- 1Key Laboratory of Tarim University Husbandry Science and Technology, College of Animal Science and Technology, Tarim University, Alar, China
- 2National Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, China
- 3State Key Laboratory of Animal Nutrition, College of Animal Science and Technology, China Agricultural University, Beijing, China
This study investigated the effects of defective pear fermentation (DPF) diets on growth performance and gastrointestinal microbial communities in 60 healthy male small-tailed Han sheep, aged 90 days. The sheep were randomly divided into four groups, each consisting of three replicates with five sheep per replicate. Initially, all groups received a basal diet for seven days during the adaptation stage. Subsequently, for 60 days, group C (control) was fed a basal diet, group X received a basal diet with 2% DPF, group Y had a basal diet with 4% DPF, and group Z was fed a basal diet with 6% DPF. The results indicated that group Y experienced a significant increase in average daily gain (ADG) and average daily feed intake (ADFI). The addition of DPF significantly elevated the levels of GSH-Px and notably reduced MDA content compared to group C. Analysis of gastrointestinal microbiota showed that groups receiving DPF had increased relative abundances of Lachnospiraceae_NK3A20_group, norank_f p-2534-18B5_gut_group, Acetitomaculum, Actinobacteriota, Bacteroidota and Ruminococcus_gauvreauii_group, and decreased abundances of Proteobacteria, Prevotella, Staphylococcus, and Psychrobacter compared to group C. Group X exhibited the highest relative abundance of Olsenella, while group Y showed a significant increase in unclassified_f Lachnospiraceae compared to the other groups. Bacterial function prediction indicated that pathways related to energy metabolism were more prevalent in group X and Y. This study preliminarily confirms the feasibility of using DPF as feed additives, providing a foundation for further research and evaluation of DPF's application in animal production.
1 Introduction
Xinjiang Uygur Autonomous Region is a prominent province for animal husbandry in China, with residents primarily consuming beef and mutton (Wang C. et al., 2023). With China's rapidly growing economy, the demand for meat products is increasing (Dawut and Tian, 2021). However, the development of animal husbandry in Xinjiang is heavily reliant on high-quality forage resources, a crucial factor that challenges the industry's growth (Thornton and Gerber, 2010; Godber and Wall, 2014).
In recent years, the cultivation of fragrant pears has become a significant sector in Xinjiang's forestry and fruit industry (Niu et al., 2019; Wang Z. et al., 2023). By the end of 2022, Bazhou's fragrant pear planting area reached 489,600 mu, yielding a total output of 386,400 tons (Ma et al., 2023). During harvesting, transportation, and marketing, a portion of the fruits, approximately 30% of total production, are deemed commercially valueless due to damage or spoilage. These are known as residual fruits and their abundance, coupled with their susceptibility to natural deterioration, poses environmental pollution risks and resource wastage (Guo, 2022).
Following China's 2020 ban on antibiotic utilization, there has been an increase in research on alternative feed additives, such as Chinese herbal additives, micro-ecological preparations, and plant essential oil extracts (Meena et al., 2022; Yang et al., 2022; Caroprese et al., 2023; Guo et al., 2023; Kiernan et al., 2023; Ramdani et al., 2023; Varga-Visi et al., 2023). While most research has focused on swine and poultry, applications in sheep are less common. At present, most of the research is to replace the traditional feed with unconventional feed to explore its effects on the growth performance and gastrointestinal function of animals. However, there are few studies on improving animal growth performance by adding fruit by-product or fermentation. The following are several studies on the use of fruit by-products in animals: Cheng (2021)'s study found that adding 8% grape pomace increased the daily feed intake of Tan sheep. Ao et al. (2022) study showed that adding 10% fermented apple pomace to the feed of weaned piglets could increase their average daily gain. As report goes, the antioxidant activity in plasma of lambs was increased after feeding 10% fermented apple pomace diet (Rodríguez-Muela et al., 2015). These few studies have revealed that fruit by-product or fermentation has a positive effect on animal growth, which is one of the reasons why our team chose to use defective pear fermentation as an additive.
In these contexts, we chose small-tail Han sheep as our study subject, one of China's superior hybrid breeds which is known for its rough feeding resistance, strong adaptability, high fecundity, and rapid growth rate (Lu et al., 2005; Jing et al., 2022). To enhance the preservation and utilization of defective pear resources in animal husbandry, this study utilized defective pear as a carrier for Lactobacillus plantarum and Bacillus subtilis to perform fermentation. The fermentation process preserved the nutrients in the pears and increased the quantity of probiotics. And then we added DPF into the diet of small-tailed Han sheep which aimed to evaluate the benefits of including fermented defective pear in the diet of small-tailed Han sheep to improve their growth, and to assess its impact on their intestinal flora using 16S rRNA analysis. The findings may establish the viability of fermented defective pear as a novel feed additive, offering insights into probiotic usage in meat sheep diets and potentially improving meat sheep productivity.
2 Materials and methods
2.1 Laboratory animals and design
This experiment was approved by the Animal Ethics Committee of the College of Animal Science and Technology of Tarim University and the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee (No. AW 72303202-1-1).
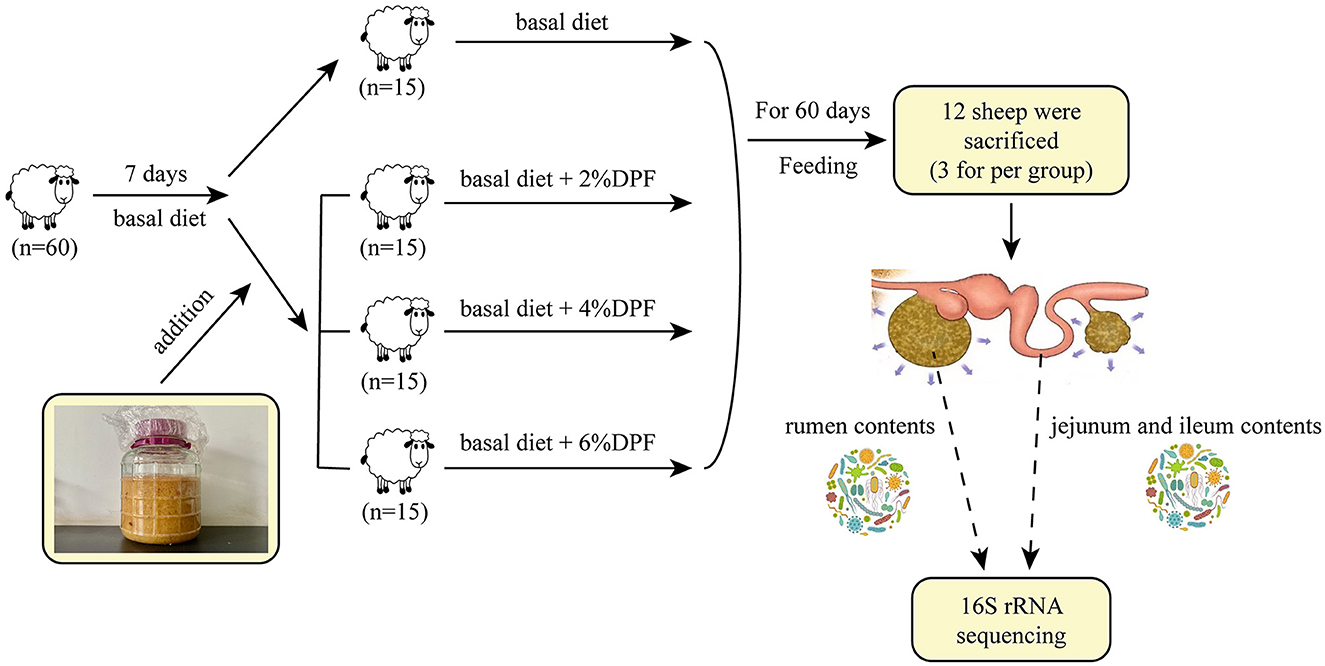
Sixty male small-tailed Han sheep, all 90 days old and similar body weights (36.03 ± 1.94 kg BW), were selected and divided into 4 groups with three replicates each and five sheep per replicate. During a 7-day adaptation stage, all groups received a basal diet. Subsequently, for 60 days, the sheep were fed different diets: group C (control) received the basal diet, group X the basal diet plus 2% DPF, group Y with 4% DPF, and group Z with 6% DPF.
The experiments were conducted at the Jin Hui Tong sheep farm (Xinjiang, China). The sheep pen was disinfected with 2% NaOH before the experiment began, and sheep were tagged and herded into the pen. The sheep were fed twice daily at 9:30 and 19:30 and had unlimited access to water, with two automatic water fountains in each pen and salt blocks provided.
The feed intake of small tail Han sheep in each treatment group was accurately recorded every day to draw the line chart of feed intake change. The BW, average daily feed intake (ADFI) and feed conversion rate were recorded every 30 days. A total of 3 sheep were sampled from each of the four groups of small-tailed Han sheep on day 60 after 12 h of fasting with the weight of each individual sheep recorded before slaughter.
2.2 Defective pear fermentation and diet
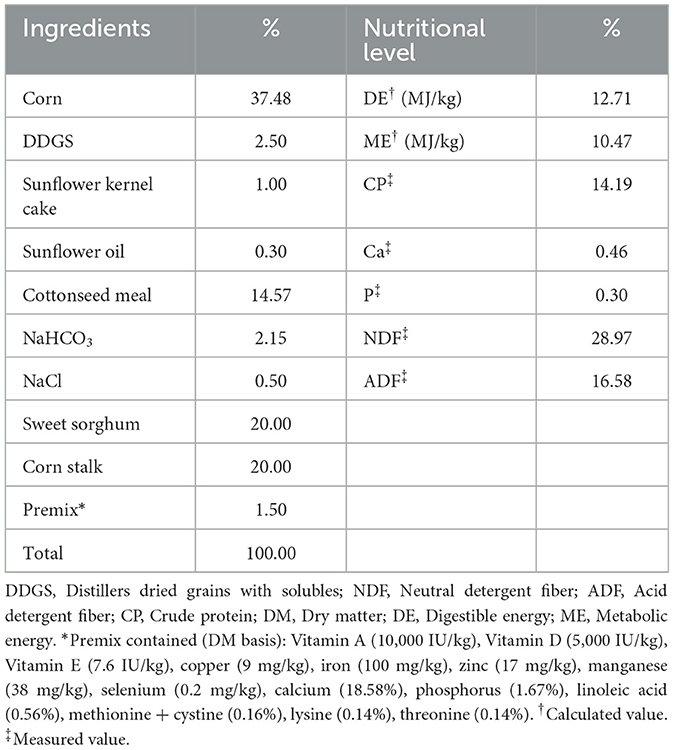
DPF was produced from defective pears with added mixed bacteria (Lactobacillus plantarum and Bacillus subtilis) and fermented for 90 days. The nutritional compositions of DPF are detailed in Table 1, and diets were formulated to meet NRC nutrient requirements, as shown in Table 2.

Table 1. The pH, nutritional compositions and antioxidant contents of defective pear fermentation (DPF).
2.3 Sample collection
Blood samples (~10 mL for each sheep) were collected from the jugular vein at 2 h before slaughtering by using the vacuum blood collection tubes and centrifuged at 3,000 rpm for 15 min and 4°C to obtain the serum sample. These sheep were euthanized and the contents of the rumen, jejunum, and ileum (at day 60) were immediately collected, snap-frozen in liquid nitrogen, and stored at −80°C for analysis of gut microorganisms. The rumen, jejunal and ileal tissues were collected and immediately fixed in 4% paraformaldehyde (Biosharp Co., Ltd., Hefei, China) for subsequent morphological analysis.
2.4 Serum analysis
The contents of a group of eight biochemical indices, including superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px), were measured using the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). An automated biochemical analyzer (BK-280, Shandong Blobase Biotechnology Co., Ltd., Shandong, China) was used to measure glucose (GLU), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC). All experiments were performed using triplicate biological replicates according to the manufacturer's recommended protocols.
2.5 Rumen, jejunum and ileum tissues staining
The pre-fixed samples were cut into small pieces of about 1 square centimeter, then they were covered and cut into thin slices and transferred to a constant temperature water bath at 40°C. After the slices had fully unfolded, let them attach to a glass slide. With 45 min baking at 55–60°C, the slices were haematoxylin-eosin (H&E) strained and measured (Liu et al., 2020). The sections with better morphology were selected and used to observe the morphological characteristics of the rumen, jejunum and ileum by using Image-Pro Plus 6.0 (Media Cybernetics Inc., Bethesda, MD).
