- Game and Wildlife Conservation Trust, Fordingbridge, United Kingdom
The gray partridge Perdix perdix was once a common breeding bird in Britain and a traditional quarry species. Its numbers have declined by over 90% over the last 50 years, and there have been similar declines across Europe. Since 1968 the Game and Wildlife Conservation Trust (GWCT) has undertaken research on this decline and identified three main reasons for it in Britain: disappearance of nesting habitat, reduction in area of brood-rearing habitat and increased pressure from predators. A nature-sparing mindset is not compatible with the conservation of a once-widespread species of farmed land such as the gray partridge, which requires a nature-sharing viewpoint. A gray partridge recovery program had to be tailored toward farmers and their advisors, requiring scientifically proven, costed, pragmatic and simple solutions. The difficulty is in convincing farmers and land managers to take up the challenge, adopt the conservation package and reverse the decline of this species. An important means of addressing this is providing demonstration sites where farmers can go to see how appropriate and practical management leads to successful restoration of gray partridge numbers. We provide two detailed examples of demonstrations in the United Kingdom, concentrating on gray partridge abundance and demography, but also considering the consequences for wider farmland biodiversity. At both demonstration sites the abundance of gray partridges was restored to abundances approaching those of 50 years ago (an average, over 10 years, of 11.3 spring pairs/km2 on one site and 13.2 pairs/km2 on the other). Obstacles to a more widespread adoption of the package among United Kingdom farmers are discussed as are signposts on how these are being addressed, both in United Kingdom and in Europe.
Introduction
Numbers of the gray partridge Perdix perdix, a once common breeding bird in Britain and a traditional quarry species, have declined by over 90% over the last 50 years (Woodward et al., 2018).
There is a similar picture across Europe, with numbers of gray partridges showing a long–term decline of 93% since 1980 (Pan European Common Bird Monitoring Scheme [PECBMS], 2020). The gray partridge was a widespread farmland bird and this led to the species’ inclusion in the European Farmland Bird Index (EFBI) as part of the EU Structural and Sustainable Development Indicators designed to measure sustainable development across Europe (EUROSTAT, 2020). In the United Kingdom, the species appears on the Red List of Birds of Conservation Concern because of its severe population decline (Eaton et al., 2015). The gray partridge was also named as a priority species in the United Kingdom Biodiversity Action Plan (UK Steering Group, 1995) and is one of 19 species contributing to the Farmland Bird Indicator, one of the United Kingdom government’s biodiversity indicators intended as a measure of the general quality of the farmed environment (DEFRA, 2011, 2019).
Declines in gray partridge numbers have continued despite the contributing factors in the United Kingdom and elsewhere being well understood—including a loss of nesting habitats due to removal of field boundary hedgerows, a decline in chick-food insects due to agricultural intensification and increased use of pesticides and, following these, increases in predation pressure linked to the land use changes and, at least in part, to a loss of wild gamebird shooting and associated predator management (Potts, 1986, 2012; Aebischer et al., 2000; Kuijper et al., 2009; Aebischer and Ewald, 2012; Bro, 2016; Brewin et al., 2020). Habitat improvements and efforts to mitigate predation are needed to address the declines in gray partridges across lowland farmland. Large-scale experiments have demonstrated how providing nesting and brood–rearing habitats and practicing legal control of generalist predators (especially during the breeding period) can reverse these declines. Gray partridge brood sizes nearly doubled where “conservation headlands” (which avoid the use of broad-spectrum pesticides in the outer 6 m of cereal fields in order to increase the insect food of young chicks) were used (Sotherton, 1991). The effect on gray partridge numbers of controlling predators during the breeding season was examined experimentally in the Salisbury Plain Experiment (Tapper et al., 1996). This used a switch-over design to show that 3 years of predator control resulted in a 2.5-fold increase in breeding density. Other habitat measures have been developed to enhance habitats for gray partridges. These include grassy mid–field strips (“beetle banks”) planted with perennial tussocky grasses such as cock’s–foot Dactylis glomerata as a means of providing mid–field nesting cover (Thomas et al., 1991; Sotherton, 1995), and wildflower/cover strips that provide both chick-food resources and overwinter food resources/cover, depending on composition and management (Buner et al., 2005; Vickery et al., 2009; Holland et al., 2014). The United Kingdom’s agri–environment schemes now include many of these habitat improvements, whereby in return for financial support, farmers are asked to deliver environmental improvements on their farm including improvements to biodiversity and the conservation of declining species of farmland wildlife. How successful these schemes are in addressing the decline in gray partridge numbers depends on whether land managers make full use of the options available to them to make sure that all the habitat requirements of the partridge are in place over its annual cycle (Ewald et al., 2010). Implementing lethal predator control often hinges on the promise of a shootable surplus to defray its costs (Draycott, 2012; Ewald et al., 2012).
Across Europe other researchers have had varying levels of success in restoring gray partridge numbers. Habitat management combined with legal predator control in Northern France has succeeded in retaining high densities of gray partridges on a small area of farmland (Bourdouxhe, 2002; Bro et al., 2005). In Germany (Gottschalk and Beeke, 2014) and Hungary (Faragó et al., 2012), substantial conservation efforts have succeeded in recovering gray partridges at lower densities, with habitat alone used in Germany and both habitat and predator control used in Hungary. In Switzerland targeted habitat management and restocking efforts were directed toward reintroducing gray partridge (Buner et al., 2005), but in the absence of predator control these efforts appear to have been in vain, with gray partridges recently declared extinct (Knaus et al., 2020).
In this paper we describe the results of targeted management on two demonstration areas in the United Kingdom. The one located near Royston, Hertfordshire—where a demonstration and reference area were set up at the start—was a classic demonstration project, illustrating the effect of combining habitat management with targeted predator control. The other demonstration was in West Sussex, within an area monitored for over 50 years by the GWCT (Potts and Vickerman, 1974; Potts, 1986; Potts, 2012); it has led to the owner re–establishing a wild gray partridge shoot, after a break of 48 years. This is an example of conservation science practiced by a landowner, who made the decisions regarding habitat management and predator control himself, converting conservation science into the restoration of a declining species on his land. Where appropriate and possible, we also provide information on the effects of gray partridge management on wider biodiversity to illustrate the broad scope and potential of such management for the conservation of farmland wildlife (Potts, 2012; Brewin et al., 2020).
Materials and Methods
Study Areas and Management
Hertfordshire Demonstration Area
The first demonstration project ran from autumn 2001 to spring 2010, on two areas of rolling arable farmland on chalk soil near Royston, Hertfordshire, about 65 km north of London. The main crops sown were cereals and break crops with some grass fields. The demonstration area comprised 996 ha (six farm holdings), while a surrounding area of 1,311 ha (seven holdings) constituted a reference area for comparison (Aebischer and Ewald, 2010, 2012). The GWCT employed a full-time gamekeeper to address the known causes of decline. These included habitat management to increase the amounts of nesting, brood-rearing and overwinter cover, lethal predation control measures targeted at reducing predation by generalist predators mainly during the gray partridge breeding season, and the provision of supplementary food over winter to augment overwinter food resources. Habitat provision relied heavily on set–aside and agri–environment schemes (Countryside Stewardship-CS, Entry Level Schemes-ELS, and Higher-Level Schemes-HLS) to cover habitat creation and management costs (Aebischer and Ewald, 2010). In the first 5 years, a third of nesting cover was created on non–rotational set–aside strips (sown to grass and not cultivated again) provided a third of the nesting cover, and agri–environment land (grass margins and beetle banks) another third, with hedgerows providing the remainder; an average of 6.8% of the demonstration area provided nesting cover. Brood–rearing cover was grown as unharvestable cereal mixes, half on rotational set–aside and half on agri–environment land, including wild bird cover. After the decoupling of production subsidies in 2005, less rotational set–aside became available, and all set–aside disappeared after the zero quota in 2008. The shortfall was made good by entering land into the UK’s Environmental Stewardship Scheme (ELS and HLS), although some brood–rearing cover in 2009 was provided by farmers without monetary assistance. This resulted in an average of 10.8% of the area providing brood-rearing cover. The predator control involved targeting mainly foxes Vulpes vulpes, stoats Mustela erminea, weasels Mustela nivalis, rats Rattus norvegicus, carrion crows Corvus corone and magpies Pica pica from February to July. Methods included shooting foxes with a rifle at night, using stopped snares (with a breakaway for non–targets), tunnel traps (for mustelids) and Larsen traps (for corvids). Predator species legally protected in the United Kingdom were not targeted. We cannot provide numbers of predators controlled for reasons of confidentiality.
Supplementary food (wheat) was provided in hoppers (25-l drums) located along field margins and cover strips from September to May, at a ratio of approximately two per partridge pair.
Sussex Demonstration Area
The second demonstration area was located on the Sussex Downs between the rivers Arun and Adur, West Sussex, near the south coast. The soil type was chiefly a thin rendzina over chalk, with clay caps on the higher ground. The land was mainly managed through arable farming (12 holdings), with cereals and break crops interspersed with pastures and patches of traditional chalk grassland managed by grazing sheep (Ewald et al., 2012). Previous to the conservation efforts described here, all cereal crops were managed intensively in terms of pesticide use, which has been shown to reduce the abundance of gray partridge chick-food insects (Potts, 1986; Ewald and Aebischer, 1999; Ewald et al., 2016). Overall, numbers of gray partridges had declined from 10.1 pairs/km2 in 1970 to 0.9 pairs/km2 in 2003 (Ewald et al., 2012).
