- 1Laboratory of Experimental and Comparative Ethology, University of Paris 13, Villetaneuse, France
- 2Laboratory of Experimental and Comparative Ethology, Institut Universitaire de France (IUF), University of Paris 13, Villetaneuse, France
Social insects use the blend of hydrocarbons present on their cuticle to efficiently distinguish nestmates from aliens. Intruders must therefore find a strategy to break the recognition code in order to exploit the colony resources. Twenty years ago, the concept of “chemical insignificance” was introduced to characterize those parasites bearing almost no recognition cues on their cuticle, thus appearing chemically undetectable to their hosts. In some cases, intruders do possess cuticular hydrocarbons, but these are present in lower amount with respect to their hosts and/or they belong to different classes than the hydrocarbons typically used as recognition cues. We propose to include these cases under the label of chemical insignificance. If chemical compounds are absent on the cuticle of the intruder, or if they are produced but not perceived by the host (e.g., below the detection threshold), or if they are perceived but not meaningful, in all cases the result is identical: the profile of the intruder appears chemically neutral; thus, it is irrelevant for the host. We also discuss the consequences of producing low amounts of cuticular hydrocarbons, given that their original function is to act as a barrier against desiccation. Clarifying the concept of chemical insignificance will help unify terminology and stimulate interdisciplinary research efforts involving simultaneous investigations of chemical profiles, behavior, and physiology to elucidate the proximate and ultimate mechanisms characterizing the co-evolutionary arms race between hosts and parasites.
Twenty years after the concept of “chemical insignificance,” characterizing insect parasites bearing almost no recognition cues on their cuticle, was introduced (Lenoir et al., 1999, 2001), several comparable chemical adaptations have been described in the literature, often using different terminology. In this perspective, we briefly review the use of these different terms and propose to include all of them under the label “chemical insignificance.” Using multiple terms to address a phenomenon that appears to have similar adaptive significance in different species can hinder the comprehension of both its underlying proximate mechanisms and evolutionary pathway.
Cuticular Hydrocarbons as Recognition Cues
Social insects use the complex blend of hydrocarbons present on their cuticle for nestmate recognition (Lenoir et al., 1999; Dani, 2006; Blomquist and Bagnères, 2010; d'Ettorre and Lenoir, 2010). This mixture is composed of different classes of hydrocarbons, such as linear alkanes, methyl-branched alkanes, and alkenes, with a chain length typically ranging from 20 to 40 carbon atoms, although in some cases, heavier hydrocarbons have been detected. The cuticular hydrocarbon (CHC) profile is species-specific, meaning that different species show qualitatively different CHC mixtures, characterized by a species-specific combination of compounds (Bagnères and Wicker-Thomas, 2010). The CHC profile is also colony-specific, implying that within a given colony, the “colony odor” is generally uniform, but different colonies of the same species show quantitatively different profiles, i.e., different relative proportions of the same hydrocarbons. The homogeneity of the colony odor is maintained by exchanges via trophallaxis, allogrooming, and/or contact with the nest material (Lenoir et al., 1999).
Deficiency of Recognition Cues in Callows and Parasites
Remarkably, some individuals do not show the typical species/colony CHC profile. Newly enclosed (callow) social insect workers usually possess very low amounts of CHCs at emergence and can be experimentally transferred from one colony to another, even a different species, without eliciting an aggressive response from adult workers (e.g., ants: Errard, 1994; wasps: Lorenzi et al., 1999; bees: Breed et al., 2004). Lenoir et al. (1999) termed this lack of chemical recognition cues “chemical insignificance.” Young workers then go through a process of chemical integration by synthesizing CHCs and also by acquiring them via interactions with colony members (allogrooming, trophallaxis) and contacts with nest material (Bos et al., 2011). This ontogeny of the CHC profile can last several days; for instance, workers of the ant Aphaenogaster senilis acquire a CHC profile typical of adults in about 20 days (Ichinose and Lenoir, 2009), while it takes about 4 days in Bombus terrestris (Sramkova and Ayasse, 2009). Polistes wasps acquire the adult CHC profile in about 3 days, during which they increase drastically the total amount of the hydrocarbons but also incur a qualitative change: the proportion of branched hydrocarbons increases at the expense of that of linear hydrocarbons and relatively long-chain hydrocarbons increase at the expense of short-chain ones (Lorenzi et al., 2004a).
