- 1Department of Radiation Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 2Department of Radiation Oncology, Hyogo Cancer Center Hospital, Hyogo, Japan
- 3Department of Medical Oncology, Cancer Center, Kobe University Hospital, Kobe, Japan
- 4Department of Head and Neck Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 5Department of Otolaryngology, Tokyo Medical Center, Tokyo, Japan
- 6Department of Otolaryngology, Head and Neck Surgery, Kyoto University Hospital, Kyoto, Japan
- 7Department of Medical Oncology, Cancer Institute Hospital of JFCR, Tokyo, Japan
- 8Department of Otorhinolaryngology-Head and Neck Surgery, Hiroshima University Hospital, Hiroshima, Japan
- 9Department of Head and Neck Surgery, National Hospital Organization Shikoku Cancer Center, Matsuyama, Japan
- 10Department of Biostatistics and Epidemiology, Yokohama City University School of Medicine, Yokohama, Japan
Background: Induction chemotherapy (IC) is a treatment option for locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). However, treatment with docetaxel, cisplatin, and 5-FU (TPF) followed by cisplatin and radiotherapy is controversial because of toxicity concerns. The aim of this phase II study was to assess the feasibility of docetaxel, cisplatin, and cetuximab (TPEx) followed by cetuximab and concurrent radiotherapy for LA SCCHN.
Patients and Methods: We enrolled patients with histological evidence of squamous cell carcinoma of the oropharynx, hypopharynx, or larynx without distant metastases. IC comprised cisplatin (75 mg/m2) and docetaxel (75 mg/m2) on day 1, repeated every 3 weeks for up to three courses. Cetuximab was initiated at 400 mg/m2, followed by 250 mg/m2 doses weekly until the end of radiotherapy. Radiotherapy (70 Gy/35 fr/7 w) was initiated after the last docetaxel administration. The primary endpoint was the rate of treatment completion.
Results: We enrolled 54 patients (median age, 58 years) between August 2013 and October 2015. Our patients were 49 males and 5 females with hypopharyngeal (n = 28), oropharyngeal (n = 19), or laryngeal (n = 7) cancers, and 48 of them had stage IV disease. The overall response rate was 72.2% with a median follow-up of 36.1 months and a 3-year overall survival of 90.7%. The treatment completion rate was 76%; 50 patients (93%) received ≥2 courses of IC, and 41 (76%) completed radiotherapy. The frequencies of grade ≥3 febrile neutropenia or allergy/infusion reactions were 39% and 11%, respectively. There was one treatment-related death.
Conclusions: IC with TPEx followed by cetuximab with concurrent radiotherapy showed acceptable compliance for the treatment of LA SCCHN. However, high frequency of febrile neutropenia remains a challenge and further improvement in the management of TPEx is necessary.
Trial Registration: UMIN000009928
Introduction
Induction chemotherapy (IC) is a treatment option for locally advanced squamous cell carcinoma of the head and neck (LA SCCHN) and allows for organ preservation. Induction cisplatin and fluorouracil (PF) has been effective for locally advanced head and neck cancers before definitive radiotherapy (1, 2). In the GORTEC 2000–2001 study (3), induction docetaxel, cisplatin, and 5-FU (TPF) was superior to induction PF regimen in terms of the overall response rate. Moreover, in the TAX323 (4) and TAX324 (5) trials, induction TPF improved survival compared with induction PF. A recent meta-analysis of chemotherapy for head and neck cancer suggested that IC may contribute to control of distant metastases (6).
A docetaxel, cisplatin, and cetuximab (TPEx) regimen was tested as a first-line treatment for recurrent/metastatic HNSCC in the GORTEC 2008-03 study and showed good efficacy and compliance (7), suggesting that the TPEx regimen might be useful as IC. The TREMPLIN study comparing the efficacy and safety of IC followed by cisplatin or cetuximab with radiotherapy for larynx preservation (LP) showed that the regimen of cetuximab with radiotherapy achieved higher compliance (even after IC) than the cisplatin regimen, suggesting that it is one of the best options for LP.
