- 1Department of Genetics, Dr A. L. Mudaliar Post Graduate Institute of Basic Medical Sciences (ALM PG IBMS), University of Madras, Chennai, India
- 2Madras Diabetes Research Foundation and Dr. Mohan’s Diabetes Specialties Centre, Chennai, India
- 3National Institute of Health–International Centre for Excellence in Research, National Institute for Research in Tuberculosis, Chennai, India
Metainflammation, as seen in chronic diabetes subjects, impairs immunity and increases the susceptibility to infections. In the present study, the effect of diabetes on immune response against filariasis was studied. Both toll-like receptor (TLR)-mediated and crude antigen-induced immune responses were quantified, in whole blood cultures from filariasis-infected subjects (LF+), with and without diabetes. Blood cultures were stimulated with TLR ligands (TLR2 and TLR4) or filarial antigen or were left unstimulated (control) for 18 h. Cytokine, chemokine, and defensin secretion was quantified by ELISA. Expression of HLA-DR, B7-1, B7-2, activation marker (CD69), and Th (Th1, Th2, Th17, and Th9) phenotypes was quantified by flow cytometry. Expression of immunomodulatory effectors (Cox-2, HO-1, IDO-1, and p47Phox) and Th-polarizing transcription factors (T-bet, GATA3, and ROR-γt) was quantified by quantitative PCR. Secretion of IL-27, IL-1Ra, IL-12, IL-33, IL-9, and SDF-1 was increased under diabetes conditions with increased Th9 polarization and increased expression of Cox-2 and IDO. Overall, diabetes was found to augment both TLR-mediated and antigen-induced inflammation, which can promote chronic pathology in LF+ subjects.
Introduction
Filarial infections, unlike viral and bacterial infections, induce immunomodulation, rather than inflammation (1). Murine studies have shown significant protection against both forms of diabetes (type 1 and type 2) by filarial pre-infections (2–4). Previously, we have shown a decreased prevalence of filariasis among both T1DM (5) and T2DM (6) subjects in the South Indian population. Serum cytokine profiling in these subjects showed significant downregulation of pro-inflammatory cytokines [interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and granulocyte–macrophage-colony stimulating factor (GM-CSF)] and upregulation of anti-inflammatory cytokine [tumor growth factor-beta (TGF-β)], in filarial-positive diabetic subjects (6). Interestingly, this effect was specific only to T1DM and T2DM and was not seen in those with coronary artery disease (CAD) (7). These observations were later replicated in other Asian countries like China (8) and Indonesia (9).
The relationship between filariasis and diabetes is bi-directional (10). While childhood filarial infection can dampen inflammation and can confer protection against diabetes, the effect of diabetes on filariasis-induced immune response is largely unknown. This is relevant in endemic zones wherein at least some of the subjects with lymphatic filariasis (LF) would develop diabetes, during the course of infection. T2DM in general, weakens the immune system and makes the patients more susceptible to infections (11). Immune response against filariasis, in general, can be broadly classified into innate and adaptive immune responses. Toll-like receptors (TLRs) serve as the first line of defense mechanism, bridging the innate and adaptive arms of the immune responses (12). They are abundantly present in cells of the innate immune system, which include macrophages, dendritic cells, neutrophils, and eosinophils (13). Previously, several filarial antigens were shown to bind directly to TLRs and activate them (14, 15). Upon TLR ligation, the professional antigen-presenting cells (APCs) that include macrophages, dendritic cells, and B cells undergo activation followed by the secretion of cytokines and chemokines (16). The cytokines secreted by the innate immune cells can be broadly classified into (1) type 1 interferons (interferon-α and β), (2) pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and GM-CSF), and (3) anti-inflammatory cytokines (IL-10, TGF-β, IL-1Ra, IL-35, and IL-27) (17). TLR ligation also upregulates the antigen processing and presentation machinery, which includes MHC (HLA) and co-stimulatory (B7-1/CD80 and B7-2/CD86) molecules (18). Furthermore, certain cell-type-specific effector functions like secretion of antibodies by B cells (19), defensin by neutrophils (20), and matrix-metalloproteinases (MMPs) by macrophages (21) are also augmented. TLR-mediated expression of Cyclooxygenase-2 (Cox-2), Heme Oxygenase-1 (HO-1), and Indoleamine-2,3-Dioxygenase (IDO) constitute a immunoregulatory circuit, which controls filariasis-mediated immunomodulation (22). The net result is the recruitment and activation of T cells, which marks the transition from innate to adaptive immune response (12).
In contrast to the cells of innate immune system, that detect pathogens through TLRs, T cells are the workhorses of the adaptive immune system, largely depending on surface T-cell receptors (TCRs), for detecting the pathogens (23). While the major function of CD4+ helper T (Th) cells is to regulate other cells, CD8+ cytotoxic T (Tc) cells are mainly involved in the elimination of virally infected cells and tumor cells (24). In contrast to the innate immune cytokines, the adaptive immune cytokines are secreted by T cells (effector cytokines) and APCs (polarizing cytokines) (25). Upon engagement of the TCRs and antigen co-receptor (CD28), the Th cells undergo differentiation into various sub-types depending on the polarizing cytokines secreted by the APCs (25). Depending on cytokine secretion, Th cells are classified into Th1 (IL-12, IFN-γ, and IL-2), Th2 (IL-33, IL-4, IL-5, and IL-13), Th9 (IL-9), and Th17 (IL-23, IL-17, and IL-17F) sub-types (26). T cell-mediated immune responses during filarial infection largely depend on the phase of the infection: (1) acute phase—skewed towards the Th2 response; (2) chronic phase—skewed towards “modified Th2 response”, with Tregs playing a more prominent role compared to Th2 cells; (3) chronic pathology phase—a drastic shift from “modified Th2” response to pro-inflammatory “Th1/Th17” response takes place and happens only in those who develop lymphatic pathology (22). Thus, in the present study, we looked at both TLR-mediated innate and filarial antigen-induced adaptive immune responses in LF+ subjects, both with and without diabetes. Cytokine/chemokine secretion, expression of immunomodulatory enzymes, upregulation of MHC and co-stimulatory molecules, T-cell activation, Th polarization, and expression of T-cell polarizing transcription factors were quantified.
Materials and Methods
Study Subjects
This study is a follow-up of our previous publication wherein we reported decreased prevalence of LF among diabetic subjects compared to control subjects (6). As a continuation of this study, 1,001 outpatients visiting Dr. Mohan’s Diabetes Specialties Centre, Chennai, India, were screened for LF. Out of 1,001, only 12 were found to be positive for LF antigen (1.2%). None of them had any clinical symptoms of LF (DM-LF+). As controls, eight normal glucose-tolerant (non-diabetic) LF+ subjects were included (NGT-LF+). The control subjects were recruited from the healthy volunteers who accompanied the diabetes patients and underwent OGTT testing, as part of this study. Institutional ethical committee approval from the Madras Diabetes Research Foundation Ethics Committee was obtained (Ref No-MDRF-EC/SOC/2009//05) and written informed consent was obtained from all the study subjects. The study was conducted as per the Declaration of Helsinki, following STROBE guidelines.
Inclusion and Exclusion Criteria
Only subjects who were LF+ (as determined by TropBio) were included in the study. None of the subjects showed any symptoms of lymphatic filariasis at the time of recruitment. The exclusion criteria were patients with type 1 diabetes and those with a previous diagnosis of urolithiasis, liver cirrhosis, congestive heart failure, chronic lung diseases, chronic infections, or viral hepatitis.
Diagnosis of T2DM
The diagnosis was done following WHO guidelines. Subjects were grouped as Control (NGT) based on the Oral Glucose Tolerance Test (OGTT) and as diabetes based on previous history (https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf) (ISBN 978-92-4-159493-6).
Detection of Bancroftian LF
To quantify the filarial antigen levels, sera were analyzed using the W. bancrofti Og4C3 antigen-capture ELISA (Tropbio, Australia) according to the manufacturer’s instructions.
Preparation of Filarial Antigen
Crude antigen extract from Brugia malayi antigen (BmA) was prepared by extracting somatic antigens from the live L3 larvae in 1× PBS, as described previously (27). The extract was concentrated, quantified, and stored at −80°C. The endotoxin levels as determined by the limulus amoebocyte lysate assay (QCL-1000 kit; BioWhittaker) was below the detection limit in these preparations. It is important to note that both W. bancrofti and Brugia malayi share several overlapping antigens, and immune cells from patients infected with W. bancrofti mount a strong immune response against Brugia malayi antigens during rechallenge experiments (28).
Peripheral Blood Leukocyte Cultures
Whole blood cultures from the study subjects were done as described previously (29). Whole blood was collected in EDTA-coated tubes. After centrifugation, the packed cell volume was diluted with RPMI medium (1:1 ratio) containing 10% FCS and was used for in vitro culture. Cells were stimulated with TLR2 ligand-PAM3CSK4 (100 ng/ml) (Invivogen, USA) or TLR4 ligand-LPS (100 ng/ml) (Invivogen, USA) or were left unstimulated for 24 h in parallel cultures. For antigen stimulation, cultures were stimulated with crude extract of BmA (10 µg/ml) for 24 h. The supernatants were harvested for cytokine/chemokine estimation. The cell pellets were solubilized in RNAzol and were stored at −80°C. Cell pellets from parallel cultures were treated with golgi stop for 6 h, washed, and stained for flow cytometry.
