- 1State Key Laboratory of Biocontrol and Guangdong Provincial Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou, China
- 2School of Ecology, Sun Yat-sen University, Shenzhen, China
- 3School of Geography, South China Normal University, Guangzhou, China
- 4State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China
Ampelopsis Michx. (Vitaceae) contains more than 30 species and is discontinuously distributed in Eurasia, North America, and Central America. China hosts an abundance of Ampelopsis species. Until now, fossil records of Ampelopsis have been reported only from the Paleocene to the Pleistocene of Europe, the Eocene to the Pliocene of Asia, and the Eocene to the Miocene of North America. Although Ampelopsis is abundant and widespread in China today, no fossils of Ampelopsis have so far been found there, except for fossil seed from the Upper Miocene of Yunnan. In this study, a fossil seed of Ampelopsis japonica (Thunb.) Makino was recovered from the Upper Pleistocene of the Maoming Basin, Guangdong province. It is the first Ampelopsis fossil found in South China. This finding shows that Ampelopsis was distributed in the low latitudes of South China in the Late Pleistocene. Global cooling during the last glaciation might have led to the southward spread of the genus to the low-latitude areas of South China. According to the structural characteristics of our fossil, it is speculated that the aborted ovule, which is common in the fruits of extant Ampelopsis, existed in this genus in the Late Pleistocene.
Introduction
The family Vitaceae currently comprises 15 genera and more than 700 species, which are distributed in tropical, subtropical, and temperate regions around the world (Chen and Manchester, 2011). Ampelopsis Michx. is one of the few genera mainly distributed in the northern temperate zone, with intercontinental discontinuous distribution in Eurasia, North America, and Central America (Chen, 2009; Nie et al., 2012; Ickert-Bond et al., 2014). There are more than 30 species of this genus, most of which are distributed in East Asia (Chen et al., 2007). In China, there are 17 Ampelopsis species, with 13 being endemic (Chen et al., 2007; Fang et al., 2009).
As the reproductive organ of plants, seeds display characteristics that are relatively stable and are often used as an important basis for taxonomic identification. Comparing with other preservation types of fossils, mummified three-dimensional fossils can provide more features conducive to reliable classification and identification. Due to the unique characteristics of Vitaceae seeds, the identification of this family is relatively reliable, and seed characteristics can be used for distinguishing genera and species (Tiffney and Barghoorn, 1976; Chen and Manchester, 2011). Chen and Manchester (2011) conducted a detailed study of the morphological characteristics of Vitaceae seeds, which provided a basis for the classification and identification of Vitaceae seed fossils through macromorphological and structural characteristics.
The seeds of Ampelopsis have an important character: in cross-section, the ventral infolds have a “keyhole” outline with an abrupt, significant thinning of the intruded seed coat (Manchester et al., 2013). Moreover, the seeds of the genus have a short, parallel to slightly divergent, pair of ventral infolds (equal-in-width or a tapered raphe between the ventral infolds), and the chalaza is oval to pyriform, close to the apical notch. These features make the seeds of this genus distinct from those of other genera of Vitaceae (Chen and Manchester, 2011; Manchester et al., 2013). The fruit of extant Ampelopsis is a berry with 1–4 seeds, and the ovary is two-locular (but the septa do not fuse) with two anatropous ovules in each chamber, and often has aborted ovules (Wen, 2007; Ickert-Bond et al., 2014; Gerrath et al., 2015). The chalaza of the seed can be clearly distinguished from the adjacent seed coat tissue and has a cavity structure.
Until now, the reported Ampelopsis fossils include seeds (e.g., Reid and Chandler, 1933; Chandler, 1961) and leaves (Bozukov, 2000; Kowalski et al., 2020). Most of the records are seed fossils, which are mainly found from the Paleocene to the Pleistocene of Europe, the Eocene to the Pliocene of Asia, and the Eocene to the Miocene of North America. Although Ampelopsis is abundant and widespread in China today (Chen et al., 2007; Fang et al., 2009), so far only one fossil record of this genus was reported from this region, i.e., fossil seeds of Ampelopsis sp. from the Upper Miocene of Yunnan (Huang et al., 2017). The Upper Pleistocene mummified fossil seed of Ampelopsis described here from the Maoming Basin of Guangdong province, South China, is the first fossil seed of Ampelopsis in South China and provides another fossil record of this genus from China. This finding sheds new light on the phylogeny and phytogeographic history of this genus.
Materials and methods
Materials
The fossil seed of Ampelopsis was recovered from the Upper Pleistocene exposed near Zhenjiang town, Maoming city, Guangdong province, South China (Figure 1A; 21°52′47.5″N; 110°40′06.3″E). Accelerator Mass Spectrometry (AMS) of 14C dating showed that the geological age of this deposit was Late Pleistocene (29–27 ka BP, 33–30 ka cal. BP) (Huang et al., 2021a). The sedimentary succession comprised yellow sands with gravel and black, gray, and grayish-yellow mudstones with a total thickness of ~2.5 m, the base of which displays an angular unconformity with the underlying upper Eocene Huangniuling Formation (Figures 1B, C). The Ampelopsis mummified three-dimensional seed fossil was found in a gray mudstone. This sedimentary succession also yielded conifers belonging to Pinus Linn. and Keteleeria Carrière (Pinaceae) and angiosperms belonging to Canarium Linn. (Burseraceae), Elaeocarpus Linn. (Elaeocarpaceae), Liquidambar Linn. (Altingiaceae), and Fagaceae (Huang et al., 2021a,b; Bazhenova et al., 2022; Xiang et al., 2022).