2.6 DNA extraction and 16S rRNA gene sequencing of rumen, jejunum and ileum contents
The total DNA of microbial community was extracted from the rumen, jejunum and ileum contents, using the E.Z.N.A.® Stool DNA Kit (Omega Bio-tek, Norcross, GA) by following the manufacturer's procedures. The quality of the extracted DNA was estimated with 1% agarose gel electrophoresis. Then this amplification protocol used the universal primers were 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The genus for key bacterial 16S rRNA in the V3–V4 regions were amplified by the PCR thermocycler (ABI GeneAmR® 9700, Foster City, CA, United States) with the following programs: Denaturation for 3 min at 95°C. Subsequently, a total of 27 cycles were performed, with denaturation for 30 s at 95°C, annealing for 30 s at 55°C, and extension for 45 s at 72°C, ended by the final extension for 10 min at 72°C. The PCR was carried out in a mixture containing 4 μL 5 × TransStart FastPfu buffer, 2 μL 2.5 mM dNTPs, 0.8 μL forward primer (5 μM) and reverse primer (5 μM), 0.4 μL TransStart FastPfu DNA Polymerase, and 10 ng template DNA, with the final volume adjusted to 20 μL using ddH2O. The PCR products were collected by 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) by following the procedures recommended by the manufacturers. The concentrations of the purified PCR products were determined using the QuantusTM Fluorometer (Promega, United States).
The paired-end sequencing (2 × 300 bp) of the equimolarly pooled purified amplicons was performed on an Illumina MiSeq platform (Illumina, San Diego, CA, United States) using standard procedures recommended by the Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Paired-end sequence reads were spliced using FLASH version 1.2.11 to generate the splicing sequences, namely raw tags (Magoč and Salzberg, 2011). QIIME version 1.9.1 (Bokulich et al., 2013) has been used for optimization of raw reads. Based on 97% identity with the representative sequences of the OTUs determined and annotated by Uparse version 11, the effective tags were clustered into the operational taxonomic units (OTUs). RDP Classifier version 2.13 based on the 16S rRNA database (Wang et al., 2007) was used to determine the taxonomy of each OTU representative sequence. The alpha diversity indexes (i.e., Chao1, Shannon, and Simpson) were calculated with Mothur version 1.30.2. The Origin software (version 2018, 64 bit) was used for relative abundance analyses at the phylum and genus level. MetaCyc pathways were predicted using PICRUSt 2 version 2.2.0 (Caspi et al., 2020).
2.7 Statistical analysis
The significant differences between groups were analyzed by one-way analysis of variance (ANOVA) and Duncan was employed in post-hoc tests using the SPSS statistical software version 22.0 (SPSS, Inc., Chicago, IL, United States). Graphs were created using Origin 2018 (OriginLab, Inc., Northampton, MA, United States). The data were shown as the mean ± standard error of the mean (SEM) with the significance levels set at P < 0.05 (*) and P < 0.01 (**), respectively.
3 Results
3.1 Effect of DPF on the growth performance of small-tailed Han sheep
The study evaluated the growth performance of small-tailed Han sheep across four groups, examining initial weights, slaughter weights, ADG, ADFI and FCR. The results, detailed in Table 3, indicated no significant differences (P > 0.05) in initial weights, slaughter weights and FCR, but there were significant differences in ADG and ADFI (P < 0.05) among the groups. The group Y exhibited the highest ADG, showing an 11.03% increase compared to group C. The ranking of average daily feed intake (ADFI) from highest to lowest was: Y, X, C, and Z, with group Y significantly higher (P < 0.01) than the other groups. The Supplementary Figure S1 illustrated that group Y maintained the highest ADFI throughout the experiment.
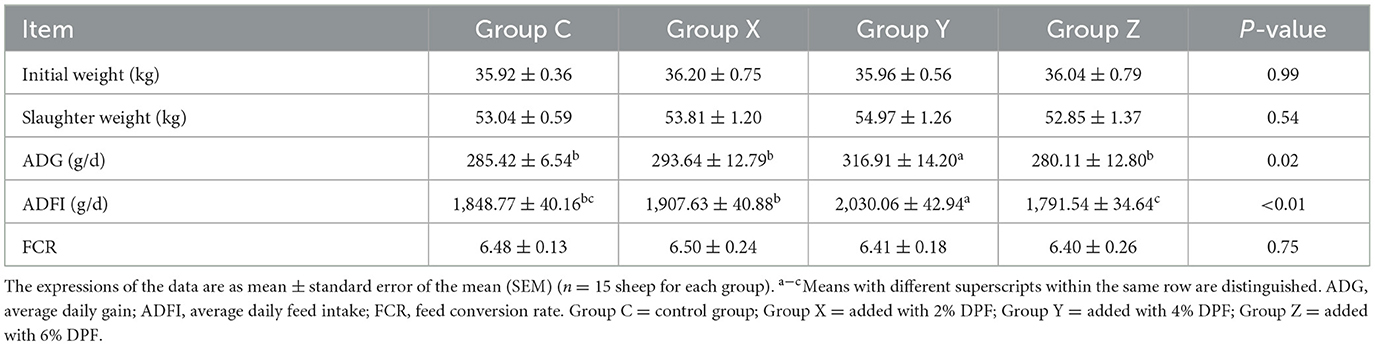
Table 3. Effects of defective pear fermentation (DPF) on the growth performance in four groups of small-tailed Han sheep (i.e., groups C, X, Y, and Z).
3.2 Effect of DPF on the serum characteristics of small-tailed Han sheep
The impact of DPF on the serum characteristics of small-tailed Han sheep is presented in Table 4. Prior to DPF supplementation, there were no significant differences (P > 0.05) in the levels of SOD, MDA, and GSH-Px among the four groups. After 60 days of DPF feeding, MDA levels in groups X, Y, and Z were significantly reduced compared to group C (P < 0.01), with the most pronounced reduction (46.53%) observed in group Y. Similarly, GSH-Px levels were significantly higher in these groups compared to group C (P < 0.01), with group Y showing the highest increase. The levels of GLU, TC, HDL-C, LDL-C, and TG did not significantly differ (P > 0.05) among the four groups.
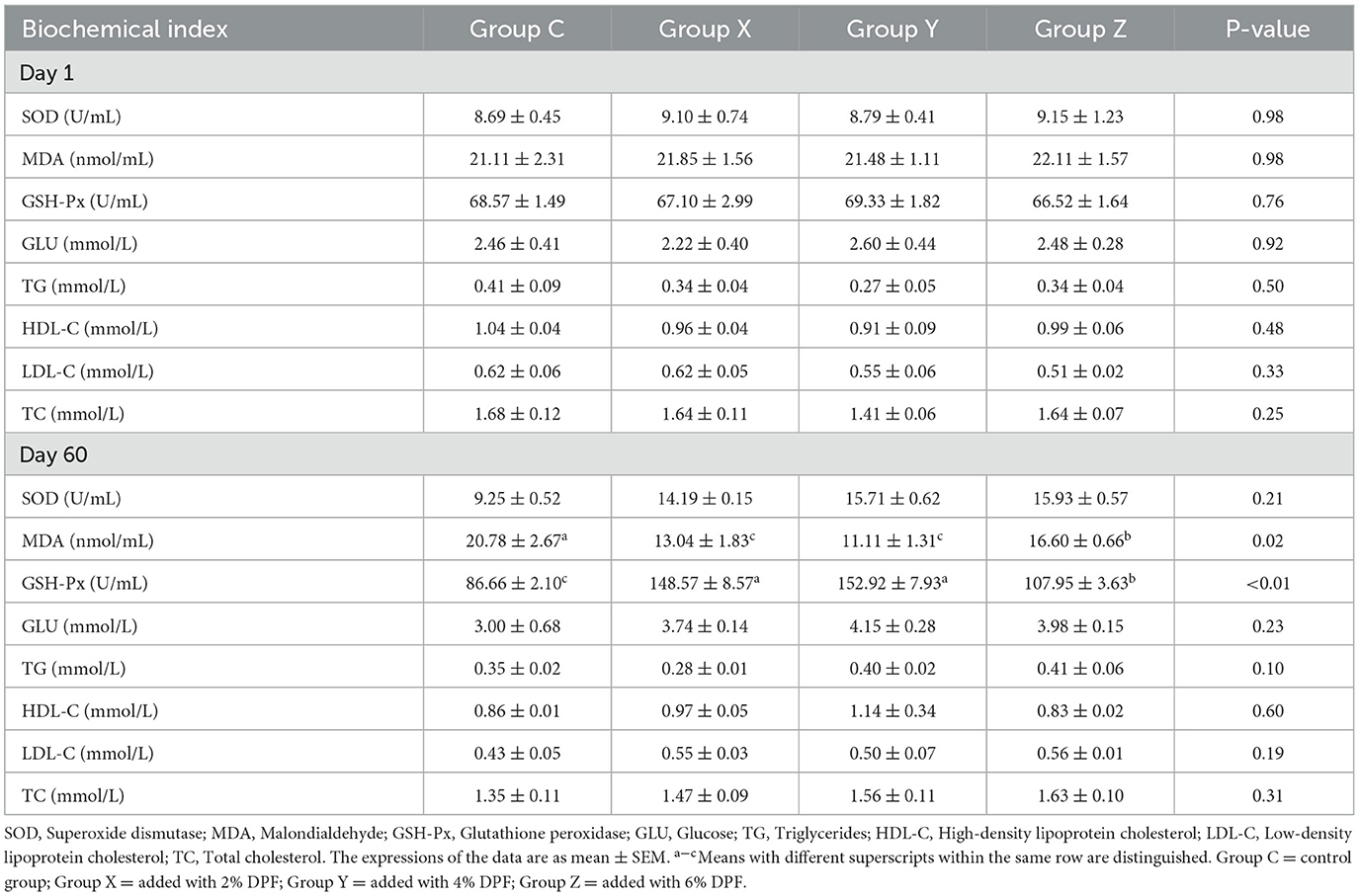
Table 4. Effects of defective pear fermentation (DPF) on the serum biochemical indices in the four groups of small-tailed Han sheep (i.e., groups C, X, Y, and Z) in 1 and 60 days.
3.3 Effect of DPF on the intestinal morphology of small-tailed Han sheep
Table 5 presents the effects of DPF on the intestinal morphology of small-tailed Han sheep over 60 days. There were no significant differences observed in the PLs, PWs, and MTs of the rumen among the four groups (P > 0.05). However, the rumen PWs tended to widen in the groups supplemented with DPF compared to group C. In the jejunum, VHs and V/C in groups X and Y were significantly increased compared to group C (P < 0.01), and CDs in group Y significantly reduced (P < 0.05). In the ileum, CDs in the DPF-supplemented groups were significantly shallower than in group C (P < 0.05), with group Y having the shallowest CDs. There were no significant differences in VHs and V/C among the four groups (P > 0.05).