Measurement of Cytokines and Chemokines by ELISA
The levels of cytokines (TNF-α, IL-6, IL-1β, GM-CSF, IL-10, TGF-β, IL-27, IL-1Ra, IFN β, IL-12p70, IFN-γ, IL-2, IL-23, IL-33, IL-4, IL-17, and IL-9), chemokines (SDF-1, IL-8, MCP-1, RANTES, and IP-10) and α-defensin-1 in the cell supernatant were quantified using ELISA (R&D, USA) following the manufacturer’s protocol. IL-35 was estimated using pre-coated ELISA plates (Immunoconcept). The lower detection limits were as follows: TNF-α = 1.95 pg/ml, IL-6 = 0.59 pg/ml, IL-1β = 0.24 pg/ml, GM-CSF = 0.67 pg/ml, IL-10 = 9.76 pg/ml, TGF-β = 1.95 pg/ml, IL-35 = 6.25 pg/ml, IL-27 = 19.53 pg/ml, IL-1Ra = 0.144 pg/ml, IL-12 = 1.95 pg/ml, IFN-γ = 1.17 pg/ml, IL-33 = 0.016 pg/ml, IL-4 = 1.9 pg/ml, IL-17, IL-9, SDF-1 = 107.2 pg/ml, IL-8 = 1.98 pg/ml, IP-10 = 0.97 pg/ml, and α-defensin = 62.50 pg/ml. The CV was found to be <10%. The list of various monoclonal antibodies used in ELISA is provided in Table S1.
Flow Cytometry
Cells were stained with fluorochrome-conjugated monoclonal antibodies specific for CD3, CD4, CD19, CD14, CD69, HLA-DR, CD80, and CD86. They were then permeabilized with saponin (0.01%), stained with antibodies specific for IFN γ, IL-4, IL-17A, and IL-9, and were analyzed on FACS Canto (BD Biosciences, USA). The lymphocytes, monocytes, and granulocytes were first gated based on the FSC vs. SSC plot. Monocytes and B cells were further gated based on CD14 and CD19 expression, respectively. T-helper cells were gated based on CD3 and CD4 expression. The expression of cytokines and other effector molecules within the gated population was analyzed as illustrated in Figures S1 and S2. Both the percentage and mean fluorescence intensity (MFI) of the gated cell population were quantified using FlowJo software, version v10 (BD Biosciences, USA). The list of various monoclonal antibodies used in flowcytometry is provided in Table S2.
Real-Time PCR Analysis
RNA extraction from the stored samples was carried out using RNeasy Mini Kit (Qiagen). The quality and quantity of the extracted RNA was quantified using nanodrop. One microgram of RNA was converted to cDNA using reverse transcription and real-time PCR was performed using TaqMan probes (Applied Biosystems, USA) specific for Cox-2, IDO, Phox P47, HO-1, T-bet, GATA-3, and ROR-γt. 18S rRNA was used as a house-keeping control. Gene expression levels (normalized to 18S rRNA) were analyzed using the StepOnePlus RT-PCR system (Applied Biosystems, Foster City, CA, USA) and 2–ΔΔCt was calculated for all samples with unstimulated sample values as reference. All the probes used in these studies were purchased from Applied Biosystems, and the list of various probes used is provided in Table S3.
Statistical Analysis
Mann–Whitney U test was used for comparing NGT versus DM groups. Multiple comparisons were corrected using Holm’s correction. All the analyses were done using GraphPad Prism version 5.0 (GraphPad Software, USA). p-value less than 0.05 was considered significant.
Results
Clinical Characteristics of the Study Subjects
Table S4 shows the clinical characteristics of the study groups. As can be seen in the table, DM-LF+ subjects had significantly increased BMI, glycemic parameters (FPG, PPPG, and HbA1c), total cholesterol, and total triglyceride lipids, compared to NGT-LF+ subjects.
Effect of Diabetes on the TLR-Induced Secretion of Pro- and Anti-Inflammatory Cytokines, Type 1 Interferons, and Defensins in LF+ Subjects
Figure 1 shows the TLR-induced secretion of pro- (TNF-α, IL-6, IL-1β, and GM-CSF) and anti-(IL-10, TGF-β, IL-1Ra, IL-35, and IL-27) inflammatory cytokines, type 1 interferons (IFN-β), and defensins (α-defensin-1) in the study groups. At the basal level, IL-1β and IL-27 levels were significantly higher in the DM-LF+ group compared to the NGT-LF+ group. TLR2 stimulation resulted in the secretion of TNF-α, IL-6, IL-1β, and IL-10. TLR4 stimulation resulted in the secretion of TNF-α, IL-6, IL-1β, IL-10, and IL-27. Significant difference was seen in the secretion of IL-10 and IL-1Ra between the NGT-LF+ and DM-LF+ groups. TLR2 and TLR4 induced α-defensin-1 secretion only in the DM-LF+ group.
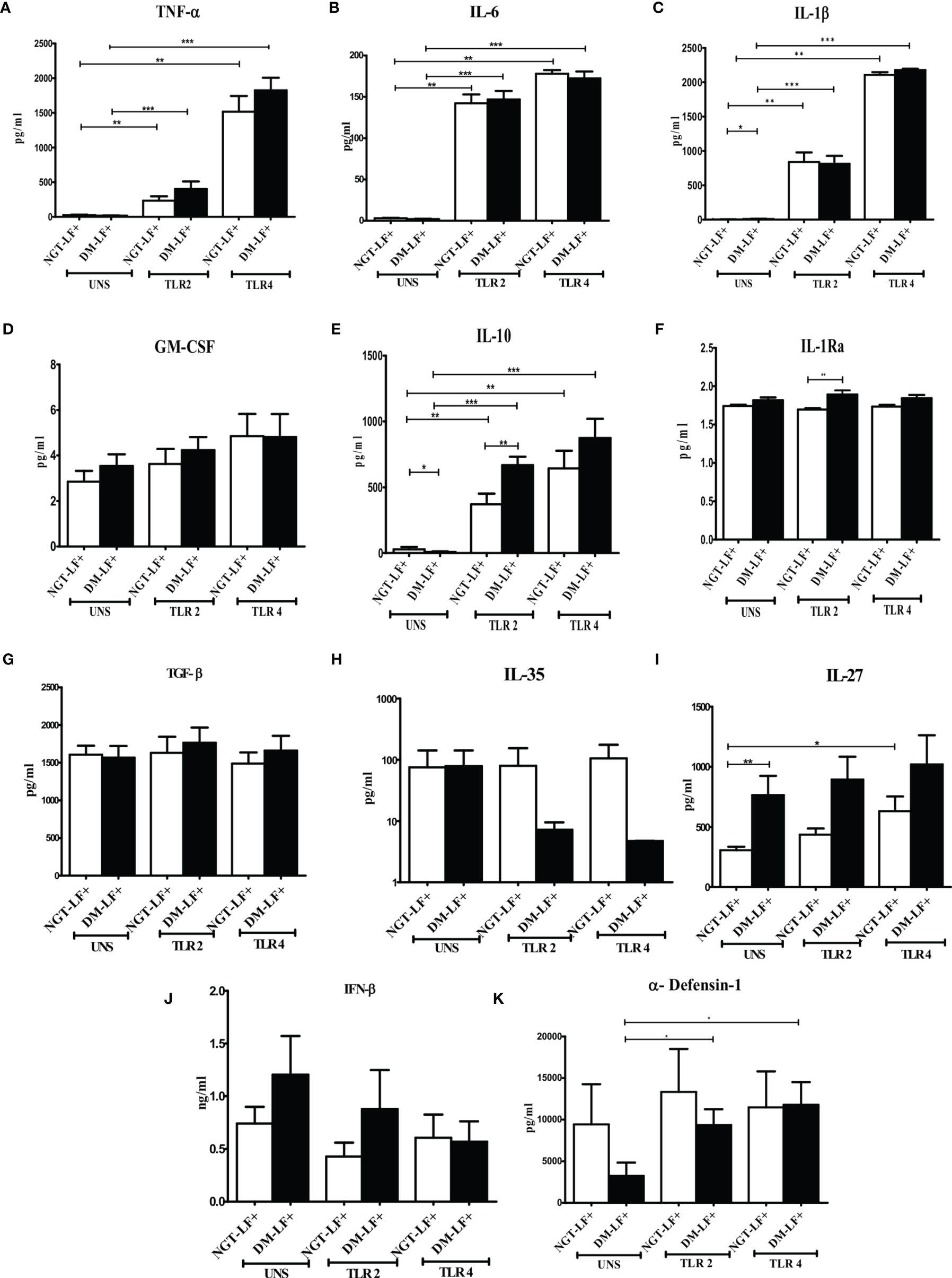
Figure 1 Effect of diabetes on the TLR-induced secretion of pro- and anti-inflammatory cytokine type 1 interferons and defensins in LF+ subjects. Bar graph showing Unstimulated (UNS), TLR2-, and TLR4-induced secretion of TNF-α (A), IL-6 (B), IL-1β (C), GM-CSF (D), IL-10 (E), IL-1Ra (F), TGF-β (G), IL-35 (H), IL-27 (I), IFN-β (J), and α-defensin (K) in the supernatants of blood cultures in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001.
