- 1Department of Radiation Oncology, UPMC Hillman Cancer Center, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Department of Radiation Medicine, University of Kentucky, Lexington, KY, United States
- 3Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, United States
Introduction: Pancreatic ductal adenocarcinoma (PDAC) commonly presents later in life with a median age at diagnosis of 70 years. Unfortunately, elderly patients are significantly underrepresented in clinical trials. Stereotactic body radiation therapy (SBRT) is a promising treatment modality in this population as it has demonstrated excellent local control with minimal toxicity. We aimed to determine prognostic factors associated with outcomes in elderly patients treated with SBRT.
Materials and Methods: Elderly patients older than 70 treated with SBRT for PDAC at our institution, from 2004 to 2014 were included. Our primary endpoints included overall survival (OS) and local-progression-free survival (LPFS). Secondary endpoints included regional-progression-free survival (RPFS), distant-progression-free-survival (DPFS) and radiation toxicity. Endpoints were analyzed with the Kaplan-Meier method. The association of these survival endpoints with risk factors was studied with Cox proportional hazards models.
Results: We identified 145 patients with 146 lesions of pancreatic adenocarcinoma with a median age at diagnosis of 79 (range, 70.1–90.3). SBRT was delivered to a median dose of 36 Gy (IQR 24–36). Surgical resection was performed on 33.8% of the total patients. Median follow-up was 12.3 months (IQR 6.0–23.3 months) and the median survival for the entire cohort 14.0 months with a 2-year OS of 27%. Multivariate analysis (MVA) demonstrated surgery [p ≤ 0.0001, HR 0.29 (95% CI, 0.16–0.51)] and post-SBRT CA19-9 [p = 0.009, HR 1.0004 (95% CI, 1.0002–1.0005)] significantly associated with overall survival. Recurrent lesions [p = 0.0069, HR 5.1 (95% CI, 1.56–16.64)] and post-SBRT CA19-9 levels [p = 0.0107, HR 1.0005 (95% CI, 1.0001–1.0008)] were significantly associated with local control on MVA. For the entire cohort, 4.1% experienced acute grade 2+ toxicity, and 2% experienced late grade 2+ toxicity at 2 years.
Conclusion: This review demonstrates prognostic factors in elderly patients with PDAC treated with SBRT. We identified surgical resection and post-SBRT CA 19-9 as predictive of overall survival in this population. Additionally, we show low acute and late toxicity following SBRT in elderly patients.
Introduction
Pancreatic adenocarcinoma is the 11th most common cause of new cancer cases, but is the third leading cause of cancer mortality in the United States. Despite aggressive multidisciplinary efforts in recent years, the 5-year mortality remains dismal at 10–30% depending on the resectable status (1–3). Currently, surgical resection remains the most significant prognostic factor, with adjuvant chemotherapy playing and important supportive role. Notably, elderly patients make up a considerable proportion of those with pancreatic cancer with a median age at diagnosis of 70 years and median age at death of 72 years (4). Unfortunately, although elderly patients account for half of this population, they are significantly underrepresented in clinical trials meant to guide treatment decisions (5). This underrepresentation makes it difficult to extrapolate the role of various treatment strategies for this specific population.
Elderly patients with pancreatic adenocarcinoma present a myriad of challenges making it difficult to administer aggressive multi-modality treatment. Standard treatment with invasive surgical procedures and multi-agent chemotherapy may not be tolerable in frail patients with significant comorbidities (6, 7). Additionally, traditional radiation therapy with 6 weeks of external beam radiation therapy (EBRT) has been associated with significant treatment-related morbidity (8). Stereotactic body radiation therapy (SBRT) was developed to deliver a high dose of radiation therapy in few treatments while minimizing dose to surrounding tissue (9). This method has demonstrated excellent local control with minimal toxicity in a variety of diseases (10, 11). Herein we aimed to determine outcomes in elderly patients treated with SBRT for pancreatic adenocarcinoma.
Methods
Patient Population
In accordance with our institutional review board, elderly patients (age >70) with histologically-proven pancreatic adenocarcinoma between 2004 and 2014 were reviewed. Patients with resectable, borderline resectable, unresectable, medically-inoperable, and recurrent tumors were included in this study. Patients were excluded if they had distant metastasis at diagnosis. SBRT was performed using either a CyberKnife® robotic radiosurgery (Accuray Inc., Sunnyvale, CA) or non-robotic linear accelerator based platforms (Trilogy® or TrueBeam®) (Varian Medical Systems, Palo Alto, CA). Patient variables included were age, race, gender, surgical status, chemotherapy treatment, prior EBRT, and SBRT dose, dosimetry, and toxicity were collected.