In 2003, one landowner trialed a restoration of gray partridge numbers on an area of 220 ha, extending this to 1,052 ha in 2007. The venture relied on fulfilling all partridge habitat requirements and applying legal predator control. The remaining 22 km2 of the study area formed the “conventional area,” where only limited or no predator and habitat management occurred (Figure 1). Habitat management on the demonstration area included creating nesting, insect-rich brood–rearing and overwinter covers. Nesting habitat was increased by planting 25 km of beetle banks and thorny hedges, amounting to 8.2 km/km2 of nesting cover, compared to the 4.3 km/km2 it had previously (Aebischer, 1991; Ewald et al., 2012). Specially grown brood–rearing cover (suitably structured insect-rich strips of vegetation) covered almost 9% of the area, including 97 ha of new conservation headlands. There was no summer use of insecticides on any part of a cereal crop and at least one third of the conservation headlands were left standing to provide cover and seed after harvest. In addition, 2.1 km/km2 of wild bird cover strips were sown, involving mixes with kale Brassica oleracea, chicory Cichorium intybus, millet Panicum miliaceum and canary grasses Phalaris canariensis and P. arundinacea. These provide cover and further food resources through the winter. Cropping patterns were adjusted so that crops and sowing times differed on either side of a field boundary or beetle bank, ensuring that on at least one side of each boundary, vegetative cover was present throughout the year (Ewald et al., 2012). Much of the new habitat was financed through agri–environment options, initially via the Higher-Level Scheme (HLS, Natural England, 2010) and then through Countryside Stewardship Higher Tier (Natural England, 2017). In addition to the habitat improvements, three gamekeepers (two full-time, one part-time) were employed. They were responsible for establishing the specially created habitats, and, as at the Hertfordshire demonstration area, for carrying out legal predation control during early spring and summer, and supplementary food overwinter; they also provided grit (1.5 mm) at each hopper.
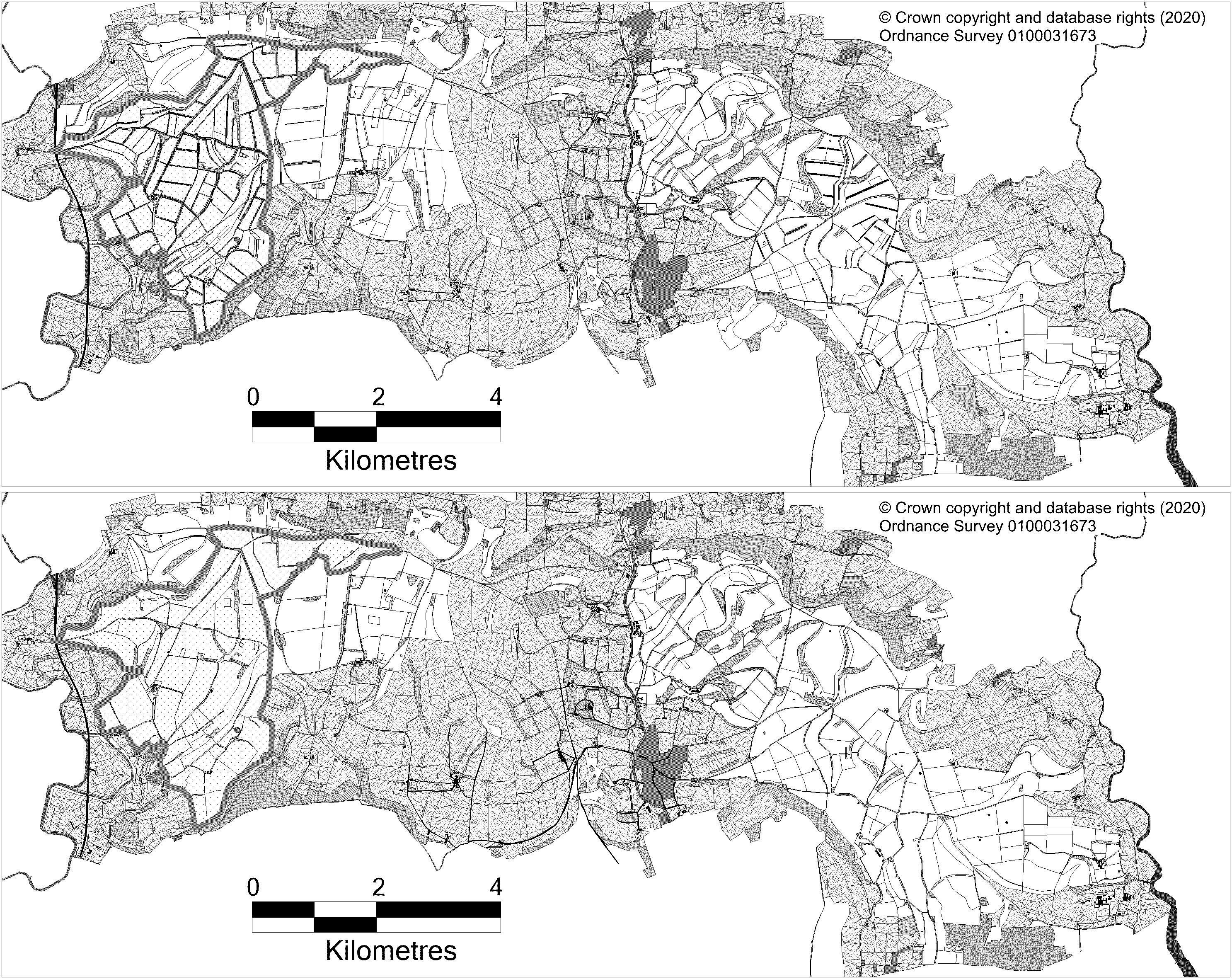
Figure 1. | Habitat on the GWCT’s Sussex Study area for the area specifically managed for gray partridges indicated by the shaded boundary, versus the remainder of the Sussex Study area not subjected to gray partridge-specific management. The fields within the Sussex Study are shaded white, with fields outside the Sussex Study shaded gray. The top habitat map is from 2013—when management was fully implemented, while the bottom map is from 1997, before management began. Gray partridge-specific management included dividing fields—either with a beetle bank or hedgerow, planting of strips of wild bird cover and having conservation headlands around every crop of cereal.
Data Collection
Gray Partridge
In Hertfordshire, GWCT staff counted and mapped gray partridges on the demonstration and reference areas in spring and autumn, following a standard method whereby they located and counted birds from a four-wheel-drive vehicle acting as a mobile hide, using binoculars, and recording observations on a map. Counts were not undertaken in rain, fog, or winds stronger than Beaufort Force 3. Spring counts recorded the number of pairs and single birds, mainly from tracks and field edges to avoid damaging crops, and took place in March/April after family groups (“coveys”) have split up, birds have paired and before crops got too high. Autumn counts took place after harvest from late August through September by quartering stubble fields, noting each covey (which often have failed breeders in attendance), and recording numbers of males, females and young in it. Breeding density was calculated from the spring counts as pairs/km2, and autumn density from the autumn counts as birds/km2.
In Sussex, the GWCT has monitored gray partridges on both managed and conventional areas since 1968 using post–harvest stubble counts, as described for Hertfordshire (Potts, 1986; Potts and Aebischer, 1991, 1995). Autumn density was again calculated as birds/km2, and, following Potts (1980), breeding density was also calculated from the autumn counts, with each adult male and any single female considered to represent a spring pair in the previous spring. Other demographic parameters calculated from the autumn counts (Potts, 1980, 1986; Ewald et al., 2012) were chick survival rate (the proportion of chicks that survived up to 6 weeks of age, calculated as 0.03665 ∗ (geometric mean brood size)1.293 if the geometric mean brood size is less than 10, or as (geometric mean brood size)/13.84 otherwise (Potts, 1986), young-to-old ratio (total number of young divided by total number of adult birds), and brood production rate (number of broods as a proportion of the combined count of adult males and single females). The young-to-old ratio is a measure of reproductive success that tends to be less sensitive than chick survival rate to the effects of low numbers of broods (Aebischer and Reitz, 2000).
Other Wildlife
Brown hares Lepus europaeus were recorded on both the Hertfordshire and the Sussex demonstration sites. The preferred method of censusing brown hare uses spotlight transects or viewpoints in winter (Frylestam, 1981; Barnes and Tapper, 1985); this was the census method used on the Hertfordshire demonstration area. In Sussex, brown hares were recorded during the annual gray partridge autumn stubble counts; this does not allow calculation of precise densities but allows comparisons through time across the area in the number seen per km2.
In Sussex, measures of the abundance of invertebrates in cereal crops and information on the presence or absence of different arable plant species has been collected each third week of June in approximately 100 cereal fields, one sample per field. Most of the arable flora was identified to species with some plants difficult to identify unless flowering or fruiting, e.g., Fumaria, identified to genus (Potts et al., 2010). We sampled invertebrates using a D-Vac suction trap (Dietrick, 1961) to take five pooled 10-s sub-samples, each of 0.092 m2, from the crop edge along a diagonal transect 12–20 m into the field. The location of invertebrate sampling reflects the typical foraging location of young gray partridge chicks, i.e., the cereal headlands (Green, 1984). Partridge chick-food invertebrates were identified to at least family level (Ewald et al., 2015). The occurrence of arable plants was recorded in the same area as was sampled for invertebrates. Roughly two-thirds of invertebrate and arable plant records were from the conventionally managed area, with the remainder from the demonstration area. We restricted our analysis of plant occurrence and invertebrate abundance to 34 arable plant and 20 invertebrate taxa that were identified in the literature as being important in the diet of declining farmland birds (Holland et al., 2006).
