- 1Department of Colorectal Surgery, Yunnan Cancer Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming, China
- 2Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 3Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Gastrointestinal Surgery, Honghe Prefecture Third People’s Hospital, Honghe Cancer Hospital, Gejiu, China
- 5Department of Imaging, Yunnan Cancer Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming, China
- 6Department of Pathology, Yunnan Cancer Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming, China
Objective: Examine patients with locally advanced rectal cancer (LARC) with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) who received neoadjuvant immunotherapy (nIT), and compare the outcomes of those who chose a watch-and-wait (WW) approach after achieving clinical complete response (cCR) or near-cCR with those who underwent surgery and were confirmed as pathological complete response (pCR).
Methods: LARC patients with dMMR/MSI-H who received nIT were retrospectively examined. The endpoints were 2-year overall survival (OS), 2-year disease-free survival (DFS), local recurrence (LR), and distant metastasis (DM). The efficacy of programmed cell death protein-1 (PD-1) inhibitor, immune-related adverse events (irAEs), surgery-related adverse events (srAEs), and enterostomy were also recorded.
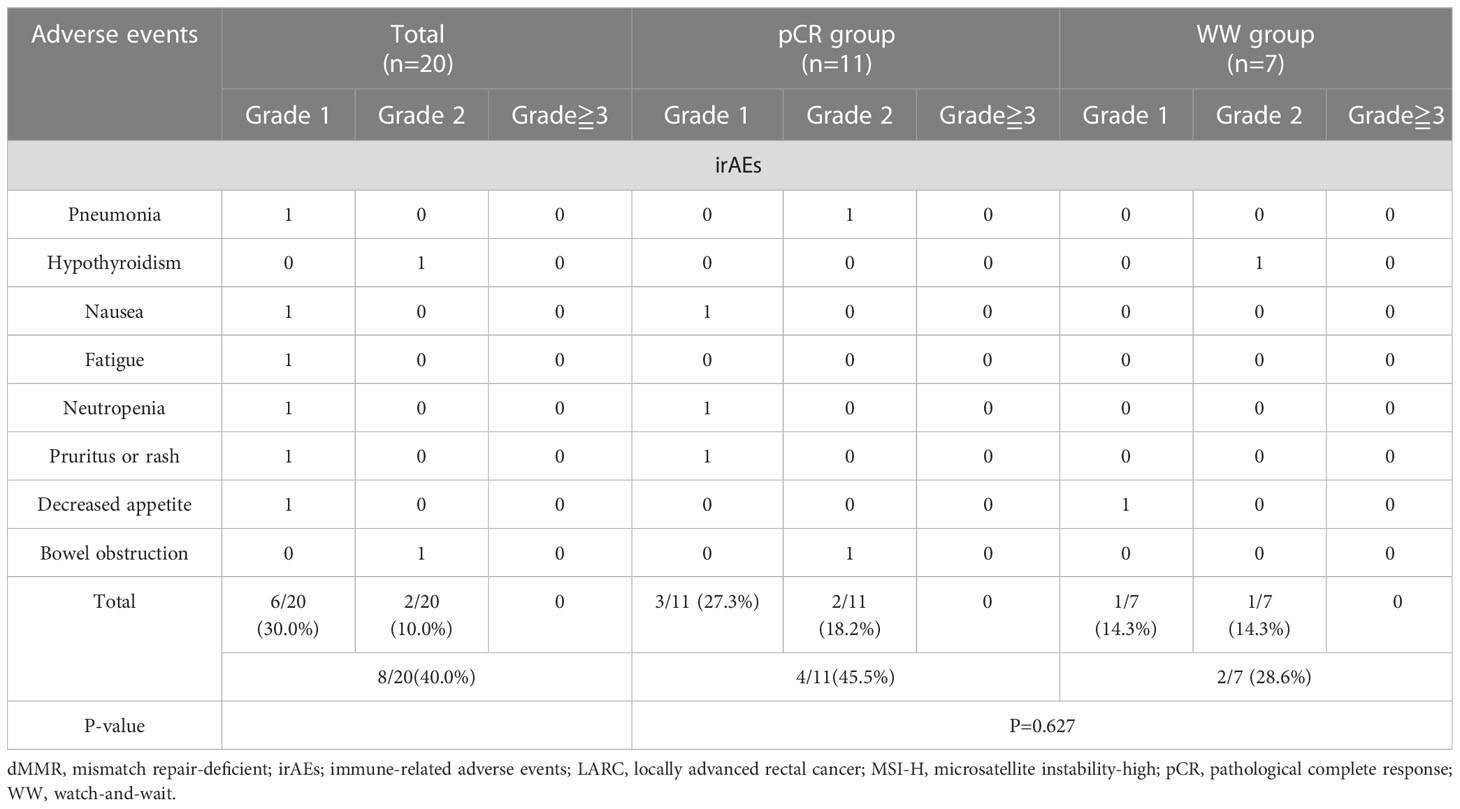
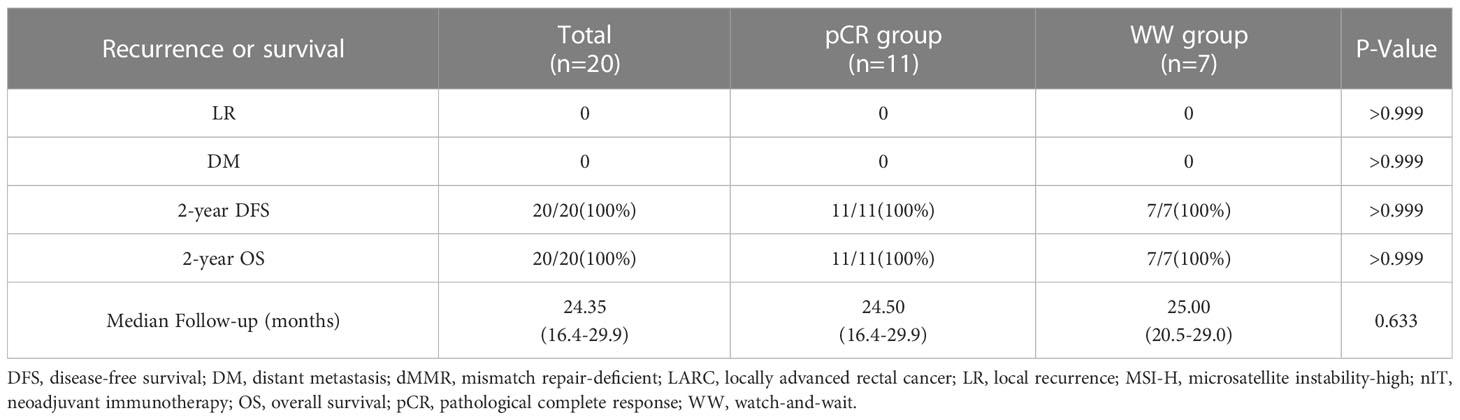
Results: Twenty patients who received a PD-1 inhibitor as initial nIT were examined. Eighteen patients (90%) achieved complete response (CR) after a median of 7 nIT cycles, including 11 with pCR after surgery (pCR group), and 7 chose a WW strategy after evaluation as cCR or near-cCR (WW group). Both groups had median follow-up times of 25.0 months. Neither group had a case of LR or DM, and the 2-year DFS and OS in each group was 100%. The two groups had similar incidences of irAEs (P=0.627). In the pCR group, however, 2 patients (18.2%) had permanent colostomy, 3 (27.3%) had temporary ileostomy, and 2 (18.2%) had srAEs.
Conclusion: Neoadjuvant PD-1 blockade had high efficacy and led to a high rate of CR in LARC patients with dMMR/MSI-H. A WW strategy appears to be a safe and reliable option for these patients who achieve cCR or near-cCR after nIT.
Introduction
Colorectal cancer (CRC) is the most common malignancy of the digestive system, and global cancer statistics for 2020 indicated it had the third-highest incidence and the second-highest mortality rate among all cancers (1). About 60% of patients with CRC have locally advanced disease upon diagnosis (2), defined as CRC stage II (clinical T3–T4, N0) or stage III (any clinical T, N1–N2). Neoadjuvant fluorouracil-based chemotherapy and radiotherapy followed by total mesorectal excision (TME), with or without postoperative chemotherapy, is the standard treatment regimen for patients with locally advanced rectal cancer (LARC) (3, 4), and this regimen enables approximately 20% of these patients to achieve a pathological complete response (pCR) (5, 6). Nevertheless, the short- and long-term toxicities from this treatment, including defecation disorders, urinary and sexual dysfunction, surgical complications, and temporary or permanent enterostomy, can seriously reduce a patient’s quality of life (7, 8). Data from a large number of studies have confirmed that adoption of a watch-and-wait (WW) strategy by patients with rectal cancer who achieved a clinical complete response (cCR) after neoadjuvant chemoradiotherapy (nCRT) prevented surgical trauma, preserved organ function, and provided a survival benefit similar to surgery (9, 10). Even for patients with near-cCR, a previous study demonstrated that more than half of them achieved organ preservation within 3 years after a WW strategy, and their local recurrence-free survival and metastasis-free survival rates were not significantly different from those who had cCR (11). However, with the increasing availability of neoadjuvant treatment options, it is uncertain whether patients with rectal cancer who achieve a cCR or near-cCR after treatment with other neoadjuvant modalities should also adopt a WW strategy.
There is evidence that CRC patients with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) receive little benefit from fluorouracil-based chemotherapy (12, 13). However, these patients typically have high sensitivity to immunotherapies, such as anti-program death-1 (PD-1) antibodies, and their responses are often long-lasting (14, 15). In addition, higher rates of pathological response were achieved when immune checkpoint inhibitors (ICIs) were used with the first-line or neoadjuvant application rather than at a later time (16–18). Although there is only no more than 10% of rectal cancers are classified as dMMR/MSI-H (19–21), a complete response (CR) rate more than 60% can be achieved from neoadjuvant immunotherapy (nIT), significantly higher than from nCRT (21–23). In addition, immunotherapy leads to fewer adverse effects and almost no damage to the sphincter, reproductive organs, sexual function, or bladder (21–23). These advantages of nIT suggest that LARC patients with dMMR/MSI-H who achieved a cCR or near-cCR after nIT might benefit from a WW strategy.
Current data regarding the effect of a WW strategy after nIT for CRC are very rare. As far as we know, this is the first study to compare the survival outcomes of MSI-H/dMMR LARC patients receiving nIT treatment who opted for a WW strategy after achieving a cCR or near-cCR with those who underwent surgery and confirmed as pCR. The present study is a preliminary evaluation of the safety and feasibility of the WW strategy after nIT in these patients using data from multiple centers.
