- 1Qinghai University Medical College, Xining, China
- 2Research Center for High Altitude Medicine, Qinghai University, Xining, China
Background: Hypoxic pulmonary hypertension (HPH) is a severe high-altitude disorder with limited therapeutic options. This study investigated the therapeutic mechanisms of Oxytropis falcata Bunge (OFB), a traditional Tibetan herbal medicine, in a rat model of HPH, focusing on its effects on endogenous metabolites and gut microbiota.
Methods: HPH was induced in male Sprague–Dawley rats exposed to chronic hypoxia. Animals were randomly assigned to normoxic control, hypoxic model, OFB-treated, or Rhodiola-treated groups. Serum metabolomics (LC-MS) and 16S rRNA sequencing of fecal microbiota were performed. Cardiopulmonary parameters including RVSP and RVHI were assessed, and pulmonary arterial ultrastructure was examined.
Results: OFB significantly attenuated HPH-induced elevations in RVSP and RVHI and mitigated pulmonary arterial remodeling. Metabolomic analysis identified 25 differentially regulated metabolites in HPH, primarily involved in pyrimidine metabolism, which were largely restored by OFB. OFB also reversed HPH-induced gut microbiota dysbiosis, restoring microbial diversity and composition toward normoxic levels. Correlation analysis revealed significant associations between specific bacterial taxa and altered metabolites.
Conclusions: These findings suggest that OFB exerts therapeutic effects against HPH by modulating gut microbiota dysbiosis and restoring metabolic homeostasis, particularly within pyrimidine metabolism. The observed gut–lung axis interactions may underlie these effects, offering novel mechanistic insights and supporting the potential clinical development of OFB as a microbiota-targeted therapy for HPH.
Introduction
Pulmonary arterial hypertension (PAH) is frequently referred to as the “cancer” of the cardiovascular system, with hypoxic pulmonary hypertension (HPH) being particularly prevalent among high-altitude populations. Chronic hypoxia has been demonstrated to induce persistent pulmonary vasoconstriction and pulmonary artery remodeling. This, in turn, has been shown to lead to increased pulmonary circulation resistance and right heart failure (Wan et al., 2024). Oxytropis falcata Bunge, a traditional Tibetan medicine known as “Edaxia,” has been widely used for its anti-inflammatory and hemostatic properties. Recent studies have indicated a close relationship between the gut microbiota and metabolites in patients with pulmonary arterial hypertension (PAH) (Gao et al., 2023; Ailizire et al., 2023; Wang et al., 2023). However, the specific mechanisms by which OFB regulates gut microbiota and metabolites in HPH remain to be elucidated. This study employs an innovative approach to investigate the therapeutic effects of OFB on HPH. It does this by analyzing changes in serum metabolites and gut microbiota composition, thus providing novel insights into the pathophysiology of HPH. Moreover, recent research has indicated that the gut-brain-lung axis may potentially have a significant role in the development of HPH (Kim, 2025). For instance, the identification of a postprandial neuroimmune axis by Chen et al. provides a link between the gastrointestinal tract and lung function via brain-coordinated sensory and motor circuits. This may offer a potential connection to the gut-lung axis in HPH (Kim, 2025). Furthermore, the role of specific gut microbiota taxa and their corresponding metabolites in modulating the overall metabolic profile in HPH warrants further investigation. The objective of this study is to address these knowledge gaps and to provide a comprehensive understanding of the therapeutic potential of Oxytropis falcata Bunge in HPH.
Materials and methods
Instruments and reagents
The following reagents and instrumentation were utilized in the study: LC–MS grade methanol, acetonitrile, ammonia, and isopropanol (CNW Technologies); Phusion Hot start flex 2X Master Mix (Shanghai Yitao Biological Instrument Co., LTD); DL2000 DNA Marker (Takara); Gene color (Beijing Jinboyi); Biowest Agarose G-10 (BIOWEST); AMPure XT beads (Beckman); NovaSeq 6000 sequencer (Illumina); centrifuge (Thermo Fisher Scientific); ultrasonic instrument (Shenzhen Redbond Electronics Co., Ltd.); homogenizer (Shanghai Jingxin Technology Co., Ltd.); freeze dryer (Sihuan Furuike Technology Development Co., Ltd.); Qubit (Invitrogen Q33226); Vanquish (Thermo Fisher Scientific).
Preparation of alcoholic extracts from the traditional Chinese medicine Oxytropis falcata Bunge
The whole grass powder of Oxytropis falcata Bunge (3,060 g, batch number T630602174) was obtained from Qinghai Tibetan Hospital and authenticated by Duojie Cairang, the chief pharmacist. The powder was reflux-extracted in a 10 L extraction device with 95% ethanol at a liquid-to-solid ratio of 10:1. The extraction was performed at 75 °C for 3 h, repeated three times. The combined extracts were concentrated using a rotary evaporator, centrifuged at 3,000 rpm for 5 min, and the supernatant was dried at 80 °C for 4 h. The final extract was freeze-dried into a powder and stored at −20 °C.
Preparation of Rhodiola alcohol extract
The Rhodiola decoction pieces were ground into powder, loaded into a 10 L extractor already containing Rhodiola rosea, and reflux-extracted three times (75 °C, 60 min each) with ethanol–water 1:100. After extraction the pooled mixture was mixed, filtered, centrifuged (3,000 rpm, 5 min) and distilled; the filtrate was transferred to a glass petri dish and dried at 80 °C for 4 h to give an alcohol extract that was kept at −20 °C, then freeze-dried in a 50 mL centrifuge tube to yield a dry powder.
Animals and grouping
Forty male Sprague–Dawley rats (weighing 130–150 g) were procured from Beijing Weitong Lihua Company (license number: SCXK (Beijing) 2021-0006). The rats were acclimatized for a period of 7 days prior to being divided into five groups: the normoxic control group (nor), the hypoxic simulation group (hyp), the hypoxic OFB group (hypofb), and the hypoxic Rhodiola group (hyprho). The normoxic control group was housed in the SPF-grade animal laboratory of Qinghai University, with a temperature of (22 ± 2)°C, humidity of (50 ± 10)%, and a 12 h light–dark cycle, with free access to food. The remaining three groups were accommodated in the low-pressure oxygen chamber (DYC3000, Guizhou Fenglei Aviation Ordnance Co., Ltd.) at Qinghai University, with a temperature of (24 ± 2)°C, humidity of (55 ± 10)%, and a 12 h light–dark cycle, with free access to food. All experimental procedures were approved by the Ethics Committee of Qinghai University Medical School (approval number: SL202401-51).
Plasma sample processing and metabolite extraction
Blood samples were collected from the aorta under urethane anesthesia and subsequently subjected to centrifugation at 3000 g for 10 min at 4 °C. The serum was then divided into 200 μL aliquots and stored at −80 °C. For the extraction of metabolites, 100 μL of plasma was mixed with 400 μL of an extraction solution (methanol: acetonitrile = 1:1, V/V) containing an internal standard. The mixture was subjected to a vortex for a period of 30 s, followed by an incubation in an ice bath for a duration of 10 min. Subsequently, the mixture was subjected to a centrifugation process at a speed of 12,000 rpm for a duration of 15 min at a temperature of 4 °C. The sample was transferred to a vial for the purpose of liquid chromatography-mass spectrometry (LC–MS) analysis.
