- 1Department of Oncology, Unit 3, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai, China
- 2Department of Oncology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Reportedly, sarcopenia is associated with prognosis in advanced colorectal cancer (CRC) patients and can lead to reduced efficacy of targeted therapy. However, studies on the relationship between sarcopenia and the prognosis (or efficacy) of advanced CRC patients receiving fruquintinib targeted therapy remains scarce. Therefore, we conducted a comprehensive assessment of the relationship between nutritional status, inflammation, immune function, and cancer-related sarcopenia. We also investigated whether sarcopenia affects the therapeutic efficacy of fruquintinib targeted therapy.
Patients and methods: In this retrospective study, sarcopenia and several markers of nutritional status and immune function were assessed in advanced CRC patients with fruquintinib therapy at the hospital. We used drug target mendelian randomization (MR) analysis to investigate the impact of fruquintinib on sarcopenia.
Results: Advanced CRC patients with sarcopenia had a poorer prognosis compared to those without sarcopenia. Furthermore, sarcopenia showed a strong correlation with various markers of nutritional status, immune function indicators, inflammation markers, quality of life scores, and the prognostic nutrition index. MR studies suggest that the spleen tyrosine kinase (SYK) gene is a key factor in the occurrence of sarcopenia associated with the use of fruquintinib.
Conclusion: Sarcopenia could be a prognostic factor in patients with advanced CRC receiving fruquintinib targeted therapy.
Introduction
Colorectal cancer (CRC) poses significant health challenges worldwide, with alarming statistics reflecting its severity. The five-year survival rate for patients with metastatic CRC stands at approximately 14% (1), underscoring the dire prognosis for this condition. Epidemiological data indicates that CRC ranks as the third most common and lethal type of cancer globally. In 2020, it was estimated that there were over 1.9 million new cases and nearly 1 million deaths attributed to this malignancy (2), highlighting the urgent need for enhanced prevention, early detection, and effective treatment strategies (3).
The etiology of CRC is multifactorial, intricately linked to genetic, environmental, and lifestyle factors, making early diagnosis essential for improving patient outcomes (4–6). The low 5-year overall survival rate in CRC is primarily due to the metastasis of CRC (7, 8). The contemporary clinical management of CRC involves a multidisciplinary approach that integrates surgical intervention, chemotherapy, targeted therapy, and immunotherapy (9, 10). Patients with tumors frequently experience a state of metabolic dysregulation, which is typified by increased catabolic processes and diminished anabolic activity (11, 12). Sarcopenia, a syndrome characterized by the progressive loss of skeletal muscle mass and function, is increasingly recognized as a significant factor affecting the prognosis of cancer patients, particularly those with CRC (13, 14). Sarcopenia can lead to metabolic disorders, along with inflammatory reactions and immune dysfunction (15–17). Recently, fruquintinib, an innovative targeted therapy for advanced CRC, has shown promise as a third-line treatment option, offering potential survival benefits for these patients (18).
The prevalence of muscle wasting among advanced CRC patients, due to impaired digestive and absorptive capacity, raises concerns regarding its impact on the efficacy of targeted therapies. In a study of colorectal cancer patients, sarcopenia was found in 18.8% of the cohort and linked to poorer progression-free and overall survival (19). A prior study demonstrated the prognostic significance of baseline skeletal muscle index (SMI) in patients with metastatic CRC undergoing treatment with fruquintinib (20). However, the impact of sarcopenia on the therapeutic efficacy of fruquintinib, a widely utilized novel targeted therapy, remains uncertain. This study aims to investigate the impact of sarcopenia on patients with advanced CRC patients undergoing targeted therapy through a retrospective analysis. Addtionally, it seeks to identify key genes between fruquintinib and sarcopenia using MR analysis. Specifically, we hypothesize that sarcopenia serves as a prognostic factor in advanced CRC patients undergoing fruquintinib treatment, and identifying the key genes associated with fruquintinib-induced sarcopenia is crucial for elucidating its underlying mechanisms and optimizing therapeutic outcomes.
Methods
Patients and study design
In this retrospective study, patients treated for advanced CRC using fruquintinib, were selected from January 2021 to January 2024 at the Shanghai Municipal Hospital of Traditional Chinese Medicine.
Measurement of muscle mass
As previously outlined, sarcopenia assessment follows a method summarized below (21). Muscle mass was quantified through cross-sectional computed tomography (CT) imaging at the third lumbar (L3) level, measuring the skeletal muscle area using hounsfield units (HU) ranging from –29 to 150. We use the Slice Omatic 5.0 image analysis software to measure the L3 level. The SMI was calculated by normalizing the muscle area to height (cm²/m²), SMI is indicative of sarcopenia if less than 40.31 cm²/m² in males and 30.88 cm²/m² in females (22). Prior to fruquintinib treatment, CRC patients underwent standardized CT evaluations, categorizing them into sarcopenia groups (SG) and non-sarcopenia groups (NSG) based on SMI values, thus enabling precise assessment of sarcopenia’s impact on treatment outcomes. Figure 1 demonstrates the comparison between CRC patients with sarcopenia (Figure 1A) and those without sarcopenia (Figure 1B), as visualized by CT scans at the L3 level, highlighting the skeletal muscle area.
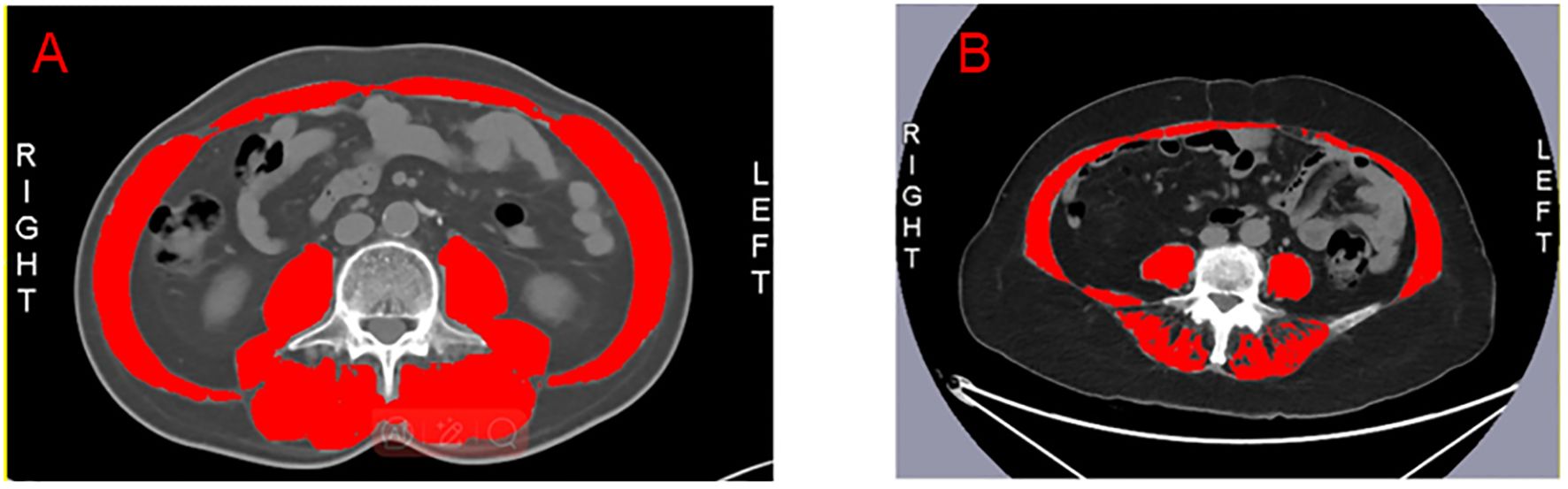
Figure 1. The red section represents the skeletal muscle at the L3 level. (A) The L3 level in patients with non-sarcopenia. (B) The L3 level in patients with sarcopenia.
Nutritional status assessment
We employed various traditional nutritional assessments, such as body mass index (BMI), SMI, albumin (ALB), prealbumin (PAB), retinol-binding protein (RBP) and the prognostic nutrition index (PNI).
Inflammatory status assessment
We assessed inflammatory markers, including interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). These markers were measured using was conducted using a fully automated flow cytometer (NovoCyte D2060R).
Immune status assessment
We assessed immune markers, including the absolute counts of CD3, CD4, CD8, and natural killer (NK) cells. These immune markers were analyzed using a fully automated flow cytometer (BD FACSCanto II).
Statistical analysis was performed using SPSS (version 29). For continuous variables that followed a normal distribution, data are presented as mean ± standard deviation (SD), and group differences were analyzed using the t-test. For continuous variables that did not follow a normal distribution, data are presented as median (interquartile range), and group differences were analyzed using the Wilcoxon test. Categorical variables are described using frequency (percentage), and group differences were compared using the chi-square test or Fisher’s exact test. To evaluate the relationships between variables, we used spearman correlation analysis, which measures the strength and direction of monotonic associations and is suitable for non-parametric data and ordinal variables.
Ethical consideration
The protocol for this investigation was approved by the Shanghai Municipal Hospital of Traditional Chinese Medicine (2021SHL-KY-04-03).
Study design of MR
To explore the causal relationship between fruquintinib and sarcopenia, we conducted a MR study. We validated the reliability of genetic variants as instrumental variables (IVs) by fulfilling three key MR assumptions. Firstly, we selected genome-wide significant variants (P < 5×10-8) strongly linked to the exposure, ensuring F-statistics > 10 to avoid weak instrument bias (20). Secondly, we screened IVs for associations with potential confounders (age, sex, BMI, smoking status, socioeconomic status) using PhenoScanner and multivariable MR adjustment. Thirdly, we confirmed that the IVs affect the outcome only through the exposure, with horizontal pleiotropy tests (MR-Egger intercept and MR-PRESSO) showing all P > 0.05 (23). In this study, gene expression quantitative trait loci (eQTLs) were employed as exposure variables, whereas indicators associated with left-hand grip strength, right-hand grip strength, appendicular muscle mass, and walking speedwere considered as outcome variables. Supplementary Figure S1 displays the study flowchart.
