- 1College of Resource and Environmental Engineering, Guizhou University, Guiyang, Guizhou, China
- 2Department of Geosciences, Trinity University, San Antonio, TX, United States
- 3Research Department of Science and Technology, Guizhou Geological Museum, Guiyang, Guizhou, China
- 4Witte Museum, San Antonio, TX, United States
Keichousaurus hui is the most abundant and representative species in the Xingyi biota in South China. K. hui has been studied in many aspects, including its functional morphology, osteology, ontogeny, allometric growth, sexual dimorphism, and reproduction. Previous studies have assumed that Keichousaurus’ anteriorly curved teeth were used to impale prey and to tear and swallow either the whole or partially fragmented prey. Prey items have been assumed to be small fish or soft-bodied invertebrates, such as squid and shrimp, that were also present in the Xingyi biota. However, there has been no direct evidence for the anatomy of the digestive tract or the diet of the Keichousaurus. There are few reports of the soft tissue preservation of K. hui. In this study, we present relatively complete preservation of the digestive tract with food remains in several well-preserved specimens of K. hui. By comparing with modern reptiles, we reconstructed the internal distribution and organ composition of the digestive tract of K. hui. Through the analysis of the alimentary canal remnants by microscope and X-ray computed microtomography, we infer that K. hui was a frequently piscivorous species that usually swallowed its prey whole.
1. Introduction
Pachypleurosaurs are considered the most primitive representatives of sauropterygians (Rieppel, 1993; Storrs, 1993), which diversified in the Triassic epicontinental seas and intraplatform basins of the Tethyan Ocean (Rieppel, 2000; Liu et al., 2011; Figure 1A). Pachypleurosauria contains the following valid taxa (Klein, 2012): Dactylosaurus gracilis (Gürich, 1884; Rieppel and Hagdorn, 1997), Anarosaurus pumilio (Dames, 1890) and A. heterodontus (Rieppel and Lin, 1995) from the Germanic Basin, Serpianosaurus mirigiolensis (Rieppel, 1989), Neusticosaurus edwardsii (Cornalia, 1854; Carroll and Gaskill, 1985), N. peyeri (Sander, 1989), and N. pusillus (Seeley, 1882) from the Alpine Triassic of Monte San Giorgio, Switzerland. These seven species of Pachypleurosauria have been reported in the western Tethyan province. Additional pachypleurosaur taxa that occur in the eastern Tethyan province (south China) include Keichousaurus hui Young 1958 and Dianopachysaurus dingi (Liu et al., 2011). The evolutionary lineage shows that the Chinese pachypleurosaurus Keichousaurus and all European Pachypleurosaurus constitute a monophyletic group (Rieppel and Lin, 1995; Liu et al., 2011; Cheng, 2015).
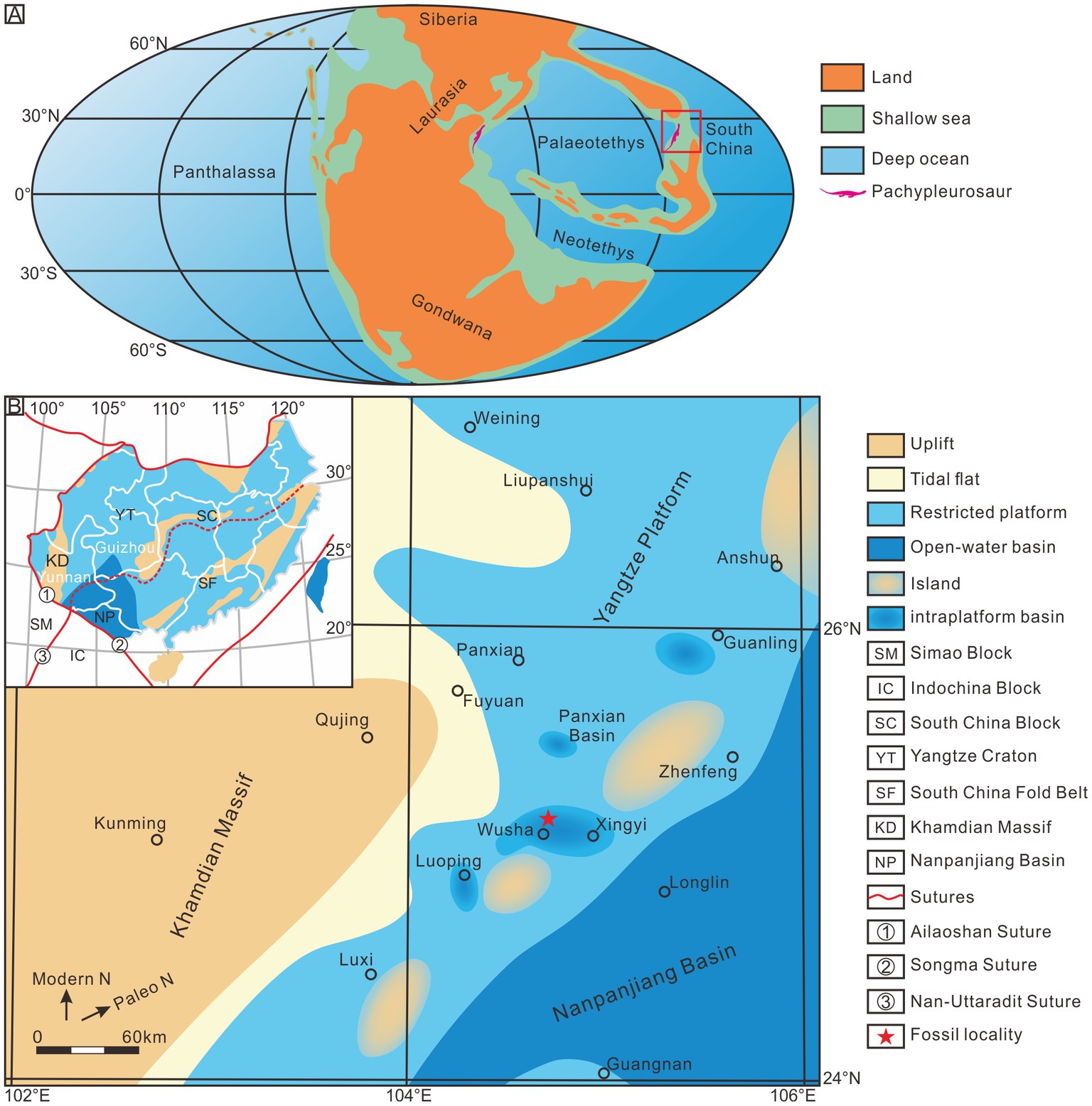
Figure 1. Maps. (A) Pachypleurosaurs palaeobiogeographical distribution in the Middle/Late Triassic [modified from Lu et al. (2018)]. (B) Palaeogeographic map showing the location of the Xingyi biota [modified from Liu et al. (2013)]. Inset is a tectonic map illustrating blocks and sutures of south China and adjacent areas [After Metcalfe (2006), Lehrmann et al. (2009), and Ma et al. (2009)].
Keichousaurus hui, as the earliest assigned to the Pachypleurosauridae (Young, 1958), is one of the most abundant Triassic marine reptiles in south China. A large number of well-preserved specimens of K. hui have provided the basis for a more comprehensive scientific study, including its skeletal system, functional morphology, ontogenesis, sexual dimorphism, allometric growth, reproductive mode, and paleoecology (Young, 1958; Lin, 1994; Lin and Rieppel, 1998; Yang, 1998; Rieppel, 1999; Cheng et al., 2004, 2009; Holmes et al., 2008; Wang et al., 2009; Fu et al., 2013; Xue et al., 2013; Qin et al., 2014; Motani et al., 2015; Zou et al., 2015; Hu et al., 2018; Lu et al., 2018). Although the stratigraphic age of K. hui is still controversial, numerous independent conodont studies support an early Late Triassic (early Carnian) (Yang et al., 1995; Wang, 1996, 2002; Wang et al., 1998), but biostratigraphy with ammonites supports a late Middle Triassic (late Ladinian) age (Chen, 1985; Li, 2006; Zou et al., 2015). Recent zircon U–Pb dating yielded an age of 240.8 ± 1.8 Ma (Li et al., 2016).