We conducted a prospective phase II study to examine the feasibility of docetaxel, cisplatin, and cetuximab (TPEx) followed by cetuximab with concurrent radiotherapy for patients with LA SCCHN.
Patients and Methods
This study was a multicenter, single-arm, phase 2 trial. Twenty-two institutions in Japan participated in this study. The study protocol was approved by the National Cancer Center Hospital Institutional Review Board. Written informed consents were obtained from all patients before enrollment in our study. This trial was registered with the UMIN clinical trials registry (UMIN000001439).
Patients
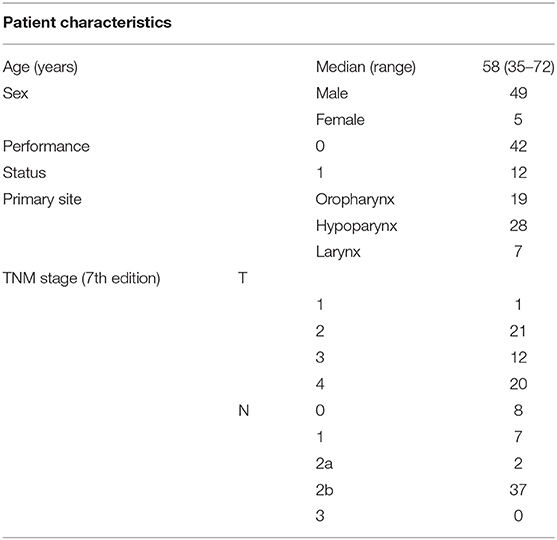
We enrolled 54 patients with stage III-IV resectable locally advanced head and neck cancer fulfilling the following criteria: (1) histologically confirmed squamous cell carcinoma of the oropharynx, hypopharynx, or larynx; (2) age between 20 and 75 years; (3) Eastern Cooperative Oncology Group performance status between 0 and 1; (4) normal organ function; and (5) hope for organ preservation.
Pretreatment Evaluation
Our pretreatment clinical evaluation included upper gastrointestinal and pharyngeal endoscopy; head and neck magnetic resonance imaging; and cervical, thoracic, and abdominal computed tomography (CT) scanning. Radiologists, surgeons, and oncologists evaluated the radiological lesion staging. We used the seventh edition of the International Union Against Cancer TNM classification for tumor staging. We did not routinely use positron emission tomography (PET) because of logistics (routine use of PET CT for staging and response evaluation was not accepted by government-issued health insurance).
Protocol Treatment
The IC comprised intravenous (IV) administration of docetaxel (75 mg/m2) on day 1 and cetuximab (400 mg/m2 IV on day 1 of cycle 1, and 250 mg/m2 IV weekly on subsequent administrations) on days 1, 8, and 15. Cisplatin (75 mg/m2, IV) was also given on day 1. Cycles were repeated every 21 days thrice, with prophylactic antibiotics on days 5 through 14. We did not administer granulocyte colony-stimulating factor (G-CSF) prophylactically until November 2014, and prescribed it only in cases with febrile neutropenia (150 g/m2 per day). After a protocol revision on December 2014, we used prophylactic G-CSF for patients considered to be at a high risk for febrile neutropenia (8).
Two weeks after the second IC cycle, patients underwent endoscopies and CT scans of the neck and chest. Those with confirmed progressive disease (PD) stopped receiving the protocol treatment and received surgery or other appropriate treatments instead. Patients with confirmed non-PD status received a third IC cycle. After three IC cycles, all patients received standard radiotherapy (total dose of 70 Gy, in 35 fractions over 7 weeks with continued weekly cetuximab 250 mg/m2) (Figure 1).
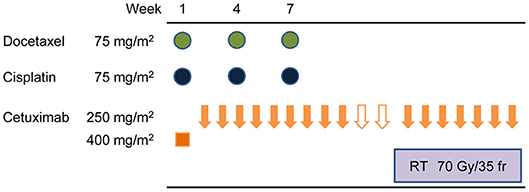
Figure 1. Protocol treatment schematic. Induction therapy comprised docetaxel (75 mg/m2) intravenously (IV) on day 1, cetuximab on days 1, 8, and 15, then cisplatin (75 mg/m2 IV) on day 1. Cycles were repeated every 21 days for three cycles. After three induction cycles, patients received standard radiotherapy, a total dose of 70 Gy in 35 fractions over 7 weeks with continued weekly cetuximab (250 mg/m2).