The concept of chemical insignificance has been extended to social parasites (Lenoir et al., 1999, 2001) that need to infiltrate and be tolerated into a host nest. Lacking conspicuous recognition cues may help both remaining undetected when entering a host colony and facilitating the acquisition of the colony odor from the nest material and the hosts. Chemical insignificance works effectively if the host recognition system is based on the undesirable-present (U-present) model, and not on a point-by-point label/template matching. In the U-present model, rejection by the discriminator is elicited only by the presence of additional/odd cues on the intruder, either different compounds or higher amounts of some compounds that are also present of the cuticle of the discriminator (Guerrieri et al., 2009; van Zweden and d'Ettorre, 2010). The underlying perceptual mechanism may act at the level of the antennae (Ozaki et al., 2005; Ozaki and Hefetz, 2014) or at higher levels (e.g., antennal lobes: Guerrieri et al., 2009; Brandstaetter et al., 2011), although these two models are not mutually exclusive (Bos and d'Ettorre, 2012). Therefore, when the incoming individual bears no detectable cues, theory predicts that the individual will not be rejected. A reduced amount of recognition cues on the cuticle (with respect to residents) has the same effect as the absence of cues, providing that the amount does not reach the detection threshold of the discriminator. Data on the detection threshold of social insects are very scarce. We know that ants can detect very low amounts of hydrocarbons, namely, a total CHC extract equivalent of 10−4 workers in A. senilis (Ichinose and Lenoir, 2010), and can discriminate different concentrations of the same hydrocarbon, with one concentration as low as 0.3 ant equivalents (Camponotus aethiops, Di Mauro et al., 2015). Foundresses of the paper wasp Polistes dominula start reacting aggressively when presented with lures carrying the two-thirds of the total surface extract obtained from one wasp, while the reaction to one-third of the extract does not differ from the reaction to the solvent alone (Cini et al., 2009), suggesting that paper wasps might have a higher detection threshold than ants.
Chemical Insignificance and Chemical Transparency
Not all the classes of hydrocarbons are good candidates to act as recognition cues. There is evidence that methyl-branched alkanes and alkenes are more important than linear alkanes in the recognition process (review in van Zweden and d'Ettorre, 2010). Consequently, a cuticular profile characterized by linear alkanes and a reduced number/amount of the other hydrocarbon classes may also be chemically undetectable in the context of nestmate recognition. The production of a profile characterized by the presence of hydrocarbons that are not used as recognition cues has been termed “chemical transparency” (Martin et al., 2008). For example, adults and eggs of the social parasite Vespa dybowskii show a chemical profile dominated by alkanes and alkenes, while methyl-branched compounds are only present in traces (<1%). These methylated compounds, which act as recognition cues in hornets, are instead present in high proportion in the host species V. simillima (41%) (Martin et al., 2008). Larvae of the paper wasp social parasite Polistes sulcifer appear to use a similar strategy, as their cuticular profile shows a higher relative proportion of linear alkanes but a lower proportion of branched and unsaturated hydrocarbons than the profile of host larvae, P. dominula (Cervo et al., 2008). Similarly, the parasite Polistes atrimandibularis larvae show higher proportion of linear alkanes compared to their host larvae (Elia et al., 2018). In these examples, even if the total amount of CHCs is similar in hosts and parasites, the relative amount of key compounds is significantly lower in parasites (see also Table 1). Therefore, these parasites produce a chemical mixture that is “invisible” to the host in the recognition context and therefore does not elicit aggression. We propose that these cases of so-called chemical transparency or chemical neutrality should be included under the broader label of chemical insignificance. Moreover, chemical insignificance, in the form of reduced total amount of CHCs, might compensate for potentially “visible” traits in the parasite CHC profile, for instance, the presence of relatively short-chain CHCs (which are in principle conspicuous) in P. atrimandibularis parasites before they invade a host nest (Uboni et al., 2012), or the “imperfect” mimicry of the cuckoo wasp, Hedychrum rutilans (Kroiss et al., 2009) (Table 1).
