- 1Department of Otorhinolaryngology, Head and Neck Surgery, Geneva University Hospital, Geneva, Switzerland
- 2Department of Otorhinolaryngology, Head and Neck Surgery, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 3Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Zurich, Zurich, Switzerland
- 4Department of Radiation Oncology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 5Department of Medical Oncology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 6Department of Medical Oncology, Hôpital Riviera-Chablais, Vevey, Switzerland
- 7Department of Radiation Oncology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 8Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital of Lausanne, Lausanne, Switzerland
- 9Department of Radiation Oncology, Cantonal Hospital Graubünden, Chur, Switzerland
- 10Department of Radiation Oncology, Cantonal Hospital of Winterthur, Winterthur, Switzerland
- 11Department of Radiation Oncology, Clinica Luganese SA, Lugano, Switzerland
- 12Department of Radiation Oncology, Cantonal Hospital Lucerne, Lucerne, Switzerland
- 13Department of Otorhinolaryngology, Head and Neck Surgery, Inselspital, Bern University Hospital, Bern, Switzerland
- 14Department of Medical Oncology, University Hospital of Basel, Basel, Switzerland
- 15Department of Otorhinolaryngology, Lindenhofspital, Bern, Switzerland
Background: The Head and Neck Cancer Working Group of Swiss Group for Clinical Cancer Research (SAKK) has investigated the level of consensus (LOC) and discrepancy in everyday practice of diagnosis and treatment in head and neck cancer.
Materials and Methods: An online survey was iteratively generated with 10 Swiss university and teaching hospitals. LOC below 50% was defined as no agreement, while higher LOC were arbitrarily categorized as low (51–74%), moderate (75–84%), and high (≥85%).
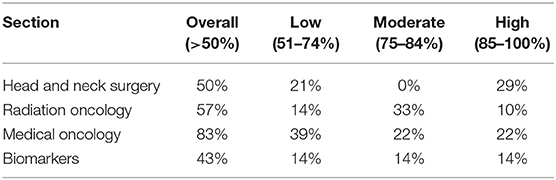
Results: Any LOC was achieved in 62% of topics (n = 60). High, moderate and low LOC were found in 18, 20, and 23%, respectively. Regarding Head and Neck Surgery, Radiation Oncology, Medical Oncology, and biomarkers, LOC was achieved in 50, 57, 83, and 43%, respectively.
Conclusions: Consensus on clinical topics is rather low for surgeons and radiation oncologists. The questions discussed might highlight discrepancies, stimulate standardization of practice, and prioritize topics for future clinical research.
Introduction
The cause of heterogeneity in the practice of diagnosis and treatment of head and neck squamous cell carcinoma (HNSCC) can be associated with multiple factors: differences in health care policies, financial and logistic factors, variations in tradition and medical culture between geographical areas, institutions, or even among physicians working in the same hospital. This heterogeneity in patterns of care is expected to be inversely correlated with the level of evidence on a given topic.
Literature on various aspects of management of HNSCC were previously published. Such reports usually focused on an anatomical site of the head and neck area (1, 2), a specific treatment approach in a clinical discipline (3–6), diagnostic modalities and strategies for diagnosis (7) and follow-up (8). Most of these survey-based studies were performed among institutions sharing the same geography or language.
The Head and Neck Cancer Working Group of Swiss Group for Clinical Cancer Research (SAKK) is a multidisciplinary collective of head and neck cancer specialists from many Swiss institutions meeting in regular intervals and collaborating in various projects. Due to the lack of a similar comprehensive work published so far, the group decided to perform a survey covering a broad spectrum of controversial topics concerning the diagnosis and the treatment of HNSCC among its member institutions.
This survey was designed to discuss current diagnostic and treatment strategies for HNSCC of all localizations undergoing within the Head and Neck Cancer Working Group of SAKK (multidisciplinary and multi-institutional) and to find out probable differences between the participating members/institutions in a pattern of care study.
Materials and Methods
In order to investigate the consensus and heterogeneity in the various aspects of diagnosis and treatment of HNSCC, an online survey via Surveymonkey® (San Mateo, CA) was generated and used by taking the following steps.
1) A steering committee of two head and neck surgeons, one medical oncologist and three radiation oncologists (P-M. P. serving as a consultant for methodology and technical issues) was founded to generate a questionnaire draft, evaluate the answers and writing the final manuscript.
2) Centers in which every patient diagnosed with a HNSCC is presented and discussed on a multidisciplinary tumor board on a regular basis, were defined and contacted through the member list of SAKK by email or phone and a local coordinator for each center was assigned. A rather balanced distribution of the specialists defined as local coordinators from the disciplines of head and neck surgery, medical oncology, and radiation oncology was encouraged. The responsibility of the local coordinator (e.g., the medical oncologist in a center) was to address the part of the questionnaire related to their specialty (medical oncology) and organize the information flow with her/his institutional colleagues from the remaining two major disciplines (head and neck surgery and radiation oncology). As a trade-off between being completely inclusive and realistically conducting the survey, specialists of the above-mentioned three disciplines were asked also to address the questions about imaging, pathology, and maxillo-facial surgery on behalf of the corresponding specialists of these disciplines.
3) The preliminary draft of the questionnaire was generated by the steering committee and sent to each local coordinator. Four categories were generated: head and neck surgery, radiation oncology, medical oncology, and biomarkers. Each center was asked to assign a numerical point for each question, proportionally reflecting its level of importance in the concerning category. Centers were also asked for feedback for any unclear questions, to suggest modifications and new questions.
4) After receiving feedbacks about the draft version, the questionnaire was finalized for improved wording as suggested and based on two criteria: (1) if a new question was suggested from more than one center in same or similar context, it was added to the final version, and (2) each category was limited with a maximal number of 20 questions, and questions with lower cumulative points were eliminated.
5) The final version (Supplementary Material) was transformed into an online survey and each center was asked to fill out the questionnaire. Each center is represented by a local coordinator as listed in the co-authors and their affiliations.
6) Answers were evaluated and discussed by the steering committee. Similar topics were grouped together.
7) For each question, an agreement per center is counted as 10 and a disagreement as 0, giving a minimal score of 0 and a maximal score of 100. Missing answers are indicated in the corresponding denominators. Level of consensus (LOC) was calculated by summing all center's answers and categorized as lack of LOC (0–50%), low LOC (51–74%), moderate (75–84%), and high (≥85%).
