- 1School of Ecological and Environment, Chengdu University of Technology, Chengdu, China
- 2Department of Environmental Science and Engineering, Fudan University, Shanghai, China
- 3Department of Environmental Engineering, Yale University, New Haven, CT, United States
“Whale Fall” is a collective term for the whale carcass, the process of dead whale fall, and the formed deep-sea ecosystem. The whale fall process produces a lot of unstable organic matter that has a significant impact on deep-sea ecosystems. Scientists speculate that organic matter input is the source of energy and material for organisms in deep-sea ecosystems. In the seafloor of the North Pacific, whale fall supports the survival of at least 12,490 organisms of 43 species, contributing to the prosperity of deep-sea life. Due to the specificity of the time and space of the formation of whale fall, there are few studies on whale fall and its impact on the deep-sea ecosystem. This article summarizes and analyses the current research status on the distribution of whale fall and its impact on the deep-sea ecosystem at home and abroad. The results show that the current distribution of whale fall is mainly concentrated in the Pacific and Atlantic regions, and the research on the impact of whale fall on deep-sea ecosystems focuses on the formation process, degradation rate and impact on deep-sea biological systems. This article has some significance to the understanding of biodiversity and ecosystem succession in the deep-sea “desert area.”
Introduction
Whale fall refers to the phenomenon in which whales die and sink to the seafloor of the marine to form an ecosystem. When a whale dies in the ocean, its carcass sinks to the bottom of the ocean. And biologists named this process after the whale fall. Whale fall, together with cold seeps and hydrothermal vents, is called an “oasis” for deep-sea life (Wang, 2020). Normally, the carcass of a whale can support a decomposing-dominated circulatory ecosystem for up to 100 years.
The unique geological features of the deep sea create a variety of habitats that support chemoautotroph communities on the seafloor (Schuller et al., 2004). The deep-sea environment has long been considered to be low-energy, nutrient-poor because the organic inputs are achieved by only a small amount of carbon produced by photosynthesis. However, there are some local areas with high microbial biomass and activity in the deep-sea, in which whale fall is like an “oasis” in the “ocean desert” and has become an important geographical station for the evolution and development of life in extreme deep-sea environments (Jorgensen and Boetius, 2007; Wang, 2021).
Most deep-sea sediments trap about 2–10 g of particulate organic carbon flux per year (Lutz et al., 2007). However, the soft tissue of a 30 t whale carcass contains about 1.2 × 103 kg of active organic carbon that is equivalent to the background organic carbon flux of 100 m2 of the deep seafloor in 1,000 years (Smith C. R. et al., 2014). It is also estimated that whale carcasses transport organic matters to the deep sea 2,000 times faster than the supply of marine snow. Marine snow is a kind of organic matter production activity mainly composed of organic matter debris, originating from the euphotic layer of the upper ocean. In the deep sea, detritus made up of organic matter is falling like snowflakes, so the process is called “marine snow.” Because sunlight cannot reach the deep sea, its creatures rely heavily on marine snow as a source of energy, so marine snow is considered as the foundation of deep-sea ecosystems (Van Dover, 2000; Smith and Baco, 2003).
Based on the studies, the sulfate reduction and sulfide levels in sediments around 0.5 m from 30 t whale carcasses were significantly increased over a period of at least 7 years (Treude et al., 2009a). The large organic inputs produced by whale fall not only show up in lipid-rich skeletons, but also create sulfur-rich habitats in the sediments surrounding the carcasses. The species richness of the biomes formed by whale fall is as high as 407 species, which is slightly lower than that of hydrothermal vents (469 species), but far greater than that of cold seeps (230 species) (Smith and Baco, 2003). Thus, whale fall may fundamentally contribute to deep-sea biodiversity and the dispersal of thiophilic species between sulfur-rich habitats (e.g., hydrothermal vents, cold seeps) and anoxic basins. Whale fall also promotes the transportation of organic matters from the upper ocean to the middle and lower layers of the ocean, playing an important role in the ocean carbon cycle (Smith et al., 1989; Bennett et al., 1994; Butman et al., 1995; Scheltema, 1996).
What is the major distribution of whale falls that have been found in the extreme environment of the deep sea? How do whale falls participate in the formation of the deep-sea ecosystem? How do the organisms interact and how much do they contribute? These are common concerns among whale fall researchers. This article systematically sorts out the relevant research results, summarizes the research related to the distribution of whale fall, analyses the research progress of the impact of whale fall on the distribution of marine organisms, deepens people’s understanding of whale fall, and provides certain basis and direction for further scientific research.
Definition of Whale Fall
“Where there is a whale falling, there is a birth.” Regarding the “whale fall,” it itself contains a literary flavor. However, there has never been a clear definition of it in the Chinese scientific community. In 2020, the Institute of Deep-Sea Science and Engineering, Chinese Academy of Sciences discovered a whale carcass about 3 m long in the South China Sea. This is the first time that China has discovered such an ecosystem as whale fall. In other related popular science articles, whale fall is defined as the carcass or remains of a whale; it is also described as a phenomenon or process that when a whale dies, the carcass will eventually sink to the seafloor. Therefore, the definition of whale fall can be expressed as follows: “Whale fall” is a general term for the whale carcass, the process of falling, and the formed deep-sea ecosystem.