Materials and methods
Patient selection
This study was a retrospective, multicenter, case series study. We reviewed patients with LARC (clinical stage T3–4/N0–2/M0) and dMMR/MSI-H who received a PD-1 inhibitor (no type limitations) alone as an initial neoadjuvant treatment from January 2019 to May 2020 at the Yunnan Cancer Hospital (Third Affiliated Hospital of Kunming Medical University), Sun Yat-sen University Cancer Center, First Affifiliated Hospital of Chongqing Medical University, or Honghe Cancer Hospital (Honghe Prefecture Third People’s Hospital). All eligible patients were 18 to 75 years-old, had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 1, and received 4 or more doses of a PD-1 inhibitor. The exclusion criteria were: suspected metastatic disease; dMMR based on immunohistochemical staining (IHC), but no evidence of MSI-H based polymerase chain reaction (PCR) testing or next-generation sequencing (NGS); active autoimmune disease or history of autoimmune disease or previous receipt of systemic biological immunotherapy.
Data collection
Standardized electronic forms were sent to physicians in each center. Complete demographic and clinicopathological information of patients were collected, including ECOG status, family and personal history of malignant tumors, serum carcinoembryonic antigen (CEA) level, clinical and pathological stage, pathological type of CRC, degree of differentiation, mismatch repair (MMR) or microsatellite status, treatment regimen, treatment response, tumor regression grade (TRG), immune-related adverse events (irAEs), surgery-related adverse events (srAEs), follow-up, and survival.
All staging was performed according to the eighth edition of the American Joint Committee on Cancer (AJCC) (24). MMR status was determined by IHC staining for mismatch repair proteins (MLH1, MSH2, MSH6, and PMS2) in biopsy tissues before treatment. Microsatellite status was determined by PCR or NGS technology. Among them, PCR was used as the “gold standard” to determine microsatellite status by analyzing the five consensus tumor microsatellite loci: two mononucleotides (BAT25 and BAT26) and three dinucleotides (D5S346, D2S123, and D17S250), and NGS was recommended as a second-line method for microsatellite status detection (25). Studies have shown that the sensitivity and specificity of IHC for MMR or PCR for MSI were both above 90%, and the concordance between the two methods is approximately 90% (26, 27). In addition, NGS-based MSI testing results to be up to 99% concordant with conventional PCR and 92.4% concordant with double confirmed IHC staining (28).
Treatment methods
All eligible patients started neoadjuvant anti-PD-1 monotherapy after diagnosis, and none of them were treated with combined radiotherapy, chemotherapy, targeted therapy, or an additional ICI. Each patient received 200 mg of a PD-1 inhibitor by intravenous infusion every 3 weeks until tumor regression to feasible R0 resection, cCR, or near-cCR. There were no limits on the type of PD-1 inhibitors, and these included pembrolizumab, sintilimab, and tislelizumab.
The timing and procedure of the operation, need for adjuvant immunotherapy (aIT), and treatment course were determined by the head surgeon after a comprehensive evaluation of the patient’s response to treatment and general condition. Patients who achieved a cCR or near-cCR after nIT were informed of the benefits and risks of the therapeutic alternatives, and most of them expressed a strong desire for the preservation of organ function and avoidance of enterostomy. Given the long-lasting response of immunotherapy and the inconsistency between imaging and pathological evaluation (for example, some patients with imaging evaluation of PR were pathologically confirmed as pCR in our previous study (22)), doctors agreed on their request for exemption from surgery. Before undergoing the WW strategy, they were informed that a WW strategy after cCR, especially after near-cCR following nIT is not currently a standard therapy and signed informed consent documents. All of them were vigilant about regular follow-up and adherence to the recommended surveillance, with consent for radical resection once the disease progresses.
Treatment response and survival outcomes
Response to treatment was assessed by magnetic resonance imaging (MRI) of the pelvic region, transrectal ultrasound (TRUS), enhanced CT examination of chest and abdomen, digital rectal examination (DRE), serum carcinoembryonic antigen (CEA), endoscopy, and selective biopsy of any residual mass or scar. The standard of efficacy evaluation was based on the Response Evaluation Criteria in Solid Tumors RECIST Version 1.1 (RECIST 1.1) (29). Because there is currently no unified international diagnostic standard for cCR and near-cCR, the standards in our study were based on the Sao Paulo criteria (30), the criteria in ESMO guidelines (4), and the Memorial Sloan Kettering Regression Schema (31), with fine-tuning according to the actual situation. The specific diagnostic criteria for cCR and near-cCR are in Supplementary Table S1.
The indicators of pathological efficacy after nIT were ypTNM stage and TRG. According to the AJCC system, TRG-0 refers to no residual tumor cells, TRG-1 refers to a single tumor cell or a small group of tumor cells, TRG-2 refers to residual cancer with desmoplastic response, and TRG-3 refers to minimal or no evidence of tumor response (24). The pCR was defined as tumor regression induced by neoadjuvant therapy, with no viable tumor cells in the resected primary tumor sample and all sampled regional lymph nodes (pCR = TRG-0 = ypT0N0M0) (24). Major pathological response (MPR) was considered to be tumor regression with 10% or less pathological residual tumor (MPR = TRG-0 + TRG-1) (24).
The primary survival outcomes were 2-year overall survival (OS), 2-year disease-free survival (DFS), local relapse (LR), and distant metastasis (DM). For DFS, the date of the last nIT treatment in the WW group and the date of surgery in the surgery group was the start date; the date of the last follow-up or the first recurrence, metastasis, or death from any cause was the termination date. OS was measured from the date of the first nIT treatment to the date of death. LR was defined as the presence of rectal adenocarcinoma inside the pelvis at the anastomosis site, presacral area, or pelvic lymph node. DM was defined by rectal adenocarcinoma recurrence that spread to an area or organ outside the pelvis (liver, lung, ovary, distant lymph node, etc.).
Treatment-related adverse events were also recorded. Immune-related adverse events (irAEs) refer to adverse events related to immunotherapy that occurred from the beginning of nIT until 90 days after the last dose of the PD-1 inhibitor, and were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (32). Surgery-related adverse events (srAEs) refer to complications directly or indirectly related to surgery from the day of surgery to 30 days after surgery, and were graded using the Clavien-Dindo grading evaluation standard (33).
Follow-up methods
For patients who underwent TME, follow-up was performed according to international guidelines (3). For patients managed by the WW strategy, a more intensive follow-up protocol was used due to the lack of uniform standards. This follow-up consisted of measurements of serum CEA and DRE every 2 to 3 months during the first two years; T2-weighted and diffusion-weighted MRI of the pelvis, TRUS, and complete colonoscopy every 3 months during the first year, and every 6 months during the subsequent 4 years; and enhanced CT examination of chest and abdomen every 6 months. Biopsy was performed selectively to examine residual nodularity or scarring from the colonoscopy examination, and abnormalities were identified on the cross-sectional imaging. The duration of follow-up was calculated as the time from the last day of the last treatment (last nIT, or aIT, or surgery) until the event of interest or the last follow-up date.
Statistical analysis
Continuous numerical variables were presented as medians and ranges, and compared using an independent samples t-test. Categorical variables were presented as numbers and percentages, and compared using the Chi-square test or Fisher’s exact test. Cumulative DFS, OS, LR, DM were presented using Kaplan-Meier curves, and the WW and pCR groups were compared using the Wilcoxon test. All statistical tests were two-sided, and results were considered statistically significant when the P value was less than 0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences Program (SPSS Inc. Chicago, IL, version 26.0 for Mac).
Results
Characteristics of patients
We identified 20 LARC patients with dMMR/MSI-H who received nIT in one of the four participating institutions from January 2019 to May 2020 (Figure 1). Three patients who achieved cCR and 4 patients who achieved near-cCR were in the WW group and the other 13 patients received TME. Among the 13 patients who underwent TME surgery, 11 patients achieved pCR and were in the pCR group, the other 2 patients had TRG-1 and were excluded from the comparative analysis of LR, DM, 2-year DFS, and 2-year OS.
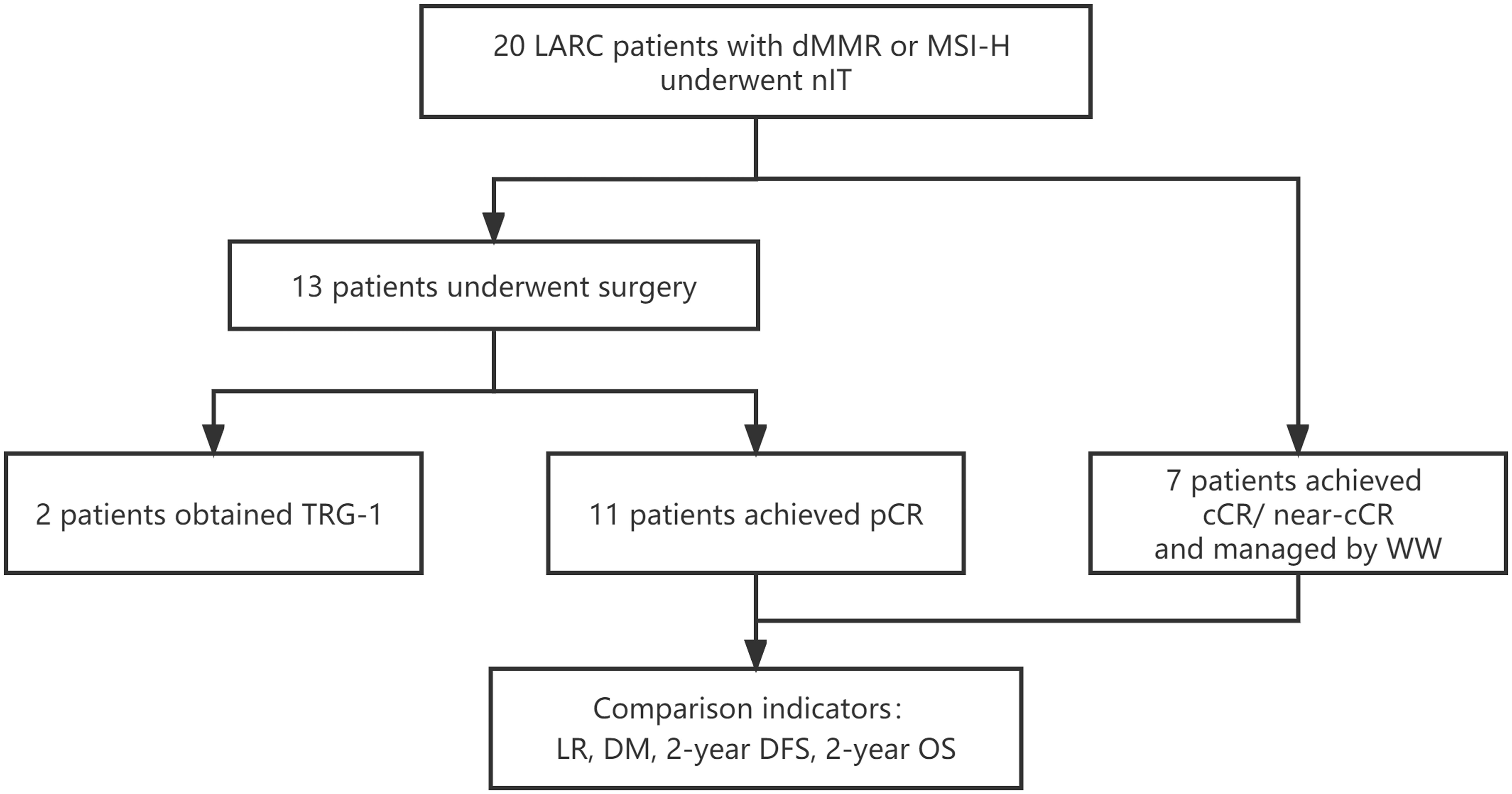
Figure 1 Study profile of nIT in LARC patients with dMMR/MSI-H. cCR, clinical complete response; DFS, disease-free survival; DM, distant metastasis; dMMR, mismatch repair-deficient; LARC, locally advanced rectal cancer; LR, local recurrence; MSI-H, microsatellite instability-high; nIT, neoadjuvant immunotherapy; near-cCR, near clinical complete response; OS, overall survival; pCR, pathological complete response; TRG, tumor regression grade; WW, watch-and-wait.
