- 1World Health Organization, Regional Office for the Eastern Mediterranean, Health Emergencies Programme, Cairo, Egypt
- 2Center for Scientific Excellence for Influenza Viruses, National Research Center, Giza, Egypt
- 3Human Link, Dubai, United Arab Emirates
Operationalizing global One Health strategies at the field level to prevent and control vector-borne and zoonotic diseases (VBZDs) is of significant public health importance. Such strategies should be coordinated at the human–animal–ecosystems interface and applied at the national, regional, and global levels through the enforcement of effective policies. We aimed to develop a regional framework that can aid countries of the World Health Organization Eastern Mediterranean region to better prevent, detect, and respond to VBZDs events. This strategic guidance is a twelve-element framework drafted using various guidance documents and peer-reviewed scientific literatures, incorporating recommendations made through expert consultations. The framework elements were then integrated within a logical framework designed for practical implementation of One Health at regional and country level.
1 Introduction
Emerging and re-emerging vector-borne and zoonotic diseases (VBZDs) are of great socio-economic and public health concern across the world. Most infectious diseases (60%) that recently emerged in humans originated in animals (1). Zoonotic diseases, defined as infectious diseases naturally transmitted from vertebrate animals to humans and vice versa, can be foodborne, waterborne, or vector- borne (2, 3). A zoonosis can also be transmitted directly (e.g. contact with contaminated fluids) and indirectly (e.g. blood transfusion). Zoonotic diseases can be classified into three classes: endemic zoonoses which are present in many geographical areas and are widespread among animal and human populations; epidemic zoonoses which are sporadic in temporal and spatial distribution; and emerging and re-emerging zoonoses which are newly appearing in a population or have existed previously but are rapidly increasing in incidence or geographical range.
Globally, more than 17% of all infectious diseases are vector-borne, and they are responsible for more than 700,000 fatalities each year (4). Vector-borne diseases are not directly communicable among humans, rather transmission occurs when favorable conditions are present (1). Previous studies reported that vector-borne human infectious diseases like yellow fever and dengue fever exhibited a wider range of distribution as several vector-borne pathogens have colonized new regions in the past two decades (1, 5).
Given the continuous increase of emerging and re-emerging VBZDs globally, there is a significant need for intersectoral coordination teams of public health practitioners, researchers, veterinarians, wildlife specialists, clinicians, environmental health specialists, and others to collaborate on emerging VBZD research and surveillance (6–8). The three international organizations, the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the World Organization for Animal Health (WOAH) recognize a joint responsibility for addressing zoonotic and other high impact diseases and have been working together for several decades to minimize the health, social, and economic impact of diseases arising at the human-animal interface (9). In 2010, the three organizations published a Tripartite Concept Note, describing their collaboration and objectives in the prevention and control of health risks at the human–animal–ecosystems interface (9).
On 17 March 2022, the heads of the three organizations and that of the United Nations Environmental Program (UNEP) signed a Memorandum of Understanding for joint One Health works, by which the UNEP joined the former Tripartite as an equal partner to form a new Quadripartite Collaboration for intersectoral coordination.
The Eastern Mediterranean Region (EMR) of the WHO remains prone to VBZD infections owing to large number of people in the region (about 9% of the world’s population) living in contact with animals and increased volume of international trade, including transboundary mass population and livestock movement between neighboring countries. International travel, globalization, and variable levels of health systems capacity to early detect epidemics have been identified as significant risk factors for emergence and rapid international spread of infectious diseases with zoonotic origin (10).
The EMR has witnessed outbreaks and increasing epidemic concerns of VBZDs including: Crimean–Congo hemorrhagic fever (CCHF) in Afghanistan, Iraq, Iran, Pakistan, Sudan and Oman, dengue in Afghanistan, Djibouti, Egypt, Oman, Pakistan, Sudan, Saudi Arabia and Yemen, MERS-CoV in Bahrain, Egypt, Iran, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Tunisia, UAE and Yemen, Yellow fever in Sudan (11), Chikungunya in Yemen, Pakistan, Somalia and Sudan (12), West Nile fever in Tunisia (13), and Q fever in Afghanistan (14, 15) and Iraq (16). To note, this is just the tip of the iceberg and there are many other VBZDs becoming a major threat to countries in the EMR (17).