Studies have shown that each whale that reaches the seafloor represents the input of energy (Lundsten et al., 2010b). In 1987, the manned submersible “Alvin” discovered a 21-m-long blue whale skeleton during a routine dive. Scientific researchers found a “biological carpet” on its skeleton, including bacteria and worms. The ecosystem that came to be known as “whale fall” was first discovered. The study found that biomes grew and thrived in sulfur-rich sediment around rotting whale carcass or remain (Smith et al., 1989). And whale falls have been found in the deep sea of the Pacific and Atlantic oceans and in fossils from the northeast Pacific Ocean 30 million years ago (Smith and Baco, 2003). Whale fall is considered a stepping stone to the growth of deep-sea creatures (Distel et al., 2000). Studies since the 1850s have shown that whale fall can support a large population of organisms. In the seafloor of the North Pacific, whale fall supports the survival of at least 12,490 organisms of 43 species, contributing to the prosperity of deep-sea life (Liu, 2015). With further research on whale fall, researchers have also carried out a series of exploration experiments. For example, try to artificially implant a whale carcass to further study the effects of whale fall on the deep-sea ecosystem.
The Characteristics and Distribution of Whale Fall
Whale’s soft tissues and bones hold huge reserves of oil. From prehistoric times to the industrial age, humans have been hunting whales for biofuels. Studying data collected by scientists during the heyday of industrial whaling, Higgs et al. (2011) found differences in the lipid content of bones in different parts of whales. Analysis of the skeletal composition of several large whales found that most of the lipids are concentrated in the skull and caudal vertebrae, while the thoracic vertebrae have relatively little lipid content. In addition, it also was found that the lipid content of whale skeleton varies greatly with the maturity and skeleton of the whale. The skeletal lipid content of young whales is much lower than that of adults. The jaw, skull, and caudal vertebrae are high in lipid (approx. 20–84%); the ribs, scapula, sternum, and lumbar vertebrae are middle (approx. 15–30%); and the thoracic and cervical vertebrae are relatively low (approx. 5–20%). In addition, seasonal changes in whales’ physiology and feeding can affect their fat storage. This is evident in the records of whales caught in different seasons. And high-lipid skeleton attracts a large number of thiophilic micro-organisms. This skeletal lipid gradient also corresponds to sites of microbial bioerosion and thus plays an important role in studying whale fall-associated microbial communities (Higgs et al., 2011).
As an energy-rich ecosystem, whale fall has several distinctive characteristics. (a) Whales usually enter the marine food web as relatively intact carcasses after death. (b) Lipid- and protein-rich whale fall contributes a large amount of energy for deep-sea organisms. (c) Whale fall is unevenly distributed in space and time.
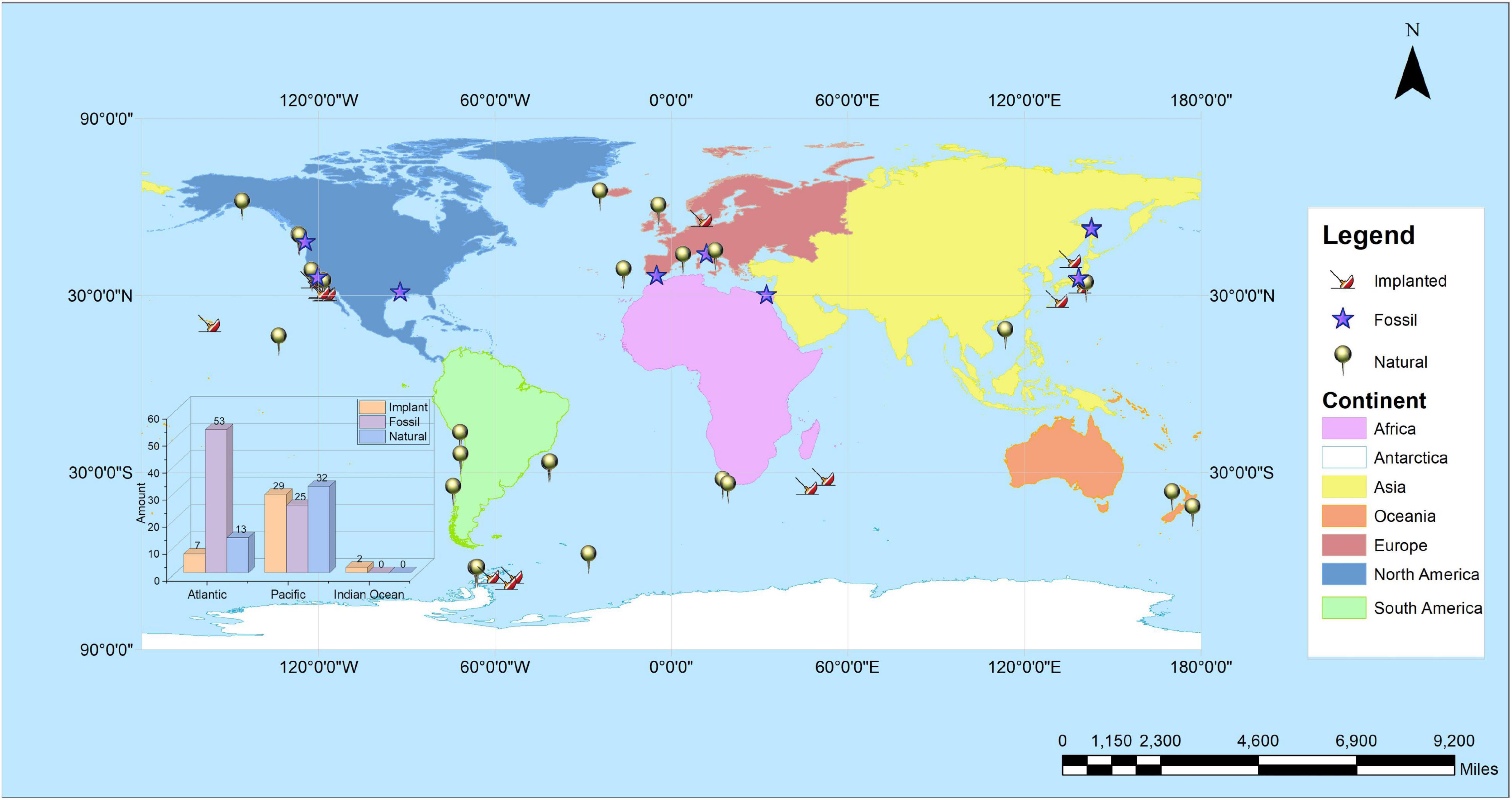
Over the past 200 years, whaling has severely reduced the population of large cetaceans, especially between 1860 and 1986. The number of all large whales has decreased sharply, and some species have vanished or are facing extinction (e.g., the North Atlantic gray whale). Whaling has dramatically changed the probability and geographic distribution of whales sinking into the deep sea (Butman et al., 1995, 1996). The decline in whale falls has reduced species diversity and may have contributed to the extinction of species in deep sea ecosystems ranging from whale falls to hydrothermal vents (Hecker, 1985; Mclean, 1985; Craddock et al., 1995). It is estimated that whaling in the 19th and 20th centuries has reduced whale fall habitats by as much as 95%, potentially