- 1Department of Pharmacy, Guangxi Hospital Division of The First Affiliated Hospital, Sun Yat-sen University, Nanning, China
- 2School of Pharmaceutical Science, Guangxi Medical University, Nanning, China
- 3Department of Pharmacy, The Second Affiliated Hospital of Guangxi Medical University, Nanning, China
- 4State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu, China
- 5Center for New Drug Safety Evaluation and Research, China Pharmaceutical University, Nanjing, Jiangsu, China
- 6School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: Emerging studies have investigated the association between acetaminophen (APAP) use and clinical outcomes in cancer patients receiving immune checkpoint inhibitors (ICIs), but their findings remain inconsistent. This meta-analysis aims to systematically synthesize available evidence to clarify this relationship and provide evidence-based guidance for clinical practice.
Methods: A systematic literature search was performed to identify studies comparing prognostic outcomes between APAP users and non-users among cancer patients treated with ICIs. Eligible studies were required to report hazard ratios (HRs) for overall survival (OS) and/or progression-free survival (PFS) with 95% confidence intervals (CIs). Meta-analyses were conducted to derive pooled effect estimates. Funnel plots and Egger’s test were used to assess publication bias, and sensitivity analyses via a leave-one-out approach were performed to evaluate the robustness of results.
Results: Five studies encompassing 7 cohorts and 2,349 patients (1,306 APAP users and 1,043 non-users) were included. Pooled analyses revealed that concomitant APAP use was significantly associated with shorter OS (HR: 1.29; 95% CI: 1.16–1.44) and PFS (HR: 1.27; 95% CI: 1.12–1.43), as well as a trend toward a lower objective response rate (RR: 0.78; 95% CI: 0.60–1.00). No significant publication bias was detected, and sensitivity analyses confirmed the robustness of these findings.
Conclusion: Current evidence indicates that APAP use is associated with poorer prognosis in cancer patients treated with ICIs. These results may inform clinical guidelines regarding concomitant APAP and ICI use. Further randomized controlled trials are warranted to validate these observations and establish causal relationships.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251118489.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment by reactivating cytotoxic T-cell responses against malignant cells (1). By targeting negative regulatory pathways such as programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), ICIs have demonstrated durable clinical benefits and survival advantages in a variety of tumor types (2). Despite these breakthroughs, a considerable proportion of patients fail to achieve sustained responses, underscoring the need to identify factors that influence immunotherapy efficacy.
One increasingly recognized yet underexplored factor is the role of concomitant medications in modulating ICI outcomes (3–5). Several classes of non-oncologic agents—including corticosteroids, antibiotics, and proton pump inhibitors—have been implicated in dampening ICI efficacy, either through direct immunosuppression or via disruption of host-microbiota interactions (6–9). Acetaminophen (APAP), one of the most frequently used antipyretic and analgesic agents in oncology, is widely regarded as safe and well-tolerated (10). However, emerging evidence suggests that APAP may exert unintended immunomodulatory effects, raising concern about its potential to compromise antitumor immune responses (11).
Mechanistic studies have shown that APAP can inhibit T-cell proliferation, suppress interferon-gamma (IFN-γ) production, and promote regulatory T cell (Treg) expansion—features that collectively impair cytotoxic immunity (11). In addition, APAP has been reported to elevate levels of immunosuppressive cytokines such as interleukin-10 (IL-10) and to attenuate antigen-presenting cell function (11). Clinical observations echo these findings: APAP exposure has been associated with reduced antibody responses to viral infections and vaccinations, prompting international health agencies to advise caution regarding its routine use in immunologically sensitive contexts (12).
Despite these signals, clinical data on APAP use during ICI therapy remain limited and inconsistent. Retrospective studies examining the association between APAP exposure and ICI outcomes have yielded conflicting results (11, 13). Given the ubiquity of APAP use in cancer care—and the increasing reliance on ICIs as a cornerstone of systemic therapy—clarifying this relationship is of substantial clinical importance.
To address this gap, we conducted a systematic review and meta-analysis of studies evaluating the prognostic impact of APAP use in cancer patients treated with ICIs. By integrating data across cohorts and tumor types, we aimed to quantify the association between APAP exposure and survival outcomes, assess potential biases, and provide evidence-based guidance for the supportive care of patients undergoing immunotherapy.
Methods
Study registration
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14). The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (identifier: CRD420251118489).
Data sources and search strategy
A systematic literature search was conducted using PubMed, Web of Science, and Embase, covering all records from their inception to August 2, 2025. The search strategy combined terms related to acetaminophen (e.g., acetaminophen, paracetamol, N-acetyl-para-aminophenol) and immune checkpoint inhibitors (e.g., immune checkpoint inhibitors, PD-1 inhibitors, PD-L1 inhibitors, CTLA-4 inhibitors, pembrolizumab, nivolumab, atezolizumab, ipilimumab), as well as terms pertaining to prognostic outcomes. The complete search strategies for each database are detailed in Supplementary Table 1.
Eligibility criteria and study selection
Studies were deemed eligible if they met the following criteria: (i) assessed the prognostic impact of APAP use in patients receiving ICIs, with comparator groups comprising patients not receiving APAP (concomitant use of other medications permitted); and (ii) reported hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for overall survival (OS) and/or progression-free survival (PFS). Eligible study designs included randomized controlled trials (RCTs) and observational cohort studies. Exclusion criteria encompassed review articles, case reports, preclinical studies, studies not evaluating APAP in relation to clinical outcomes, and those comparing high-dose versus low-dose APAP without a non-APAP control group.