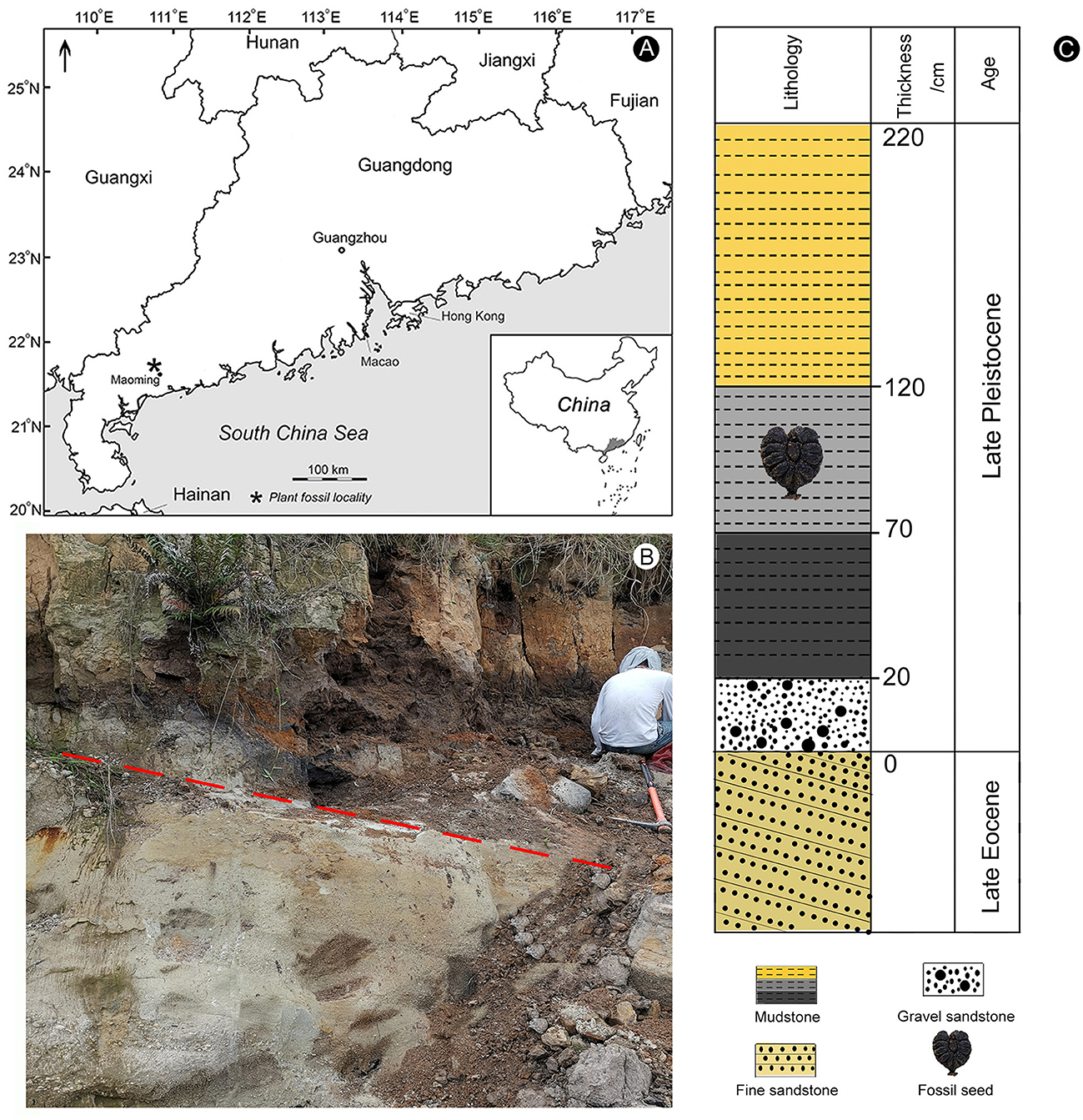
Figure 1. Location and lithology of the fossil locality (modified from Huang et al., 2021a; Bazhenova et al., 2022). (A) Map indicates the location of the Zhenjiang opencast mine within the Maoming Basin in Guangdong province of South China. (B) Lithological characteristics of sediments. The red dashed line indicates the unconformity between the Upper Pleistocene deposits and the upper Eocene Huangniuling Formation. (C) The lithological column of the studied succession shows the position of the fossil seed.
The fossil described here was deposited in the Museum of Biology, Sun Yat-sen University, Guangzhou, China.
Methods
To clearly show the surface characteristics of the fossil seed, we immersed it in clean water and together with gentle brushing using an ultrasonic cleaner (Jiemeng JP−880) at a frequency of 42 kHz to remove adhered sediment particles. After cleaning, the seed was placed on absorbent paper and allowed to dry naturally and slowly in shade. Photographs of the seed were taken using a Nikon SMZ25 stereo microscope at the Museum of Biology, Sun Yat-sen University, Guangzhou, China. Length, width, length of ventral infold, and other morphological data were measured. A micro-computed tomography (Micro-CT) system was used to reveal the internal three-dimensional structure of the seed (Zeiss Xradia 520 Versa X-ray microscope (CT) housed at the University of Science and Technology of China in Hefei, Anhui province). Image data were processed by Dragonfly v.4.1 (https://www.theobjects.com/dragonfly) software.
Morphological terms were employed here to describe the seed of Ampelopsis [follow Tiffney and Barghoorn (1976) and Chen and Manchester (2011)] (Figure 2).
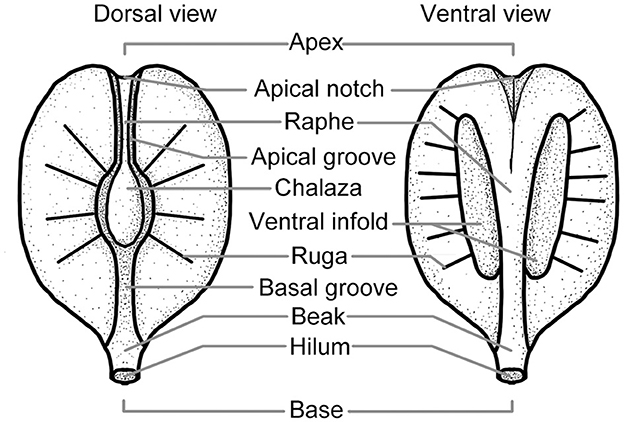
Figure 2. The surface structure of the Vitaceae seed (modified from Tiffney and Barghoorn, 1976; Chen and Manchester, 2011).
Results
Systematic paleontology
Order: Vitales Juss. ex Bercht. and J. Presl.
Family: Vitaceae Juss., nom. cons.
Genus: Ampelopsis Michx.
Species: Ampelopsis japonica (Thunb.) Makino (Figures 3A–K).
Specimen: MMRH-236.
Locality and age: Maoming Basin, Guangdong; Late Pleistocene.
Repository: The Museum of Biology of Sun Yat-sen University, Guangzhou, China.
Description: This is a three-dimensional mummified seed, with a black surface, 4.38 mm in length, and 4.22 mm in width, and the seed coat is 0.25 mm in thickness. The seed is heart-shaped in dorsal and ventral views (Figures 3A, B) and has bilateral symmetry. It is nearly elliptical in the side view (Figures 3E, F), apical view (Figure 3H), and basal view (Figure 3I). At the base of the seed, there is a prominent beak (hilum), with a depressed center in its skirt-shaped tip (Figures 3A, B, E, F, I). An apical notch is obvious (Figures 3A, B), and the apical groove is connected with the ventral raphe (Figure 3H). The dorsal chalaza is nearly elliptical, close to the apical notch (Figures 3B–D), and obviously lower than the dorsal plane (Figures 3E, F, H, I). Dorsal six pairs of rugas are arranged radially and symmetrically around the chalaza (Figures 3B–D). The basal groove is obvious and narrow, extending from the chalaza to the base (Figure 3B). The apical groove is almost invisible (Figures 3B, H). A ventral infold is located in the middle of the seed, nearly semicircular, cracked along with the peripheral rugas (Figure 3A), 1.42 mm long, and accounts for approximately one-third of the total seed length. The ventral-infold cavity intrudes into the seed at a slightly oblique angle (Figure 3G), and the cavities are relatively round (Figure 3J). The nearly equal-in-width raphe is located between the ventral infolds, and a dent extends from the base to the apex of the raphe (Figure 3A). The ventral surface is relatively flat, and the dorsal surface is obviously raised on the side view. The ventral and dorsal rugas are different (Figures 3E, F), and the dorsal rugas are more obvious than the ventral ones (Figures 3A, B, E, F). A sunken chalaza (Figure 3J) and ventral infold can be seen in the cross-section. In the cross-section, the seed coat in ventral-infold cavities is significantly thinner than other parts and has an abrupt “keyhole” outline (Figure 3K).