Liquid chromatography-mass spectrometry conditions
The separation of polar metabolites was achieved through the utilization of a Waters ACQUITY UPLC BEH Amide column (2.1 mm × 50 mm, 1.7 μm), employing a Vanquish ultra-high-performance liquid chromatograph. The mobile phase A solution comprised 25 mmol/L ammonium acetate and 25 mmol/L ammonia, while the mobile phase B solution was acetonitrile. The temperature of the sample tray was maintained at 4 °C, and the injection volume was 2 μL. The acquisition of mass spectrometry data was conducted using an Orbitrap Exploris 120 mass spectrometer, which was controlled by Thermo’s Xcalibur software (version 4.4).
Processing of intestinal contents samples and 16S rRNA sequencing
Intestinal contents were collected from the cecum of rats that had been anesthetized and stored at −80 °C. The DNA was extracted using the CTAB method, and its purity and concentration were assessed by agarose gel electrophoresis and UV spectrophotometry, respectively. The 16S rRNA V3-V4 region was subjected to PCR amplification using specific primers, and the resulting PCR products were purified and sequenced on a NovaSeq 6000 platform (Illumina) with a 2 × 250 bp paired-end configuration.
Data processing and statistical analysis
The raw LC–MS data were converted to mzXML format using the ProteoWizard program and processed using the R package. A total of 30,346 peaks were extracted from 5 QC samples and 40 experimental samples. Following the implementation of data preprocessing procedures, incorporating the removal of outliers, the imputation of missing values, and normalization, a total of 18,871 peaks were retained for subsequent analysis. The 16S rRNA sequencing data were processed using DADA2 for denoising and ASV identification. Alpha and beta diversity analyses were performed using various indices and distance metrics. The identification of species was conducted using the SILVA database. Statistical significance was determined using Fisher’s exact test, Mann–Whitney U test, or Kruskal-Wallis test, with p < 0.05 considered significant.
Results
Right ventricular systolic blood pressure (RVSP) and right cardiac hypertrophy index (RVHI)
In comparison with the normoxic control group, the hypoxic simulation group demonstrated a marked increase in RVSP (p < 0.001) and RVHI (p < 0.001). The findings revealed that both OFB and Rhodiola treatments significantly reduced RVSP and RVHI in HPH rats (p < 0.001 for OFB, p < 0.01 for Rhodiola), thereby indicating their protective effects against HPH-induced right heart damage (Figure 1).
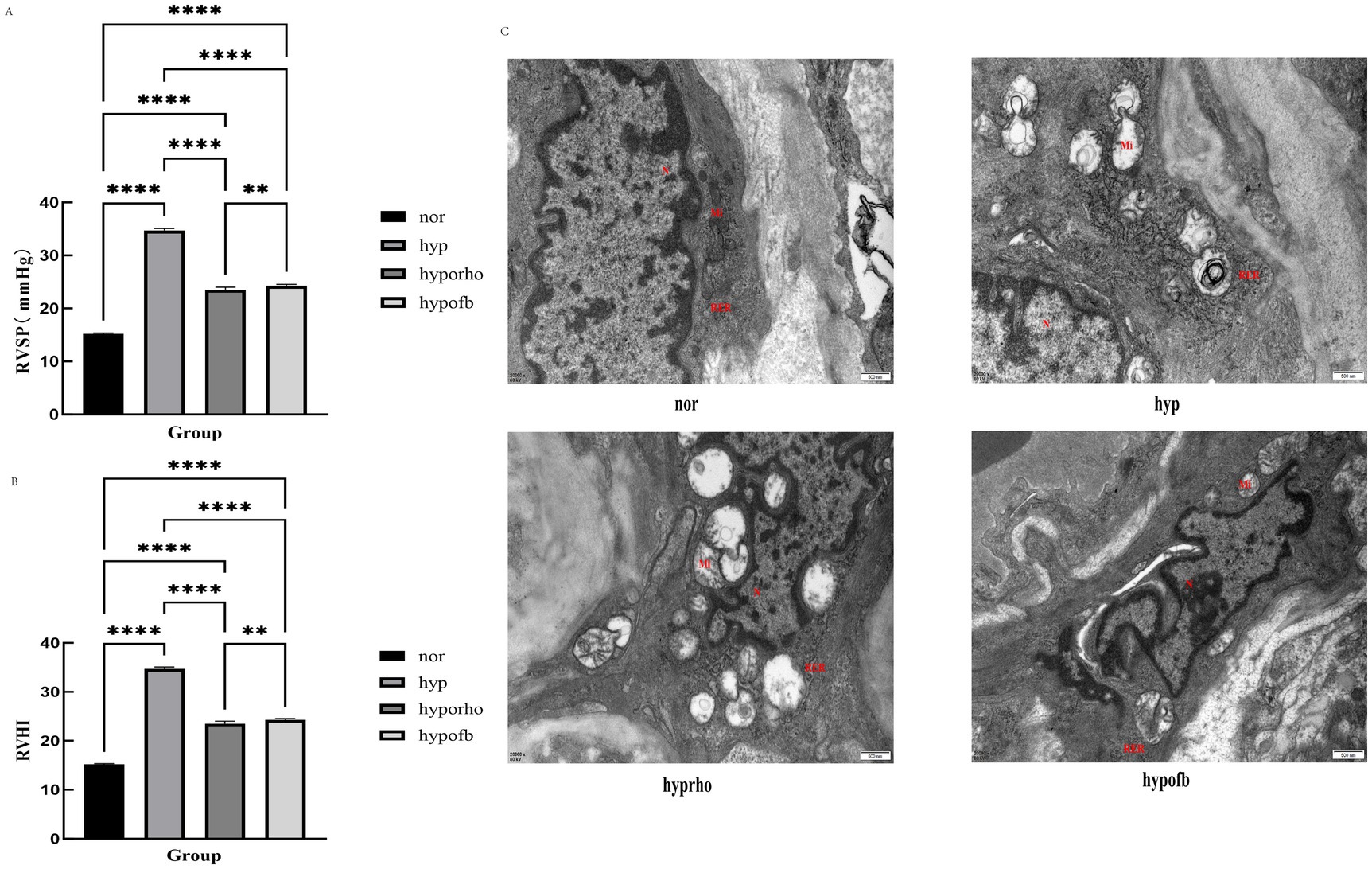
Figure 1. Effects of Oxytropis falcata Bunge on cardiopulmonary pathological indicators in rats with hypoxic pulmonary hypertension (HPH). (A) Right ventricular systolic blood pressure (RVSP) in rats; (B) right ventricular hypertrophy index (RVHI) in rats; (C) transmission electron microscopy results of pulmonary artery smooth muscle cells.
Transmission electron microscopy of rat lung smooth muscle cells
Transmission electron microscopy revealed significant remodeling of pulmonary artery smooth muscle cells in the hypoxic simulation group, characterized by mitochondrial proliferation, swelling, and rough endoplasmic reticulum expansion. The administration of OFB treatment resulted in a significant alleviation of these pathological changes, with a more pronounced effect than Rhodiola (Figure 1).