exterminating up to half of marine basin species that feed on whale carcasses. Some insight into the impact of fluctuations in whale carcass numbers can be gained by studying whale fall ecology and biogeography. However, even such studies fail to shed light on the characteristics of species endangered by whaling. If we hope to explore deep sea wonders that understand ecology and evolution, it is essential to explore distant and unknown deep sea ecosystems to further reduce the impact of anthropogenic factors on marine ecosystems, such as pollution and overfishing. The distribution of currently known whale falls is shown in Figure 1. As seen in the Figure, whale falls are mainly distributed in the Atlantic Ocean and the Pacific Ocean. Among the oceans, the Atlantic Ocean has the largest number of whale falls including natural whale falls, implanted whale falls, and fossil whale falls. And the inset shows that the distribution of whale falls in the Atlantic Ocean has a significantly higher number of fossil whale falls than the others; in the Pacific Ocean, the number of whale falls of all three properties is approximately the same, with a higher number of natural whale falls; while the number of whale falls in the Indian Ocean is low, and so far there is no report of whale fall in the Arctic Ocean. With the progress of science and technology, whale falls have been discovered continuously (Smith et al., 1989, 2015; Mclean, 1992; Baco and Smith, 2003; Smith and Baco, 2003; Goffredi et al., 2004; Braby et al., 2007; Lundsten et al., 2010a,b; Smith C. R. et al., 2014; Smith K. E. et al., 2014; Alfaro-Lucas et al., 2017). At the same time, scientists have also implanted whale carcasses to study the community ecology and phylogenetics of the whale fall to further investigate the species supported by whale falls and their impact on deep-sea ecosystems.
Effects of Whale Fall on the Deep-Sea Biomes
It takes approximately 2 years for the soft tissues of whale carcasses to decompose completely. However, exposed skeletons can support specific populations for decades due to their high lipid content (Baco and Smith, 2003; Schuller et al., 2004). The duration for which whale falls maintain their chemosynthetic fauna depends on their lipid content, supporting specific organisms for about 10 years in an oxygen-rich environment and up to 50 years or more in an oxygen-poor environment (Smith and Baco, 2003; Fujiwara et al., 2007; Glover et al., 2010; Lundsten et al., 2010b). Whale skeletons are decomposed by bacteria, producing sulfide that continues to flow into the surrounding seawater and sediments (Treude et al., 2009b). Sulfides provide energy for the chemoautotrophic fauna living on or around whale remains. Whale falls act as carriers for the spread and succession of these organisms.
Smith and Baco (2003), pointed out that there were differences in whale falls formed by different growth stages. The fauna found in young whale skeletons does not appear to be as dependent on chemical autotrophy as larger skeletons (Smith and Baco, 2003). Related studies have shown that the distribution of red bone marrow is inversely proportional to the lipid content of the skeleton. There are more red bone marrow in young whales, and the decrease of lipid content in it will shorten the survival time of whale (Ohe, 1950). Feltmann et al. (1948) pointed out that fragmented whale vertebrae showed “blood spots,” which are red bone marrow, the site of mammalian blood cell production, and that bones filled with yellow bone marrow had high lipid content. Tont et al. (1977) also showed that vertebrae filled with red bone marrow had a low lipid content. Honda et al. (1984a,b) found that in young whales, even the caudal vertebrae contained red bone marrow, but as the whales aged, the red bone marrow was gradually replaced by yellow bone marrow and the process started in the caudal vertebrae.