Results and Discussion
Ten centers participated in the survey. The survey was completed on 13 September 2017. Possible practice changes which may have occurred after this date were not reflected in this manuscript. Union for International Cancer Control (UICC) 7th edition (9) was used for discussions related to staging.
Some LOC was achieved in 62% of all topics of interest, while no LOC was found in 38% of questions. High, moderate and low LOC were 18, 20, and 23%, respectively. LOC in each section is summarized in Table 1.
Following section provides the results for the items concerning head and neck surgery discipline, each followed by a short discussion if deemed relevant.
Head and Neck Surgery
Diagnostic Measures
➢ Routine use of diagnostic panendoscopy: high LOC (100%).
During the diagnosis and baseline workup, all (10/10) centers routinely performed an endoscopy of the upper aerodigestive tract under general anesthesia to detect synchronous secondary malignancies. In one center, panendoscopy was not part of the routine workup for patients without history of tobacco or alcohol abuse.
The incidence of synchronous HNSCC around 5–6% (10, 11) is considered high enough to require a diagnostic panendoscopy. Usually, the second primary is of small size and thus curable. Hence, the diagnosis of synchronous lesions usually alters the therapeutic approach. Since 18F-fluorodeoxyglucose positron emission tomography combined with computerized tomography (18FDG-PET/CT) is often performed during the evaluation or treatment planning, some have suggested that a 18FDG-PET/CT scan could replace endoscopy (12). However, 18FDG-PET/CT will not detect small superficial lesions which are main focus of endoscopy (13, 14) and Swiss centers are unanimous in using panendoscopy during the initial evaluation. However, this practice can be questioned in non-smoker patients who are diagnosed with a Human Papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) due to the decreased rates of secondary malignancies (15–17).
➢ Routine diagnostic use of 18FDG-PET/CT to address loco-regional extension: low LOC (60%).
➢ Routine diagnostic use of 18FDG-PET/CT to address distant metastases or second primary tumors is preferred: high LOC (100%).
The use of 18FDG-PET/CT for the purpose of determining the extent of the loco-regional disease is being used in 6/10 centers. In all centers 18FDG-PET/CT was undergone to detect/rule out distant metastases or locate the primary tumor in the staging of a clinical carcinoma of unknown primary (CUP).
There is no high-level evidence for or against the value of the 18FDG-PET/CT for an accurate estimation of the extent of the disease, especially for the primary site. Since the gold standard is the assessment of the surgical specimen, a correlation between parameters such as dimensions, volume, depth, or involvement of critical structures obtained radiologically and pathologically is sought (18). Because of the distortions and shrinkage of surgical specimen, few studies have been undertaken especially for 18FDG-PET/CT. The available data for 18FDG-PET/CT is restricted to laryngo-hypopharyngeal primaries and is based on a total of 19 patients (19, 20): tumor volume estimation seems accurate but the superficial extension was inaccurate. While surgeons possibly have the direct estimation of the superficial spread to complement the radiologic findings, the widespread use of 18FDG-PET/CT on target volume delineation for radiation could be questioned.
In some series, the sensitivity of 18FDG-PET/CT is shown to be superior to CT and MRI for the identification of occult neck lymph node metastases (21). However, the sensibility of all techniques remains low in this setting, around 60% (22). 18FDG-PET/CT seems to accurately estimate volumes of metastatic neck lymph nodes (23), but adds marginal value to the information obtained from standard imaging modalities, such as CT or MRI in clinically N+ patients (24).
The role of 18FDG-PET/CT for the diagnosis of distant metastases seems more straightforward, but because of the low incidence of distant metastases from HNSCC at initial presentation, it should be restricted to advanced N stages. Furthermore, 18FDG-PET/CT is useful to diagnose synchronous cancers such as lung or abdominal primaries, although the superiority over chest CT has not been demonstrated (25).
For unknown primaries, the added diagnostic value of 18FDG-PET/CT in the pre-HPV era was about 20% (26), while small recent studies and imaging modalities might increase the yield to 50% (27).
Management of the Neck
➢Use of sentinel lymph node biopsy (SLNB) in cN0 oral cavity tumors: no consensus.
In cN0 oral cavity primaries, SLNB is performed only in 4/10 centers. The reasons not to perform the technique were not queried.
The only randomized prospective study in cN0 oral cavity management concluded that neck exploration during the initial treatment resulted in better overall and disease-free survival than observation followed by therapeutic neck dissection for nodal recurrences. This study validated elective neck dissection, not sentinel neck biopsy (28).
Proponents of SLNB in cN0 neck stress that many patients (70%) will have a non-metastatic neck and therefore will be overtreated by a surgery associated with a substantial morbidity. If this line of arguments is followed, omitting SLNB in oral cavity primaries could be seen as suboptimal surgical oncology management.
The arguments against a SLNB approach when comparing it to the traditional elective neck dissection include: (1) oncologic inferiority, (2) unavailability or unreliability of frozen sections in SLNB, (3) need of a second procedure in case of SLNB positivity, (4) technical challenges and learning curve of the procedure, (5) lack of conviction in the difference in morbidity between the two approaches. The arguments for a SLNB include (1) less invasive approach, (2) second stage completion neck dissection only necessary in the minority of patients (25–30%), (3) selective detection of the lymph nodes of highest risk to harbor metastatic disease, (4) the pathologic workup of sentinel lymph nodes allows for the detection of small metastatic disease such as isolated tumor cells and micrometastases rather than macrometastases only leading to a more accurate staging of the neck.
Because of the pathology processing, most pathologists are reluctant to recommend frozen sections in a sentinel lymph node approach. Since frozen section of a sentinel lymph node usually consists in the examination of a single section, several studies have found this technique is suboptimal or unreliable (29). The unavailability of frozen sections or their lack of reliability makes most centers use SLNB during one procedure, with a subsequent neck dissection performed during a second operation. If the initial panendoscopy is performed as a separate procedure, this could make three general anesthesia for the treatment of a T1 carcinoma.
An elective neck dissection approach with frozen sections of lymph nodes appearing suspicious during the procedure allows for definitive neck management by completing the dissection in the same surgical setting (therapeutic neck dissection) when frozen section yields occult nodal metastasis.
