- Comparative BioCognition, Institute of Cognitive Science, University of Osnabrück, Osnabrück, Germany
How human language evolved remains one of the most intriguing questions in science, and different approaches have been used to tackle this question. A recent hypothesis, the Interaction Engine Hypothesis, postulates that language was made possible through the special capacity for social interaction involving different social cognitive skills (e.g., joint attention, common ground) and specific characteristics such as face-to-face interaction, mutual gaze and turn-taking, the exchange of rapid communicative turns. Recently, it has been argued that this turn-taking infrastructure may be a foundational and ancient mechanism of the layered system of language because communicative turn-taking has been found in human infants and across several non-human primate species. Moreover, there is some evidence for turn-taking in different mammalian taxa, especially those capable of vocal learning. Surprisingly, however, the existing studies have mainly focused on turn-taking production of adult individuals, while little is known about its emergence and development in young individuals. Hence, the aim of the current paper was 2-fold: First, we carried out a systematic review of turn-taking development and acquisition in mammals to evaluate possible research bias and existing gaps. Second, we highlight research avenues to spur more research into this domain and investigate if distinct turn-taking elements can be found in other non-human animal species. Since mammals exhibit an extended development period, including learning and strong parental care, they represent an excellent model group in which to investigate the acquisition and development of turn-taking abilities. We performed a systematic review including a wide range of terms and found 21 studies presenting findings on turn-taking abilities in infants and juveniles. Most of these studies were from the last decade, showing an increased interest in this field over the years. Overall, we found a considerable variation in the terminologies and methodological approaches used. In addition, studies investigating turn-taking abilities across different development periods and in relation to different social partners were very rare, thereby hampering direct, systematic comparisons within and across species. Nonetheless, the results of some studies suggested that specific turn-taking elements are innate, while others are acquired during development (e.g., flexibility). Finally, we pinpoint fruitful research avenues and hypotheses to move the field of turn-taking development forward and improve our understanding of the impact of turn-taking on language evolution.
Introduction
Language has been proposed to be uniquely human (Christiansen and Kirby, 2003a; Corballis, 2009; McNeill, 2010) because it involves specific characteristics such as high variation, complexity, open-endedness, and the use of linguistic and socially learned symbols to direct the attentional and mental states of others (e.g., Christiansen and Kirby, 2003a; Pika et al., 2005). However, although the evolution of language has intrigued scientific scholars for centuries (Darwin, 1859) and across scientific disciplines (e.g., Christiansen and Kirby, 2003b; Fitch, 2010; Corballis, 2011; Hauser et al., 2014; Killin, 2017), it still remains a mystery (Knight et al., 2000). Attempts to shed light on language evolution have used different approaches and methods (e.g., comparative approach and purpose of language), focused on different research disciplines (e.g., biology, linguistics, and neuroscience), and used different model systems (e.g., songbirds, great apes; for an overview see Fitch, 2010). In addition, several hypotheses have been postulated ranging from different communicative modalities as starting points (e.g., Hewes et al., 1973; Armstrong and Sherman, 2007; Cheney and Seyfarth, 2010; McNeill, 2010), proto-languages (e.g., Wray, 1998) to the purpose of language (e.g., Shannon, 1948; Hauser et al., 2010; Seyfarth et al., 2010a).
One important approach to investigating the evolution of language is the comparative approach, which investigates similarities and differences between human and non-human animal species, especially non-human primates (hereafter primates) to then draw informed inferences about the abilities of our extinct ancestors (Pika, 2015). Due to the analogy to speech, first comparative studies investigated the vocal abilities of primates with a special focus on Hocketts' design features of language (e.g., interchangeability, semanticity, displacement, flexibility, learnability; Kellogg and Kellogg, 1933; Hayes and Hayes, 1951; Hockett, 1960, 1963). The first studies investigating vocalizations of different monkey species showed that distinct call types are characterized by “semanticity” and “arbitrariness,” while there is no evidence yet for the features “displacement” and “traditional transmission” (e.g., Hockett and Hockett, 1960; Seyfarth et al., 2010b; Slocombe et al., 2011; Pika and Fröhlich, 2019; Janik and Knörnschild, 2021). Furthermore, the majority of studies on primate vocalizations provided evidence that call morphology and usage seem to have limited flexibility, with learning playing a relatively small role only (e.g., Tomasello and Zuberbühler, 2002; Hammerschmidt and Fischer, 2008; Seyfarth and Cheney, 2010; but see Crockford et al., 2004; Schel et al., 2013).
In parallel, researchers also examined gestural abilities of primates in interactions with humans (e.g., Ladygina-Kohts, 1935), by for instance teaching great ape individuals American Sign language (Gardner and Gardner, 1969; Patterson, 1978) and observing natural communicative interactions between conspecifics (e.g., Call and Tomasello, 2007; Liebal et al., 2012; Schel et al., 2022). The studies showed that gestural signaling of primates involves distinct design features such as interchangeability, semanticity, and arbitrariness (e.g., Pika et al., 2003; Cartmill and Byrne, 2007; Hobaiter and Byrne, 2014; Fröhlich et al., 2016a,b). In contrast to vocalizations, however, some studies also provided evidence for the features productivity, traditional transmission, and flexibility (e.g., Leavens and Hopkins, 1998; Call and Tomasello, 2007; Hobaiter et al., 2017; Pika and Deschner, 2019; Prieur et al., 2020).
However, currently no consensus has been reached concerning the evolutionary trajectory of language (Arbib et al., 2008; Cheney and Seyfarth, 2010; Slocombe et al., 2011; Fischer, 2017; Fröhlich et al., 2019a). In addition, Levinson (2006, 2016) recently proposed the “Interaction Engine” hypothesis which suggests that it is not language that makes human communication possible but a special capacity for social interaction. This capacity rests on a layered assemblage of different social cognitive skills, including joint attention (see Box 1 for definitions), common ground, collaboration, and reasoning about communicative intent (Clark, 1996). It also deploys the specific characteristics of face-to-face interaction, frequent employment of mutual gaze, and the exchange of rapid communicative turns—conversational turn-taking (Sacks et al., 1974; Levinson, 2006).
The first systematic framework of conversational turn-taking has been provided by Sacks et al. (1974) in the last century. It consists of an exchange of communicative turns with at least two interlocutors and is governed by specific rules (e.g., avoidance of overlaps, specific temporal relationships, adjacency pairs, communicative repair; see Box 1 for definitions). A recent study by Stivers et al. (2009) investigated the temporal relationships of turns across 10 languages of varied types, geographical locations, and cultural settings and showed that they were all characterized by a similar distribution of response offsets (unimodal peak of response within 200 ms of the end of a given question). The study, therefore, suggested a strong universal basis for turn-taking behavior and emphasizes the antiquity of the turn-taking system. Furthermore, studies on human infants revealed that turn-taking interactions first start around the age of 3 months with infants coordinating actions and signals with caretakers (such as smiles, gaze looking, and facial expressions; Bates et al., 1975; Bateson, 1975; Gratier et al., 2015).
In addition, Levinson and Holler (2014) and Levinson (2016) proposed that the turn-taking infrastructure for conversations may be one of the most ancient layer of the language system with evolutionary precursors present in all the major primate branches. Subsequently, Pika et al. (2018) provided a comprehensive overview of the existing research on turn-taking and related phenomena in the animal kingdom. They showed that although the study of turn-taking abilities in animal species has been growing in the last decades (e.g., Miller et al., 2004; Méndez-Cárdenas and Zimmermann, 2009; Morisaka et al., 2013; Takahashi et al., 2015; Terleph et al., 2018; see Figure 1), the field is strongly biased toward investigations involving primates (e.g., Rossano, 2013; Takahashi et al., 2013; Fröhlich et al., 2016c; Snowdon, 2017; Pougnault et al., 2020).
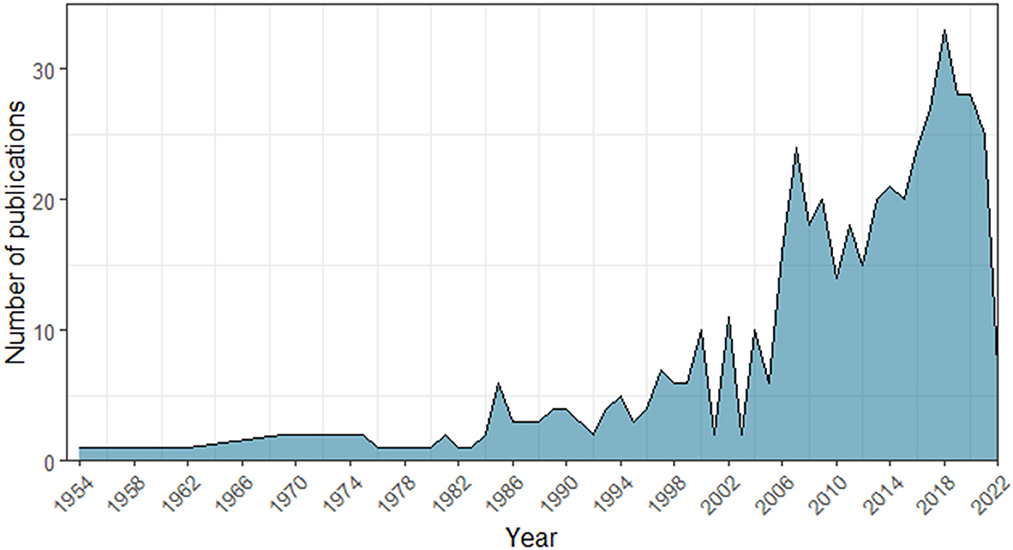
Figure 1. Number of studies indexed in the Scopus and Web of Science databases that included the terms “duet” or “turn-taking” or “antiphonal calling” combined with the words “animal” or “non-human” or “Animalia” or “fauna” across years (1954–2022).
Furthermore, Vanderhoff and Bernal Hoverud (2022) recently provided an overview of communicative exchanges in non-primate mammals with a special focus on antiphonal calling, duetting, and counter-singing (for definitions of terms, see Box 1) and showed that some singing species also possess turn-taking abilities. For instance, Alston's signing mice (Scotinomys teguina) show the ability to combine singing with turn-taking behaviors. These results are aligned with studies on several ape and monkey species, which exhibit some aspects of conversational turn-taking involving signal-signal and signal-action exchanges (Callithrix jacchus: Chow et al., 2015; Takahashi et al., 2015; Gorilla g. gorilla: Luef et al., 2016; Pan paniscus and Pan troglodytes: Fröhlich et al., 2016c; Cercocebus torquatus: Aychet et al., 2021; Alouatta pigra: Briseño-Jaramillo et al., 2021; Pan troglodytes: Pougnault et al., 2021a), distinct temporal relationships (avoiding overlap and presence of gaps between signal-response; e.g., Callithrix jacchus: Yamaguchi et al., 2009; Macaca fuscata: Katsu et al., 2018; Hylobates lar: Terleph et al., 2018; Indri indri: De Gregorio et al., 2019), and adjacency pair-like sequences (Hylobates agilis: Koda et al., 2013; Macaca fuscata: Bouchet et al., 2017; Ateles geoffroyi: Briseño-Jaramillo et al., 2018). Other mammal species and groups such as bats, cetaceans, meerkats, and Alston's singing mice, also show some elements of conversational turn-taking. For instance, they interact and exchange signals, mainly vocal ones, and these turn-exchanges adhere to specific temporal relationships (e.g., Loxodonta africana: Leighty et al., 2008; O'Connell-Rodwell et al., 2012; Physeter macrocephalus: Schulz et al., 2008; Diaemus youngi: Carter et al., 2009; Delphinapterus leucas: Morisaka et al., 2013; Suricata suricatta: Demartsev et al., 2018; Lagenorhynchus obliquidens: Mishima et al., 2018; Scotinomys teguina: Okobi et al., 2019; for a review see Vernes, 2017).
Overall, specific temporal relationships in turn-taking interactions have been found in a variety of non-mammal taxa including amphibians, birds, and insects (see for a recent review Pika et al., 2018; Pougnault et al., 2020; de Reus et al., 2021). Pika et al. (2018), however, concluded that considerable methodological confounds and the employment of different terminologies in the existing studies (e.g., antiphonal calling and duetting) have significantly hampered insightful comparisons across species and an in-depth understanding of turn-taking complexity. To counteract these problems, they proposed a new comparative framework focusing particularly on four key elements of human social action during conversations: involved flexibility, adjacency-pair like sequences, temporal relationships, and participation-framework (Pika et al., 2018; see Box 1 for definitions).
Furthermore, relatively little is also known about the development of turn-taking skills and the acquisition of involved elements (Levinson, 2016; Pika et al., 2018). This is surprising since especially long-living mammal species exhibit extended developmental periods, including social learning, strong parental care, and cooperative behaviors (e.g., Kappeler and Van Schaik, 2005; Yamamoto, 2005; Hudson and Trillmich, 2008; Kerth, 2008; Clutton-Brock, 2009; Kölliker et al., 2012; Rosenbaum and Gettler, 2018; Janik and Knörnschild, 2021). These characteristics make them an excellent group to investigate and understand the acquisition and development of turn-taking abilities and draw inferences about its phylogenetic trajectory.
To date, most studies on turn-taking development have concerned two cooperative breeding species, humans (Homo sapiens; e.g., Henrich et al., 2010; Hilbrink et al., 2015; Nomikou et al., 2017) and common marmosets (Callithrix jacchus), a New World monkey (Chow et al., 2015; Takahashi et al., 2016). The studies on human children were strongly biased toward individuals living in western, educated, industrialized, rich and democratic (WEIRD) societies (Henrich et al., 2010). Furthermore, investigations in both species focused on production rather than comprehension of turn-taking (e.g., Chow et al., 2015; Hilbrink et al., 2015; Takahashi et al., 2015) and specifically examined the onset of turn-taking, temporal relationships, and the role of learning (Snow, 1977; Jaffe et al., 2001; Casillas et al., 2016; Takahashi et al., 2016). Overall, turn-taking production in these two highly social primate species seemed to start relatively early during development (e.g., 0–2 months: Takahashi et al., 2016; 4–6 months: Nomikou et al., 2017), with some studies suggesting that distinct elements (such as temporal relationships and adjacency pair-like sequences) are learned and rely on input and active shaping by caretakers (Chow et al., 2015).
Concerning other species and taxa, however, an in-depth understanding of the acquisition and development of turn-taking and involved elements is currently missing (but see Briseño-Jaramillo et al., 2018; Fröhlich et al., 2019b; Araya-Salas et al., 2020; Ames et al., 2021; Dafreville et al., 2021). Moreover, the few studies available have used different terms, research approaches, and focused on non-comparable age classes, thereby hampering cross-species comparisons and a general understanding of the learning processes involved. Hence, the goal of the current review was 2-fold: First, we aimed to provide a comprehensive overview of the current knowledge of turn-taking acquisition and development in non-human mammals with a special focus on methodologies employed, distribution of studies across species and modalities and components of communication investigated to identify current gaps and research biases. Second, we pinpoint fruitful research avenues to spur more research into this domain and to gain a better understanding of the role of learning, shaping and social tradition for turn-taking development and involved elements.
Methods
Search protocol
We applied the PRISMA search protocol (O'Dea et al., 2021) and used the online search engines Scopus and Web of Science. The following terms were utilized to search titles, abstracts, and keywords of publications: “turn-taking,” “taking turns,” “conversation,” “duet*,” “antiphon*,” “chorus*,” “communicative interaction,” “communicative interactions,” “interactive communication,” “interactive communications,” “dialog*,” “vocal exchang*,” “vocal cooperation,” “vocal production,” “vocal sequence,” “vocal interact*,” “vocal timing,” “vocal overlap,” “verbal exchang*,” “verbal cooperation,” “verbal production,” “verbal sequence,” “verbal interact*,” “verbal timing,” “verbal overlap,” “call exchang*,” “call cooperation,” “call production,” “call sequence,” “call interact*,” “call timing,” “call overlap,” “signal exchang*,” “signal cooperation,” “signal production,” “signal sequence,” “signal interact*,” “signal timing,” “signal overlap,” “gesture exchang*,” “gesture cooperation,” “gesture production,” “gesture sequence,” “gesture interact*,” “gesture timing” and “gesture overlap” combined with the words “develop*,” “learn*,” “ontogen*,” “age,” “offspring,” “cub,” “infant,” “calf,” “group,” “descendant,” “young*,” “litter,” “progeny,” “bab*,” “pup*,” “calves,” “piglet*,” “juvenile” and “immature.” We also combined all these terms with “mammal*,” “primate,” “monkey,” “chiroptera,” “bat,” “rodent*,” “rat,” “soricomorpha,” “carnivora,” “fox,” “wolf,” “bear,” “racoon,” “dog,” “cat,” “mongoose,” “hyena,” “bear,” “weasel,” “pinniped,” “seal,” “ungulate,” “cetacean,” “whales,” “dolphin,” “porpoise,” “beluga,” “pig,” “hippopotamus,” “antelope,” “deer,” “giraffe,” “camel,” “llama,” “alpaca,” “sheep,” “goat,” “cattle,” “marsupial,” “kangaroo,” “koala,” “wallaby,” “wombat,” “possum,” “lagomorph,” “pika,” “rabbit,” “marsupial,” “opossum,” “mole,” “hedgehogs,” “armadillos,” “shrew,” “horse,” “zebra,” “rhinoceroses,” “tapir,” “elephant,” “sloth,” “echidna.” The search (December 2021) returned a total of 2098 manuscripts (without duplicates).