Relevant studies have shown that the enrichment capacity of whale skeleton to nearby sediments is lower than that of other tissues, and that skeletons inhibit microbial lipase activity and reduce bioturbation rates in their surroundings, but increase species diversity of larger animals (Smith et al., 1998). It is worth noting that some deep-sea animals can change their own metabolism, growth rate, feeding behavior, and reproduction to better utilize whale carcasses (Gage and Tyler, 1991; Levin, 2000). The decomposition of whale carcasses delivers large amounts of organic matters to the seafloor and serves as a unique habitat for deep-sea life, contributing to the complexity and biodiversity of the deep-sea environment (Levin, 2000).
Smith et al., collected a total of 2,649 macrofauna individuals from 17 whale fall samples discovered in 1988, of which 143 species were identified, consisting of 1% of macrofauna gathered around the skeleton. Seven of these species were not collected in previous intensive sampling of sediment macrofauna in the Santa Catalina Basin (SCB) and are presumed to be possible alien species, only Mitrella permodesta was collected on the whale bones, and the other six species were found within 1 m of the whale skeleton, suggesting the response to the habitat conditions created by the whale fall (Bennett et al., 1994). The average abundance of the macrofaunal community was found to be the highest among the whale fall samples; species diversity showed a clear pattern around the whale skeleton: species richness increased with decreasing distance from the skeleton (Pettibone, 1993).
The local diversity of background sediments in the SCB is low, while the biodiversity near the whale skeleton is significantly increased, with a significant increase in rare species diversity (Connell, 1978; Petraitis et al., 1989). Stockton and DeLaca (1982) hypothesized that concentrated organic inputs from whale falls would lead to the local development of dense communities on the deep seafloor, producing characteristic species structures. Such change in the local benthic population takes many years (Stockton and DeLaca, 1982). So far, only 21 species larger deep-sea animals are found around the whale fall, including 11 species identical to hydrothermal vents and 20 species identical to cold seeps. In addition to the typical molluscan and serpulid communities, whale fall has recorded many new species and evolutionary peculiarities, including Osedax, gastropods, and a variety of animals living on sulfur bacteria. The ecosystem formed by whale fall facilitated the development of a deep-sea characteristic fauna, and high species abundance could be reached and maintained for decades.
Biologists have found that scavengers on the seafloor also use lipid-rich bones from whale carcasses or remains for organic synthesis, including many organisms that have been discovered for the first time in science, some of which can directly utilize whale bones as food. Sipuncula worm Phascolosoma saprophagicum feed on lipids in the skeleton (Gibbs, 1987), limpet of Osteopeltidae feeds on bacteria that grow on bone tissue (Marshall, 1987), Osedax degrades bones for nutrients with the help of endosymbiotic heterotrophic bacteria (Rouse et al., 2004; Goffredi et al., 2010).
Effects of Whale Fall on the Deep-Sea Microbes
Microorganisms account for a significant portion of the Earth’s biodiversity (Van Der Heijden et al., 2008). Due to evolutionary pressures, they exist in almost all known environments, some of which are harsh, forcing them to adapt to specific ecological niches (Coughlan et al., 2015). The deep-sea ecosystem is unusual in that it is a high-pressure, low-temperature, low-oxygen environment, where organic matter is mainly low concentrations of stable carbon. Despite the physical and biochemical limitations of the deep-sea, microbial communities still exist in the deep-sea sediments (Li et al., 1999; Vetriani et al., 1999; Dhillon et al., 2005; Knittel et al., 2005; Inagaki et al., 2006). Whale falls deliver large amounts of organic material to the seafloor, and the microbes of the deep-sea habitat into which this organic material enters have unique ecological potential, particularly in terms of interactions between microbial populations and specific pathways that facilitate nutrient cycling. Whale falls provide a source of material for the deep sea. At the same time, the organic enrichment produced by the fall of oil-rich whales stimulates the degradation of oil by bacteria in the sediment (Schuller et al., 2004). To assess the effects of organic enrichment on deep-sea microbial communities, (Goffredi et al., 2010), investigated bacterial diversity in sediments around two whale colonies located in Monterey Canyon at depths of 1,820 m and 2,893 m. Bacteroidetes, Epsilonproteobacteria and Firmicutes were found mainly in the sediments where the whales landed, compared with the surrounding control sediments 20 m away. In comparison with control sediments 20 m away, Bacteroidetes, Epsilonproteobacteria, and Firmicutes were found mainly in the sediments below the whale fall, while Gammaproteobacteria and Planctomycetes were found mainly in the control sediments. A large number of Deltaproteobacteria were found in both sediments, with Desulfobacteraceae and Desulfobulbaceae mainly distributed under the whale fall. The bacterial community at 1,820 m depth 7 months after whale fall deposition was less diverse than the reference sediment, with Deltaproteobacteria, Epsilonproteobacteria, and Bacteroidetes accounting for 89% of the community biodiversity (Goffredi and Orphan, 2010). At 70 months, bacterial diversity in the reference sediment near the whale fall at a depth of 2,893 m decreased. In the long term, the impact of the whale fall was also manifested by an increase in total organic carbon and enhanced protein hydrolysis activity, which lasts for at least 17–70 months. The analysis found no significant differences between the bacterial communities gathered around the two whale falls, but differed from the control group, suggesting that the deposition of whale fall biomass has a greater impact on the deep-sea microbial community than a specific benthic location (Smith et al., 1998). Whale falls, as discrete resource blocks, are thought to contribute significantly to deep-sea habitat heterogeneity and may contribute to the proliferation of unique microbial assemblages (Grassle and Morse-Porteous, 1987; Baco and Smith, 2003).