Definition of Parameters
Resectable status was determined by a multidisciplinary case review using National Comprehensive Cancer Network (NCCN) guidelines for resectable, borderline resectable, and unresectable disease. Prophylactic proton-pump inhibitors was not routinely recommended for patients. Local, regional, and distant progression were determined based on radiographic findings on follow up and/or confirmatory biopsy if done. Local progression was identified as progressive disease (PD) using RECIST 1.1 criteria which is characterized by at least a 20% increase in the sum of diameters of the tumor and a minimum of a 5 mm increase (12). Regional failure was defined as disease progression to the regional nodes defined as n1, n2, or n3 by the Japanese Pancreas Society (JPS) classification (13, 14) (or new tumor growth within the pancreas outside of the radiation field). Toxicity was graded retroactively with the Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE 4.0). Patients included in this review were simulated in the supine position using four-dimensional CT-scan, utilizing 1.25 mm slices, with IV contrast in a vacuum lock bag and wingboard. The GTV was determined based on the simulation CT scan and diagnostic CT scans. The PTV margin was added to be ~3 mm from GTV. When the GTV or PTV abutted the GI luminal structures, we cropped the PTV out the bowel and accepted underdosing of the GTV and PTV with no specific target volume criteria at those areas. Patients included in the study had fiducials placed before CT-simulation to assist with target delineation during treatment. Patients were treated to either 36 Gy in 3 fractions or 24 Gy in one fraction. The bowel was our major dose limiting structured and was limited to no more than 20 Gy (single fraction) and 30 Gy (multi-fraction) maximum dose. The max dose for the kidneys, liver, and cord were limited to 10 Gy, 20 Gy, 5 Gy (single fraction), and 15 Gy, 50 Gy, and 15 Gy (multi-fraction) respectively. Notably one patient exceeded the max dose for the left and right kidney (28.8 Gy and 29.0 Gy respectively).
Endpoints
Our primary endpoints included overall survival (OS) from diagnosis and local-progression-free survival (LPFS) from SBRT. Secondary endpoints included regional-progression-free-survival (RPFS), distant-progression-free-survival (DPFS), and acute and late toxicity.
Statistical Analysis
Continuous variables were summarized with median and inter-quartile range (IQR). Categorical variables were summarized with frequency and percentage. The survival endpoints, LPFS, OS, RPFS, and DPFS, were analyzed with the Kaplan-Meier method. Patients were censored at last medical follow-up. The association of these survival endpoints with risk factors was studied with univariate Cox proportional hazards models. To build multivariable Cox models for the survival endpoints, the stepwise variable selection was performed. All the variables from univariate models that had a p-value of < 0.1 were included as potential predictors. Variables were subsequently removed from the multivariable model if the p-value was > 0.05. All p-values reported are two-sided. Acute toxicity was reported as crude rates occurring within 3 months of treatment. Late toxicity was considered toxicity that occurred >3 months following treatment. Actuarial late toxicity estimates were calculated by the Kaplan-Meier methods. The effect of factors on grade 2+ and grade 3+ toxicities were analyzed with logistic regression models.