Since the use of the term “turn-taking” has only increased in the last decade (see Figure 2), with former studies applying different terms to refer to turn-taking abilities (such as antiphonal conversation or duetting; Pika et al., 2018; Ravignani et al., 2019), we designed our search protocol as broad as possible. Similarly, we also used both the taxonomic order and common names to search for the groups of organisms of our interest (e.g., bats and chiropteran).
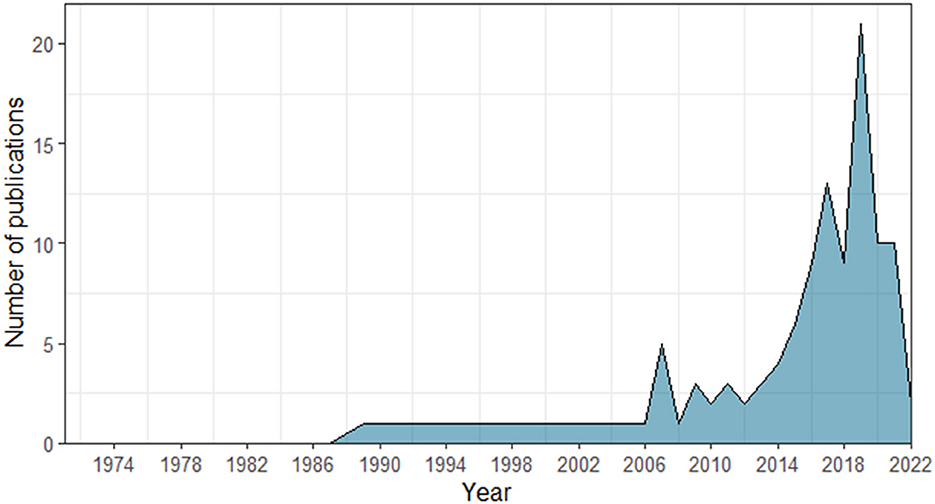
Figure 2. Number of non-human animal studies indexed in the Scopus and Web of Science databases, including the term “turn-taking” across years (1971–2022).
Eligibility criteria and data extraction
To evaluate the manuscripts, we first read the manuscript titles and abstracts and selected only those studies that presented empirical findings on the acquisition and development of coordinated exchanges in non-human mammal species. At this stage, we included in our review all those publications that investigated communicative interactions, defined as an exchange of signals or actions between at least two individuals (where one of them needed to be an infant/juvenile). We also included studies in this review that did not (1) use the term “turn-taking”; (2) focus specifically on turn-taking interactions, and (3) measure specific elements involved in conversational turn-taking (see for definitions Pika et al., 2018). Since the field of comparative turn-taking is a new one, we aimed to provide a relatively broad overview to inform this useful research avenue and inspire future research. We excluded event publications, theses, reviews, methodological articles, and publications containing only human findings.
This method resulted in a total of 74 manuscripts, which were then screened to assess whether they matched the criteria outlined above. We excluded 45 manuscripts and maintained a total of 29 studies matching the selection criteria. To expand our search, we applied the PRISMA protocol (O'Dea et al., 2021) and verified the bibliographic references of the articles chosen. This method resulted in a total of five additional articles. These contained relevant information about turn-taking development and acquisition but did not appear in our search protocol due to the lack of key search terms in the title, abstract, and keywords (for more details on the extraction process, see Supplementary Figure 1).
The articles selected in the previous stage were then systematically screened and read to enable data extraction. Here, we followed a specific “protocol” spreadsheet (e.g., O'Dea et al., 2021; Rodrigues et al., 2021; Ferreira et al., 2022; Supplementary material) to extract the following data from text, figures, tables, or Supplementary material: (i) article information; (ii) subjects studied; (iii) methodology used; (iv) components of the turn-taking investigated; (v) modalities studied and (vi) elements of turn-taking explored following the framework proposed by Pika et al. (2018; Table 1). The information from i to vi was always available in the main manuscript text or Supplementary material. Therefore, we did not contact any authors requesting additional data.
Data analysis
Since we included all publications that presented findings on communicative exchanges, we decided to provide as a first step descriptive statistics to better assess the existing knowledge on the acquisition and development of turn-taking abilities in mammals. Therefore, we clustered the publications into two main categories: Studies that (1) failed or (2) succeeded to present findings that could increase our knowledge of turn-taking abilities in infant/juvenile individuals. Following Pika et al. (2018; Box 1) we defined turn-taking as “purely communicative signals or behaviors between individuals characterized by principles for the coordination of turn transfer, which result in observable temporal regularities. The communicative signals delivered by turns can vary, as can the size and the order of turns, and techniques used to allocate turns to specific individuals.” Second, the studies included in the second category also required to include assessments of at least one element characterizing human social action during conversations (see Pika et al., 2018). For all publications incorporated into this category, we used descriptive statistics (absolute number, frequency, etc.) to compare trends and biases according to (i) terminology used; (ii) taxa used in studies of the development of turn-taking abilities; (iii) research design; (iv) components and modalities of communication; (v) elements of the comparative turn-taking framework; (vi) social factors investigated, and (vii) number of studies that investigated the development of turn-taking abilities over time. We also included and descriptively reported the results of studies that showed a link between turn-taking elements and development.
Results
Overall, we found 34 studies that reported findings concerning communicative interactions (signal-signal or signal-action) between at least one infant or juvenile individual and another conspecific. Thirteen of these studies (38%) were categorized as studies that failed to provide information about turn-taking abilities. They were biased toward species of the primate order (62%), followed by species of the order artiodactyla (14%), chiroptera (8%), rodentia (8%), and proboscidea (8%). Five of these studies focused on the development of communicative interactions (38%), while eight did not address this aspect (62%). None of these studies investigated any developmental markers important to perform comparisons across species such as weaning, locomotion, or feeding independence (see Supplementary Table 1). Some of these studies addressed distinct elements characterizing human social action during conversations (Pika et al., 2018) such as flexibility of turns and temporal relationships. For instance, one study focused specifically on assessing intentionality, thereby also enabling inferences about the flexibility involved in communicative interactions (chimpanzee Pan troglodytes: Bard et al., 2014). Another one examined the timing between the onset of a vocalization and the onset of an action (baboons Papio cynocephalus ursinus: Fischer et al., 2000). The remaining studies focused on vocal recognition and interactions between mother-infant dyads (38%, e.g., Cow Bos taurus: Marchant-Forde et al., 2002; Sheep Ovis aries: Sèbe et al., 2010) or investigated which elements characterized coordinated communicative exchanges [46%, e.g., Gibbons, duets: Nomascus gabriellae (formerly genus Hylobates): Merker and Cox, 1999; Hylobates agilis and Hylobates lar: Koda et al., 2014; Nomascus leucogenys: Hradec et al., 2016; antiphonal calling: Bulldog bat Noctilio albiventris, Brown et al., 1983, see Box 1 for definitions]. For instance, Elowson et al. (1998) observed in a group of pygmy marmosets (Cebuella pygmea) in captivity that until the age of 20 months, crying infants were more likely to change the behavior of a given adult individual (by being carried, being groomed, or getting the opportunity to climb on the back more often) than non-crying infants. Moreover, an experimental study performed on mice (Mus musculus) in captivity reported that mothers responded to the calls of their newborn offspring by increasing the frequency of their maternal behavior (e.g., licking and changing their body position: Ehret and Bernecker, 1986).
The remaining 21 studies involved findings to aid in increasing our knowledge of turn-taking skills in infant or juvenile individuals (62%; see Supplementary Table 1 for more details). In the following paragraphs, we will introduce and discuss these studies in more detail by paying specific attention to (i) terminology used, (ii) distribution across taxa, (iii) research design utilized, (iv) social factors, (v) development, (vi) components and modalities of communication investigated, and (vii) involved turn-taking elements.
Terminology
The first scientific article addressing some elements characterizing turn-taking was published in the 1970's (Matsumura, 1979). It did not explicitly use the term “turn-taking,” but described behaviors exchanged between mother-infant dyads of horseshoe bats (Rhinolophus ferrumequinum nippon). The first paper using the term “turn-taking” was published in the twenty-first century by Lemasson et al. (2011). It focused on the production and comprehension of communicative turn-taking in one group of Japanese macaques (Macaca fuscata) in captivity involving five juvenile individuals. From the 21 citations extracted, only six studies used the term “turn-taking” (29%). Moreover, only one study (5%) defined the terminology in the methods section (Chow et al., 2015), and only one paid attention to specific elements of turn-taking (Fröhlich et al., 2016c).
In sum, the majority of articles found and extracted did not use the term turn-taking but referred indirectly to turn-taking interactions by utilizing terms such as “exchanges” and/or “interactions.” The few studies that specifically used the term “turn-taking” were conducted on primate species and were published in the last decade. This is probably due to the coining of the term and predominant usage in the field of conversational analysis (Sacks et al., 1974), with comparative researchers only recently grasping its importance and implications for language evolution (Pika et al., 2018; Rossano, 2018; Ravignani et al., 2019).
Distribution across taxa
Across all 21 studies, a total of 12 different species were investigated. Most of the studies focused on primates (81%), followed by chiropterans (14%) and cetaceans (5%). Within the primate studies, the majority focused on great ape species (59%), with a strong bias toward chimpanzees (Pan troglodytes; 60%). Turn-taking interactions of Old and New World monkeys were reported in a comparable number of studies (17%), followed by small apes (6%; see Figure 3). Only one study investigated turn-taking abilities in more than one species (chimpanzees and bonobos Pan paniscus: Fröhlich et al., 2016c).
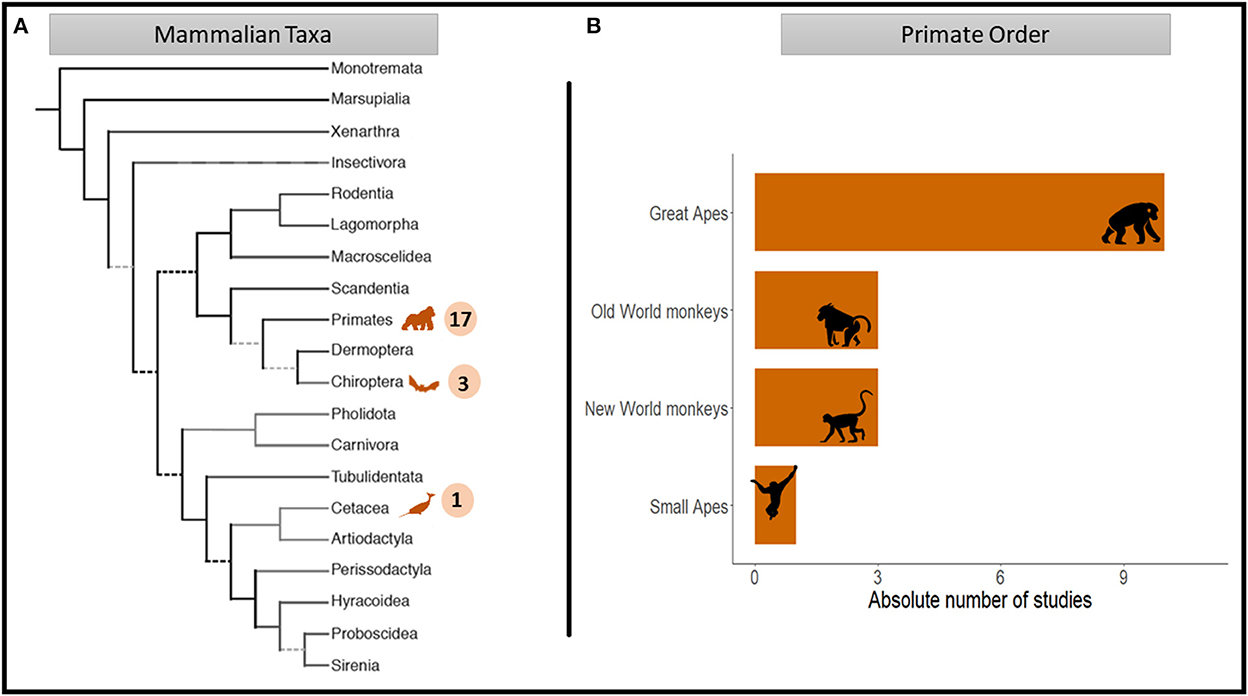
Figure 3. Mammal orders represented in the studies reviewed as a function of the total numbers of turn-taking studies distributed across (A) mammal orders (n = 21) and (B) the primate order (n = 17; adapted from Figure 1A in Springer et al., 2004).
Research designs used
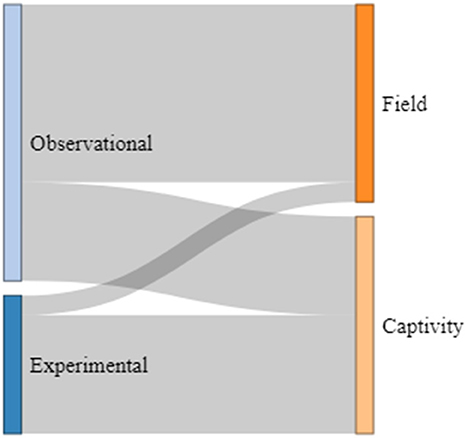
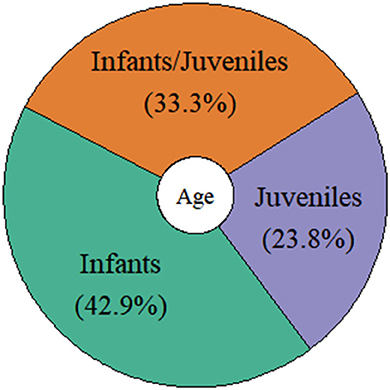
Of the 21 studies considered here, 14 (67%) used observational methods, and seven (33%) applied experimental set-ups. More than half of the observational studies were performed with individuals and species living in their natural environments (64%), while the others were carried out in captive settings (36%). Of those studies using experimental designs, only one was conducted with a species living in its natural environment (14%), while all other studies were performed with species living in captive settings (86%; see Figure 4). The number of individuals observed and tested showed a relatively high variation: Nine studies included < 10 individuals (43%), nine between 10 and 13 individuals (43%), and three studies included more than 13 individuals (14%). For example, studies on narwhals (Monodon monoceros: cetacean) and bonobos (primates) used only one and two individuals, respectively, while the study on horseshoe bats (chiropteran) included a total of 26 individuals. The ages of the individuals investigated in the studies were also relatively broad. For instance, nine studies included infant individuals (ranging from 4 days to 2 years depending on the species, 43%), five investigated turn-taking abilities in juvenile individuals (ranging from 4 months to 4 years, 24%), and seven included both infant and juvenile individuals in their studies (33%; see Figure 5). From those, five compared infant and juvenile individuals or younger and older infant age classes (71%). Only four studies investigated the use and onset of turn-taking abilities of individuals directly after birth (but see for common marmosets, Takahashi et al., 2016; Spix's disc-winged bats Thyroptera tricolor, Araya-Salas et al., 2020).
In sum, we found a wide variety concerning the research designs employed, age groups and number of individuals tested, and very little research concerning turn-taking skills of very young and newborn individuals.