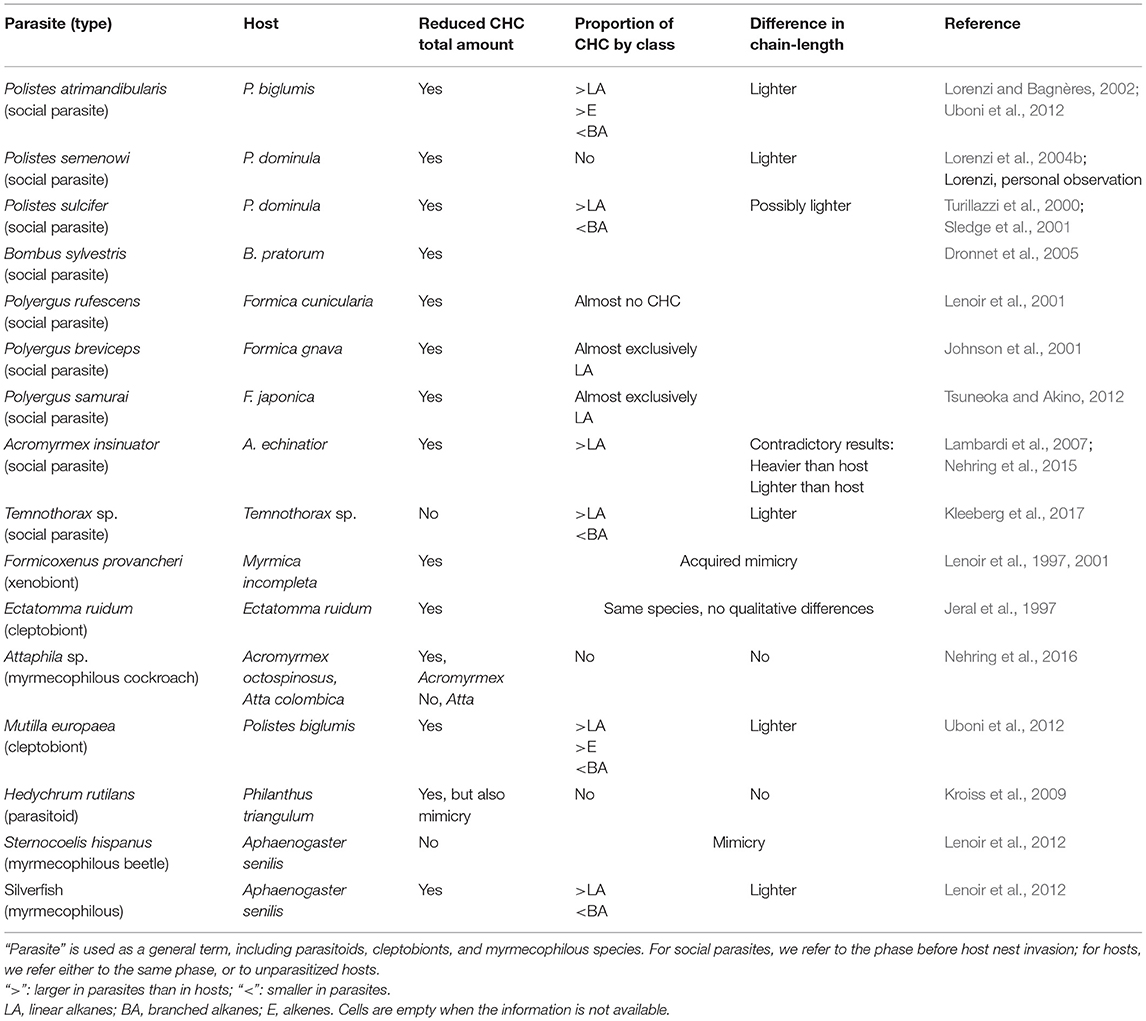
Table 1. Characteristics of the CHC profile of parasites that differ from that of hosts (calculated as “parasites minus hosts”).
Another aspect to be considered is the hydrocarbon chain length. Very-long-chain hydrocarbons are characterized by low volatility and thus are relatively more difficult to perceive for the discriminator by olfaction, which is the usual way of hydrocarbon detection (Brandstaetter et al., 2008). Producing a CHC profile shifted toward long-chain hydrocarbons could be a form of chemical insignificance (Lambardi et al., 2007). However, this does not seem to be the general case, at least for the examples listed in Table 1, in which—if anything—we observe sometimes the opposite trend, with parasites having a profile characterized by lighter compounds than their hosts. An intriguing case is that of parabiotic ant species, in which a large-scale comparison highlighted that associated species show significantly longer CHCs and higher proportions of methyl-branched alkenes (very rare compounds among insects) and alkadienes than non-associated species, suggesting that the evolution of interspecific amicable associations is linked to a shift toward higher chain lengths (Menzel and Schmitt, 2012).
Why Produce Hydrocarbons?