Results
Patient Characteristics
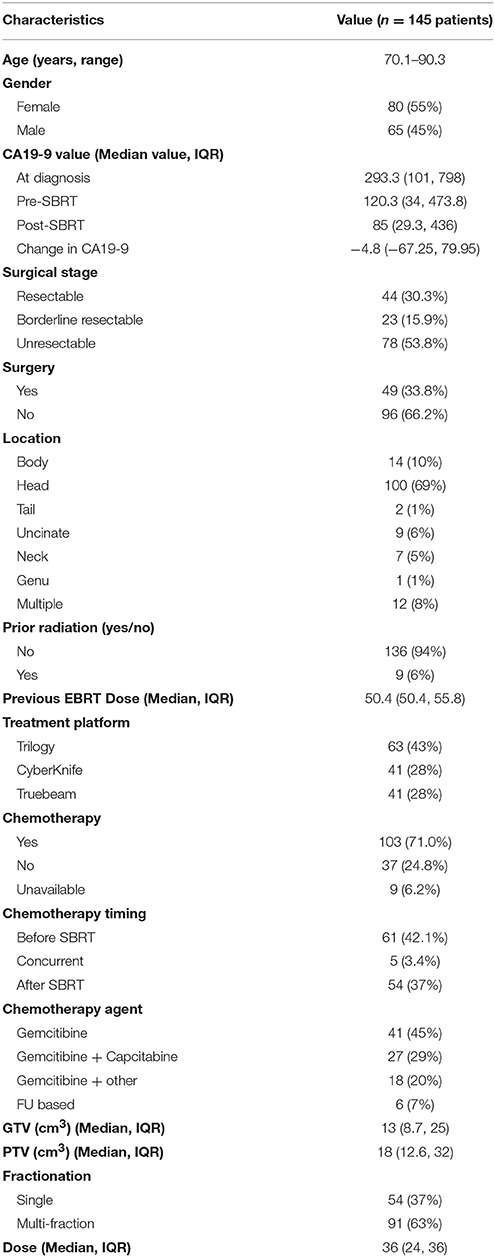
A detailed list of patient characteristics can be found in Table 1. We identified 145 patients with 146 lesions of pancreatic adenocarcinoma with a median age at diagnosis of 79 (range 70.1–90.3) with 55% female and 45% male. Tumors were most commonly located in the head (69%) of the pancreas. Nine patients (6%) had prior radiation with a median dose of 50.4 Gy (IQR, 50.4–55.8) in a median of 14.9 months prior to SBRT. Surgical stage at diagnosis, deemed in a multidisciplinary case review, included, resectable (30.3%), borderline resectable (15.9%), and unresectable (53.8%). Surgical resection was performed on 33.8% of the total patients. No patients with unresectable disease at diagnosis received a resection. Chemotherapy was given prior to (42.1%), concurrent with (4.1%) or follow SBRT (37.2%) Chemotherapy regimens included gemcitabine alone (45%), gemcitabine + capecitabine (29%), gemcitabine + other additional chemotherapy (20%), and FU based chemotherapy regimens (7%). Prior to SBRT, CA19-9 was elevated in 63.7%% (n = 93) of patients.
SBRT Treatment Characteristics
SBRT was delivered by either Trilogy® (43%), Truebeam® (28%), or CyberKnife® (28%) in either one (37%) or multiple fractions (63%). Median BED10 and EQD2 were 81.6 (range 50.40–87.5) Gy and 68 (range 42–72.9) Gy (single fraction) and 79.2 (range 51.3–79.2) Gy and 66 (range 42.8–66) Gy (multi-fraction) respectively. Patients received SBRT as either definitive treatment (65.8%), or as neoadjuvant (8.3%) or adjuvant (25.5%) therapy in resected patients. Median dose was 36 Gy (IQR 24–36). For the entire cohort median gross tumor volume (GTV) was 13 cm3 (IQR 8.7–25) and planning target volume (PTV) was 18 cm3 (IQR 12.6–32). Following SBRT, CA19-9 levels were remeasured in a median of 1.9 months (IQR 1.2–2.9). Of the 93 patients with elevated baseline CA19-9 levels, 6 patients returned to normal levels following SBRT.
Overall Survival
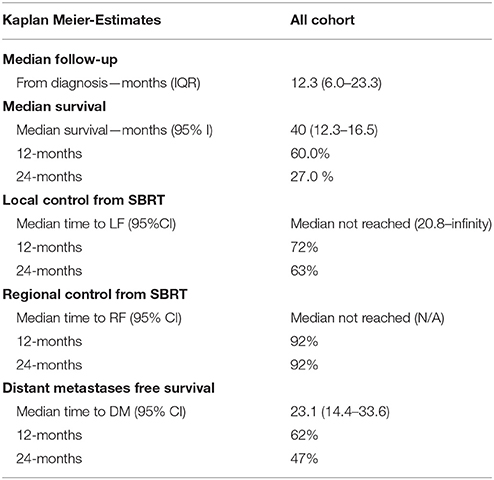
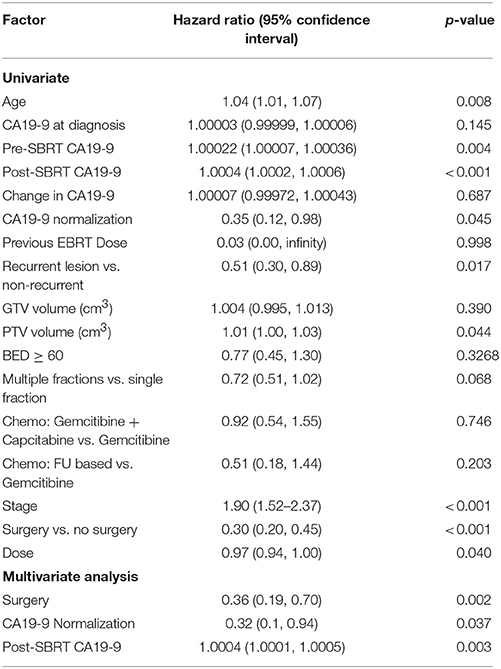
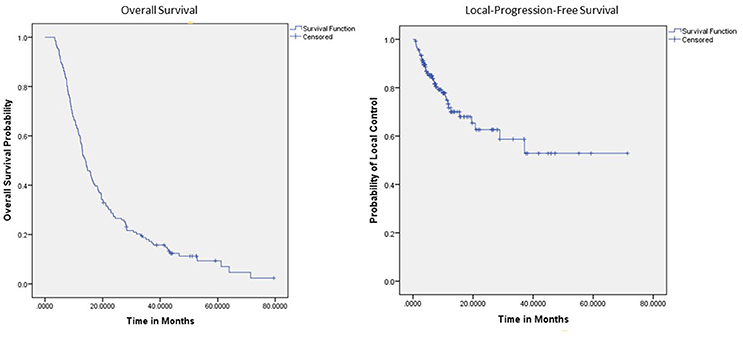