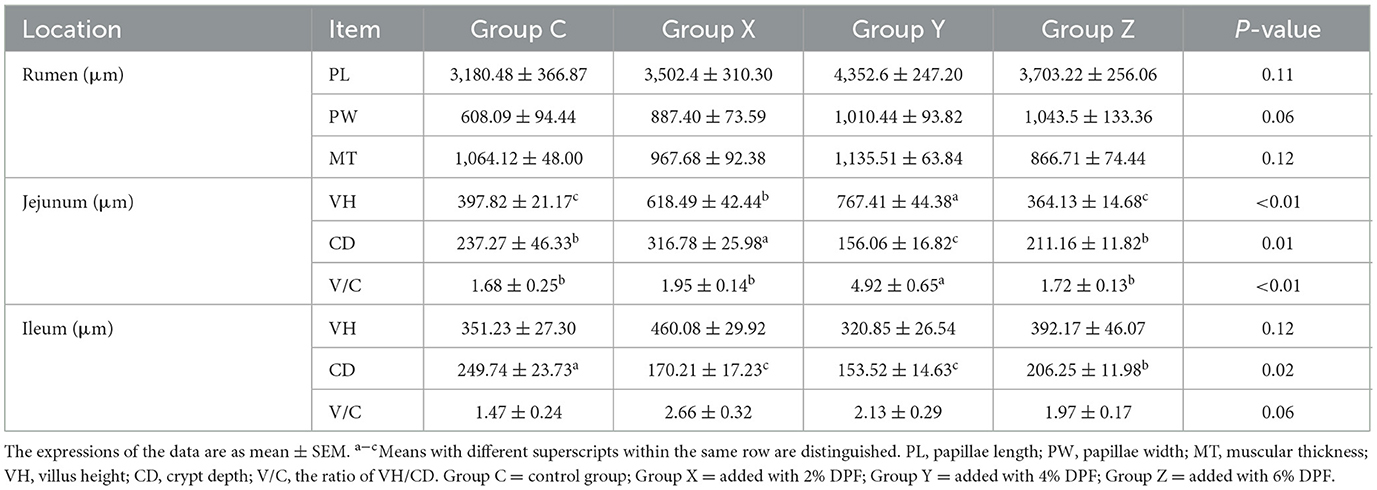
Table 5. Effects of defective pear fermentation (DPF) on the gastrointestinal morphology of the four groups of small-tailed Han sheep (i.e., groups C, X, Y, and Z).
3.4 Effect of DPF on the rumen microbiota of small-tailed Han sheep
Figure 1 shows the impact of DPF on the rumen microbiota of small-tailed Han sheep after 60 days. Sequencing of the V3–V4 regions of the 16S rRNA gene yielded 263 shared bacterial OTUs, with group C having 142 unique OTUs and group Z 70 in Figure 1D. The Chao1 index, reflecting community richness, was significantly higher in the DPF groups compared to group C (P < 0.05; Figure 1A), indicating a substantial increase in rumen community richness following DPF feeding. The Shannon and coverage indexes showed no significant differences among the groups (P > 0.05; Figures 1B, C).
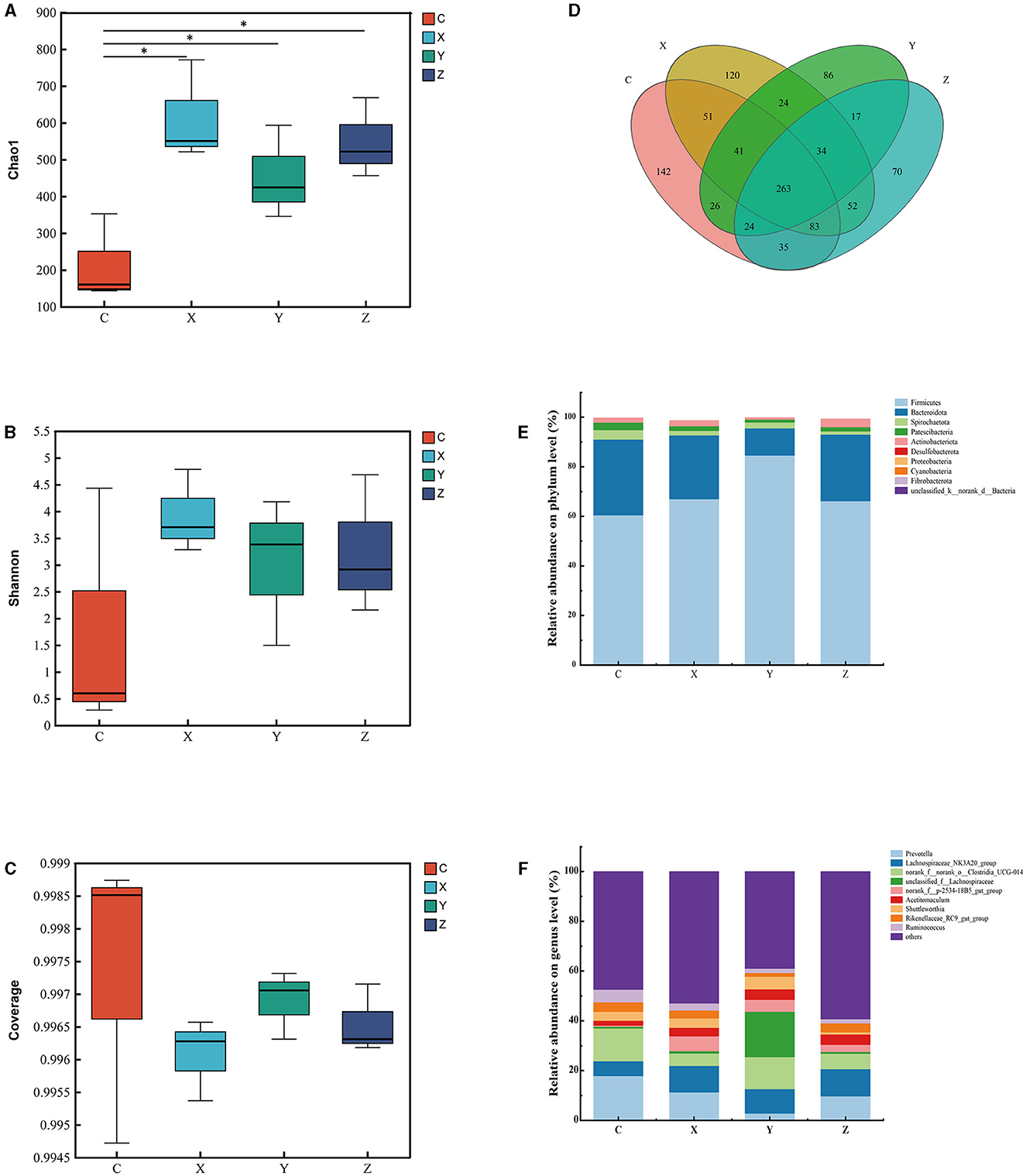
Figure 1. Effect of defective pear fermentation (DPF) on the rumen microbiota in small-tailed Han sheep in 60 days. (A) The boxplot of Chao1 index. (B) The boxplot of Shannon index. (C) The boxplot of coverage index. (D) The shared and individual species number on the rumen detected in four groups were showed at Venn diagram in small-tailed Han sheep. (E) The relative abundances of the top ten bacterial phyla in the rumen microbiome of small-tailed Han sheep on day 60. (F) The relative abundances of the top ten bacterial taxa in the rumen microbiome of small-tailed Han sheep on day 60. Symbol “*” is respectively the expression of the significant differences at P < 0.05. Group C = control group; Group X = added with 2% DPF; Group Y = added with 4% DPF; Group Z = added with 6% DPF.
At the phylum levels, Table 6 showed that Firmicutes and Bacteroidota were the most abundant in the rumen microbiota. The relative abundance of Bacteroidota decreased as Firmicutes increased. In group Y, Bacteroidota had a significantly lower abundance than in the other groups (P < 0.01; Figure 1E). At the genus level, Prevotella was significantly lower in group Y (P < 0.01), while Lachnospiraceae_NK3A20_group was significantly higher in DPF groups (P < 0.05; Figure 1F; Table 6) compared to group C. The abundances of norank_f_norank_o_Clostridia_UCG-014 were higher in groups C and Y (P < 0.05; Figure 1F; Table 6) than in groups X and Z. Group Y showed a notably higher abundance of unclassified_f_Lachnospiraceae (P < 0.01), and groups X and Y had significantly higher levels of norank_f_p-2534-18B5_gut_group (P < 0.01; Figure 1F) compared to group C. Besides, there were significantly higher levels of Acetitomaculum in groups Y and Z compared to group C.
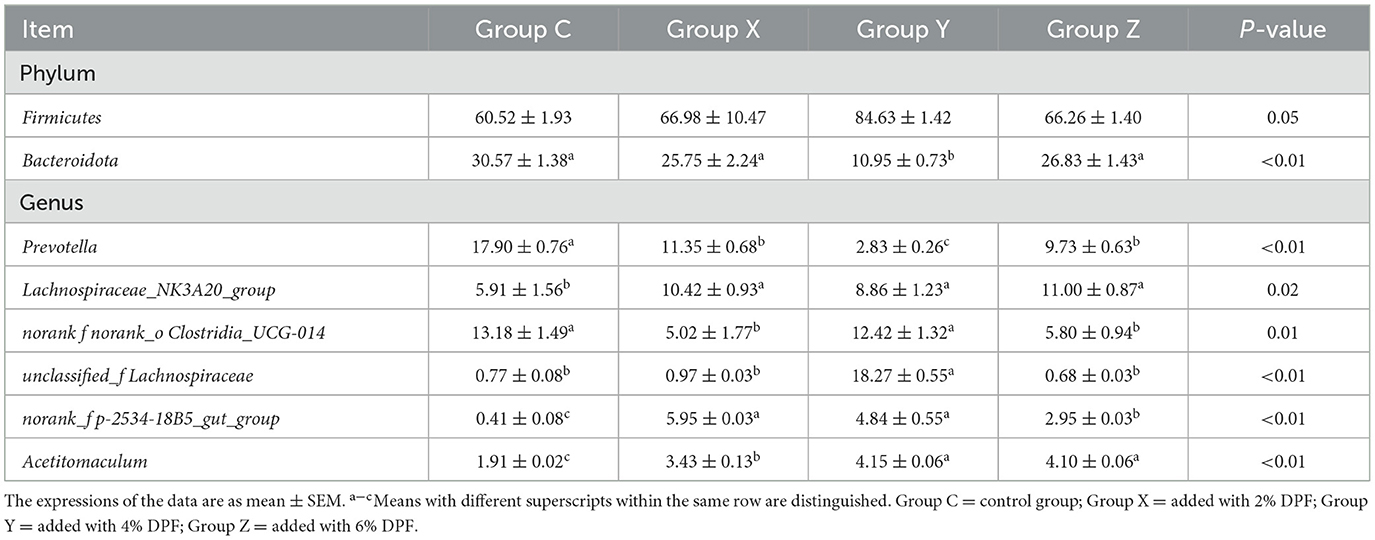
Table 6. Species with different relative abundance of rumen bacteria in different treatments at phylum and genus levels (%).
Functional prediction analysis using PICRUSt2 software identified 388 MetaCyc pathways. T-tests revealed no significant pathway differences between most groups; however, Pyruvate Fermentation to Isobutanol (PWY-7111) was significantly higher in group Y compared to group Z (P < 0.05; Figure 2A).
