable.
We believe that our results provide several interesting insights, both at the taxon-specific level (Polistes paper wasps) and at a wider perspective. First, at the taxon-specific level, we demonstrated that visual cues alone can allow NMR in specific context, i.e., in small groups, which are interestingly those in which eusociality evolved in wasps. In this case, this type of social recognition can be considered familiar recognition. This is the first such finding for polistine wasps, as so far NMR based on visual cues has been shown only for hover wasps (Baracchi et al., 2015). The relevance of visual cues in NMR opens interesting perspectives on the highly debated topic of the use of visual communication in Polistes paper wasps, in which the absence, presence, and different level in the use of visual cues are demonstrated in different species and populations for a wide range of social recognition processes, from familiar recognition to gender recognition (reviewed in Cervo et al., 2015; Cappa et al., 2016). Moreover, we unexpectedly found that chemical cues alone are not sufficient in pre-emergence to allow accurate NMR, which suggests that the long-lasting tenet that NMR in social insects is governed by chemicals not necessarily holds true for all species in all contexts.
Under a wider perspective, our results also suggest an important concept. We argue that NMR can take the shape of familiar recognition in small groups and of NMR in large societies. It is possible that, within animal groups that shift from being small associations to large societies, group members first learn to recognize individual by familiarity (and possibly by individual recognition, also suggested for P. fuscatus and other social insects; Tibbetts, 2002; d'Ettorre and Heinze, 2005) and then, when colonies grow, shift to NMR. In the latter, individuals are recognized as nestmate if they bear the specific colonial label (Gamboa et al., 1986; Dani, 2006; van Zweden and d'Ettorre, 2010). This is a drastically different process from what occurs in the perennial large societies of many ants, termites, and bees, in which colony foundation by swarming or colony fission prevents the “small society” phase, thus precluding the possibility of a familiar recognition.
In conclusion, our results demonstrate an underestimated plasticity in the mechanisms of social recognition within the same species across different contexts. The same kind of social recognition (i.e., NMR) can be based on very different cues (visual and chemical ones) in different social environments and, at the same time, the same cues (i.e., clypeus patterns) can mediate two different social recognition processes (NMR and, putatively, mutual assessment of agonistic ability). Ultimately, this highlights the limitations of communication studies focusing on a single and/or specific context, life stage, or phenotype. Unfortunately, there is a dramatic lack of replication studies in animal (and especially insects) communication studies. Given its biological features and the easiness of manipulation, P. dominula will certainly represent a fruitful model to assess these topics in the future.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
AC and RC planned the experiment. AC, FC, IP, LP, LD, and RC performed the experiment and analyzed behavioral data. AC analyzed data and wrote the paper. All authors read, commented, and finally agreed on the MS.
Funding
Research was supported by funds from the University of Florence (to RC) and by the Marie Sklodowska-Curie Action, grant No. 706208 SocParPhenoEvol, to AC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank the members of Gruppo Vespe and ZenLab, Università di Firenze, for the fruitful discussion and Seirian Sumner and Maria Cristina Lorenzi for their useful comments on an earlier draft.
References
Aquiloni, L., and Tricarico, E. (2015). Social Recognition in Invertebrates: The Knowns and the Unknowns. Springer International Publishing. doi: 10.1007/978-3-319-17599-7
Baracchi, D., Petrocelli, I., Chittka, L., Ricciardi, G., and Turillazzi, S. (2015). Speed and accuracy in nest-mate recognition: a hover wasp prioritizes face recognition over colony odour cues to minimize intrusion by outsiders. Proc. R. Soc. Lond. B Biol. Sci. 282:20142750. doi: 10.1098/rspb.2014.2750
Baracchi, D., Petrocelli, I., Cusseau, G., Pizzocaro, L., Teseo, S., and Turillazzi, S. (2013). Facial markings in the hover wasps: quality signals and familiar recognition cues in two species of Stenogastrinae. Anim. Behav. 85, 203–212. doi: 10.1016/j.anbehav.2012.10.027
Baracchi, D., Turillazzi, S., and Chittka, L. (2016). Facial patterns in a tropical social wasp correlate with colony membership. Sci. Nat. 103:80. doi: 10.1007/s00114-016-1406-8
Beani, L. (1996). “Lek-like courtship in paper-wasps: a prolonged, delicate, and troublesome affair,” in Natural History and Evolution of Paper-Wasps, eds S. Turillazzi and M. J. West-Eberhard (Oxford: Oxford University Press), 113–125.