Within a median follow-up of 12.3 months (IQR 6.0–23.3 months) the median survival from diagnosis for the entire cohort 14.0 months (95% CI: 12.3–16.5) with 1- and 2-year OS of 60% and 27.0%, respectively (Table 2). Median OS by resectability status was 24.4 months (95%CI: 15.6–33.1), 15.9 months (95%CI: 3.4–18.3), and 10.0 months (95%CI: 7.8–12.3) for resectable, borderline resectable, and unresectable, respectively. Univariate analysis demonstrated worse OS was significantly associated with increased age [p < 0.0081, HR 1.04 (95% CI, 1.01–1.070)], elevated pre-SBRT CA19-9 [p = 0.004, HR 1.00022 (95% CI, 1.00007–1.000360)], elevated post-SBRT CA19-9 [p < 0.001, HR 1.0004 (95% CI, 1.0002–1.00060)], increased stage [p < 0.001, HR 1.90 (95% CI, 1.52–2.37)], and increased PTV [p = 0.044, HR 1.01 (95% CI, 1.00–1.030)]. Improved OS was observed with recurrent lesions [p = 0.0173, HR 0.51 (95% CI, 0.30–0.89)], surgery [p < 0.001, HR 0.30 (95% CI, 0.20–0.45)], increased SBRT dose [p = 0.040, HR 0.97 (95% CI, 0.94–1.00)], and post-SBRT CA19-9 normalization [p = 0.045, HR 0.35 (95% CI, 0.12–0.980)]. On multivariate analysis, only surgery [p = 0.002, HR 0.36 (95% CI, 0.19–0.70)], post-SBRT CA19-9 normalization [p = 0.037, HR 0.32 (95%CI, 0.10–0.94)], and post-SBRT CA19-9 [p = 0.003, HR 1.0004 (95% CI, 1.0001–1.0005)] maintained significance on multivariate analysis (Table 3). Median survival for patients receiving resection was 28.3 months (95% CI: 16.2–40.5) vs. 11.4 months (95% CI: 9.3–13.5) in those without resection.
Local Control
One- and 2-year LPFS is 72 and 63%, respectively for the entire cohort (Figure 1). Univariate analysis demonstrated significantly worse 2-year LPFS associated with elevated post-SBRT CA19-9 [p = 0.0216, HR 1.0038 (95% CI, 1.00006–1.00071)], and recurrent lesions [p < 0.005, HR 3.31 (95% CI, 1.42–7.68)]. Improved 2-year LPFS was observed with multi-fraction SBRT [p = 0.0189, HR 0.45 (95% CI, 0.23–0.88)]. On multivariate analysis, multi-fraction SBRT did not hold significance for superior local control. Only recurrent lesions [p = 0.0069, HR 5.1 (95% CI, 1.56–16.64)], and post-SBRT CA19-9 levels [p = 0.0107, HR 1.0005 (95% CI, 1.0001–1.0008)] maintained significance on multivariate analysis (Table 4). Within this cohort, 6.9% (n = 10) died of local progression.