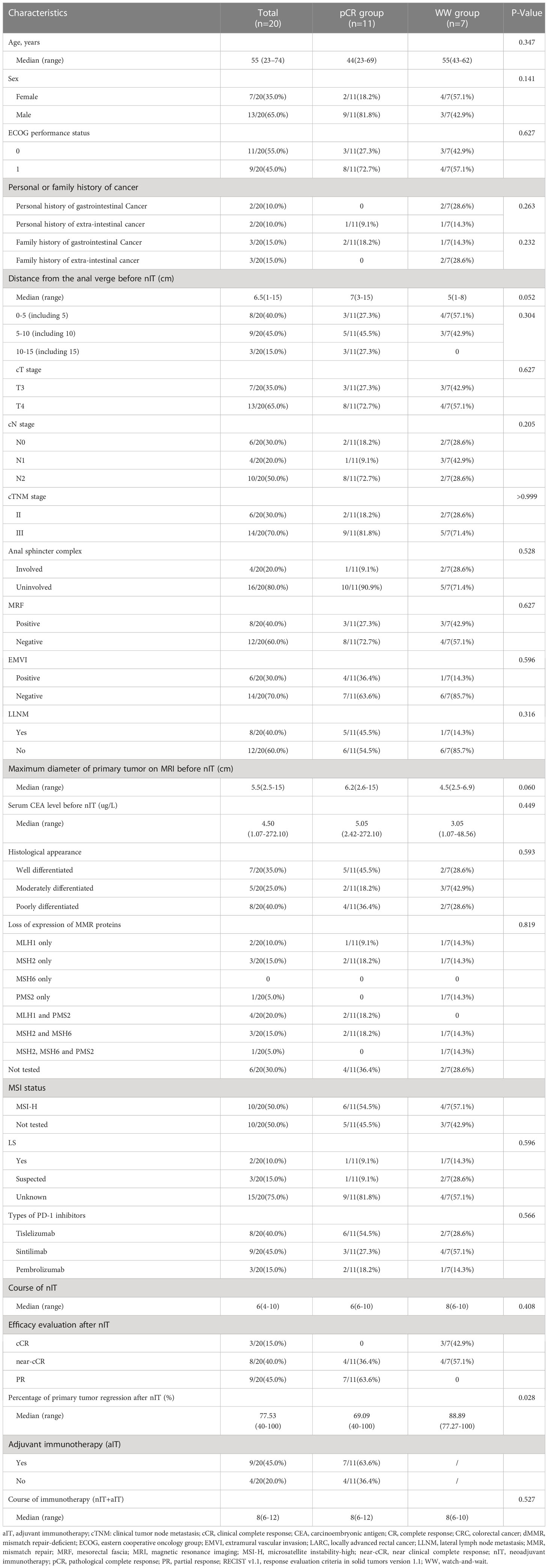
We compared the baseline demographic and clinicopathological characteristics of all 20 patients, the 11 patients in the pCR group, and the 7 patients in the WW group (Table 1). We also recorded the individual details of each patient in the TME group (Table 2) and the WW group (Table 3). Patients in the pCR and WW groups were similar in terms of age, gender, ECOG performance status, personal or family history of cancer, distance of tumor from the anal verge before nIT, cT stage, cN stage, cTNM stage, maximum diameter of the primary tumor on MRI, serum CEA level before nIT, anal complex invasion, mesorectal fascia invasion (MRF+), extramural vascular invasion (EMVI+), and degree of tissue differentiation (all P > 0.05). The median age of all 20 patients was 55 years old (range: 23–74), and the median distance of the tumor from the anal verge before nIT was 6.5 cm. Patients in the WW group were slightly older (55 vs. 44 years, P = 0.347) and had tumors that were located closer to the anal verge (5 vs. 7 cm, P = 0.052), although these differences were not significant.
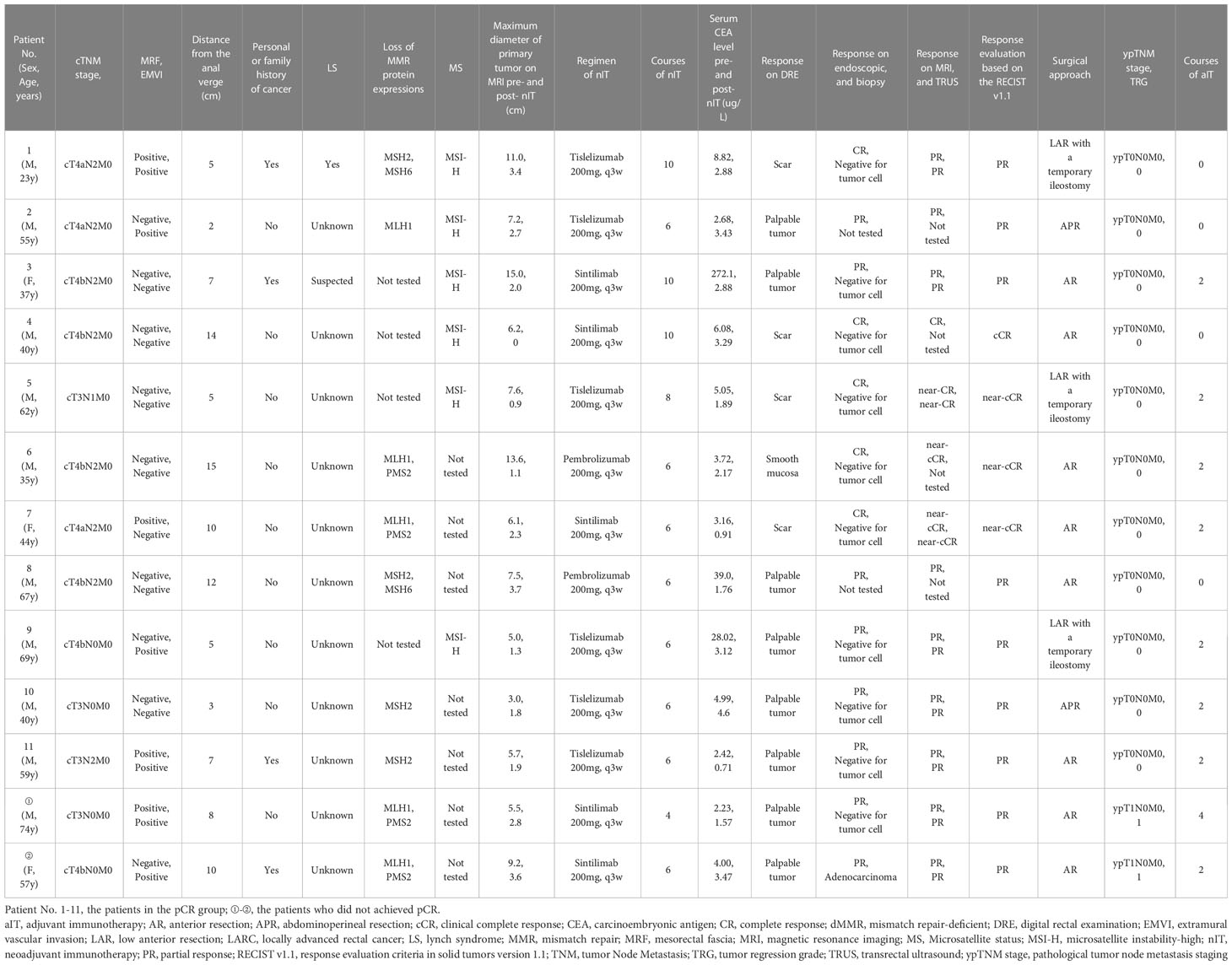
Among all 20 patients, 7 had a family or personal history of malignancy. In the latter group, the 3 patients with a personal and family history of cancer and the 2 with only a family history of gastrointestinal malignancy were suspected to have Lynch syndrome, but only 2 of them received tests for the relevant germline genes and had confirmed Lynch syndrome: patient III in the WW group (Table 3) and patient 1 in the pCR group (Table 3). Patient III in the WW group received surgery and chemotherapy for jejunal well-differentiated adenocarcinoma at the age of 30s and ovarian clear cell carcinoma at the age of 40s, and the mother of this patient died from ovarian cancer; genetic testing results indicated this patient had an exon 1 germline mutation in the MLH1 gene. Patient 1 in the pCR group had a grandmother who died from gastric cancer, and the father of this patient had rectal cancer at the time of this study; genetic testing identified a germline mutation in exon 7 of MSH2 gene.
Analysis of microsatellite status indicated 1 patient had MSI-H based on PCR, and 5 patients had MSI-H based on NGS. IHC results on pre-treatment tumor specimens in 10 patients confirmed dMMR status, and the remaining 4 patients had both dMMR (by IHC) and MSI-H (by NGS or PCR). Among the 14 patients identified as dMMR, one had losses of MSH2, MSH6, and PMS2; 4 had losses of MLH1 and PMS2; 3 had losses of MSH2 and MSH6; 2 had a loss of MLH1; 1 had a loss of PMS2; and 3 had a loss of MSH2. The pCR and WW groups had no statistically significant difference in MMR protein deletions (P = 0.819).