Social factors
The majority of studies that investigated turn-taking acquisition and development focused solely on mother-infant dyads (48%), followed by interactions with conspecifics (individuals from the whole social group; 43%), and parents and siblings (9%; see Figure 6). All species were observed/tested interacting only with their close family members (mothers, fathers, and siblings). However, the studies examining interactions between infants/juveniles and their group members mainly concerned primate species (but see the study on Spix's disc-winged bats, Araya-Salas et al., 2020). For instance, Fröhlich et al. (2016a), who studied the communicative behavior of mother-infant dyads of two different chimpanzee communities (Kanyawara, Kibale National Park, Uganda; Taï South, Taï National Park, Côte D'Ivoire) belonging to two different subspecies (Pan troglodytes schweinfurthii; Pan troglodytes verus) observed that the majority of play initiations by infants were produced toward mothers than toward other individuals. Similarly, in a subsequent paper, Fröhlich et al. (2019b) showed that the likelihood of receiving an inappropriate response across the contexts of joint travel, social play, and food sharing was higher when chimpanzee infants interacted with non-maternal conspecifics than with their mothers. Moreover, Chow et al. (2015) studied communicative interactions of two groups of common marmosets in captivity. They showed that juveniles aged 10–12 months started to interact with their mothers much earlier than their fathers. In addition, the juveniles were more likely to interrupt their fathers but not their mothers' vocalization during Phee call exchanges (used for group coordination; Bezerra and Souto, 2008). Also, the frequency of vocal exchanges decreased in the 1st year of life when exchanging signals with parents but remained constant in sibling-sibling interactions (Chow et al., 2015).
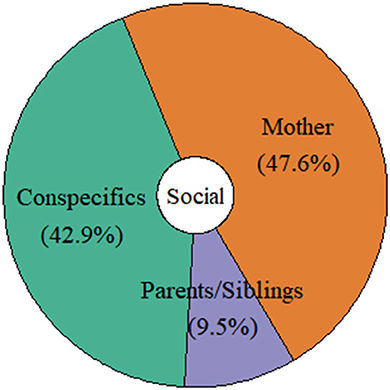
Figure 6. Distribution of the distinct social partners interacting with infants/juveniles in the reviewed studies.
In sum, these studies suggest that parents in some pair-bonded primate species (e.g., owl monkeys and common marmosets) and mothers in polygamous species (e.g., gorillas and chimpanzees) seem to play a crucial role in the acquisition and shaping of distinct turn-taking elements. They act as role models, and shape their infants' responses by providing them with appropriate responses, possibly qualifying as teaching (Musgrave et al., 2016). Teaching—high-fidelity social learning—occurs in the presence of a naïve learner, involves some cost or at least no benefit to the teacher, and facilitates learning in another individual (Caro and Hauser, 1992).
Development of turn-taking
Across all studies considered here, eight studies did not address the development of turn-taking abilities and elements (38%). In contrast, they reported turn-taking skills at a specific time point (e.g., juveniles) or a specific age (e.g., 2 months). For instance, Matsumura (1979) found that captive 1-week-old horseshoe bats, when separated from their mothers, emitted “attractive” calls that only ceased after the mothers approached and took their infants under the wings. Furthermore, the only two studies that addressed turn-taking comprehension showed that juvenile individuals did not adhere to the respective “turn-taking rules” more (e.g., call matching) in comparison to adult individuals (Campbell's monkeys: Lemasson et al., 2011; Japanese macaques: Bouchet et al., 2017). However, the authors did not evaluate the development of turn-taking skills across different age classes. Similarly, Ames et al. (2021) and Knörnschild and von Helversen (2008) observed the behavior of wild narwhals at 5 months and wild greater sac-winged bats at the age of 6 weeks (Saccopteryx bilineata) respectively and showed that at this young age infants already replied vocally to the vocalizations of their mothers.
Additionally, 13 of the 21 studies addressed the development of turn-taking abilities across different age classes (62%). Seven of these studies investigated the development of turn-taking skills continuously (54%), followed by distinctions between age classes (e.g., “infant-juvenile-adolescent” or “baby-younger-older;” 31%) and two specific developmental time points (e.g., “volants and non-volants” which refers to the ability to fly; 15%). The studies addressing turn-taking interactions continuously across a specific ontogenetic time period showed that younger individuals seemed to improve certain elements (e.g., temporal relationships and adjacency pair-like sequences) across ages by stopping to overlap parents' vocalizations and increase call matching. They, therefore, argued that these elements might be shaped and adjusted during ontogeny (e.g., common marmosets: Chow et al., 2015; Takahashi et al., 2016). Furthermore, one of the studies that compared turn-taking skills across different age classes reported that young and old immature spider monkeys living in their natural environments replied less frequently and answered less with the same call type than adults (Briseño-Jaramillo et al., 2018).
Only a minor proportion of these articles investigated developmental milestones with regard to turn-taking development (23%). The examined developmental milestones were locomotion independence (“volant and non-volant:” if an individual can fly; Araya-Salas et al., 2020) and independence from the mother (breastfeeding and locomotion; Genty, 2019 and Dafreville et al., 2021). For instance, Araya-Salas et al. (2020) showed that very young bats (around 5 days of age) living in their natural environments that could not yet fly produced response calls. They also uttered first inquiry calls at the age of 40 days (vocalizations produced when already volant and only during flight) when held on the experimenter's hand, mimicking flight conditions. These findings may suggest that the motivation to respond and engage in turn-taking interactions may be present in some species from very early on, and may not be learned from conspecifics, and only produced in different stages of the development or in the presence of a specific stimulus.
Furthermore, two studies on the development of two great apes' species (chimpanzees and bonobos) provided insights that may be useful to draw inferences to the development of turn-taking skills. For instance, Dafreville et al. (2021), re-using a data set collected on a chimpanzee community in Uganda, showed that it is only when chimpanzees gain full independence from their mothers (around 103–180 months) that they are capable of adjusting the type of gestural signals to the mother's visual attention (considered by the authors when the mother had a full view of the infant) during a signal-action turn-taking interaction. Similarly, Genty (2019), who studied the behavior of seven bonobo infants living in “Lola ya Bonobo” sanctuary, DRC, reported that as infants become more independent from their mothers, their gesture specificity during signal-action turn-taking interaction also increases, showing a developing of adjacency pair-like sequences element across ages. These studies suggest that distinct communication modalities can have different developing times and a need for different cognitive capacities, possibly with vocal responses preceding gestural ones (Fröhlich et al., 2016b). Therefore, the first appearance of turn-taking skills may change according to the modality observed (see Box 1 for definitions of bimodal and multimodality).
In sum, although almost none of the previous studies specifically addressed the development of turn-taking abilities, they suggested that individuals at relatively early ages are capable of engaging in some form of turn-taking with closely related individuals, mainly mothers. However, full-blown adult-like turn-taking abilities may only be present with increasing age.
Components and modalities of turn-taking
Almost all studies investigated the production (90%) but not the comprehension of turn-taking (5%). One study examined both the production and the comprehension (5%). Of the 19 studies that addressed the production of turn-taking, 10 focused solely on the behavior of the initiator of the interaction (53%), and one addressed the recipient's behavior (5%). The remaining studies investigated both the signalers' and the recipients' behavior (42%).
The majority of studies investigated one modality of turn-taking interactions (e.g., gestural or vocal; 67%) only. Of the 10 studies that addressed the signalers' behavior, three investigated vocalizations only (30%), three gestures only (30%), and one focused on gestures and facial expressions (10%). The other three studies examined multimodal communication (30%) in different ape species (chimpanzees: Fröhlich et al., 2019b; bonobos: Genty, 2019; siamang: Liebal et al., 2004). The study investigating the behavior of the recipient considered vocalizations only (100%). Concerning the studies that did not discriminate between signalers and recipients, the modalities investigated were mainly vocal (50%), followed by gestural signals and actions (38%) and multimodal signaling (12%; see Figure 7).
The comparative turn-taking framework
Adopting a previously proposed framework by Pika et al. (2018), we analyzed which papers examined four main key elements of human conversational turn-taking with regards to development: Flexibility of turn-taking organization, temporal relationships, adjacency pair-like sequences, and participation framework (presented below in more detail). In sum, from the 21 studies reviewed here, six measured a single element (29%), seven two elements (33%), and four investigated three elements (19%). All four elements were only investigated in four of the 21 studies (19%). The results also showed that all studies that paid attention to all four elements were carried out in the current century (2010 forward, see Figure 8).
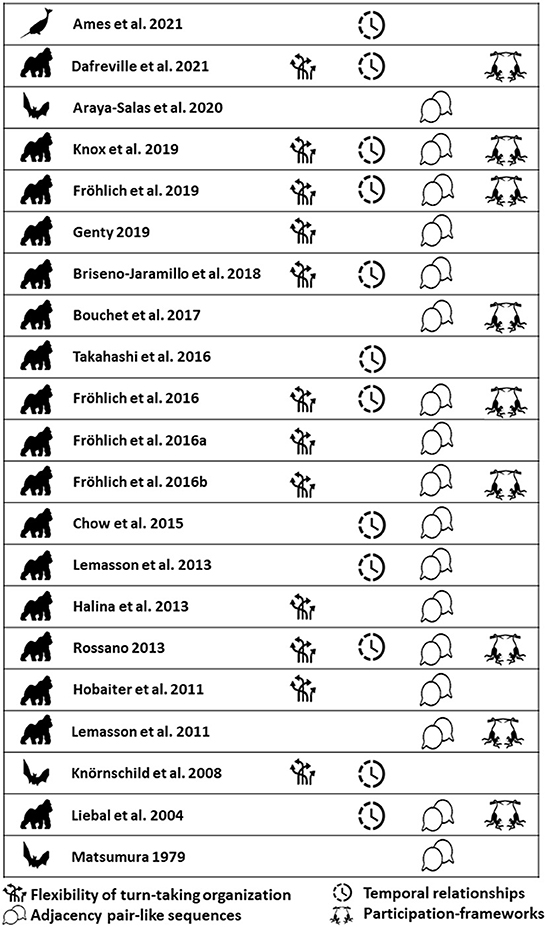
Figure 8. Overview of the four key elements of the comparative framework proposed by Pika et al. (2018) to enable systematic comparisons across species.
Flexibility of turn-taking organization
Twelve of the 21 articles investigated abilities crucial to voluntarily influence and adjust signals/actions (57%). Moreover, only four studies investigated interactions using a multimodal approach (14%). Although not explicitly focusing on turn-taking interactions, they may help inform future studies on turn-taking development. We therefore provide a brief overview here. The 12 studies focused on four specific parameters (57%): audience checking (e.g., directing eyes, head, or body at the recipient before signaling), response waiting (e.g., visual attention on the recipient after signaling), goal persistence (e.g., using same signal when the first one did not result in the desired interaction), and elaboration of signaling (e.g., using different signals when the first one did not result in the desired interaction), representing key criteria of flexibility. For instance, Fröhlich et al. (2019b), studying the communicative development of wild chimpanzees at the Taï and Kanyawara communities, found that audience checking and goal persistence but not sensitivity to the recipient's attentional state increased with age. The recipient's attentional state already occurred at 9 months, while goal persistence and audience checking were only fully developed at the age of 70 months. The authors argued that even at a relatively young age, chimpanzees need to be attentive to the visual orientation of their mothers because it is crucial for mother-infant coordination. However, around 15 months of age, when chimpanzee infants start to interact with other group members, audience checking and goal persistence also begin to play a crucial role (Bründl et al., 2021). In contrast, Dafreville et al. (2021), also working with wild chimpanzees, showed that the adjustment in the use of gestures in relation to the mother's attentional state was age-dependent, with only adolescent chimpanzees adjusting their communication appropriately. The authors explain the difference between their results and Fröhlich et al. (2019b) findings because, in their study, maternal visual attention was considered only when the mother had a full view of the infant.
Moreover, two studies on mother-infant interactions in seven pairs of orangutans living in the Sabangau peat-swamp forest, Borneo, Indonesia (Pongo pygmaeus wurmbii, Knox et al., 2019) and two pairs of bonobos living in the Leipzig Zoo in Germany (Rossano, 2013) showed that individuals aged three and 2 years, respectively, changed and adjusted their signals during communicative exchanges. Rossano (2013) also showed that the use of response waiting started around the age of 2 years in captive bonobos.
A small number of studies examined the flexibility involved in communicative exchanges with a special focus on the variability of signals used during turn-taking interactions (23%). For instance, Genty (2019) showed that the number and specificity of signals by infant bonobos living in captivity to request different actions from their mothers increased with age. Similarly, Briseño-Jaramillo et al. (2018) found that age positively influenced call rates during turn-taking interactions in one group of wild spider monkeys. In sum, the reported findings, provided by studies that did not directly investigate turn-taking interactions, can be helpful to gain knowledge about the development of distinct cognitive skills thereby helping to formulate hypotheses to be tested in future studies.
Overall, the reviewed studies suggested that key criteria of interactional flexibility such as response waiting, goal persistence, elaboration of signaling, and adjustment to audience effects may be acquired in non-human primates during interactions with mothers and other group members. These may act as models, nurturing and even actively influencing the learning process.
Temporal relationships
Twelve studies investigated the temporal relationships present in communicative interactions (57%) by measuring the time span between the onset of the first signal and the response of the recipient. However, the majority of studies did not measure the time between signal and response. Instead, they delimit the time between the first signal and the answer as a turn-taking event. For example, Lemasson et al. (2013) and Briseño-Jaramillo et al. (2018) considered that a call was emitted as a response if it occurred within 2 and 3 s, respectively. Lemasson et al. (2013), who studied a social group of captive Japanese macaques (Macaca fuscata), found that response rates (coo calls given as a response to others and uttered <2 s after the initial call) were less frequently produced by juvenile females aged 8–10 months than by adult females. Moreover, they also found that juvenile females did not adhere to the commonly used temporal relationships in the group (2 s) and produced several consecutive calls repeatedly disrespecting turn-taking principal. Similarly, Briseño-Jaramillo et al. (2018) found that the response rates of wild spider monkeys to calls of conspecifics (calls made within 3 s in response to another call) increased with age.
Furthermore, a study by Takahashi et al. (2016) on three family groups of common marmosets living in captivity showed that individuals avoided overlapping the vocalizations of their parents with increasing age. Moreover, during the 1st month, the infants already engaged in vocal turn-taking interactions with their parents. Similarly, Fröhlich et al. (2016c), who studied bonobos and chimpanzees living in four different communities (LuiKotale at the fringe of Salonga National Park, DRC; Wamba in the Luo Scientific Reserve, DRC, and Kanyawara in Kibale National Park, Uganda; Taï South in Taï National Park, Côte d'Ivoire) in the wild, investigated temporal relationship in interactions by assessing the timing between signals and the respective responses. They divided them into three categories: Immediate responses (<2 s), delayed responses (>2 s), and overlapping responses (<0 s or <1 s). The authors found that overlapping responses were more frequent in interactions between younger infants than between older infants of both species and that chimpanzees, but not bonobos, produced more delayed responses across ages.
Ames et al. (2021) also reported that a narwhal calf of 5 months living in the Scoresby Sound fjord was able to produce a call that either overlapped or occurred within 1 s after the mother's call (78%). The mother only replied to the calf's vocalizations in 16.7% of the time. Since the authors considered a response as “a signal that occurred overlapping or within 1 s of an initiating call,” the mother could be replying after this predetermined time. Thus, it was not considered by the authors as a response. However, to verify whether narwhales learn to engage in turn-taking interactions and the species-typical response times, a better sample size and investigations across development will be needed. In addition, Rossano (2013) showed that the temporal relationships of young bonobos (between the age of 1- and 2-years) in captivity when replying to gestures are very similar to those of their mothers. However, this study did not address the development of the temporal relationship element and just compared this element between infants and adults.