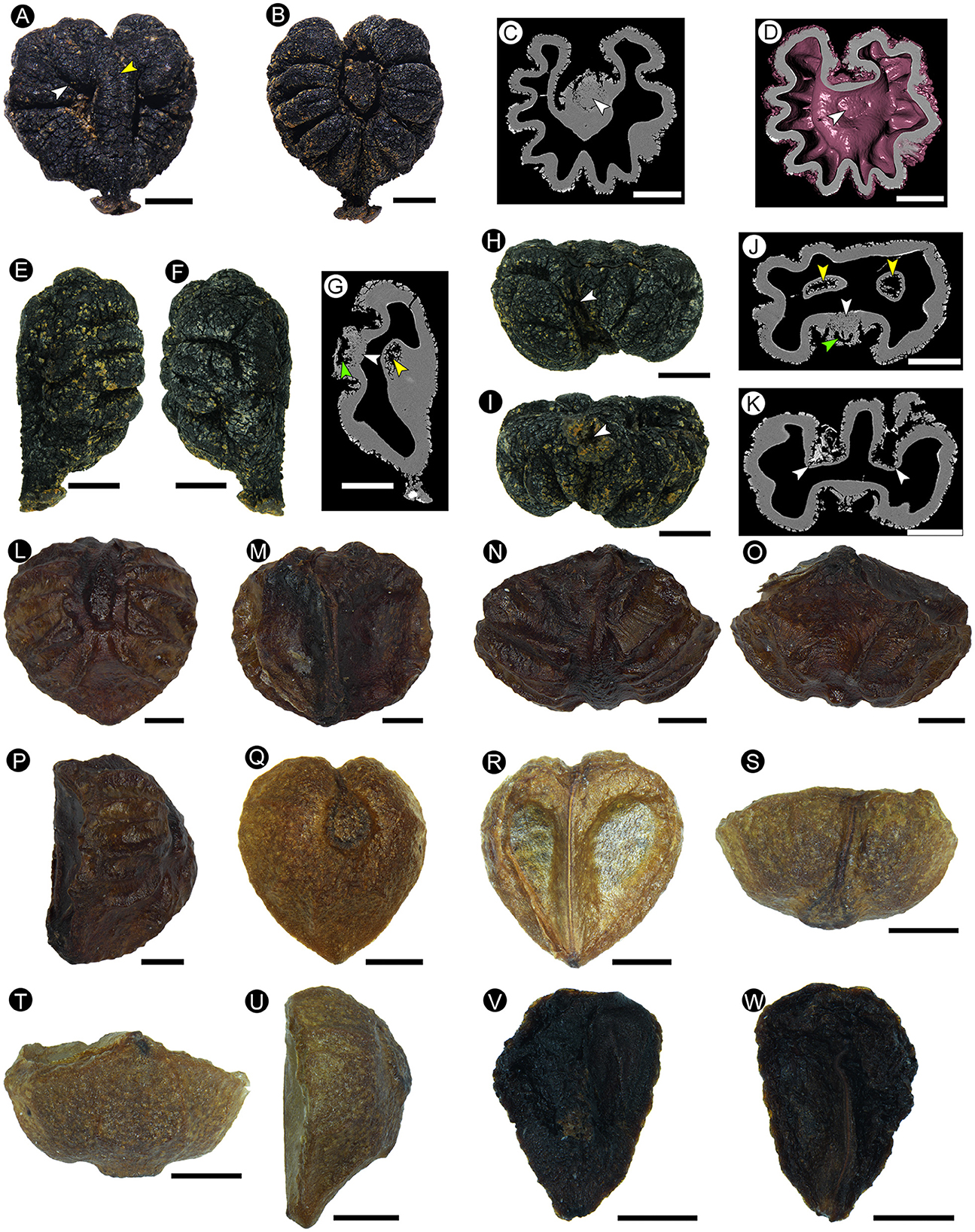
Figure 3. The morphological characters of the fossil seed from Maoming (A–K) (MMRH-236), extant Ampelopsis cantoniensis (Hook. et Arn.) Planch. (L–P), and Ampelopsis grossedentata (Hand.-Mazz.) W. T. Wang (Q–W). (A) Ventral view showing the ventral infold crack along with the peripheral rugas (white arrow), and a dent on the raphe (yellow arrow). (B) Dorsal view showing the nearly elliptical chalaza, and rugas arranged symmetrically. (C) Micro-CT of the longitudinal section shows the loose tissue of the chalaza (arrow), which can be distinguished clearly from the surrounding seed coat tissue. (D) Micro-CT of the longitudinal section showing the structure of the chalaza (arrow). (E, F) Side view showing the non-corresponding ventral and dorsal rugas. (G) Micro-CT of the longitudinal section showing the cavity structure (green arrow) and loose tissue (white arrow) of the chalaza, and the ventral-infold cavity that intrudes into the seed at a slightly oblique angle (yellow arrow). (H) Apical view showing the apical notch (arrow). (I) Basal view showing the skirt-shaped tip of the beak with a depressed center (arrow). (J) Micro-CT transverse section showing the cavity structure (green arrow) and loose tissue (white arrow) of the chalaza, and nearly round ventral-infold cavity (yellow arrows). (K) Micro-CT transverse section showing the significantly thinner seed coat in ventral-infold cavities (arrows). (L) Dorsal view showing the nearly elliptical chalaza, and rugas arranged symmetrically. (M) Ventral view showing the indistinct ventral infold and the nearly equal-in-width raphe between that. (N) Apical view showing two planes and a corner of the ventral surface. (O) Basal view showing two planes and a corner of the ventral surface. (P) Side view. (Q–W) Two seeds in the same fruit. (Q–U) Normally developed seed. (Q) Dorsal view. (R) Ventral view. (S) Apical view. (T) Basal view. (U) Side view. (V, W) Aborted ovule. Scale bars = 1 mm.
Comparison of the fossil seed from Maoming with extant species of Ampelopsis
The identification of Vitaceae seeds is relatively reliable because of the characteristic pair of ventral infolds and a dorsal chalaza that is absent in other families (Chen and Manchester, 2011). The morphological structure of Vitaceae seeds is important in classification, and the variation of the shape and position of features such as the chalaza, the ventral infold, and the raphe has great value in the taxonomy of Vitaceae (Chen et al., 2007). Based on the external morphology, the seeds of extant Vitaceae can be identified to a specific genus and even a species (Tiffney and Barghoorn, 1976). A long or linear chalaza can be seen in seeds of Leea L., Cissus L., Cyphostemma Alston, Tetrastigma K. Schum., Rhoicissus Planch., and Cayratia Juss. (Chen and Manchester, 2011), however, our seed has a nearly elliptical chalaza. The whorled, spiny rugas are a distinctive feature present only in Acareosperma Gagnep. but absent in our fossil. In addition, Acareosperma has an extremely thickened endotesta in certain parts of the seeds (C41 > 0.15), but the maximum thickness (C41) of the endotesta of our fossil is only 0.08 (Chen and Manchester, 2011). Unlike our fossil, Ampelocissus Planch., Pterisanthes Blume, and Nothocissus Latiff are distinguished from those of all other genera of Vitaceae by having ventral infolds that are almost as long as the full length of the seed and chalaza that is located in the center of the dorsal side; the chalaza of Parthenocissus Planch is round and centrally positioned, a distinct rib, groove-like infold, and a raphe that tapers from the apex to the base can be seen in the ventral side; Yua C. L. Li has a pear-shaped seed that is flat on the dorsal and ventral sides, linear ventral infolds that extend two-thirds of the length of the seed from the base, ventral-infold circularity (C8) < 0.4 (up to 0.58 in our fossil); Clematicissus Planch. only has one long and linear ventral infold and a chalaza that is located centrally (Chen and Manchester, 2007, 2011; Chen et al., 2007).