The advocates of SLNB consider that 20–50 cases are necessary during the learning phase of the technique, while most head and neck surgeons dealing with cancer are quite proficient in elective neck dissection. Other problems include the necessity of a nuclear medicine exam, the necessity of the surgeon to be available for the intraoral injection, the pain associated with the awake intraoral injection, and difficulties of scheduling an operating theater with a specific delay after the injection.
Beyond difficulties accepting new techniques, if the morbidity associated with elective supra-omohyoid selective neck dissection was considerable, oncologic head and neck surgeons would have had adopted SLNB readily. However, around half of the centers probably consider that convincing data of such superiority is lacking (30, 31). Probably the main advantage of SLNB is the more thorough pathologic examination of the lymph nodes most at risk, but the exact oncologic significance of micro-metastasis in HNSCC remains to be determined.
Whether, an N0 neck is treated by elective neck dissection or SLNB, follow-up is essential, especially for necks not requiring adjuvant therapy. Radiologic surveillance could be accomplished by various modalities (CT, MRI, and US) with US-FNAC being the most accurate and cost-effective (32, 33). This neck follow-up policy is valid in other situations where the primary is treated surgically and the neck not treated, for example an early laryngeal primary.
➢Standard use of any up-front neck dissection strategy for advanced neck stages: no consensus.
In the chemoradiotherapy (CRT) setting, 4/8 centers pursue a systematic elective neck dissection strategy. Three of those 4 perform an up-front neck dissection in case of a cN2/3 disease, whereas a planned neck dissection 8–12 weeks after CRT is preferred in the fourth center.
CRT has become the preferred strategy for pharyngeal (34) and laryngeal (35) primaries in some centers. Advanced stage disease is often associated with bulky (N3) or multiple (N2b/c, N3) neck lymph node metastasis and the optimal strategy to treat these metastatic neck diseases remains controversial. Possible strategies include: (1) up-front neck dissection before CRT; (2) planned neck dissection after CRT; or (3) radiologic surveillance. Several Swiss centers have pursued the up-front neck dissection since the 1990's (36, 37) and have not found convincing arguments to change their strategy (38). Until recently, the debate has been centered on whether a planned neck dissection after CRT is necessary and whether a post-treatment 18FDG-PET/CT scan can be used to select patients needing surgery. This has been settled in a randomized controlled trial showing that a post-treatment 18FDG-PET/CT scan would safely identify patients not requiring neck dissection after CRT (39). The question of up-front neck dissection vs. post-CRT treatment is the subject of an ongoing prospective multicenter study in Switzerland (NCT02918955).
➢Systematic division and reporting of lymph node levels after a neck dissection: no consensus.
When performing a neck dissection, 4/8 centers systematically mark the lymph node stations before sending the material to pathology.
Whether neck dissection is therapeutic (cN+) or elective (cN0), one of its main purposes is to determine which patients are candidates for adjuvant therapy (40). Since neck irradiation is no longer performed by lateral opposed fields but by intensity modulated radiotherapy optimized via inverse planning, precise knowledge of the metastatic groups is crucial to the radiation oncologist. The American Head & Neck Society recommends that neck contents should be divided into levels and sublevels by the surgeon in the operating room immediately after the specimen is removed, each level being placed into a separate container and labeled appropriately (41, 42). One possible exception to these guidelines is obtaining negative margins on bulky and obviously metastatic nodes, which might require keeping two or three adjacent levels together. Even a pathologist specialized in HNSCC has trouble deciding on the limits of individual groups without the orienting presence of the hyoid bone and of the cricoid cartilage, especially on a neck dissection specimen fixed in formalin.
➢Impact of depth of tongue infiltration on the decision to perform a neck dissection: no consensus.
For the carcinoma of the lateral side of the tongue, the depth of invasion does not influence the decision to perform a neck dissection in two centers. In five centers, 2–8 mm depth of invasion (mean and median 4 mm) would change the treatment strategy. Three centers did not provide any answer.
Convincing data on the relationship between tumor thickness and prognosis in oral cavity squamous cell carcinoma date back to the 1980's (43). Recently and after this questionnaire was completed, depth of invasion was incorporated in the T staging system for oral cavity carcinoma and validated in recent studies (44, 45). The treatment strategy, especially for neck management, should be more aggressive with depth of invasion >4 mm (44, 46).
Management of Bone and Peri-Neural Invasion
➢Adequate resection margin of mandible in case of bone invasion: no consensus.
In case the CT and/or MRI suggest a 2 cm long cortical defect on the body of the mandible with a 5 mm depth of invasion without any enhancement of the mandibular nerve, resection margins of 1, 2, and 3 centimeters would be used in 3, 2, and 1 centers, respectively. Four centers did not provide any answer.
Three decades ago, Slootweg and Muller (47) described two patterns of mandibular invasion: an “erosive pattern” carrying a good prognosis and associated with direct bone infiltration by the carcinoma, on a broad front, without infiltration of the periodontal ligament and of the inferior alveolar nerve. The “infiltrative pattern” carries a worse prognosis and histologically exhibits an aggressive invasion of mandibular cancellous marrow, periodontal ligament, as well as a frequent perineural invasion of the inferior alveolar nerve. Subsequent series (48, 49) have confirmed two- to three-fold higher recurrence rates and approximately halved survival in the infiltrative pattern of invasion. Furthermore, because cortical bone invasion does not carry a poor prognosis, it has been suggested that to stage it as T3 (50).
The literature rarely speaks of “erosive” and “infiltrative” pattern but often refers to cortical vs. marrow infiltration. Preoperative performance for mandibular marrow invasion of MRI carries a high sensitivity (95–100%) but a lower specificity (60–70%) (51, 52).
According to the Dutch Guidelines Database (53), in the erosive pattern a bony margin of 1 cm is sufficient, while the infiltrative pattern requires bony margins of 1.5 cm and invasion within the canal of the mandibular nerve 2 cm. While these recommendations are cited in the literature, their exact scientific foundation is unclear.
➢The indication to perform a mandibulectomy in case of mandibular nerve invasion: no consensus.
In case of an oral cavity tumor where the MRI suggests an enhancement of the mandibular nerve and the CT shows no erosion of the mandible, 2/8 centers would perform a mandibulectomy, whereas the rest would not or decide based on the intraoperative assessment.