Statistical Analysis
We compared the yearly mean spring and autumn gray partridge density and the yearly brown hare density recorded at the demonstration site in Hertfordshire with those on the reference site using a generalized linear model (overdispersed Poisson error and logarithmic link) with the number of animals counted each year on each management area (demonstration/reference) as dependent variable, ln(area counted) as offset, and year and management area as factors (McCullagh and Nelder, 1989). The significance of management area was tested using a deviance ratio statistic, which follows an F distribution.
For Sussex, we compared yearly gray partridge spring and autumn density and productivity (measured as brood production rate, chick survival rate and young-to-old ratio), yearly density of brown hares, annual plant occurrence (proportion of fields where each taxon was recorded) and average annual invertebrate abundance per sample on the managed area with those on the conventional area in two time-periods, the 10 years before (1993–2002) and the 10 years after (2009–2018) gray partridge management was implemented fully across the managed area. In each case, the unit for analysis was the value recorded in each year in each management area (managed/conventional); we fitted a generalized linear model comprising the factors time-period, management area, their interaction and year nested within time-period, and tested the interaction between time-period and management area for significance using a deviance ratio statistic. We used an overdispersed Poisson error and logarithmic link when analyzing number of spring pairs, number of partridges counted in the autumn and number of hares counted, all with an offset of ln(area counted). A normal error and identity link were used when analyzing chick survival rate (logit transformed, restricted to years when more than two broods of chicks were counted), young-to-old ratio (ln-transformed), and average number of invertebrates per sample. We used an overdispersed binomial error and a logit link function when analyzing plant occurrence at the field level and brood production rate (with number of fields sampled and combined count of male and single female adult partridges, respectively, as binomial denominator).
In the case of the Sussex invertebrates, multiple testing increased the likelihood that some statistically significant results arose by chance. Therefore, in addition to considering the significance levels for individual taxa, we also report on the overall pattern of effects across all taxa, whether statistically significant or not.
We carried out all the statistical analysis in Genstat Release 19.1 for Windows (VSN International, Hemel Hempstead, United Kingdom).
Results
Gray Partridge
On the Hertfordshire area, gray partridge breeding density reached a high of 18.4 pairs/km2 in 2007 and, over the 10 years of the demonstration, breeding density was higher on the demonstration area than the reference area (an average of 11.3 pairs/km2 compared to 3.1 pairs/km2, [F(1, 8) = 182.01, P < 0.001, Figure 2]. Autumn densities showed a similar pattern [an average of 55.0 birds/km2 compared to 16.5 birds/km2, F(1, 9) = 97.53, P < 0.001].
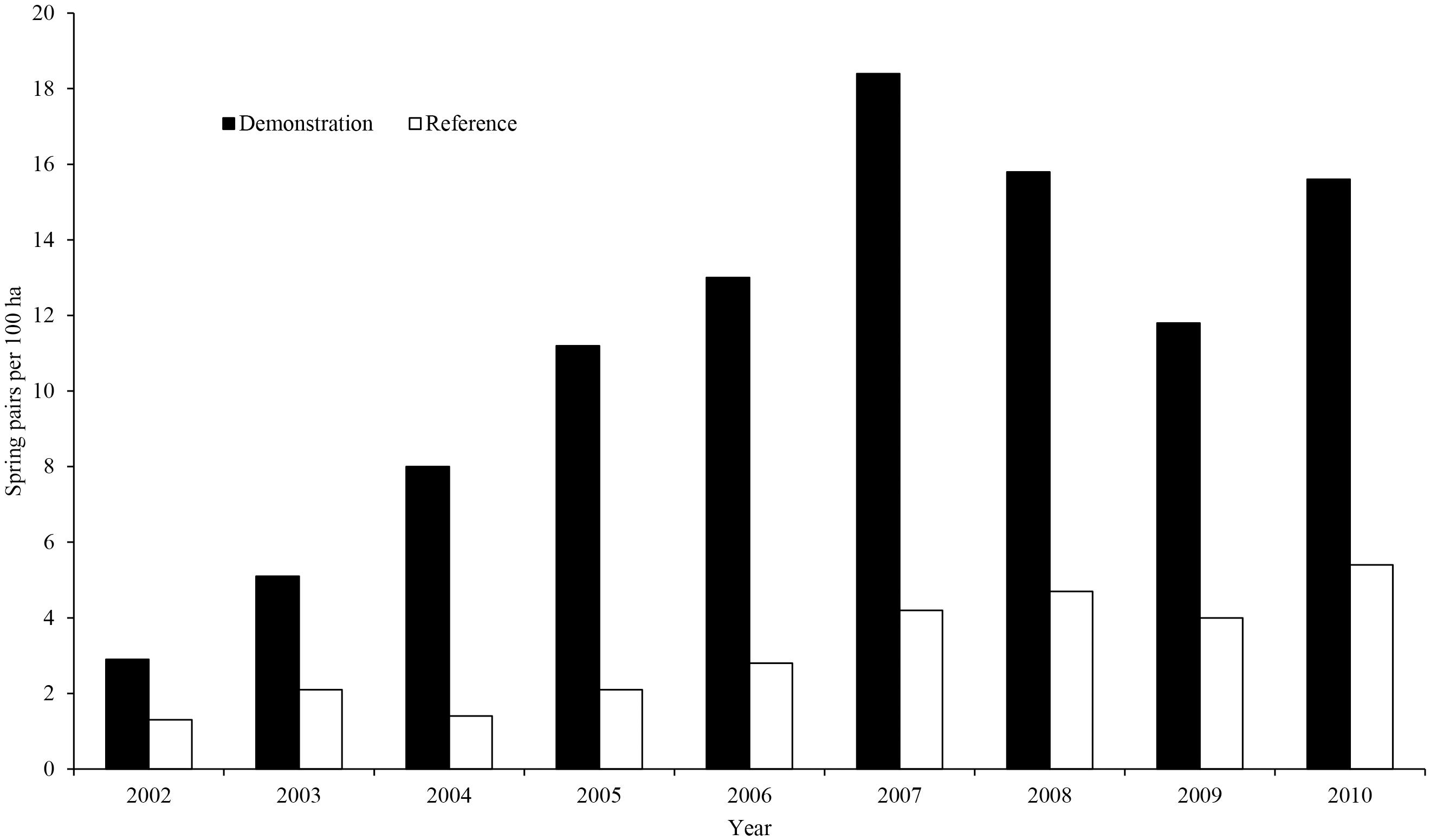
Figure 2. | Spring pair counts (pairs/100 ha) of gray partridges, on the GWCT’s Royston demonstration and reference areas 2002–2010. Filled bars are from the demonstration area specifically managed for gray partridges, open bars from the reference area not subjected to gray partridge-specific management.
Within Sussex, the breeding pair density on the conventional area averaged 4.0 pairs/km2 in the 10 years preceding gray partridge management (1993–2002), while the managed area averaged 3.0 pairs/km2 over the same time period. Breeding density on the managed area increased to an average of 13.2 pairs/km2 from 2009 to 2018, while on the conventionally managed area pair density declined to 1.3 pairs/km2. There was a significant increase on the managed area compared to the conventional area [F(1, 18) = 355.84, P < 0.001, Figure 3]. Autumn densities in the 10 years before management averaged 9.8 birds/km2 on the area that was subsequently managed, with 12.5 birds/km2 on the area that remained conventionally managed. In the 10 years after management was established, autumn density on the managed area averaged 93.6 birds/km2, compared to 6.2 birds/km2 on the conventionally managed area, with management significantly affecting autumn density [F(1, 18) = 296.14, P < 0.001, Figure 4]. Looking at productivity, brood production rate on the managed area before management began (1993–2002) averaged 26% compared with 25% on the conventional area; after management, a combination of increased nesting habitat and predator control on the managed area resulted in an average brood production rate of 71% over 2009–2018 compared to 46% on the conventional area, with a significant difference in the change on the two areas [F(1, 18) = 15.80, P < 0.001]. Chick survival rate over the managed area compared to that on the conventional area from 1993 to 2002 averaged 27% versus 23%, rising to 46% on average on the managed area in 2009–2018 compared to 33% on the conventional area, though there was no significant effect of management [F(1, 16) = 1.02, P = 0.327]. Before management, the conventionally managed area averaged a young-to-old ratio of 0.78, compared to 0.79 on the subsequently managed area. In the 10 years after management was established the managed area averaged a young-to-old ratio of 2.72, compared to 1.46 on the conventionally managed area, with a significant effect of management [F(1, 18) = 9.14, P = 0.007, Figure 5].