Efficacy of nIT with PD-1 inhibitors and adjuvant therapy
All 20 patients received PD-1 inhibitor monotherapy as the initial treatment (8 patients with tislelizumab, 9 with sintilimab, and 3 with pembrolizumab) and there were no significant differences in the type of drug used in the WW and the pCR groups (Table 1). After completing a median of 6 cycles (range: 4–10) of nIT, the objective response rate (ORR) was 100% (20/20), the cCR rate was 15% (3/20), the near-cCR rate was 40.0% (8/20), and the partial response (PR) rate was 45.0% (9/20) (Table 1). Representative MRI, endoscopic and pathological images of patients with dMMR/MSI-H LARC who achieved cCR and near-cCR after nIT are shown in Figures 2, 3.

Figure 2 Representative radiologic, colonoscopic and pathological response to nIT in one patient with cCR (patient II in Table 3). (A) Sagittal plane MR views of the pelvis: pre-nIT VS post-nIT VS 25.5 months after WW; (B) Axial plane MR views of the pelvis: pre-nIT VS post-nIT VS 25.5 months after WW; (C) Colonoscopy: pre-nIT VS post-nIT VS 25.5 months after WW; (D) Pathology: tumor biospy of pre-nIT (HE x40) VS re-biospy of post-nIT (HE x40) VS re-biospy of 25.5 months after WW (HE x40). cCR, clinical complete response; HE, hematoxylin-eosin; MR, Magnetic resonance; nIT, neoadjuvant immunotherapy.
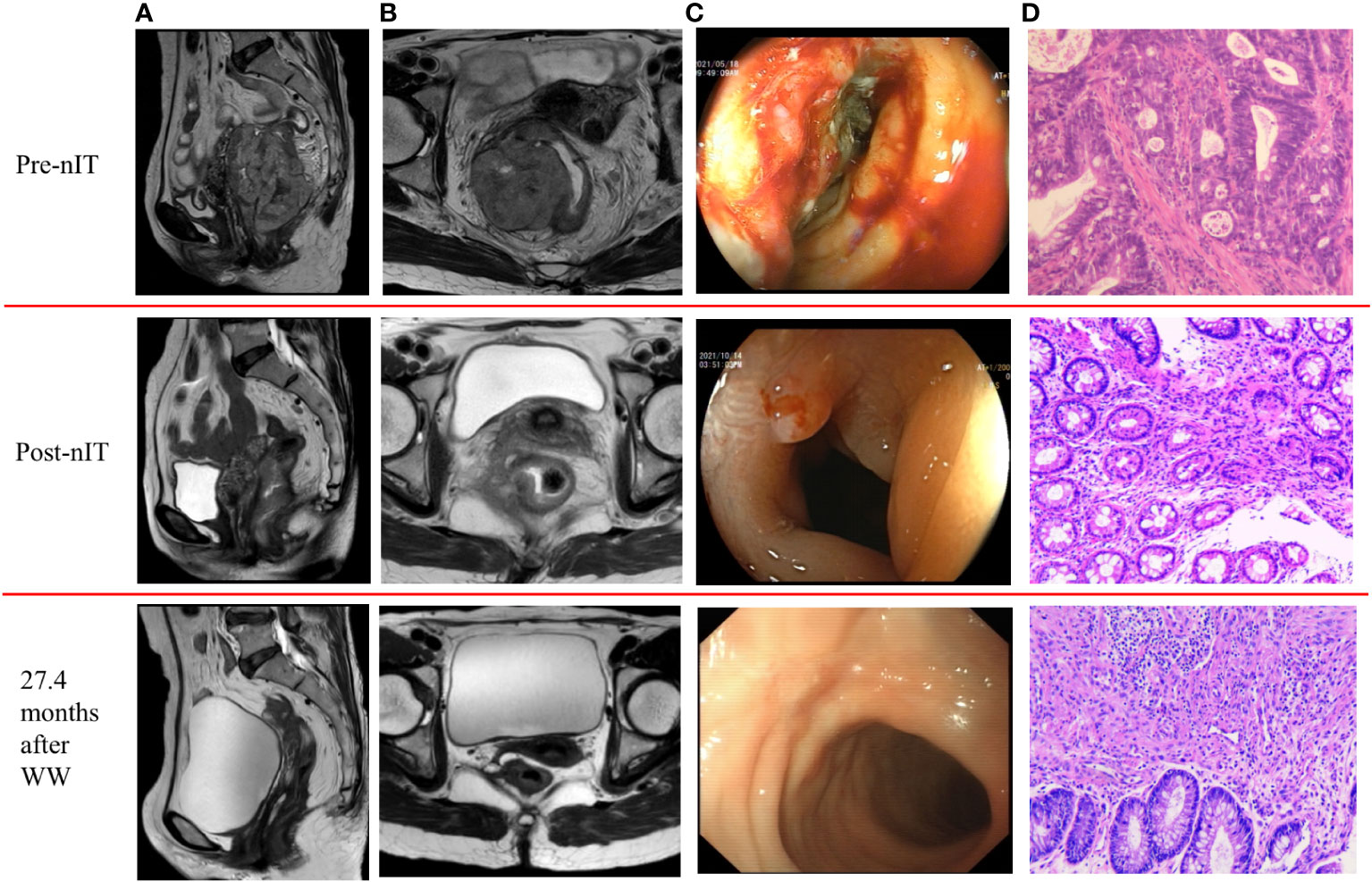
Figure 3 Representative radiologic, colonoscopic and pathological response to nIT in one patient with near-cCR (patient VI in Table 3). (A) Sagittal plane MR views of the pelvis: pre-nIT VS post-nIT VS 27.4 months after WW; (B) Axial plane MR views of the pelvis: pre-nIT VS post-nIT VS 27.4 months after WW; (C) Colonoscopy: pre-nIT VS post-nIT VS 27.4 months after WW; (D) Pathology: tumor biospy of pre-nIT (HE x40) VS re-biospy of post-nIT (HE x40) VS re-biospy of 27.4 months after WW (HE x40). HE, hematoxylin-eosin; MR, magnetic resonance; nIT, neoadjuvant immunotherapy;near-cCR, near clinical complete response.
The pCR and WW groups had no significant difference in the median number of cycles of nIT (6 vs. 8, P = 0.408), however, patients in the WW group exhibited greater radiographic regression in the primary lesion (P = 0.028; Table 1 and Figure 4). For all 20 patients, the WW group, and the pCR group, there was a median of 3 cycles of nIT (range: 2–6) from treatment initiation to PR, corresponding to 2.25 months (range: 1.5–4.5); and there was a median of 8 cycles of nIT (range: 6–10) needed to achieve cCR or near-cCR.
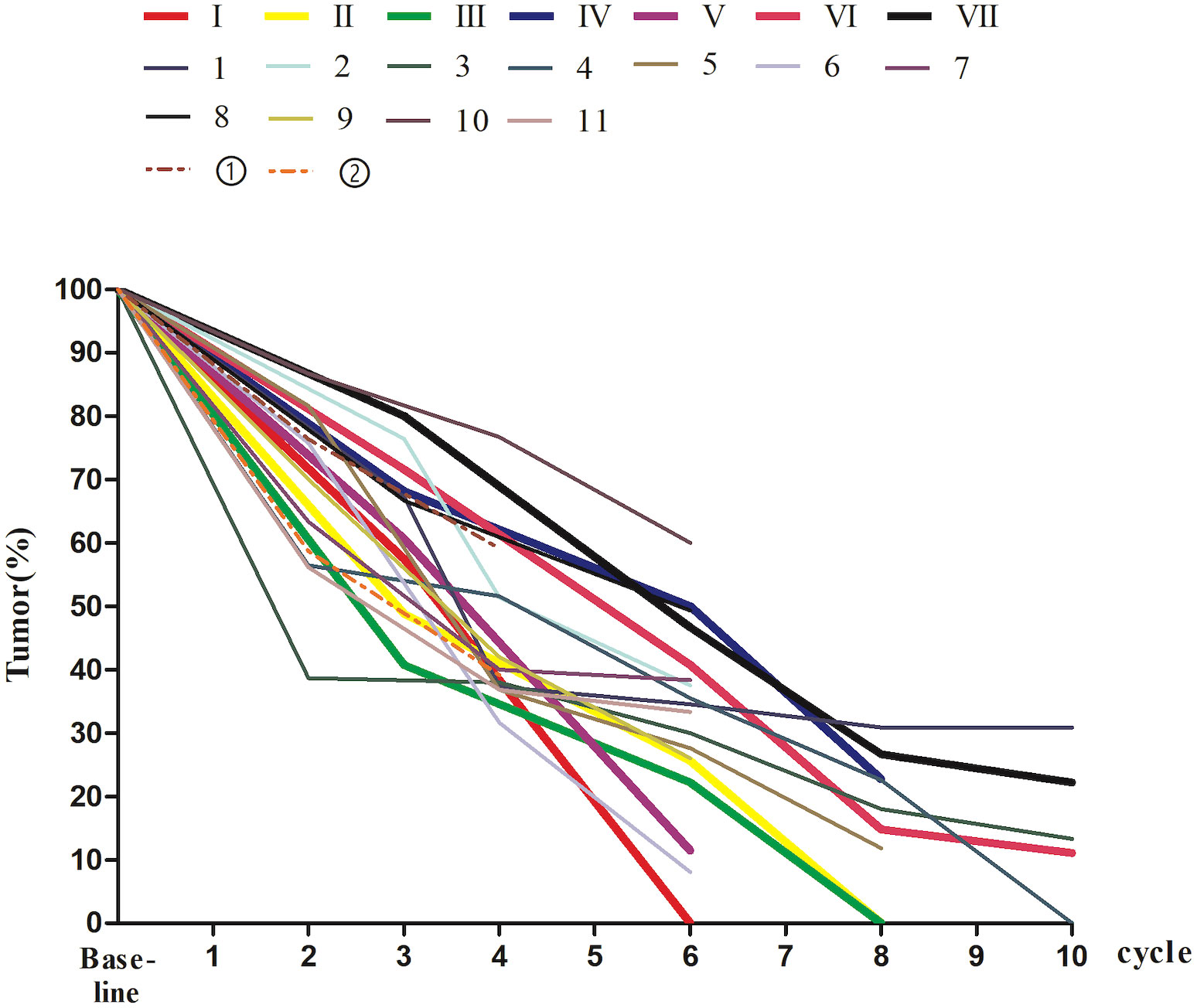
Figure 4 The percentage of tumor size on MRI at baseline and during nIT in 20 dMMR/MSI-H LARC patients. I-VII, the patients in the WW group; 1-11, the patients in the pCR group; ①-②, the patients who did not achieved pCR. dMMR, mismatch repair-deficient; LARC, locally advanced rectal cancer; MRI, magnetic resonance imaging; MSI-H, microsatellite instability-high; nIT, neoadjuvant immunotherapy; pCR, pathological complete response; WW, watch-and-wait.