Sources
Three genes were identified from DrugBank and seven from the DGIdb database for fruquintinib. The SMILES structures of the compounds were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Target gene prediction using SwissTargetPrediction (http://www.swisstargetprediction.ch/) yielded 117 potential target genes. After merging and deduplicating results from the three databases, a final list of 111 target genes for fruquintinib was established.
The diagnosis of sarcopenia was referred to the criteria established by the European Working Group on Sarcopenia in Older People (EWGSOP) (24). This study includes 461,089 European samples with 9,851,867 single nucleotide polymorphisms (SNPs) associated with grip strength, including both left and right hand grip strength. For appendicular lean mass, 450,243 European samples and 18,071,518 SNPs were analyzed. Usual walking pace is associated with 459,915 European samples and 9,851,867 SNPs. A detailed description of the data used in this study is provided in Supplementary Table S1.
Statistical analysis of MR
To address horizontal pleiotropy in MR analyses, MR-PRESSO detected no outliers with Distortion test P-values < 0.05. Five methods—IVW, MR-Egger, Weighted Median, Simple Mode, and Weighted Mode (25)—were applied, prioritizing IVW results. Heterogeneity tests guided the use of random effects (P < 0.05) or fixed effects (P > 0.05) in IVW. The robustness of the results was ensured through sensitivity analyses, which included heterogeneity testing using mr_heterogeneity(), the application of MR-Egger regression to assess pleiotropy, and the implementation of a leave-one-out analysis (26). Causal effects were reported as odds ratios (OR) with 95% confidence intervals (CI). Analyses were performed using R packages “TwoSampleMR” (v 0.6.0) and “MRPRESSO” (v 1.0).
Results
Patients’ characteristics
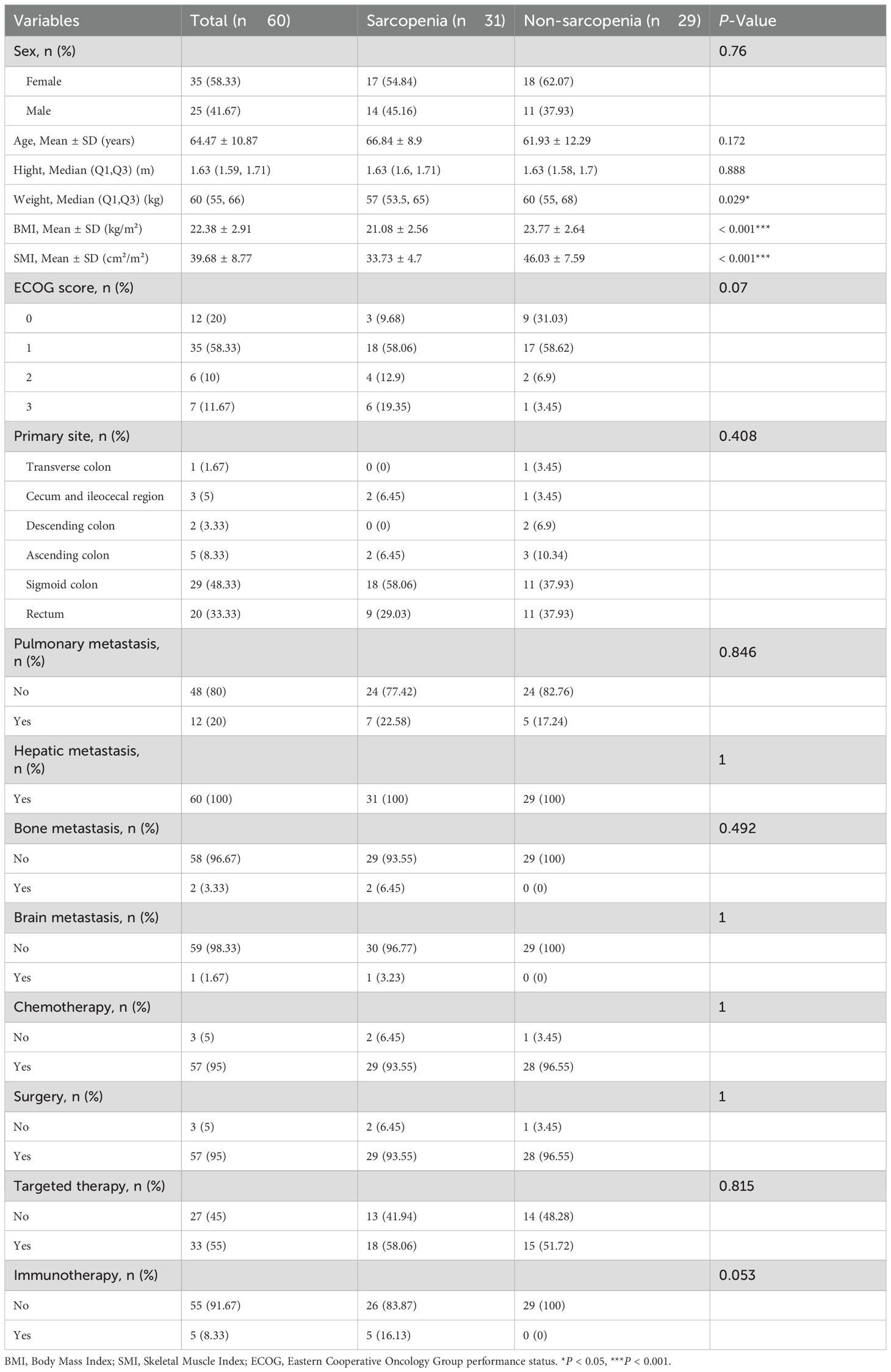
Of the 60 eligible patients, 31 were categorized into the sarcopenia group and 29 into the non-sarcopenia group. Table 1 details patients’ background.
Comparison of nutritional, inflammatory, and immune indexes
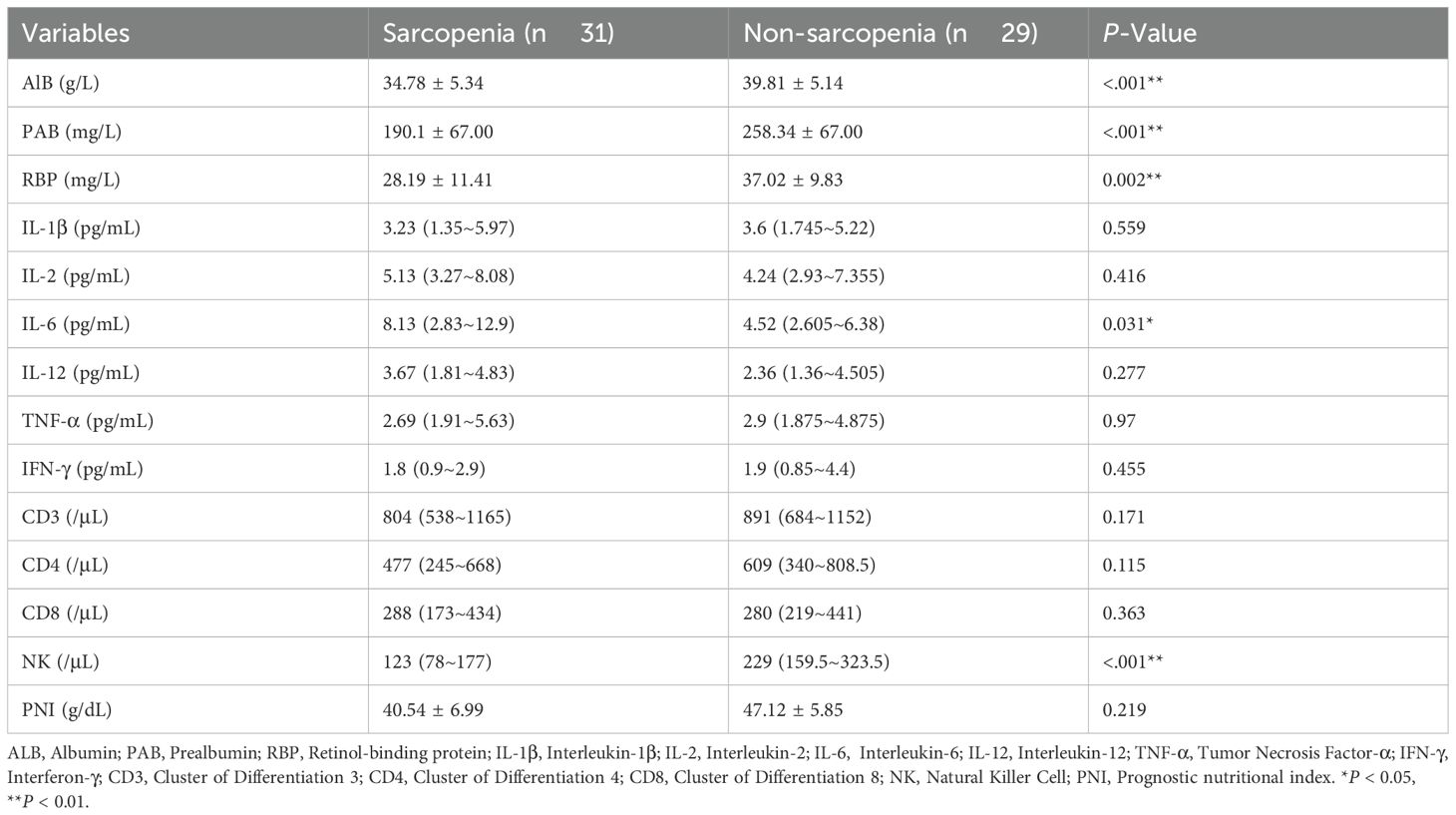
Previous study found that sarcopenia correlates with the nutrition indicators (27). We assessed the relationship between nutritional markers such as albumin, prealbumin, retinol-binding protein, and sarcopenia in our study. Table 2 presents that compared to the NSG, patients in the SG had significantly lower levels of albumin, prealbumin, and retinol-binding protein. As a proinflammatory cytokine, IL-6, along with IL-6 signaling, plays a crucial role in the inflammatory microenvironment linked to CRC (28, 29). Furthermore, the progression of sarcopenia is associated with elevated serum levels of IL-6 (30). We found that IL-6 level was significantly higher in the SG than in the NSG (P < 0.05). Sarcopenia may impair myokine signaling, shift membrane-bound factors to a pro-inflammatory state, and reduce immune cell regeneration, leading to immune dysfunction (31). We compared absolute counts of CD3, CD4, CD8, and NK cells between the two groups. Our results revealed that, compared to the NSG, patients in the SG had a significant decrease in the absolute count of NK cells (P < 0.01). Thus, it is evident that tumor-associated sarcopenia may lead to alterations in the patient’s nutritional, inflammatory, and immune status.