Our fossil displays a distinctive feature of the seeds of Ampelopsis that the seed coat is well-developed near the ventral-infold opening, as seen in the cross-section, but it is significantly thinner in the ventral-infold cavities and have an abrupt “keyhole” outline, which readily distinguishes it from Vitis L. and Ampelocissus (Manchester et al., 2013). Our fossil is consistent with Ampelopsis seeds, which have a pair of ventral infolds that are short and parallel to slightly divergent (equal-in-width or the raphe tapers between the ventral infolds), and a chalaza that is close to the notch and oval to pyriform in shape (Chen and Manchester, 2011; Manchester et al., 2013). Based on macroscopic morphology and structural characteristics, our fossil can be reliably assigned to Ampelopsis. We compared our fossil with some Asian species of Ampelopsis based on research of Chen et al. (2007), Chen and Manchester (2011), and our observation of specimens.
Both Ampelopsis grossedentata (Hand.-Mazz.) W. T. Wang (Figures 3Q–W) and A. chaffanjonii (H. Léveillé and Vaniot) Rehder have an oval chalaza, but the ventral infold in our specimen is nearly semicircular, which is different from the inverted oval of A. grossedentata. The skirt-shaped tip of the beak in our specimen is also different from the sharp beak of A. chaffanjonii. Our specimen is heart-shaped with an obvious apical notch, which is also different from the inverted oval seeds with a circular apex as shown in A. grossedentata and A. chaffanjonii.
Just like Ampelopsis gongshanensis C. L. Li and A. hypoglauca (Hance) C. L. Li, our specimen also has obvious rugas on the dorsal and ventral surfaces, but the raphe between the ventral infolds of our specimen is nearly equal-in-width along with its length, while it tapers from the apex to the base in A. gongshanensis. The chalaza of our specimen is close to the apical notch and different from that of A. hypoglauca in which the chalaza is in the middle of the dorsal surface. In addition, A. gongshanensis and A. hypoglauca differ from our specimen in having obovoid seeds with round and blunt apices and sharp basal beaks.
The chalaza of our specimen and that of Ampelopsis rubifolia (Wall.) Planch. is elliptical, with obvious rugas on the dorsal and ventral surfaces, but A. rubifolia has a prominent upper raphe, an obovoid seed, and an inconspicuous ventral infold. Both our specimen and A. tomentosa Planch. have short ventral infolds, but the seeds of A. tomentosa are inverted triangular oval in shape, and the dorsal raphe of the seed is prominent.
Both our specimen and Ampelopsis cantoniensis (Hook. et Arn.) Planch. (Figures 3L–P) have an oval chalaza, as well as obvious dorsal and ventral rugas. However, the seeds of A. cantoniensis are obovate, the apex is round, the ventral infolds are not obvious, and the tip of the beak is sharp, while our specimen does not have these characteristics.
Unlike our fossil seed, in Ampelopsis acutidentata W. T. Wang, the chalaza is located in the center, and the ventral surface has a prominent rib. Seeds of A. bodinieri (Levl. et Vant.) Rehd. have an acute beak. Seeds of A. acutidentata and A. bodinieri are obovate with a rounded apex and a smooth surface. A. aconitifolia Bge., A. megalophylla Diels et Gilg, and A. delavayana Planch have obovoid seeds with centrally positioned chalaza and ventral groove-like infolds. A. heterophylla (Thunb.) Sieb. et Zucc. seed is oblong with a nearly round top, and the ventral infold is oval and extends diagonally from the base to the top of the seed. A. humulifolia Bge. has a rounded seed apex, a chalaza located in the center, and the ventral infold is oval. The seed of A. glandulosa (Wall.) Momiy is narrowly elliptic. Seeds of A. vitifolia (Boiss.) Planch. have rounded tops, smooth surfaces, and distinctive ventral ribs.
The seed of Ampelopsis japonica (Thunb.) Makino is obovoid to heart-shaped, the surface of the seed is smooth or has obvious rugas, the chalaza is elliptical, the beak is prominent, has a nearly semicircular ventral infold located in the middle of the seed, and a nearly equal-in-width raphe is located between the ventral infolds. Our fossil shows the greatest similarity with this species in terms of all the above characteristics.
Comparison of the fossil seed from Maoming with other fossil species of Ampelopsis
Chen (2009) divided Vitaceae fossil seeds into 14 types based on morphological measurement data. After measurement and comparison, our fossil seed can be classified as seed type st-Ampelopsis-rugose. Therefore, we compared our fossil with the seeds of Ampelopsis belonging to this type and the newly reported seeds of Ampelopsis which have obvious rugas on the surface (Table 1).
