- Department of Ecology and Evolutionary Biology, University of California, Irvine, Irvine, CA, United States
1. Introduction
Metabolic heat may play an important role in microbial conflict and cooperation. On the conflict side, microbes often differ in their temperature optima (Alster et al., 2018). A microbe that raises the local temperature closer to its own optimum gains a growth advantage over relatively thermophobic competitors (Goddard, 2008).
On the cooperative side, aggregates may retain metabolic heat and gain a growth rate advantage (Tabata et al., 2013). Internal cells in an aggregate potentially benefit by generating excess heat, the energy cost reducing their own growth but stimulating faster growth among neighboring genetic relatives. Cool environments with slow heat dissipation favor cooperative thermogenesis.
The theory builds on three assumptions. First, temperature influences fitness. Second, individual and group traits can modulate heat production and heat flow. Third, heat flow affects local temperature and thus the fitness of neighbors.
I discuss each assumption. I then turn to predictions.
2. Fitness Consequences: Competition
Competition requires that taxa differ in their temperature response. Types with relatively higher temperature optima or greater tolerance to heat can potentially gain an advantage by warming the local environment.
Alster et al. (2018) quantified temperature response in terms of thermodynamic variables that determine reaction rates. Thermodynamic measures of reaction rates are not direct measures of fitness. However, differences in the temperature sensitivity of metabolic processes likely influence temperature differences in growth rate and metabolic efficiency.
Alster et al. (2018) focused on three variables. The optimum temperature maximizes reaction rate. The heat capacity determines the breadth of the temperature response curve, with greater heat capacity corresponding to a broader temperature response curve and less overall sensitivity to temperature variability. Maximum temperature sensitivity defines the point at which the reaction rate changes most rapidly with respect to temperature.
Literature meta-analysis yielded 353 response curves across diverse microbial groups (Alster et al., 2018). The distribution of optimum temperature is approximately a right-skewed Gaussian shape with a mean and standard deviation of 29.4 ± 10.1°C. Heat capacity and maximum temperature sensitivity also vary widely.
Many laboratory studies have measured fitness at different temperatures (Bennett and Lenski, 2007; Chen and Shakhnovich, 2010; Caspeta and Nielsen, 2015; Yung et al., 2015). Growing microbes at higher or lower temperatures often causes an evolutionary shift in temperature response, demonstrating lability of thermotolerance. Protein thermosensitivity likely explains a significant part of the variability in the temperature response curves of taxa (Ghosh and Dill, 2010; Chen et al., 2017).
Overall, variation in observed temperature response suggests wide scope for using metabolic heat as a competitive weapon.
3. Fitness Consequences: Cooperation
The potential for cooperation requires that microbes sometimes live in habitats below their optimum temperature. When below the optimum, excess heat can be a shareable public good that is costly to produce and potentially beneficial to neighbors. I found only one study of metabolic heat used to raise local temperature for colony benefit (Tabata et al., 2013).
Several examples suggest that increased local temperature could be advantageous in cold environments.
Some organisms use dark pigmentation to raise cellular temperature. Cordero et al. (2018) showed that pigmentation increases in yeast with latitude. That increase suggests that high latitude taxa gain from raising their temperature above the ambient level. When grown in the lab at 4°C under light, melanized Crytococcus neoformans gained a growth advantage relative to non-melanized variants, but at 23°C the melanized form suffered increased thermal stress.
Most of the biosphere is permanently cold, including alpine, arctic, and oceanic habitats (Rodrigues and Tiedje, 2008). Cold-adapted microbes (D'Amico et al., 2006) occupy these habitats down to about −20°C. Estimates suggest counts of approximately 105 and 106 cells ml-1 in Arctic ice pack and Antarctic sea ice, respectively (Brinkmeyer et al., 2003). Smaller counts have been observed in deep ice cores (Price and Sowers, 2004).