Regarding the irradiation technique, both three-dimensional multi-beam irradiation (3D-RT) and intensity-modulated radiotherapy (IMRT) were accepted. We determined the gross tumor volume (GTV) by endoscopic or radiographic examination before the IC initiation. Clinical target volume (CTV) was defined as the GTV plus the volumes of all lesions considered at risk of containing microscopic disease. We further categorized the CTVs into two volumes, (1) a therapeutic CTV, including the primary tumor with a 1-cm margin craniocaudally and any metastatic nodes within a 0.5–2-cm margin, and (2) a prophylactic CTV, including a therapeutic CTV plus regional nodes. The planning target volume (PTV) was defined as the CTV plus a 1–3-mm margin that we adjusted as necessary when considering organ risk. The therapeutic and prophylactic PTVs received 70 and 40 Gy, respectively. We used five daily fractions of 2 Gy.
Endpoints and Statistical Analyses
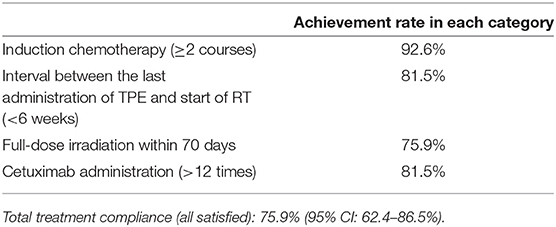
The primary endpoint was the treatment completion rate, which we identified in cases satisfying all of the following criteria: (1) patients received two or more IC courses; (2) irradiation was initiated within 6 weeks between the last IC course and the start of the radiotherapy; (3) full-dose irradiation was completed within 10 weeks; and (4) received cetuximab administration >12 times during their treatment.
In TAX 323 (4) and 324 (5) studies, the complete rates of induction chemotherapy in TPF group were 76 and 73%, respectively. Bonner et al. (9) reported the completion rate of cetuximab with radiotherapy was 90%.
Considering these and on the basis of 5% dropped out because of progressive disease between induction chemotherapy and radiotherapy, with regard to treatment completion rate as the primary endpoint in our phase 2 study, expected and threshold values for exact binomial test were 65 and 40%, respectively, and a total of 50 was required with a power of 90% and one-sided significance level of 2.5%.
We calculated the overall survival times from the date of study registration to the date of death, or the last confirmed survival date. We defined the progression-free survival (PFS) time from the study registration date until the first day of confirmation of PD at any site or of death by any cause.
Events for laryngo-esophageal dysfunction-free survival (LEDFS) included death, local relapse, total or partial laryngectomy or tracheotomy, and chronic enteral nutrition. We estimated binominal confidence intervals (CIs) for the overall response rate (ORR) by using the exact method and assessed the differences in these rates among subgroups using Fisher's exact test. We estimated survival curves using the Kaplan–Meier method.
We conducted primary analysis on the full analysis set population, defined as all registered patients excluding those ineligible after enrollment (i.e., those who did not receive any study treatment). We performed safety analysis for all registered patients who received at least one dose of study treatment. We performed all statistical analyses using the SAS software version 9.4.