Effect of Diabetes on the TLR-Induced Secretion of Adaptive Immune Cytokines and Chemokines in LF+ Subjects
Figure 2 shows the TLR-induced secretion of adaptive immune cytokines (IL-12, IFN-γ, IL-2, IL-23, IL-17, IL-9, IL-33, and IL-4) and chemokines (IL-8, IP-10, RANTES, MCP-1, and SDF-1) in the study groups. TLR2 stimulation resulted in the secretion of IL-12 and IL-8 and decreased the secretion of IL-23. TLR4 stimulation resulted in the secretion of IL-12, IL-23, IL-8, and IP-10. The basal secretion of IL-12 and IL-8 was decreased while that of IL-17, IL-9, IL-33, and SDF-1 was augmented in the DM-LF+ compared to the NGT-LF+ group. TLR2 and TLR4 induced MCP-1 and downregulated IL-33 secretion only in the DM-LF+ group.
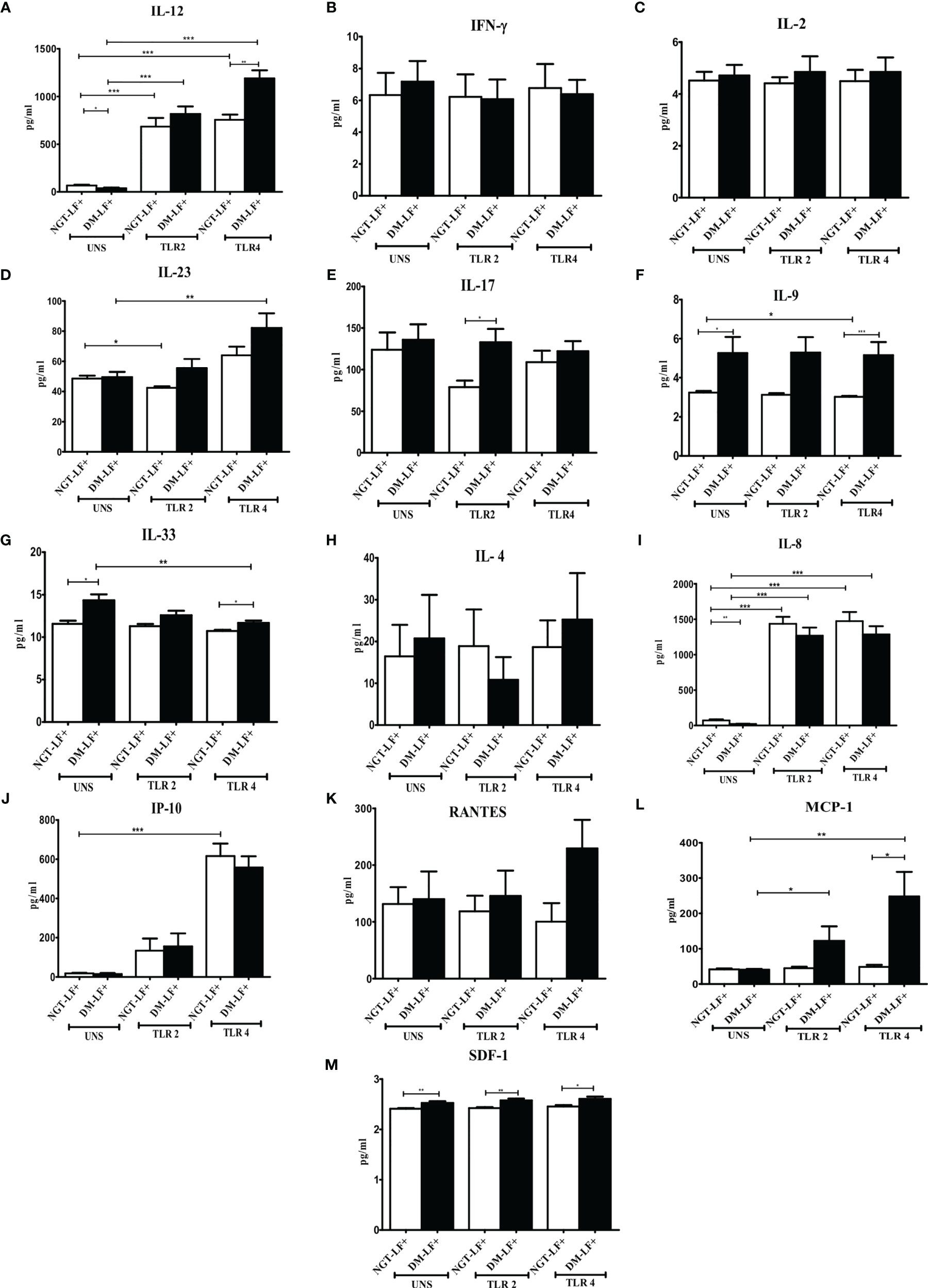
Figure 2 Effect of diabetes on the TLR-induced secretion of adaptive immune cytokines and chemokines in LF+ subjects. Bar graph showing Unstimulated (UNS), TLR2-, and TLR4-induced secretion of IL-12 (A), IFN-γ (B), IL-2 (C), IL-23 (D), IL-17 (E), IL-9 (F), IL-33 (G), IL-4 (H), IL-8 (I), IP-10 (J), RANTES (K), MCP-1 (L), and SDF-1 (M) in the supernatants of blood cultures in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001.
Effect of Diabetes on the TLR-Induced Expression of MHC and Costimulatory Molecules in LF+ Subjects
Figure S3 shows the expression of HLA-DR, CD80, and CD86 in B cells, monocytes, and granulocytes in the study groups. No significant difference was seen in the expression of these molecules between the groups.
Effect of Diabetes on the TLR-Induced Expression of Immunomodulatory Effectors in LF+ Subjects
Figure 3 shows the TLR-induced expression of immunomodulatory effectors (Cox-2, HO-1, Phox47, and IDO), in the study groups. TLR-induced expression of Cox-2 and IDO was significantly augmented in the diabetic group (DM-LF+).
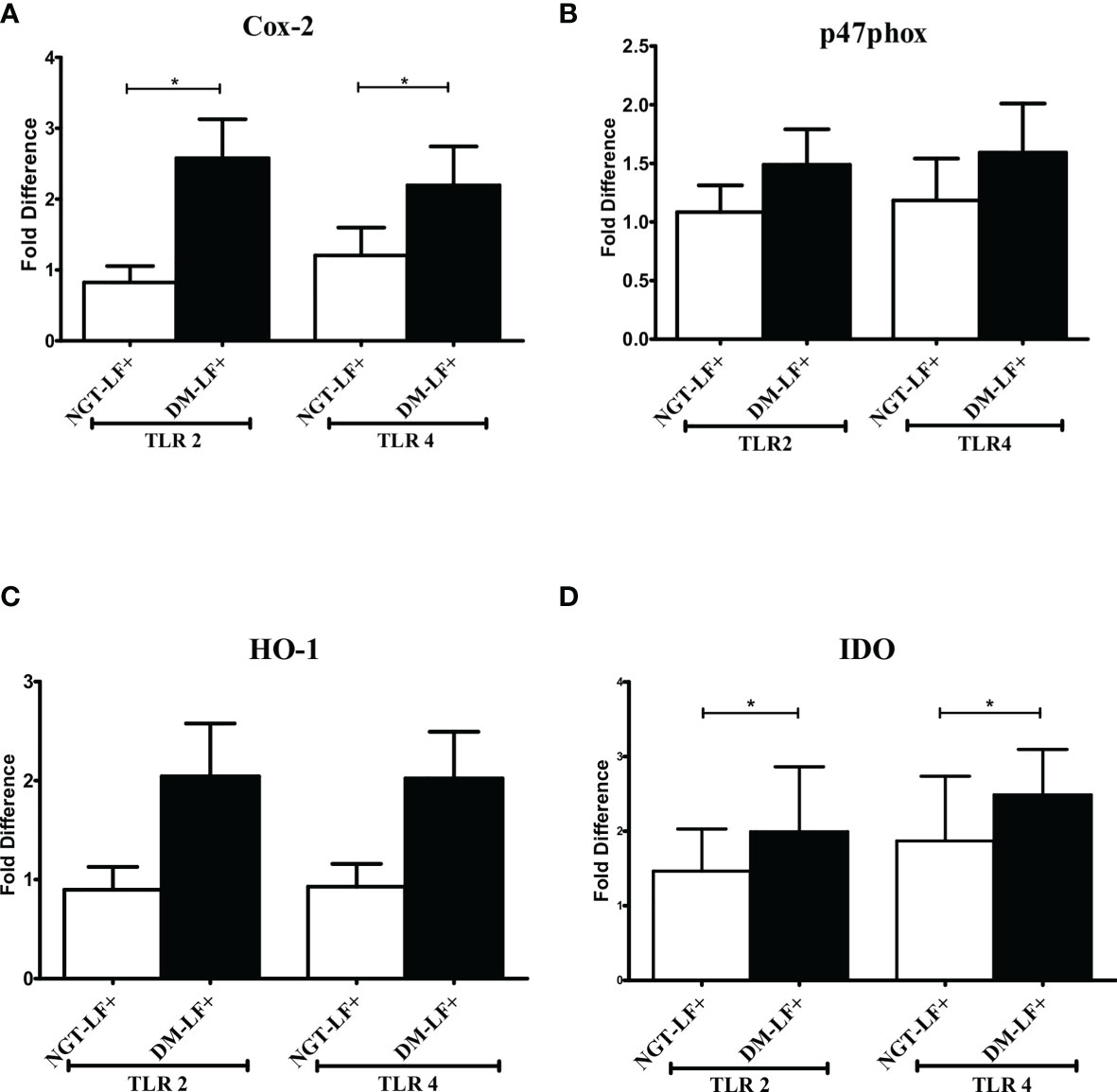
Figure 3 Effect of diabetes on the TLR-induced expression of immunomodulatory effectors in LF+ subjects. Bar graph showing TLR2- and TLR4-induced expression of Cyclooxygenase (Cox)-2 (A), p47Phox (B), Heme Oxygenase (HO)-1 (C), and Indoleamine 2,3-dioxygenase (IDO) (D) in the immune cells in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05. NGT, normal glucose tolerance; DM, diabetes mellitus.