For most Mesozoic marine reptiles, dietary inference relies primarily on indirect evidence from dentition and jaw architecture (Massare, 1987). In the case of the Keichousaurus and other pachypleurosaurs, the anterior teeth on the upper and lower jaws become gradually curved as they taper anteriorly to their tips, and this has been interpreted to enable the animal to impale its prey and manipulate either the torn prey or whole prey item into position to swallow (Sander, 1989; Holmes et al., 2008; Fu et al., 2013; Liao et al., 2021). In the case of the Keichousaurus, prey items have been assumed to be small fishes or soft-bodied invertebrates, such as squid and shrimp, that were also present in the Xingyi Lagerstatte deposit (Su, 1959; Jin, 2001; Liu et al., 2002, 2003; Li, 2006; Xu et al., 2012, 2015; Tintori et al., 2015; Zou et al., 2015; Sun et al., 2016; Ni et al., 2017; Xu and Ma, 2018; Li et al., 2019; Xu, 2020). However, there has been no direct evidence for diet or mode of feeding from stomach contents.
In some apex predators, such as ichthyosaurs and plesiosaurs, preserved gut contents provide direct evidence for prey preferences (Pollard, 1968; Kear et al., 2003; Cheng and Chen, 2007; O’Keefe et al., 2009; Jiang et al., 2020). Nevertheless, well-preserved and identifiable gastric contents are relatively rare in small tetrapods (Xing et al., 2019), not to mention relatively complete digestive tracts. Because taphonomy usually limits soft parts preservation, there is little documented direct evidence about soft tissue preservation in pachypleurosaurs. Only in Neusticosaurus of Monte San Giorgio has preservation of soft tissue anatomy been reported (Sander, 1989). In the present study, we show the occurrence of spectacularly preserved specimens with soft part fossilization of the alimentary canal in Keichousaurus hui from South China and from the Triassic period. These remarkably well-preserved specimens not only provide the first direct and unequivocal evidence of prey preference and feeding strategy but also help in the reconstruction of the alimentary canal of this species.
2. Materials and methods
The nine exceptionally preserved specimens of Keichousaurus hui, reposited in the Paleontological Collection of Guizhou University (PCGU4281-PCGU4289), were collected from Wusha Town, Xingyi, and Guizhou, China (Figure 1B) (104°47′36.83″E, 25°9′32.87″N). The deposits from which they were found are those of the Zhuganpo Member of the Falang Formation. Rich invertebrates, bony fishes, and several other types of marine reptiles in this stratum were also found (Liao et al., 2021). The whole fossil assemblage represents the renowned Xingyi biota (Su, 1959; Jin, 2001; Liu et al., 2002, 2003; Li, 2006; Xu et al., 2012, 2015; Tintori et al., 2015; Zou et al., 2015; Sun et al., 2016; Ni et al., 2017; Xu and Ma, 2018; Li et al., 2019; Xu, 2020).
The preparatory work of specimens was completed using pneumatic air scribe tools and fine steel needles under a stereo microscope SZM7045–STL2 (magnification of 45×, viewable range of 4.4 mm, and working distance of 100 mm) with Multi-Position Fiber Optic Illuminator System. We precluded any potentially corrosive methods. Six separate measurements (Supplementary Table 1) were taken from each specimen using the criteria of Sander (1989). Entire fossil specimens were photographed using a Canon 200D camera (60 mm f/2.8 Macro USM lens). The trunk regions of the specimens were magnified by a Ultra-depth-of-field 3D microscopic system KEYENCE VHK–2000, and digitized by a CMOS camera (DVI–1) with a 1920 × 1,200 pixel chip and 16-bit dynamic range. The fine structures of each specimen were examined by a stereo microscope Leica M205C (optical resolution of 0.952 μm and a working distance of 61.5 mm), and images were recorded with a 20 Megapixel color CMOS camera DMC5400 using the Leica Application Suite X software. Computed tomography images of specimen PCGU4281 were conducted using a non-destructive high-resolution X-ray CT scanner Nano Voxel 4,000 (Tianjin Sanying Precision Instruments Co., Ltd., China). Scan parameters used for the specimen were: voxel size = 2 μm and voltage = 240 kV.
3. Results
3.1. Description of specimens
PCGU4281 is a well-preserved adult, male individual exposed in ventral view. Its total length over the preserved portions is 222.67 mm (Supplementary Table 1). Postmortem disturbance appears to be minimal, only the manus and tail tip were affected (Figure 2A). Of particular interest were well-preserved organic remains occurring within the trunk region of this specimen (Figures 2B,C). The remains can be divided into four types: (1) A black, amorphous matter encrusting the 5th–9th dorsal vertebrae (Figure 2B wa1 and Figure 3A wa1). The presence of the amorphous matter prevents the marginal boundaries of the 5th-9th vertebrae from being observed (compare with the rest of the vertebrae, for example, 10th-14th vertebrae) (Figure 2C). This black matter is covered by the posterior coracoid and a central V-shape element of gastralia at the position of the 8th dorsal centrum (Figure 3B arrowhead); (2) Within the right thoracic cavity, a black organic film can be seen covering the dorsal ribs along the 8th to 10th dorsal vertebrae. Additionally, some gastralia are draped over this film (Figure 2B wa2 and Figures 3A,B wa2). This phenomenon is in sharp contrast to the corresponding position of the left rib cage. It is worth noting that a black organic mass is preserved in the vicinity of the right humerus (Figure 2B wa3 and Figure 3A wa3). It is inferred to be caused by post-depositional compression of gut remains within the right body cavity. A natural connection at the position of the 8th dorsal vertebra is preserved and leads to the amorphous matter along the trunk axis to turn to the anterior margin of the film (Figure 2B ya1 and Figure 3A ya1); (3) The right side of the abdominal cavity is filled with a J-shaped mass preserved in three dimensions (Figures 2B wa4, and Figure 3B wa4). The mass is wrapped among the dorsal ribs and gastralia along the 11th to 15th dorsal vertebrae (Figure 2C). A natural transition is present between the abovementioned film and the J-shaped mass (Figure 2B ya2 and Figure 3B ya2); and (4) A large number of black phosphatic masses are situated in the posterior section of the body cavity (Figures 2B,C, 3C,D). These masses contact the posterior margin of the J-shaped mass at the position of the 16th dorsal vertebrae (Figure 2B ya3; Figure 3B ya3). In the left side of the abdominal cavity, portions of these phosphatic masses along the 15th–17th dorsal vertebrae are preserved between the dorsal ribs and gastralia (Figure 2B wa5; Figure 3C wa5). In addition, the entire region of the pelvic girdle is nearly filled by these phosphatic masses (Figure 2B wa6; Figure 3D wa6). Two masses can be observed on both sides of the base of the tail, and are made up of numerous small phosphatic nodules (Figure 2B wa7; Figure 3D wa7). Ordinarily, the presence of phosphatic material is characteristic of both marine reptilian bromalites and coprolites (Kear, 2006). The ilium of the left side has been pushed outward (Figures 2B,C, 3C). Similarly, Evans and Wang (2012) recorded extraversive ribs associated with the gut contents from an Early Cretaceous lizard and interpreted them to be caused by either decay gases of the bacterial degradation in the gut or post-depositional compression.