Among taxa that could be cultured (Rodrigues and Tiedje, 2008), most isolates from cold habitats survived or grew at cold temperatures but reproduced most quickly at 20–25°C. Only a few isolates grew fastest at cool temperatures of 10–15°C. Thus, the capture and sharing of local metabolic heat may be particularly valuable in cold habitats.
4. Individual and Group Traits
Individuals may contribute heat by excess thermogenesis. Groups may retain heat by aggregation and by insulation.
Cellular aggregation is perhaps the simplest trait. I did not find studies of microbes that consider individual and group traits in terms of conflicting and cooperative aspects of thermoregulation. The closest analogy to my argument comes from huddling behavior in birds and mammals to retain heat.
Haig (2008, 2010a) noted that heat is a public good in a vertebrate huddle. Heat generators pay the cost of production. The benefit is shared by all neighbors. Individuals can exploit warm neighbors by reducing their own heating budget. In broods, siblings and parents have various conflicting and cooperative interests with regard to heat.
Familial conflicts over physiological traits often associate with genomic imprinting in mammals (Haig, 2010b). Several imprinted genes in mice and humans influence thermogenesis and follow the common pattern for familial conflict (Haig, 2008; Crespi, 2020).
In microbes, aggregation by intercellular adhesion occurs widely. Cells may also aggregate by surface attachment and by active movement toward groups. Many possible costs and benefits of cellular aggregation occur (Grosberg and Strathmann, 2007; Koschwanez et al., 2011; Smith et al., 2014; Ratcliff et al., 2015; Kuzdzal-Fick et al., 2019). However, heat in microbial aggregates has not been widely discussed.
In addition to aggregation, groups may also retain heat by secreting extracellular insulation. The idea that extracellular secretions function as insulation for microbial thermoregulation has not been widely discussed.
Biofilms combine aggregation and insulation. Apart from the one study mentioned above noting that aggregates may beneficially raise their temperature (Tabata et al., 2013), I did not find discussion of increased temperature as an adaptive benefit of biofilms. The idea has likely been mentioned, but is not widely considered.
Heat may be used as a weapon against relatively thermophobic competitors or invaders. Goddard's (2008) suggestion that Saccharomyces cerevisiae may use metabolic heat as a competitive weapon against other species is the only clear statement that I found. I did not find any literature on microbes that use metabolic heat as a defense against invading bacteriophage.
With regard to defense, fever in vertebrates (Evans et al., 2015) and social insects (Starks et al., 2000) provides an analogy. Those organisms sometimes raise their temperature to control microbial invaders. Observations suggest that Japanese honey bees surround invading Asian giant hornets (Vespa mandarinia) and generate excess heat and CO2 to kill their relatively thermophobic enemy (Sugahara and Sakamoto, 2009).
These various benefits of excess heat require that some individuals in the group spend metabolic energy on heat production (Figure 1). Microbes have metabolic flux pathways that seem designed to dissipate excess ATP without driving any anabolic processes. Those energy spilling reactions release significant heat, sometimes associated with a futile cycle of proton flux through the cell membrane (Russell, 2007).