Involvement of the inferior alveolar nerve is associated with a worse prognosis and requires more extensive resection (54). The question thus addresses the possibility of assessing perineural spread in mandibles with an intact bony cortex. Techniques derived from MR neurography using special sequences, such as 3D double-echo steady-state with water excitation have been shown to have high sensitivity (95–100%) for detection perineural spread (55, 56). While the radiological results have been pathologically validated for HNSCC in general, no publication has specifically targeted the inferior alveolar nerve.
Optimal Resection Margins
➢Adequate resection margin should be 5 mm in T1-2 oral cavity tumors: high LOC (89%).
In a T1-2 oral cavity tumor, the adequate resection margin was defined as 5 mm in 8 centers. For one center, it was defined as 10 mm. One center did not provide an answer.
A “sufficient” pathological margin implies a low risk for tumor recurrence and possibly makes adjuvant treatment redundant. However, this issue for oral squamous cell carcinoma is still a subject to debate. Combined analysis (57) of the EORTC 22931 (58) and the RTOG 9501 (59) trials concluded that the adverse prognostic factors requiring adjuvant CRT following surgical resection included extracapsular extension (ECE) of metastatic lymph nodes and positive margins. Somewhat provocative results were published from the Toronto group evaluating oral cavity pN0 patients with margins smaller than 5 mm, treated only surgically: negative margins of 1–5 mm were not associated with inferior local control; while tumor thickness, perineural invasion, and pattern of invasion were predictive of local recurrence (60). The data are in agreement with other studies, establishing pathological scores for resected oral squamous cell carcinoma (61). A review of the literature on the subject seems to confirm that most studies consider 5 mm as a negative margin (60), following the Guideline of the UK Royal College of Pathologists: >5 mm clear, 1–5 mm close, and <1 mm positive margin (62). This discussion pertains to margins assessed by the pathologist and given about 50% shrinkage of the specimen (63), resection should start about 10 mm from the tumor edge.
Treatment of Laryngo-Hypopharyngeal Primaries
➢The status of vocal cord mobility is a key criterion for primary treatment decision: low LOC (63%).
In glottic larynx cancer, vocal cord mobility affects the treatment decision in 5/8 centers.
The presence of vocal cord mobility indicates that there is probably an infiltration of the vocal muscle or in rare cases of the crico-arytenoid joint. This is a well-recognized adverse prognostic factor and has been incorporated in the TNM classification for glottic cancer since 1988: an otherwise T1 carcinoma would become a T2 in case of hypomobility, and T3 for complete immobility (64).
The main implication of vocal cord mobility impairment is that the tumor is much bulkier (65) and has extended laterally. Because of this, endoscopic surgery will be more extensive (66) and thus result in more important functional voice and swallowing impairment. Furthermore, especially for T3 cases, the resection might not be possible endoscopically and open partial laryngectomy might become the procedure of choice (67). Even if radiation is the chosen treatment modality, impaired vocal cord mobility carries the main adverse prognostic factor in T2 glottic cancers (68) and is associated with suboptimal cure rates (69).
Why vocal cord mobility does not bring a consensus higher than 63% is difficult to understand. Since vocal cord mobility clearly influences the surgical approach, only possibility is that in some centers, all low stage (T1–T2) carcinoma are treated with radiation therapy and surgeons do not see the mobility as a decisive factor.
➢Radiologic imaging is reliable to assess laryngeal cartilage invasion: high LOC (86%).
Radiologic imaging modalities are considered to be reliable to assess cartilage invasion of larynx cancer in 6/7 centers.
Cartilage invasion has a major impact in the optimal management of laryngeal cancer (see the following question). Cartilage invasion cannot be assessed clinically and therefore, a reliable diagnostic test is essential. The main options are CT and MRI.
It should be kept in mind that the gold standard of evaluating performance of radiological exams is definitive pathology and thus studies evaluating CT and MRI only include patients that underwent surgery which is often total laryngectomy. Thus, compared to the general population of patients with laryngeal cancer, cartilage invasion is probably over-represented, and this bias probably leads to an overestimated positive predictive value (PPV) and to an underestimated negative predictive value (NPV) for the diagnostic modality under investigation.
A recent meta-analysis of CT shows a prevalence of cartilage invasion between 19 and 27%, a PPV ranging between 44 and 80%, and relatively high NPVs ranging between 85 and 100% (70). In other words, false positive CT scans are frequent, while false negative CT scans infrequent and according to the authors, false negative cases stem from minor cartilage invasion, which might not be a contra-indication to conservative treatments, being CRT or partial laryngectomies. Similar results were found in classical studies on the subject (71). However, the performance of CT for extralaryngeal spread is insufficient with NPVs of only 71% (72), making CT not reliable for selecting patients for organ preservation strategies.
MRI can improve the NPVs of CT above 95% in experienced hands (18) and because of its excellent soft tissue evaluation, is the preferred evaluation method for extralaryngeal spread (73). The PPVs are however not better than CT.
➢To prioritize larynx preservation strategies in cT4a laryngeal primaries or not: no consensus.
The first choice of treatment in cT4a laryngeal primaries is always to pursue a larynx preservation strategy in one center. Four centers prefer CRT only if the cartilage is not destructed. Other five centers always prefer total laryngectomy followed by adjuvant treatment.
T4a laryngeal carcinoma by definition invades the cartilaginous framework of the larynx and remains best treated by a multimodality regimen, starting with total laryngectomy (74). This has been reemphasized in the recently updated guidelines from the American Society of Clinical Oncology: for “extensive T3 or large T4a lesions and/or poor pretreatment laryngeal function, better survival rates and quality of life may be achieved with total laryngectomy rather than with organ-preservation approaches and may be the preferred treatment strategy” (74).
The debate originated after the VA trial (75) demonstrating that some T4a larynx tumors could be preserved by a CRT protocol. However, in this study, 56% of T4a patients underwent total laryngectomy, especially in glottic primaries with cartilage invasion. Because of that, this population was specifically excluded from the RTOG 91–11 trial (76). This trial was based on the 5th UICC classification of 1992, and the change in the T3–T4 larynx T-staging introduced in the 6th UICC edition added to the confusion. Small inner cortex erosion was classified as T4a in the 5th edition and as T3 in the 6th edition. It is probably safe to say that present day T4 patients were not included in the RTOG 91–11 trial.
As discussed in detail in the guideline of the American Society of Clinical Oncology (74), several high-quality retrospectives studies (77–82) support the better survival of T4a laryngeal cancer patients treated with total laryngectomy, rather than CRT protocols.