Experiences from the EMR have shown that managing VBZD infections poses significant challenges due to the fragmented and insufficient surveillance system, lack of appropriate and safe sample shipment mechanisms, insufficient on-site or in-country laboratory diagnostic facilities, and limited capacity of countries to plan, mobilize, and implement appropriate control measures in geographically dispersed areas. These challenges can hinder effectiveness and performance of control measures. Other major limitations in controlling the VBZD infections in the region include the lack of effective collaboration between the animal and human health sectors, integrated surveillance and reporting systems, limited institutionalized inter-sectoral collaboration mechanisms between key sectors, and inadequate communication between Ministries of Health and Agriculture (1).
International non-governmental organizations and lead experts in the field have combined several efforts to address VBZDs within the One Health context. However, there are no existing strategic frameworks or guidance for prevention and control of emerging and re-emerging VBZDs in the EMR that can support strengthening joint response and the collaboration and information sharing among human and animal sectors.
The urgent public health concern of emerging and re-emerging VBZDs in the region necessitated us to develop a Strategic Guidance Framework in order to enhance the country and pan-regional capacity for the prevention, detection, and response to those rising threats. This framework includes a logical framework (Appendix A) as well as a narrative document that explains all key elements and disease-specific annexes. The Framework and its elements were discussed and validated by a group of representatives from the EMR countries and international experts during a VBZD consultative meeting in January 2023.
2 Development of the framework for strategic guidance
The objective was to develop a comprehensive framework that could enhance collaboration and information sharing between human and animal sectors. Additionally, we sought to identify best practices across multidisciplinary teams, prioritize key VBZDs in the EMR, and establish research priorities.
The strategic guidance is the final product of an extensive process which involved an in-depth understanding of existing evidence through systematic literature review (17), an extensive stakeholder consultation process which involved a regional stakeholder workshop with multiple working group and round table meetings, and review of the draft document by experts globally and regionally. Finally, the document was reviewed by the member states.
The literature review (17) analyzed articles in PubMed, Embase, and WHO Global Index Medicus published between 1st of January 2011 and 27th of June 2022. The review included 295 studies; 55% on leishmaniasis and dengue combined, and 75% studies from Pakistan, Kingdom of Saudi Arabia, and Iran combined. The review showed substantial burden of VBZDs in the region with significant differences between countries in terms of availability of data. Besides, we found scarcity of data for several countries with complex humanitarian emergencies. Even with limited data on the determinants of these VBZDs, we observed profound differences in burden across socioeconomic groups.
Expert consultation was done thorough a consultative meeting for prevention and control of emerging and re-emerging VBZDs in the EMR. The meeting was jointly held by WHO, FAO and WOAH in Amman, Jordan 29-31 January 2023, which brought together more than 100 experts from the member states, WHO headquarters, and regional and country offices, international agencies including the United States Centers for Disease Control and Prevention (US CDC), European Centre for Disease Prevention and Control (ECDC) and academic and research institutions.
The meeting resulted in a number of outcomes and recommendations that were used to refine the framework. Some of the key outcomes are addressed below:
1. Concrete and actionable recommendations that guide countries in information sharing mechanism of surveillance and laboratory data among animal and human sectors;
2. Identification and consensus on best practices across multidisciplinary teams to enhance joint and timely response by human and animal sectors;
3. Identification and consensus on key priority VBZDs in the region that can also serve as case demonstrations for integrated data sharing among human and animal sectors and coordinated outbreak response;
4. Identification of research priorities in the region for the next few years;
5. Technical recommendations for a regional strategic guidance development that encompasses identified data sharing mechanisms, best practices and disease/research priorities in control and response to VBZDs in the region;
6. Enhanced support for the development or modification of national VBZDs strategies that integrate surveillance and laboratory data between human and animal sectors in collaboration with line ministers and stakeholders;
7. Strengthen national, regional, and international technical coordination, collaboration and information-sharing among stakeholders on concerned VBZDs prevention, control and response in the region.