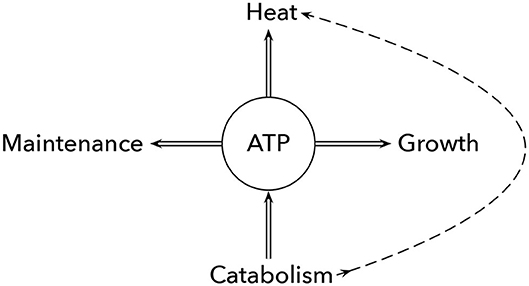
Figure 1. Intrinsic vs. excess metabolic heat production. Catabolism of food yields ATP and intrinsically generated metabolic heat (dashed curve). A cell may use the free energy added to the ATP/ADP disequilibrium to drive cellular maintenance and growth. Alternatively, the ATP/ADP disequilibrium can drive futile biochemical processes that may function to relieve biochemical imbalances or to generate excess beneficial heat. Any benefit of excess heat trades off against lost ATP/ADP disequilibrium to drive maintenance and growth. Alternatively, cells can generate excess metabolic heat by reducing the ATP produced during catabolism and increasing the direct heat generated along the dashed curve. For example, allowing proton motive force to cross the membrane without driving ATP synthase increases the heat production associated with the ion flow, as can happen in the electron transport chain or similar processes. Approximate calculations intended only to provide a rough sense of magnitude suggest that a cell aerobically respiring its own weight in glucose produces enough intrinsic metabolic heat to raise cellular temperature by about 2°C and enough ATP that could be used to generate additional excess metabolic heat to raise temperature by another 2°C. Cells that bypass ATP production and act almost purely as excess heat producers may be able to increase maximum metabolic rate because they have a larger free energy gradient between glucose and the final metabolic products. Modified from Figure 1 of Russell (2007).
A few possible functions for futile cycles have been mentioned, such as correcting thermodynamic imbalance (Von Stockar and Liu, 1999). However, the Tabata et al. (2013) article is the only one I found that suggests heat generation may itself be a benefit.
If free energy limits reproduction, then individuals that generate excess heat may be reducing their own reproduction in favor of the group-level benefit shared by neighbors. With heat as a public good (West et al., 2007), competitive non-producers could gain a growth advantage against cooperative heat producers.
In dense, energy-rich environments that dissipate heat relatively slowly, metabolic heat can raise local temperatures beyond the optimum for growth. Excreting catabolic intermediates such as lactate, acetate or ethanol may reduce heat production to keep temperatures below stressful levels. Protection against overheating provides an alternative explanation for the puzzle of overflow metabolism (Warburg, 1956; Postma et al., 1989; Wolfe, 2005).
5. Heat Flow and Spatial Scale
Metabolic heat can alter local temperature and potentially be important in conflict and cooperation. However, local heat must dissipate sufficiently slowly to play an important role. Here, “slowly” means the scaling of heat dissipation relative to the rate of other processes.
For example, does excess heat dissipate slowly enough that it can raise the rates of metabolic reactions and the growth rate of neighbors? Can excess heat be sufficiently concentrated to be used as a weapon that reduces the growth rate of relatively thermophobic competitors? What aspects of cellular aggregation and biofilm properties retain heat sufficiently to raise growth rate? How do changes in heat flow trade off against changes in the flow of other resources? How do larger-scale biophysical aspects of a habitat interact with smaller-scale intercellular processes to affect overall heat conductance?
Habitats vary in thermal properties. For example, water content and particle size significantly influence heat flow in soils (Usowicz et al., 2013). Water absorbs and dissipates heat more rapidly than does air. Convective flow may often dominate in the movement of heat. Still habitats may therefore be better candidates for local concentration of heat.
Many articles consider whether temperature gradients can be maintained within single cells (Inada et al., 2019; Suzuki and Plakhotnik, 2020). The current consensus suggests that significant intracellular gradients are unlikely because heat diffuses too quickly within cells and across membranes (Balaban, 2020; Oyama et al., 2020).
My arguments concern multicellular interactions and insulated environments, so the single-cell controversy is not directly relevant. But the technical issues of measurement (Braissant et al., 2010) may be important for studies of heat flow within multicellular aggregations. Future improvements in technology will likely enhance spatial resolution, which may improve the tracking of heat flow over the spatial scales at which conflict and cooperation play out.
With regard to the single-cell scale and cooperation, Dunn (2017) suggested that the first step toward eukaryotes arose when an archaeal cell acquired a bacterial symbiont as a source of internal cellular heat. The additional intracellular heat might have allowed thermophilic archaea to migrate to colder environments by a form of endothermy. However, the most recent single-cell studies mentioned above tend toward rejecting significant temperature differences between organelles, the cytosol, and the extracellular environment.