➢The preferred treatment of cT1/2 hypopharyngeal cancer is non-surgical: low LOC (60%).
A cT1/2 hypopharyngeal primary is never treated surgically in 6/10 centers.
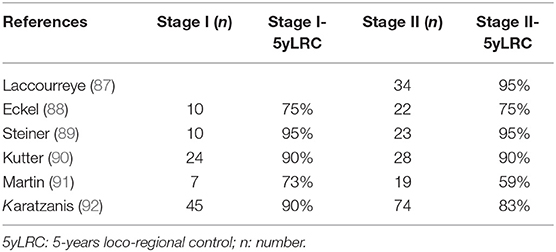
Hypopharyngeal primaries are associated with low survival (5-year overall survival about 30%) that has barely improved over the years (83). Radiotherapy or CRT are often considered as the standard treatment for hypopharyngeal primaries (84, 85), whereas surgical series with voice preservation are not new (86). No randomized trial has addressed early hypopharyngeal carcinoma. For early T1–T2 primaries, small series with surgical resection, often endoscopic and without adjuvant irradiation, provide encouraging results (Table 2).
Conclusion
The findings of our survey indicate a low LOC among head and neck oncologists working in academic and multidisciplinary setting in 10 Swiss institutions. Regarding the results and the discussion concerning the specialties other than head and neck surgery, the reader is advised to read the corresponding parts of this article. The highest LOC was achieved among medical oncologists, whereas the lowest was observed among head and neck surgeons. On the other hand, this level of disagreement may also depend on the topics chosen for the survey, and not necessarily the heterogeneity within the disciplines. It is also interesting to witness a low LOC regarding topics, where a high level of evidence actually does exist, and vice versa. This article is expected to serve the head and neck oncologists to be aware of their discrepancies and to stimulate discussion toward standardization of practice and prioritize topics of future clinical research.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
GH, MB, OE, PD, and PP: conception and design. OE and PP: collection of data. Generation of the initial and final versions of the questions, drafting of the manuscript, and approval of the final version by all co-authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank each of our colleagues working with the local coordinators for filling out the part of the questionnaire corresponding to their area of expertise in their institution.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01125/full#supplementary-material
References
1. Makki FM, Williams B, Rajaraman M, Hart RD, Trites J, Brown T, et al. Current practice patterns in the management of glottic cancer in Canada: results of a national survey. J Otolaryngol Head Neck Surg. (2011) 40:205–10. doi: 10.2310/7070.2011.100228
2. Müller von der Grün J, Bon D, Rödel C, Balermpas P. Patterns of care analysis for head & neck cancer of unknown primary site: a survey inside the German society of radiation oncology (DEGRO). Strahlenther Onkol. (2018) 194:750–8. doi: 10.1007/s00066-018-1308-0
3. Thariat J, Hamoir M, Garrel R, Cosmidis A, Dassonville O, Janot, et al. Management of the neck in the setting of definitive chemoradiation: is there a consensus? A GETTEC study. Ann Surg Oncol. (2012) 19:2311–9. doi: 10.1245/s10434-012-2275-9
4. Bisase B, Kerawala C, Skilbeck C, Spencer C. Current practice in management of the neck after chemoradiotherapy for patients with locally advanced oropharyngeal squamous cell carcinoma. Br J Oral Maxillofac Surg. (2013) 51:14–8. doi: 10.1016/j.bjoms.2012.02.017
5. Pettit L, Hartley A, Bowden SJ, Mehanna H, Glaholm J, Cashmore J, et al. Variation in volume definition between UK head and neck oncologists treating oropharyngeal carcinoma. Clin Oncol. (2011) 23:654–5. doi: 10.1016/j.clon.2011.07.006
6. Kansy K, Mueller AA, Mücke T, Koersgen F, Wolff KD, Zeilhofer HF, et al. Microsurgical reconstruction of the head and neck region: current concepts of maxillofacial surgery units worldwide. J Cranio Maxillofac Surg. (2015) 43:1364–8. doi: 10.1016/j.jcms.2015.06.034
7. Norling R, Grau C, Nielsen MB, Homøe P, Sørensen JA, Lambertsen K, et al. Radiological imaging of the neck for initial decision-making in oral squamous cell carcinomas–a questionnaire survey in the Nordic countries. Acta Oncol. (2012) 51:355–61. doi: 10.3109/0284186X.2011.640346
8. Madana J, Morand GB, Barona-Lleo L, Black MJ, Mlynarek AM, Hier MP. A survey on pulmonary screening practices among otolaryngology-head & neck surgeons across Canada in the post treatment surveillance of head and neck squamous cell carcinoma. J Otolaryngol Head Neck Surg. (2015) 44:1–5. doi: 10.1186/s40463-015-0057-7
9. Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer (2010).