Blomquist, G. J., and Bagnères, A. G. (2010). Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press.
Bonavita-Cougourdan, A., Theraulaz, G., Bagnères, A. G., Roux, M., Pratte, M., Provost, E., et al. (1991). Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. B Comp. Biochem. 100, 667–680. doi: 10.1016/0305-0491(91)90272-F
Branconi, R., Baracchi, D., Turillazzi, S., and Cervo, R. (2018). Testing the signal value of clypeal black patterning in an Italian population of the paper wasp Polistes dominula. Insectes Soc. 65, 161–169. doi: 10.1007/s00040-017-0598-z
Bruschini, C., Cervo, R., Cini, A., Pieraccini, G., Pontieri, L., Signorotti, L., et al. (2011). Cuticular hydrocarbons rather than peptides are responsible for nestmate recognition in Polistes dominulus. Chem. Senses 36, 715–723. doi: 10.1093/chemse/bjr042
Bruschini, C., Cervo, R., and Turillazzi, S. (2010). “Pheromones in social wasps,” in Vitamins and Hormones: Pheromones, ed G. Litwack (Elsevier Academic Press).
Buczkowski, G., Kumar, R., Suib, S. L., and Silverman, J. (2005). Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression. J. Chem. Ecol. 31, 829–843. doi: 10.1007/s10886-005-3547-7
Cappa, F., Beani, L., and Cervo, R. (2016). The importance of being yellow: visual over chemical cues in gender recognition in a social wasp. Behav. Ecol. 27, 1182–1189. doi: 10.1093/beheco/arw025
Cervo, R., Cini, A., and Turillazzi, S. (2015). “Visual recognition in social wasps,” in Social Recognition in Invertebrates, eds L. Aquiloni and E. Tricarico (Springer International Publishing).
Cervo, R., Dapporto, L., Beani, L., Strassmann, J. E., and Turillazzi, S. (2008). On status badges and quality signals in the paper wasp Polistes dominulus: body size, facial colour patterns and hierarchical rank. Proc. R. Soc. Lond. B Biol. Sci. 275, 1189–1196. doi: 10.1098/rspb.2007.1779
Chittka, L., Skorupski, P., and Raine, N. E. (2009). Speed–accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. doi: 10.1016/j.tree.2009.02.010
Cini, A., Bruschini, C., Poggi, L., and Cervo, R. (2011a). Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim. Behav. 81, 1139–1145. doi: 10.1016/j.anbehav.2011.02.017
Cini, A., Bruschini, C., Signorotti, L., Pontieri, L., Turillazzi, S., and Cervo, R. (2011b). The chemical basis of host nest detection and chemical integration in a cuckoo paper wasp. J. Exp. Biol. 214, 3698–3703. doi: 10.1242/jeb.059519
Cini, A., and Dapporto, L. (2009). Autumnal helpers of Polistes dominulus represent a distinct behavioural phenotype. Ann. Zool. Fenn. 46, 423–431. doi: 10.5735/086.046.0603
Cini, A., Ortolani, I., Zecchini, L., and Cervo, R. (2015). Facial markings in the social cuckoo wasp Polistes sulcifer: no support for the visual deception and the assessment hypotheses. Behav. Processes. 111, 19–24. doi: 10.1016/j.beproc.2014.11.010
Cini, A., Sumner, S., and Cervo, R. (2019). Inquiline social parasites as tools to unlock the secrets of insect sociality. Philos. Trans. B Biol. Sci. 374:20180193. doi: 10.1098/rstb.2018.0193
Crozier, R. H., and Pamilo, P. (1996). Evolution of Socialinsect Colonies. Oxford: Oxford University Press.