The amount of organic carbon available in most deep-sea microbial communities is very low. However, the level of organic carbon obtained by microorganisms was significantly higher than the amount of primary organic matter produced in surface waters in the local habitats of whale fall. Whale falls thus provide a unique ecological niche that dynamically affects microbial diversity and activity in the associated sediments over time. Whale falls and surrounding macrofaunal communities reinforce the bioturbation of their surroundings. Bacteria are commonly enriched around whale fall habitats, including Bacteroidetes, Firmicutes, Epsilonproteobacteria, Desulfobacteraceae, and Desulfobulbaceae of the Deltaproteobacteria, suggesting that eutrophic deep-sea bacterial communities may lead to biotic community changes. Due to the high oxygen consumption of microorganisms, which is conducive to the formation of anoxic conditions for anaerobic processes such as sulfate reduction and methanogenesis. Whale fall and its surrounding sediments are suitable habitats for sulfide chemical synthesis communities and sulfate reduction and methanogenesis bacteria (Allison et al., 1991). Microorganisms found in deep-sea whale falls are mainly heterotrophic, such as Vesicomyid clams, Bathymodioline mussels, and Vestimentiferan tubeworms, which cover the skeletal surface of whales and form “oases of life” similar to deep-sea hydrothermal vents (Bennett et al., 1994; Deming et al., 1997; Goffredi et al., 2004). The initial formation of whale falls can cause great perturbations in natural environmental conditions, even resulting in a short-term reduction in surrounding diversity (Danise et al., 2012). Over time, the impact of nutrients released from whale carcasses or remains expanded laterally, with some researchers observing an increase in bacterial diversity and total organic carbon (TOC) content at distances of up to 20 m from the whale bones (Goffredi et al., 2010). Related studies have shown that, methanogenic bacteria are predominantly archaea (98%) in whale fall sediments, including Methanomicrobiales and Methanosarcinales (Onishi et al., 2018). Temporal changes in this archaeal community included the early establishment of methylotrophic methanogens followed by development of methanogens thought to be hydrogenotrophic, as well as members related to the newly described methanotrophic lineage, ANME-3. Chemical analysis revealed elevated methane and depleted sulfate concentrations in the sediments under the whale-fall, as compared to surrounding sediments. Carbon was enriched (up to 3.5%) in whale-fall sediments, as well as the surrounding sea floor to at least 10 m, forming a “bulls eye” of elevated carbon (Goffredi et al., 2008). Studying the diversity and distribution of natural microbial communities in sediments of permanently low-temperature deep-sea environments is crucial to our understanding of global biogeochemical cycles, especially the decomposition and cycling of organic carbon.
Conclusion
Whale fall has a significant effect on the deep-sea biological community, which is beneficial to the diffusion and succession of deep-sea organisms. Whale fall also supports special fauna and provides the basis for the reproduction, survival and evolution of some deep-sea sulfur-loving microorganisms. And whale skeletons have played an important role in speciation and greatly enhance biodiversity. Despite great advances in whale fall research, there are still significant gaps in our understanding of the microbial processes, reproductive strategies, population genetics, and biogeography that contribute to whale fall. Therefore, it is important for us to explore a series of mysterious and unknown deep-sea ecosystems such as whale falls and understand the complex dynamics of community change in the deep-sea environment.
Author Contributions
QhL: conceptualization, data curation, and writing – original draft. YL: methodology and software. GL: conceptualization, methodology, and resources. ZW: investigation and data curation. ZZ: investigation and supervision. YS: conceptualization and resources. NL: methodology and data curation. QiL: conceptualization and supervision. WZ: conceptualization, methodology, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Chengdu University of Technology Research Startup Fund (10912-KYQD2020-08431) and the National Environmental Protection Key Laboratory of Cooperative Control and Joint Remediation of Water and Soil Pollution Open Fund Project (GHB-2020-010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alfaro-Lucas, J. M., Shimabukuro, M., Ferreira, G. D., Kitazato, H., Fujiwara, Y., and Sumida, P. (2017). Bone-eating Osedax worms (Annelida: Siboglinidae) regulate biodiversity of deep-sea whale-fall communities. Deep-Sea Res. Pt. II. 146, 4–12. doi: 10.1016/j.dsr2.2017.04.011
Allison, P. A., Smith, C. R., Kukert, H., Deming, J. W., and Bennett, B. A. (1991). Deep-water taphonomy of vertebrate carcasses: a whale skeleton in the bathyal Santa Catalina Basin. Paleobiology 17, 78–89. doi: 10.1017/S0094837300010368
Baco, A. R., and Smith, C. R. (2003). High species richness in deep-sea chemoautotrophic whale skeleton communities. Mar. Ecol. Prog. Ser. 260, 109–114. doi: 10.3354/meps260109
Bennett, B. A., Smith, C. R., Glaser, B., and Maybaum, H. L. (1994). Faunal community structure of a chemoautotrophic assemblage on whale bones in the deep northeast Pacific ocean. Mar. Ecol. Prog. Ser. 108, 205–223.