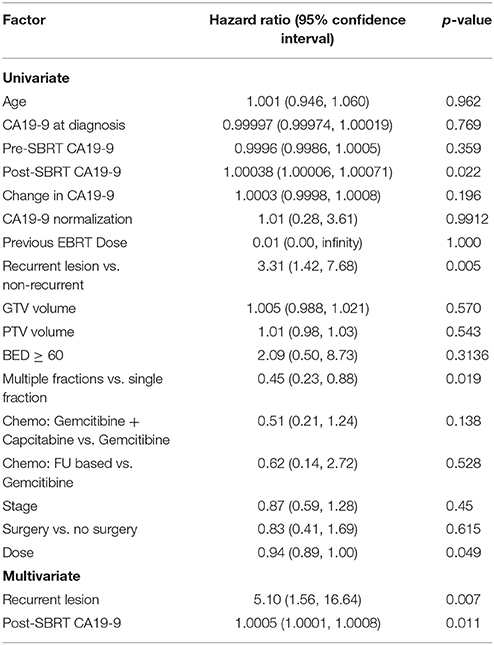
Table 4. Results of univariate and multivariate Cox regression models for time to local progression.
Regional and Distant Progression Free Survival
One- and 2-year RPFS rates were both 92%. None of the variables analyzed were found to be significantly associated with inferior regional control on univariate or multivariate analysis (Table 6s supplementary). At 1 and 2 years, the Kaplan-Meier estimated rate of DPFS was 62 and 47%, respectively. Univariate analysis identified elevated CA19-9 at diagnosis [p = 0.0006, HR 1.00015 (95% CI, 1.00006–1.00023)], and elevated pre-SBRT CA 19-9 [p = 0.0016, HR 1.00034 (95% CI, 1.00031–1.00056)] associated with inferior DPFS and surgery [p = 0.0493, HR 0.55 (95% CI, 0.31–1.00)] associated with superior DPFS (Table 7s in Supplementary Material). None of these variables maintained significance on multivariate analysis. Treatment fractionation was not found to be associated with either regional control or distant metastases.
Radiation Toxicity
For the entire cohort, 4.1 and 0.7% of patients experienced acute grade 2+ and 3+ toxicity respectively. Acute grade 2 toxicity included gastritis (n = 1), nausea (n = 2), and maculopapular rash (n = 2). One patient experienced acute grade 3 nausea requiring hospitalization. At 2-years late grade 2+ and 3+ toxicity was 2 and 1% respectively. One patient experienced a late grade 4 duodenal stenosis (8 months after SBRT) requiring urgent operative intervention. This patient was treated in 3 fractions and received a max dose to the small bowel of 25.5 Gy. This was likely a result of radiation and not tumor progression as there were no signs of local or regional progression. Two patients experienced late grade 3 toxicity which included nausea (n = 1) and enteritis (n = 1). None of the variables analyzed with univariate logistic regression were found to be significantly associated with acute or late grade 2+ or grade 3+ toxicity.
Discussion
Pancreatic adenocarcinoma is primarily a malignancy of the elderly; however, this geriatric population is frequently underrepresented in clinic trials that aim to guide treatment decisions (5). As these patients are frequently more medically complex, special considerations need to be given when determining treatment planning. The dearth of evidence however makes this task especially challenging as the benefit of various treatment options is unclear. This retrospective review aimed to look for prognostic factors associated with outcomes for elderly patients treated with SBRT for pancreatic adenocarcinoma.
Our results did not identify any differences in overall survival, local, regional, or distant control, or toxicity between single or multi-fraction regimens. In contrast, our previous work looking at 289 patients (291 lesions) of all ages with pancreatic adenocarcinoma identified multi-fraction SBRT associated with improved local control on multivariate analysis [p = 0.009, HR 0.53 (95% CI, 0.33–0.85)] with a 2-year local control of 69.7 and 56.8% for multi-fraction and single fraction, respectively. It is possible that our lack of statistical significance on multivariate analysis is the smaller sampler size of the present study (n = 145) compared to our much larger previous report on all ages (n = 289).