Correlation analysis
Subsequently, Our correlation analysis demonstrated a significant association between sarcopenia and several biomarkers, including albumin, prealbumin, retinol-binding protein, NK cells, BMI, and PNI. Based on these results, sarcopenia showed the strongest correlation with nutritional markers overall (Figure 2).
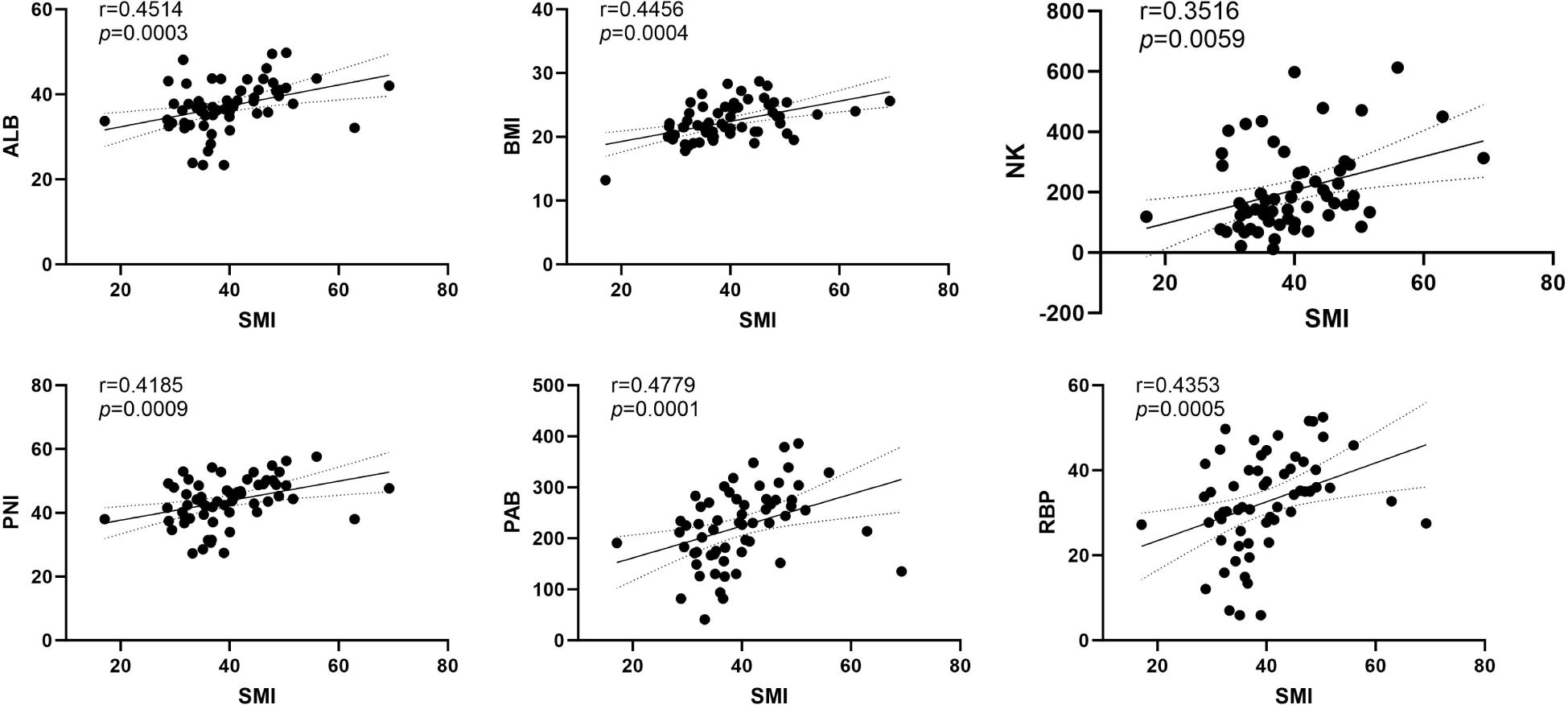
Figure 2. The correlation analysis between SMI and ALB, PAB, RBP, NK, BMI and PNI. ALB, Albumin (g/L); PAB, Prealbumin (mg/L); RBP, Retinol-binding protein (mg/L); NK, Natural Killer Cell (/μL); BMI, Body Mass Index (kg/m²); PNI, Prognostic nutritional index (g/dL).
Efficacy for all patients
In SG the median progression-free survival (PFS) was 5.9 months (95% CI=0.3644-1.454, HR=1.655). In NSG the median PFS was 8.4 months (95% CI=0.71386-2.744, HR=0.6042). The median time to treatment failure (TTF) in SG was 59 days (95% Cl=0.4412-1.440, HR=1.571) while in NSG this value was 69 days (95% Cl=0.6943-2.267, HR=0.6364). Figure 3 demonstrates the Kaplan-Meier curves of PFS and TTF. In terms of PFS and TTF, no significant differences were observed between SG and NSG.
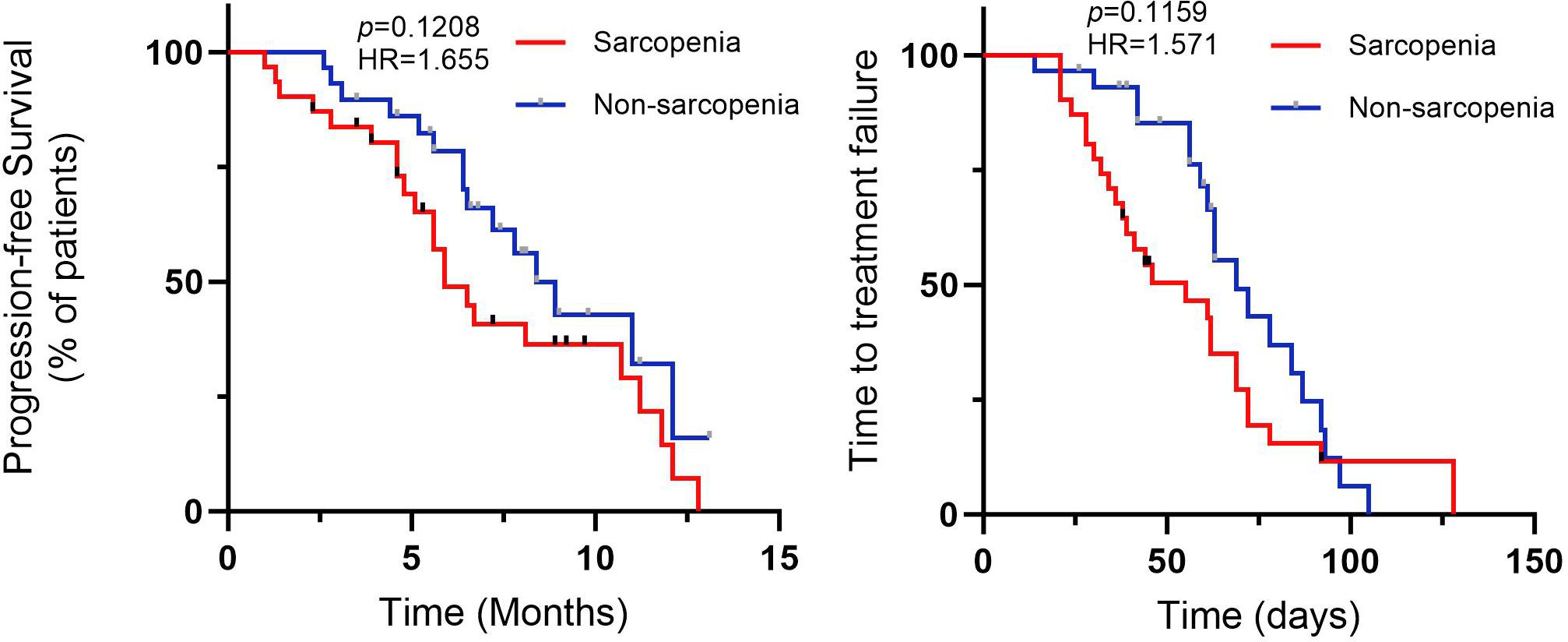
Figure 3. Profession-free survival (PFS), and time to treatment failure (TTF) for CRC patients with fruquintinib therapy.
Safety analysis
According to reports, the main side effects of fruquintinib include hypertension, gastrointestinal reactions such as nausea, vomiting, and diarrhea, abnormal bleeding such as epistaxis and hematuria, as well as hand-foot syndrome and abnormalities in liver and kidney function (32–34). We analyzed the occurrence of adverse effects during fruquintinib targeted therapy in patients with and without sarcopenia (Figure 4). Our results indicated that patients in the SG presented the higher incidence of nausea than in the NSG (P<0.05). Patients in the SG exhibited higher levels of hypertension, hand-foot syndrome, and severity of symptoms compared to the NSG. Additionally, the SG had a higher incidence of diarrhea, and thrombocytopenia. However, there were no significant inter-group differences in these indicators.