Results
Patient Characteristics
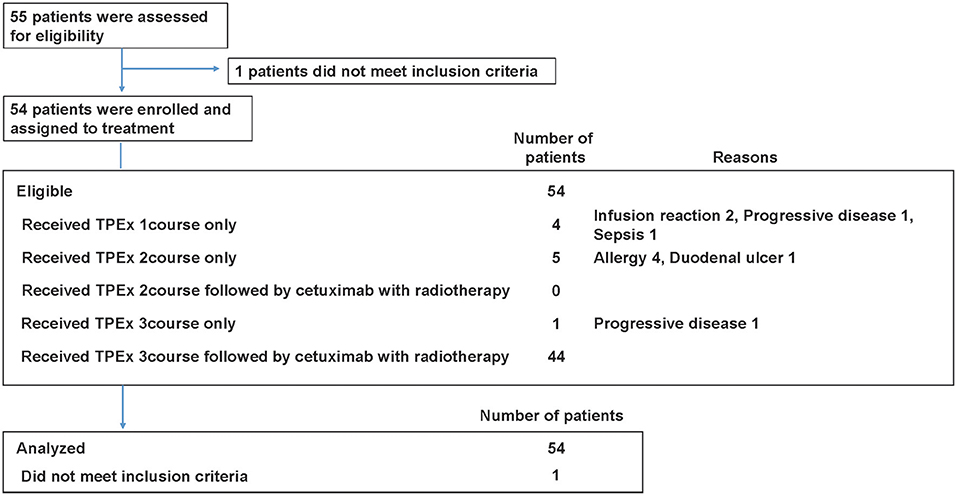
Patient characteristics are summarized in Table 1 and Table S1. Total 54 eligible patients with a median age of 58 years participated in the study (49 males and 5 females, 48 with stage IV disease) between August 2013 and October 2015 (Figure 2). The numbers of patients with hypopharyngeal, oropharyngeal, and laryngeal cancers were 28, 19, and 7, respectively. Of the 19 patients with oropharyngeal cancer, 14 had p16 positive oropharyngeal cancer.
Treatment Compliance
The mean treatment completion rate was 75.9% (95% CI, 62.4–86.5%). The rate of patients receiving two or more TPEx cycles was 92.6% (50/54). Forty-four patients (44/54, 81.5%) received three TPEx cycles, and of those, 23 completed the planned TPEx.
The relative dose intensities of cisplatin and docetaxel were 0.90 (95% CI, 0.86–0.94) and 0.84 (95% CI, 0.80–0.88), respectively. The reasons for treatment interruption included seven severe adverse events (four allergies, two infusion reactions, and one sepsis), two PD cases, and other reasons (one duodenal ulcer). As a result, 10 patients could not receive radiotherapy with cetuximab within 6 weeks after the last course of TPEx.
Forty-four patients received radiotherapy, and of those, 41 patients (93.2%) completed the planned irradiation. The median radiotherapy duration was 51 days (range, 46–60). Reasons for treatment interruption included sepsis, local infection, and protocol deviation. Through IC and radiotherapy, the median times of cetuximab administration was 17 (range, 2–19), and the rate of patients receiving ≥12 administrations was 81.5% (44/54). Table 2 summarizes the treatment compliance results.
Toxicities
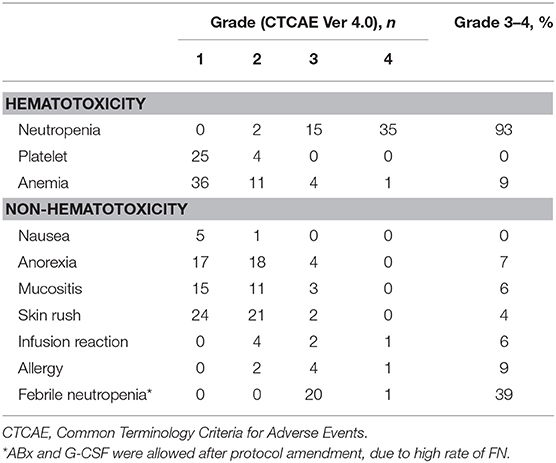
During the TPEx, the most frequent grade ≥3 toxicities were neutropenia (93%) and febrile neutropenia (39%). We modified our protocol during the study owing to the high frequency of grade ≥3 neutropenia and febrile neutropenia, and thus, initiated the administration of prophylactic G-CSF. The rate of febrile neutropenia dropped from 41.2 (14/34) to 35.0% (7/20) post protocol modification. Toxicity profile in TPEx is shown in Table 3. During radiotherapy, the most frequent grade ≥3 toxicities were mucositis (45%) and radiation dermatitis (48%). We did not find any infusion reactions or allergies during the radiotherapy phase, and we did not observe severe late toxicities during the follow-ups. Table 4 presents all grade toxicities during the radiotherapy phase.