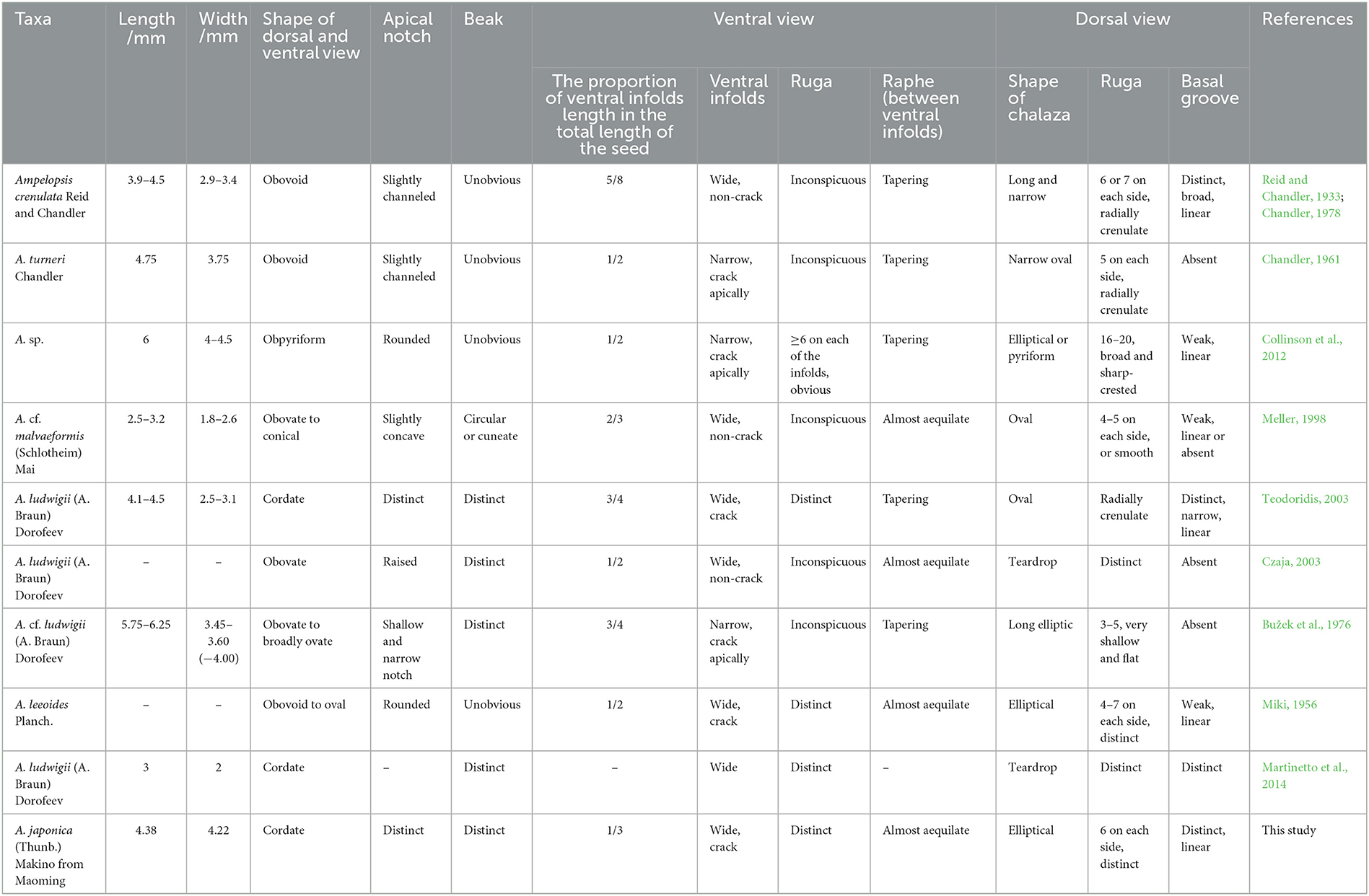
Table 1. Morphological comparison of the fossil seed from the Upper Pleistocene of the Maoming Basin with related fossil species of Ampelopsis.
Our specimen is similar to Ampelopsis crenulata (Reid and Chandler, 1933; Chandler, 1978) and A. turneri (Chandler, 1961) is known from the lower Eocene of the United Kingdom. They have a dorsal surface that is obviously raised on the side view, rugas arranged radially around the chalaza, and there are obvious grooves around the chalaza. However, the ventral infold of our specimen is wide and our specimen has a basal groove, while that of A. turneri is narrow without a basal groove. The apex of our specimen has an obvious notch, and the ventral infold accounts for approximately one-third of the total length, while that of A. crenulata is only slightly grooved and accounts for five-eighths of the total length. In addition, our fossil is heart-shaped, and the nearly equal-in-width raphe is located between the ventral infolds, while the shapes of A. crenulate and A. turneri are obovoid, and the raphe tapers from the apex to the base.
A fossil seed of Ampelopsis sp. reported from the middle Eocene of Germany (Collinson et al., 2012) is obovoid in dorsal and ventral views and lacks a prominent beak, while our specimen is heart-shaped with a prominent beak. Moreover, the seed of Ampelopsis sp. is 6 mm long, and it is longer than our specimen (4.38 mm). The ventral infold length of Ampelopsis sp. accounts for approximately one-half of the total seed length, while in our specimen, it only accounts for one-third. In addition, our fossil does not have 16–20 wide, sharp-crested ridges on the dorsal surface like those possessed by Ampelopsis sp.
Like Ampelopsis cf. malvaeformis (Schlotheim) Mai (Meller, 1998) known from the upper and middle Eocene of Austria, our specimen has nearly the same width of the raphe between the ventral infolds and has obvious dorsal rugas. However, the length (4.38 mm) and width (4.22 mm) of our specimen exceed those of A. cf. malvaeformis (2.5–3.2 mm in length and 1.8–2.6 mm in width). In addition, the seed of A. cf. malvaeformis is obovate to conical, with a prominent dorsal chalaza, while our specimen is heart-shaped, and the dorsal chalaza is obviously lower than the dorsal plane.
Both Ampelopsis ludwigii (A. Braun) Dorofeev found in the Lower Miocene of the Czech Republic (Teodoridis, 2003; Martinetto et al., 2014) and our specimen are heart-shaped, and both have a prominent beak at the base, with obvious radial rugas around the chalaza. However, in A. ludwigii, the raphe between the ventral infolds tapers from the apex to the base, which is different from our specimen. Moreover, the tip of the beak in our specimen is skirt-shaped, while that of A. ludwigii is sharp.
As with the Ampelopsis ludwigii reported from the Lower/Middle Miocene of Germany (Czaja, 2003), our specimen has a nearly semicircular ventral infold in the middle, a prominent beak at the base, and the raphe between the ventral infolds is nearly equal-in-width throughout its length. However, our specimen is heart-shaped, the ventral surface also has obvious rugas, and the chalaza is obviously lower than the dorsal plane, while A. ludwigii is obovoid, the ventral infolds are almost invisible, and the chalaza is convex.
There are 3–5 shallow and flat rugas on the dorsal surface of Ampelopsis cf. ludwigii, which was found in the Miocene of the Czech Republic. Unlike our specimen, this fossil has a narrow ventral infold, its basal groove is missing, and the raphe gradually narrows from the apex to the base (Bužek et al., 1976).
Miki (1956) reported Ampelopsis leeoides Planch. from the Pliocene of Japan. This fossil has 4–7 pairs of rugas arranged symmetrically on the dorsal surface, its raphe is nearly equal in width, and the nearly semicircular ventral infold with cracks along with the peripheral rugas is located in the middle of the seed. Our specimen is similar to it. However, the beak of A. leeoides is not obvious, the apex is nearly round, and the ventral infold occupies ~1/2 of the total length of the seed. Moreover, the overall shape of A. leeoides is from obovate to oval. Our fossil does not have these characteristics.
To sum up, the Ampelopsis fossil seed described here is different from all the above fossils and most extant species of Ampelopsis, but it shows the greatest similarity with extant Ampelopsis japonica, so our fossil seed can be assigned to this living species.
Discussion
Phylogenetic implications
The Ampelopsis fruit is a berry with 1–4 seeds, the ovary is two-locular (but the septa do not fuse) with two anatropous ovules in each chamber, typically has the aborted ovule (Figures 3V, W) and possesses a funiculus extending into the chalazal end of the ovule (Wen, 2007; Ickert-Bond et al., 2014; Gerrath et al., 2015).