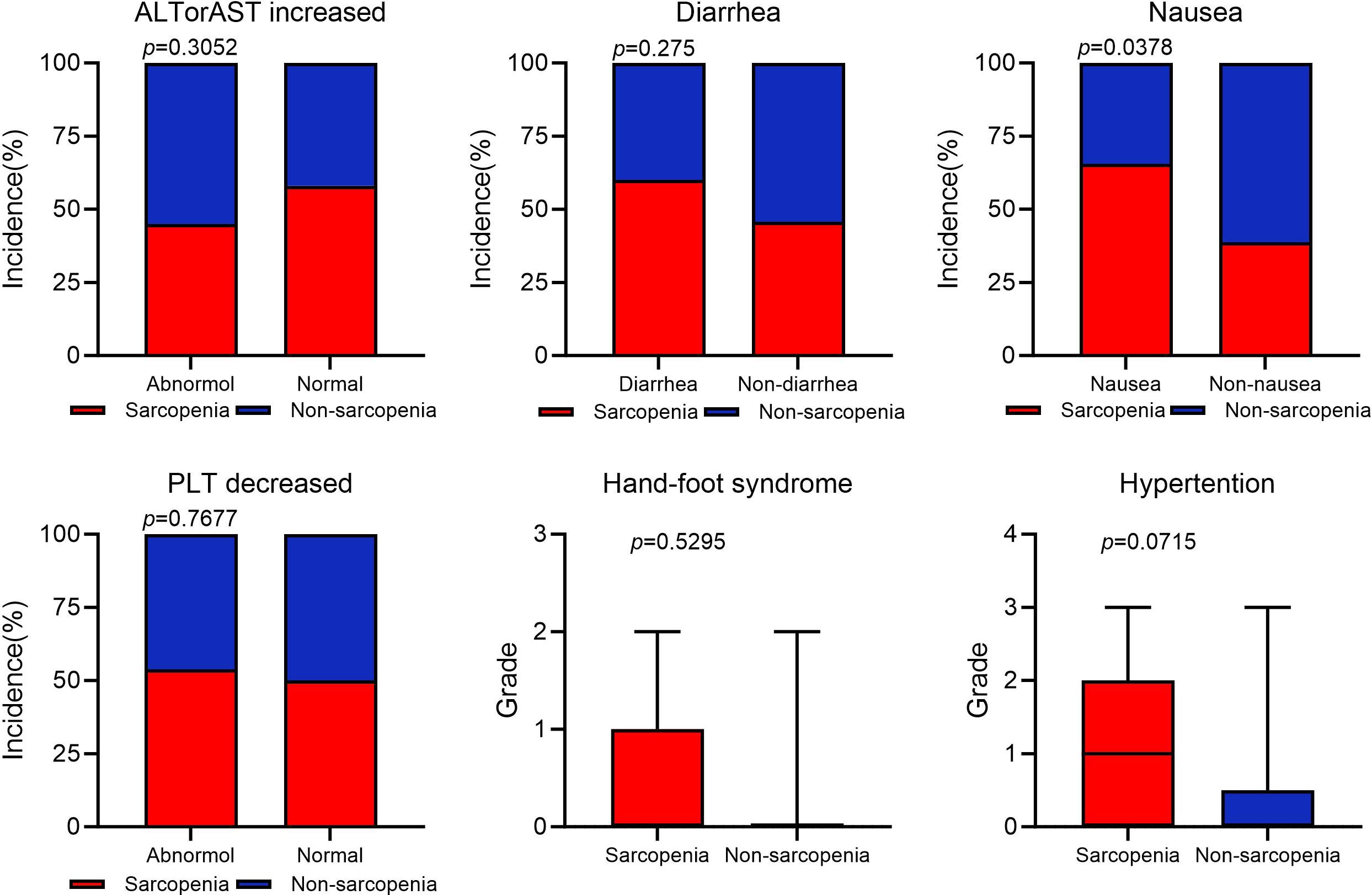
Figure 4. Adverse event occurrence statistics. AST, aspirate aminotransferase (U/L); ALT, alanine aminotransferase (U/L); PLT, platelet (×10^9^/L).
MR results between the target genes of fruquintinib and left-hand grip strength
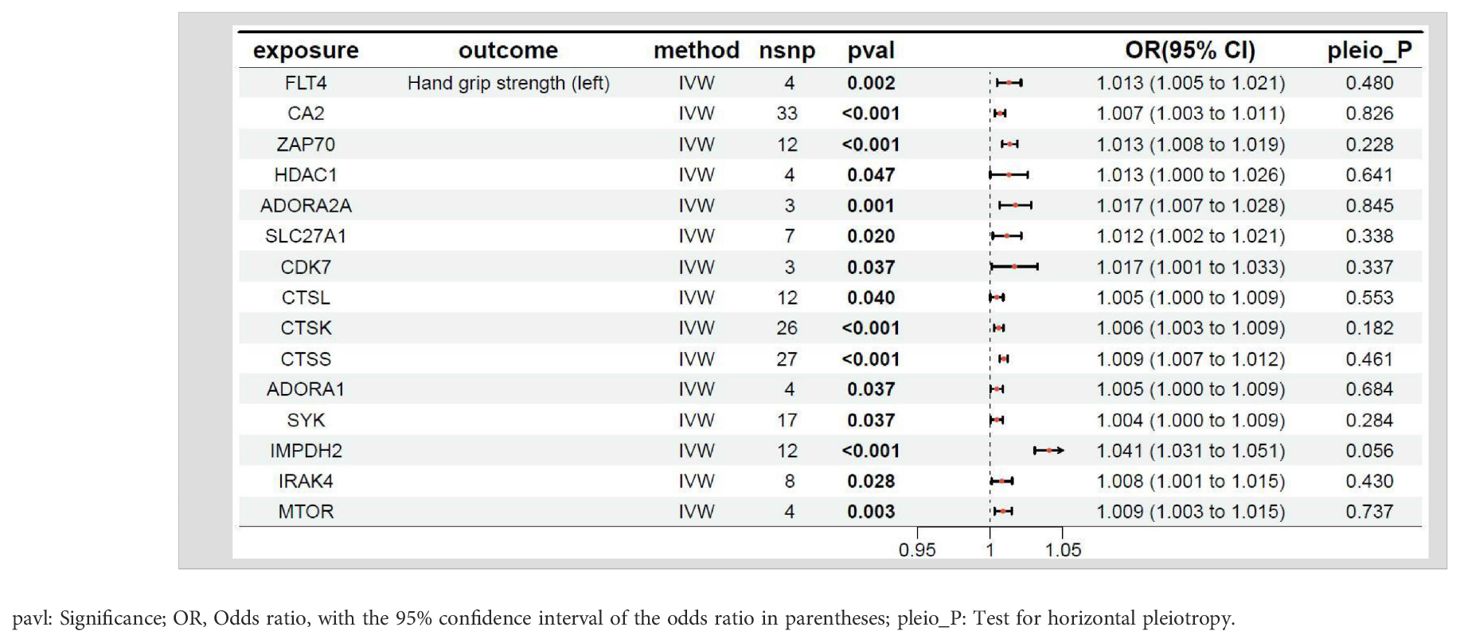
The relationship between the target genes of fruquintinib and left-hand grip strength are illustrated in Table 3. A p-value < 0.05 in MR analysis signifies a significant causal link. If the OR is > 1, it suggests that the gene is a risk factor for the outcome, meaning that an increase in the expression level of this gene is associated with a higher risk of the outcome. Conversely, if the OR is < 1, the gene is considered a protective factor, indicating that higher expression levels of this gene are associated with a decreased risk of the outcome. A total of 28 genes exhibited P-values < 0.05 between the target genes of fruquintinib and left-hand grip strength. The following drug target genes are correlated with an elevated risk (OR > 1) of adverse outcomes: FLT4, CA2, ZAP70, HDAC1, ADORA2A, SLC27A1, CDK7, CTSL, CTSK, CTSS, ADORA1, SYK, IMPDH2, IRAK4, MTOR.