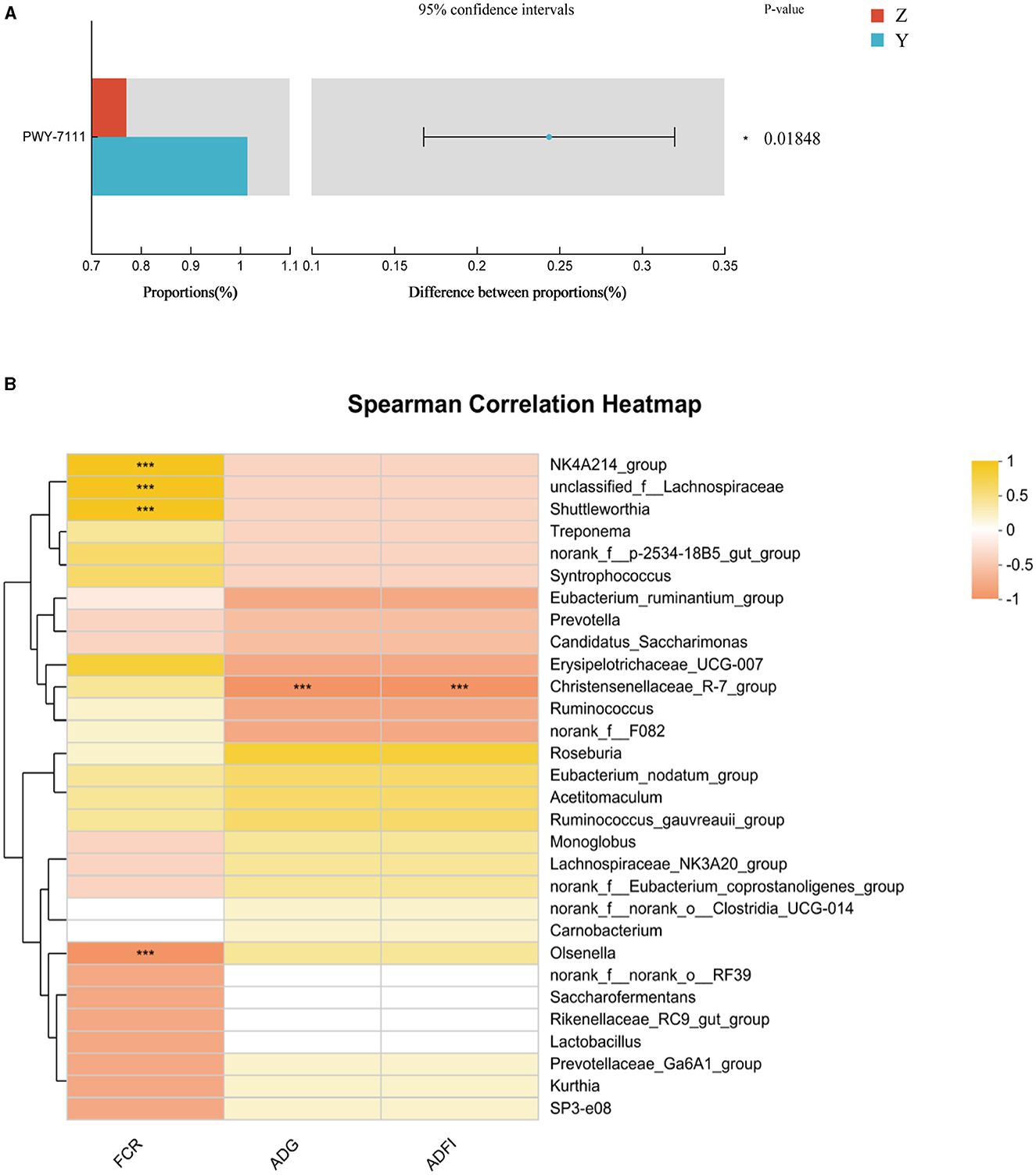
Figure 2. (A) Differential MetaCyc pathways of rumen bacteria in different treatment groups of small-tailed Han sheep. (B) The bacterial taxa in rumen correlated with growth performance of small-tailed Han sheep. The colors of the squares correspond to correlations: Yellow is positive, but red is negative. Symbol “***” represents a highly significant difference, i.e. P < 0.01.
Correlation analysis at the genus level showed significant associations between specific microbial species and growth performance indicators. The Figure 2B showed that the relative abundances of NK4A214_group, unclassified_f_Lachnospiraceae, and Shuttleworthia had significant positive correlations (P < 0.001) with FCR. Olsenella showed a significant negative correlation with FCR (P < 0.001), and Christensenellaceae_R-7_group had a significant negative correlation (P < 0.001) with ADG and ADFI.
3.5 Effect of DPF on the jejunum microbiota of small-tailed Han sheep
Figure 3 illustrates the impact of DPF on the jejunum microbiota of small-tailed Han sheep over a period of 60 days. Sequencing the V3–V4 regions of the 16S rRNA gene in jejunum content samples from the four groups identified 50 shared bacterial OTUs, with 34 unique OTUs in group C and 170 in group X (Figure 3D). The Chao1 index, which indicates community richness, was significantly higher in the DPF-supplemented groups compared to group C (P < 0.05; Figure 3A). Notably, groups X and Z had significantly higher Chao1 indices than group Y (P < 0.05), with group X also surpassing group Z. The Shannon index, representing community diversity, was significantly higher in groups X and Z compared to group C, while no significant difference was observed between groups Y and C. The coverage index was significantly lower in the DPF groups compared to group C (P < 0.05; Figures 3B, C).
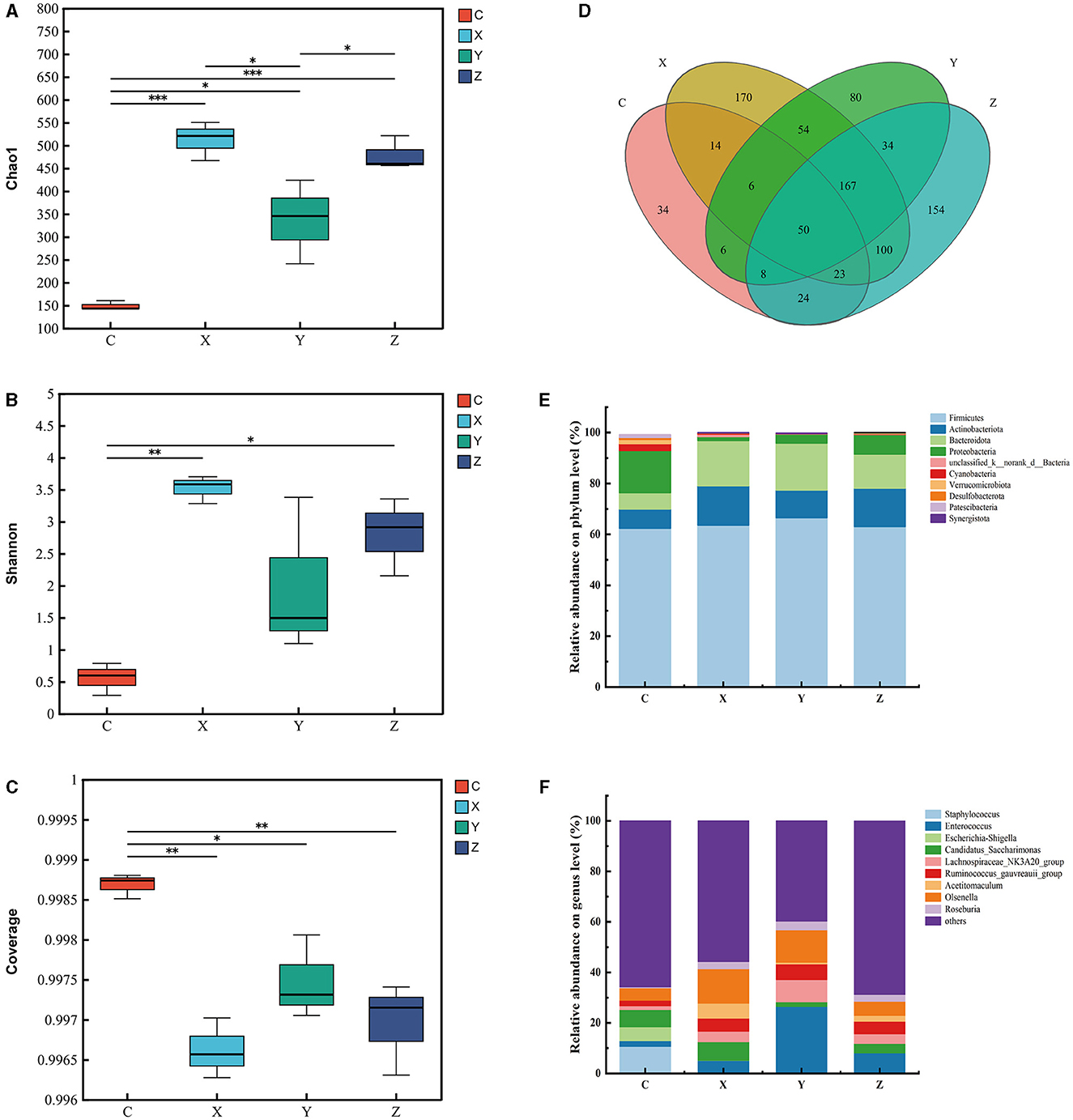
Figure 3. Effect of defective pear fermentation (DPF) on the jejunum microbiota in small-tailed Han sheep in 60 days. (A) The boxplot of Chao1 index. (B) The boxplot of Shannon index. (C) The boxplot of coverage index. (D) The shared and individual species number on the jejunum detected in four groups were showed at Venn diagram in small-tailed Han sheep. (E) The relative abundances of the top ten bacterial phyla in the jejunum microbiome of small-tailed Han sheep on day 60. (F) The relative abundances of the top ten bacterial taxa in the jejunum microbiome of small-tailed Han sheep on day 60. Symbol “*,” “**,” and “***” are respectively the expression of the significant differences at P < 0.05, P < 0.01 and P < 0.001. Group C = control group; Group X = added with 2% DPF; Group Y = added with 4% DPF; Group Z = added with 6% DPF.
Regarding the relative abundances at the phylum level, Actinobacteriota and Bacteroidota were significantly higher in the DPF groups than in group C (P < 0.01; Figure 3E; Table 7) and Proteobacteria was significantly lower in the addition groups compared with group C (P < 0.05; Figure 3E; Table 7). At the genus level, Staphylococcus was significantly more abundant in group C than in the other groups (P < 0.01; Figure 3F). Notably, Enterococcus and Howardella were most abundant in group Y, showing significant differences (P < 0.01; Figure 3F; Table 7) compared to the other groups. The relative abundances of Olsenella were significantly higher in groups X and Y than in groups C and Z (P < 0.01). Group Z had a significantly higher abundance of Family_XIII_AD3011_group compared to the other groups.
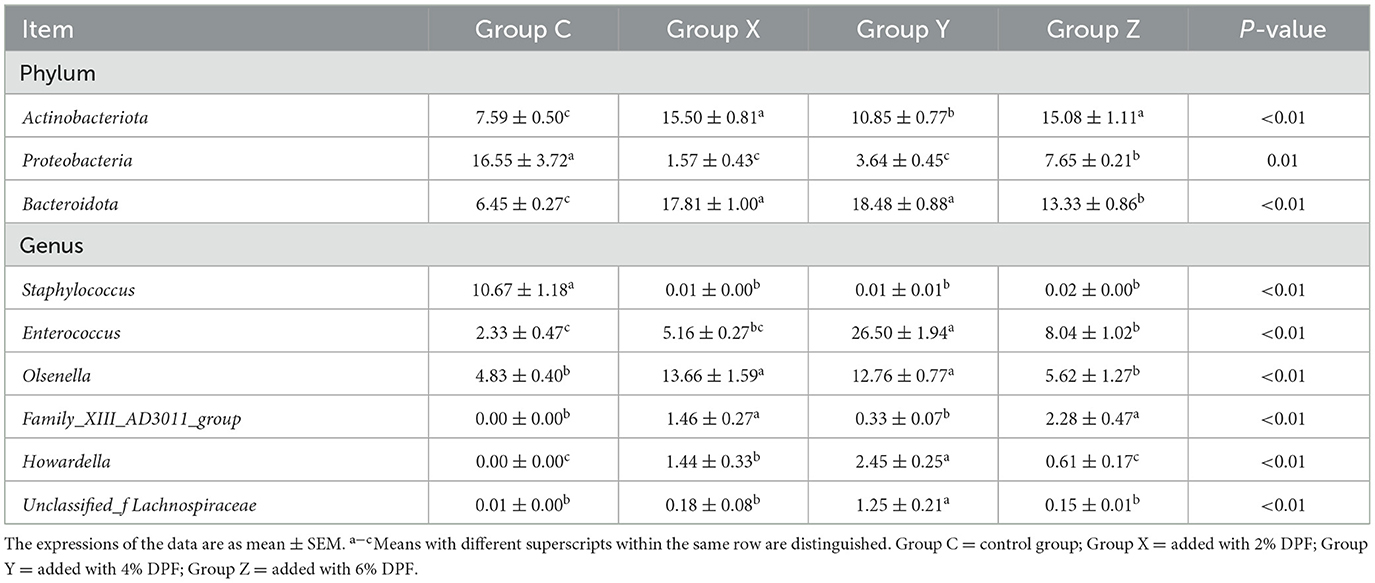
Table 7. Species with different relative abundance of jejunum bacteria in different treatments at phylum and genus levels (%).
Functional prediction analysis using PICRUSt2 software identified 404 MetaCyc pathways. T-tests indicated significant differences (P < 0.05; Figure 4A) in four pathways between groups C and X: CDP-diacylglycerol biosynthesis I (PWY-5667), glycolysis III (ANAGLYCOLYSIS-PWY), the Calvin-Benson-Bassham cycle (CALVIN-PWY), and the non-oxidative branch of the pentose phosphate pathway (NONOXIPENT-PWY), with higher proportions in group X. Group Z showed significantly higher proportions of CALVIN-PWY compared to group C (P < 0.05; Figure 4B). Between groups X and Z, four pathways showed significant differences (P < 0.05; Figure 4C): Adenosine ribonucleotides de novo biosynthesis (PWY-7219), Superpathway of branched amino acid biosynthesis (BRANCHED-CHAIN-AA-SYN-PWY), CALVIN-PWY, and NONOXIPENT-PWY, with higher proportions in group X.