3 Possible implementation of the framework in the EMR
We first plan to propose and discuss the framework with public health services and policy makers, and make adaptations to meet the country specific contexts. One of the potential barriers that could hamper its implementation is the lack of effective collaboration between the animal and human health sectors in the EMR. There are limited institutionalized inter-sectoral collaboration mechanisms between these sectors, including inadequate communication between Ministries of Health and Agriculture (Veterinary Services). Finally, there is limited harmonization of the various regulatory public health frameworks.
Aiming to operationalize One Health, similar endeavors are ongoing in different parts of the world (18, 19). Latest survey and research (18, 19) show that major advantages in operationalizing One Health are not much different from the EMR. However, the major challenge that is specific to the EMR and could hamper operationalizing One Health is complex emergencies hence conflicting priorities in member states.
Over the past year, the EMR has seen a substantial increase in the number of emergencies due to multiple hazards. Across the Region, WHO responded to 55 disease outbreaks in 2022, compared to 31 in 2021 and 14 in 2020, and the number of people requiring humanitarian assistance has increased from 102.3 million to 127.3 million (20). Worsening insecurity, major natural disasters (floods in Pakistan, food security crisis in Somalia and Greater Horn of Africa), disruptions due to the COVID-19 pandemic and severe economic decline (e.g., Lebanon, the Syrian Arab Republic) have contributed to an unprecedented level of humanitarian need and vulnerability across the Region. There is also a convergence of crises in many countries with protracted humanitarian needs, e.g., Afghanistan, Somalia and Sudan have all experienced multiple outbreaks, natural disasters, insecurity and displacement throughout 2022. Political tensions and instability conspire to constrain the response to health emergencies. On contrary, the Region is also home to Gulf Cooperation Council (GCC); Saudi Arabia, Kuwait, the United Arab Emirates, Qatar, Bahrain, and Oman; and the GCC is making a great progress in One Health implementation (21).
Considering different priorities and public health challenges among countries in the EMR, there is a need to develop an approach to address underlying drivers across sectors to prevent or mitigate human, animal, and environmental health outcomes proactively. This can help reduce reliance on response which is resource intensive and difficult considering the particular health systems and socio-political and humanitarian challenges in the region. Therefore, we opted to develop this strategic guidance framework for VBZDs from a One Health perspective.
4 Framework elements
Based on the mentioned developmental process, twelve elements were identified as essential to be included in the strategic guidance framework. Refer to Supplementary Material for descriptions of the framework elements.
4.1 Leadership and governance
Clear leadership role identification, a well-defined chain of command and control, and clear mechanisms of coordination, collaboration, and communication between various sectors is key to optimum response. This can be achieved by 1) identifying the principal assigned authorities and mapping additional authorities who could be involved; 2) establishing a high-level committee with membership of all related authorities (virologists, immunologists, entomologists, infectious disease specialists, and veterinarians); 3) agreement on escalation of command system in case of alert; and 4) mapping of all available laws and regulations and deciding how to overcome inactivated laws and legislations to increase political commitment and supportive policies, laws, and regulations, and actions to be performed.
4.2 Operationalizing intersectoral coordination
Operationalization requires integrative approaches with the active engagement of the most affected stakeholders in their individual contexts. The following steps are recommended: 1) agreeing on the need for a multisectoral coordination mechanism for VBZDs and mapping of existing coordination mechanisms by assessing the available capacity and developing a stepwise operational guidance; 2) convening and endorsing an intersectoral coordination mechanism for VBZDs by performing experts and stakeholders mapping; 3) identifying best practices for intersectoral coordination; 4) institutionalization of intersectoral coordination communication, and information dissemination by involving academic institutions, private sector, and Non-Governmental Organizations (NGOs) in collaborative networks, ensuring transparency between human and animal health sectors, and developing intersectoral data sharing strategy; 5) identify social, cultural, political, financial, logistic, and legal determinants affecting the intersectoral coordination.