MR results between the target genes of fruquintinib and right-hand grip strength
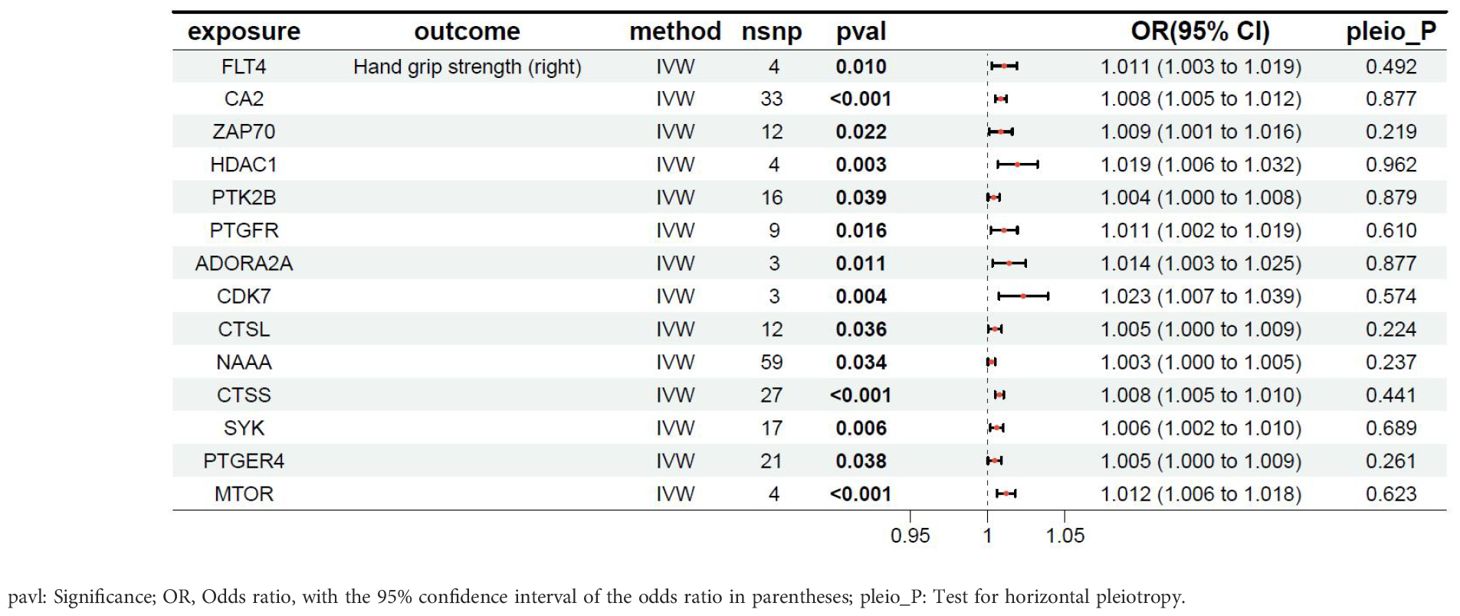
The relationship between the target genes of fruquintinib and right-hand grip strength are illustrated in Table 4. A total of 25 genes exhibited P-values < 0.05. The following drug target genes are associated with an increased risk of adverse outcomes: FLT4, CA2, ZAP70, HDAC1, PTK2B, PTGFR, ADORA2A, CDK7, CTSL, NAAA, CTSS, SYK, PTGER4, MTOR.
MR results between the target genes of fruquintinib and muscle mass
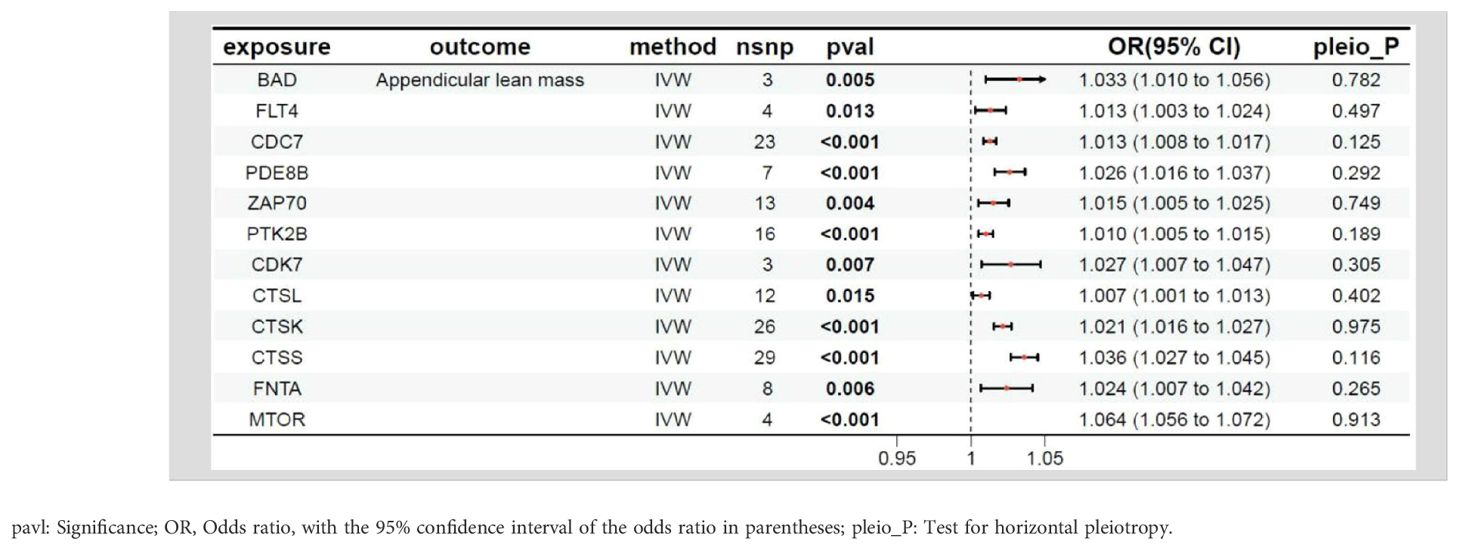
The relationship between the target genes of fruquintinib and limb muscle mass are illustrated in Table 5. A total of 21 genes exhibited P-values < 0.05. The following drug target genes are associated with an increased risk of adverse outcomes: BAD, FLT4, CDC7, PDE8B, ZAP70, PTK2B, CDK7, CTSL, CTSK, CTSS, FNTA, MTOR.
MR results between the target genes of fruquintinib and walking speed
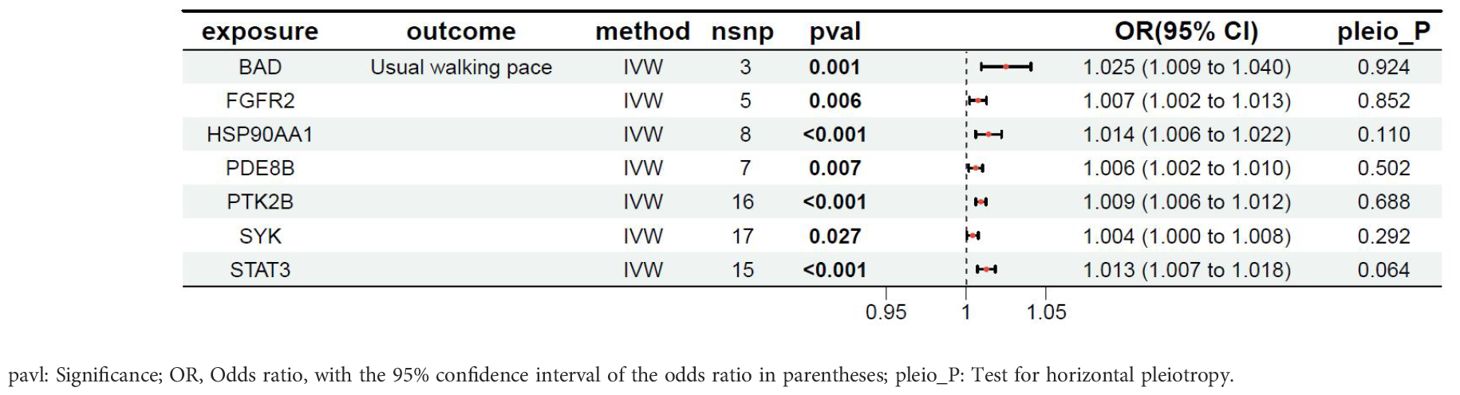
The relationship between the target genes of fruquintinib and walking speed are illustrated in Table 6. A total of 12 genes exhibited P-values < 0.05. The following drug target genes are associated with an increased risk of adverse outcomes: BAD, FGFR2, HSP90AA1, PDE8B, PTK2B, SYK, STAT3.
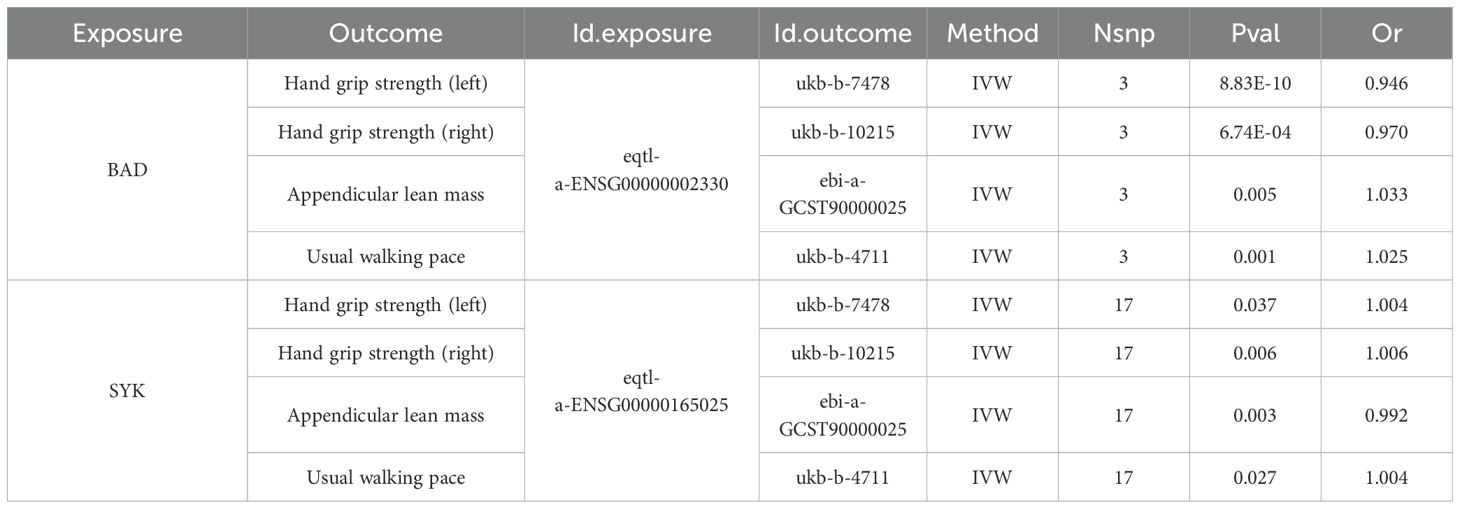
The intersection of MR results for drug target genes and the four outcome variables mentioned above was visualized, as shown in Supplementary Figure S2. Two genes, the BCL2 associated agonist of cell death (BAD) gene and the spleen tyrosine kinase (SYK) gene, were found to have a significant causal relationship with all four outcome variables (Table 7). The SYK gene is considered to be the most likely candidate gene associated with an increased risk of sarcopenia.