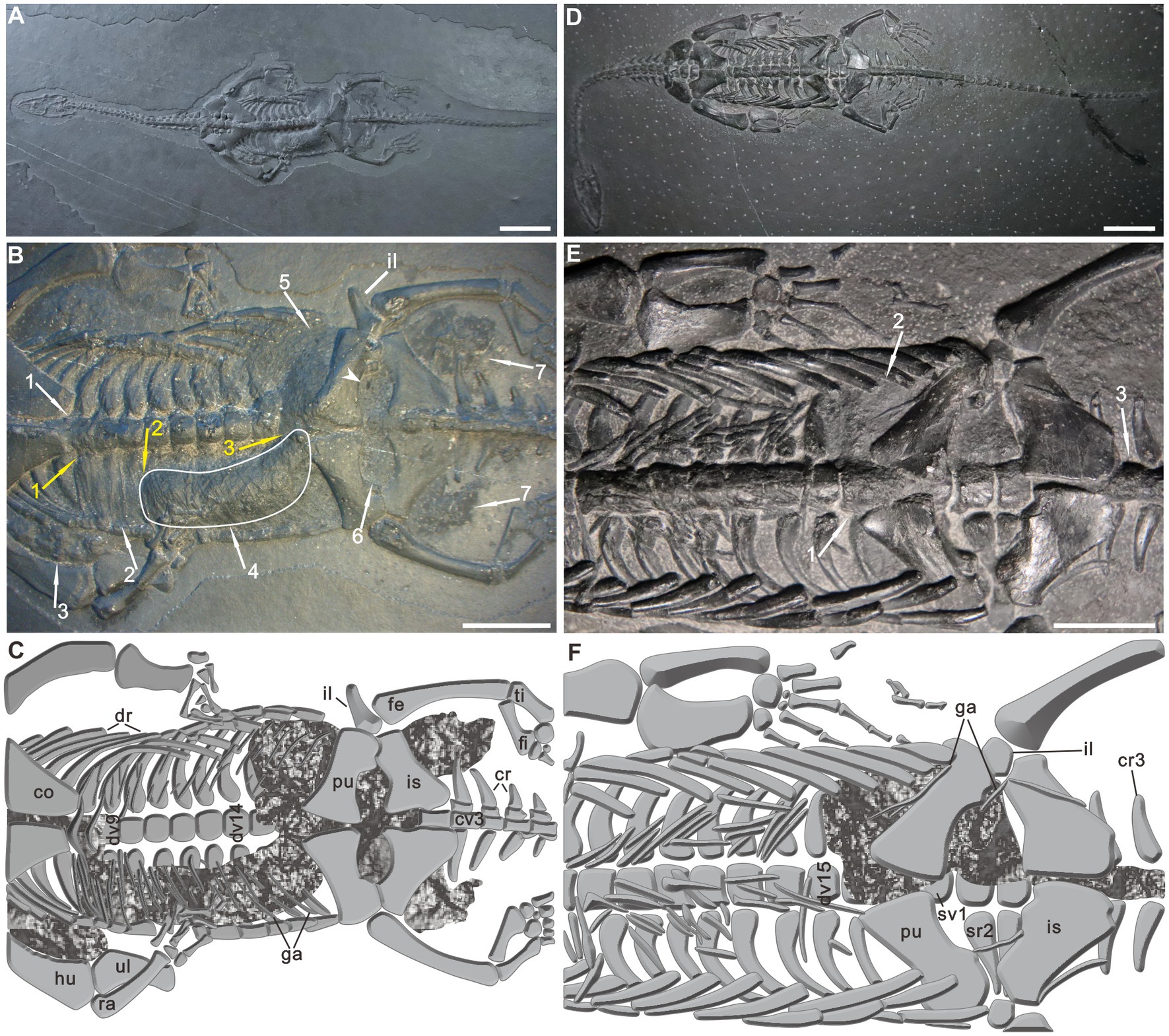
Figure 2. Keichousaurus hui with remains of the alimentary canal. (A) Specimen PCGU4281. (B) Specimen PCGU4281, a photograph of the trunk region. Arrowhead and white arrow 4 indicate enlargements shown in Figures 4A, 5A. The white line indicates a J-shaped three-dimensional preserved organic mass wrapped between the dorsal ribs and gastralia. Note caudal extremity of the J-shaped organic mass gradually narrows. (C) Specimen PCGU4281, interpretive drawing of the trunk region. Note the ilium outward-dipping [compare with panels (E,F)]. Figure 3 presents enlarged photographs of the trunk of PCGU4281. (D) Specimen PCGU4282. (E) Specimen PCGU4282, a photograph of the trunk region. (F) Specimen PCGU4282, interpretive drawing of the trunk region. Note gut remains wrapped within the left abdominal cavity. White arrows (wa) indicate the alimentary canal remains, and yellow arrows (ya) indicate the positions of junctions between different types of remains. Scale bars, 2 cm (A,D), 1 cm (B,E). co, coracoid; cr, caudal rib; cv., caudal vertebra; dr, dorsal rib; dv, dorsal vertebra; fe, femur; fi, fibula; ga, gastralia; hu, humerus; il, ilium; is, ischium; pu, pubis; ra, radius; sr, sacral rib; sv, sacral vertebra; ti, tibia; ul, ulna.

Figure 3. Details of the remains of the alimentary canal in PCGU4281. (A) Esophagus remains within the thoracic cavity. Note the remains are covered by the posterior coracoid and a central V-shape element of gastralia [arrowhead in panel (B)] and encrusting the 5th–9th dorsal vertebrae. (B) Stomach remains within the right abdominal cavity. Note organic film wrapped among dorsal ribs and gastralia along the 8th–10th dorsal vertebrae. The white line indicates a J-shaped three-dimensional preserved organic mass wrapped between the dorsal ribs and gastralia. Note caudal extremity of the J-shaped organic mass gradually narrows. (C) Intestine remains within the posterior region of the left abdominal cavity. Note the ilium outward-dipping. (D) Intestine remains within the pelvic girdle region. The arrowhead indicates enlargements shown in Figure 4A. The arrows are the same as in Figure 2. Scale bars, 1 cm.
PCGU4282 is a full-grown male individual exposed as a complete articulated skeleton in ventral view (Figure 2D), with a total length of about 274 mm (Supplementary Table 1). The forelimbs and tail lack some distal elements. A certain degree of disorganization is obvious in the gastralia. A black organic mass, covering the ribs of the left abdominal cavity and 16th dorsal vertebra (Figures 2E wa1, F), reaches the 15th dorsal rib anteriorly and the third sacral rib posteriorly (Figures 2E wa2, F), and it also covers the 1st–3rd caudal vertebrae (Figures 2E wa3, F). The mass is partly overlain by some gastralia and the bones of the pelvic girdle. The rest of the six specimens (PCGU4283–4288) show soft tissue preservation (Supplementary Figures 1–6) with anatomical features similar to those observed in PCGU4282.
