- Wildlife Institute of India, Dehradun, India
Geographical isolation can often lead to speciation, and two disconnected populations of the same species living in drastically different bioclimatic regions provide an opportunity to understand the process of speciation. The Woolly wolf is found in the cold-arid, Trans-Himalayan landscape, while the Indian wolf inhabits the semi-arid grasslands of Central India. Both the lineages of wolves from India have generated scientific debate on their taxonomic status in recent years. In this study, we collected data and reviewed published literature to document the ecological and behavioral differences between the Woolly wolf and the Indian wolf. Most studies have used genetic data; hence we discuss variation in spatial ecology, habitat preferences, vocalization, diet diversity and cranial measurements of these two subspecies. The spatial ecology of two lineages was compared from the data on three Woolly and ten Indian wolves tagged with GPS collars. The telemetry data shows that there has been no difference in the day-night movement of Woolly wolves, whereas Indian wolves show significant high displacement during the night. The BBMM method indicated that Woolly wolf home ranges were three times larger than the Indian wolf. The Woolly wolf diet is comprised of 20 different types of food items, whereas the Indian wolf diet consists of 17 types. The Woolly and Indian wolf largely depend upon domestic prey base, i.e., 48.44 and 40.34%, respectively. We found no differences in the howling parameters of these subspecies. Moreover, the Woolly wolf skull was significantly longer and broader than the Indian wolf. Wolves of India are ancient and diverged from the main clade about 200,000–1,000,000 years ago. Their genetic and ecological evolution in different bioclimatic zones has resulted in considerable differences as distinct subspecies. The present study is a step in understanding ecological differences between two important, genetically unique subspecies of wolves.
Introduction
Divergent selection can occur among populations with varying environmental conditions, habitats, climates, resource types and phylogeographic patterns (Schluter, 2000; Dieckmann et al., 2004). The phylogeographic patterns isolate populations that inhabit same geographic range but are unable to interact due to the presence of natural barriers, unsuitable habitat or a combination of these factors that could hinder movement (Avise et al., 1987). The long isolation of closely related populations could eventually lead to speciation by accumulating genetic changes over time. Studying and tracking the differences in species ecology, climatic conditions, and reproductive isolation between populations is essential to understand the formation of incipient species and plan appropriate conservation strategies (Moritz, 1994; Rundell and Price, 2009; Sobel et al., 2010). The mechanism of geographic isolation bringing about divergent evolution is well known in the birds, e.g., Darwin’s finches in the subfamily Geospizinae (Sulloway, 1982) and five sub-species of the masked yellowthroat (Geothlypis aequinoctialis) in Central and South America (Curson et al., 1994). Among mammals too, four subspecies of Cheetah (Acinonyx jubatus) (Kitchener et al., 2017) and three subspecies of snow leopard (Panthera uncia) (Janecka et al., 2018) have evolved in different geographical areas. While speciation is an outcome of long isolation, shorter time-scale could set the populations of the same species on different evolutionary paths. The wolves in British Columbia showed strong genetic differentiation between adjacent populations, explained by habitat discontinuity between the coastal and inland regions (Muñoz-Fuentes et al., 2009).
The gray wolf (Canis lupus), one of the most widely distributed mammals and most studied species across the world (Mech and Boitoni, 2003), has had several populations worldwide that evolved in isolation. The wolves are considered one of the most resilient carnivores that have adapted and succeeded in a wide range of habitats from tundra to the deserts (Mech and Boitoni, 2003). Currently, at least forty-four wolf subspecies are described based on the variations in morphological features, behavioral aspects, and geographical distribution (Wozencraft, 2005). However, the status of many of these subspecies is uncertain and contested among scientists (Busch, 2018), making gray wolf taxonomy highly debatable (Shrotriya et al., 2012; Pilot et al., 2014; Ersmark et al., 2016). Several taxonomic resolutions are pending validation and revision at the species as well as subspecies level. Amid a muddled account of the number of species and sub-species, studies suggested that wolves from the Indian regions could form the oldest lineages (Aggarwal et al., 2003, 2007; Sharma et al., 2004; Shrotriya et al., 2012; Werhahn et al., 2017, 2018; Hennelly et al., 2021).
There are two major wolf populations in India, i.e., Woolly wolf (C. l. chanco) (also known as Tibetan or Himalayan wolf) and Indian wolf (C. l. pallipes). These wolves are supposed to have diverged from the main clade about 800,000 and 400,000 years ago, respectively (Sharma et al., 2004; Aggarwal et al., 2007; Shrotriya et al., 2012; Werhahn et al., 2017; Joshi et al., 2020). However, a recent study suggested a contradicting possibility that the Indian wolf could be basal to the Woolly wolf (Hennelly et al., 2021). The study stated that Indian and Woolly/Tibetan wolves shared the most common ancestors with Holarctic wolves 200,000 and 500,000 years, respectively (Hennelly et al., 2021). These differences in the year of divergence are mainly due to the selection of genes and the method used for the analysis. Isolated evolution of the Woolly wolf corresponds with rapid uplift of the Tibetan plateau and associated habitat modifications (Sun and Liu, 2000), which may have endowed them with the genetic adaptation for the cold and arid landscape of the Trans-Himalayas (Werhahn et al., 2018). The Indian wolf is believed to have evolved during the drier period of the Pleistocene and adapted to survive in the arid zones (Sharma et al., 2004; Singh and Kumara, 2006). After the extinction of Cheetah from India, it became the top carnivore species of the Indian open plains (e.g., semi-arid grasslands, scrublands, and grazing lands). Some of the studies proposed that the wolves of India were not subspecies but qualified as separate species viz. Canis himalayensis and Canis indica (Aggarwal et al., 2007). The debate regarding their taxonomic status is unsettled yet (Alvares et al., 2019). Nonetheless, both wolves could be presumed two distinct lineages (Pocock, 1941) and are geographically isolated, having allopatric distribution in India (Aggarwal et al., 2003). The Woolly wolf is found in the cold-arid zone of the upper Trans-Himalayas of India, covering the state of Himachal Pradesh and in two union territories, Ladakh and Jammu and Kashmir, with sightings recorded from Uttarakhand and Sikkim (Bhattacharya and Sathyakumar, 2010; Habib et al., 2013; Choudhury, 2015). The population estimates for both the lineages are not very accurate. As per the last available estimate, only 350 individuals of the Woolly wolf were found (Fox and Chundawat, 1995) which is a rough estimate for the Woolly wolf as it was assessed for the Ladakh and Spiti regions of India covering an area of 60,000 km2. The Indian wolf is distributed across 13 states of India, primarily in the semi-arid zone with the latest population estimate of 1200–1800 packs (Jhala et al., 2013). Both the wolves inhabit open and grassland habitats and survive primarily outside protected areas. However, the Woolly wolf is functioning in far less anthropogenic pressure compared to the Indian wolf. Wolves in India are majorly found in non-protected areas with low natural prey populations (Habib, 2007; Maurya et al., 2011; Shrotriya et al., 2015; Habib et al., 2021b). Therefore, in most of their range in India, wolves primarily subsist on domestic livestock (Shahi, 1982; Mishra, 1997; Jethva and Jhala, 2004; Habib, 2007; Werhahn et al., 2019; Lyngdoh et al., 2020; Habib et al., 2021b) and are severely persecuted as a consequence. Although both these wolves are protected as Schedule I species under the Indian Wildlife (Protection) Act 1972. In spite of protection, conserving a species that resides in a human-dominated landscape and comes in direct conflict is a challenge.
The two lineages of wolves from India are unique and require focused conservation measures. Since both the lineages have remained isolated for a long period and functioning in two different bioclimatic zones, biological, ecological, and behavioral differences are expected to arise in these two populations. Understanding the differences is important because both subspecies are subject to management actions as they survive in a human-dominated landscape in India. In this study, we provide a comparative ecological perspective on spatial ecology, vocalization, food habits and cranial morphometry of these two lineages. The current study will help plan and implement better conservation and management actions. Moreover, information incorporating genetic analyses, morphology, and behavior using a comparative approach will help understanding key differences between the two lineages.
Materials and Methods
We used primary as well as secondary data to elucidate differences in movement, space use, habitat use, howling characteristics, cranial morphology and diet.
Habitat Preferences, Movement, and Space Use Using Primary Data
A total of 14 individuals (11 Indian wolves and three Woolly wolves) were captured, radio-collared, and monitored from 2015 to 2021 to understand the movement, space use, and habitat preferences of two wolf lineages (Table 1). The Woolly wolves were captured in Himachal Pradesh and Indian wolves in Maharashtra, states of India. These wolves were captured using soft leg-hold traps. The traps (n = 25) were placed in a circular setup (radius = 70–80 cm), placed ∼20 cm apart from each other and tied with each other using rope (Habib, 2007). The wolf gland lure No. 100 (Stanley Hawbaker and Sons, Fort London, Pennsylvania) was used to attract the wolves toward traps. We also used fresh scats collected from different areas considering the scats were of different packs and placed around the traps to attract the wolf. The traps were connected to GSM-based device called “MinkPolice.” This device operates with a magnetic switch and triggers a message on the registered email and phone numbers. Trapped wolves were held using double threaded nylon hockey net. The captured wolves were immobilized by injecting Ketamine–Xylazine (dosages based on the weight of the captured wolf) intramuscularly on their hind leg (Habib, 2007; Habib et al., 2021a). The immobilized wolves were fitted with 10 iridium, 3 Globalstar and 1 VHF collars.
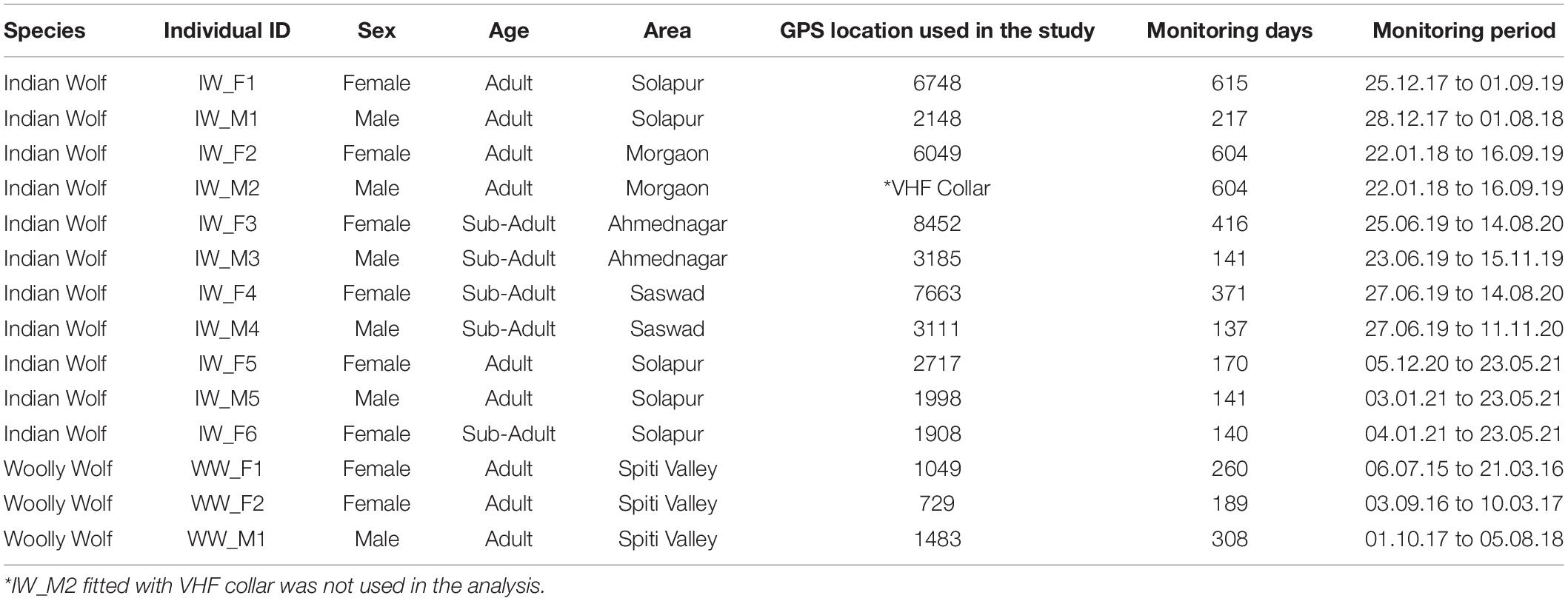
Table 1. Detail of each individual’s characteristics and number of locations used to study the movement, space use and habitat use of wolf in India.