Dani, F. R. (2006). Cuticular lipids as semiochemicals in paper wasps and other social insects. Ann. Zool. Fennici 43, 500–514.
Dani, F. R., Fratini, S., and Turillazzi, S. (1996). Behavioural evidence for the involvement of Dufour's gland secretion in nestmate recognition in the social wasp Polistes dominulus (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 38, 311–319. doi: 10.1007/s002650050247
Dani, F. R., Jones, G. R., Destri, S., Spencer, S. H., and Turillazzi, S. (2001). Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 62, 165–171. doi: 10.1006/anbe.2001.1714
Dapporto, L., and Palagi, E. (2006). Wasps in the shadow: looking at the pre-hibernating clusters of Polistes dominulus. Ann. Zool. Fennici 43, 583–594.
Dapporto, L., Pansolli, C., and Turillazzi, S. (2004a). Hibernation clustering and its consequences for associative nest foundation in Polistes dominulus (Hymenoptera Vespidae). Behav. Ecol. Sociobiol. 56, 315–321. doi: 10.1007/s00265-004-0800-y
Dapporto, L., Sledge, F. M., and Turillazzi, S. (2005). Dynamics of cuticular chemical profiles of Polistes dominulus workers in orphaned nests (Hymenoptera, Vespidae). J. Insect Physiol. 51, 969–973. doi: 10.1016/j.jinsphys.2005.04.011
Dapporto, L., Theodora, P., Spacchini, C., Pieraccini, G., and Turillazzi, S. (2004b). Rank and epicuticular hydrocarbons in different populations of the paper wasp Polistes dominulus (Christ) (Hymenoptera, Vespidae). Insectes Soc. 51, 279–286. doi: 10.1007/s00040-004-0738-0
d'Ettorre, P., and Heinze, J. (2005). Individual recognition in ant queens. Curr. Biol. 15, 2170–2174. doi: 10.1016/j.cub.2005.10.067
d'Ettorre, P., and Lenoir, A. (2009). “Nestmate and kin recognition,” in Ant Ecology, eds L. Lach, C. L. Parr, and K. L. Abbott (Oxford: Oxford University Press), 194–209.
Fishwild, T. G., and Gamboa, G. J. (1992). Colony defence against conspecifics: caste-specific differences in kin recognition by paper wasps, Polistes fuscatus. Anim. Behav. 43, 95–102. doi: 10.1016/S0003-3472(05)80075-2
Fürst, M. A., Durey, M., and Nash, D. R. (2011). Testing the adjustable threshold model for intruder recognition on Myrmica ants in the context of a social parasite. Proc. Biol. Sci. 279, 516–522. doi: 10.1098/rspb.2011.0581
Gamboa, G. J., Reeve, H. K., and Pfennig, D. W. (1986). The evolution and ontogeny of nestmate recognition in social wasps. Annu. Rev. Entomol. 31, 431–454. doi: 10.1146/annurev.en.31.010186.002243
Gherardi, F., Aquiloni, L., and Tricarico, E. (2012). Revisiting social recognition systems in invertebrates. Anim. Cogn. 15, 745–762. doi: 10.1007/s10071-012-0513-y
Green, J. P., and Field, J. (2011). Interpopulation variation in status signalling in the paper wasp Polistes dominulus. Anim. Behav. 81, 205–209. doi: 10.1016/j.anbehav.2010.10.002
Green, J. P., Leadbeater, E., Carruthers, J. M., Rosser, N. S., Lucas, E. R., and Field, J. (2013). Clypeal patterning in the paper wasp Polistes dominulus: no evidence of adaptive value in the wild. Behav. Ecol. 24, 623–633. doi: 10.1093/beheco/ars226
Green, J. P., Rose, C., and Field, J. (2012). The role of climatic factors in the expression of an intrasexual signal in the paper wasp Polistes dominulus. Ethology 118, 766–774. doi: 10.1111/j.1439-0310.2012.02067.x
Hamilton, W. D. (1987). “Kinship, recognition, disease, and intelligence: constraints of social evolution,” in Animal Societies: Theories and Facts, eds Y. Ito, J. L. Brown, and J. Kikkawa (Tokyo: Japanese Scientific Society), 81–102.