Serum differential metabolite sceening
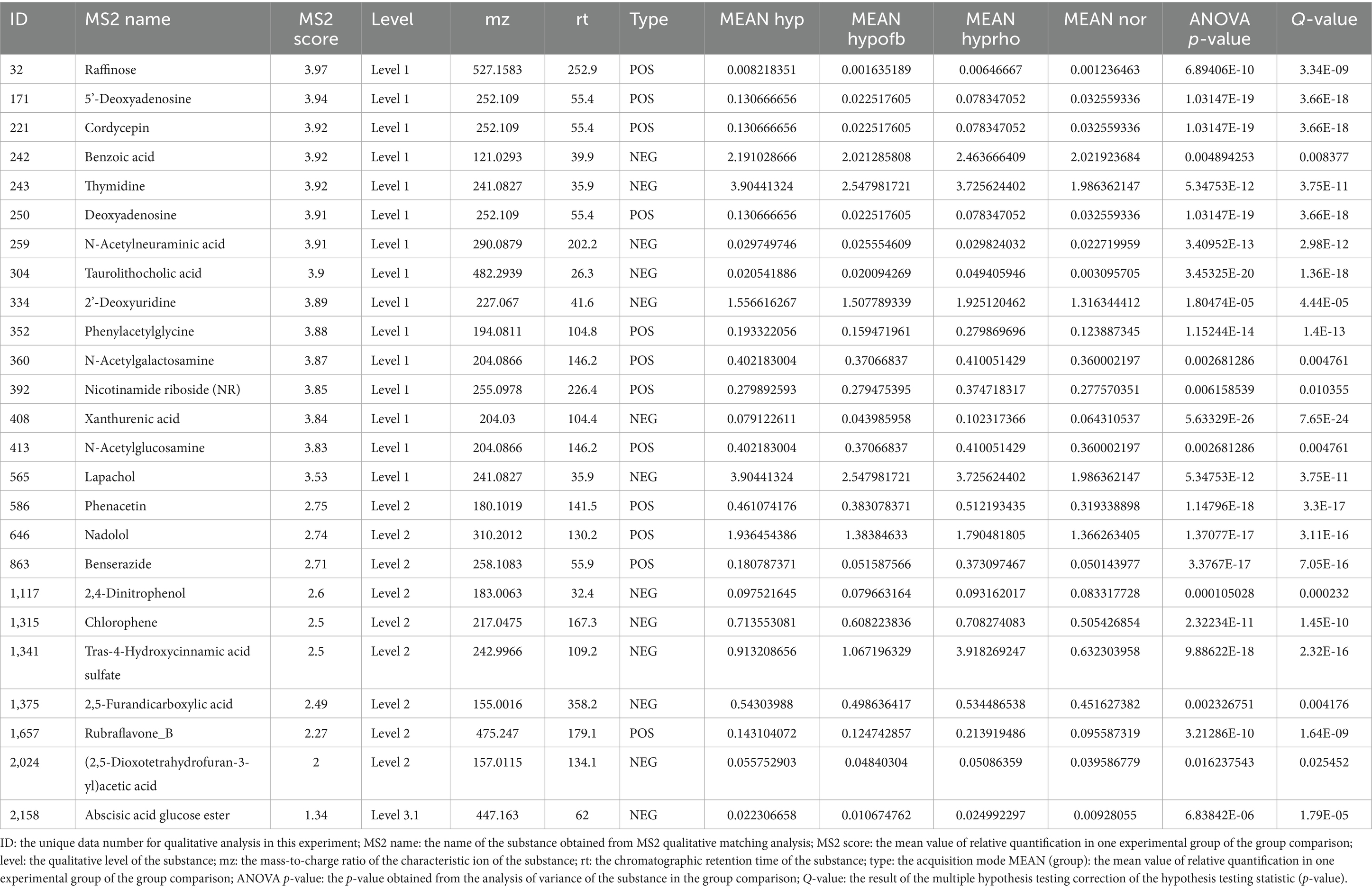
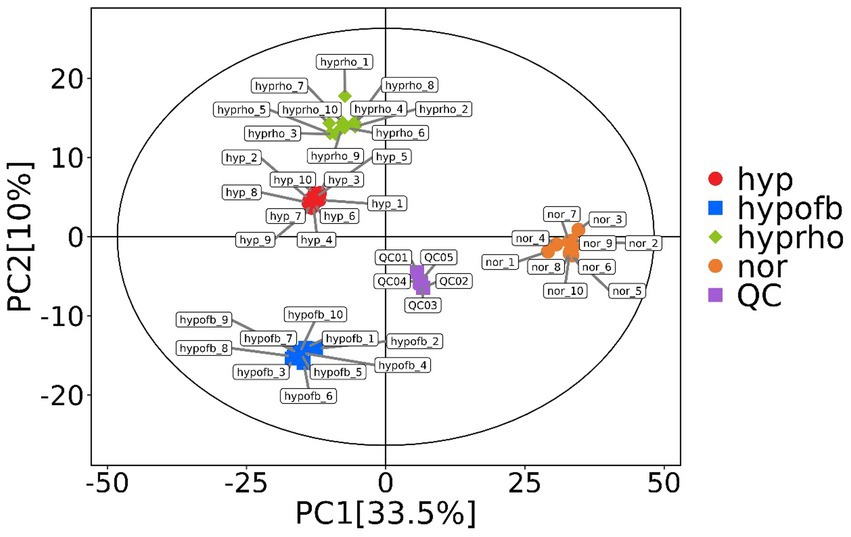
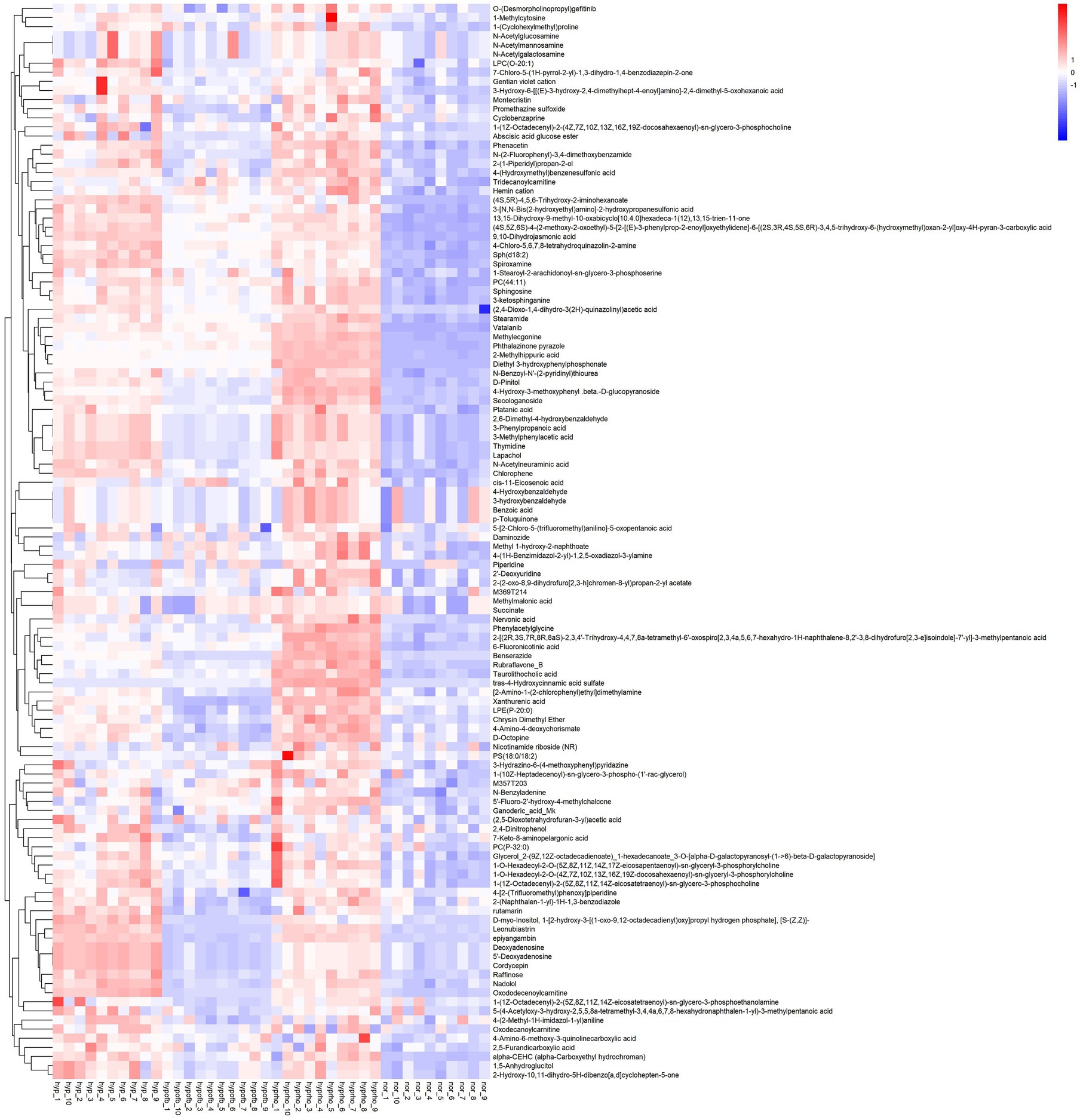
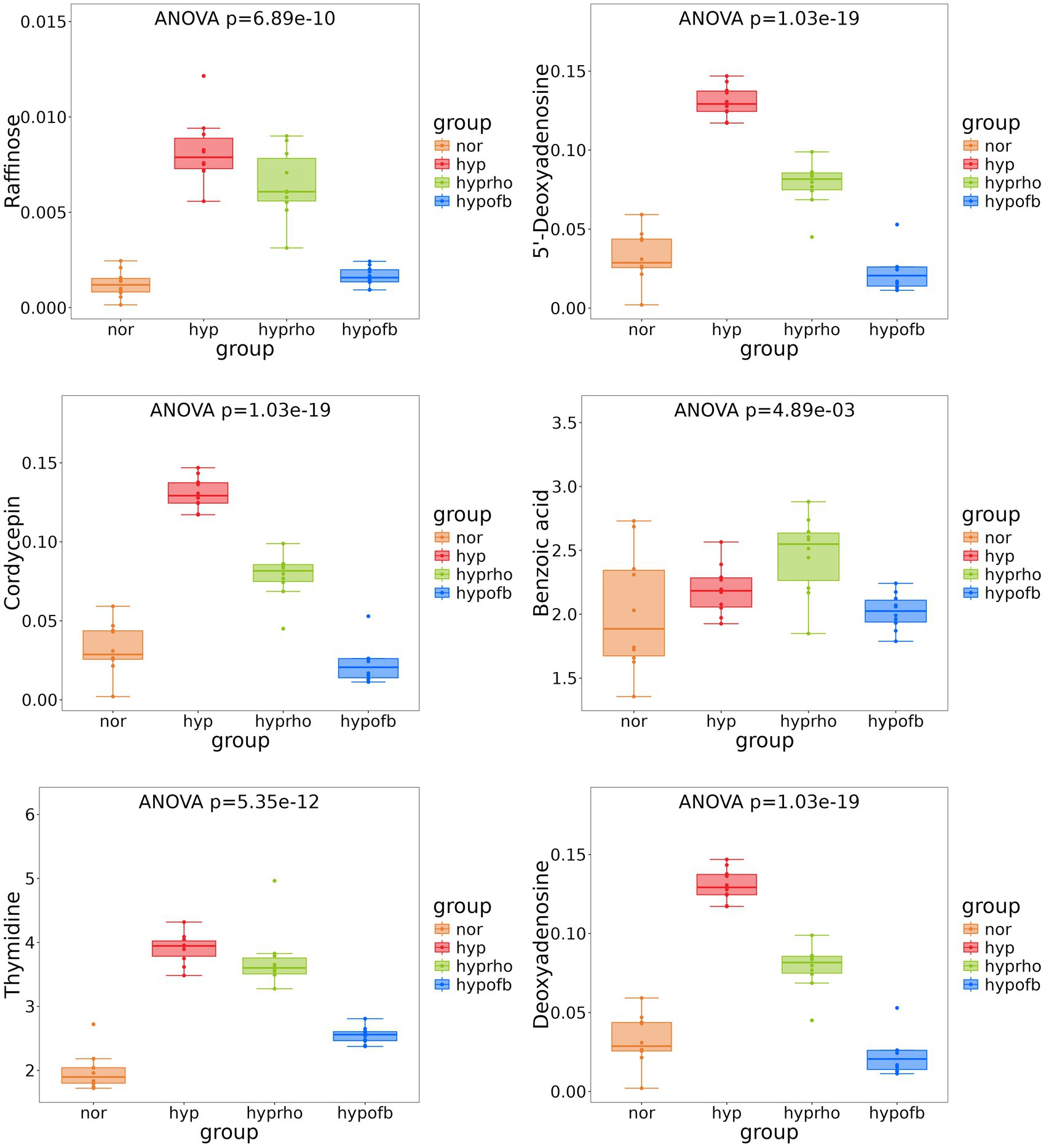
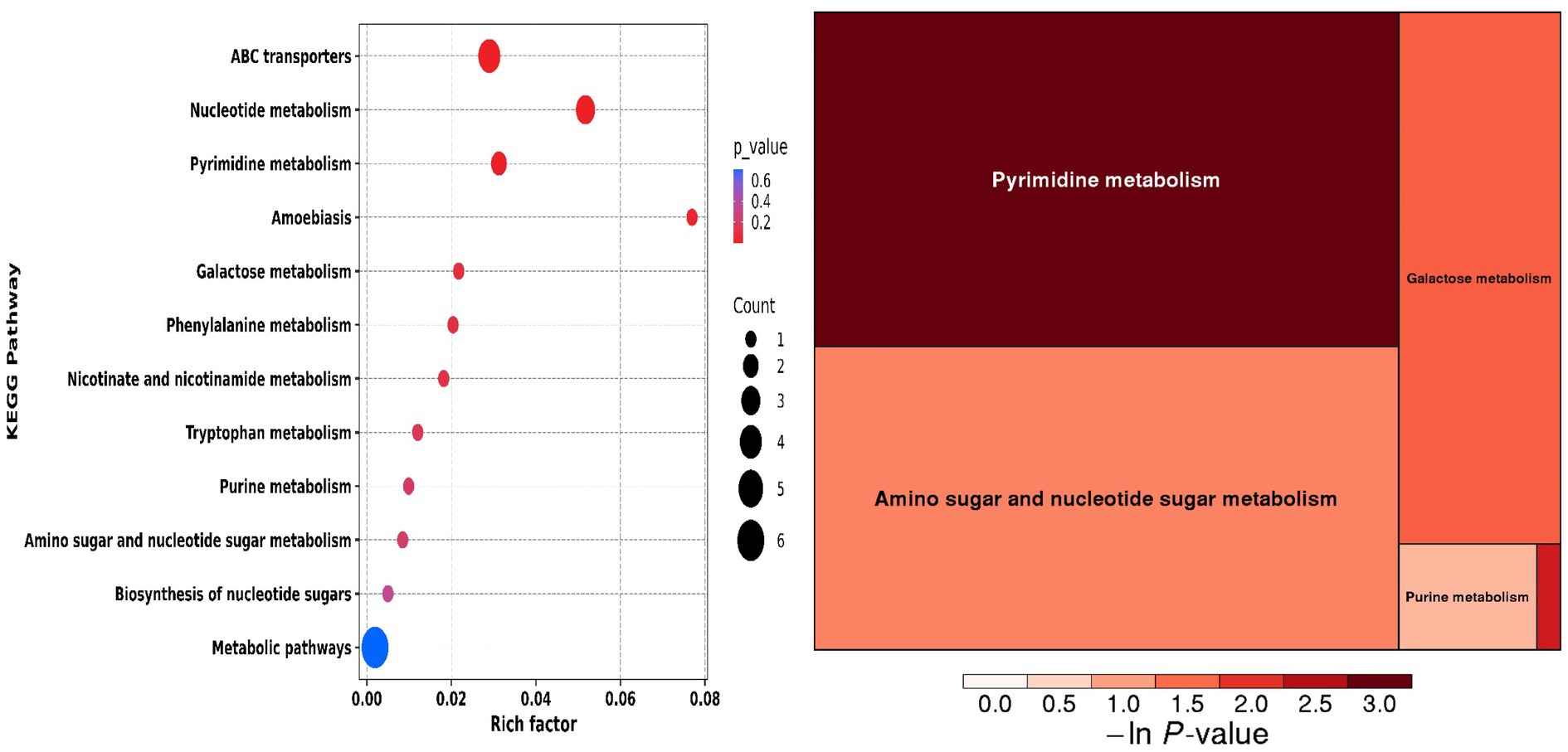
Principal component analysis (PCA) and clustering heatmap analysis revealed significant disparities in endogenous metabolites between the hyp and the nor groups. A total of 25 differential metabolites were identified, primarily involved in the pyrimidine metabolism pathway. Treatment with OFB resulted in the restoration of metabolite levels to those observed in the normoxic control group (see Table 1; Figures 2–5).