3.2. Interpretations of soft tissue preservation
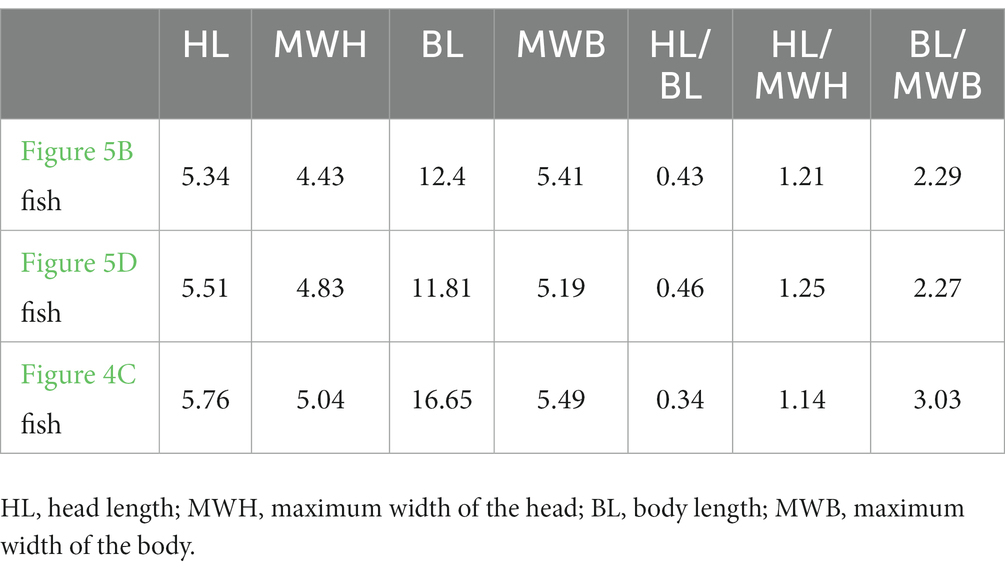
The organic remains described in this paper are interpreted as food matter and remains of the alimentary canal. Several lines of evidence support the interpretation: (1) The most obvious evidence is the occurrence of the organic residue within the thoracoabdominal skeleton (Figures 1, 2; Supplementary Figures 1–6). (2) Two obvious ridges or wrinkles of the organic residue are preserved in the right posterior abdominal cavity of the specimen PCGU4289 (Figure 4E), which indicates that the remains of this organic matter exist objectively and are not artificially sculpted. These ridges or wrinkles closely resemble those that have been observed to distort decomposing integuments from a Jurassic ichthyosaur fossil and a postmortal modern dolphin (Lindgren et al., 2018). These ridges or wrinkles were formed by the contraction of the digestive tract tissue of Keichousaurus hui following the loss of structural integrity after it was decomposed (3) An image acquired by X-ray computed microtomography of the right abdominal cavity of PCGU4281 (Figure 5A) exhibited a relatively complete articulated fish (Figures 5B,C), implying that it must have been swallowed whole rather than being torn during feeding. The shape and dimensions of this fish within the stomach are nearly identical to fossil fish commonly found within the enclosing strata and frequently next to specimens of Keichousaurus hui (Figures 4C,D, 5; Table 1). Since the fish is enclosed by organic remains in the abdominal cavity (Figure 5A) it is not possible at this time to verify the presence of bones or scales. A degree of corrosion by gastric acid prevents the ability to identify the fish as belonging to a known genus or species. Although its tail fin is partially missing due to corrosion, the fusiform outline of the fish is clear (Figures 5B,C). Further, the skull and the body can still be distinguished and several gaps between the scales are observed on the medial body of the fish. A fish Peltopleurus Orientalis (Su, 1959) from the same deposit of Keichousaurus hui shows a similar preservation state to the fish in Figure 5B (Figure 5D). Moreover, the dimensions and proportions of the skull and body of the two fish fossils are similar (Table 1). It is also noteworthy that an incomplete carcass of a fish is exposed adjacent to Keichousaurus hui in specimen PCGU4284 (Figures 4C,D). The fish has a well-preserved body with elongated scales in the left lateral view, but most of the bones of the fins and the skull are missing. However, the assignment of this specimen to the species Peltopleurus orientalis is most probable on account of its gracile scales (Su, 1959). Peltopleurus orientalis is thus interpreted to have been a likely food item for Keichousaurus hui in terms of size. (4) It is difficult to identify fish skeletal elements within the organic masses of the body cavity of K. hui, but at least one fish scale in the left region of the pelvic girdle of specimen PCGU4281 can be identified (Figure 4A) and bone fragments were identified within the intestine (Figure 4F). The absence of distinct ornamentation on the scale surface indicates the erosion from stomach acid. The nature of this scale precludes assignment to genus or species. However, a survey of the literature revealed a strong similarity between this fish scale and the reported scale of the fish Crenilepis sandbergeri from the Middle Triassic, Würzburg of Germany (Mutter, 2004; Figure 4B), both of which show an identical structure of the peg-and-socket articulation with three variably well-developed processes. (5) Widespread corrosive damage (e.g., pitting) occurred on the surface of bone fragments, as indicated by rounded edges (Figure 4F arrow), during the process of digestion by stomach acid. (6) The absence of these organic masses on the opposing side in the thoracoabdominal cavity implies that the residue is within the gut. This is supported by the presence of embryos in the gravid Keichousaurus hui that show paired oviducts are always distributed on each side of the coelom (Cheng et al., 2004).
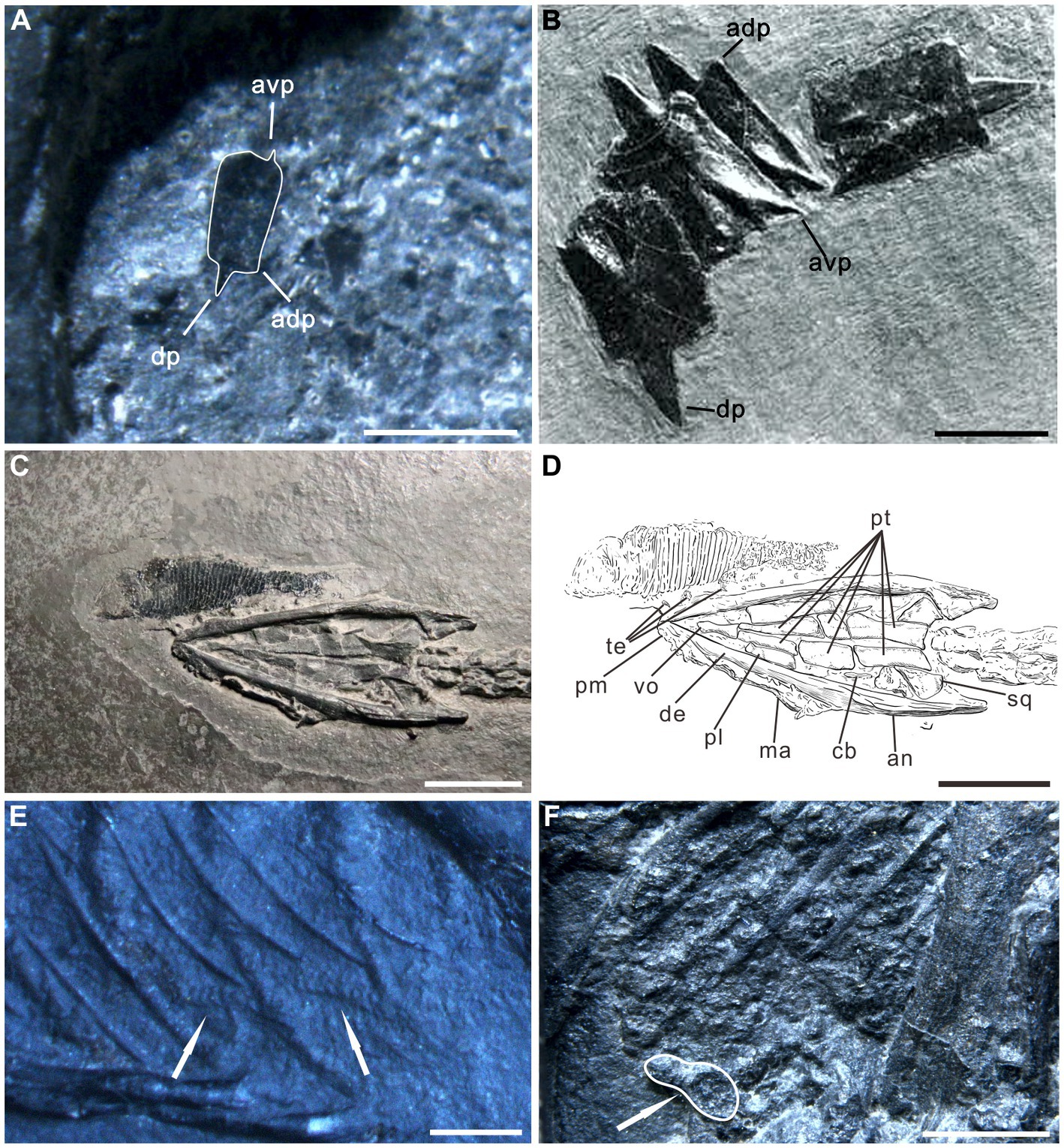
Figure 4. Evidence of soft tissue preservation in Keichousaurus hui. (A) Enlargement of left pelvis region of specimen PCGU4281. A fish scale with peg-and-socket articulation is preserved in the gut contents (compare with B). avp, antero-ventral process; adp, antero-dorsal process; dp, dorsal process. (B) Specimen PIMUZ T 32, scales of the fish Crenilepis sandbergeri [modified from Mutter (2004)]. (C,D) Enlargement and interpretive drawing of the skull of specimen PCGU4284. Note ventral region of a fish Peltopleurus orientalis overlaps the teeth of the premaxilla of Keichousaurus hui. an, angular; cb, first ceratobranchial; de, dentary; ma, maxilla; pl., palatine; pm, premaxilla; pt., pterygoid; sq., squamosal; te, teeth; vo, vomer. (E) Two obvious ridges or wrinkles (arrows) of the organic residue in the right posterior abdominal cavity of the specimen PCGU4289. (F) Enlargement of the left abdominal cavity of specimen PCGU4285. Gut contents that have undergone a certain degree of digestion. Note that the white line indicates a bone fragment with a rounded edge, indicating corrosion. Scale bars, 10 mm (B,C,D), 2 mm (E), 1 mm (A,F).