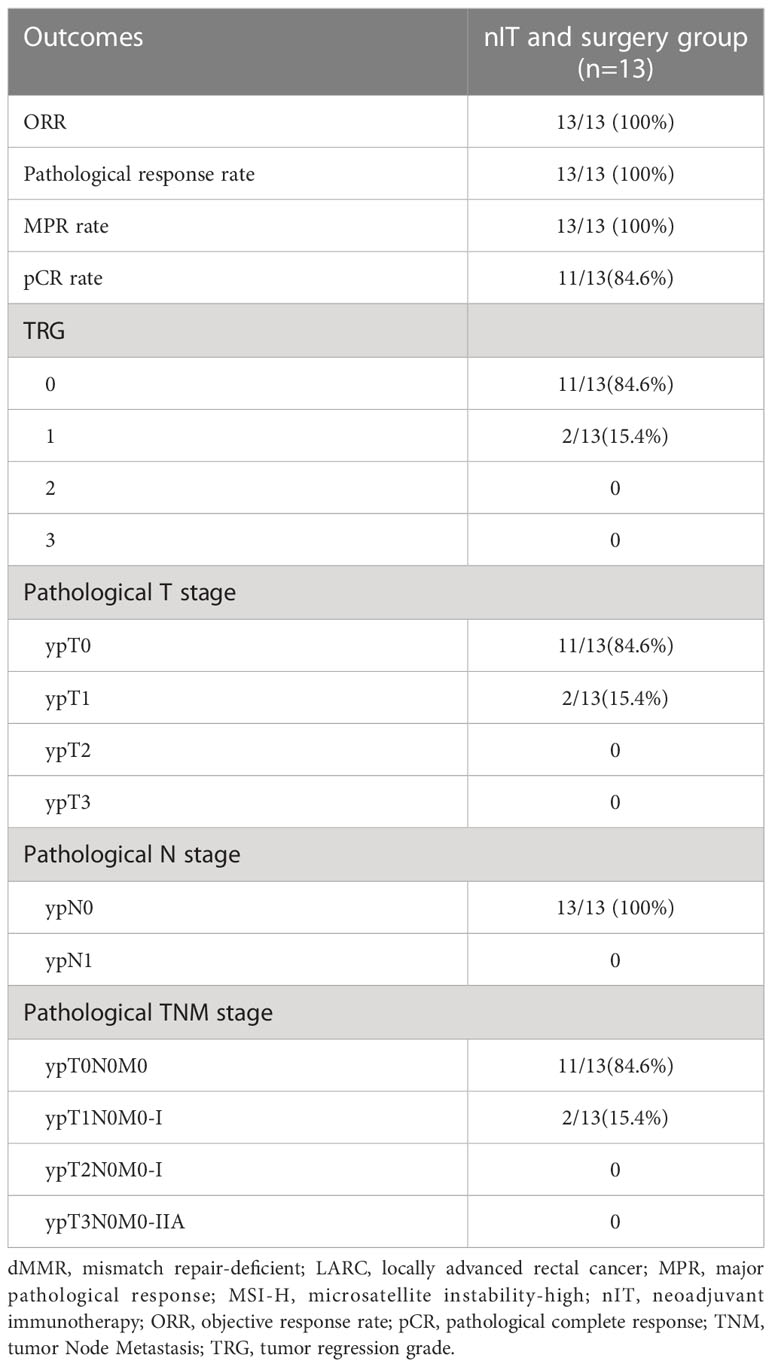
For the 13 patients who underwent TME after nIT (9 with PR, 1 with cCR, 3 with near-cCR), the ORR, pathological response rate, and MPR were all 100% (13/13), and the pCR was 84.6% (11/13). Typical images from MRI, post-nIT resection specimens, and pathologic response of patients with dMMR/MSI-H LARC who achieved a pCR are shown in Figure 5. The two patients who did not achieve pCR had TRG-1 and pathological stage of ypT1N0M0 (Table 4). Notably, 3 patients achieved a pCR after 6 to 10 cycles of nIT, despite their very large tumors (>10 cm) and very late clinical stage (cT4N2M0) (Table 2).
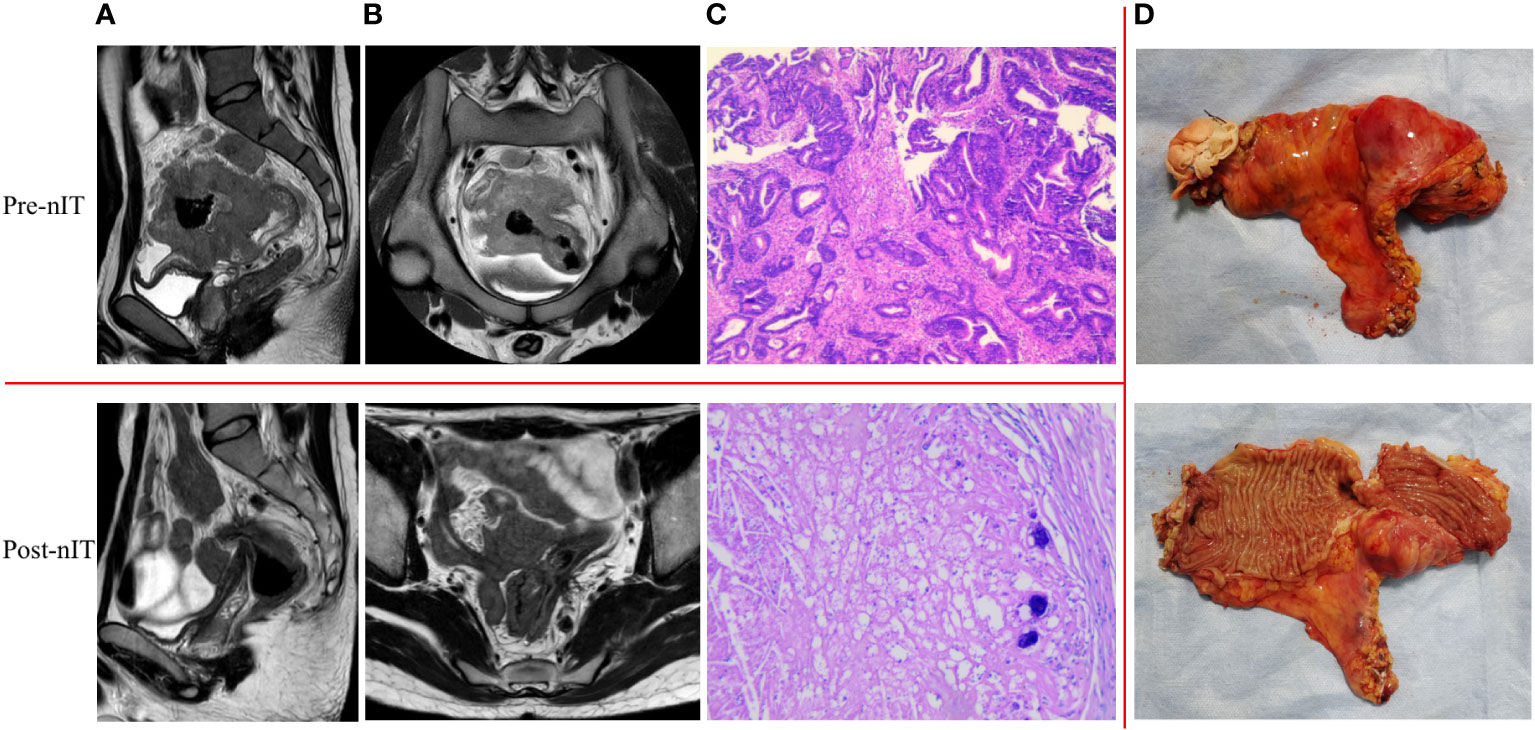
Figure 5 Representative radiologic, resection specimen and pathological response to nIT in one patient with pCR (patient 1 in Table 2). (A) Sagittal plane MR views of the pelvis: pre- VS post- nIT; (B) Axial plane MR views of the pelvis: pre- VS post- nIT; (C) Pathology: tumor biospy of pre-nIT (HE x40) VS postoperative specimen of post-nIT (HE x40); (D) Specimen: Resection specimen of post-nIT. HE, hematoxylin-eosin; MR, magnetic resonance; nIT, neoadjuvant immunotherapy; pCR, pathological complete response.
Adjuvant anti-PD-1 monotherapy was administered to 69.2% (9/13) of patients with surgery, including 7 patients with pCR and 2 with TRG-1. Most of them received 2 cycles, only 1 patient with TRG-1 who completed just 4 cycles of nIT received 4 cycles of aIT. None of the patients in the WW group continued to use PD-1 inhibitors after achieving cCR or near-cCR. There was a median of 8 cycles (6 months) of immunotherapy (nIT + aIT) in all 20 patients, in the pCR group, and in the WW group (Table 1).
Treatment-related adverse events and enterostomy
We evaluated treatment-related adverse events (including those related to immunotherapy and surgery) and enterostomy (Tables 2, 3). During the neoadjuvant and adjuvant phases, the incidence of irAEs was 35.0% (7/20) for all 20 patients, 45.5% (4/11) in the pCR group, and 28.6% (2/7) in the WW group (P = 0.627). Most of the irAEs were grade 1, but one patient developed a grade 2 hypothyroidism that was significantly relieved by low-dose thyroxine supplementation. There were no grade 3 or higher irAEs (Table 5).
Among the 13 patients who received TME, the median time from the last nIT to surgery was 25 days (18–42), and none of these patients had a delay of surgery due to an irAE. All 13 of these patients achieved R0 resection: 2 (15.4%) received abdominoperineal resection (APR) and 11 (84.6%) received sphincter-saving surgery. In the latter group, 3 (23.1%) patients received low anterior resection (LAR) with a temporary ileostomy. There were srAEs in 3 (23.1%) of these patients: a grade 1 surgical incision with poor healing, a grade 1 postoperative anastomotic bleeding, and a grade 2 anastomotic leak. No patient experienced perioperative mortality or severe surgery-related morbidity requiring re-operation (Table 6).