Gut microbiota composition
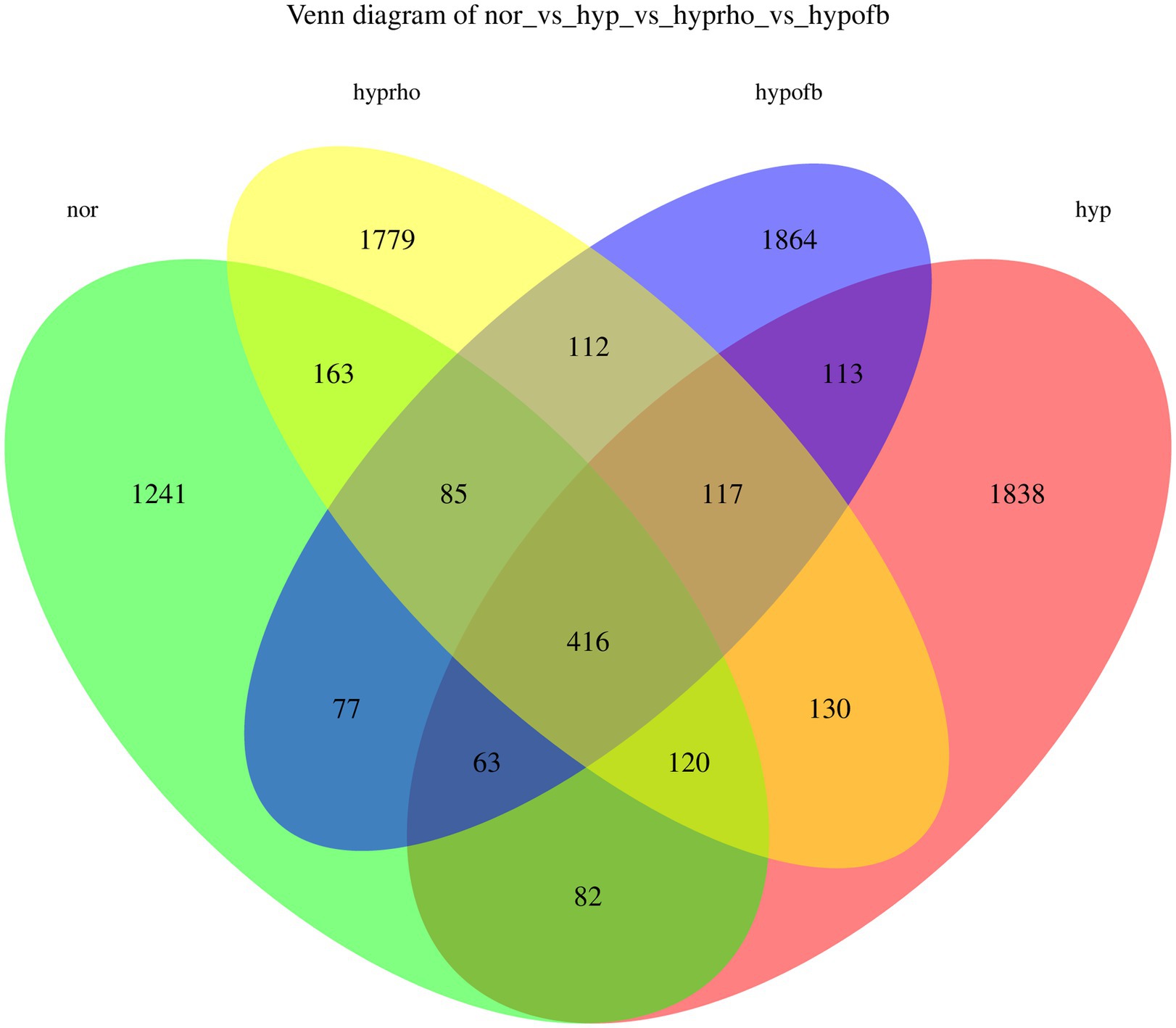
The results from the Venn diagram indicate significant differences in microbial community composition among the various treatment groups, with certain species being more abundant or unique to specific treatment groups. The violin plots for the Shannon and Simpson diversity indices demonstrate that the hypoxic group exhibits significantly higher microbial diversity (richness and evenness) compared to other groups (p < 0.05). In contrast, the violin plots for the Goods Coverage, Chao1 index, and Observed Species index demonstrate no significant differences in species coverage, total species count, and the number of observed species among the different groups (p > 0.05). Combining the Venn diagram analysis with previous findings, it is hypothesized that OFB treatment may contribute to restoring the diversity of the gut microbiota, bringing it closer to that of the normoxic control group (see Figures 6, 7).