Howard, R. W., and Blomquist, G. J. (2005). Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. doi: 10.1146/annurev.ento.50.071803.130359
Injaian, A., and Tibbetts, E. A. (2014). Cognition across castes: individual recognition in worker Polistes fuscatus wasps. Anim. Behav. 87, 91–96. doi: 10.1016/j.anbehav.2013.10.014
Jandt, J. M., Tibbetts, E. A., and Toth, A. L. (2014). Polistes paper wasps: a model genus for the study of social dominance hierarchies. Insectes Soc. 61, 11–27. doi: 10.1007/s00040-013-0328-0
Leadbeater, E., Carruthers, J. M., Green, J. P., Rosser, N. S., and Field, J. (2011). Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876. doi: 10.1126/science.1205140
Leonhardt, S. D., Brandstaetter, A. S., and Kleineidam, C. J. (2007). Reformation process of the neuronal template for nestmate-recognition cues in the carpenter ant Camponotus floridanus. J. Comp. Physiol. A 193, 993–1000. doi: 10.1007/s00359-007-0252-8
Liang, D., and Silverman, J. (2000). “You are what you eat”: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87, 412–416. doi: 10.1007/s001140050752
Lorenzi, M.-C., Bagnères, A.-G., and Clément, J.-L. (1996). “The role of cuticular hydrocarbons in social insects: is it the same in paper-wasps?” in Natural History and Evolution of Paper Wasps, eds S. Turillazzi and M. J. West-Eberhard (Oxford: Oxford University Press), 178–189.
Ortolani, I., Zechini, L., Turillazzi, S., and Cervo, R. (2010). Recognition of a paper wasp social parasite by its host: evidence for a visual signal reducing host aggressiveness. Anim. Behav. 80, 683–688. doi: 10.1016/j.anbehav.2010.07.003
Pardi, L. (1946). Ricerche sui Polistini. 7. Poliginia eccezionale in Polistes (Leptopolistes) omisseis. Weyrauch Processi Verbali. Società Toscana di Scianze. Naturali 54, 3–7.
Pardi, L. (1948). Dominance order in Polistes wasps. Physiol. Zool. 21, 1–13. doi: 10.1086/physzool.21.1.30151976
Pardi, L. (1996). “Polistes: analysis of a society,” in Natural History and Evolution of Paper-Wasps, eds S. Turillazzi and M. J. West-Eberhard (Oxford: Oxford University Press), 1–17.
Pratte, M. (1989). Foundress association in the paper wasp Polistes dominulus Christ. (Hymen. Vesp.). Effects of dominance hierarchy on the division of labour. Behaviour 111, 208–219. doi: 10.1163/156853989X00664
Queller, D. C., Zacchi, F., Cervo, R., Turillazzi, S., Henshaw, M. T., Santorelli, L. A., et al. (2000). Unrelated helpers in a social insect. Nature 405, 784–787. doi: 10.1038/35015552
Reeve, H. K. (1989). The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. doi: 10.1086/284926
Reeve, H. K. (1991). “Polistes,” in The Social Biology of Wasps, eds K. G. Ross and R. W. Metthews (Ithaca, NY: Cornell University Press), 99–148.
Rehan, S. M., and Toth, A. L. (2015). Climbing the social ladder: the molecular evolution of sociality. Trends Ecol. Evol. 30, 426–433. doi: 10.1016/j.tree.2015.05.004
Richard, F. J., and Hunt, J. H. (2013). Intracolony chemical communication in social insects. Insectes Soc. 60, 275–291. doi: 10.1007/s00040-013-0306-6
Röseler, P. F. (1991). “Reproductive competition during colony establishment,” in The Social Biology of Wasps, eds K. G. Ross and R. W. Metthews (Ithaca, NY: Comstock Publishing Associates), 309–335.