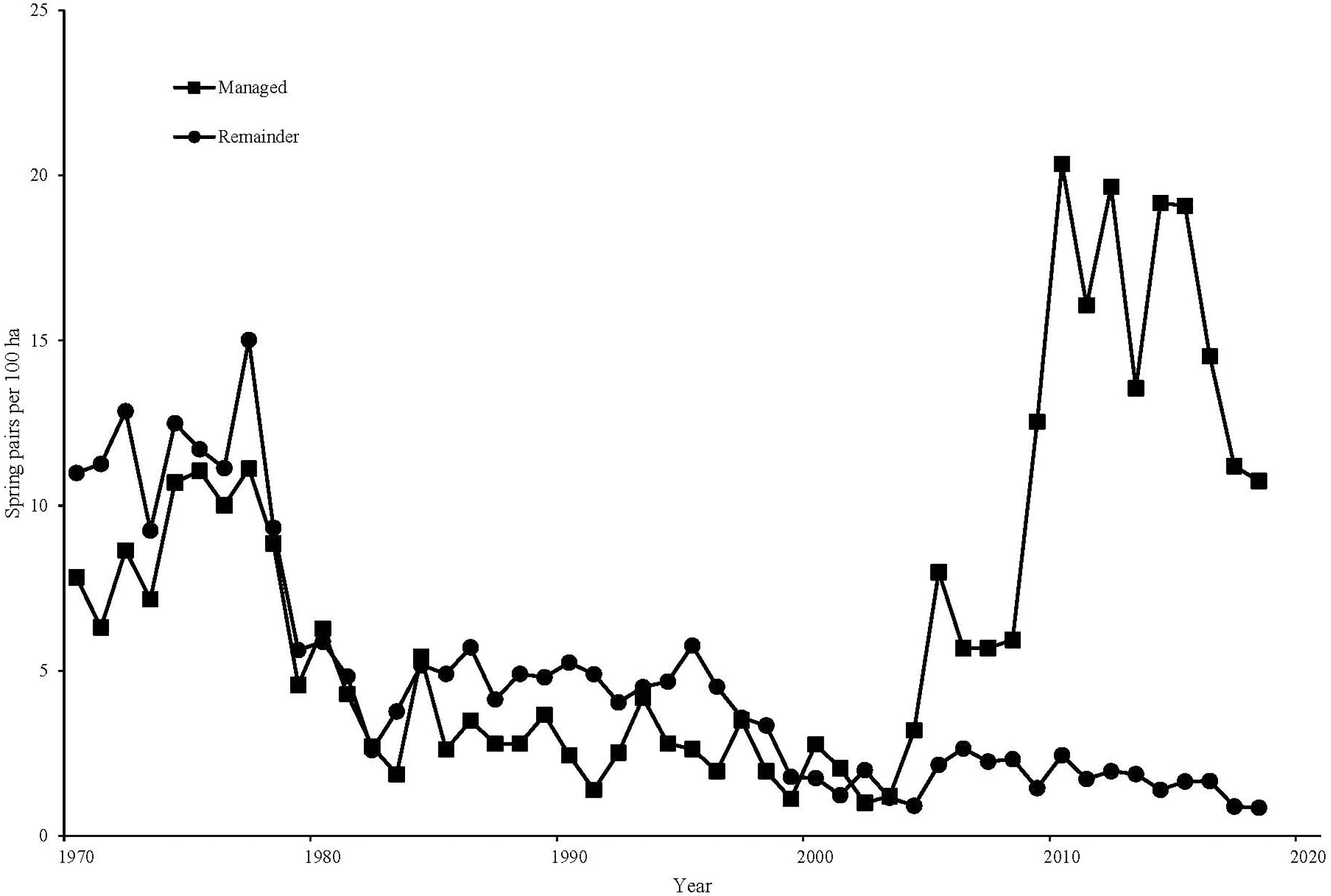
Figure 3. | Spring pair counts (pairs/100 ha) of gray partridges, on the GWCT’s Sussex Study area, 1970–2018. Squares represent the land specifically managed for gray partridges from the mid-2000s. Circles represent land not subjected to partridge-specific management.
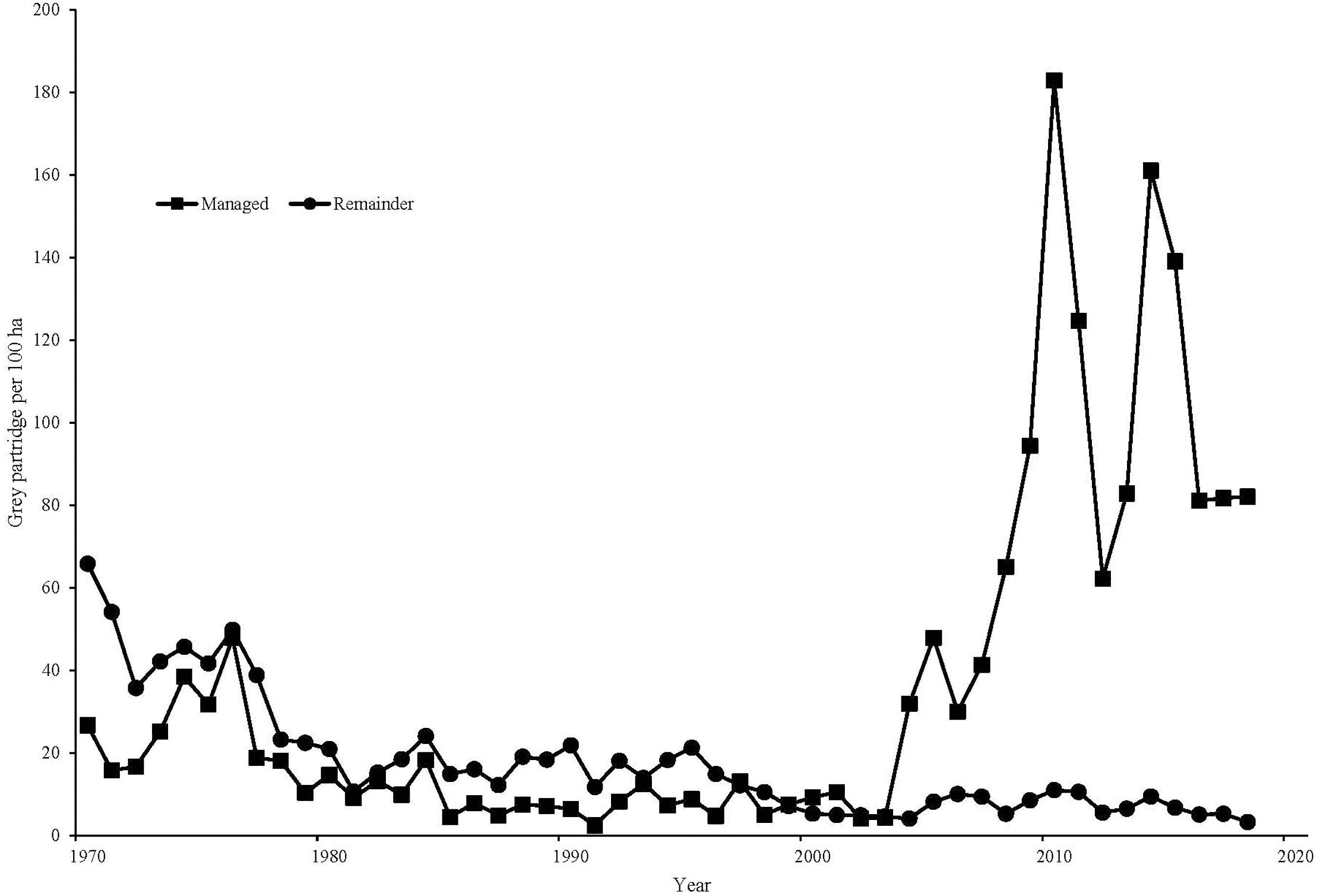
Figure 4. | Autumn counts (pairs/100 ha) of gray partridges, on the GWCT’s Sussex Study area, 1970–2018. Squares represent the land specifically managed for gray partridges from the mid-2000s. Circles represent land not subjected to partridge-specific management.