Adjacency pair-like sequences
This element was investigated in the majority of studies (81%) by focusing on the presence of “signal-response” pairs (82%). For instance, Briseño-Jaramillo et al. (2018) found that call matching increased across ages in wild spider monkeys. The authors suggested that this turn-taking element may be learned during development. In a similar vein, two studies on two groups of common marmosets in captivity also showed that younger individuals (age 4–6 months) replied to the Phee calls of their parents by using a non-matching call type, a Twitter call. However, at the age of 8 months they started to use the matching call type to engage in turn-taking interactions with their parents (Chow et al., 2015, but see Takahashi et al., 2016 for another explanation of turn-taking development in common marmosets). Moreover, the authors suggested that some turn-taking elements—adjacency pair-like sequences and temporal relationships—in common marmosets seem to be learned during ontogeny and are actively shaped by the parents. A study by Fröhlich et al. (2016c) investigating bonobos and chimpanzees in natural environments found that age influenced specific turn-taking elements, including adjacency pair-like sequences (considered as the number of gesture-response pairs and response waiting). They found that the number of gesture-response pairs (defined by the authors as the number of gestures produced by the signaler and replied by the recipient with a minimum interval of 1 s) decreased with age in bonobos, whereas it increased with age in chimpanzees. The infants of both species were also more likely to wait for a response than their mothers. Moreover, a study performed by Araya-Salas et al. (2020) showed that wild individuals of Spix's disc-winged bats living in a natural environment already produced matched response calls at the age of 4–6 days. This species is known to engage in antiphonal calling by producing an inquiry call that is usually replied with a “response” call during the flight in a roost (Chaverri and Gillam, 2010; Chaverri et al., 2013).
Additionally, the two comprehension studies (Lemasson et al., 2011; Bouchet et al., 2017) that investigated the behavior of Campbell's monkeys (around 2–3 years) in captivity (Rennes University, France), and captive females of Japanese macaques (12–16 months) in captivity (Primate Research Institute, Japan), found that individuals did not pay attention to species-specific turn-taking rules during playback experiments. For instance, juvenile Campbell's monkeys, exposed to two different stimuli (appropriate vocal exchange: A1BA2 and inappropriate vocal exchange: BA1A2), did not show differences in their looking behavior toward the loudspeaker. Similarly, juvenile Japanese macaques were exposed to matching calls (e.g., AbBb) and non-matching calls (e.g., AbBw) via loudspeakers. Their response showed a random distribution regardless of the type of stimulus. The authors attributed these results to the lack of experience of the young individuals in turn-taking interactions, thus, suggesting a possible role of social learning for the production and comprehension of different turn-taking elements. Furthermore, they also argued that participating and being exposed to turn-taking interactions may be a necessary step for fully understanding the turn-taking rules.
Other studies addressed if the signaler was “satisfied” with the response from the recipient (gestures or actions) by investigating intentional gesturing and ontogenetic ritualization or included this parameter as a requirement in the study methods. For instance, Hobaiter and Byrne (2011) and Halina et al. (2013), who studied one community of chimpanzees at the Budongo Forest Reserve (Uganda) and 10 mother-infant bonobo dyads from six zoos, respectively, focused only on signals produced by infants and juveniles that presented a satisfactory outcome to the signaler. However, they did not investigate the changes across development. Similarly, some studies examined the use of intentional signals by chimpanzee infants and juveniles to start interactions—for example, playing and traveling—but did not specifically address the questions of distinct gesture-response pairs (Fröhlich et al., 2016a,b).
Although some of the presented studies did not provide detailed information concerning the development of adjacency pair-like sequences in mammals, they seem to suggest that this ability may be learned in some orders (e.g., primates) and present in others from birth (e.g., bats; Montero and Gillam, 2015; Araya-Salas et al., 2020). However, further research is needed to rule out the possibility of fast learning (Knörnschild, 2014). Moreover, this element may be crucial in species where communicative responses increase survival and reproductive success (e.g., Montero and Gillam, 2015; Araya-Salas et al., 2020).
Participation-frameworks
Nine of the 21 articles addressed the element of participation frameworks in communicative interactions (43%). All these studies used gaze and body orientation as testing parameter (100%). One also measured the distance between the signaler and the receiver. For instance, Rossano (2013) found that 2-year-old captive bonobos established participation frameworks by looking toward their mothers before signaling. Dafreville et al. (2021) and Fröhlich et al. (2019b) included in their studies “eye gaze toward a recipient” as a parameter to be able to consider an exchange of signal-action as an interaction. However, the authors did not measure this element across ages nor did they present analyses of this specific element in their results. Moreover, the studies suggest the presence of this capability at younger ages in distinct chimpanzee communities. Finally, a study conducted by Fröhlich et al. (2016c) with wild bonobos and chimpanzees highlighted that body orientation and initiation distance increased with infant age in both species. These results suggested that similar to the participation-framework element, other turn-taking elements also improve during ontogeny in these two primate species.
In sum, the presented studies suggest that the elements flexibility, and participation-frameworks seem to have a strong learning component, in which full-blown adult-like behavior only appears with increasing age, especially in different primate species. In contrast, other studies suggested that some turn-taking elements, such as temporal relationship and adjacency pair-like sequences, seem to show distinct developmental trajectories according to the non-human species, with some mammal species presenting developed turn-taking elements early in life (e.g., Matsumura, 1979; Rossano, 2013; Araya-Salas et al., 2020). For instance, bats of the species Thyroptera tricolor seem to be able to use adjacency pair-like sequences directly after birth. It may be possible that this feature is present since birth, but due to the lack of systematic investigations, the possibility of fast learning cannot be ruled out (Knörnschild, 2014). In contrast, some primate species (such as common marmosets or spider monkeys) appear to learn how to match calls across their development. However, the majority of studies that enabled insights into species' capacities to engage in turn-taking interactions at early ages did not investigate the development of turn-taking longitudinally or during the 1st days of life.
Discussion
The present review aimed to summarize the current knowledge of turn-taking acquisition and development in non-human mammals by carrying out a systematic review of the existing body of research. This approach resulted in a total of 21 studies using experimental and observational methods to investigate the development and acquisition of turn-taking abilities in infant and juvenile individuals of a total of 12 mammal species, mostly primates. Overall, the studies showed considerable variation in methodological approaches and terminologies and were biased toward specific model species (e.g., chimpanzees, common marmosets, and bonobos), and social factors (e.g., mother-infant interactions). As a result, systematic comparisons across species and a detailed understanding of the acquisition and development of turn-taking abilities across mammals is currently not yet possible. In the following paragraphs, we will highlight and discuss the existing gaps and biases in more detail with a special focus on species, developmental milestones, social factors, and turn-taking elements. We will also pinpoint fruitful research avenues to spur more research into this intriguing and new research domain.
Terminologies used
Similar to a recent cross-species review on turn-taking skills by Pika et al. (2018), we found a high degree of heterogeneity concerning the terminologies used. Quite naturally, the term has mainly been used by linguists since the first systematic framework originated in this field (e.g., Sacks et al., 1974; de Ruiter et al., 2006; Stivers et al., 2009). In the twenty-first century, Levinson (2006) stirred considerable interest in turn-taking and involved cognitive processes, particularly in the fields of cognitive science and animal communication (e.g., Logue and Stivers, 2012; Wilkinson et al., 2012; Levinson and Torreira, 2015). Hence, the field of comparative turn-taking is just emerging, and the term may be embraced more in future studies and research. Furthermore, many studies used the term when referring to and investigating temporal relationships only or utilized traditional ethological terms such as “antiphonal calling” and “duetting” (e.g., for recent overviews Pika et al., 2018; Ravignani et al., 2019).
Species and methodological bias
We found a strong research bias toward non-human primates, specifically great ape species (e.g., chimpanzees and bonobos). Furthermore, we found some evidence for turn-taking in infants of other mammalian taxa, especially those capable of vocal learning (e.g., bats and cetaceans). Due to their close phylogenetic proximity to humans (Langergraber et al., 2012; Prüfer et al., 2012), great apes and particularly chimpanzees (Beck, 1982; Gruber and Clay, 2016; Bezanson and McNamara, 2019) have been the focus of a lot of research studies (e.g., Lemasson et al., 2018; Dezecache et al., 2019; Miglietta et al., 2021). For several decades, great apes have been investigated regarding their gestural, vocal, and bimodal communication (e.g., Call and Tomasello, 2007; Genty et al., 2009; Slocombe et al., 2011). Consequently, this attention has also resulted in several studies addressing turn-taking skills in great apes, with a considerable research bias on chimpanzees and bonobos (e.g., Rossano, 2013; Fröhlich et al., 2016c; Genty, 2019) as well as adult individuals (e.g., Luef and Pika, 2017; Levréro et al., 2019; Pougnault et al., 2021b; Rodrigues et al., 2021; Cornec et al., 2022). While studying turn-taking in our closest living relatives is crucial and may aid in developing, in comparison with data from modern humans, more accurate estimates of our extinct ancestors (e.g., Wrangham, 1987; Gruber and Clay, 2016; Muller, 2018), these studies offer only limited insight into abilities derived by convergent evolution (e.g., Emery and Clayton, 2004). A better understanding of the role of turn-taking for sophisticated communication systems and the selective pressures involved can therefore only be gained by studying and comparing turn-taking skills also in and across more distantly related species that live in comparable social settings or show some comparable social aspects (e.g., corvids, cetaceans, New World primates, and Strepsirrhines).
The assessment of research designs employed (e.g., age, the number of individuals, and study design) showed that research interest in this new field increased considerably. However, the studies were unevenly distributed between and within mammal species. In addition, the majority of studies conducted in captivity used experimental designs, while studies carried out in natural settings applied both observational and experimental designs. Moreover, we found a wide variety on the sample size and the age of investigated individuals. Given that infants of different mammal species develop at different rates and show distinct time dependencies and attachments to their mothers (e.g., gorillas: Hoff et al., 1983; common marmosets: Schiel and Huber, 2006; Wang et al., 2014; dolphins: von Streit et al., 2013; bats: Mehdizadeh et al., 2018; chimpanzees: Bründl et al., 2021), future longitudinal studies (infancy to adulthood) could be useful to better understand the linkage between turn-taking skills and developmental milestones thereby avoiding that age becomes a confounding factor. For instance, in marmosets, weaning and locomotory independence starts at the age of ~1 and 3 months, respectively (Tardif et al., 2003; Schultz-Darken et al., 2016). In contrast, chimpanzees start to walk independently only after the age of 6 months (Goodall, 1986; Bründl et al., 2021) and stop breastfeeding after ~4 years of age (Samuni et al., 2020). Moreover, offspring of species with prolonged periods to gain independence and extended attachment periods with their mothers are exposed to more learning opportunities and interaction partners, teaching and scaffolding to learn, develop and fine-tune their turn-taking skills. In support of this hypothesis, mother-infant bonding has been shown to be correlated with social communication (e.g., interacting with others in adult life, affective communication) and relationship preferences in different long-living mammal species (e.g., Boccia et al., 1991; Suomi, 2005; Maestripieri, 2018; Verderane et al., 2020). In addition, mothers in these species are quite naturally the first role models for social learning (Whiten and van de Waal, 2018).
Moreover, social and ecological factors have been shown to affect and shape communicative repertoires, usage and, consequently, the exchange of signals (e.g., Fröhlich et al., 2019b, 2021; Pika and Fröhlich, 2019; Roberts and Roberts, 2020). For instance, Fröhlich et al. (2019b) showed that gesture frequency and repertoire size in wild chimpanzees increased with higher interaction rates with non-maternal conspecifics. Thus, future developmental studies could pay special attention to species' biology and control for the influence of social and ecological factors (see also Bräuer et al., 2020).
The influence of social factors
Overall, half of the studies that investigated the role of social factors focused on interactions between mothers and their infants, whereas a smaller proportion also investigated interactions with non-related group members (e.g., Hobaiter and Byrne, 2011; Briseño-Jaramillo et al., 2018). For instance, Hobaiter and Byrne (2011) and Liebal et al. (2004) examined the understanding of intentionally produced signals of infants/juveniles with members of their social group in chimpanzees living in a natural environment and siamangs living in captivity, respectively. Studies focusing on adult individuals have, however, already shown the influence of distinct social factors on turn-taking skills (e.g., Leong et al., 2003; Digweed et al., 2007; Lemasson et al., 2010; Arlet et al., 2015; Levréro et al., 2019; Jenikejew et al., 2020; Pougnault et al., 2021b). For instance, Levréro et al. (2019) showed that social affinity (measured by spatial proximity) influenced the response rate of vocal calls (mainly Peep yelps and Peeps) in captive bonobos. Lemasson et al. (2010) found that captive Campbell's monkeys replied vocally more frequently to older individuals. Moreover, the strength of social bonds seems to be the best predictor of vocal and gestural exchanges in adult individuals of different mammal species (e.g., Fedurek et al., 2013; Roberts and Roberts, 2016; Fröhlich et al., 2017; Toarmino et al., 2017; Kavanagh et al., 2021; Chereskin et al., 2022).
Therefore, it may be possible that some of these factors also shape the communicative development of infants/juveniles. For instance, parents (mother or father) seem to have different influences on the learning processes involved to acquire distinct turn-taking skills. For instance, Chow et al. (2015) showed that in common marmosets living in captivity, parents play essential roles in the development of turn-taking, with juveniles replying differently to the vocalizations of their mothers and fathers compared to their siblings. Moreover, it may be possible that distinct turn-taking elements develop at different developmental rates in cooperative, solitary, or pair-bonding living species due to the number of individuals available to interact with infants/juveniles. Social learning opportunities are provided mainly through mothers in many mammal species (e.g., Bender et al., 2009; van Schaik et al., 2017; Whiten and van de Waal, 2018). However, other group members can also act as role models (e.g., Thornton and Clutton-Brock, 2011; Allen, 2019). Van Boekholt et al. (2021) suggested that a higher number of individuals in the group positively influences learning opportunities in a wide range of behaviors. Similarly, in humans, variability of the interactions (e.g., heterogeneity and numerosity) positively affect learning in different domains, including language (Raviv et al., 2022). Moreover, Fröhlich et al. (2017) demonstrated that in chimpanzees in the wild, interaction rates with other group members crucially influenced communicative exchanges of infants and resulted in a higher number of gestures used in their interactions and hence their gestural repertoires. In the present review, we also found that success and frequency of turn-taking interactions were more common with mothers (Fröhlich et al., 2016c, 2019b). Overall, it seems that different role models (mother, father, or non-related group members) provide crucial but also different learning opportunities to infants. Thus, observing the development of turn-taking with regards to the whole complexity of the respective social group may significantly strengthen our understanding of how and which social factors influence the acquisition and development of turn-taking abilities.
Linkage to developmental milestones
Only relatively little research focused on the acquisition and development of turn-taking and a possible linkage to developmental milestones (Genty, 2019; Araya-Salas et al., 2020; Dafreville et al., 2021). For instance, Genty (2019) and Dafreville et al. (2021) investigated turn-taking abilities of bonobos living in captive settings and chimpanzees living in natural environment and correlated the ages to two developmental milestones, breastfeeding and locomotion. Araya-Salas et al. (2020) investigated the developmental milestone “volant and non-volant” in Spix's disc-winged bats in the wild to assess whether they are able to engage in call-response exchanges. Since the life cycles of mammals can be very different (Western, 1979), the linkage between developmental milestones and turn-taking abilities is essential to enable systematic comparisons across mammal species. Moreover, it is also crucial to better understand how turn-taking abilities and involved elements correlate with the social development of a given species. For instance, some studies showed that several social behaviors in chimpanzees only start later in life and are shaped and scaffolded during ontogeny (e.g., mutual grooming, nut-cracking; Boesch and Boesch, 1983; Goodall, 1986; Matsuzawa, 1994). However, some skills crucial to engage in turn-taking may develop earlier (e.g., goal persistence and audience checking in chimpanzees: Plooij, 1978; Fröhlich et al., 2019b).
In sum, some turn-taking skills and underlying cognitive prerequisites are acquired before mammals engage in frequent social interactions with mothers, possibly shaping and scaffolding these learning processes (e.g., Luef and Pika, 2013; Chow et al., 2015; Musgrave et al., 2016; Whiten and van de Waal, 2018). Thus, linking developmental milestones to turn-taking skills may offer crucial insights into similarities and differences of turn-taking skills and involved elements between mammal species and beyond.
Components and modalities of turn-taking
The investigated components and modalities of communication (production vs. comprehension, signaler vs. recipient, type of signals used) also diverged across studies. Most studies investigated the development of turn-taking production but not comprehension. Although this bias is probably due to studying comprehension being more complicated than production, experimental field studies and current advancements in technology (e.g., observer gaze paradigm and cognitive field experiments using tablets; Hayashi et al., 2020; Lewis and Krupenye, 2022) may enable a methodological balance and a systematic understanding of the cognitive processes needed to understand turn-taking in others.