Effect of Diabetes on the Antigen-Induced Secretion of Pro- and Anti-Inflammatory Cytokines, Type 1 Interferons, and Defensins in LF+ Subjects
Figure 4 shows the antigen-induced secretion of pro- (TNF-α, IL-6, IL-1β, and GM-CSF) and anti- (IL-10, TGF-β, IL-1Ra, IL-35, and IL-27) inflammatory cytokines, and type 1 interferon (IFN-β) and defensin (α-defensin-1) cytokines in the study groups. BmA stimulation resulted in the secretion of TNF-α, IL-6, IL-1β, IL-10, and IL-27. No significant difference was seen in the secretion of these cytokines between the groups. Thus, with respect to pro- and anti-inflammatory cytokines, antigen-induced secretion of these cytokines remains largely unaffected by diabetes.
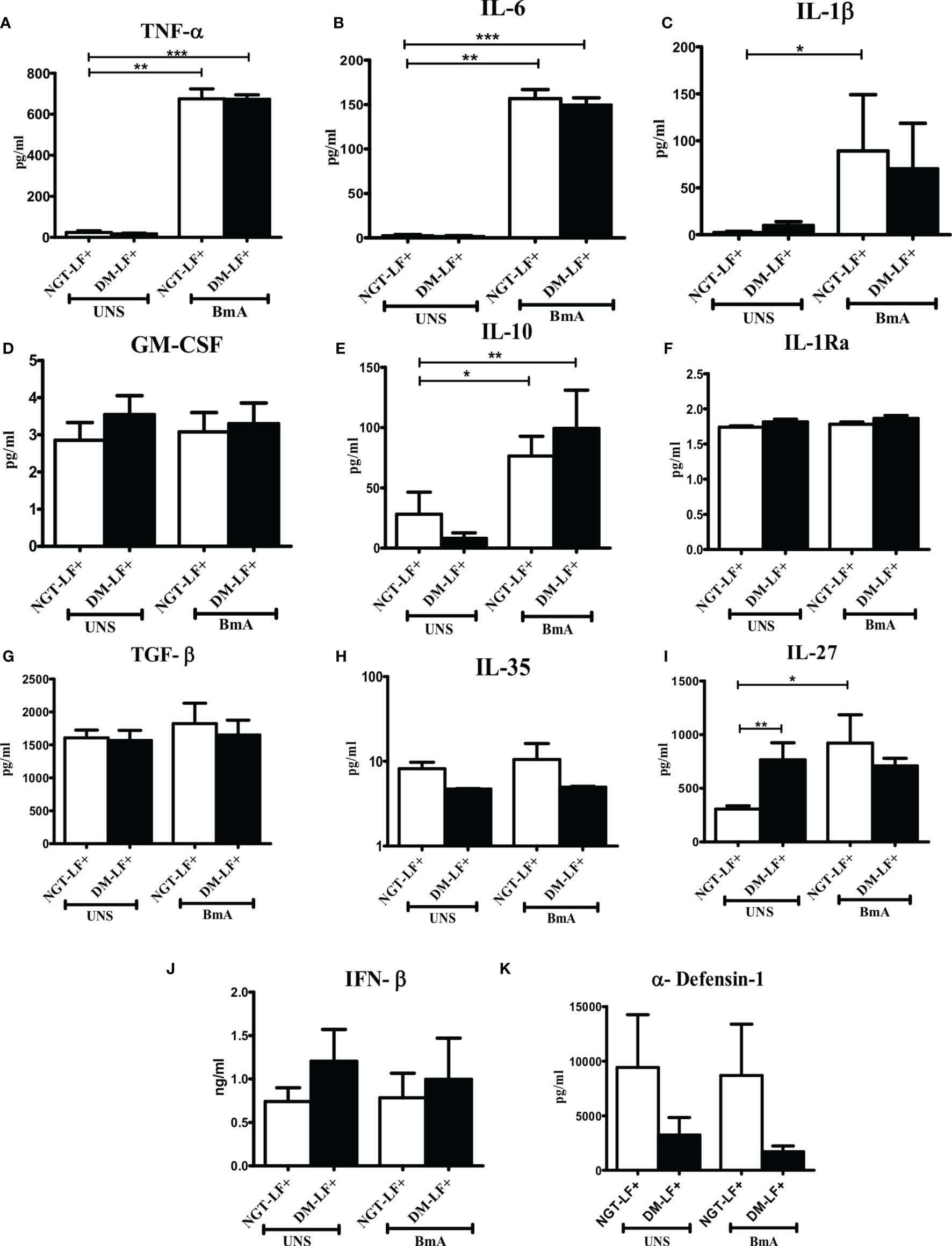
Figure 4 Effect of diabetes on the antigen-induced secretion of pro- and anti-inflammatory cytokines, type 1 interferons, and defensins in LF+ subjects. Bar graph showing Unstimulated (UNS) and filarial antigen (BmA)-induced secretion of TNF-α (A), IL-6 (B), IL-1β (C), GM-CSF (D), IL-10 (E), IL-1Ra (F), TGF-β (G), IL-35 (H), IL-27 (I), IFN-β (J), and α-defensin (K) in the supernatants of blood cultures in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001.
Effect of Diabetes on the Antigen-Induced Secretion of Adaptive Immune Cytokines and Chemokines in LF+ Subjects
Figure 5 shows the antigen-induced secretion of adaptive immune cytokines (IL-12, IFN-γ, IL-2, IL-23, IL-17, IL-9, IL-33, and IL-4) and chemokines (IL-8, IP-10, RANTES, MCP-1, and SDF-1) in the study groups. BmA stimulation resulted in the secretion of IL-8. BmA-induced secretion of IL-12 and IL-33 was seen only in the DM-LF+ group.