Diet Diversity, Howling Parameters and Cranial Measurements Using Secondary Data
Data on the diet of both the wolf lineages were collected from published sources using specific keyword searches in Google scholar such as Tibetan wolf, Tibetan wolf diet, Himalayan wolf diet, Woolly wolf diet, Canis lupus chanco, Indian wolf diet, Indian gray wolf diet, Canis lupus pallipes. We found seven studies (two from India, three from Nepal, and two from Pakistan) to understand the Woolly wolf. For the Indian wolf diet, we found seven exclusive diet studies from Maharashtra, Gujarat, and Bihar States of India.
To understand the food habits of the Woolly and Indian wolves, we collected published data from different sources (detail provided in Supplementary Table 1). Different studies presented results in various forms, such as absolute frequency and relative frequency. To reduce the effect of study-specific variability, we first calculated the number of food items in the number of scats (Supplementary Tables 2, 3) and then calculated the relative frequency of occurrence (%RFO).
So far, there are two published studies on the howling parameter of the wolves from the Indian sub-continent (Hennelly et al., 2017; Sadhukhan et al., 2019). Hennelly et al. (2017) compared the vocalization of multiple wolf populations across the world, including the Woolly wolf and the Indian wolf and Sadhukhan et al. (2019) characterized the vocalization of the Indian wolves exclusively. Both the studies used nine parameters, such as mean frequency, maximum frequency, minimum frequency, frequency range, and end frequency, to understand the howling behavior of these two lineages. In these studies, the acoustic data were collected in the Trans-Himalayan region for Woolly wolf and Maharashtra for the Indian wolf.
The data on cranial morphometry of 14 Woolly and 20 Indian wolves were obtained from three studies, Allen (1938), Pocock (1941), and Srinivas and Jhala (2021). Data on total length (maximum length of skull from tip of rostrum to the nuchal crest); condyle basal length (distance from posterior projection of the occipital condyles to the anterior edge of pre-maxillary bones); zygomatic width (distance between outermost points of zygomatic arches), mandibular length (the distance from the jaw bone between the lower incisor of the anterior border of condyle); interorbital width (least distance between anterior orbits); post-orbital width (least distance between posterior orbits) and maxillary width (distance between the central fosse of the right and left first maxillary molars), PM4 (Pre-molar 4) and M1 (molar 1) were compared to understand the differences between the two subspecies (Supplementary Figure 1). The details of studies used for comparative ecological perspectives are provided in Supplementary Table 1.
Data Analyses
The movement patterns of wolves were assessed using parameters such as daily average displacement (straight-line distance between two consecutive fixes) and average displacement during day and night. The varying inter-fix intervals were made uniform by post-processing all the data into an hourly data format (Abrahms, 2015; Habib et al., 2021a). Average displacement during the day and night were calculated by classifying locations. Average displacements of both the lineages were compared using the Wilcoxon rank sum test. Home ranges were calculated using minimum convex polygon (MCP) and Brownian bridge movement model (BBMM) (Horne et al., 2007). To understand the habitat preferences of Woolly and Indian wolves the landuse data for the states of Himachal Pradesh and Maharashtra were acquired from Bhuvan’s open-source website (NRSA, 2016).1 Habitat use and preference of the Woolly and Indian wolf lineages were analyzed using Manly’s resource selection function (Manly et al., 2002). The design-III study framework of habitat use, where the animals are identified individually and both the use and the availability are measured for each one, was applied (Thomas and Taylor, 1990). The data on “use” was calculated from the number of GPS fixes and “availability” of land-use categories was calculated within the 100% MCP home range. All the measurements of movement parameters and analyses were carried out using ArcMET tool (Wall, 2014) in ArcGIS 10.6 and package “adehabitatLT,” version 0.3.25 (Calenge, 2015a) and animal movement tool (amt, version 0.1.3) (Signer et al., 2017) in program R 4.0.3 (R Core Team, 2021). Habitat use analysis was performed using the package “adehabitatHS,” version 0.3.15 in the program R, version 4.0.3 (Calenge, 2015b; R Core Team, 2021). For the comparison of cranial measurement of Woolly and Indian wolf we used two sampled unequal variance T-test and hedge’s g test to evaluate the effect size.
We combined the food items consumed in all the studies to represent the overall diet of wolves in the landscape and calculated the relative frequency of occurrence (RO) of each food item. We tabulated the RO of each item (number of occurrences of each prey item in scats/total number of scats × 100). We categorized the food items into wild and domestic prey and also in the body-weight classes (0–10 kg small prey; 11–70 kg medium-sized prey; >70 kg large prey) to compare the food habits. The details of studies used to comprehend the feeding habits of the two subspecies is provided in Supplementary Tables 2, 3.
Results
Spatial Ecological Perspectives Based on the Movement Characteristics and Home Ranges of Woolly and Indian Wolves
A total of 47,240 fixes across 13 individuals (five males and eight females) of the wolves were analyzed (Table 1) to examine daily movement, movement during day and night time, space use, and habitat use. The average hourly displacement of the Indian wolf (527.68 ± 111.52 m/hr) was significantly (W = 29, p = 0.01) higher than the Woolly wolf (338.54 ± 34.82 m/hr). The mean hourly displacement during the day for Indian wolf (393.71 ± 172.43 m/hr) and Woolly wolf (316.70 ± 18.7 m/hr) was not significantly different (W = 12; p = 0.6). However, the mean hourly displacement during nighttime was 53% higher for the Indian wolf than the Woolly wolf and the result was significantly different (W = 28, p = 0.02) (Figure 1).
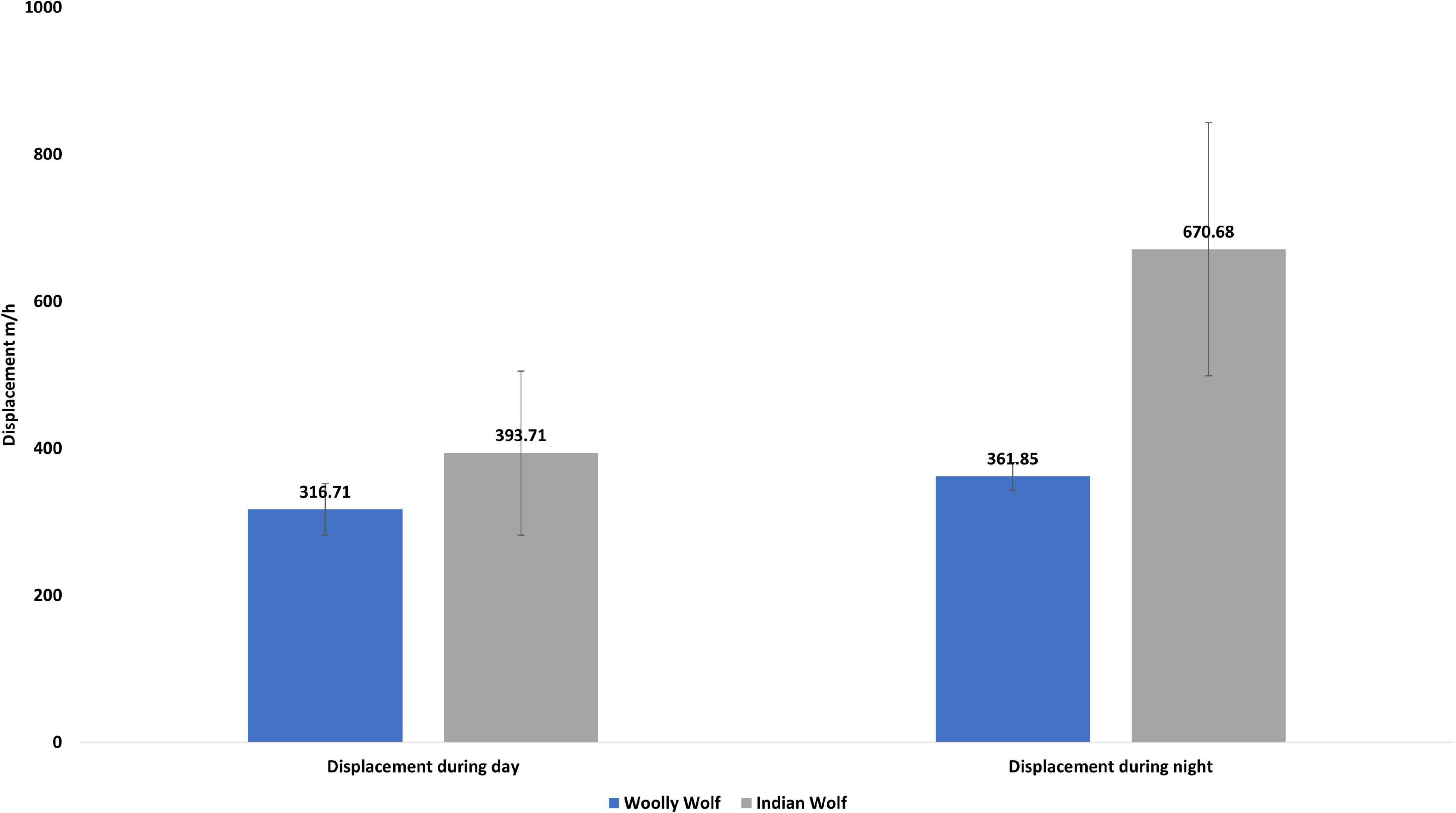
Figure 1. Daily overall displacement, average daytime and nighttime displacement (meter per hour; m/h) of the Woolly and Indian wolf.
We evaluated the home range and core area sizes of both the lineages (Woolly wolf n = 3; Indian wolf n = 10) to compare the space use (Figure 2). The Woolly wolf home range was significantly larger (1689.80 km2, range 827.54–3055.45 km2) than the Indian wolf home range (212.26 km2, range 4.92–981.48 km2) based on 95% MCP (W = 1; p = 0.01). The core area (50% MCP) for the Woolly wolf was also larger (352.71 km2, range 273.18–477.51 km2) than that of the Indian wolf (20.37 km2, range 0.53–51.05 km2) (Figure 3). The 95% BBMM of the Woolly wolf was significantly larger (374.17 km2, range 283.93–422.17 km2) than that of the Indian wolf (130.86 km2, range 4.7- 399.56 km2) (W = 3; p = 0.04). The core area (50% BBMM) for Woolly wolf was also larger (37.74 km2, range 37.74–28.37 km2) than the Indian wolf (13.07 km2, range 0.63–54.43 km2), but values were not statistically significant (W = 4; p = 0.07) (Figure 3).