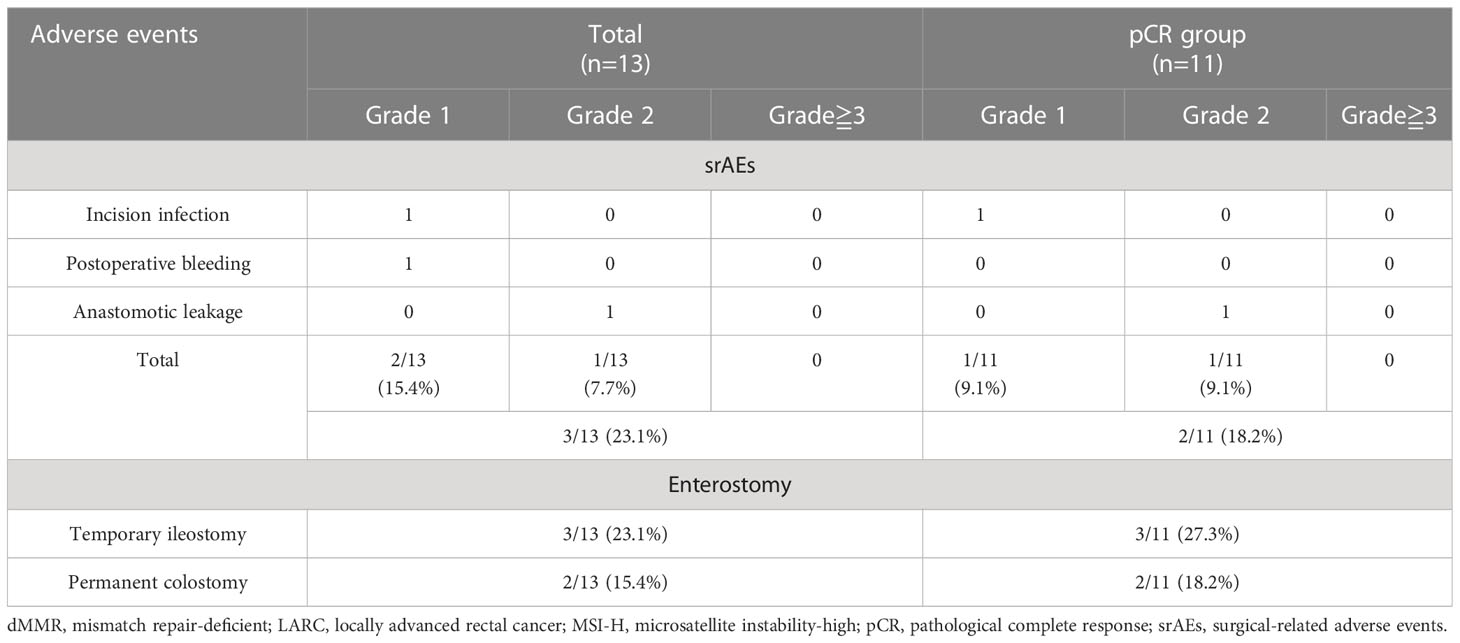
Table 6 Surgical-related adverse events (srAEs) and enterostomy of patients with dMMR/MSI-H LARC ungerwent surgery.
Recurrence and survival outcomes
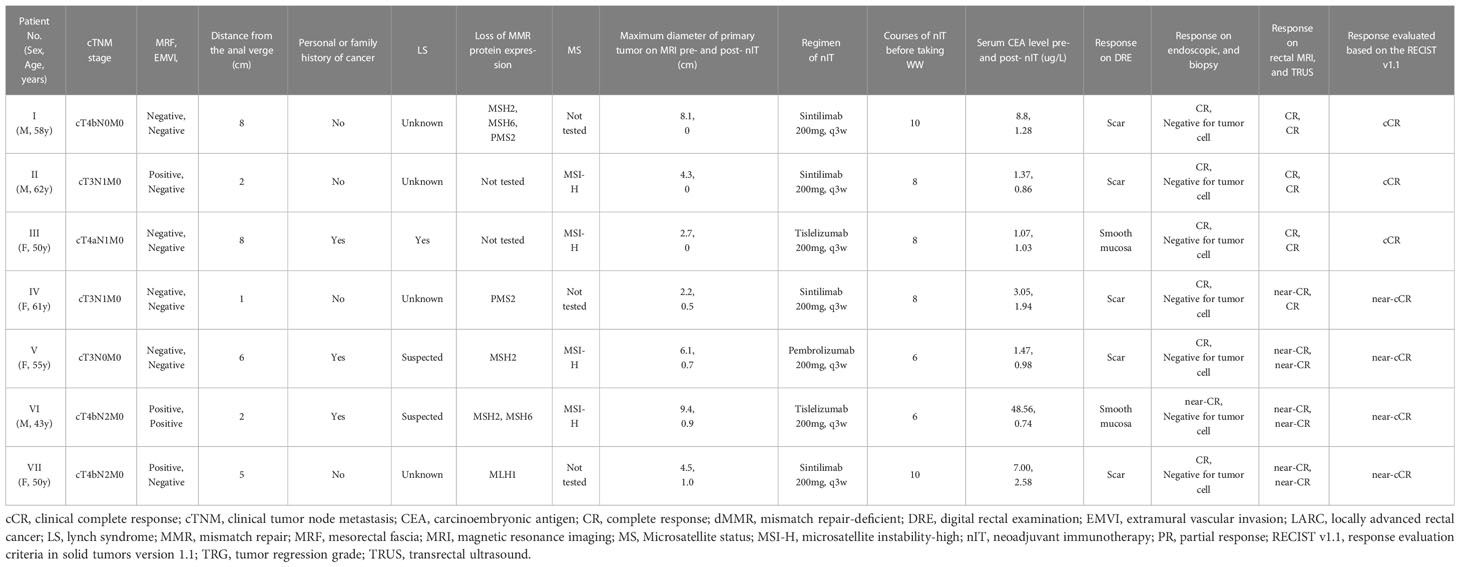
The median follow-up time was 24.35 months (range: 16.4–29.9) in all 20 patients, 24.5 months (range: 16.4–29.9) in the pCR group, and 25.0 months (range: 20.5–29.0) in the WW group (P = 0.633; Table 7). None of the 20 patients (including the 2 with TRG-1) experienced LR, DM, or death as of the last follow-up date (June 15, 2022). Thus, the pCR group and WW group had 2-year DFS rates and 2-year OS rates of 100%, and LR rates and DM rates of 0% (Table 7 and Figure 6). Remarkably, 4 patients who adopted a WW strategy after achieving near-cCR and did not continue anti-PD-1 therapy did not experience LR or DM, even though some of them had high-risk factors at baseline, such as EMVI+, MRF+, and T4 stage (Table 3).
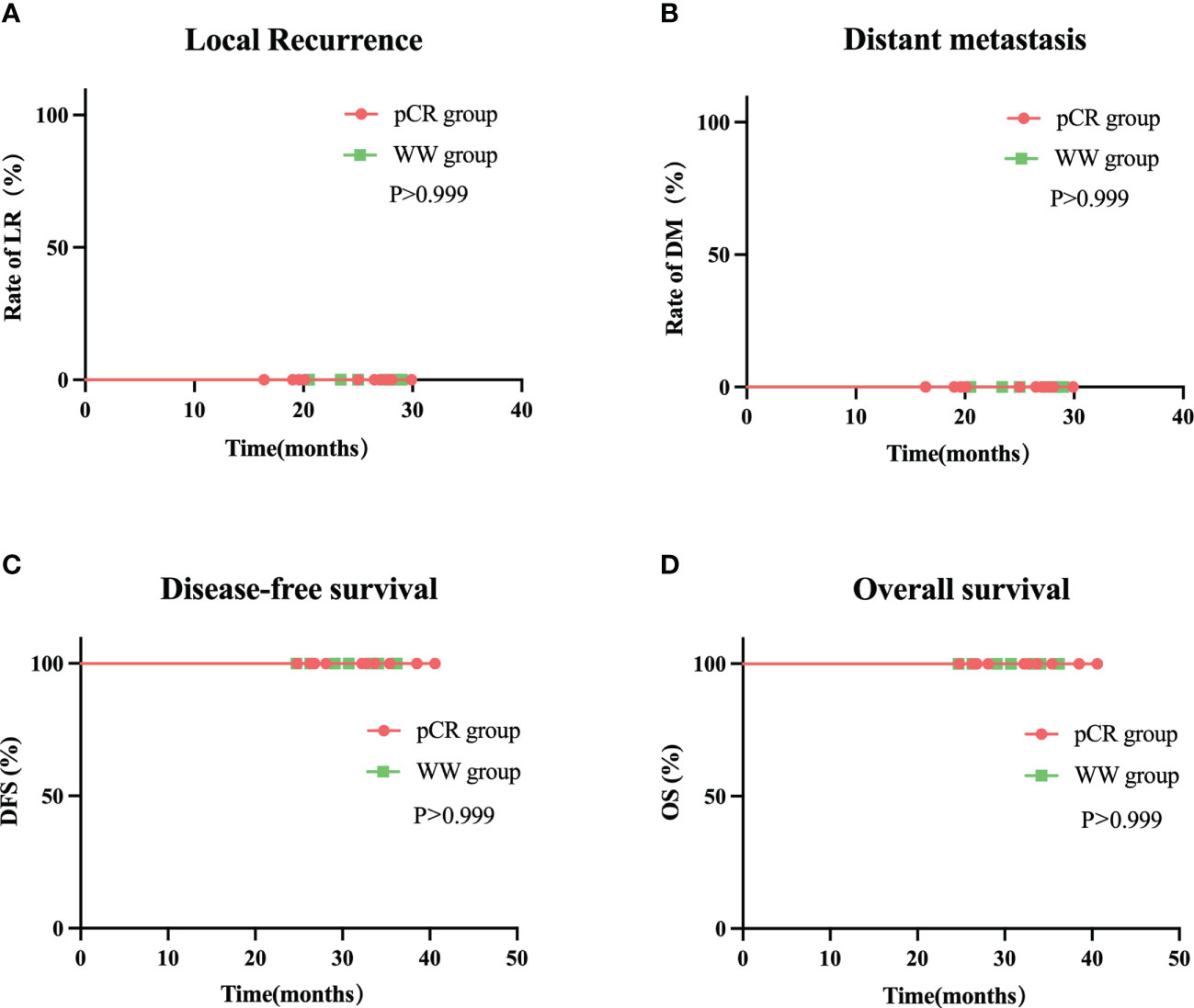
Figure 6 Rate of local recurrence (A) distant metastasis (B) disease-free survival (C) and overall survival (D) in WW group and pCR group during follow-up.
Discussion
Our real-world study examined a multicenter cohort of LARC patients with dMMR/MSI-H who were treated with neoadjuvant PD-1 inhibitor monotherapy. During the 2-year follow-up period, 100% (7/7) of patients who were managed with a WW strategy after achieving a cCR or near-cCR had a comparable oncological safety profile as those who accepted TME surgery. More importantly, patients in the WW group did not experience a reduced quality of life associated with surgical complications, enterostomies, or deterioration of the bowel, urinary system, or sexual function. Moreover, in the WW group, the 4 patients with near-cCR achieved the same rate of organ sparing and oncological safety as the other 3 patients with cCR (Table 3), similar to the results of the OPERA study (11). Thus, for LARC patients with dMMR/MSI-H who achieve cCR or near-cCR after nIT, the WW strategy is a safe and beneficial option.