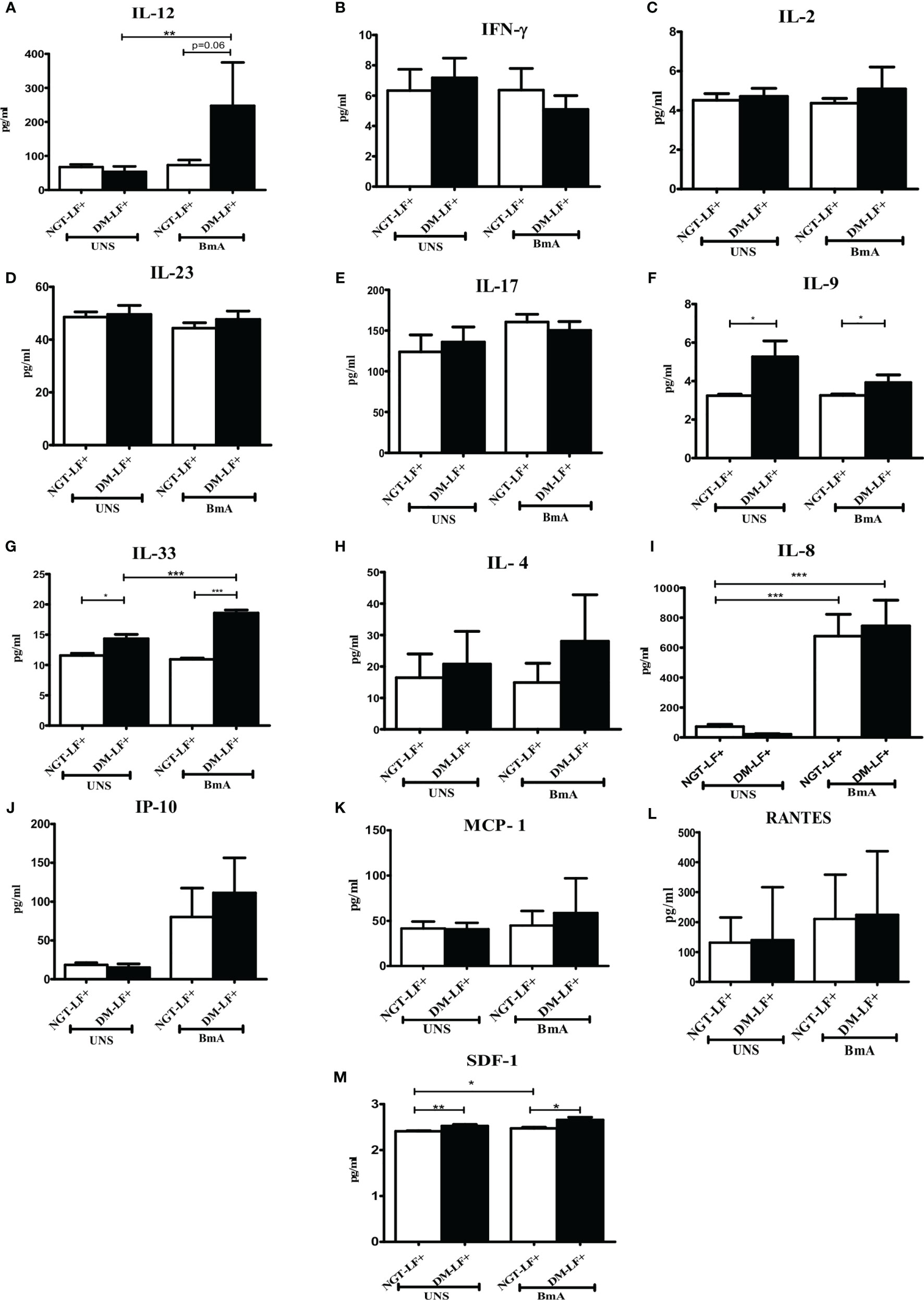
Figure 5 Effect of diabetes on the antigen-induced secretion of adaptive immune cytokines, and chemokines in LF+ subjects. Bar graph showing Unstimulated (UNS) and filarial antigen (BmA)-induced secretion of IL-12 (A), IFN-γ (B), IL-2 (C), IL-23 (D), IL-17 (E), IL-9 (F), IL-33 (G), IL-4 (H), IL-8 (I), IP-10 (J), RANTES (K), MCP-1 (L), and SDF-1 (M) in the supernatants of blood cultures in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001.
Effect of Diabetes on T-Cell Activation, Th Polarization, Expression of Th Master Regulators, and Immunomodulatory Enzymes in LF+ Subjects
Figure 6 shows the antigen-induced T-cell activation (expression of CD69) and Th polarization (Th1, Th2, Th9, and Th17) in the study groups. There was no significant difference in T-cell activation (as determined by CD69 expression) between the groups. However, in accordance to antigen-induced cytokine secretion, the percentage of Th9 cells was significantly higher in the DM-LF+ compared to the NGT-LF+ group. Figure S4 shows the expression of Th polarizing transcriptional regulators (T-bet, GATA3, and ROR-γt) and immunomodulatory enzymes (Phox47 and HO-1) between diabetic and non-diabetic LF+ subjects. No significant difference was seen in the expression of T-bet, GATA-3, and ROR-γT between the groups. The expression of Phox47 and HO-1 was not significantly different between the groups.
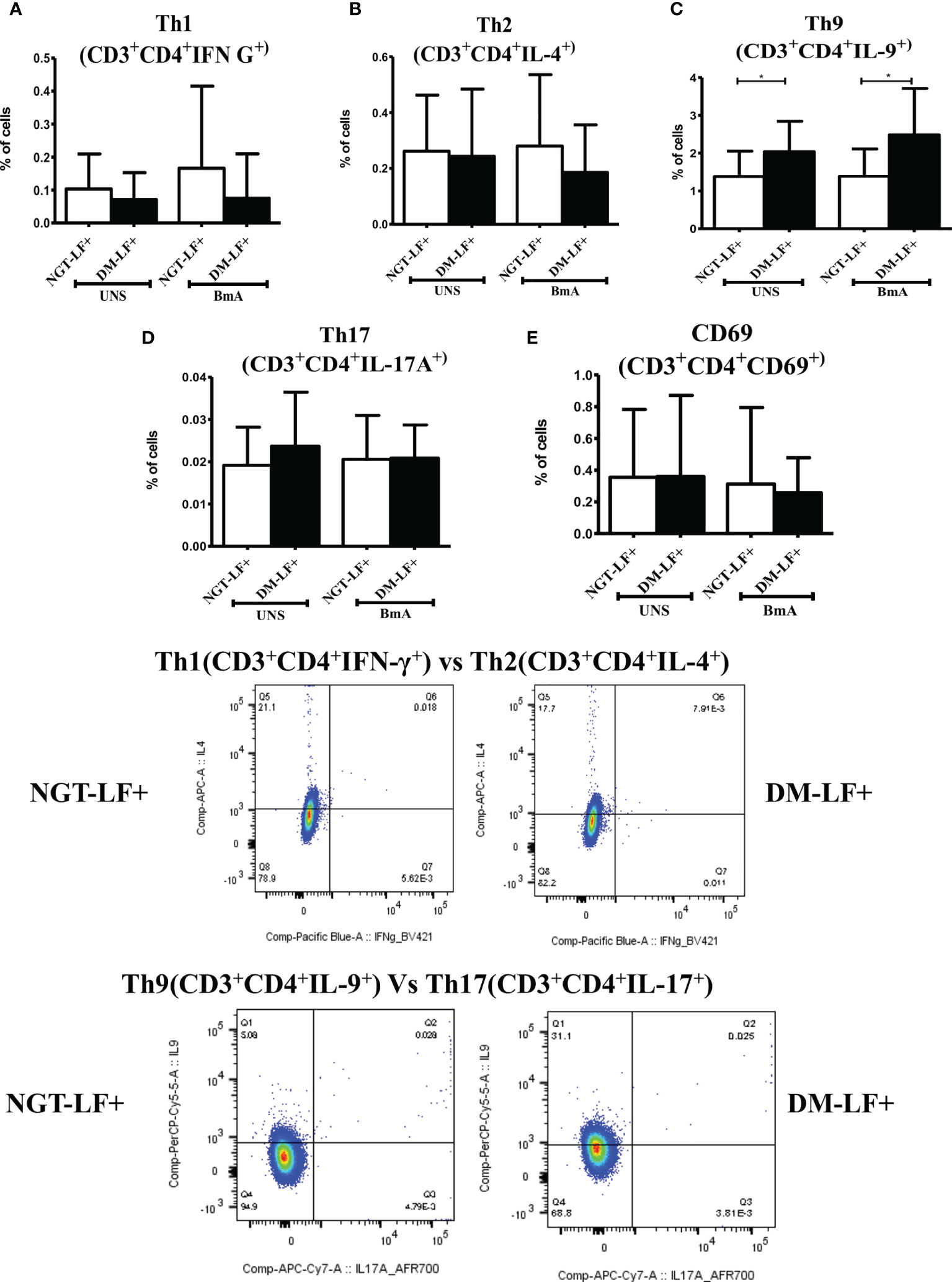
Figure 6 Effect of diabetes on T-cell activation and Th polarization in LF+ subjects. Bar graph showing Unstimulated (UNS) and filarial antigen (BmA)-induced Th1 (A), Th2 (B), Th9 (C), Th17 (D), and activated T cells (E) in NGT-LF+ and DM-LF+ subjects. Statistical significance was determined by non-parametric Mann–Whitney U test and p < 0.05 was considered significant. *p < 0.05.