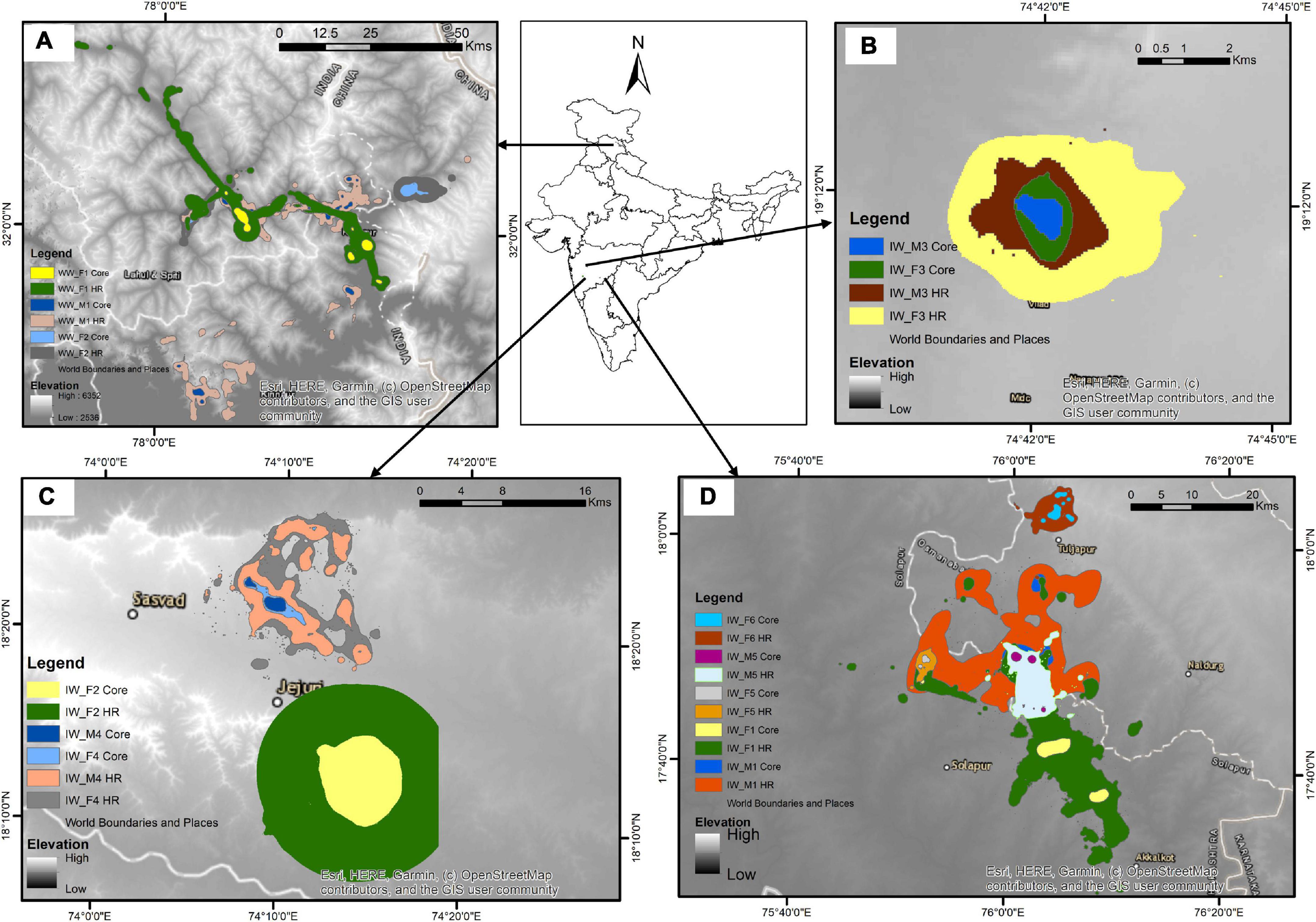
Figure 2. Distribution pattern of the collared individuals [(A) Woolly wolf, (B–D) Indian Wolf] using BBMM (50% contour- core area; 95% contour- home range) from Himachal Pradesh, and Maharashtra, India, respectively (BBMM: Brownian bridge movement model).
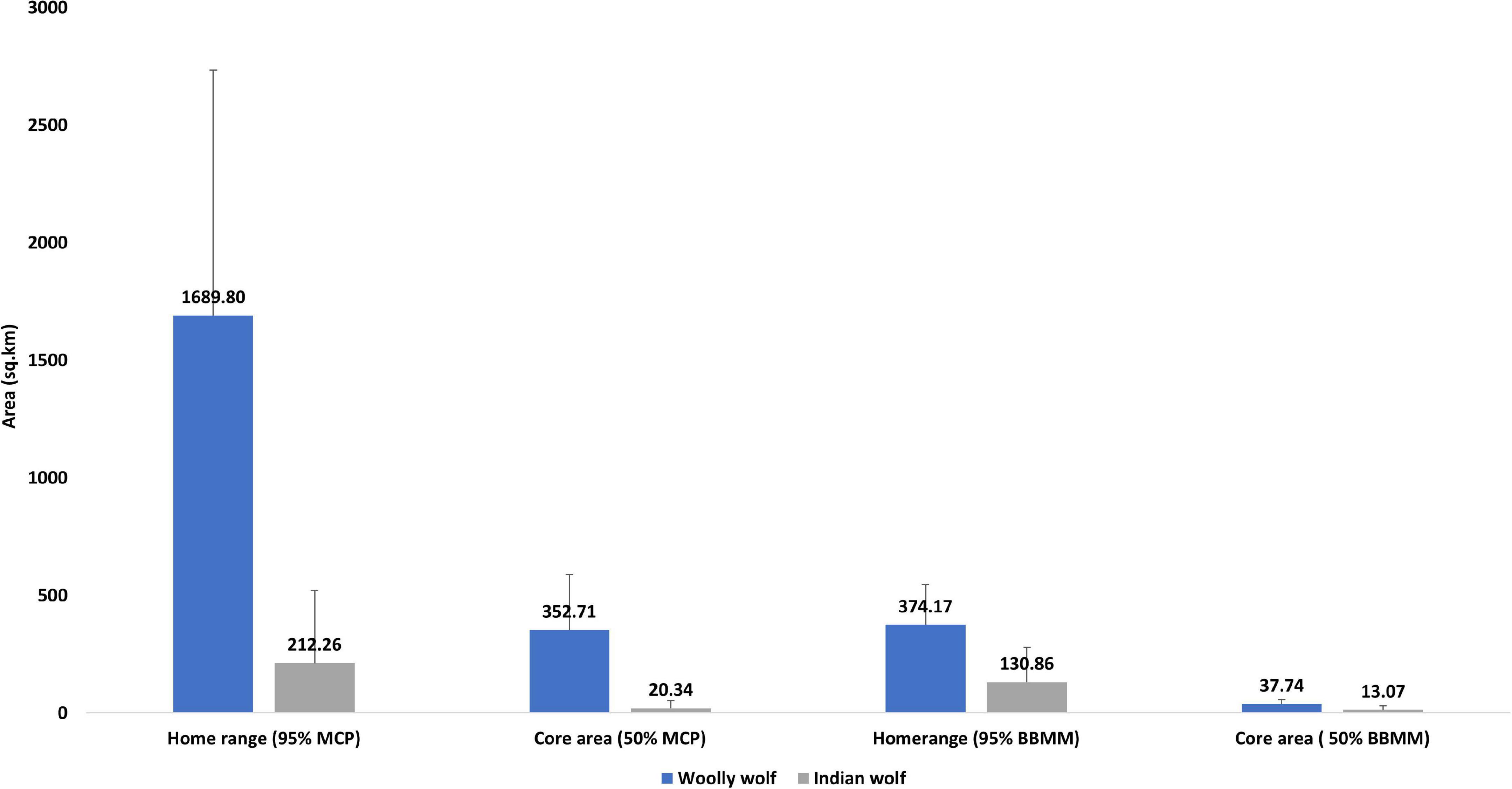
Figure 3. Comparison of the home ranges (95% MCP and BBMM) and core area (50% MCP and BBMM) used by the Woolly and Indian wolf (MCP; minimum convex polygon; BBMM: brownian bridge movement model).
Habitat Ecology Based on the Land Use Preferences of Woolly and Indian Wolves
Resource selection function revealed a preferential selection for landuse categories by the Woolly wolf (Khi2L = 77.26, df = 6, p < 0.001) and the Indian wolf lineages (Khi2L = 520.3487, df = 35, p < 0.001). While riversides, marshes (valley habitat) (Wi = 6.44 ± 3.86SE) and scrub forests (Wi = 2.58 ± 3.39SE) were preferred by the Woolly wolf whereas the Indian wolf preferred grassland areas (Wi = 2.86 ± 0.37SE) and plantations (Wi = 2.71 ± 1.25SE). Builtup and agriculture areas were very less used in proportion to its availability by both the wolves (Figure 4A). In addition, the Woolly wolf and the Indian wolf avoided snow cover and waterbodies, respectively (Figure 4B).
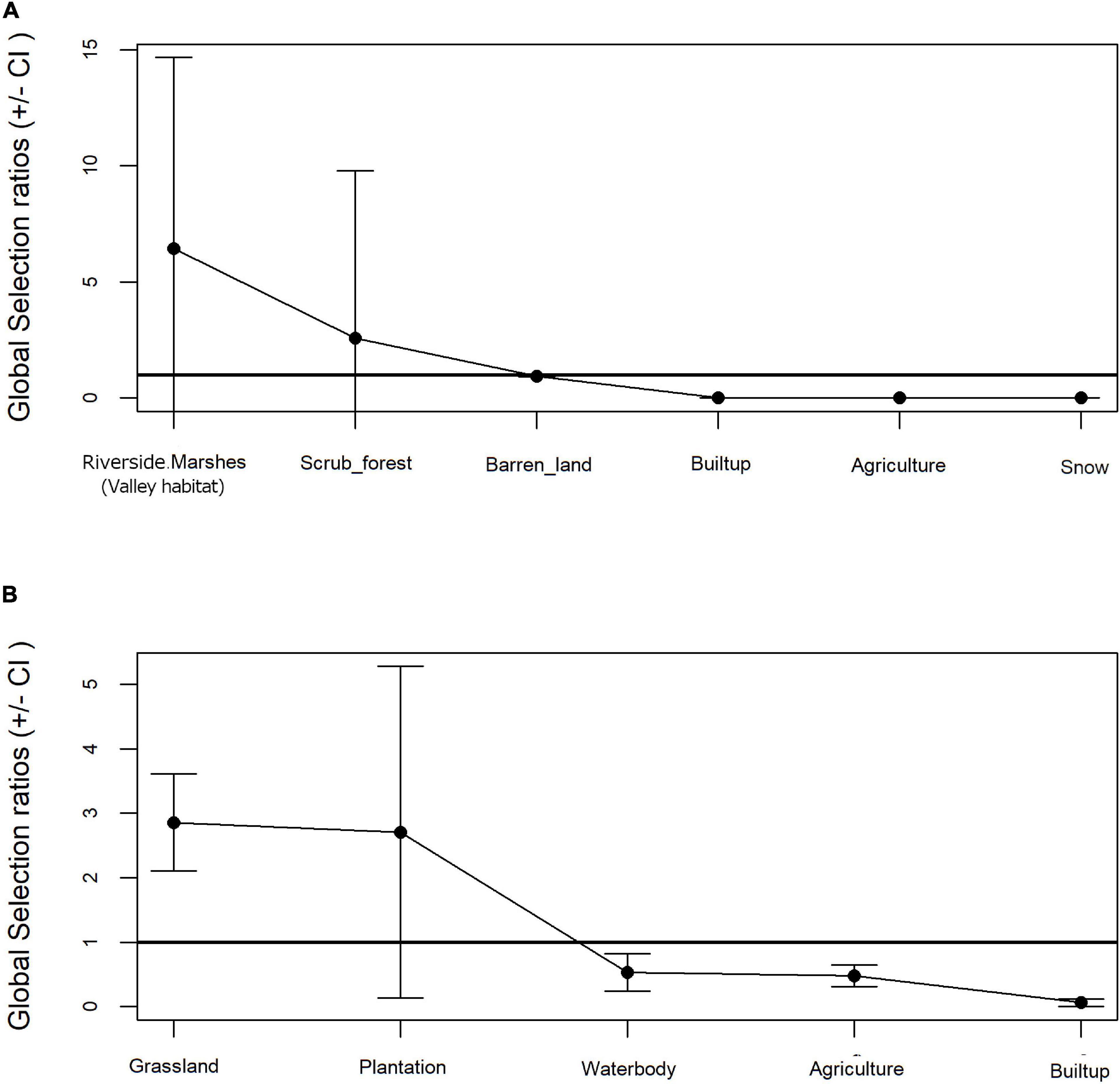
Figure 4. Land use proportion in relation to availability vs. used based on Manly’s test for (A) Woolly wolf and (B) Indian wolf.
Comparison of Howling Parameters of Woolly and Indian Wolves
Hennelly et al. (2017) suggested that the Indian wolf exhibited higher mean frequencies (593.5 Hz ± 211.4SE), wider frequency ranges (197.1 Hz ± 137.4SE), and longer duration (2.71 s ± 2.11SE) based on 117 howl recordings. On the other hand, 301 howls of the Woolly wolf characteristically had a lower mean frequency (428.5 Hz ± 125.7SE), shorter duration (2.56 s ± 1.68SE), and unmodulated frequency variation (101.8 Hz ± 107.1SE) (Table 2). In contrast to Hennelly et al. (2017), the Indian wolf howl parameters reported by Sadhukhan et al. (2019) were broader in range and overlapped with the howling parameters of the Woolly wolf (Table 2). Sadhukhan et al. (2019) reported 422.2 Hz ± 126.40SE mean frequency and longer duration 5.21 s ± 2.49 for the Indian wolf howl (Table 2). Sadhukhan et al. (2019) collected data on 238 howling records of the Indian wolves from Maharashtra, in the same landscape where Hennelly et al. (2017) collected their samples of 117 Indian wolf howls.