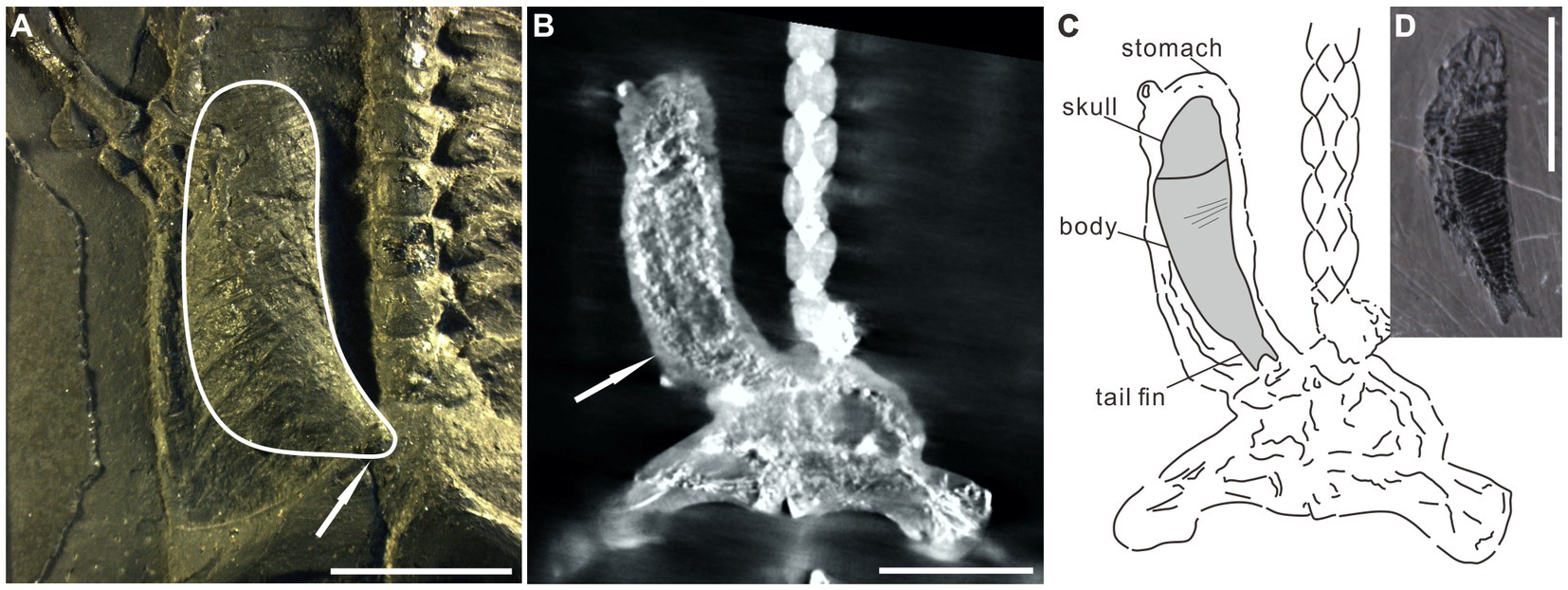
Figure 5. Specimen PCGU4281. (A) Photograph of the right abdominal cavity. The white line indicates a J-shaped three-dimensional preserved organic mass wrapped between the dorsal ribs and gastralia. Note that the caudal extremity of the J-shaped organic mass gradually narrows (white arrow). (B) X-ray CT scanning image shows an articulated fusiform fish (arrow) within the stomach [compare with the fish in panel (D)]. Note that panel (B) indicates the same area as panel (A). (C) Interpretive line drawing of panel (B). The similarity of shape and dimensions to fish within the Xingyi biota indicates that it is the carcass of a fish that was preserved in the stomach of Keichousaurus hui. Several gaps between the scales occur on the medial body of the fish in panel (B). (D) A fish Peltopleurus Orientalis from the same deposit containing Keichousaurus hui. Note the similarity in shape and dimensions to the fish in panel (B). Scale bars, 10 mm (A,B,D).
4. Discussion
4.1. Feeding strategy and prey preference
The direct evidence of diet put forward here offers insight into the feeding strategy of pachypleurosaurs and indicates that they may have been frequent piscivores. This is consistent with existing studies of the tooth morphology of the Keichousaurus and other pachypleurosaurs (Sander, 1989; Fu et al., 2013; Liao et al., 2021). The most significant feature is that the anterior teeth of the jaws become gradually recurved as they taper anteriorly to their tips (Holmes et al., 2008). We interpret that the prey was caught with these functional teeth and manipulated into a position to be swallowed whole. In specimen PCGU4281, the gastrointestinal contents occupy most of the region of the body cavity, potentially representing a very large meal. It would suggest that, when the animal ate large quantities of food at once, the esophagus and stomach may have served as storage compartments until the food was sufficiently digested. It is a reasonable conjecture that the Keichousaurus hui may have been an opportunistic predator.
4.2. Reconstruction of the alimentary canal
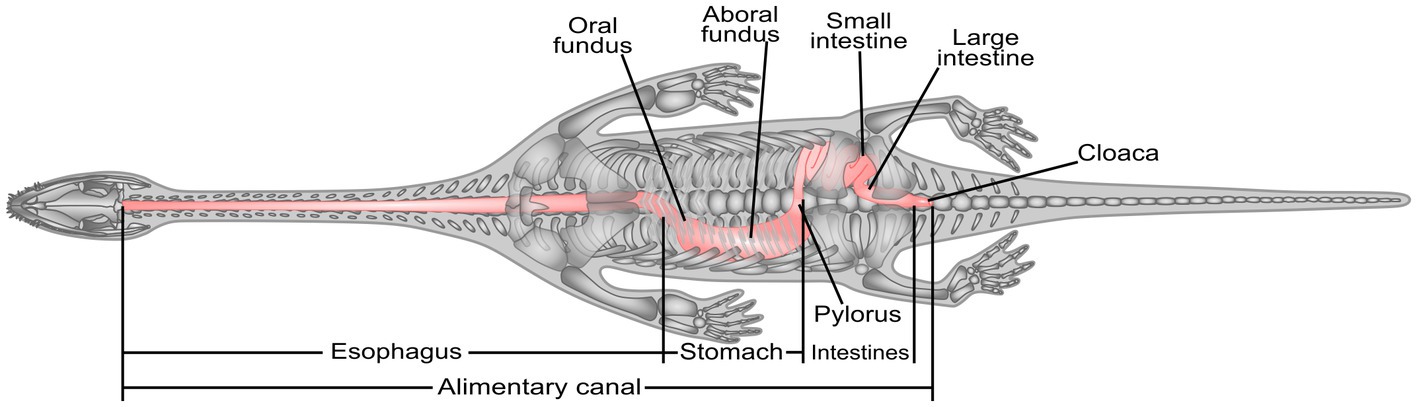
The fact that the residue occupied different positions in the body cavity can help reconstruct the distribution of the alimentary canal. Thus, the alimentary canal of the Keichousaurus hui is divided into four regions based on a comparison with similar modern animals (Kardong, 2018): esophagus, stomach, intestines, and cloaca (Figure 6). The esophagus would have been a straight-lined tube along the long axis based on the slender neck. The esophagus connected the pharynx with the stomach, with its caudal extremity reaching the 8th dorsal vertebra and then turning to the right side of the body cavity to meet the esophagogastric transition (Figures 2B,C). This is a reasonable inference that, after swallowing the prey whole, the esophagus would become distended to accommodate the prey size and secreting mucus to lubricate the food (Ahmed et al., 2009; Zaher et al., 2012). The stomach of reptiles is divided into two distinct regions: the fundic region and the pyloric region (Luppa, 1977). According to the gut remnant within the right abdomen of the specimen PCGU4281, we interpret that the stomach is slightly right-curved and J-shaped (Figures 2B, 3B, 5A white line). The stomach of the Keichousaurus hui is comprised of an oral fundus, aboral fundus, and pylorus as in living the gecko Hemidactylus mabouia (Sartori et al., 2011). The caudal extremity of the stomach gradually narrows to a pylorus (Figures 2B, 3B ya3, and Figure 5A arrow), whose secretions help to neutralize the acidic chyme as it moves next into the intestine, the pylorus plays an important role in preventing the retrograde transfer of food. The pylorus then shifts toward the left side of the abdominal cavity, across the 16th dorsal vertebra to extend next to the intestine, like many living reptiles such as lizards (O’Malley, 2005). There are two major regions of the intestines, the small and large intestines. The small intestine is inferred as a coiled tube for slowing the passage of digesta, because it is the primary site for absorption of the protein and digestible nutrients, while the large intestine is a straight tube passing to the cloaca. A caecum between the transitional area of the small and large intestines is not discernible. Diet is not the only predictor of the presence of a cecum (Lönnenberg, 1902). Beyond that, a colon is not present in theory, due to Keichousaurus hui being a carnivorous marine reptile, as in the aquatic lizard Varanus salvator (Srichairat et al., 2018). However, herbivorous reptiles usually depend on a colonic microflora to facilitate the fermentation of non-digestible cellulose into usable volatile fatty acids (Diaz Figueroa and Mitchell, 2006). According to the location of the alimentary canal remains in the specimens PCGU4282–4288, the small and large intestines of the Keichousaurus hui should be concentrated in the posterior region of the left abdomen. Consequently, the gut residue of the pelvic girdle region in specimen PCGU4281 may have been exaggerated by dorsoventral compression postmortem. The posterior large intestine narrows into the cloaca, a common chamber that receives products from the intestines and urogenital tracts. It may reach the second caudal vertebra posteriorly (Cheng et al., 2004), or even further.