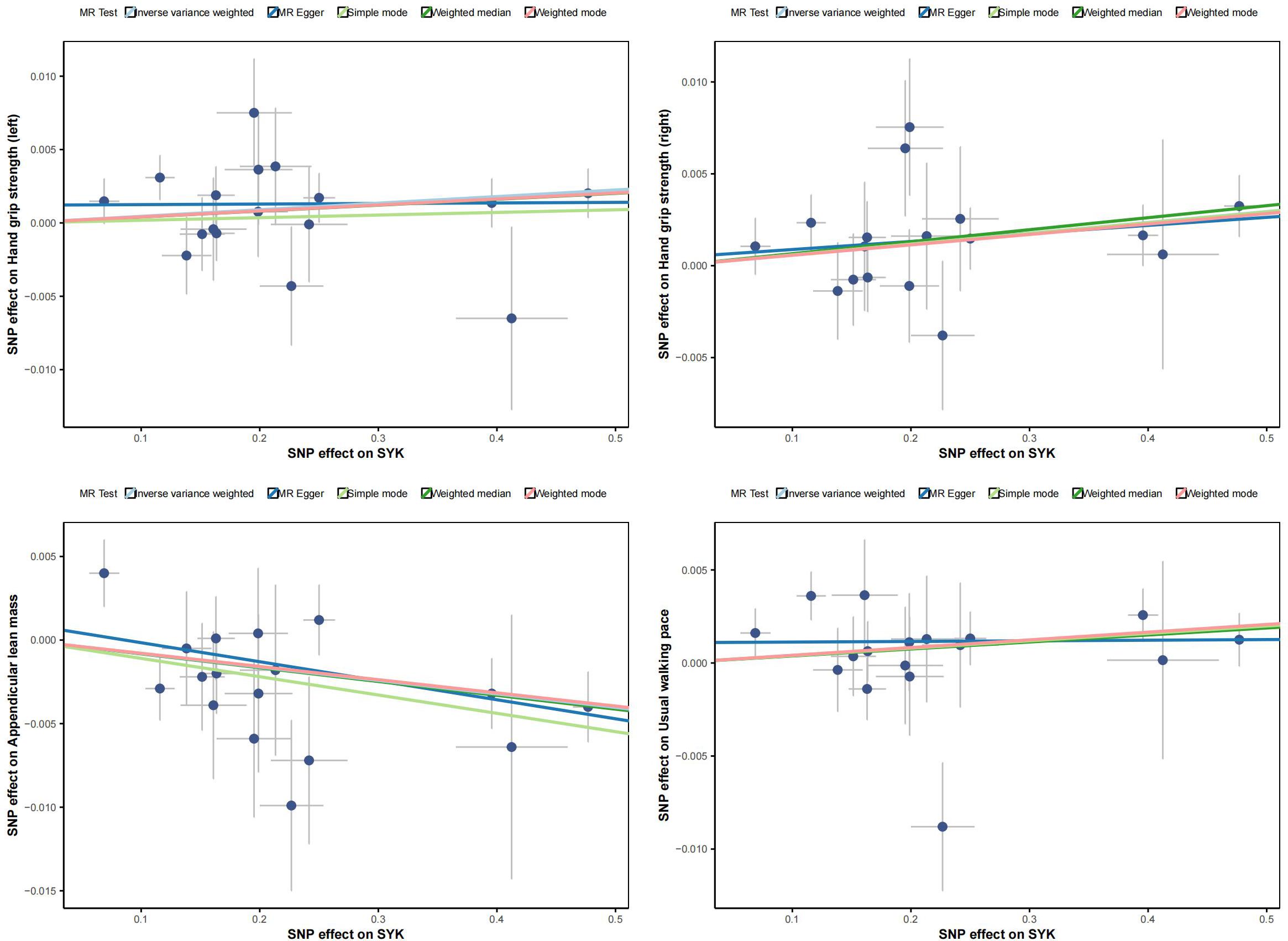
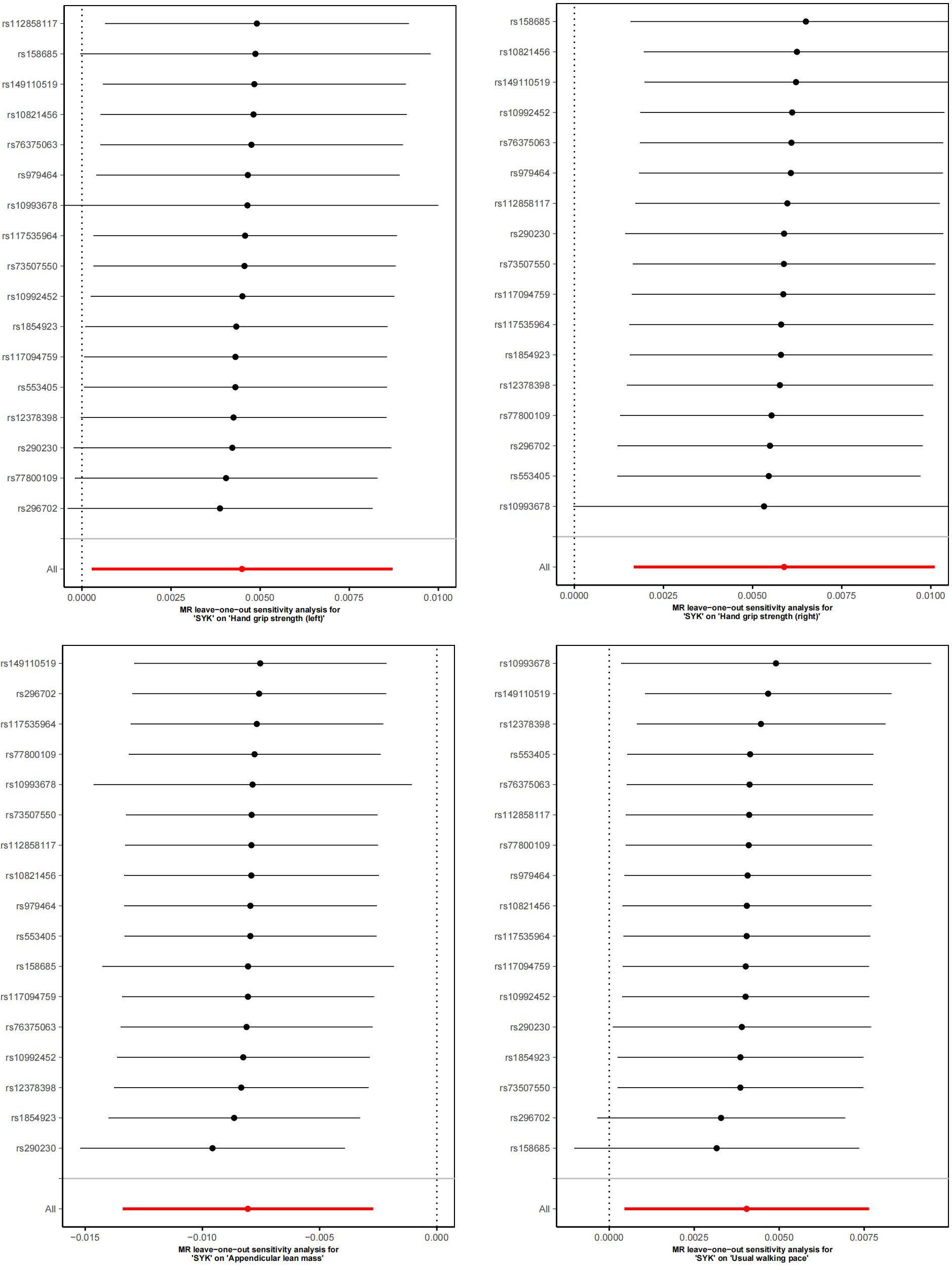
Figure 5 displays scatter plots of the associations between exposure factors and outcome variables obtained from five MR algorithms. The leave-one-out sensitivity analysis, shown in Figure 6, demonstrates that the removal of each SNP individually does not significantly affect the outcome variable, indicating that the results of this MR analysis are reliable and stable.
Discussion
This study provides significant insights into the relationship between sarcopenia and the clinical outcomes of fruquintinib targeted therapy in advanced CRC patients. Recently, poor baseline nutrition has been viewed as a predictor of cancer outcomes, particularly in gastrointestinal tumors (35, 36). A decline in SMI, BMI, and nutritional markers, including albumin, prealbumin, and the PNI, may serve as indicators for assessing the risk of sarcopenia (37–39). Moreover, the presence of sarcopenia can diminish the effectiveness of tumor-targeted therapies (40, 41). We observed that sarcopenic patients exhibited a significant decline in nutritional markers, including albumin, prealbumin, and retinol-binding protein, in comparison to non-sarcopenic patients (Table 2). Studies have shown that IL-6 levels correlate with muscle wasting and atrophy, which are key features of sarcopenia (42, 43). IL-6 is a cytokine that plays a significant role in inflammation and the immune response (44). In particular, IL-6 can suppress the function of NK cells, leading to a reduced ability to target and eliminate cancer cells (45, 46). Patients with sarcopenia exhibited increased concentrations of IL-6 and a significant decrease in NK cell counts, indicating a potential impairment in immune function (Table 2). Sarcopenic patients had a shorter duration of fruquintinib treatment and faced more adverse events, particularly gastrointestinal symptoms like nausea, and diarrhea. These symptoms can decrease appetite and food intake, worsening nutritional status and nutrient absorption, which in turn aggravates sarcopenia by hindering muscle maintenance (Figure 4).This highlights the critical importance of monitoring sarcopenia in CRC patients undergoing targeted therapies, as it not only affects nutritional and immune status but also impacts treatment efficacy and toxicity.