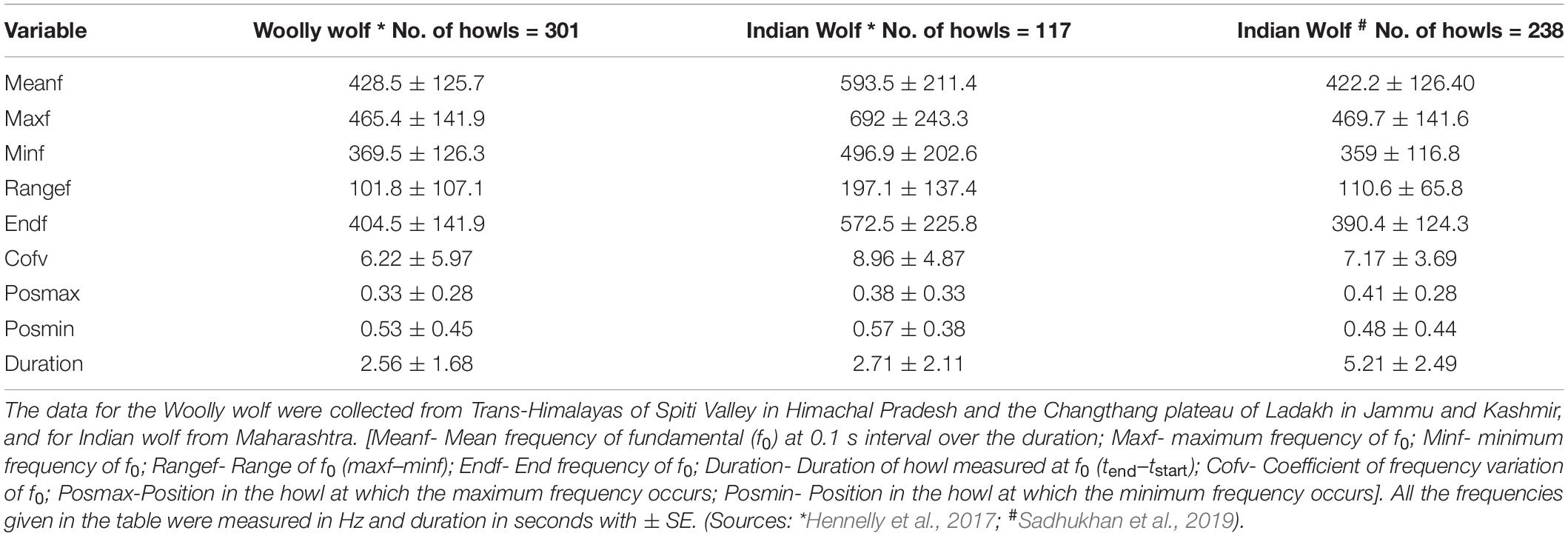
Table 2. Comparison of the vocalization parameters of the Woolly and Indian wolf based on the published literature.
Comparative Food Habits of Woolly and Indian Wolf
We found seven studies exclusive on the diet of Woolly wolf covering the Himalayan region in India, Pakistan and Nepal. There were 20 different items (Supplementary Table 2) reported in Woolly wolf diet from 869 scats with 124.14 (± 190.94 SD) scats per study. The relative frequency of occurrences (RO) in scats for wild prey, domestic prey and vegetative matter were 41.93, 48.44, and 6.46%, respectively. The RO in scats of Woolly wolves for large, medium and small-sized prey were 32.32, 33.87, and 24.18%, respectively (Figure 5A). About 70% of the Woolly diet consisted of goat, marmot, blue sheep, and pika, with a major contribution of cattle.
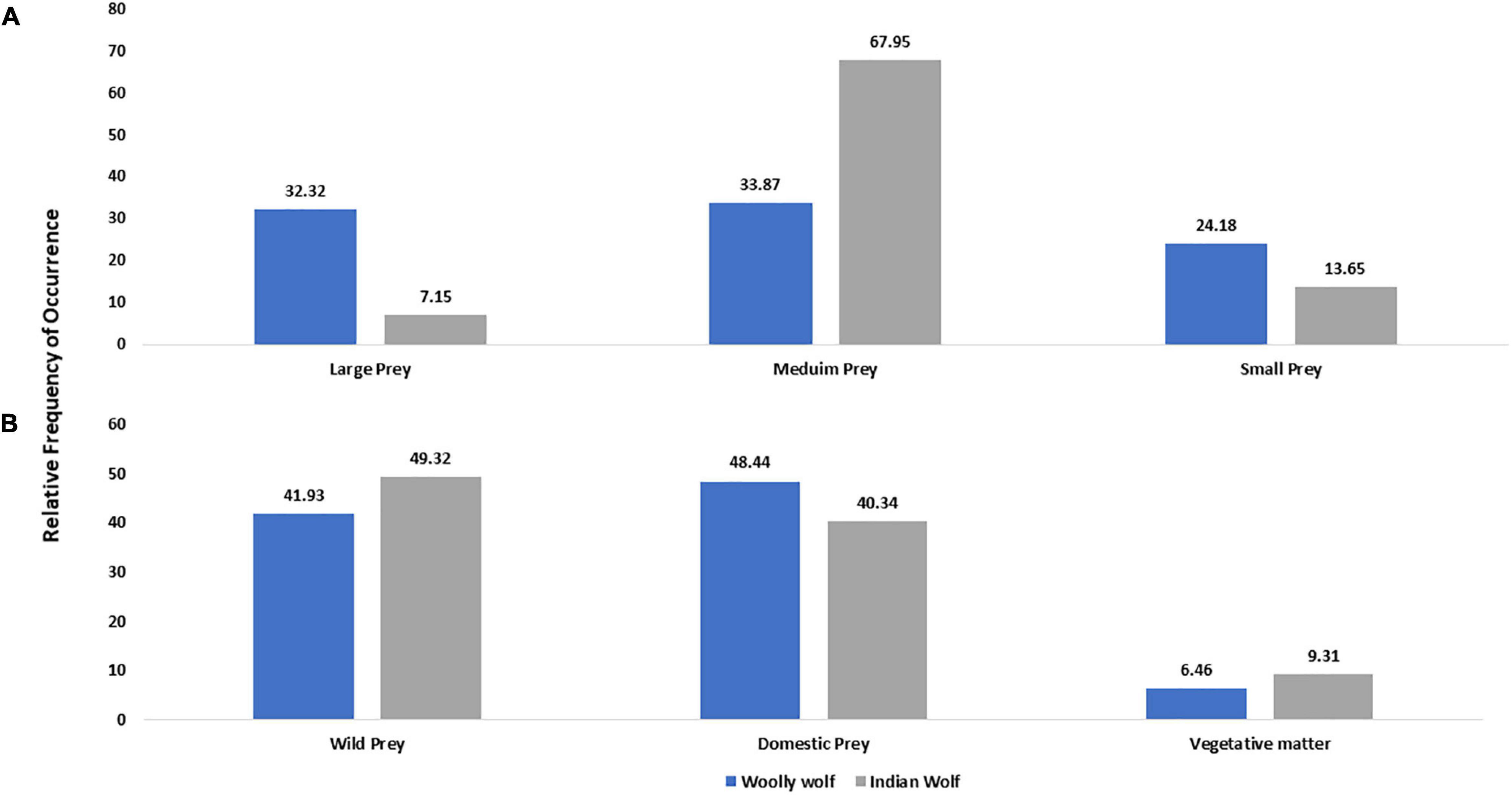
Figure 5. Comparison of the relative frequency of occurrence of different food types of Woolly and Indian wolf; based on (A) prey size (0–10 kg small prey; 11–70 kg medium-sized prey; >70 kg large prey) and (B) prey types in the diet.
Another seven studies exclusively on the diet of Indian wolves from Rajasthan, Bihar and Maharashtra states of India revealed 17 different prey items from 6,877 scats with 982.42 (± 1363.67 SD) scats per study (Supplementary Table 3). The RO in scats for wild prey, domestic prey and vegetative matter was found 49.32, 40.34, and 9.31%, respectively. The Indian wolf diet primarily consisted of 67.95% of medium-sized prey followed by small (13.65%) and large-sized prey (7.15%) (Figure 5B). 75% of food items consisted of blackbuck, goat, sheep and hare in the Indian wolf diet with the major contribution of livestock.
Cranial Measurement of Woolly and Indian Wolves
We re-examined the data and compared the morphological differences between the two sub species. We found a significant difference between the two subspecies in the total length of the skull (p = 0.004; hedge’s g = 2.15), condyle basal length (p = 0.003; hedge’s g = 1.21), zygomatic width (p < 0.001; hedge’s g = 1.31) and mandibular length (p = 0.04; hedge’s g = 1.002) (Figure 6A), interorbital width (p < 0.001; hedge’s g = 1.79), post-orbital width (p < 0.001; hedge’s g = 1.12), maxillary width (p = 0.001; hedge’s g = 1.29), pre-molar4 (p < 0.001; hedge’s g = 1.52), and molar1 (p < 0.001; hedge’s g = 1.65) (Figure 6B).
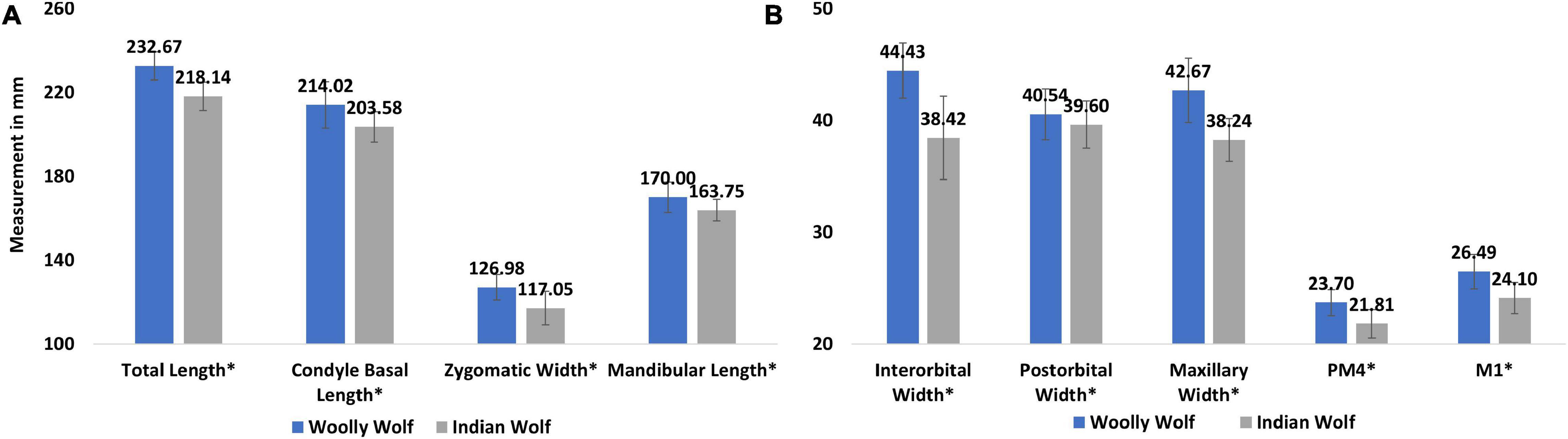
Figure 6. Comparison of different cranial measurement of eight Woolly and eight Indian wolves obtained from Allen (1938), Pocock (1941), and Srinivas and Jhala (2021) (A) measurement of total length, condyle basal length, zygomatic width and mandibular length, (B) measurement of inter and post-orbital, mandibular width, length of pre-molar4 and molar 1. *The difference between the sub species of wolves were found statistically significant.
Discussion
Spatial Ecological Differences Based on Home Range and Core Area Size in Woolly and Indian Wolf
The home range of Woolly wolf (95% MCP) was found eight times larger than the Indian wolf home range. Moreover, the extensive use area within the home range (50% MCP or core area) of the Woolly wolf was 17 times larger than the core area of the Indian wolf home range. The difference between the home ranges of these two lineages narrowed down in home range estimation by the advanced method of BBMM. The 95% isopleth of BBMM and 50% isopleth of BBMM of Woolly wolf were found only three times larger than those of the Indian wolf. The Woolly wolf resides in high-altitude rugged terrain areas with harsh climatic conditions. They usually used gentle slopes and valleys to move from one place to another (Lyngdoh, 2020), while MCP included cliff areas that the wolves could not use due to inaccessibility (Kenward et al., 2001; Silva et al., 2018). Different methods provided different information about home range size (Swihart and Slade, 1985; Gese et al., 1990; Silva et al., 2018). However, the MCP is widely used and can be useful to compare the home ranges of wolves from different studies. The BBMM provides reliable home range estimation, especially for central-place foragers and territorial carnivore species (Börger et al., 2008). This method is also reliable when the animals used several core areas or important travel corridors (Bullard, 1999; Horne et al., 2007; Kie et al., 2010).