We also found that not all studies analyzed both the signalers' and recipients' perspectives (e.g., Briseño-Jaramillo et al., 2018; Knox et al., 2019). Although all studies focused on interactions between signalers and recipients, the analyses were biased toward signalers. However, investigating both the behavior of the signaler and the recipient is crucial since some aspects of human conversational turn-taking, such as temporal relationships and communicative repair (Sacks et al., 1974), can only be explored when focusing on both interlocutors (Heesen et al., 2022; Kolff and Pika, 2022).
Furthermore, studies investigating multimodal turn-taking exchanges were relatively limited (e.g., Fröhlich, 2017; Fröhlich et al., 2019b), mirroring a general bias in animal communication research (e.g., Slocombe et al., 2011; Liebal et al., 2012; Prieur et al., 2020; but see Genty et al., 2014). All studies investigated either the vocal or the gestural modality only and the modality changed according to specific model systems studied. For example, gestural interactions were mainly investigated in great apes (e.g., Bard et al., 2014; Knox et al., 2019; Dafreville et al., 2021) whereas vocal exchanges were studied in other primate species and non-primate mammals (e.g., Chow et al., 2015; Araya-Salas et al., 2020). However, as already reported in human children, the use of distinct modalities and multimodal combinations may have different developmental trajectories (e.g., Bates et al., 1975; Holler et al., 2015; Fröhlich et al., 2016c), thereby affecting the first onset and appearance of turn-taking skills. Thus, unimodal and multimodal turn-taking, with the later probably reflecting a higher degree of cognitive flexibility, may also be characterized by different acquisition and developmental times in other mammal species. Hence, using a more holistic approach onto communicative signaling may be important to gain a better understanding of the acquisition and development of turn-taking skills and the importance of turn-taking for language to evolve (Levinson and Holler, 2014; Fröhlich et al., 2019a; Holler and Levinson, 2019). It may also enable better comparisons between mammal species, including humans.
The comparative framework
Recently, Pika et al. (2018) developed a systematic framework to enable systematic comparisons of turn-taking abilities across different species (Pika et al., 2018). Although, they pointed out already that scholars used a wide variety of different terms to describe similar phenomena, we still found considerable variation of terminologies used across recent mammal studies focusing on communicative exchanges. Moreover, the studies were biased toward specific turn-taking elements such as temporal relationships and adjacency pair-like sequences (e.g., Matsumura, 1979; Lemasson et al., 2013; Takahashi et al., 2016; Ames et al., 2021). One explanation for this finding is that temporal relationships can be reliably and consistently measured in both captive and wild individuals and settings and across communicative modalities (e.g., Wong et al., 2004; Kondo et al., 2010; Ames et al., 2021). In addition, measuring the temporal aspects of signals has a long tradition in ethology (Catchpole and Slater, 2008; Pika et al., 2018; Ravignani et al., 2019; de Reus et al., 2021).
The few studies that examined all four elements of the comparative framework involved only older individuals (around 1 year old or more; Rossano, 2013; Fröhlich et al., 2016a,b, 2019b; Knox et al., 2019) and did not always measure each element using the same parameters. For instance, parameters used to assess temporal relationship and flexibility of turns were very broad and were investigated from different approaches. While some studies provided a limited interval time between the offset of the first signal and the onset of the response to consider the exchange as a turn-taking interaction (e.g., Briseño-Jaramillo et al., 2018), others measured the time between the two signals providing the mean of all interval times (e.g., Rossano, 2013). One explanation may be that studies collecting data and measuring and analyzing all involved elements are very time-consuming. In addition, reliable assessments of specific parameters underlying some elements, such as intentionality (Dennett, 1983), may be difficult (Rodrigues and Fröhlich, 2021). However, the quickly developing field of machine learning may offer new solutions to overcome these challenges in the future.
In sum, although the form of turn-taking exchanges of young individuals differed from full-blown turn-taking interactions in adults—similarly to human children—they were characterized by the elements of flexibility, participation frameworks, and temporal relationships. For instance, the element flexibility seems to be positively correlated with age, indicating a possible learning process involved. In addition, studies examining participation frameworks and adjacency pair-like sequences showed that these elements are learned during development in primates (Fröhlich et al., 2016c; Takahashi et al., 2016; Briseño-Jaramillo et al., 2018). However, it is important to note that adjacency pair-like sequences in some non-primate species may already be present at birth (Montero and Gillam, 2015; Araya-Salas et al., 2020). For instance, Araya-Salas et al. (2020) showed that newborn non-volant bats already produced inquiry calls (only produced during flight) when mimicking flying conditions. Further studies could focus on these elements and investigate them across different ages and mammal species. Moreover, the use of signals in appropriate contexts and circumstances may change with regards to the involved costs, benefits, and survival risk (Krebs and Dawkins, 1985; Zeifman, 2001; Laidre and Johnstone, 2013). For example, the survival of young individuals of species where the mothers leave their offspring for considerable time periods to collect food (e.g., seals, bats) relies heavily on correctly replying to their mother's signal. Therefore, this capacity and the intrinsic motivation to reply but also to recognize the mother's call needs to be present early in life in these species.
On the other hand, although the element of temporal relationships is one of the most frequent elements investigated in the existing literature, the results are also the most contradictory in both human and non-human species. For instance, some studies showed that younger animals use response times similar to those of adult individuals (e.g., bonobos: Rossano, 2013; belugas Delphinapterus leucas: Vergara et al., 2010), while other studies revealed that young individuals changed their response times with increasing age by decreasing the time of overlap between signals and converging to adult response times (e.g., common marmosets: Takahashi et al., 2016). Similarly, studies investigating temporal relationships in turn-taking interactions of human children also produced mixed results (e.g., Hilbrink et al., 2015; Dominguez et al., 2016; but see Nguyen et al., 2022 for a systematic review in the development of timing in adult–child turn-taking interactions). However, the differences may be due to comparisons between subjects and study groups of different ages (e.g., 1- to 2-year-old children) with older individuals possibly having learned the temporal relationships via active and passive shaping in interactions with their caretakers. Thus, further studies may investigate individuals at younger ages to gain a better understanding of the evolution and development of this element and the linkage to the ecology of a given species (see also Bräuer et al., 2020).
Limitations, future directions, and concluding remarks
One of the major limitations of the current review was the lack of available studies investigating interactions of individuals at early ages in different mammal species (e.g., newborns or individuals aged 1–6 months of life), longitudinal studies as well as studies linking turn-taking abilities to developmental milestones (Dafreville et al., 2021). Even when the focus was on newborns, the observational periods were restricted to 4 weeks and 2 months, respectively (e.g., Matsumura, 1979; Takahashi et al., 2016). In contrast, studies that addressed longer developmental time spans did not observe individuals of younger ages (e.g., 10–24 months: Halina et al., 2013; 9–36 months: Fröhlich et al., 2016a) or included a limited number of individuals [e.g., 11 individuals distributed in three age classes (infants-juveniles-adolescents): Dafreville et al., 2021].
However, this research field is still very new, with few published data but with a high potential to help us gain a better understanding of turn-taking, the impact of prosociality on turn-taking evolution and cooperation and the role for language evolution (Yoshida and Okanoya, 2005; Pika et al., 2018). We thus hope to have stirred interest in this new research field to increase future research efforts, and longitudinal studies. Moreover, the review also revealed the challenges of collecting behavioral data, especially when filming interactions with infants in natural settings due to poor visibility, restricted access, and difficulties in following animals through longer time-periods. Thus, one possible solution is to join forces and work collaboratively with other researchers to create large datasets, as has already been done in projects such as ManyPrimates, ManyBirds, ManyBabies, and 1000PAN (Primates et al., 2019; Lambert et al., 2021; Comparative BioCognition, 2022).
Although we found a considerable number of cross-sectional studies, these were biased toward great ape species (e.g., chimpanzees and bonobos). While studying turn-taking abilities in our closest living relatives has key importance, these findings offer only limited insights into the selective pressures favoring the onset and development of cooperative communication (e.g., Vygotsky, 1978; Tomasello, 2008; Pika and Bugnyar, 2011). Carrying out systematic investigations of turn-taking abilities and their development across selected mammal species differing in distinct ecological and social factors will contribute to a more profound knowledge of involved elements and the evolutionary precursors and trajectory of skills constituting “the interaction engine” (Levinson, 2010, 2016). Moreover, we also found a high variation in the elements investigated and a lack of essential measurable variables in each turn-taking element in most of the considered studies, preventing us from drawing reliable conclusions regarding a possible evolutionary trajectory of turn-taking elements.
Nonetheless, the results of the present review suggest that turn-taking abilities and involved elements may have different evolutionary and ontogenetic trajectories depending on the species, social and ecological factors. These findings enable the formation of predictions and hypotheses that can be addressed and tested in future studies to move the field of comparative turn-taking forward (Pika et al., 2018).
For instance, we hypothesize that the onset of cooperative communication is tightly linked to the ecology of a given species and arose to increase reproductive fitness. If this hypothesis is true, mammal species with feeding ecologies that require the mothers to leave their offspring for extended periods and then locate them again respond to contact calls earlier than species where the offspring grows up clinging to the mother's body (e.g., primates) or stays in nests or close proximity (e.g., mice).
We also predict that the onset of the turn-taking elements flexibility of turn-taking organization and adjacency pair-like sequence are correlated with developmental milestones (e.g., timing of weaning, feeding and spatial independence). These elements may be present earlier in species that are characterized by shorter rates of independence, weaning, or that possess shorter periods in close body contact and proximity with their mothers/caretakers.
Moreover, the speed of development of turn-taking elements might be linked to demographic and social factors (e.g., sex, mating system, and parental care strategies). For instance, infants of mammal species that live in large groups or groups that possess cooperative parental care and provide their infants with a higher number of interactions and learning possibilities may show faster learning processes than solitary or pair-bonded species. The possible influence from social and parental care systems on turn-taking abilities has also been supported by Ravignani et al. (2022). Furthermore, since the singing behavior of mammals may also be influenced by the social system and the degree of territoriality (e.g., De Gregorio et al., 2022), future studies into turn-taking skills and acquisition patterns of singing mammals may be crucial to test whether evolutionary new inferential processes ensue when communication becomes governed by more cooperative motives (Vygotsky, 1978; Pika and Bugnyar, 2011).
In conclusion, we highlight five “take-home messages” to nurture the design, implementation, and comparisons of future studies when investigating the acquisition and development of turn-taking abilities: (1) The “turn-taking” terminology should be included in the abstract, title, or keywords of the manuscript; (2) data are needed in systematically selected model systems of mammals differing with regards to social system, parental care and ecology; (3) different social factors, and more extensive developmental periods should be investigated; (4) a more holistic approach to communicative interactions is needed involving different communicative modalities and multi-modal interactions; (5) given the variation across the elements used, the inclusion and usage of specific, measurable variables for non-human animals can be of extreme relevance. These five bullet points will hopefully open up future opportunities for this research field, allowing a better assessment and comparison of the acquisition and development of turn-taking abilities in mammal species. Moreover, based on recent experimental and conceptual studies that investigated the neural circuit mechanisms of vocal turn-taking in different mammal and bird species (e.g., Banerjee and Vallentin, 2022; Ravignani et al., 2022), non-invasive neuroethological approaches may also be very fruitful to move the field forward.
We thus hope that the present review served to highlight the gaps and trends in the study of the acquisition and development of turn-taking in mammal species and pinpointed the challenges and difficulties of this research field.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SP had the idea for the present paper and designed it with FA. FA collected and analyzed the data and wrote the first draft. FA and SP edited and finalized the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This paper is part of a project that was funded by an EU-Consolidator grant (772000, TurnTaking) to SP of the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme.
Acknowledgments
We are grateful to S. Cosper for his help editing the manuscript and constructive discussions. We also thank the entire Comparative BioCognition (CBC) team for all support and helpful discussions and the reviewers for very constructive comments to increase the quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.987253/full#supplementary-material
References
Allen, J. A. (2019). Community through culture: from insects to whales. BioEssays 41, 1900060. doi: 10.1002/bies.201900060
Ames, A. E., Blackwell, S. B., Tervo, O. M., and Heide-Jørgensen, M. P. (2021). Evidence of stereotyped contact call use in narwhal (Monodon monoceros) mother-calf communication. PLoS ONE 16, 1–19. doi: 10.1371/journal.pone.0254393
Araya-Salas, M., Hernández-Pinsón, H. A., Rojas, N., and Chaverri, G. (2020). Ontogeny of an interactive call-and-response system in Spix's disc-winged bats. Anim. Behav. 166, 233–245. doi: 10.1016/j.anbehav.2020.05.018
Arbib, M. A., Liebal, K., and Pika, S. (2008). Primate vocalization, gesture, and the evolution of human language. Curr. Anthropol. 49, 1053–1076. doi: 10.1086/593015
Arlet, M., Jubin, R., Masataka, N., and Lemasson, A. (2015). Grooming-at-a-distance by exchanging calls in non-human primates. Biol. Lett. 11, 711. doi: 10.1098/rsbl.2015.0711
Armstrong, D. F., and Sherman, E. W. (2007). The Gestural Origin of Language. New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780195163483.001.0001
Aychet, J., Blois-heulin, C., and Lemasson, A. (2021). Sequential and network analyses to describe multiple signal use in captive mangabeys. Anim. Behav. 9, 5. doi: 10.1016/j.anbehav.2021.09.005
Bakeman, R., and Adamson, L. B. (1984). Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Dev. 55, 1278. doi: 10.2307/1129997
Banerjee, A., and Vallentin, D. (2022). Convergent behavioral strategies and neural computations during vocal turn-taking across diverse species. Curr. Opin. Neurobiol. 73, 102529. doi: 10.1016/j.conb.2022.102529
Bard, K. A., Dunbar, S., Maguire-Herring, V., Veira, Y., Hayes, K. G., and Mcdonald, K. (2014). Gestures and social-emotional communicative development in chimpanzee infants. Am. J. Primatol. 76, 14–29. doi: 10.1002/ajp.22189
Bates, E., Camaioni, L., and Volterra, V. (1975). The acquisition of performatives prior to speech. Merrill. Palmer. Q. 21, 205–226.
Bateson, M. C. (1975). Mother-infant exchanges: the epigenesis of conversational interaction. Ann. N. Y. Acad. Sci. 263, 101–113. doi: 10.1111/j.1749-6632.1975.tb41575.x
Beck, B. B. (1982). Chimpocentrism: bias in cognitive ethology. J. Hum. Evol. 11, 3–17. doi: 10.1016/S0047-2484(82)80027-4
Bender, C. E., Herzing, D. L., and Bjorklund, D. F. (2009). Evidence of teaching in atlantic spotted dolphins (Stenella frontalis) by mother dolphins foraging in the presence of their calves. Anim. Cogn. 12, 43–53. doi: 10.1007/s10071-008-0169-9
Bezanson, M., and McNamara, A. (2019). The what and where of primate field research may be failing primate conservation. Evol. Anthropol. 28, 166–178. doi: 10.1002/evan.21790
Bezerra, B. M., and Souto, A. (2008). Structure and usage of the vocal repertoire of Callithrix jacchus. Int. J. Primatol. 29, 671–701. doi: 10.1007/s10764-008-9250-0
Boccia, M. L., Reite, M., and Laudenslager, M. L. (1991). Early social environment may alter the development of attachment and social support: two case reports. Infant Behav. Dev. 14, 253–260. doi: 10.1016/0163-6383(91)90009-H
Boesch, C., and Boesch, H. (1983). Optimisation of nut-cracking with natural hammers by wild chimpanzees. Behaviour 83, 265–286. doi: 10.1163/156853983X00192
Bouchet, H., Koda, H., and Lemasson, A. (2017). Age-dependent change in attention paid to vocal exchange rules in Japanese macaques. Anim. Behav. 129, 81–92. doi: 10.1016/j.anbehav.2017.05.012
Bräuer, J., Hanus, D., Pika, S., Gray, R., and Uomini, N. (2020). Old and new approaches to animal cognition: there is not “one cognition.” J. Intell. 8, 1–25. doi: 10.3390/jintelligence8030028
Briseño-Jaramillo, M., Berthet, M., Estrada, A., Biquand, V., and Lemasson, A. (2021). Socially mediated overlap in vocal interactions between free-ranging black howler monkeys. Am. J. Primatol. 83, 1–14. doi: 10.1002/ajp.23297
Briseño-Jaramillo, M., Ramos-Fernández, G., Palacios-Romo, T. M., Sosa-López, J. R., and Lemasson, A. (2018). Age and social affinity effects on contact call interactions in free-ranging spider monkeys. Behav. Ecol. Sociobiol. 72, 2. doi: 10.1007/s00265-018-2615-2
Brown, P. E., Brown, T. W., and Grinnell, A. D. (1983). Echolocation, development, and vocal communication in the lesser bulldog bat, Noctilio albiventris. Behav. Ecol. Sociobiol. 13, 287–298. doi: 10.1007/BF00299676
Bründl, A. C., Tkaczynski, P. J., Nohon Kohou, G., Boesch, C., Wittig, R. M., and Crockford, C. (2021). Systematic mapping of developmental milestones in wild chimpanzees. Dev. Sci. 24, 1–13. doi: 10.1111/desc.12988
Call, J., and Tomasello, M. (2007). The Gestural Communication of Apes and Monkeys. Oxfordshire: Taylor & Francis Group/Lawrence Erlbaum Associates.