The literature was managed and deduplicated using EndNote. Two independent reviewers conducted the initial screening based on titles and abstracts, followed by full-text evaluations for final inclusion. Any discrepancies were resolved through discussion with a third reviewer.
Data extraction and quality assessment
Two reviewers independently extracted data using a standardized data collection form. Any discrepancies were resolved through consensus or, if necessary, by consultation with a third reviewer. The following information was extracted:
Study characteristics: First author, publication year, country, study design, and sample size.
Patient characteristics: Cancer type, ICI regimen, and number of patients receiving APAP.
Outcome data: HRs with corresponding 95% CIs for OS and PFS, as well as objective response rate (ORR). When both unadjusted and adjusted HRs were reported, adjusted estimates controlling for potential confounders were prioritized.
The methodological quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) for cohort studies, which evaluates study quality across three domains: selection of study groups, comparability of groups, and ascertainment of outcomes (maximum score = 9 stars). Studies with a NOS score ≥ 7 were be considered high quality (15).
Statistical analysis
Meta-analyses were conducted using R software (version 4.5.1). Heterogeneity among studies was assessed using the I² statistic and Cochran’s Q test. An I² value greater than 50% along with a Q-test p-value less than 0.10 was considered indicative of significant heterogeneity. The DerSimonian–Laird random-effects model was applied when substantial heterogeneity was present, whereas the Mantel–Haenszel fixed-effects model was used for homogeneous data. Pooled effect sizes were calculated for each outcome. HRs with 95% CIs were used for OS and PFS. Odds ratios (OR) with 95% CIs were used for ORR. Publication bias was assessed using funnel plots and Egger’s regression test, with a p-value less than 0.05 indicating significant bias. Sensitivity analyses were performed by sequentially excluding each study to evaluate the robustness of the results. All statistical tests were two-sided, and a p-value less than 0.05 was considered statistically significant.
Results
Study selection
The systematic literature search initially identified 96 potentially relevant records. After removing duplicates, 84 unique publications remained for preliminary eligibility assessment. Title and abstract screening led to the exclusion of 75 articles that were not relevant to the research objectives. As a result, 9 articles proceeded to full-text evaluation. Following rigorous application of the predefined inclusion criteria, 5 studies were deemed eligible and included in the final quantitative synthesis (11, 13, 16–18). The complete study selection process is illustrated in Figure 1.
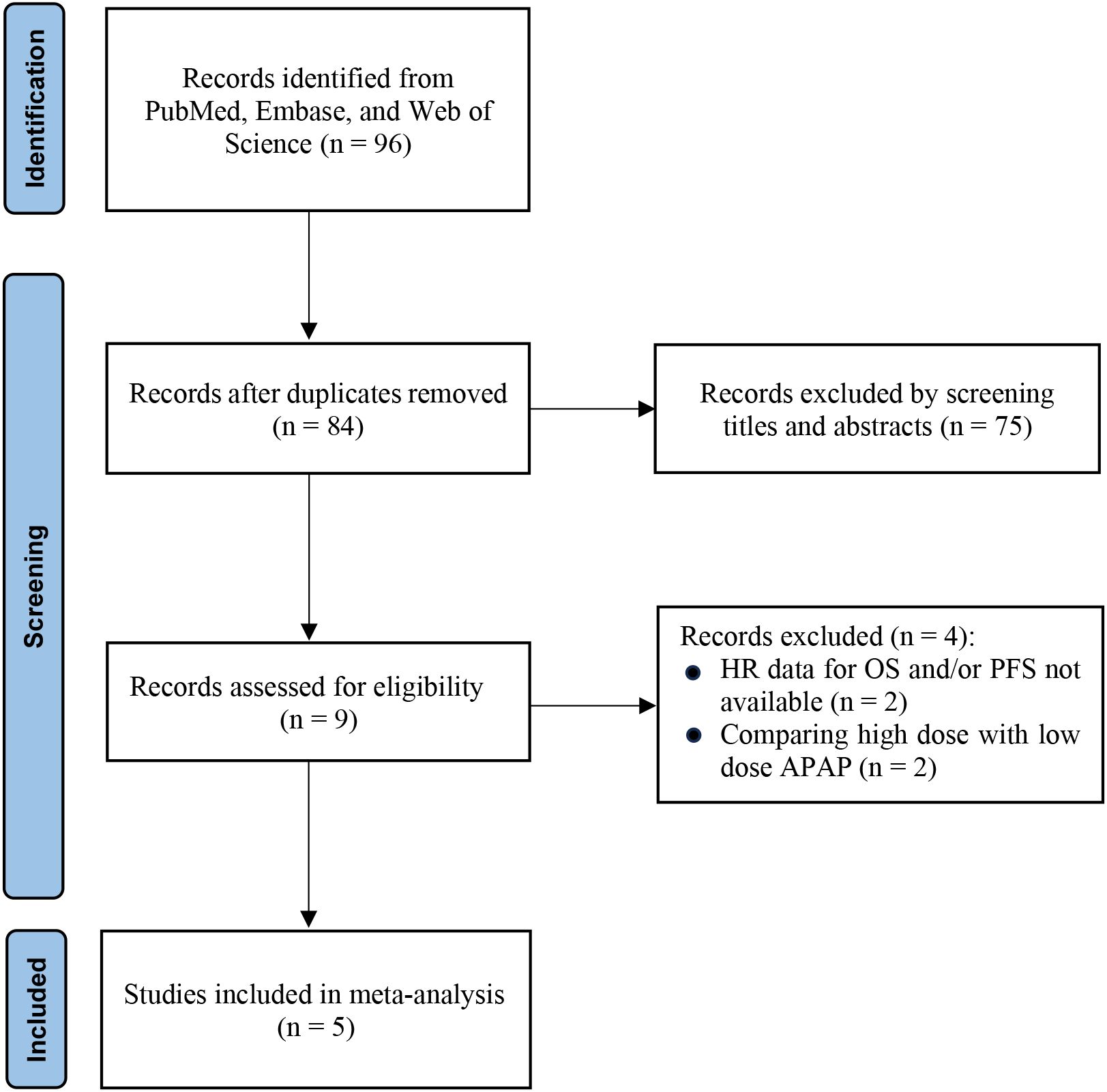
Figure 1. PRISMA flow diagram of study selection. Flowchart summarizing the literature search and selection process according to PRISMA 2020 guidelines. The diagram shows the number of records identified, screened, excluded (with reasons), and finally included in the meta-analysis.