Moreover, we also compared the home ranges and core areas of Indian wolf (MCP) with the previous studies and found that the home ranges in the previous studies were smaller (148.49 km2) (Jethva et al., 1997; Habib, 2007). The increase in the Indian wolf home ranges may probably be due to increased human pressure, habitat fragmentation, and prey depletion.
Large home ranges and core areas of the Woolly wolf could result from sparsely distributed prey species (Shrotriya et al., 2015) and suitable habitat continuity (Belongie, 2008). Wolves in mountainous regions selected low elevations, moderate slope and southwest aspect, probably to avoid snow cover and prey availability (Whittington et al., 2005). The home range for Mongolian wolves, which are closely related to the Woolly wolf, were among the largest home ranges (26,619 km2) of the wolves worldwide (Kaczensky et al., 2008). Kaczensky et al. (2008) argue that long-range seasonal shift in the used area could dramatically increase the overall home-ranges. Therefore, the reason of large home range of Woolly wolf may due to the seasonal variation of space use. In contrast, the Indian wolf is surviving in patchy, fragmented habitat within high human-dominated landscape. In such landscapes where wild prey is almost absent, factors such as the type and distribution of food resources, human interference, and topography may have played a significant role in determining home-range size. The human disturbance could also be the reason for having multiple core areas in Indian wolf, as they use small undisturbed patches for resting, mating, and rearing pups (Habib, 2007).
Spatial Ecological Differences Based on Movement Characteristics of Woolly and Indian Wolf
The mean displacement (m/h) of the Indian wolves was 35.2% higher than the Woolly wolves’ daily per hour movement. The daytime and nighttime displacement of the Indian wolves was 19.5 and 46.0% higher than the displacement of the Woolly wolf, respectively. The wolf is a widely distributed carnivore and its survival tactics involve a fine-tuned adaptation to local conditions. Their movement decisions are often the best functional compromise between finding food and avoiding humans (Ciucci et al., 1997). Some studies suggested that the wolf was primarily nocturnal in some areas, which allowed it to visit and move in intensive human-use areas without confrontation (Ciucci et al., 1997; Theurkauf et al., 2007). The Woolly wolf showed no difference between the day and night movement. In contrast, the Indian wolf movement aligned with the previous studies as their nighttime movement was almost twice the daytime movement. The no difference in the day night movement pattern of the Woolly wolf agreed with the findings of Vilà et al. (1995), who found that the wolf in Spain tended toward bimodal activity where nocturnality developed to cope up with human presence. Some studies suggested that wolves were most active during the night in summer (Fancy and Ballard, 1995) and during the daytime in winter (Mech, 1970).
As discussed above, the Woolly wolf home range was much larger than that of the Indian wolf. The functioning of the Woolly wolf in large home ranges but having less daily movement might be due to their use of valleys which makes the home range more linear, less compact, and larger. The higher movement of the Indian wolf in our study is in line with Daan (1981), who suggested a temporal shift in movement due to human disturbance. The other reason might be habitat fragmentation as the Indian wolves occupied fragmented areas, and the Woolly wolf inhibited continuous habitat. The results showed that the Indian wolf holds multiple core areas (Figure 2). Therefore, they need to travel more to find a secure area for resting and mating, as also supported by Ciucci et al., 1997. stated that the wolf activity mostly involved daily round-trip traveling from retreat areas to the core area of home ranges for some sort of social activity, which was also observed in previous studies (Carbyn, 1974).
Habitat Use and Preferences of Woolly and Indian Wolf
Wolves are known as grassland and openland species (Pocock, 1941; Mech, 1970). While the Indian wolves clearly showed a preference toward the grasslands and used the grassland patches more than its availability, the Woolly wolves did not show much preference toward grassland and openland (included in the barren land category) and used it according to the availability. This is due to the high availability of openland in continuity in the Trans-Himalayan region. The Woolly wolf showed an affinity to the riverside and marshes (valley habitat), which is energetically economic travel routes along with the refuge areas. Moreover, these areas are often also more sheltered from the harsh conditions inclusing cold wind. Avoidance of waterbodies by the Indian wolf could be better explained by the association of water presence with the proximity of humans in the peninsular Indian landscape. Both the wolves showed avoidance toward human-influenced areas such as agriculture and builtup more strongly so in the case of the Indian wolf. Paquet et al. (1996) found that mountain wolves did not have many options to avoid humans due to the selection of valley habitats which is often used by humans. The Indian wolf also faces higher human pressure than the Trans-Himalayan population because of the higher human population density in plains than in the Trans-Himalayan region (Mishra et al., 2009).
Differences in Howling Parameters of Woolly and Indian Wolf
Based on published literature, we compared the magnitude and pattern of howl acoustic structure to evaluate whether the long-range vocalizations showed acoustic differences between two wolf lineages in India. It is well understood that howl characteristics vary among different wolf subspecies (Kershenbaum et al., 2016). Due to the unique features of high amplitude and low frequency, a howl can travel for six kilometers or more and can be used to identify individuals for population estimation (Sadhukhan et al., 2021). Hennelly et al. (2017), compared the differences in the howls of the Himalayan wolf (aka Woolly wolf), Indian wolf, North African wolf (now African golden jackal; Sarabia et al., 2021), and Holarctic clades. The study found that small wolf subspecies, North African, Indian, and Israeli wolf had higher mean frequencies than the large wolf subspecies. Hence the body size could affect acoustic parameters.
In contrast, the study by Sadhukhan et al. (2019) reported lower mean frequencies for the Indian wolf. The difference between the Woolly wolf and the Indian wolf howls in Hennelly et al. (2017) could be an artifact of sub-sampling bias as Hennelly et al., 2017 used 117 howl recordings. In contrast, Sadhukhan et al., 2019 used 238 howl recordings. Although both the studies sampled in the state of Maharashtra, yet their sampling years and locations varied. Both the studies sampled only a section of the Indian wolf population rendering the Indian wolf with a larger variation in mean frequencies overall.
Further, earlier studies have shown the similarities between the howls of various canid species and subspecies. Canis rufus showed similar howl type as coyotes Canis latrans, while European wolf (C. l. lupus) and Iberian wolf (C. l. signatus) howls were similar in signatures (Kershenbaum et al., 2016). The Indian wolf, Mackenzie Valley wolf (C. l. occidentalis) and Mexican wolf (C. l. baileyi) also showed similar howling signatures (Kershenbaum et al., 2016). The similarities in acoustic signatures between the two lineages may advocate the evolutionary history playing a role in howling behavior (Kershenbaum et al., 2016). We also second that canid howling is not an arbitrary signal but possesses species-specific information, reflecting that the adaptive processes of isolation or habitat features play a key role in howling behavior (Hennelly et al., 2017). Habitat and temporal variability might be the reason for different outcomes from the two studies. More studies are recommended to understand the differences in the howling parameters of these two lineages.
Differences in the Food Preferences of Woolly and Indian Wolf
Wolves are pack hunters and are known to feed on a variety of different food items. They choose their prey based on availability, abundance, pack stability, season, and habitat accessibility in the human-dominated landscapes (Imbert et al., 2016; Lyngdoh et al., 2020). Our study revealed that the diet of Woolly wolf from the Himalayan region consisted of 20 different food items from small birds, reptiles to large mammals and domestic animals such as cattle and yak. A review study on the Woolly wolf dietary spectrum across their global distribution, including Tibet and China, reported 39 different food items (Lyngdoh et al., 2020). The energy requirement of large carnivores makes them prone to conflict with humans as they need a large prey base (Carbone et al., 1999; Mech and Boitoni, 2003). The Woolly wolf also consumed a sufficient proportion of the large-sized prey (32.32%) and medium-sized prey (33.87%) with a considerable quantity of small prey (24.18%) consumption in their diet (Figure 5). Various studies across the wolf distribution range confirm that large prey forms the major part of the wolf diet (Imbert et al., 2016; Mengulluoglu et al., 2019; Petridou et al., 2019; Sin et al., 2019). The dependency on small prey might be because of the scarcity of the large and medium-sized animals to avoid interactions with humans or to gain and fulfill energy requirements in the harsh climatic condition. The Woolly wolf is also heavily dependent upon the domestic prey items in the Himalayan region. Livestock (Yak, Dzo cow, Goat, and Sheep) were the most consumed mammals in the Woolly wolf diet compared to sparsely distributed wild prey. Hence, it is not surprising that Woolly wolf showed a marginal preference for domestic prey than wild prey. Many studies from the Trans-Himalayas accounted that low abundance of wild prey and poor management of livestock resulted in depredation by snow leopard and Woolly wolf (Jackson and Ahlborn, 1984; Mishra, 1997; Namgail et al., 2007; Anwar et al., 2012; Suryawanshi et al., 2013; Chetri et al., 2017).
Despite the Woolly wolf’s significant domestic animal consumption, they are being tolerated by the locals in some regions (Rangarajan, 2001; Lyngdoh et al., 2020). At the same time, they are persecuted in many areas for the same reason (Mishra, 1997). Apart from domestic animal consumption, major wild prey species were Asiatic ibex (Capra sibirica), Urial (Ovis vignei), Tibetan wild ass (Equus kiang), Tibetan argali (Ovis ammon hodgsoni) and Blue sheep (Pseudois navaur) (Anwar et al., 2012; Subba, 2012; Ahmed et al., 2017; Bocci et al., 2017; Chetri et al., 2017; Werhahn et al., 2019; Lyngdoh et al., 2020; Habib et al., 2021b). However, their combined contribution was as low as 16.77% of the total food items. It could be related to the small population size and sparse distribution of the ungulate species in the region (Shrotriya et al., 2015).
The Indian wolf also primarily preyed upon domestic animals, followed by wild prey items. The Indian wolf consumed a considerable amount of vegetative material (fruits and plant items, etc.), which was absent in the Woolly wolf diet. The absence of vegetative matter in the diet of the Woolly wolf may be due to the scarcity of wild fruiting plants in the Trans-Himalayan region or missed reporting in the studies due to difficulties in identification in the studies we reviewed in this study. The Indian wolf primarily preyed upon medium-sized mammals relating to the availability of blackbuck, chinkara, and especially livestock such as sheep and goat. The Indian wolf-bearing states (Rajasthan, Andhra Pradesh, Maharashtra, Telangana) have the highest goat and sheep population (20th Livestock Census: All India Report, 2019). The average pack size of the Indian wolf varied from 1.5 to 4.7 individuals in the breeding and non-breeding season (Kumar, 1998). The preference toward medium-sized prey could be due to the smaller pack size and body size of the Indian wolf. Small packs would require less food and find it difficult to prey upon large prey species. The studies used to understand the food habits were from different protection regimes, such as the protected area of Velvadar Blackbuck Sanctuary (Jhala, 1991) and the human-dominated landscape of Maharashtra (Habib, 2007). The diet of the wolves from Velvadar Blackbuck Sanctuary was dominantly comprised of wild prey base (91.8%), whereas Maharashtra wolves’ diet was dominated by domestic prey (47.8%). This shows that the wolf may prefer wild ungulates over domestic prey, depending upon the availability. Consequences of human-wolf conflict due to low prey abundance and unavailability can hinder conservation measures. Therefore, it is essential to address prey restoration and livestock security to reduce conflict and achieve better conservation management for wolves in the Himalayas and in the plains of India.
Cranial Morphometric Differences Between Woolly and Indian Wolf
The Woolly wolf exists at 3,900–5,600 m elevation across the low-oxygen region of the Himalayas (Habib et al., 2013; Werhahn et al., 2018). Survival in such a landscape requires the Woolly wolf to face metabolic challenges such as severe oxidative stress and increased metabolic rates (Beall, 2007; Hassanin et al., 2009). Several studies have conducted a genetic analysis of the Woolly wolf, identified the genes facilitating their adaptation to cope with hypoxia (Zhang et al., 2014; Werhahn et al., 2018; Wang et al., 2020). However, their morphological adaptations against hypoxic conditions have not been paid much attention yet. Butaric and Klocke (2018) studied the adaptation of upper respiratory structures to hypoxic and cold dry air in humans occupying high and low altitudes. These adaptations in skull structures help in increased uptake and air conditioning processes. The Woolly wolf exhibited longer (total length) and broader (post and inter-orbital width) skulls than the Indian wolf. Hence, their skull size and structure could be an adaptation to meet respiratory demand of more oxygen (Butaric and Klocke, 2018). However, the wolves are known to conform Bergmann’s rule, that is the wolves in the northern latitude are generally larger in body size (Meiri et al., 2007).