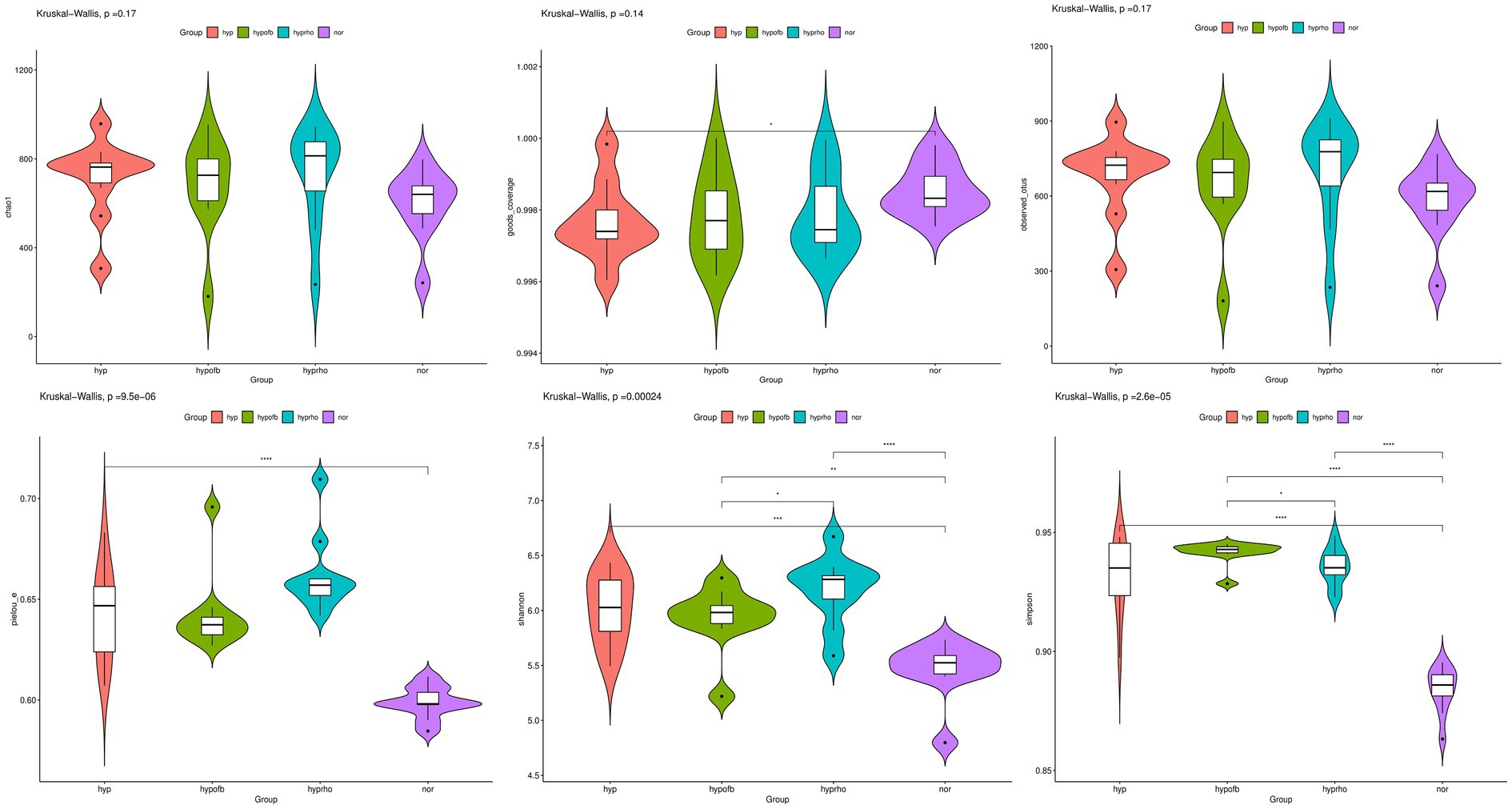
Figure 7. Distribution of alpha diversity indices (Chao1 Index, Goods Coverage, Observed Species Index, Simpson Diversity Index, and Shannon Diversity Index) among different treatment groups.
Beta diversity and species analysis
PCA and PCoA analysis revealed substantial disparities in the composition of gut microbiota between the normoxic and hypoxic groups. The hypofb group demonstrated a microbiota composition that was more akin to that of the normoxic group, thus suggesting that OFB exerts a therapeutic effect on gut microbiota dysbiosis in HPH rats (Figure 8).
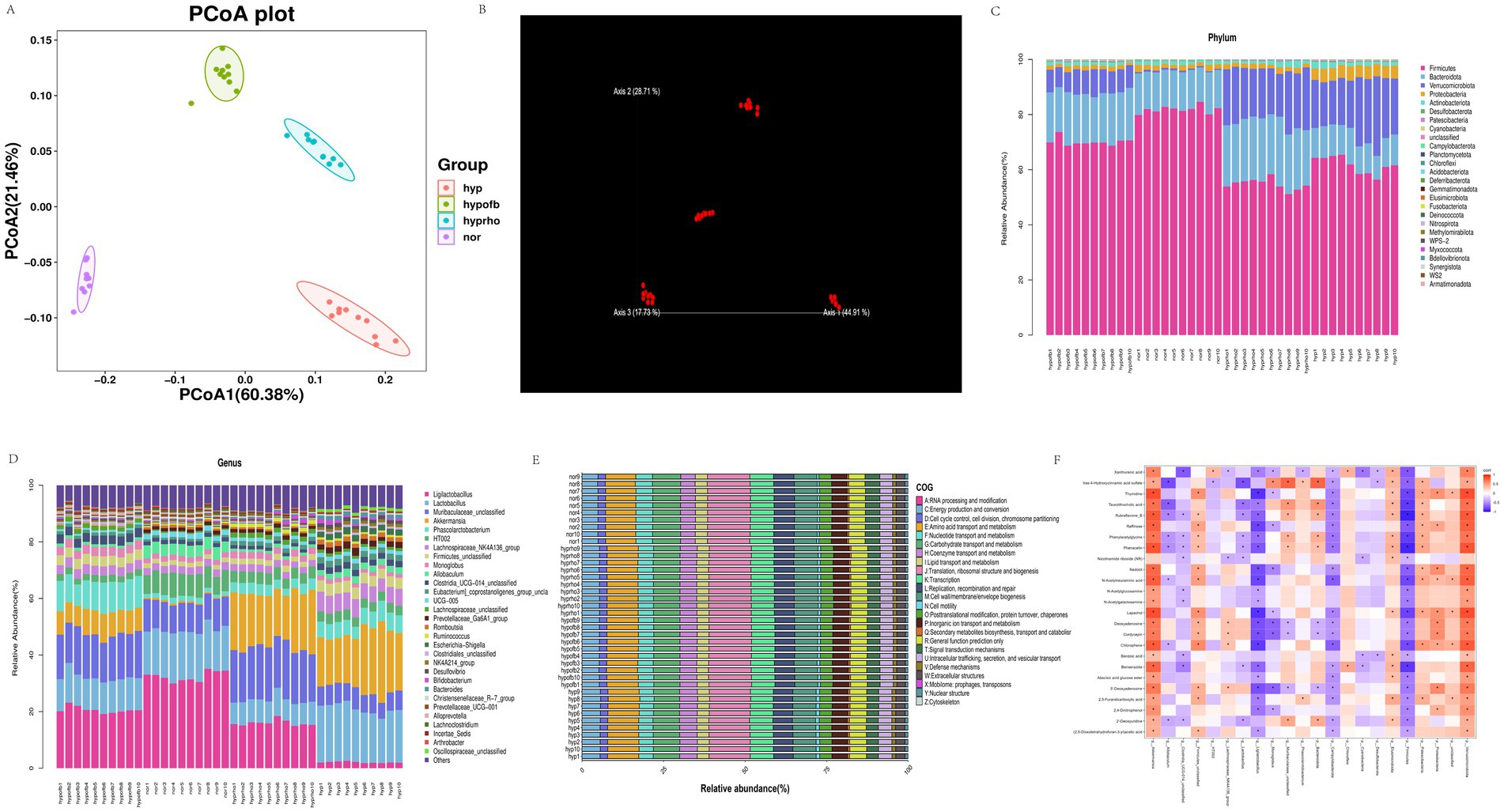
Figure 8. (A) β diversity analysis of gut microbiota using Principal Coordinate Analysis (PCoA); (B) β diversity analysis of gut microbiota using Non-metric Multidimensional Scaling (NMDS); (C) stratified map of the top 30 intestinal flora richness of phylum and genus; (D) stratified map of the top 30 intestinal flora richness of phylum and genus; (E) COG functional classification of gut microbiota; (F) heat map of correlation analysis between differential metabolites and intestinal flora.