Characteristics of the included studies
All five included studies employed retrospective cohort designs, collectively comprising seven independent cohorts and a total of 2,349 patients (1,306 APAP users and 1,043 non-users). These studies were published between 2022 and 2025 and represented diverse geographic regions, including France (4 cohorts), China (Hong Kong; 1 cohort), Italy (1 cohort), Japan (1 cohort), and one multinational cohort. Considerable heterogeneity was observed in cohort sizes, which ranged from 34 to 753 patients, and in the prevalence of APAP exposure, which varied from 26.0% to 74.8%.
The included cohorts investigated a range of cancer types. Four focused exclusively on non-small cell lung cancer (NSCLC), one on renal cell carcinoma (RCC), and two included patients with various solid tumors. ICI regimens consisted of anti-PD-1/PD-L1 monotherapy (e.g., nivolumab, pembrolizumab, atezolizumab) or combination therapy with anti-CTLA-4 agents (e.g., ipilimumab). Detailed study characteristics are summarized in Table 1.
Methodological quality, as assessed using the NOS, was uniformly high. Six cohorts received the maximum score of 9 out of 9, and one cohort received a score of 8, as presented in Supplementary Table 2.
APAP use and prognosis in patients receiving ICIs
All studies included in this meta-analysis comprehensively examined the association between APAP use and OS in patients treated with ICIs, encompassing a total of 2,349 patients (APAP users: 1,306; non-users: 1,043). Notably, no heterogeneity was observed across studies (I²=0%), allowing for the application of a fixed-effect model to estimate the pooled HR. The analysis demonstrated that APAP use was significantly associated with a 29% increased risk of death (HR: 1.29; 95%CI: 1.16–1.44; Figure 2A), suggesting a potential detrimental effect of APAP on the efficacy of ICIs.
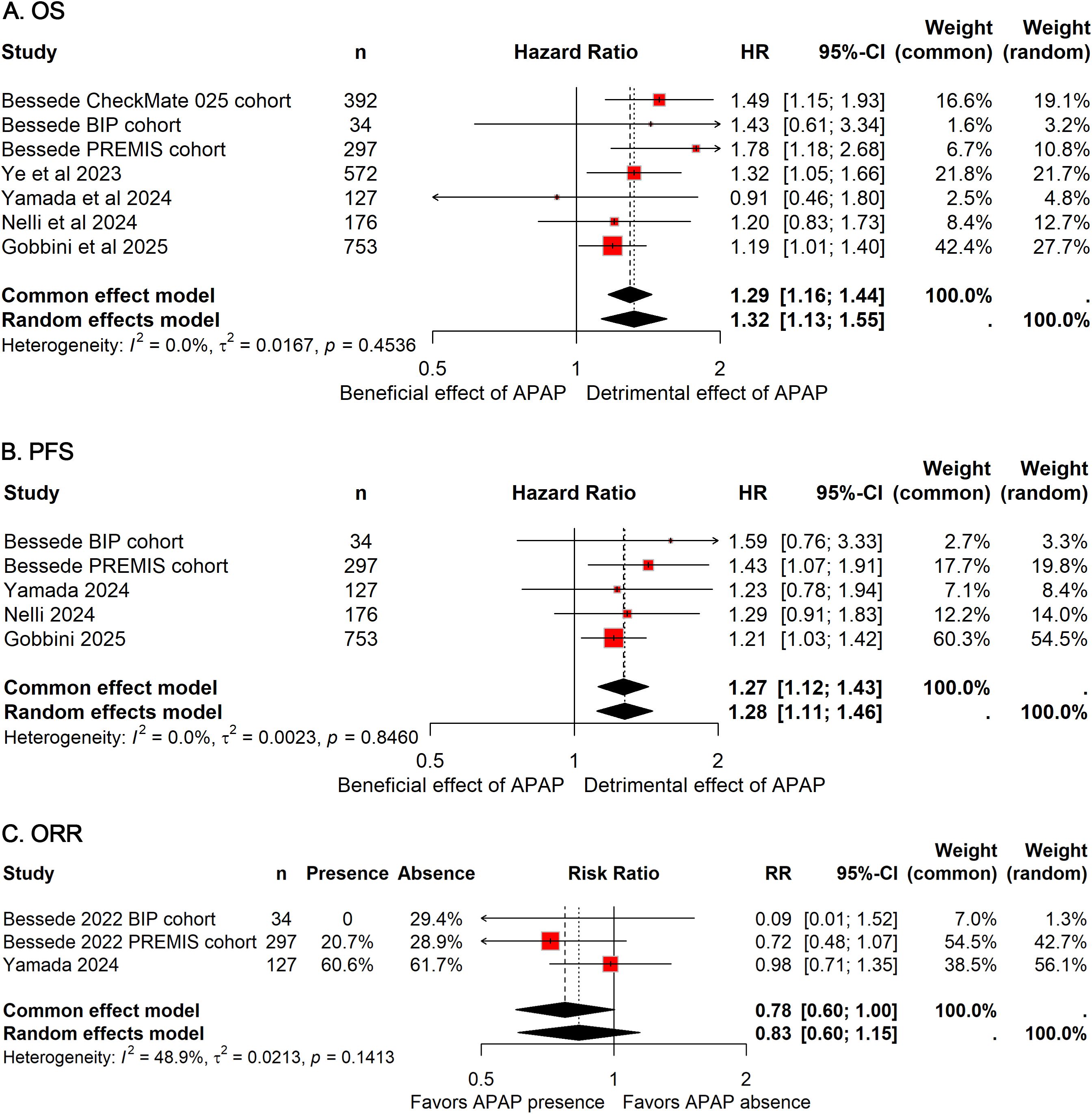
Figure 2. Forest plots of the prognostic impact of APAP use in patients receiving ICIs. Pooled HRs for OS (A) and PFS (B), and pooled OR for ORR (C) are shown. Squares represent individual study estimates, with size proportional to study weight; horizontal lines indicate 95% CIs. Diamonds represent pooled estimates derived from random-effects models. HRs greater than 1 or OR less than 1 indicate worse outcomes in APAP users compared with non-users.