Taxonomic Dilemma
The systematics of wolves from the Indian subcontinent is less studied, remains controversial and confusing regarding their taxonomic status as sub-species and species. Indian wolves gathered the interest of the scientific community for their unique evolutionary history and uncertain taxonomic status. Although the Indian wolf was first described by Hodgson (1847), a consensus on its nomenclature is yet to be reached after 175 years with several attempts made to clear their taxonomy (Aggarwal et al., 2003; Sharma et al., 2004; Werhahn et al., 2017; Alvares et al., 2019; Joshi et al., 2020; Wang et al., 2020, 2021). Hodgson (1847) described the Himalayan wolf as separate species, C. laniger, noting its appearances. Later, Blanford (1888) rejected Hodgson’s proposal and combined C. laniger with C. lupus, and elevated the Indian wolf to C. pallipes. However, after 50 years, Pocock (1941) described both the taxa as subspecies of C. lupus. In Pocock’s scheme, C. pallipes became C. l. pallipes, and C. laniger merged with C. l. chanco. Later on, more studies suggested the uniqueness of these two taxa and identified them as the oldest lineage of wolves but did not reach any conclusion (Sharma et al., 2004; Aggarwal et al., 2007; Werhahn et al., 2017). A recently conducted study of the Woolly wolf by Joshi et al., 2020 found no evidence for C. l. chanco to be distinct species. They suggested the acceptance of Woolly wolf as C. l. chanco and not as C. langier or C. himalayensis. Their findings were additionally supported by Wang et al. (2020), who found that wolves across the Himalayas and Tibetan plateau are closely related. The Woolly wolf adapted to survive in a low oxygen environment and the Indian wolf represents two of the most endangered wolf populations (Joshi et al., 2020; Hennelly et al., 2021; Wang et al., 2021). Therefore, the wolves from India have been identified as unique and qualified as an important population and proposed as Evolutionary Significant Units (ESUs) due to their distinct evolution and adaptation (Hennelly et al., 2021).
Conclusion
Identifying the ecological and behavioral differences in closely related species provides understanding in the evolutionary process of speciation and helps identify a species or subspecies (Arnegard et al., 2010; Ramasindrazana et al., 2011). The literature clearly highlights the uniqueness of these two wolves from India (Sharma et al., 2004; Aggarwal et al., 2007; Shrotriya et al., 2012; Joshi et al., 2020). The genetic study strongly suggested that both the subspecies were distinct and the most ancient lineages. These lineages did not show any genetic admixture or geographic overlap with other wolves from rest of the world (Aggarwal et al., 2003, 2007; Sharma et al., 2004). Geographical isolation and differential habitat selection of closely related adjacent wolf populations is the leading mechanism of the evolution of genetically and ecologically different subspecies (Leonard, 2014), for example, Mexican wolf, North American wolf, Italian, Iberian and Scandinavian wolf (Wayne et al., 1991; Vilà et al., 1999, 2003; Lucchini et al., 2004). The two geographically non-overlapping wolf subspecies from India also showed genetic as well as ecological differences exhibiting their evolutionary divergence. In this study, we found a clear difference in the spatial ecology of both the wolves.
Wolves are considered a typical grassland species in Asia and Europe (Mech, 1970). Both the lineages in India primarily choose grassland or openlands. The Woolly wolf lives in a landscape with vast openlands, hence the habitat type did not show up in preferential analysis. Plantations by the Indian wolf and marshes/riversides and shrubs by the Woolly wolf were preferred as potential refuge. The wolves are known to live in variety of habitats and are considered ecological tolerant animals (Jedrzejewski et al., 2004). The habitat use by wolves can be influenced by many factors, e.g., habitat type, prey availability and anthropogenic pressure (Meriggi et al., 1996; Ciucci and Boitoni, 1998; Mech and Boitoni, 2003). We postulate that the ecological differences between Woolly wolf and Indian wolf could be mainly due to their functioning in entirely different habitats. The home range of the Woolly wolf was significantly larger than that of the Indian wolf. The differences in home range sizes and movement patterns could be because of the Trans-Himalayan region having low prey base, less anthropogenic pressure and connected suitable patches compared to the Peninsular India having disturbed landscape with patchily distributed suitable habitats. However, to understand the ranging pattern and habitat use of Woolly wolf, we used only three individual data collared from Spiti region of trans-Himalayas. More data from different regions could help us better understanding of wolf ecology as the space use may vary with different regions based on prey base and habitat/landscape characteristics. There was no significant difference in the daytime and nighttime movement of the Woolly wolf, whereas the Indian wolf traversed more during the nighttime. Variation in the level of human disturbance could be the reason for Indian wolf being more nocturnal than Woolly wolf.
Both the lineages had significant differences in their cranial measurement, and we found that the Woolly wolf has longer and broader skulls than the Indian wolf. Moreover, the data presented in two studies on the vocalization characteristics were conflicting and did not show a clear picture of the difference. The canid howling may vary species-wise or depend upon the environment, still it possesses species-specific information which may reflect adaptive and or neutral processes of isolation (Kershenbaum et al., 2016). The studies used in this review showed contrasting results. Hennelly et al. (2017) suggested a clear difference but the results of Sadhukhan et al., 2019 suggested that howling parameters of both the lineages overlapped. The differences in their feeding ecology occurred primarily due to the availability of landscape-specific prey species. Both the wolves of India depended on the livestock for more than 50% of their diet. The wolves across the world have been reported to feed on a wide variety of food items from animal matter to vegetative matter. Their main prey in most of the areas are large and medium-sized prey depending upon the availability (Jhala, 1991; Meriggi et al., 1996; Jethva and Jhala, 2004; Chavez and Gese, 2005; Stahler and Smith, 2006; Habib, 2007; Hosseini-Zavarei et al., 2013; Newsome et al., 2016). Nevertheless, since these populations are geographically isolated, genetic, morphological data and ecological requirements suggest apparent distinctiveness. The Indian and Woolly/Tibetan wolves shared the most common ancestors with Holarctic wolves 0.2 mya (0.17–0.3 mya) and 0.5 mya (0.38–0.64 mya), respectively (Hennelly et al., 2021). These two lineages diverged and become incipient species with genetic distinctiveness a long time ago. Their geographic isolation is persistent and would facilitate the behavioral and ecological changes to intensify over time. The wolves of Asia are paid less academic attention compared to their counterparts in Europe and America. This study sheds light on the ecological and behavioral differences of the two oldest wolf lineages of the world found in India. We further suggest detailed morphological analysis and further studies should be conducted to understand the in-depth differences in ecological requirements of the subspecies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Wildlife Institute of India, Animal Ethics Committee.
Author Contributions
SK collected data, performed analysis, reviewed all the potential manuscript and reports that were included in the meta-analysis, and drafted the manuscript. SL and SoS collected data. ShS guided in analysis. BH, ShS, SG, and SL reviewed the manuscript. BH conceptualized and acquired funding of the study. All authors contributed to the article and approved the final version of the manuscript.
Funding
Department of Science and Technology (DST) Govt. of India, Mohd Bin Zayed Species Conservation Grant, WII and Grant in Aid are duly acknowledged for providing funding for the projects in respected areas.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to the Ministry of Environment, Forest and Climate Change (MoEFCC), Himachal Pradesh and Maharashtra Forest Department for the permissions to collar animals and conduct research. Department of Science and Technology (DST) Govt. of India, MBZ Species Conservation Grant, WII Grant in Aid and MFD are duly acknowledged for funding provided to carry out the research. We are also grateful to the Field Directors, DFOs, RFOs, and especially our field assistants for providing help to track collared animal. We thank the Director, Dean, and Research Coordinator of the Wildlife Institute of India for supporting the study. We thank Zehidul Hussain and Hussain Saifi Reshamwala for their valuable comments and suggestions to improve the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.775612/full#supplementary-material
Footnotes
References
20th Livestock Census: All India Report (2019). Ministry of Agricultural Department of Animal Husbandry. Krishi Bhawan: Dairying and Fisheries.
Abrahms, B. (2015). The ecology and conservation of animal movement in changing land and seascapes. [Ph.D. Thesis]. California: University of California.
Aggarwal, R. K., Kivisild, T., Ramadevi, J., and Singh, L. (2007). Mitochondrial DNA coding region sequences support the phylogenetic distinction of two Indian wolf species. J. Zool. Syst. Evol. Res. 45, 163–172. doi: 10.1111/j.1439-0469.2006.00400.x
Aggarwal, R. K., Ramadevi, J., and Singh, L. (2003). Ancient origin and evolution of the Indian wolf: evidence from mitochondrial DNA typing of wolves from Trans-Himalayan region and Pennisular India. Genome Biol. 4, 2–16. doi: 10.1186/gb-2003-4-6-p6
Ahmed, T., Khan, A., and Chandan, P. (2017). Dietary spectrum of two sympatric canid species in Ladakh, India. Proc. Zool. Soc. 71, 320–326. doi: 10.1007/s12595-017-0212-4
Allen, G. M. (1938). Zoological results of the second dolan expidition to western china and eastern tibet, 1934-1936. Part III: Mammals. Proc. Acad, Nat. Sci. Philadel. 90, 261–294. doi: 10.5962/bhl.title.123689
Alvares, F., Sillero-Zubiri, C., Jhala, Y. V., Viranta, S., Koepfli, K.-P., Godinho, R., et al. (2019). Old World Canis spp. with taxonomic ambiguity. Workshop conclusions and recommendations. Vairão
Anwar, M. B., Nadeem, M. S., Shah, S. I., Kiayani, A. R., and Mushtaq, M. (2012). A note on the diet of Indian wolf. Pakistan. Pak. J. Zool. 2012, 588–591.
Arnegard, M. E., Zwiskl, D. J., Lu, Y., and Zakon, H. H. (2010). Old gene duplication facilitates origin and diversification of an innovative communication system- twice. PNAS 107, 22172–22177. doi: 10.1073/pnas.1011803107
Avise, J. C., Arnold, J., Ball, R. M., Bermingham, E., Lamb, T., Neigel, J. E., et al. (1987). Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 18, 489–522. doi: 10.1146/annurev.ecolsys.18.1.489
Beall, C. M. (2007). Two routes to functional adaptation: Tibetan and andean high-altitude natives. PNAS 104, 8655–8660. doi: 10.17226/11790
Belongie, C. C. (2008). Using GIS to create a gray wolf habitat suitability model and to assess wolf pack ranges in the western upper peninsula of michigan. Resour. Anal. 10:15.
Bhattacharya, T., and Sathyakumar, S. (2010). Sighting of Tibetan Wolf. J. Threat. Taxa. 2010, 1345–1348. doi: 10.11609/jott.o2423.1345-8
Blanford, W. T. (1888). Fauna of British India inculding Ceylon and Burma. London: Thacker, Spink & Co.
Bocci, A., Lovari, S., Khan, M. Z., and Mori, E. (2017). Sympatric snow leopards and Tibetan wolves: coexistence of large carnivores with human-driven potential competition. Eur. J. Wildl. Res 63:1151. doi: 10.1007/s10344-017-1151-0
Börger, L., Dalziel, B. D., and Fryxell, J. M. (2008). Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 11, 637–650. doi: 10.1111/j.1461-0248.2008.01182.x
Bullard, F. (1999). Estimating the home range of an animal: a Brownian bridge approach. [Master Thesis]. Maryland: Johns Hopkins University.
Busch, R. (2018). Wolf Almanac: A Celebration Of Wolves And Their World. Maryland: Rowman & Littlefield publisher.
Butaric, L. N., and Klocke, R. P. (2018). Nasal variation in relation to high-altitude adaptations among Tibetans and Andeans. Am. J. Hum. Biol. 30:23104. doi: 10.1002/ajhb.23104
Calenge, C. (2015a). Analysis of animal movements in R: the adehabitatLT package. Auffargis: Office national de la chasse et de la faune sauvage. 1–82.