Discussion
In the present study, we elucidated the effect of diabetes on anti-filarial response, in LF+ subjects. Anti-filarial response is complex and multifaceted and involves both the innate and adaptive arms of the immune system (30). For innate immunity, we studied TLR signaling, and for adaptive immunity, we studied filarial antigen-induced responses. Under TLR signaling, we studied (1) secretion of cytokines (IFN-β, TNF-α, IL-6, IL-1β, GM-CSF, IL-10, TGF-β, IL-1Ra, IL-35, IL-27, IL-1Ra, IL-12, IFN-γ, IL-2, IL-33, IL-4, IL-9, IL-23, and IL-17) and chemokines (RANTES, MCP-1, IL-8, IP-10, and SDF-1); (2) upregulation of MHC (HLA-DR) and co-stimulatory molecules (B7-1/CD80 and B7-2/CD86); and (3) effector functions, which include secretion of defensins (α-Defensin-1) and upregulation of immunomodulatory enzymes (Cox-1, HO-1, IDO-1, and p47Phox). Under antigen-induced responses, we studied (1) secretion of cytokines (IFN-β, TNF-α, IL-6, IL-1β, GM-CSF, IL-10, TGF-β, IL-1Ra, IL-35, IL-27, IL-1Ra, IL-12, IFN-γ, IL-2, IL-33, IL-4, IL-9, IL-23, and IL-17) and chemokines (RANTES, MCP-1, IL-8, IP-10, and SDF-1); (2) Th immunophenotype (Th1, Th2, Th9, and Th17); (3) T-cell activation (upregulation of CD69); and (4) expression of master controllers of Th polarization (T-bet, GATA3, and RORγt). Our major findings were as follows: (1) Even in the absence of TLR- or antigen-induced activation, the basal level secretion of few cytokines was significantly altered in the DM-LF+ compared to the NGT-LF+ group. (2) TLR2 stimulation induced the secretion of TNF-α, IL-6, IL-1β, IL-10, IL-12, and IL-8; TLR4 stimulation induced the secretion of TNF-α, IL-6, IL-1β, IL-10, IL-27, IL-12, IL-23, IL-8, and IP-10; both TLR2 and 4 induced the secretion of α-defensin-1 and MCP-1 only in the DM-LF+ group. A significant difference was seen in the TLR2-induced secretion of IL-10, IL-1Ra, IL-17, and SDF-1 and TLR4-induced secretion of IL-9, IL-33, MCP-1, and SDF-1, between the NGT-LF+ and DM-LF+ groups. (3) TLR-induced expression of Cox-2 and IDO was significantly augmented by diabetes. (4) BmA stimulation induced the secretion of TNF-α, IL-6, IL-1β, IL-10, IL-27, IL-8, and SDF-1. In the presence of diabetes, the basal secretion of IL-1β, IL-27, IL-33, and SDF-1 is augmented while that of IL-10, IL-12, and IL-8 was downregulated. The augmented secretion of IL-9 in the diabetic group (DM-LF+) was associated with increased Th9 polarization. While we expected downregulation of many of these responses, most of these effectors were upregulated under diabetes conditions.
The basal level secretion of IL-12 and IL-8 was decreased while that of IL-1β, IL-27, IL-17, IL-9, IL-33, and SDF-1 was increased in the DM-LF+ compared to the NGT-LF+ group. This could be due to epigenetic modifications induced due to chronic hyperglycemia, onto the immune cells (31). It is well known that chronic hyperglycemia, as seen in diabetes, can induce epigenetic modifications onto immune cells, which can permanently alter their function, which is referred to as metabolic memory (32). TLRs serve as a first line of defense mechanism against pathogen invasion, linking innate and adaptive arms of the immune response (33). TLR2 polymorphisms were shown to be associated with bancroftian filariasis (34). In the present study, we studied TLR-induced secretion of (1) type 1 interferon (IFN-β), (2) pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and GM-CSF), (3) anti-inflammatory cytokines (IL-10, TGF-β, IL-1Ra, IL-35, IL-27, and IL-1Ra), (4) adaptive immune cytokines (Th1 cytokines—IL-12, IFN-γ, and IL-2; Th2 cytokines—IL-33 and IL-4; Th9 cytokines—IL-9; Th17 cytokines—IL-23 and IL-17), and (5) chemokines (RANTES, MCP-1, IL-8, IP-10, and SDF-1). Out of all the cytokines, chemokines, and defensins, diabetes specifically altered (1) TLR2-induced secretion of IL-10, IL-1Ra, IL-17, and SDF-1; and (2) TLR4-induced secretion of IL-12, IL-9, IL-33, MCP-1, and SDF-1. The expression of MHC (HLA-DR) and co-stimulatory (B7-1 and B7-2) molecules on monocytes, B cells, and granulocytes was largely unaffected. Finally, TLR2- and TLR4-induced expression of immunomodulatory effectors Cox-2 and IDO was significantly augmented by diabetes. Previously, it was shown that baseline expression of TLRs was significantly lower in B cells (but not in monocytes) of the LF+ subjects, compared to uninfected individuals (35). TLR stimulation of B cells from these subjects showed diminished activation and antibody secretion, indicating a state of immune unresponsiveness (35). Filarial parasite was shown to actively inhibit the expression and function of TLRs in human dendritic cells (36). In contrast, diabetes is largely a chronic inflammatory state that augments TLR expression and increases the activation in various cell types (37). Recently, fetuin-A was found to act as an endogenous ligand for TLR4 to promote free fatty acid-induced insulin resistance, in the adipose tissue (38). Several filarial antigens are known to directly bind to TLR4 and down-modulate their effect (39, 40). However, few filarial antigens were also found to augment TLR-induced inflammation, especially in macrophages and dendritic cells, indicating that not all filarial antigens have an anti-inflammatory effect (14, 15). Thus, under conditions of diabetes–filariasis comorbidity, one can expect either augmentation or inhibition of TLR responses, depending on the expression pattern of the filarial antigens, under the given set of conditions. In the present study, at least some of the TLR-induced responses were found to be augmented under conditions of diabetes–filariasis comorbidity (22). Interestingly, the TLR downregulation gets reversed when filarial patients develop chronic pathology, with augmented secretion of pro-inflammatory cytokines (41). They also show enhanced TLR-induced secretion of the pro-angiogenic factor, which promotes neo lymphangiogenesis leading to lymphedema and elephantiasis, a hallmark feature of lymphatic filariasis (42). As per the above model, diabetes can at least partially augment chronic pathology in LF+ subjects by enhancing TLR-mediated immune responses.
Compared to TLR-induced cytokine/chemokine secretion, the expression of immunomodulatory effectors like Cox-2, HO-1, and IDO-1 is poorly studied in filariasis and diabetes. These effectors produce second messengers like prostaglandin H, CO, and kynurenine, respectively, which have an immunomodulatory effect. In the present study, diabetes specifically augmented TLR-induced expression of Cox-2 and IDO in LF+ subjects. While Cox-2 expression induces a pro-inflammatory milieu, IDO expression induces an anti-inflammatory environment, as a counter mechanism (43). At least in a tumor microenvironment, this positive feedback loop augments inflammation and inhibits T-cell response, augmenting tumor growth and immune evasion, respectively (44). Whether the same strategy has been hijacked by filariasis and is augmented by diabetes, aiding pathology, is an interesting possibility that needs further exploration.
Next to TLRs, T cells play a vital role in orchestrating the immune response against filariasis. Antigen stimulation induced the secretion of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), anti-inflammatory cytokines (IL-10 and IL-27), and chemokines (IL-8 and SDF-1). Out of all the cytokines, chemokines, and defensins secreted, diabetes specifically altered antigen-induced secretion of IL-12, IL-9, and SDF-1. T-cell-mediated immune responses during filariasis depend on the phase of the infection: (1) the acute phase is characterized by skewed Th2 response (with enhanced secretion of IL-4, IL-5, and IL-13); (2) the chronic phase is associated with “modified Th2 response” with Tregs (with enhanced IL-10 and TGF-β secretion) playing a major role compared to Th2 cells; and (3) the chronic pathology phase is characterized by a shift towards “Th1/Th17” (enhanced secretion of IFN-γ, IL-2, and IL-17) response, which takes place in individuals who develop pathology (45). Stimulation of T cells from LF+ subjects with B. malayi antigen showed impaired Th1 response with decreased expression of T-bet, Suppressor of cytokine signaling-1 (SOCS-1), SOCS-5, and SOCS-7 (27). Furthermore, in vitro culture of PBMCs from LF+ subjects with live larval stage 3 (L3) and microfilarial (mf) worms showed impaired expression of T-bet and GATA-3 and augmented expression of FoxP3 (46). In accordance with these reports, no significant Th1 or Th17 polarization was seen in LF+ subjects. While TLR-induced IL-12 and basal IL-33 secretion was augmented under diabetes condition, this was not seen at the level of Th1 and Th2 polarization. This indicates that, at least under in vivo condition, apart from IL-12 and IL-33, other factors might play a significant role in Th1/Th2 polarization.
Compared to TLR stimulation, antigen stimulation induced the secretion of only pro-inflammatory cytokines and chemokines. Compared to Th1, Th2, and Th17 cell types, Th9 cells were recently characterized and were found to be involved in allergies, autoimmunity, and tumor evasion (47, 48). Their role in both diabetes and filariasis is largely unknown. Recently, expansion of filarial antigen-specific Th9 cells in whole blood culture was found to be associated with chronic pathology (49). In the present study, diabetes augmented IL-9 secretion and increased Th9 population. Like IL-9, the basal level of SDF-1α was found to be significantly high in LF+ subjects with diabetes. Previously, SDF-1/CXCR4 was found to promote chronic pathology in LF+ subjects (50). Thus, diabetes, by augmenting IL-9 and SDF-1, can promote chronic pathology in LF+ subjects.