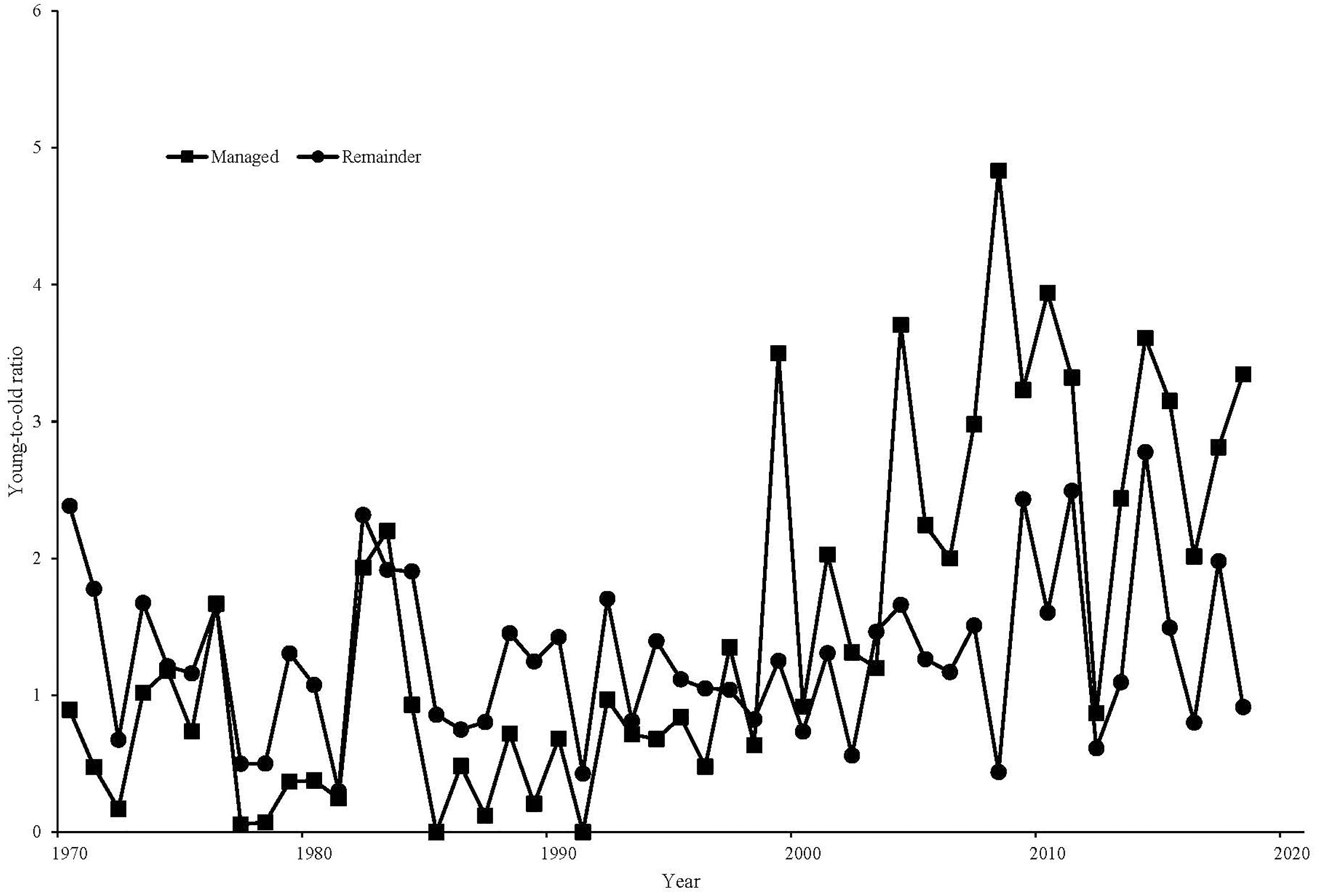
Figure 5. | Young-to-old ratios of gray partridges on the GWCT’s Sussex Study area, 1970–2018. Squares represent the land specifically managed for gray partridges from the mid-2000s. Circles represent land not subjected to partridge-specific management.
Since 2009, the landowner who undertook the management has started shooting gray partridges sustainably on the area (avoiding shooting in 2012 where chick production was low owing to an extremely wet summer). The bag averaged 21% of the autumn stock from 2009 to 2018. The revenue from shooting, together with that received through agri–environment payments, helped defray the cost of management. In the view of the landowner, these two income streams together with the enjoyment derived from his shooting justify his investment in gray partridge conservation.
Other Flora and Fauna
At the Hertfordshire site the density of hares on the demonstration area was higher (60.9 hares/km2) than the density on the reference area [26.4 hares/km2, F(1, 8) = 44.16, P < 0.001]. In Sussex, before management began, 2.6 hares/km2 were counted during autumn partridge counts on the area to be managed, with 1.0 hares/km2 counted on the conventionally managed area. From 2009 to 2018, an average of 7.3 hares/km2 were counted on the managed area, compared to 2.8 hares/km2 on the conventional area. There was no significant effect found due to the management [F(1, 18) = 0.02, P = 0.946], though one should bear in mind that the method used to census hares is not the preferred one using transects and lamps that was employed on the Hertfordshire demonstration area.
On the Sussex Study site, for 23 plant taxa (68% of those considered), there was a significant increase in occurrence associated with management for gray partridges (Table 1). This included 14 taxa whose percentage occurrence before management began was lower on the area that was subsequently managed than on the conventional area. After management, only one taxon, common mugwort Artemisia vulgaris, occurred more frequently on the conventional area than on the managed area. Individual species recorded on the managed area after 2009 included dwarf spurge Euphorbia exigua (near-threatened on the British red list), few-flowered fumitory Fumaria vaillantii (a nationally scarce species) and night-flowering catchfly Silene noctiflora (a species of Conservation Concern).
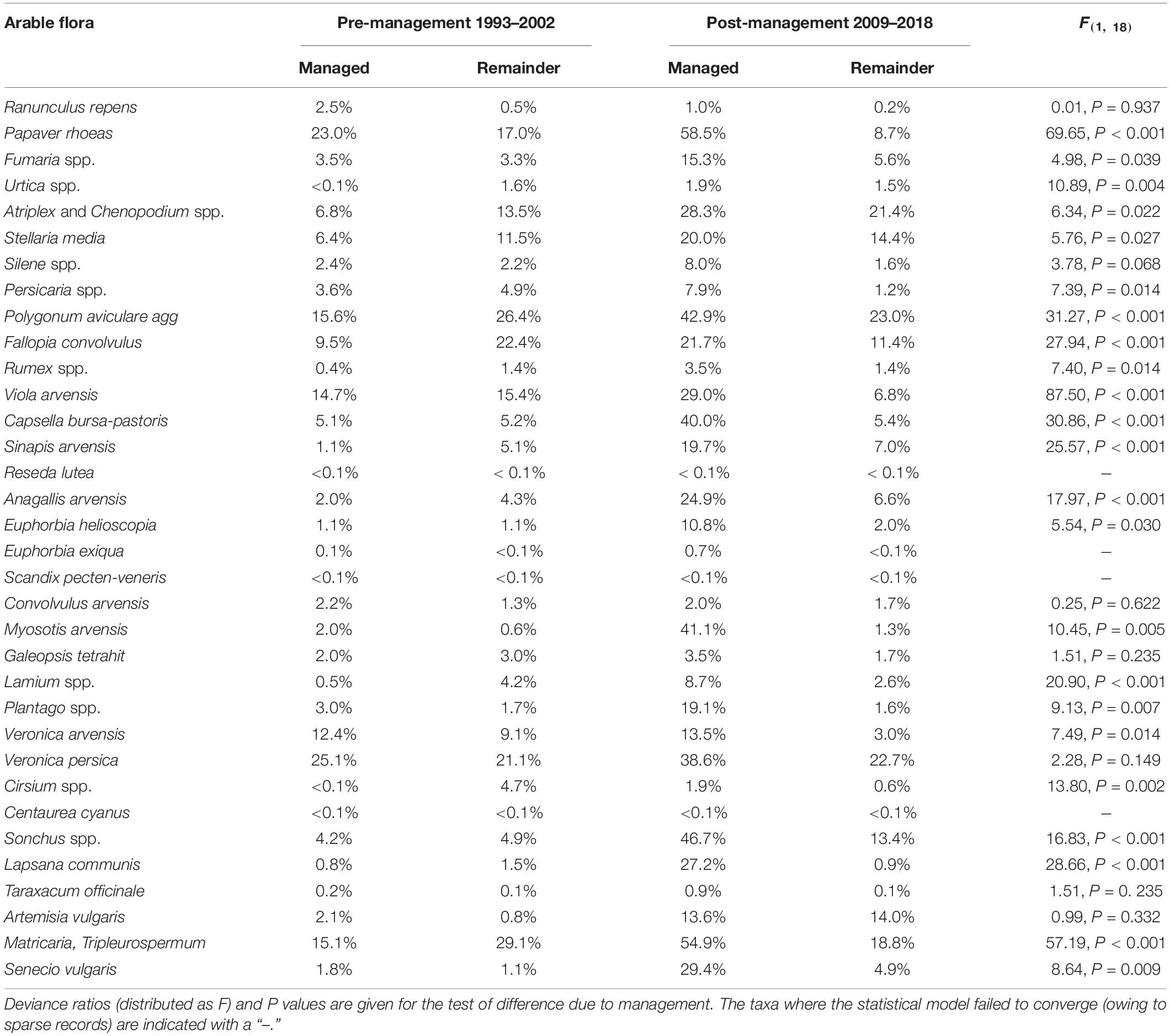
Table 1. | Occurrence of arable plant taxa associated with the diet of farmland birds in the area under gray partridge shoot management (managed) and the conventionally managed area of the Sussex Study (remainder), pre- and post-management.
Changes in the abundance of twenty invertebrate taxa important in the diet of farmland birds in Sussex (Table 2) were not as straightforward as those in the occurrence of the arable flora. The results for the invertebrate abundance can be divided into five groups. Firstly there were seven taxa where the average abundance increased between the two time periods, but the increase on the managed area was relatively higher than that on the conventionally managed area—with a significant effect associated with management for three of these taxa (Collembola, Delphacidae, and Nitidulidae). There were four taxa (Araneae, Opiliones, Formicoidea, and Tipulidae) where the average abundance increased on both areas but where the increase on the conventionally managed area was relatively higher than on the managed area; one of these changes was statistically significant (Tipulidae). There were two taxa where the average abundance on the managed area increased or stayed the same while it decreased on the conventionally managed area (Neuroptera and Curculionidae), for both there was a significant positive effect associated with management. Conversely the abundance of Syrphidae increased on the conventionally managed area but declined on the managed area. There were two taxa (Heteroptera and Cryptophagidae) where declines occurred on both areas, with less of a decline on the managed area. Finally, there were four taxa where the average abundance declined on both areas, but less so on the conventional area than on the managed area (Aphididae, Carabidae, Staphylinidae, and Chrysomelidae).