The relative abundance and functional analysis of gut microbiota
The application of the COG database to the functional classification of gut microbiota has revealed that the microbiota in HPH rats is involved in a variety of biological processes, including energy metabolism, RNA processing, and cell cycle regulation. The administration of OFB treatment resulted in a substantial alteration of the functional profile of the gut microbiota, leading to its restoration to a state resembling normoxia (Figure 8).
Correlation analysis between gut microbiota and metabolites
Spearman correlation analysis revealed significant correlations between specific gut microbiota taxa and serum metabolites. For instance, N-acetylglucosamine demonstrated a positive correlation with Verrucomicrobiota and a negative correlation with Firmicutes. These correlations highlight the interplay between gut microbiota and host metabolites in HPH (Figure 8).
Discussion
This study explores the therapeutic effects of Oxytropis falcata Bunge on hypoxic pulmonary hypertension (HPH) by analyzing changes in serum metabolites and gut microbiota. The findings demonstrate that OFB can regulate gut microbiota dysbiosis and restore metabolic homeostasis in HPH rats. As demonstrated in preceding studies, an imbalance in the composition of the gut microbiota has been demonstrated to be associated with the progression of pulmonary hypertension (Callejo et al., 2018; Baix et al., 2022; Everard et al., 2013). The present study revealed that the abundance of Firmicutes and Lactobacillus, two dominant phyla in the gut microbiota, was significantly reduced in the hypoxic group. However, OFB treatment was found to restore their abundance to normal levels. In this study, researchers observed that treatment with OFB significantly restored the diversity of the gut microbiota and the levels of metabolites in a rat model of HPH. This finding is in accordance with the results obtained by Rastogi et al., who proposed that gut microbes ferment dietary fibers to produce metabolites, particularly short-chain fatty acids (SCFAs) (Rastogi et al., 2022). These SCFAs have been shown to migrate directly to lung tissues through the bloodstream, modulating pulmonary immune responses, or promoting the differentiation and activation of immune cells to produce cytokines and IgA. In this experiment, the administration of OFB treatment resulted in a significant reduction in HPH-induced damage, which may be partly attributable to the effects of SCFAs. It is evident that SCFAs exert their influence not only within the gastrointestinal tract but also potentially on the pulmonary immune environment via the gut-lung axis (Rastogi et al., 2022; Dumas et al., 2018; Anand and Mande, 2018; Price and O'Toole, 2021; Ashique et al., 2022). In the lungs, IgA has been shown to promote the clearance of pathogens, regulatory T cells (Tregs) have been demonstrated to reduce pulmonary inflammation and injury, and certain cytokines (such as TNF-α, IL-4, etc.) have been observed to alter the immune environment, thereby affecting lung health. These results suggest that OFB may improve the imbalance of gut microbiota and metabolite levels in HPH rats by regulating the gut microbiota and its metabolites, thereby positively impacting lung health through the gut-lung axis. It can be concluded from these findings that OFB may exert its therapeutic effects by modulating the gut microbiota (Luo et al., 2023; Sanada et al., 2020; Liao et al., 2023; Khanna et al., 2021; Luo et al., 2024).
The association between lung injury and neurocognitive dysfunction is a subject that is currently receiving increased attention from the scientific community. The “triple-hit” hypothesis is a theoretical model that has been postulated as a potential underlying mechanism (Cheng et al., 2024). This hypothesis elucidates a cascade of events triggered by lung injury, including immune dysregulation, inflammation, and microbiota changes, which collectively activate the “lung-gut axis,” leading to the onset or exacerbation of cognitive impairments. The “gut-lung axis” and “gut-brain axis” are used to denote the complex interactions between the gut microbiota and the lungs, as well as between the gut microbiota and the brain, respectively. Dysbiosis of the gut microbiota has been demonstrated to facilitate the migration of pathogenic bacteria to the lungs and modulate lung immune responses, potentially contributing to or aggravating lung injury. Concurrently, the “gut-brain axis” theory posits that the gut microbiota can influence brain cognitive functions and behaviors through neural, immune and endocrine pathways.
The “triple-hit” hypothesis, which is based on these regulatory mechanisms, offers a novel perspective for understanding cognitive impairments caused by lung injury. The present study emphasizes the multifaceted role of the gut microbiota within the “gut-lung-brain axis” and demonstrates its capacity to impact cognitive functions through various mechanisms.
Recent studies have highlighted the role of specific metabolites in the pathogenesis of HPH. For instance, trimethylamine N-oxide (TMAO) has been demonstrated to intensify pulmonary hypertension (Huang et al., 2022). In the present study, 25 differential metabolites associated with HPH were identified, primarily involving the pyrimidine metabolism pathway. The administration of OFB treatment resulted in a substantial restoration of these metabolite levels, suggesting its potential as a metabolic regulator in HPH. Furthermore, correlation analysis revealed significant associations between gut microbiota and serum metabolites, thereby providing further evidence to support the hypothesis that gut microbiota dysbiosis contributes to metabolic alterations in HPH (Luo et al., 2023; Sanada et al., 2020).
It can be hypothesized that the therapeutic effects of OFB on HPH may also be related to its antioxidant and anti-inflammatory properties (Everard et al., 2013; Liao et al., 2023). As demonstrated in earlier research, OFB has been found to ameliorate hypoxia-induced lung injury by means of downregulating glucose-6-phosphate dehydrogenase (G6PD) and reducing oxidative stress (Xuefeng, 2025). In the present study, it was demonstrated that OFB treatment was able to restore redox homeostasis by means of regulating the levels of N-acetylglucosamine, a metabolite which has been identified as being involved in antioxidant defense (Ou et al., 2021). Furthermore, the administration of OFB treatment resulted in a reduction in the proliferation of endoplasmic reticulum and mitochondria in pulmonary artery smooth muscle cells, thereby indicating its protective effects against pulmonary artery remodeling.
In conclusion, this study provides valuable insights into the therapeutic potential of OFB in alleviating HPH by modulating gut microbiota and metabolites. It is recommended that future research endeavors focus on a more comprehensive exploration of the molecular mechanisms that underpin the therapeutic effects of OFB on HPH. For instance, the identification of a postprandial neuroimmune axis by Kim (2025), which links the gastrointestinal tract to type 2 immunity in the lung via brain-coordinated sensory and motor circuits, suggests a possible connection to the gut-lung axis. Further investigation is warranted to elucidate whether this gut-brain-lung axis plays a crucial role in the pathogenesis of HPH. Furthermore, the conduction of clinical trials is imperative to substantiate the therapeutic efficacy of OFB in human HPH patients, thereby facilitating its potential implementation in clinical settings.
Conclusion
This study demonstrates that Oxytropis falcata Bunge can regulate gut microbiota dysbiosis and restore metabolic homeostasis in rats with hypoxic pulmonary hypertension. These findings highlight the potential therapeutic effects of OFB on HPH and provide a basis for further exploration of its underlying mechanisms.
Data availability statement
The datasets presented in this study can be found in online repositories and the Supplementary materials. The raw 16S rRNA sequencing data have been deposited into the NCBI Sequence Read Archive (SRA) under BioProject ID PRJNA1348596. The processed metabolomics dataset, which includes all peak intensities and compound identifications supporting the conclusions of this article, is available as Supplementary Material.
Ethics statement
The animal study was reviewed and approved by the Ethics Committee of Qinghai University (Approval Number: PJ202401-51).