Through the analysis of the above specimens, the Keichousaurus hui was a frequently piscivorous species that usually swallowed its prey whole. These results, which are consistent with previous dental studies (Sander, 1989; Fu et al., 2013; Liao et al., 2021), provide insights into the food chain of the Xinyi biota. By comparing with modern reptiles, we reconstructed the internal distribution and organ composition of the digestive tract of the Keichousaurus hui, which is very similar to that of modern carnivorous reptiles. By extension, exceptional soft tissue preservation provides abundant geological information. The discovery of the digestive tract of the Keichousaurus hui indicates that the Xingyi biota was capable of preserving soft tissue rather than just skeletal materials. Pachypleurosaurs are now generally accepted as having lived in the shallow epicontinental seas and intraplatform basins of the Tethyan Ocean (Rieppel, 2000; Liu et al., 2011; Klein, 2012). Phylogenetic analyses have shown that the Chinese pachypleurosaur Keichousaurus forms a monophyletic group with all European pachypleurosaurs (Rieppel and Lin, 1995; Liu et al., 2011; Cheng, 2015). It is conceivable that other pachypleurosaurs have the potential for similar digestive organ distribution and composition because of their similar body configurations and paleoecology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JL, DL, Y-mL, TA, M-yY, and YW conceived and designed the research. Y-mL and M-yY collected and prepared these specimens. JL, J-lL, and Y-jQ examined and took photographs of the specimens. JL and Y-lL prepared the interpretive drawings. JL assembled the all figures and wrote the manuscript. All authors revised the manuscript.
Funding
This study was financially supported by the National Natural Sciences Foundation of China (grant number 41762001) and the Science and Technology Planning Project of Guizhou Province (grant number [2020]4Y033).
Acknowledgments
We would like to express our heartfelt thanks to Professor Jonathan L. Payne for his advice on this study. Ming–kun Wang of Northwest University and Fei Su of China University of Geosciences are thanked for their advice that improved the manuscript. We thank Dong Liu of Tianjin Sanying Precision Instruments Co., Ltd. for her help in the acquisition of the X-ray CT scanning images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1186354/full#supplementary-material
References
Ahmed, Y., Aae, E., and Zayed, A. (2009). Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. J. Vet. Anat. 2, 35–48. doi: 10.48550/arXiv.1610.01717
Carroll, R. L., and Gaskill, P. (1985). The nothosaur Pachypleurosaurus and the origin of plesiosaurs. Phil. Trans. R. Soc. Lond. B 309, 343–393. doi: 10.1098/rstb.1985.0091
Chen, Z. F. (1985). Stratigraphical position of kueichousaurus hui young of Middle Triassic and itssingificance in southwestern Guizhou. Guiz. Geol. 2, 289–290.
Cheng, L. (2015) The succession of marine reptiles from the middle to late Triassic of Guizhou and Yunnan provinces, Southwest China. dissertation’s thesis Wuhan: China University of Geosciences.
Cheng, L., and Chen, X. H. (2007). Gut contents in the Triassic ichthyosaur Panjiangsaurus from the Guanling biota in Guizhou. Chin. Geol. 34, 61–65. (in Chinese with English abstract). doi: 10.3969/j.issn.1000-3657.2007.01.009
Cheng, Y. N., Holmes, R., Wu, X. C., and Alfonso, N. (2009). Sexual dimorphism and life history of Keichousaurus hui (Reptilia: Sauropterygia). J. Vertebr. Paleontol. 29, 401–408. doi: 10.1671/039.029.0230
Cheng, Y. N., Wu, X. C., and Ji, Q. (2004). Triassic marine reptiles gave birth to live young. Nature 432, 383–386. doi: 10.1038/nature03050
Cornalia, E. (1854). Notizie zoologiche sul Pachypleura edwardsii Cor. Nuovo sauro acrodonte degli strati triassici di Lombardia. Gior. Ist. Lombardo di Sci. Lett. 6, 1–46.
Diaz Figueroa, O., and Mitchell, M. A. (2006). “Gastrointestinal anatomy and physiology” in Reptile medicine and surgery. ed. D. R. Mader. 2nd ed (St. Louis: Elsevier Press), 145–162.
Evans, S. E., and Wang, Y. (2012). New material of the early cretaceous lizard yabeinosaurus from China. Cretac. Res. 34, 48–60. doi: 10.1016/j.cretres.2011.10.004
Fu, W. L., Zhang, X., Ji, C., Jiang, D. Y., Sun, Z. Y., and Hao, W. C. (2013). Morphology of Keichousaurus hui from the middle Triassic of Xingyi, Guizhou Province with comments on its reproduction mode. Acta Sci. Natur. Univ. Pekinensis. 49, 102–109.
Gürich, G. J. E. (1884). Über einige Saurier des oberschlesischen Muschelkalkes. Z. Dtsch. Geol. Ges. 36, 125–144.