The shapes of Ampelopsis seeds can be significantly affected by the number of seeds per berry (Chandler, 1961; Tiffney and Barghoorn, 1976) (Figure 4). If a berry contains several seeds, they will squeeze each other during the growth and development process, forming multiple planes with edges and corners on the ventral surface (Figures 3N, O, 4A, B). If a berry contains only one seed in a locule, the seeds usually have a flat surface (Figures 3S–U, 4B–D). However, our fossil has a flat ventral surface without edges and corners, and the edge is rounded (Figures 3E, F, H, I). We, therefore, infer that there may have one to three seeds in the berry from which our fossil seed is derived, which are in one (Figure 4D) or two locules (Figures 4B, C), respectively, indicating that at least one ovule in one locule of the fruit is aborted. Aborted ovules commonly occur in the fruits of extant Ampelopsis. According to the structural characteristics of our fossil, we speculate that this phenomenon of Ampelopsis fruit development existed in the Late Pleistocene.
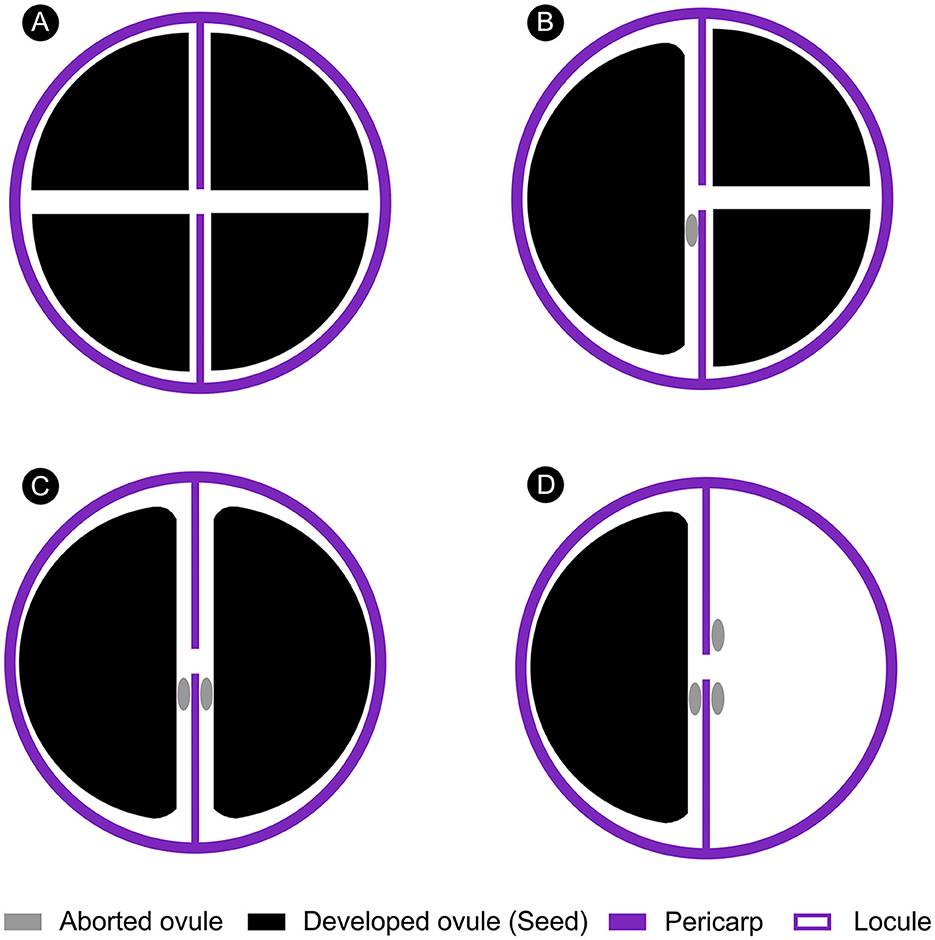
Figure 4. Schematic diagram of the effect of the number of seeds on seed shape in an Ampelopsis fruit (Apical view). (A) All ovules developed. (B) One ovule in one locule of the fruit is aborted. (C) Two ovules in two locules of the fruit are aborted. (D) One ovule in one locule and two ovules in another locule of the fruit are aborted.
The vascular strand that connects the seed with the berry culminates in a relatively dark knot on the upper part of the seeds, marking the chalaza (Gerrath et al., 2015). There is a cavity structure at the chalaza in extant Ampelopsis seeds, and the chalaza can be clearly distinguished from the adjacent seed coat tissue. This special structure is also found in our fossil seed. The obvious cavity structure at the chalaza can be seen in the seed section imaged by Micro-CT (Figures 3G, J), and this part of the tissue is relatively loose, clearly distinguishing it from the surrounding seed coat tissue (Figures 3C, D, G, J). This indicates that the special chalazal structure of Ampelopsis seeds existed in the Late Pleistocene, and there has been little change in this structure since Late Pleistocene. To a certain extent, it reflects the stability of plant reproductive organs' structure.
Paleophytogeographical implications
Ampelopsis is mainly distributed in Eurasia, North America, and Central America (Chen, 2009; Nie et al., 2012), with most occurring in East Asia and China [17 species (13 endemic species)] (Chen et al., 2007; Fang et al., 2009) (Figure 5). Ampelopsis has abundant fossil records (Figure 5), which are mainly from the Paleocene to Pleistocene in Europe. The earliest fossil record of this genus is Ampelopsis cf. monasteriensis Kirchheimer, a seed reported from the Paleocene of Germany (Mai, 1987). In addition, fossils ascribed to this genus have been reported from the Eocene of Britain and Germany, the Oligocene of Poland, the Miocene of Austria, Germany, Poland, the Czech Republic, and Bulgaria, as well as the Pliocene to the Pleistocene of Italy (e.g., Reid and Chandler, 1933; Teodoridis, 2003; Martinetto et al., 2014; Kowalski et al., 2020). A. rooseae Manchester was discovered in the middle and upper Eocene of Oregon (Manchester, 1994; Manchester and McIntosh, 2007), and a seed fossil of the genus was also reported from the Miocene of Washington state (Tcherepova and Pigg, 2005). In Asia, Ampelopsis fossils are only reported from the Eocene to the Miocene of Siberia, Russia (e.g., Dorofeev, 1963; Nikitin, 2006), the Upper Miocene of China (Huang et al., 2017), and the Pliocene of Japan (Miki, 1956).