Efficacies
The ORR during the TPEx was 72.2% (95% CI, 58.4–83.5%). We observed complete responses (CR) in nine patients (16.7%), and one patient developed PD. The ORR after the radiotherapy was 75.9% (95% CI, 62.4–86.5%). We observed CR in 26 patients (48.1%), and 1 patient had PD. With a median follow-up period of 36.1 months, the 3-year overall survival and PFS rates were 90.7 and 58.2%, respectively. Twenty-six patients received second-line treatment: 11 patients underwent laryngectomy, 4 underwent neck dissection, 1 underwent surgery for lung metastases, 7 underwent chemoradiotherapy, 2 underwent chemotherapy, and 1 had incomplete data. The 2- and 3-year LEDFS were 64.8 and 60.1%, respectively (Figure 3).
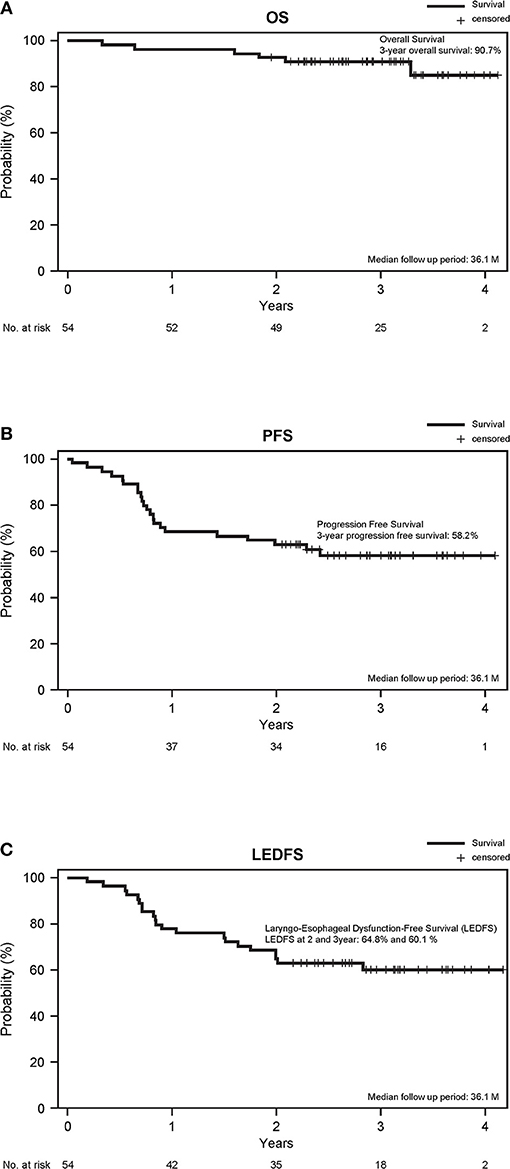
Figure 3. (A) Overall survival, (B) PFS, and (C) Laryngo-esophageal dysfunction-free survival. The survival time was calculated from the study registration date.
Discussion
TPEx followed by cetuximab with concurrent radiotherapy demonstrated an acceptable compliance for the treatment of LA SCCHN. However, high frequency of febrile neutropenia remains a challenge.
We used a cetuximab-based regimen instead of a platinum-based one. Induction TPF followed by chemoradiotherapy (TPF-CRT) seems to be the strongest regimen among the currently available regimens. However, patients have difficulty completing it, and thus, TPF-CRT is not the standard of care in the 2016 National Comprehensive Cancer Network guidelines due to concerns of toxicity and low compliance. Kim et al. (10) reported a meta-analysis of prospective trials (11–14), including TPF IC and chemoradiotherapy, stating that CRT treatment completion rate with this particular regimen was only 63.4% (478/651), and there was no statistically significant overall survival (OS) advantage for TPF prior to CRT (TPF/CRT) over CRT alone (hazard ratio [HR] 0.92; 95% confidence interval [CI], 0.79–1.09; p = 0.339).