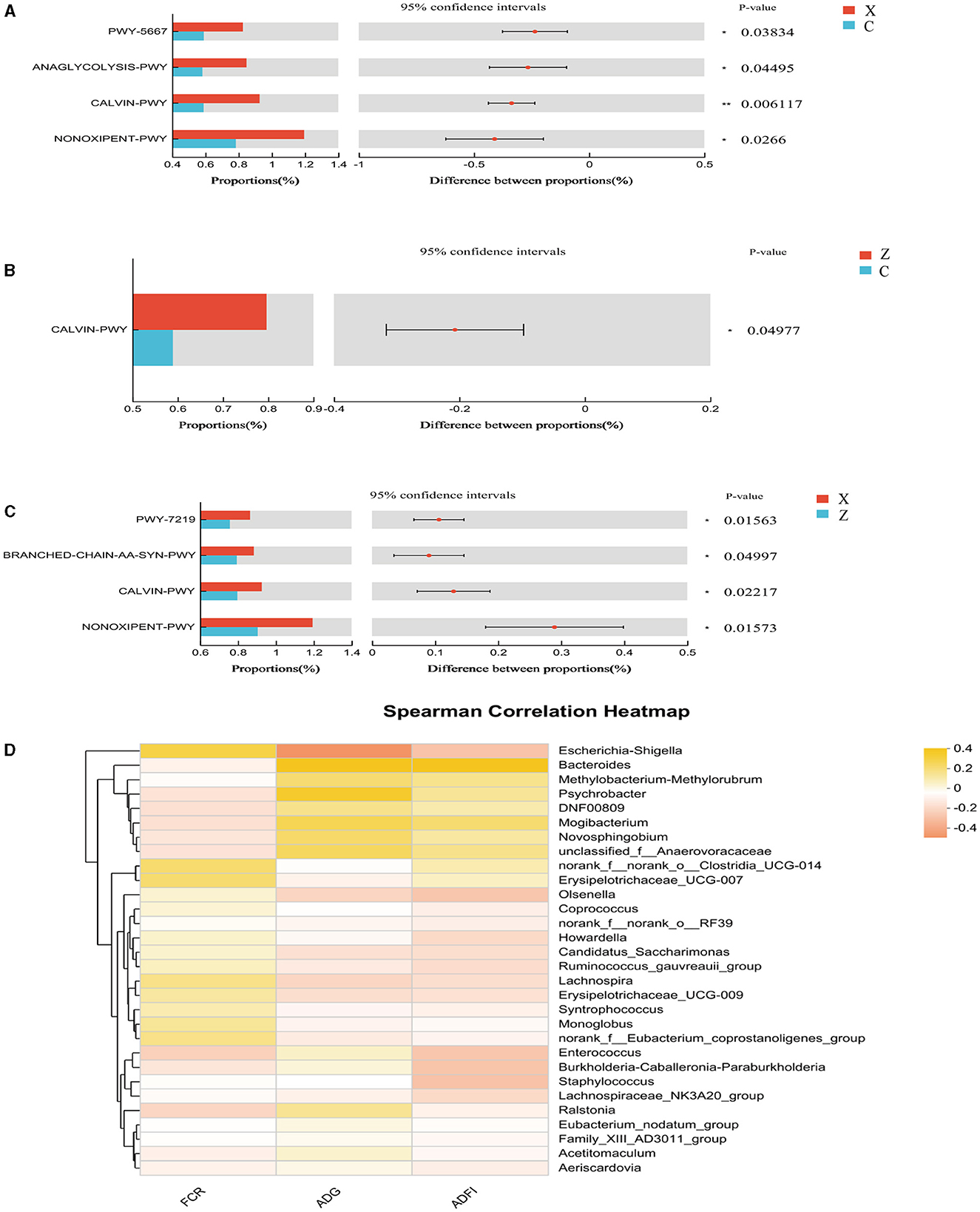
Figure 4. Differential MetaCyc pathways of jejunum bacteria in different treatment groups of small-tailed Han sheep: (A) group C and X; (B) group C and Z; (C) group X and Z. (D) The bacterial taxa in jejunum correlated with growth performance of small-tailed Han sheep. The colors of the squares correspond to correlations: Yellow is positive, but red is negative. The significant difference is set at P < 0.05 (*), P < 0.01 (**), respectively.
Spearman correlation analysis revealed no significant correlations between species at the genus level in the jejunum microbiota and growth performance indicators in small-tailed Han sheep (Figure 4D).
3.6 Effect of DPF on the ileum microbiota of small-tailed Han sheep
Figure 5 displays the effects of DPF on the ileum microbiota of small-tailed Han sheep over 60 days. Sequencing the V3–V4 regions of the 16S rRNA gene in ileum content samples from the four groups identified 257 shared bacterial OTUs, with group C having 51 unique OTUs and group Z 211 (Figure 5D). The Chao1 index, indicating community richness, showed no significant differences among the treatment groups (P > 0.05; Figure 5A). However, group X exhibited a significantly higher Shannon index (reflecting community diversity) compared to group C (P < 0.05; Figure 5B), and its Coverage index was also significantly higher than that of group Z (P < 0.05; Figure 5C).
Figure 5. Effect of defective pear fermentation (DPF) on the ileum microbiota in small-tailed Han sheep in 60 days. (A) The boxplot of Chao1 index. (B) The boxplot of Shannon index. (C) The boxplot of coverage index. (D) The shared and individual species number on the ileum detected in four groups were showed at Venn diagram in small-tailed Han sheep. (E) The relative abundances of the top ten bacterial phyla in the ileum microbiome of small-tailed Han sheep on day 60. (F) The relative abundances of the top ten bacterial taxa in the ileum microbiome of small-tailed Han sheep on day 60. Symbol “*” is respectively the expression of the significant differences at P < 0.05. Group C = control group; Group X = added with 2% DPF; Group Y = added with 4% DPF; Group Z = added with 6% DPF.
The analysis revealed that the relative abundances of Firmicutes were significantly higher in groups C and Y than in groups X and Z (P < 0.05; Figure 5E; Table 8). Actinobacteriota was significantly less abundant in groups X, Y, and Z compared to group Z (P < 0.05), while group X had the highest relative abundance of Bacteroidota (P < 0.01; Figure 5E; Table 8). At the genus level, Psychrobacter was significantly less abundant in the DPF-supplemented groups than in group C (P < 0.01; Figure 5F; Table 8). Additionally, the relative abundances of Olsenella, Howardella, DNF00809, and Monoglobus were significantly higher in group X compared to the other groups (P < 0.01; Figure 5F; Table 8). Group Y had the highest relative abundances of Romboutsia, Ruminococcus_gauvreauii_group, and Clostridium_sensu_stricto_13 among four groups (P < 0.01; Figure 5F; Table 8).
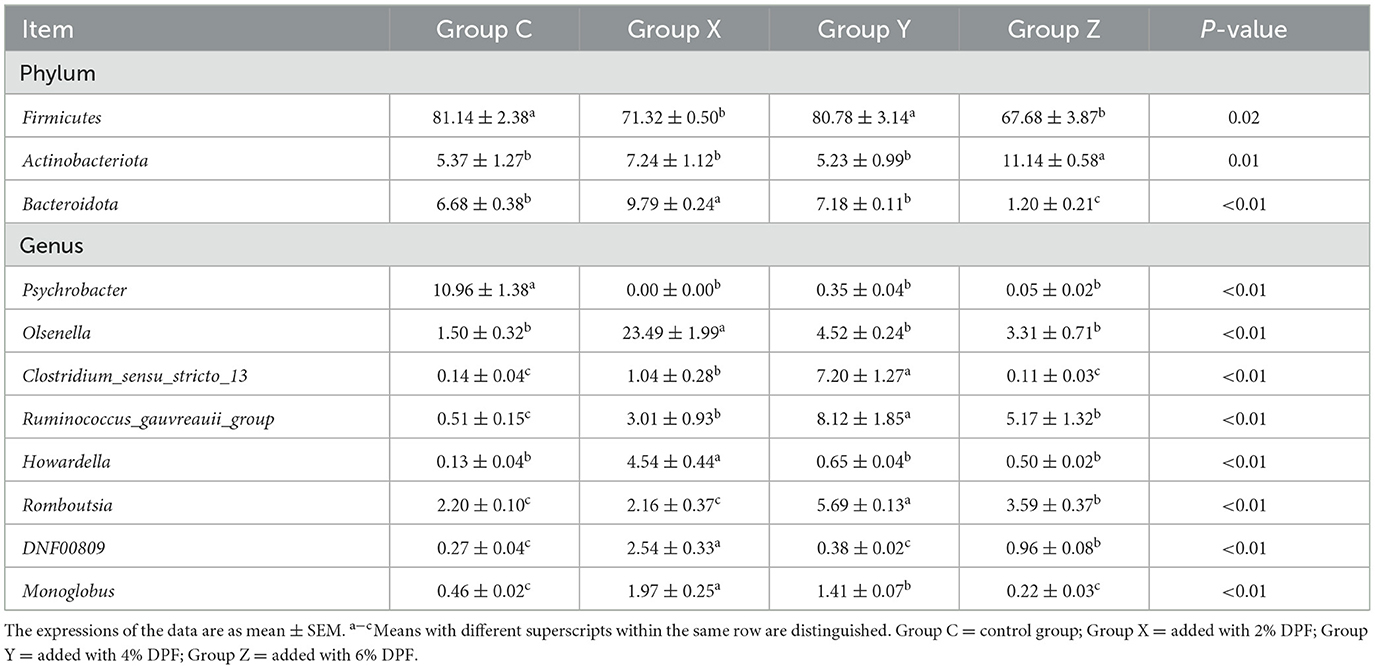
Table 8. Species with different relative abundance of ileum bacteria in different treatments at phylum and genus levels (%).
Functional prediction analysis using PICRUSt2 software identified 403 MetaCyc pathways. T-tests indicated significant differences in six pathways between groups C and X: Superpathway of pyrimidine nucleobases salvage (PWY-7208), CDP-diacylglycerol biosynthesis II (PWY0-1319), CDP-diacylglycerol biosynthesis I (PWY-5667), CALVIN-PWY, Adenosine ribonucleotides de novo biosynthesis (PWY-7219), and NONOXIPENT-PWY, with group X showing higher proportions (Figure 6A). Group Z had significantly higher proportions in five pathways compared to group C: L-lysine biosynthesis III (PWY-2942), L-isoleucine biosynthesis I (ILEUSYN-PWY), L-valine biosynthesis (VALSYN-PWY), L-isoleucine biosynthesis II (PWY-5101), and PWY-7111 (Figure 6B). Ten pathways were significantly more abundant in group X compared to group Z: PWY-5667, L-isoleucine biosynthesis IV (PWY-5104), UMP biosynthesis (PWY-5686), PWY-7219, ILEUSYN-PWY, VALSYN-PWY, CALVIN-PWY, PWY-7111, PWY-5101, and NONOXIPENT-PWY (Figure 6C).