4.3 Prioritizing vector-borne and zoonotic diseases
While there is an extensive list of VBZDs in the EMR that are affecting both humans and animals, or only humans or animals, prioritizing both VBZDs and associated activities (e.g., aligning surveillance, developing a multisectoral preparedness plan) using a multisectoral approach is required to focus actions towards addressing VBZDs. It is also necessary to prioritize VBZDs aligning to other national health concerns.
Agreeing on priority VBZDs per country is an essential activity to be done by all relevant national sectors using a multisectoral approach (22). The set of priority diseases will inform joint action planning for capacity building including conducting efficient and effective disease surveillance, and building laboratory capacity, and creating prevention and control strategies and sharing data across all relevant sectors.
We used existing VBZD prioritization tools such as WHO OneHealth Tool (23) and CDC One Health Zoonotic Disease Prioritization (OHZDP) (24) to identify high-priority VBZDs for pan-regional use and multisectoral engagement in the EMR. The draft regional priority list was extensively discussed during the consultative meeting and the priority emerging and re-emerging VBZDs list in Table 1 was agreed by the participants.
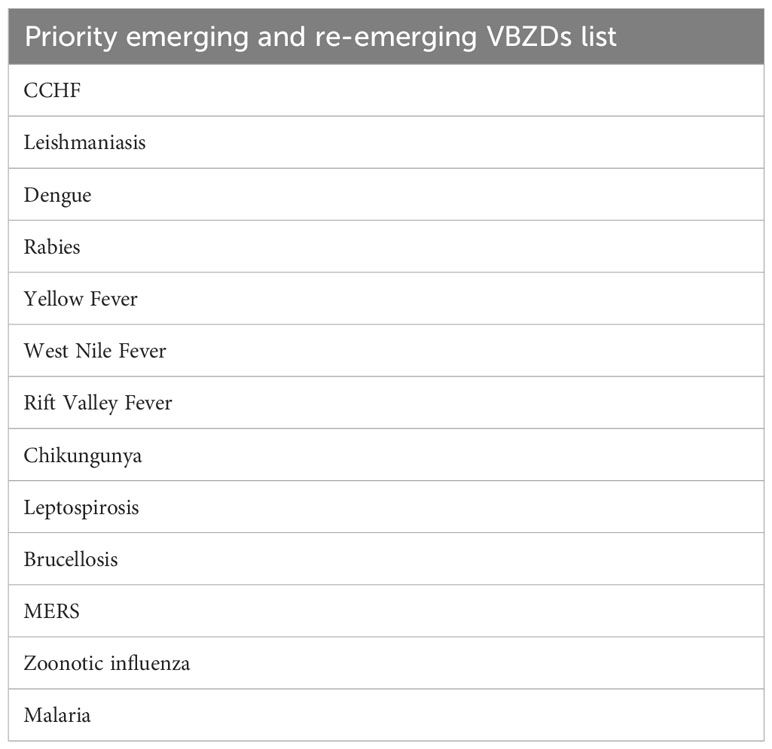
Table 1 High-priority vector borne and zoonotic diseases in the EMR (22).
4.4 Vector-borne and zoonotic diseases research
Emerging infectious disease research should rest on four pillars: enhanced communications across disciplinary and agency boundaries, the assessment and development of surveillance and disease detection tools, the examination of linkages between animal health determinants of human health outcomes, and finally, cross-disciplinary training and research (25).
Most current VBZDs research focuses on the interface of the pathogen and the clinically ill person, emphasizing microbial detection, mechanisms of pathogenicity and clinical intervention strategies, rather than examining the causes of emergence, persistence and spread of new zoonoses. Many research approaches are needed to achieve transdisciplinary research and fill the current gaps. The following steps are recommended:
• Conducting cross sectional research at the human-animal interface.
• Build up a joint research agenda by creating joint task forces around specific public health emergencies (26).
• Mapping of the available capacities in relevant sectors such as subnational government structures, national public health and animal health laboratories, and academic and research institutions.