6. Discussion
The potential role of metabolic heat in microbial conflict and cooperation follows from basic observations and simple ideas. However, no evidence directly supports the theory, perhaps because the problem has rarely been discussed or studied. Several broad predictions summarize key points and future applications.
Relatively cold habitats more strongly favor excess metabolic heat to raise local temperature.
Habitats that dissipate heat more slowly favor the benefits of local heat production more strongly.
Cellular aggregation and extracellular insulation to retain local heat are more strongly favored as the growth rate benefits from heat become more valuable competitively.
The more genetically distinct cells are in an aggregation, the more likely that some cells do not contribute to costly heat production (the public goods dilemma).
In cellular aggregations, internal cells are more likely to generate excess heat because their heat production is protected by greater insulation than peripheral cells.
In biofilms, some of the observed spatial variation in gene expression between cells (Lenz et al., 2008; Besharova et al., 2016) may associate with internal heat production and external insulation.
Species with broader thermal tolerance and higher optimum temperature are more likely to use local heat as a competitive weapon.
Competitive heat generation favors competitors to raise their thermotolerance, which alters the selective pressure on competitive heat production, leading to game-like dynamics.
Species with broader thermal tolerance are more likely to use excess heat generation as a defensive fever response against invading bacteriophage.
The benefit of competitive and defensive heat generation may often increase with the number of cells that cooperate to create a thermal weapon, suggesting a link to quorum sensing.
The benefit of competitive and defensive heat generation rises with the tendency of the aggressors to surround their foe.
In dense environments that tend to overheat, microbes may secrete catabolic intermediates in overflow metabolism to reduce heat generation.
In summary, heat plays a primary role in the rate processes of life. Various individual and group traits of heat generation, cellular aggregation, and extracellular insulation may influence aspects of conflict and cooperation in microbial communities.
Author Contributions
SF developed the concepts and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Donald Bren Foundation, NSF grant DEB-1939423, and DoD grant W911NF2010227 support my research. This manuscript has been released as a pre-print at bioRxiv (Frank, 2020a) and preprints.org (Frank, 2020b).
References
Alster, C. J., Weller, Z. D., and von Fischer, J. C. (2018). A meta-analysis of temperature sensitivity as a microbial trait. Glob. Change Biol. 24, 4211–4224. doi: 10.1111/gcb.14342
Balaban, R. S. (2020). How hot are single cells? J. Gen. Physiol. 152:e202012629. doi: 10.1085/jgp.202012629
Bennett, A. F., and Lenski, R. E. (2007). An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl. Acad. Sci. U.S.A. 104(suppl 1):8649–8654. doi: 10.1073/pnas.0702117104
Besharova, O., Suchanek, V. M., Hartmann, R., Drescher, K., and Sourjik, V. (2016). Diversification of gene expression during formation of static submerged biofilms by Escherichia coli. Front. Microbiol. 7:1568. doi: 10.3389/fmicb.2016.01568
Braissant, O., Wirz, D., Gopfert, B., and Daniels, A. U. (2010). Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 303, 1–8. doi: 10.1111/j.1574-6968.2009.01819.x