Caro, T., and Hauser, M. (1992). Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174. doi: 10.1086/417553
Carter, G. G., Fenton, M. B., and Faure, P. A. (2009). White-winged vampire bats (Diaemus youngi) exchange contact calls. Can. J. Zool. 87, 604–608. doi: 10.1139/Z09-051
Cartmill, E. A., and Byrne, R. W. (2007). Orangutans modify their gestural signaling according to their audience's comprehension. Curr. Biol. 17, 1345–1348. doi: 10.1016/j.cub.2007.06.069
Casillas, M., Bobb, S. C., and Clark, E. V. (2016). Turn-taking, timing, and planning in early language acquisition. J. Child Lang. 43, 1310–1337. doi: 10.1017/S0305000915000689
Catchpole, C. K., and Slater, P. J. B. (2008). Bird Song: Biological Themes and Variations, 2nd Edn. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511754791
Chaverri, G., and Gillam, E. H. (2010). Cooperative signaling behavior of roost location in a leaf-roosting bat. Commun. Integr. Biol. 3, 599–601. doi: 10.4161/cib.3.6.13277
Chaverri, G., Gillam, E. H., and Kunz, T. H. (2013). A call-and-response system facilitates group cohesion among disc-winged bats. Behav. Ecol. 24, 481–487. doi: 10.1093/beheco/ars188
Cheney, D. L., and Seyfarth, R. M. (2010). “Primate communication and human language: continuities and discontinuities,” in Mind the Gap, eds P. Kappeler, J. Silk (Berlin; Heidelberg, Springer), 13. doi: 10.1007/978-3-642-02725-3_13
Chereskin, E., Connor, R. C., Friedman, W. R., Sørensen, P. M., Kru, M., and King, S. L. (2022). Allied male dolphins use vocal exchanges to “bond at a distance”. Curr. Biol. 1657–1663. doi: 10.1016/j.cub.2022.02.019
Chow, C. P., Mitchell, J. F., and Miller, C. T. (2015). Vocal turn-taking in a non-human primate is learned during ontogeny. Proc. R. Soc. B. 282, 69. doi: 10.1098/rspb.2015.0069
Christiansen, M. H., and Kirby, S. (2003a). Language evolution: consensus and controversies. Trends Cogn. Sci. 7, 300–307. doi: 10.1016/S1364-6613(03)00136-0
Christiansen, M. H., and Kirby, S. (2003b). Language Evolution. New York, NY: Oxford University Press.
Clark, H. H. (1996). Using Language. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511620539
Clutton-Brock, T. (2009). Structure and function in mammalian societies. Philos. Trans. R. Soc. B Biol. Sci. 364, 3229–3242. doi: 10.1098/rstb.2009.0120
Comparative BioCognition (2022). 100pan. Available online at: https://www.comparative-biocognition.de/1000pan/about-1000pan (accessed June 18, 2022).
Corballis, M. C. (2009). The evolution of language. Ann. N. Y. Acad. Sci. 1156, 19–43. doi: 10.1111/j.1749-6632.2009.04423.x
Cornec, C., Ngofuna, M., Lemasson, A., Monghiemo, C., Narat, V., and Levréro, F. (2022). A pilot study of calling patterns and vocal turn-taking in wild bonobos Pan paniscus. Ethol. Ecol. Evol. 2022, 1–18. doi: 10.1080/03949370.2022.2044387
Crockford, C., Herbinger, I., Vigilant, L., and Boesch, C. (2004). Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110, 221–243. doi: 10.1111/j.1439-0310.2004.00968.x
Dafreville, M., Hobaiter, C., Guidetti, M., Sillam-Dussès, D., and Bourjade, M. (2021). Sensitivity to the communicative partner's attentional state: a developmental study on mother–infant dyads in wild chimpanzees (Pan troglodytes schweinfurthii). Am. J. Primatol. 2021, ajp.23339. doi: 10.1002/ajp.23339
Davila-Ross, M., Jesus, G., Osborne, J., and Bard, K. A. (2015). Chimpanzees (Pan troglodytes) produce the same types of “laugh faces” when they emit laughter and when they are silent. PLoS ONE 10, 1–11. doi: 10.1371/journal.pone.0127337
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 34, 205–219. doi: 10.1080/03949370.2021.2015451
De Gregorio, C., Zanoli, A., Valente, D., Torti, V., Bonadonna, G., Randrianarison, R. M., et al. (2019). Female indris determine the rhythmic structure of the song and sustain a higher cost when the chorus size increases. Curr. Zool. 65, 89–97. doi: 10.1093/cz/zoy058
de Reus, K., Soma, M., Anichini, M., Gamba, M., De Heer Kloots, M., Lense, M., et al. (2021). Rhythm in dyadic interactions. Philos. Trans. R. Soc. B Biol. Sci. 376, 337. doi: 10.1098/rstb.2020.0337
de Ruiter, J. P., Mitterer, H., and Enfield, N. J. (2006). Projecting the end of a speaker's turn: a cognitive cornerstone of conversation. Language 82, 515–535. doi: 10.1353/lan.2006.0130
Demartsev, V., Strandburg-Peshkin, A., Ruffner, M., and Manser, M. (2018). Vocal turn-taking in meerkat group calling sessions. Curr. Biol. 28, 3661–3666.e3. doi: 10.1016/j.cub.2018.09.065
Dennett, D. C. (1983). Intentional systems in cognitive ethology: the “Panglossian paradigm” defended. Behav. Brain Sci. 6, 343–355. doi: 10.1017/S0140525X00016393
Dezecache, G., Crockford, C., and Zuberbühler, K. (2019). The development of communication in alarm contexts in wild chimpanzees. Behav. Ecol. Sociobiol. 73, 6. doi: 10.1007/s00265-019-2716-6
Digweed, S. M., Fedigan, L. M., and Rendall, D. (2007). Who cares who calls? Selective responses to the lost calls of socially dominant group members in the white-faced capuchin (Cebus capucinus). Am. J. Primatol. 69, 829–835. doi: 10.1002/ajp.20398
Dingemanse, M., Roberts, S. G., Baranova, J., Blythe, J., Drew, P., Floyd, S., et al. (2015). Universal principles in the repair of communication problems. PLoS ONE 10, 1–15. doi: 10.1371/journal.pone.0136100
Dominguez, S., Devouche, E., Apter, G., and Gratier, M. (2016). The roots of turn-taking in the neonatal period. Infant Child Dev. 25, 240–255. doi: 10.1002/icd.1976
Ehret, G., and Bernecker, C. (1986). Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Anim. Behav. 34, 821–830. doi: 10.1016/S0003-3472(86)80067-7
Elowson, A. M., Snowdon, C. T., and Lazaro-Perea, C. (1998). Infant “babbling” in a nonhuman primate: complex vocal sequences with repeated call types. Behaviour 135, 643–664. doi: 10.1163/156853998792897905
Emery, N. J., and Clayton, N. S. (2004). The mentality of crows: convergent evolution of intelligence in corvids and apes. Science 306, 1903–1907. doi: 10.1126/science.1098410
Fedurek, P., Machanda, Z. P., Schel, A. M., and Slocombe, K. E. (2013). Pant hoot chorusing and social bonds in male chimpanzees. Anim. Behav. 86, 189–196. doi: 10.1016/j.anbehav.2013.05.010
Ferreira, M. J., Levis, C., Chaves, L., Clement, C. R., and Soldati, G. T. (2022). Indigenous and traditional management creates and maintains the diversity of ecosystems of south american tropical savannas. Front. Environ. Sci. 10, 1–18. doi: 10.3389/fenvs.2022.809404
Fischer, J. (2017). Primate vocal production and the riddle of language evolution. Psychon. Bull. Rev. 24, 72–78. doi: 10.3758/s13423-016-1076-8
Fischer, J., Cheney, D. L., and Seyfarth, R. M. (2000). Development of infant baboons' responses to graded bark variants. Proc. R. Soc. B Biol. Sci. 267, 2317–2321. doi: 10.1098/rspb.2000.1285
Fitch, W. T. (2010). The Evolution of Language. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511817779
Fröhlich, M. (2017). Taking turns across channels: conversation-analytic tools in animal communication. Neurosci. Biobehav. Rev. 80, 201–209. doi: 10.1016/j.neubiorev.2017.05.005
Fröhlich, M., Bartolotta, N., Fryns, C., Wagner, C., Momon, L., Jaffrezic, M., et al. (2021). Orangutans have larger gestural repertoires in captivity than in the wild - a case of weak innovation? iScience 24, 103304. doi: 10.1016/j.isci.2021.103304
Fröhlich, M., Kuchenbuch, P., Müller, G., Fruth, B., Furuichi, T., Wittig, R. M., et al. (2016c). Unpeeling the layers of language: bonobos and chimpanzees engage in cooperative turn-taking sequences. Sci. Rep. 6, srep25887. doi: 10.1038/srep25887
Fröhlich, M., Müller, G., Zeiträg, C., Wittig, R. M., and Pika, S. (2017). Gestural development of chimpanzees in the wild: the impact of interactional experience. Anim. Behav. 134, 271–282. doi: 10.1016/j.anbehav.2016.12.018
Fröhlich, M., Sievers, C., Townsend, S. W., Gruber, T., van Schaik, C. P., Fröhlich, M., et al. (2019a). Multimodal communication and language origins: integrating gestures and vocalizations. Biol. Rev. 94, 1809–1829. doi: 10.1111/brv.12535
Fröhlich, M., Wittig, R. M., and Pika, S. (2016a). Play-solicitation gestures in chimpanzees in the wild: flexible adjustment to social circumstances and individual matrices. R. Soc. Open Sci. 3, 160278. doi: 10.1098/rsos.160278
Fröhlich, M., Wittig, R. M., and Pika, S. (2016b). Should I stay or should I go? Initiation of joint travel in mother–infant dyads of two chimpanzee communities in the wild. Anim. Cogn. 19, 483–500. doi: 10.1007/s10071-015-0948-z
Fröhlich, M., Wittig, R. M., and Pika, S. (2019b). The ontogeny of intentional communication in chimpanzees in the wild. Dev. Sci. 22, 1–14. doi: 10.1111/desc.12716
Gardner, R. A., and Gardner, B. T. (1969). Teaching sign language to a chimpanzee. Science 165, 664–672. doi: 10.1126/science.165.3894.664
Genty, E. (2019). Vocal–gestural combinations in infant bonobos: new insights into signal functional specificity. Anim. Cogn. 22, 505–518. doi: 10.1007/s10071-019-01267-0
Genty, E., Breuer, T., Hobaiter, C., and Byrne, R. W. (2009). Gestural communication of the gorilla (Gorilla gorilla): repertoire, intentionality and possible origins. Anim. Cogn. 12, 527–546. doi: 10.1007/s10071-009-0213-4
Genty, E., Clay, Z., Hobaiter, C., and Zuberbühler, K. (2014). Multi-modal use of a socially directed call in bonobos. PLoS ONE 9, e84738. doi: 10.1371/journal.pone.0084738
Gibson, D. R. (2003). Participation shifts: order and differentiation in group conversation. Soc. Forces 81, 1335–1381. doi: 10.1353/sof.2003.0055
Goodall, J. (1986). The Chimpanzees of Gombe: Patterns of Behavior. Cambridge: Cambridge University Press.
Goodwin, C., and Heritage, J. (1990). Conversation analysis. Annu. Rev. Anthropol. 19, 283–307. doi: 10.1146/annurev.an.19.100190.001435
Gratier, M., Devouche, E., Guellai, B., Infanti, R., Yilmaz, E., and Parlato-Oliveira, E. (2015). Early development of turn-taking in vocal interaction between mothers and infants. Front. Psychol. 6, 1–10. doi: 10.3389/fpsyg.2015.01167
Gruber, T., and Clay, Z. (2016). A comparison between bonobos and chimpanzees: a review and update. Evol. Anthropol. 25, 239–252. doi: 10.1002/evan.21501
Halina, M., Rossano, F., and Tomasello, M. (2013). The ontogenetic ritualization of bonobo gestures. Anim. Cogn. 16, 653–666. doi: 10.1007/s10071-013-0601-7
Hammerschmidt, K., and Fischer, J. (2008). Constraints in primate vocal production. Evol. Commun. Flex. 5, 92–119. doi: 10.7551/mitpress/9780262151214.003.0005
Hauser, M. D., Chomsky, N., and Fitch, W. T. (2010). The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579. doi: 10.1126/science.298.5598.1569
Hauser, M. D., Yang, C., Berwick, R. C., Tattersall, I., Ryan, M. J., Watumull, J., et al. (2014). The mystery of language evolution. Front. Psychol. 5, 1–12. doi: 10.3389/fpsyg.2014.00401
Hayashi, T., Akikawa, R., Kawasaki, K., Egawa, J., Minamimoto, T., Kobayashi, K., et al. (2020). Macaques exhibit implicit gaze bias anticipating others' false-belief-driven actions via medial prefrontal cortex. Cell Rep. 30, 4433–4444.e5. doi: 10.1016/j.celrep.2020.03.013
Hayes, K. J., and Hayes, C. (1951). The intellectual development of a home-raised chimpanzee. Proc. Am. Philos. Soc. 95, 105–109. doi: 10.1037/e533882004-001
Hebets, E. A., and Papaj, D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. doi: 10.1007/s00265-004-0865-7
Heesen, R., Fröhlich, M., Sievers, C., Woensdregt, M., and Dingemanse, M. (2022). Coordinating social action: a primer for the cross- species investigation of communicative repair. Philos. Trans. R. Soc B. 2021, 110. doi: 10.1098/rstb.2021.0110
Henrich, J., Heine, S. J., and Norenzayan, A. (2010). The weirdest people in the world? Behav. Brain Sci. 33, 61–83. doi: 10.1017/S0140525X0999152X
Hewes, G. W., Andrew, R. J., Carini, L., Choe, H., Gardner, R. A., Kortlandt, A., et al. (1973). Primate communication and the gestural origin of language [and Comments and Reply]. Curr. Anthropol. 14, 5–24. doi: 10.1086/201401
Hilbrink, E. E., Gattis, M., and Levinson, S. C. (2015). Early developmental changes in the timing of turn-taking: a longitudinal study of mother–infant interaction. Front. Psychol. 6, 1–12. doi: 10.3389/fpsyg.2015.01492
Hobaiter, C., and Byrne, R. W. (2011). Serial gesturing by wild chimpanzees: its nature and function for communication. Anim. Cogn. 14, 827–838. doi: 10.1007/s10071-011-0416-3
Hobaiter, C., and Byrne, R. W. (2014). The meanings of chimpanzee gestures. Curr. Biol. 24, 1596–1600. doi: 10.1016/j.cub.2014.05.066
Hobaiter, C., Byrne, R. W., and Zuberbühler, K. (2017). Wild chimpanzees' use of single and combined vocal and gestural signals. Behav. Ecol. Sociobiol. 71, 1. doi: 10.1007/s00265-017-2325-1
Hobaiter, C., Graham, K. E., and Byrne, R. W. (2022). Are ape gestures like words? Outstanding issues in detecting similarities and differences between human language and ape gesture. Philos. Trans. R. Soc. B Biol. Sci. 377, 301. doi: 10.1098/rstb.2021.0301
Hockett, C. D., and Hockett, C. F. (1960). The origin of speech. Sci. Am. 203, 88–96. doi: 10.1038/scientificamerican0960-88
Hockett, C. F. (1960). “Logical considerations in the study of animal communication,” in Animal Sounds and Communication, eds W. E. Lanyon and W. N. Tavolga (Washington, DC, American Institute of Biological Sciences), 392–430.