If the lack of CHCs facilitates integration into the host colony, one should expect the totality of parasites being denuded of these cues. Yet, we observe the majority of them producing at least a minimum amount (e.g., 20% of the amount found in the hosts, Kroiss et al., 2009; Uboni et al., 2012). The original function of hydrocarbons is protection against desiccation and also pathogens and toxins; hence, parasites might be obliged to produce them. However, not all hydrocarbon classes are suitable to prevent desiccation. Linear alkanes (n-C20-C40), especially with long chain, are better than other classes in limiting dehydration as they are solid and in a relatively impermeable state at temperatures <40°C, while they begin melting and become more water permeable at higher temperatures (Gibbs and Pomonis, 1995). When alkanes are blended with unsaturated CHC (alkenes) or methylated (branched) alkanes, the resulting blend is characterized by a lower melting temperature than a blend composed exclusively of linear alkanes, and thus it becomes more permeable and less effective as a waterproofing barrier (Gibbs and Rajpurohit, 2010). The cuticular layer of social parasites is typically relatively rich in linear alkanes with respect to their hosts (Table 1), which may increase cuticle impermeability and counterbalance the overall low amount of CHCs. However, the relationships between CHC composition and their physical properties may be more complex: the physical properties of complex blends, such as those that characterize the cuticular layer of insects, are poorly understood and their waterproofing properties are rarely studied (Gibbs and Rajpurohit, 2010). Even the presumptive correlation between amount of hydrocarbons and waterproofing (Hadley, 1981) does not always hold. For instance, several species of Cataglyphis desert ants, which forage at high temperature, have considerably lower total amount of CHCs (about ¼) than Myrmica ants, which live in humid boreal forests, but Cataglyphis ants lose less water than Myrmica ants due to a lower transpiration rate (Lenoir et al., 2009). The profile of Cataglyphis is characterized by a higher proportion of saturated compounds and a lower proportion of alkenes compared to that of Myrmica. Therefore, physiological adaptations may contribute to water balance and counteract effectively a low amount of CHCs, as well as morphological adaptations, such us a thicker cuticle in parasites compared to their hosts (Cervo, 1994). In addition, chemically insignificant parasites might compensate for the costs of a higher potential for desiccation through specific behavioral strategies. For instance, they might spend more time than their hosts in less dry, shadowy, parts of the nest, or increase the ingestion of liquids, e.g., by soliciting trophallaxis from the hosts. Preliminary field data on the parasitic wasp P. atrimandibularis are consistent with this hypothesis: parasites appear to avoid direct sunlight by seeking the shade (they are less active than the hosts and rest more time behind the nest in the part not exposed to the sun), and they are more involved than their hosts in trophallactic exchanges as recipients, rather than as donors (Lorenzi, personal observation).
Discussion
We show that there are at least three different ways in which parasitic insects can wear a cloak of chemical invisibility in order to facilitate the intrusion and tolerance in a host nest: (i) the total absence of CHCs, (ii) the production of a reduced amount of CHCs (below the discriminator's detection threshold), and (iii) the production of hydrocarbons that are not used as recognition cues.
In the first case, CHCs are virtually totally lacking from the cuticle of the intruder, thus preventing hosts from detecting any chemical cue that might inform them about the presence of the intruder. While this was the original observation that prompted the emergence of the term chemical insignificance (Lenoir et al., 1999, 2001) in some social parasites of ants, subsequent discoveries in other insects have offered less drastic examples of paucity of recognition cues (reviewed in Bagnères and Lorenzi, 2010). Bearing a reduced amount of CHCs with respect to the host target, the second case that we highlight is a relatively more common adaptation and may result in intruders sneaking into host nests undetected, or at least not detected enough to elicit the most violent rejection. If the amount of cues falls below or around the detection threshold of the host, the amount of aggression will be limited, as suggested by the observation that the level of aggressive responses is positively correlated to the total amount of recognition cues on lures in wasps and ants (Cini et al., 2009; Ichinose and Lenoir, 2010).
Finally, the chemical profile of intruders may be composed of hydrocarbons that are not primarily important in the nestmate recognition process. For instance, bearing a higher proportion of linear alkanes, rather than branched alkanes, is a common characteristic among social parasites. If only part of the chemical profile informs about the identity of the bearer, lacking that part is equivalent to being chemically insignificant.