Holmes, R., Cheng, Y. N., and Wu, X. C. (2008). New information on the skull of Keichousaurus hui (Reptilia: Sauropterygia) with comments on sauropterygian interrelationships. J. Vertebr. Paleontol. 28, 76–84. doi: 10.1671/0272-4634(2008)2876:NIOTSO2.0.CO;2
Hu, Z., Xie, T., and Yin, F. (2018). Carbon and oxygen isotopic studies of the horizon of Kueichousaurus Fauna. Geol. China 45, 1039–1048. doi: 10.12029/gc20180511
Jiang, D. Y., Motani, R., Tintori, A., Rieppel, O., and Li, Z. G. (2020). Evidence supporting predation of 4-m marine reptile by Triassic megapredator. iScience 23:101347. doi: 10.1016/j.isci.2020.101347
Jin, F. (2001). Notes on the discovery of Birgeria in China. Vertebr. Palasiat. 39, 168–176. doi: 10.3969/j.issn.1000-3118.2001.03.002
Kardong, K. V. (2018). “The digestive system” in vertebrates comparative anatomy, function, and evolution 8th. McGraw–Hill Press, New York, 504–545
Kear, B. P. (2006). First gut contents in a cretaceous sea turtle. Biol. Lett. 2, 113–115. doi: 10.1098/rsbl.2005.0374
Kear, B. P., Boles, W. E., and Smith, E. T. (2003). Unusual gut contents in a cretaceous ichthyosaur. Proc. R. Soc. Lond. B 270, S206–S208. doi: 10.1098/rsbl.2003.0050
Klein, N. (2012). Postcranial morphology and growth of the pachypleurosaur Anarosaurus heterodontus (Sauropterygia) from the lower Muschelkalk of Winterswijk, the Netherlands. Paläontol. Z. 86, 389–408. doi: 10.1007/s12542-012-0137-1
Lehrmann, D. J., Minzon, I. M., Enos, P., Yu, Y. Y., Wei, J. Y., and Li, R. X. (2009). Triassic depositional history of the Yangtze Platform and Great Bank of Guizhou in the Nanpanjiang Basin of South China. J. Eart. Sci. Enviro. 31, 344–367.
Li, J. L. (2006). A brief summary of the Triassic marine reptiles of China. Vertebr. Palasiat. 44, 99–108. doi: 10.3969/j.issn.1000-3118.2006.01.006
Li, J., Luo, Y. M., Wang, Y., Xu, G. F., and Ma, Z. H. (2019). A new discovery of Colobodus Agassiz, 1844 (Colobodontidae) from the Carnian (Upper Triassic) of Guizhou, South China. Acta Geol. Sin. 93, 1967–1968. doi: 10.1111/1755-6724.13832
Li, Z. G., Sun, Z. Y., Jiang, D. Y., and Ji, C. (2016). LA-ICP-MS zircon U-Pb age of the fossil layer of Triassic Xingyi Fauna from Xingyi, Guizhou, and its significance. Geol. Rev. 62, 779–790. doi: 10.16509/j.georeview.2016.03.018
Liao, J. L., Lan, T., Xu, G. H., Li, J., Qin, Y. J., Zhao, M. S., et al. (2021). Tooth structure and replacement of the Triassic Keichousaurus (Sauropterygia, Reptilia) from South China. Front. Ecol. Evol. 9:741851. doi: 10.3389/fevo.2021.741851
Lin, K. (1994). Functional morphology and phylogeny of Keichousaurus hui (Sauropterygia, Reptilia). dissertation’s thesis Montreal: McGill University.
Lin, K., and Rieppel, O. (1998). Functional morphology and ontogeny of Keichousaurus hui (Reptilia, Sauropterygia). Fieldiana Geol. 39, 1–35. doi: 10.5962/bhl.title.5174
Lindgren, J., Sjovall, P., Thiel, V., Zheng, W., Ito, S., Wakamatsu, K., et al. (2018). Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 564, 359–365. doi: 10.1038/s41586-018-0775-x
Liu, J., Motani, R., Jiang, D. Y., Hu, S. X., Aitchison, J. C., Rieppel, O., et al. (2013). The first specimen of the Middle Triassic Phalarodon atavus (Ichthyosauria: Mixosauridae) from South China, showing postcranial anatomy and peri-Tethyan distribution. Palaeontology 56, 849–866. doi: 10.1111/pala.12021
Liu, J., Rieppel, O., Jiang, D. Y., Aitchison, J. C., Motani, R., Zhang, Q. Y., et al. (2011). A new pachypleurosaur (Reptilia: Sauropterygia) from the lower middle Triassic of southwestern China and the phylogenetic relationships of Chinese pachypleurosaurs. J. Vertebr. Paleontol. 31, 292–302. doi: 10.1080/02724634.2011.550363
Liu, G. B., Yin, G. Z., and Wang, X. H. (2002). On the most primitive amid fish from Upper Triassic of Xingyi, Guizhou. Acta. Palaeontolo. Sin. 41, 461–463. doi: 10.3969/j.issn.0001-6616.2002.03.015
Liu, G. B., Yin, G. Z., Wang, X. H., Luo, Y. M., and Wang, S. Y. (2003). New discovered fishes from Keichousaurus bearing horizon of late Triassic in Xingyi of Guizhou. Acta. Palaeontol. Sin. 42, 346–366.
Lönnenberg, E. (1902). On some points of relation between the morphological structure of the intestine and the diets of reptiles. Bih. Svensk. Vet. Ak. Handl. 28, 1–51.
Lu, H., Jiang, D. Y., Motani, R., Ni, P. G., Sun, Z. Y., Tintori, A., et al. (2018). Middle Triassic Xingyi Fauna: showing turnover of marine reptiles from coastal to oceanic environments. Palaeoworld 27, 107–116. doi: 10.1016/j.palwor.2017.05.005
Luppa, H. (1977). “Histology of the digestive tract” in Biology of the Reptilia. eds. C. Gans and T. S. Parsons (London: Academic Press), 225–302.
Ma, Y. S., Chen, H. D., and Wang, G. L. (2009). Atlas of tectonics and sequence lithofacies palaeogeography in southern China(Sinian-Neogene). (Beijing: Science Press), 1–603.
Massare, J. A. (1987). Tooth morphology and prey preference of Mesozoic marine reptiles. J. Vertebr. Paleontol. 7, 121–137. doi: 10.1080/02724634.1987.10011647
Metcalfe, I. (2006). Palaeozoic and Mesozoic tectonic evolution and palaeogeography of east Asian crustal fragments: the Korean Peninsula in context. Gondwana Res. 9, 24–46. doi: 10.1016/j.gr.2005.04.002
Motani, R., Jiang, D. Y., Rieppel, O., Xue, Y. F., and Tintori, A. (2015). Adult sex ratio, sexual dimorphism and sexual selection in a Mesozoic reptile. Proc. R. Soc. B Biol. Sci. 282:20151658. doi: 10.1098/rspb.2015.1658
Mutter, R. J. (2004). “The “perleidiform” family colobodontidae in a review” in Mesozoic fishes 3–systematics, paleoenvironments and biodiversity. eds. G. Arratia and A. Tintori (Munich: Verlag Dr Friedrich Pfeil Press), 197–208.
Ni, P. G., Tintori, A., Sun, Z. Y., and Jiang, D. Y. (2017). A new specimen of Birgeria liui (Osteichthyes, Actinopterygii) from the Longobardian (Ladinian, Middle Triassic) of Xingyi, Guizhou Province, South China. Research 3, 55–58. doi: 10.14456/randk.2017.27
O’Keefe, F. R., Street, H. P., Cavigelli, J. P., Socha, J. J., and O’Keefe, R. D. (2009). A plesiosaur containing an ichthyosaur embryo as stomach contents from the Sundance Formation of the Bighorn Basin, Wyoming. J. Vertebr. Paleontol. 29, 1306–1310. doi: 10.1671/039.029.0403
O’Malley, B. (2005). “Lizards” in Clinical anatomy and physiology of exotic species (London: Elsevier Press), 58–75.
Pollard, J. E. (1968). The gastric contents of an ichthyosaur from the Lower Lias of Lyme Regis, Dorset. Palaeontology 11, 376–388.
Qin, Y. J., Yu, M. Y., and Luo, Y. M. (2014). Ontogenesis of Keichousaurus hui. Guizhou Geol. 120, 210–214.
Rieppel, O. (1989). A new pachypleurosaur (Reptilia: Sauropterygia) from the Middle Triassic of Monte San Giorgio, Switzerland. Phil. Trans. R. Soc. Lond. B 323, 1–73. doi: 10.1098/rstb.1989.0001
Rieppel, O. (1993). Euryapsid relationships: a preliminary analysis. Neues Jahrb. Geol. Palaontol. Abh. 188, 241–264.