Hockett, C. F. (1963). “The problem of universals in language,” in Universals of Language, ed J. H. Green-berg (Cambrigde, MIT Press), 1–22.
Hoff, M. P., Nadler, R. D., and Maple, T. L. (1983). Maternal transport and infant motor development in a captive group of lowland gorillas. Primates 24, 77–85. doi: 10.1007/BF02381455
Holler, J., Kendrick, K. H., Casillas, M., and Levinson, S. C. (2015). Editorial: turn-taking in human communicative interaction. Front. Psychol. 6, 1–4. doi: 10.3389/fpsyg.2015.01919
Holler, J., and Levinson, S. C. (2019). Multimodal language processing in human communication. Trends Cogn. Sci. 23, 639–652. doi: 10.1016/j.tics.2019.05.006
Hradec, M., Bolechová, P., and Svobodová, I. (2016). Production of a female-specific great call in an immature male gibbon, the Nomascus genus. Primates 57, 445–448. doi: 10.1007/s10329-016-0569-4
Hudson, R., and Trillmich, F. (2008). Sibling competition and cooperation in mammals: challenges, developments and prospects. Behav. Ecol. Sociobiol. 62, 299–307. doi: 10.1007/s00265-007-0417-z
Jaffe, J., Beebe, B., Feldstein, S., Crown, C. L., and Jasnow, M. D. (2001). Rhythms of dialogue in infancy: coordinated timing in development. Monogr. Soc. Res. Child Dev. 66, 1–132. doi: 10.1111/1540-5834.00137
Janik, V. M., and Knörnschild, M. (2021). Vocal production learning in mammals revisited. Philos. Trans. R. Soc. B Biol. Sci. 376, 244. doi: 10.1098/rstb.2020.0244
Jenikejew, J., Chaignon, B., Linn, S., and Scheumann, M. (2020). Proximity-based vocal networks reveal social relationships in the Southern white rhinoceros. Sci. Rep. 10, 72052. doi: 10.1038/s41598-020-72052-0
Kappeler, P. M., and Van Schaik, C. P. (2005). Cooperation in Primates and Humans: Mechanisms and Evolution. Berlin; Heidelberg: Springer. doi: 10.1007/3-540-28277-7
Katsu, N., Yamada, K., Okanoya, K., and Nakamichi, M. (2018). Temporal adjustment of short calls according to a partner during vocal turn-taking in Japanese macaques. Curr. Zool. 65, 99–105. doi: 10.1093/cz/zoy077
Kavanagh, E., Street, S. E., Angwela, F. O., Bergman, T. J., Blaszczyk, M. B., Bolt, L. M., et al. (2021). Dominance style is a key predictor of vocal use and evolution across nonhuman primates. R. Soc. Open Sci. 8, 210873. doi: 10.1098/rsos.210873
Kellogg, W. N., and Kellogg, L. A. (1933). The Ape and the Child. New York, NY: McGraw Hill. doi: 10.1097/00006324-193311000-00009
Kerth, G. (2008). Causes and consequences of sociality in bats. Bioscience 58, 737–746. doi: 10.1641/B580810
Killin, A. (2017). Where did language come from? Connecting sign, song, and speech in hominin evolution. Biol. Philos. 32, 759–778. doi: 10.1007/s10539-017-9607-x
Knight, C., Studdert-Kennedy, M., and Hurford, J. (2000). The Evolutionary Emergence of Language. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511606441
Knörnschild, M. (2014). Vocal production learning in bats. Curr. Opin. Neurobiol. 28, 80–85. doi: 10.1016/j.conb.2014.06.014
Knörnschild, M., and von Helversen, O. (2008). Nonmutual vocal mother-pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009. doi: 10.1016/j.anbehav.2008.05.018
Knox, A., Markx, J., How, E., Azis, A., Hobaiter, C., van Veen, F. J. F., et al. (2019). Gesture use in communication between mothers and offspring in wild orangutans (Pongo pygmaeus wurmbii) from the Sabangau peat-swamp forest, Borneo. Int. J. Primatol. 40, 393–416. doi: 10.1007/s10764-019-00095-w
Koda, H., Lemasson, A., Oyakawa, C., Rizaldi, Pamungkas, J., and Masataka, N. (2013). Possible role of mother-daughter vocal interactions on the development of species-specific song in gibbons. PLoS ONE 8, 71432. doi: 10.1371/journal.pone.0071432
Koda, H., Oyakawa, C., Kato, A., Shimizu, D., Rizaldi, Koyama, Y., et al. (2014). Immature male gibbons produce female-specific songs. Primates 55, 13–17. doi: 10.1007/s10329-013-0390-2
Kolff, K., and Pika, S. (2022). “The evolutionary roots of human communicative repair: the case of chimpanzee grooming,” The Evolution of Language: Proceedings of the Joint Conference on Language Evolution (JCoLE), Japan, eds A. Ravignani, R. Asano, D. Valente, F. Ferretti, S. Hartmann, M. Hayashi, et al. (Kanazawa).
Kölliker, M., Royle, N., and Smiseth, P. T. (2012). The Evolution of Parental Care. New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780199692576.001.0001
Kondo, N., Watanabe, S., and Izawa, E. I. (2010). A temporal rule in vocal exchange among Large-billed Crows Corvus macrorhynchos in Japan. Ornithol. Sci. 9, 83–91. doi: 10.2326/osj.9.83
Krebs, J. R., and Dawkins, R. (1985). “Animal signals: mind-reading and manipulation,” in Behavioral Ecology: An Evolutionary Approach, eds J. R. Krebs and N. B. Davies (Oxford; London; Blackwell Scientific Publications), 381–403.
Ladygina-Kohts, N. N. (1935). Infant Chimpanzee and Human Child. A Classic Comparative Study of Ape Emotions and Intelligence. New York, NY: Oxford University Press.
Laidre, M. E., and Johnstone, R. A. (2013). Animal signals. Curr. Biol. 23, 829–833. doi: 10.1016/j.cub.2013.07.070
Lambert, M. L., Farrar, B. G., Garcia-Pelegrin, E., Reber, S., and Miller, R. (2021). ManyBirds: a multi-site collaborative open science approach to avian cognition and behaviour research. Anim. Behav. Cogn. 9, 133–152. doi: 10.26451/abc.09.01.11.2022
Langergraber, K. E., Prüfer, K., Rowney, C., Boesch, C., Crockford, C., Fawcett, K., et al. (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc. Natl. Acad. Sci. U. S. A. 109, 15716–15721. doi: 10.1073/pnas.1211740109
Leavens, D. A., and Hopkins, W. D. (1998). Intentional communication by chimpanzees: a cross-sectional study of the use of referential gestures. Dev. Psychol. 34, 813–822. doi: 10.1037/0012-1649.34.5.813
Leighty, K. A., Soltis, J., Leong, K., and Savage, A. (2008). Antiphonal exchanges in African elephants (Loxodonta africana): collective response to a shared stimulus, social facilitation, or true communicative event? Behaviour 145, 297–312. doi: 10.1163/156853908783402885
Lemasson, A., Gandon, E., and Hausberger, M. (2010). Attention to elders' voice in non-human primates. Biol. Lett. 6, 325–328. doi: 10.1098/rsbl.2009.0875
Lemasson, A., Glas, L., Barbu, S., Lacroix, A., Guilloux, M., Remeuf, K., et al. (2011). Youngsters do not pay attention to conversational rules: is this so for nonhuman primates? Sci. Rep. 1, srep00022. doi: 10.1038/srep00022
Lemasson, A., Guilloux, M., Rizaldi, Barbu, S., Lacroix, A., and Koda, H. (2013). Age- and sex-dependent contact call usage in Japanese macaques. Primates 54, 283–291. doi: 10.1007/s10329-013-0347-5
Lemasson, A., Pereira, H., and Levréro, F. (2018). Social basis of vocal interactions in western lowland gorillas (Gorilla g. gorilla). J. Comp. Psychol. 132, 141–151. doi: 10.1037/com0000105
Leong, K. M., Ortolani, A., Burks, K. D., Mellen, J. D., Savage, A., Leong, K. M., et al. (2003). Quantifying acoustic and temporal characteristics of vocalizations for a group of captive african elephants Loxodonta africana. Bioacoustics 13, 213–231. doi: 10.1080/09524622.2003.9753499
Levinson, S. C. (2006). On the human “interaction engine”. Roots Hum. Soc. Cult. Cogn. Interact. 3, 39–69. doi: 10.4324/9781003135517-3
Levinson, S. C. (2010). Interactional foundations of language: the interaction engine hypothesis. Hum. Lang. From Genes Brain Behav. 18, 189–200. doi: 10.7551/mitpress/10841.003.0018
Levinson, S. C. (2016). Turn-taking in human communication - origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Levinson, S. C., and Holler, J. (2014). The origin of human multi-modal communication. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130302. doi: 10.1098/rstb.2013.0302
Levinson, S. C., and Torreira, F. (2015). Timing in turn-taking and its implications for processing models of language. Front. Psychol. 6, 731. doi: 10.3389/fpsyg.2015.00731
Levréro, F., Touitou, S., Frédet, J., Nairaud, B., Guéry, J.-P., and Lemasson, A. (2019). Social bonding drives vocal exchanges in bonobos. Sci. Rep. 9, 9. doi: 10.1038/s41598-018-36024-9
Lewis, L. S., and Krupenye, C. (2022). Eye-tracking as a window into primate social cognition. Am. J. Primatol. 2022, 1–18. doi: 10.1002/ajp.23393
Liebal, K., Pika, S., and Tomasello, M. (2004). Social communication in siamangs (Symphalangus syndactylus): use of gestures and facial expressions. Primates 45, 41–57. doi: 10.1007/s10329-003-0063-7
Liebal, K., Waller, B. M., Burrows, A. M., and Slocombe, K. E. (2012). Primate Communication: A Multimodal Approach. Cambridge: Cambridge University Press.
Liebal, K., Waller, B. M., Burrows, A. M., and Slocombe, K. E. (2013). Primate Communication: A Multimodal Approach. Cambridge: Cambridge University Press. doi: 10.1017/CBO9781139018111
Logue, D. M., and Stivers, T. (2012). Squawk in interaction: a primer of conversation analysis for students of animal communication. Behaviour 149, 1283–1298. doi: 10.1163/1568539X-00003031
Luef, E. M., Breuer, T., and Pika, S. (2016). Food-associated calling in gorillas (Gorilla g. gorilla) in the wild. PLoS One. 11. doi: 10.1371/journal.pone.0144197
Luef, E. M., and Pika, S. (2013). Gorilla mothers also matter! New insights on social transmission in gorillas (Gorilla gorilla gorilla) in captivity. PLoS ONE 8, 79600. doi: 10.1371/journal.pone.0079600
Luef, E. M., and Pika, S. (2017). Reciprocal greeting in chimpanzees (Pan troglodytes) at the Ngogo community. J. Neurolinguistics 43, 263–273. doi: 10.1016/j.jneuroling.2016.11.002
Maestripieri, D. (2018). Maternal influences on primate social development. Behav. Ecol. Sociobiol. 72, 2547. doi: 10.1007/s00265-018-2547-x
Marchant-Forde, J. N., Marchant-Forde, R. M., and Weary, D. M. (2002). Responses of dairy cows and calves to each other's vocalisations after early separation. Appl. Anim. Behav. Sci. 78, 19–28. doi: 10.1016/S0168-1591(02)00082-5
Matsumura, S. (1979). Mother-infant communication in a horseshoe bat (Rhinolophus ferrumequinum nippon): development of vocalization. J. Mammal. 60, 76–84. doi: 10.2307/1379760
Matsuzawa, T. (1994). “Field experiments on use of stone tools by chimpanzees in the wild,” in Chimpanzee Cultures, eds R. W. Wrangham, W. C. McGrew, F. de Wall, and P. G. Heltne (Cambridge, MA: Harvard University Press), 351–370.
McNeill, D. (2010). How Language Began: Gesture and Speech in Human Evolution. Cambridge: Cambridge University Press.
Mehdizadeh, R., Eghbali, H., and Sharifi, M. (2018). Postnatal growth and vocalization development in the long-fingered bat, Myotis capaccinii (Chiroptera, vespertilionidae). Zool. Stud. 57, 37. doi: 10.6620/ZS.2018.57-37
Méndez-Cárdenas, M. G., and Zimmermann, E. (2009). Duetting - a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. doi: 10.1002/ajpa.21017
Merker, B., and Cox, C. (1999). Development of the female great call in Hylobates gabriellae: a case study. Folia Primatol. 70, 97–106. doi: 10.1159/000021680
Miglietta, S., Bardino, G., Sotto-Mayor, A., Galli, A. S., Meulman, E., Breuer, T., et al. (2021). Absence of specific individuals and high food abundance elicit food calls in wild western gorillas. Behav. Ecol. Sociobiol. 75, 3027. doi: 10.1007/s00265-021-03027-y
Miller, P. J. O., Shapiro, A. D., Tyack, P. L., and Solow, A. R. (2004). Call-type matching in vocal exchanges of free-ranging resident killer whales, Orcinus orca. Anim. Behav. 67, 1099–1107. doi: 10.1016/j.anbehav.2003.06.017
Mishima, Y., Morisaka, T., Mishima, Y., Sunada, T., and Miyamoto, Y. (2018). Redefinition and sexual difference of contact calls in belugas (Delphinapterus leucas). Aquat. Mamm. 44, 538–554. doi: 10.1578/AM.44.5.2018.538
Mocha, Y., Ben Mundry, R., and Pika, S. (2019). Joint attention skills in wild Arabian babblers (Turdoides squamiceps): a consequence of cooperative breeding? Proc. R. Soc. B Biol. Sci. 286, 147. doi: 10.1098/rspb.2019.0147
Montero, K., and Gillam, E. H. (2015). Geographic variation in contact calls emitted by a leaf-roosting bat suggest distinct modes of vocal transmission. J. Acoust. Soc. Am. 138, 1932–1932. doi: 10.1121/1.4934089
Morisaka, T., Yoshida, Y., Akune, Y., Mishima, H., and Nishimoto, S. (2013). Exchange of “signature” calls in captive belugas (Delphinapterus leucas). J. Ethol. 31, 141–149. doi: 10.1007/s10164-013-0358-0
Muller, M. N. (2018). “Introduction: chimpanzees and human evolution,” in Chimpanzees and Human Evolution, eds N. M. Muller, R. W. Wrangham and D. R. Pilbeam (Cambridge, MA; London: Harvard University Press), 3–21. doi: 10.4159/9780674982642
Mundy, P., and Newell, L. (2007). Attention, joint attention & social cognition. Soc. Cogn. 16, 269–274. doi: 10.1111/j.1467-8721.2007.00518.x
Musgrave, S., Morgan, D., Lonsdorf, E., Mundry, R., and Sanz, C. (2016). Tool transfers are a form of teaching among chimpanzees. Sci. Rep. 6, 1–7. doi: 10.1038/srep34783
Nguyen, V., Versyp, O., Cox, C., and Fusaroli, R. (2022). A systematic review and Bayesian meta- - analysis of the development of turn taking in adult - child vocal interactions. Child Dev. 2022, 1–20. doi: 10.1111/cdev.13754
Nomikou, I., Leonardi, G., Radkowska, A., Raczaszek-Leonardi, J., and Rohlfing, K. J. (2017). Taking up an active role: emerging participation in early mother–infant interaction during peekaboo routines. Front. Psychol. 8, 1–19. doi: 10.3389/fpsyg.2017.01656
O'Connell-Rodwell, C. E., Wood, J. D., Wyman, M., Redfield, S., Puria, S., and Hart, L. A. (2012). Antiphonal vocal bouts associated with departures in free-ranging African elephant family groups (Loxodonta africana). Bioacoustics 21, 215–224. doi: 10.1080/09524622.2012.686166
O'Dea, R. E., Lagisz, M., Jennions, M. D., Koricheva, J., Noble, D. W. A., Parker, T. H., et al. (2021). Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol. Rev. 96, 1695–1722. doi: 10.1111/brv.12721
Okobi, D. E., Banerjee, A., Matheson, A. M. M., Phelps, S. M., and Long, M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. doi: 10.1126/science.aau9480
Partan, S., and Marler, P. (1999). Communication goes multimodal. Science 1272–1273. doi: 10.1126/science.283.5406.1272
Pika, S. (2015). Gestural communication in nonhuman species. Emerg. Trends Soc. Behav. Sci. 2015, 1–11. doi: 10.1002/9781118900772.etrds0147
Pika, S., and Bugnyar, T. (2011). The use of referential gestures in ravens (Corvus corax) in the wild. Nat. Commun. 2, 1567. doi: 10.1038/ncomms1567
Pika, S., and Deschner, T. (2019). A new window onto animal culture: the case of chimpanzee gesturing. Gesture 18, 239–260. doi: 10.1075/gest.19012.pik
Pika, S., and Fröhlich, M. (2019). Gestural acquisition in great apes: the Social Negotiation Hypothesis. Anim. Cogn. 22, 551–565. doi: 10.1007/s10071-017-1159-6
Pika, S., Liebal, K., Call, J., and Tomasello, M. (2005). The gestural communication of apes and human infants. Gesture 5, 41–56. doi: 10.1075/gest.5.1.05pik
Pika, S., Liebal, K., and Tomasello, M. (2003). Gestural communication in young gorillas (Gorilla gorilla): gestural repertoire, learning, and use. Am. J. Primatol. 60, 95–111. doi: 10.1002/ajp.10097
Pika, S., Wilkinson, R., Kendrick, K. H., and Vernes, S. C. (2018). Taking turns: bridging the gap between human and animal communication. Proc. R. Soc. B Biol. Sci. 285, 598. doi: 10.1098/rspb.2018.0598
Plooij, F. (1978). “Some basic traits of language in wild chimpanzees?” in Action, Gesture and Symbol: The Emergence of Language, ed A. Lock (London: Academic Press), 111–131.