In conclusion, in the present report, the effect of diabetes on TLR pathway and antigen-induced immune response in LF+ subjects was studied. Several parameters including cytokines and chemokines, antigen processing and presentation molecules, T-cell activation marker, Th polarization, expression of master regulators of Th polarization, and immunomodulatory effectors were studied. The most interesting and unexpected finding of this study is that, while we expected downregulation of TLR and antigenic responses, diabetes had a modest but significant augmenting effect on specific TLR-mediated inflammatory markers. Most of the inflammatory markers that were found to be augmented by diabetes have previously been shown to promote chronic pathology in LF+ subjects (51, 52). The major limitations of this study are the limited sample size and the cross-sectional study design, which makes it impossible to decipher the cause–effect phenomenon. However, the major strength of the study is that it was conducted in a filarial endemic population wherein the prevalence of diabetes is rapidly increasing (53). Overall, diabetes was found to augment certain inflammatory components that can promote chronic pathology in LF+ subjects, and this needs further validation in a large cohort study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the MDRF Institutional Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VM, SB, and VA conceived and designed the experiment. JS and SM performed the experiment. JS and VA analyzed the data. JS and VA drafted the manuscript. All the authors contributed to the discussion and reviewed the manuscript. VA is the guarantor of the data. All authors contributed to the article and approved the submitted version.
Funding
The Department of Genetics, University of Madras, has received funds for infrastructural support from DST-FIST and UGC-SAP programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.716515/full#supplementary-material
Supplementary Figure 1 | Gating strategy used for the analysis ofHLA-DR, B7-l(eD80), B7-2 (eD86) on Monocytes and Granulocytes and B cells. FSe vs SSe plot showing monocyte, lymphocyte and granulocyte gates (A). FSe-A vs FSe-H plot showing gated single cells oflymphocytes (B), monocytes (C), Granulocytes (D). CD19 vs FSC-H plot showing the gated CD19+ B cells (E). CD14 vs FSC-H plot showing the gated CD14+ Monocytes (F). Expression of HLA DR on lymphocytes (G), monocytes (H), Granulocytes (I). Expression of B7-1 (CD80) on lymphocytes (J), monocytes (K), Granulocytes (L). Expression ofB7-2 (CD86) on lymphocytes (M), monocytes (N), Granulocytes (O).
Supplementary Figure 2 | Gating strategy used for analysis of T helper cell subtype analysis. FSC vs SSC plot showing the gated lymphocyte population (A). FSC-A vs FSC-H plot showing gated single cells of lymphocytes (B). CD3 vs CD4 plot showing the gated CD3+CD4+ T helper cells (C). IFN-y Vs IL-4 plot showing the gated Thl and Th2 population (E). IL-9 Vs IL-17 plot showing the gated Th 17 and Th9 population (F).
Supplementary Figure 3 | Effect of diabetes on the TLR induced expression of MHC and costimulatory molecules in LF+ subjects.
Supplementary Figure 4 | Effect of diabetes on expression of Th master regulators and immunomodulatory enzymes in LF+ subjects.
References
1. Evans H, Mitre E. Worms as Therapeutic Agents for Allergy and Asthma: Understanding Why Benefits in Animal Studies Have Not Translated Into Clinical Success. J Allergy Clin Immunol (2015) 135:343–53. doi: 10.1016/j.jaci.2014.07.007
2. Hubner MP, Stocker JT, Mitre E. Inhibition of Type 1 Diabetes in Filaria-Infected Non-Obese Diabetic Mice is Associated With a T Helper Type 2 Shift and Induction of FoxP3+ Regulatory T Cells. Immunology (2009) 127:512–22. doi: 10.1111/j.1365-2567.2008.02958.x
3. Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, et al. Helminth Protection Against Autoimmune Diabetes in Nonobese Diabetic Mice Is Independent of a Type 2 Immune Shift and Requires TGF-Beta. J Immunol (2012) 188:559–68. doi: 10.4049/jimmunol.1100335
4. Berbudi A, Surendar J, Ajendra J, Gondorf F, Schmidt D, Neumann AL, et al. Filarial Infection or Antigen Administration Improves Glucose Tolerance in Diet-Induced Obese Mice. J Innate Immun (2016) 8:601–16. doi: 10.1159/000448401
5. Aravindhan V, Mohan V, Surendar J, Rao MM, Ranjani H, Kumaraswami V, et al. Decreased Prevalence of Lymphatic Filariasis Among Subjects With Type-1 Diabetes. Am J Trop Med Hyg (2010) 83:1336–9. doi: 10.4269/ajtmh.2010.10-0410
6. Aravindhan V, Mohan V, Surendar J, Muralidhara Rao M, Pavankumar N, Deepa M, et al. Decreased Prevalence of Lymphatic Filariasis Among Diabetic Subjects Associated With a Diminished Pro-Inflammatory Cytokine Response (CURES 83). PloS Negl Trop Dis (2010) 4:e707. doi: 10.1371/journal.pntd.0000707
7. Aravindhan V, Mohan V, Surendar J, Rao MM, Anuradha R, Deepa M, et al. Effect of Filarial Infection on Serum Inflammatory and Atherogenic Biomarkers in Coronary Artery Disease (CURES-121). Am J Trop Med Hyg (2012) 86:828–33. doi: 10.4269/ajtmh.2012.11-0773
8. Chen Y, Lu J, Huang Y, Wang T, Xu Y, Xu M, et al. Association of Previous Schistosome Infection With Diabetes and Metabolic Syndrome: A Cross-Sectional Study in Rural China. J Clin Endocrinol Metab (2013) 98:E283–7. doi: 10.1210/jc.2012-2517
9. Wiria AE, Hamid F, Wammes LJ, Prasetyani MA, Dekkers OM, May L, et al. Infection With Soil-Transmitted Helminths Is Associated With Increased Insulin Sensitivity. PloS One (2015) 10:e0127746. doi: 10.1371/journal.pone.0127746
10. Raizada N, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, et al. Endotext (Internet). Raizada N, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. eds. South Dartmouth, MA: Endotext (2000).
11. Unnikrishnan R, Misra A. Infections and Diabetes: Risks and Mitigation With Reference to India. Diabetes Metab Syndr (2020) 14:1889–94. doi: 10.1016/j.dsx.2020.09.022
12. Mukherjee S, Huda S, Sinha Babu SP. Toll-Like Receptor Polymorphism in Host Immune Response to Infectious Diseases: A Review. Scand J Immunol (2019) 90:e12771. doi: 10.1111/sji.12771
13. Tartey S, Takeuchi O. Pathogen Recognition and Toll-Like Receptor Targeted Therapeutics in Innate Immune Cells. Int Rev Immunol (2017) 36:57–73. doi: 10.1080/08830185.2016.1261318
14. Mukherjee S, Mukherjee S, Maiti TK, Bhattacharya S, Sinha Babu SP. A Novel Ligand of Toll-Like Receptor 4 From the Sheath of Wuchereria Bancrofti Microfilaria Induces Proinflammatory Response in Macrophages. J Infect Dis (2017) 215:954–65. doi: 10.1093/infdis/jix067
15. Mukherjee S, Karnam A, Das M, Babu SPS, Bayry J. Wuchereria Bancrofti Filaria Activates Human Dendritic Cells and Polarizes T Helper 1 and Regulatory T Cells via Toll-Like Receptor 4. Commun Biol (2019) 2:169. doi: 10.1038/s42003-019-0392-8
16. Zeytun A, Chaudhary A, Pardington P, Cary R, Gupta G. Induction of Cytokines and Chemokines by Toll-Like Receptor Signaling: Strategies for Control of Inflammation. Crit Rev Immunol (2010) 30:53–67. doi: 10.1615/CritRevImmunol.v30.i1.40
17. Lata S, Raghava GP. CytoPred: A Server for Prediction and Classification of Cytokines. Protein Eng Des Sel (2008) 21:279–82. doi: 10.1093/protein/gzn006
18. Guo X, Wu N, Shang Y, Liu X, Wu T, Zhou Y, et al. The Novel Toll-Like Receptor 2 Agonist SUP3 Enhances Antigen Presentation and T Cell Activation by Dendritic Cells. Front Immunol (2017) 8:158. doi: 10.3389/fimmu.2017.00158
19. Buchta CM, Bishop GA. Toll-Like Receptors and B Cells: Functions and Mechanisms. Immunol Res (2014) 59:12–22. doi: 10.1007/s12026-014-8523-2
20. Sabroe I, Dower SK, Whyte MK. The Role of Toll-Like Receptors in the Regulation of Neutrophil Migration, Activation, and Apoptosis. Clin Infect Dis (2005) 41(Suppl 7):S421–6. doi: 10.1086/431992