It could be argued that alternative explanations exist for the observed large difference in the total amount of CHCs between social parasites and hosts. For instance, hosts may not necessarily express a minimum amount of CHCs to avoid dehydration, they could have an excessive amount of CHCs on their cuticle to serve recognition purposes (the “host chemical over-significance” hypothesis). In this view, the amount of CHCS on the social parasite cuticle, despite being lower than that of the host, might be enough to prevent dehydration. In our opinion, this hypothesis is less parsimonious than that of chemical insignificance, since CHCs are supposed to be costly to produce and therefore hosts might incur high costs for overexpressing them (e.g., d'Ettorre and Heinze, 2001; Holman et al., 2010). Moreover, the chemical over-significance hypothesis calls for different explanations even among closely related species of social parasites. Young queen of Polyergus rufescens social parasites have virtually no CHCs, while Polyergus breviceps and P. samurai have some but less CHCs than their hosts. Under the chemical insignificance hypothesis, the three Polyergus species employ the same strategy, and differ only in the how extreme their strategy is; under the chemical over-significance hypothesis, the lack of CHC in P. rufescens is not easily explained. Finally, there is evidence for a gradual mode of evolution of CHCs in ants (van Wilgenburg et al., 2011), which also contradicts this alternative hypothesis.
A total lack of recognition cues, a diluted chemical profile, or the lack of hydrocarbons relevant in recognition processes may be parsimoniously interpreted as parasite adaptation that serve the same function: sneaking into host nests undetected. In a review about terminology, von Beeren et al. (2012) suggest the term “chemical hiding” when an organism shows the total absence of recognition cues or the presence of cues below the discriminator detection threshold (thus not detectable), as opposed, for instance, to “chemical masquerade” (detected but misidentified as uninteresting entity, eliciting no reaction) and “chemical mimicry” (resembling an interesting entity, such as a nestmate). However, one can hide by resembling something else; therefore, we believe that a functional approach might be more practical than focusing on terms. Furthermore, “chemical insignificance” is now a term widely employed in the social insect literature, and it might be more difficult to replace it than to clarify its meaning by including the three functional categories presented here.
The different ways of being chemically insignificant are not mutually exclusive: for instance, invading females of the social parasite P. atrimandibularis, as well as females of the cleptoparasite Mutilla europaea, are chemical insignificant by producing only 20% of the total amount of CHCs compared to their host, and also express primarily linear alkanes, at the expense of the more informative branched alkane component of the profile (Uboni et al., 2012). Chemical insignificance offers a protection against detection, but it might also be a way to facilitate the acquisition of host colony odor (Lenoir et al., 2001; Lorenzi et al., 2004b; Lorenzi, 2006). This hypothesis stems from the observation that newly eclosed social insects are typically chemical insignificant (i.e., poor in recognition cues) and acquire the colony odor with time (Lenoir et al., 1999). Chemical insignificance may be not completely effective: Polyergus queens, as well as P. atrimandibularis parasites or Hedychrum, are regularly attacked when they infiltrate the host colony, which means that other cues elicit the reaction of the hosts. For instance, visual cues might be used by residents (e.g., visual cues have a role in interactions among nestmates in wasps) as well as tactile cues, which might help distinguish a chemically insignificant parasite from the background.
Finally, what is especially surprising is that, at least in social insects, chemical insignificant social parasites share the same habitat as their hosts: they live in the same colony and are exposed to the same physical stressors (e.g., solar radiation, heat, dryness) as their hosts, and therefore are likely to share similar physiological requirements to cope for water loss (Lorenzi, 2006). If CHCs act as a protective barrier, chemical insignificant parasites have to find a solution to reduce the impact of these stressors. One testable hypothesis is that behavioral modifications (e.g., minimizing direct exposure to sunlight, reducing general activity, and increasing introduction of liquids) may compensate for the deficiency of CHCs.
The data are currently too scanty to draw conclusions, yet preliminary measures of total CHC quantities and environmental parameters suggest that the association between the amount of CHCs and dehydration risk may be mediated by modifications involving other traits, such as behavior and physiology. We hope that clarifying the concept of chemical insignificance by using a functional approach will help both unify terminology and broaden interdisciplinary research effort involving simultaneous investigations on chemical ecology, behavioral adaptations, and water balance physiology.
Author Contributions
ML and PE conceived and wrote the paper.
Funding
PE was supported by a grant from the French National Research Agency (Project PheroMod ANR-14-CE18-003) and by the Institut Universitaire de France (IUF).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the editors of this Research Topic for inviting us to contribute. We are grateful to Professor Alain Lenoir for comments and suggestions.