Previously, Zhu et al. reported on outcomes of pancreatic cancer patients aged over 65 years treated with SBRT. They reported on 417 patients with advanced and medically inoperable pancreatic cancer with a median age of 73 years. Patients were treated with 30-46.8 Gy in 5–8 fractions. One-year OS, progression free survival (PFS), local-recurrence free survival (LRFS), and distant metastasis free survival (DMFS) were 35.5, 18.2, 26.6, and 27.1% respectively. Tumor stage, tumor response at 6 months, and CA19-9 level normalization at 3 months were all identified as predictors for OS, PFS, LRFS, and DMFS. Additionally, patients receiving 5-FU demonstrated improved survival compared to gemcitabine based chemotherapy. Finally, patients with BED10 ≥ 60 Gy achieved better tumor response as compared to those who received BED10 < 60 Gy (15). Compared to the present study, Zhu et al. reported worse outcomes for 1-year OS (35.5 vs. 60.0%), LRFS (26.6 vs. 72.0%), and DMFS (27.1 vs. 62%). These differences were likely a result of our report including patients with resectable disease. As such, 33.8% of patients in our cohort received surgical resection compared to 12.8% leading to the disparity in outcomes. Regarding prognostic factors, we also identified CA19-9 normalization to predict overall survival but not local control, regional control, or freedom from distant metastasis.
Unfortunately, as with, non-elderly patients, only 10–20% of patients are deemed to have resectable disease at the time of diagnosis (16–18). In addition to a large proportion of elderly patients having unresectable disease, elderly patients fare even worse as they are more likely to be medically-inoperable due to significant comorbidities. This has generated interest in radiation as definitive treatment for these patients. Specifically, SBRT has been the modality of choice for definitive treatment as its shorter duration allows patients to receive full dose systemic therapy with less delay (19). Burton et al. reported on 26 patients ≥ 80 years with pancreatic adenocarcinoma treated with definitive SBRT (24 Gy/ 1 fraction or 30-36 Gy/ 3 fractions) +/–chemotherapy. Median overall survival was 7.6 months with 34.6% 1-year survival and median local control was 11.5 months with 41.2% 1-year local control. This cohort exhibited no acute or late grade 3+ toxicity (20). The results of our study compare favorably with improved survival and local control however it is important to note our study included surgical patients which accounted for 34% (n = 49) of our cohort. Additionally, our cohort included younger patients than those above.
Compared to historical controls, our outcomes with SBRT appear to have a greater impact on resected patients than when used as definitive treatment. Prior reports have demonstrated a median OS of 16.1–23.4 months for resected patients treated with adjuvant chemotherapy alone or chemoradiation with EBRT (6, 7). Our results compare favorably with median OS of 28.1 months for resected patients treated with SBRT and chemotherapy. However, for patients with locally advanced disease, receiving either chemotherapy alone or chemoradiation with EBRT, median OS ranges from 8.6 to 11.4 months which is similar to our reported 11.4 months for patients receiving definitive SBRT (21–23).
The present study continues to support the prognostic role of CA19-9. Overall survival has been associated with both kinetic changes during chemotherapy as well as static values post operatively (24, 25). Additionally, as we have shown in our previous report of all ages, post-SBRT CA19-9 was associated with inferior overall survival and local control on multivariate analysis (26). Although numerous reports have demonstrated the prognostic importance of CA19-9 in the entire population, few studies have assessed if this holds true for elderly patients. Frakes et al. assessed pancreatic cancer outcomes in the elderly and found post-operative CA19-9 greater than 90 (p < 0.001, HR 2.81) was associated with worse survival (6).
The present study also identified recurrent pancreatic adenocarcinoma significantly associated with inferior local control. Treatment of recurrent pancreatic cancer has been challenging due to limited therapeutic options (27, 28). Surgical re-resection, EBRT, SBRT, and systemic chemotherapy have all been used and have their limitations (29–32). The precision SBRT provides is especially useful in recurrent patients as they have often received prior radiation therapy. Previous reports have identified SBRT to be a safe and reasonable treatment option for locally recurrent pancreatic cancer capable of providing symptoms palliation (33, 34). Within our cohort, recurrent patients were often treated with reirradiation, and it is therefore possible that their SBRT treatment plan was more conservative to reduce potential toxicity. This could have led to the observed inferior local control compared to non-recurrent tumors. Additionally, it is possible that recurrent tumors are more locally aggressive and therefore likely to re-recur.
Here we add to currently limited literature of pancreatic cancer treatment in elderly patients. This identified no differences in outcomes or toxicity between single fraction and multi-fraction SBRT for this cohort. Further, we confirmed SBRT for pancreatic adenocarcinoma in elderly patients to be a safe and effective treatment modality for this challenging population with very low rates of toxicity. This study however was limited by its retrospective nature. Firstly, patients were treated on three different treatment platforms and received a non-standardized treatment regimen. The patients included in this study also represented a heterogeneous population including resectable, borderline resectable, and unresectable patients that received various surgical and chemotherapeutic treatments. Additionally, we did not have any object data regarding pain control or symptom palliation following treatment which represents a critically important aspect among elderly patients. Finally, our toxicity may be underrepresented due to poor follow-up with radiation oncology. Prospective studies will be needed for a more rigorous assessment of the role of fractionation on patient outcomes and the role of SBRT in the elderly more generally.