One of the innovative aspects of this research is the identification of the SYK gene as a risk factor for sarcopenia development in patients treated with fruquintinib. The SYK is a non-receptor protein tyrosine kinase involved in immune signaling and tumor metastasis initiation (47). Meanwhile, the SYK is a cytoplasmic enzyme that links immune cell receptors to intracellular signaling, crucial for adaptive and inflammatory immune responses (48, 49). The SYK gene exhibits dual functionality, acting as both a promoter and a suppressor of tumorigenesis (50). SYK gain-of-function variants found in patients with immune deficiency and systemic inflammation boost immune signaling and elevate inflammatory cytokines. A SYK-S544Y knock-in mouse model mirrored human disease symptoms, which were partially relieved by SYK inhibitors (51). These findings emphasize the pivotal role of SYK in amplifying inflammatory and immune responses, and underscore the potential of targeting SYK as a fundamental therapeutic strategy for the management of related disorders. However, no studies have identified a correlation between the SYK gene and cancer-associated sarcopenia, indicating a need for further in-depth investigation in future research. Our study found that sarcopenia is closely related to inflammation and immune responses in advanced CRC patients treated with fruquintinib. We identified SYK as a risk gene for fruquintinib-induced sarcopenia through MR analysis. This discovery offers a novel perspective on the genetic basis of sarcopenia in cancer patients and may guide future precision medicine approaches. From a clinical perspective, our findings underscore the importance of early detection and management of sarcopenia, as addressing this condition may enhance the efficacy of treatments such as fruquintinib and mitigate associated adverse side effects. By improving nutritional status and enhancing immunity, nutrition intervention can help alleviate symptoms and improve the prognosis for patients with CRC (52, 53). Thus, we can conclude that nutritional interventions and physical rehabilitation should be integrated into the standard care of CRC patients, especially those at risk for sarcopenia, to optimize outcomes.
This study has several limitations, including its retrospective design, which limits causal inference. Further prospective studies are needed to confirm these findings and explore the underlying mechanisms. Additionally, factors such as muscle strength, physical activity, and exercise interventions were not evaluated, and should be considered in future research. Further validation of the SYK gene in larger cohorts is also needed.
In conclusion, sarcopenia may be an important prognostic factor for patients with advanced colorectal cancer undergoing fruquintinib targeted therapy. Early identification and management of sarcopenia could potentially enhance treatment outcomes and reduce the risk of complications in these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Our study is a retrospective study and does not involve specific drug intervention protocols. Therefore, no written informed consent was required.
Author contributions
YW: Funding acquisition, Writing – original draft, Writing – review & editing. DM: Conceptualization, Investigation, Writing – review & editing. SQ: Conceptualization, Methodology, Writing – review & editing. TY: Data curation, Formal analysis, Writing – review & editing. GS: Data curation, Writing – review & editing. HS: Data curation, Methodology, Writing – review & editing. WH: Data curation, Methodology, Writing – review & editing. ZT: Data curation, Methodology, Project administration, Writing – review & editing. WC: Writing – review & editing, Data curation, Conceptualization. HF: Funding acquisition, Methodology, Writing – review & editing. YX: Conceptualization, Project administration, Resources, Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82174188, 82405498) and Shanghai Municipal Health Commission Talent Program (2022YQ032).
Acknowledgments
The GWAS summary data were obtained from the online public platform (https://gwas.mrcieu.ac.uk/). The analyses of GWAS summary data were performed under application R version 4.0.2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1671178.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1582308/full#supplementary-material
References
1. Fleischer JR, Schmitt AM, Haas G, Xu X, Zeisberg EM, Bohnenberger H, et al. Molecular differences of angiogenic versus vessel co-opting colorectal cancer liver metastases at single-cell resolution. Mol Cancer. (2023) 22:17. doi: 10.1186/s12943-023-01713-1, PMID: 36691028
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660, PMID: 33538338
3. Wang Q, Huang X, Zhou S, Ding Y, Wang H, Jiang W, et al. IL1RN and PRRX1 as a prognostic biomarker correlated with immune infiltrates in colorectal cancer: evidence from bioinformatic analysis. Int J Genomics. (2022) 2022:2723264. doi: 10.1155/2022/2723264, PMID: 36483329
4. Ying P, Chen C, Lu Z, Chen S, Zhang M, Cai Y, et al. Genome-wide enhancer-gene regulatory maps link causal variants to target genes underlying human cancer risk. Nat Commun. (2023) 14:5958. doi: 10.1038/s41467-023-41690-z, PMID: 37749132
5. Lu L, Mullins CS, Schafmayer C, Zeißig S, and Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41:1137–51. doi: 10.1002/cac2.12220, PMID: 34563100
6. Shen G, Wang Q, Li Z, Xie J, Han X, Wei Z, et al. Bridging chronic inflammation and digestive cancer: the critical role of innate lymphoid cells in tumor microenvironments. Int J Biol Sci. (2024) 20:4799–818. doi: 10.7150/ijbs.96338, PMID: 39309440
7. Xia J, Ma N, Shi Q, Liu QC, Zhang W, Cao HJ, et al. XAF1 promotes colorectal cancer metastasis via VCP-RNF114-JUP axis. J Cell Biol. (2024) 223. doi: 10.1083/jcb.202303015, PMID: 38095639
8. Biller LH and Schrag D. Diagnosis and treatment of metastatic colorectal cancer: A review. Jama. (2021) 325:669–85. doi: 10.1001/jama.2021.0106, PMID: 33591350
9. Singh M, Morris VK, Bandey IN, Hong DS, and Kopetz S. Advancements in combining targeted therapy and immunotherapy for colorectal cancer. Trends Cancer. (2024) 10:598–609. doi: 10.1016/j.trecan.2024.05.001, PMID: 38821852
10. Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, et al. Recent advances in the treatment of colorectal cancer: A review. J Nippon Med Sch. (2022) 89:246–54. doi: 10.1272/jnms.JNMS.2022_89-310, PMID: 35082204
11. Feng L, Zhang W, Shen Q, Miao C, Chen L, Li Y, et al. Bile acid metabolism dysregulation associates with cancer cachexia: roles of liver and gut microbiome. J Cachexia Sarcopenia Muscle. (2021) 12:1553–69. doi: 10.1002/jcsm.12798, PMID: 34585527
12. Halma MTJ, Tuszynski JA, and Marik PE. Cancer metabolism as a therapeutic target and review of interventions. Nutrients. (2023) 15. doi: 10.3390/nu15194245, PMID: 37836529
13. Souza BU, Souza NCS, Martucci RB, Rodrigues VD, Pinho NB, Gonzalez MC, et al. Factors associated with sarcopenia in patients with colorectal cancer. Nutr Cancer. (2018) 70:176–83. doi: 10.1080/01635581.2018.1412480, PMID: 29351494
14. Xie H, Wei L, Gao S, Liu M, Liang Y, Yuan G, et al. Prognostic significance of sarcopenia diagnosed based on the anthropometric equation for progression-free survival and overall survival in patients with colorectal cancer. Front Nutr. (2023) 10:1076589. doi: 10.3389/fnut.2023.1076589, PMID: 36819674
15. Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, and Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. (2021) 13(10):3159. doi: 10.3390/nu13103519, PMID: 34684520
16. Aluganti Narasimhulu C and Singla DK. Amelioration of diabetes-induced inflammation mediated pyroptosis, sarcopenia, and adverse muscle remodelling by bone morphogenetic protein-7. J Cachexia Sarcopenia Muscle. (2021) 12:403–20. doi: 10.1002/jcsm.12662, PMID: 33463042
17. Nicholson T, Dhaliwal A, Quinlan JI, Allen SL, Williams FR, Hazeldine J, et al. Accelerated aging of skeletal muscle and the immune system in patients with chronic liver disease. Exp Mol Med. (2024) 56:1667–81. doi: 10.1038/s12276-024-01287-y, PMID: 39026032
18. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. Jama. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855, PMID: 29946728
19. Loeliger J, Edbrooke L, Daly RM, Stewart J, Bucci L, Puskas C, et al. Development and feasibility of an inpatient cancer-related sarcopenia pathway at a major cancer centre. Int J Environ Res Public Health. (2022) 19(7):4038. doi: 10.3390/ijerph19074038, PMID: 35409719
20. Tang W, Li F, Zheng H, Zhao J, Wei H, Xiong X, et al. Prognostic Value of Baseline Skeletal Muscle Index in Colorectal Cancer Patients Treated with Fruquintinib: A multi-center real world analysis. Int J Colorectal Dis. (2024) 39:186. doi: 10.1007/s00384-024-04747-z, PMID: 39565368
21. Aminianfar A, Hashemi R, Emami F, Heshmat R, Motlagh AD, and Esmaillzadeh A. Associations between dietary total antioxidant capacity and sarcopenia: a cross-sectional study. Nutr J. (2024) 23:87. doi: 10.1186/s12937-024-00933-5, PMID: 39085886