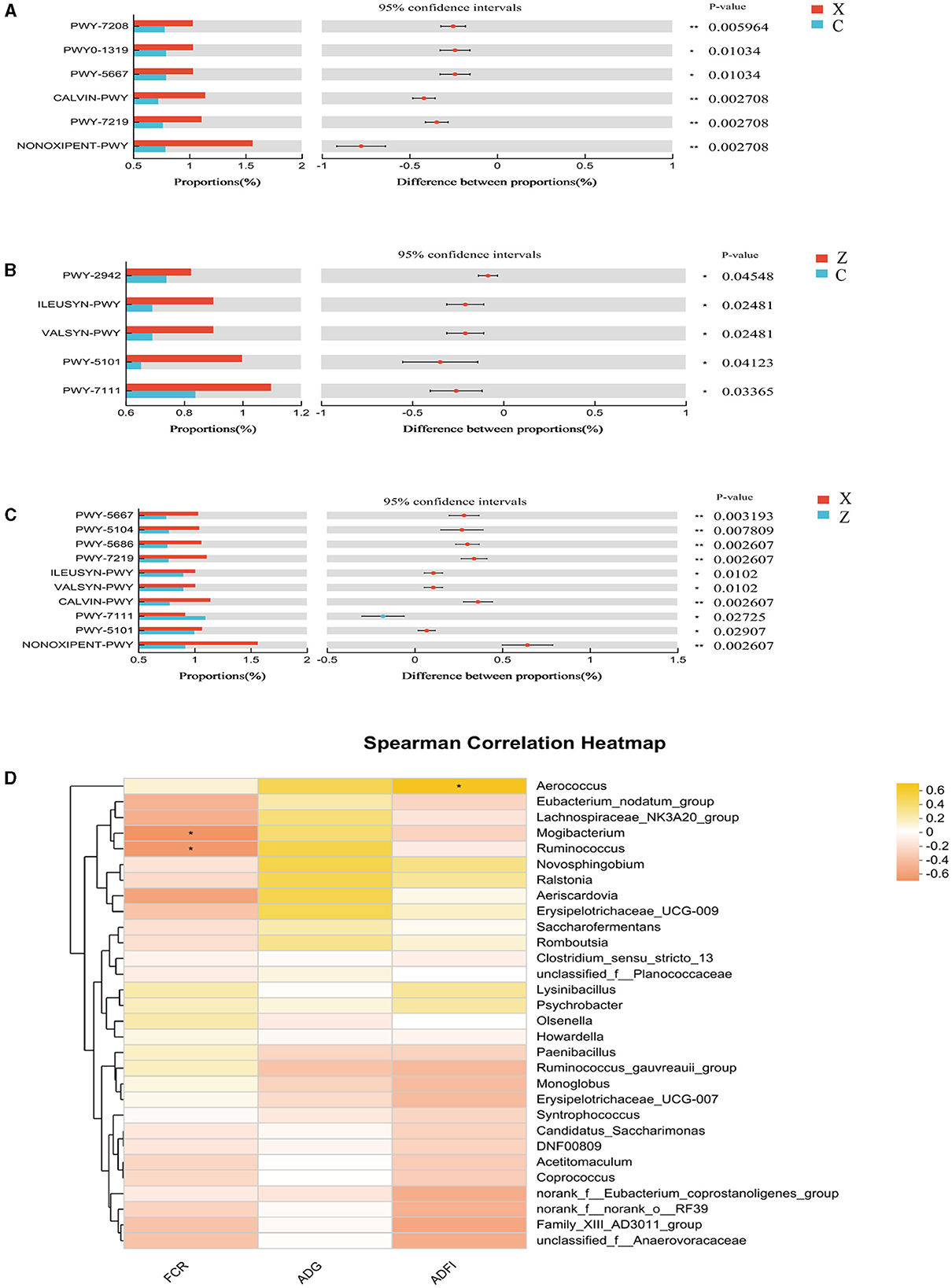
Figure 6. Differential MetaCyc pathways of ileum bacteria in different treatment groups of small-tailed Han sheep: (A) group C and X; (B) group C and Z; (C) group X and Z. (D) The bacterial taxa in ileum correlated with growth performance of small-tailed Han sheep. The colors of the squares correspond to correlations: Yellow is positive, but red is negative. Symbol “*” represents a significant difference, i.e. P < 0.05.
Spearman correlation analysis found significant associations between certain genus-level species in the ileal microbiota and growth performance indicators. Figure 6D revealed that the abundance of Aerococcus (P < 0.05) was positively correlated with ADFI, while Mogibacterium and Ruminococcus showed negative correlations with FCR (P < 0.05).
4 Discussion
4.1 Effects of DPF on the growth performance of small-tailed Han sheep
The nutritional value of feed is primarily determined by its chemical composition, but its effectiveness depends on how it's digested and metabolized by animals, thereby fulfilling their physiological needs (Gao, 2023). In this study, the inclusion of group Y's diet in small-tailed Han sheep led to a significant increase in ADFI compared to group C. This suggested that the fermentation process, which included specific probiotics and a distinctive fruit flavor (Guo, 2022), may have enhanced the palatability of the total mixed ration (TMR). Similar findings, such as the unique probiotic fermentation aroma of Brewers' spent grain improving feed intake and weight gain in lambs, support this observation (Feksa Frasson et al., 2018). Group Z showed the lowest ADFI, likely due to an excessive amount of defective pear fermentation and high sugar content, increasing the TMR's viscosity and altering its texture, thus reducing feed intake.
ADG increased with the increase of ADFI, and there was a positive correlation between them (Foote et al., 2017; Saleem et al., 2017; Flohr et al., 2018; Devyatkin et al., 2021; Zhou et al., 2023). However, the increase of ADFI does not necessarily increase FCR. For example, as reported by Ferronato et al. (2024), the feed intake of broilers in the grower period increased significantly after vitamin C and acetylsalicylic acid were added, but there was no significant change in FCR. The influence of feed pH on feed intake is mainly due to the acidity impacting the palatability of the feed itself. For example, Wang (2022) reported that when excessive fermented pomegranate peel was added to the feed of broilers, high tannic acid content made the feed itself sour and palatability decreased, resulting in lower feed intake and weight gains in the treated group. In this study, although ADG and ADFI in groups X and Y were significantly increased, slaughter weight was not affected. The reason for this phenomenon may be that the pH of TMR decreased slightly after the addition of DPF, while the internal environment of the rumen was in a relatively stable neutral environment for a long time, and the decrease of feed pH may have a certain impact on rumen digestion and absorption, which resulted in no significant difference in slaughter weight and FCR.
4.2 Effects of DPF on the serum characteristics in small-tailed Han sheep
Pears, as one of the most well-known fruits around the world, contain a certain amount of flavonoids and phenolic compounds in their flesh, skin, and core, which are known to have antioxidant properties (Teixeira et al., 2023). However, an animal's antioxidant defense system is integral to its health. The primary roles of GSH-Px and SOD are to eliminate free radicals (Song and Shen, 2020; Zheng et al., 2023). Malondialdehyde, a byproduct of lipid peroxidation in cells, can damage cell membrane structure and function (Meng et al., 2023). In this study, the defective pears still retained a certain amount of flavonoids and phenolic compounds after they were made into fermentation (Table 1). So the antioxidant capacity of sheep in the groups with added DPF were better than that in the group without added DPF.
GLU is an energy source crucial for the growth of small-tailed Han sheep, reflecting their energy metabolism state (Graugnard et al., 2012). TC, HDL-C, LDL-C, and TG are important lipid markers (Agongo et al., 2022). Since the sheep in this study were raised in captivity, these indicators were monitored to ensure normal growth without excessive fat accumulation. The results indicated no abnormal fat accumulation in any group.
4.3 Effects of DPF on the gastrointestinal morphology of small-tailed Han sheep
The rumen of ruminants is a complex ecosystem vital for the initial digestion and absorption of nutrients such as protein, starch, and fiber in feed (Norouzian et al., 2011; Zeitz et al., 2016). Key structural aspects of the rumen, such as papilla height (PL), width, and muscle layer thickness (MT), influence its digestive and absorptive functions. These structures enhance feed nutrient digestion and absorption by increasing the surface area in contact with feed (Górka et al., 2018; Zhang K. et al., 2021). In this study, while there were no significant differences in various indicators, group Y exhibited the highest levels of PLs and MTs. A higher PL increases the contact area with ingested feed, and a thicker MT indicates stronger rumen peristalsis ability in group Y, leading to improved rumen digestion and absorption. This correlates with the observed changes in ADG and ADFI in group Y, which were the highest among the four groups, due to the better development of ruminal PLs and MTs.
Typically, an animal's gut is divided into anterior segment and posterior segments. The anterior segment, including the jejunum and ileum, is the primary site for digestion and absorption. Feed initially digested in the rumen turns into chyme, which is mainly digested and absorbed in the jejunum and ileum (Wang et al., 2020). Villus height (VH), crypt depth (CD), and the villus-to-crypt ratio (V/C) are critical indicators of the small intestine's health and the digestive and absorptive capacity of sheep (Han et al., 2022). VH determines the contact area between the intestinal lining and chyme and is positively correlated with this area (Awad et al., 2011). As CD increases, villi shrink, reducing digestion. V/C ratio is an indicator of small intestine function (Yu et al., 2022).
In this study, group Y showed the highest VH and V/C ratios and the lowest CD in the jejunum, indicating superior intestinal health and digestive and absorptive capabilities compared to other groups. Group X's VH was second only to group Y, but its CD was the highest, leading to a V/C ratio comparable to groups C and Z. Although the VH in group Z's jejunum was not significantly different from group C, it was the lowest among all groups. Thus, excessive DPF addition (6%) may negatively impact intestinal health and function.
The CD of the ileum in each treatment group merits attention. As DPF amounts increased, CD initially decreased then increased, particularly at a 6% supplementation level. These structural changes suggest that the DPF amount should be optimal. This also explains the poor growth performance of group Z, likely due to diminished intestinal health and reduced digestive and absorptive functions.
4.4 Effects of DPF on the rumen microbiota in small-tailed Han sheep
The rumen, a crucial digestive organ in ruminants, significantly impacts their digestive system, particularly through its microbial structure. Understanding the relationship between rumen microbiota and growth performance is essential for enhancing the growth of small-tailed Han sheep. In this study, we compared and explored the bacterial communities in rumen content samples across four treatment groups. The results indicated a significant increase in species richness of bacterial communities with the addition of DPF, although the Shannon and coverage indices showed no significant differences. At the phylum level, the microbial composition of the rumen is relatively stable, predominantly comprising Firmicutes, Bacteroidota, Fibrobacteres, and Proteobacteria (McCann et al., 2014; Deusch et al., 2017; Ge et al., 2023). In line with previous studies, the rumen microbiota in our study was dominated by Firmicutes and Bacteroidota, collectively accounting for over 90% of the total abundance in each treatment group. Common dominant genera included unidentified Prevotellaceae, Fibrobacter, unidentified Lachnospiraceae, Saccharofermentans, and Succinivibrio (Dai et al., 2015; Zhang Y. K. et al., 2021). Our findings (Figure 1F; Table 6) mirrored these trends, showing high relative abundances of Prevotella, Lachnospiraceae_NK3A20_group, norank_f_norank_o_Clostridia_UCG-014, and unclassified_f_Lachnospiraceae at the genus level. Notably, the relative abundance of Prevotella was significantly lower in the DPF-supplemented groups compared to group C. This could be due to the inhibition of Prevotella growth by higher abundances of Lachnospiraceae_NK3A20_group in the supplemented groups. Specifically, group Y had the highest abundance of unclassified_f_Lachnospiraceae but the lowest of Prevotella, suggesting a possible antagonistic relationship between Prevotella and Lachnospiraceae. unclassified_f_Lachnospiraceae, a potential probiotic, played a role in the metabolism of various carbohydrates and the fermentation of intermediate lactate and acetate to butyrate, aiding in maintaining rumen homeostasis and feed digestion (Paz et al., 2018; Zhang et al., 2022). This aligns with our findings, which revealed a significant positive correlation between unclassified_f_Lachnospiraceae and FCR, but slightly different, the increase of ADG and ADFI in group Y did not have significant effects on slaughter weight and FCR. Therefore, we can only assume that DPF has the potential to improve the growth performance of small-tailed Han sheep. Additionally, bacterial function prediction indicated that Pyruvate Fermentation to Isobutanol pathway was enriched in group Y (Figure 2A). Given that isobutanol production requires one NADPH molecule per molecule, higher isobutanol levels can enhance animals' antioxidant capacity (Sauer and Eikmanns, 2005). Consequently, the antioxidant capacity of sheep in group Y was stronger than that in group Z.