Rieppel, O. (1999). Phylogeny and paleobiogeography of Triassic Sauropterygia: problems solved and unresolved. Palaeogeogr. Palaeocl. Palaeoel. 153, 1–15. doi: 10.1016/S0031-0182(99)00067-X
Rieppel, O. (2000). “Sauropterygia I: Placodonita, Pachypleurosauria, Nothosauroidea, Pistosauroidea” in Encyclopedia of paleoherpetology. ed. P. Wellnhofer, vol. 12A (Munich: Verlag Dr Friedrich Pfeil), 1–134.
Rieppel, O., and Hagdorn, H. (1997). “Paleobiogeography of Middle Triassic Sauropterygia in central and western Europe” in Ancient Marine Reptiles. eds. M. J. Callaway and L. E. Nicholls (San Diego: Academic Press), 121–144.
Rieppel, O., and Lin, K. (1995). Pachypleurosaurs (Reptilia: Sauropterygia) from the lower Muschelkalk, and a review of the Pachypleurosauroidea. Fieldiana Geol. 32, 1–44. doi: 10.5962/bhl.title.3474
Sander, P. M. (1989). The pachypleurosaurids (Reptilia: Nothosauria) from the Middle Triassic of Monte San Giorgio (Switzerland) with the description of a new species. Phil. Trans. R. Soc. Lond. B 325, 561–666. doi: 10.1098/rstb.1989.0103
Sartori, S. S. R., Nogueira, K. D. O. P. C., Rocha, A. D. S., and Neves, C. A. (2011). Morphology of the stomach of the tropical house gecko Hemidactylus mabouia (Squamata: Gekkonidae). Acta Zool. 92, 179–186. doi: 10.1111/j.1463-6395.2010.00451.x
Seeley, H. G. (1882). On Neusticosaurus pusillus (Fraas), an amphibious reptile having affinities with terrestrial Nothosauria and with marine Plesiosauria. Q. J. Geol. Soc. Lond. 38, 350–366. doi: 10.1144/GSL.JGS.1882.038.01-04.39
Srichairat, N., Taksintum, W., and Chumnanpuen, P. (2018). Gross morphological structure of digestive system in water monitor lizard Varanus salvator (Squamata: Varanidae). Walailak J. Sci. Tech. 15, 245–253. doi: 10.48048/wjst.2018.3356
Storrs, G. W. (1993). The systematic position of Silvestrosaurus and a classification of Triassic sauropterygians (Neodiapsida). Paläontol. Z. 67, 177–191. doi: 10.2475/ajs.293.A.63
Sun, Z. Y., Jiang, D. Y., Ji, C., and Hao, W. C. (2016). Integrated biochronology for Triassic marine vertebrate faunas of Guizhou Province, South China. J. Asian Earth Sci. 118, 101–110. doi: 10.1016/j.jseaes.2016.01.004
Tintori, A., Sun, Z. Y., Ni, P. G., Lombardo, C., Jiang, D. Y., and Ryosuke, M. (2015). Oldest stem Teleostei from the late Ladinian (Middle Triassic) of southern China. Riv. Ital. Paleontolo. Stratigrafia. 121, 285–296. doi: 10.13130/2039-4942/651
Wang, L. T. (1996). A discussion on horizon and age of Keichousaurus hui occurrence. Guiz. Geol. 3, 209–216.
Wang, L. T. (2002). Study advances on triassic marine reptiles in Guizhou. Guiz. Geol. 19, 6–9. doi: 10.3969/j.issn.1000-5943.2002.01.002
Wang, X. F., Chen, X. H., Cheng, L., Wang, C. S., Bachmann, G. H., Sander, M., et al. (2009). Sedimentary and palaeoecological environments of the Guanling and related biotas. Acta Palaeontol. Sin. 48, 509–526.
Wang, C. Y., Kang, P. Q., and Wang, Z. H. (1998). Conodont based age of the Keichousaurus hui Yang, 1958. Acta Micropalaeontol. Sin. 15, 886–888. doi: 10.1088/0256-307X/15/12/010
Xing, L., Niu, K., and Evans, S. E. (2019). Inter–amphibian predation in the early cretaceous of China. Sci. Rep. 9:7751. doi: 10.1038/s41598-019-44247-7
Xu, G. H. (2020). A new species of Luganoia (Luganoiidae, Neopterygii) from the Middle Triassic Xingyi Biota, Guizhou, China. Vert. Palasiat. 58, 267–282. doi: 10.19615/j.cnki.1000-3118.200624
Xu, G. H., and Ma, X. Y. (2018). Redescription and phylogenetic reassessment of Asialepidotus shingyiensis (Holostei: Halecomorphi) from the Middle Triassic (Ladinian)of China. Zool. J. Linnean Soc. 184, 95–114. doi: 10.1093/zoolinnean/zlx105
Xu, G. H., Zhao, L. J., Gao, K. Q., and Wu, F. X. (2012). A new stem-neopterygian fish from the Middle Triassic of China shows the earliest over-water gliding strategy of the vertebrates. Proc. R. Soc. B Biol. Sci. 280:20122261. doi: 10.1098/rspb.2012.2261
Xu, G. H., Zhao, L. J., and Shen, C. C. (2015). A Middle Triassic thoracopterid from China highlights the evolutionary origin of over-water gliding in early ray-finned fishes. Biol. Lett. 11:20140960. doi: 10.1098/rsbl.2014.0960
Xue, Y. F., Jiang, D. Y., Motani, R., Rieppel, O., Sun, Y. L., Sun, Z. Y., et al. (2013). New information on sexual dimorphism and allometric growth in Keichousaurus hui, a pachypleurosaur from the Middle Triassic of Guizhou, South China. Acta Palaeontol. Pol. 60, 681–687. doi: 10.4202/app.00006.2013
Yang, R. D. (1998). On Paleoecological environment of Kueichousaurus Fauna in Dingxiao of Xingyi area, Guizhou. Guiz. Geol. 14, 35–39.
Yang, S. R., Liu, J., and Zhang, M. F. (1995). Conodonts of the Falang formation of southwestern Guizhou and their age. J. Stratigr. 19, 161–170.
Young, C. C. (1958). On the new Pachypleurosauroidea from Keichow, South-West China. Ver. PalAsiat. 2, 69–81.
Zaher, M., El Ghareeb, A., Hamdi, H., Essa, A., and Lahsik, S. (2012). Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I–Uromastyx aegyptiaca. Life Sci. J. 9, 84–104. doi: 10.7537/marslsj090312.13
Zou, X., Balini, M., Jiang, D. Y., Tintori, A., Sun, Z. Y., and Sun, Y. L. (2015). Ammonoids from the Zhuganpo Member of the Falang Formation at Nimaigu and their relevance for dating the Xingyi fossil-lagerstätte (late Ladinian, Guizhou, China). Riv. Ital. Paleontol. Strat. 2, 135–161. doi: 10.13130/2039-4942/6511
Keywords: soft tissue preservation, digestive tract anatomy, feeding strategy, prey preference, digestive tract reconstruction, Keichousaurus, Triassic
Citation: Li J, Lehrmann DJ, Luo Y-m, Adams TL, Yu M-y, Liao J-l, Qin Y-j, Li Y-l and Wang Y (2023) Soft tissue preservation in the Triassic pachypleurosaur Keichousaurus hui: evidence for digestive tract anatomy, diet, and feeding behavior. Front. Ecol. Evol. 11:1186354. doi: 10.3389/fevo.2023.1186354
Edited by:
Jürgen Kriwet, University of Vienna, AustriaReviewed by:
Benjamin P. Kear, Uppsala University, SwedenTetsuto Miyashita, Canadian Museum of Nature (CMN), Canada
Copyright © 2023 Li, Lehrmann, Luo, Adams, Yu, Liao, Qin, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel J. Lehrmann, ZGxlaHJtYW5uQHRyaW5pdHkuZWR1; Yue Wang, Z3p5dWV3YW5nQDEyNi5jb20=
 Ji Li
Ji Li Daniel J. Lehrmann
Daniel J. Lehrmann Yong-ming Luo3
Yong-ming Luo3 Jun-ling Liao
Jun-ling Liao Yu-lan Li
Yu-lan Li