Conclusion
Surgical resection, post-SBRT CA 19-9, and post-SBRT CA 19-9 normalization appears to be predictive of clinical outcomes in elderly patients with pancreatic adenocarcinomas. SBRT can be delivered with minimal acute and late toxicity and therefore little impact on patients' quality of life. Future studies should further refine treatments based on these characteristics.
Author Contributions
PS: data collection, data analysis, wrote, and prepared manuscript; MB and DH: development of project, review and editing of final manuscript; Provided insight into how results relate to radiation oncology. HW: data analysis, ran all statistical tests, review, and editing of final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00282/full#supplementary-material
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. Cancer J Clin. (2007) 57:43–66. doi: 10.3322/canjclin.57.1.43
2. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet (2017) 389:1011–24. doi: 10.1016/S0140-6736(16)32409-6
3. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA (2007) 297:267–77. doi: 10.1001/jama.297.3.267
4. Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, et al. editors. SEER Cancer Statistics Review, 1975-2014, Bethesda, MD: National Cancer Institute. Available online at: https://seer.cancer.gov/csr/1975_2014/
5. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Eng J Med. (1999) 341:2061–7. doi: 10.1056/NEJM199912303412706
6. Frakes JM, Strom T, Springett GM, Hoffe SE, Balducci L, Hodul P. Resected pancreatic cancer outcomes in the elderly. J Geriatr Oncol. (2015) 6:127–32. doi: 10.1016/j.jgo.2014.11.005
7. Nagrial AM, Chang DK, Nguyen NQ, Johns AL, Chantrill LA, Humphris JL. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br J Cancer (2014) 110:313–9. doi: 10.1038/bjc.2013.722
8. Milano MT, Chmura SJ, Garofalo MC, Rash C, Roeske JC, Connell PP. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. (2004) 59:445–53. doi: 10.1016/j.ijrobp.2003.11.003
9. Rubio C, Morera R, Hernando O, Leroy T, Lartigau SE. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. (2013) 18:387–96. doi: 10.1016/j.rpor.2013.09.009
10. Brunner TB, Nestle U, Grosu AL, Partridge M. SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol. (2015) 114:109–16. doi: 10.1016/j.radonc.2014.10.015
11. Kim SK, Wu CC, Horowitz DP. Stereotactic body radiotherapy for the pancreas: a critical review for the medical oncologist. J Gastrointest Oncol. (2016) 7:479–86. doi: 10.21037/jgo.2015.10.01
12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
13. Yamada S, Fujii T, Hirakawa A, Kanda M, Sugimoto H, Kodera Y. Lymph node ratio as parameter of regional lymph node involvement in pancreatic cancer. Langenbecks Arch Surg. (2016) 401:1143–52. doi: 10.1007/s00423-016-1412-5
14. Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas (2004) 28:231–4. doi: 10.1097/00006676-200404000-00003
15. Zhu X, Li F, Ju X, Cao F, Cao Y, Fang F. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. (2017) 6:2263–70. doi: 10.1002/cam4.1164
16. Yechieli RL, Robbins JR, Mahan M, Siddiqui F, Ajlouni M. Stereotactic body radiotherapy for elderly patients with medically inoperable pancreatic cancer. Am J Clin Oncol. (2017) 40:22–6. doi: 10.1097/COC.0000000000000090
17. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Eng J Med. (2014) 371:1039–49. doi: 10.1056/NEJMra1404198
18. Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. (2015) 22:2352–8. doi: 10.1245/s10434-014-4274-5
19. Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. (2010) 78:735–42. doi: 10.1016/j.ijrobp.2009.08.046
20. Kim CH, Ling DC, Wegner RE, Flickinger JC, Heron DE, Zeh H. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. (2013) 8:240. doi: 10.1186/1748-717X-8-240
21. Morizane C, Okusaka T, Ito Y, Ueno H, Ikeda M, Takezako Y. Chemoradiotherapy for locally advanced pancreatic carcinoma in elderly patients. Oncology (2005) 68:432–7. doi: 10.1159/000086985