4.5 Effects of DPF on the jejunum microbiota in small-tailed Han sheep
The jejunum is a critical site for nutrient absorption in animals, and a healthy jejunal environment is essential for their growth and development. Our analysis of alpha diversity indicated a significant increase in species richness in the groups supplemented with DPF. Microbial community diversity was notably higher in groups X and Z compared to groups Y and C (Figure 3B). The coverage for each group exceeded 0.99, ensuring the reliability of the detection results (Figure 3C). At the phylum level, an important observation (Figure 3E; Table 7) was made regarding the relative abundances of bacteria in the jejunum. Group C exhibited the highest relative abundance of Proteobacteria but the lowest of Bacteroidota among all groups (Pitta et al., 2014). Previous research suggests that Bacteroidota are more efficient in degrading carbohydrates and polysaccharides than Proteobacteria, which could explain the reduced digestion and absorption capabilities, and consequently poorer growth performance, in group C. Additionally, the presence of Staphylococcus, of which more than half are pathogenic, was noted in group C (Hindieh et al., 2022). In contrast, only minimal amounts of Staphylococcus were detected in the DPF-supplemented groups, indicating that DPF addition to the diet of small-tailed Han sheep could effectively reduce Staphylococcus levels in the jejunum (Figure 3F; Table 7). Moreover, group Y had the highest relative abundance of Enterococcus (Figure 3F; Table 7), a potential probiotic known to withstand digestive stress and benefit host health, possibly contributing to the superior growth performance of sheep in this group (Siddique et al., 2021). Bacterial function prediction revealed that most metabolic pathways were enriched in group X (Figures 4A–C), with significant differences from groups C and Z. For instance, the NONOXIPENT-PWY pathway, closely associated with glycolysis in the body, suggests that stronger glycolysis capability, as seen in group X, yields more energy conducive to bodily activities. Hence, the proportion of NONOXIPENT-PWY in group X was slightly higher than in groups C and Z (Figures 4A–C).
4.6 Effects of DPF on the ileum microbiota in small-tailed Han sheep
The ileum, situated after the jejunum, plays a crucial role in digestion and absorption. Alpha diversity analysis showed that group X had the highest Shannon index, indicating a more diverse bacterial community in this group (Figure 5B). The bar chart representing genus-level relative abundance revealed that group C had the highest abundance of Psychrobacter (Figure 5F). Some research suggests that Psychrobacter may be a potential probiotic positively affecting animal growth (Yang et al., 2011; Makled et al., 2017). However, Psychrobacter was not detected in the groups supplemented with DPF in this study, likely due to the inhibitory effect of other bacteria in these groups. Notably, group Y had the highest abundance of the Ruminococcus_gauvreauii_group (Figure 5F; Table 8), crucial in starch degradation (Flint et al., 2008; Chassard et al., 2012). Group Y exhibited the highest abundance (Figure 5F; Table 8) of Clostridium_sensu_stricto_13, a member of the Clostridium genus and a symbiotic bacterium in the animal gut known for producing spores that withstand environmental stress, thus aiding gut health (Guo et al., 2020). There is varied research regarding Olsenella. Some studies identify it as an anaerobic bacterium that produces lactic acid from glucose fermentation, similar to Lactobacillus, and is considered a probiotic aiding feed digestion (Gaowa et al., 2021). However, McLoughlin et al. (2020) found a negative correlation between Olsenella and FCR. Our study differs from this finding, Mogibacterium and Ruminococcus were negatively correlated with FCR (Figure 6D). However, given the growth performance of DPF2, Olsenella appeared to positively influence the growth and development of sheep in our study, tentatively categorizing it as a potential probiotic. Like in the jejunum, group X had more enriched MetaCyc pathways, with no significant differences in pathway proportions between groups X and Y (Figures 6A–C).
5 Conclusion
In conclusion, our study demonstrated the positive effects of using 2% to 4% DPF as feed additives on the growth performance of small-tailed Han sheep. This supplementation notably increased both the ADG and ADFI of these sheep. Furthermore, incorporating DPF into the diet significantly enhanced the antioxidant capacity of the small-tailed Han sheep. The gastrointestinal microbiota compositions were notably altered by DPF addition, primarily elevating the relative abundance of Firmicutes and suppressing Gram-negative bacteria, including Prevotella. The addition of 4% DPF notably increased the relative abundance of unclassified_f_Lachnospiraceae in the rumen. Concurrently, there was a marked increase in the relative abundance of Bacteroidota and a decrease in Proteobacteria, with a significant increase in Enterococcus, especially in group Y within the jejunum. Additionally, the relative abundances of Olsenella and Ruminococcus_gauvreauii_group in jejunum segment and ileum segment were increased after the addition of DPF. At the same time, an increase of the relative abundance of Olsenella in group X in the ileum segment was particularly prominent. Bacterial function prediction indicated that energy metabolism-related pathways, such as NONOXIPENT-PWY, were more prevalent in groups X and Y, potentially boosting the sheep's metabolic capacity. These findings preliminarily confirm the viability of using DPF as a feed additive, suggesting a 2% to 4% inclusion rate in the diet of small-tailed Han sheep. However, further research is needed to explore the broader implications of DPF use in animal production, particularly in regions lacking high-quality feed.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1050385.
Ethics statement
The animal studies were approved by the Animal Ethics Committee of the College of Animal Science and Technology of Tarim University and the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee (No. AW 72303202-1-1). Tarim University is my affiliation, and China Agricultural University is the cooperative institution of the project. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. PC: Methodology, Writing – review & editing. RG: Methodology, Writing – review & editing. ZZ: Methodology, Writing – review & editing. YC: Methodology, Writing – review & editing. PX: Methodology, Writing – review & editing. HS: Conceptualization, Resources, Writing – review & editing. YX: Writing – review & editing. HJ: Conceptualization, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Joint Research Foundation of China Agricultural University, Tarim University (ZNLH202305), and Research and Demonstration of Forage High-Yield Cultivation and Forage Combination to Improve Quality and Efficiency in Southern Xinjiang (2021DB015-03).
Acknowledgments
The authors would like to thank Xu Wang and Shijie Li at the Huazhong Agricultural University for their laboratory assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1358033/full#supplementary-material
Supplementary Figure S1. Effect of defective pear fermentation (DPF) on average daily feed intake of small-tailed Han sheep in 60 days. Group C = control group; Group X = added with 2% DPF; Group Y = added with 4% DPF; Group Z = added with 6% DPF.
Abbreviations
DPF, defective pear fermentation; ADG, average daily gain; ADFI, average daily feed intake; SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; GLU, glucose; TG, triglycerides; TC, total cholesterol; HDL-C, high density liptein cholesterol; LDL-C, low density liptein cholesterol; PL, papillae length; PW, papillae width; MT, muscular thickness; VH, villus height; CD, crypt depth; V/C, villus height/crypt depth.
References
Agongo, G., Raal, F., Nonterah, E. A., Debpuur, C., Oduro, A. R., Ramsay, M., et al. (2022). Non-HDL-C and LDL-C/HDL-C are associated with self-reported cardiovascular disease in a rural west african population: analysis of an array of lipid metrics in an AWI-gen sub-study. PLoS ONE 17:e278375. doi: 10.1371/journal.pone.0278375
Ao, W., Cheng, M., Chen, Y., Sun, J., Zhang, C., Zhao, X., et al. (2022). Fermented apple pomace improves plasma biochemical and antioxidant indicators and fecal microbiota of weaned pigs. Agriculture 12:1603. doi: 10.3390/agriculture12101603
Awad, W. A., Ghareeb, K., and Bohm, J. (2011). Evaluation of the chicory inulin efficacy on ameliorating the intestinal morphology and modulating the intestinal electrophysiological properties in broiler chickens. J. Animal Physiol. Animal Nutr. 95, 65–72. doi: 10.1111/j.1439-0396.2010.00999.x
Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., et al. (2013). Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Caroprese, M., Ciliberti, M. G., Marino, R., Santillo, A., Sevi, A., and Albenzio, M. (2023). Essential oil supplementation in small ruminants: a review on their possible role in rumen fermentation, microbiota, and animal production. Dairy 4:497. doi: 10.3390/dairy4030033
Caspi, R., Billington, R., Keseler, I. M., Kothari, A., Krummenacker, M., Midford, P. E., et al. (2020). The MetaCyc database of metabolic pathways and enzymes - a 2019 update. Nucleic Acids Res. 48, D445–D453. doi: 10.1093/nar/gkz862
Chassard, C., Delmas, E., Robert, C., Lawson, P. A., and Bernalier-Donadille, A. (2012). Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int. J. System. Evolut. Microbiol. 62, 138–143. doi: 10.1099/ijs.0.027375-0
Cheng, X. (2021). Effect of Grape pomace on growth performance, the morphological, function and microbiota of the gastrointestinal tract in Tan sheep. Ningxia University.
Dai, X., Tian, Y., Li, J., Su, X., Wang, X., Zhao, S., et al. (2015). Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 81, 1375–1386. doi: 10.1128/AEM.03682-14
Dawut, A., and Tian, Y. (2021). Competitiveness of Xinjiang's mutton industry based on diamond model. PLoS ONE 16:e0257669. doi: 10.1371/journal.pone.0257669
Deusch, S., Camarinha-Silva, A., Conrad, J., Beifuss, U., Rodehutscord, M., and Seifert, J. (2017). A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 8:1605. doi: 10.3389/fmicb.2017.01605
Devyatkin, V., Mishurov, A., and Kolodina, E. (2021). Probiotic effect of bacillus subtilis B-2998D, B-3057D, and bacillus licheniformis B-2999D complex on sheep and lambs. J. Adv. Veter. Animal Res. 8, 146–157. doi: 10.5455/javar.2021.h497
Feksa Frasson, M., Carvalho, S., Jaurena, G., Menegon, A. M., Machado Severo, M., Henriques da Motta, J., et al. (2018). Intake and performance of lambs finished in feedlot with wet brewer's grains. J. Animal Sci. Technol. 60:12. doi: 10.1186/s40781-018-0166-8
Ferronato, G., Tavakoli, M., Bouyeh, M., Seidavi, A., and Lourdes Suárez Ramírez, Prandini, A. (2024). Effects of combinations of dietary vitamin C and acetylsalicylic acid on growth performance, carcass traits and, serum and immune response parameters in broilers. Animals 14:649. doi: 10.3390/ani14040649
Flint, H. J., Bayer, E. A., Rincon, M. T., Lamed, R., and White, B. A. (2008). Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6, 121–131. doi: 10.1038/nrmicro1817
Flohr, J. R., DeRouchey, J. M., Dritz, S. S., Goodband, R. D., Tokach, M. D., and Woodworth, J. C. (2018). Development of equations to predict the influence of floor space on average daily gain, average daily feed intake and gain: Feed ratio of finishing pigs. Animal 12, 1022–1029. doi: 10.1017/S1751731117002440
Foote, A. P., Keel, B. N., Zarek, C. M., and Lindholm-Perry, A. (2017). Beef steers with average dry matter intake and divergent average daily gain have altered gene expression in the jejunum. J. Animal Sci. 95, 4430–4438. doi: 10.2527/jas2017.1804
Gao, X. (2023). Study on the Nutritional Value of Silage Type Fermented TMR and Its Effects on Fattening Sheep. Lanzhou University.
Gaowa, N., Li, W., Gelsinger, S., Murphy, B., and Li, S. (2021). Analysis of host jejunum transcriptome and associated microbial community structure variation in young calves with feed-induced acidosis. Metabolites 11:414. doi: 10.3390/metabo11070414
Ge, T., Chen, Y., Li, B., Huang, X., Zhao, L., Zhang, X., et al. (2023). High-energy diet modify rumen microbial composition and microbial energy metabolism pattern in fattening sheep. BMC Veter. Res. 19, 1–12. doi: 10.1186/s12917-023-03592-6
Godber, O. F., and Wall, R. (2014). Livestock and food security: Vulnerability to population growth and climate change. Global Change Biol. 20, 3092–3102. doi: 10.1111/gcb.12589
Górka, P., Sliwiński, B., Flaga, J., Olszewski, J., Wojciechowski, M., Krupa, K., et al. (2018). Effect of exogenous butyrate on the gastrointestinal tract of sheep. I. structure and function of the rumen, omasum, and abomasum. J. Animal Sci. 96, 5311–5324. doi: 10.1093/jas/sky367
Graugnard, D. E., Bionaz, M., Trevisi, E., Moyes, K. M., Salak-Johnson, J., Wallace, R. L., et al. (2012). Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 95, 1749–1758. doi: 10.3168/jds.2011-4579
Guo, P., Zhang, K., Ma, X., and He, P. (2020). Clostridium species as probiotics: Potentials and challenges. J. Animal Sci. Biotechnol. 11, 1–10. doi: 10.1186/s40104-019-0402-1
Guo, R. (2022). Selecting and evaluating of mixed fermentation feed of defective pear and corn stalks. Tarim University.