Previous studies indicated that the 2-year LR rate of LARC patients who achieved cCR and adopted a WW strategy after nCRT was 19 to 25% (9, 34). In our study, a WW approach, even for patients with near-cCR or high-risk factors for LR and DM (EMVI+, MRF+, or T4 stage), led to a 2-year LR rate and DM rate of 0%. This remarkable efficacy may be because the nIT can convert the high level of tumor antigens produced by the primary tumor with dMMR/MSI-H into “autologous vaccines”, which activate and recruit more tumor-specific T cells and promote the formation of long-term immune memory, rather than simply killing tumor cells. This kind of systemic and persistent immunity against tumors enables greater clearance of micrometastases that cannot be eliminated by surgery or radiotherapy and reduces the rate of LR and DM (35–37).
The WW strategy described herein is indeed promising, but accurate assessment of cCR and near-cCR remains a difficult problem. Due to the lack of more sensitive assessment methods, we used the same diagnostic criteria for cCR and near-cCR as used in patients after traditional nCRT. However, the mechanism of action and response of immunotherapy are markedly different from those of conventional therapy with cytotoxic drugs and radiation (38). These could lead to inconsistent clinical and pathological evaluations, as indicated by an underestimated efficacy of immunotherapy based on imaging. In this study, although the imaging results demonstrated that the 3 patients with near-cCR and the 7 patients with PR in the pCR group still had residual mass, the pathology results revealed that none of their surgical resection specimens had residual tumor cells, but instead consisted of massive inflammatory cells or mucus lakes (Table 2). The recent PICC study found that 28 of 35 patients who were evaluated as PR preoperatively had pCR based on postoperative pathology (23). This inconsistency also occurred in studies of nIT for other solid malignancies (39, 40). A possible explanation is that radiology cannot easily distinguish masses consisting of inflammatory cells, necrotic tissue, and/or fibrous tissues from masses consisting of tumor cells. This issue appears to challenge the routine use of established morphological-based response evaluation criteria.
Fortunately, tests other than conventional imaging examinations have gradually been used for efficacy evaluation in patients with malignancy. For example, 18F-fluorodeoxyglucose-positron emission computed tomography (18F-FDG-PET/CT), a valuable tool that combines anatomic morphologic imaging with functional metabolic imaging, can help to distinguish malignant and non-malignant masses. Goldfarb et al. first proposed the immune PET response criteria in solid tumors (iPERCIST) in 2019. They reported that this criterion could compensate for about 39% of the response underestimated by the RECIST 1.1 criteria in non-small cell lung cancer patients who received ICIs (41). In the 2022 study of Cercek et al., 12 rectal cancer patients with dMMR/MSI-H who were evaluated as cCR by traditional anatomical imaging combined with 18F-FDG-PET/CT after 6 months of neoadjuvant dostarlimab treatment and adopted a WW strategy did not develop recurrence during the 6 to 25-month follow-up period (21). In addition, other studies found that changes in the level of circulating tumor DNA (ctDNA) could be used to predict response to immunotherapy (42, 43). Bratman et al. reported a 100% OS rate of patients with solid tumors who had ctDNA elimination after pembrolizumab treatment during a median follow-up time of 25.4 months (43). Hence, a regimen that combines radiological, metabolic, and hematological parameters might improve the accuracy of the efficacy evaluations of immunotherapy, and this could facilitate early identification of patients who would benefit most from a WW strategy and prevent over-treatment.
Furthermore, an underestimation of the efficacy of immunotherapy from imaging suggests that rectal cancer patients with dMMR/MSI-H who were treated with ICIs may have achieved pCR before being evaluated as near-cCR, cCR, or even PR. Another consideration is that patients who receive immunotherapy have unique remission patterns, such as delayed response, pseudo-progression (38), and long-lasting efficacy. We therefore suggest that LARC patients who are considering a WW strategy after nIT — even if imaging does not yet indicate cCR — should be offered the option of more time for observations or more cycles of ICIs followed by re-evaluation, rather than early surgical resection. Transanal local tumor resection, another organ-sparing option, may be preferred to proctectomy for rectal cancer patients who have a persistent clinical stage of ycT1N0M0 after nIT.
In terms of optimal efficacy, our results demonstrated a 90% CR rate (55% pCR, 15% cCR, and 20% near-cCR) was achieved after 6 cycles (range 4–10) of a neoadjuvant PD-1 inhibitor. This is better than reported in the NICHE study (17), the NICHE-2 study (18), and the PICC study (23), but worse than in the study of Cercek et al. (21). We believe that these differences in efficacy can be partly explained by differences in the dose and duration of the ICIs. In particular, the NICHE study and NICHE-2 study reported pCR rates of dMMR/MSI-H colon cancer patients were 69% and 67% after one dose of ipilimumab (1 mg/kg on day 1) and two doses of nivolumab (3 mg/kg on days 1 and 15). The PICC study reported the pCR rate in LARC patients with dMMR/MSI-H after 6 cycles of toripalimab (3 mg/kg) was 88% with celecoxib and 65% without celecoxib. However, the Cercek et al. study of LARC patients with dMMR/MSI-H reported the cCR rate was 100% after neoadjuvant treatment with 9 cycles of dostarlimab (500 mg every 3 weeks for 6 months).
Another consideration is that these previous studies examined tumors at different sites. The Cercek et al. (21). study and our study examined LARC patients and achieved CR rates of 90% or more; this is higher than in the NICHE study (17) and the NICHE-2 study (18), which examined patients with colon cancer. The PICC study reported a slightly higher pCR rate in rectal cancer patients (83.33%) than in colon cancer patients (78.57%) (23), and Liu et al. reported similar results (pCR rate, rectal cancer: 100%, colon cancer: 50%) (44). These differences in efficacy suggest that rectal cancer patients with dMMR/MSI-H appear to benefit more from nIT than colon cancer patients with dMMR/MSI-H. There is also evidence of differences in the distribution of gut microbiota in different parts of the colorectum. In particular, rectal cancer patients have significantly more diverse gut microbiota than colon cancer patients (45, 46). Furthermore, several gut microbes can affect the efficacy of ICIs (39, 47), such as Fusobacterium nucleatum, which induces various immune responses in CRCs with distinct microsatellite states, and can enhance the efficacy of ICIs (48, 49).
However, LARC patients with dMMR/MSI-H achieved a 75% CR rate (lower than our study) after a median of 8 cycles of nIT with sintilimab (higher than our study), and a case of primary resistance to ICIs was reported in a recent study (50). It indicates that factors other than those mentioned above influence patient efficacy, among which tumor mutation burden (TMB), gene status and immune microenvironment are several research hotspots. For instance, dMMR/MSI-H CRC patients with low TMB, immunosuppressive tumor microenvironment, or mutation in the PTEN gene or PIK3CA gene are resistant to ICIs (51, 52), while pMMR/MSS CRC patients with a mutation in POLE or POLD1 gene are sensitive to ICIs (53). Therefore, despite nIT being highly effective for CRC patients with dMMR/MSI-H, the inclusion of the analysis of the molecular profile as well as the immune contexture remains imperative.
It is currently uncertain whether continuous ICI treatment is required for CRC patients who achieve a cCR or pCR after nIT. In the NICHE study (17) and the NICHE-2 study (18), aIT was not offered to 69% (22/32) and 67% (72/107) of dMMR colon cancer patients who achieved pCR. The 12 LARC patients with dMMR/MSI-H in the study of Cercek et al. (21). who achieved a cCR also did not continue ICI treatment after adopting a WW strategy. Notably, patients in all three previous studies who attained pCR or cCR did not develop LR or DM during the follow-up period (median: 25 months for NICHE, 13 months for NICHE-2, and 12 months for Cercek et al.). However, the PICC study recommended adjuvant use of toripalimab with or without celecoxib for all patients, regardless of whether they attained pCR, until completion of 6 months of perioperative anti-PD-1 therapy (23). In our study, the median time from treatment initiation to CR was 5.25 months (range 4.5–7.5), the median time of the entire perioperative period was 6 months (range 4.5–9.0), and we observed no recurrence or metastasis during the 2-year follow-up. The value of aIT is currently unknown, indicating the need for large prospective studies.
Several studies reported that ctDNA is associated with an increased risk of recurrence (54, 55). This ctDNA reflects minimal residual disease (MRD), which is responsible for the most postoperative recurrences (56). Henriksen et al. studied patients with stage III colon cancer and compared ctDNA-negative and ctDNA-positive patients after surgery and adjuvant chemotherapy. They found a 7-fold increased risk of recurrence for patients who were ctDNA-positive after surgery and a 50-fold increased risk of recurrence for patients who were ctDNA-positive after adjuvant therapy, while patients with continuous negative ctDNA during and after adjuvant therapy had no recurrence (57). Therefore, a ctDNA-based MRD assay may help to identify patients with high risk of relapse, and provide more personalized suggestions for adjuvant treatment and surveillance (54, 57). Three ongoing prospective clinical studies (NCT04761783; NCT05198154; NCT04636047) are examining the use of ctDNA-based MRD assays for guiding immunotherapy.
Finally, nIT for LARC is still in its infancy, and there is no unified standard regarding the therapeutic dose, course, efficacy evaluation criteria, or the need for aIT. Although outcomes appear promising, this study was limited by its retrospective design, small sample size, and short follow-up time. Firstly, a WW strategy after obtaining cCR or near-cCR following nIT is not currently a standard therapy, and the decision to circumvent proctectomy was mostly driven by an individual patient’s strong desire for preservation of organ function and avoidance of enterostomy. This is why there were only 7 cases in our WW group. Secondly, the median follow-up time of our study was only 24.35 months. However, this was the longest follow-up time of any study that examined the WW strategy after nIT. In addition, due to economic and other reasons, only two patients in our study had a detection of germline genes and confirmed a diagnosis of Lynch syndrome. Given the familial heritability of Lynch syndrome and the potential for multiple primary malignancies, we will continue to encourage subjects who are young or suspected of Lynch syndrome to perform germline genetic testing for better long-term management and follow-up.
Conclusions
In conclusion, our study verified the feasibility and safety of a WW strategy for LARC patients with dMMR/MSI-H who achieved cCR or near-cCR after nIT. Our results suggest that a WW strategy for these patients could help to preserve sphincter function and improve long-term survival. Longer follow-up studies and prospective trials are needed to evaluate this promising treatment option.
Data availability statement
All datasets supporting the conclusions of this article are included within the article and its Supplementary files.The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics statement
This retrospective study was conducted according to the Declaration of Helsinki, and approved to waive informed patient consent by the ethics review boards of the Yunnan Cancer Hospital (Third Affiliated Hospital of Kunming Medical University) (approval number KYLX2022053) because it was an observational and non-interventional study.