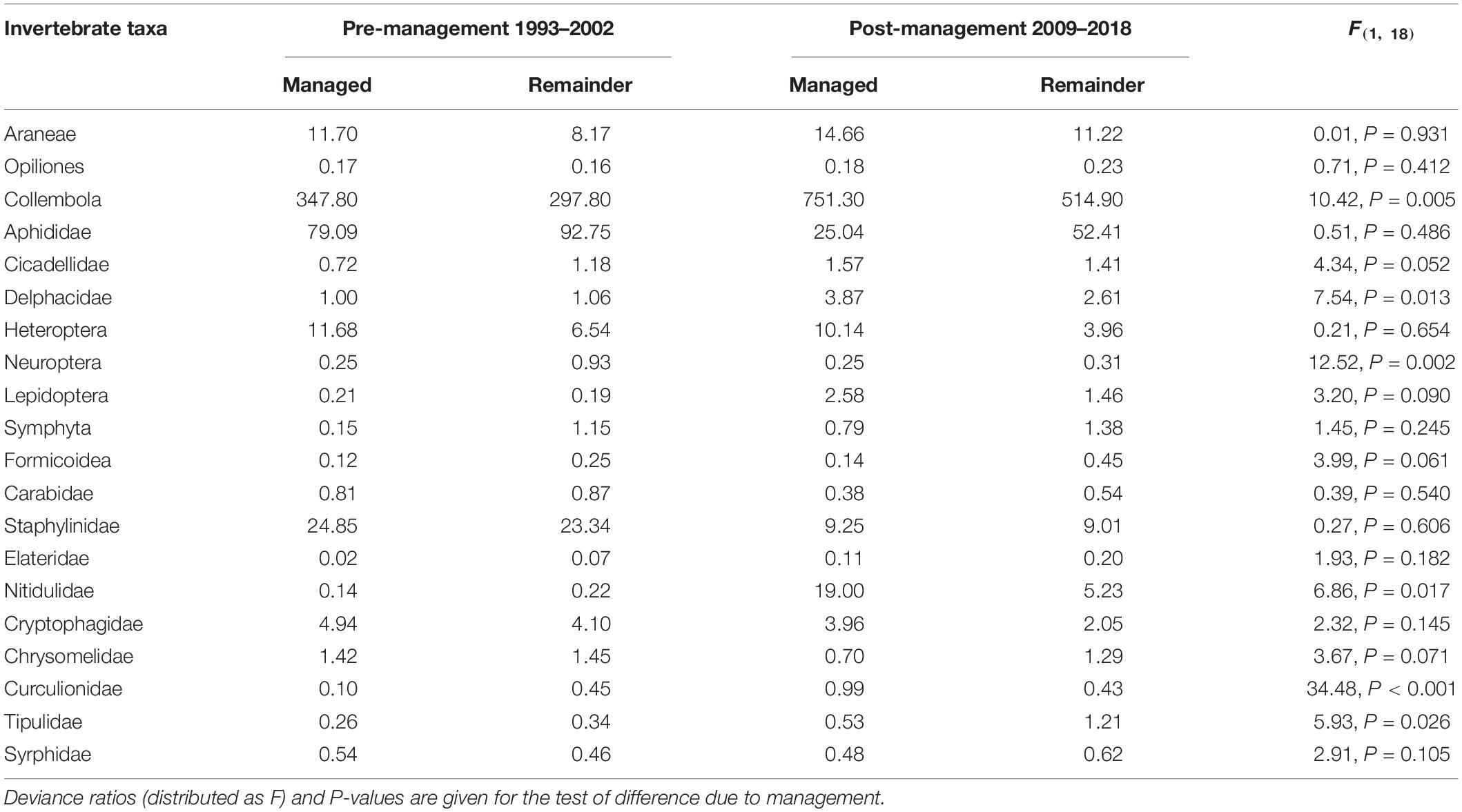
Table 2. | Average abundance of invertebrate taxa associated with the diet of farmland birds in the area under gray partridge shoot management (managed) and the conventionally managed area of the Sussex Study (remainder), pre-and post-management.
Discussion
Both of these demonstration projects illustrate the ability of targeted predator control and well-directed habitat improvement to increase numbers of gray partridges on conventionally managed farmland in the United Kingdom. The densities in the later years of the Hertfordshire demonstration mirror those found in the landowner-led project in Sussex, indicating that dedicated organizations and individuals can turn around the fortunes of gray partridges. The turnaround in gray partridge numbers in Sussex reflects the hard work and vision of the landowner and his staff, given that the gray partridge was close to extinction. On the managed area, densities recovered to levels that enabled sustainable driven shooting of wild gray partridges, with over 200 birds sustainably harvested on a shoot day. The landowner sees his project as a means of encouraging other landowners, showing how government funding for agri–environmental options would also allow other land managers to implement the habitat management needed to restore gray partridge numbers on a much wider scale.
Comparison With Other Places Where Partridge-Specific Habitat Management Was Implemented
The average spring pair density on the managed area in Sussex (15.4 pairs/km2) was higher than the average across the Hertfordshire demonstration area (10.1 pairs/km2)– though the average in Hertfordshire includes the years when the habitat and predator management was just beginning. The density on Sussex was over 1.5 times that reported on the Salisbury Plain experimental area, where predator control on its own was used experimentally to increase gray partridge numbers (Tapper et al., 1996). It was higher than the densities seen in Germany, where habitat management for gray partridges was installed across a large area but where there was little predator control (Gottschalk and Beeke, 2014). The German project managed to hold the gray partridge population stable at 2 spring pairs/km2 across a large area (1,00,000 ha), though densities did reach 5.6 spring pairs/km2 on an area of 600 ha with 7% of high-quality habitat. The densities reported on our areas were, however, far below the densities of 80 pairs/km2 reached locally on some hunting grounds in northern France. Their densities were over five times higher despite the use of similar habitat management and predator control (Bourdouxhe, 2002; Bro et al., 2005).
The GWCT’s long-running gray partridge research resulted in the construction of an English gray partridge population model (Potts, 1986), which can be used to explore how habitat management and predator control affects gray partridge abundance. This model predicts that predator control roughly triples the equilibrium population level expected from increasing brood-rearing and nesting habitat alone, which itself is predicted to double numbers (Aebischer, 1991). At 15 pairs/km2, the results on the managed area in Sussex were close to what the model predicted for a population managed for shooting using full habitat management and predator control. Without predator control (and without shooting), the model predicts around 5 pairs/km2, similar to densities recorded in Germany where habitat management was initiated (Gottschalk and Beeke, 2014). The average chick survival rate observed on the conventional part of the Sussex study area was similar to the long–term average reported for the post–decline period (32.3%; Potts and Aebischer, 1995). The average chick survival rate on the managed area in Sussex was close to the level recorded before gray partridge numbers began to decline when herbicide use in cereals became widespread (48.6%; Potts and Aebischer, 1995). They exceed those of the original experiments that confirmed the value of conservation headlands as chick–rearing habitat (gray partridge chick survival rates increased to an average of 39.1%, Sotherton et al., 1993). The cereal area occupied by conservation headlands in the managed area was higher than the original recommendation for the use of conservation headlands (6%; Boatman and Sotherton, 1988), which may explain the higher chick survival rates.
The results from Sussex show that arable flora can be restored using conservation headlands (Albrecht et al., 2016). The restoration of invertebrates in cereal crops appears to be more difficult – even though the original work on conservation headlands in the United Kingdom demonstrated their usefulness in restoring both some chick-food insects (Sotherton, 1991) and butterflies (Dover, 1997). A recent interest in long-term declines in invertebrate abundance associated with agricultural intensification (Hallmann et al., 2017; Harvey et al., 2020, but see Bell et al., 2020) indicates a need for further research on methods to conserve their abundance in arable crops.
Encouraging Uptake of the Management Described Here Across England
As long as the relevant agri-environment options (beetle banks, conservation headlands, wild bird cover, winter cover/food provision) remain available in the future, the habitats established in Hertfordshire and Sussex could be used by arable farmers across England through the government’s agri-environment stewardship scheme. Results from these demonstrations can be compared with the GWCT’s national database on partridges called the Partridge Count Scheme (PCS). The PCS is a voluntary, non-random recording scheme whereby participants (farmers/gamekeepers) are asked to count the partridges on their land twice a year to enable the GWCT to monitor numbers of breeding pairs (from spring counts) and their productivity (chick, autumn counts). Members of the PCS are informed about methods to improve the provision of nesting and brood-rearing habitats, methods of legally and humanely controlling predators, best use of agri-environmental subsidies and guidelines on shooting to ensure that the numbers harvested are sustainable (Aebischer, 2009; Ewald et al., 2009, with information provided all publicly available at http://www.gwct.org.uk/partridge). For many PCS members, the opportunity to shoot wild gray partridges while conserving the species has been a strong incentive to undertake the requisite management, with an emphasis on sustainable levels of harvest. This offers an example of species conservation that is in accordance with the Addis Ababa Principles and Guidelines for the Sustainable Use of Biodiversity (CBD, 2004).