Five cohorts, including 1,387 patients (APAP users: 777; non-users: 610), assessed the impact of APAP use on PFS (11, 13, 17, 18). Consistent with OS findings, no heterogeneity was detected (I²=0%), and a fixed-effect model was utilized. The pooled results indicated a 27% reduction in PFS among patients receiving APAP concomitantly with ICIs (HR: 1.27; 95% CI: 1.12–1.43; Figure 2B), further supporting the notion that APAP use may negatively influence survival outcomes in this population.
Regarding ORR, data from three cohorts comprising 458 patients (APAP users: 217; non-users: 241) were analyzed (11, 13). The combined OR suggested a non-significant trend toward reduced ORR with concurrent APAP use compared to non-use (OR: 0.78; 95% CI: 0.60-1.00; Figure 4C).
Publication bias and sensitivity analysis
Funnel plots and Egger’s regression tests revealed no significant publication bias for OS and PFS outcomes (Figure 3). Furthermore, sensitivity analyses employing a leave-one-out method confirmed the stability and robustness of the pooled estimates for both OS and PFS (Figure 4).
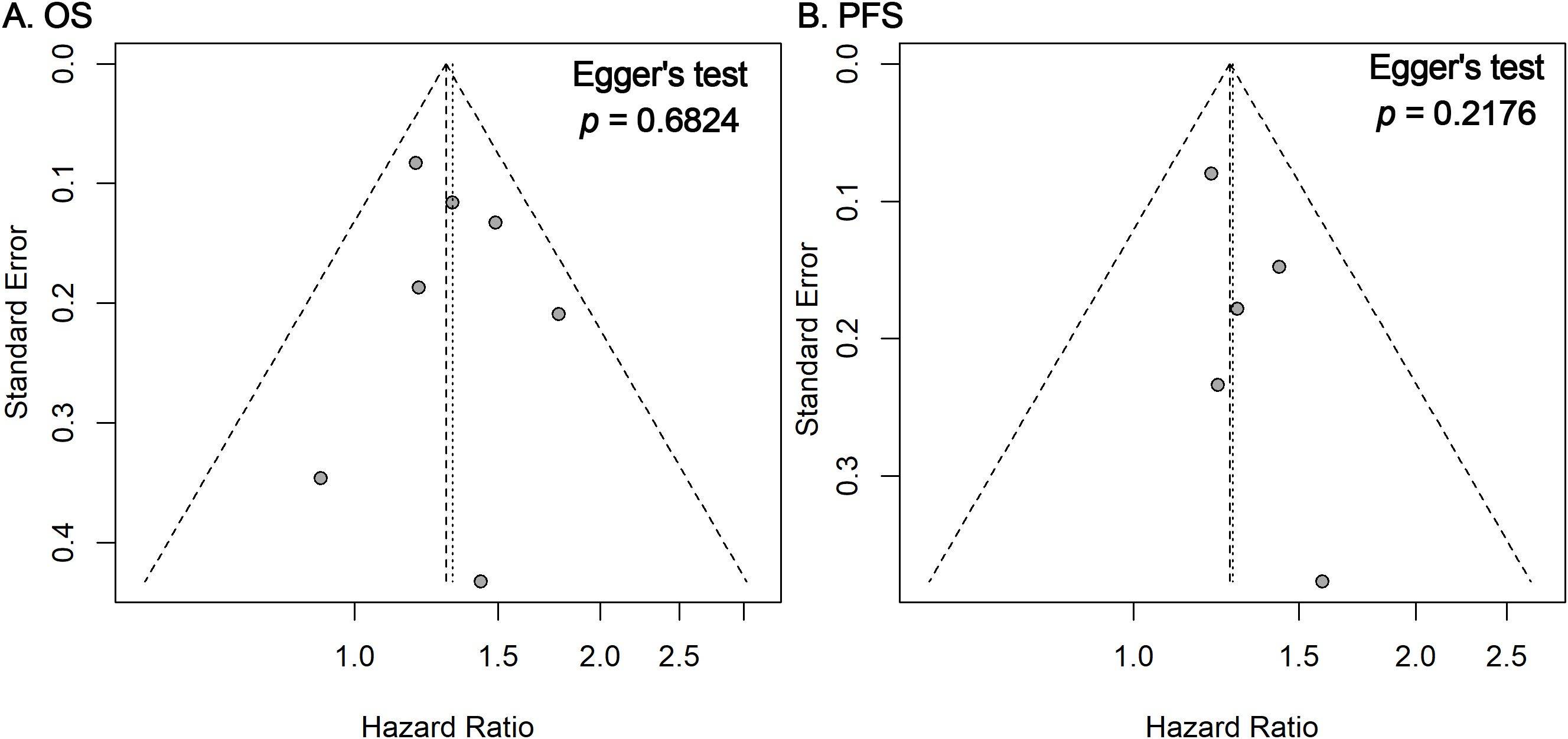
Figure 3. Funnel plots and Egger’s tests for publication bias. Funnel plots for OS (A) and PFS (B) outcomes showing the distribution of study-specific log HRs against their standard errors. Visual symmetry suggests a low likelihood of publication bias, which was further evaluated using Egger’s regression test (p-values shown).