Braby, C. E., Rouse, G. W., Johnson, S. B., Jones, W. J., and Vrijenhoek, R. C. (2007). Bathymetric and temporal variation among osedax boneworms and associated megafauna on whale-falls in monterey bay, California. Deep-Sea Res. Pt. I. 54, 1773–1791. doi: 10.1016/j.dsr.2007.05.014
Butman, C. A., Carlton, J. T., and Palumbi, S. R. (1995). Whaling effects on deep-sea biodiversity. Conserv. Biol. 9, 462–464. doi: 10.1046/j.1523-1739.1995.9020462.x
Butman, C. A., Carlton, J. T., and Palumbi, S. R. (1996). Whales don’t fall like snow: reply to Jelmert. Conserv. Biol. 10, 655–656. doi: 10.1046/j.1523-1739.1996.10020655.x
Connell, J. (1978). Diversity in tropical rain forests and coral reefs: high diversity of trees and corals is maintained only in non-equilibrium state. Science 199, 1302–1310. doi: 10.1126/science.199.4335.1302
Coughlan, L. M., Cotter, P. D., Hill, C., and Alvarez-Ordóñez, A. (2015). Biotechnological applications of functional metagenomics in the food and pharmaceutical industries. Front. Microbiol. 6:672. doi: 10.3389/fmicb.2015.00672
Craddock, C., Hoeh, W. R., Gustafson, R. G., Lutz, R. A., and Vrijenhoek, R. C. (1995). Evolutionary relationships among deep-sea mytilids (Bivalvia: Mytilidae) from hydrothermal vents and cold-water methane/sulfide seeps. Mar. Biol. 121, 477–485. doi: 10.1007/BF00349456
Danise, S., Cavalazzi, B., Dominici, S., Westall, F., Monechi, S., and Guioli, S. (2012). Evidence of microbial activity from a shallow water whale fall (Voghera, northern Italy). Palaeogeogr. Palaeocol. 317-318, 13–26. doi: 10.1016/j.palaeo.2011.12.001
Deming, J. W., Reysenbach, A. L., And, S., and Smith, C. R. (1997). Evidence for the microbial basis of a chemoautotrophic invertebrate community at a whale fall on the deep seafloor: bone-colonizing bacteria and invertebrate endosymbionts. Microsc. Res. Techniq. 37, 162–170. doi: 10.1002/(SICI)1097-0029(19970415)37:2<162::AID-JEMT4>3.0.CO;2-Q
Dhillon, A., Lever, M., Lloyd, K. G., Albert, D. B., Sogin, M. L., and Teske, A. (2005). Methanogen diversity evidenced by molecular characterization of methyl coenzyme m reductase a (mcra) genes in hydrothermal sediments of the Guaymas basin. Appl. Environ. Microb. 71, 4592–4601. doi: 10.1128/AEM.71.8.4592-4601.2005
Distel, D. L., Baco, A. R., Chuang, E., Morrill, W., Cavanaugh, C., and Smith, C. R. (2000). Do mussels take wooden steps to deep-sea vents? Nature 403, 725–726. doi: 10.1038/35001667
Feltmann, C. F., Slijper, E. J., and Vervoort, W. (1948). Preliminary researches on the fat-content of meat and bone of blue and fin whales. Proc. R. Neth. Acad. Arts Sci. 51, 604–615.
Fujiwara, Y., Kawato, M., Yamamoto, T., Yamanaka, T., and Okutani, T. (2007). Three-year investigations into sperm whale-fall ecosystems in Japan. Mar. Ecol. 28, 219–232. doi: 10.1111/j.1439-0485.2007.00150.x
Gage, J. D., and Tyler, P. A. (1991). Deep-sea Biology: a Natural History of Organisms at the Deep-sea Floor. Cambridge: Cambridge University Press.
Gibbs, P. E. (1987). A new species of Phascolosoma (Sipuncula) associated with a decaying whale’s skull trawled at 880 m depth in the South-west Pacific. New Zeal. J. Zool. 14, 135–137. doi: 10.1080/03014223.1987.10422691
Glover, A. G., Gooday, A. J., Bailey, D. M., Billett, D., and Vanreusel, A. (2010). Temporal change in deep-sea benthic ecosystems: a review of the evidence from recent time-series studies. Adv. Mar. Biol. 58, 1–95. doi: 10.1016/B978-0-12-381015-1.00001-0
Goffredi, S. K., and Orphan, V. J. (2010). Bacterial community shifts in taxa and diversity in response to localized organic loading in the deep sea. Environ. Microbiol. 12, 344–363. doi: 10.1111/j.1462-2920.2009.02072.x
Goffredi, S. K., Orphan, V. J., Rouse, G. W., Jahnke, L., Embaye, T., Turk, K., et al. (2010). Evolutionary innovation: a bone-eating marine symbiosis. Environ. Microbiol. 7, 1369–1378. doi: 10.1111/j.1462-2920.2005.00824.x
Goffredi, S. K., Paull, C. K., Fulton-Bennett, K., Hurtado, L. A., and Vrijenhoek, R. C. (2004). Unusual benthic fauna associated with a whale fall in Monterey Canyon, California. Deep-Sea Res. Pt. I. 51, 1295–1306. doi: 10.1016/j.dsr.2004.05.009
Goffredi, S. K., Wilpiszeski, R., Lee, R., and Orphan, V. J. (2008). Temporal evolution of methane cycling and phylogenetic diversity of archaea in sediments from a deep-sea whale-fall in Monterey Canyon, California. ISME J. 2, 204–220. doi: 10.1038/ismej.2007.103
Grassle, J. F., and Morse-Porteous, L. S. (1987). Macrofaunal colonization of disturbed deep-sea environments and the structure of deep-sea benthic communities. Deep-Sea Res. Pt. I. 34, 1911–1915, 1917–1950.