Calenge, C. (2015b). adehabitatHS: analysis of habitat selection by animals. R Packag. version 0.3.12.
Carbone, C., Mace, G. M., Roberts, S. C., and Macdonald, D. W. (1999). Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288. doi: 10.1038/46266
Carbyn, L. N. (1974). Wolf population fluctuations in Jasper National Park. Alberta Can. Biol. Conserv. 6, 94–101. doi: 10.1016/0006-3207(74)90020-2
Chavez, A. S., and Gese, E. M. (2005). Food habits of wolves in relation to livestock depredations in northwestern Minnesota. Am. Midl. Nat. 154, 253–263. doi: 10.1674/0003-00312005154
Chetri, M., Odden, M., and Wegge, P. (2017). Snow leopard and himalayan Wolf: Food habits and prey selection in the central Himalayas. Nepal. PLoS One 12:549. doi: 10.1371/journal.pone.0170549
Choudhury, A. (2015). The tibetan wolf. India. J. Threat. Taxa. 2015, 7475–7476. doi: 10.11609/jott.o4206.7475-6
Ciucci, P., and Boitoni, L. (1998). Wolf and dog depredationo livestock in Central Italy. Wildlife Soc. Bull. 26, 504–514. doi: 10.1016/j.fsigen.2012.11.001
Ciucci, P., Boitani, L., Francisci, F., and Andreoli, G. (1997). Home range, activity and movements of a wolf pack in central Italy. J. Zool. 243, 803–819. doi: 10.1111/j.1469-7998.1997.tb01977.x
Curson, J., Quinn, D., and Beadle, D. (1994). Warblers of the Americas: an identification guide. California: Houghton Mifflin Harcourt.
Daan, S. (1981). “Adaptive daily strategies in behavior,” in Biological rhythms, ed. J. Aschoff (Boston: Springer), 275–298. doi: 10.1007/978-1-4615-6552-9_15
Dieckmann, U., Metz, J. A. J., Doebeli, M., and Tautz, D. (2004). Adaptive Speciation: Introduction. Laxenburg: Intrim Report.
Ersmark, E. Klütsch, C. F. C., Chan, Y. L., Sinding, M. H. S., Fain, S. R., Illarionova, N. A., et al. (2016). From the past to the present: wolf phylogeography and demographic history based on the mitochondrial control region. Front. Ecol. Evol. 4:134. doi: 10.3389/fevo.2016.00134
Fancy, S. G., and Ballard, W. B. (1995). “Monitoring wolf activity by satellite,” in Ecology and conservation of wolves in a changing world, eds L. N. Carbyn, S. H. Fritts, and D. R. Seip Edmonton (Edmonton: Canadian Circumpolar Institute, University of Alberta), 329–333.
Fox, J. L., and Chundawat, R. S. (1995). “Wolves in the Transhimalayan region of India: the continued survival of a low density population,” in Ecology and conservation of wolves in a changing world- Proceedings of the 2nd North American Symposium on wolves held in Edmonton, Alberta, Canada, eds L. N. Crabyn, S. H. Fritts, and D. R. Seip (Edmonton: University of Alberta)
Gese, E. M., Andersen, D. E., and Rongstad, O. J. (1990). Determining home-range size of resident coyotes from point and sequential locations. J. Wildl. Manage. 54, 501–506. doi: 10.2307/3809665
Habib, B. (2007). Ecology of Indian wolf (Canis lupus pallipes sykes. 1831), and modeling its potential habitat in the great Indian bustard sanctuary, Maharashtra, India. [Ph.D. Thesis]. Aligarh: Aligarh Muslim University.
Habib, B., Ghaskadbi, P., Khan, S., Hussain, Z., and Nigam, P. (2021a). Not a cakewalk: Insights into movement of large carnivores in human-dominated landscapes in India. Ecol. Evol. 11, 1653–1666. doi: 10.1002/ece3.7156
Habib, B., Jhala, Y. V., Lyngdoh, S., Shrotriya, S., and Reshamwala, S. R. (2021b). Ecology and conservation of Himalayan wolf. Final Project Report. Dehradun: Wildlife Institute of India.
Habib, B., Shrotriya, S., and Jhala, Y. V. (2013). Ecology and Conservation of Himalayan Wolf. Technical Report. Dehradun: Wildlife Institute of India, doi: 10.13140/RG.2.2.36012.87685
Hassanin, A., Ropiquet, A., Couloux, A., and Cruaud, C. (2009). Evolution of the mitochondrial genome in mammals living at high altitude: new insights from a study of the Tribe Caprini (Bovidae. Antilopinae). J Mol Evol 68, 293–310. doi: 10.1007/s00239-009-9208-7
Hennelly, L. M., Habib, B., Modi, S., and Gaubert, P. (2021). Ancient divergence of Indian and Tibetan wolves revealed by recombination-aware phylogenomics. Mol. Ecol. 2021, 1–14. doi: 10.1111/mec.16127
Hennelly, L., Habib, B., Root-Gutteridgec, H., Palaciosd, V., Passilongo, D., Root-Gutteridge, H., et al. (2017). Howl variation across Himalayan, North African, Indian, and Holarctic wolf clades: Tracing divergence in the world’s oldest wolf lineages using acoustics. Curr. Zool. 63, 341–348. doi: 10.1093/cz/zox001
Hodgson, B. H. (1847). Description of the wild ass and wolf of Tibet, with illustrations. Calcutta J. Nat. Hist. 7, 469–477.
Horne, J. S., Garton, E. O., Krone, S. M., and Lewis, J. S. (2007). Analyzing animal movements using Brownian bridges. Ecology 88, 2354–2363. doi: 10.1890/06-0957.1
Hosseini-Zavarei, F., Farhadinia, M. S., Beheshti-Zavareh, M., and Abdoli, A. (2013). Predation by grey wolf on wild ungulates and livestock in central Iran. J. Zool. 290, 127–134. doi: 10.1111/jzo.12022
Imbert, C., Caniglia, R., Fabbri, E., Milanesi, P., Randi, E., Serafini, M., et al. (2016). Why do wolves eat livestock: Factors influencing wolf diet in northern Italy. Biol. Conserv. 195, 156–168. doi: 10.1016/j.biocon.2016.01.003
Jackson, R. M., and Ahlborn, G. (1984). A Preliminary Habitat Suitability Model for the Snow Leopard (Panthera uncia). Int. Pedig. B. Snow Leop. 2, 43—52.
Janecka, J. E., Janecka, M. J., Helgen, K. M., and Murphy, W. J. (2018). The validity of three snow leopard subspecies: Response to Senn, et al. Heredity 120, 586–590. doi: 10.1038/s41437-018-0052-7
Jedrzejewski, W., Niedziallkowska, M., Nowak, S., and Jedrzejewski, B. (2004). ‘Habitat variables associated with wolf. Divers. Distrib. 2004, 225–233. doi: 10.1111/j.1472-4642.2011.00782.x
Jethva, B. D., and Jhala, Y. V. (2004). Foraging ecology, economics and conservation of Indian wolves in the Bhal region of Gujarat, Western India. Biol. Conserv. 116, 351–357. doi: 10.1016/S0006-3207(03)00218-0
Jethva, B. D., Jhala, Y. V., and Rajvanshi, A. (1997). Ecological impact of lignite mining in Kutch with special emphasis on the Indian grey wolf and its habitats. Technical Report. Dehradun: Wildlife Institute of India.
Jhala, Y. V. (1991). Habitat & population dynamics of wolves and Blackbuck in Velvadar National Park. [Ph.D. Thesis]. Virginia: Virginia Polytechnic Institute and State University.
Jhala, Y. V., Habib, B., Shrotriya, S., and Lyngdoh, S. (2013). Status of wolves in India. Proceeding in International Wolf Symposium: Wolves and Humans at the Crossroads. Duluth: Minnesota.
Joshi, B. D., Lyngdoh, S., Singh, S. K., Sharma, R., Kumar, V., Prakash, T. V., et al. (2020). Revisiting the Woolly wolf (Canis lupus chanco) phylogeny in Himalaya: Addressing taxonomy, spatial extent and distribution of an ancient lineage in Asia. PLoS One 15:231621. doi: 10.1371/journal.pone.0231621
Kaczensky, P., Enkhsaikhan, N., Ganbaatar, O., and Walzer, C. (2008). The Great Gobi B strictly protected area in Mongolia - refuge or sink for wolves. Wildlife Biol. 444–456. doi: 10.2981/0909-6396-14.4.444
Kenward, R. E., Clarke, R. T., Hodder, K. H., and Walls, S. S. (2001). Density and linkage estimators of home range: Nearest-neighbor clustering defines multinuclear cores. Ecology 82, 1905–1920. doi: 10.1890/0012-96582001082
Kershenbaum, A., Root-Gutteridge, H., Habib, B., Koler-Matznick, J., Mitchell, B., Palacios, V., et al. (2016). Disentangling canid howls across multiple species and subspecies: Structure in a complex communication channel. Behav. Proc. 124, 149–157. doi: 10.1016/j.beproc.2016.01.006
Kie, J. G., Matthiopoulos, J., Fieberg, J., Powell, R. A., Cagnacci, F., Mitchell, M. S., et al. (2010). The home-range concept: Are traditional estimators still relevant with modern telemetry technology? Philos. Trans. R. Soc. B Biol. Sci. 365, 2221–2231. doi: 10.1098/rstb.2010.0093
Kitchener, A. C., Breitenmoser-Würsten, C., Eizirik, E., Gentry, A., Werdelin, L., Wilting, A., et al. (2017). A revised taxonomy of the Felidae: the final report of the Cat Classification Task Force of the IUCN Cat Specialist Group. Cat News 2017, 1–80.
Kumar, S. (1998). Ecology and behaviour of indian grey wolf (Canis lupus pallipes Sykes, 1831) in the deccan grasslands of Solapur, Maharashtra. [Ph.D. Thesis]. Aligarh: Aligarh Muslim University.
Lucchini, V., Galov, A., and Randi, E. (2004). Evidence of genetic distinction and long-term population decline in wolves. Mol. Ecol. 523–536. doi: 10.1046/j.1365-294X.2004.02077.x
Lyngdoh, S. (2020). Spatial ecology and predation pattern of wolf in Spiti Valley, Himachal Pradesh, India. [Ph.D. Thesis], Rajkot. Saurashtra University.
Lyngdoh, S. B., Habib, B., and Shrotriya, S. (2020). Dietary spectrum in Himalayan wolves: comparative analysis of prey choice in conspecifics across high-elevation rangelands of Asia. J. Zool. 310, 24–33. doi: 10.1111/jzo.12724
Manly, B. F. J., McDonald, L. L., Thomas, D. L., McDonald, T. L., and Erickson, W. P. (2002). Resource Selection by Animals: Statistical Design and Analysis for Field Studies. Netherlands: Kluwer Academic Publisher.
Maurya, K., Habib, B., and Kumar, S. (2011). Food Habits of Indian Wolf. India. J. Zool. 318–322. doi: 10.1093/oxfordjournals.aje.a114807
Mech, L. D. (1970). The Wolf: ecology and behavior of an endangered species. Minneapolis: University of Minnesota Press.
Mech, L. D., and Boitoni, L. (2003). Wolves: Behaviour, Ecology and Conservation. Chicago: University of Chicago Press.