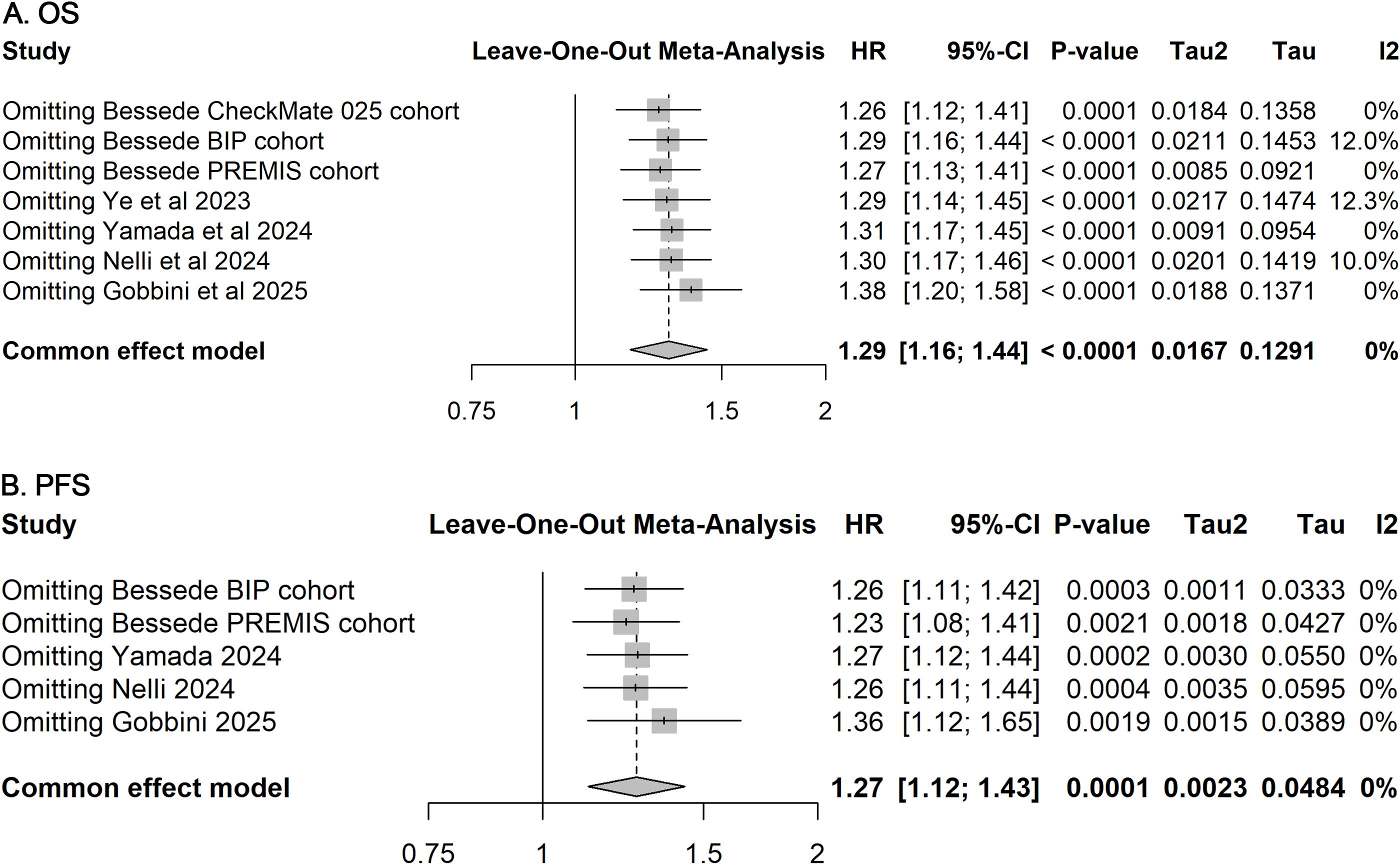
Figure 4. Sensitivity analyses for included studies on OS and PFS. Leave-one-out sensitivity analyses assessing the influence of each individual study on the pooled HR for OS (A) and PFS (B). The stability of pooled estimates upon sequential exclusion of single studies indicates the robustness of the overall results.