22. Miyamoto DT, Mamon HJ, Ryan DP, Willett CG, Ancukiewicz M, Kobayashi WK, et al. Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys. (2010) 77:1171–7. doi: 10.1016/j.ijrobp.2009.06.020
23. Hentic O, Dreyer C, Rebours V, Zappa M, Lévy P, Raymond E. Gemcitabine in elderly patients with advanced pancreatic cancer. World J Gastroenterol. (2011) 17:3497–502. doi: 10.3748/wjg.v17.i30.3497
24. Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. (2008) 26:5918–22. doi: 10.1200/JCO.2008.18.6288
25. Boeck S, Haas M, Laubender RP, Kullmann F, Klose C, Bruns CJ. Application of a time-varying covariate model to the analysis of CA 19-9 as serum biomarker in patients with advanced pancreatic cancer. Clin Cancer Res. (2010) 16:986–94. doi: 10.1158/1078-0432.CCR-09-2205
26. Sutera P, Bernard M, Quan K, Gill BS, Heron DE. One versus 3 fraction pancreatic SBRT for pancreatic adenocarcinoma: single institution retrospective review. Int J Radiat Oncol Biol Phys. 99:S193. doi: 10.3389/fonc.2017.00272
27. Koong AJ, Toesca DAS, von Eyben R, Pollom EL, Chang DT. Reirradiation with stereotactic body radiation therapy after prior conventional fractionation radiation for locally recurrent pancreatic adenocarcinoma. Adv Radiat Oncol. (2017) 2:27–36. doi: 10.1016/j.adro.2017.01.003
28. Kyriazanos ID, Tsoukalos GG, Papageorgiou G, Verigos KE, Miliadis L, Stoidis CN. Local recurrence of pancreatic cancer after primary surgical intervention: how to deal with this devastating scenario? Surg Oncol. (2011) 20:e133–42. doi: 10.1016/j.suronc.2011.04.004
29. Nakamura A, Itasaka S, Takaori K, Kawaguchi Y, Shibuya K, Yoshimura M. Radiotherapy for patients with isolated local recurrence of primary resected pancreatic cancer. Prolonged disease-free interval associated with favorable prognosis. Strahlentherapie Onkol. (2014) 190:485–90. doi: 10.1007/s00066-014-0610-8
30. Dagoglu N, Callery M, Moser J, Tseng J, Kent T, Bullock A. stereotactic body radiotherapy (SBRT) reirradiation for recurrent pancreas cancer. J Cancer (2016) 7:283–8. doi: 10.7150/jca.13295
31. Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. (2007) 245:566–72. doi: 10.1097/01.sla.0000245845.06772.7d
32. Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y. Comparative outcomes between initially unresectable and recurrent cases of advanced pancreatic cancer following palliative chemotherapy. Pancreas (2014) 43:411–6. doi: 10.1097/MPA.0000000000000050
33. Ryan JF, Groot VP, Rosati LM, Hacker-Prietz A, Narang AK, McNutt TR. Stereotactic body radiation therapy for isolated local recurrence after surgical resection of pancreatic ductal adenocarcinoma appears to be safe and effective. Ann Surg Oncol. (2018) 25:280–9. doi: 10.1245/s10434-017-6134-6
34. Wild AT, Hiniker SM, Chang DT, Tran PT, Khashab MA, Limaye MR. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: experience from two institutions. J Gastrointest Oncol. (2013) 4:343–51. doi: 10.3978/j.issn.2078-6891.2013.044
Keywords: pancreatic cancer, elderly population, stereotactic body radiation therapy, prognostic factors, radiation toxicity
Citation: Sutera PA, Bernard ME, Wang H and Heron DE (2018) Prognostic Factors for Elderly Patients Treated With Stereotactic Body Radiation Therapy for Pancreatic Adenocarcinoma. Front. Oncol. 8:282. doi: 10.3389/fonc.2018.00282
Received: 15 May 2018; Accepted: 05 July 2018;
Published: 27 July 2018.
Edited by:
Charles A. Kunos, Investigational Drug Branch, National Cancer Institute (NIH), United StatesReviewed by:
Michael Chuong, Baptist Health South Florida, United StatesShahed Nicolas Badiyan, Washington University in St. Louis, United States
Copyright © 2018 Sutera, Bernard, Wang and Heron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dwight E. Heron, aGVyb25kMkB1cG1jLmVkdQ==
 Philip A. Sutera1
Philip A. Sutera1 Hong Wang
Hong Wang Dwight E. Heron
Dwight E. Heron