10. Jain KS, Sikora AG, Baxi SS, Morris LGT. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. (2013) 119:1832–7. doi: 10.1002/cncr.27988
11. Haughey BH, Gates GA, Arfken CL, Harvey J. Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol. (1992) 101(2 Pt 1):105–12. doi: 10.1177/000348949210100201
12. Haerle SK, Strobel K, Hany TF, Sidler D, Stoeckli SJ. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck. (2010) 32:319–25. doi: 10.1002/hed.21184
13. Hanamoto A, Takenaka Y, Shimosegawa E, Ymamamoto Y, Yoshii T, Nakahara S, et al. Limitation of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography (FDG-PET) to detect early synchronous primary cancers in patients with untreated head and neck squamous cell cancer. Ann Nucl Med. (2013) 27:880–5. doi: 10.1007/s12149-013-0765-x
14. Suzuki H, Hasegawa Y, Terada A, Ogawa T, Hyodo I, Suzuki M, et al. Limitations of FDG-PET and FDG-PET with computed tomography for detecting synchronous cancer in pharyngeal cancer. Arch Otolaryngol Head Neck Surg. (2008) 134:1191–5. doi: 10.1001/archotol.134.11.1191
15. Martel M, Alemany L, Taberna M, Mena M, Tous S, Bagué S, et al. The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol. (2017) 64:37–43. doi: 10.1016/j.oraloncology.2016.11.011
16. Diaz DA, Reis IM, Weed DT, Elsayyad N, Samuels M, Abramowitz MC. Head and neck second primary cancer rates in the human papillomavirus era: a population-based analysis. Head Neck. (2016) 38(Suppl. 1):E873–83. doi: 10.1002/hed.24119
17. Morris LGT, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. (2011) 29:739–46. doi: 10.1200/JCO.2010.31.8311
18. Becker M, Zbären P, Casselman JW, Kohler R, Dulguerov P, Becker CD. Neoplastic invasion of laryngeal cartilage: reassessment of criteria for diagnosis at MR imaging. Radiology. (2008) 249:551–9. doi: 10.1148/radiol.2492072183
19. Caldas-Magalhaes J, Kasperts N, Kooij N, van den Berg CAT, Terhaard CHJ, Raaijmakers CPJ, et al. Validation of imaging with pathology in laryngeal cancer: accuracy of the registration methodology. Int J Radiat Oncol Biol Phys. (2012) 82:e289–98. doi: 10.1016/j.ijrobp.2011.05.004
20. Daisne J-F, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. (2004) 233:93–100. doi: 10.1148/radiol.2331030660
21. Ng S-H, Yen T-C, Chang JT-C, Chan S-C, Ko S-F, Wang H-M, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol. (2006) 24:4371–6. doi: 10.1200/JCO.2006.05.7349
22. Liao L-J, Lo W-C, Hsu W-L, Wang C-T, Lai M-S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer. (2012) 12:236. doi: 10.1186/1471-2407-12-236
23. Schinagl DAX, Span PN, van den Hoogen FJA, Merkx MAW, Slootweg PJ, Oyen WJG, et al. Pathology-based validation of FDG PET segmentation tools for volume assessment of lymph node metastases from head and neck cancer. Eur J Nucl Med Mol Imaging. (2013) 40:1828–35. doi: 10.1007/s00259-013-2513-9
24. Sohn B, Koh YW, Kang WJ, Lee JH, Shin NY, Kim J. Is there an additive value of 18 F-FDG PET-CT to CT/MRI for detecting nodal metastasis in oropharyngeal squamous cell carcinoma patients with palpably negative neck? Acta Radiol. (2016) 57:1352–9. doi: 10.1177/0284185115587544
25. Brouwer J, Senft A, de Bree R, Comans EFI, Golding RP, Castelijns JA, et al. Screening for distant metastases in patients with head and neck cancer: is there a role for (18)FDG-PET? Oral Oncol. (2006) 42:275–80. doi: 10.1016/j.oraloncology.2005.07.009
26. Strojan P, Ferlito A, Medina JE, Woolgar JA, Rinaldo A, Robbins KT, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck. (2013) 35:123–32. doi: 10.1002/hed.21898
27. Noij DP, Martens RM, Zwezerijnen B, Koopman T, de Bree R, Hoekstra OS, et al. Diagnostic value of diffusion-weighted imaging and 18F-FDG-PET/CT for the detection of unknown primary head and neck cancer in patients presenting with cervical metastasis. Eur J Radiol. (2018) 107:20–5. doi: 10.1016/j.ejrad.2018.08.009
28. D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. (2015) 373:521–9. doi: 10.1056/NEJMoa1506007
29. Vorburger MS, Broglie MA, Soltermann A, Haerle SK, Haile SR, Huber GF, et al. Validity of frozen section in sentinel lymph node biopsy for the staging in oral and oropharyngeal squamous cell carcinoma. J Surg Oncol. (2012) 106:816–9. doi: 10.1002/jso.23156
30. Schilling C, Shaw R, Schache A, McMahon J, Chegini S, Kerawala C, et al. Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? Br J Oral Maxillofac Surg. (2017) 55:757–62. doi: 10.1016/j.bjoms.2017.07.007
31. Yang Y, Zhou J, Wu H. Diagnostic value of sentinel lymph node biopsy for cT1/T2N0 tongue squamous cell carcinoma: a meta-analysis. Eur Arch Otorhinolaryngol. (2017) 274:3843–52. doi: 10.1007/s00405-017-4740-3
32. van den Brekel MW, Castelijns JA, Reitsma LC, Leemans CR, van der Waal I, Snow GB. Outcome of observing the N0 neck using ultrasonographic-guided cytology for follow-up. Arch Otolaryngol Head Neck Surg. (1999) 125:153–6. doi: 10.1001/archotol.125.2.153
33. de Bondt RBJ, Nelemans PJ, Hofman PAM, Casselman JW, Kremer B, van Engelshoven JMA, et al. Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol. (2007) 64:266–72. doi: 10.1016/j.ejrad.2007.02.037
34. Kuo P, Sosa JA, Burtness BA, Husain ZA, Mehra S, Roman SA, et al. Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: analysis of the National Cancer Data Base. Cancer. (2016) 122:1853–60. doi: 10.1002/cncr.29962
35. Timmermans AJ, van Dijk BAC, Overbeek LIH, van Velthuysen M-LF, van Tinteren H, Hilgers FJM, et al. Trends in treatment and survival for advanced laryngeal cancer: a 20-year population-based study in The Netherlands. Head Neck. (2016) 38(Suppl. 1):E1247–55. doi: 10.1002/hed.24200
36. Allal A, Dulguerov P, Bieri S, Lehmann W, Kurtz JM. A conservation approach to pharyngeal carcinoma with advanced neck disease: optimizing neck management. Head Neck. (1999) 21:217–22.
37. Elicin O, Albrecht T, Haynes AG, Bojaxhiu B, Nisa L, Caversaccio M, et al. Outcomes in advanced head and neck cancer treated with up-front neck dissection prior to (chemo)radiotherapy. Otolaryngol Head Neck Surg. (2016) 154:300–8. doi: 10.1177/0194599815608370
38. Elicin O, Nisa L, Dal Pra A, Bojaxhiu B, Caversaccio M, Schmücking M, et al. Up-front neck dissection followed by definitive (chemo)-radiotherapy in head and neck squamous cell carcinoma: Rationale, complications, toxicity rates, and oncological outcomes–A systematic review. Radiother Oncol. (2016) 119:185–93. doi: 10.1016/j.radonc.2016.03.003
39. Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. (2016) 374:1444–54. doi: 10.1056/NEJMoa1514493
40. National Comprehensive Cancer Network. National Comprehensive Cancer Network Guidelines for Head and Neck Cancers (Version 2.2018). (2018). p. 536–8.
41. Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. (2008) 134:536–8. doi: 10.1001/archotol.134.5.536
42. Miller MC, Goldenberg D, Education Committee of the American Head and Neck Society (AHNS). AHNS Series: do you know your guidelines? Principles of surgery for head and neck cancer: a review of the National Comprehensive Cancer Network guidelines. Head Neck. (2016) 36:1391. doi: 10.1002/hed.24654
43. Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. (1986) 152:345–50. doi: 10.1016/0002-9610(86)90302-8
44. Almangush A, Mäkitie AA, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, et al. Small oral tongue cancers. (≤ 4 cm in diameter) with clinically negative neck: from the 7th to the 8th edition of the American Joint Committee on Cancer. Virchows Arch. (2018) 473:481–7. doi: 10.1007/s00428-018-2417-y
45. Kano S, Sakashita T, Tsushima N, Mizumachi T, Nakazono A, Suzuki T, et al. Validation of the 8th edition of the AJCC/UICC TNM staging system for tongue squamous cell carcinoma. Int J Clin Oncol. (2018) 23:844–50. doi: 10.1007/s10147-018-1276-5
46. Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. (2009) 115:1489–97. doi: 10.1002/cncr.24161
47. Slootweg PJ, Müller H. Mandibular invasion by oral squamous cell carcinoma. J Craniomaxillofac Surg. (1989) 17:69–74. doi: 10.1016/S1010-5182(89)80048-4
48. Wong RJ, Keel SB, Glynn RJ, Varvares MA. Histological pattern of mandibular invasion by oral squamous cell carcinoma. Laryngoscope. (2000) 110:65–72. doi: 10.1097/00005537-200001000-00013
49. Shaw RJ, Brown JS, Woolgar JA, Lowe D, Rogers SN, Vaughan ED. The influence of the pattern of mandibular invasion on recurrence and survival in oral squamous cell carcinoma. Head Neck. (2004) 26:861–9. doi: 10.1002/hed.20036
50. Ebrahimi A, Murali R, Gao K, Elliott MS, Clark JR. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer. (2011) 117:4460–7. doi: 10.1002/cncr.26032
51. Chung TS, Yousem DM, Seigerman HM, Schlakman BN, Weinstein GS, Hayden RE. MR of mandibular invasion in patients with oral and oropharyngeal malignant neoplasms. AJNR Am J Neuroradiol. (1994) 15:1949–55.
52. Kim M, Higuchi T, Arisaka Y, Achmad A, Tokue A, Tominaga H, et al. Clinical significance of 18F-α-methyl tyrosine PET/CT for the detection of bone marrow invasion in patients with oral squamous cell carcinoma: comparison with 18F-FDG PET/CT and MRI. Ann Nucl Med. (2013) 27:423–30. doi: 10.1007/s12149-013-0701-0
53. Dutch Guidelines: Treatment of Oral Cavity Carcinoma. Available online at: https://richtlijnendatabase.nl/richtlijn/hoofd-halstumoren/behandeling_mondholtecarcinoom.html (accessed August 21, 2018).
54. Niu LX, Feng ZE, Wang DC, Zhang JY, Sun ZP, Guo CB. Prognostic factors in mandibular gingival squamous cell carcinoma: a 10-year retrospective study. Int J Oral Maxillofac Surg. (2017) 46:137–43. doi: 10.1016/j.ijom.2016.09.014
55. Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol. (2015) 59:578–85. doi: 10.1111/1754-9485.12338
56. Gandhi MR, Panizza B, Kennedy D. Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: comparing “targeted” MRI with the histologic findings following surgery. Head Neck. (2011) 33:469–75. doi: 10.1002/hed.21470
57. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. (2005) 27:843–50. doi: 10.1002/hed.20279
58. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre J-L, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. (2004) 350:1945–52. doi: 10.1056/NEJMoa032641
59. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. (2004) 350:1937–44. doi: 10.1056/NEJMoa032646
60. Ch'ng S, Corbett-Burns S, Stanton N, Gao K, Shannon K, Clifford A, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer. (2013) 119:2427–37. doi: 10.1002/cncr.28081
61. Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. (2005) 29:167–78. doi: 10.1097/01.pas.0000149687.90710.21
62. Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. (2005) 41:1034–43. doi: 10.1016/j.oraloncology.2005.06.008
63. Shah AK. Postoperative pathologic assessment of surgical margins in oral cancer: a contemporary review. J Oral Maxillofac Pathol. (2018) 22:78–85. doi: 10.4103/jomfp.JOMFP_185_16
64. Hermanek P, Scheibe O, Spiessl B, Wagner G. [TNM classification of malignant tumors: the new 1987 edition]. Rontgenblatter. (1987) 40:200. doi: 10.1055/s-2007-1020216
65. Kocatürk S, Han U, Yilmazer D, Onal B, Erkam U. A hystopathological study of thyroarytenoid muscle invasion in early. (T1) glottic carcinoma. Otolaryngol Head Neck Surg. (2005) 132:581–3. doi: 10.1016/j.otohns.2004.09.133
66. Peretti G, Piazza C, Mensi MC, Magnoni L, Bolzoni A. Endoscopic treatment of cT2 glottic carcinoma: prognostic impact of different pT subcategories. Ann Otol Rhinol Laryngol. (2005) 114:579–86. doi: 10.1177/000348940511400801
67. Chevalier D, Laccourreye O, Brasnu D, Laccourreye H, Piquet JJ. Cricohyoidoepiglottopexy for glottic carcinoma with fixation or impaired motion of the true vocal cord: 5-year oncologic results with 112 patients. Ann Otol Rhinol Laryngol. (1997) 106:364–9. doi: 10.1177/000348949710600502
68. McCoul ED, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch Otolaryngol Head Neck Surg. (2009) 135:479–86. doi: 10.1001/archoto.2009.47
69. Bhateja P, Ward MC, Hunter GH, Greskovich JF, Reddy CA, Nwizu TI, et al. Impaired vocal cord mobility in T2N0 glottic carcinoma: suboptimal local control with radiation alone. Head Neck. (2016) 38:1832–6. doi: 10.1002/hed.24520