Brinkmeyer, R., Knittel, K., Jurgens, J., Weyland, H., Amann, R., and Helmke, E. (2003). Diversity and structure of bacterial communities in arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 69, 6610–6619. doi: 10.1128/AEM.69.11.6610-6619.2003
Caspeta, L., and Nielsen, J. (2015). Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses. mBio 6:e00431-15. doi: 10.1128/mBio.00431-15
Chen, K., Gao, Y., Mih, N., O'Brien, E. J., Yang, L., and Palsson, B. O. (2017). Thermosensitivity of growth is determined by chaperone-mediated proteome reallocation. Proc. Natl. Acad. Sci. U.S.A. 114, 11548–11553. doi: 10.1073/pnas.1705524114
Chen, P., and Shakhnovich, E. I. (2010). Thermal adaptation of viruses and bacteria. Biophys. J. 98, 1109–1118. doi: 10.1016/j.bpj.2009.11.048
Cordero, R. J., Robert, V., Cardinali, G., Arinze, E. S., Thon, S. M., and Casadevall, A. (2018). Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr. Biol. 28, 2657–2664. doi: 10.1016/j.cub.2018.06.034
Crespi, B. J. (2020). Why and how imprinted genes drive fetal programming. Front. Endocrinol. 10:940. doi: 10.3389/fendo.2019.00940
D'Amico, S., Collins, T., Marx, J.-C., Feller, G., and Gerday, C. (2006). Psychrophilic microorganisms: challenges for life. EMBO Rep. 7, 385–389. doi: 10.1038/sj.embor.7400662
Dunn, C. D. (2017). Some liked it hot: a hypothesis regarding establishment of the proto-mitochondrial endosymbiont during eukaryogenesis. J. Mol. Evol. 85, 99–106. doi: 10.1007/s00239-017-9809-5
Evans, S. S., Repasky, E. A., and Fisher, D. T. (2015). Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 15, 335–349. doi: 10.1038/nri3843
Frank, S. A. (2020a). Metabolic heat in microbial conflict and cooperation. arXiv [Preprint] arXiv:2005.04796.
Frank, S. A. (2020b). Metabolic heat in microbial conflict and cooperation. arXiv [Preprint] arXiv:2005.04796. doi: 10.20944/preprints202005.0211.v1
Ghosh, K., and Dill, K. (2010). Cellular proteomes have broad distributions of protein stability. Biophys. J. 99, 3996–4002. doi: 10.1016/j.bpj.2010.10.036
Goddard, M. R. (2008). Quantifying the complexities of Saccharomyces cerevisiae's ecosystem engineering via fermentation. Ecology 89, 2077–2082. doi: 10.1890/07-2060.1
Grosberg, R. K., and Strathmann, R. R. (2007). The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654. doi: 10.1146/annurev.ecolsys.36.102403.114735
Haig, D. (2008). Huddling: brown fat, genomic imprinting and the warm inner glow. Curr. Biol. 18, R172–R174. doi: 10.1016/j.cub.2007.12.040
Haig, D. (2010a). “The huddler's dilemma: a cold shoulder or a warm inner glow,” in Social Behaviour: Genes, Ecology and Evolution, eds T. Székely, A. J. Moore and J. Komdeur (Cambridge: Cambridge University Press), 107–109. doi: 10.1017/CBO9780511781360.010
Haig, D. (2010b). Transfers and transitions: parent-offspring conflict, genomic imprinting, and the evolution of human life history. Proc. Natl. Acad. Sci. U.S.A. 107, 1731–1735. doi: 10.1073/pnas.0904111106
Inada, N., Fukuda, N., Hayashi, T., and Uchiyama, S. (2019). Temperature imaging using a cationic linear fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Protoc. 14, 1293–1321. doi: 10.1038/s41596-019-0145-7
Koschwanez, J. H., Foster, K. R., and Murray, A. W. (2011). Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. PLoS Biol. 9:e1001122. https://doi.org/10.1371/journal.pbio.1001122
Kuzdzal-Fick, J. J., Chen, L., and Balazsi, G. (2019). Disadvantages and benefits of evolved unicellularity versus multicellularity in budding yeast. Ecol. Evol. 9, 8509–8523. doi: 10.1002/ece3.5322