Author contributions
Drafting the work and/or revising it critically: RY, TW and JY. Final approval of the version to be published: PD, XZ, YL, and RY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Scientific Research Fund of Yunnan Provincial Education Department (2022J0227 to XZ), and the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University (202201AY070001-149 to XZ).
Acknowledgments
We thank the patients and families who made this study possible, clinical study teams, and Medjaden Inc. All authors made efforts and contributions to this article. Writing and editorial assistance were provided by Medjaden Inc.
Conflict of interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer WX declared a shared affiliation with the authors JY and PD to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1182299/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Shi JF, Wang L, Ran JC, Wang H, Liu CC, Zhang HZ, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: overall design and results from a multicenter retrospective epidemiologic survey. Cancer (2021) 127(11):1880–93. doi: 10.1002/cncr.33445
3. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(10):1139–67. doi: 10.6004/jnccn.2022.0051
4. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv263. doi: 10.1093/annonc/mdy161
5. Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol (2010) 11(3):241–8. doi: 10.1016/S1470-2045(09)70381-X
6. Wilkins S, Haydon A, Porter I, Oliva K, Staples M, Carne P, et al. Complete pathological response after neoadjuvant long-course chemoradiotherapy for rectal cancer and its relationship to the degree of T3 mesorectal invasion. Dis Colon Rectum (2016) 59(5):361–8. doi: 10.1097/DCR.0000000000000564
7. Wallner C, Lange MM, Bonsing BA, Maas CP, Wallace CN, Dabhoiwala NF, et al. Causes of fecal and urinary incontinence after total mesorectal excision for rectal cancer based on cadaveric surgery: a study from the cooperative clinical investigators of the Dutch total mesorectal excision trial. J Clin Oncol (2008) 26(27):4466–72. doi: 10.1200/JCO.2008.17.3062
8. Hendren SK, O'Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg (2005) 242(2):212–23. doi: 10.1097/01.sla.0000171299.43954.ce
9. Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, et al. Assessment of a watch-and-Wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol (2019) 5(4):e185896. doi: 10.1001/jamaoncol.2018.5896
10. Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol (2011) 29(35):4633–40. doi: 10.1200/JCO.2011.37.7176
11. Myint AS, Thamphya B, Gerard J-P. Does non-TME surgery of rectal cancer compromise the chance of cure? preliminary surgical salvage data from OPERA phase III randomized trial. J Clin Oncol (2021) 39(3_suppl):12–. doi: 10.1200/JCO.2021.39.3_suppl.12
12. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol (2010) 28(20):3219–26. doi: 10.1200/JCO.2009.27.1825
13. Seligmann JF, Group FC. FOxTROT: neoadjuvant FOLFOX chemotherapy with or without panitumumab (Pan) for patients (pts) with locally advanced colon cancer (CC). J Clin Oncol (2020) 38(15_suppl):4013–. doi: 10.1200/JCO.2020.38.15_suppl.4013
14. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
15. Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol (2022) 33(9):929–38. doi: 10.1016/j.annonc.2022.05.519
16. Diaz LA Jr., Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol (2022) 23(5):659–70. doi: 10.1016/S1470-2045(22)00197-8
17. Verschoor YL, Jvd B, Beets G, Sikorska K, Aalbers A, Av L, et al. Neoadjuvant. nivolumab, ipilimumab, and celecoxib in MMR-proficient and MMR-deficient colon cancers: final clinical analysis of the NICHE study. J Clin Oncol (2022) 40(16_suppl):3511–. doi: 10.1200/JCO.2022.40.16_suppl.3511
18. Chalabi M, Verschoor YL, van den Berg J, Sikorska K. Neoadjuvant immune checkpoint. inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study [abstract]. Ann Oncol (2022) 33(suppl_7):Abstract LBA7. doi: 10.1016/j.annonc.2022.08.016
19. Papke DJ Jr., Yurgelun MB, Noffsinger AE, Turner KO, Genta RM, Redston M. Prevalence of mismatch-repair deficiency in rectal adenocarcinomas. N Engl J Med (2022) 387(18):1714–6. doi: 10.1056/NEJMc2210175
20. Ye SB, Cheng YK, Zhang L, Zou YF, Chen P, Deng YH, et al. Association of mismatch. repair status with survival and response to neoadjuvant chemo(radio)therapy in rectal cancer. NPJ Precis Oncol (2020) 4:26. doi: 10.1038/s41698-020-00132-5
21. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1. blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med (2022) 386(25):2363–76. doi: 10.1056/NEJMoa2201445
22. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI⁃H locally advanced colorectal cancer: a single-center real-world study. Front Immunol (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
23. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol (2022) 7(1):38–48. doi: 10.1016/S2468-1253(21)00348-4
24. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
25. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116
26. Saeed OAM, Mann SA, Luchini C, Huang K, Zhang S, Sen JD, et al. Evaluating mismatch repair deficiency for solid tumor immunotherapy eligibility: immunohistochemistry versus microsatellite molecular testing. Hum Pathol (2021) 115:10–8. doi: 10.1016/j.humpath.2021.05.009
27. Zito Marino F, Amato M, Ronchi A, Panarese I, Ferraraccio F, De Vita F, et al. Microsatellite status detection in gastrointestinal cancers: PCR/NGS is mandatory in Negative/Patchy MMR immunohistochemistry. Cancers (Basel) (2022) 14(9):2204. doi: 10.3390/cancers14092204
28. Zhu L, Huang Y, Fang X, Liu C, Deng W, Zhong C, et al. A novel and reliable method to detect microsatellite instability in colorectal cancer by next-generation sequencing. J Mol Diagn (2018) 20(2):225–31. doi: 10.1016/j.jmoldx.2017.11.007
29. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
30. Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum (2013) 56(10):1109–17. doi: 10.1097/DCR.0b013e3182a25c4e
31. Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer (2015) 15:767. doi: 10.1186/s12885-015-1632-z
32. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common. terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) (2021) 112(1):90–2. doi: 10.1016/j.ad.2019.05.009
33. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
34. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): an international multicentre registry study. Lancet (2018) 391(10139):2537–45. doi: 10.1016/S0140-6736(18)31078-X
35. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol (2019) 16(6):361–75. doi: 10.1038/s41575-019-0126-x
36. O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res (2019) 25(19):5743–51. doi: 10.1158/1078-0432.CCR-18-2641
37. Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov (2016) 6(12):1382–99. doi: 10.1158/2159-8290.CD-16-0577
38. Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol (2019) 30(3):385–96. doi: 10.1093/annonc/mdz003
39. Cascone T, William WN Jr., Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
40. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med (2018) 24(11):1655–61. doi: 10.1038/s41591-018-0198-0
41. Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res (2019) 9(1):8. doi: 10.1186/s13550-019-0473-1
42. Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol (2017) 28(8):1996–2001. doi: 10.1093/annonc/mdx212
43. Bratman SV, Yang SYC, Iafolla MAJ, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer (2020) 1(9):873–81. doi: 10.1038/s43018-020-0096-5
44. Liu DX, Li DD, He W, Ke CF, Jiang W, Tang JH, et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology (2020) 9(1):1711650. doi: 10.1080/2162402X.2020.1711650
45. Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA (2014) 111(51):18321–6. doi: 10.1073/pnas.1406199111
46. Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut (2017) 66(4):633–43. doi: 10.1136/gutjnl-2015-309595
47. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359(6371):97–103. doi: 10.1126/science.aan4236
48. Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res (2018) 6(11):1327–36. doi: 10.1158/2326-6066.CIR-18-0174
49. Gao Y, Bi D, Xie R, Li M, Guo J, Liu H, et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct Target Ther (2021) 6(1):398. doi: 10.1038/s41392-021-00795-x
50. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol (2023) 8(5):422–31. doi: 10.1016/S2468-1253(22)00439-3
51. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, et al. A low. tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res (2021) 27(13):3714–24. doi: 10.1158/1078-0432.CCR-21-0401
52. Conciatori F, Bazzichetto C, Falcone I, Ciuffreda L, Ferretti G, Vari S, et al. PTEN function at the interface between cancer and tumor microenvironment: implications for response to immunotherapy. Int J Mol Sci (2020) 21(15):5337. doi: 10.3390/ijms21155337
53. Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol (2019) 5(10):1504–6. doi: 10.1001/jamaoncol.2019.2963
54. Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol (2019) 5(12):1710–7. doi: 10.1001/jamaoncol.2019.3616
55. Chen G, Peng J, Xiao Q, Wu HX, Wu X, Wang F, et al. Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J Hematol Oncol (2021) 14(1):80. doi: 10.1186/s13045-021-01089-z
56. Tarazona N, Gimeno-Valiente F, Gambardella V, Zuñiga S, Rentero-Garrido P, Huerta M, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol (2019) 30(11):1804–12. doi: 10.1093/annonc/mdz390
57. Henriksen TV, Tarazona N, Frydendahl A, Reinert T, Gimeno-Valiente F, Carbonell-Asins JA, et al. Circulating tumor DNA in stage III colorectal cancer, beyond minimal residual disease detection, toward assessment of adjuvant therapy efficacy and clinical behavior of recurrences. Clin Cancer Res (2022) 28(3):507–17. doi: 10.1158/1078-0432.CCR-21-2404
Keywords: locally advanced rectal cancer, neoadjuvant immunotherapy, programmed cell death protein-1 inhibitor, mismatch repair-deficient, clinical complete response, watch-and-wait strategy
Citation: Yang R, Wu T, Yu J, Cai X, Li G, Li X, Huang W, Zhang Y, Wang Y, Yang X, Ren Y, Hu R, Feng Q, Ding P, Zhang X and Li YF (2023) Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front. Immunol. 14:1182299. doi: 10.3389/fimmu.2023.1182299
Received: 08 March 2023; Accepted: 13 June 2023;
Published: 27 June 2023.
Edited by:
Ganesan Ramamoorthi, Moffitt Cancer Center, United StatesReviewed by:
Zhaohui Jin, Mayo Clinic, United StatesWeiwei Xiao, Sun Yat-sen University Cancer Center (SYSUCC), China
Wenjun Meng, Sichuan University, China
Copyright © 2023 Yang, Wu, Yu, Cai, Li, Li, Huang, Zhang, Wang, Yang, Ren, Hu, Feng, Ding, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfeng Li, bGl5dW5mZW5nQGttbXUuZWR1LmNu; Xuan Zhang, MTAyNTQxNTA4NUBxcS5jb20=; Peirong Ding, ZGluZ3ByQHN5c3VjYy5vcmcuY24=
†These authors contributed equally to this work and share first authorship
 Renfang Yang1†
Renfang Yang1† Tao Wu
Tao Wu Jiehai Yu
Jiehai Yu Ya Zhang
Ya Zhang Yongping Ren
Yongping Ren Peirong Ding
Peirong Ding Xuan Zhang
Xuan Zhang Yunfeng Li
Yunfeng Li