70. Adolphs APJ, Boersma NA, Diemel BDM, Eding JEC, Flokstra FE, Wegner I, et al. A systematic review of computed tomography detection of cartilage invasion in laryngeal carcinoma. Laryngoscope. (2015) 125:1650–5. doi: 10.1002/lary.25145
71. Becker M, Zbären P, Laeng H, Stoupis C, Porcellini B, Vock P. Neoplastic invasion of the laryngeal cartilage: comparison of MR imaging and CT with histopathologic correlation. Radiology. (1995) 194:661–9. doi: 10.1148/radiology.194.3.7862960
72. Beitler JJ, Muller S, Grist WJ, Corey A, Klein AM, Johns MM, et al. Prognostic accuracy of computed tomography findings for patients with laryngeal cancer undergoing laryngectomy. J Clin Oncol. (2010) 28:2318–22. doi: 10.1200/JCO.2009.24.7544
73. Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol. (2008) 66:460–79. doi: 10.1016/j.ejrad.2008.03.027
74. Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. (2017) 24:JCO2017757385. doi: 10.1200/JCO.2017.75.7385
75. Wolf GT. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. (1991) 324:1685–90. doi: 10.1056/NEJM199106133242402
76. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. (2003) 349:2091–8. doi: 10.1056/NEJMoa031317
77. Rosenthal DI, Mohamed ASR, Weber RS, Garden AS, Sevak PR, Kies MS, et al. Long-term outcomes after surgical or nonsurgical initial therapy for patients with T4 squamous cell carcinoma of the larynx: a 3-decade survey. Cancer. (2015) 121:1608–19. doi: 10.1002/cncr.29241
78. Vengalil S, Giuliani ME, Huang SH, McNiven A, Song Y, Xu W, et al. Clinical outcomes in patients with T4 laryngeal cancer treated with primary radiotherapy versus primary laryngectomy. Head Neck. (2016) 38(Suppl. 1):E2035–40. doi: 10.1002/hed.24374
79. Gourin CG, Conger BT, Sheils WC, Bilodeau PA, Coleman TA, Porubsky ES. The effect of treatment on survival in patients with advanced laryngeal carcinoma. Laryngoscope. (2009) 119:1312–7. doi: 10.1002/lary.20477
80. Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. (2014) 140:855–60. doi: 10.1001/jamaoto.2014.1671
81. Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. (2007) 133:1270–6. doi: 10.1001/archotol.133.12.1270
82. Grover S, Swisher-McClure S, Mitra N, Li J, Cohen RB, Ahn PH, et al. Total laryngectomy versus larynx preservation for T4a larynx cancer: patterns of care and survival outcomes. Int J Radiat Oncol Biol Phys. (2015) 92:594–601. doi: 10.1016/j.ijrobp.2015.03.004
83. Petersen JF, Timmermans AJ, van Dijk BAC, Overbeek LIH, Smit LA, Hilgers FJM, et al. Trends in treatment, incidence and survival of hypopharynx cancer: a 20-year population-based study in the Netherlands. Eur Arch Otorhinolaryngol. (2018) 275:181–9. doi: 10.1007/s00405-017-4766-6
84. Lefebvre J-LL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. (1996) 88:890–9. doi: 10.1093/jnci/88.13.890
85. Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope. (2014) 124:2064–9. doi: 10.1002/lary.24651
86. Freeman RB, Marks JE, Ogura JH. Voice preservation in treatment of carcinoma of the pyriform sinus. Laryngoscope. (1979) 89:1855–63. doi: 10.1288/00005537-197911000-00021
87. Laccourreye O, Mérite-Drancy A, Brasnu D, Chabardes E, Cauchois R, Ménard M, et al. Supracricoid hemilaryngopharyngectomy in selected pyriform sinus carcinoma staged as T2. Laryngoscope. (1993) 103:1373–9. doi: 10.1288/00005537-199312000-00010
88. Eckel HE, Staar S, Volling P, Sittel C, Damm M, Jungehuelsing M. Surgical treatment for hypopharynx carcinoma: feasibility, mortality, and results. Otolaryngol Head Neck Surg. (2001) 124:561–9. doi: 10.1067/mhn.2001.115060
89. Steiner W, Ambrosch P, Hess CF, Kron M. Organ preservation by transoral laser microsurgery in piriform sinus carcinoma. Otolaryngol Head Neck Surg. (2001) 124:58–67. doi: 10.1067/mhn.2001.111597
90. Kutter J, Lang F, Monnier P, Pasche P. Transoral laser surgery for pharyngeal and pharyngolaryngeal carcinomas. Arch Otolaryngol Head Neck Surg. (2007) 133:139–44. doi: 10.1001/archotol.133.2.139
91. Martin A, Jäckel MC, Christiansen H, Mahmoodzada M, Kron M, Steiner W. Organ preserving transoral laser microsurgery for cancer of the hypopharynx. Laryngoscope. (2008) 118:398–402. doi: 10.1097/MLG.0b013e31815aeda3
Keywords: consensus, head and neck cancer, patterns of care, practice patterns, survey
Citation: Dulguerov P, Broglie MA, Henke G, Siano M, Putora PM, Simon C, Zwahlen D, Huber GF, Ballerini G, Beffa L, Giger R, Rothschild S, Negri SV and Elicin O (2019) A Review of Controversial Issues in the Management of Head and Neck Cancer: A Swiss Multidisciplinary and Multi-Institutional Patterns of Care Study—Part 1 (Head and Neck Surgery). Front. Oncol. 9:1125. doi: 10.3389/fonc.2019.01125
Received: 26 April 2019; Accepted: 09 October 2019;
Published: 24 October 2019.
Edited by:
Jeroen Meulemans, University Hospitals Leuven, BelgiumReviewed by:
Alberto Deganello, University of Brescia, ItalyPietro Perotti, Ospedale Santa Chiara, Italy
Copyright © 2019 Dulguerov, Broglie, Henke, Siano, Putora, Simon, Zwahlen, Huber, Ballerini, Beffa, Giger, Rothschild, Negri and Elicin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olgun Elicin, b2xndW4uZWxpY2luQGluc2VsLmNo
 Pavel Dulguerov
Pavel Dulguerov Martina A. Broglie
Martina A. Broglie Guido Henke
Guido Henke Marco Siano
Marco Siano Paul Martin Putora4,7
Paul Martin Putora4,7 Christian Simon
Christian Simon Daniel Zwahlen
Daniel Zwahlen Roland Giger
Roland Giger Sandro V. Negri
Sandro V. Negri Olgun Elicin
Olgun Elicin