References
Bagnères, A.-G., and Lorenzi, M. C. (2010). “Chemical deception/mimicry using cuticular hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. J. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press), 282–324. doi: 10.1017/CBO9780511711909.015
Bagnères, A.-G., and Wicker-Thomas, C. (2010). “Chemical taxonomy with hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. J. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press), 121–162. doi: 10.1017/CBO9780511711909.008
Blomquist, G. J., and Bagnères, A.-G. (2010). Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology. Cambridge: Cambridge University Press.
Bos, N., and d'Ettorre, P. (2012). Recognition of social identity in ants. Front. Psychol. 3:83. doi: 10.3389/fpsyg.2012.00083
Bos, N., Grinsted, L., and Holman, L. (2011). Wax on, wax off: nest soil facilitates indirect transfer of recognition cues between ant nestmates. PLoS ONE 6:e19435. doi: 10.1371/journal.pone.0019435
Brandstaetter, A. S., Endler, A., and Kleineidam, C. J. (2008). Nestmate recognition in ants is possible without tactile interaction. Naturwissenschaften 95, 601–608. doi: 10.1007/s00114-008-0360-5
Brandstaetter, A. S., Rössler, W., and Kleineidam, C. J. (2011). Friends and foes from an ant brain's point of view – neuronal correlates of colony odors in a social insect. PLoS ONE 6:e21383. doi: 10.1371/journal.pone.0021383
Breed, M. D., Perry, S., and Bjostad, L. B. (2004). Testing the blank slate hypothesis: why honey bee colonies accept young bees. Insect. Soc. 51, 12–16. doi: 10.1007/s00040-003-0698-9
Cervo, R. (1994). Morphological adaptations to the parasitic life in Polistes sulcifer and Polistes atrimandibularis (Hymenoptera, Vespidae). Ethol. Ecol. Evol. Special Issue 3, 61–66. doi: 10.1080/03949370.1994.10721975
Cervo, R., Dani, F. R., Cotoneschi, C., Scala, C., Lotti, I., Strassmann, J. E., et al. (2008). Why are larvae of the social parasite wasp Polistes sulcifer not removed from the host nest? Behav. Ecol. Sociobiol. 62, 1319–1331. doi: 10.1007/s00265-008-0560-1
Cini, A., Gioli, L., and Cervo, R. (2009). A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 5, 459–461. doi: 10.1098/rsbl.2009.0140
Dani, F. R. (2006). Cuticular lipids as semiochemicals in paper wasps and other social insects. Annal. Zool. Fenn. 43, 500–514.
d'Ettorre, P., and Heinze, J. (2001). Sociobiology of slave-making ant. Acta Ethol. 3, 67–82. doi: 10.1007/s102110100038
d'Ettorre, P., and Lenoir, A. (2010). “Nestmate recognition,” in Ant Ecology, eds L. Lach, C. Parr, and K. Abbott (Oxford: Oxford University Press, 194–209.
Di Mauro, G., Perez, M., Lorenzi, M. C., Guerrieri, F. J., Millar, J. G., and d'Ettorre, P. (2015). Ants discriminate between different hydrocarbon concentrations. Front. Ecol. Evol. 3:133. doi: 10.3389/fevo.2015.00133
Dronnet, S., Simon, X., Verhaeghe, J.-C., Rasmont, P., and Errard, C. (2005). Bumblebee inquilinism in Bombus (Fernaldaepsithyrus) sylvestris (Hymenoptera, Apidae): behavioural and chemical analyses of host-parasite interactions. Apidologie 36, 59–70. doi: 10.1051/apido:2004070
Elia, M., Khalil, A., Bagnères, A.-G., and Lorenzi, M. C. (2018). Appeasing their hosts: a novel strategy for parasite brood. Anim. Behav. 146, 123–134. doi: 10.1016/j.anbehav.2018.10.011
Errard, C. (1994). Development of interspecific recognition behavior in the ants Manica rubida and Formica selysi (Hymenoptera: Formicidae) reared in mixed-species groups. J. Insect. Behav. 7, 83–99. doi: 10.1007/BF01989829
Gibbs, A. G., and Pomonis, J. G. (1995). Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. B 112, 243–249. doi: 10.1016/0305-0491(95)00081-X
Gibbs, A. G., and Rajpurohit, S. (2010). “Cuticular lipids and water balance” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. J. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press, 100–120.