• Strengthen routine surveillance including passive lab-based surveillance by conducting active surveillance projects (clinical-based, field-based, high-risk population) and animal value chain analysis.
• Applying research translation to develop evidence-based policies and programs for the prevention and control of infectious diseases (27).
• Enhance collaboration between the transdisciplinary experienced researchers who can collaborate across disciplines (28).
• Enabling environments and funding (29) by developing funding sources and advocate for funding opportunities from national organizations.
• Engage researchers with funders as partners (30) and involve them in advisory and grant review boards.
4.5 Risk assessment
Conducting periodic joint risk assessments (JRA) is essential in bringing together national information and expertise from all relevant sectors to manage shared risks at the human-animal-environment interface. The WHO JRA Operation tool provides a 10-step approach to conducting joint qualitative risk assessments to support policy communication, risk mitigation, planning, and preparedness for VBZDs, contributing to health security at the national, regional, and global levels. Joint risk assessments should be conducted routinely for contingency planning, after VBZDs prioritization to agree on implementation measures, and during an emergency event. The JRA outcomes should be considered to determine the likelihood and impact of events on public health so that action can be taken to manage and reduce the negative consequences.
4.6 Joint surveillance and response
An effective global, integrated VBZDs surveillance system requires surveillance at national, regional, and international levels. Event-based surveillance (EBS) allows the detection of signals of public health importance outside of routine surveillance systems.
Building a coordinated surveillance system involves establishing networks and partnerships, joint training, reinforcing surveillance at points of entry, and sharing protocols and resources between animal and human health laboratories. Surveillance plans for VBZDs should include options for active surveillance, passive surveillance, syndromic surveillance, sentinel surveillance, indicator-based surveillance (IBS), and EBS and should evolve based on the results obtained or on new threats that arise, with regular revisions and updates of plans. Linking the coordinated surveillance system with other intersectoral coordination activities is crucial for effective joint risk assessment and guiding future surveillance.
4.7 Laboratory capacity
Successful and sustainable laboratory systems require strategic interagency planning across sectors and building on existing capacities in country to standardize laboratory methods, prioritize laboratory resources, and develop information sharing channels. Central, regional, private, and research laboratories should be expanded for animal and environmental samples capacity and diagnostic techniques should be standardized and aligned with international standards. Procurement and supply chain should be strengthened to ensure availability of reagents and supplies. Notification systems involving all sectors should be established in case of emergencies. To guarantee testing quality, laboratory staff must be trained, and policies and procedures should be in place, while biosafety and biosecurity practices must be followed. A reliable system for specimen collection, transportation, storage, and management is essential, and mentoring partnerships should be encouraged for joint training and procurement of reagents and laboratory consumables. Standardization of diagnostic techniques and national laboratory capacity should be ensured and involve all sectors in the notification system by collecting daily reports. Opportunities for relationships with reference laboratories or private partnerships should be encouraged. As a technical resource toward harmonized laboratory and surveillance efforts, the utilization of existing tools such as the FAO Assessment Tool for Laboratories and AMR Surveillance Systems (FAO-ATLASS) (31) should be encouraged.
4.8 Information sharing
To effectively address new or emerging VBZDs, it is crucial to establish a robust system for sharing surveillance information across all relevant sectors. This includes routine and timely sharing of laboratory and clinical information and other data from different sectors and partners to identify clusters of illness or death. Additionally, it is essential to establish systems for regular information sharing with partners such as neighboring countries, regional partners, and non-governmental stakeholders. Notifiable VBZDs must be reported to appropriate international and regional authorities in compliance with international or regional codes of practice. Establishing regional networks for sharing information using a multisectoral approach is recommended.