As regards the GWCT’s Partridge Count Scheme (PCS), increases in chick survival rates on the area managed by PCS members are associated with their use of the same in–field agri–environment options found to be useful in both demonstration areas described here—conservation headlands and beetle banks—that provide chick-food insects and nesting sites (Ewald et al., 2010). Our demonstration sites show that new partridge recovery areas could be land owned by one landowner (as in Sussex) or consist of several farmers and landowners working together (as in Hertfordshire). However, to reach the densities recorded in the demonstration projects presented here, it is necessary, in our experience, to employ at least one dedicated gamekeeper (in Sussex there was one full-time gamekeeper for 400 ha, while in Hertfordshire there was one full-time keeper per 1,000 ha). The Salisbury Plain experiment has shown that, in the absence of habitat management, predation control alone on an area of between 4 and 5 km2 can increase densities to a level allowing some gray partridge shooting (an average of 23% of autumn numbers counted; Tapper et al., 1996), but in that experiment the numbers in the autumn were too low to provide a reasonable revenue and support the employment of a dedicated gamekeeper. Having a shootable surplus of partridges allows some return (both monetarily and socially through shoot days) on a landowner’s dedication to gray partridge conservation and ensures the long–term viability of management. The Hertfordshire demonstration showed that it was possible to recover partridges on modern farmland, but the gamekeeper was funded through the GWCT. Often, in order for private landowners to undertake partridge management on the scale seen in Sussex there needs to be additional motivation beyond conservation and gray partridge shooting provides this. This combination of incentives is what is behind the success of the Sussex project. We note that, although all the habitat and feeding requirements for gray partridge management can be funded by agri-environmental subsidy, the control of predators receives no publicly funded support at this time. On the Sussex area the landowner reports that the price paid for shooting compensates sufficiently—though perhaps not totally—for the costs of the gamekeeping.
The GWCT’s PCS encourages its members across the United Kingdom to do both habitat management and predator control in aid of partridge conservation. It is possible to restore a wild gray partridge shoot on modern farmland based on the results of the two demonstration sites detailed here, the findings from the Salisbury plain predation experiment (Tapper et al., 1996) and the Gray Partridge model (Potts, 1986). This is predicated on providing suitable nesting, chick-rearing and overwinter habitat over 9% of the area of that farmland and undertaking legal, targeted predator control mainly during the gray partridge nesting season. In England the current agri-environmental schemes include the options needed to do this; those farmers and landowners who have been inspired by the demonstrations outlined here can take advantage of the scheme to cover the costs of implementing the necessary habitat improvements. The results described here indicate how useful these options can be in restoring gray partridge numbers, emphasizing the need to include them in the United Kingdom new farm subsidy system post-Brexit.
In Britain, despite a farm subsidy system geared to deliver biodiversity, a body of evidence showing the positive impacts of legal seasonal predator control, and a willingness among land managers to restore a much-loved quarry species, gray partridge recovery still faces an uncertain future. Gray partridge restoration is long-term, requires landowner commitment, needs high levels of skill and management from the team on the ground, and an agriculture with a stable future. Without a Brexit deal we face an uncertain future where the prospect of a farmer taking between 7 and 10% of arable land out of production for conservation could seem foolhardy. Once this uncertainty is addressed, however, the science is in place, results from the demonstration projects reported here show what can be done, and the PARTRIDGE project illustrates how to roll this out across Europe (see below). In 2020 there appears to be an increased interest in a green recovery from the SARS-CoV-2 pandemic1; the approaches used to restore gray partridge numbers on farmland should be part of that recovery.
Expanding the Management Described Here Across Europe
An earlier review of EU–wide agri–environment options showed that AES options similar to those available in England and designed to provide chick- food and nesting habitat, are not commonly available across Europe, with the programs in the newer EU countries particularly lacking them (Keenleyside et al., 2011). The need to expand the uptake of options directed at providing food, nesting and breeding areas across Europe is being addressed by an EU-funded Interreg North Sea Region Project, PARTRIDGE, which has set up 10 paired demonstration and comparison sites (each 500 ha in size) across five countries in northern Europe (Brewin et al., 2020)2. The PARTRIDGE project, begun in 2017 and due to finish in 2023, has set out to increase biodiversity (gray partridge, brown hare, songbirds) by 30% across these areas of arable farmland, using techniques developed for gray partridge restoration—providing nesting, brood-rearing and winter cover and food. Legal lethal predator control is practiced where it is allowed and customary (e.g., England, Scotland), while in other countries (Germany, the Netherlands) non-lethal methods of limiting predation are used to address predator pressure on nesting partridges; sites in Belgium use a mixture of both methods (Brewin et al., 2020). One of the stated aims of the project is to ensure that the measures used to restore gray partridges in each country are funded through that country’s agri-environmental program. The demonstrations established through the PARTRIDGE project will point the way forward for gray partridge conservation (and conservation for other farmland flora and fauna) across Europe.
Author Contributions
NS was the Director of Research at the GWCT and oversees all the research output. NA was our senior Biometrician and has overseen the analysis of the data. JE was responsible for the partridge counting and data compilation. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the landowners, farmers and gamekeepers within the Hertfordshire and Sussex study areas. For over half a century they have welcomed us onto their farms, allowed us to count partridges, collect invertebrates, record arable plants and responded to our requests for information on their management. The fate of gray partridges and other farmland flora and fauna rests in their hands, and in others like them across Europe. The production of this paper was supported by a generous grant from Game Conservancy USA.
Footnotes
- ^ https://www.gov.uk/government/speeches/george-eustice-speech-on-environmental-recovery-20-july-2020
- ^ https://northsearegion.eu/partridge
References
Aebischer, N. J. (1991). Sustainable yields: gamebirds as a harvestable resource. Gibier Faune Sauv. 8, 335–351.
Aebischer, N. J. (2009). “The GWCT grey partridge recovery programme: a species action plan in action,” in Gamebird 2006: Quail VI and Perdix XII: 291-301, eds S. B. Cederbaum, B. C. Faircloth, T. M. Terhune, J. J. Thompson, and J. P. Carroll (Athens, GA: Warnell School of Forestry and Natural Resources).
Aebischer, N. J., and Ewald, J. A. (2010). Grey partridge Perdix perdix in the UK: recovery status, set–aside and shooting. Ibis 152, 530–542. doi: 10.1111/j.1474-919x.2010.01037.x
Aebischer, N. J., and Ewald, J. A. (2012). The grey partridge in the UK: population status, research, policy and prospects. Anim. Biodivers. Conserv. 35, 353–362.
Aebischer, N. J., Green, R. E., and Evans, A. D. (2000). “From science to recovery: four case studies of how research has been translated into conservation action in the UK,” in Ecology and Conservation of Lowland Farmland Birds, eds N. J. Aebischer, A. D. Evans, P. V. Grice, and J. A. Vickery (Tring: British Ornithologists’ Union), 43–54.
Aebischer, N. J., and Reitz, F. (2000). Estimating brood production and chick survival rates of grey partridges: an evaluation. Hungar. Small Game Bull. 5, 191–210.
Albrecht, H., Cambecèdes, J., Lang, M., and Wagner, M. (2016). Management options for the conservation of rare arable plants in Europe. Bot. Lett. 163, 389–415. doi: 10.1080/23818107.2016.1237886
Barnes, R. F. W., and Tapper, S. C. (1985). A method for counting hares by spotlight. J. Zool. 206, 273–276. doi: 10.1111/j.1469-7998.1985.tb05653.x
Bell, J. R., Blumgart, D., and Shortall, C. R. (2020). Are insects declining and at what rate? An analysis of standardised, systematic catches of aphid and moth abundances across Great Britain. Insect Conserv. Divers. 13, 115–126. doi: 10.1111/icad.12412
Boatman, N. D., and Sotherton, N. W. (1988). The agronomic consequences and costs of managing field margins for game and wildlife conservation. Aspects Appl. Biol. 17, 47–56.
Brewin, J., Buner, F., and Ewald, J. (2020). Farming with Nature – Promoting Biodiversity Across Europe Through Partridge Conservation. Fordingbridge: The Game & Wildlife Conservation Trust.
Bro, E. (2016). La Perdrix Grise: Biologie, Écologie, Gestion et Conservation. Mèze: Biotope Editions.
Bro, E., Reitz, F., and Landry, P. (2005). Grey partridge Perdix perdix population status in central northern France: spatial variability in density and 1994–2004 trend. Wildlife Biol. 11, 287–298. doi: 10.2981/0909-6396(2005)11[287:gpppps]2.0.co;2
Buner, F. D., Jenny, M., Zbinden, N., and Naef-Daenzer, B. (2005). Ecologically enhanced areas - a key habitat structure for re-introduced grey partridges Perdix perdix. Biol. Conserv. 124, 373–381. doi: 10.1016/j.biocon.2005.01.043
CBD (2004). Addis Ababa Principles and Guidelines for the Sustainable Use of Biodiversity. CBD Guidelines. Rio de Janeiro: Secretariat of the Convention on Biological Diversity.
DEFRA (2011). UK Biodiversity Indicators in Your Pocket 2011. London: Department for Environment, Food and Rural Affairs.
DEFRA (2019). Wild Bird Populations in the UK, 1970 to 2018. London: Department for Environment, Food and Rural Affairs.
Dietrick, E. J. (1961). An improved backpack motorised fan for suction of insects. J. Econ. Entomol. 54, 394–395. doi: 10.1093/jee/54.2.394
Dover, J. W. (1997). Conservation headlands: effects on butterfly distribution and behaviour. Agricult. Ecosyst. Environ. 63, 31–49. doi: 10.1016/s0167-8809(96)01120-6
Draycott, R. A. H. (2012). Restoration of a sustainable wild grey partridge shoot in eastern England. Anim. Biodivers. Conserv. 35, 381–386.