Induction TPF with cetuximab (TPFE) was tested in the EORTC phase II study (15), showing a severe toxicity profile with only 63.8% (30/47) of patients reaching the radiotherapy phase. Therefore, the question of which IC is the best for the following chemoradiotherapy remains unanswered.
Considering the results of previous trials, we should consider the treatment compliance before discussing about efficacy.
Thus, the primary endpoint of this study was the treatment completion rate.
In this study, the rate of patients receiving two or more TPEx cycles reached 92.6%, and relative dose intensity of cisplatin and docetaxel were 0.90 (95% CI, 0.86–0.94) and 0.84 (95% CI, 0.80–0.88), respectively.
We observed a high frequency (39%) of febrile neutropenia in our patients, which appears to be one of the most important factors to be considered in a TPEx regimen. Because of this, we modified our protocol during the study and initiated the administration of primary and secondary prophylactic G-CSF. However, considering the small sample size, the frequency of febrile neutropenia appeared to be high even after protocol amendment. Thus, primary prophylactic G-CSF should be considered to manage the TPEx regimen.
Almost all of our patients completed planned irradiation. We encountered frequent cases of severe mucositis and dermatitis that we were able to control with standard oral care (16, 17) and nursing (18–20). We found no cases of infusion reaction or allergy during the radiotherapy phase and believe this may have been due to the gap of 2 months between the initial cetuximab administration and the radiotherapy initiation. The mean treatment completion rate was 75.9% (95% CI, 62.4–86.5%). As a result, seven patients of 10 patients who couldn't receive full dose radiotherapy had some trouble in TPEx section. Then, the management of TPEx section is of upmost importance.
A previous phase II study of TPEx conducted by Argiris et al. (21) showed similar results to ours and reported good compliance. A total of 39 patients were enrolled and of those, 35 patients (90%) received three cycles of cisplatin and docetaxel. A total of 34 patients (87%) received all planned doses of cetuximab during induction TPE, and 33 patients (85%) received full dose radiotherapy.
Several reports suggest that cetuximab with radiotherapy is not less toxic than chemoradiotherapy. However, the toxicity profile of cetuximab with radiotherapy was different from that of CDDP with radiotherapy and this point is important in considering the adjunctive treatment to IC.
The LP Consensus Panel recommended using LEDFS as a composite endpoint in preservation studies (22). In our phase II study, the 2-year LEDFS was 64.8%, greater than that reported in other studies (23, 24).
The 3-year OS and PFS rates were excellent at 90.7 and 58.2%, respectively. However, the efficacy of this regimen couldn't be discussed from these results, because 14 patients with p16 positive oropharyngeal cancer were also included.
Two recent phase III trials showed cetuximab with radiotherapy to be inferior to CDDP with radiotherapy in efficacy (25, 26); therefore, re-evaluation of TPEx followed by cetuximab with radiotherapy in efficacy is mandatory.
In conclusion, IC with TPEx followed by cetuximab with concurrent radiotherapy showed acceptable compliance for the treatment of LA SCCHN. However, high frequency of febrile neutropenia remains a challenge and further improvement in the management of TPEx is necessary.
Author Contributions
SZ, NK, MF, TY, and MT: study concept. SZ, NK, TY, and MT: study design. SZ, YO, NK, SO, MF, MK, ST, TU, NM, and MT: acquisition of data. TY: analysis of data. SZ, NK, ST, and MT: interpretation of data. NK, ST, TY, and MT: drafting of the manuscript. SZ, YO, SO, MF, MK, TU, and NM: critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by partial funding by the Comprehensive Support Project of the Public Health Research Foundation (CSPOR), with support from Merck Serono (Japan), an affiliate of Merck KGaA, Darmstadt, Germany.