Röseler, P. F., Röseler, I., and Strambi, A. (1980). The activity of corpora allata in dominant and subordinated females of the wasp Polistes gallicus. Insectes Soc. 27, 97–107. doi: 10.1007/BF02229247
Signorotti, L., Cervo, R., and d'Ettorre, P. (2015). “Ontogeny of nestmate recognition in social Hymenoptera,” in Social Recognition in Invertebrates, eds L. Aquiloni and E. Tricarico (Springer International Publishing), 165–191.
Sledge, M. F., Boscaro, F., and Turillazzi, S. (2001). Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol. 49, 401–409. doi: 10.1007/s002650000311
Standage, D. S., Berens, A. J., Glastad, K. M., Severin, A. J., Brendel, V. P., and Toth, A. L. (2016). Genome, transcriptome and methylome sequencing of a primitively eusocial wasp reveal a greatly reduced DNA methylation system in a social insect. Mol. Ecol. 25, 1769–1784. doi: 10.1111/mec.13578
Sturgis, S. J., and Gordon, D. M. (2012). Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol News 16, 101–110.
Tibbetts, E. A. (2002). Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B Biol. Sci. 269, 1423–1428. doi: 10.1098/rspb.2002.2031
Tibbetts, E. A., and Curtis, T. R. (2007). Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav. Ecol. 18, 602–607. doi: 10.1093/beheco/arm013
Tibbetts, E. A., and Dale, J. (2004). A socially enforced signal of quality in a paper wasp. Nature 432, 218–222. doi: 10.1038/nature02949
Tibbetts, E. A., and Lindsay, R. (2008). Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biol. Lett. 4, 237–239. doi: 10.1098/rsbl.2008.0048
Tibbetts, E. A., Mettler, A., and Levy, S. (2010). Mutual assessment via visual status signals in Polistes dominulus wasps. Biol. Lett. 6, 10–13. doi: 10.1098/rsbl.2009.0420
van Zweden, J. S., and d'Ettorre, P. (2010). “Nestmate recognition in social insects and the role of hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, ed G. J. Blomquist (Reno: University of Nevada), 222–243.
Waldman, B. (1988). The ecology of kin recognition. Annu. Rev. Ecol. Syst. 19, 543–571. doi: 10.1146/annurev.es.19.110188.002551
Ward, A., and Webster, M. (2016). Sociality: The Behaviour of Group-Living Animals. Berlin: Springer.
Wittwer, B., and Elgar, M. A. (2018). Cryptic castes, social context and colony defence in a social bee, Tetragonula carbonaria. Ethology 124, 617–622. doi: 10.1111/eth.12765
Keywords: cuticular hydrocarbons, multimodal communication, paper wasps, familiar recognition, phenotypic plasticity
Citation: Cini A, Cappa F, Pepiciello I, Platania L, Dapporto L and Cervo R (2019) Sight in a Clique, Scent in Society: Plasticity in the Use of Nestmate Recognition Cues Along Colony Development in the Social Wasp Polistes dominula. Front. Ecol. Evol. 7:444. doi: 10.3389/fevo.2019.00444
Received: 31 May 2019; Accepted: 31 October 2019;
Published: 25 November 2019.
Edited by:
Mark A. Elgar, The University of Melbourne, AustraliaReviewed by:
Aurore Avargues-Weber, UMR5169 Centre de Recherches sur la Cognition Animale (CRCA), FranceEmma I. K. Vitikainen, University of Helsinki, Finland
Copyright © 2019 Cini, Cappa, Pepiciello, Platania, Dapporto and Cervo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Cini, Y2luaS5hbGVzQGdtYWlsLmNvbQ==
 Alessandro Cini
Alessandro Cini Federico Cappa
Federico Cappa Irene Pepiciello
Irene Pepiciello Leonardo Platania
Leonardo Platania Leonardo Dapporto1
Leonardo Dapporto1 Rita Cervo
Rita Cervo