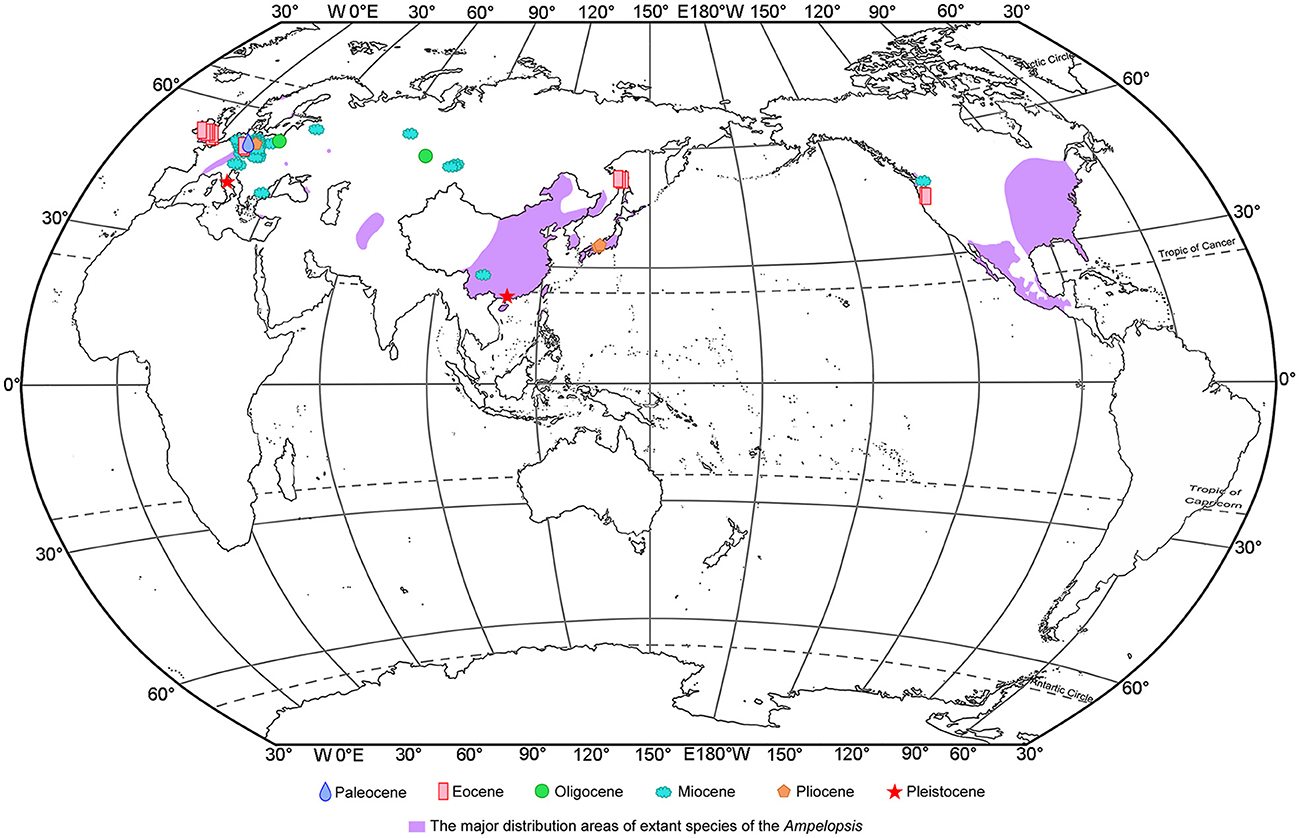
Figure 5. Megafossil records of Ampelopsis and the major native ranges of its extant species. The extant species ranges of Ampelopsis are derived from Global Biodiversity Information Facility [GBIF.org (16 June 2022) GBIF Occurrence Download https://doi.org/10.15468/dl.mzuqcs], Fang et al. (2009), Villaseñor (2016).
To date, apart from the fossil seeds from the Upper Miocene of Zhaotong Basin, Yunnan province (Huang et al., 2017), there have been no reports of Ampelopsis fossils in China. The Ampelopsis fossil seed found in Maoming Basin is the first fossil record of the genus from South China, indicating that Ampelopsis had reached South China by the Late Pleistocene. Previous fossil records of Ampelopsis have mostly been distributed in the mid-latitudes (Figure 5), but our specimen was discovered in the low-latitude area of South China. Four earth's climate states are identified during the past 66 Ma: Warmhouse (66–56 and 47–34 Ma), Hothouse (56–47 Ma), Coolhouse (34–3.3 Ma), and Icehouse (3.3–0 Ma) (Westerhold et al., 2020). During the Pleistocene, especially during the last glaciation, the global temperature experienced a rapid decline (Liu et al., 2001), and the temperature decline also occurred in the Maoming (Huang et al., 2023), the climate in Maoming might become suitable for the Ampelopsis. Thus, we infer that global cooling during the last glaciation led to the southward spread of the genus into the low-latitude area of South China.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JJ, LH, and HX conceived and designed the project and contributed to initial manuscript preparation. JJ, LH, XL, and XW organized field work and the collection of fossils. HX and SX prepared, imaged fossil and modern specimens, and processed data. All authors discussed results, read, revised, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 42102004 and 41820104002], State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Paleontology, CAS) [grant number 223118], and the Fundamental Research Funds for the Central Universities [grant number 22qntd2606]. All the above funders provided the funding for the implementation of this research, including the field work. National Natural Science Foundation of China [grant numbers 42102004 and 41820104002] pay for Open Access publication fees.
Acknowledgments
We sincerely thank R. A. Spicer (The Open University, UK) for the linguistic improvement of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bazhenova, N. V., Wu, X. K., Kodrul, T. M., Maslova, N. P., Tekleva, M. V., Xu, S. L., et al. (2022). Mummified seed cones of Pinus prehwangshanensis sp. nov. (subgenus Pinus, Pinaceae) from the Upper Pleistocene of Guangdong, South China: taxonomical significance and implication for phytogeography and ecology. Front. Ecol. Evol. 10, 900687. doi: 10.3389/fevo.2022.900687
Bozukov, V. (2000). Miocene macroflora of the Satovcha Graben (Western Rhodopes). I. Systematics. 5. Magnoliophyta: Araliaceae, Aquifoliaceae, Celastraceae, Rhamnaceae, Vitaceae, Apocynaceae, Caprifoliaceae, Convolvulaceae, Macclintockia; Smilacaceae, Cyperaceae, Sparganiaceae, Typhaceae. Phytol. Balcan. 6, 15–30.
Bužek, C., Holý, F., and Kvaček, Z. (1976). Tertiary flora from the Volcanogenic Series at Markvartice and Veseličko near Česká Kamenice (České středohoři Mts.). Palaeontology 18, 69–132.
Chandler, M. E. J. (1961). The Lower Tertiary Floras of Southern England. I Paleocene Floras. London Clay flora (Supplement). Text and Atlas. London: The British Museum (Natural History).
Chandler, M. E. J. (1978). Supplement to the Lower Tertiary Floras of Southern England, Part 5. London: The Tertiary Research Group.
Chen, I. (2009). History of Vitaceae inferred from morphology-based phylogeny and the fossil record of seeds (PhD diss.). University of Florida, Gainesville.
Chen, I., and Manchester, S. R. (2007). Seed morphology of modern and fossil Ampelocissus (Vitaceae) and implications for phytogeography. Am. J. Bot. 94, 1534–1553. doi: 10.3732/ajb.94.9.1534
Chen, I., and Manchester, S. R. (2011). Seed morphology of Vitaceae. Int. J. Plant Sci. 172, 1–35. doi: 10.1086/657283
Chen, Z. D., Ren, H., and Wen, J. (2007). “Vitaceae,” in Flora of China, ed C. Li (St. Louis: Science Press, Beijing and Missouri Botanical Garden Press), 173–222
Collinson, M. E., Manchester, S. R., and Wilde, V. (2012). Fossil fruits and seeds of the middle Eocene Messel biota, Germany. Abhandlungen der Senckenberg Gesellschaft für Naturforschung 570, 1–249.