Pougnault, L., Lemasson, A., Mulot, B., and Levréro, F. (2021a). Temporal calling patterns of a captive group of chimpanzees (Pan troglodytes). Int. J. Primatol. doi: 10.1007/s10764-021-00262-y
Pougnault, L., Levréro, F., Leroux, M., Paulet, J., Bombani, P., Dentressangle, F., et al. (2021b). Social pressure drives “conversational rules” in great apes. Biol. Rev. doi: 10.1111/brv.12821
Pougnault, L., Levréro, F., Mulot, B., and Lemasson, A. (2020). Breaking conversational rules matters to captive gorillas: a playback experiment. Sci. Rep. 10, 7. doi: 10.1038/s41598-020-63923-7
Prieur, J., Barbu, S., Blois-Heulin, C., and Lemasson, A. (2020). The origins of gestures and language: history, current advances and proposed theories. Biol. Rev. 95, 531–554. doi: 10.1111/brv.12576
Primates, M., Altschul, D. M., Beran, M. J., Bohn, M., Call, J., DeTroy, S., et al. (2019). Establishing an infrastructure for collaboration in primate cognition research. PLoS ONE 14, e0223675. doi: 10.1371/journal.pone.0223675
Prüfer, K., Munch, K., Hellmann, I., Akagi, K., Miller, J. R., Walenz, B., et al. (2012). The bonobo genome compared with the chimpanzee and human genomes. Nature 486, 527–531. doi: 10.1038/nature11128
Ravignani, A., Lumaca, M., and Kotz, S. A. (2022). Interhemispheric brain communication and the evolution of turn-taking in mammals. Front. Ecol. Evol. 10, 1–7. doi: 10.3389/fevo.2022.916956
Ravignani, A., Verga, L., and Greenfield, M. D. (2019). Interactive rhythms across species: the evolutionary biology of animal chorusing and turn-taking. Ann. N. Y. Acad. Sci. 1453, 12–21. doi: 10.1111/nyas.14230
Raviv, L., Lupyan, G., and Green, S. C. (2022). How variability shapes learning and generalization. Trends Cogn. Sci. 26, 462–483. doi: 10.1016/j.tics.2022.03.007
Roberts, A. I., and Roberts, S. G. B. (2016). Wild chimpanzees modify modality of gestures according to the strength of social bonds and personal network size. Sci. Rep. 6, 1–13. doi: 10.1038/srep33864
Roberts, S. G. B., and Roberts, A. I. (2020). Social and ecological complexity is associated with gestural repertoire size of wild chimpanzees. Integr. Zool. 15, 276–292. doi: 10.1111/1749-4877.12423
Rodrigues, E. D., and Fröhlich, M. (2021). Operationalizing intentionality in primate communication: social and ecological considerations. Int. J. Primatol. 21, 248. doi: 10.1007/s10764-021-00248-w
Rodrigues, E. D., Santos, A. J., Hayashi, M., Matsuzawa, T., and Hobaiter, C. (2021). Exploring greetings and leave-takings: communication during arrivals and departures by chimpanzees of the Bossou community, Guinea. Primates 21, 957. doi: 10.1007/s10329-021-00957-z
Rosenbaum, S., and Gettler, L. T. (2018). With a little help from her friends (and family) part II: non-maternal caregiving behavior and physiology in mammals. Physiol. Behav. 193, 12–24. doi: 10.1016/j.physbeh.2017.12.027
Rossano, F. (2013). Sequence organization and timing of bonobo mother-infant interactions. Interact. Stud. 14, 160–189. doi: 10.1075/is.14.2.02ros
Rossano, F. (2018). Social manipulation, turn-taking and cooperation in apes: implications for the evolution of language-based interaction in humans. Interact. Stud. 19, 151–166. doi: 10.1075/is.17043.ros
Sacks, H., Schegloff, E. A., and Jefferson, G. (1974). A simplest systematics for the organization of turn- taking in conversation. Language 50, 696–735. doi: 10.1353/lan.1974.0010
Samuni, L., Tkaczynski, P., Deschner, T., Löhrrich, T., Wittig, R. M., and Crockford, C. (2020). Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front. Zool. 17, 1–12. doi: 10.1186/s12983-019-0343-8
Schegloff, E. A. (2007). Sequence Organization in Interaction: A Primer in Conversation Analysis I. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511791208
Schel, A. M., Bono, A., Aychet, J., Pika, S., and Lemasson, A. (2022). Intentional gestural communication amongst red-capped mangabeys (Cercocebus torquatus). Anim. Cogn. 2022, 7. doi: 10.1007/s10071-022-01615-7
Schel, A. M., Machanda, Z., Townsend, S. W., Zuberbühler, K., and Slocombe, K. E. (2013). Chimpanzee food calls are directed at specific individuals. Anim. Behav. 86, 955–965. doi: 10.1016/j.anbehav.2013.08.013
Schiel, N., and Huber, L. (2006). Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus). Am. J. Primatol. 68, 1150–1160. doi: 10.1002/ajp.20284
Schultz-Darken, N., Braun, K. M., and Emborg, M. E. (2016). Neurobehavioral development of common marmoset monkeys. Dev. Psychobiol. 58, 141–158. doi: 10.1002/dev.21360
Schulz, T. M., Whitehead, H., Gero, S., and Rendell, L. (2008). Overlapping and matching of codas in vocal interactions between sperm whales: insights into communication function. Anim. Behav. 76, 1977–1988. doi: 10.1016/j.anbehav.2008.07.032
Sèbe, F., Duboscq, J., Aubin, T., Ligout, S., and Poindron, P. (2010). Early vocal recognition of mother by lambs: contribution of low- and high-frequency vocalizations. Anim. Behav. 79, 1055–1066. doi: 10.1016/j.anbehav.2010.01.021
Seyfarth, R. M., and Cheney, D. L. (2010). Production, usage, and comprehension in animal vocalizations. Brain Lang. 115, 92–100. doi: 10.1016/j.bandl.2009.10.003
Seyfarth, R. M., Cheney, D. L., Bergman, T., Fischer, J., Zuberbühler, K., and Hammerschmidt, K. (2010a). The central importance of information in studies of animal communication. Anim. Behav. 80, 3–8. doi: 10.1016/j.anbehav.2010.04.012
Seyfarth, R. M., Cheney, D. L., and Marler, P. (2010b). Vervet monkey alarm calls: Semantic communication in a free-ranging primate. Anim. Behav. 28, 1070–1094. doi: 10.1016/S0003-3472(80)80097-2
Shannon, C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x
Slocombe, K. E., Waller, B. M., and Liebal, K. (2011). The language void: the need for multimodality in primate communication research. Anim. Behav. 81, 919–924. doi: 10.1016/j.anbehav.2011.02.002
Snow, C. E. (1977). “Mothers' speech research: from input to interaction,” in Talking to Children, eds C. E. Snow and C. A. Ferguson (Cambridge: Cambridge University Press), 31–49.
Snowdon, C. T. (2017). Learning from monkey “talk.” Science 355, 1120–1122. doi: 10.1126/science.aam7443
Springer, M. S., Stanhope, M. J., Madsen, O., and Jong, W. W. (2004). Molecules consolidate the placental mammal tree. Trends Ecol. Evol. 19, 430–438. doi: 10.1016/j.tree.2004.05.006
Stivers, T., Enfield, N. J., Brown, P., Englert, C., Hayashi, M., Heinemann, T., et al. (2009). Universals and cultural variation in turn-taking in conversation. Proc. Natl. Acad. Sci. U. S. A. 106, 10587–10592. doi: 10.1073/pnas.0903616106
Suomi, S. J. (2005). Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum. Dev. 48, 67–79. doi: 10.1159/000083216
Takahashi, D. Y., Fenley, A. R., and Ghazanfar, A. A. (2016). Early development of turn-taking with parents shapes vocal acoustics in infant marmoset monkeys. Philos. Trans. R. Soc. B Biol. Sci. 2015, 371. doi: 10.1098/rstb.2015.0370
Takahashi, D. Y., Fenley, A. R., Teramoto, Y., Narayanan, D. Z., Borjon, J. I., Holmes, P., et al. (2015). The developmental dynamics of marmoset monkey vocal production. Science 349, 734–738. doi: 10.1126/science.aab1058
Takahashi, D. Y., Narayanan, D. Z., and Ghazanfar, A. A. (2013). Coupled oscillator dynamics of vocal turn-taking in monkeys. Curr. Biol. 23, 2162–2168. doi: 10.1016/j.cub.2013.09.005
Tardif, S. D., Smucny, D. A., Abbott, D. H., Mansfield, K., Schultz-Darken, N., and Yamamoto, M. E. (2003). Reproduction in captive common marmosets (Callithrix jacchus). Comp. Med. 53, 364–368.
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2018). An analysis of white-handed gibbon male song reveals speech-like phrases. Am. J. Phys. Anthropol. 166, 649–660. doi: 10.1002/ajpa.23451
Thornton, A., and Clutton-Brock, T. (2011). Social learning and the development of individual and group behaviour in mammal societies. Philos. Trans. R. Soc. B Biol. Sci. 366, 978–987. doi: 10.1098/rstb.2010.0312
Toarmino, C. R., Wong, L., and Miller, C. T. (2017). Audience affects decision-making in a marmoset communication network. Biol. Lett. 13, 20160934. doi: 10.1098/rsbl.2016.0934
Tomasello, M., and Zuberbühler, K. (2002). “Primate vocal and gestural communication,” in The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition, eds M. Bekoff, C. S. Allen and G. Burghardt (Cambridge: MIT Press), 293–299.
Townsend, S. W., Koski, S. E., Byrne, R. W., Slocombe, K. E., Bickel, B., Boeckle, M., et al. (2017). Exorcising Grice's ghost: an empirical approach to studying intentional communication in animals. Biol. Rev. 92, 1427–1433. doi: 10.1111/brv.12289
Van Boekholt, B., van de Waal, E., and Sterck, E. H. M. (2021). Organized to learn: the influence of social structure on social learning opportunities in a group. iScience 24, 102117. doi: 10.1016/j.isci.2021.102117
van Schaik, C., Graber, S., Schuppli, C., and Burkart, J. (2017). The ecology of social learning in animals and its link with intelligence. Span. J. Psychol. 19, 1–12. doi: 10.1017/sjp.2016.100
Vanderhoff, E. N., and Bernal Hoverud, N. (2022). Perspectives on antiphonal calling, duetting and counter-singing in non-primate mammals: an overview with notes on the coordinated vocalizations of bamboo rats (Dactylomys spp., Rodentia: Echimyidae). Front. Ecol. Evol. 10, 1–10. doi: 10.3389/fevo.2022.906546
Verderane, M. P., Aguiar, R. M., and Izar, P. (2020). Face–to–face interactions between mothers and female infants in wild bearded capuchin monkeys (Sapajus libidinosus). Dev. Psychobiol. 62, 941–949. doi: 10.1002/dev.21948
Vergara, V., Michaud, R., and Barrett-Lennard, L. G. (2010). What can captive whales tell us about their wild counterparts? Identification, usage, and ontogeny of contact calls in belugas (Delphinapterus leucas). Int. J. Comp. Psychol. 23, 278–309. doi: 10.46867/IJCP.2010.23.03.08
Vernes, S. C. (2017). What bats have to say about speech and language. Psychon. Bull. Rev. 24, 111–117. doi: 10.3758/s13423-016-1060-3
von Streit, C., Ganslosser, U., and von Fersen, L. (2013). Behavioral development of two captive mother-calf dyads of bottlenose dolphins (Tursiops truncatus) in the calves' first year. Int. J. Comp. Psychol. 26, 5. doi: 10.46867/ijcp.2013.26.03.05
Vygotsky, L. (1978). Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press.
Wang, Y., Fang, Q., and Gong, N. (2014). Motor assessment of developing common marmosets. Neurosci. Bull. 30, 387–393. doi: 10.1007/s12264-013-1395-y
Western, D. (1979). Size, life history and ecology in mammals. Afr. J. Ecol. 17, 185–204. doi: 10.1111/j.1365-2028.1979.tb00256.x
Whiten, A., and van de Waal, E. (2018). The pervasive role of social learning in primate lifetime development. Behav. Ecol. Sociobiol. 72, 80. doi: 10.1007/s00265-018-2489-3
Wilkinson, R., Leudar, I., and Pika, S. (2012). “Requesting behaviours within episodes of active sharing. A new look on chimpanzee signalling,” in Developments in Primate Gesture Research, eds S. Pika and K. Liebal (Amsterdam: John Benjamins Publishing Company), 199–221. doi: 10.1075/gs.6.12wil
Wong, B. B. M., Cowling, A. N. N., Cunningham, R. B., Donnelly, C. F., and Cooper, P. D. (2004). Do temperature and social environment interact to affect call rate in frogs (Crinia signifera)? Aust. Ecol. 29, 209–214. doi: 10.1111/j.1442-9993.2004.01338.x
Wrangham, R. W. (1987). “The significance of African apes for reconstructing human social evolution,” in Primate Models of Hominid Evolution, ed W. G. Kinzey (Albany: SUNY Press), 51–71.
Wray, A. (1998). Protolanguage as a holistic system for social interaction. Lang. Commun. 1, 47–67. doi: 10.1016/S0271-5309(97)00033-5
Yamaguchi, C., Izumi, A., and Nakamura, K. (2009). Temporal rules in vocal exchanges of phees and trills in common marmosets (Callithrix jacchus). Am. J. Primatol. 71, 617–622. doi: 10.1002/ajp.20697
Yamamoto, M. E. (2005). Infant care in callitrichids: cooperation and competition. Annu. Rev. Biomed. Sci. 7, 149–160. doi: 10.5016/48
Yoshida, S., and Okanoya, K. (2005). Evolution of turn-taking: a bio-cognitive perspective. Cogn. Stud. 12, 153–165. doi: 10.11225/jcss.12.153
Keywords: language evolution, social interaction, development, learning, turn-taking, mammals, primates, infants
Citation: Abreu F and Pika S (2022) Turn-taking skills in mammals: A systematic review into development and acquisition. Front. Ecol. Evol. 10:987253. doi: 10.3389/fevo.2022.987253
Received: 06 July 2022; Accepted: 14 November 2022;
Published: 01 December 2022.
Edited by:
Patrice Adret, Universidad Autónoma Gabriel René Moreno, BoliviaReviewed by:
Koen de Reus, Vrije University Brussel, BelgiumAsif A. Ghazanfar, Princeton University, United States
Copyright © 2022 Abreu and Pika. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filipa Abreu, ZmlsaXBhLmFicmV1QHVuaS1vc25hYnJ1ZWNrLmRl; Simone Pika, c2ltb25lLnBpa2FAdW5pLW9zbmFicnVlY2suZGU=
 Filipa Abreu
Filipa Abreu Simone Pika
Simone Pika