Discussion
This meta-analysis consolidates evidence from 2,349 patients across seven cohorts, revealing a consistent association between APAP use and diminished clinical outcomes in cancer patients receiving ICIs. The observed HRs—1.29 for OS and 1.27 for PFS—translate to a nearly 30% increased risk of death or disease progression among APAP-exposed individuals. These findings assume critical significance in the context of immuno-oncology, where ICIs rely on robust T-cell-mediated antitumor responses, and even subtle immune modulation by concomitant medications can undermine therapeutic efficacy. The homogeneity of effects across diverse populations (I²=0%) and robustness to sensitivity analyses further underscore the clinical relevance of this association.
Although no significant statistical heterogeneity was detected, underlying variability in patient characteristics, tumor type, and APAP exposure definitions may have contributed to subtle clinical differences among studies. For example, four included cohorts exclusively enrolled NSCLC patients, while others involved RCC or mixed tumor populations, potentially influencing immune responsiveness. Moreover, some studies quantified plasma APAP levels, reflecting direct systemic exposure, whereas others relied on prescription data, which may underestimate biologically relevant dosing. Despite these variations, the direction of effect remained remarkably consistent across cohorts, suggesting a genuine association rather than a population-specific artifact. Future prospective analyses with standardized exposure definitions are warranted to validate this relationship.
The biological plausibility of the detrimental impact of APAP on ICI efficacy is supported by converging mechanistic evidence that elucidates how APAP interferes with the immune pathways targeted by checkpoint blockade. The therapeutic activity of ICIs depends on restoring cytotoxic CD8+ T-cell function and overcoming immunosuppressive barriers within the tumor microenvironment. APAP appears to counteract these processes through several complementary mechanisms. First, APAP fosters an immunosuppressive milieu by promoting regulatory T-cell (Treg) expansion and elevating interleukin-10 (IL-10) levels (11). These expanded Tregs further suppress effector T-cell proliferation and cytotoxic activity, partly through the release of inhibitory cytokines such as TGF-β (19). This immune shift toward tolerance has been observed in patients with detectable plasma APAP at ICI initiation, who exhibited increased circulating Tregs and IL-10 concentrations correlated with inferior survival outcomes (11). Second, APAP directly impairs effector T-cell function by suppressing interferon-gamma (IFN-γ) production—a key cytokine essential for antitumor immunity, antigen presentation, and tumor cell killing (11, 20). Experimental studies have demonstrated that APAP reduces IFN-γ secretion in anti–PD-1–stimulated peripheral blood mononuclear cells, indicating a direct antagonistic effect on the immune activation intended by PD-1 blockade (11). Beyond this, emerging evidence suggests that APAP may disrupt mitochondrial function and cellular energy metabolism, potentially compromising the bioenergetic demands of T-cell activation and proliferation, though this pathway warrants further investigation in the context of immunotherapy (21–23). Furthermore, APAP has been shown to induce neutrophil extracellular trap (NET) formation (24). These NETs not only hinder T-cell infiltration and cytotoxic function but may also promote tumor progression (25). In APAP-induced liver injury models, NETosis has been associated with AIM2 inflammasome activation and inflammatory cell death (PANoptosis), potentially exacerbating tissue injury and reinforcing an immunosuppressive microenvironment (26). This mechanism, already linked to reduced response to neoadjuvant chemoimmunotherapy in NSCLC (24), represents a novel physical and functional barrier through which APAP may diminish immunotherapy efficacy. Collectively, these findings highlight that APAP compromises multiple arms of antitumor immunity—including amplification of immunosuppressive circuits, suppression of effector T-cell signaling, potential disruption of T-cell metabolism, and induction of NET-mediated barriers—offering a coherent and multifaceted biological rationale for the poorer survival outcomes observed in APAP-exposed patients.
A critical consideration in interpreting these findings is the potential for confounding by indication. Patients requiring APAP may have a higher baseline symptom burden, such as pain from bone metastases or cancer-related fever, which itself reflects more aggressive disease biology and poorer prognosis. While most included studies employed multivariate analyses adjusting for key confounders—such as performance status, tumor burden, and line of therapy—the possibility of residual confounding from unmeasured factors (e.g., systemic inflammation or concurrent infection) cannot be fully excluded. Nevertheless, the consistency of the detrimental association across multiple independent cohorts, even after statistical adjustment, supports the likelihood of an independent effect of APAP. Future prospective studies with detailed documentation of indication-for-use and concomitant medications are warranted to further disentangle this relationship.
The dose–response relationship between APAP exposure and ICI outcomes has begun to emerge, lending further support to a potential causal link. Although high doses (e.g., 4 g/day) have been shown to induce Treg expansion in healthy volunteers (11), the immunologic effects of typical oncologic doses (1–2 g/day) require further clarification. Clinically, studies stratifying patients by plasma APAP concentration have demonstrated that higher levels at ICI initiation are associated with significantly worse survival outcomes (24, 27). Similarly, in real-world cohorts, high cumulative APAP exposure (e.g., ≥60 doses of 1000 mg) was identified as an independent predictor of shorter PFS and OS in NSCLC patients receiving ICIs, whereas low or short-term exposure showed no significant impact (27). Although the precise threshold for clinically relevant risk remains undefined, these findings suggest that both the magnitude and timing of APAP exposure—particularly sustained, high-dose use during early immune activation—may be key determinants of immunotherapy efficacy.
Notably, studies stratifying patients by APAP plasma concentration support a potential dose-response gradient, with higher levels at ICI initiation linked to significantly worse survival (24, 27). This implies that not only the presence but also the timing, dose, and systemic availability of APAP may modulate immunotherapy efficacy. In real-world settings, high cumulative APAP exposure (≥60 doses of 1000 mg) independently predicts shorter PFS and OS in NSCLC patients on ICIs, whereas low exposure (<24 hours or <60 doses) shows no significant impact, offering preliminary guidance for clinical practice (27).