Conflict of Interest Statement
SZ reports personal fees from Merck Serono during the conduct of the study. MT reports personal fees from Merck Serono, grants and personal fees from MSD, Bayer, Eisai, Pfizer, Astra Zeneca, Ono Pharmaceutical and Bristol-Myers Squibb, personal fees from Otsuka, grants from Boehringer-Ingelheim, Novartis and NanoCarrier, outside the submitted work. NK reports honoraria from Merck Serono, grants from research funding from Ono Pharmaceutical, and Astra Zeneca, and honoraria from Ono Pharmaceutical, Bristol-Meyers Squibb, Astra Zeneca, Eisai and Bayer. Outside the submitted work, ST reports grants and personal fees from Takeda, grants and personal fees from Taiho, personal fees from Boehringer-Ingelheim, personal fees from Chugai. Outside the submitted work, ST reports honoraria from Merck Serono during the conduct of the study; grants and personal fees from Bristol-Meyers Squibb and MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the participating investigators and patients. A full list of the investigators in this study is shown in the Supplementary Appendix, available at Frontier in Oncology online.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00006/full#supplementary-material
Abbreviations
CI, Confidence intervals; CR, Complete responses; CSPOR, Comprehensive Support Project of the Public Health Research; CT, Computed tomography; CTV, Clinical target volume; GETTEC, Groupe d'Etude des Tumeurs de la Tete et du Cou; GTV, Gross tumor volume; IC, Induction chemotherapy; LEDFS, Laryngo-esophageal dysfunction-free survival; LP, Larynx preservation; ORR, Overall response rate; PD, Progressive disease; PET, Positron emission tomography; PFS, Progression-free survival; PTV, Planning target volume.
References
1. Domenge C, Hill C, Lefebvre JL, De Raucourt D, Rhein B, Wibault P, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC). Br J Cancer (2000) 83:1594–8. doi: 10.1054/bjoc.2000.1512
2. Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. (1994) 86:265–72. doi: 10.1093/jnci/86.4.265
3. Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. (2009) 101:498–506. doi: 10.1093/jnci/djp007
4. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. (2007) 357:1695–704. doi: 10.1056/NEJMoa071028
5. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. (2007) 357:1705–15. doi: 10.1056/NEJMoa070956
6. Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. (2009) 92:4–14. doi: 10.1016/j.radonc.2009.04.014
7. Guigay J, Fayette J, Dillies AF, Sire C, Kerger JN, Tennevet I, et al. Cetuximab, docetaxel, and cisplatin as first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma: a multicenter, phase II GORTEC study. Ann Oncol. (2015) 26:1941–7. doi: 10.1093/annonc/mdv268
8. Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of wbc growth factors: american society of clinical oncology clinical practice guideline update. J Clin Oncol. (2015) 33:3199–212. doi: 10.1200/JCO.2015.62.3488
9. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New Engl J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
10. Kim R, Hahn S, Shin J, Ock CY, Kim M, Keam B, et al. The effect of induction chemotherapy using docetaxel, cisplatin, and fluorouracil on survival in locally advanced head and neck squamous cell carcinoma: a meta-analysis. Cancer Res Treat. (2016) 48:907–16. doi: 10.4143/crt.2015.359
11. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. (2014) 32:2735–43. doi: 10.1200/JCO.2013.54.6309
12. Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. (2013) 14:257–64. doi: 10.1016/S1470-2045(13)70011-1
13. Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, García-Girón C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. (2014) 25:216–25. doi: 10.1093/annonc/mdt461
14. Paccagnella A, Ghi MG, Loreggian L, Buffoli A, Koussis H, Mione CA, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol. (2010) 21:1515–22. doi: 10.1093/annonc/mdp573