Hecker, B. (1985). Fauna from a cold sulfur-seep in the Gulf of Mexico: comparison with hydrothermal vent communities and evolutionary implications. Bull. Biol. Soc. Washington 6, 465–473.
Higgs, N. D., Little, C. T. S., and Glover, A. G. (2011). Bones as biofuel: a review of whale bone composition with implications for deep-sea biology and palaeoanthropology. P. Roy. Soc. B-Biol. Sci. 278, 9–17. doi: 10.1098/rspb.2010.1267
Honda, K., Fujise, Y., Itano, K., and Tatsukawa, R. (1984a). Composition of chemical components in bone of striped Dolphin, Stenella coeruleoalba: distribution characteristics of heavy metals in various bones. Agr. Biol. Chem. 48, 677–683. doi: 10.1080/00021369.1984.10866203
Honda, K., Fujise, Y., Tatsukawa, R., and Miyazaki, N. (1984b). Composition of chemical components in bone of striped dolphin, Stenella coeruleoalba: distribution characteristics of major inorganic and organic components in various bones, and their age-related changes. Agr. Biol. Chem. 48, 409–418. doi: 10.1080/00021369.1984.10866136
Inagaki, F., Nunoura, T., Nakagawa, S., Teske, A., Lever, M., Lauer, A., et al. (2006). Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl. Acad. Sci. U S A. 103, 2815–2820. doi: 10.1073/pnas.0511033103
Jorgensen, B. B., and Boetius, A. (2007). Feast and famine–microbial life in the deep-sea bed. Nat. Rev. Microbiol. 5, 770–781. doi: 10.1038/nrmicro1745
Knittel, K., Lösekann, T., Boetius, A., Kort, R., and Amann, R. (2005). Diversity and distribution of methanotrophic archaea at cold seeps. Appl. Environ. Microb. 71, 467–479. doi: 10.1128/AEM.71.1.467-479.2005
Levin, L. A. (2000). Polychaetes as environmental indicators: response to low oxygen and organic enrichment. B. Mar. Sci. 67:668.
Li, L., Kato, C., and Horikoshi, K. (1999). Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar. Biotechnol. 1, 391–400. doi: 10.1007/PL00011793
Liu, J. L. (2015). Whale fall, an alternative ecosystem in the deep sea. Sci. Public: Middle School Students China 6, 41–43.
Lundsten, L., Schlining, K. L., Frasier, K., Johnson, S. B., Kuhnz, L. A., Harvey, J., et al. (2010b). Time-series analysis of six whale-fall communities in Monterey Canyon, California, USA. Deep-Sea Res. Pt. I. 57, 1573–1584. doi: 10.1016/j.dsr.2010.09.003
Lundsten, L., Paull, C. K., Schlining, K. L., Mcgann, M., and Iii, W. U. (2010a). Biological characterization of a whale-fall near Vancouver Island, British Columbia, Canada. Deep-Sea Res. Pt. I. 57, 918–922. doi: 10.1016/j.dsr.2010.04.006
Lutz, M. J., Caldeira, K., Dunbar, R. B., and Behrenfeld, M. J. (2007). Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J. Geophys. Res-Oceans. 112:C10. doi: 10.1029/2006JC003706
Marshall, B. A. (1987). Osteopeltidae (Mollusca: Gastropoda): a new family of limpets associated with whale bone in the deep-sea. J. Mollus. Stud. 53, 121–127. doi: 10.1093/mollus/53.2.121
Mclean, J. H. (1985). Preliminary report on the limpets at hydrothermal vents. Bull. Biol. Soc. Washington 6, 159–166.
Mclean, J. H. (1992). Cocculiniform limpets (Curculionidae and Pyropeltidae) living on whale bone in the deep sea off California. J. Mollus. Stud. 58, 401–414. doi: 10.1093/mollus/58.4.401
Ohe, T. (1950). Distribution of the red marrow in bones of the fin whale. Sci. Rep. Whale Res. Inst. Tokyo 3, 17–22.
Onishi, Y., Shimamura, S., Yamanaka, T., Nakayama, R., Ozaki, K. I., Miyazaki, M., et al. (2018). Variation of geochemical environments associated with whale-fall biomass mineralization processes in the sediment during the mobile scavenger, enrichment opportunist, and sulfophilic stages. Mar. Biol. 165, 1–17.
Petraitis, P. S., Latham, R. E., and Niesenbaum, R. A. (1989). The maintenance of species diversity by disturbance. Q. Rev. Biol. 64, 393–418. doi: 10.1086/416457
Pettibone, M. H. (1993). Polynoid polychaetes associated with a whale skeleton in the bathyal Santa Catalina Basin. P. Biol. Soc. Wash. 106, 678–688.