21. McCoy CE, O’Neill LA. The Role of Toll-Like Receptors in Macrophages. Front Biosci (2008) 13:62–70. doi: 10.2741/2660
22. Aravindhan V, Anand G. Cell Type-Specific Immunomodulation Induced by Helminthes: Effect on Metainflammation, Insulin Resistance and Type-2 Diabetes. Am J Trop Med Hyg (2017) 97:1650–61. doi: 10.4269/ajtmh.17-0236
23. Milam AV, Allen PM. Functional Heterogeneity in CD4(+) T Cell Responses Against a Bacterial Pathogen. Front Immunol (2015) 6:621. doi: 10.3389/fimmu.2015.00621
24. Taniuchi I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu Rev Immunol (2018) 36:579–601. doi: 10.1146/annurev-immunol-042617-053411
25. Beadling C, Slifka MK. Regulation of Innate and Adaptive Immune Responses by the Related Cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp (Warsz) (2006) 54:15–24. doi: 10.1007/s00005-006-0002-6
26. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol (2020) 11:2025. doi: 10.3389/fimmu.2020.02025
27. Babu S, Kumaraswami V, Nutman TB. Transcriptional Control of Impaired Th1 Responses in Patent Lymphatic Filariasis by T-Box Expressed in T Cells and Suppressor of Cytokine Signaling Genes. Infect Immun (2005) 73:3394–401. doi: 10.1128/IAI.73.6.3394-3401.2005
28. Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C, et al. Filarial Lymphedema is Characterized by Antigen-Specific Th1 and Th17 Proinflammatory Responses and a Lack of Regulatory T Cells. PloS Negl Trop Dis (2009) 3:e420. doi: 10.1371/journal.pntd.0000420
29. Madhumitha H, Mohan V, Babu S, Aravindhan V. TLR-Induced Secretion of Novel Cytokine IL-27 Is Defective in Newly Diagnosed Type-2 Diabetic Subjects. Cytokine (2018) 104:65–71. doi: 10.1016/j.cyto.2017.09.032
30. Kwarteng A, Ahuno ST. Immunity in Filarial Infections: Lessons From Animal Models and Human Studies. Scand J Immunol (2017) 85:251–7. doi: 10.1111/sji.12533
31. Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic Histone H3 Lysine 9 Methylation in Metabolic Memory and Inflammatory Phenotype of Vascular Smooth Muscle Cells in Diabetes. Proc Natl Acad Sci USA (2008) 105:9047–52. doi: 10.1073/pnas.0803623105
32. Mossel DM, Moganti K, Riabov V, Weiss C, Kopf S, Cordero J, et al. Epigenetic Regulation of S100A9 and S100A12 Expression in Monocyte-Macrophage System in Hyperglycemic Conditions. Front Immunol (2020) 11:1071. doi: 10.3389/fimmu.2020.01071
33. Venugopal PG, Nutman TB, Semnani RT. Activation and Regulation of Toll-Like Receptors (TLRs) by Helminth Parasites. Immunol Res (2009) 43:252–63. doi: 10.1007/s12026-008-8079-0
34. Junpee A, Tencomnao T, Sanprasert V, Nuchprayoon S. Association Between Toll-Like Receptor 2 (TLR2) Polymorphisms and Asymptomatic Bancroftian Filariasis. Parasitol Res (2010) 107:807–16. doi: 10.1007/s00436-010-1932-9
35. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Diminished Expression and Function of TLR in Lymphatic Filariasis: A Novel Mechanism of Immune Dysregulation. J Immunol (2005) 175:1170–6. doi: 10.4049/jimmunol.175.2.1170
36. Semnani RT, Venugopal PG, Leifer CA, Mostbock S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 Function and Expression in Human Dendritic Cells by Helminth Parasites. Blood (2008) 112:1290–8. doi: 10.1182/blood-2008-04-149856
37. Aravindhan V, Madhumitha H. Metainflammation in Diabetic Coronary Artery Disease: Emerging Role of Innate and Adaptive Immune Responses. J Diabetes Res (2016) 2016:6264149. doi: 10.1155/2016/6264149
38. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat Med (2012) 18:1279–85. doi: 10.1038/nm.2851
39. Panda SK, Kumar S, Tupperwar NC, Vaidya T, George A, Rath S, et al. Chitohexaose Activates Macrophages by Alternate Pathway Through TLR4 and Blocks Endotoxemia. PloS Pathog (2012) 8:e1002717. doi: 10.1371/journal.ppat.1002717
40. Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, et al. Immunomodulation via Novel Use of TLR4 by the Filarial Nematode Phosphorylcholine-Containing Secreted Product, ES-62. J Immunol (2005) 174:284–93. doi: 10.4049/jimmunol.174.1.284
41. Babu S, Anuradha R, Kumar NP, George PJ, Kumaraswami V, Nutman TB. Filarial Lymphatic Pathology Reflects Augmented Toll-Like Receptor-Mediated, Mitogen-Activated Protein Kinase-Mediated Proinflammatory Cytokine Production. Infect Immun (2011) 79:4600–8. doi: 10.1128/IAI.05419-11
42. Babu S, Anuradha R, Kumar NP, George PJ, Kumaraswami V, Nutman TB. Toll-Like Receptor- and Filarial Antigen-Mediated, Mitogen-Activated Protein Kinase- and NF-kappaB-Dependent Regulation of Angiogenic Growth Factors in Filarial Lymphatic Pathology. Infect Immun (2012) 80:2509–18. doi: 10.1128/IAI.06179-11
43. Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, et al. The Immune Tolerance of Cancer Is Mediated by IDO That is Inhibited by COX-2 Inhibitors Through Regulatory T Cells. J Immunother (2009) 32:22–8. doi: 10.1097/CJI.0b013e31818ac2f7
44. Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma Interplay via Cyclooxygenase-2 and Indoleamine 2,3-Dioxygenase Promotes Breast Cancer Progression. Breast Cancer Res (2014) 16:410. doi: 10.1186/s13058-014-0410-1
45. Bancroft AJ, Grencis RK, Else KJ, Devaney E. The Role of CD4 Cells in Protective Immunity to Brugia Pahangi. Parasite Immunol (1994) 16:385–7. doi: 10.1111/j.1365-3024.1994.tb00364.x
46. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory Networks Induced by Live Parasites Impair Both Th1 and Th2 Pathways in Patent Lymphatic Filariasis: Implications for Parasite Persistence. J Immunol (2006) 176:3248–56. doi: 10.4049/jimmunol.176.5.3248
47. He Y, Dong L, Cao Y, Bi Y, Liu G. IL-9 and Th9 Cells in Tumor Immunity. Adv Exp Med Biol (2020) 1240:35–46. doi: 10.1007/978-3-030-38315-2_3
48. Badolati I, Sverremark-Ekstrom E, van der Heiden M. Th9 Cells in Allergic Diseases: A Role for the Microbiota? Scand J Immunol (2020) 91:e12857. doi: 10.1111/sji.12857
49. Anuradha R, George PJ, Hanna LE, Chandrasekaran V, Kumaran P, Nutman TB, et al. IL-4-, TGF-Beta-, and IL-1-Dependent Expansion of Parasite Antigen-Specific Th9 Cells Is Associated With Clinical Pathology in Human Lymphatic Filariasis. J Immunol (2013) 191:2466–73. doi: 10.4049/jimmunol.1300911
50. Nathan AA, Dixit M, Babu S, Balakrishnan AS. Comparison and Functional Characterisation of Peripheral Blood Mononuclear Cells Isolated From Filarial Lymphoedema and Endemic Normals of a South Indian Population. Trop Med Int Health (2017) 22:1414–27. doi: 10.1111/tmi.12969
51. Satapathy AK, Sartono E, Sahoo PK, Dentener MA, Michael E, Yazdanbakhsh M, et al. Human Bancroftian Filariasis: Immunological Markers of Morbidity and Infection. Microbes Infect (2006) 8:2414–23. doi: 10.1016/j.micinf.2006.05.003
52. Anuradha R, George PJ, Pavan Kumar N, Fay MP, Kumaraswami V, Nutman TB, et al. Circulating Microbial Products and Acute Phase Proteins as Markers of Pathogenesis in Lymphatic Filarial Disease. PloS Pathog (2012) 8:e1002749. doi: 10.1371/journal.ppat.1002749
Keywords: diabetes, filariasis, TLR, immunomodulation, Th cell, inflammation
Citation: Sibi JM, Mohan V, Munisankar S, Babu S and Aravindhan V (2021) Augmented Innate and Adaptive Immune Responses Under Conditions of Diabetes–Filariasis Comorbidity. Front. Immunol. 12:716515. doi: 10.3389/fimmu.2021.716515
Received: 28 May 2021; Accepted: 23 August 2021;
Published: 10 September 2021.
Edited by:
Manuel Ritter, University Hospital Bonn, GermanyReviewed by:
Suprabhat Mukherjee, Kazi Nazrul University, IndiaDominik Rückerl, The University of Manchester, United Kingdom
Copyright © 2021 Sibi, Mohan, Munisankar, Babu and Aravindhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vivekanandhan Aravindhan, Y3ZhcmF2aW5kaGFuQGdtYWlsLmNvbQ==
 Joy Manohar Sibi1
Joy Manohar Sibi1 Vivekanandhan Aravindhan
Vivekanandhan Aravindhan