Author contributions
YH: Writing – original draft, Writing – review & editing, Investigation, Formal analysis, Visualization, Project administration. ZG: Investigation, Methodology, Validation, Writing – review & editing. HX: Investigation, Resources, Writing – review & editing. XZ: Investigation, Data curation, Writing – review & editing. TL: Investigation, Software, Writing – review & editing. ZB: Supervision, Funding acquisition, Writing – review & editing. LM: Supervision, Funding acquisition, Writing – review & editing. XC: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Applied Basic Research Program of Qinghai Province, China (Project No. 2023-ZJ-773), the Kunlun Talents Program of Qinghai Province (Project No. K9924072) and the Science and Technology Project of Qinghai Province (grant No. 2025-ZJ-748, “Chronic High-Altitude Hypoxic Stress Injury: Mechanisms and Intervention Strategies”, 2025.01-2029.12).
Acknowledgments
We are grateful for the support of the Altitude Medical Research Center and the assistance of the study team members.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HL declared a shared affiliation with the authors YH, ZG, HX, XZ, TL, ZB, LM and XC to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1538260/full#supplementary-material
References
Ailizire, A., Wang, X., Ma, Y., Yan, X., Li, S., Wu, Z., et al. (2023). How hypoxia affects microbiota metabolism in mice. Front. Microbiol. 14:1244519. doi: 10.3389/fmicb.2023.1244519
Anand, S., and Mande, S. S. (2018). Diet, microbiota and gut-lung connection. Front. Microbiol. 9:2147. doi: 10.3389/fmicb.2018.02147
Ashique, S., De Rubis, G., Sirohi, E., Mishra, N., Rihan, M., Garg, A., et al. (2022). Short chain fatty acids: fundamental mediators of the gut-lung axis and their involvement in pulmonary diseases. Chem Biol Interact 368:110231. doi: 10.1016/j.cbi.2022.110231
Baix, L. G., Yang, J., Zhu, J., Wang, Q., Zhou, Y., Gu, W., et al. (2022). Changes in the gut microbiota of rats in high-altitude hypoxic environments. Microbiol. Spectr. 10:e0162622. doi: 10.1128/spectrum.01626-22
Callejo, M., Mondejar-Parreno, G., Barreira, B., Izquierdo-Garcia, J. L., Morales-Cano, D., Esquivel-Ruiz, S., et al. (2018). Pulmonary arterial hypertension affects the rat gut microbiome. Sci. Rep. 8:9681. doi: 10.1038/s41598-018-27682-w
Cheng, Y., Hu, G., Deng, L., Zan, Y., and Chen, X. (2024). Therapeutic role of gut microbiota in lung injury-related cognitive impairment. Front. Nutr. 11:1521214. doi: 10.3389/fnut.2024.1521214
Dumas, A., Bernard, L., Poquet, Y., Lugo-Villarino, G., and Neyrolles, O. (2018). The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 20:e12966. doi: 10.1111/cmi.12966
Everard, A., Belzer, C., Geurts, L., Ouwerkerk, J. P., Druart, C., Bindels, L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 110, 9066–9071. doi: 10.1073/pnas.1219451110
Gao, J., Zhao, M., Cheng, X., Yue, X., Hao, F., Wang, H., et al. (2023). Metabolomic analysis of human plasma sample after exposed to high altitude and return to sea level. PLoS One 18:e0282301. doi: 10.1371/journal.pone.0282301
Huang, Y., Lin, F., Tang, R., Bao, C., Zhou, Q., Ye, K., et al. (2022). Gut microbial metabolite trimethylamine N-oxide aggravates pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 66, 452–460. doi: 10.1165/rcmb.2021-0414OC
Khanna, K., Mishra, K. P., Chanda, S., Ganju, L., Singh, S. B., and Kumar, B. (2021). Effect of synbiotics on amelioration of intestinal inflammation under hypobaric hypoxia. High Alt. Med. Biol. 22, 32–44. doi: 10.1089/ham.2020.0062
Kim, B. S. (2025). Fighting off a gut feeling: a gut-brain-lung neuroimmune circuit. Neuron 113, 641–643. doi: 10.1016/j.neuron.2025.01.029
Liao, Y., Chen, Z., Yang, Y., Shen, D., Chai, S., Ma, Y., et al. (2023). Antibiotic intervention exacerbated oxidative stress and inflammatory responses in SD rats under hypobaric hypoxia exposure. Free Radic. Biol. Med. 209, 70–83. doi: 10.1016/j.freeradbiomed.2023.10.002
Luo, D., Yan, L., Wang, Z., Ji, X., Pei, N., Jia, J., et al. (2024). Pulchinenoside B4 ameliorates oral ulcers in rats by modulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 108:292. doi: 10.1007/s00253-024-13099-1
Luo, L., Yin, H., and Gou, D. (2023). Gut microbiota and metabolome changes in three pulmonary hypertension rat models. Microorganisms 11:472. doi: 10.3390/microorganisms11020472
Ou, W., Liang, Y., Qin, Y., Wu, W., Xie, M., Zhang, Y., et al. (2021). Hypoxic acclimation improves cardiac redox homeostasis and protects heart against ischemia-reperfusion injury through upregulation of O-GlcNAcylation. Redox Biol. 43:101994. doi: 10.1016/j.redox.2021.101994
Price, C. E., and O'Toole, G. A. (2021). The gut-lung axis in cystic fibrosis. J. Bacteriol. 203:e0031121. doi: 10.1128/JB.00311-21
Rastogi, S., Mohanty, S., Sharma, S., and Tripathi, P. (2022). Possible role of gut microbes and host's immune response in gut-lung homeostasis. Front. Immunol. 13:954339. doi: 10.3389/fimmu.2022.954339
Sanada, T. J., Hosomi, K., Shoji, H., Park, J., Naito, A., Ikubo, Y., et al. (2020). Gut microbiota modification suppresses the development of pulmonary arterial hypertension in an SU5416/hypoxia rat model. Pulm Circ 10, 1–10. doi: 10.1177/2045894020929147
Wan, J. J., Yi, J., Wang, F. Y., Zhang, C., and Dai, A. G. (2024). Expression and regulation of HIF-1a in hypoxic pulmonary hypertension: focus on pathological mechanism and pharmacological treatment. Int. J. Med. Sci. 21, 45–60. doi: 10.7150/ijms.88216
Wang, J., Liu, S., Xie, Y., and Xu, C. (2023). Association analysis of gut microbiota-metabolites-neuroendocrine changes in male rats acute exposure to simulated altitude of 5500 m. Sci. Rep. 13:9225. doi: 10.1038/s41598-023-35573-y
Keywords: hypoxic pulmonary hypertension, Oxytropis falcata Bunge, intestinal microbiota, metabolomics, correlation, mechanism of action
Citation: He Y, Guo Z, Xue H, Zhu X, Luo T, Bai Z, Ma L and Cao X (2025) Tibetan herbal medicine Oxytropis falcata Bunge ameliorates hypoxic pulmonary hypertension in rats via regulation of intestinal microbiota and metabolites. Front. Microbiol. 16:1538260. doi: 10.3389/fmicb.2025.1538260
Edited by:
Elisavet Stavropoulou, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Mario Juan Simirgiotis, Austral University of Chile, ChileJames David Adams, Independent Researcher, Benicia, United States
Hao Lizhuang, Qinghai University, China
Copyright © 2025 He, Guo, Xue, Zhu, Luo, Bai, Ma and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng Cao, MjAwMjk4MDAwMUBxaHUuZWR1LmNu
†These authors share first authorship
 Yuxin He
Yuxin He Zixu Guo1†
Zixu Guo1†