4.9 Case management
To effectively control and prevent the spread of VBZDs, primary, secondary, and tertiary healthcare centers must be equipped to promptly diagnose and manage cases. Case definition standardization and training for responders on timely case identification and proper management is essential for the case management of infected people with VBZDs and mitigating the mortality. Facilities must also have guidelines for infection prevention and control, including hand hygiene, proper use of personal protective equipment, isolation of patients, injection safety, vector control, proper ventilation, and safe waste disposal. Patients suspected of possible exposure to VBZDs should be provided with user-friendly evidence-based guidance on prevention and control. Establishing a timely notification system between health facilities and surveillance sectors, and agreement on prepositioning supplies including rapid diagnostic test (RDT) and defining supply chains are also necessary steps for effective control and prevention of VBZDs.
4.10 Vector control
Vector management is the main method for controlling many vector-borne diseases, but it faces significant challenges due to the nature of these diseases and their transmission intensity being primarily driven by wildlife reservoirs. Integrated vector management (IVM) is a decision-making process that promotes efficient, cost-effective, and sustainable vector control. Environmental management, which involves modifying the environment to minimize vector productivity and human contact with the vector-pathogen, can also be a useful strategy. The use of vector insecticides such as acaricides, larvicides, adulticides, baits, and insecticide impregnated bed nets, although effective, can have negative effects on the environment and non-target organisms. The choice of insecticide needs to be based on the results of susceptibility and effectiveness testing. Biological control of vectors, using safe and sustainable methods, can help reduce the prolonged application of insecticides. Finally, capacity building for remote sensing, vector surveillance, risk-based mapping, and modeling can support vector control efforts (32).
4.11 Risk communication
The effective assessment of outcomes is crucial for managing risks and communicating decisions to stakeholders. Risk communication is an important component of this process, as it involves the exchange of information, advice, and opinions among experts, community leaders, officials, and those at risk. Developing a joint risk communication and community engagement strategy and plan involves identifying all relevant stakeholders and affected communities and establishing mechanisms for communication between relevant sectors. Ongoing evaluation of communication strategies is also essential to continuously gather data and adapt and improve activities. To fully mitigate risks, a risk communication plan should identify the purpose of communication, the affected populations, the best way to reach them, the best spokespeople to communicate key messages, and provide adequate resources and training for communication staff.
4.12 Evaluation and monitoring
By continuously monitoring, evaluating, and updating all elements and actions, countries can improve the effective VBZDs control, response, and coordination systems. It is also important to regularly conduct simulation exercises and after-action reviews to identify strengths, weaknesses, and areas for improvement. Lessons learned from these activities should be reflected in updated strategies and plans.
5 Conclusion
The regional strategic guidance for prevention and control of emerging and re-emerging VBZDs in the EMR is designed to serve as a Standard Operational Procedure (SOP) that outlines the necessary and practical processes and actions in prevention, control and response to the increasing threat of VBZDs in the region. The guidance provides a set of processes and actions including identifying the risks, developing strategies to mitigate them, and building capacity to timely respond to epidemics and outbreaks of VBZDs.
Emerging and re-emerging VBZDs continue to pose a significant threat to public health globally, and the EMR is no exception. In fact, the region is vulnerable to a range of VBZDs, which can draw severe consequences for public health, animal health, environment, economy, and social stability. The guidance provides a strategic SOP for countries in the region to address these challenges and build resilience against VBZDs.
Finally, the guidance stresses the importance of interagency planning and collaboration across sectors including surveillance, laboratory, case management, vector control and risk communication. The regional guidance supports EMR countries in operationalizing One Health at regional and national level - to be better equipped and prepared for the control and response to VBZDs epidemics and outbreaks - in order to save lives, reduce the economic burden, and enhance social stability.
Author’s note
The views and opinions expressed are those of the authors and do not reflect the official policy or position of World Health Organization.
Author contributions
CK, AA and GK contributed to conception of manuscript. AR, RB and RS contributed to the design. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2023.1237688/full#supplementary-material
References
1. Chala B, Hamde F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front Public Health (2021) 9:715759. doi: 10.3389/fpubh.2021.715759
2. Wang LF, Crameri G. Emerging zoonotic viral diseases. Rev Sci Tech (2014) 33(2):569–81. doi: 10.20506/rst.33.2.2311
3. Rahman MT, Sobur MA, Islam MS, Ievy S, Hossain MJ, El Zowalaty ME, et al. Zoonotic diseases: etiology, impact, and control. Microorganisms (2020) 8(9):1405. doi: 10.3390/microorganisms8091405
4. WHO. Vector-borne diseases. Available at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
5. Institute of Medicine (US) Forum on Microbial Threats. Vector-borne diseases: understanding the environmental, human health, and ecological connections: workshop summary. Washington, DC: National Academies Press (US).
6. Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun (2017) 8(1):1124. doi: 10.1038/s41467-017-00923-8
7. Stephen C, Stemshorn B. Leadership, governance and partnerships are essential One Health competencies. One Health (Amsterdam Netherlands) (2016) 2:161–3. doi: 10.1016/j.onehlt.2016.10.002
8. Dasgupta R, Tomley F, Alders R, Barbuddhe SB, Kotwani A. Adopting an intersectoral One Health approach in India: Time for One Health Committees. Indian J Med Res (2021) 153(3):281–6. doi: 10.4103/ijmr.IJMR_537_21
9. FAO-OIE-WHO, The FAO-OIE-WHO Collaboration. A Tripartite Concept Note (2010). Available at: https://www.who.int/publications/m/item/the-fao-oie-who-collaboration.
10. WHO. Regional Committee Sixty-First Session. Zoonotic disease: emerging public health threats in the Region. Available at: https://www.emro.who.int/about-who/rc61/zoonotic-diseases.html.
11. Markoff L. Yellow fever outbreak in Sudan. New Engl J Med (2013) 368(8):689–91. doi: 10.1056/NEJMp1300772
12. Malik MR, Mnzava A, Mohareb E, Zayed A, Al Kohlani A, Thabet AA, et al. Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: epidemiological characterization and key lessons learned for early detection and control. J Epidemiol Global Health (2014) 4(3):203–11. doi: 10.1016/j.jegh.2014.01.004
13. EpiSouth weekly epi bulletin – number 243; (7 November – 14 November 2012). In: EMHJ-Eastern Mediterranean Health Journal, vol. 19. p. S31–8. (2013). Available at: http://www.episouthnetwork.org/sites/default/files/bulletin_file/eweb_243_15_11_12.pdf.
14. Aronson NE. Infections associated with war: the American forces experience in Iraq and Afghanistan. Clin Microbiol Newslett (2008) 30(18):135–40. doi: 10.1016/j.clinmicnews.2008.08.004
15. Hartzell JD, Peng SW, Wood-Morris RN, Sarmiento DM, Collen JF, Robben PM, et al. Atypical Q fever in US soldiers. Emerging Infect Dis (2007) 13(8):1247. doi: 10.3201/eid1308.070218
16. Leung-Shea C, Danaher PJ. Q fever in members of the United States armed forces returning from Iraq. Clin Infect Dis (2006) 43(8):e77–82. doi: 10.1086/507639
17. Fazaludeen Koya S, Abdalla SM, Kodama C, Keita M, Abubakar A. Vector-borne and zoonotic diseases in the Eastern Mediterranean Region: a systematic review. J Epidemiol Glob Health (2023) 13(1):105–14. doi: 10.1007/s44197-023-00091-7
18. Cediel Becerra NM, Olaya Medellin AM, Tomassone L, Chiesa F, De Meneghi D. A survey on one health approach in Colombia and some latin American countries: from a fragmented health organization to an integrated health response to global challenges. Front Public Health (2021) 9:649240. doi: 10.3389/fpubh.2021.649240
19. Chiesa F, Tomassone L, Savic S, Bellato A, Mihalca AD, Modry D, et al. A survey on one health perception and experiences in europe and neighboring areas. Front Public Health (2021) 9:609949. doi: 10.3389/fpubh.2021.609949
20. WHO. WHO EMRO Regional Appeal (2023). Available at: https://www.who.int/emergencies/funding/outbreak-and-crisis-response-appeal/2023/2023-appeals/appeal-region–emro.