Guo, Y., Hu, Y., Liu, S., Wang, M., Xian, Z., Liu, D., et al. (2023). Effects of the oat hay feeding method and compound probiotic supplementation on the growth, antioxidant capacity, immunity, and rumen bacteria community of dairy calves. Antioxidants 12:1851. doi: 10.3390/antiox12101851
Han, H., Zhang, L., Shang, Y., Wang, M., Phillips, C. J. C., Wang, Y., et al. (2022). Replacement of maize silage and soyabean meal with mulberry silage in the diet of hu lambs on growth, gastrointestinal tissue morphology, rumen fermentation parameters and microbial diversity. Animals 12:1406. doi: 10.3390/ani12111406
Hindieh, P., Yaghi, J., Khoury, A. E., Chokr, A., Atoui, A., Louka, N., et al. (2022). Lactobacillus rhamnosus and Staphylococcus epidermidis in gut microbiota: In vitro antimicrobial resistance. AMB Expr. 12:128. doi: 10.1186/s13568-022-01468-w
Jing, X., Guo, Y., Degen, A., Wang, W., Kang, J., Ding, L., et al. (2022). Hypothalamic regulation of energy homoeostasis when consuming diets of different energy concentrations: comparison between tibetan and small-tailed han sheep. Br. J. Nutr. 127, 1132–1142. doi: 10.1017/S0007114521001902
Kiernan, D. P., John, V., and O'Doherty, Sweeney, T. (2023). The effect of maternal probiotic or synbiotic supplementation on sow and offspring gastrointestinal microbiota, health, and performance. Animals 13:2996. doi: 10.3390/ani13192996
Liu, W., Sun, J., Guo, Y., Liu, N., Ding, X., Zhang, X., et al. (2020). Calhex231 ameliorates myocardial fibrosis post myocardial infarction in rats through the autophagy-NLRP3 inflammasome pathway in macrophages. J. Cell. Molec. Med. 24, 13440–13453. doi: 10.1111/jcmm.15969
Lu, S., Chang, H., Du, L., Tsunoda, K., Sun, W., Yang, Z., et al. (2005). Phylogenetic relationships of sheep populations from coastal areas in east asia. Biochem. Genet. 43, 251–260. doi: 10.1007/s10528-005-5217-5
Ma, W., Yang, Z., Qi, X., Xu, Y., Liu, D., Tan, H., et al. (2023). Study on the fragrant pear-picking sequences based on the multiple weighting method. Agriculture 13:1923. doi: 10.3390/agriculture13101923
Magoč, T., and Salzberg, S. L. (2011). FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Makled, S. O., Hamdan, A. M., El-Sayed, A., and Hafez, E. E. (2017). Evaluation of marine psychrophile, psychrobacter namhaensis SO89, as a probiotic in nile tilapia (oreochromis niloticus) diets. Fish Shellfish Immunol. 61, 194–200. doi: 10.1016/j.fsi.2017.01.001
McCann, J. C., Wiley, L. M., Forbes, T. D., Rouquette, F. M., and Tedeschi, L. O. (2014). Relationship between the rumen microbiome and residual feed intake-efficiency of brahman bulls stocked on bermudagrass pastures. PloS ONE 9:e91864. doi: 10.1371/journal.pone.0091864
McLoughlin, S., Spillane, C., Claffey, N., Smith, P. E., O'Rourke, T., Diskin, M. G., et al. (2020). Rumen microbiome composition is altered in sheep divergent in feed efficiency. Front. Microbiol. 11:1981. doi: 10.3389/fmicb.2020.01981
Meena, M. K., Kumar, H., and Meena, K. (2022). Effect of feeding allium sativum herb and probiotic (Saccharomyces cerevisiae) alone or in combination with ground nut straw on nutrient utilization efficiency in sonadi sheep. J. Animal Res. 12, 879–884. doi: 10.30954/2277-940X.06.2022.8
Meng, M., Zhao, X., Huo, R., Li, X., Chang, G., and Shen, X. (2023). Disodium fumarate alleviates endoplasmic reticulum stress, mitochondrial damage, and oxidative stress induced by the high-concentrate diet in the mammary gland tissue of hu sheep. Antioxidants 12:223. doi: 10.3390/antiox12020223
Niu, Y., Chen, X., Dong, S., Li, W., Liao, K., Nasir, M., et al. (2019). Genetic relationship between the ‘Korla fragrant pear' and local pear varieties in xinjiang based on floral organ characteristics. Scientia Hortic. 257:108621. doi: 10.1016/j.scienta.2019.108621
Norouzian, M. A., Valizadeh, R., and Vahmani, P. (2011). Rumen development and growth of balouchi lambs offered alfalfa hay pre- and post-weaning. Trop. Animal Health Prod. 43, 1169–1174. doi: 10.1007/s11250-011-9819-z
Paz, H. A., Hales, K. E., Wells, J. E., Kuehn, L. A., Freetly, H. C., Berry, E. D., et al. (2018). Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Animal Sci. 96, 1045–1058. doi: 10.1093/jas/skx081
Pitta, D. W., Kumar, S., Vecchiarelli, B., Shirley, D. J., Bittinger, K., Baker, L. D., et al. (2014). Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J. Animal Sci. 92, 4014–4022. doi: 10.2527/jas.2014-7621
Ramdani, D., Yuniarti, E., Jayanegara, A., and Abdul, S. C. (2023). Roles of essential oils, polyphenols, and saponins of medicinal plants as natural additives and anthelmintics in ruminant diets: a systematic review. Animals 13:767. doi: 10.3390/ani13040767
Rodríguez-Muela, C., Rodríguez, H. E., Arzola, C., Díaz-Plascencia, D., Ramírez-Godínez, J. A., Flores-Mariñelarena, A., et al. (2015). Antioxidant activity in plasma and rumen papillae development in lambs fed fermented apple pomace. J. Animal Sci. 93, 2357–2362. doi: 10.2527/jas.2014-8670
Saleem, A. M., Zanouny, A. I., and Singer, A. M. (2017). Growth performance, nutrients digestibility, and blood metabolites of lambs fed diets supplemented with probiotics during pre- and post-weaning period. Asian-Austral. J. Animal Sci. 30, 523–530. doi: 10.5713/ajas.16.0691
Sauer, U., and Eikmanns, B. J. (2005). The PEP—pyruvate—oxaloacetate node as the switch point for carbon flux distribution in bacteria: We dedicate this paper to rudolf K. thauer, director of the max-planck-institute for terrestrial microbiology in marburg, germany, on the occasion of his 65th birthday. FEMS Microbiol. Rev. 29, 765–794. doi: 10.1016/j.femsre.2004.11.002
Siddique, A., Azim, S., Ali, A., Adnan, F., Arif, M., Imran, M., et al. (2021). Lactobacillus reuteri and enterococcus faecium from poultry gut reduce mucin adhesion and biofilm formation of cephalosporin and fluoroquinolone-resistant salmonella enterica. Animals 11:3435. doi: 10.3390/ani11123435
Song, C., and Shen, X. (2020). Effects of environmental zinc deficiency on antioxidant system function in Wumeng semi-fine wool sheep. Biol. Trace Element Res. 195, 110–116. doi: 10.1007/s12011-019-01840-1
Teixeira, J. D., Soares Mateus, A. R., Sanchez, C., Parpot, P., Almeida, C., and Ana, S. S. (2023). Antioxidant capacity and phenolics profile of portuguese traditional cultivars of apples and pears and their by-products: on the way to newer applications. Foods 12:1537. doi: 10.3390/foods12071537
Thornton, P. K., and Gerber, P. J. (2010). Climate change and the growth of the livestock sector in developing countries. Mitigat. Adapt. Strat. Global Change 15, 169–184. doi: 10.1007/s11027-009-9210-9
Varga-Visi, É., Nagy, G., Csivincsik, Á., and Tóth, T. (2023). Evaluation of a phytogenic feed supplement containing carvacrol and limonene on sheep performance and parasitological status on a hungarian milking sheep farm. Veter. Sci. 10:369. doi: 10.3390/vetsci10060369
Wang, C., Zhang, J., and Zhou, W. (2023). The effect of animal husbandry on economic growth: evidence from 13 provinces of north China. Front. Environ. Sci. 10:1085219. doi: 10.3389/fenvs.2022.1085219
Wang, J. (2022). Application of pomegranate peel on growth performance of broilersand its resistance to chickens with colibacillosis. Huazhong Agricultural University.
Wang, J., Yu, X., Bai, Y., Wang, P., and Liu, C. (2020). Effects of grazing and confinement on the morphology and microflora of the gastrointestinal tract of small-tailed han sheep. Livestock Sci. 241:104208. doi: 10.1016/j.livsci.2020.104208
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wang, Z., Liu, R., Fu, L., Tao, S., and Bao, J. (2023). Effects of orchard grass on soil fertility and nutritional status of fruit trees in korla fragrant pear orchard. Horticulturae 9:903. doi: 10.3390/horticulturae9080903
Yang, C., Wang, F., Wu, S., Wu, Z., Qin, L., Wan, H., et al. (2022). Effects of Chinese herbal medicine feed additives on growth performance and dietary nutrient metabolism of black-bone chickens. Asian Agric. Res. 14, 86–91. doi: 10.19601/j.cnki.issn1943-9903.2022.11.018
Yang, H., Sun, Y., Ma, R., Li, J., and Huang, K. (2011). Probiotic psychrobacter sp. improved the autochthonous microbial diversity along the gastrointestinal tract of grouper epinephelus coioides. J. Aquac. Res. Dev. S1:001. doi: 10.4172/2155-9546.S1-001
Yu, J., Zheng, C., Zheng, J., Duan, G., Guo, Q., Zhang, P., et al. (2022). Development of intestinal injury and restoration of weaned piglets under chronic immune stress. Antioxidants 11:2215. doi: 10.3390/antiox11112215
Zeitz, J. O., Tschuor, A., Soliva, C. R., Leiber, F., Clauss, M., Kreuzer, M., et al. (2016). Variability in microbial population and fermentation traits at various sites within the forestomach and along the digestive tract as assessed in goats fed either grass or browse. Small Rumin. Res. 136, 7–17. doi: 10.1016/j.smallrumres.2015.12.029
Zhang, K., Qian, Q., Mao, Y., Xu, Y., Yang, Y., Chen, Y., et al. (2021). Characterization of growth phenotypes and gastrointestinal tract microbiota in sheep fed with caragana. J. Appl. Microbiol. 131, 2763–2779. doi: 10.1111/jam.15138
Zhang, Y. K., Zhang, X. X., Li, F. D., Li, C., Li, G. Z., Zhang, D. Y., et al. (2021). Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 15:100161. doi: 10.1016/j.animal.2020.100161
Zhang, Z., Huang, B., Shi, X., Wang, T., Wang, Y., Zhu, M., et al. (2022). Comparative analysis of bacterial diversity between the liquid phase and adherent fraction within the donkey caeco-colic ecosystem. Animals 12:1116. doi: 10.3390/ani12091116
Zheng, M., Liu, Y., Zhang, G., Yang, Z., Xu, W., and Chen, Q. (2023). The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants 12:1675. doi: 10.3390/antiox12091675
Keywords: defective pear fermentation, small-tailed Han sheep, growth performance, serum antioxidant, gastrointestinal microbiota community
Citation: Peng H, Chen P, Guo R, Zhou Z, Chen Y, Xu P, Su H, Xiao Y and Jiang H (2024) Regulation of the growth performance and the gastrointestinal microbiota community by the addition of defective pear fermentation to feed of small-tailed Han sheep. Front. Microbiol. 15:1358033. doi: 10.3389/fmicb.2024.1358033
Received: 19 December 2023; Accepted: 18 March 2024;
Published: 04 April 2024.
Edited by:
Qing Zhang, South China Agricultural University, ChinaReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceChirasak Phoemchalard, Mahidol University, Amnat Charoen Campus, Thailand
Copyright © 2024 Peng, Chen, Guo, Zhou, Chen, Xu, Su, Xiao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Jiang, amlhbmdodWkzMDhAdGFydS5lZHUuY24=
 Hongxin Peng
Hongxin Peng Pinpin Chen
Pinpin Chen Rui Guo1
Rui Guo1 Huawei Su
Huawei Su Yuncai Xiao
Yuncai Xiao