15. Specenier PM, Remenar E, Buter J, Schrijvers DL, Bergamini C, Licitra LF, et al. TPF plus cetuximab induction chemotherapy followed by biochemoradiation with weekly cetuximab plus weekly cisplatin or carboplatin: a randomized phase II EORTC trial. Ann Oncol. (2017) 28:2219–24. doi: 10.1093/annonc/mdx300
16. Zenda S, Matsuura K, Tachibana H, Homma A, Kirita T, Monden N, et al. Multicenter phase II study of an opioid-based pain control program for head and neck cancer patients receiving chemoradiotherapy. Radiother Oncol. (2011) 101:410–4. doi: 10.1016/j.radonc.2011.09.016
17. Yokota T, Tachibana H, Konishi T, Yurikusa T, Hamauchi S, Sakai K, et al. Multicenter phase II study of an oral care program for patients with head and neck cancer receiving chemoradiotherapy. Supportive Care Cancer (2016) 24:3029–36. doi: 10.1007/s00520-016-3122-5
18. Zenda S, Ishi S, Akimoto T, Arahira S, Motegi A, Tahara M, et al. DeCoP, a Dermatitis Control Program using a moderately absorbent surgical pad for head and neck cancer patients receiving radiotherapy: a retrospective analysis. J Clin Oncol. (2015) 45:433–8. doi: 10.1093/jjco/hyv010
19. Zenda S, Ishi S, Kawashima M, Arahira S, Tahara M, Hayashi R, et al. A Dermatitis Control Program (DeCoP) for head and neck cancer patients receiving radiotherapy: a prospective phase II study. Int J Clin Oncol. (2013) 18:350–5. doi: 10.1007/s10147-012-0385-9
20. Zhu G, Lin JC, Kim SB, Bernier J, Agarwal JP, Vermorken JB, et al. Asian expert recommendation on management of skin and mucosal effects of radiation, with or without the addition of cetuximab or chemotherapy, in treatment of head and neck squamous cell carcinoma. BMC Cancer (2016) 16:42. doi: 10.1186/s12885-016-2073-z
21. Argiris A, Heron DE, Smith RP, Schrijvers DL, Bergamini C, Licitra LF, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. (2010) 28:5294–300. doi: 10.1200/JCO.2010.30.6423
22. Lefebvre JL, Ang KK. Larynx preservation clinical trial design: key issues and recommendations–a consensus panel summary. Head Neck (2009) 31:429–41. doi: 10.1002/hed.21081
23. Gorphe P, Matias M, Even C, Ferte C, Bidault F, Garcia G, et al. Laryngo-esophageal dysfunction-free survival in a preservation protocol for T3 laryngeal squamous-cell carcinoma. Anticancer Res. (2016) 36:6625–30. doi: 10.21873/anticanres.11269
24. Timme DW, Jonnalagadda S, Patel R, Rao K, Robbins KT. Treatment selection for T3/T4a laryngeal cancer: chemoradiation versus primary surgery. Ann Otol Rhinol Laryngol. (2015) 124:845–51. doi: 10.1177/0003489415588130
25. Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet (2019) 393:51–60. doi: 10.1016/S0140-6736(18)32752-1
26. Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet (2019) 393:40–50. doi: 10.1016/S0140-6736(18)32779-X
Keywords: head and neck cancer, induction chemotherapy, cetuximab, clinical trial, endpoint
Citation: Zenda S, Ota Y, Kiyota N, Okano S, Fujii M, Kitamura M, Takahashi S, Ueda T, Monden N, Yamanaka T and Tahara M (2019) A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab (TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR HN01: ECRIPS Study). Front. Oncol. 9:6. doi: 10.3389/fonc.2019.00006
Received: 01 November 2018; Accepted: 02 January 2019;
Published: 22 January 2019.
Edited by:
Athanassios Argiris, Thomas Jefferson University, United StatesReviewed by:
Jordi Giralt, Hospital Universitari Vall d'Hebron, SpainPanagiotis Balermpas, UniversitätsSpital Zürich, Switzerland
Copyright © 2019 Zenda, Ota, Kiyota, Okano, Fujii, Kitamura, Takahashi, Ueda, Monden, Yamanaka and Tahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadamoto Zenda, c3plbmRhQGVhc3QubmNjLmdvLmpw
Makoto Tahara, bWF0YWhhcmFAZWFzdC5uY2MuZ28uanA=
 Sadamoto Zenda
Sadamoto Zenda Yosuke Ota
Yosuke Ota Naomi Kiyota
Naomi Kiyota Susumu Okano
Susumu Okano Masato Fujii5
Masato Fujii5 Makoto Tahara
Makoto Tahara