The pooled hazard ratios of 1.29 for OS and 1.27 for PFS translate to an approximately 30% increase in the risk of death or disease progression among APAP users. This effect size is not only statistically significant but also clinically meaningful. In the field of immuno-oncology, a 30% increase in mortality risk represents a substantial effect, comparable to the detrimental impact observed with baseline corticosteroid or antibiotic use in patients receiving ICIs. This magnitude of risk underscores the need for heightened awareness and strategic management of concomitant APAP exposure. These findings also emphasize that even a widely perceived “safe” medication may compromise immunotherapy efficacy, highlighting the importance of incorporating medication review and pharmacovigilance into routine immunotherapy management to identify and mitigate modifiable risk factors.
Given these complexities, a risk-adapted approach to symptom management is warranted. For mild symptoms, non-pharmacologic measures such as physical therapy or cooling should be prioritized. When analgesia is necessary, short-term APAP use (<72 hours) may be acceptable in low-risk settings (e.g., during ICI maintenance or among strong responders), whereas NSAIDs with gastroprotection or low-dose opioids may be preferable in high-risk contexts (e.g., during early ICI cycles or in patients with low PD-L1 expression). Importantly, symptom control should not be compromised; analogous to WHO recommendations discouraging prophylactic APAP before vaccination to preserve immune responses, similar principles of timing and selective avoidance may apply in immunotherapy (28). NSAIDs, in particular, have shown improved OS compared to APAP in NSCLC patients receiving ICIs, though their use requires caution in those with renal or gastrointestinal comorbidities (16).
Several limitations of the primary studies merit consideration. First, retrospective designs risk residual confounding by indication, as patients requiring APAP may have higher symptom burdens reflective of aggressive disease. Second, variability in APAP exposure metrics—from plasma quantification to prescription records—precludes definitive dose-response conclusions. Third, insufficient data on concomitant medications (e.g., corticosteroids or antibiotics) limits assessment of drug-drug interactions. Finally, the predominance of NSCLC cohorts raises questions about generalizability to other malignancies.
Future research should address these gaps. Prospective studies quantifying APAP dose, timing, and indication relative to ICI cycles could identify vulnerability thresholds (e.g., >3 g/day within 48 hours of infusion). Biomarker-integrated studies measuring Treg or IL-10 dynamics in response to APAP may identify high-risk patients. Pragmatic trials comparing APAP-restrictive and permissive strategies—stratified by cancer type and co-medications—are ethically feasible using endpoints like pain control and ICI response. Regulatory updates, such as requiring drug labels to highlight APAP-ICI interactions, and documenting APAP exposure in electronic medical records, could facilitate real-world risk assessments and guide clinical decision-making.
Conclusion
Current evidence indicates that APAP use is associated with poorer outcomes in ICI-treated cancer patients, likely via suppression of antitumor immunity. Clinicians should minimize unnecessary or prolonged APAP use during critical periods of immune activation, prioritizing alternative symptom management strategies when feasible. Further research is needed to establish causal relationships and define safe thresholds to guide evidence-based stewardship.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YuL: Conceptualization, Data curation, Writing – original draft, Visualization, Formal Analysis. YoL: Writing – original draft, Visualization, Formal Analysis. JS: Writing – review & editing, Supervision, Data curation, Project administration, Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors extended their appreciation to the databases that provided the invaluable data resources for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Polish the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1682686/full#supplementary-material
References
1. Ribas A and Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
2. Morad G, Helmink BA, Sharma P, and Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. (2021) 184:5309–37. doi: 10.1016/j.cell.2021.09.020
3. Shen J, Hou H, Liang B, Guo X, Chen L, Yang Y, et al. Effect of renin-angiotensin-aldosterone system inhibitors on survival outcomes in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1155104. doi: 10.3389/fimmu.2023.1155104
4. Yu J, Meng F, Sui W, Yu J, and Shen J. Concomitant use of renin-angiotensin system inhibitors augments the efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Front Pharmacol. (2024) 15:1378577. doi: 10.3389/fphar.2024.1378577
5. Liao Y, Lin Y, Ye X, and Shen J. Concomitant statin use and survival in patients with cancer on immune checkpoint inhibitors: A meta-analysis. JCO Oncol Pract. (2025) 21:989–1000. doi: 10.1200/OP-24-00583
6. Li N, Zheng X, Gan J, Zhuo T, Li X, Yang C, et al. Effects of glucocorticoid use on survival of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors. Chin Med J (Engl). (2023) 136:2562–72. doi: 10.1097/CM9.0000000000002544