Eaton, M. A., Aebischer, N. J., Brown, A. F., Hearn, R. D., Lock, L., Musgrove, A. J., et al. (2015). Birds of conservation concern 4: the population status of birds in the UK, Channel Islands and Isle of Man. Br. Birds 108, 708–746.
EUROSTAT (2020). Sustainable Development in the European Union: Monitoring Report on Progress Towards the SDGs in an EU Context, 2020 Edition. Luxembourg: EUROSTAT.
Ewald, J. A., and Aebischer, N. J. (1999). Pesticide Use, Avian Food Resources and Bird Densities in Sussex. JNCC Report No. 296. Peterborough: Joint Nature Conservation Committee.
Ewald, J. A., Aebischer, N. J., Richardson, S. M., Grice, P. V., and Cooke, A. I. (2010). The effect of agri–environment schemes on grey partridges at the farm level in England. Agricult. Ecosyst. Environ. 138, 55–63. doi: 10.1016/j.agee.2010.03.018
Ewald, J. A., Kingdon, N. G., and Santin–Janin, H. (2009). “The GWCT partridge count scheme: a volunteer–based monitoring and conservation promotion scheme,” in Gamebird, 2006, eds S. B. Cederbaum, B. C. Faircloth, T. M. Terhune, J. J. Thompson, and J. P. Carroll (Athens, GA: Warnell School of Forestry), 27–37.
Ewald, J. A., Potts, G. R., and Aebischer, N. J. (2012). Restoration of a wild grey partridge shoot: a major development in the Sussex study, UK. Anim. Biodivers. Conserv. 35, 363–369.
Ewald, J. A., Wheatley, C. J., Aebischer, N. J., Duffield, S. J., and Heaver, D. (2016). Investigation of the Impact of Changes in Pesticide Use on Invertebrate Populations. Natural England Commissioned Report, NECR182. York: Natural England.
Ewald, J. A., Wheatley, C. J., Aebischer, N. J., Moreby, S. J., Duffield, S. J., Crick, H. Q. P., et al. (2015). Influences of extreme weather, climate and pesticide use on invertebrates in cereal fields over 42 years. Glob. Change Biol. 21, 3931–3950. doi: 10.1111/gcb.13026
Faragó, S., Dittrich, G., Horváth–Hangya, K., and Winkler, D. (2012). Twenty years of the grey partridge population in the LAJTA Project (Western Hungary). Anim. Biodivers. Conserv. 35, 311–319.
Frylestam, B. (1981). Studies on the European Hare. XXXVII. Estimating by spotlight the populations density of the European hare. Acta Theriol. 26, 419–427. doi: 10.4098/at.arch.81-35
Gottschalk, E., and Beeke, W. (2014). How can the drastic decline in the grey partridge (Perdix perdix) be stopped? Lessons from ten years of the Grey Partridge Conservation Project in the district of Göttingen. Berichte zum Vogelschutz 51, 95–116.
Green, R. (1984). The feeding ecology and survival of partridge chicks (Alectoris rufa and Perdix perdix) on arable farmland in East Anglia. J. Appl. Ecol. 21, 817–830. doi: 10.2307/2405049
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12:e0185809. doi: 10.1371/journal.pone.0185809
Harvey, J. A., Heinen, R., Armbrecht, I., Basset, Y., Baxter-Gilbert, J. H., Bezemer, T. M., et al. (2020). International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 4, 174–176. doi: 10.1038/s41559-019-1079-8
Holland, J. M., Hutchison, M. A. S., Smith, B., and Aebischer, N. J. (2006). A review of invertebrates and seed bearing plants as food for farmland birds in Europe. Ann. Appl. Biol. 148, 49–71. doi: 10.1111/j.1744-7348.2006.00039.x
Holland, J. M., Storkey, J., Lutman, P. J. W., Birkett, T. C., Simper, J. N., and Aebischer, N. J. (2014). Utilisation of agri-environment scheme habitats to enhance invertebrate ecosystem service providers. Agricult. Ecosyst. Environ. 183, 103–109. doi: 10.1016/j.agee.2013.10.025
Keenleyside, C., Allen, B., Hart, K., Menadue, H., Stefanova, V., Prazan, J., et al. (2011). Delivering Environmental Benefits Through Entry Level Agri–Environment Schemes in the EU. Report Prepared for DG Environment, Project ENV.B.1/ETU/2010/0035. London: Institute for European Environmental Policy.
Knaus, P., Sattler, T., Schmid, H., Strebel, N., and Volet, B. (2020). Zustand der Vogelwelt in der Schweiz: Bericht 2020. Sempach: Schweizerische Vogelwarte.
Kuijper, D. P. J., Oosterveld, E., and Wymenga, E. (2009). Decline and potential recovery of the European grey partridge (Perdix perdix) population – a review. Eur. J. Wildlife Res. 55, 455–463. doi: 10.1007/s10344-009-0311-2
McCullagh, P., and Nelder, J. A. (1989). Generalised Linear Models, 2nd Edn. Boca Raton, FL: Chapman & Hall.
Natural England (2010). Higher Level Stewardship, Environmental Stewardship Handbook, 3rd Edn. Peterborough: Natural England.
Pan European Common Bird Monitoring Scheme [PECBMS] (2020). Trends of Common Birds in Europe, 2020 Update. Prague: European Bird Census Council.
Potts, G. R. (1980). The effects of modern agriculture, nest predation and game management on the population ecology of partridges (Perdix perdix and Alectoris rufa). Adv. Ecol. Res. 11, 1–79. doi: 10.1016/s0065-2504(08)60266-4
Potts, G. R. (2007). “Global biodiversity conservation: We need more managers and better theorists,” in Wildlife Science: Linking Ecological Theory and Management Applications, eds T. E. Fulbright and D. G. Hewitt (Boca Raton, FL: CRC Press), 43–64. doi: 10.1201/9781420007619.ch3
Potts, G. R., and Aebischer, N. J. (1991). “Modelling the population dynamics of the grey partridge: conservation and management,” in Bird Population Studies: Relevance to Conservation and Management, eds C. M. Perrins, J. –D. Lebreton, and G. J. M. Hirons (Oxford: Oxford University Press), 373–390.
Potts, G. R., and Aebischer, N. J. (1995). Population dynamics of the Grey Partridge Perdix perdix 1793–1993: monitoring, modelling and management. Ibis 137, (Suppl. 1), S29–S37.
Potts, G. R., Ewald, J. A., and Aebischer, N. J. (2010). Long-term changes in the flora of the cereal ecosystem on the Sussex Downs, England, focusing on the years 1968-2005. J. Appl. Ecol. 47, 215–226. doi: 10.1111/j.1365-2664.2009.01742.x
Potts, G. R., and Vickerman, G. P. (1974). Studies on the cereal ecosystem. Adv. Ecol. Res. 8, 107–197. doi: 10.1016/s0065-2504(08)60278-0
Sotherton, N. W. (1991). “Conservation headlands: a practical combination of intensive cereal farming and conservation,” in The Ecology of Temperate Cereal Fields, eds L. G. Firbank, N. Carter, J. F. Derbyshire, and G. R. Potts (Oxford: Blackwell Scientific Publications), 373–397.
Sotherton, N. W., Robertson, P. A., and Dowell, S. D. (1993). “Manipulating pesticide use to increase the production of wild game birds in Britain,” in Quail III: National Quail Symposium, eds K. E. Church and T. V. Dailey (Pratt: Kansas Department of Wildlife and Parks), 92–101. doi: 10.2307/1363574
Tapper, S. C., Potts, G. R., and Brockless, M. H. (1996). The effect of an experimental reduction in predation pressure on the breeding success and population density of grey partridges Perdix perdix. J. Appl. Ecol. 33, 965–978. doi: 10.2307/2404678
Thomas, M. B., Wratten, S. D., and Sotherton, N. W. (1991). Creation of island habitats in farmland to manipulate populations of beneficial arthropods – predator densities and emigration. J. Appl. Ecol. 28, 906–917. doi: 10.2307/2404216
UK Steering Group (1995). Biodiversity: The UK Steering Group Report: Action Plans, Vol. 2. London: Her Majesty’s Stationery Office.
Vickery, J. A., Feber, R. E., and Fuller, R. J. (2009). Arable field margins managed for biodiversity conservation: a review of food resource provision for farmland birds. Agricult. Ecosyst. Environ. 133, 1–13. doi: 10.1016/j.agee.2009.05.012
Keywords: farmland birds, biodiversity, sustainable use, Sussex study, agri-environment
Citation: Ewald JA, Sotherton NW and Aebischer NJ (2020) Research Into Practice: Gray Partridge (Perdix perdix) Restoration in Southern England. Front. Ecol. Evol. 8:517500. doi: 10.3389/fevo.2020.517500
Received: 04 December 2019; Accepted: 22 October 2020;
Published: 26 November 2020.
Edited by:
Sumeet Gulati, University of British Columbia, CanadaReviewed by:
András Báldi, Hungarian Academy of Science, HungaryJeremy Wilson, Royal Society for the Protection of Birds (RSPB), United Kingdom
Copyright © 2020 Ewald, Sotherton and Aebischer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie A. Ewald, amV3YWxkQGd3Y3Qub3JnLnVr; N. W. Sotherton, bnNvdGhlcnRvbkBnd2N0Lm9yZy51aw==
 Julie A. Ewald*
Julie A. Ewald* N. W. Sotherton
N. W. Sotherton