21. Bansal D, Jaffrey S, Al-Emadi NA, Hassan M, Islam MM, Al-Baker WAA, et al. A new One Health Framework in Qatar for future emerging and re-emerging zoonotic diseases preparedness and response. One Health (2023) 16:100487. doi: 10.1016/j.onehlt.2023.100487
22. FAO, OIE, WHO. Taking a multisectoral one health approach: a tripartite guide to addressing zoonotic diseases in countries (2019). Available at: https://www.who.int/publications/i/item/9789241514934.
23. WHO. The OneHealth tool. Available at: https://www.who.int/tools/onehealth.
24. US CDC. One Health Zoonotic Disease Prioritization (OHZDP). Available at: https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/index.html.
25. Stephen C, Artsob H, Bowie WR, Drebot M, Fraser E, Leighton T, et al. Perspectives on emerging zoonotic disease research and capacity building in Canada. Can J Infect Dis Med Microbiol (2004) 15(6):339–44. doi: 10.1155/2004/238126
26. Bronzwaer S, Catchpole M, de Coen W, Dingwall Z, Fabbri K, Foltz C, et al. One health collaboration with and among EU Agencies – bridging research and policy. One Health (2022) 15:100464. doi: 10.1016/j.onehlt.2022.100464
27. Min B, Allen-Scott LK, Buntain B. Transdisciplinary research for complex One Health issues: a scoping review of key concepts. Prev Vet Med (2013) 112(3-4):222–9. doi: 10.1016/j.prevetmed.2013.09.010
28. Allen-Scott LK, Buntain B, Hatfield JM, Meisser A, Thomas CJ. Academic institutions and one health: building capacity for transdisciplinary research approaches to address complex health issues at the animal-human-ecosystem interface. Acad Med (2015) 90(7):866–71. doi: 10.1097/ACM.0000000000000639
29. Stokols D, Fuqua J, Gress J, Harvey R, Phillips K, Baezconde-Garbanati L, et al. Evaluating transdisciplinary science. Nicotine Tob Res (2003) 5 Suppl 1:S21–39. doi: 10.1080/14622200310001625555
30. Landrum JP, Hudson CG, Close SL, Knight E, Paquin R-M, Bell V, et al. Grant-making criteria for developing useful and usable marine science: A philanthropic perspective. Front Mar Sci (2022) 8. doi: 10.3389/fmars.2021.809953
31. FAO. FAO Assessment Tool for Laboratories and AMR Surveillance Systems (FAO-ATLASS). Available at: https://www.fao.org/antimicrobial-resistance/resources/tools/fao-atlass/en/.
32. WHO. Handbook for integrated Vector Management (2012). Available at: https://www.who.int/publications/i/item/9789241502801.
Keywords: vector borne disease, zoonotic disease, emerging disease outbreak, WHO Eastern Mediterranean Region, One Health, operationalizing one health, strategic guidance, prevention and control
Citation: Kodama C, El Rifay AS, Badra R, Abu Salbi R, Abubakar A and Kayali G (2023) Operationalizing One Health: strategic guidance for prevention and control of emerging and re-emerging vector-borne and zoonotic diseases in the Eastern Mediterranean Region. Front. Trop. Dis 4:1237688. doi: 10.3389/fitd.2023.1237688
Received: 09 June 2023; Accepted: 20 October 2023;
Published: 07 November 2023.
Edited by:
Soewarta Kosen, Consultant, IndonesiaReviewed by:
Moses Muia Masika, University of Nairobi, KenyaLaura Tomassone, University of Turin, Italy
Copyright © 2023 Kodama, El Rifay, Badra, Abu Salbi, Abubakar and Kayali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiori Kodama, a29kYW1hY0B3aG8uaW50; Ghazi Kayali, Z2hhemlAaHVtYW4tbGluay5vcmc=
†These authors share first authorship
 Chiori Kodama
Chiori Kodama Amira S. El Rifay
Amira S. El Rifay Rebecca Badra3
Rebecca Badra3 Abdinasir Abubakar
Abdinasir Abubakar Ghazi Kayali
Ghazi Kayali