22. Sharma V, Fadel A, Tollefson MK, Psutka SP, Blezek DJ, Frank I, et al. Artificial intelligence-based assessment of pre-operative body composition is associated with early complications after radical cystectomy. J Urol. (2025) 213(2):2828–237. doi: 10.1097/JU.0000000000004292., PMID: 39392812
23. Du D, Jing Z, Zhang G, Dang X, Liu R, and Song J. The relationship between central obesity and bone mineral density: a Mendelian randomization study. Diabetol Metab Syndr. (2022) 14:63. doi: 10.1186/s13098-022-00840-x, PMID: 35501835
24. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034, PMID: 20392703
25. Mavromatis LA, Rosoff DB, Cupertino RB, Garavan H, Mackey S, and Lohoff FW. Association between brain structure and alcohol use behaviors in adults: A mendelian randomization and multiomics study. JAMA Psychiatry. (2022) 79:869–78. doi: 10.1001/jamapsychiatry.2022.2196, PMID: 35947372
26. Li Z, Huang X, Wang Q, and Gopinath D. Causal association between microbiome and oral-oropharyngeal cancer: A mendelian randomization study. Int Dent J. (2025) 75(3):1897–1905. doi: 10.1016/j.identj.2025.01.017, PMID: 39986982
27. Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, et al. Poor nutritional status and sarcopenia influences survival outcomes in gastric carcinoma patients undergoing radical surgery. Eur J Surg Oncol. (2020) 46:1963–70. doi: 10.1016/j.ejso.2020.04.044, PMID: 32402508
28. Pucci M, Raimondo S, Urzì O, Moschetti M, Di Bella MA, Conigliaro A, et al. Tumor-derived small extracellular vesicles induce pro-inflammatory cytokine expression and PD-L1 regulation in M0 macrophages via IL-6/STAT3 and TLR4 signaling pathways. Int J Mol Sci. (2021) 22(22):12118. doi: 10.3390/ijms222212118, PMID: 34829995
29. Jain SM, Deka D, Das A, Paul S, Pathak S, and Banerjee A. Role of interleukins in inflammation-mediated tumor immune microenvironment modulation in colorectal cancer pathogenesis. Dig Dis Sci. (2023) 68:3220–36. doi: 10.1007/s10620-023-07972-8, PMID: 37277647
30. Zanders L, Kny M, Hahn A, Schmidt S, Wundersitz S, Todiras M, et al. Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J Cachexia Sarcopenia Muscle. (2022) 13:713–27. doi: 10.1002/jcsm.12867, PMID: 34821076
31. Nelke C, Dziewas R, Minnerup J, Meuth SG, and Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. (2019) 49:381–8. doi: 10.1016/j.ebiom.2019.10.034, PMID: 31662290
32. Li J, Wang Z, Zhong H, He Y, Zhang C, Niu Z, et al. A phase IV study to evaluate the safety of fruquintinib in Chinese patients in real-world clinical practice. Oncologist. (2024) 29:e1012–e9. doi: 10.1093/oncolo/oyae073, PMID: 38642091
33. Li J, Guo W, Bai Y, Deng Y, Yang L, Chen Z, et al. Safety profile and adverse events of special interest for fruquintinib in chinese patients with previously treated metastatic colorectal cancer: analysis of the phase 3 FRESCO trial. Adv Ther. (2020) 37:4585–98. doi: 10.1007/s12325-020-01477-w, PMID: 32901330
34. Tan S, Zhang S, Zhou N, Cai X, Yi C, and Gou H. Efficacy and safety of fruquintinib dose-escalation strategy for elderly patients with refractory metastatic colorectal cancer: A single-arm, multicenter, phase II study. Cancer Med. (2023) 12:22038–46. doi: 10.1002/cam4.6786, PMID: 38063405
35. Zhou J, Fang P, Li X, Luan S, Xiao X, Gu Y, et al. Prognostic value of geriatric nutritional risk index in esophageal carcinoma: A systematic review and meta-analysis. Front Nutr. (2022) 9:831283. doi: 10.3389/fnut.2022.831283, PMID: 35399659
36. Cai H, Li J, Chen Y, Zhang Q, Liu Y, and Jia H. Preoperative inflammation and nutrition-based comprehensive biomarker for predicting prognosis in resectable colorectal cancer. Front Oncol. (2023) 13:1279487. doi: 10.3389/fonc.2023.1279487, PMID: 38074640
37. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr. (2021) 40:40–6. doi: 10.1016/j.clnu.2020.04.043, PMID: 32563598
38. Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. Combination of sarcopenia and prognostic nutritional index to predict long-term outcomes in patients undergoing initial hepatectomy for hepatocellular carcinoma. Asian J Surg. (2023) 46:816–23. doi: 10.1016/j.asjsur.2022.07.122, PMID: 35961897
39. Yin M, Zhang H, Liu Q, Ding F, Hou L, Deng Y, et al. Determination of skeletal muscle mass by aspartate aminotransferase/alanine aminotransferase ratio, insulin and FSH in Chinese women with sarcopenia. BMC Geriatr. (2022) 22:893. doi: 10.1186/s12877-022-03491-9, PMID: 36419004
40. Nishiyama A, Staub Y, Suga Y, Fujita M, Tanimoto A, Ohtsubo K, et al. Sarcopenia may influence the prognosis in advanced thyroid cancer patients treated with molecular targeted therapy. In Vivo. (2021) 35:401–10. doi: 10.21873/invivo.12271, PMID: 33402489
41. Hiroshige T, Ogasawara N, Kumagae H, Ueda K, Chikui K, Uemura KI, et al. Sarcopenia and the therapeutic effects of androgen receptor-axis-targeted therapies in patients with castration-resistant prostate cancer. In Vivo. (2023) 37:1266–74. doi: 10.21873/invivo.13204, PMID: 37103069
42. Riviati N, Legiran L, Saleh I, Indrajaya T, Ali Z, Irfannuddin, et al. Ophiocephalus striatus extract supplementation decreases serum IL-6 levels in older people with sarcopenia-A single-center experience. Geriatr (Basel). (2024) 9:35. doi: 10.3390/geriatrics9020035, PMID: 38525752
43. Dupont J, Vercauteren L, Amini N, Lapauw L, De Schaepdryver M, Poesen K, et al. Are inflammatory markers associated with sarcopenia-related traits in older adults with sarcopenia? - A cross-sectional analysis of the ENHANce study. Exp Gerontol. (2023) 178:112196. doi: 10.1016/j.exger.2023.112196, PMID: 37156446
44. Orange ST, Leslie J, Ross M, Mann DA, and Wackerhage H. The exercise IL-6 enigma in cancer. Trends Endocrinol Metab. (2023) 34:749–63. doi: 10.1016/j.tem.2023.08.001, PMID: 37633799
45. Patel SA, Nilsson MB, Yang Y, Le X, Tran HT, Elamin YY, et al. IL6 mediates suppression of T- and NK-cell function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin Cancer Res. (2023) 29:1292–304. doi: 10.1158/1078-0432.CCR-22-3379, PMID: 36595561
46. Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, et al. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. (2019) 10:273. doi: 10.1038/s41419-019-1435-2, PMID: 30894509
47. Biswas P, Dey D, Rahman A, Islam MA, Susmi TF, Kaium MA, et al. Analysis of SYK gene as a prognostic biomarker and suggested potential bioactive phytochemicals as an alternative therapeutic option for colorectal cancer: an in-silico pharmaco-informatics investigation. J Pers Med. (2021) 11:888. doi: 10.3390/jpm11090888, PMID: 34575665
48. Mansueto MS, Reens A, Rakhilina L, Chi A, Pan BS, and Miller JR. A reevaluation of the spleen tyrosine kinase (SYK) activation mechanism. J Biol Chem. (2019) 294:7658–68. doi: 10.1074/jbc.RA119.008045, PMID: 30923129
49. Canton J, Blees H, Henry CM, Buck MD, Schulz O, Rogers NC, et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat Immunol. (2021) 22:140–53. doi: 10.1038/s41590-020-00824-x, PMID: 33349708
50. Buffard M, Naldi A, Freiss G, Deckert M, Radulescu O, Coopman PJ, et al. Comparison of SYK signaling networks reveals the potential molecular determinants of its tumor-promoting and suppressing functions. Biomolecules. (2021) 11:308. doi: 10.3390/biom11020308, PMID: 33670716
51. Wang L, Aschenbrenner D, Zeng Z, Cao X, Mayr D, Mehta M, et al. Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice. Nat Genet. (2021) 53:500–10. doi: 10.1038/s41588-021-00803-4, PMID: 33782605
52. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. (2019) 19:98. doi: 10.1186/s12885-018-5232-6, PMID: 30670009
53. Ye J, Hu Y, Chen X, Chang C, and Li K. Comparative effects of different nutritional supplements on inflammation, nutritional status, and clinical outcomes in colorectal cancer patients: A systematic review and network meta-analysis. Nutrients. (2023) 15:2772. doi: 10.3390/nu15122772, PMID: 37375676
Keywords: sarcopenia, immune, nutrition, advanced colorectal cancer, fruquintinib, mendelian randomization study
Citation: Wang Y, Meilu D, Qi S, Yufei T, Song G, Susu H, Haotian W, Tingting Z, Chao W, Fenggang H and Xiaoling Y (2025) The impact of sarcopenia on prognosis and fruquintinib efficacy in advanced colorectal cancer: a retrospective and mendelian randomization study. Front. Immunol. 16:1582308. doi: 10.3389/fimmu.2025.1582308
Received: 24 February 2025; Accepted: 10 June 2025;
Published: 16 July 2025.
Edited by:
Antonio Mario Scanu, University of Sassari, ItalyReviewed by:
Namuunaa Juramt, Harvard Medical School, United StatesWenfeng He, Macau University of Science and Technology, China
Copyright © 2025 Wang, Meilu, Qi, Yufei, Song, Susu, Haotian, Tingting, Chao, Fenggang and Xiaoling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Chao, cGQzNjIwNjBAc2hzbXUuZWR1LmNu; Hou Fenggang, Zmdob3U1NTVAMTI2LmNvbQ==; Yin Xiaoling, eWlueGlhb2xpbmcyMDE1QDE2My5jb20=
†These authors have contributed equally to this work
 Yao Wang
Yao Wang Du Meilu1†
Du Meilu1† Shi Qi
Shi Qi Gao Song
Gao Song Han Susu
Han Susu Wang Chao
Wang Chao Hou Fenggang
Hou Fenggang Yin Xiaoling
Yin Xiaoling