Guerrieri, F. J., Nehring, V., Jørgensen, C. G., Nielsen, J., Galizia, C. G., and d'Ettorre, P. (2009). Ants recognize foes and not friends. Proc. R. Soc. London B 276, 2461–2468. doi: 10.1098/rspb.2008.1860
Hadley, N. F. (1981). Cuticular lipids of terrestrial plants and arthopods: a comparison of their structure, composition, and waterproofing function. Biol. Rev. 56, 23–47. doi: 10.1111/j.1469-185X.1981.tb00342.x
Holman, L., Jørgensen, C. G., Nielsen, J., and d'Ettorre, P. (2010). Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. Lond. B, 277, 3793–3800. doi: 10.1098/rspb.2010.0984
Ichinose, K., and Lenoir, A. (2009). Ontogeny of hydrocarbon profiles in the ant Aphaenogaster senilis and effects of social isolation. C.R. Biologies 332, 697–703. doi: 10.1016/j.crvi.2009.04.002
Ichinose, K., and Lenoir, A. (2010). Hydrocarbons detection level in ants. Ins. Soc. 57, 453–455. doi: 10.1007/s00040-010-0103-4
Jeral, J. M., Breed, M. D., and Hibbard, B. E. (1997). Thief ants have reduced quantities of cuticular compounds in a ponerine ant, Ectatomma ruidum. Physiol. Entomol. 22, 207–211. doi: 10.1111/j.1365-3032.1997.tb01160.x
Johnson, C. A., Vander Meer, R. K., and Lavine, B. (2001). Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a Formica host queen (Hymenoptera: Formicidae). J Chem. Ecol. 27, 1787–1804. doi: 10.1023/A:1010456608626
Kleeberg, I., Menzel, F., and Foitzik, S. (2017). The influence of slave making lifestyle, caste and sex on chemical profiles in Temnothorax ants: insights into the evolution of cuticular hydrocarbons. Proc. R. Soc. Lond. B 284:20162249. doi: 10.1098/rspb.2016.2249
Kroiss, J., Schmitt, T., and Strohm, E. (2009). Low level of cuticular hydrocarbons in a parasitoid of a solitary digger wasp and its potential for concealment. Entomol. Sci. 12, 9–16. doi: 10.1111/j.1479-8298.2009.00300.x
Lambardi, D., Dani, F. R., Turillazzi, S., and Boomsma, J. J. (2007). Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 61, 843–851. doi: 10.1007/s00265-006-0313-y
Lenoir, A., Aron, S., Xím Cerdá, X., and Hefetz, A. (2009). Cataglyphis desert ants: a good model for evolutionary biology in Darwin's anniversary year–a review. Israel J. Entomol. 39, 1–32.
Lenoir, A., Chalon, Q., Carvajal, A., Ruel, C., Barroso, A., Lackner, T., et al. (2012). Chemical integration of myrmecophilous guests in Aphaenogaster ant nests. Psyche 2012:840860. doi: 10.1155/2012/840860
Lenoir, A., d'Ettorre, P., Errard, C., and Hefetz, A. (2001). Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. doi: 10.1146/annurev.ento.46.1.573
Lenoir, A., Fresneau, D., Errard, C., and Hefetz, A. (1999). “The individuality and the colonial identity in ants: the emergence of the social representation concept,” in Information Processing in Social Insects, eds C. Detrain, J. L. Deneubourg, and J. Pasteels (Basel: Birkhauser, 219–237.
Lenoir, A., Malosse, C., and Yamaoka, R. (1997). Chemical mimicry between parasitic ants of the genus Formicoxenus and their host Myrmica (Hymenoptera, Formicidae). Biochem. Syst. Ecol. 25, 379–389. doi: 10.1016/S0305-1978(97)00025-2
Lorenzi, M. C. (2006). The result of an arms race: the chemical strategies of Polistes social parasites. Ann. Zool. Fenn. 43, 550–563.