Meiri, S., Yom-Tov, Y., and Geffen, E. (2007). What determines conformity to Bergmann’s rule? Glob. Ecol. Biogeogr. 16, 788–794. doi: 10.1111/j.1466-8238.2007.00330.x
Mengulluoglu, D., Ilaslan, E., Emir, H., and Berger, A. (2019). Diet and wild ungulate preferences of wolves in northwestern Anatolia during winter. PeerJ 7:7446. doi: 10.7717/peerj.7446
Meriggi, A., Brangi, A., Sacchi, O., and Matteucci, C. (1996). The feeding habits of wolves in relation to large prey availability in northern Italy. Ecography 19, 287–295. doi: 10.1111/j.1600-0587.1996.tb01256.x
Mishra, C. (1997). Livestock depredation by large carnivores in the Indian trans-Himalaya: Conflict perceptions and conservation prospects. Environ. Conserv. 24, 338–343. doi: 10.1017/S0376892997000441
Mishra, C., Bagchi, S., Namgail, T., and Bhatnagar, Y. V. (2009). “Multiple use of Trans-Himalayan rangelands: Reconciling human livelihoods with wildlife conservation,” in Wild rangelands: Conserving wildlife while maintaining livestock in semi-arid ecosystems, eds J. T. du Toit, R. Kock, and J. C. Deutsch (Hoboken: Blackwell Publishing Ltd), 291–311. doi: 10.1002/9781444317091.ch11
Moritz, C. (1994). Defining ‘Evolutionarily Significant Units’ for conservation. Trends Ecol. Evol. 9, 373–375. doi: 10.1016/0169-5347(94)90057-4
Muñoz-Fuentes, V., Darimont, C. T., Wayne, R. K., Paquet, P. C., and Leonard, J. A. (2009). Ecological factors drive differentiation in wolves from British Columbia. J. Biogeogr. 36, 1516–1531. doi: 10.1111/j.1365-2699.2008.02067.x
Namgail, T., Fox, J. L., and Bhatnagar, Y. V. (2007). Carnivore- caused livestock mortality in Trans-Himalaya. Environ. Manage. 39, 490–496. doi: 10.1007/s00267-005-0178-2
Newsome, T. M., Boitani, L., Chapron, G., Ciucci, P., Dickman, C. R., Dellinger, J. A., et al. (2016). Food habits of the world’s grey wolves. Mamm. Rev. 46, 255–269. doi: 10.1111/mam.12067
NRSA (2016). Manual on National Landuse/land cover mapping on 1:250,000 using multi- temporal IRS P6-AwiFS data. Balanagar: NRSA.
Paquet, P. C., Wierzchowski, J., and Callagan, C. (1996). “Effects of Human Activity on Gray Wolves in the Bow River Valley, Banff National Park, Alberta,” in A Cumulative Effects Assesment and Futures Outlook for the Banff Bow Valley, eds L. C. J. Green, C. Pacas, and S. Bayley (Ottawa: Banff Bow Valley Study, Department of Canadian Heritage).
Petridou, M., Youlatos, D., Lazarou, Y., Selinides, K., Pylidis, C., Giannakopoulos, A., et al. (2019). Wolf diet and livestock selection in central Greece. Mammalia 2019:21. doi: 10.1515/mammalia-2018-0021
Pilot, M., Dąbrowski, M. J., Hayrapetyan, V., Yavruyan, E. G., Kopaliani, N., Tsingarska, E., et al. (2014). Genetic variability of the grey wolf Canis Lupus in the Caucasus in comparison with Europe and the Middle East: distinct or intermediary population? PLoS One 9:e93828. doi: 10.1371/journal.pone.0093828
Pocock, R. I. (1941). The Fauna of British India, Including Ceylon and Burma: Mammalia. II. London: Taylor & Francis.
R Core Team (2021). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ramasindrazana, B., Goodman, S. M., Schoeman, M. C., and Appleton, B. (2011). Identification of cryptic species of Miniopterus bats (Chiroptera: Miniopteridae) from Madagascar and the Comoros using bioacoustics overlaid on molecular genetic and morphological characters. Biol. J. Linn. Soc. 104, 284–302. doi: 10.1111/j.1095-8312.2011.01740.x
Rundell, R. J., and Price, T. D. (2009). Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol. Evol. 24, 394–399. doi: 10.1016/j.tree.2009.02.007
Sadhukhan, S., Gutteridge, H. R., and Habib, B. (2021). Identifying unknown Indian wolves by their distinctive howls: its potential as a non - invasive survey method. Sci. Rep. 2021, 1–13. doi: 10.1038/s41598-021-86718-w
Sadhukhan, S., Hennelly, L., and Habib, B. (2019). Characterising the harmonic vocal repertoire of the Indian wolf (Canis lupus pallipes). PLoS One 14:1–16. doi: 10.1371/journal.pone.0216186
Sarabia, C., vonHoldt, B., Larrasoana, J. C., Urios, V., and Leonard, J. A. (2021). Pleistocene climate fluctuations drove demographic history of African golden wolves (Canis lupaster). Mol. Ecol. 30, 6101–6120. doi: 10.1111/mec.15784
Schluter, D. (2000). Ecological Character Displacement in Adaptive Radiation. Am. Nat. 156, 4–16. doi: 10.1086/303412
Sharma, D. K., Maldonado, J. E., Jhala, Y. V., and Fleischer, R. C. (2004). Ancient wolf lineages in India. Proc. R. Soc. B Biol. Sci. 271, 2–5. doi: 10.1098/rsbl.2003.0071
Shrotriya, S., Lyngdoh, S., and Habib, B. (2012). Wolves in Trans-Himalayas: 165 Years of taxonomic confusion. Curr. Sci. 103, 885–887.
Shrotriya, S., Reshamwala, H. S., Mahar, N., Habib, B., Suhail, I., and Takpa, J. (2015). Distribution and Population Estimation of Ungulates in Changthang Region, Ladakh, Jammu & Kashmir, India. [Technical Report]. Jammu and Kashmir: Department of Wildlife Protection, Govt. of J&K.
Signer, J., Fieberg, J., and Avgar, T. (2017). Estimating utilization distributions from fitted step-selection functions. Ecosphere 8:1771. doi: 10.1002/ecs2.1771
Silva, I., Crane, M., Suwanwaree, P., Strine, C., and Goode, M. (2018). Using dynamic Brownian Bridge Movement Models to identify home range size and movement patterns in king cobras. PLoS One 13:1–20. doi: 10.1371/journal.pone.0203449
Sin, T., Gazzola, A., Chiriac, S., and Rîşnoveanu, G. (2019). Wolf diet and prey selection in the SouthEastern Carpathian Mountains. Romania PLoS One 14, 1–15. doi: 10.1371/journal.pone.0225424
Singh, M., and Kumara, H. N. (2006). Distribution, status and conservation of Indian gray wolf. India. J. Zool. 164–169. doi: 10.1111/j.1469-7998.2006.00103.x
Sobel, J. M., Chen, G. F., Watt, L. R., and Schemske, D. W. (2010). The biology of speciation. Evolution 64, 295–315. doi: 10.1111/j.1558-5646.2009.00877.x
Srinivas, Y., and Jhala, Y. (2021). Morphometric variation in wolves and golden jackal in India (Mammalia,Carnivora). Biodivers. Data J. 9:e67677. doi: 10.3897/BDJ.9.e67677
Stahler, D. R., and Smith, D. W. (2006). The Waltham, International nutritional sciences symposia foraging and feeding ecology of the Gray wolf (Canis lupus): Lessons. J. Nutr. 136, 1923–1926.
Subba, S. A. (2012). Assessing the genetic status, distribution, prey selection and conservation issues of Himalayan wolf (Canis himalayensis) in Trans- Himalayan Dolpa, Nepal. [Ph.d. Thesis]. Sweden: Lund University.
Sulloway, F. J. (1982). Darwin and his finches: The evolution of a legend. J. Hist. Biol. 15, 1–53. doi: 10.1007/BF00132004
Sun, J., and Liu, T. (2000). Stratigraphic evidence for the uplift of the Tibetan Plateau between 1.1 and 0.9 myr Ago. Quat. Res. 54, 309–320. doi: 10.1006/qres.2000.2170
Suryawanshi, K. R., Bhatnagar, Y. V., Redpath, S., and Mishra, C. (2013). People, predators and perceptions: Patterns of livestock depredation by snow leopards and wolves. J. Appl. Ecol. 50, 550–560. doi: 10.1111/1365-2664.12061
Swihart, R. K., and Slade, N. A. (1985). Influence of Sampling Interval on Estimates of Home-Range Size. J. Wildl. Manage. 49, 1019–1025.
Theurkauf, J., Gula, R., Pirga, B., Tsunoda, H., Eggermann, J., Brzezowska, B., et al. (2007). Human impact on wolf activity in the Bieszczady Mountains, SE Poland. Ann. Zool. Fennici. 44, 225–231.
Thomas, D. L., and Taylor, E. J. (1990). Study design and test for comparing resourse use and availibility. J. Wildl. Manage 54, 322–330.
Vilà, C., Amorim, I. R., Leonard, J. A., Posada, D., Castroviejo, J., Petrucci-Fonseca, F., et al. (1999). Mitochondrial DNA phylogeography and population history of the grey wolf Canis lupus. Mol. Ecol. 8, 2089–2103. doi: 10.1046/j.1365-294X.1999.00825.x
Vilà, C., Sundqvist, A. K., Flagstad, O., Seddon, J., Bjornerfeldt, S., Kojola, I., et al. (2003). Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc. R. Soc. B Biol. Sci. 270, 91–97. doi: 10.1098/rspb.2002.2184
Vilà, C., Urios, V., and Castroviejo, J. (1995). “Observations on the daily activity patterns in the Iberian wolf,” in Ecology and conservation of wolves in a changing world, eds L. N. Carbyn, S. H. Fritts, and D. R. Seip (Edmonton: University of Alberta), 335–340.
Wang, M. S., Thakur, M., Jhala, Y. V., Wang, S., Srinivas, Y., Liu, Z., et al. (2021). Genome sequencing a gray wolf from peninsular india provides new insights into the evolution and hybridization of gray wolves. Genome Biol. Evol. 14:12. doi: 10.1093/gbe/evac012
Wang, M. S., Wang, S., Li, Y., Jhala, Y., Thakur, M., Otecko, N., et al. (2020). Ancient Hybridization with Unknown Population Facilitated High Altitude Adaptation of Canids. Mol. Biol. Evol. 37, 2616–2629. doi: 10.2139/ssrn.3456297
Wayne, R. K., Lehman, N., Girman, D., Gogan, P. J. P., Gilbert, D. A., Hansen, K., et al. (1991). Conservation genetics of the endangered isle royale gray wolf. Conserv. Biol. 5, 41–51. doi: 10.1111/j.1523-1739.1991.tb00386.x
Werhahn, G., Kusi, N., Li, X., Chen, C., Zhi, L., Lázaro Martín, R., et al. (2019). Himalayan wolf foraging ecology and the importance of wild prey. Glob. Ecol. Conserv. 20:780. doi: 10.1016/j.gecco.2019.e00780
Werhahn, G., Senn, H., Ghazali, M., Karmacharya, D., Sherchan, A. M., Joshi, J., et al. (2018). The unique genetic adaptation of the Himalayan wolf to high-altitudes and consequences for conservation. Glob. Ecol. Conserv. 16:e00455. doi: 10.1016/j.gecco.2018.e00455
Werhahn, G., Senn, H., Kaden, J., Joshi, J., Bhattarai, S., Kusi, N., et al. (2017). Phylogenetic evidence for the ancient himalayan wolf: Towards a clarification of its taxonomic status based on genetic sampling from Western Nepal. R. Soc. Open Sci. 4:186. doi: 10.1098/rsos.170186
Whittington, J., Clair, C. C., and Mercer, G. (2005). Spatial responces of wolves to raods and trails in mountain valleys. Ecol. Appl. 15, 543–553. doi: 10.5958/0976-5506.2017.00102.4
Wozencraft, W. C. (2005). in Mammal species of the world. A taxonomic and geographic reference. 3rd edition, eds D. E. Wilson and D. E. Reeder (Washington: John Hopkins University Press).
Keywords: howling, peninsular India, spatial ecology, Trans-Himalayas, conservation, food habit, movement ecology
Citation: Khan S, Shrotriya S, Sadhukhan S, Lyngdoh S, Goyal SP and Habib B (2022) Comparative Ecological Perspectives of Two Ancient Lineages of Gray Wolves: Woolly Wolf (Canis lupus chanco) and Indian Wolf (Canis lupus pallipes). Front. Ecol. Evol. 10:775612. doi: 10.3389/fevo.2022.775612
Received: 14 September 2021; Accepted: 16 February 2022;
Published: 15 March 2022.
Edited by:
Miguel Ferrer, Spanish National Research Council (CSIC), SpainReviewed by:
Vicente Urios, University of Alicante, SpainGeraldine Werhahn, University of Oxford, United Kingdom
Copyright © 2022 Khan, Shrotriya, Sadhukhan, Lyngdoh, Goyal and Habib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bilal Habib, YmhAd2lpLmdvdi5pbg==
 Shaheer Khan
Shaheer Khan Shivam Shrotriya
Shivam Shrotriya Sougata Sadhukhan
Sougata Sadhukhan Salvador Lyngdoh
Salvador Lyngdoh Surendra P. Goyal
Surendra P. Goyal Bilal Habib
Bilal Habib