Czaja, A. (2003). Paleocarpological investigations of the taphocoenoses of the Lower- and Middle Miocene from the opencast mine Berzdorf/Upper Lusatica (Saxony). Palaeontogr. Abteilung B 265, 1–148. doi: 10.1127/palb/265/2003/1
Dorofeev, P. I. (1963). Tertiary Floras of Western Siberia. Komarov Botanical Institute. Moscow: Academy of Sciences of the U.S.S.R.
Fang, J. Y., Wang, Z. H., and Tang, Z. Y. (2009). Atlas of Woody Plants in China. Distribution and Climate, Vol. 2. Beijing: Higher Education Press.
Gerrath, J., Posluszny, U., and Melville, L. (2015). “Reproductive features of the Vitaceae,” in Taming the Wild Grape: Botany and Horticulture in the Vitaceae (Cham: Springer International Publishing), 45–64. doi: 10.1007/978-3-319-24352-8_3
Huang, L., Li, S., Huang, W., Xiang, H., Jin, J., and Oskolski, A. A. (2023). Glacial expansion of cold-tolerant species in low latitude: megafossil evidence and distribution modelling. Natl. Sci. Rev. 10, nwad038. doi: 10.1093/nsr/nwad038
Huang, L. L., Jin, J. H., and Oskolski, A. A. (2021a). Mummified fossil of Keteleeria from the Late Pleistocene of Maoming Basin, South China, and its phytogeographical and paleoecological implications. J. Syst. Evol. 59, 198–215. doi: 10.1111/jse.12540
Huang, L. L., Jin, J. H., Quan, C., and Oskolski, A. A. (2021b). New occurrences of Altingiaceae fossil woods from the Miocene and Upper Pleistocene of South China with phytogeographic implications. J. Palaegeogr. 10, 482–493. doi: 10.1016/j.jop.2021.11.001
Huang, Y. J., Ji, X. P., Su, T., Deng, C. L., Ferguson, D. K., Yu, T. S., et al. (2017). Habitat, climate and potential plant food resources for the late Miocene Shuitangba hominoid in Southwest China: insights from carpological remains. Palaeogeogr. Palaeoclimatol. Palaeoecol. 470, 63–71. doi: 10.1016/j.palaeo.2017.01.014
Ickert-Bond, S. M., Gerrath, J., and Wen, J. (2014). Gynoecial structure of Vitales and implications for the evolution of placentation in the Rosids. Int. J. Plant Sci. 175, 998–1032. doi: 10.1086/678086
Kowalski, R., Worobiec, G., Worobiec, E., and Krajewska, K. (2020). Oligocene plant assemblage from Rebiszów, Lower Silesia: first “volcanic flora” from Poland. Acta Palaeontol. Pol. 65, 273–290. doi: 10.4202/app.00686.2019
Liu, J. Q., Ni, Y. Y., and Chu, G. Q. (2001). Main palaeoclimatic events in the Quaternary. Quat. Sci. 21, 239–248.
Mai, D. H. (1987). Neue Früchte und Samen aus paläozänen Ablagerungen Mitteleuropas. Feddes Rep. 98, 197–229.
Manchester, S. R. (1994). Fruits and seeds of the middle Eocene Nut Beds flora, Clarno Formation, Oregon. Palaeontogr. Am. 58, 1–205.
Manchester, S. R., Kapgate, D. K., and Wen, J. (2013). Oldest fruits of the grape family (Vitaceae) from the Late Cretaceous Deccan Cherts of India. Am. J. Bot. 100, 1849–1859. doi: 10.3732/ajb.1300008
Manchester, S. R., and McIntosh, W. C. (2007). Late Eocene silicified fruits and seeds from the John Day Formation near Post, Oregon. PaleoBios 27, 7–17.
Martinetto, E., Bertini, A., Basilici, G., Baldanza, A., Bizzarri, R., Cherin, M., et al. (2014). The plant record of the Dunarobba and Pietrafitta sites in the Plio-Pleistocene palaeoenvironmental context of central Italy. Alpine Mediter. Quat. 27, 29–72.
Meller, B. (1998). Systematic-taxonomic and palaeoecological investigations of the Carpo-Taphocoenoses from the Köflach-Voitsberg lignite mining area (Styria, Austria; Early Miocene). Jahrbuch der Geologischen Bundesanstalt 140, 497–655.
Nie, Z. L., Sun, H., Manchester, S. R., Meng, Y., Luke, Q., and Wen, J. (2012). Evolution of the intercontinental disjunctions in six continents in the Ampelopsis clade of the grape family (Vitaceae). BMC Evolut. Biol. 12, 17. doi: 10.1186/1471-2148-12-17
Nikitin, V. P. (2006). Paleocarpology and Stratigraphy of the Paleogene and Neogene Strata in Asian Russia. Novosibirsk: Academic Publishing House.
Reid, E. M., and Chandler, M. E. J. (1933). The London Clay Flora. London: British Museum (Natural History).
Tcherepova, M., and Pigg, K. B. (2005). Vitaceae: Fossil Seeds and Wood From the Middle Miocene of Yakima Canyon, Washington, USA. Austin: Botany.
Teodoridis, V. (2003). Early Miocene carpological material from the Czech part of the Zittau Basin. Acta Palaeobot. 43, 9–49.
Tiffney, B. H., and Barghoorn, E. S. (1976). Fruits and seeds of the brandon lignite. I. Vitaceae. Rev. Palaeobot. Palynol. 22, 169–191.
Villaseñor, J. L. (2016). Checklist of the native vascular plants of Mexico. Revista mexicana de biodiversidad. 87, 559902. doi: 10.1016/j.rmb.2016.06.017
Wen, J. (2007). “Flowering plants eudicots,” in The Families and Genera of Vascular Plants, ed K. Kubitzki (New York, NY: Springer), 1–509.
Westerhold, T., Marwan, N., Drury, A. J., Liebrand, D., Agnini, C., Anagnostou, E., et al. (2020). An astronomically dated record of Earth's climate and its predictability over the last 66 million years. Science 369, 1383–1387. doi: 10.1126/science.aba6853
Keywords: Ampelopsis, fossil seed, Maoming Basin, South China, Late Pleistocene
Citation: Xiang H, Wu X, Liu X, Xu S, Jin J and Huang L (2023) The first fossil seed of Ampelopsis (Vitaceae) in South China. Front. Ecol. Evol. 11:1130586. doi: 10.3389/fevo.2023.1130586
Received: 23 December 2022; Accepted: 20 February 2023;
Published: 22 March 2023.
Edited by:
Zhifei Zhang, Northwest University, ChinaReviewed by:
Ning Tian, Shenyang Normal University, ChinaShufeng Li, Xishuangbanna Tropical Botanical Garden (CAS), China
Copyright © 2023 Xiang, Wu, Liu, Xu, Jin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luliang Huang, NTcxODU0MjAyQHFxLmNvbQ==
 Helanlin Xiang
Helanlin Xiang Xinkai Wu2
Xinkai Wu2 Xiaoyan Liu
Xiaoyan Liu Shenglan Xu
Shenglan Xu Jianhua Jin
Jianhua Jin Luliang Huang
Luliang Huang