Lorenzi, M. C., and Bagnères, A.-G. (2002). Concealing identity and mimicking hosts: a dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 125, 507–512. doi: 10.1017/S003118200200238X
Lorenzi, M. C., Cervo, R., Zacchi, F., Turillazzi, S., and Bagnères, A.-G. (2004b). Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera: Vespidae). Parasitology 129, 643–651. doi: 10.1017/S0031182004005992
Lorenzi, M. C., Cometto, I., and Marchisio, G. (1999). Species and colony components in the recognition odor of young wasps: their expression and learning (Polistes biglumis and P. atrimandibularis; Hymenoptera: Vespidae). J. Insect Behav. 12,147–158. doi: 10.1023/A:1020906631121
Lorenzi, M. C., Sledge, M. F., Laiolo, P., and Turillazzi, S. (2004a). Cuticular hydrocarbon dynamics in young adult Polistes dominulus (Hymenoptera: Vespidae) and the role of linear hydrocarbons in nestmate recognition systems. J. Insect Physiol. 50, 935–941. doi: 10.1016/j.jinsphys.2004.07.005
Martin, S. J., Takahashi, J., Onob, M., and Drijfhout, F. P. (2008). Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 54, 700–707. doi: 10.1016/j.jinsphys.2008.01.010
Menzel, F., and Schmitt, T. (2012). Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66, 896–904. doi: 10.1111/j.1558-5646.2011.01489.x
Nehring, V., Dani, F. R., Turillazzi, S., Bohn, H., Klass, K.-D., and d'Ettorre, P. (2016). Chemical disguise of myrmecophilous cockroaches and its implications for understanding nestmate recognition mechanisms in leaf-cutting ants. BMC Ecol. 16:35. doi: 10.1186/s12898-016-0089-5
Nehring, V., Dani, F. R., Turillazzi, S., Boomsma, J. J., and d'Ettorre, P. (2015). Integration strategies of a leaf-cutting ant social parasite. Anim. Behav. 108, 55–65. doi: 10.1016/j.anbehav.2015.07.009
Ozaki, M., and Hefetz, A. (2014). Neural mechanisms and information processing in recognition systems. Insects 5, 722–741. doi: 10.3390/insects5040722
Ozaki, M., Wada-Katsumata, A., Fujikawa, K., Iwasaki, M., Yokohari, F., Satoji, Y., et al. (2005). Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314. doi: 10.1126/science.1105244
Sledge, M. F., Dani, F. R., Cervo, R., Dapporto, L., and Turillazzi, S. (2001). Recognition of social parasites as nest-mates: adoption of colony specific host cuticular odors by the paper wasp parasite Polistes sulcifer. Proc. R. Soc. London B 268, 2253–2260. doi: 10.1098/rspb.2001.1799
Sramkova, A., and Ayasse, M. (2009). Chemical ecology involved in invasion success of the cuckoo bumblebee Psithyrus vestalis and in survival of workers of its host Bombus terrestris. Chemoecology 19, 55–62. doi: 10.1007/s00049-009-0009-7
Tsuneoka, Y., and Akino, T. (2012). Chemical camouflage of the slave-making ant Polyergus samurai queen in the process of the host colony usurpation (Hymenoptera: Formicidae). Chemoecology 22, 89–99. doi: 10.1007/s00049-012-0101-2
Turillazzi, S., Sledge, M. F., Dani, F. R., Cervo, R., Massolo, A., and Fondelli, L. (2000). Social hackers: integration in the host chemical recognition system by a paper wasp social parasite. Naturwissenschaften 87, 172–176. doi: 10.1007/s001140050697
Uboni, A., Bagnères, A.-G., Christidès, J.-P., and Lorenzi, M. C. (2012). Cleptoparasites, social parasites and a common host: chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 58, 1259–1264. doi: 10.1016/j.jinsphys.2012.06.013
van Wilgenburg, E., Symonds, M. R., and Elgar, M. A. (2011). Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol. 24, 1188–1198. doi: 10.1111/j.1420-9101.2011.02248.x
van Zweden, J. S., and d'Ettorre, P. (2010). “The role of hydrocarbons in nestmate recognition,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. J. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press, 222–243.
Keywords: ants, bees, wasps, cuticular hydrocarbons, social parasites
Citation: Lorenzi MC and d'Ettorre P (2020) Nestmate Recognition in Social Insects: What Does It Mean to Be Chemically Insignificant? Front. Ecol. Evol. 7:488. doi: 10.3389/fevo.2019.00488
Received: 26 June 2019; Accepted: 29 November 2019;
Published: 09 January 2020.
Edited by:
Mark A. Elgar, The University of Melbourne, AustraliaReviewed by:
Qike Wang, The University of Melbourne, AustraliaFabio Santos Nascimento, University of São Paulo Ribeirão Preto, Brazil
Copyright © 2020 Lorenzi and d'Ettorre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia d'Ettorre, ZC1ldHRvcnJlQHVuaXYtcGFyaXMxMy5mcg==
 Maria Cristina Lorenzi
Maria Cristina Lorenzi Patrizia d'Ettorre
Patrizia d'Ettorre