Lenz, A. P., Williamson, K. S., Pitts, B., Stewart, P. S., and Franklin, M. J. (2008). Localized gene expression in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 74, 4463–4471. doi: 10.1128/AEM.00710-08
Oyama, K., Gotoh, M., Hosaka, Y., Oyama, T. G., Kubonoya, A., Suzuki, Y., et al. (2020). Single-cell temperature mapping with fluorescent thermometer nanosheets. J. Gen. Physiol. 152:e201912469. doi: 10.1085/jgp.201912469
Postma, E., Verduyn, C., Scheffers, W. A., and Van Dijken, J. P. (1989). Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 55, 468–477. doi: 10.1128/AEM.55.2.468-477.1989
Price, P. B., and Sowers, T. (2004). Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. U.S.A. 101, 4631–4636. doi: 10.1073/pnas.0400522101
Ratcliff, W. C., Fankhauser, J. D., Rogers, D. W., Greig, D., and Travisano, M. (2015). Origins of multicellular evolvability in snowflake yeast. Nat. Commun. 6, 1–9. doi: 10.1038/ncomms7102
Rodrigues, D. F., and Tiedje, J. M. (2008). Coping with our cold planet. Appl. Environ. Microbiol. 74, 1677–1686. doi: 10.1128/AEM.02000-07
Russell, J. B. (2007). The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 13, 1–11. doi: 10.1159/000103591
Smith, J., Queller, D. C., and Strassmann, J. E. (2014). Fruiting bodies of the social amoeba Dictyostelium discoideum increase spore transport by Drosophila. BMC Evol. Biol. 14:105. doi: 10.1186/1471-2148-14-105
Starks, P. T., Blackie, C. A., and Seeley, T. D. (2000). Fever in honeybee colonies. Naturwissenschaften 87, 229–231. doi: 10.1007/s001140050709
Sugahara, M., and Sakamoto, F. (2009). Heat and carbon dioxide generated by honeybees jointly act to kill hornets. Naturwissenschaften 96, 1133–1136. doi: 10.1007/s00114-009-0575-0
Suzuki, M., and Plakhotnik, T. (2020). The challenge of intracellular temperature. Biophys. Rev. 12, 593–600. doi: 10.1007/s12551-020-00683-8
Tabata, K., Hida, F., Kiriyama, T., Ishizaki, N., Kamachi, T., and Okura, I. (2013). Measurement of soil bacterial colony temperatures and isolation of a high heat-producing bacterium. BMC Microbiol. 13:56. doi: 10.1186/1471-2180-13-56
Usowicz, B., Lipiec, J., Usowicz, J. B., and Marczewski, W. (2013). Effects of aggregate size on soil thermal conductivity: Comparison of measured and model-predicted data. Int. J. Heat Mass Transfer 57, 536–541. doi: 10.1016/j.ijheatmasstransfer.2012.10.067
Von Stockar, U., and Liu, J.-S. (1999). Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth. Biochim. Biophys. Acta Bioenerget. 1412, 191–211. doi: 10.1016/S0005-2728(99)00065-1
Warburg, O. (1956). On the origin of cancer cells. Science 123, 309–314. doi: 10.1126/science.123.3191.309
West, S. A., Diggle, S. P., Buckling, A., Gardner, A., and Griffin, A. S. (2007). The social lives of microbes. Annu. Rev. Ecol. Systemat. 38, 53–77. doi: 10.1146/annurev.ecolsys.38.091206.095740
Wolfe, A. J. (2005). The acetate switch. Microbiol. Mol. Biol. Rev. 69, 12–50. doi: 10.1128/MMBR.69.1.12-50.2005
Keywords: thermoregulation, biofilms, social evolution, ecological competition, fever, bacteriophage defense, public goods
Citation: Frank SA (2020) Metabolic Heat in Microbial Conflict and Cooperation. Front. Ecol. Evol. 8:275. doi: 10.3389/fevo.2020.00275
Received: 15 June 2020; Accepted: 03 August 2020;
Published: 10 September 2020.
Edited by:
Heikki Helanterä, University of Oulu, FinlandReviewed by:
Rolf Kümmerli, University of Zurich, SwitzerlandSara Mitri, University of Lausanne, Switzerland
Copyright © 2020 Frank. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven A. Frank, c2FmcmFua0B1Y2kuZWR1
 Steven A. Frank
Steven A. Frank