7. Zhou J, Huang G, Wong WC, Hu DH, Zhu JW, Li R, et al. The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2022) 13:968729. doi: 10.3389/fimmu.2022.968729
8. Lopes S, Pabst L, Dory A, Klotz M, Gourieux B, Michel B, et al. Do proton pump inhibitors alter the response to immune checkpoint inhibitors in cancer patients? A meta-analysis. Front Immunol. (2023) 14:1070076. doi: 10.3389/fimmu.2023.1070076
9. Shen J, Ye X, Hou H, and Wang Y. Clinical evidence for the prognostic impact of metformin in cancer patients treated with immune checkpoint inhibitors. Int Immunopharmacol. (2024) 134:112243. doi: 10.1016/j.intimp.2024.112243
10. Wiffen PJ, Derry S, Moore RA, McNicol ED, Bell RF, Carr DB, et al. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. (2017) 7:Cd012637. doi: 10.1002/14651858.CD012637.pub2
11. Bessede A, Marabelle A, Guégan JP, Danlos FX, Cousin S, Peyraud F, et al. Impact of acetaminophen on the efficacy of immunotherapy in cancer patients. Ann Oncol. (2022) 33:909–15. doi: 10.1016/j.annonc.2022.05.010
12. Saleh E, Moody MA, and Walter EB. Effect of antipyretic analgesics on immune responses to vaccination. Hum Vaccin Immunother. (2016) 12:2391–402. doi: 10.1080/21645515.2016.1183077
13. Yamada J, Fukui T, Yatani A, Mimura C, Fukuda K, Hazama D, et al. Impact of concurrent medications on the outcome of immunotherapy in non-small cell lung carcinoma. Thorac Cancer. (2024) 15:1228–36. doi: 10.1111/1759-7714.15294
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Ye ZH, Wang S, Zhang J, Lee TTL, Wai AKC, and Kong F. 2098P The impact of concomitant use of non-opioid analgesics and immune checkpoint inhibitors on survival in lung cancer patients: A Hong Kong population-based cohort study. Ann Oncol. (2023) 34:S1101. doi: 10.1016/j.annonc.2023.09.880
17. Nelli F, Ruggeri EM, Schirripa M, Virtuoso A, Giannarelli D, Raso A, et al. Longitudinal assessment of FT3 to FT4 conversion ratio in predicting the efficacy of first-line pembrolizumab-based therapy in advanced non-small cell lung cancer: A propensity-score matching analysis of data from the national drug monitoring agency. Curr Oncol. (2024) 31:7647–62. doi: 10.3390/curroncol31120564
18. Gobbini E, Langlais A, Missy P, Chanoine S, Besse B, Souquet PJ, et al. Morphine and metformin impact immunotherapy benefit in patients with NSCLC: Results of the real-world study IFCT-1502 CLINIVO-SNDS. Eur J Cancer. (2025) 225:115560. doi: 10.1016/j.ejca.2025.115560
19. Ohue Y and Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. (2019) 110:2080–9. doi: 10.1111/cas.14069
20. Wawrzyniak P and Hartman ML. Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors. Mol Cancer. (2025) 24:89. doi: 10.1186/s12943-025-02294-x
21. Jaeschke H and Ramachandran A. Acetaminophen hepatotoxicity: paradigm for understanding mechanisms of drug-induced liver injury. Annu Rev Pathol. (2024) 19:453–78. doi: 10.1146/annurev-pathmechdis-051122-094016
22. Du K, Ramachandran A, and Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. (2016) 10:148–56. doi: 10.1016/j.redox.2016.10.001
23. Fu X, Xu M, Zhang H, Li Y, Li Y, and Zhang C. Staphylococcal enterotoxin C2 mutant-directed fatty acid and mitochondrial energy metabolic programs regulate CD8(+) T cell activation. J Immunol. (2020) 205:2066–76. doi: 10.4049/jimmunol.2000538
24. Li C, Wu J, Zhang L, Wang F, Xu L, Zhao Y, et al. Brief report: acetaminophen reduces neoadjuvant chemoimmunotherapy efficacy in patients with NSCLC by promoting neutrophil extracellular trap formation: analysis from a phase 2 clinical trial. JTO Clin Res Rep. (2023) 4:100556. doi: 10.1016/j.jtocrr.2023.100556
25. Zuo H, Yang M, Ji Q, Fu S, Pu X, Zhang X, et al. Targeting neutrophil extracellular traps: A novel antitumor strategy. J Immunol Res. (2023) 2023:5599660. doi: 10.1155/2023/5599660
26. Zeng FL, Zhang Y, Wang ZH, Zhang H, Meng XT, Wu YQ, et al. Neutrophil extracellular traps promote acetaminophen-induced acute liver injury in mice via AIM2. Acta Pharmacol Sin. (2024) 45:1660–72. doi: 10.1038/s41401-024-01239-2
27. Nelli F, Virtuoso A, Giannarelli D, Fabbri A, Giron Berrios JR, Marrucci E, et al. Effects of acetaminophen exposure on outcomes of patients receiving immune checkpoint inhibitors for advanced non-small-cell lung cancer: A propensity score-matched analysis. Curr Oncol. (2023) 30:8117–33. doi: 10.3390/curroncol30090589
28. CDC. Vaccine administration (2024). Available online at: https://www.cdc.gov/vaccines/hcp/imz-best-practices/vaccine-administration.html?CDC_AAref_Val=https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/administration.htmlprint (Accessed October 12, 2025).
Keywords: acetaminophen, cancer, immune checkpoint inhibitors, prognosis, meta-analysis
Citation: Lin Y, Liao Y and Shen J (2025) Acetaminophen use and prognosis in cancer patients treated with immune checkpoint inhibitors: evidence from a meta-analysis. Front. Immunol. 16:1682686. doi: 10.3389/fimmu.2025.1682686
Received: 09 August 2025; Accepted: 06 November 2025; Revised: 12 October 2025;
Published: 21 November 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesCopyright © 2025 Lin, Liao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhai Shen, c2hlbmpoX3BoYXJtQDEyNi5jb20=
†ORCID: Jinhai Shen, orcid.org/0000-0001-8673-087X
 Yuxuan Lin
Yuxuan Lin Yonghe Liao
Yonghe Liao Jinhai Shen
Jinhai Shen