Rouse, G. W., Goffredi, S. K., and Vrijenhoek, R. C. (2004). Osedax: bone-eating marine worms with dwarf males. Science 305, 668–671. doi: 10.1126/science.1098650
Scheltema, R. S. (1996). Understanding marine biodiversity — a research agenda for the nation : by committee on biological diversity in marine systems, etc.; national academy press, washington, dc; 1995; ii + 114 pp.; GBP 24.95; ISBN 0-309-05225-4. J. Exp. Mar. Biol. Ecol. 196, 389–390. doi: 10.1016/0022-0981(96)80608-3
Schuller, D., Kadko, D., and Smith, C. R. (2004). Use of 210Pb/226Ra disequilibria in the dating of deep-sea whale falls. Earth Planet. Sc. Lett. 218, 277–289. doi: 10.1016/s0012-821x(03)00690-3
Smith, C. R., and Baco, A. R. (2003). Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. biol. 41, 311–354.
Smith, C. R., Bernardino, A. F., Baco, A., Hannides, A., and Altamira, I. (2014). Seven-year enrichment: macrofaunal succession in deep-sea sediments around a 30 tonne whale fall in the Northeast Pacific. Mar. Ecol. Prog. Ser. 515, 133–149. doi: 10.3354/meps10955
Smith, C. R., Glover, A. G., Treude, T., Higgs, N. D., and Amon, D. J. (2015). Whale-fall ecosystems: recent insights into ecology, paleoecology, and evolution. Annu. Rev. Mar. Sci. 7, 571–596. doi: 10.1146/annurev-marine-010213-135144
Smith, C. R., Kukert, H., Wheatcroft, R. A., Jumars, P. A., and Deming, J. W. (1989). Vent fauna on whale remains. Nature 341, 27–28. doi: 10.1038/341027a0
Smith, C. R., Maybaum, H. L., Baco, A. R., Pope, R. H., and Deming, J. W. (1998). Sediment community structure around a whale skeleton in the deep northeast pacific: macrofaunal, microbial and bioturbation effects. Deep-Sea Res. Pt. II. 45, 335–364. doi: 10.1016/s0967-0645(97)00043-x
Smith, K. E., Thatje, S., Singh, H., Amsler, M. O., Vos, S. C., Mcclintock, J. B., et al. (2014). Discovery of a recent, natural whale fall on the continental slope off Anvers Island, western Antarctic Peninsula. Deep-Sea Res. Pt. I. 90, 76–80. doi: 10.1016/j.dsr.2014.04.013
Stockton, W. L., and DeLaca, T. E. (1982). Food falls in the deep sea: occurrence, quality, and significance. Deep-Sea Res. Pt. I. 29, 157–169. doi: 10.1016/0198-0149(82)90106-6
Tont, S. A., Pearcy, W. G., and Arnold, J. S. (1977). Bone structure of some marine vertebrates. Mar. biol. 39, 191–196. doi: 10.1007/BF00387004
Treude, T., Smith, C. R., Wenzhöfer, F., Carney, E., Bernardino, A. F., Hannides, A. K., et al. (2009a). From whale to chemosynthesis: energy and carbon fluxes at a deep-sea whale fall. Geochim. Cosmochim. Ac. Suppl. 73:A1346. doi: 10.1016/j.gca.2009.03.006
Treude, T., Smith, C. R., Wenzhöfer, F., Carney, E., Bernardino, A. F., Hannides, A. K., et al. (2009b). Biogeochemistry of a deep-sea whale fall: sulfate reduction, sulfide efflux and methanogenesis. Mar. Ecol. Prog. Ser. 382, 1–21. doi: 10.3354/meps07972
Van Der Heijden, M. G., Bardgett, R. D., and Van Straalen, N. M. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310. doi: 10.1111/j.1461-0248.2007.01139.x
Van Dover, C. L. (2000). The Ecology of Deep-Sea Hydrothermal Vents. Princeton, NJ: Princeton University Press.
Vetriani, C., Jannasch, H. W., MacGregor, B. J., Stahl, D. A., and Reysenbach, A. L. (1999). Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl. Environ. Microb. 65, 4375–4384. doi: 10.1128/AEM.65.10.4375-4384.1999
Keywords: whale fall, deep-sea, impact, biodiversity, ecosystems
Citation: Li Q, Liu Y, Li G, Wang Z, Zheng Z, Sun Y, Lei N, Li Q and Zhang W (2022) Review of the Impact of Whale Fall on Biodiversity in Deep-Sea Ecosystems. Front. Ecol. Evol. 10:885572. doi: 10.3389/fevo.2022.885572
Received: 28 February 2022; Accepted: 09 March 2022;
Published: 18 May 2022.
Edited by:
Chao Wang, Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Kunlun Yang, Jiangnan University, ChinaQixuan Song, Nanjing University, China
Yanqiang Du, Nanjing Agricultural University, China
Copyright © 2022 Li, Liu, Li, Wang, Zheng, Sun, Lei, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Li, MTgxMTA3NDAwNzVAZnVkYW4uZWR1LmNu; Weizhen Zhang, emhhbmd3ejE1QGZ1ZGFuLmVkdS5jbg==
 Qihui Li1
Qihui Li1 Yaping Liu
Yaping Liu Weizhen Zhang
Weizhen Zhang